Introduction
Hypertension is a condition of major concern for
health care that markedly increases the risk of death from stroke,
myocardial infarction, arteriosclerosis, cerebral hemorrhage and
other vascular diseases (1). The
renin-angiotensin system is an endogenic regulator of blood
pressure, water electrolyte homeostasis and cell growth balance
(2). In particular, it plays a
key role in the pathology of hypertension (3). In the renin-angiotensin system,
angiotensin I (Ang I)-converting enzyme (ACE) hydrolyzes the
inactive decapeptide Ang I by cleaving a dipeptide from the
C-terminus to produce the potent vasoconstrictor Ang II, which is
responsible for increasing arterial pressure (4,5).
Therefore, the modulation of the renin-angiotensin system has
become a promising approach for controlling blood pressure in the
treatmente of cardiovascular disorders, for example by using ACE
inhibitors as therapeutic agents.
Many synthetic ACE inhibitors have been used
extensively in the treatment of hypertension and cardiovascular
disorders; these inhibitors include the drugs captopril, enalapril,
alacepril and lisinopril (6,7).
However, they have been reported to have some side-effects,
including coughing, a disrupted sense of taste, azotemia,
angioedema and skin rashes (7).
Therefore, ACE inhibitors from natural sources are currently being
considered as alternative therapeutic agents for controlling blood
pressure (2–4,6,7).
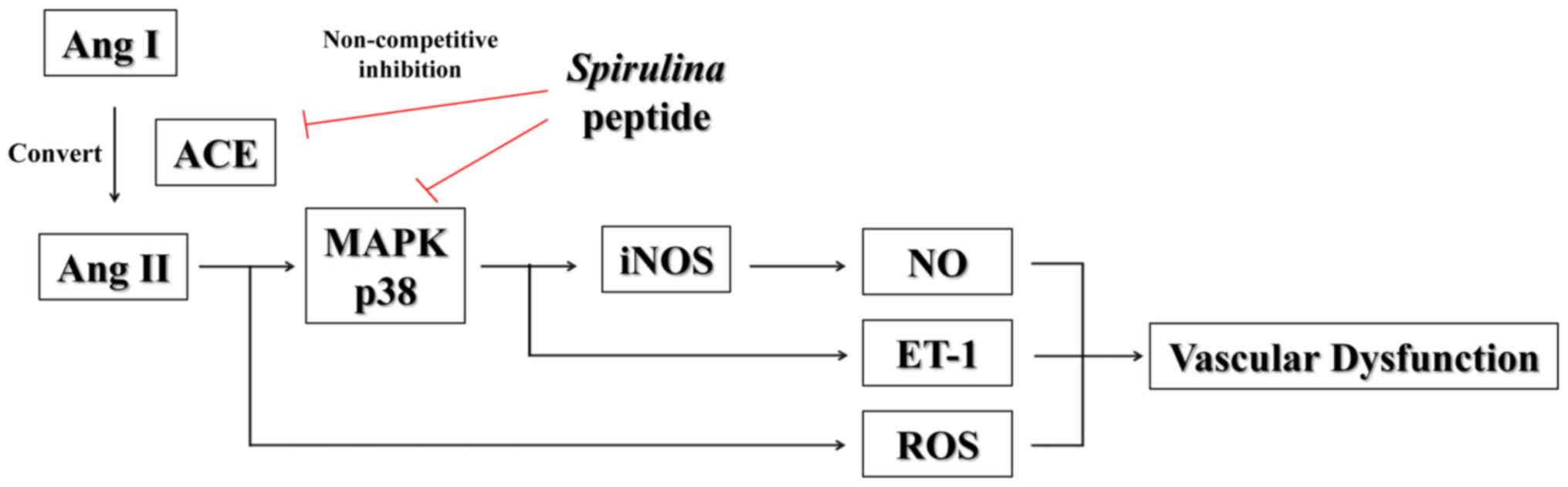
Ang II has pleiotropic acute and chronic effects on
vascular smooth muscle and plays an important role in
cardiovascular diseases, including hypertension, atherosclerosis
and heart failure (8,9). In this regard, it has been suggested
that Ang II induces the production of other vascular
dysfunction-related factors, such as free radicals [nitric oxide
(NO) and reactive oxygen species (ROS)] and vasoconstrictors
[endothelin-1 (ET-1) and vasopressin] (10–12). These factors can promote cellular
oxidative stress and induce vascular endothelial dysfunction,
hypertension and atherosclerosis (11). Therefore, research into ACE
inhibitors has focused on decreasing the production of Ang II to
control the systemic balance between the potent vasoactive
regulators, ET-1 and free radicals.
Microalgae contain various valuable natural
compounds, such as pigments, β-carotenes, polysaccharides and
peptides that have uses in the pharmaceutical, cosmetic and
nutraceutical industries (13,14). Therefore, microalgae are
potentially an excellent source of natural compounds that may be
used as ingredients for preparing functional foods and
nutraceuticals. Although microalgae possess >50% protein in
their biomass, they are usually used as animal feed (6). However, microalgal proteins can be
converted into value-added products with improved functional
properties by enzymatic hydrolysis. As a result, researchers are
interested in obtaining effective bioactive peptides from marine
microalgae (13,15–17).
Among the microalgae, Spirulina is a
blue-green alga appertaining to the Oscillatoraceae family. It is
grown in marine and freshwater environments and is referred to as a
'superfood' which has several biological activities, such as
antioxidant, anti-diabetic, cholesterol-controlling and insulin
resistance effects (18).
Although, Suetsuna and Chen reported thye anti-hypertensive effect
of Spirulina (16), no
study to date has shown an inhibitory effect of Spirulina on
Ang II-induced endothelial dysfunction, at least to the best of our
knowledge.
The principal objective of the present study was to
purify and identify an ACE inhibitory peptide from Spirulina
sp., and to determine the mechanisms underlying the effects of the
purified peptide on ACE and on the production of vascular
dysfunction-related factors by Ang II-stimulated human endothelial
cells.
Materials and methods
Materials
ACE (from rabbit lung), N-Hippuryl-His-Leu
tetrahydrate (HHL), Griess reagent,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
gastrointestinal enzymes (pepsin, α-chymotrypsin and trypsin) and
Ang II were all purchased from Sigma-Aldrich (St. Louis, MO, USA).
Specific antibodies against inducible nitric oxide synthase (iNOS;
sc-7271), GAPDH (sc-25778), p-p38 (sc-7973), p38 (sc-7149), p-c-Jun
N-terminal kinase (JNK; sc-6254), JNK (sc-7345), p-extracellular
signal-regulated kinase (ERK; sc-7383), ERK (sc-292838) and ET-1
(sc-21625) were all purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA), and 2′,7′-dichlorodihydrofluorescein
diacetate (DCFH-DA) and Hoechst 33342 were obtained from Invitrogen
Life Technologies (Carlsbad, CA, USA). The HiPrep™ 16/10 DEAE FF
anion exchange column was purchased from GE Healthcare
(Buckinghamshire, UK). The other chemicals and reagents were of
analytical grade.
Preparation of enzymatic hydrolysate of
marine microalga, Spirulina sp
The enzymatic hydrolysis of the marine microalga,
Spirulina sp., was performed using gastrointestinal enzymes,
as previously described (19). A
total of 100 ml of 4% (w/v) Spirulina sp. solution was
brought to pH 2.2 in gastric digestion using HCl or NaOH under
rigorous mixing. Pepsin (EC 3.4.23.1) was added at the enzyme to
substrate ratio of 1/100 (w/w), followed by incubation at 37°C on a
shaker for 2 h. The pH was set to 6.5 to obtain the conditions of
small intestinal digestion. Trypsin (EC 3.4.21.4) and
α-chymotrypsin (EC 3.4.21.1) were both supplemented at an enzyme to
substrate ratio of 1/100 (w/w), then incubated at 37°C for 2.5 h.
The hydrolysate was then boiled for 10 min at 100°C to inactivate
the enzyme reaction. The collected sample was clarified by
centrifugation (10,000 × g for 15 min at 4°C) for removal of the
residue. The hydrolysate was passed through ultrafiltration (UF)
membranes [molecular weight (MW) cut-off of 5, 10 and 100 kDa]
using Millipore's UF system (Millipore, Bedford, MA, USA). The
gastrointestinal hydrolysate was subjected to molecular weight
fractionation to obtain the peptide with a molecular weight of
<5 kDa (5 kDa or smaller), 5–10 kDa (between 5 and 10 kDa),
10–100 kDa (between 10 and 100 kDa) and >100 kDa (100 kDa and
larger). All recovered fractions were lyophilized and store −80°C
until use.
Purification of ACE inhibitory
peptide
The highest ACE inhibitory fraction was loaded onto
an HiPrep DEAE FF ion-exchange column using fast protein liquid
chromatography (FPLC ÄKTA; Amersham Biosciences, Uppsala, Sweden).
The column was equilibrated in 20 mM sodium phosphate buffer (pH
7.0) previously, and eluted with a linear gradient of NaCl (0–2 M)
at a flow rate of 2 ml/min. Elution peaks were monitored at 280 nm.
The fractions were further purified on a PrimeSphere ODS
C18 column permeation reversed-phase high-performance
liquid chromatography (RP-HPLC) with a linear gradient of
acetonitrile (0–35% in 35 min) containing 0.1% trifluoroacetic acid
(TFA) at a flow rate of 0.8 ml/min. Finally, the fraction with the
ACE inhibitory activity was collected and lyophilized; this was
followed by identification of the amino acid sequence.
