Introduction
Vascular smooth muscle cells (VSMCs) are one of the
major cellular components of the blood vessel wall, and are mainly
responsible for the regulation of blood flow distribution and blood
pressure (1,2). Under physiological conditions, VSMCs
maintain an extremely low proliferation rate, but they are highly
plastic and can convert from a differentiated phenotype to
dedifferentiated phenotype as adaptive responses to environmental
changes (2–4). In the process of phenotypic
modulation, VSMCs are characterized by an increased abilities of
proliferation and migration, as well as an increase in
extracellular matrix protein deposition, which collectively can
accelerate atherosclerosis, hypertension, and diabetic vascular
complications (5,6). An increasing number of studies have
demonstrated a variety of factors including growth factors,
cytokines, mitogens, cell adhesion, cell-cell contact, mechanical
influences, extracellular matrix interactions that may control the
phenotypic modulation of VSMCs (7). In diabetics, accumulating evidence
has demonstrated that the production and accumulation of advanced
glycation end products (AGEs) play an important role in regulating
the proliferation and migration of VSMCs (8–11),
indicating that AGEs are an important mediator in various vascular
diseases, particularly diabetic vascular complications.
AGEs result from a slow nonenzymatic glycation
reaction between sugars and amine groups present in proteins,
lipids or DNA, and can form and accumulate in diabetics (12). AGE formation can activate the
receptor (RAGE) and furthermore lead to an aberrant activation of
multiple signaling pathways including nuclear factor-κB (NF-κB)
(11), mitogen-activated protein
kinases (MAPK) (12) and PI3K/AKT
(13). Our previous study also
indicated that AGEs could promote proliferation and suppress
autophagy via reduction of cathepsin D in VSMCs (14). Notably, several pathways are
involved in oxidative stress via an increased production of
reactive oxygen species (ROS) (14–17), suggesting that oxidative stress
could be an important contributor to the proliferation and
migration of VSMCs induced by AGEs. However the underlying
mechanisms are so complex that there is still much to be
explored.
Bcl-2-associated athanogene 3 (BAG3) is a member of
the BAG family and plays an important role in diverse cellular
behaviors including cell apoptosis, autophagy, proliferation,
adhesion, migration, and differentiation (18–20). As a previous study summarized,
BAG3 expression could be upregulated in a varieties of human
primary tumors (21). Normal
tissues seldom express BAG3, except for cardiomyocytes and skeletal
muscle cells, but its expression is induced upon exposure to
various stressful stimuli (22).
In recent years, accumulating evidence indicates that BAG3 is also
associated with various cardiovascular diseases such as myocardial
hypertrophy, dilated cardiomyopathy, Takotsubo cardiomyopathy and
chronic heart failure (23–26). To date, the role of BAG3 in the
proliferation and migration of VSMCs has not been explored.
Therefore, the present study was aimed to
investigate the role of ROS in AGE-induced proliferation and
migration of VSMCs and whether BAG3 is involved in the process.
Materials and methods
Ethics statement
Animals used in this study were treated in
accordance with the NIH Guide for the Care and Use of Laboratory
Animals. The procedures were in accordance with the Ethical
Standards of the Committee on Animal Experimentation of China
Medical University (project identification code,
SCXK-2013-0001).
Materials
The Real-Time polymerase chain reaction (qPCR)
system was purchased from Applied Biosystems (Foster City, CA,
USA). Click-iT Nascent RNA Capture kit was purchased from
Invitrogen (Carlsbad, CA, USA). Western blot analysis-related
equipment was purchased from Invitrogen. EdU Alexa Fluor 555
Imaging kit was purchased from Invitrogen. Tetramethylrhodamine
methyl ester (TMRE) was purchased from Molecular Probes (Eugene,
OR, USA). 2′,7′-Dichlorofluorescein diacetate (DCFH-DA),
N-acetylcysteine (NAC), cycloheximide (CHX), and actinomycin
D were purchased from Sigma (St. Louis, MO, USA). BSA and AGEs-BSA
were obtained from Merck-Millipore (Darmstadt, Germany). A
fluorescence microscope (CKX41-F32FL) was purchased from Olympus
(Tokyo, Japan). Microchemi 4.2 was purchased from DNR Bio-Imaging
Systems, Ltd. (Jerusalem, Israel). Transwell-related equipment
(8-µm pore) was purchased from BD Biosciences (Franklin
Lakes, NJ, USA). Microplate reader was purchased from Bio-Rad
(Hercules, CA, USA). Antibodies for BAG3 (sc-292154) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sc-47724) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Lentiviral vectors were purchased from GeneChem (Shanghai,
China).
Isolation and culture of primary rat
VSMCs
Neonatal rats (1–2 days old) were sacrificed by
cervical dislocation, disinfected with 75% alcohol, and then moved
to a clean bench. The thoracic aorta was excised and the
inner/outer layers of blood vessels were removed. Primary neonatal
rat VSMCs were then isolated as described in our previous study
(14). VSMCs were cultured in
complete medium including 10% fetal bovine serum (FBS) and
penicillin-streptomycin (100 U/ml-100 µg/ml) at 37°C, in 5%
CO2 and a humidified atmosphere as previously described
(14,27). Media were changed every other day.
After primary cells achieved 80–90% confluence, 0.25% trypsin was
added into the culture plate for digestion. Thirty seconds later,
serum-containing medium was used to terminate the digestion.
Subsequently, a part of the cells was moved into a new culture
dish. After attachment to the wall, the cells were incubated with
complete medium, which was passage 2 of VSMCs. Using the methods,
cells between passages 2 to 8 were obtained and applied for the
next experiments.
Construction of BAG3 plasmid and cell
transfection
The construction of the BAG3 plasmids was carried
out by GeneChem. The cells were transfected with Lipofectamine 2000
reagent (Invitrogen) as previously described (28). The lentiviral plasmids which
contained short hairpin RNA (shRNA) against rat BAG3 or control
shRNA were labelled with green fluorescent protein (GFP) and used
in the knockdown experiments. There were five shRNA
oligo-nucleotides specific for rat BAG3, i.e. shBAG3#1, #2, #3, #4
and #5. The titers of control shRNA and shBAG3#1, #2, #3, #4 and #5
were 8×108, 4×108, 4×108,
3×108, 3×108 and 4×108 TU/ml,
respectively. Cells were seeded into 6-well plate and incubated
with vector supernatants at a multiplicity of infection (MOI) of
100 for 12 h. Then the old culture medium was removed and replaced
with DMEM with 10% FBS. After being cultured for 2 days, the cells
were observed under fluorescence microscopy and transduction
efficiency was calculated using the following formula: Transduction
efficiency = GFP+ cells/total cells. After digestion,
transduced cells were cultured for another 2 days. To confirm that
the transduction was successful, the mRNA and protein expression
levels of BAG3 were analyzed by quantitative PCR (qPCR) and western
blot analysis, respectively.
Western blot analysis
Western blot analysis was performed as described in
our previous studies (14,29).
Briefly, the cells were solubilized in a radio-immunoprecipitation
assay (RIPA) lysis buffer for 30 min, and then total protein
concentrations were measured by a BCA protein assay kit (Beyotime,
Shanghai, China). After heat denaturation, the samples were
analyzed on a 12 or 14% Tris-glycine gradient gel, and then
transferred to PVDF membranes and blocked with 5% nonfat milk in
Tris-buffered solution (TBS) for 1.5 h at room temperature. The
membranes were incubated with primary antibody overnight at 4°C.
