Introduction
Wound healing is a dynamic and complex process
through which the skin repairs itself following injury. It involves
a series of coordinated phases, including hemostasis, inflammation,
proliferation and remodeling (1).
The inflammatory response is essential for removing the bacteria
and cell debris from the wound. Additionally, different types of
cells coordinate to influence wound repair and re-establish barrier
function (2). Epithelial cells
migrate across the surface of the wound bed and form a new
epithelial layer (3). Mesenchymal
stem cells (MSCs) play an important role in the inflammation,
proliferation and remodeling phase of normal wound healing
(4). In the proliferation phase,
revascularization of the wound bed is a crucial stage of wound
healing, which can reconstruct the circulation and nutrition
supplement in the wound tissue. Therefore, endothelial cells are
also needed to play an important role to break through the dermis
of the wound and form tubes in the newly developing tissue
(5,6). In the final stage of wound healing,
fibroblasts are attracted into the wound bed and begin to secrete
collagenous extracellular matrix (ECM) that constitutes the newly
formed granulation tissue, thereby providing structural integrity
to the wound (5). Remodeled
collagen promotes the maturation of the wound.
It is known that mechanical stress can influence
wound healing by affecting the behavior of cells within the dermis
(3). In wound treatment, vacuum
sealing drainage (VSD), also known as negative pressure (NP) wound
therapy, has been extensively researched (7) and has been proven to be markedly
effective in wound healing (8,9).
VSD has been certified to decrease bacterial colony formation
(10,11), edema (12,13), the permeability of vessels
(11,14,15), and to increase angiogenesis and
blood flow to the wound margins (12,13). It was originaly considered that
VSD exerts NP on the wound. However, in recent years, some
researches have demonstrated that VSD exerts positive pressure (PP)
on the wound rather than NP (16). In the present study, we created a
homemade device and found that it could maintain NP on the wound.
We wished to determine whether VSD-induced PP or the NP induced by
our homemade device was more efficient on wound healing.
In this study, we examined the effects and
mechanisms of PP and NP using an animal wound healing model. The
infiltration of inflammatory cells and growth factors were first
analyzed. The distribution scale of different cell types in wound
tissue following treatment with the pressure devices was then
detected by histological analysis, including endothelial cells,
MSCs, epithelial cells and fibroblasts. Furthermore, different
types of collagen deposition were measured by western blot
analysis. Our data demonstrate that the NP induced by our homemade
device was more effective in promoting wound healing than the
VSP-induced PP.
Materials and methods
Ethics statement
All methods used in this study were carried out in
accordance with the approved ethical guidelines of Central South
University. The study protocol was approved by the Central South
University Institutional Review Board.
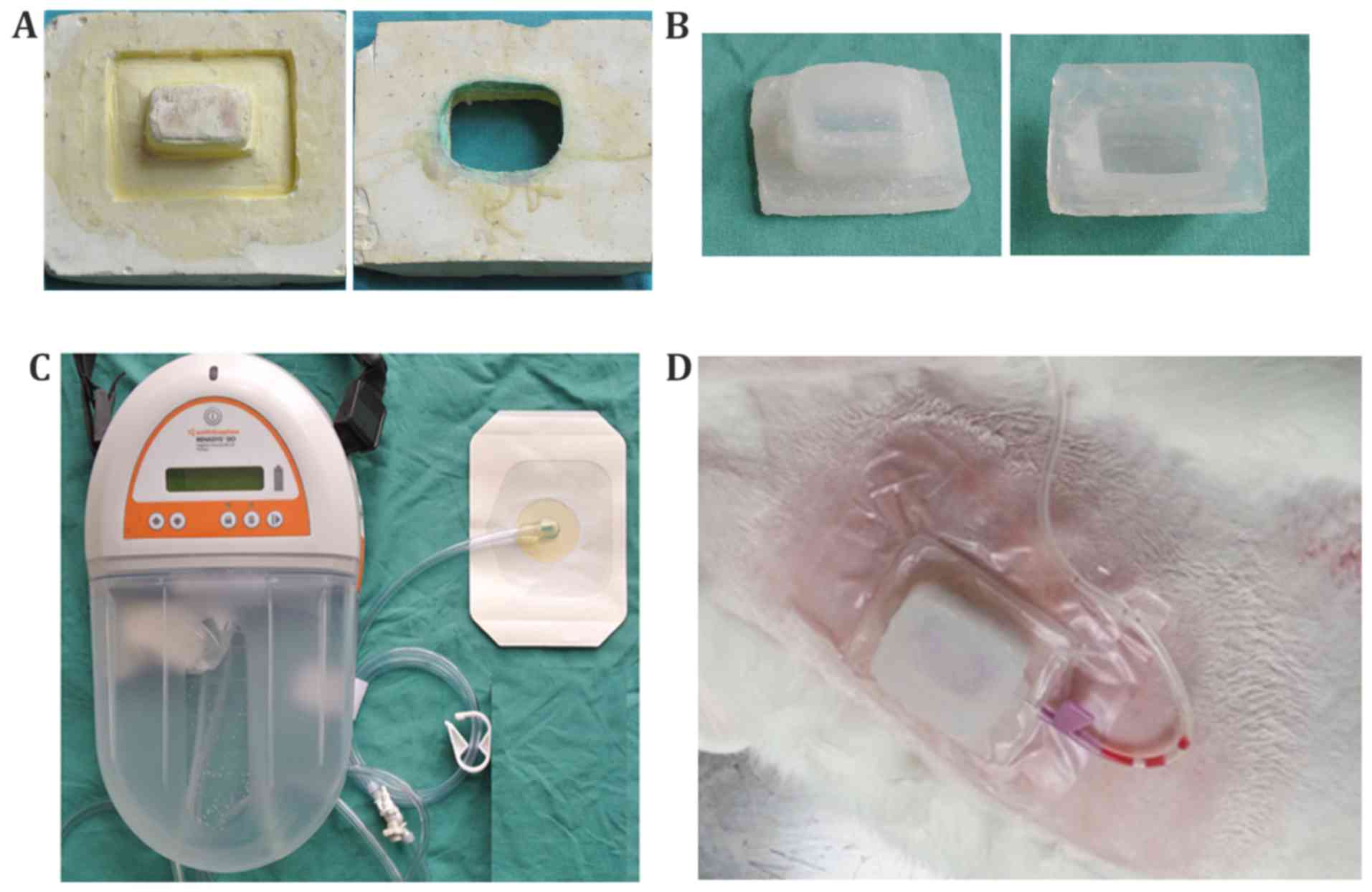
Homemade NP device
The NP device with a cavity was made with medical
silica gel by our laboratory. Briefly, we firstly used paraffin to
carve a model with a square-hat-like structure. The model was then
placed in a glass container, and surrounded by dental gypsum
suspension (special plaster used in the Department of Stomatology).
After the plaster water had evaporated, the paraffin model was
dissolved and removed in hot water to obtain the plaster mold
(Fig. 1A). Subsequently, the
medical silica gel A and B components were mixed at the proportion
of 1:1, and injected into the plaster mold. After being shaped at
room temperature, the plaster mold was removed, and the silicone NP
device (Fig. 1B; length, 4.5 cm;
width, 3.5 cm; height, 2 cm) was ready for use in the following
experiments.
