Introduction
The skin is composed of two layers: the epidermis
and the dermis. Mesenchymal cells, known as dermal fibroblasts,
reside within the dermis and generate the major components of the
extracellular matrix (ECM), which organizes the dermal layer
(1–3). Dermal fibroblasts have been reported
to play a key role in controlling skin physiology, and their
proliferation is also crucial for skin structural homeostasis and
wound healing (4–6).
The epidermal growth factor receptor (EGFR), a
glycoprotein on the cell surface membrane, acts as a signaling
initiator. EGFR is composed of an extracellular receptor domain, an
intracellular domain, and a transmembrane region. The intracellular
domain acts as a tyrosine kinase, and activated EGFR initiates
signal transduction pathways that regulate cellular proliferation,
differentiation and survival (7,8).
EGFR is bound by epidermal growth factor (EGF), a small peptide,
which stimulates EGFR to induce cell proliferation and migration
(2,8,9).
As a cellular signal transduction molecule,
phytosphingosine-1-phosphate (PhS1P) is not abundant in animals.
However, previous studies suggest that it is highly expressed in
plants and fungi (3). PhS1P is
generated by sphingosine kinase through phosphorylation of
phytosphingosine. PhS1P has been shown to have a high affinity for
the sphingosine-1-phosphate (S1P) receptor and shows structural
similarity to S1P (3).
The aim of the present study was to ascertain
whether PhS1P and EGF display synergistic effects, using in
vitro assays and an in vivo clinical trial. Our results
demonstrated that treatment with PhS1P and EGF induced an
anti-aging effect in cell culture, as well as in study subjects,
further suggesting that PhS1P holds promise as a potential
anti-aging cosmetic ingredient.
Materials and methods
Cell culture
Human dermal fibroblasts (HDFs; Lonza, Basel,
Switzerland) were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco/Life Technologies, Carlsbad, CA, USA), supplemented
with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO,
USA) and 1% penicillin/streptomycin (Gibco/Life Technologies) at
37°C in an atmosphere of 5% CO2. PhS1P (Avanti Polar
Lipids, Alabaster, AL, USA) and EGF (R&D Systems, Minneapolis,
MN, USA) were dissolved in dimethyl sulfoxide (DMSO).
Cell viability assay
HDFs were seeded at a density of 3×103
cells/well in 96-well plates and incubated for 24 h. The cells were
then either left untreated, pretreated with a range of PhS1P
concentrations (0–5 µM), or pretreated with both PhS1P (5
µM) and EGF (10 ng/ml) for 3 h, prior to irradiation with
UVB (50 mJ/cm2). UVB irradiated cells were incubated for
24 h, and cytotoxicity was evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, MTT tetrazolium salt (0.5 mg/ml; Sigma-Aldrich) was
added to the cells for 4 h. The medium was then replaced with DMSO,
and the absorbance of each sample was measured at 595 nm using a
plate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Western blotting
Western blotting was performed as described in our
previous study (2). Cells were
collected and washed with cold phosphate-buffered saline (PBS). The
cell pellets were lysed using modified radioimmunoprecipitation
assay (RIPA) buffer [50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40,
0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS)] containing
protease inhibitors (Complete Tablets, Mini, EDTA-free, EASYpack;
Roche Applied Science, Mannheim, Germany) at 4°C for 20 min. The
lysates were then centrifuged at 12,000 × g for 30 min, and the
supernatant was decanted and saved. The concentration of total
protein was determined using the Bradford assay (Bio-Rad
Laboratories), and 50 µg of total cellular protein was
solubilized in SDS sample buffer and resolved by SDS-PAGE. Anti-ERK
(M5670), anti-p-ERK (M9692), anti-AKT (SAB4500797) and anti-p-AKT
(SAB4504332) primary antibodies for immunoblotting were purchased
from Sigma-Aldrich.
Isolation of total RNA and quantitative
(real-time) polymerase chain reaction (qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) as previously described (10), and RNA purity and concentration
were evaluated using MaestroNano® microspectrophotometer
(Maestrogen, Las Vegas, NV, USA). Equal amounts of RNA were used
for cDNA synthesis, and this was then used as a PCR template to
examine the expression of EGFR, matrix metalloproteinase 1 (MMP1),
and COL1A1. PCR primers were designed by Primer3 software
(http://frodo.wi.mit.edu) (EGFR forward,
5′-CAGCGCTACCTTGTCATTCA-3′ and reverse, 5′-TGCACTCAGAGACCTCAGGA-3′;
MMP1 forward, 5′-GGTCTCTGAGGGTCAAGCAG-3′ and reverse,
5′-AGTTCATGGCTGCAACACG-3′; β-actin forward,
5′-GGATTCCTATGTGGGCGACGA-3′ and reverse,
5′-CGCTCGGTGAGGATCTTCATG-3′); and qPCR was performed using EvaGreen
dye (Solis BioDyne, Tartu, Estonia) and Line-Gene K software
(Bioer, Hangzhou, China). The Ct value for each gene was normalized
to that of β-actin.
Subjects for clinical evaluation
The study protocol was approved by the Institutional
Review Board of the Korea Institute for Skin and Clinical Sciences
(KISCS) Incorporated. Forty women over the age of 35 were
registered in a randomized, double-blind clinical trial (control
group: 43.85±5.07 years; experimental group: 46.40±5.75 years). The
subjects were selected based on age and the absence of skin
conditions other than those determined to be age-related; none were
pregnant or nursing. All subjects were informed about the objective
of the study, provided signed informed consent, and agreed to use
only study-associated products for skin care during the duration of
the study. Exclusion criteria included itching, erythema, or
excessive drinking or smoking. Subjects were divided into a control
and an experimental group consisting of 20 subjects each. All
conditions were identical for both groups, other than the exposure
of the experimental group to the test material. The study lasted
for 6 weeks, with none of the study subjects dropping out. Clinical
parameters were evaluated three times, namely, before use, and
after 3 and 6 weeks of use. At each evaluation, the investigator
asked subjects about the condition of their skin and performed a
visual examination of their skin condition, to assess any erythema,
itching, scaling, edema, tingling, and burning sensation. The cream
given to the experimental group contained 30 ppm PhS1P and 2 ppm
EGF, whereas the cream given to the control group was prepared
using the same volume of water in place of PhS1P and EGF.
Experimental procedures
To investigate the improvement in skin barrier
function, subjects were instructed to apply 2 g of the test
material to the face every morning and night for 4 weeks. Subjects
and investigators were blinded as to which group received the
experimental and control treatments. Moisture and transepidermal
water loss (TEWL) were measured on the right cheek, and skin
texture was evaluated on the left side of the forehead. All
subjects washed with the cleanser provided and lay quietly in a
room with constant temperature (22±1°C) and humidity (45±5%) before
assessments were performed, so that they all would be evaluated
under the same conditions.