After washing three times with TBS, the membranes were incubated
with secondary antibodies for 1.5 h at room temperature. After the
washing steps, immunoreactive binding was detected with enhanced
chemiluminescence (ECL) (Amersham Biosciences, Piscataway, NJ,
USA). The band intensity was quantified using ImageJ 1.47 software
and GAPDH served as a control.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
MTT assay was used to determined cell viability.
VSMCs were seeded in a 96-well plate at a density of
4×103 cells/well. After being cultured for 48 h, the
cells were incubated with MTT solution (final concentration, 5
mg/ml) for 4 h at 37°C. Then the culture media containing MTT were
removed and replaced with 100 µl DMSO. Then the plate was
gently rotated on a linear and orbital shaker for 5 min to
completely dissolve the precipite. The absorbance was measured with
a microplate reader at a wavelenght of 570 nm. The percentage of
cell viability was calculated according to the following formula:
Cell viability (%) = optical density (OD) of the treatment group/OD
of the control group ×100%.
EdU incorporation analysis
As described in our previous studies (14,30) and according to the manufacturer's
instructions, the DNA synthesis rate in VSMCs was determined by EdU
incorporation analysis using Click-iT™ EdU Alexa Fluor 555 Imaging
kit. Briefly, the cells were incubated with EdU-labeling solution
for 8 h at 37°C, and then fixed with 4% cold formaldehyde for 30
min at room temperature. After permeabilization with 1% Triton
X-100, the cells were reacted with Click-iT reaction cocktails
(Invitrogen) for 30 min. Subsequently, the DNA contents of the
cells were stained with Hoechst 33258 for 30 min. Finally,
EdU-labeled cells were counted using ImageJ 1.47 software and
normalized to the total number of Hoechst-stained cells. At least
500 cells in each experiment were counted, and EdU-positive cells
are expressed as a percentage of the total cells.
Wound-healing assay
Cells at 80–90% confluence were wounded with a
200-µl pipette tip and incubated with BSA or AGEs for 24 h.
Multiple views of the leading edge of the scratch were photographed
under a microscope at 0 and 24 h. The experiments were performed
three times independently.
Transwell migration assay
For the Transwell migration assay, cells were seeded
at a density of 2×106 cells in the upper chamber. The
lower chamber was filled with BSA or AGEs. After being cultured for
24 h, the cells on the upper chamber were removed by gentle
abrasion with a cotton swab, and the cells on the lower chamber
were fixed and stained with Hoechst 33258. Three experiments were
performed independently. The cells that had passed through the
filter were photographed under a fluorescence microscope with
ultraviolet light. Hoechst-labeled cells in five representative
microscopic fields were counted using ImageJ 1.47 software.
RNA extraction and qPCR
The total RNA was extracted using the Qiagen RNeasy
Mini kit. After the determination of concentration, the synthesis
of cDNA was performed. With a primer design software we synthesized
the sense and antisense primers of each fragment: BAG3 sense,
5′-CATCCAGGAGTGCTGAAAGTG-3′ and antisense primer,
5′-TCTGAACCTTCCTGACACCG-3′; GAPDH sense, 5′-GCACCGTCAAGGCTGAGAAC-3′
and antisense primer, 5′-TGGTGAAGACGCCAGTGGA-3′. qPCR was run and
analyzed with the 7500 Real-Time-PCR system. Results were
normalized against those of GAPDH and are presented as arbitrary
unit.
Labeling and capture of nascent RNA
Click-iT Nascent RNA capture kit was used to detect
newly synthesized RNA according to the manufacturer's instructions.
5-Ethymyl uridine (EU) is an alkyne-modified uridine analog and it
is efficiently and naturally incorporated into nascent RNA. Cells
were incubated in 0.2 mM of EU for 4 h and total RNA labeled with
EU was isolated using Trizol reagent (Invitrogen). Then EU-labeled
RNA was biotinylated in a Click-iT reaction buffer with 0.5 mM of
biotin azide and subsequently captured on streptavidin magnetic
beads.
Measurement of mitochondrial membrane
potential
We used TMRE (Ex/Em, 549/573 nm) to detect changes
in mitochondrial membrane potential, as described in our previous
study (31). Briefly, unfixed
live cells were incubated with 100 nM TMRE in the dark for 30 min
at 37°C in 5% CO2. After being washed, the cells were
analyzed under a fluorescence microscope. The fluorescence
intensity of TMRE staining was quantified using ImageJ.
Measurement of intracellular ROS
The formation of intracellular ROS was measured with
the DCFH-DA (Ex/Em, 485/530 nm) method, as described in our
previous study (31). DCFH-DA
transforms into the fluorescent compound dichlorofluorescein (DCF)
upon oxidation by ROS. Briefly, the cells were incubated with
DCFH-DA at a final concentration of 5 mM at 37°C in 5%
CO2 in darkness for 40 min. After being washed, the
cells were analyzed under a fluorescence microscope. The
fluorescence intensity of DCFH-DA staining was quantified using
ImageJ.
Statistical analysis
Data were obtained from at least three individual
experiments. Continuous variables were expressed as the mean ± SD
and tested by one-way ANOVA or Student's t-test. All the
statistical analyses were performed using SPSS statistics for
Windows (version 17.0; SPSS, Chicago, IL, USA), and p-values
<0.05 were considered statistically significant.
Results
AGEs increase the expression of BAG3 in
primary rat VSMCs
The cells were treated with different concentrations
of AGEs (25, 50, 100 and 200 µg/ml) and BSA (2.5, 5, 10 and
20 µg/ml) for 24 h, respectively. We found that AGEs
increased the BAG3 mRNA (Fig. 1A)
and protein (Fig. 1B) expression
in the VSMCs in a dose-dependent manner using qPCR and western blot
analysis, respectively. Next, we addressed whether transcriptional
and translational inhibitors (actinomycin D and CHX, respectively)
could modulate the effect of AGEs on the expression of BAG3 in
VSMCs. The results of qPCR showed that actinomycin D significantly
reduced the expression of BAG3 mRNA induced by AGEs while CHX had
no effects (Fig. 1C), which
indicated that AGEs mainly increased the expression of BAG3 mRNA by
increasing the RNA synthesis rather than inhibiting the RNA
translation. Next, to further support these observations, we
evaluated the effect of AGEs on BAG3 mRNA expression using Click-iT
Nascent RNA capture kit to isolate newly synthesized RNA. The
results of qPCR indicated that AGEs significantly increased BAG3
mRNA synthesis at 24 h with the maximal stimulation effect at a
concentration of 100 µg/ml (Fig. 1D). Then, the cells were incubated
with acti-nomycin D and 100 µg/ml AGEs or 10 µg/ml
BSA for different times (0, 1, 2, 4, 8 and 24 h). We found that
AGEs increased the expression of BAG3 nascent mRNA in a
time-dependent manner (Fig. 1E).
Therefore, in the next experiment, we used the dose (100
µg/ml AGEs/10 µg/ml BSA) and time (24 h) to determine
the molecular mechanism of AGE-induced proliferation and migration
of VSMCs.