The profile of the homemade silicone NP device could
be punctured by an infusion needle which was connected to a
portable suction pump with an accurate pressure gauge (AZ 8205; AZ
Instrument Corp., Taichung, Taiwan) with a tube, and can aspirate a
pressure of −37.5 mmHg (also known as −5 kPa). The pressure beneath
the homemade device was measured by an intracranial tissue pressure
micro-sensor (Codman; Johnson and Johnson Professional, Inc.,
Raynham, MA, USA) (16), which is
pasted on the skin surface and is small enough to be covered by the
homemade device. It measures both PP and NP in gas and liquids or
any compliant substances, such as soft tissue. The cavity was
sealed to maintain the corresponding pressure.
The VSD device was used as a control device in this
study (Fig. 1C) (RENASYS; Smith
& Nephew, Inc., Andover, MA, USA). VSD is a type of device that
has been widely used in the treatment of trauma wounds. The device
is comprised of a transparent film with the permeability of
biological seal, a drainage tube and drainage device.
The main difference between the VSD and our homemade
device was that the material used was different, and that there was
no cavity between the VSD and the skin, as opposed to the cavity
with our homemade device. In other words, the VSD is placed close
onto the wounded skin and has a vacuum suction effect, while the
homemade device is not placed so close to the skin due to the
existence of a cavity beneath the device.
Animal care and surgical procedure
The skin trauma model was established using 12
female New Zealand rabbits (purchased from SLACCAS, Shanghai,
China) each weighing 2.5–3.0 kg. The dorsum of the animal was
cleaned, shaved and treated with Veet (Reckitt Benckiser,
Parsippany, NJ, USA) for hair removal. After weighing the animals,
1 ml/kg 3% pentobarbital (P3761; Sigma, St. Louis, MO, USA) sodium
was injected intravenously for anesthetization. A circular area of
1 cm in diameter was marked using a pen at both sides of the back
of the rabbits. After disinfecting with chlorhexadine and alcohol 3
times, lidocaine hydrochloride (H37022147; Shandong Hualu
Pharmaceutical Co., Ltd., Liaocheng, China) was injected
subcutaneously in the circular area mentioned above. Subsequently,
a sterile surgical blade was used to make a surgical incision on
the skin along the marked line, followed by blunt separation of the
subcutaneous superficial layer of the fascia using sterile
scissors. Finally, the 1 cm-diameter-circular skin was fully cut
off to construct the wound model of full-thickness skin defect.
Corresponding therapies were randomized on the left
or right side of the animal wound (12 for the control group and 12
for the experimental group). For the control group, each site was
disinfected with iodine and alcohol complex 3 times each day. For
the experimental group, as shown in Fig. 1D, each site was covered by the
homemade NP device mentioned above or the VSD device, and pumped
using a vacuum device to maintain a pressure of −37.5 mmHg. The
pressure that the skin received was detected using the intracranial
tissue pressure microsensor and monitored using an ICP express
monitoring system (Codman; Johnson and Johnson Professional, Inc.).
The animals of experimental groups receive a 2-h treatment each day
for 7 days. The rabbits were euthanized at tbhe indicated time
points (days 3, 7, 10 and 14), and the complete area of the wounds
was harvested and fixed in 4% paraformaldehyde for use in
histological analysis.
Immunohistochemical analysis
Ki67 was used to determine cell proliferation; CD31
for endothelial cells; CK10 for epithelial subset; and Masson's
staining for collagen fibers. The rabbits were euthanized at the
indicated time points. The wounds were excised and fixed in 4%
paraformaldehyde overnight at 4°C, embedded in paraffin, divided
into sections and stained with antibodies to CK10 (bs-11186R;
Bioss, China), CD31 (ab199012; Abcam, Cambridge, USA) and Ki67
(ab15580; Abcam), or Masson's trichrome stain (ab150686; Abcam),
and imaged using an Olympus BX50 microscope with a DP71 camera and
Software cellSens Standard 1.6 (Olympus Corp., Center Valley, PA,
USA). The integrated option density (IOD) of the digital images was
evaluated using Image-Pro Plus software 5.1 (Media Cybernetics,
Inc, Rockville, MD, USA).
Immunofluorescence staining
CD29 and CD90 were used to label MSCs; CK14 and CK19
for different epithelial subsets; and S-100A4 for fibroblasts. The
rabbits were euthanized at the indicated time points. The wounds
were soaked in 30% sucrose in PBS overnight at 4°C and then
embedded in Tissue-Tek O.C.T (Sakura Finetek USA, Inc., Torrance,
CA, USA). Serial 8 mm thick sections were cut at −20°C and placed
on poly-L-lysine-coated microscopic slide. Non-specific labeling
was blocked with 10% normal goat serum for 30 min at room
temperature. The sections were incubated with primary antibodies
against CD29 (bs-3973R; Bioss), CD90 (ab225; Abcam), CK14 (ab77684;
Abcam), CK19 (ab84632; Abcam), and S-100A4 (bs-3759R; Bioss) at 4°C
overnight. Omitting primary antibodies was performed as control
staining. After several washes, the secondary antibodies (ab150077;
Abcam) were applied followed by incubation for 60 min at room
temperature. An Olympus BX51 microscope equipped with fluorescence
and a CCD camera was used to capture the fluorescence images. The
IOD was evaluated using Image-Pro Plus 5.1 software (Media
Cybernetics, Inc.).
Quantitative polymerase chain reaction
(qPCR) for the analysis of mRNA expression
Total RNA was extracted from the wound tissues at
different time points with the use of TRIzol reagent (Life
Technologies, Shanghai, China) according to the manufacturer's
instructions. cDNA was synthesized using the TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems Life Technologies,
Foster City, CA, USA). qPCR was performed with the use of a
fluorescence quantitative PCR instrument (ABI 7500 thermocycler;
Life Technologies) and SYBR-Green Universal PCR Master Mix
(Bio-Rad, Hercules, CA, USA). The oligonucleotide sequences of the
primer sets used were as follows: interleukin (IL)-1β (sense, ACC
AAC AAG TGG TGT TCT CC; antisense, TCT TTG GGT AAC GGT TGG GG),
IL-10 (sense, TCA CCG ATT TCT CCC CTG TG; antisense, GAA GAT GTC
AAA CTC ACT CAT GC), basic fibroblast growth factor (bFGF) (sense,
AGC GGC TGT ACT GCA AAA AC; antisense, AAC GGT TTG CAC ACA CAC CT),
epidermal growth factor (EGF) (sense, AGT GCT CGT ATG TGC TCT TGT
G; antisense, ATT CTA ACC ATT TCC TTC CCA GT), vascular endothelial
growth factor (VEGF) (sense, GCC AGC ACA TAG GGG AGA TG; antisense,
GCT TTC GTT TTT GCC CCT T), insulin-like growth factor-1 (IGF-1)
(sense, CAT GCC CAA GAC TCA GAA GT; antisense, CAA ATG TAC TTC CTT
TCC TTC TC), platelet-derived growth factor (PDGF) (sense, AAG TGT
GAG ACG GTG GCA G; antisense, TGT GCT TGA ACT TGT GGT GC),
transforming growth factor (TGF)-β (sense, ACA GCA TGA ACC GAC CCT
TC; antisense, GGT CCT TGC GGA AGT CAA TG), GAPDH (sense, TTT GTG
ATG GGC GTG AAC C; antisense, CCC TCC ACA ATG CCG AAG T). PCR was
performed in a total volume of 20 µl, including 10 µl
of 2X SYBR-Green qPCR Mix, 1 µl of each forward and reverse
primer (10 µmol/l), 1 µl each cDNA sample, and 7
µl H2O. Amplifications were carried out in
triplicate in 96-well microtiter plates. The thermal cycling
conditions were as follows: 95°C for 5 min, followed by 65 cycles
of 95°C for 10 sec, and 60°C for 10 sec, and finally followed by
72°C for 10 sec.