Determination of molecular weight and
amino acid sequence
An accurate molecular mass and amino acid sequence
of the purified peptide was determined using a quadrupole
time-of-flight mass spectroscopy (Micromass, Altrincham, UK)
coupled with an electrospray ionization (ESI) source. The purified
peptide was separately infused into the electrospray source after
being dissolved in methanol/water (1:1, v/v), and the molecular
mass was determined by the doubly charged [M+2H]2+ state
in the mass spectrum. Following molecular mass determination, the
peptide was automatically selected for fragmentation, and sequence
information was obtained by tandem MS analysis.
Measurement of ACE inhibitory
activity
The ACE inhibitory activity assay was performed
according to the method of Cushman and Cheung (20) with slight modifications. For each
assay, 50 µl of the hydrolysate solution with 50 µl
of ACE solution (25 mU/ml) was pre-incubated at 37°C for 30 min,
and then incubated with 100 µl of substrate (25 mM HHL in 50
mM sodium borate buffer at pH 8.3) at 37°C for 60 min. The reaction
was terminated by the addition of 250 µl of 1 N HCl.
Hippuric acid was extracted with 500 µl of ethyl acetate.
Subsequently, a 200 µl aliquot of the extract was removed by
evaporation in a dry oven at 80°C. The residue was dissolved in 1
ml distilled water and its UV spectra density was measured at 228
nm using a microplate reader (PowerWave XS2; BioTek Instruments,
Inc., Winooski, VT, USA).
Determination of ACE inhibition
pattern
Various concentrations of ACE inhibitory peptide
were added to each reaction mixture, as previously described by
Bush et al (21). The
enzyme activity was measured with various concentrations of the
substrate. The ACE inhibitory pattern in the presence of the
inhibitor was obtained by the Lineweaver-Burk plots.
Molecular modeling
In order to find the binding site of the purified
peptide and to understand the mixed non-competitive inhibition
mechanism further, a three-stage molecular modeling was carried out
by Monte Carlo (MC) and molecular dynamics (MD) simulations. The
simulation system was composed of ACE, Ang II and the purified
peptide. The crystal structure of ACE in complex with Ang II (PDB
ID, 4APH) was used to set up the system. Ang II is the principal
end-product of the enzyme function. Since the purified peptide is a
mixed non-competitive inhibitor, Ang II was kept in the docking
simulation system to avoid overlapping between the binding site of
the purified peptide and the binding site of the substrate. Ang II
shares the binding site with Ang I, the substrate of the
enzyme.
In the first stage, docking of the purified peptide
around the ACE complex was performed, and the resulting model
structures were then clustered based on the location and the
orientation of the peptide relative to the ACE complex. In four
clusters, we selected the structure with the lowest score of each
cluster to run the second stage of docking for further sampling. In
the final stage, MD simulation was carried out using the structure
with the lowest score of the models generated in the second stage
in order to relax the structure further with explicit water
molecules.
Docking simulations
The binding site of the purified peptide on the ACE
complex was searched by docking simulations using the FlexPepDock
protocol in the Rosetta program, which is designed for modeling
complexes between a flexible peptide and a globular protein
(22). Using the MC with
minimi-zation approach in the main part of FlexPepDock protocol
(22), backbone torsion angles
and rigid body orientations of the peptide were optimized relative
to the protein. In the first stage of docking, 555 simulations were
performed with different initial positions and orientations of the
peptide around the ACE protein to cover the inside and the outside
of the cleft of two sub domains. The lowest energy scores of each
docking resulting 111,000 model structures simulation on the
outside of the cleft were higher than those on the inside of the
cleft. Based on the location and the orientation of the purified
peptide inside the cleft, resulting docking structures were
categorized into four clusters. The structure with the lowest score
of each cluster was selected, and the second stage of the docking
simulations was carried out with these four initial conformations
of the peptide. Using the 4 models with the lowest score of each
cluster as initial structures, the second stage of docking
simulations were carried out to sample 8,000 models further.
MD simulation
The structure of the complex of ACE, Ang II and the
purified peptide with the lowest score of the second stage of
docking simulations was selected to run MD simulation. The complex
system was solvated in a truncated octahedral box with 23,410 TIP3P
water molecules (23) and 0.1 M
NaCl ions. All MD simulations were carried out using NAMD software
(24) with CHARMM force field
(25) under the periodic boundary
condition. After removing bad contacts with 1,000 steps of energy
minimization, the MD simulation began by gradually heating up the
system from 10 to 310 K for 60 psec under the constant volume
condition. Afterward, all simulation was switched to constant
temperature and pressure conditions (NPT). The simulation system
was equilibrated for 200 psec. All Cα atoms in the steps
of energy minimization, heating and equilibration were restrained
with harmonic constant 1 kcal/mol•A2, and the following
MD simulation was continued without any restraint. All bonds
involving hydrogen atoms were constrained, allowing an integration
time step of 2 fsec, and the average pressure and temperature were
maintained at 1 bar and 310 K. The non-bonded interactions (Van der
Waals and electrostatic interactions) were smoothly truncated from
10 to 12 Å cut-off, and the particle-mesh Ewald method was used to
treat long-range electrostatic interactions (26). The simulation system was
stabilized at 36.57 nsec, and the total length of MD simulation was
123.83 nsec. The trajectory from 36.57 to 123.83 nsec, the end of
the simulation time, was analyzed in the study.
Cell culture analysis of the purified
peptide
Cell culture and cell viability
Human umbilical vein endothelial cells (EA.hy926)
were kindly provided by Professor S.K. Kim from Pukyong National
University, Busan, Korea. The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), penicillin (100 U/ml) and streptomycin (100
µg/ml) at 37°C in 5% CO2 humidified air
environment.
Cytotoxic assessment by MTT assay
Cell viability was determined by MTT reduction
assay. The EA.hy926 cells were plated on 96-well plates, which were
pre-incubated and subsequently treated with Ang II (1 µM)
coupled with aliquots of the purified peptide (62.5, 125 and 250
µM) at 37°C for 24 h. MTT stock solution was then added to
each well. Following incubation for 4 h, the plates were
centrifuged (800 × g, 5 min), and the supernatants were aspirated.
The formazan crystals in each well were dissolved in DMSO, and the
absorbance was measured using a microplate reader (PowerWave XS2;
BioTek Instruments, Inc.) at 540 nm.
Determination of NO and ROS
levels
NO levels in the culture supernatants were
determined by measuring nitrite, which is a major stable product of
NO, using Griess reagent. Following the pre-incubation of the
EA.hy926 cells with the purified peptide and Ang II for 24 h, the
quantity of nitrite which had accumulated in the culture medium was
measured as an indicator of NO production. Briefly, a 100 µl
of cell culture medium was mixed with 100 µl of Griess
reagent (1% sulfanilamide and 0.1% naphthylenthylenediamine
dihydrocholoride in 2.5% phosphoric acid), the mixture was
incubated at room temperature for 10 min, and the absorbance at 540
nm was measured using a microplate reader. Fresh culture medium was
used as a blank in every experiment.
The intracellular generation of ROS was then
detected by oxidation of the cell permeable fluorescence dye
5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate
(carboxy-DCFH-DA) to the fluorescent DCF by Ang II. The EA.hy926
cells were seeded on 24-well plate. After 24 h, the cells were
pre-treated with various concentrations of the purified peptide.
After 30 min, Ang II was added at a concentration of 1 µM,
and the cells were thyen incubated for an additional 30 min at 37°C
under a humidified atmosphere. Finally, 40 µM
carboxy-DCFH-DA was introduced to the cells, and carboxy-DCFH-DA
and DAPI was imaged using a fluorescence microscope (Axio Observer
A1; Zeiss, Jena, Germany).
Western blot analysis
The cells were lysed in lysis buffer (20 mM Tris, 5
mM EDTA, 10 mM Na4P2O7, 100 mM
NaF, 2 mM Na3VO4, 1% NP-40, 10 mg/ml
aprotinin, 10 mg/ml leupeptin and 1 mM PMSF) for 60 min and then
centrifuged at 12,000 rpm and 4°C for 15 min. The protein
concentrations were determined using the BCA™ protein assay kit.
The lysate containing 40 µg of protein was subjected to
electrophoresis on a sodium dodecyl sulfate (SDS)-polyacrylamide
gel, and the gel was transferred onto nitrocellulose membranes. The
membranes were blocked with 5% non-fat dry milk in TBS-T (25 mM
Tris-HCl, 137 mM NaCl, 2.65 mM KCl and 0.05% Tween-20, pH 7.4) for
2 h. The primary antibodies were used at a 1:1,000 dilution. The
membranes were incubated with the primary antibodies at 4°C
overnight, washed with TBS-T and then incubated with the secondary
antibodies (G21040 and G21234; Invitrogen Life Technologies) at
1:3,000 dilutions. The signals were developed using an ECL western
blotting detection kit and quantified using an LAS3200®
luminescent image analyzer and protein expression was quantified by
Multi Gauge V3.0 software (Fujifilm Life Science, Tokyo,
Japan).
Statistical analysis
All quantitative data are presented as the means ±
standard deviation (SD) with least 3 individual experiments that
were conducted using fresh reagents. Significant differences
between the groups were determined using the unpaired Student's
t-test. The differences were considered statistically significant
at P<0.05.