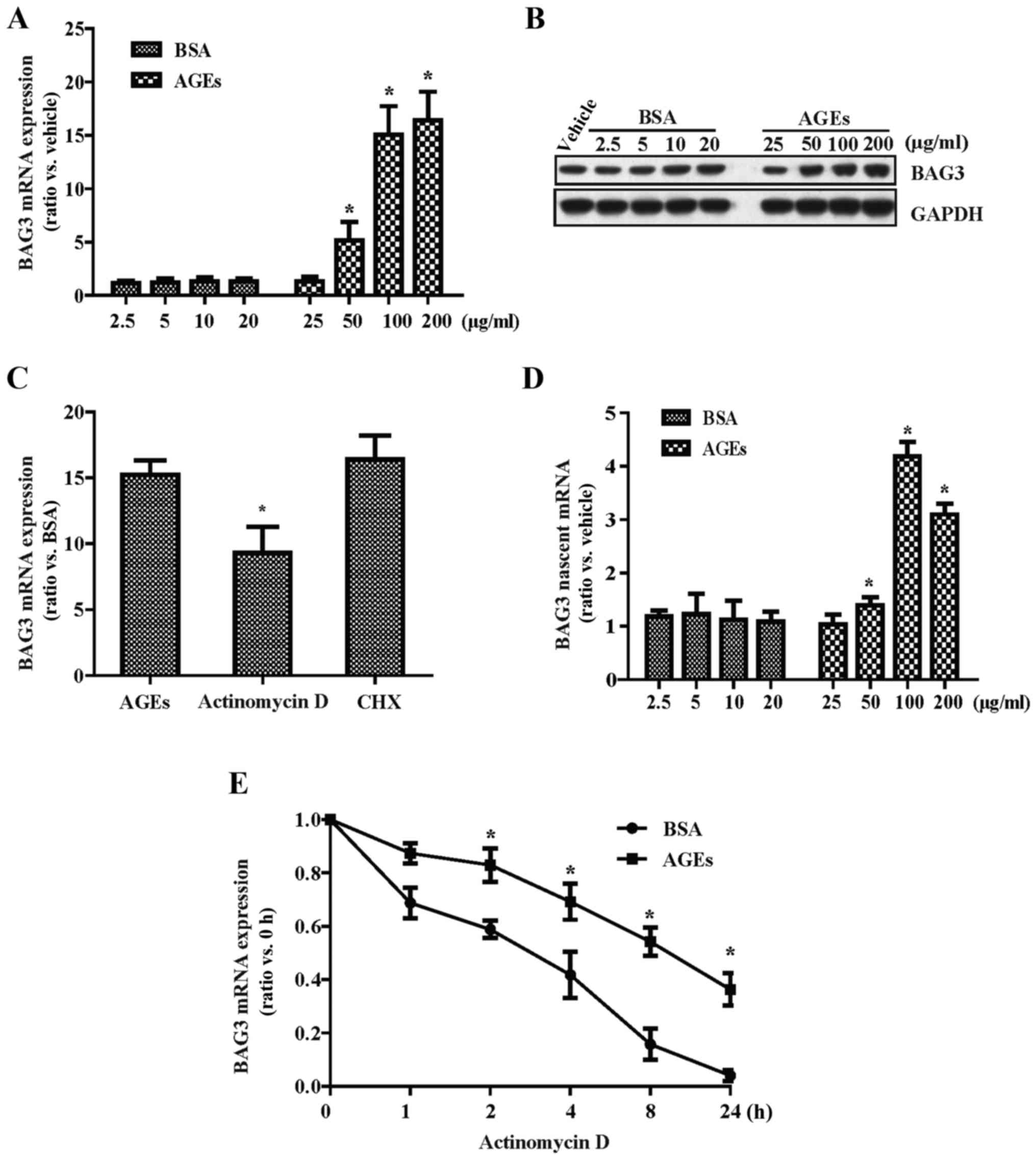 | Figure 1Advanced glycation end products
(AGEs) increase the expression of Bcl-2-associated athanogene 3
(BAG3) in cultured primary rat vascular smooth muscle cells
(VSMCs). Cells were treated with different concentrations of AGEs
(25, 50, 100 and 200 µg/ml) and BSA (2.5, 5, 10 and 20
µg/ml), respectively. The mRNA and protein expression levels
of BAG3 were detected by (A) RT-PCR and (B) western blotting,
respectively. (C) Actinomycin D (10 µg/ml), a
transcriptional inhibitor, and cycloheximide (CHX) (20
µg/ml), a translational inhibitor, were used to study the
effect of AGEs on the mRNA expression of BAG3. (D) Click-iT nascent
RNA capture kit was used to label and isolate newly synthesized
RNA. (E) Cells were incubated with 10 µg/ml actinomycin D
and 100 µg/ml AGEs or 10 µg/ml BSA for different
times (0, 1, 2, 4, 8 and 24 h), and then the mRNA expression of
BAG3 was detected by RT-PCR. The experiments were repeated three
times with reproducible results. *p<0.05 compared
with the control. |
BAG3 promotes the proliferation of
primary rat VSMCs
To further investigate the involvement of BAG3 in
VSMC proliferation, we generated lentiviral vectors containing
shRNAs against BAG3 (shBAG3) to knock down BAG3 expression in the
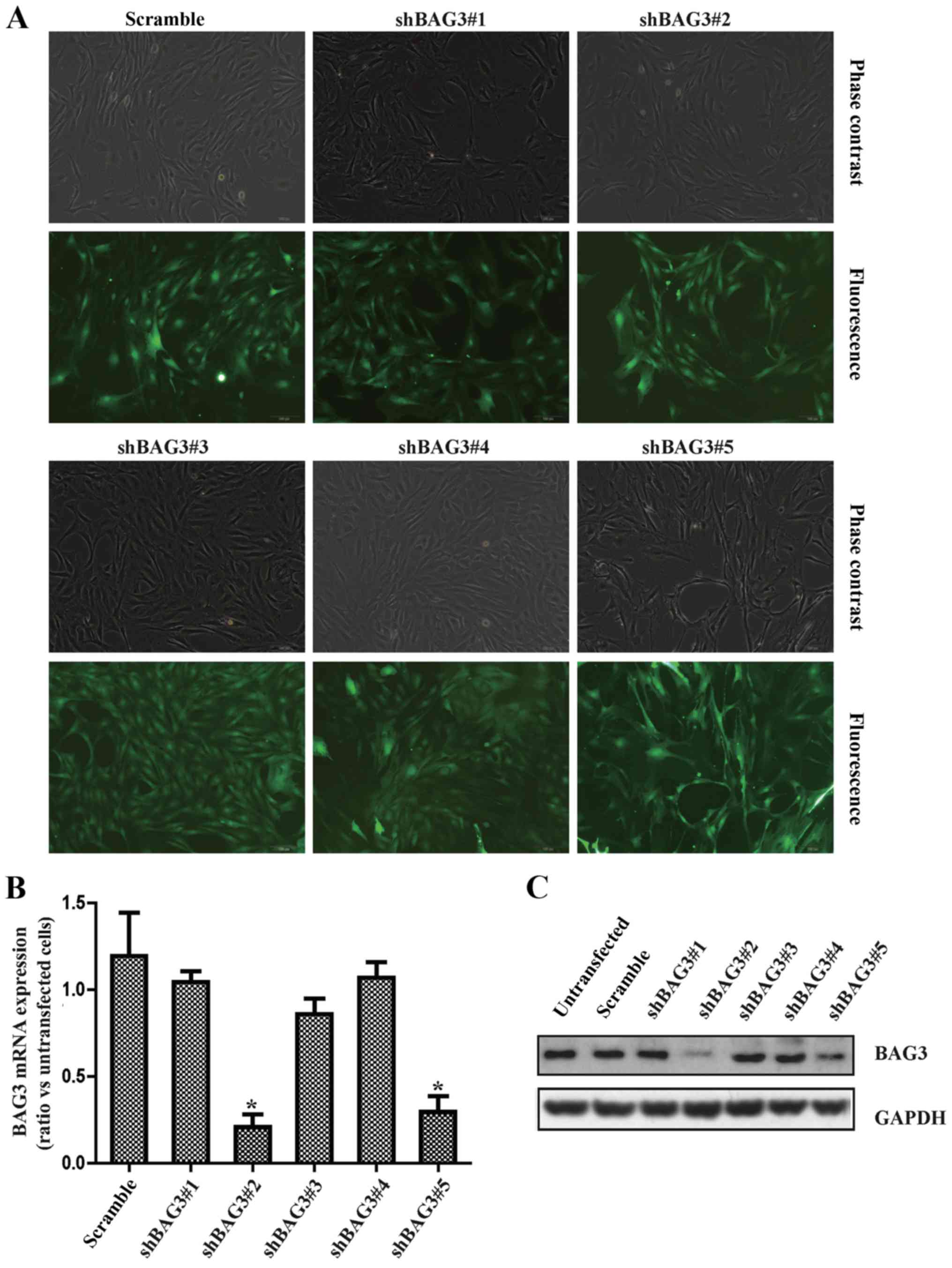
VSMCs. Measurement of the GFP+ cells under fluorescence
microscopy demonstrated that the transduction efficiency by
lentiviral vectors at 100 MOI was 80–90% (Fig. 2A). The results of qPCR (Fig. 2B) and western blot analysis
(Fig. 2C) demonstrated that two
shRNAs (shBAG3#3 and shBAG3#5) significantly decreased the mRNA and
protein expression of BAG3 in VSMCs, respectively. Importantly,
results from MTT assay (Fig. 2D)
and EdU staining (Fig. 2E and F)
consistently demonstrated that forced knockout of BAG3
significantly reduced the cell viability and proliferation of
VSMCs.