Western blot analysis
Western blot analysis was performed to detect the
protein expression levels. In brief, tissues and cells were
solubilized in cold RIPA lysis buffer. Proteins were separated by
10% SDS-PAGE, and transferred onto PVDF membranes, which were then
incubated with PBS containing 5% milk overnight at 4°C. The PVDF
membranes were then incubated with primary antibodies [collagen I,
(2 µg/ml, ab6308); collagen III (1:8,000, ab7778); FN, (3
mg/ml, ab6328); MMP13 (1 µg/ml, ab84594); TIMP-1 (0.2
µg/ml, ab126847); TIMP-2 (1:1,000, ab1828); all from Abcam]
at room temperature for 3 h, respectively, and then secondary
antibodies [goat anti-mouse (ab6785, 1:10,000); goat anti-rabbit
(ab6721, 1:20,000); donkey anti-goat (ab6881, 1:10,000); all from
Abcam] at room temperature for 1 h. An ECL kit was then used to
perform chemiluminent detection. Relative protein expression was
analyzed using Image-Pro Plus software 6.0, represented as the
density ratio vs. GAPDH.
Statistical analysis
All experiments were repeated 3 times. All data are
presented as the mean values ± SEM. Two-way ANOVA was used to
compare the differences between groups. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Homemade device-induced NP promotes wound
healing and reduces the wound proportion area more efficiently than
VSD-induced PP
We first measured the pressure generated by the
homemade NP device and VSD, and found that the home-made NP device
generated NP on the skin (Fig.
2A), whereas the VSD device exerted PP on the skin (Fig. 2B). Additionally, we compared the
effects of NP and PP on wound healing. As shown in Fig. 2C, both types of pressure
efficiently promoted wound healing compared with the controls (NP,
P<0.05; PP, P<0.05). Of note, NP was shown to promote wound
healing within a shorter period of time compared to PP (P<0.05).
Additionally, the wound healing rate was measured at different time
points during wound healing (Fig.
2D). The proportion of wound area was significantly reduced in
both pressure groups compared to the control group on day 7 (NP,
P<0.001; PP, P<0.01) and day 10 (NP, P<0.001; PP,
P<0.001); however, exposure to NP led to a more significant
reduction in the wound proportion area than PP (P<0.001)
(Fig. 2E). On the whole, NP more
efficiently promoted wound healing than PP.
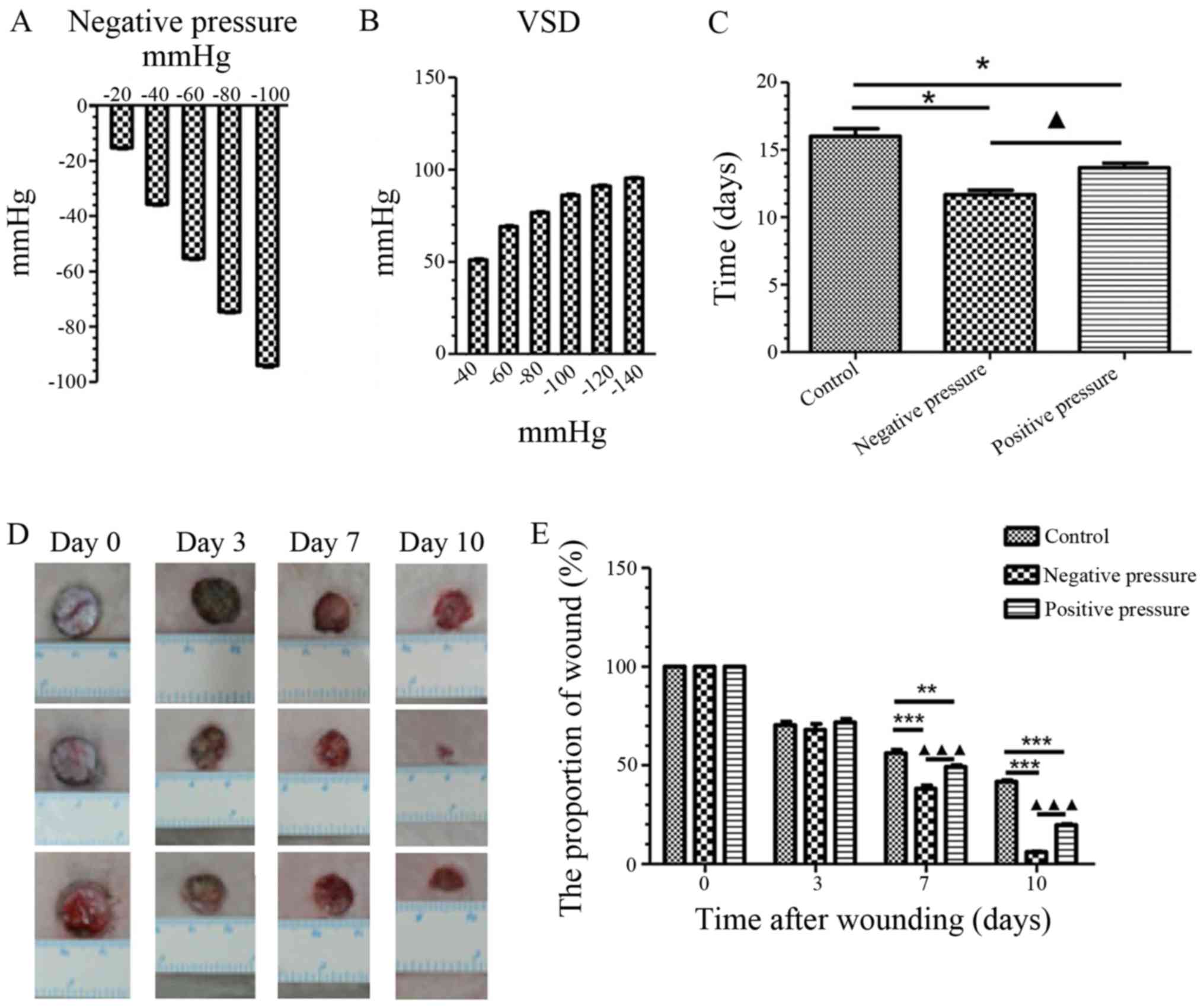 | Figure 2NP promotes wound healing and reduces
the wound proportion area more efficiently than PP. (A) Detection
of pressure on skin that was induced by the homemade device on
wound healing. (B) Detection of pressure on skin that was induced
by the VSD. (C) Statistical analysis of the required time for wound
healing in the different treatment groups. Data are presented as
the means ± SEM, n=3. *P<0.05, compared with the
control; ▲P<0.05, compared with NP. (D)
Representative images of wound healing on days 0, 3, 7, and 10 in
the different treatment groups. (E) Statistical analysis of the
wound proportion area in the different treatment groups. Data are
presented as the means ± SEM, n=3. **P<0.01,
***P<0.001, compared with the control;
▲▲▲P<0.001, compared with NP. NP, negative pressure;
PP, positive pressure; VSD, vacuum sealing drainage. |
NP and PP promote the inflammatory
response and the expression of growth factors in wound healing
The infiltration of macrophages and neutrophils was
examined by tissue analysis following exposure to NP or PP
(Fig. 3A). We found that the
infiltration of macrophages was significantly reduced from days 3
to 10 in both the NP and PP treatment goups compared to the
controls (Fig. 3B). The number of
neutrophils was significantly increased by NP from day 3 compared
with the controls (day 3, P<0.01; day 7, P<0.001; day 10,
P<0.05); however, in the PP group, the number of neutrophils was
increased from day 7 compared to the controls (Fig. 3C). Subsequently,
inflammation-related factors were analyzed in the wound tissue
exposed to NP and PP. During the early stages of wound healing
(from day 0 to day 10), NP treatment promoted the expression of
pro-inflammatory IL-1β on day 3, which was earlier than PP
(Fig. 3D). The expression of
anti-inflammatory factor IL-10 was only increased on day 3 in both
the NP and PP groups (Fig. 3E).