Results
Fractionation of Spirulina sp. protein
hydrolysate to produce an ACE inhibitory peptide
Spirulina was hydrolyzed using
gastrointestinal enzymes that increase the absorption of active
peptides, including pepsin, α-chymotrypsin and trypsin, under
simulated physiological conditions. The obtained hydroly-sate was
tested for its potential to inhibit ACE. In the ACE inhibitory
assay (Table I), the hydrolysate
IC50 value was exhibited by the gastrointestinal
hydrolysate of Spirulina sp. at 0.98 mg/ml. The hydrolysate
was fractionated using an ultrafiltration membrane system into 4
individual fractions with MWs of <5, 5–10, 10–100 and >100
kDa, respectively, and the resultant fractions were also analyzed
for their abilities to inhibit ACE. Among all the MW groups, the
<5 kDa fraction exhibited the highest ACE inhibitory activity
and had an IC50 value of 0.66 mg/ml (Table I). Therefore, we selected the
<5 kDa fraction for further purification of an ACE inhibitory
peptide.
 | Table IACE inhibitory activity of molecular
weight fractions from gastrointestinal hydrolysate of
Spirulina sp. |
Table I
ACE inhibitory activity of molecular
weight fractions from gastrointestinal hydrolysate of
Spirulina sp.
| Fraction | IC50
value (mg/ml)a |
|---|
|
Unfractionatedb | 0.98±0.02 |
| >100 kDac | 1.06±0.03 |
| 10–100 kDa | 0.96±0.02 |
| 5–10 kDa | 0.97±0.01 |
| <5 kDa | 0.66±0.03 |
Purification of ACE inhibitory
peptide
An ACE inhibitory peptide was purified from the
marine Spirulina sp. by using chromatographic methods,
combining FPLC on a HiPrep™ DEAE FF 16/10 anion-exchange column (GE
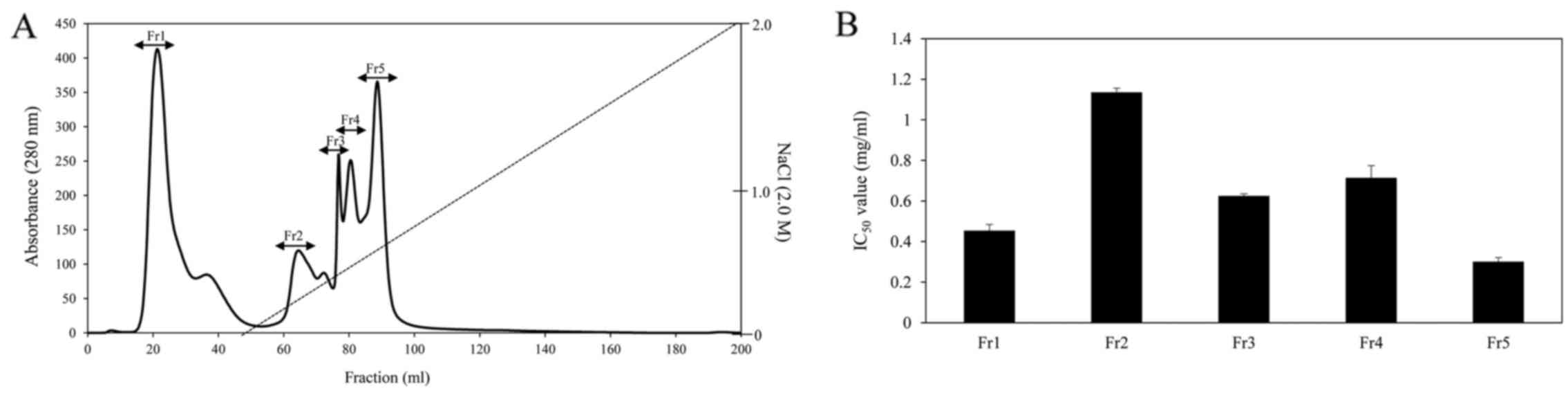
Healthcare). As shown in Fig. 1A,
there were 4 major absorbance peaks at 280 nm and 5 fractions
associated with the peaks were pooled and lyophilized for ACE
inhibitory activity. Among the fractions, fraction Fr5 exhibited
the strongest ACE inhibitory activity and had an IC50
value of 0.3 mg/ml (Fig. 1B). The
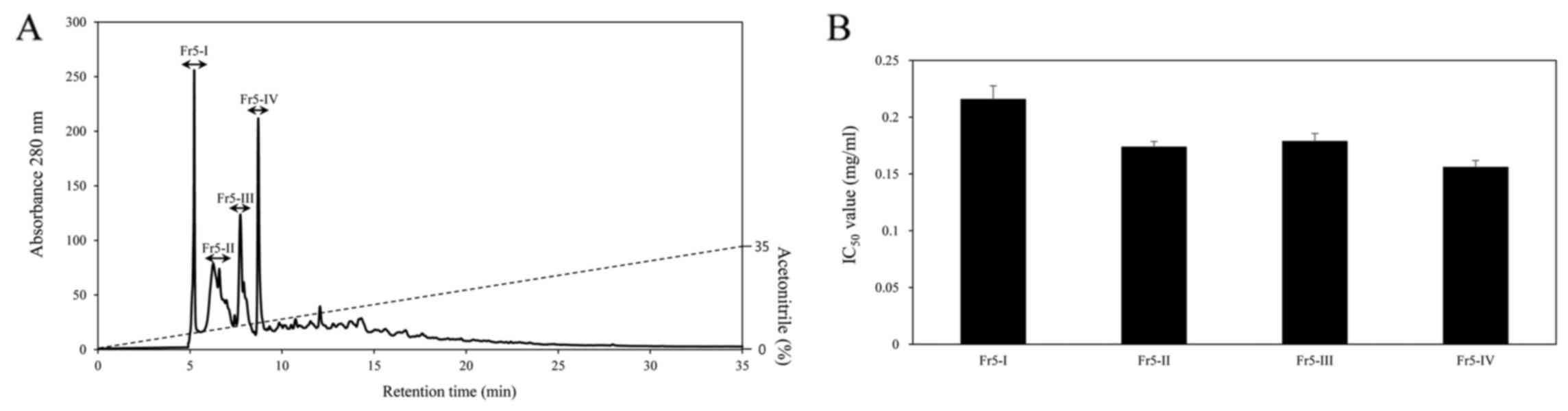
active fraction was further separated by RP-HPLC on a PrimeSphere™
ODS column (Phenomenex, Torrance, CA, USA) with a linear gradient
of acetotitrile (0–35% for 35 min) containing 0.1% TFA. The elusion
profiles of the peaks are shown in Fig. 2A. The peaks were separated into 4
peaks (Fr5-I to Fr5-IV) and each peak was pooled for ACE inhibitory
activity. The Fr5-IV peak exhibited the most potent ACE inhibitory
activity, with an IC50 value of 0.1 mg/ml (Fig. 2B). The typical results
investigated during the purification processes are summarized in
Table II. The ACE inhibitory
peptide was purified 10.2-fold from the enzymatic hydrolysate using
a 3-step purification procedure.
 | Table IIPurification of ACE inhibitory
peptide of gastrointestinal hydrolysate from Spirulina
sp. |
Table II
Purification of ACE inhibitory
peptide of gastrointestinal hydrolysate from Spirulina
sp.
| Purification
step | IC50
value (mg/ml)a | Purification
fold |
|---|
| Gastrointestinal
hydrolysate | 0.98±0.02 | 1 |
| Ultrafiltration
(<5 kDa) | 0.66±0.03 | 1.48 |
| FPLCb | 0.30±0.01 | 2.97 |
| RP-HPLCc | 0.10±0.02 | 9.80 |
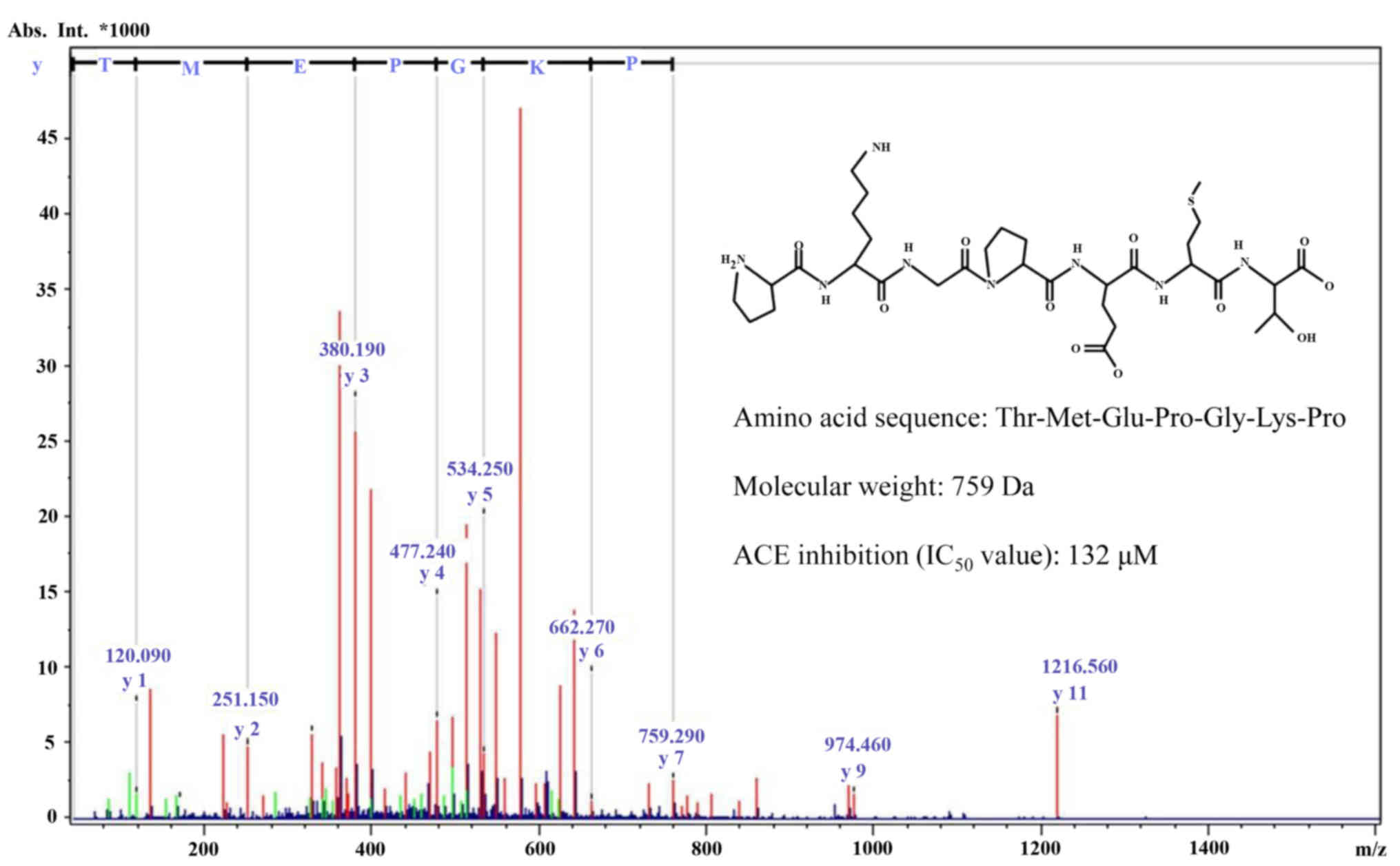
The molecular mass of the purified ACE inhibitory
peptide was determined to be 759 Da by quadrupole time-of-flight
mass spectroscopy coupled to an electrospray ionization source. Its
full amino acid sequence was determined to be
Thr-Met-Glu-Pro-Gly-Lys-Pro (Fig.
