Introduction
Skin wound healing begins immediately following an
injury and consists of several overlapping stages: hemostasis,
inflammation, proliferation and remodeling (1). These phases proceed with
well-organized interaction among various types of cells, including
platelets, keratinocytes, fibroblasts, endothelial cells and
macrophages.
Keratinocytes, the major cellular component of the
epidermis, are critical to wound re-epithelialization. Under normal
conditions, several layers of keratinocytes form the integumentary
system, providing a physical barrier between the environment and
the organism, thereby protecting the body from external agents and
pathogens (2). Once injured,
keratinocytes undergo migration, proliferation and differentiation
in order to maintain the integrity of the skin barrier.
Stimuli that prompt keratinocyte activation include
growth factors, cytokines and chemokines (3–5).
Epidermal growth factor (EGF) may be one of the most
well-characterized growth factors in skin wound healing. In acute
wounds, EGF is mainly secreted by platelets, macrophages and
fibroblasts and is upregulated within a short period of time
following injury (6,7). The release of EGF stimulates
epithelial cell migration and proliferation, thus contributing to
re-epithelialization (8).
A great deal of research efforts have focused on
understanding the mechanisms of EGF responses in keratinocytes.
Increased integrin expression can be detected in EGF-stimulated
corneal keratinocytes, and has been proven to be responsible for
the downstream events triggered by EGF (9). As regards re-epithelialization,
integrins serve as cell attachment receptors and mediators of
cellular signaling. Compared to the other subunits, the β1 family
integrins are relatively well established: the increased expression
of α2β1, α3β1, α9β1, α5β1 and αVβ1 can be detected in the epidermis
after wounding (10,11); the loss of β1 integrins in
keratinocytes results in defective migration (12); the conditional deletion of the β1
subunit in the mouse epidermis has been shown to prevent
re-epithelialization and inhibit the closure of excisional wounds
(13). All these clues point to
the vital role of β1 integrins in wound healing.
To fully understand integrin bidirectional
signaling, numerous researchers have focused on integrin binding
proteins, which are critical in bridging integrin to various
signaling pathways. For example, talin and intergrin-linked kinases
(ILK) (14). Recent studies
reported that Kindlins, a protein family structurally related to
talin, also act as adaptor molecules by ⅰ) cooperating with talin
to activate integrin (15), and
ii) linking integrin with the cytoskeleton, scaffolding and
signaling proteins by interacting with ILK, and recruiting proteins
to focal adhesions (16,17). Given such findings, we attempted
to explore the potential role of Kindlin-1 in EGF-induced wound
healing.
Kindlins are evolutionary conserved,
FERM-domain-containing adaptor proteins which play a vital role in
integrin activation (18,19). All three members of the Kindlin
family can directly bind to the membrane distal NPxY motifs within
cytoplasmic integrin β tails, recruit cytoskeletal proteins and
therefore activate intracellular cell signal transduction, as well
as integrin binding to extracellular ligands (20). Among the Kindlins, Kindlin-1 is
widely expressed in murine and human tissues, including the skin,
heart, lungs, liver, kidneys, colon, prostate, ovaries and
pancreas, and is predominantly distributed in epithelia (21,22). Mutations in the Kindlin-1 gene
have been shown to cause Kindler syndrome, a rare human disease
characterized by a variety of skin abnormalities, including skin
blisters, photosensitivity, mucosal erosion and skin cancer
(23), indicating that Kindlin-1
is critical in maintaining the normal function of the skin.
However, the functions of Kindlin-1 in skin wound healing are not
yet fully understood, although it has been previously demonstrated
that a deficiency in Kindlin-1 results in reduced cell adhesion and
increased apoptosis in keratinocytes (24).
In the present study, we first demonstrate that
Kindlin-1 is involved in the natural skin wound healing process and
in particular, it contributes to EGF-induced re-epithelialization
in skin wound healing. The use of shRNA targeting Kindlin-1
efficiently suppressed EGF-induced HaCaT activation, including
migration and proliferation by suppressing integrin β1 activation,
focal adhesion kinase (FAK) phosphorylation and actin
re-organization. Our data not only provide new mechanistic insight
into the regulation of re-epithelialization in skin wound healing,
but also lead to an improved understanding of the molecular basis
underlying the application of EGF in the treatment of wounds.
Materials and methods
Ethics statement
Animal experiments were performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health,
which conforms to the provisions of the Declaration of Helsinki.
The protocol was approved by the Ethics Committee of Fudan
University, Shanghai, China. All surgerical procedures were
performed under sodium pentobarbital anesthesia, and all efforts
were made to minimize animal suffering.
Wound model
Balb/c mice (Slac Laboratory Animal Co., Ltd.,
Shanghai, China) were deeply anesthetized before shaving the dorsal
hair and cleaning the exposed skin with 70% ethanol. Subsequently,
5 mm full thickness punch wounds were created through the skin and
the panniculus carnosus on the dorsal surface (one wound each on
the left and right side) of each mouse. For further experiments,
including immunohistochemical anlaysis, real-time PCR and western
blot analysis, the healed tissues were removed en bloc with a 7 mm
punch to include a margin of unwounded skin at the indicated time
intervals; the unwounded back skin tissues removed at day 0 were
used as controls [in the following results, the controls are
presented as 'normal', EGF(−) day 0 or EGF(+) day 0].
Experimental groups and treatment
A total of 60 Balb/c mice (8–9 weeks old) were
randomly divided into 10 groups as follows: i) EGF(−) (wound model,
but no EGF treatment) 1 day group; ii) EGF(−) 3 day group; iii)
EGF(−) 7 day group; iv) EGF(−) 10 day group; v) EGF(−) 14 day
group; vi) EGF(+) (wound model with EGF treatment) 1 day group;
vii) EGF(+) 3 day group; viii) EGF(+) 7 day group; ix) EGF(+) 10
day group; and x) EGF(+) 14 day group. In the EGF(+) group, the
wounds were treated with EGF (recombinant human epidermal growth
factor derivative for external use, liquid (I), 2,000 IU/ml,
S20010038; Shenzhen Watsin Genetech Co., Ltd. Shenzhen, China) once
a day (spray once on each wound). In each group, 6 animals were
included. At the end of the experiment, mice were sacrificed by
cervical dislocation under anesthesia.
Immunohistochemistry and
immunofluorescence staining
For immunohistochemical staining, fresh skin tissues
were embedded by optimal cutting temperature (OCT) compound. Frozen
sections of skin (10-µm-thick) were stained with rabbit
polyclonal Kindlin-1 antibody (22215-1-AP; Proteintech Group Inc.,
Chicago, IL, USA) at a dilution of 1:100 overnight (4°C) and then
incubated with Dako REAL™ EnVision™/HRP, Rabbit/Mouse (ENV) reagent
(K5007; DAKO, Glostrup, Denmark). For tissue immunofluorescence
staining (25,26), the frozen tissues were stained
with F4/80 (a marker of macrophages), Keratin 6 (K6, a marker of
keratinocytes), FSP1 (a marker of fibroblasts) and Kindlin-1
antibodies. For cell immunofluorescence staining, the cells were
first fixed with 4% paraformaldehyde for 10 min at room
temperature, rinsed with PBS and incubated in blocking solution for
60 min at room temperature. For Kindlin-1, activated integrin β1
[(integrin β1 (ac)] and phospho-FAK (p-FAK) labeling, the cells
were incubated with the primary antibody for 2 h at room
temperature, rinsed with PBS and incubated with the relevant
secondary antibody for 1 h at room temperature; For actin staining,
the cells were incubated with Phalloidin-iFluor 594 Conjugate (cat.
no. 23122; 1:1,000; AAT Bioquest, Sunnyvale, CA, USA) followed by
DAPI staining (Sigma, St. Louis, MO, USA). The following antibodies
were used: rabbit polyclonal Kindlin-1 antibody (cat. no.
22215-1-AP; 1:50; Proteintech Group Inc.), mouse monoclonal
activated integrin β1 antibody (cat. no. MAB2079Z; 1:300,
Millipore, Bedford, MA, USA), mouse monoclonal Kindlin-1 antibody
(cat. no. SAB4200465; 1:250; Sigma), rabbit monoclonal phospho-FAK
(Tyr397) antibody (cat. no. EP2160Y; 1:400; Abcam, Cambridge, UK),
DyLight 405 affinipure goat anti-rabbit IgG (H+L) (cat. no. A23120;
1:800; Abbkine, Los Angeles, CA, USA), DyLight 405 affinipure goat
anti-mouse IgG (H+L) (cat. no. A23110; 1:800; Abbkine), Alexa Fluor
594 affinipure goat anti-mouse IgG (H+L) (cat. no. 115-585-003;
1:800; Jackson ImmunoResearch, West Grove, PA, USA), Alexa Fluor
594 affinipure goat anti-rabbit IgG (H+L) (cat. no. 111-585-003;
1:800; Jackson ImmunoResearch). Images were captured using a Nikon
Eclipse 660 microscope (Nikon, Tokyo, Japan) or a confocal laser
scanning microscope (Leica Microsystems, Wetzlar, Germany). A total
of 6 samples from each group was selected. For each sample, at
least 4 fields were captured.
Cell culture and RNA interference-induced
knockdown of Kindlin-1
The immortalized keratinocyte cell line, HaCaT, (KCB
200442YJ, Kunming Cell Bank of Chinese Academy of Science, Kunming,
China), were kept in Dulbecco's modified Eagle's medium (DMEM),
containing 10% fetal bovine serum and 1% penicillin/streptomycin.