Evaluation of skin moisture
To evaluate improvement in skin moisture, the
DermaLab USB moisture probe (Cortex Technology, Inc., Hadsund,
Denmark) was applied, and data were analyzed using the associated
application software, version 1.09. All subjects were measured on
the same region of the right cheek, five consecutive times, and the
mean, maximum, and minimum values were determined. Measurements
were taken three times, namely, before application, and after 3 and
6 weeks of use. The device applies the conductance measurement
principle to measure the water-binding capacity of the stratum
corneum; this conductance value correlates with skin moisture and
is expressed in microsiemens (µS).
Measurement of dermal density and
thickness
To evaluate dermal density, a DUB skin scanner (tpm
taberna pro medicum, Lueneburg, Germany) was used. Dermal density
was measured 3 cm from the left eye, applying the couplant for
ultrasonic examination. The analysis range was limited to the area
between the dermis and upper panniculus. The measurements were
taken three times, namely, before application, and after 3 and 6
weeks of use.
Measurement of length and evenness of
crow's feet
Clinical images for the measurement of facial skin
evenness were obtained by a PRIMOS Lite (field of view 45×30;
GFMesstechnik GmbH, Teltow, Germany), and captured images were
analyzed using the associated imaging software, PRIMOS Lite,
version 5.6E. Three consecutive clinical images of the subject's
crow's feet were captured. Facial skin roughness was assessed based
on the Ra value, which is the average of all heights and depths
relative to the reference plane. The Ra value is the most widely
used parameter of facial skin roughness and is the arithmetic mean
of the maximum values of all measurements. To evaluate improvement
in wrinkles, especially at the eye rim, a Robo skin analyzer CS50
(Inforward Inc., Tokyo, Japan) was used. The facial images were
captured from each subject under identical positions, and with
equal lighting, on the front, left, and right sides of the face. To
evaluate improvement, measurements were taken three times, namely,
before application, and after 3 and 6 weeks of use. We analyzed the
captured images, matching the facial feature points accurately, and
the unit of measurement for crow's feet length was mm.
Measurement of skin elasticity
To evaluate improvement in skin elasticity, a
DermaLab USB elasticity probe (Cortex Technology, Inc.) was
applied, and the results were analyzed using the associated
application software, version 1.09. Measurements were obtained
using a fixed elasticity probe on the left cheek of each subject.
To analyze the elasticity measurements, Young's modulus (E) was
calculated as a dose-dependent representation of skin elasticity.
The measurements of elasticity were taken three times, namely,
before application, and after 3 and 6 weeks of use. The unit of
measurement was MPa.
Statistical and mathematical
analysis
In the cellular efficacy tests, all results are
presented as the mean percentage ± standard deviation (SD) of three
independent experiments. Differences with a P-value <0.05 or
0.001, as determined by Student's t-test, were considered
statistically significant. In clinical efficacy tests, statistical
analyses were conducted using SPSS software (SPSS, version 17.0 for
Windows; IBM, Armonk, NY, USA). Paired t-tests were performed in
cases of repeated measurements on the same subject. To analyze
subject questionnaires, the mean values, standard deviation, and
percentages were used. The formula used to measure the percent
change for each skin parameter was 'Percent change = [(A − B)/B]
×100', where A is defined as the individual value of any parameter
at the 3- and 6-week visits, and B represents the zero hour of the
assessed parameter.
Results and Discussion
PhS1P induces HDF proliferation and acts
as a cognate ligand for EGFR
The PhS1P molecule is structurally similar to S1P,
suggesting they perform similar functions. S1P is known as a signal
transduction molecule that induces cellular proliferation (11). We therefore, aimed to determine
whether PhS1P would function similarly to induce the proliferation
of HDFs. We first assessed the cytotoxicity of this molecule by
treating HDFs with various PhS1P concentrations, ranging from 0 to
5 µM, for 24 h. Cell viability was then measured using the
MTT assay. Importantly, we observed that cell viability increased
gradually with the increased dosage of PhS1P, up to a 23% increase
at 5 µM PhS1P (Fig. 1A),
suggesting that PhS1P induced HDF proliferation. Subsequently, in
order to determine whether PhS1P affects EGFR expression, EGFR mRNA
levels were measured in cells treated with PhS1P using qPCR. We
observed that the relative expression of EGFR mRNA was increased
2-fold when cells were treated with 2.5 µM PhS1P and
4.5-fold when they were treated with 5 µM PhS1P (Fig. 1B). These results revealed that
PhS1P induced EGFR mRNA expression in HDFs, and demonstrated that
this molecule has properties that suggest it may act as a ligand
for EGFR.
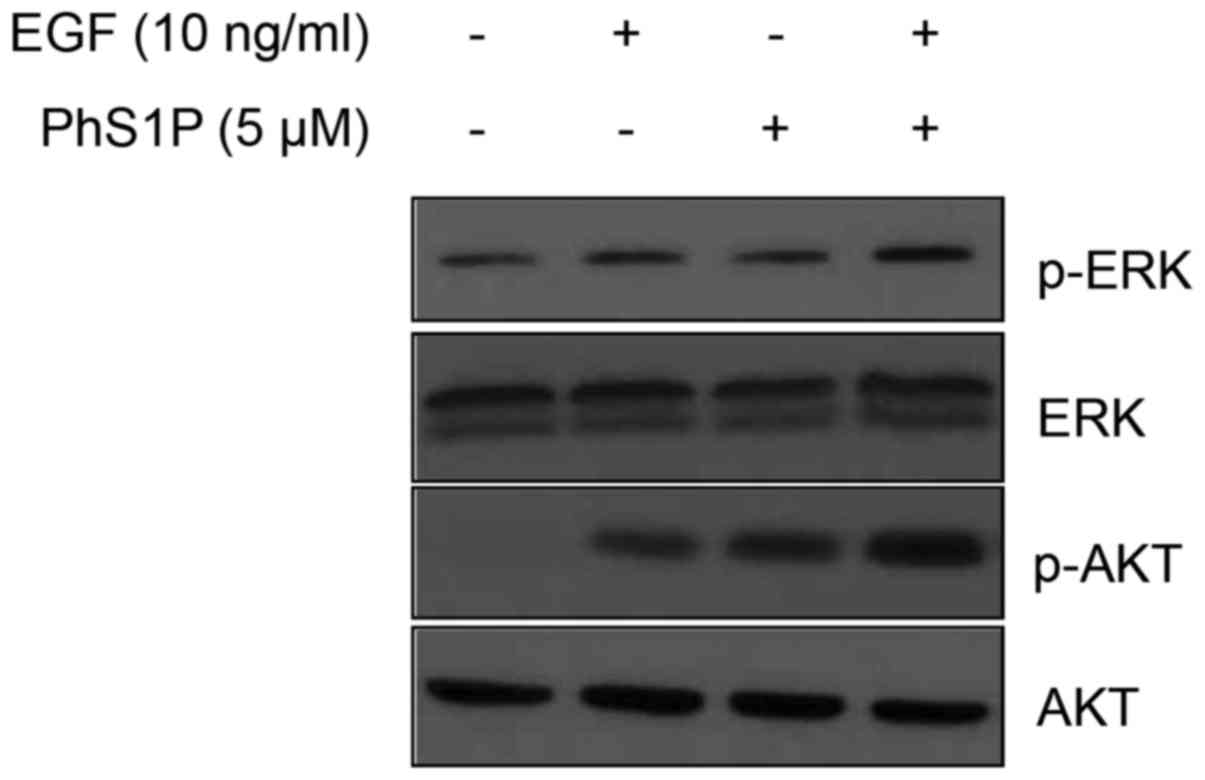
Co-treatment with PhS1P and EGF induces
phosphorylation of ERK and AKT in HDFs
The binding of EGF to EGFR activates downstream
signaling proteins, such as ERK and AKT, by phosphorylation
(12). Furthermore, S1P, a
molecule that has structural and functional similarity to PhS1P,
also induces cellular proliferation and growth through the ERK and
AKT pathways (13), suggesting
that PhS1P may have a similar function. To test this, HDFs were
treated with PhS1P (5 µM) and EGF (10 ng/ml), alone and in
combination, and the protein levels of ERK, phospho-ERK (p-ERK),
AKT, and phospho-AKT (p-AKT) were measured using western blotting.
