Introduction
The heme-heme oxygenase (HO) system is comprised of
constitutive HO-2 and inducible HO-1 isoforms, which play a key
role in defense mechanisms against a series of external stimuli,
including cytokines, oxidants, hypoxia and pharmacological agents
(1,2). HO-1 is a rapidly inducible
stress-responsive enzyme that degrades heme to carbon monoxide
(CO), biliverdin and ferrous iron (3). Induction of HO-1 provides a
protective function in the cardiovascular system through the
effects of CO and bilirubin on various cells. HO-1 executes
anti-apoptotic, anti-inflammatory, anti-hypertensive and
anti-oxidant functions, and protects the cardiovascular system by
the activation of the p55/TNFR-1, p38 MAPK and PI3K/AKT signaling
pathways (4–6). Importantly, the HO-1 gene promoter
sequence contains a large quantity of regulatory
cis-elements, such as stress-responsive, hypoxia-responsive,
and cadmium responsive elements and NF-κB binding sites (7–9).
Obesity is a risk factor leading to heart disease, including an
increase in the incidence of cardiac diseases such as coronary
artery disease, heart failure and cardiomyopathy (10–12). Metabolic syndrome induced by
excessive intake of cholesterol is a pathological condition. A
long-term high-fat diet results in systemic inflammation and
oxidative stress. The risk factor induces the disruption of
metabolic homeostasis and promotes progressive cardiovascular
dysfunction (13,14). HO-1 overexpression in macrophages
can significantly improve the function of adipose cells and enhance
insulin sensitivity. Meanwhile, HO-1 upregulation in the failing
heart is a cardioprotective adaptation for improving left
ventricular (LV) function.
Clinical studies have shown that a long-term
high-fat diet may be a critical factor in the occurrence and
progression of a series of cardiovascular diseases, including
hypertension, coronary atherosclerosis and heart failure. However,
the protective mechanism underlying how HO-1 antagonizes the
excessive intake of cholesterol is still obscure. The aim of the
present study was to elucidate the mechanisms of the anti-oxidative
damage effects of HO-1 against the cholesterol stimulation
condition.
Here, we first demonstrated that 137 HO-1
interactive proteins, especially a novel interactor
emopamil-binding protein (EBP), may influence the intracellular
lipid metabolism. We constructed an HO-1 interactive network using
bioinformatic analysis. We also confirmed that cholesterol
stimulation could induce myocardial hypoxia, activate the PI3K/AKT
and nuclear factor erythroid 2-like 2 (Nrf2)/mTOR signaling
pathways, and subsequently upregulate the expression of HO-1,
binding the increasing number of EBP to restore the balance of
intracellular lipid metabolism and cardiomyocyte function in in
vivo and in vitro experiments.
Materials and methods
Materials
High-glucose Dulbecco's modified Eagle's medium
(H-DMEM) was obtained from Basalmedia Technologies (Shanghai,
China) and fetal bovine serum (FBS) was obtained from Bailing
Biotechnology (Lanzhou, China). TurboFect™ transfection reagent
(R0531) and Pierce™ Protein G Magnetic Beads were from Thermo
Fisher Scientific (Waltham, NY, USA). Cholesterol was obtained from
Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies, rabbit
monoclonal anti-AKT1 (ab81283), anti-AKT-1/2/3 (ab179463),
anti-mTOR/p-mTOR (ab32028, ab109268), anti-HO-1 (ab52947),
anti-Aconitase2 (Aco2) (ab129069), anti-lactate dehydrogenase (LDH)
(ab134187), anti-pyruvate dehydrogenase E-1β (PDHE-1β) (ab155996),
anti-nuclear factor erythroid 2-like 2 (Nrf2) (ab62352) and
anti-EBP (ab135745) were purchased from Abcam (Cambridge, MA, USA).
Anti-histone H3.1 was obtained from Abmart. Anti-malic
dehydrogenase (MDH2) (#8610) was obtained from Cell Signaling
Technology. The anti-rabbit HRP-labeling and the anti-rabbit
DyLight™ 488- and anti-mouse DyLight™ 633-labeled secondary
antibodies were from KPL.
Cell culture
293T and H9c2 cells (Cat. nos. GNHu17 and GNR5;
obtained from Shanghai Cell Resource Center of Chinese Academy of
Sciences, Shanghai, China) were cultured in H-DMEM containing 10%
FBS at 37°C in a humidified 5% CO2 atmosphere. According
to a previous study (15),
5×105 H9c2 cells were plated into 100-mm culture dishes,
and cultured for 48 h. For cell viability following cholesterol
treatment, the cells were washed with phosphate-buffered saline
(PBS) prior to removing the additional cholesterol.
Ethics statement
The experiments were approved by the institution's
Ethics Committee for Investigation of Logistics University of the
Chinese People's Armed Police Force, and all of the 20 animals were
treated under humane care in compliance with the Public Health
Service Policy on Humane Care and Use of Laboratory Animals.
Animals and treatments
Ten 24-week old male wild-type (WT) C57BL/6 mice
were acquired from the Laboratory Animal Center of the Academy of
Military Medical Sciences, and 10 24-week old male
HO-1+/− mice were acquired from the South Model Animal
Center of China. As a low HO-1 protein level is associated with
high abortion rates (16), only 2
homozygote HO-1−/− mice survived out of 200 newborn
HO-1-knockdown mice. Therefore, this study used HO-1+/−
mice to perform the subsequent experiment.
All mice were raised in a controlled environment at
a temperature of 25°C and a 40–70% humidity with a 12-h light and
dark cycle. Among the WT and HO-1+/− mice, 5 were fed
with a normal diet while 5 were fed with a normal diet and added
cholesterol (3.5 mg/kg) (17).
Sixty days later, the weights of the mice were calculated, and the
cardiac functions of the mice were assessed using cardiac
ultrasound. Finally, the mice were sacrificed using cervical
dislocation to collect samples for the Oil Red O staining and laser
scanning confocal microscope image experiments.
Co-immunoprecipitation (Co-IP)
Cells (1×105) were seeded into 100-mm
culture dish, and transfection was performed when cells reached
30–50%. According to the transfection protocol, 10 µg of
pCDNA3.1-HO-1 and pCDNA3.1-blank vectors were respectively
transferred into the 293T cells using the TurboFect transfection
reagent. After they were transfected for 24 h, the cells were lysed
with a buffer (150 mmol/l NaCl, 1 mmol/l EDTA, 50 mmol/l Tris-HCl,
5% glycerol, 0.1% NP-40 and cocktails) at 4°C. The supernatants
were collected by centrifugation at 4°C. The concentration of
protein was determined by the BCA assay. Co-IP was performed
according to a previous study (18). Briefly, 10 µg of rabbit
anti-HO-1 anti-body was added into 1 mg supernatants of 293T/HO-1
cells and then incubated overnight at 4°C. This was followed by
incubation with 25 µl Pierce™ Protein G Magnetic Beads for 2
h at 4°C. After the cells were washed completely, the Co-IP product
was obtained with a 50 µl elution buffer [1.74 mmol/l sodium
dodecyl sulfate (SDS), 0.037 mmol/l bromphenol blue, 50% glycerol,
0.25 mol/l Tris-HCl and 2.5 mmol/l DTT]. Then the eluent was
reduced with 10 mmol/l DTT for 10 min at 100°C and alkylated with
50 mmol/l IAA at room temperature in the dark for 30 min.
Additionally, 10 µl of the Co-IP products was used to
perform western blot analysis using an anti-EBP antibody to
validate the interaction between HO-1 and EBP.
Protein digestion and LC-MS/MS
analysis
The Co-IP products were separated with 10% SDS-PAGE
gel and then the gel was stained with Coomassie Blue G-250. The gel
lane was then sliced into 8 bands followed by destaining and in-gel
digestion with 10 ng/µl of trypsin (Promega, Madison, WI,
USA) at 37°C overnight according to a previous study (19). The peptides of bands were
extracted by the addition of 25 mmol/l ammonium bicarbonate and 5%
v/v FA (formic acid), and dried in a vacuum concentrator. The
samples were later subjected to mass spectrometric analysis, and
MS/MS analysis was performed as described previously (20). The raw data obtained were searched
via the MaxqQuant (version 1.5.3.28 build) search engine against a
decoy database/composite target to appraise the false discovery
rate (FDR) (21). The target
proteins were acquired from the Swiss-Prot Homo sapiens
reference protein database (release 2015_08, 69787 query number)
and the decoy proteins were produced from pseudo-reversed sequences
of the target proteins. Precursor ions were searched with an
initial mass tolerance of 20 ppm. Only b and y ions
were considered during the database search. Enzyme specificity was
trypsin, with two missed cleavages allowed and peptides with at
least 6 amino acids. The dynamic modifications for methionine
oxidation (+15.99492) and the static modification for cysteine
carbamidomethylation (57.021465) were allowed. The FDR of peptides
and proteins <1% was accepted after appraisal based on the
number of accepted decoy hits.
Profiling of the global HO-1 interactome
network
The Gene Ontology (GO) information for the HO-1
interaction proteins was submitted to DAVID for biological process
(BP) and cellular component (CC) analysis (22), and the Homo sapiens species
was selected as the background and species. The CluePedia plugin of
the Cytoscape version 3.3 was used to generate the protein-protein
interaction (PPI) network.
MTT assay
The MTT assay was carried out as referred to in a
previous study (23). Briefly, a
concentration of 1×104 cells was seeded into each well
of a 96-well plate (Corning, 3599). The cells were incubated with
10, 100 and 500 mmol/l cholesterol for 12, 24, 36 and 48 h, and
then were incubated with 5 mg/ml MTT buffer for 5 h. The optical
density at 450 nm was measured using the SpectraMax Series
microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Quantification of adipose droplets by
staining lipids with Oil Red O
H9c2 cells were seeded onto a 6-well plate at a
density of 3×104/ml in H-DMEM medium. After the cells
grew to 60–70% confluence, the cells were treated with 100 mmol/l
cholesterol for 12, 24, 36 and 48 h, according to a previous study
(24), followed by incubation
with staining buffer (0.5% Oil Red O in pentanediol) for 15 min,
and then incubated at room temperature with hematoxylin for 2 min.