To inhibit the expression of Kindlin-1 in the HaCaT cells, shRNA
targeting Kindlin-1 [EGFP-Kindlin-1 shRNA (hu)] lentiviral
particles (Genechem, Shanghai, China), a pool of viral particles
containing 3 target-specific constructs that encode 19 nt shRNA
designed to knock down Kindlin-1 expression in the HaCaT cells,
were transduced into the cells according to the manufacturer's
instructions. Control lentiviral particles (Genechem) containing a
scrambled shRNA sequence, were used as a negative control. The
knockdown efficiency was confirmed by western blot analysis.
Real-time PCR
Real-time PCR was performed using the SYBR Primer Ex
Taq™ II kit (Takara, Dalian, China). Data were obtained using the
StepOne plus system (Applied Bio-systems, Foster City, CA, USA).
The relative change in Kindlin-1 mRNA expression was determined by
the fold change. The sequences of the primers used were as follows:
Mouse Kindlin-1 sense primer (5′→3′), GGAGAGCAGCAGACAGAGAT;
antisense primer (5′→3′), TGCTGAGGGGTGAAGAGAAG. Mouse GAPDH sense
primer (5′→3′), GAGCGAGACCCCACTAACAT; antisense primer (5′→3′):
TCTCCATGGTGGTGAAGACA.
Western blot analysis
Western blot analysis was carried out as previously
described (25). Briefly, tissues
and cell lysates were collected, and the protein concentrations
were determined using a BCA™ Protein Assay kit (Pierce, Chemical
Co., Rockford, IL, USA). Proteins were separated by 10% SDS-PAGE
and electro-transferred onto PVDF membranes (Millipore). The
membranes were first incubated with the indicated primary
antibodies overnight (4°C), followed by HRP-conjugated secondary
antibodies for 2 h (room temperature). Blots were visualized using
an enhanced chemiluminescence detection kit (Tiangen Biotech Co.,
Ltd. Beijing, China). The following antibodies were used: mouse
monoclonal Kindlin-1 antibody (cat. no. SAB4200465; 1:1,000,
Sigma), rabbit monoclonal integrin β1 antibody (cat. no.
NB110-57123; 1:1,000, Novus Biologicals, Littleton, CO, USA), mouse
monoclonal activated integrin β1 antibody (cat. no. MAB2079Z;
1:1,000, Millipore), rabbit polyclonal FAK antibody (cat. no. 3285;
1:1,000, Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
monoclonal Phospho-FAK (Tyr397) antibody (cat. no. 8556; 1:1,000,
Cell Signaling Technology, Inc.), rabbit monoclonal GAPDH antibody
(cat. no. 3683, 1:1,000; Cell Signaling Technology, Inc.). All
experiments were repeated at least 4 times.
Induction of FAK activation in
HaCaTs
Fibronectin (FN, PHE0023; Gibco, Frederick, MD,
USA), was used to induce cell adhesion. Briefly, dishes were coated
with FN (10 µg/ml, 37°C) overnight. Detached serum-starved
control HaCaT cells and Kindlin-1 shRNA-transfected HaCaT cells
were stimulated with or without EGF (10 ng/ml) for 15 min and were
then subjected to FN-coated dishes. Cells were harvested at 30 min,
1 h and 3 h, respectively, and FAK phosphorylation was detected by
western blot analysis.
Migration assay
Migration assay was performed using Transwell cell
culture systems (3422; Corning, NY, USA), as previously described
(26). Briefly, the HaCaT cells
were added into the upper chamber. In order to verify the role of
FAK in HaCaT cell migration, normal cells were first treated with
or without FAK inhibitor, PF573228 (S2013; Selleck, Houston, TX,
USA) (1 µM, 30 min) and then treated with or without
recombinant human EGF (236-EG; R&D Systems, Minneapolis,
MN,USA) (10 ng/ml) for 24 h. In order to investigate the role of
Kindlin-1 in HaCaT cell migration, normal, Kindlin-1 shRNA and
control shRNA cells were treated with or without recombinant human
EGF (10 ng/ml) for 24 h. The infiltrating cells were stained with
crystal violet (Beyotime, Shanghai, China), and cell numbers were
counted from 5 wells. All experiments were repeated 3 times.
Cell proliferation assay
Cell proliferation was determined using the Cell
Counting Kit-8 (CCK-8; Dojindo, Kunamoto, Japan) following the
instruction manual. In brief, the cells were treated with or
without EGF (10 ng/ml) for 24 h and then incubated with CCK-8
solution for 1.5 h. The absorbance was determined at 450 nm using a
Universal Microplate Reader (Bio-Tek Instruments Inc., Winooski,
VT, USA). All assays were performed in triplicate, and the
experiment was repeated 5 times.
Statistical analysis
The results are presented as the means ± SEM.
Statistical analysis was performed using One-way ANOVA. Statistical
significance was determined at the level of P<0.05.
Results
Dynamic expression of Kindlin-1 in
EGF-induced skin wound healing in mice
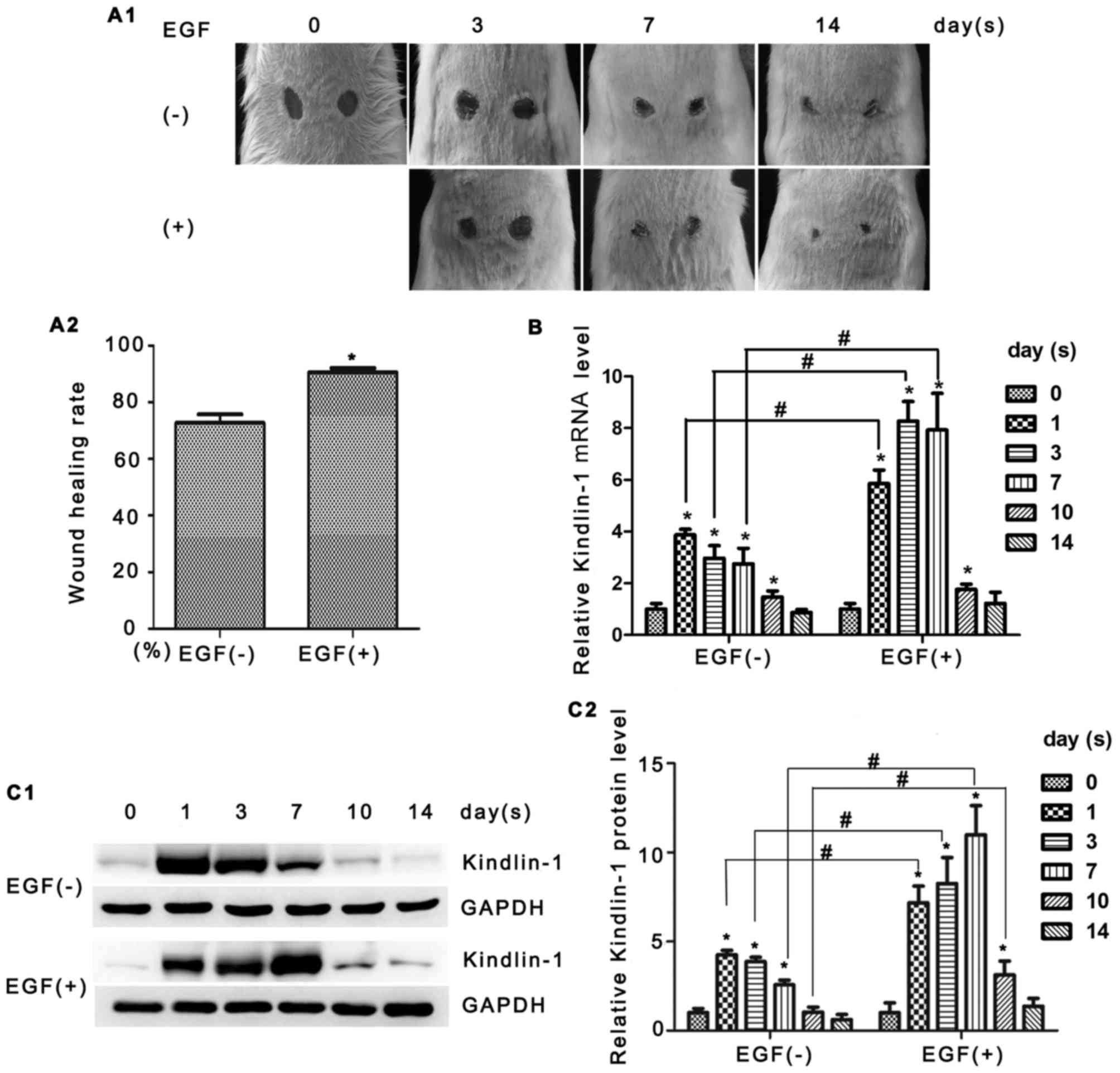
In our initial set of experiments, we examined
Kindlin-1 expression in skin tissues. Animals that underwent
incisions were randomly divided into 2 main groups (with or without
EGF treatment) and raised for different periods of time (Fig. 1A1). As shown in Fig. 1A2, the topical application of EGF
improved the healing rate (72.73% vs. 90.54% on the 14th day).
Kindlin-1 mRNA expression was assessed by real-time PCR. As shown
in Fig. 1B, the mRNA expression
of Kindlin-1 reached the highest level at 1 day post-injury. The
administration of EGF resulted in the more rapid and prolonged
increase of Kindlin-1 mRNA expression. Similar alterations were
observed by western blot analysis (Fig. 1C1 and C2). The dynamic expression
of Kindlin-1 suggested that it is involved in the process of skin
wound healing and is very likely to contribute to EGF
signaling.