Treatment with 10 ng/ml EGF or 5 µM PhS1P induced
phosphorylation of ERK and AKT (Fig.
2). Importantly, in cells co-treated with PhS1P and EGF, the
p-AKT and p-ERK levels were significantly higher than these levels
in the cells treated with either molecule alone. These results
suggest that PhS1P exerts a synergistic effect on EGF-induced
expression of p-ERK and p-AKT, and may function to induce cellular
proliferation through these pathways.
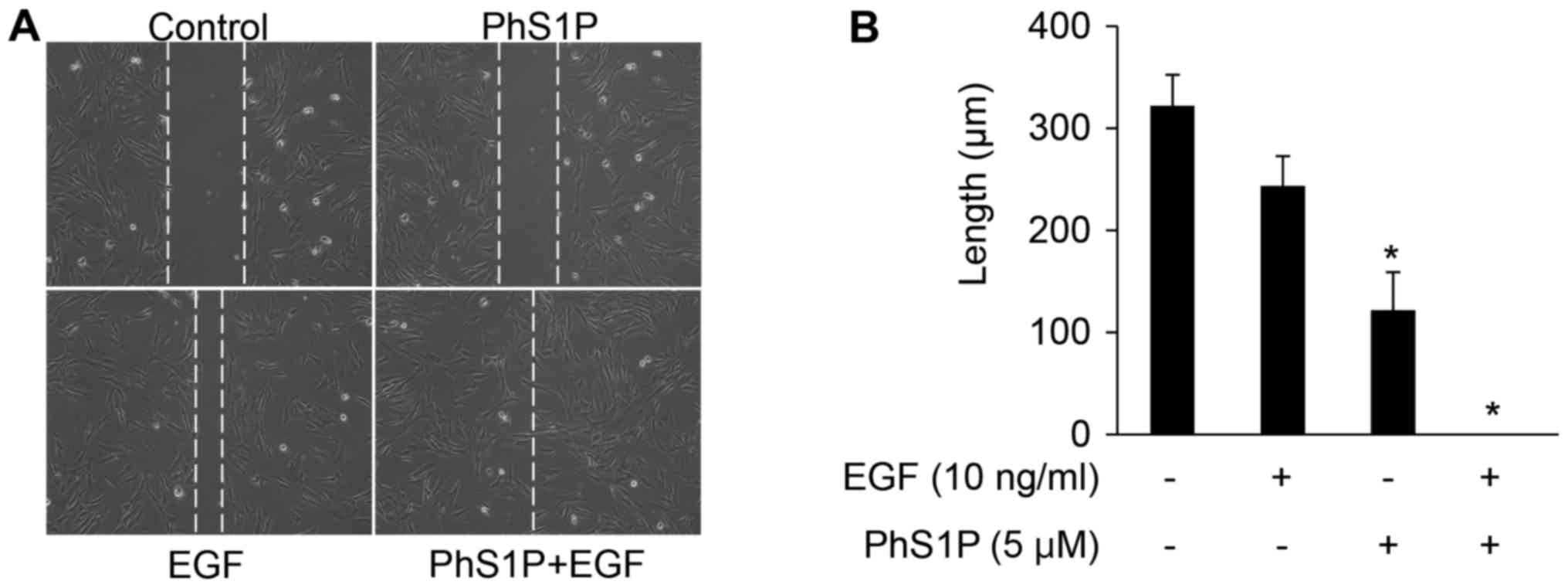
Treatment with PhS1P and EGF promotes
wound healing in a scratch plate assay
Both the proliferation and migration of HDFs are
essential for cutaneous wound healing (14), and treatment with a combination of
EGF and EGFR activates the AKT and ERK signaling pathways, inducing
cellular migration (12,15). Here, we observed that co-treatment
with EGF and PhS1P also significantly increased AKT and ERK
phosphorylation in HDFs (Fig. 2).
In order to determine whether the enhanced phosphorylation of AKT
and ERK observed in cells treated with EGF and PhS1P affects HDF
migration, we utilized a scratch wound healing assay. As shown in
Fig. 3A and B, the wound distance
was decreased in the HDFs treated with either PhS1P or EGF.
Additionally, when cells were co-treated with PhS1P and EGF, the
wound distance decreased further, as compared to the HDFs treated
with either molecule alone (Fig. 3A
and B). These results demonstrated that PhS1P induced cellular
migration in HDFs, and did so more efficiently in combination with
EGF.