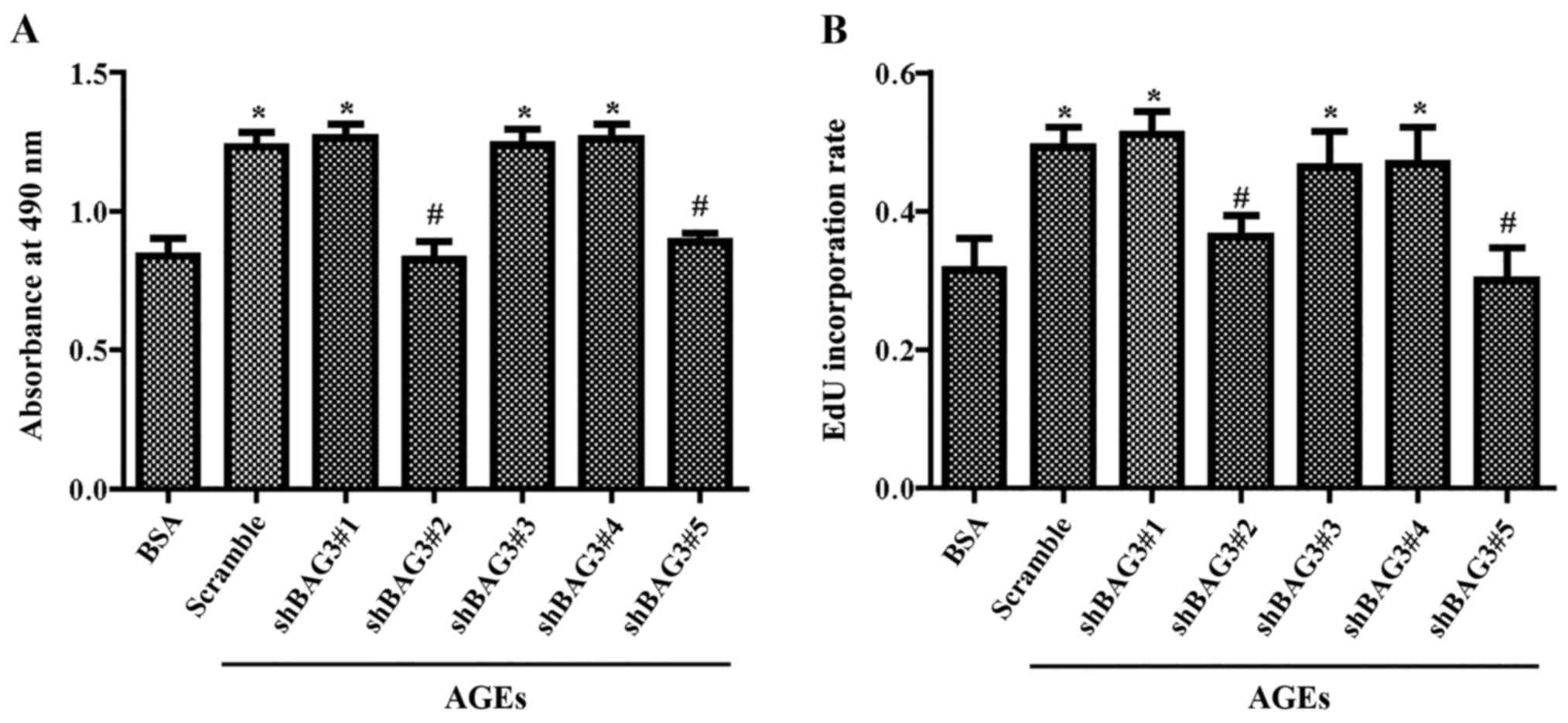
AGEs promote the proliferation of primary
rat VSMCs via BAG3
To investigate the potential involvement of BAG3 in
the proliferation of VSMCs induced by AGEs, VSMCs containing shRNA
against BAG3 were treated with 100 µg/ml AGEs or 10
µg/ml BSA for 24 h. The results of the MTT assay (Fig. 3A) and EdU staining (Fig. 3B) demonstrated that BAG3 knockout
could significantly decrease the proliferation of VSMCs induced by
AGEs.