3).
ACE inhibition pattern and molecular
modeling of the purified ACE inhibitory peptide
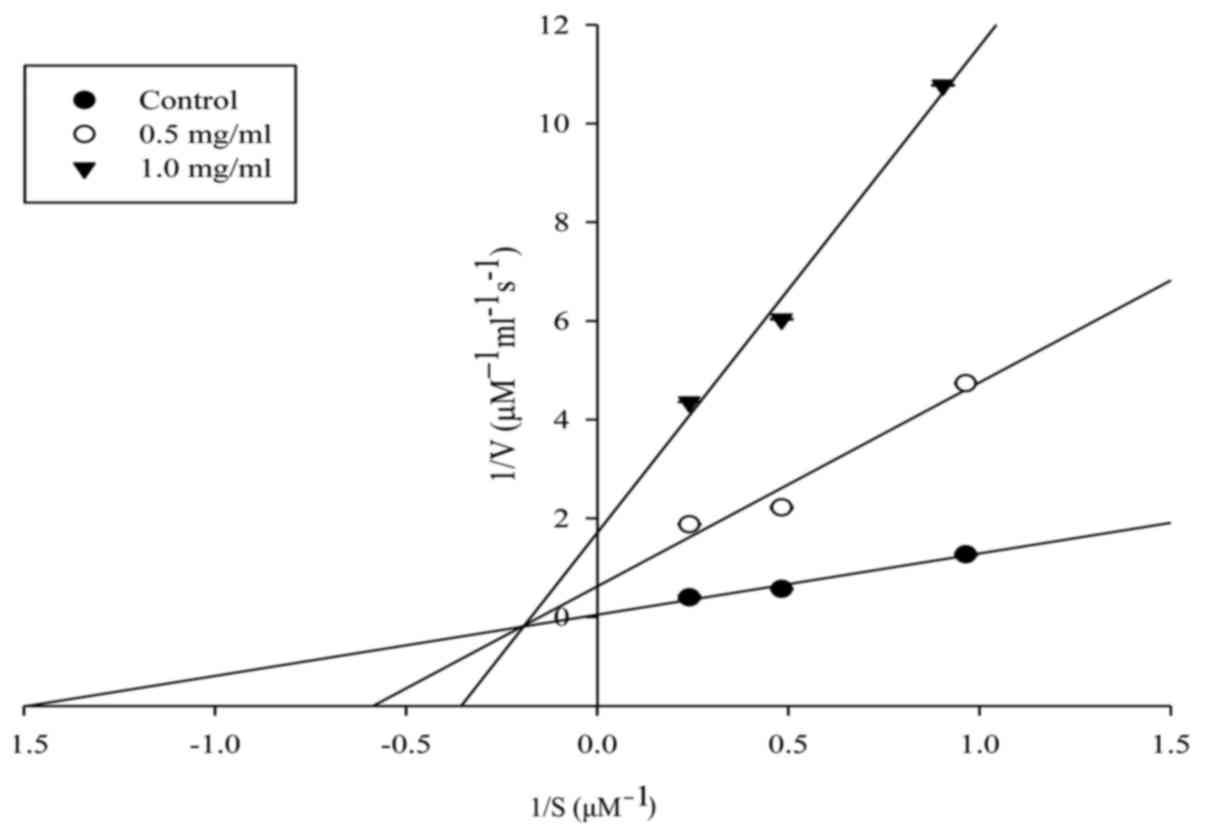
The ACE inhibition pattern of the purified peptide
was investigated by Lineweaver-Burk plots and was found to show a
mixed non-competitive inhibition pattern (Fig. 4). Moreover, based on molecular
modeling, we were able to determine a mixed non-competitive
inhibition mechanism of ACE by the purified peptide (Fig. 5). The ACE binding site of the
purified peptide was predicted by 3-stage molecular modeling. The
peptide bound in the N-terminal side of the cleft formed by two
sub-domains of ACE and next to the binding site of Ang II (Fig. 5A and B). The binding of the
purified peptide was stabilized by hydrogen bond interactions with
ACE and Ang II. We investigated the presence of hydrogen bonds by
performing MD simulations using a distance of 3.4 Å and a rotation
angle of 30°. On average, during MD simulation, the purified
peptide formed 5.76±1.50 and 2.58±0.83 pairs of hydrogen bonds with
ACE and Ang II, respectively, at the same time (data expressed as
the means ± SD). The most frequently formed pairs of hydrogen bonds
during the MD simulation are listed in Fig. 5C. In addition to hydrogen bonds,
binding of the peptide was stabilized by van der Waals
interactions. The MD simulation results revealed that Pro4 of the
purified peptide was bound in the hydrophobic pocket formed by
Ile204, Ala208 and Trp220 of ACE. This result indicates that the
purified peptide can combine with ACE to produce a dead-end
complex, regardless of whether a substrate molecule is bound or
not. Thus, the purified peptide must be bound to an alternative
site of the enzyme. Taken together, the results suggest that the
peptide acts as an ACE inhibitor by forming
enzyme-substrate-inhibitor and enzyme-inhibitor complexes to
suppress the catalytic activity of ACE.