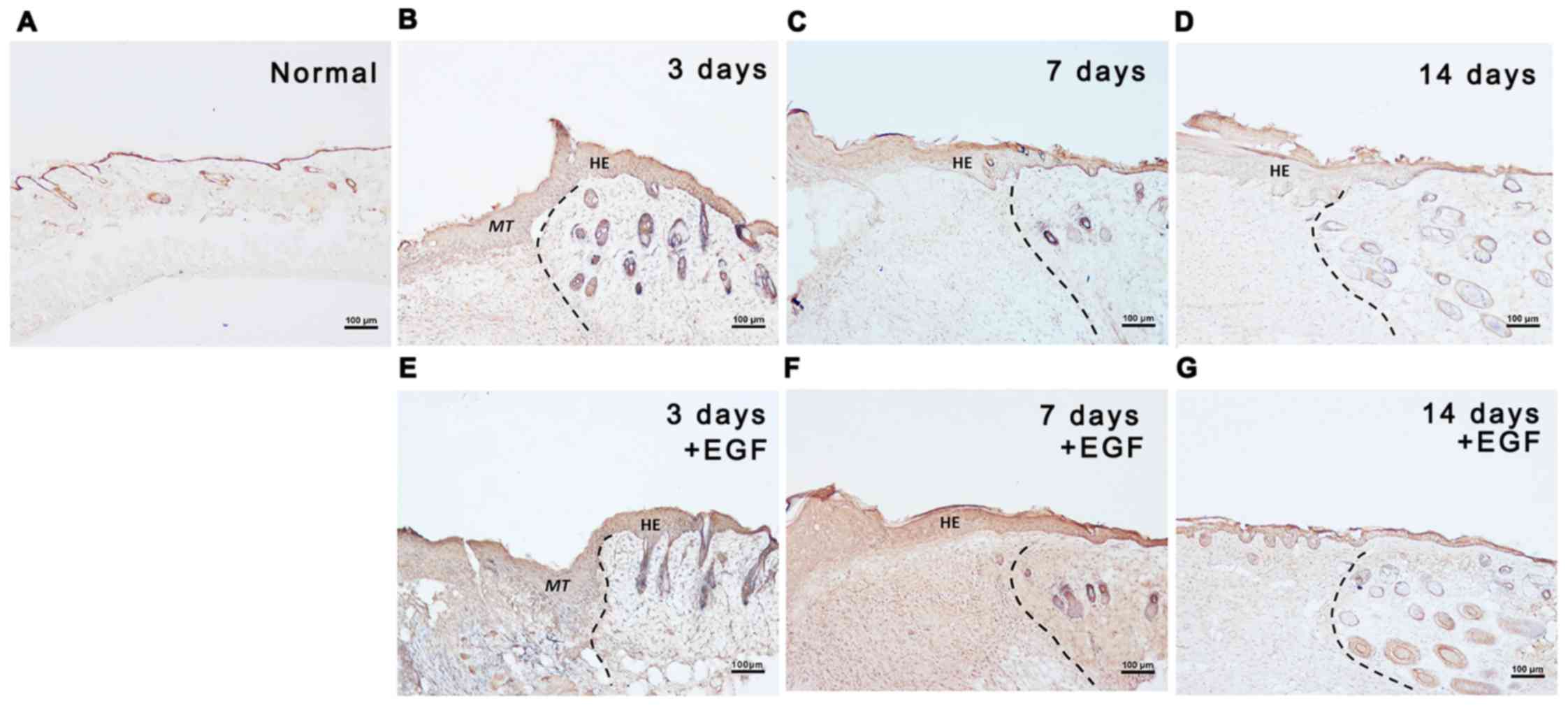
Distribution of Kindlin-1 in skin
wounds
The distribution of Kindlin-1 was detected by
immunostaining. As shown in Fig.
2A, in normal skin, Kindlin-1 was mainly located in the
epidermis with low intensity. During the natural healing process
[EGF(−) group, Fig. 2B–D], strong
Kindlin-1 staining was found 3 days after wounding, mainly
distributed in the epidermis and sporadically in the new dermal
fibroblasts (Fig. 2B). On the 7th
day, moderate Kindlin-1-positive staining was observed in the
regenerated epidermis, which decreased with the distance from the
wound center (Fig. 2C). On the
14th day, only weak Kindlin-1 staining was observed in the wound
epidermis (Fig. 2D). A more
intense kindlin-1-positive signal was observed in the EGF(+) group
(Fig. 2E–G); the most intense
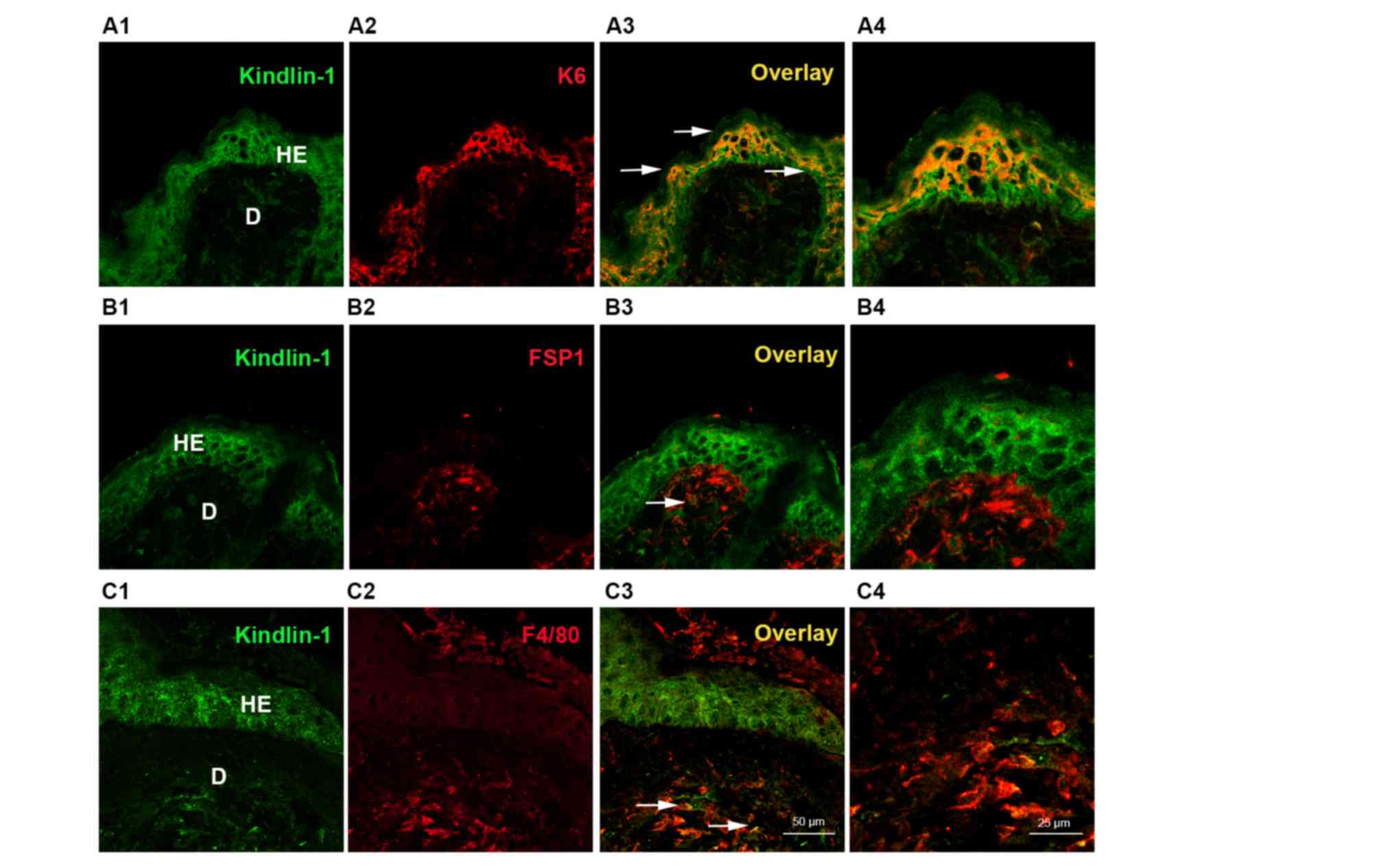
staining was found in the epidermal cells post-injury. Double
labeling immunofluorescence (Fig.
3) further indicated that most of Kindlin-1 was located in the
keratinocytes (Fig. 3A4), and to
a lesser extent in fibroblasts (Fig.
3B4) and macrophages (Fig.
3C4). The specific accumulation of Kindlin-1 in keratinocytes
encouraged us to further investigate the possible role of Kindlin-1
in re-epithelialization.