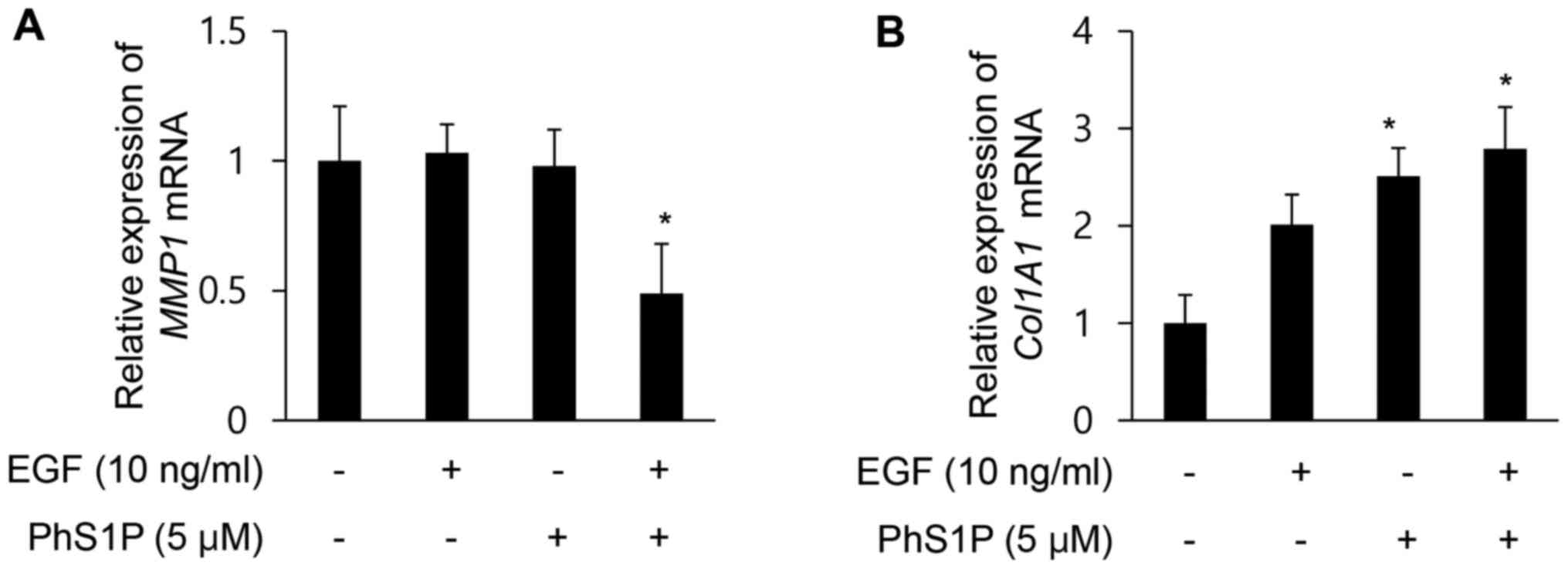
PhS1P promotes EGF-induced ECM-related
gene expression
Fibroblasts can alter the ECM by degrading fibrin
and promoting the production of collagen (16,17) and components of the ECM,
particularly collagen I and III, are essential for wound healing
(17,18). Collagen is the major structural
component of dermal tissue, and therefore, structural changes in
this molecule result in typical signs of aging, such as wrinkling
(19,20). One member of the MMP family, MMP1
(collagenase-1), is generated in fibroblasts and is also capable of
degrading type I and III fibrillar collagen (21). Members of the MMP family have also
been shown to be capable of degrading all components of the ECM,
including collagen, elastin, laminin, proteoglycans, and
fibronectin (21,22). To investigate the effects of PhS1P
on the expression of MMP1 and COL1A1, qPCR was performed on HDFs
treated with EGF (10 ng/ml) and/or PhS1P (5 µM). We observed
that the relative expression of MMP1 decreased 0.49-fold in the
HDFs treated with both PhS1P and EGF (Fig. 4A). Conversely, treatment with both
molecules increased COL1A1 mRNA levels 2.79-fold (Fig. 4B). These results demonstrated that
PhS1P promoted EGF-induced COL1A1 mRNA expression, and together,
these molecules suppressed MMP1 expression, two genes that regulate
the ECM and influence skin elasticity.
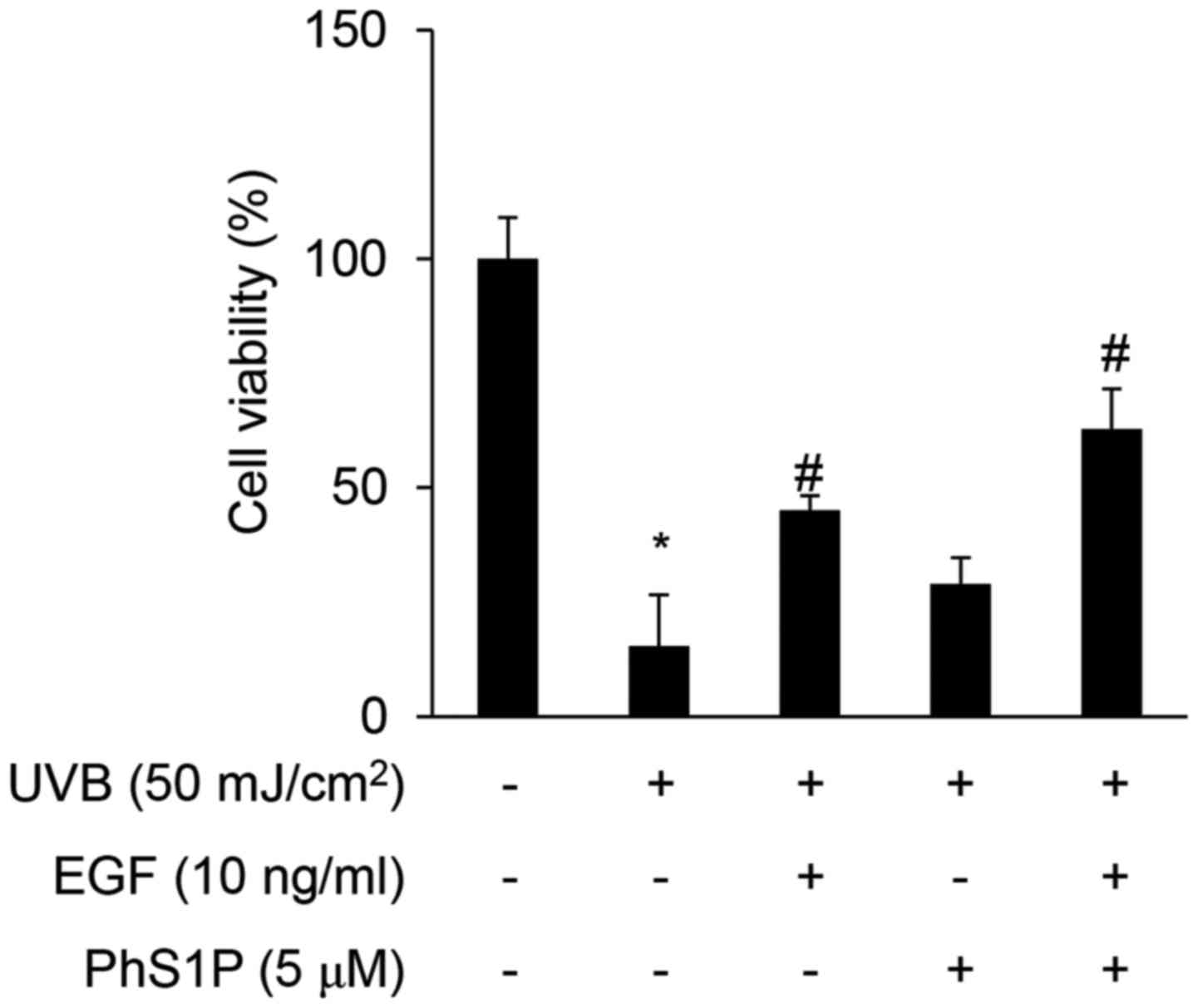
PhS1P protects against UVB-induced
decreased cell viability and stimulates EGF-induced cell
viability
Ultraviolet (UV) radiation is a well-characterized
mutagen that induces both skin aging and cancer. Here, we showed
that PhS1P stimulated cell migration and promoted the expression of
cellular proliferation markers. We, therefore, aimed to determine
whether PhS1P can protect HDFs from UVB-induced damage. HDFs were
irradiated with UVB at 50 mJ/cm2, and under these
conditions, only 15.37% of the cells remained viable. However, the
cells treated with either EGF or PhS1P prior to UVB irradiation
retained a viability of 45.12 and 28.73%, respectively. Notably,
HDFs co-treated with both PhS1P and EGF before UVB irradiation
maintained a cell viability of 62.78% (Fig. 5). These data suggest that PhS1P
protects against UVB-induced decreased cell viability, and further
indicate that its protective ability is increased in the presence
of EGF.