TGF-β was significantly upregulated in the NP and PP groups,
although the effect of NP was more long lasting (Fig. 3F). As regards growth factors in
the promotion of wound healing, the expression levels of EGF and
IGF-1 were both upregulated in the NP and PP groups on days 7 and
10; however, the changes in bFGF expression were more complex
(Fig. 3G–I). NP and PP both led
to an earlier expression peak in PDGF and VEGF, although in the
later stages of wound healing (day 14) a lower expression of VEGF
was observed in the NP and PP groups than in the control (Fig. 3J and K). On the whole, NP and PP
promoted the inflammatory response and the secretion of growth
factors in the earlier stages of wound healing.
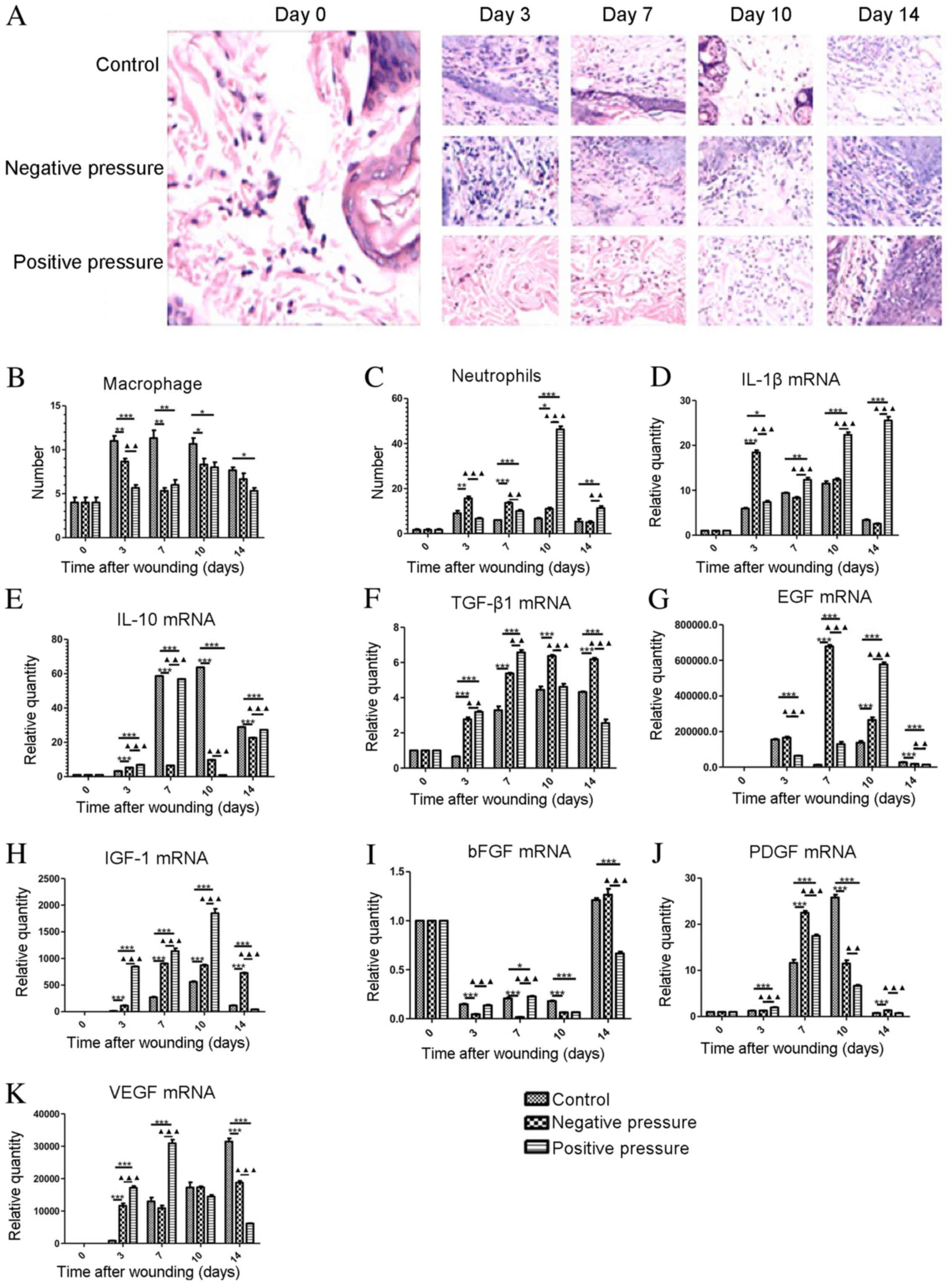 | Figure 3Analysis of inflammatory and growth
factor secretion in wound tissue in the NP and PP groups. (A)
Representative images of H&E staining of inflammatory cells in
wound tissues following pressure treatment on days 3, 7, 10, and
14. The upper lane indicates the control group, the middle lane
indicates the NP group, and the lower lane indicates the PP group.
(B) Statistical quantification of macrophage infiltration in wound
tissue at different time points. (C) Statistical quantification of
neutrophil infiltration in wound tissue at different time points.