After washing the cells with pentanediol twice, the density of
adipose droplets was measured by Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Bethesda, MD, USA).
Nuclear protein extraction
H9c2 cells were treated with 100 mmol/l cholesterol
for 12, 24, 36 and 48 h, washed with PBS twice, and then the cells
were suspended in lysis buffer A (10 mmol/l HEPES pH 7.9, 10 mmol/l
KCl, 0.1 mmol/l EDTA, 0.1 mmol/l EGTA, 0.5% NP-40, 1 mmol/l DTT and
protease inhibitor) and collected in tubes. The supernatants were
collected after centrifugation. Buffer B [20 mmol/l HEPES (pH 7.9),
0.4 mol/l NaCl, 1 mmol/l EDTA, 1 mmol/l EGTA, 1 mmol/l DTT and
protease inhibitor] was added into the nuclear pellet, and
incubated on ice for 30 min. After centrifugation for 15 min, the
supernatant was collected.
Western blot analysis
H9c2 cells were treated with 100 mmol/l cholesterol
for 12, 24, 36 and 48 h, and cells were lysed with RIPA buffer at
4°C. The concentration of protein was measured using the BCA assay.
Then 60 µg of the samples of the different groups was
separated by 10% SDS-PAGE and then were transferred onto a
0.22-µm nitrocellulose membrane by a semi-dry
electroblotter. The membranes were respectively incubated with the
primary antibodies (1:1,000) overnight at 4°C followed by
incubation with the secondary antibody (1:5,000) for 1 h at room
temperature. The concentration of protein was detected using the
ECL immunoblotting reagent. The gray values of the bands were
quantified with the Scion Image software and were normalized with
β-actin and histone.
Measurements of the cardiac function of
the mice using cardiac ultrasound
The mice were divided into groups as previously
described. After they were fed for 60 days, the weights of the mice
were measured. Then the cardiac function was measured using the
Vevo 2100 Imaging System. A MS-400 scan head (30 MHz) was used to
acquire the left ventricular short axis (SAX) and left ventricular
long axis (PSLAX) views of the mouse ventricle in M-mode. The left
ventricular short axis shortening (LVFS%), left ventricular
ejection fraction (LVEF%) and left ventricular (LV) mass were
analyzed by Visual Sonic Vevo 2100 software, and LV masses were
normalized with the corresponding weight of the mice.
Quantification of adipose droplets by
staining tissue slices with Oil Red O
Myocardial tissues of the four previously described
groups were fixed in 4% paraformaldehyde for 24 h and dehydrated in
30% sucrose for 3 h. After they were embedded in tissue freezing
medium (Leica, 14020108926), they were processed into 7-µm
frozen sections using a freezing microtome (Leica, CM1860). Oil Red
O staining was performed as previous described (25). Briefly, five slices of each group
were first soaked in 60% isopropanol for 10 min followed by
incubation with staining buffer (60% Oil Red O in water) for 25
min, and the slices were later differentiated in 75% ethanol
containing 1% hydrochloric acid for 2 min. The slices were
incubated with hematoxylin buffer at room temperature for 2 min.
After washing them with pentanediol twice, the lipid accumulation
in myocardial tissues was observed using the Image-Pro Plus 6.0
software (Media Cybernetics, Inc.).
Laser scanning confocal microscope
image
A total of 1×104 cells were seeded onto a
confocal plate (Nest, 801002) and treated with cholesterol as
previously described. The ICC was performed as in a previous study
(26). Briefly, the cells and 5
frozen slices from four groups were fixed in pre-cooling methanol
for 10 min and then permeabilized with 0.5% Triton X-100 for 20 min
at 37°C. Then they were incubated with the anti-HO-1 and anti-EBP
primary antibody (1:100) at 4°C for 12 h followed by incubation
with the DyLight™ 488- and DyLight™ 633-labeled secondary antibody
for 1 h at 37°C, as well as dyed in 1 µg/ml DAPI for 5 min.
Cells and the slices were visualized using confocal microscopy
(objective, 20X; Leica TCS SP8). The images were analyzed using IPP
6.0 software.
Statistical analysis
Data are expressed as the means ± SEM of three
independent experiments. The differences between groups of the
in vivo experiments were analyzed using one-way ANOVA.
Differences between groups of the in vitro experiments were
analyzed using multi-way ANOVA. A P-value <0.05 was used to
determine a statistically significant difference.
Results
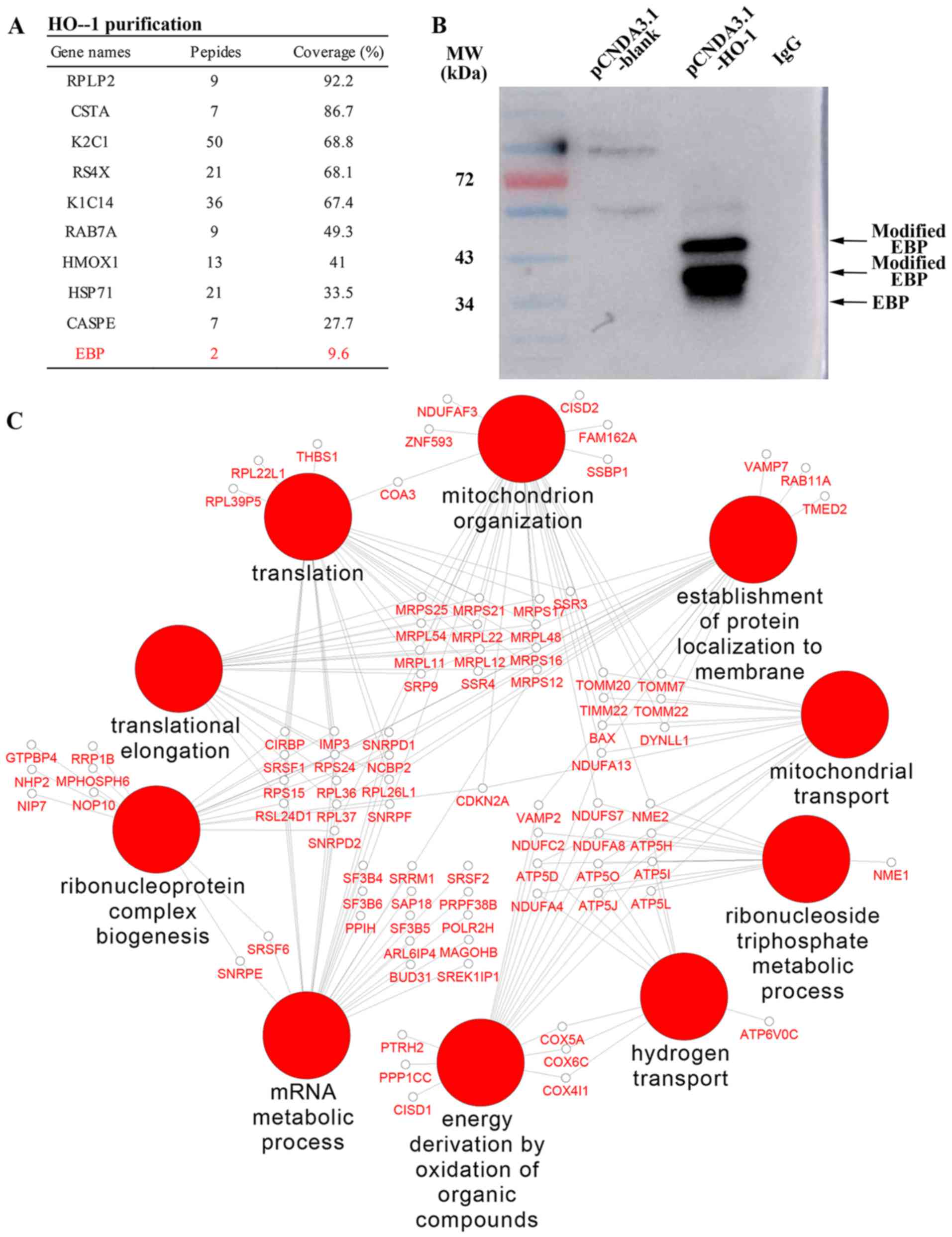
Interaction between HO-1 and EBP
A total of 137 HO-1 interactive proteins were
identified using overlap of two repeated filtering with control
groups, and several of the interactive proteins are listed in
Fig. 1A. In particular, a sterol
isomerase named EBP, an integral membrane protein of the
endoplasmic reticulum, catalyzes the conversion of Delta (8)-sterols to their corresponding Delta
(7)-isomers for involvement in
the cholesterol metabolism. The western blot analysis result of the
Co-IP products confirmed that HO-1 and EBP indeed were interactive
(Fig. 1B). Based on the results
of the interactome, we speculated that overexpression of HO-1 may
cause a cardioprotective adaptation for improvement under a high
concentration cholesterol environment.
Global profiling and mapping of the
HO-1-interacting protein network
By GO enrichment analysis, the HO-1-interacting
proteins were found to be mainly involved in BPs (Fig. 1C), including oxidative
phosphorylation, ATP synthesis-coupled proton transport,
mitochondrion organization and nitrogen catabolic process.
Meanwhile, these proteins were also found to participate in CCs,
such as mitochondrion, endoplasmic reticulum and transport
vesicles.