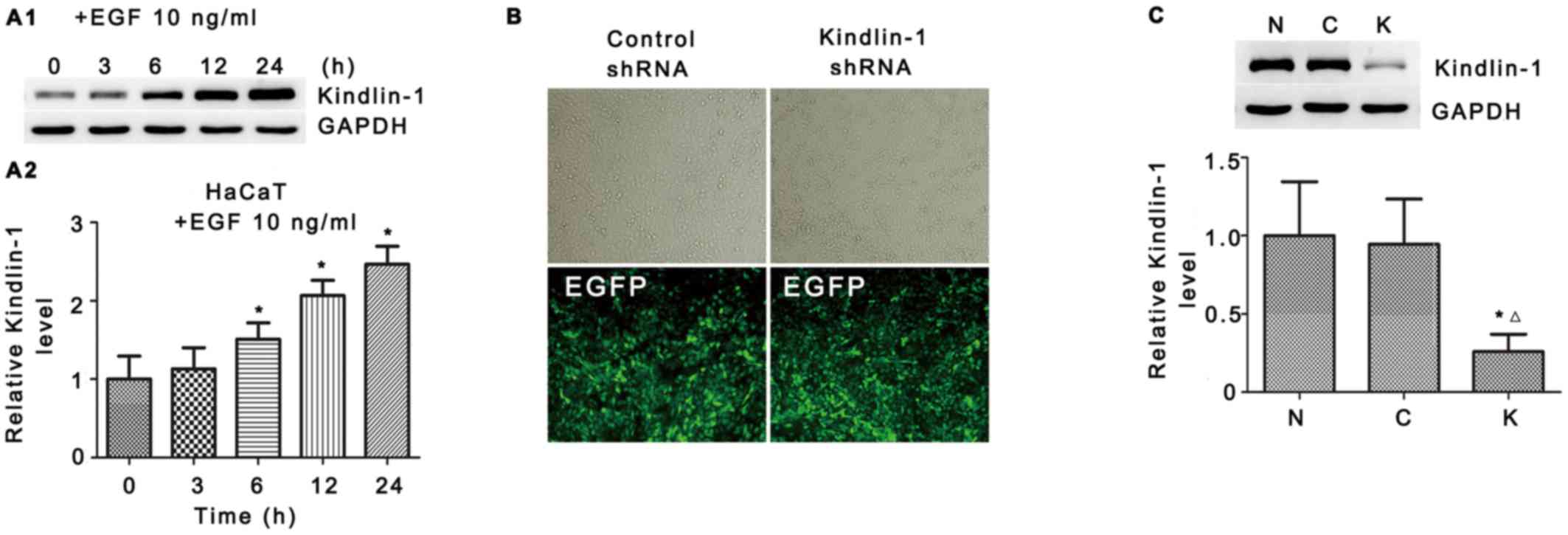
Expression of Kindlin-1 in EGF induces
HaCaT cell activation in vivo
As mentioned above, keratinocytes are major cell
components in re-epithelialization, EGF accelerates keratinocyte
migration, induces rapid proliferation and exerts its effects in a
paracrine manner (7,8). Therefore, in the following in
vitro experiments, we used EGF and HaCaT cells to mimic the
in vivo conditions of re-epithelization.
As shown in Fig.
4A1, in response to EGF stimuli, upregulated Kindlin-1
expression was detected 3 h after and sustained until the end of
the observation (24 h, 2.47-fold vs. 0 h, Fig. 4A2). These data confirmed our
hypothesis that Kindlin-1 may contribute to EGF-induced
re-epithelialization through keratinocytes.
Kindlin-1 shRNA suppresses EGF-induced
integrin β1-FAK activation and actin recruitment
It has been documented that there is a highly
complex regulation of cell signaling in re-epithelialization
(2). To further explore the
signal transdution of EGF-Kindlin-1 in HaCaT cells, we employed
Kindlin-1 shRNA to search for downstream regulators. The
interference and knockdown efficiency was confirmed by fluorescence
microscopy (Fig. 4B) and western
blot analysis (Fig. 4C). The
Kindlin-1 knockdown population (Kindlin-1 shRNA) and the
scrambled-RNAi population (Control shRNA) of HaCaT cells were used
in the following assays.
Integrin β1 is one the best candidates of the
downstream regulators either for its potential binding sites of
Kindlin-1 or for its critical role in promoting
re-epithelialization (27,28).
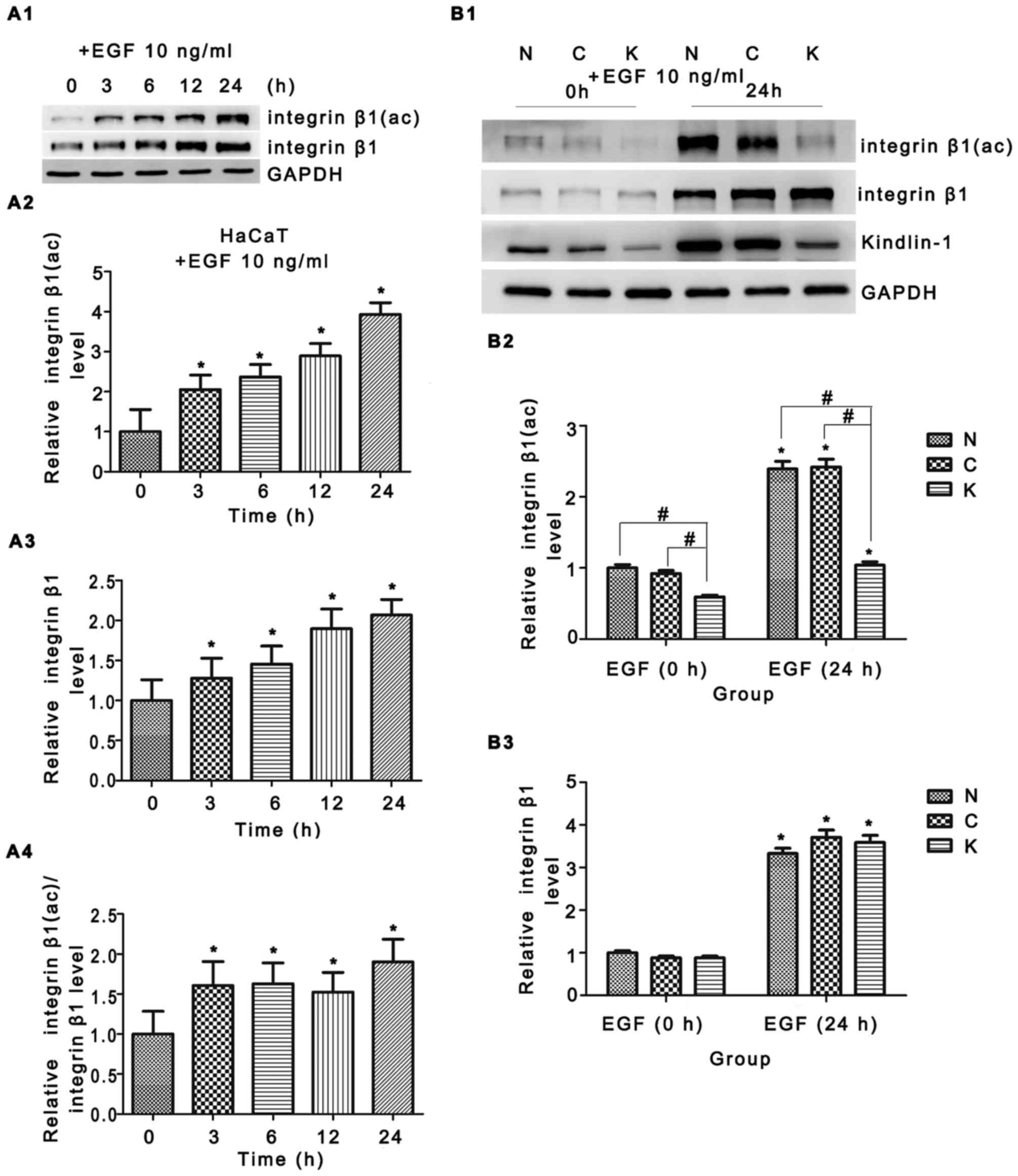
In our study, EGF triggered integrin β1 expression and its
activation was confirmed in Fig.
5A1–A4. Although Kindlin-1 knockdown yielded no obvious changes
in integrin β1 protein expression (Fig. 5B1 and B3), reduced integrin β1
activation [integrin β1 (ac)] was observed in the EGF 0 h group,
approximately 36% (Fig. 5B2, lane
3 vs. lane 2); a greater reduction was observed in the EGF 24 h
group, an approximately 58% decrease when compared to the control
shRNA group (Fig. 5B2, lane 6 vs.
