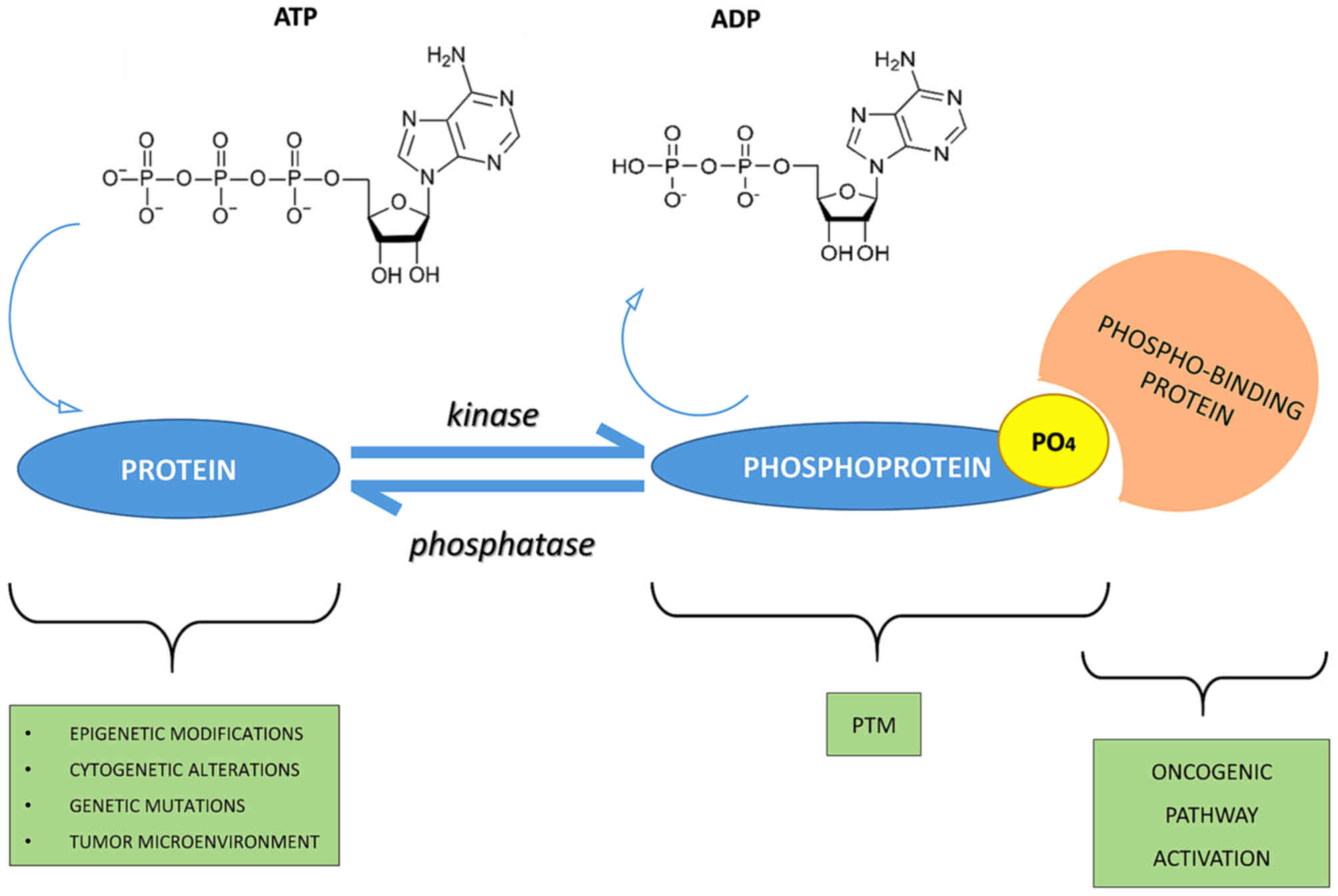
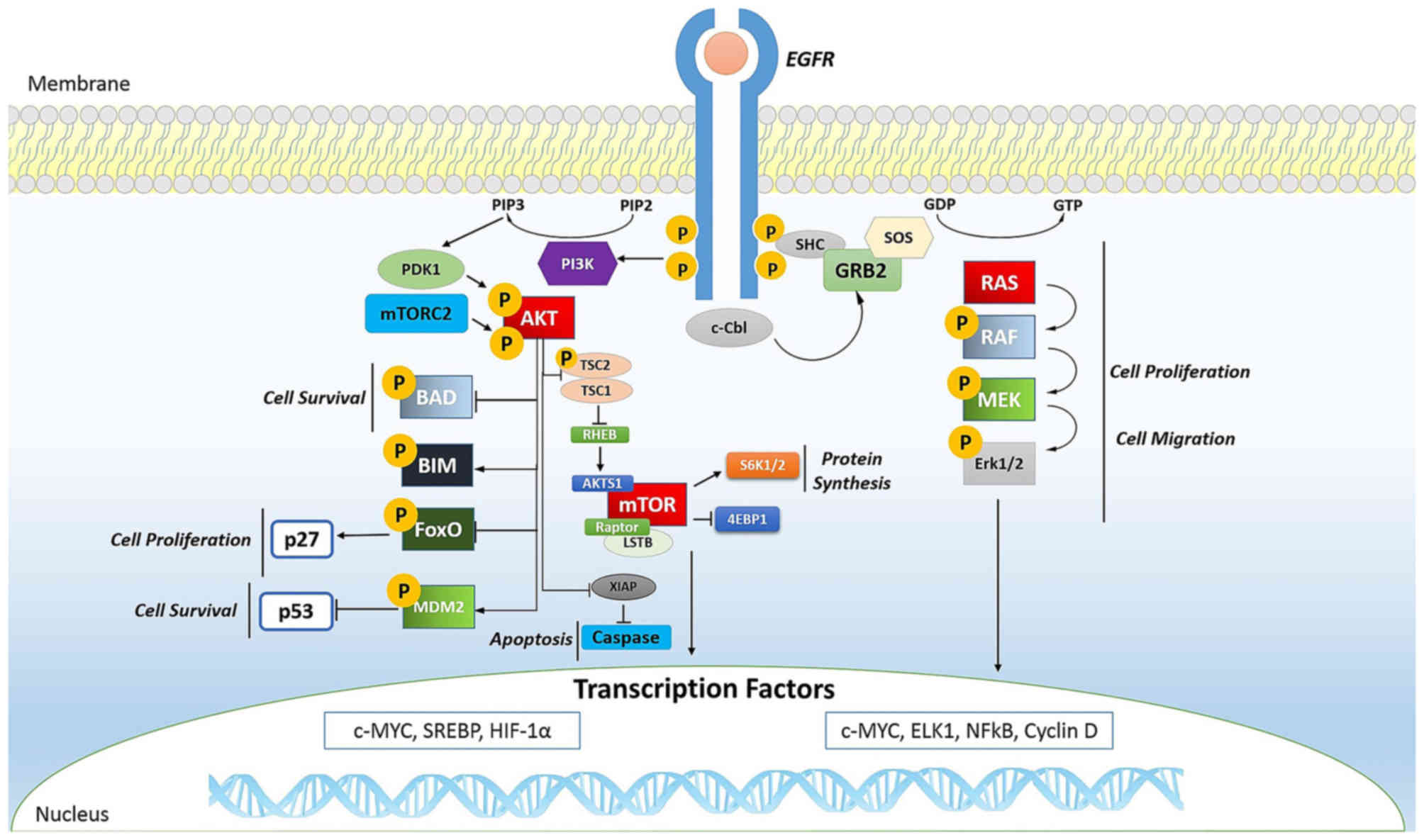
|
1
|
Li X, Wilmanns M, Thornton J and Köhn M:
Elucidating human phosphatase-substrate networks. Sci Signal.
6:rs102013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sacco F, Perfetto L, Castagnoli L and
Cesareni G: The human phosphatase interactome: an intricate family
portrait. FEBS Lett. 586:2732–2739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alberts B, Johnson A, Lewis J, Raff M,
Roberts K and Walter P: Molecular Biology of the Cell. Anderson M
and Granum S: 5th edition. Garland Science; New York, NY: pp.
1752007
|
|
4
|
Hunter T: Why nature chose phosphate to
modify proteins. Philos Trans R Soc Lond B Biol Sci. 367:2513–2516.
2009. View Article : Google Scholar
|
|
5
|
Fukami Y and Lipmann F: Reversal of Rous
sarcoma-specific immunoglobulin phosphorylation on tyrosine (ADP as
phosphate acceptor) catalyzed by the src gene kinase. Proc Natl
Acad Sci USA. 80:1872–1876. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kole HK, Abdel-Ghany M and Racker E:
Specific dephosphorylation of phosphoproteins by protein-serine and
-tyrosine kinases. Proc Natl Acad Sci USA. 85:5849–5853. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roskoski R Jr: ERK1/2 MAP kinases:
structure, function, and regulation. Pharmacol Res. 66:105–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwartz PA and Murray BW: Protein kinase
biochemistry and drug discovery. Bioorg Chem. 39:192–210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishi H, Shaytan A and Panchenko AR:
Physicochemical mechanisms of protein regulation by
phosphorylation. Front Genet. 5:2702014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCance KL and Huether SE:
Pathophysiology: The Biologic Basis for Disease in Adults and
Children. Brashers VL and Rote NS: 7th edition. Elsevier; 2014
|
|
11
|
Hornberg JJ, Bruggeman FJ, Binder B, Geest
CR, de Vaate AJ, Lankelma J, Heinrich R and Westerhoff HV:
Principles behind the multifarious control of signal transduction.
ERK phosphorylation and kinase/phosphatase control. FEBS J.
272:244–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tonks NK: Protein tyrosine phosphatases:
from genes, to function, to disease. Nat Rev Mol Cell Biol.