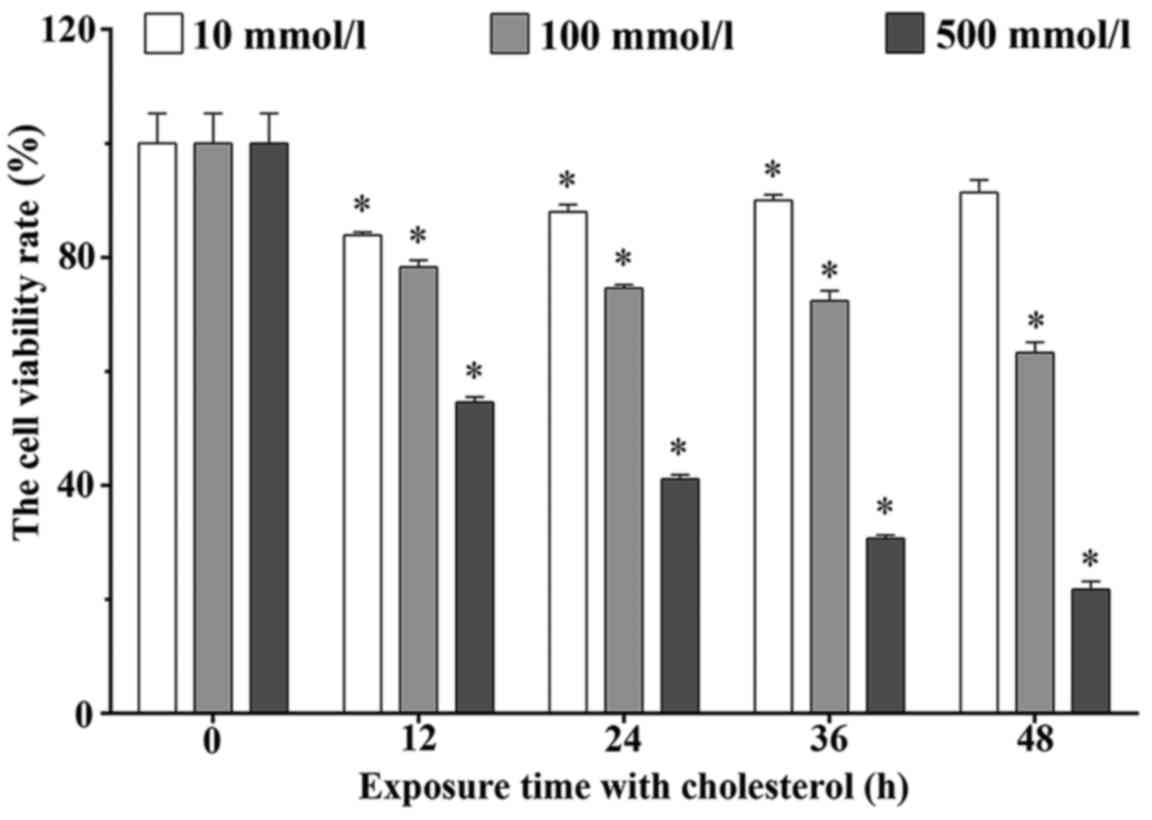
Effects of cholesterol on the growth of
H9c2 cells
The MTT assay was performed to determine the
viability rates of the H9c2 cells treated with 10, 100 and 500
mmol/l cholesterol for 12, 24, 36 and 48 h. The viability rates
were 83.92±0.51, 87.99±1.22, 90.00±0.96 and 91.35±2.22% in the 10
mmol/l cholesterol treatment group. These rates were 78.33±1.14,
74.62±0.54, 72.36±1.78 and 63.26±1.86% in the 100 mmol/l
cholesterol treatment groups, and these rates were 54.60±0.89,
41.12±0.74, 30.69±0.59 and 21.76±1.39% in the 500 mmol/l
cholesterol treatment groups, respectively. As shown in Fig. 2, cholesterol significantly
inhibited the H9c2 cell viability in a dose- and time-dependent
manner (P<0.05).
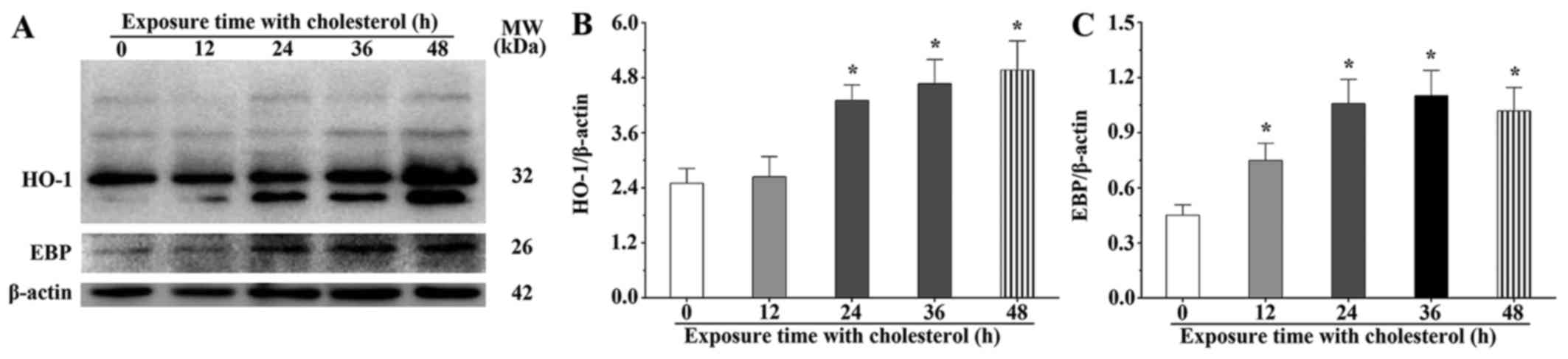
Upregulated expression of HO-1 and EBP
plays a protective role during cholesterol stimulation
We speculated that a high concentration of
cholesterol could result in the overexpression of HO-1 by
activation of the Nrf2, AKT and mTOR signaling pathways. The
western blot results showed that the HO-1 expression levels in the
H9c2 cells treated with cholesterol for 24, 36 and 48 h were 1.72-,
1.87- and 2.00-fold, respectively, compared with those of the
control group (P<0.05) (Fig. 3A
and B). We also found that the abundance of post-translational
modified and degraded HO-1 was gradually increased in the
cholesterol treatment groups. The western blot results showed that
the EBP expression levels in the H9c2 cells treated with
cholesterol for 12, 24, 36 and 48 h were 1.66-, 2.34-, 2.44-and
2.26-fold, respectively, compared with that of the control group
(P<0.05) (Fig. 3A and C).
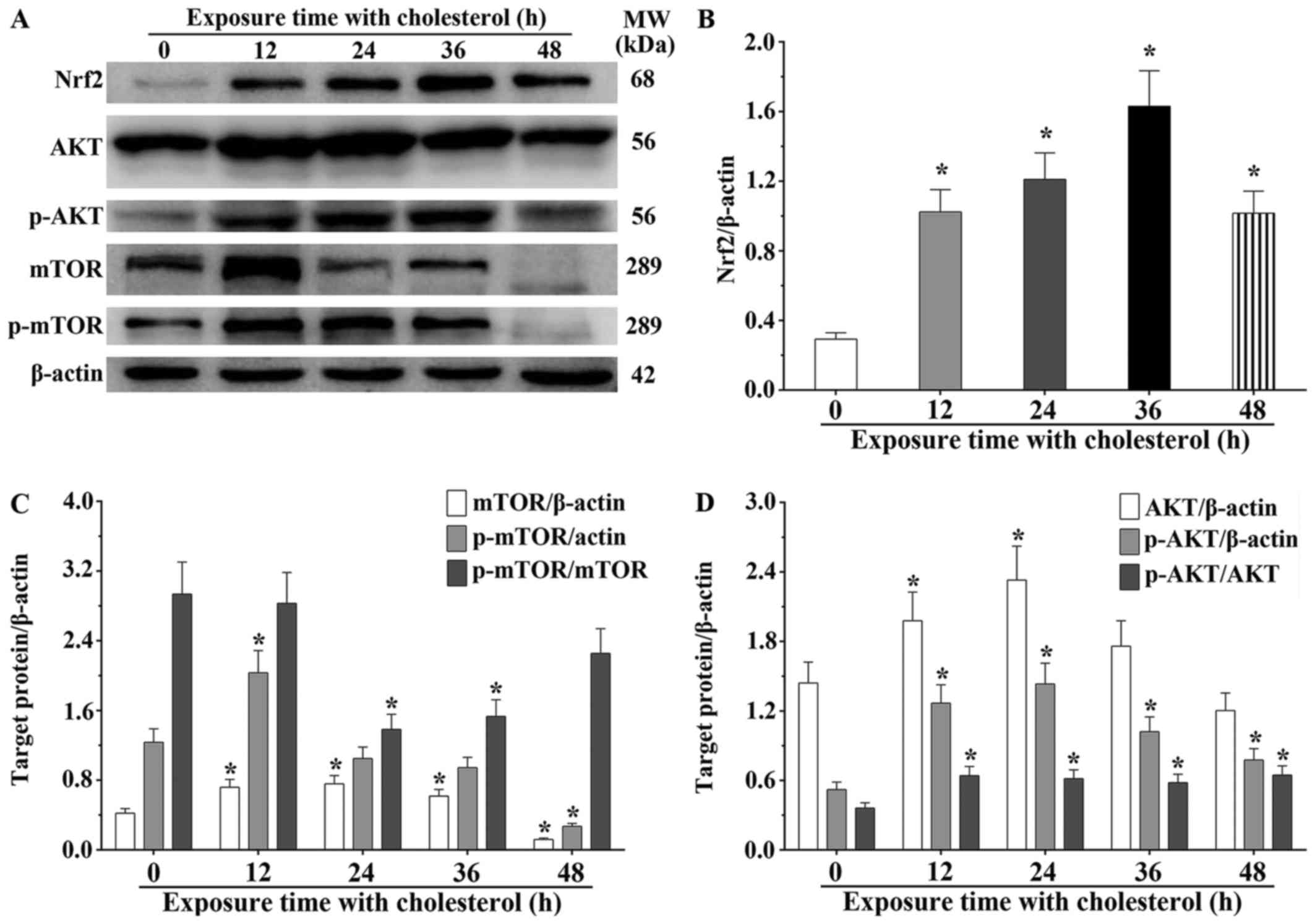
We also found that expression of several critical
molecules in lipid metabolism, Nrf2, AKT and mTOR, was
significantly altered. Nrf2 is a type of transcription activator
that binds to antioxidant response (ARE) elements in the promoter
regions of downstream target genes. Nrf2 expression in H9c2 cells
treated with cholesterol was significantly increased compared to
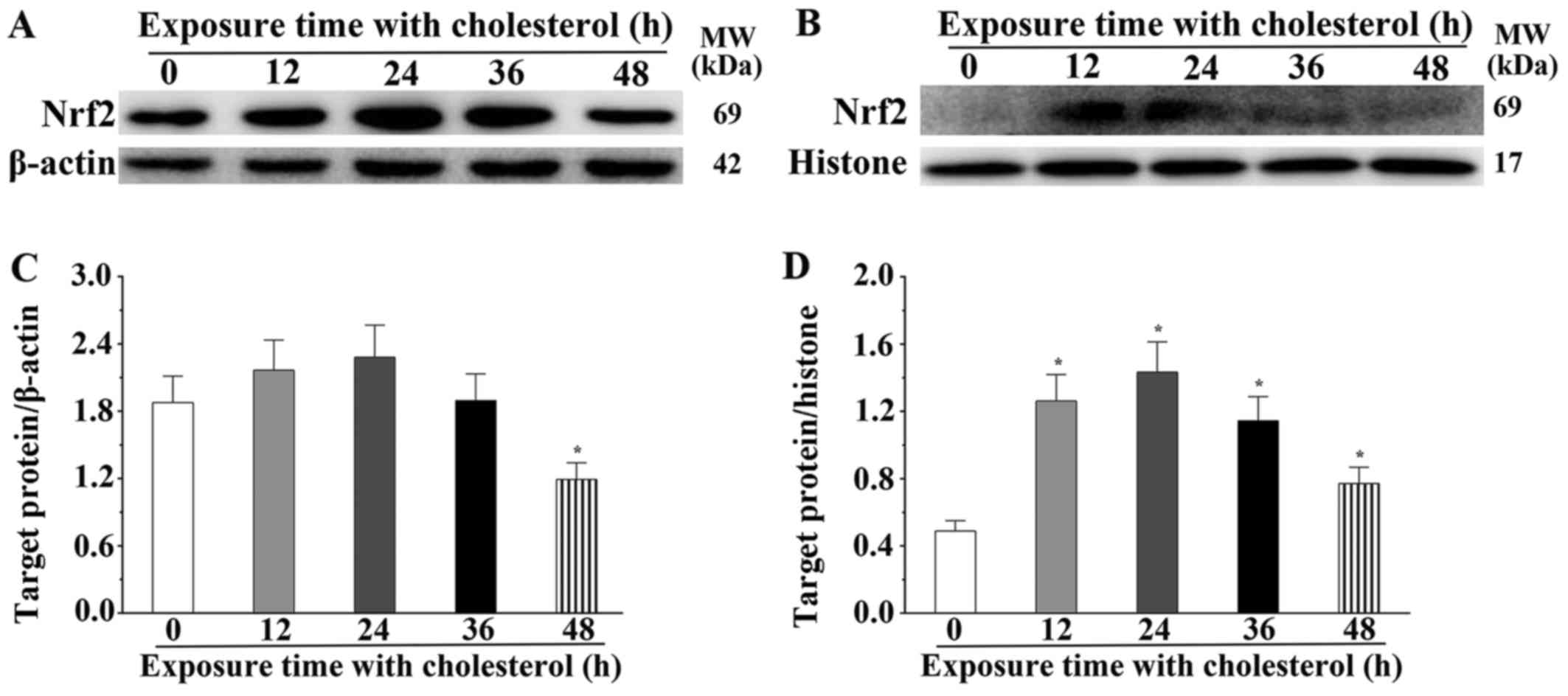
that noted in the control group (P<0.05) (Fig. 4A and B). We also determined the
expression level of Nrf2 in the cytoplasm and nucleus. The