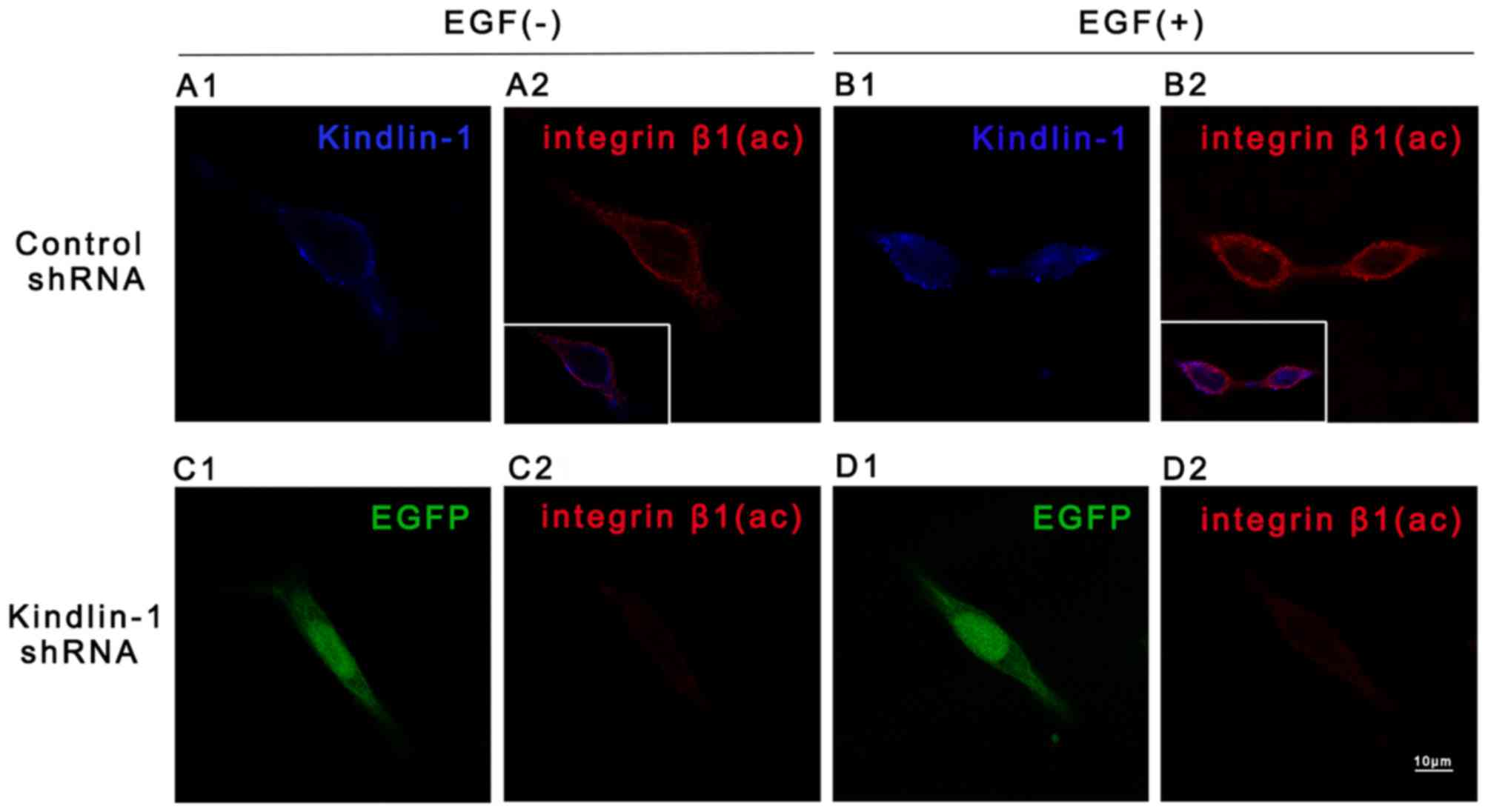
lane 5). Such findings were confirmed by immunofluorescence
staining. Enhanced Kindlin-1 and integrin β1 (ac) staining was
observed in the cells in the control shRNA group in response to EGF
stimuli (Fig. 6B1 vs. A1 and B2 vs.
A2), particularly at the cell peripheral sites. Intensive
violet fluorescence in the inlays (Fig. 6B2 vs. A2) indicated the increased
co-localization of Kindlin-1 and integrin β1 (ac) in the
EGF-treated control shRNA-transfected cells. However in the cells
transfected with Kindlin-1 shRNA, integrin β1 (ac) staining was
less than that of the control shRNA-transfected cells, whether EGF
was present in culture or not. These results implied that EGF
evoked both the expression and binding of Kindlin-1 and integrin β1
(ac), while Kindlin-1 shRNA suppressed this effect. Together with
the regulatory role of Kindlin-1 in integrin β1 trafficking
(29), our data indicated that
Kindlin-1 was very likely to be a mediator of EGF-induced integrin
β1 signaling.
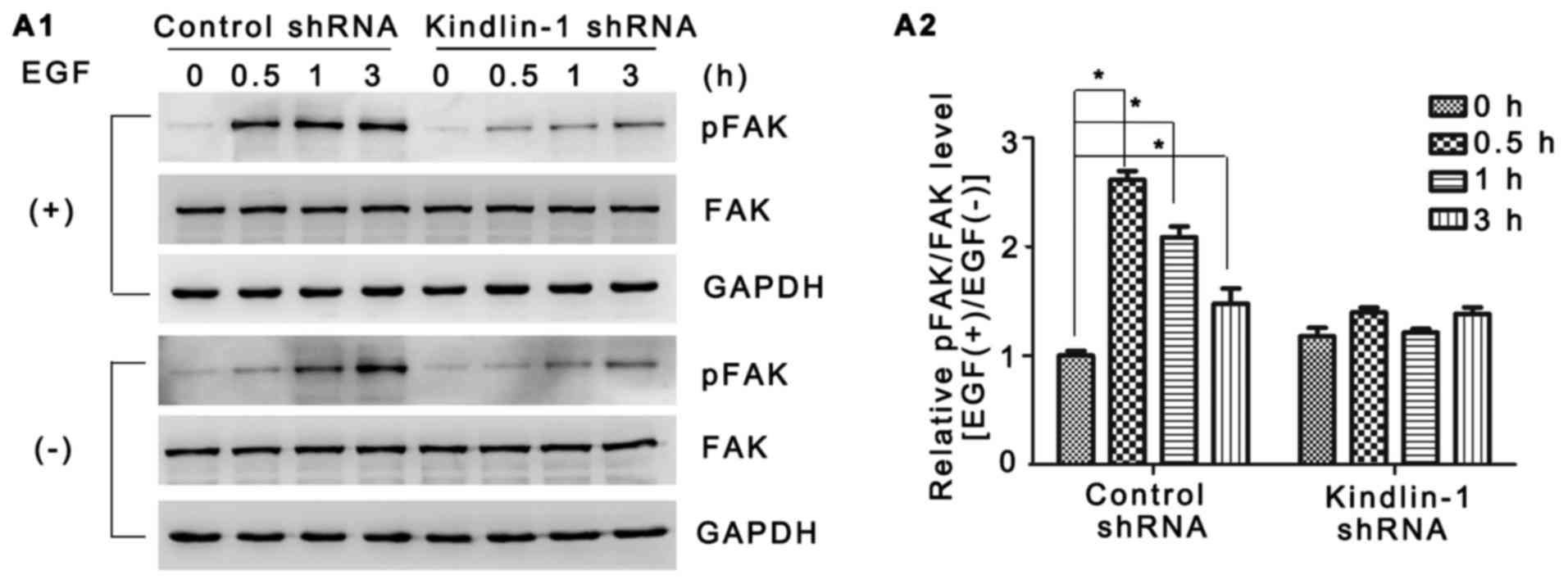
FAK is a widely expressed non-receptor tyrosine
kinase which is responsible for multiple cell functions by
mediating integrin signaling transduction (30–32). In our study, of note, although no
obvious difference was observed in FAK expression between the
groups, EGF-induced FAK activation was confirmed in the control
shRNA-transfected HaCaT cells by both western blot analysis and
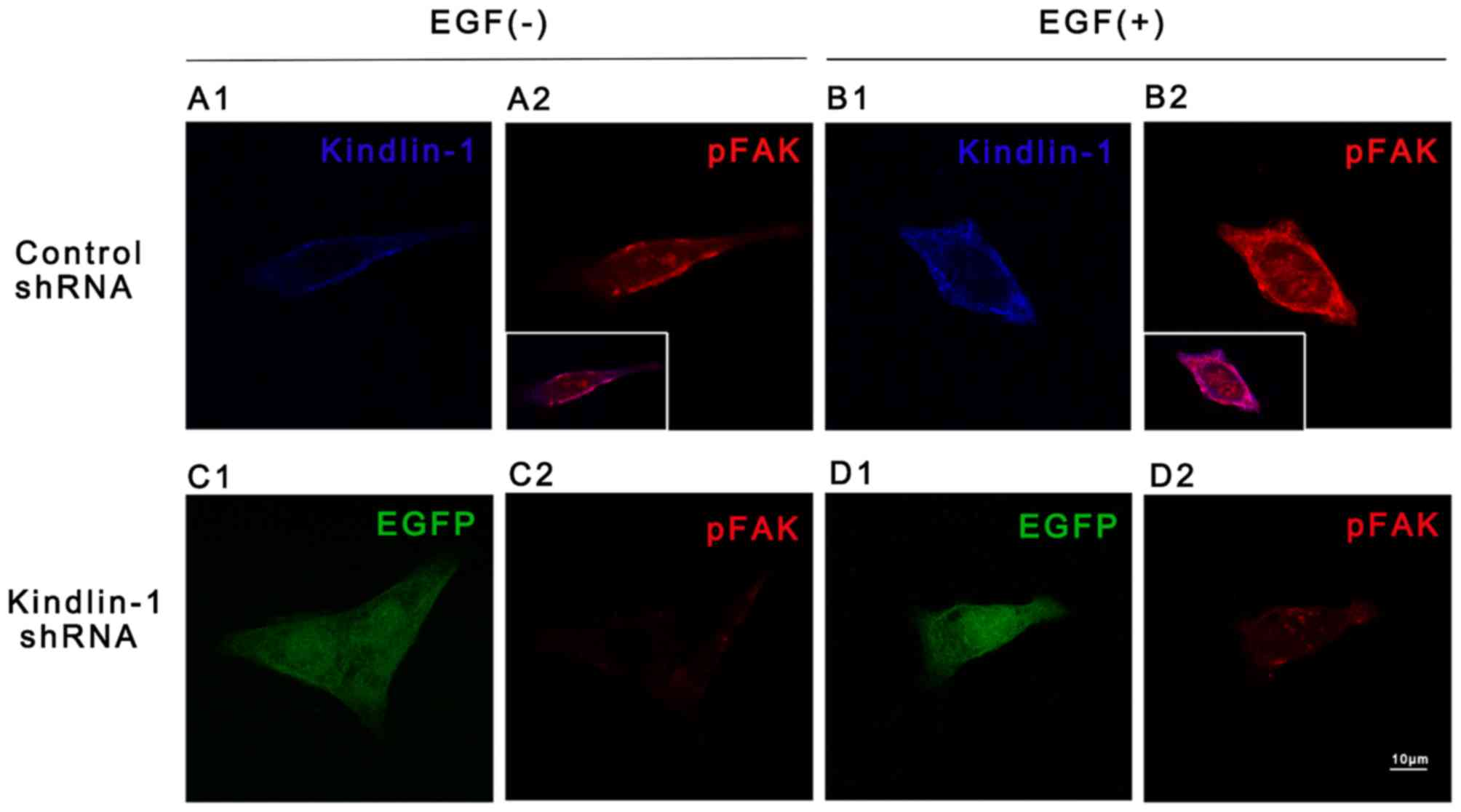
immunofluorescence staining (Figs
7A1 and 8B2 vs. A2). In
contrast, both adhesion-dependent FAK autophosphorylation (Figs 7A1 and 8C2 vs. A2,) and EGF-stimulated FAK
phosphorylation were partly abolished in the Kindlin-1 knockdown
group (Figs 7A1 and 8D2 vs. B2). Fold changes in FAK
phosphorylation following exposure to EGF were quantified as shown
in Fig. 7A2. Furthermore,
exposure to EGF increased the co-localization of Kindlin-1 and
p-FAK in the control HaCaT cells (inlay of Fig. 8B2 vs. A2). Combined with our
findings shown in Fig. 6, the
above-mentioned data proved that the Kindlin-1-integrin β1-FAK
complex formed at cell peripheral sites and suggested the existence
of an EGF-Kindlin-1-integrin β1-FAK signaling pathway in HaCaT
cells.