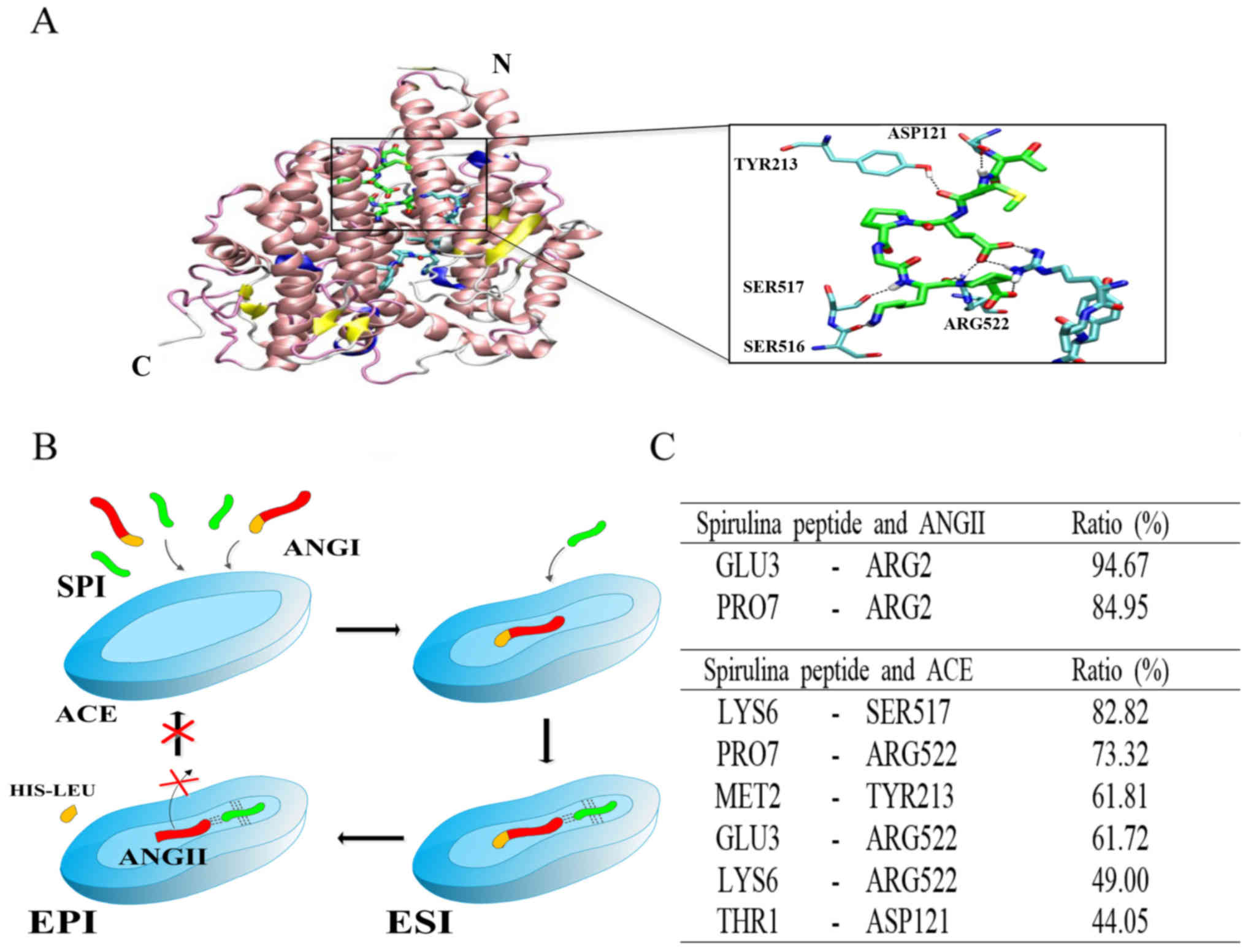 | Figure 5The binding site of the purified
peptide in the cleft of ACE. ACE is represented by ribbons, and
zinc atom in active site is represented by a gray sphere. Ang II
and the purified peptide are represented by thick sticks, and amino
acids of ACE involved in hydrogen bonds are represented by thin
sticks. (A) Carbon, nitrogen, oxygen, sulfur and hydrogen atoms are
colored by cyan, blue, red, yellow and white, respectively. Carbon
atoms of the purified peptide are colored by green. N and C
indicate the N- and C-terminus of the enzyme, respectively. The
snapshot is taken at 121.6 nsec. (B) Mixed non-competitive
inhibition mechanism of ACE by the purified peptide. The broken
lines indicate hydrogen bonds (I, inhibitor, or the purified
peptide; ACE, angiotensin I-converting enzyme; Ang I, angiotensin
I, or substrate; Ang II, angiotensin II, or product; ESI,
enzyme-substrate-inhibitor complex; EPI, enzyme-product-inhibitor
complex, dead-end complex). (C) Ratio of the presence of hydrogen
bond pairs during the simulation time. |
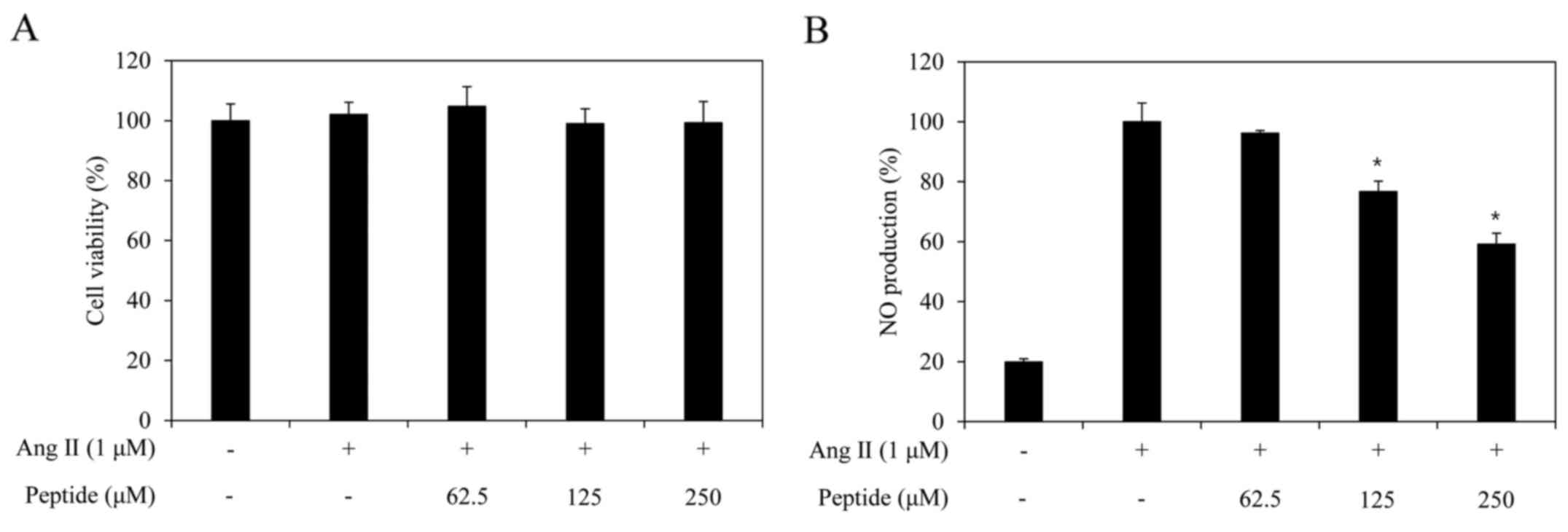
Cytotoxicity of the purified peptide and
its inhibitory effect on NO and intracellular ROS generation
The cytotoxic effect of the purified peptide in
EA.hy926 cells was assessed by MTT assay at multiple purified
peptide concentrations (62.5, 125 and 250 µM). No
significant toxic effects were observed in the cells, which were
treated with concentrations of up to 250 µM of the purified
peptide in the presence or absence of Ang II (Fig. 6A). Thus, based on this result, an
NO production assay was performed at the peptide concentration of
250 µM.
In order to determine whether the purified peptide
inhibited the generation of Ang II-induced NO and intracellular
ROS, which play central roles in endothelial dysfunction, the
EA.hy926 cells were stimulated with Ang II in the absence or
presence of the purified peptide. Ang II treatment alone markedly
induced NO generation by the treated cells compared with the
untreated cells. The purified peptide significantly inhibited NO
generation by the Ang II-stimulated EA.hy926 cells (Fig. 6B). Subsequently, in order to
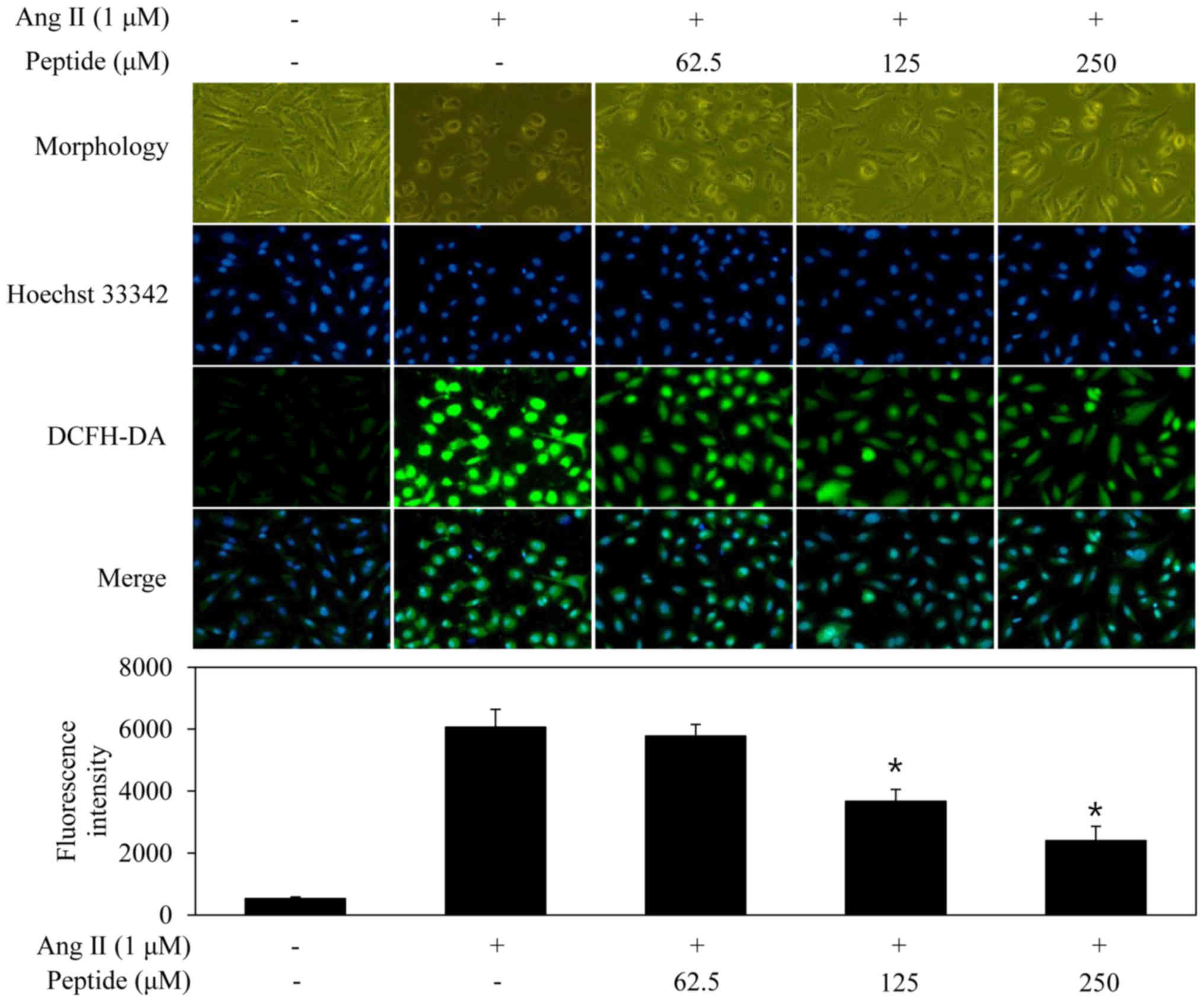
confirm the inhibition of Ang II-induced oxidative stress in the
EA.hy926 cells treated with the purified peptide, intracellular ROS
generation was evaluated by monitoring DCFH-DA fluorescence
(Fig. 7). The fluorescence
intensity in the EA.hy926 cells increased markedly following
exposure to Ang II compared with that in the untreated cells.