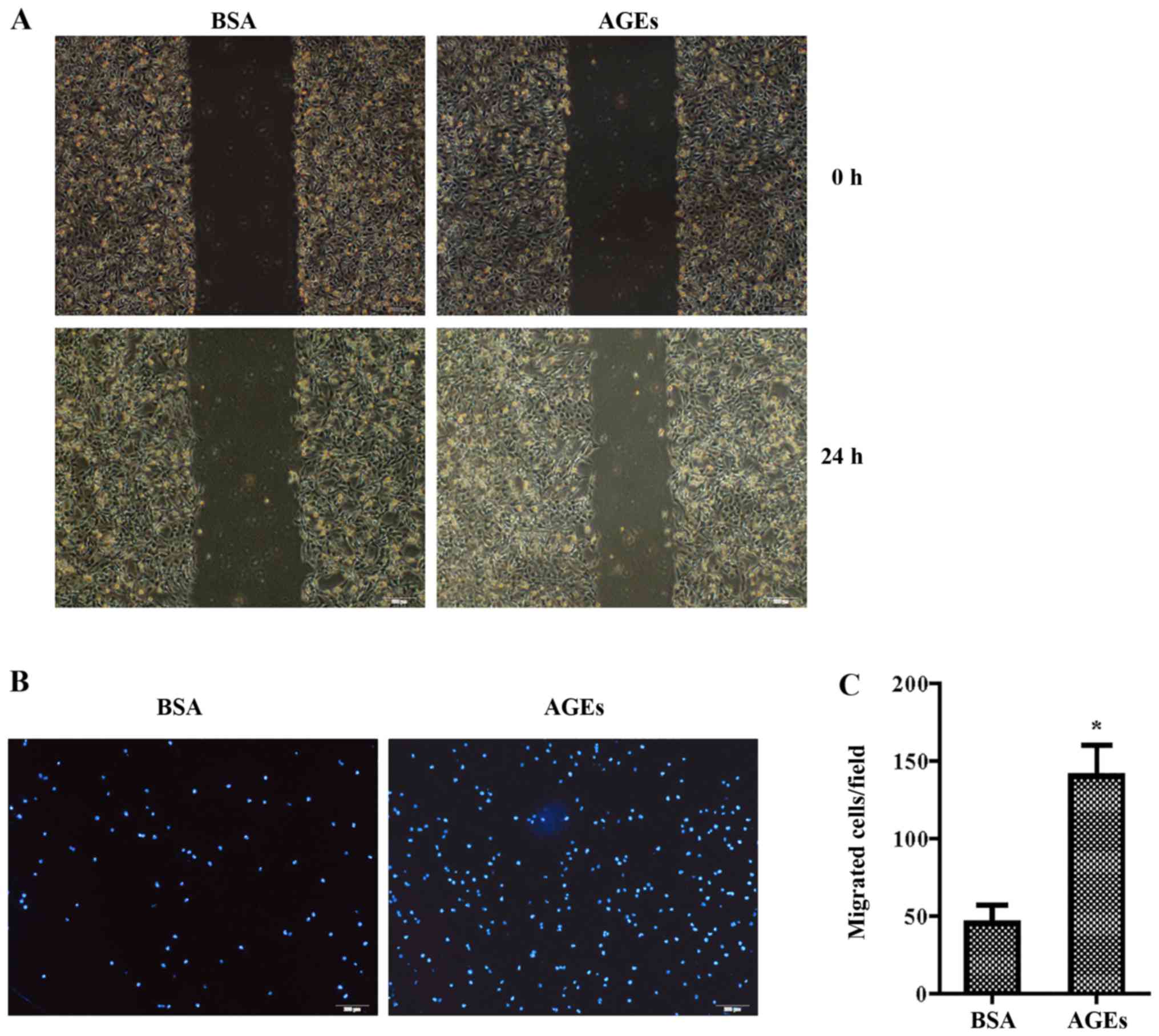
AGEs promote the migration of primary rat
VSMCs via BAG3
Cells were treated with 100 µg/ml AGEs or 10
µg/ml BSA for 24 h. The wound-healing assay showed that AGEs
significantly increased the migration of VSMCs compared with that
noted in control group (Fig. 4A).
The Transwell assay demonstrated that the number of invasive cells
in the AGE-treated group was significantly higher than the number
in the control group (Fig. 4B and
C). We then continued to investigate the potential involvement
of BAG3 in the migration of VSMCs. The results of the wound-healing
assay (Fig. 4D) and Transwell
assay (Fig. 4E) demonstrated that
BAG3 knockout significantly decreased the migration of the
VSMCs.
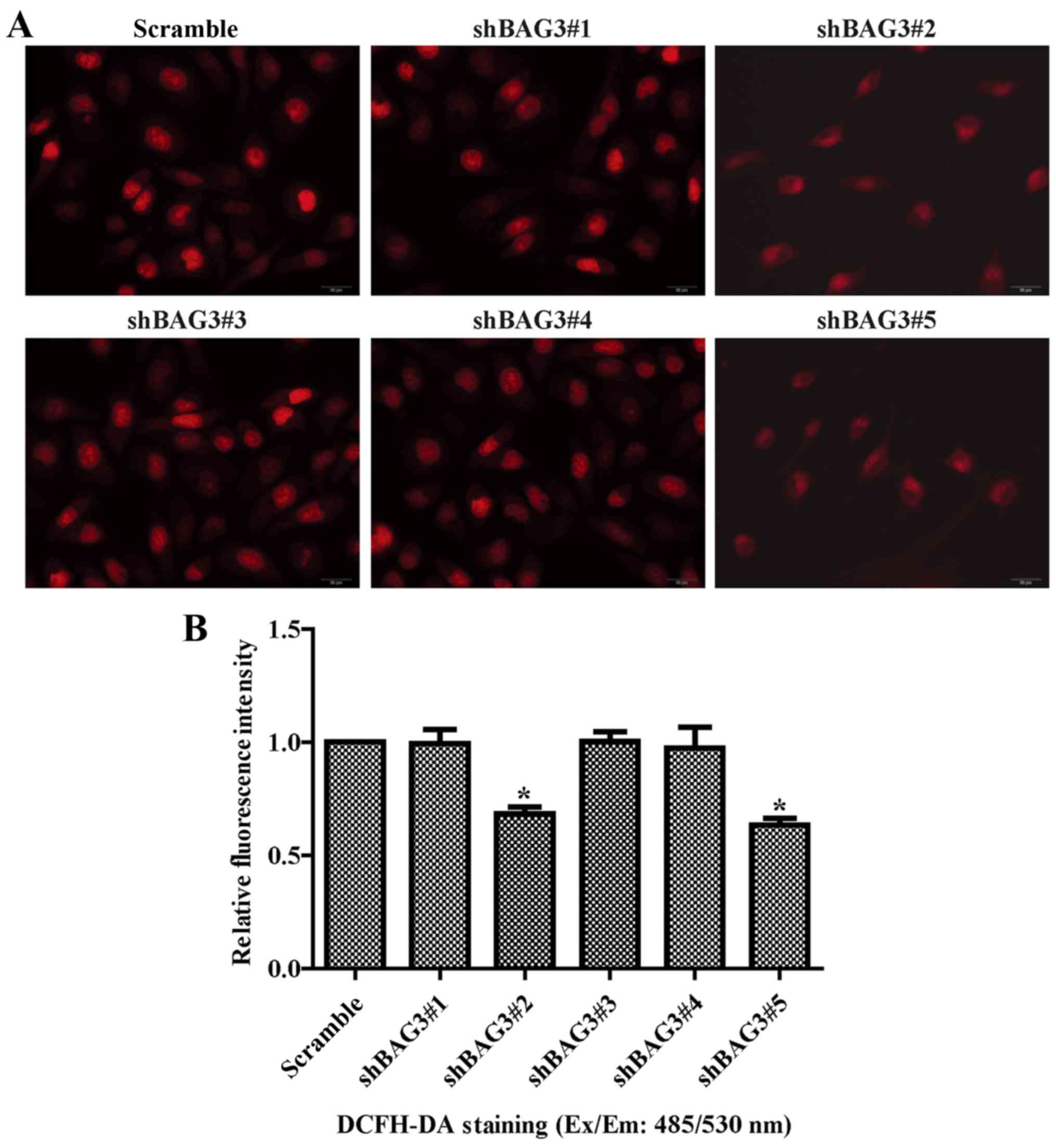
Knockout of BAG3 reduces the oxidative
stress and maintains the mitochondrial membrane potential of
VSMCs
The results of DCHF-DA assay indicated that BAG3
knockout obviously reduced the ROS generation in the VSMCs
(Fig. 5A and B). ROS mainly
result from mitochondrial respiratory chain complexes in
mitochondria (32). We then
investigated the potential effect of BAG3 on the mitochondria. The
results of TMRE showed that BAG3 knockout reversed the decrease in
mitochondrial membrane potential induced by AGEs (Fig. 5C and D).