Actin is another candidate of downstream regulators
of EGF-Kindlin-1 either for its indirect interaction to Kindlin-1
through migfilin, or its direct binding to the intracellular part
of integrin β1 (18,33). Through the adjustment of its
organization, the actin cytoskeleton contributes to
integrin-mediated outside-in and inside-out signal transduction.
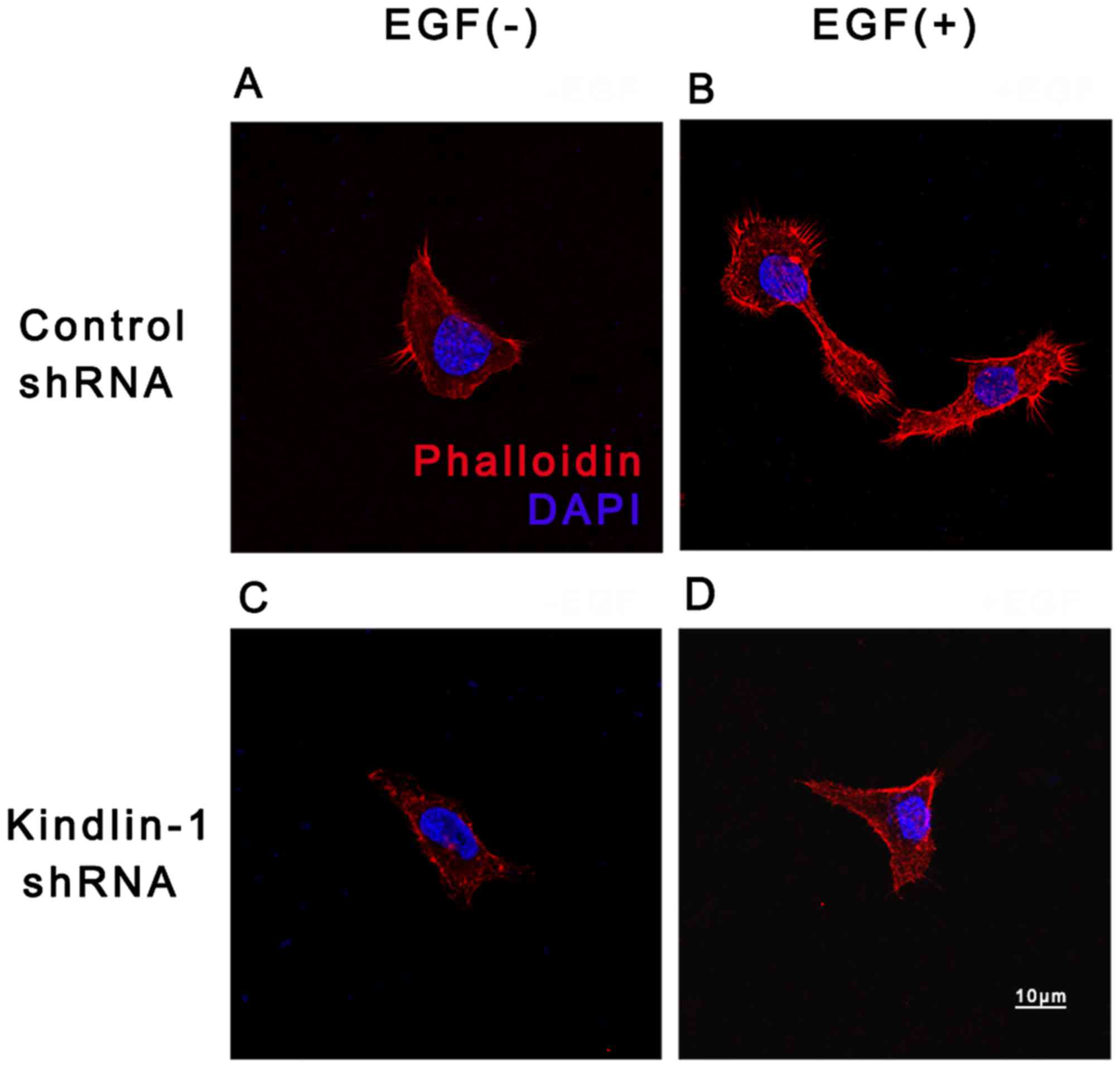
Our results demonstrated that, following culture with EGF, more
obvious cell actin was concentrated at cell extensions and/or cell
borders (Fig. 9B) when compared
with EGF(−) deprived control cultures (Fig. 9A). However, in the Kindlin-1
knockdown group, the actin network was disorganized and disrupted
(Fig. 9D vs. C), suggesting that
EGF-induced actin reorganization may be partly dependent on
Kindlin-1.
Although previous studies have proven that there is
no catalytic activity, Kindlin-1 may regulate cell functions by
integrating and transmitting cell signals (16,17,34). In this study, in the presence of
EGF, the potential role of Kindlin-1 in actin re-organization and
its increased binding to integrin β1, as well as FAK, strongly
suggested that Kindlin-1 may influence cell behavior in wound
healing by directly or indirectly binding to signaling proteins and
mediating protein interactions.