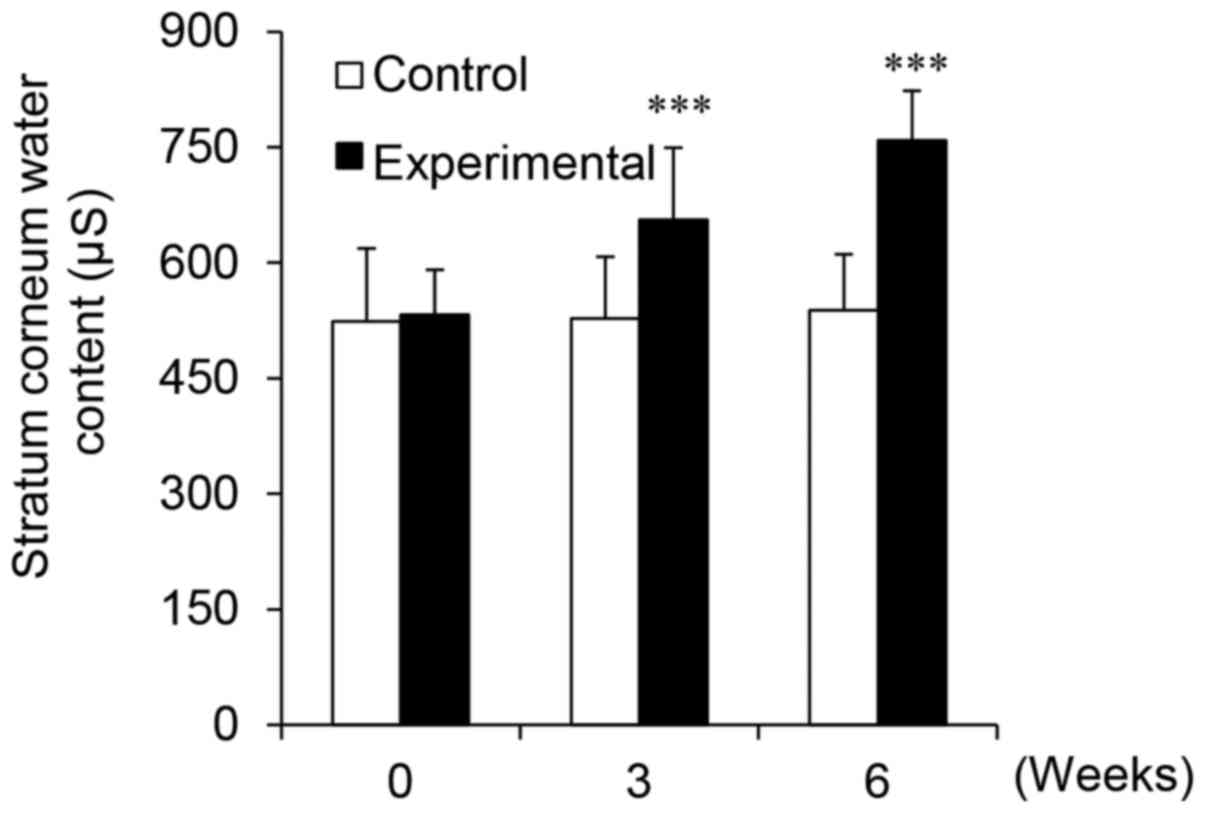
PhS1P improves skin moisture content
Collectively, our in vitro data suggest that
PhS1P, in combination with EGF, can promote pathways that are
protective against aging-associated skin damage. We therefore,
designed a double-blind clinical trial to test the efficacy of a
cream containing PhS1P and EGF in women >35 years of age. Skin
dryness is one of the hallmarks of skin aging (23), and therefore, we analyzed skin
moisture content in the study subjects using the DermaLab USB
moisture probe. We observed that in the control group, which used a
cream that did not contain either PhS1P or EGF, the skin
conductance (which directly reflects moisture content) was 523.93
µS at the beginning of the study, and 527.45 µS and
538.09 µS after 3 and 6 weeks of application, respectively
(Fig. 6). We then calculated the
degree of improvement (Fig. 6)
and found that the skin conductance of the control group increased
by 0.67 and 2.70% after 3 and 6 weeks, respectively. These changes
were not statistically significant (P>0.05), indicating that the
control cream had no measurable effect on moisture content.
Conversely, the skin conductance in the experimental group, which
used the PhS1P and EGF-containing cream, was 532.37 µS
before use, 655.95 µS after 3 weeks, and 758.88 µS
after 6 weeks (Fig. 6). Notably,
the use of the PhS1P and EGF-containing cream significantly
improved skin conductance by 23.21 and 42.55% after 3 and 6 weeks,
respectively (P<0.001). These experiments demonstrated that the
use of the PhS1P and EGF-containing cream improved skin moisture
content in the study subjects.