AGEs promote the proliferation and
migration of primary rat VSMCs via oxidative stress
ROS, such as superoxide anions and hydrogen
peroxide, play a crucial role in regulating the proliferation and
migration of VSMCs. We tested the hypothesis that the stimulative
effect of AGEs on the proliferation and migration of VSMCs involved
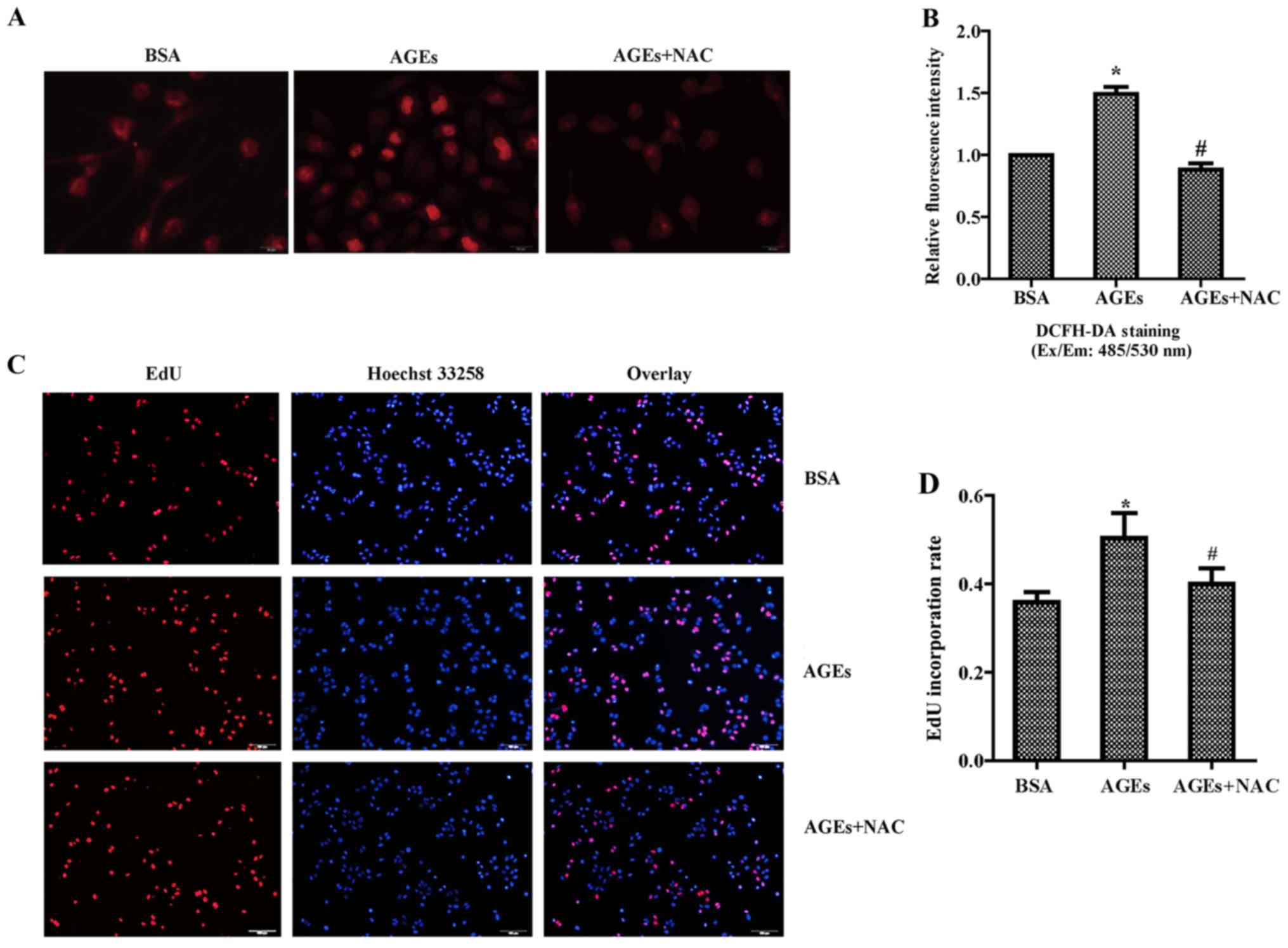
ROS production. We next used NAC, a potent antioxidant, to
investigate the potential effect of oxidative stress on the VSMCs.
Cells were incubated with 100 µg/ml NAC and 100 µg/ml
AGEs or 10 µg/ml BSA for 24 h. The results of the DCFH-DA
assay indicated that NAC could completely prevented the generation
of the intracellular ROS level after AGE stimulation (Fig. 6A and B). Furthermore, the results
from the EdU staining (Fig. 6C and
D) and MTT assay (Fig. 6E)
consistently demonstrated that NAC significantly reduced the
proliferation of VSMCs induced by AGEs. Results from the wound
healing (Fig. 6F) and Transwell
assays (Fig. 6G and H)
consistently demonstrated that NAC significantly reduced the
migration of VSMCs induced by AGEs. Overall, our results indicated
that intracellular ROS generation has an essential role in
AGE-induced proliferation and migration of VSMCs.
Discussion
The present study demonstrated a novel role for BAG3
in regulating the proliferation and migration of VSMCs induced by
AGEs. We found that AGEs promoted the proliferation and migration
of VSMCs via upregulation of BAG3 expression, in which ROS played
an important role.
In vivo, AGEs slowly form in hyperglycemic
environments and during aging, and play role in a variety of
microvascular and macrovascular complications in diabetes (13). Accumulating evidence suggests that
AGEs promote the proliferation and migration of VSMCs, which
accelerate atherosclerosis and restenosis after percutaneous
coronary intervention (9,33,34). Our results were consistent with
these previous studies. The interaction between AGEs and its
receptor RAGE plays an important role in AGE-induced cell injury.
It is well accepted that overexpression of RAGE is activated by
AGEs in vascular dysfunction and resulting in apoptosis, oxidative
stress and inflammation responses (35). There is a growing body of evidence
that shows that AGE-RAGE interaction with a positive feedback loop
is related to the dysfunction of VSMCs (10,36–38). A previous study found that 100
µg/ml AGEs enhanced vascular calcification through a
RAGE/oxidative stress pathway (38). In the process of vascular
calcification, the proliferation and migration of VSMCs play a
crucial role. Based on these previous studies, the present study
continued to explore the mechanisms underlining the promotion of
the proliferation and migration of VSMCs by AGEs. We found that
AGEs significantly promoted the expression of BAG3 and knockout of
BAG3 reduced the proliferation and migration of VSMCs induced by
AGEs.
BAG3 plays an important role in a series of cellular
processes, including cell proliferation, migration, apoptosis,
autophagy, adhesion and cell cycle progression (18–20,39). Several lines of evidence suggest
that the expression of BAG3 is elevated in various tumors including
glioblastoma, acute lymphoblastic leukemia, and prostate carcinoma
(40–42). However, normal tissues seldom
express BAG3, except for cardiomyocytes and skeletal muscle cells
(22). The expression of BAG3 can
be induced by a variety of stimuli such as the early growth
responsive gene-1 (Egr-1) (43),
heat shock factor-1 (HSF-1) (44), proteasome inhibitors (45), heavy metals as well as heat stress
(46,47). BAG3 is seldom expressed in normal
tissues, but its expression is induced upon exposure to various
stressful stimuli, which appears to be a protective mechanism
(22). Previous studies indicated
that BAG3 involved in various CVD such as myocardial hypertrophy,
dilated cardiomyopathy, Takotsubo cardiomyopathy and chronic heart
failure (23–26). However, the relationship between
the expression of BAG3 and the proliferation and migration of VSMCs
is still unclear.
This study, for the first time, found the dynamic
alterations of BAG3 expression in VSMCs treated with AGEs and
demonstrated that shBAG3 could reduce the proliferation and
migration of VSMCs induced by AGEs. As far as we know, currents
there are scarce data on AGEs and BAG3. However, it is reasonable
to speculate that unfolded protein response (UPR) is a main
mediator. Endoplasmic reticulum (ER) is responsible for the
post-translational modification, folding and trafficking of
approximately one-third of all cellular proteins (48). Under physiological conditions, ER
can maintain a balance between folded and misfolded proteins.