expression level of Nrf2 in the cytoplasm was significantly
decreased after treatment with cholesterol for 48 h (P<0.05)
(Fig. 5A and C) and the
expression level of Nrf2 in the nucleus was significantly increased
after treatment with cholesterol (P<0.05) (Fig. 5B and D). AKT and mTOR belong to
the serine/threonine protein kinases, and are central regulators of
cellular metabolism and growth in response to energy supplements
and stress signals. We also found that the mTOR expression levels
in the 12, 24 and 36 h cholesterol treatment groups were
significantly increased compared with that of the control group.
However, the expression level in the 48-h cholesterol treatment
group was significantly decreased (P<0.05). Notably, p-mTOR in
the 12-h cholesterol treatment group was increased (P<0.05).
Meanwhile, we also observed that the mTOR phosphorylation process
was not obviously changed at 24 and 36 h. However, p-mTOR was
significantly decreased in the H9c2 cells treated with cholesterol
for 48 h compared with that of the control group (P<0.05)
(Fig. 4C). This study confirmed
that AKT expression in the H9c2 cells treated with cholesterol for
12 and 24 h was significantly increased compared to that of the
control group (P<0.05), and the p-AKT level in all cholesterol
treatment groups was significantly increased compared to that of
the control group (P<0.05). The ratio of p-AKT/AKT in the
cholesterol treatment groups was significantly increased compared
to that of the control group (P<0.05) (Fig. 4D). These results suggest that
cholesterol stimulation induces an upregulated expression of HO-1
and EBP by activation of the Nrf2, AKT and mTOR signaling
pathways.
Interaction between HO-1 and EBP
alleviates the lipid accumulation in H9c2 cells as determined using
laser scanning confocal microscope image
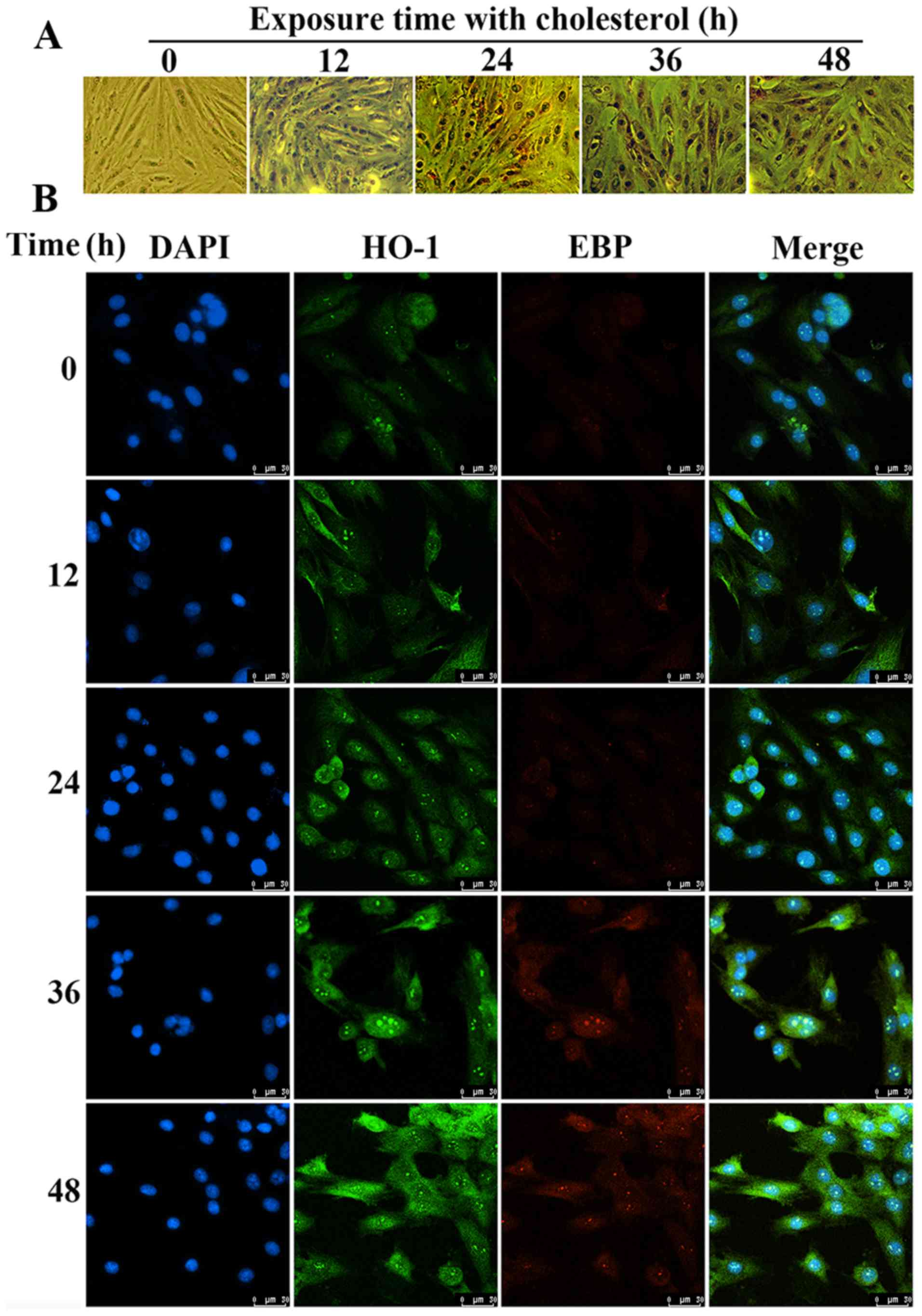
Using Oil Red O staining, we found that the amount
of lipid droplets in the H9c2 cells treated with cholesterol was
significantly increased compared to that of the control group
(Fig. 6A). The results of the
confocal microscopy showed that the expression levels of HO-1 and
EBP were significantly upregulated under cholesterol stimulation.