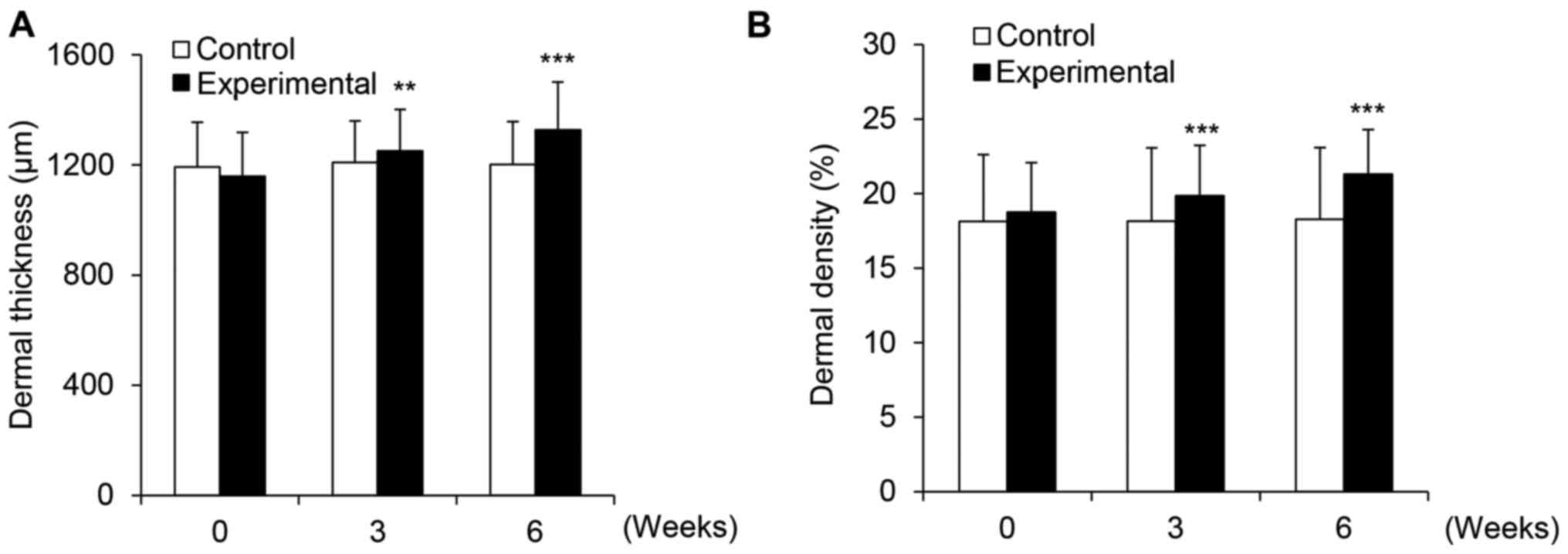
PhS1P improves EGF-induced dermal
thickness and density
During the aging process, dermal thickness and
density are reduced over time. Concurrently, MMP proteins show
increased expression and degrade skin substrate proteins (24). Clinically, it has been shown that
dermal thickness and density decrease with increasing
concentrations of MMPs, as these proteins degrade albuminoids and
the collagen layer (24). Here,
we showed that PhS1P and EGF downregulated MMP1 and promoted
expression of the collagen-encoding gene, COL1A1. We, therefore,
evaluated the synergistic efficacy of PhS1P and EFG on dermal
thickness and density in aging skin in vivo. In the control
subjects, dermal thickness measurements were 94.00 µm before
use, and 96.85 and 94.75 µm after 3 and 6 weeks of use,
respectively. Thickness measurements in the subjects using the
PhS1P and EGF-containing cream were 115.00 µm before use,
and 125.45 and 147.15 µm after 3 and 6 weeks of application,
respectively; these data were statistically significant
(P<0.001). We then calculated the improvement in dermal
thickness as a percentage, based on the values before and after
application. Notably, the dermal thickness improvement was 3.03%
after 3 weeks and 0.80% after 6 weeks in the control group, whereas
the experimental group showed improvements in dermal thickness of
9.09 and 27.96% after 3 and 6 weeks of use, respectively (Fig. 7A).
Dermal density measurements are represented as
percentages, which are proportional to the skin density. In the
subjects using the control cream, density measurements were 18.13%
before application, and 18.16 and 18.28% after 3 and 6 weeks of
use, respectively. Conversely, the subjects using the experimental
cream displayed an average density of 18.76% before application,
and densities of 19.85 and 21.30% after 3 and 6 weeks of use,
respectively (Fig. 7B). The
results for the experimental group were statistically significant
(P<0.001). We then calculated the improvement in dermal density
and found that application of the experimental cream resulted in
improvements of 5.82 and 13.57% after 3 and 6 weeks, respectively,
whereas those using the control cream improved by only 0.20 and
0.84%, respectively. These results demonstrated that PhS1P, in
combination with EGF, improved both dermal thickness and
density.
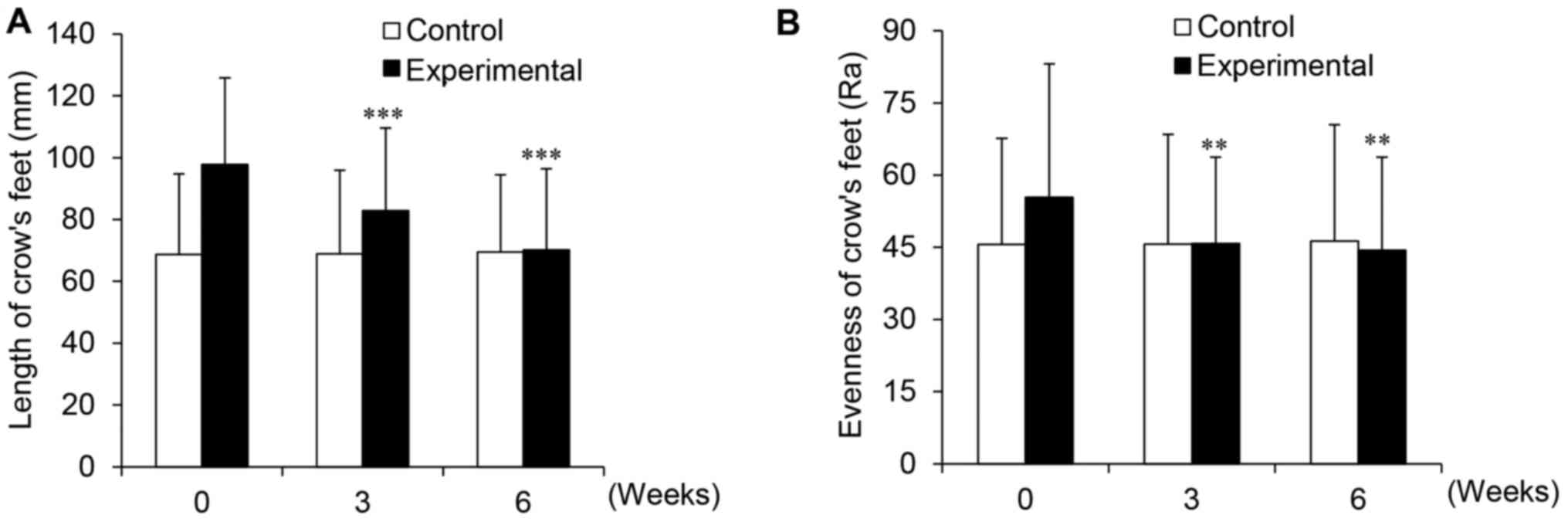
Cream containing PhS1P and EGF improves
the length and evenness of crow's feet
Wrinkles occur in response to structural changes in
cells and tissues, and arise due to intrinsic aging factors,
including decreases in collagen and elasticity, denaturalization of
elastic fibers and the stratum corneum, and loss of skin moisture
(25). Wrinkles can also form as
a result of extrinsic aging factors, such as reactive oxygen
species (ROS), which damage lipids and proteins in the skin via
inflammatory cytokine production (26,27). We, therefore, determined whether
the PhS1P and EGF-containing cream can relieve wrinkles through
clinical efficacy experiments. As shown in Fig. 8A, in the control group, the mean
length of crow's feet was 68.90 mm before application, and 68.90
and 69.45 mm after 3 and 6 weeks, respectively. Conversely, for the
experimental group, the mean length was 97.75 mm before
application, and 82.80 and 70.20 mm after 3 and 6 weeks
application, respectively; these values were statistically
significant (P<0.001). When improvement over time was
calculated, for the experimental group, the length of crow's feet
improved markedly by 15.29 and 28.18% after 3 and 6 weeks,
respectively. In the control group, improvement was only 0.29%
after 3 weeks and 1.09% after 6 weeks. Based on these results, we
concluded that the cream containing both PhS1P and EGF decreased
wrinkle length in vivo.