Statistical analysis of the mRNA expression of (D) IL-1β, (E)
IL-10, (F) EGF, (G) TGF-β1, (H) IGF-1, (I) bFGF, (J) PDGF, (K) VEGF
in wound tissue examined by qPCR. Data are presented as the means ±
SEM, n=3 experiments. ***P<0.001,
**P<0.01, *P<0.05, compared with the
control. ▲▲▲P<0.001, ▲▲P<0.01, compared
with NP. NP, negative pressure; PP, positive pressure. |
Changes in the proliferation and
distribution of different cell types in wound tissue exposed to NP
and PP
Tissue staining was carried out to examine the
effects of NP and PP on the proliferation and distribution of
different cell types in the wound tissue. As shown in Fig. 4, Ki67 was used to detect cell
proliferation, endothelial cells were labeled with CD31, while MSCs
were labeled with CD90 and CD29; CK10, CK14 and CK19 were used to
stain the different epithelial cell subsets; S-100A4 and Masson's
trichrome staining were used to mark the fibroblasts and collagen
fibers. The quantified results of the histological analysis in
Fig. 4 are all shown in Fig. 5. The expression of Ki67 was used
for the detection of proliferation. As shown in Fig. 5A, NP and PP both significantly
increased cell proliferation in the wound tissue on day 10 compared
to the controls (NP, P<0.001; PP, P<0.01); however, the
effect of NP on proliferation was more potent than that of PP
(P<0.001). There was no significance among the groups at the
other time points examined. These data indicated that the wound
tissue was in a proliferative state, and that exposure to NP led to
significantly greater proliferation on day 10, as evidenced by the
increase in Ki67 expression.
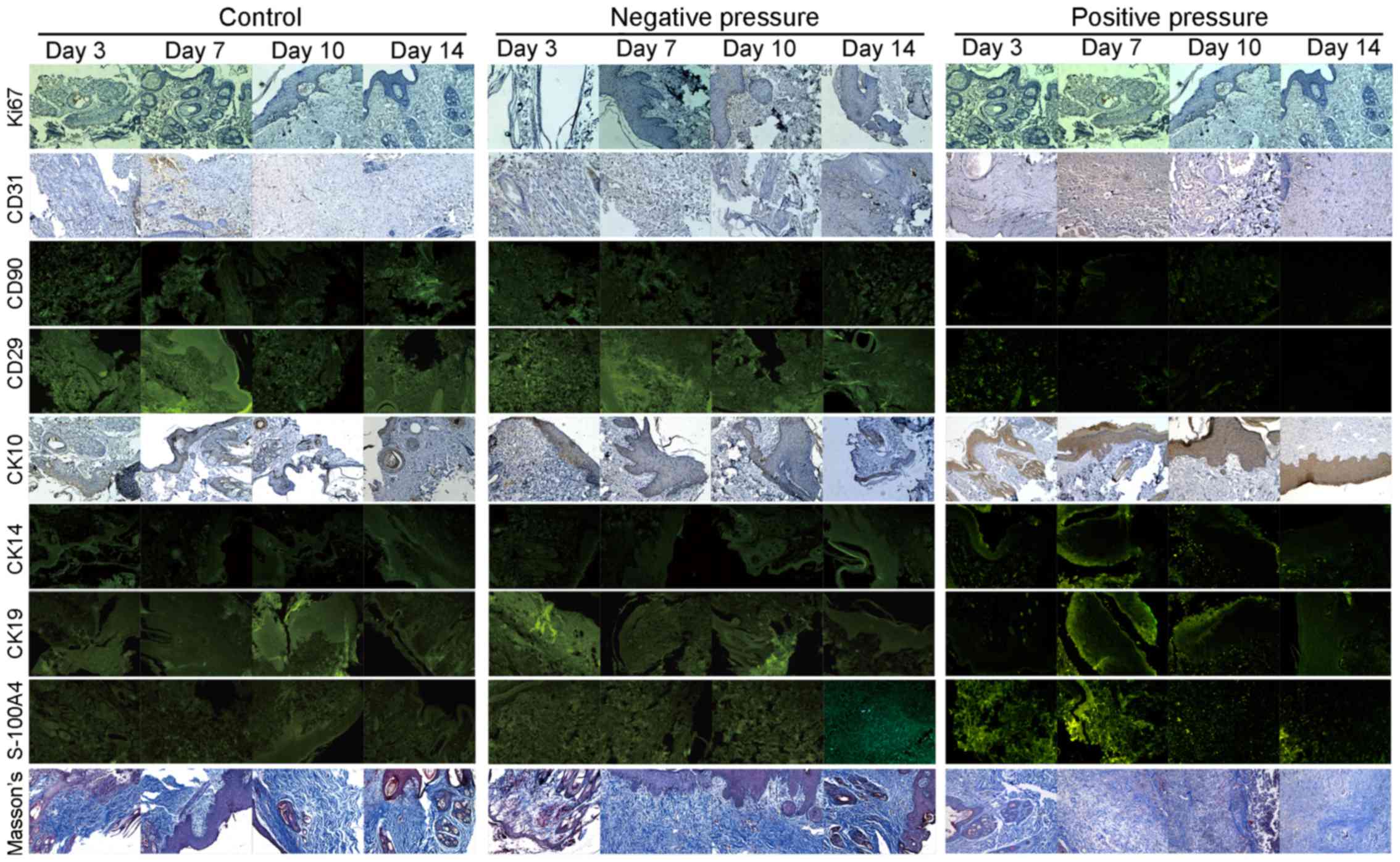 | Figure 4Histological analysis of different
cell markers in wound tissue in the different pressure treatment
groups. Representative images of H&E staining and
immunofluorescence staining in wound tissues following pressure
treatment on days 3, 7, 10, and 14. Ki67, CD31, CD90, CD29, CK10,
CK14, CK19, S-100A4, and Masson's staining were all used for
histological analysis. |
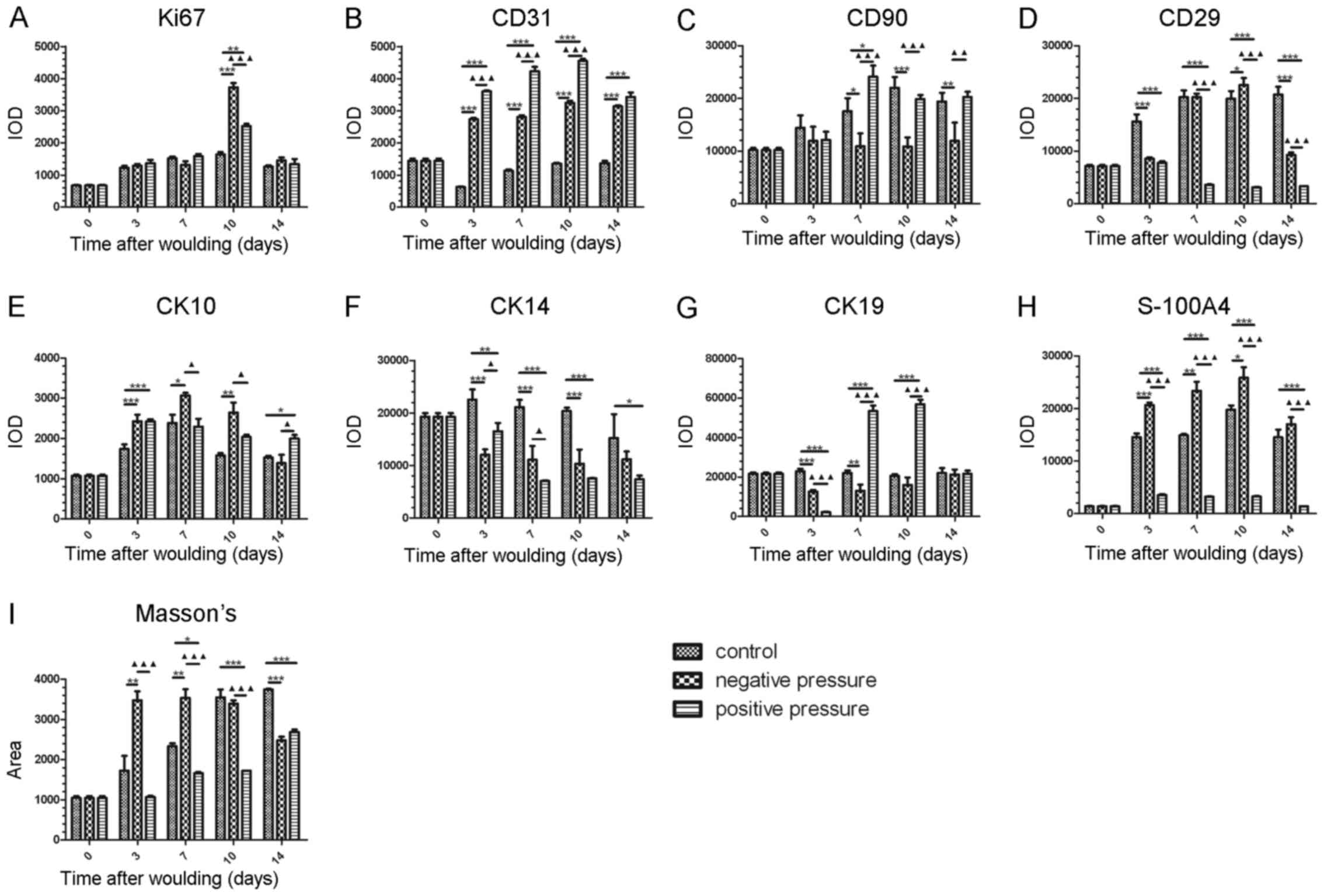 | Figure 5Statistical analysis of the
histological results of different cell markers in wound tissue in
the different pressure treatment groups. Statistical quantification
of the IOD value of (A) Ki67, (B) CD31, (C) CD90, (D) CD29, (E)
CK10, (F) CK14, (G) CK19, (H) S-100A4, (I) Masson's staining. Data
are presented as the means ± SEM, n=3 experiments.