We also confirmed that HO-1 and EBP have the same location in H9c2
cells according to the confocal image (Fig. 6B). These results also confirmed
the interaction between HO-1 and EBP to alleviate lipid
accumulation.
HO-1 overexpression alleviates H9c2 cell
hypoxia by inhibition of the glucose metabolism process
Hypoxia inducible factor-1 (HIF-1) is a critical
regulatory factor for stress reactions, which usually degrades in
the normal oxygen status; however, it maintains its stable state
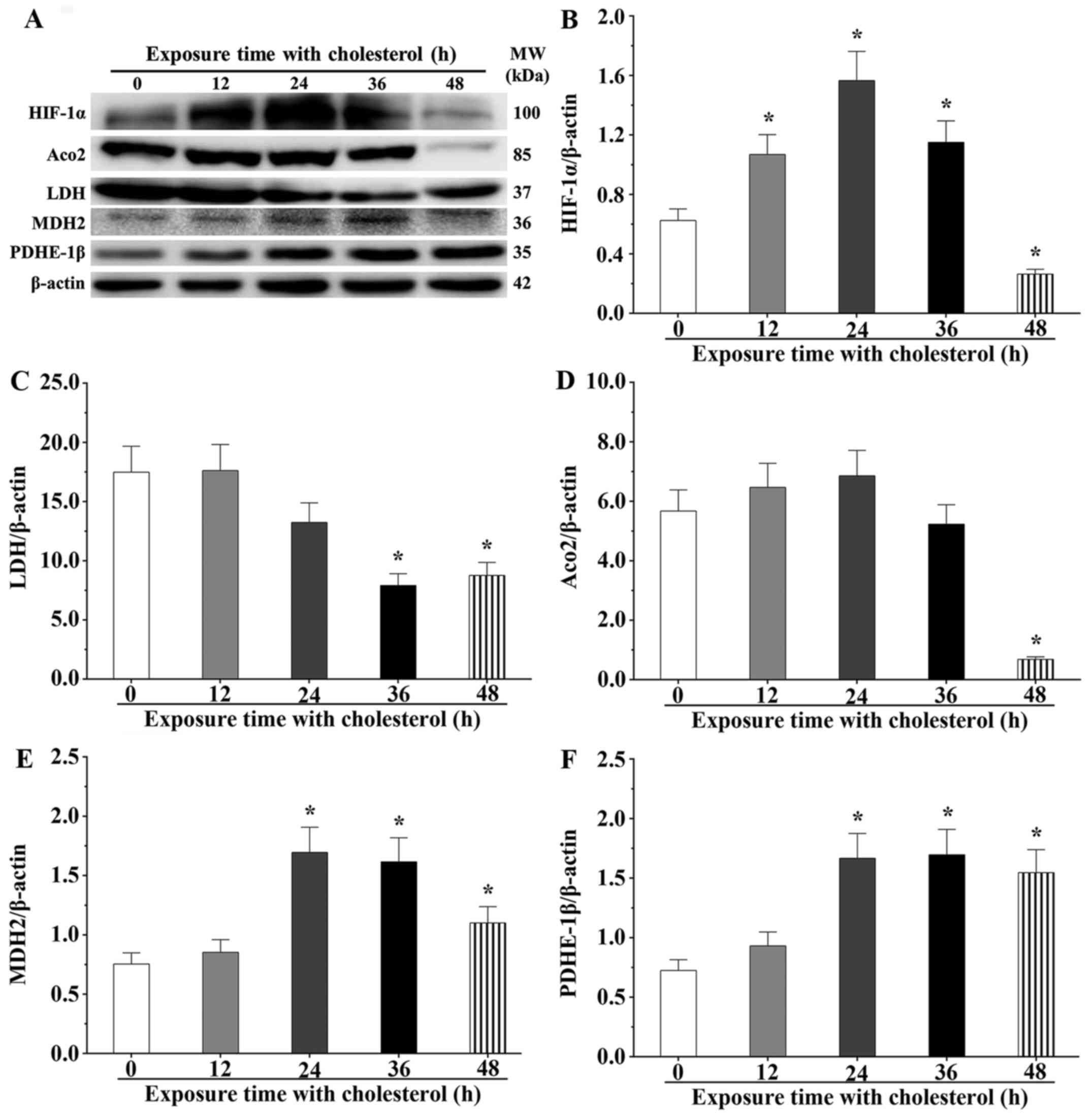
under a hypoxic status. After cholesterol stimulation for 12, 24
and 36 h, we noted that expression levels of HIF-1α were
significantly increased compared with the control group (P<0.05)
(Fig. 7A and B). However, the
expression level of HIF-1α reverted to the normal status after
cholesterol stimulation for 48 h. LDH, involved in the final step
of aerobic glycolysis, catalyzes the conversion of L-lactate and
NAD to pyruvate and NADH. After cholesterol stimulation for 36 and
48 h, LDH expression levels were significantly downregulated
(P<0.05). However, LDH expression levels in the 12 and 24 h
cholesterol treatment groups were not obviously different compared
with that of the control group (P<0.05) (Fig. 7C). Aco2, a critical enzyme in the
Krebs cycle, catalyzes citric acid into aconitic acid. After
cholesterol stimulation for 48 h, Aco2 expression levels were
significantly downregulated (P<0.05) (Fig. 7D). MDH2, a crucial enzyme of malic
acid shuttle, transferred malic acid into the mitochondria matrix.
The results showed that the expression level of MDH2 was
significantly upregulated after cholesterol stimulation for 24, 36
and 48 h (P<0.05) (Fig. 7E).
PDHE-1β catalyzes pyruvate acid in the mitochondria matrix into
acetyl-CoA. Our experiment showed that the expression level of
PDHE-1β was significantly increased at 24, 36 and 48 h (P<0.05)
(Fig. 7F). These findings
indicate that overexpression of HO-1 reduced the oxygen consumption
to alleviate the hypoxic effect by inhibition of glycolysis and
aerobic oxidation processes after cholesterol treatment. Hence, we
confirmed that HO-1 displayed cardioprotective effects against the
oxidative damage induced by cholesterol in cardiomyocytes.
HO-1 plays a protective role during
cholesterol stimulation in an in vivo experiment
We found that the mouse weight in all groups was not
significantly different between the normal diet and high-fat diet
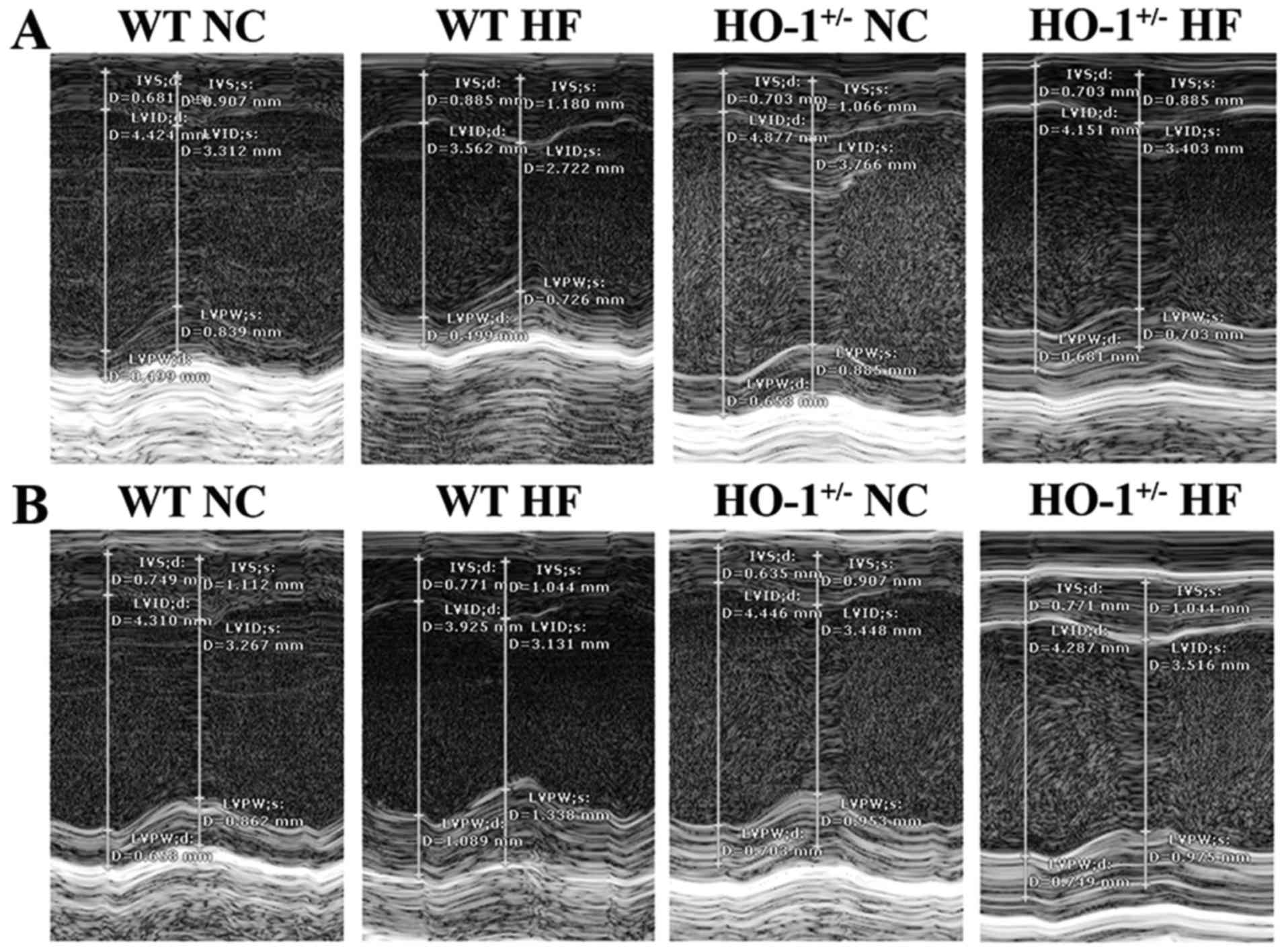
groups (P>0.05). Using cardiac ultrasound, we determined that
the values for LVEF% in the WT mice with a normal diet, the WT mice
with a high fat diet, the HO-1+/− mice with a normal
diet and the HO-1+/− mice with a high-fat diet were
1.51±0.11, 1.34±0.20, 1.66±0.08 and 1.21±0.09, respectively. The
values of LVFS% in these four groups were 0.75±0.06, 0.64±0.11,
0.84±0.05 and 0.57±0.05, and the LV mass in these four groups were
2.49±0.30, 2.89±0.37, 3.52±0.29 and 3.03±0.32, respectively,
according to the data obtained from the SAX view (Fig. 8A). The values of LVEF% of mice in
these four groups were 1.46±0.07, 1.27±0.19, 1.69±0.08 and
1.19±0.08, respectively. The values of LVFS% of these four groups
were 0.73±0.04, 0.61±0.10, 0.86±0.05 and 0.56±0.04, and the LV mass
of these four groups were 3.01±0.18, 3.34±0.41, 3.62±0.30 and
3.56±0.28, respectively, according to the data obtained from the
PSLAX view (Fig. 8B). In
addition, all values were normalized with the corresponding weight