When we evaluated the evenness of crow's feet, we
found that in the control group, this was 45.57 Ra before
application, and 45.66 and 46.27 Ra after 3 and 6 weeks,
respectively. However, in the experimental group, the evenness was
55.37 Ra before application, and 45.81 and 44.39 Ra after 3 and 6
weeks of application, respectively (Fig. 8B); these values were statistically
significant (P<0.001). To compare improvement in crow's feet
evenness between the control and experimental group, we calculated
the percentage of improvement using the values before application,
and after 3 and 6 weeks of use. The control group showed an
improvement of 0.20% after 3 weeks and 1.54% after 6 weeks
application, whereas for the experimental group, improvement was
17.27% after 3 weeks and 19.83% after 6 weeks of use. Based on
these data, we concluded that the cream containing PhS1P and EGF
improved facial wrinkles, particularly in regards to the length and
evenness of the crow's feet.
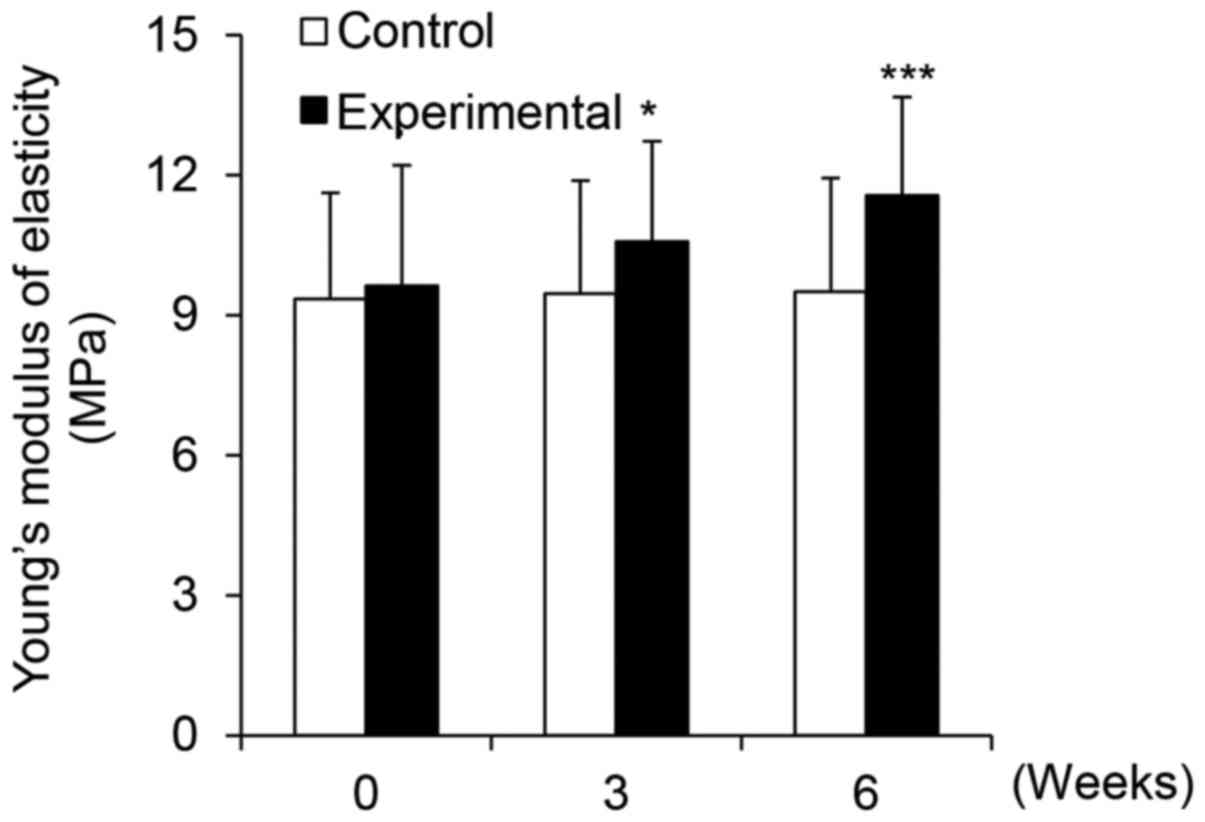
PhS1P and EGF-containing cream improves
skin elasticity
The dermis is composed of dermal fibroblasts that
exist within the extracellular matrix consisting of fibrous
proteins, and it is largely involved in the regulation of skin
elasticity. Various factors cause wrinkle formation and decrease
skin elasticity through the denaturation of dermal structural
components, including collagen and elastin (28). Therefore, in this experiment, we
investigated the effects of the PhS1P and EGF-containing cream on
skin elasticity. Using a DermaLab USB, we measured elasticity in
our study subjects and found that before application, the mean
elasticity was 9.35, whereas it was 9.46 and 9.51 after 3 and 6
weeks, respectively. For the experimental group, mean elasticity
was 9.63 before application, and 10.58 and 11.57 after 3 and 6
weeks of application, respectively (Fig. 9); these values were statistically
significant (P<0.001). When assessing improvement in skin
elasticity over time, the control group improved 1.18 and 1.71%
after 3 and 6 weeks application, respectively. Conversely, the
experimental group showed an improvement of 9.81 and 20.15% after 3
and 6 weeks, respectively. From these data, we concluded that cream
containing PhS1P and EGF significantly improved skin
elasticity.
Analysis of adverse effects of the PhS1P
and EGF-containing cream
In the present study, investigators asked the
subjects individually regarding the condition of their skin and
performed a visual evaluation to assess any possible skin
reactions, such as erythema, itching, scaling, tingling, tightness,
prickling and burning sensation at every visit. No unusual
reactions were reported, based on either the visual evaluation or
the questionnaire.
In the present study, we showed that PhS1P can act
as a stimulator of EGFR, inducing signal transduction to promote
cell proliferation and migration. Our in vitro experiments
demonstrated that PhS1P upregulated EGFR mRNA expression and
displayed a synergistic effects with EGF at the cellular level.
Co-treatment of HDFs with PhS1P and EGF enhanced cellular
migration, protected against UVB-induced decreased cell viability,
and influenced both the phosphorylation and expression of molecules
critical for regulating cellular proliferation and the ECM.
Furthermore, our in vivo experiments demonstrated that
application of the cream containing PhS1P and EGF improved skin
hydration, dermal density and thickness, evenness of crow's feet,
and skin elasticity. EGF is a well-known and highly valued cosmetic
ingredient that has shown efficacy as an anti-aging agent (29). This study suggests that PhS1P may
represent a new efficacious anti-aging cosmetic ingredient, which
displays synergistic effects with EGF in aged skin efficacy
trials.
Acknowledgments
This study was supported by the KU Research
Professor (H.-J.C.) Program of Konkuk University. Funding was also
provided by a grant from the Ministry of Science, ICT and Future
Planning (no. 20110028646) of the Republic of Korea and a grant
from the Korean Health Technology R&D Project, Ministry of
Health and Welfare, Republic of Korea (grant no. HN13C0075).