***P<0.001, **P<0.01,
*P<0.05, compared with the control.
▲▲▲P<0.001, ▲▲P<0.01, compared with NP.
IOD, integrated option density; NP, negative pressure. |
We then measured the number of endothelial cells in
wound tissue by CD31-positive staining. As shown in Fig. 5B, the IOD value of CD31 was
significantly increased in the NP and PP groups compared with the
controls from day 3 (P<0.001); however, PP increased the number
of CD31-positive cells more significantly than NP in the wound
tissue from days 3 to 10 (P<0.001).
The number of MSCs in the wound tissue was then
detected by CD29- and CD90-double-positive staining. Our data
indicated that the CD29- and CD90-positive area in the NP group was
significantly reduced at the later stage of healing, while the
CD90-positive area in the PP group showed no reduction when
compared with the control group from days 0 and 14, although the
CD29-positive area in the PP group was reduced from days 3 to day
14 (Fig. 5C and D). In general, a
lower amount of MSCs was detected following exposure to NP in wound
healing.
In order to examine the distribution of different
epithelial cell subsets in wound healing, CK10, CK14 and CK19 were
used to stain the slides. CK10 was mainly secreted by the
keratinized stratified epithelium, and CK14 and CK19 were used to
identify the basal layer epithelia and the epithelia with stemness,
respectively. Our data demonstrated that NP induced a more
significant increase in the number of CK10-positive cells in wound
tissue from days 3 to 10; PP increased the number of CK10-positive
cells more significantly on day 14 (Fig. 5E). Additionally, cells positive
for CK14 were significantly decreased following exposure to NP and
PP (Fig. 5F). PP induced a
significant increase in the number of CK19-positive cells on days 7
and 10; however, NP induced a decreased in the number of
CK19-positive cells on day 3 and 7 (Fig. 5G). This indicated that NP
treatment generated more keratinized stratified epithelium, and
less basal layer epithelia and epithelia with stemness in the early
stages of wound healing, al no difference was observed on day 14.
PP treatment led to an increase of epithelia with stemness in the
early stages of wound healing, which differed from the effects of
NP.
For the measurement of fibroblasts and collagen
fibers in the wound tissue, S-100A4 and Masson's trichrome were
used to stain the tissues, respectively. We found that NP
significantly increased the proportion of fibroblasts (P<0.001)
and collagen fibers (P<0.01) in wound tissue, while PP induced
lowr amounts of these markers compared to NP (P<0.001) (Fig. 5H and I).
NP and PP modulate the generation of ECM
components
In order to examine the structural recovery effect
of NP and PP on wound healing, we analyzed the dermal papilla
structure. As shown in Fig. 6A,
the dermal papilla structure was flat and the arrangement of
collagen fibers was disrupted in the 3 groups. Additionally, wound
healing-related protein expression levels were measured following
exposure to NP and PP (Fig. 6B).
As shown in Fig. 6B–D, the levels
of collagen I and III were significantly decreased in the NP and PP
groups compared with the controls (P<0.001 and P<0.001). NP
induced a more significant increase in the expression of FN on day
3 (P<0.001) and day 10 (P<0.001) compared to PP (Fig. 6B and E). In addition, MMP-13
expression increased in the NP group (P<0.001), but decreased in
the PP group (P<0.001) on day 3 (Fig. 6B and F). NP also induced a
decrease in TIMP-1 (P<0.001) and TIMP-2 (P<0.001) expression
on days 7 and 3, respectively; this effect was significantly
different from the upregulatory effect of PP on TIMP-1 (P<0.001)
and TIMP-2 (P<0.001) at the corresponding time points (Fig. 6B, G and H). Collectively, NP and
PP did not alter the dermal papilla structure and the arrangement
of collagen fibers. NP induced a greater increase in MMP-13
expression and decreased TIMP-1/2 expression during the early
stages of wound healing.