Pre-treatment with the purified peptide inhibited the induction of
increased fluorescence intensity induced by Ang II.
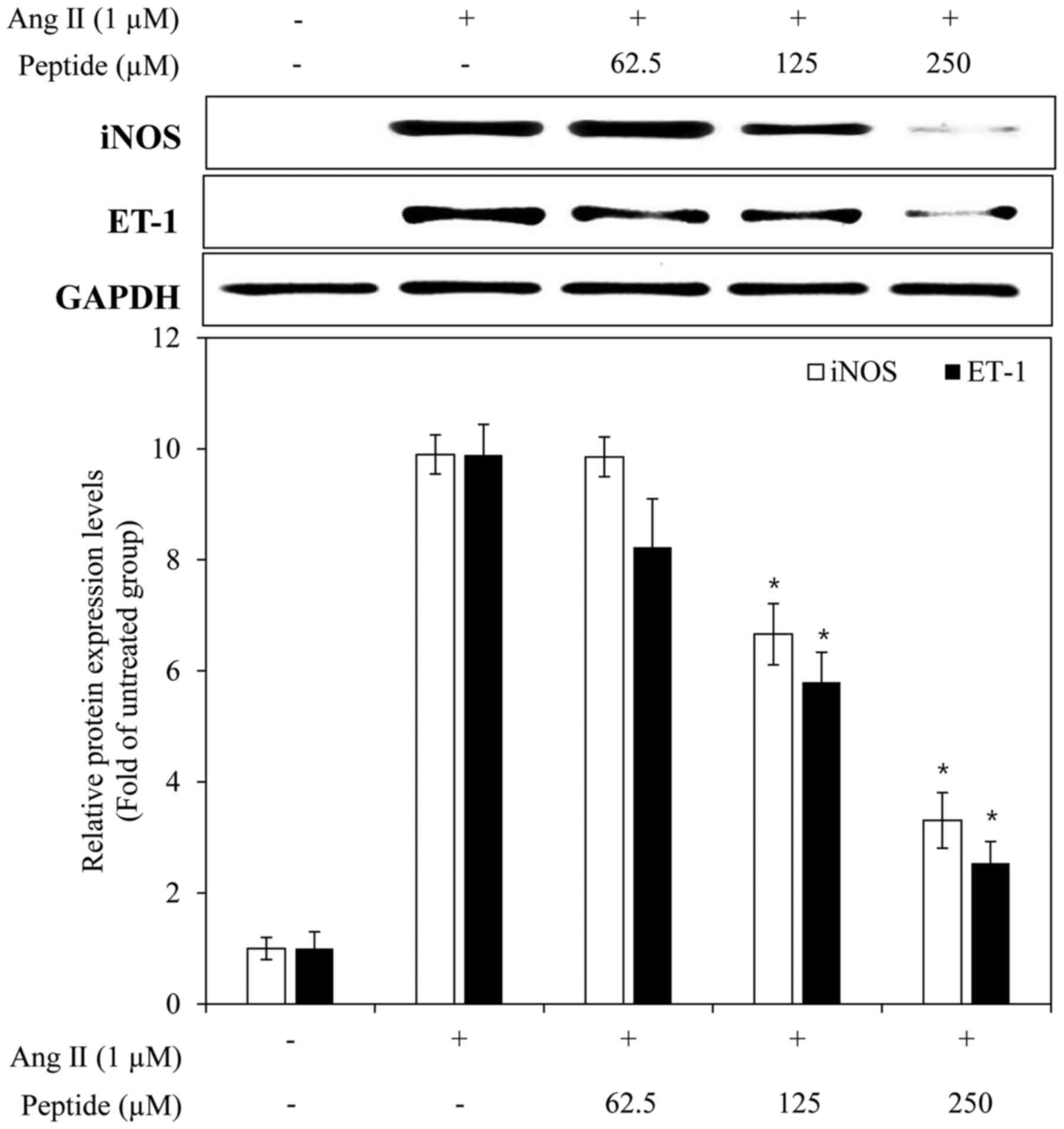
Effects of the purified peptide on
endothelial dysfunction-related factors and the activation of
mitogen-activated protein kinases (MAPKs) in Ang II-stimulated
EA.hy926 cells
To examine the ability of the purified peptide to
inhibit Ang II-mediated endothelial dysfunction, its effects on the
expression of iNOS and ET-1 were determined in the presence of Ang
II. In response to Ang II, the expression of iNOS and ET-1 was
markedly increased, and the purified peptide inhibited the iNOS and
ET-1 expression levels in a dose-dependent manner (Fig. 8). To elucidate the mechanisms of
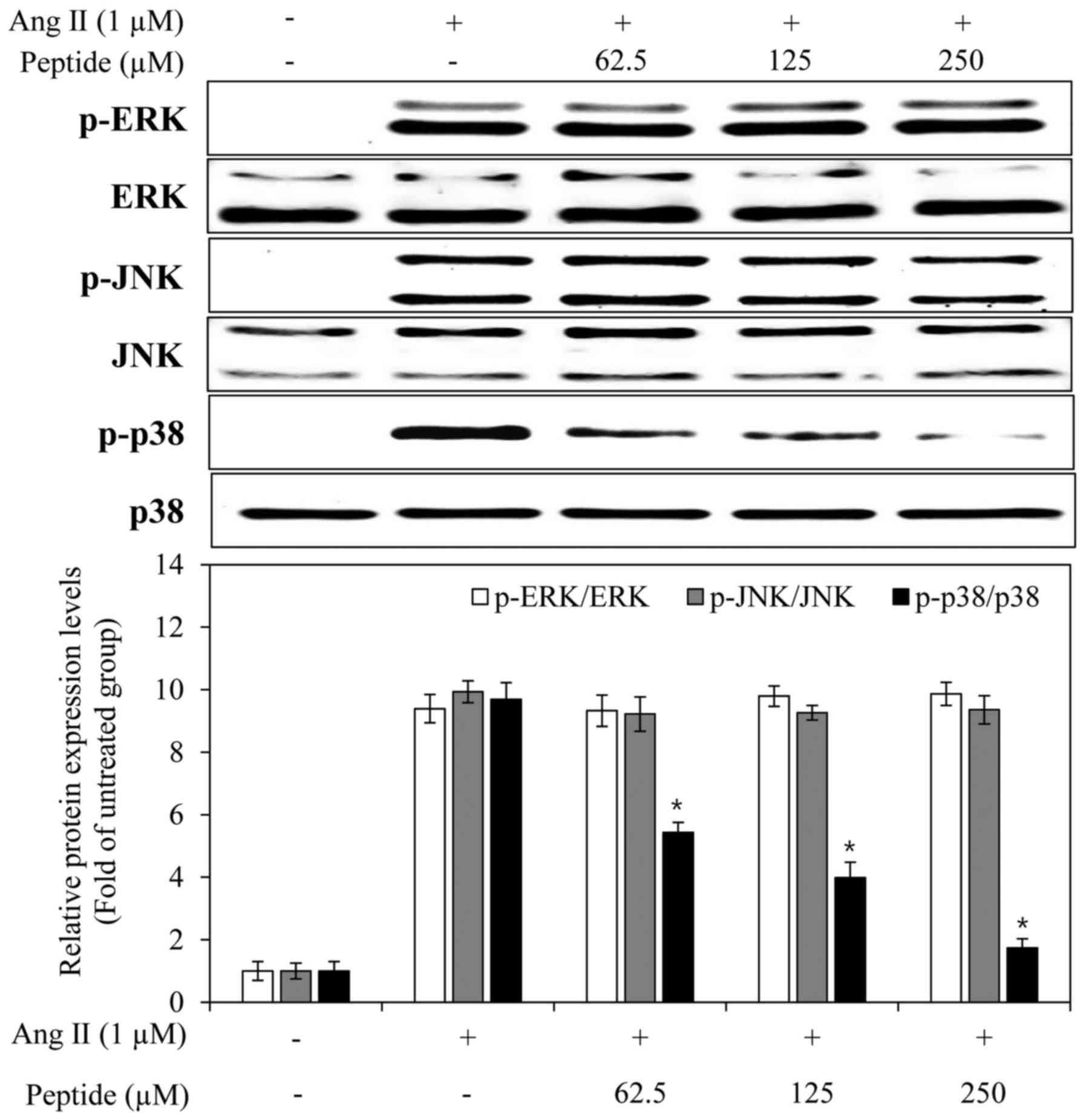
action of the purified peptide, we examined its effects on the
phosphorylation of MAPKs in Ang II-stimulated endothelial cells.
The levels of phosphorylated ERK, JNK and p38 MAPK were elevated in
the EA.hy926 cells following treatment with Ang II alone. The
purified peptide attenuated the Ang II-induced phosphorylation of
p38 MAPK, but did not affect the phosphorylation of ERK and JNK
(Fig. 9). These results suggested
that the peptide inhibited the expression of iNOS and ET-1 by
reducing the phosphorylation of p38 MAPK.