However, when unfolded/misfolded protein accumulation impairs ER
homeostasis, ER stress occurs, which could further activate UPR
(48). As summarized (49), AGEs induce the UPR in different
cell types including endothelial, neuronal, pancreatic cells and
podocytes, suggesting this crosstalk as an underlying pathological
mechanism that contributes to metabolic diseases. At the same time,
BAG3, as a molecular chaperones, plays a major role in protein
quality control and could sense misfolded proteins and direct them
to protein degradation systems (50). Therefore, the increased expression
of BAG3 seems to protect against cell death under extreme stimuli.
However, the exact mechanism by which AGEs induce the expression of
BAG3 in VSMCs warrants further investigation.
In addition, our data demonstrated that shBAG3
reduced the proliferation and migration of VSMCs and ROS
production; while reducing ROS production by NAC also inhibited the
proliferation and migration of VSMCs. These results indicated that
BAG3 is a regulator of ROS. A previous study (51) found that BAG3 overexpression
significantly decreased lipid peroxidative product MDA content but
increased SOD and GSH-Px activity (two important anti-oxidases) in
cardiomyocytes after anoxia/reoxygenation injury, which indicated
that BAG3 plays an important role in reducing ROS generation of
cardiomyocytes. The difference between these two studies indicate
that the mechanism by which BAG3 regulates ROS may be different in
different cell type and is due to various signaling pathways. In
VSMCs, ROS are mainly produced by NOX activity and mitochondrial
respiratory electron transport chain during oxidative respiration
(32). ROS include superoxide
anion, hydroxyl radicals and hydrogen peroxide, which are the
destructive feature of oxidative stress (17). Previous research indicates that
ROS are involved in various vascular cell signaling via modulating
redox-sensitive transcription and transduction pathways (52). Furthermore, increasing evidence
has demonstrated that ROS accumulation plays an important role in
the proliferation and migration of VSMCs (15–17). Thereby, attenuating ROS production
may be a promising therapeutic stategy for preventing the
proliferation and migration of VSMCs in the process of vascular
complications of diabetes.
In conclusion, the present study demonstrated for
the first time that AGEs increase ROS production and promote the
proliferation and migration of VSMCs by upregulating BAG3
expression. This study suggests that BAG3 is a potential target for
the prevention and/or treatment of the vascular complications of
diabetes.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81470417 and 81670231).
References
|
1
|
Kudryavtseva O, Aalkjaer C and Matchkov
VV: Vascular smooth muscle cell phenotype is defined by
Ca2+-dependent transcription factors. FEBS J.
280:5488–5499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang DD and Anfinogenova Y: Physiologic
properties and regulation of the actin cytoskeleton in vascular
smooth muscle. J Cardiovasc Pharmacol Ther. 13:130–140. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bochaton-Piallat ML and Gabbiani G:
Modulation of smooth muscle cell proliferation and migration: Role
of smooth muscle cell heterogeneity. Handb Exp Pharmacol.
170:645–663. 2005. View Article : Google Scholar
|
|
5
|
Orr AW, Hastings NE, Blackman BR and
Wamhoff BR: Complex regulation and function of the inflammatory
smooth muscle cell phenotype in atherosclerosis. J Vasc Res.
47:168–180. 2010. View Article : Google Scholar :
|
|
6
|
Rzucidlo EM, Martin KA and Powell RJ:
Regulation of vascular smooth muscle cell differentiation. J Vasc
Surg. 45(Suppl A): A25–A32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis-Dusenbery BN, Wu C, Hata A and Sessa
WC: Micromanaging vascular smooth muscle cell differentiation and
phenotypic modulation. Arterioscler Thromb Vasc Biol. 31:2370–2377.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao LM, Su XL, Wang Y, Li GR and Deng XL:
KCa3.1 channels mediate the increase of cell migration and
proliferation by advanced glycation endproducts in cultured rat
vascular smooth muscle cells. Lab Invest. 93:159–167. 2013.
View Article : Google Scholar
|
|
9
|
Yuan X, Zhang Z, Gong K, Zhao P, Qin J and
Liu N: Inhibition of reactive oxygen species/extracellular
signal-regulated kinases pathway by pioglitazone attenuates
advanced glycation end products-induced proliferation of vascular
smooth muscle cells in rats. Biol Pharm Bull. 34:618–623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nam MH, Son WR, Lee YS and Lee KW:
Glycolaldehyde-derived advanced glycation end products
(glycol-AGEs)-induced vascular smooth muscle cell dysfunction is
regulated by the AGES-receptor (RAGE) axis in endothelium. Cell
Commun Adhes. 22:67–78. 2015. View Article : Google Scholar
|
|
11
|
Simard E, Söllradl T, Maltais JS, Boucher
J, D'Orléans-Juste P and Grandbois M: Receptor for advanced
glycation end-products signaling interferes with the vascular
smooth muscle cell contractile phenotype and function. PLoS One.
10:e01288812015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh R, Barden A, Mori T and Beilin L:
Advanced glycation end-products: A review. Diabetologia.
44:129–146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: Sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma M, Guo X, Chang Y, Li C, Meng X, Li S,
Du ZX, Wang HQ and Sun Y: Advanced glycation end products promote
proliferation and suppress autophagy via reduction of Cathepsin D
in rat vascular smooth muscle cells. Mol Cell Biochem. 403:73–83.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu L, Li X, Li Y, Wang L, Tang Y and Xue
M: Proliferative inhibition of danxiongfang and its active
ingredients on rat vascular smooth muscle cell and protective
effect on the VSMC damage induced by hydrogen peroxide. J
Ethnopharmacol. 126:197–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panchenko MP, Silva N and Stone JR:
Up-regulation of a hydrogen peroxide-responsive pre-mRNA binding
protein in atherosclerosis and intimal hyperplasia. Cardiovasc
Pathol. 18:167–172. 2009. View Article : Google Scholar
|
|
17
|
Zhou Y, Zhang MJ, Li BH, Chen L, Pi Y, Yin
YW, Long CY, Wang X, Sun MJ, Chen X, et al: PPARγ inhibits VSMC
proliferation and migration via attenuating oxidative stress
through upregulating UCP2. PLoS One. 11:e01547202016. View Article : Google Scholar
|
|
18
|
Iwasaki M, Homma S, Hishiya A, Dolezal SJ,
Reed JC and Takayama S: BAG3 regulates motility and adhesion of
epithelial cancer cells. Cancer Res. 67:10252–10259. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu BQ, Du ZX, Zong ZH, Li C, Li N, Zhang
Q, Kong DH and Wang HQ: BAG3-dependent noncanonical autophagy
induced by proteasome inhibition in HepG2 cells. Autophagy.
9:905–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosati A, Graziano V, De Laurenzi V,
Pascale M and Turco MC: BAG3: A multifaceted protein that regulates
major cell pathways. Cell Death Dis. 2:e1412011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi H, Xu H, Li Z, Zhen Y, Wang B, Huo S,
Xiao R and Xu Z: BAG3 regulates cell proliferation, migration, and
invasion in human colorectal cancer. Tumour Biol. 37:5591–5597.
2016. View Article : Google Scholar
|
|
22
|
Hishiya A, Kitazawa T and Takayama S: BAG3
and Hsc70 interact with actin capping protein CapZ to maintain
myofibrillar integrity under mechanical stress. Circ Res.