References
|
1
|
Sorrell JM and Caplan AI: Fibroblast
heterogeneity: More than skin deep. J Cell Sci. 117:667–675. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cha HJ, Lee JP, Lee KS, Lee KK, Choi MJ,
Lee DK, Kim KN and An S: Phytosphigosine-1-phosphate increases
sensitivity of EGF-dependent cell proliferation. Int J Mol Med.
33:649–653. 2014.PubMed/NCBI
|
|
3
|
Lee JP, Cha HJ, Lee KS, Lee KK, Son JH,
Kim KN, Lee DK and An S: Phytosphingosine-1-phosphate represses the
hydrogen peroxide-induced activation of c-Jun N-terminal kinase in
human dermal fibroblasts through the phosphatidylinositol
3-kinase/Akt pathway. Arch Dermatol Res. 304:673–678. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crigler L, Kazhanie A, Yoon TJ, Zakhari J,
Anders J, Taylor B and Virador VM: Isolation of a mesenchymal cell
population from murine dermis that contains progenitors of multiple
cell lineages. FASEB J. 21:2050–2063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giro MG, Oikarinen AI, Oikarinen H, Sephel
G, Uitto J and Davidson JM: Demonstration of elastin gene
expression in human skin fibroblast cultures and reduced
tropoelastin production by cells from a patient with atrophoderma.
J Clin Invest. 75:672–678. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schreier T, Degen E and Baschong W:
Fibroblast migration and proliferation during in vitro wound
healing. A quantitative comparison between various growth factors
and a low molecular weight blood dialysate used in the clinic to
normalize impaired wound healing. Res Exp Med (Berl). 193:195–205.
1993. View Article : Google Scholar
|
|
7
|
Herbst RS: Review of epidermal growth
factor receptor biology. Int J Radiat Oncol Biol Phys. 59(Suppl 2):
21–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carpenter G and Cohen S: Epidermal growth
factor. J Biol Chem. 265:7709–7712. 1990.PubMed/NCBI
|
|
9
|
Allen G: Cosmetics - chemical technology
or biotechnology? Int J Cosmet Sci. 6:61–69. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cha HJ, Lee OK, Kim SY, Ko JM, Kim SY, Son
JH, Han HJ, Li S, Kim SY, Ahn KJ, et al: MicroRNA expression
profiling of p-phenylenediamine treatment in human keratinocyte
cell line. Mol Cell Toxicol. 11:19–28. 2015. View Article : Google Scholar
|
|
11
|
Kim MK, Park KS, Lee H, Kim YD, Yun J and
Bae YS: Phytosphingosine-1-phosphate stimulates chemotactic
migration of L2071 mouse fibroblasts via pertussis toxin-sensitive
G-proteins. Exp Mol Med. 39:185–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar
|
|
13
|
Pan HY, Yang H, Shao MY, Xu J, Zhang P,
Cheng R and Hu T: Sphingosine-1-phosphate mediates AKT/ERK
maintenance of dental pulp homoeostasis. Int Endod J. 48:460–468.
2015. View Article : Google Scholar
|
|
14
|
Stevenson S and Thornton J: Effect of
estrogens on skin aging and the potential role of SERMs. Clin
Interv Aging. 2:283–297. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Puccinelli TJ, Bertics PJ and Masters KS:
Regulation of keratinocyte signaling and function via changes in
epidermal growth factor presentation. Acta Biomater. 6:3415–3425.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ivarsson M, McWhirter A, Borg TK and Rubin
K: Type I collagen synthesis in cultured human fibroblasts:
Regulation by cell spreading, platelet-derived growth factor and
interactions with collagen fibers. Matrix Biol. 16:409–425. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDougall S, Dallon J, Sherratt J and
Maini P: Fibroblast migration and collagen deposition during dermal
wound healing: Mathematical modelling and clinical implications.
Philos Trans A Math Phys Eng Sci. 364:1385–1405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ho MT, Kang HS, Huh JS, Kim YM, Lim Y and
Cho M: Effects of the novel compound DK223
([1E,2E-1,2-Bis(6-methoxy-2H-chromen-3-yl)methylene]hydrazine) on
migration and proliferation of human keratinocytes and primary
dermal fibroblasts. Int J Mol Sci. 15:13091–13110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HM, Lee DE, Park SD, Kim YT, Kim YJ,
Jeong JW, Jang SS, Ahn YT, Sim JH, Huh CS, et al: Oral
administration of Lactobacillus plantarum HY7714 protects hairless
mouse against ultraviolet B-induced photoaging. J Microbiol
Biotechnol. 24:1583–1591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung JH, Seo JY, Choi HR, Lee MK, Youn
CS, Rhie G, Cho KH, Kim KH, Park KC and Eun HC: Modulation of skin
collagen metabolism in aged and photoaged human skin in vivo. J
Invest Dermatol. 117:1218–1224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho S, Won CH, Lee DH, Lee MJ, Lee S, So
SH, Lee SK, Koo BS, Kim NM and Chung JH: Red ginseng root extract
mixed with Torilus fructus and Corni fructus improves facial
wrinkles and increases type I procollagen synthesis in human skin:
A randomized, double-blind, placebo-controlled study. J Med Food.
12:1252–1259. 2009. View Article : Google Scholar
|
|
22
|
Inomata S, Matsunaga Y, Amano S, Takada K,
Kobayashi K, Tsunenaga M, Nishiyama T, Kohno Y and Fukuda M:
Possible involvement of gelatinases in basement membrane damage and
wrinkle formation in chronically ultraviolet B-exposed hairless
mouse. J Invest Dermatol. 120:128–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asserin J, Lati E, Shioya T and Prawitt J:
The effect of oral collagen peptide supplementation on skin
moisture and the dermal collagen network: Evidence from an ex vivo
model and randomized, placebo-controlled clinical trials. J Cosmet
Dermatol. 14:291–301, Epub ahead of print. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nwomeh BC, Liang HX, Diegelmann RF, Cohen
IK and Yager DR: Dynamics of the matrix metalloproteinases MMP-1
and MMP-8 in acute open human dermal wounds. Wound Repair Regen.
6:127–134. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parrado J, Bougria M, Ayala A, Castaño A
and Machado A: Effects of aging on the various steps of protein
synthesis: Fragmentation of elongation factor 2. Free Radic Biol
Med. 26:362–370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fisher GJ, Wang ZQ, Datta SC, Varani J,
Kang S and Voorhees JJ: Pathophysiology of premature skin aging
induced by ultraviolet light. N Engl J Med. 337:1419–1428. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kimura Y and Sumiyoshi M: Olive leaf
extract and its main component oleuropein prevent chronic
ultraviolet B radiation-induced skin damage and carcinogenesis in
hairless mice. J Nutr. 139:2079–2086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schouest JM, Luu TK and Moy RL: Improved
texture and appearance of human facial skin after daily topical
application of barley produced, synthetic, human-like epidermal
growth factor (EGF) serum. J Drugs Dermatol. 11:613–620.
2012.PubMed/NCBI
|