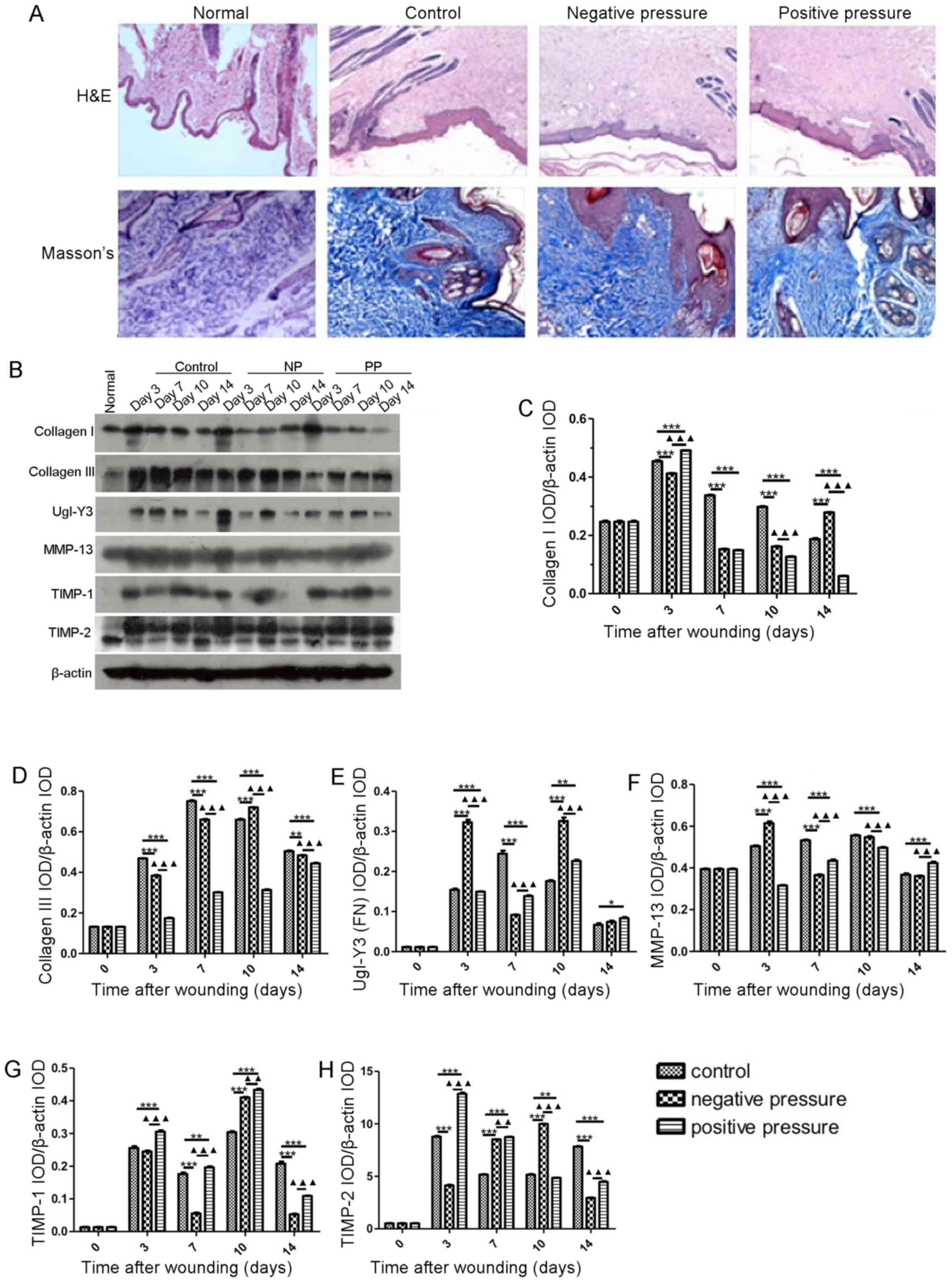 | Figure 6NP and PP affect the generation of
extracellular matrix components. (A) Representative images of
dermal papilla structure in the different pressure treatment
groups. (B) Protein expression of different matrix components in
wound tissues treated with different pressure types. Different time
points (day 3, 7, 10 and 14) were recorded. Statistical
quantification of IOD value of (C) collagen I, (D) collagen III,
(E) UgI-Y3 (FN), (F) MMP-13, (G) TIMP-1, (H) TIMP-2. Data are
presented as the means ± SEM, n=3 experiments.
***P<0.001, **P<0.01,
*P<0.05, compared with the control.
▲▲▲P<0.001, ▲▲P<0.01, compared with NP.
NP, negative pressure; PP, positive pressure; IOD, integrated
option density. |
Discussion
The VSD technique is a therapeutic strategy which is
widely used to promote rapid wound healing in traumatic soft wounds
and chronic infections. In this study, we compared the VSD to a
homemade device we created. The material used to create our
homemade device differed from that used for the VSD. In addition,
the main difference between the VSD and our homemade device in this
study, was that in the VSD, there was no cavity between the device
and the skin. As a result, the VSD is placed very close to the skin
wound for vacuum suction, and the pressure on the skin is thus not
negative, but positive (16). In
other words, the pressure is perpendicular to the skin and is
directed downwards. Additionally, as shown in our study, the
existence of the cavity between the homemade device and the skin
meant that NP was exerted on the skin after vacuum suction, the
pressure being perpendicular to the skin and directed upwards.
Furthermore, we examined the effects and differences between PP and
NP on wound healing. Both types of pressure promoted the wound
healing process and reduced the proportion of the wound. However,
NP exerted more prominent wound healing effects. NP induced greater
neutrophil infiltration and more pro-inflammatory factor secretion
during the early stages of healing. Both NP and PP upregulated the
expression of growth factors, such as EGF, VEGF, PDGF and TGF-β1.
Furthermore, we found that cell proliferation in the wound tissue
was significantly increased by NP on day 10. The distribution scale
of different cell types and the generation of collagen in wound
tissue were also altered following pressure treatment.
Inflammation is an essential stage of wound healing.
Within hours of wounding, neutrophils are recruited to the wound
site, and phagocytose debris and kill bacteria (17,18). In this study, we detected the
infiltration of neutrophils in wound tissues, and found that NP
increased neutrophil infiltration to a greater extent than PP on
day 3, which may enhance clearance at the early stages of wound
healing and may be helpful in accelerating the healing process. In
the NP group, the level of neutrophils from day 10 was similar to
that of the controls, suggesting that this may help diminish the
continuous or extended inflammation that often impedes the
following stage of healing; this effect was confirmed by the
results of the expression of IL-1β on day 10 and 14, which was
similar between the control and NP group on these days. This was
one of the key differences between NP and PP.
During the wound healing process, endothelial cells,
fibroblasts and collagen significantly infiltrated the wound tissue
following pressure treatment, while MSCs in the wound tissue in the
NP group were lower in number compared to the control. In addition,
NP promoted more keratinized stratified epithelium, and less basal
layer of stratified epithelia and epithelia with stemness. These
data are in accordance with those of other studies, that have
proven that wound tissue treated with negative pressure wound
therapy (NPWT) increases epithelial cell migration (19), endothelial migration (20,21) and dermal fibroblast migration
(22). Resident MSCs and
circulating progenitor cells have also been observed to migrate
into granulation tissue following the application of NPWT (23). Of note, the differentiation of
epithelial cells during NPWT has been found to decrease (19). Cytokeratin (CK) is a member of
intermediate filaments found in the intra-cytoplasmic cytoskeleton
of epithelial tissue. It is well known that CK plays a critical
role in cell structure integrity, and the expression of CK is
remarkably tissue- and differentiation-specific. Each type of
epithelium has its specific pattern of CK expression. CK10 is a
specific keratinization marker; CK14 is a marker of basal cells
layer; CK19 is a biochemical marker of skin stem cells in
vivo (24). Our data
indicated that NP treatment caused more keratinized epithelium, but
less basal cell layer and skin stem cells. Epithelial cells play a
critical role in wound healing, and their migration across the
wound tissue to form a keratinized epithelium between the wound and
the environment is one of the most important processes in wound
healing. This may lead to the efficient healing effect of NPWT.