Kindlin-1 shRNA suppresses EGF-induced
HaCaT cell migration and proliferation
To verify the above hypothesis, we then focused on
examining the influence of Kindlin-1 on integrin β1 and FAK-related
cell functions. During re-epithelization, keratinocytes migrate to
the wound center and proliferate to fill the skin defect (35,36). Indeed, Kindlin-1 shRNA-mediated
abnormal integrin regulation caused cell behavior defects:
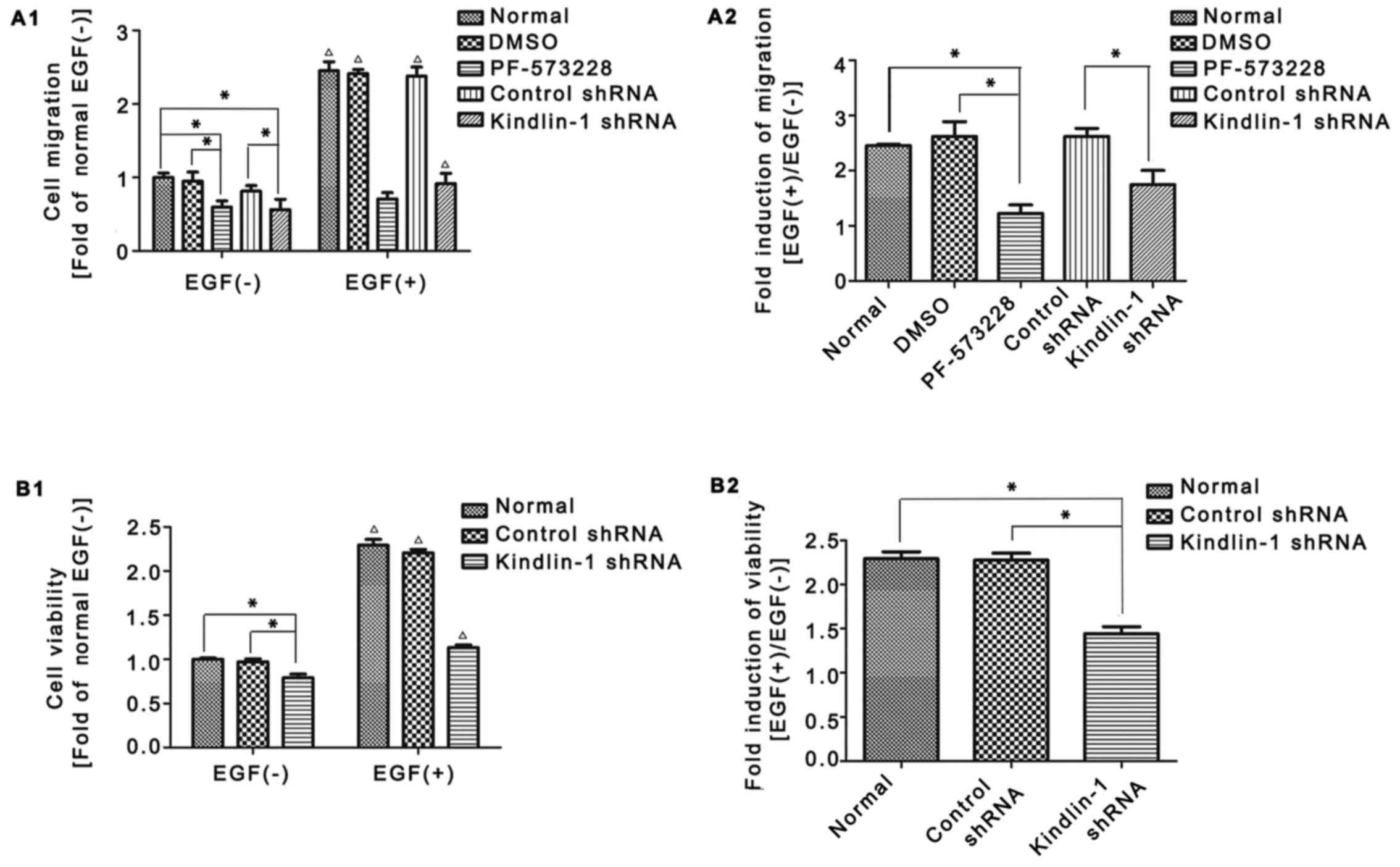
Transwell assay revealed that in the absence of EGF, PF-573228
suppressed EGF-induced HaCaT cell migration (Fig. 10A1, lane 3 vs. lane 2); likewise,
Kindlin-1 shRNA attenuated HaCaT cell migration compared to the
control shRNA group (Fig. 10A1,
lane 5 vs. lane 4). More marked differences were observed between
the Kindlin-1 shRNA- and control shRNA-transfected cells following
stimulation with EGF (Fig. 10A1,
lane 10 vs. lane 9). The fold induction of cell migration following
exposure to EGF was quantified as shown in Fig. 10A2 [EGF(+)/EGF(−)], Kindlin-1
shRNA restrained cell migration compared to the control shRNA group
(approximately 35% reduction, lane 5 vs. lane 4), indicating
Kindlin-1 shRNA partly abolished EGF induced migration.
Similar changes were observed for cell
proliferation. Under normal conditions, slightly impaired cell
proliferation was observed in the Kindlin-1 shRNA-transfected cells
(Fig. 10B1, lane 3 vs. lane 2),
while in the presence of EGF, a more rapid difference was observed
between the groups (Fig. 10B1,
lane 6 vs. lane 5). The fold induction of cell viability following
exposure to EGF was quantified as shown in Fig. 10B2 [EGF(+)/EGF(−)]; Kindlin-1
shRNA repressed cell proliferation compared to the control shRNA
group (approximately 45% reduction, lane 3 vs. lane 2).
Taken together, the inhibition of Kindlin-1
expression in HaCaT cells partially weakened EGF-induced integrin
β1-FAK activation and the recruitment of cytoskeletal proteins,
which finally led to impaired cell functions, including migration
and proliferation. These results confirm the role of Kindlin-1 in
transmitting EGF activation signals in skin wound healing.
Discussion
Multiple stimuli are responsible for inducing cell
migration and proliferation during re-epithelialization, including
an absence signal transmitted from the free edge of the wound and
the increasing expression of growth factors (e.g., EGF and TGF).
Clinical applications of growth factors in wound healing have been
rapidly developed. In China, human recombinant EGF and basic
fibroblast growth factor (bFGF) have been commercially available,
and the topical administration of these growth factors has been
proven to be effective for wound healing in clinical practice.
Fully understanding the downstream signaling of EGF will allow for
the more precise control of skin wound healing.
It has been reported that the major function of EGF
in acute wounds is re-epithelialization (4). Keratinocytes are the main cell
components of the epithelium, and also the main executors of
re-epithelialization, considering the specific distribution of
Kindlin-1 in EGF-induced re-epithelialization. In the present
study, we proposed a novel hypothesis (to the best of our
knowledge) that in skin wound healing, EGF induced kindlin-1, which
in turn activated intergrin β1 signaling to promote HaCaT
keratinocyte activation.
By using full-thickness excisional wound models, the
upregulated expression of kindlin-1 was observed in the epidermis.
Indeed, the increased expression was not only detected in the
EGF-treated group, but also in the untreated controls. This may at
least partly be due to endogenic EGF. In the normal healing
process, following injury, EGF is synthesized by platelets,
lymphocytes, macrophages and fibroblasts, which acts on
keratinocytes in a paracrine manner and finally contributes to
keratinocyte activation (7,8).
By contrast, in our study, the topical application of EGF directly
acted on wounds at a higher concentration, thus accelerating the
above-mentioned bio-effects. This may explain why the more rapid
and durable upregulation of Kindlin-1 was found in the EGF-treated
group (Fig. 1).
Considering the expression of Kindlin-1 in the
epidermis and its enrichment in response to EGF, we then sought to
explore its role in re-epithelialization, particularly in
keratinocyte bioactivity. It has been demonstrated that Kindlins
are co-activator of integrins (37). Evidence of the role of integrins
in cell signal transmitting has accumulated. In the wound
epidermis, inter-grin β1 has been reported to serve as a
bidirectional signal transduction molecule by binding to various
signaling effectors directly or indirectly (38). These intergrin β1-related protein
complexes convey signals, regulating gene expression and cell
behavior, such as cell proliferation, migration and adhesion, as
well as cell survival (39).
Based on such observations, we hypothesized that Kindlin-1 may
affect cell bioactivity through integrin β1. As expected, EGF
stimuli evoked integrin β1 activation in HaCaT keratinocytes, and
the blockade of Kindlin-1 led to decreased integrin β1 activation
compared to the normal and control group (Fig. 5).
The activation of integrins allows the binding of
intracellular signaling molecules, such as FAK, Src, p130C and
other structural proteins. Up to 180 different scaffold and
signaling proteins have been reported to be included in this
network (40). As regards FAK for
example, integrin activation can promote the combination of
integrin and the ECM ligand and helps to form focal adhesion, which
recruits focal adhesion proteins, including FAK. The β subunit of
integrins can bind to the N-terminal regions of FAK to induce a
structural change. FAK then switches to an active state
(autophosphorylation of Tyr397) and triggers the downstream
signaling pathway (41). FAK
activation often occurs as a result of growth factor stimulation
(42,43). In this study, we also observed FAK
phosphorylation in response to EGF stimuli in serum-starved normal
HaCaT cells, (Fig. 7), which is
consistent with the study by Kim and Kim (44). However, in the Kindlin-1 shRNA
group, the upregulation of FAK was attenuated. One reason may
explain this phenomenon: in nomal HaCaT cells, integrin-mediated
cell adhesion through focal adhesion provided the signaling
transduction platform to activate FAK, and EGF accelerated this
process. However, in Kindlin-1 shRNA-transfected HaCaT cells, the
depletion of Kindlin-1 resulted in the impaired activation of
integrin β1, thus affecting the subsequent 'platform' foundation
and FAK activation.
The activation of integrin-FAK and changes in actin
conformation are related to several keratinocyte functions
associated with wound healing. In this study, we first evaluated
the role of Kindlin-1 in EGF-induced HaCaT cell migration and
proliferation, two striking features of keratinocytes during
re-epithelialization. Our results revealed that Kindlin-1
inhibition significantly attenuated EGF-induced HaCaT cell
migration (Fig. 10A1 and A2) and
proliferation (Fig. 10B1 and
B2). These results indicated the existence of an
EGF-Kindlin-1-integrin β1-FAK axis in triggering HaCaT cell
activation.
It worth determining whether Kindlin-1 alone
fulfilled the above function or cooperated with other molecules. In
a previous study, Kindlin-2, another member of the Kindlins, was
implicated in wound closure and tissue repair by acting on
fibroblasts (45). Moreover,
functional similarities between Kindlin-1 and Kindlin-2 have been
reported, as they are localized in epidermal keratinocytes and have
overlapping functions in adhesion and survival (46). Thus, Kindlin-1 and Kindlin-2 may
function together in keratinocyte integrin activation during wound
healing. However, this hypothesis and the exact mechanisms warrant
further investigation.
In conclusion, our data provide evidence that in
HaCaT keratinocytes, Kindlin-1 acts on integrin β1, FAK and actin
to trigger cell activation. The inhibition of Kindlin-1 expression
in HaCaT cells significantly attenuates the EGF-induced migration
and proliferation. Given the essential role of keratinocyte
activation in wound healing, Kindlin-1 may be a promising
therapeutic target to promote wound healing.
Acknowledgments
The present study was supported by the National
High-tech R&D program (863 program, 2014AA020705) and the
National Natural Scientific Foundation of China (grant no.
81101178).