of the mice. The LVEF% and the LVFS% of the HO-1+/− mice
fed the high-fat diet was significantly decreased compared to the
WT mice fed a normal diet (P<0.05). The LV mass of the
HO-1+/− mice fed a normal diet was significantly higher
than the WT mice fed a normal diet (P<0.05) according to the
data obtained from the SAX view. The LVEF% and the LVFS% of the
HO-1+/− mice fed a normal diet were significantly
decreased compared to those of the WT mice fed a normal diet
(P<0.05), and the LVEF% and LVFS% of the HO-1+/− mice
fed a high fat diet were significantly decreased compared to those
of the HO-1+/− mice fed a normal diet (P<0.05). The
LV mass of the HO-1+/− mice fed a normal diet was
significantly higher than the WT mice fed a normal diet (P<0.05)
according to the data obtained from the PSLAX view.
Interaction between HO-1 and EBP
alleviates lipid accumulation in the in vivo experiment
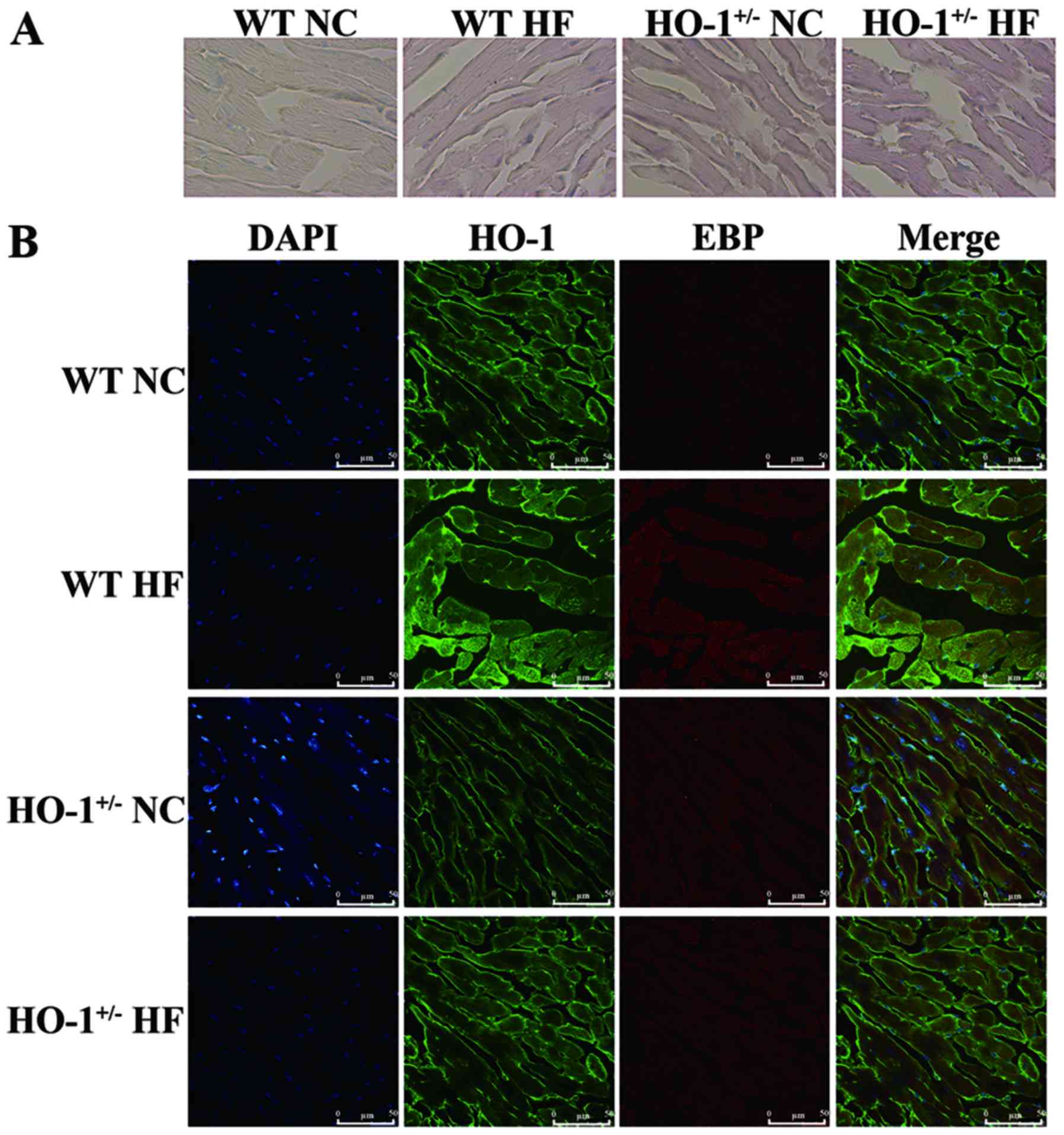
Using Oil Red O staining, we found that the amount
of lipid droplets in the myocardium of mice treated with
cholesterol was also significantly increased compared with that in
the mice fed a normal diet. The amount of lipid droplets in the
HO-1+/− mice was significantly increased compared with
that noted in the WT mice (Fig.
9A). We also confirmed that HO-1 and EBP are co-located in the
myocardium according to the confocal image (Fig. 9B).
Discussion
HO-1, an essential enzyme in heme catabolism, is
activated at high concentrations of heme and during a certain
pathophysiological status, including oxidative stress, high glucose
and viral infection, and exhibits cytoprotective effects (27,28). However, the detailed mechanism
remains obscure. Our experiment showed that the signaling network
of 137 HO-1 interactive proteins was mainly distributed in
oxidative phosphorylation, mitochondrion organization processes and
endoplasmic reticulum and transport vesicle cellular components
(29). Interestingly, we found
that EBP is a HO-1 interactive proteins and is a sterol isomerase,
closely related to lipid metabolisms, endoplasmic reticulum and
vesicular fraction. The HO-1/EBP interaction was also confirmed
both in vivo and in vitro using confocal microscopy.
Moreover, we found that HO-1/EBP interaction not only occurred in
the cytoplasm, but also in the nucleus and membrane. Meanwhile, our
results also showed that HO-1 and EBP expression had a trend of
consistency, presenting upregulated expression under cholesterol
stimulation. Hence, we suggested that HO-1 interacts with EBP and
are involved in lipid metabolism, consume redundant cholesterol,
and alleviate oxidative stress effects caused by cholesterol.
HO-1 usually is induced by a variety of signal
transduction pathways that activate different transcription
factors, including Bach2, p53, CREB and Nrf2 (30). Nrf2 recognizes specific
DNA-binding elements of the HO-1 promoter (31–33). This study showed that cholesterol
stimulation induced the upregulation of Nrf2 expression in
cardiomyocytes, and then caused the upregulation of HO-1
expression. Cholesterol simulation not only markedly evaluated the
AKT expression, but also increased Nrf2 expression (34,35). Our results suggested that
cholesterol may increase the intercellular reactive oxygen species
(ROS) level and activate the PI3K/AKT signaling pathway, and then
increase the Nrf2 expression and promote its separation from the
Keap1-Nrf2 complex, which translocates to the nucleus and binds the
promoter of HO-1 and plays an antioxidant role in cardiomyocytes.