Discussion
Spirulina is one of the edible microalgae and
contains a high amount of protein. It also contains all of the
essential amino acids, which makes it a complete protein source
(17). Thus, Spirulina is
considered a useful material from which bioactive peptides can be
obtained. Potent bioactive peptides have attracted attention in
research that aims to promote human health and reduce disease risk
(17). Bioactive peptides have
previously been produced via the enzymatic hydrolysis of various
bioresources that contain a high protein concentration (17,27). Simulated gastrointestinal
digestion can be used as a production process for potent bioactive
peptides, with the advantage that the obtained peptides will resist
physiological digestion after oral intake (28). As previously reported, digestion
by gastrointestinal proteases provides more potent bioactive
peptides compared with those obtained using other types of enzyme
digestions (29). Recently, a
number of studies have found that specific bioactive peptides
obtained from gastrointestinal hydrolysates have anti-hypertensive
(30), antioxidative (19), anticancer (31) and anti-inflammatory (32) effects. In the present study, we
isolated an ACE inhibitory peptide from a gastrointestinal digest
of Spirulina sp. protein, and the peptide was then
investigated for its potential inhibitory effect on endothelial
dysfunction in a human umbilical vein cell line.
Compared to a previous study, the ACE inhibitory
activity of gastrointestinal hydrolysate from Spirulina sp.
was more effective than that of gastrointestinal hydrolysate from
silkworm pupa protein (33).
Moreover, gastrointestinal hydrolysate of silkworm pupa protein was
fractionated into 3 fractions (>10, 5–10 and <5 kDa) by UF
according to molecular weights and the <5 kDa fraction exhibited
strongest ACE inhibitory activity and similar with our results
(33). Following purification, an
ACE inhibitory peptide composed of 7 amino acids was obtained.
According to previous findings, the inhibitory efficiency of ACE
inhibitory peptides is highly influenced by their molecular weight
and structure (6). Generally, ACE
inhibitors contain structurally and functionally homologous
features, such as dipeptidyl carboxypeptidase activity and zinc
coordination geometry. Among ACE inhibitory peptides, the most
potent and specific peptide inhibitors have similar structures, and
ACE activity is strongly affected by the C-terminal tripeptide
sequence of the substrate. The main substrates of peptide
composition, such as Trp, Tyr, Phe, Pro and a hydrophobic amino
acid at the C-terminal were found to be effective for ACE
inhibitory activity due to the interaction among 3 sub-sites:
S1 (antepenultimate), S′1 (penultimate) and
S′2 (ultimate), the active ACE site. In this study, the
ACE inhibitory peptide obtained from a Spirulina sp. protein
hydrolysate contained a Pro residue within its C-terminal
tripeptide sequence, which may contribute to its ACE inhibitory
activity. In addition, kinetic analysis by Lineweaver-Burk plots
revealed that the purified peptide acts as a mixed non-competitive
inhibitor. Moreover, based on molecular modeling, we were able to
determine a mixed non-competitive inhibition mechanism of ACE by
the purified peptide. This inhibition pattern type was similar to
hard clam (34) and Styela
plicata (35). Since the
purified peptide does not share its binding site with Ang II, it
does not compete for binding with the substrate. The peptide,
however, binds in the cleft of the enzyme and next to the binding
site of the substrate. The bound peptide may inhibit the
conformational changes required to release Ang II, the end product,
by forming about 6 hydrogen bonds with the enzyme. In addition, the
purified peptide forms hydrogen bonds with Ang II and holds it in
the active site of the enzyme. Therefore, the peptide, enzyme, and
Ang II form a dead-end complex, making it difficult for Ang II to
leave the enzyme. Indeed, in this scenario, Ang II, the end product
of catalysis by ACE, becomes a competitive inhibitor. Thus, the
active site is occupied and another substrate cannot bind for the
next enzymatic action.
In addition, the purified peptide also inhibited the
Ang II-induced production of NO and intracellular ROS. Moreover,
changes in cell morphology correlated with intracellular ROS
production were recovered by treatment with the purified peptide.
Ang II has been widely considered to be a systemic regulator of
blood pressure (36). Moreover,
it is known to be a strong vasoconstriction factor and a regulator
of cardiac function (37). The
excessive generation of intracellular ROS has been implicated in
the pathogenesis of many cardiovascular diseases, including
atherosclerosis, hypertension, heart failure and the associated
endothelial dysfunction (38).
According to a previous study, Ang II induces NO production via the
expression of iNOS in the vascular smooth muscle cell (39). In addition, it does not affect the
expression of endothelial nitric oxide synthase (eNOS) (40). Increased NO production via the
expression of iNOS has been observed to trigger apoptotic and
necrotic cell death in the neighboring tissue, for instance in
atherosclerosis (41). ET-1 is
another known vasoconstriction factor that, similar to Ang II, can
induce endothelial dysfunction (42). Our results demonstrated that the
purified ACE inhibitory peptide reduced the expression of iNOS and
ET-1 in the Ang II-stimulated EA.hy926 cells.
MAPKs, including ERK, JNK and p38 kinase have been
proposed to be activated in response to Ang II stimulation.
According to previous studies, signaling by MAPKs promotes iNOS and
ET-1 expression in Ang II-stimulated endothelial cells (43,44). In addition, the activation of
MAPKs promotes the production of endothelial dysfunction-related
factors in Ang II-stimulated endothelial cells (8). Thus, the inhibition of MAPK
phosphorylation is a key mechanism. In the present study, we
examined the effects of the purified ACE inhibitory peptide on the
activation of MAPKs and found that the purified peptide attenuated
the Ang II-induced phosphorylation of p38 MAPK.
In conclusion, we evaluated the effects of the ACE
inhibitory peptide purified from a hydrolysate produced by the
gastrointestinal digestion obtained from Spirulina sp. The
ACE inhibitory pattern of the purified peptide was determined by
Lineweaver-Burk plots and a mixed non-competitive inhibition
mechanism of this peptide was shown at the molecular level by
molecular modeling. The purified peptide potently inhibited NO
production, intracellular ROS, iNOS and ET-1 production and
decreased the levels of phosphorylated p38 MAPK in Ang
II-stimulated endothelial cells (Fig. 10). Consequently, it appears that
this purified peptide has a beneficial effect on anti-hypertension
and related diseases by inhibiting ACE and the cellular response to
Ang II.
Abbreviations:
|
ACE
|
angiotentin I-converting enzyme
|
|
Ang
|
angiotensin
|
|
NO
|
nitric oxide
|
|
ROS
|
reactive oxygen species
|
|
ET-1
|
endothelin-1
|
|
iNOS
|
inducible nitric oxide synthase
|
|
MAPKs
|
mitogen-activated protein kinases
|
Acknowledgments
This study was supported by a grant from the Marine
Biotechnology Program (20150220) funded by the Ministry of Oceans
and Fisheries, Republic of Korea.
References
|
1
|
Yamada Y, Kato K, Yoshida T, Yokoi K,
Matsuo H, Watanabe S, Ichihara S, Metoki N, Yoshida H, Satoh K, et
al: Association of polymorphisms of ABCA1 and ROS1 with
hypertension in Japanese individuals. Int J Mol Med. 21:83–89.
2008.
|
|
2
|
Ko SC, Jung WK, Kang SM, Lee SH, Kang MC,
Heo SJ, Kang KH, Kim YT, Park SJ, Jeong Y, et al: Angio-tensin
I-converting enzyme (ACE) inhibition and nitric oxide (NO)-mediated
antihypertensive effect of octaphlorethol A isolated from Ishige
sinicola: in vitro molecular mechanism and in vivo SHR model. J
Funct Foods. 18:289–299. 2016. View Article : Google Scholar
|
|
3
|
Sanae M and Yasuo A: Green asparagus
(Asparagus officinalis) prevented hypertension by an inhibitory
effect on angiotensin-converting enzyme activity in the kidney of
spontaneously hypertensive rats. J Agric Food Chem. 61:5520–5525.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahn CB, Jeon YJ, Kim YT and Je JY:
Angiotensin I converting enzyme (ACE) inhibitory peptides from
salmon byproduct protein hydrolysate by alcalase hydrolysis.
Process Biochem. 47:2240–2245. 2012. View Article : Google Scholar
|
|
5
|
Tomita N, Yamasaki K, Izawa K, Kunugiza Y,
Osako MK, Ogihara T and Morishita R: Improvement of organ damage by
a non-depressor dose of imidapril in diabetic spontaneously
hypertensive rats. Int J Mol Med. 19:571–579. 2007.PubMed/NCBI
|
|
6
|
Ko SC, Kang N, Kim EA, Kang MC, Lee SH,
Kang SM, Lee JB, Jeon BT, Kim SK, Park SJ, et al: A novel
angiotensin I-converting enzyme (ACE) inhibitory peptide from a
marine Chlorella ellipsoidea and its antihypertensive effect in
spontaneously hypertensive rats. Process Biochem. 47:2005–2011.
2012. View Article : Google Scholar
|
|
7
|
Qian ZJ, Je JY and Kim SK:
Antihypertensive effect of angiotensin I converting
enzyme-inhibitory peptide from hydrolysates of Bigeye tuna dark
muscle, Thunnus obesus. J Agric Food Chem. 55:8398–8403. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Li D, Schaefer R and Mehta JL:
Cross-talk between dyslipidemia and renin-angiotensin system and
the role of LOX-1 and MAPK in atherogenesis studies with the
combined use of rosuvastatin and candesartan. Atherosclerosis.
184:295–301. 2006. View Article : Google Scholar
|
|
9
|
Li JM and Shah AM: Mechanism of
endothelial cell NADPH oxidase activation by angiotensin II. Role
of the p47phox subunit. J Biol Chem. 278:12094–12100.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alonso-Galicia M, Maier KG, Greene AS,
Cowley AW Jr and Roman RJ: Role of 20-hydroxyeicosatetraenoic acid
in the renal and vasoconstrictor actions of angiotensin II. Am J
Physiol Regul Integr Comp Physiol. 283:R60–R68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gragasin FS, Xu Y, Arenas IA, Kainth N and
Davidge ST: Estrogen reduces angiotensin II-induced nitric oxide
synthase and NAD(P)H oxidase expression in endothelial cells.