References
|
1
|
Greaves NS, Ashcroft KJ, Baguneid M and
Bayat A: Current understanding of molecular and cellular mechanisms
in fibroplasia and angiogenesis during acute wound healing. J
Dermatol Sci. 72:206–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pastar I, Stojadinovic O, Yin NC, Ramirez
H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR and
Tomic-Canic M: Epithelialization in Wound Healing: A Comprehensive
Review. Adv Wound Care (New Rochelle). 3:445–464. 2014. View Article : Google Scholar
|
|
3
|
Werner S and Grose R: Regulation of wound
healing by growth factors and cytokines. Physiol Rev. 83:835–870.
2003.PubMed/NCBI
|
|
4
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar
|
|
5
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shiraha H, Glading A, Gupta K and Wells A:
IP-10 inhibits epidermal growth factor-induced motility by
decreasing epidermal growth factor receptor-mediated calpain
activity. J Cell Biol. 146:243–254. 1999.PubMed/NCBI
|
|
7
|
Schultz G, Rotatori DS and Clark W: EGF
and TGF-alpha in wound healing and repair. J Cell Biochem.
45:346–352. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haase I, Evans R, Pofahl R and Watt FM:
Regulation of keratinocyte shape, migration and wound
epithelialization by IGF-1- and EGF-dependent signalling pathways.
J Cell Sci. 116:3227–3238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eberwein P, Laird D, Schulz S, Reinhard T,
Steinberg T and Tomakidi P: Modulation of focal adhesion
constituents and their down-stream events by EGF: On the cross-talk
of integrins and growth factor receptors. Biochim Biophys Acta.
1853(10 Pt A): 2183–2198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh P, Chen C, Pal-Ghosh S, Stepp MA,
Sheppard D and Van De Water L: Loss of integrin alpha9beta1 results
in defects in proliferation, causing poor re-epithelialization
during cutaneous wound healing. J Invest Dermatol. 129:217–228.
2009. View Article : Google Scholar
|
|
11
|
Kenny FN and Connelly JT:
Integrin-mediated adhesion and mechano-sensing in cutaneous wound
healing. Cell Tissue Res. 360:571–582. 2015. View Article : Google Scholar
|
|
12
|
Grose R, Hutter C, Bloch W, Thorey I, Watt
FM, Fässler R, Brakebusch C and Werner S: A crucial role of beta 1
integrins for keratinocyte migration in vitro and during cutaneous
wound repair. Development. 129:2303–2315. 2002.PubMed/NCBI
|
|
13
|
Chen L, Hughes RA, Baines AJ, Conboy J,
Mohandas N and An X: Protein 4.1R regulates cell adhesion,
spreading, migration and motility of mouse keratinocytes by
modulating surface expression of beta1 integrin. J Cell Sci.
124:2478–2487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calderwood DA, Campbell ID and Critchley
DR: Talins and kindlins: Partners in integrin-mediated adhesion.
Nat Rev Mol Cell Biol. 14:503–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kahner BN, Kato H, Banno A, Ginsberg MH,
Shattil SJ and Ye F: Kindlins, integrin activation and the
regulation of talin recruitment to αIIbβ3. PLoS One. 7:e340562012.
View Article : Google Scholar
|
|
16
|
Montanez E, Ussar S, Schifferer M, Bösl M,
Zent R, Moser M and Fässler R: Kindlin-2 controls bidirectional
signaling of integrins. Genes Dev. 22:1325–1330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu Y, Wu S, Shi X, Chen K and Wu C:
Migfilin and Mig-2 link focal adhesions to filamin and the actin
cytoskeleton and function in cell shape modulation. Cell.
113:37–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Larjava H, Plow EF and Wu C: Kindlins:
Essential regulators of integrin signalling and cell-matrix
adhesion. EMBO Rep. 9:1203–1208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meves A, Stremmel C, Gottschalk K and
Fässler R: The Kindlin protein family: New members to the club of
focal adhesion proteins. Trends Cell Biol. 19:504–513. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harburger DS, Bouaouina M and Calderwood
DA: Kindlin-1 and -2 directly bind the C-terminal region of beta
integrin cytoplasmic tails and exert integrin-specific activation
effects. J Biol Chem. 284:11485–11497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel DH, Ashton GH, Penagos HG, Lee JV,
Feiler HS, Wilhelmsen KC, South AP, Smith FJ, Prescott AR,
Wessagowit V, et al: Loss of kindlin-1, a human homolog of the
Caenorhabditis elegans actin-extracellular-matrix linker protein
UNC-112, causes Kindler syndrome. Am J Hum Genet. 73:174–187. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ussar S, Wang HV, Linder S, Fässler R and
Moser M: The Kindlins: Subcellular localization and expression
during murine development. Exp Cell Res. 312:3142–3151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ashton GH, McLean WH, South AP, Oyama N,
Smith FJ, Al-Suwaid R, Al-Ismaily A, Atherton DJ, Harwood CA, Leigh
IM, et al: Recurrent mutations in kindlin-1, a novel keratinocyte
focal contact protein, in the autosomal recessive skin fragility
and photosensitivity disorder, Kindler syndrome. J Invest Dermatol.
122:78–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herz C, Aumailley M, Schulte C,
Schlötzer-Schrehardt U, Bruckner-Tuderman L and Has C: Kindlin-1 is
a phosphoprotein involved in regulation of polarity, proliferation,
and motility of epidermal keratinocytes. J Biol Chem.
281:36082–36090. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang D, Sun L, Zhu H, Wang L, Wu W, Xie J
and Gu J: Microglial LOX-1 reacts with extracellular HSP60 to
bridge neuroinflammation and neurotoxicity. Neurochem Int.
61:1021–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia D, Duan F, Peng P, Sun L, Ruan Y and
Gu J: Pyrroloquinoline-quinone suppresses liver fibrogenesis in
mice. PLoS One. 10:e01219392015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li JF, Duan HF, Wu CT, Zhang DJ, Deng Y,
Yin HL, et al: HGF accelerates wound healing by promoting the
dedifferentiation of epidermal cells through β1-integrin/ILK
pathway. BioMed Res Int. 470418:20132013.
|
|
28
|
Iwata Y, Akamatsu H, Hasegawa S, Takahashi
M, Yagami A, Nakata S and Matsunaga K: The epidermal Integrin
beta-1 and 75NTR positive cells proliferating and migrating during
wound healing produce various growth factors, while the expression
of P75N TR is decreased in patients with chronic skin ulcers. J
Dermatol Sci. 71:122–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Margadant C, Kreft M, Zambruno G and
Sonnenberg A: Kindlin-1 regulates integrin dynamics and adhesion
turnover. PLoS One. 8:e653412013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parsons JT: Focal adhesion kinase: The
first ten years. J Cell Sci. 116:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: In command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Geiger B, Spatz JP and Bershadsky AD:
Environmental sensing through focal adhesions. Nat Rev Mol Cell
Biol. 10:21–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda
K, Qin J, Plow EF and Wu C: The MIG-2/integrin interaction
strengthens cell-matrix adhesion and modulates cell motility. J
Biol Chem. 282:20455–20466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frank DE and Carter WG: Laminin 5
deposition regulates keratinocyte polarization and persistent
migration. J Cell Sci. 117:1351–1363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Danussi C, Petrucco A, Wassermann B,
Pivetta E, Modica TM, Del Bel Belluz L, Colombatti A and Spessotto
P: EMILIN1-α4/α9 integrin interaction inhibits dermal fibroblast
and keratinocyte proliferation. J Cell Biol. 195:131–145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Böttcher RT, Lange A and Fässler R: How
ILK and kindlins cooperate to orchestrate integrin signaling. Curr
Opin Cell Biol. 21:670–675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Longmate WM and Dipersio CM: Integrin
Regulation of Epidermal Functions in Wounds. Adv Wound Care (New
Rochelle). 3:229–246. 2014. View Article : Google Scholar
|
|
39
|
Koivisto L, Heino J, Häkkinen L and
Larjava H: Integrins in Wound Healing. Adv Wound Care (New
Rochelle). 3:762–783. 2014. View Article : Google Scholar :
|
|
40
|
Zaidel-Bar R and Geiger B: The switchable
integrin adhesome. J Cell Sci. 123:1385–1388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cooper LA, Shen TL and Guan JL: Regulation
of focal adhesion kinase by its amino-terminal domain through an
autoinhibitory interaction. Mol Cell Biol. 23:8030–8041. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Golubovskaya V, Beviglia L, Xu LH, Earp HS
III, Craven R and Cance W: Dual inhibition of focal adhesion kinase
and epidermal growth factor receptor pathways cooperatively induces
death receptor-mediated apoptosis in human breast cancer cells. J
Biol Chem. 277:38978–38987. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK,
Schaefer E, Damsky CH and Schlaepfer DD: FAK integrates
growth-factor and integrin signals to promote cell migration. Nat
Cell Biol. 2:249–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim SH and Kim SH: Antagonistic effect of
EGF on FAK phosphorylation/dephosphorylation in a cell. Cell
Biochem Funct. 26:539–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He Y, Esser P, Schacht V,
Bruckner-Tuderman L and Has C: Role of kindlin-2 in fibroblast
functions: Implications for wound healing. J Invest Dermatol.
131:245–256. 2011. View Article : Google Scholar
|
|
46
|
He Y, Esser P, Heinemann A,
Bruckner-Tuderman L and Has C: Kindlin-1 and -2 have overlapping
functions in epithelial cells implications for phenotype
modification. Am J Pathol. 178:975–982. 2011. View Article : Google Scholar : PubMed/NCBI
|