Our results also indicate that cholesterol simulation significantly
increased the expression levels of AKT and p-AKT in cardiomyocytes
by activation of the PI3K/AKT signaling pathway, which could not
only evaluate the AKT expression, but it also increased the
intercellular Ca2+ ([Ca2+]i)
concentration. [Ca2+]i plays a critical role
in translocation of Nrf2 to the nucleus to perform the
transcriptional factor function (36–38). Meanwhile, we also noted that
cholesterol increased the expression levels of mTOR and p-mTOR in
cardiomyocytes. mTOR, a type of serine/threonine protein kinase,
mediates cellular responses to stresses such as DNA damage and
nutrient deprivation. It acts as a part of two structurally and
functionally distinct signaling complexes, mTORC1 and mTORC2.
Research has confirmed that mTOR inhibitor molecules (TSC1/TSC2)
inhibit mTOR activity. Activation of PI3K and AKT, involved in the
phosphorylation of TSC1/TSC2 promoted mTOR activity (39). In this study, the expression
levels of Nrf2, AKT, p-AKT, mTOR and p-mTOR following cholesterol
stimulation for 48 h were significantly decreased compared to those
following cholesterol stimulation for 24 and 36 h. However, the
expression levels of HO-1 and EBP were not different in any of the
cholesterol stimulation groups.
Hyperlipidemia usually alters the balance of
cellular oxygen levels and induces hypoxia (40). HIF-1 is a critical regulatory
factor for stress reaction and belongs to downstream molecules of
the mTOR signaling pathway. It is activated by oxygen-mediated
protein post-translational modification and cytoplasm/nucleus
translocation under hypoxic condition. HIF-1α is usually degraded
in a normal oxygen condition; however, it maintains its stable
state under hypoxia (41). The
present study showed that the expression level of HIF-1α following
cholesterol stimulation for 12, 24 and 36 h was significantly
increased compared to the control group. However, in the 48 h
group, there was no difference. The result confirmed that H9c2
cells recovered from a hypoxic condition to a normal oxygen status
after cholesterol treatment for 48 h, and the expression level of
HIF-1α was significantly decreased compared with that of the other
groups.
Based on carbohydrate metabolism, we aimed to
explain that HO-1 plays a cardioprotective role and improves the
cholesterol-induced hypoxic status in H9c2 cells (42). Our results showed that expression
levels of tricarboxylic acid (TCA) cycle-related molecules, Aco2,
malate MDH2 and PDHE-1β, were obviously changed. Of these, Aco2
catalyzes the conversion of citrate to isocitrate via
cis-aconitate in the second step of the TCA cycle (43). Aco2 was significantly decreased in
the H9c2 cells under cholesterol stimulation. MDH2 catalyzes the
reversible oxidation of malate to oxaloacetate, utilizing the
NAD/NADH cofactor system in the TCA cycle (44). PDHE-1β is located in the
mitochondrial matrix and catalyzes the conversion of pyruvate to
acetyl coenzyme A (Acyl-CoA) (45). In the present study, the
expression levels of MDH2 and PDHE-1β were significantly increased
in the H9c2 cells treated with cholesterol. We suggest that
cholesterol may destroy the balance of the TCA cycle and induce the
excessive accumulation of Acyl-CoA by downregulation of Aco2
expression and upregulation of MDH2 and PDHE-1β expression.
Otherwise, LDH catalyzes the conversion of L-lactate and NAD to
pyruvate and NADH in the final step of anaerobic glycolysis
(46). The expression level of
LDH was significantly decreased in the H9c2 cells treated with
cholesterol. These findings indicate that cholesterol could inhibit
the glycolysis and TCA cycle in H9c2 cells.
In addition, hyperlipidemia can cause a vascular
inflammation reaction, leading to the occurrence of
atherosclerosis. Atherosclerotic plaque blocks blood vessels,
resulting in myocardial infarction, stroke and other serious
diseases (47). In addition,
hyperglycemia also causes an increase in ROS in clinical
hypercholesterolemia patients, which generates the oxidative stress
response resulting in the abnormal systolic function of
cardiomyocytes (48,49). The results of the cardiac
ultrasound indicated that the LV systolic score and ejection
fraction of WT mice fed a high-fat diet were significantly
decreased compared to that of the WT mice fed a normal diet.
Meanwhile, the injury level of cardiac function in the
HO-1+/− mice was significantly increased compared to
that in the WT mice fed a high-fat diet. Meanwhile, the Oil Red O
staining results also showed that the lipid accumulation phenomenon
in cardiac muscle tissues of the HO-1+/− mice was more
obvious. These findings indicate that HO-1 protects the systolic
function of the heart, improves the blood supply of the coronary
artery and target organs, and alleviates the occurrence and
development of cardiovascular diseases.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81570335), the Tianjin
Natural Science Foundation (nos. 15ZXJZSY00010 and 12JCYBJC15900),
the Opening Funding for Tianjin Key Laboratory of Cardiovascular
Remodeling and Target Organ Injury (no. TJC1401) and intramural
research grants from Pingjin Hospital (FYM201538).
References
|
1
|
Gozzelino R, Jeney V and Soares MP:
Mechanisms of cell protection by heme oxygenase-1. Annu Rev
Pharmacol Toxicol. 50:323–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klickovic U, Doberer D, Gouya G, Aschauer
S, Weisshaar S, Storka A, Bilban M and Wolzt M: Human
pharmacokinetics of high dose oral curcumin and its effect on heme
oxygenase-1 expression in healthy male subjects. BioMed Res Int.
2014:4585922014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chau LY: Heme oxygenase-1: Emerging target
of cancer therapy. J Biomed Sci. 22:222015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yen TL, Chen RJ, Jayakumar T, Lu WJ, Hsieh
CY, Hsu MJ, Yang CH, Chang CC, Lin YK, Lin KH, et al:
Andrographolide stimulates p38 mitogen-activated protein
kinase-nuclear factor erythroid-2-related factor 2-heme oxygenase 1
signaling in primary cerebral endothelial cells for definite
protection against ischemic stroke in rats. Transl Res. 170:57–72.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lv H, Yu Z, Zheng Y, Wang L, Qin X, Cheng
G and Ci X: Isovitexin exerts anti-inflammatory and anti-oxidant
activities on lipopolysaccharide-induced acute lung injury by
inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int J
Biol Sci. 12:72–86. 2016. View Article : Google Scholar :
|
|
6
|
Wang J, Yang H, Hu X, Fu W, Xie J, Zhou X,
Xu W and Jiang H: Dobutamine-mediated heme oxygenase-1 induction
via PI3K and p38 MAPK inhibits high mobility group box 1 protein
release and attenuates rat myocardial ischemia/reperfusion injury
in vivo. J Surg Res. 183:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YC, Lii CK, Wei YL, Li CC, Lu CY, Liu
KL and Chen HW: Docosahexaenoic acid inhibition of inflammation is
partially via cross-talk between Nrf2/heme oxygenase 1 and
IKK/NF-κB pathways. J Nutr Biochem. 24:204–212. 2013. View Article : Google Scholar
|
|
8
|
Zhang L, Gan ZK, Han LN, Wang H, Bai J,
Tan GJ, Li XX, Xu YP, Zhou Y, Gong ML, et al: Protective effect of
heme oxygenase-1 on Wistar rats with heart failure through the
inhibition of inflammation and amelioration of intestinal
microcirculation. J Geriatr Cardiol. 12:353–365. 2015.PubMed/NCBI
|
|
9
|
Seo YJ, Lee KT, Rho JR, Choi JH and
Phorbaketal A: Phorbaketal A, isolated from the Marine Sponge
Phorbas sp., exerts its anti-inflammatory effects via NF-κB
inhibition and heme oxygenase-1 activation in
lipopolysaccharide-stimulated macrophages. Mar Drugs. 13:7005–7019.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miele L, Giorgio V, Alberelli MA, De
Candia E, Gasbarrini A and Grieco A: Impact of gut microbiota on
obesity, diabetes, and cardiovascular disease risk. Curr Cardiol
Rep. 17:1202015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo F and Garvey WT: Cardiometabolic
disease risk in metabolically healthy and unhealthy obesity:
Stability of metabolic health status in adults. Obesity (Silver
Spring). 24:516–525. 2016. View Article : Google Scholar
|
|
12
|
Melgar-Lesmes P, Garcia-Polite F,
Del-Rey-Puech P, Rosas E, Dreyfuss JL, Montell E, Vergés J, Edelman
ER and Balcells M: Treatment with chondroitin sulfate to modulate
inflammation and atherogenesis in obesity. Atherosclerosis.
245:82–87. 2016. View Article : Google Scholar :
|
|
13
|
Huang ZP, Kataoka M, Chen J, Wu G, Ding J,
Nie M, Lin Z, Liu J, Hu X, Ma L, et al: Cardiomyocyte-enriched
protein CIP protects against pathophysiological stresses and
regulates cardiac homeostasis. J Clin Invest. 125:4122–4134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimizu I, Yoshida Y and Minamino T:
Maintenance of subcutaneous fat homeostasis improves systemic
metabolic dysfunction in obesity. Diabetes. 64:3984–3986. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Ren Y, Sorokin V, Poh KK, Ho HH, Lee
CN, de Kleijn D, Lim SK, Tam JP and Sze SK: Quantitative profiling
of the rat heart myoblast secretome reveals differential responses
to hypoxia and re-oxygenation stress. J Proteomics. 98:138–149.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schumacher A, Wafula PO, Teles A,
El-Mousleh T, Linzke N, Zenclussen ML, Langwisch S, Heinze K,
Wollenberg I and Casalis PA: Blockage of heme oxygenase-1 abrogates
the protective effect of regulatory T cells on murine pregnancy and
promotes the maturation of dendritic cells. PLoS One. 7:e423012012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Das S, Datta A, Bagchi C, Chakraborty S,
Mitra A and Tripathi SK: A comparative study of lipid-lowering
effects of guggul and atorvastatin monotherapy in comparison to
their combination in high cholesterol diet-induced hyperlipidemia
in rabbits. J Diet Suppl. 13:495–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
López-Rosas I, Marchat LA, Olvera BG,
Guillen N, Weber C, Hernández de la Cruz O, Ruíz-García E,
Astudillo-de la Vega H and López-Camarillo C: Proteomic analysis
identifies endoribouclease EhL-PSP and EhRRP41 exosome protein as
novel interactors of EhCAF1 deadenylase. J Proteomics. 111:59–73.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang T, Xie N, He W, Liu R, Lei Y, Chen
Y, Tang H, Liu B, Huang C and Wei Y: An integrated proteomics and
bioinformatics analyses of hepatitis B virus X interacting proteins
and identification of a novel interactor apoA-I. J Proteomics.