Arterioscler Thromb Vasc Biol. 23:38–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hahn AW, Resink TJ, Scott-Burden T, Powell
J, Dohi Y and Bühler FR: Stimulation of endothelin mRNA and
secretion in rat vascular smooth muscle cells: a novel autocrine
function. Cell Regul. 1:649–659. 1990.PubMed/NCBI
|
|
13
|
Qian ZJ, Heo SJ, Oh CH, Kang DH, Jeong SH,
Park WS, Choi IW, Jeon YJ and Jung WK: Angiotensin I-converting
enzyme (ACE) inhibitory peptide isolated from biodiesel byproducts
of marine microalgae, Nannochloropsis oculata. J Biobased Mater
Bioenergy. 7:135–142. 2013. View Article : Google Scholar
|
|
14
|
Servaes K, Maesen M, Prandi B, Sforza S
and Elst K: Polar lipid profile of Nannochloropsis oculata
determined using a variety of lipid extraction procedures. J Agric
Food Chem. 63:3931–3941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oh GW, Ko SC, Heo SY, Nguyen VT, Kim G,
Jang CH, Park WS, Choi IW, Qian ZJ and Jung WK: A novel peptide
purified from the fermented microalga Pavlova lutheri attenuates
oxidative stress and melanogenesis in B16F10 melanoma cells.
Process Biochem. 50:1318–1326. 2015. View Article : Google Scholar
|
|
16
|
Suetsuna K and Chen JR: Identification of
antihypertensive peptides from peptic digest of two microalgae,
Chlorella vulgaris and Spirulina platensis. Mar Biotechnol (NY).
3:305–309. 2001. View Article : Google Scholar
|
|
17
|
Vo TS, Ryu B and Kim SK: Purification of
novel anti-inflammatory peptides from enzymatic hydrolysate of the
edible microalgal Spirulina maxima. J Funct Foods. 5:1336–1346.
2013. View Article : Google Scholar
|
|
18
|
Samuels R, Mani UV, Iyer UM and Nayak US:
Hypocholesterolemic effect of Spirulina in patients with
hyperlipidemic nephrotic syndrome. J Med Food. 5:91–96. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jung WK, Qian ZJ, Lee SH, Choi SY, Sung
NJ, Byun HG and Kim SK: Free radical scavenging activity of a novel
anti-oxidative peptide isolated from in vitro gastrointestinal
digests of Mytilus coruscus. J Med Food. 10:197–202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cushman DW and Cheung HS:
Spectrophotometric assay and properties of the
angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol.
20:1637–1648. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bush K, Henry PR and Slusarchyk DS:
Muraceins - muramyl peptides produced by Nocardia orientalis as
angiotensin-converting enzyme inhibitors. I. Taxonomy, fermentation
and biological properties. J Antibiot (Tokyo). 37:330–335. 1984.
View Article : Google Scholar
|
|
22
|
Raveh B, London N and Schueler-Furman O:
Sub-angstrom modeling of complexes between flexible peptides and
globular proteins. Proteins. 78:2029–2040. 2010.PubMed/NCBI
|
|
23
|
Jorgensen WL, Chandrasekhar J, Madura JD,
Impey RW and Klein ML: Comparison of simple potential functions for
simulating liquid water. J Chem Phys. 79:926–935. 1983. View Article : Google Scholar
|
|
24
|
Phillips JC, Braun R, Wang W, Gumbart J,
Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L and Schulten K:
Scalable molecular dynamics with NAMD. J Comput Chem. 26:1781–1802.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
MacKerell AD Jr, Bashford D, Bellott M,
Dunbrack RL Jr, Evanseck JD, Field MJ, Fischer S, Gao J, Guo H, Ha
S, et al: All-atom empirical potential for molecular modeling and
dynamics studies of proteins. J Phys Chem B. 102:3586–3616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toukmaji A, Sagui C, Board J and Darden T:
Efficient particle-mesh Ewald based approach to fixed and induced
dipolar interactions. J Chem Phys. 113:10913–10927. 2000.
View Article : Google Scholar
|
|
27
|
Ko SC, Lee JK, Byun HG, Lee SC and Jeon
YJ: Purification and characterization of angiotensin I-converting
enzyme inhibitory peptide from enzymatic hydrolysates of Styela
clava flesh tissue. Process Biochem. 47:34–40. 2012. View Article : Google Scholar
|
|
28
|
Qian ZJ, Jung WK, Byun HG and Kim SK:
Protective effect of an antioxidative peptide purified from
gastrointestinal digests of oyster, Crassostrea gigas against free
radical induced DNA damage. Bioresour Technol. 99:3365–3371. 2008.
View Article : Google Scholar
|
|
29
|
Himaya SWA, Ngo DH, Ryu B and Kim SK: An
active peptide purified from gastrointestinal enzyme hydrolysate of
Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme
(ACE) activity and cellular oxidative stress. Food Chem.
132:1872–1882. 2012. View Article : Google Scholar
|
|
30
|
Jung WK, Mendis E, Je JY, Park PJ, Son BW,
Kim HC, Choi YK and Kim SK: Angiotensin I-converting enzyme
inhibitory peptide from yellow sole (Limanda aspera) frame protein
and its antihypertensive effect in spontaneously hypertensive rats.
Food Chem. 94:26–32. 2006. View Article : Google Scholar
|
|
31
|
Nguyen VT, Qian ZJ, Ryu B, Kim KN, Kim D,
Kim YM, Jeon YJ, Park WS, Choi IW, Kim GH, et al: Matrix
metalloproteinases (MMPs) inhibitory effects of an octameric
oligopeptide isolated from abalone Haliotis discus hannai. Food
Chem. 141:503–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qian ZJ, Ryu B, Park WS, Choi IW and Jung
WK: Inhibitory effects and molecular mechanism of an
anti-inflammatory peptide isolated from intestine of abalone,
Haliotis discus hannai on LPS-induced cytokine production via the
p-38/p-JNK pathways in RAW264.7 macrophages. J Food Biochem.
4:690–698. 2016.
|
|
33
|
Wu Q, Jia J, Yan H, Du J and Gui Z: A
novel angiotensin-I converting enzyme (ACE) inhibitory peptide from
gastrointestinal protease hydrolysate of silkworm pupa (Bombyx
mori) protein: biochemical characterization and molecular docking
study. Peptides. 68:17–24. 2015. View Article : Google Scholar
|
|
34
|
Tsai JS, Chen JL and Pan BS:
ACE-inhibitory peptides identified from the muscle protein
hydrolysate of hard clam (Meretrix lusoria). Process Biochem.
43:743–747. 2008. View Article : Google Scholar
|
|
35
|
Ko SC, Kang MC, Lee JK, Byun HG, Kim SK,
Lee SC, Jeon BT, Park PJ, Jung WK and Jeon YJ: Effect of
angiotensin I-converting enzyme (ACE) inhibitory peptide purified
from enzymatic hydrolysates of Styela plicata. Eur Food Res
Technol. 233:915–922. 2011. View Article : Google Scholar
|
|
36
|
Griendling KK, Murphy TJ and Alexander RW:
Molecular biology of the renin-angiotensin system. Circulation.
87:1816–1828. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kagiyama S, Eguchi S, Frank GD, Inagami T,
Zhang YC and Phillips MI: Angiotensin II-induced cardiac
hypertrophy and hypertension are attenuated by epidermal growth
factor receptor antisense. Circulation. 106:909–912. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: the role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Millatt LJ, Abdel-Rahman EM and Siragy HM:
Angiotensin II and nitric oxide: a question of balance. Regul Pept.
81:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Olson SC, Dowds TA, Pino PA, Barry MT and
Burke-Wolin T: ANG II stimulates endothelial nitric oxide synthase
expression in bovine pulmonary artery endothelium. Am J Physiol.
273:L315–L321. 1997.PubMed/NCBI
|
|
41
|
Hemmrich K, Suschek CV, Lerzynski G and
Kolb-Bachofen V: iNOS activity is essential for endothelial stress
gene expression protecting against oxidative damage. J Appl
Physiol. 95:1937–1946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ito H, Hirata Y, Adachi S, Tanaka M,
Tsujino M, Koike A, Nogami A, Murumo F and Hiroe M: Endothelin-1 is
an autocrine/paracrine factor in the mechanism of angiotensin
II-induced hypertrophy in cultured rat cardiomyocytes. J Clin
Invest. 92:398–403. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jung WK, Choi I, Lee DY, Yea SS, Choi YH,
Kim MM, Park SG, Seo SK, Lee SW and Lee CM: Caffeic acid phenethyl
ester protects mice from lethal endotoxin shock and inhibits
lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric
oxide synthase expression in RAW 264.7 macrophages via the p38/ERK
and NF-kappaB pathways. Int J Biochem Cell Biol. 40:2572–2582.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kyaw M, Yoshizumi M, Tsuchiya K, Kirima K,
Suzaki Y, Abe S, Hasegawa T and Tamaki T: Antioxidants inhibit
endothelin-1 (1–31)-induced proliferation of vascular smooth muscle
cells via the inhibition of mitogen-activated protein (MAP) kinase
and activator protein-1 (AP-1). Biochem Pharmacol. 64:1521–1531.
2002. View Article : Google Scholar : PubMed/NCBI
|