107:1220–1231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Falco A, Festa M, Basile A, Rosati A,
Pascale M, Florenzano F, Nori SL, Nicolin V, Di Benedetto M,
Vecchione ML, et al: BAG3 controls angiogenesis through regulation
of ERK phosphorylation. Oncogene. 31:5153–5161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Norton N, Li D, Rieder MJ, Siegfried JD,
Rampersaud E, Züchner S, Mangos S, Gonzalez-Quintana J, Wang L,
McGee S, et al: Genome-wide studies of copy number variation and
exome sequencing identify rare variants in BAG3 as a cause of
dilated cardiomyopathy. Am J Hum Genet. 88:273–282. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Citro R, d'Avenia M, De Marco M, Giudice
R, Mirra M, Ravera A, Silverio A, Farina R, Silvestri F, Gravina P,
et al: Polymorphisms of the antiapoptotic protein bag3 may play a
role in the pathogenesis of tako-tsubo cardiomyopathy. Int J
Cardiol. 168:1663–1665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Marco M, Falco A, Basile A, Rosati A,
Festa M, d'Avenia M, Pascale M, Dal Piaz F, Bisogni R, Barcaroli D,
et al: Detection of soluble BAG3 and anti-BAG3 antibodies in
patients with chronic heart failure. Cell Death Dis. 4:e4952013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salabei JK, Cummins TD, Singh M, Jones SP,
Bhatnagar A and Hill BG: PDGF-mediated autophagy regulates vascular
smooth muscle cell phenotype and resistance to oxidative stress.
Biochem J. 451:375–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Li S, Kong DH, Meng X, Zong ZH, Liu
BQ, Guan Y, Du ZX and Wang HQ: BAG3 is upregulated by c-Jun and
stabilizes JunD. Biochim Biophys Acta. 1833:3346–3354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo L, Guo X, Chang Y, Li Z, Yu S, Yang H
and Sun Y: Modified ideal cardiovascular health status is
associated with lower prevalence of stroke in rural northeast
China. Int J Environ Res Public Health. 13:2072016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen S, Liu B, Kong D, Li S, Li C, Wang H
and Sun Y: Atorvastatin calcium inhibits phenotypic modulation of
PDGF-BB-induced VSMCs via down-regulation the Akt signaling
pathway. PLoS One. 10:e01225772015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang Y, Li Y, Ye N, Guo X, Li Z, Sun G
and Sun Y: Atorvastatin inhibits the apoptosis of human umbilical
vein endothelial cells induced by angiotensin II via the
lysosomal-mitochondrial axis. Apoptosis. 21:977–996. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Pietro M, Filardo S, De Santis F,
Mastromarino P and Sessa R: Chlamydia pneumoniae and oxidative
stress in cardiovascular disease: State of the art and prevention
strategies. Int J Mol Sci. 16:724–735. 2014. View Article : Google Scholar
|
|
33
|
Crauwels HM, Herman AG and Bult H: Local
application of advanced glycation end products and intimal
hyperplasia in the rabbit collared carotid artery. Cardiovasc Res.
47:173–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Z, Wang K, Penn MS, Marso SP, Lauer
MA, Forudi F, Zhou X, Qu W, Lu Y, Stern DM, et al: Receptor for AGE
(RAGE) mediates neointimal formation in response to arterial
injury. Circulation. 107:2238–2243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coughlan MT, Thorburn DR, Penfold SA,
Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K,
Thallas-Bonke V, Nawroth PP, et al: RAGE-induced cytosolic ROS
promote mitochondrial superoxide generation in diabetes. J Am Soc
Nephrol. 20:742–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kay AM, Simpson CL and Stewart JA Jr: The
role of AGE/RAGE signaling in diabetes-mediated vascular
calcification. J Diabetes Res. 2016:68097032016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu X, Liu K, Wang Z, Liu C, Han Z, Tao J,
Lu P, Wang J, Wu B, Huang Z, et al: Advanced glycation end products
accelerate arteriosclerosis after renal transplantation through the
AGE/RAGE/ILK pathway. Exp Mol Pathol. 99:312–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei Q, Ren X, Jiang Y, Jin H, Liu N and Li
J: Advanced glycation end products accelerate rat vascular
calcification through RAGE/oxidative stress. BMC Cardiovasc Disord.
13:132013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bloemberg D, McDonald E, Dulay D and
Quadrilatero J: Autophagy is altered in skeletal and cardiac muscle
of spontaneously hypertensive rats. Acta Physiol (Oxf).
210:381–391. 2014. View Article : Google Scholar
|
|
40
|
Gentilella A and Khalili K: BAG3
expression in glioblastoma cells promotes accumulation of
ubiquitinated clients in an Hsp70-dependent manner. J Biol Chem.
286:9205–9215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu H, Wu W, Fu Y, Shen W, Miao K, Hong M,
Xu W, Young KH, Liu P and Li J: Overexpressed BAG3 is a potential
therapeutic target in chronic lymphocytic leukemia. Ann Hematol.
93:425–435. 2014. View Article : Google Scholar
|
|
42
|
Staibano S, Mascolo M, Di Benedetto M,
Vecchione ML, Ilardi G, Di Lorenzo G, Autorino R, Salerno V, Morena
A, Rocco A, et al: BAG3 protein delocalisation in prostate
carcinoma. Tumour Biol. 31:461–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gentilella A, Passiatore G, Deshmane S,
Turco MC and Khalili K: Activation of BAG3 by Egr-1 in response to
FGF-2 in neuroblastoma cells. Oncogene. 27:5011–5018. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Franceschelli S, Rosati A, Lerose R, De
Nicola S, Turco MC and Pascale M: Bag3 gene expression is regulated
by heat shock factor 1. J Cell Physiol. 215:575–577. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Du ZX, Meng X, Zhang HY, Guan Y and Wang
HQ: Caspase-dependent cleavage of BAG3 in proteasome
inhibitors-induced apoptosis in thyroid cancer cells. Biochem
Biophys Res Commun. 369:894–898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liao Q, Ozawa F, Friess H, Zimmermann A,
Takayama S, Reed JC, Kleeff J and Büchler MW: The anti-apoptotic
protein BAG-3 is overexpressed in pancreatic cancer and induced by
heat stress in pancreatic cancer cell lines. FEBS Lett.
503:151–157. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pagliuca MG, Lerose R, Cigliano S and
Leone A: Regulation by heavy metals and temperature of the human
BAG-3 gene, a modulator of Hsp70 activity. FEBS Lett. 541:11–15.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fiorentino TV, Prioletta A, Zuo P and
Folli F: Hyperglycemia-induced oxidative stress and its role in
diabetes mellitus related cardiovascular diseases. Curr Pharm Des.
19:5695–5703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Adamopoulos C, Mihailidou C, Grivaki C,
Papavassiliou KA, Kiaris H, Piperi C and Papavassiliou AG: Systemic
effects of AGEs in ER stress induction in vivo. Glycoconj J.
33:537–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum
U, Hartl FU and Behl C: Protein quality control during aging
involves recruitment of the macroautophagy pathway by BAG3. EMBO J.
28:889–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ma Y, Gai Y, Yan J, Li J and Zhang Y:
Puerarin attenuates anoxia/reoxygenation injury through enhancing
Bcl-2 associated athanogene 3 expression, a modulator of apoptosis
and autophagy. Med Sci Monit. 22:977–983. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kunsch C and Medford RM: Oxidative stress
as a regulator of gene expression in the vasculature. Circ Res.
85:753–766. 1999. View Article : Google Scholar : PubMed/NCBI
|