However, our data demonstrated that the infiltration of MSCs was
decreased in the NP group, which may indicate some disadvantages of
NPWT. Firstly, the major mechanisms of the effects of MSCs on the
wound repair process are thought to be structural repair via
cellular differentiation, immune modulation and the secretion of
cytokines, which may promote angiogenesis and the recruitment of
fibroblasts and other cells (25). The low level of MSC infiltration
in wound tissue will lead to chronic wounds and will delay the
repair process. In addition, another consideration in the repair of
wounds is the formation of scars, caused by deposition of excess
ECM by fibroblasts in the wound bed. The cytokines and growth
factors secreted by MSCs have been proved to reduce scar formation
at the site of injury (26). The
low level of MSCs infiltration in wound tissue will lead to an
increased scar formation. Accordingly, although NPWT in promoting
wound healing has been largely accepted by clinicians, the number
of high-level clinical studies demonstrating its effectiveness is
still limited (27). Thus, our
data may indicate some disadvantages of NPWT that may need to be
improved.
Collagen deposition was also influenced by NP and PP
treatment. The significantly increased levels of collagen I in the
NP group indicated that NP promoted the maturation of wounds on day
14. In addition, the significant increase in MMP-13 levels and the
decrease in TIMP-1/2 levels on days 3 or 7 in the NP vs. the PP
group may be helpful for completing the matrix remodeling in the
early stages of healing, which may promote the wound healing
effect.
In conclusion, our study demonstrates that NP is
more effective than PP for wound healing by promoting the
inflammation during the early stages of healing, increasing
proliferation in the wound tissue, increasing the number of
endothelial cells, epithelial cells and fibroblasts, and
strengthening the remodeling process and matrix maturation.
Acknowledgments
This study was supported by the Key Projects of the
Fund of Science and Technology Department of Hunan Province
(2014SK2018) and the Scientific Research Fund of the Health
Department of Hunan Province (132013-026).
References
|
1
|
Watterson KR, Lanning DA, Diegelmann RF
and Spiegel S: Regulation of fibroblast functions by
lysophospholipid mediators: Potential roles in wound healing. Wound
Repair Regen. 15:607–616. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ko SH, Nauta A, Wong V, Glotzbach J,
Gurtner GC and Longaker MT: The role of stem cells in cutaneous
wound healing: What do we really know? Plast Reconstr Surg.
127(Suppl 1): 10S–20S. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans ND, Oreffo RO, Healy E, Thurner PJ
and Man YH: Epithelial mechanobiology, skin wound healing, and the
stem cell niche. J Mech Behav Biomed Mater. 28:397–409. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maxson S, Lopez EA, Yoo D,
Danilkovitch-Miagkova A and Leroux MA: Concise review: Role of
mesenchymal stem cells in wound repair. Stem Cells Transl Med.
1:142–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Darby IA and Hewitson TD: Fibroblast
differentiation in wound healing and fibrosis. Int Rev Cytol.
257:143–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nuschke A: Activity of mesenchymal stem
cells in therapies for chronic skin wound healing. Organogenesis.
10:29–37. 2014. View Article : Google Scholar :
|
|
7
|
Shi J, Xi W, Yi C, Wang Z, Guo S and Han
Y: Vacuum sealing drainage promotes experimental pig explosive
abdomen wound healing. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
30:312–315. 2014.In Chinese. PubMed/NCBI
|
|
8
|
Argenta LC, Morykwas MJ, Marks MW,
DeFranzo AJ, Molnar JA and David LR: Vacuum-assisted closure: State
of clinic art. Plast Reconstr Surg. 117(Suppl): 127S–142S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banwell P and Téot L: Topical negative
pressure (TNP): The evolution of a novel wound therapy. J Tissue
Viability. 16:16–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morykwas MJ, Simpson J, Punger K, Argenta
A, Kremers L and Argenta J: Vacuum-assisted closure: State of basic
research and physiologic foundation. Plast Reconstr Surg.
117(Suppl): 121S–126S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banwell PE: Topical negative pressure
therapy in wound care. J Wound Care. 8:79–84. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fonder MA, Lazarus GS, Cowan DA,
Aronson-Cook B, Kohli AR and Mamelak AJ: Treating the chronic
wound: A practical approach to the care of nonhealing wounds and
wound care dressings. J Am Acad Dermatol. 58:185–206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hunter JE, Teot L, Horch R and Banwell PE:
Evidence-based medicine: Vacuum-assisted closure in wound care
management. Int Wound J. 4:256–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen SZ, Li J, Li XY and Xu LS: Effects of
vacuum-assisted closure on wound microcirculation: An experimental
study. Asian J Surg. 28:211–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans D and Land L: Topical negative
pressure for treating chronic wounds: A systematic review. Br J
Plast Surg. 54:238–242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kairinos N, Solomons M and Hudson DA: The
paradox of negative pressure wound therapy - in vitro studies. J
Plast Reconstr Aesthet Surg. 63:174–179. 2010. View Article : Google Scholar
|
|
17
|
Muller MJ, Hollyoak MA, Moaveni Z, Brown
TL, Herndon DN and Heggers JP: Retardation of wound healing by
silver sulfadiazine is reversed by Aloe vera and nystatin. Burns.
29:834–836. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greenhalgh DG: The role of apoptosis in
wound healing. Int J Biochem Cell Biol. 30:1019–1030. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nuutila K, Siltanen A, Peura M, Harjula A,
Nieminen T, Vuola J, Kankuri E and Aarnio P: Gene expression
profiling of negative-pressure-treated skin graft donor site
wounds. Burns. 39:687–693. 2013. View Article : Google Scholar
|
|
20
|
Baldwin C, Potter M, Clayton E, Irvine L
and Dye J: Topical negative pressure stimulates endothelial
migration and proliferation: A suggested mechanism for improved
integration of Integra. Ann Plast Surg. 62:92–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Potter MJ, Banwell P, Baldwin C, Clayton
E, Irvine L, Linge C, Grobbelaar AO, Sanders R and Dye JF: In vitro
optimisation of topical negative pressure regimens for angiogenesis
into synthetic dermal replacements. Burns. 34:164–174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McNulty AK, Schmidt M, Feeley T and
Kieswetter K: Effects of negative pressure wound therapy on
fibroblast viability, chemotactic signaling, and proliferation in a
provisional wound (fibrin) matrix. Wound Repair Regen. 15:838–846.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu F, Ogawa R, Nguyen DT, Chen B, Guo D,
Helm DL, Zhan Q, Murphy GF and Orgill DP: Microdeformation of
three-dimensional cultured fibroblasts induces gene expression and
morphological changes. Ann Plast Surg. 66:296–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Michel M, Török N, Godbout MJ, Lussier M,
Gaudreau P, Royal A and Germain L: Keratin 19 as a biochemical
marker of skin stem cells in vivo and in vitro: Keratin 19
expressing cells are differentially localized in function of
anatomic sites, and their number varies with donor age and culture
stage. J Cell Sci. 109:1017–1028. 1996.PubMed/NCBI
|
|
25
|
Balaji S, Keswani SG and Crombleholme TM:
The role of mesenchymal stem cells in the regenerative wound
healing phenotype. Adv Wound Care (New Rochelle). 1:159–165. 2012.
View Article : Google Scholar
|
|
26
|
Ankrum J and Karp JM: Mesenchymal stem
cell therapy: Two steps forward, one step back. Trends Mol Med.
16:203–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang C, Leavitt T, Bayer LR and Orgill
DP: Effect of negative pressure wound therapy on wound healing.
Curr Probl Surg. 51:301–331. 2014. View Article : Google Scholar : PubMed/NCBI
|