84:92–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu Z, Wang F, Fan F, Gu Y, Shan N, Meng X,
Cheng S, Liu Y, Wang C, Song Y, et al: Quantitative proteomics
reveals that the inhibition of Na(+)/K(+)-ATPase activity affects
S-phase progression leading to a chromosome segregation disorder by
attenuating the aurora a function in hepatocellular carcinoma
cells. J Proteome Res. 14:4594–4602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramus C, Hovasse A, Marcellin M, Hesse AM,
Mouton-Barbosa E, Bouyssié D, Vaca S, Carapito C, Chaoui K, Bruley
C, et al: Benchmarking quantitative label-free LC-MS data
processing workflows using a complex spiked proteomic standard
dataset. J Proteomics. 132:51–62. 2016. View Article : Google Scholar
|
|
22
|
Dutta M, Subramani E, Taunk K, Gajbhiye A,
Seal S, Pendharkar N, Dhali S, Ray CD, Lodh I, Chakravarty B, et
al: Investigation of serum proteome alterations in human
endometriosis. J Proteomics. 114:182–196. 2015. View Article : Google Scholar
|
|
23
|
Li J, Song J, Bi S, Zhou S, Cui J, Liu J
and Wu D: Electrochemical estrogen screen method based on the
electrochemical behavior of MCF-7 cells. J Hazard Mater.
313:238–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhan Y, Zhao F, Xie P, Zhong L, Li D, Gai
Q, Li L, Wei H, Zhang L and An W: Mechanism of the effect of
glycosyltransferase GLT8D2 on fatty liver. Lipids Health Dis.
14:432015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Ji J, Song Z, Zhang W, He X, Li F,
Zhang C, Guo C, Wang C and Yuan C: Hypocholesterolemic effect of
emodin by simultaneous determination of in vitro and in vivo bile
salts binding. Fitoterapia. 110:116–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu F, Tang W, Chen D, Li M, Gao Y, Zheng
H and Chen S: Expression of TGF-β1 and CTGF is associated with
fibrosis of denervated sternocleidomastoid muscles in mice. Tohoku
J Exp Med. 238:49–56. 2016. View Article : Google Scholar
|
|
27
|
Abraham NG and Kappas A: Pharmacological
and clinical aspects of heme oxygenase. Pharmacol Rev. 60:79–127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Ye F, Li L, Chang W, Wu X and Chen
J: The role of HO-1 in protection against lead-induced
neurotoxicity. Neurotoxicology. 52:1–11. 2016. View Article : Google Scholar
|
|
29
|
Gil-Bona A, Parra-Giraldo CM, Hernáez ML,
Reales-Calderon JA, Solis NV, Filler SG, Monteoliva L and Gil C:
Candida albicans cell shaving uncovers new proteins involved in
cell wall integrity, yeast to hypha transition, stress response and
host-pathogen interaction. J Proteomics. 127:340–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ning W, Song R, Li C, Park E, Mohsenin A,
Choi AM and Choi ME: TGF-beta1 stimulates HO-1 via the 38
mitogen-activated protein kinase in A549 pulmonary epithelial
cells. Am J Physiol Lung Cell Mol Physiol. 283:L1094–L1102. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang JQ, Zhang JQ, Fang LZ, Liu L, Fu WP
and Dai LM: Effect of oral N-acetylcysteine on COPD patients with
microsatellite polymorphism in the heme oxygenase-1 gene promoter.
Drug Des Devel Ther. 9:6379–6387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu MM, Lee CH, Hsu LI, Cheng WF, Lee TC,
Wang YH, Chiou HY, Chen CJ and Meei-Maan Wu: Effect of heme
oxygenase-1 gene promoter polymorphism on cancer risk by
histological subtype: A prospective study in arseniasis-endemic
areas in Taiwan. Int J Cancer. 138:1875–1886. 2016. View Article : Google Scholar
|
|
33
|
Daenen KE, Martens P and Bammens B:
Association of HO-1 (GT)n promoter polymorphism and cardiovascular
disease: A reanalysis of the literature. Can J Cardiol. 32:160–168.
2016. View Article : Google Scholar
|
|
34
|
Shibata T, Saito S, Kokubu A, Suzuki T,
Yamamoto M and Hirohashi S: Global downstream pathway analysis
reveals a dependence of oncogenic NF-E2-related factor 2 mutation
on the mTOR growth signaling pathway. Cancer Res. 70:9095–9105.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pang C, Zheng Z, Shi L, Sheng Y, Wei H,
Wang Z and Ji L: Caffeic acid prevents acetaminophen-induced liver
injury by activating the Keap1-Nrf2 antioxidative defense system.
Free Radic Biol Med. 91:236–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JH, Park GY, Bang SY, Park SY, Bae SK
and Kim Y: Crocin suppresses LPS-stimulated expression of inducible
nitric oxide synthase by upregulation of heme oxygenase-1 via
calcium/calmodulin-dependent protein kinase 4. Mediators Inflamm.
2014:7287092014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao H, Feng Y, Ning Y, Zhang Z, Li W and
Li Q: Edaravone protects rats and human pulmonary alveolar
epithelial cells against hyperoxia injury: Heme oxygenase-1 and
I3K/Akt pathway may be involved. Exp Lung Res. 41:404–414. 2015.
View Article : Google Scholar
|
|
38
|
Barbagallo I, Parenti R, Zappalà A,
Vanella L, Tibullo D, Pepe F, Onni T and Li Volti G: Combined
inhibition of Hsp90 and heme oxygenase-1 induces apoptosis and
endoplasmic reticulum stress in melanoma. Acta Histochem.
117:705–711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen B, Lu Y, Chen Y and Cheng J: The role
of Nrf2 in oxidative stress-induced endothelial injuries. J
Endocrinol. 225:R83–R99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akhtar S, Hartmann P, Karshovska E,
Rinderknecht FA, Subramanian P, Gremse F, Grommes J, Jacobs M,
Kiessling F, Weber C, et al: Endothelial hypoxia-inducible
factor-1α promotes atherosclerosis and monocyte recruitment by
upregulating MicroRNA-19a. Hypertension. 66:1220–1226.
2015.PubMed/NCBI
|
|
41
|
Mao X, Wang T, Liu Y, Irwin MG, Ou JS,
Liao XL, Gao X, Xu Y, Ng KF and Vanhoutte PM: N-acetylcysteine and
allopurinol confer synergy in attenuating myocardial ischemia
injury via restoring HIF-1α/HO-1 signaling in diabetic rats. PLoS
One. 8:e689492013. View Article : Google Scholar
|
|
42
|
Chang SH, Barbosa-Tessmann I, Chen C,
Kilberg MS and Agarwal A: Glucose deprivation induces heme
oxygenase-1 gene expression by a pathway independent of the
unfolded protein response. J Biol Chem. 277:1933–1940. 2002.
View Article : Google Scholar
|
|
43
|
Li C, Lönn ME, Xu X, Maghzal GJ, Frazer
DM, Thomas SR, Halliwell B, Richardson DR, Anderson GJ and Stocker
R: Sustained expression of heme oxygenase-1 alters iron homeostasis
in nonerythroid cells. Free Radic Biol Med. 53:366–374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Menckhoff L, Mielke-Ehret N, Buck F,
Vuletić M and Lüthje S: Plasma membrane-associated malate
dehydrogenase of maize (Zea mays L.) roots: Native versus
recombinant protein. J Proteomics. 80:66–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mitra A, Basak T, Ahmad S, Datta K, Datta
R, Sengupta S and Sarkar S: Comparative proteome profiling during
cardiac hypertrophy and myocardial infarction reveals altered
glucose oxidation by differential activation of pyruvate
dehydrogenase E1 component subunit β. J Mol Biol. 427:2104–2120.
2015. View Article : Google Scholar
|
|
46
|
Zaimoku Y, Takahashi W, Iwaki N, Saito C,
Yoshida A, Aoki G, Yamaguchi M and Nakao S: Human
herpesvirus-8-unrelated primary effusion lymphoma of the elderly
not associated with an increased serum lactate dehydrogenase level:
A benign sub-group of effusion lymphoma without chemotherapy. Leuk
Lymphoma. 57:1625–1632. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pechlaner R, Willeit P, Summerer M, Santer
P, Egger G, Kronenberg F, Demetz E, Weiss G, Tsimikas S, Witztum
JL, et al: Heme oxygenase-1 gene promoter microsatellite
polymorphism is associated with progressive atherosclerosis and
incident cardiovascular disease. Arterioscler Thromb Vasc Biol.
35:229–236. 2015. View Article : Google Scholar :
|
|
48
|
Song G, Zong C, Zhang Z, Yu Y, Yao S, Jiao
P, Tian H, Zhai L, Zhao H, Tian S, et al: Molecular hydrogen
stabilizes atherosclerotic plaque in low-density lipoprotein
receptor-knockout mice. Free Radic Biol Med. 87:58–68. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang CM, Lin CC and Hsieh HL:
High-glucose-derived oxidative stress-dependent heme oxygenase-1
expression from astrocytes contributes to the neuronal apoptosis.
Mol Neurobiol. 54:470–483. 2017. View Article : Google Scholar
|