7:833–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heinrich R, Neel BG and Rapoport TA:
Mathematical models of protein kinase signal transduction. Mol
Cell. 9:957–970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liberti S, Sacco F, Calderone A, Perfetto
L, Iannuccelli M, Panni S, Santonico E, Palma A, Nardozza AP,
Castagnoli L, et al: HuPho: the human phosphatase portal. FEBS J.
280:379–387. 2013. View Article : Google Scholar
|
|
15
|
Hatzihristidis T, Liu S, Pryszcz L,
Hutchins AP, Gabaldón T, Tremblay ML and Miranda-Saavedra D:
PTP-central: a comprehensive resource of protein tyrosine
phosphatases in eukaryotic genomes. Methods. 65:156–164. 2014.
View Article : Google Scholar
|
|
16
|
Miller ML, Jensen LJ, Diella F, Jørgensen
C, Tinti M, Li L, Hsiung M, Parker SA, Bordeaux J, Sicheritz-Ponten
T, et al: Linear motif atlas for phosphorylation-dependent
signaling. Sci Signal. 1:–ra2. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hunter T: Tyrosine phosphorylation: thirty
years and counting. Curr Opin Cell Biol. 21:140–146. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Capra M, Nuciforo PG, Confalonieri S,
Quarto M, Bianchi M, Nebuloni M, Boldorini R, Pallotti F, Viale G,
Gishizky ML, et al: Frequent alterations in the expression of
serine/threonine kinases in human cancers. Cancer Res.
66:8147–8154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin J and Pawson T: Modular evolution of
phosphorylation-based signalling systems. Philos Trans R Soc Lond B
Biol Sci. 367:2540–2555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pearce LR, Komander D and Alessi DR: The
nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol.
11:9–22. 2010. View Article : Google Scholar
|
|
21
|
Wayman GA, Tokumitsu H, Davare MA and
Soderling TR: Analysis of CaM-kinase signaling in cells. Cell
Calcium. 50:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eide EJ and Virshup DM: Casein kinase I:
another cog in the circadian clockworks. Chronobiol Int.
18:389–398. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sundaram MV: RTK/Ras/MAPK signaling.
WormBook: pp. 1–19. 2006, View Article : Google Scholar
|
|
24
|
Cohen P and Goedert M: GSK3 inhibitors:
development and therapeutic potential. Nat Rev Drug Discov.
3:479–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moeslein FM, Myers MP and Landreth GE: The
CLK family kinases, CLK1 and CLK2, phosphorylate and activate the
tyrosine phosphatase, PTP-1B. J Biol Chem. 274:26697–26704. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Müller-Taubenberger A, Ishikawa-Ankerhold
HC, Kastner PM, Burghardt E and Gerisch G: The STE group kinase
SepA controls cleavage furrow formation in Dictyostelium. Cell
Motil Cytoskeleton. 66:929–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdi AI, Carvalho TG, Wilkes JM and Doerig
C: A secreted Plasmodium falciparum kinase reveals a signature
motif for classification of tyrosine kinase-like kinases.
Microbiology. 159:2533–2547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barford D: Molecular mechanisms of the
protein serine/thre-onine phosphatases. Trends Biochem Sci.
21:407–412. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang ZY: Protein tyrosine phosphatases:
structure and function, substrate specificity, and inhibitor
development. Annu Rev Pharmacol Toxicol. 42:209–234. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mackintosh C: Protein Phosphorylation: A
Practical Approach. Hardie GD: IRL Press; New York, NY: pp.
3281993
|
|
31
|
Thingholm TE, Larsen MR, Ingrell CR,
Kassem M and Jensen ON: TiO(2)-based phosphoproteomic analysis of
the plasma membrane and the effects of phosphatase inhibitor
treatment. J Proteome Res. 7:3304–3313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stern DF: Phosphoproteomics for oncology
discovery and treatment. Expert Opin Ther Targets. 9:851–860. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y and Chance MR: Integrating
phosphoproteomics in systems biology. Comput Struct Biotechnol J.
10:90–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moorhead GB, De Wever V, Templeton G and
Kerk D: Evolution of protein phosphatases in plants and animals.
Biochem J. 417:401–409. 2009. View Article : Google Scholar
|
|
35
|
Das AK, Helps NR, Cohen PT and Barford D:
Crystal structure of the protein serine/threonine phosphatase 2C at
2.0 A resolution. EMBO J. 15:6798–6809. 1996.PubMed/NCBI
|
|
36
|
Shi Y: Serine/threonine phosphatases:
mechanism through structure. Cell. 139:468–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Virshup DM and Shenolikar S: From
promiscuity to precision: protein phosphatases get a makeover. Mol
Cell. 33:537–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guan KL and Dixon JE: Evidence for
protein-tyrosine-phosphatase catalysis proceeding via a
cysteine-phosphate intermediate. J Biol Chem. 266:17026–17030.
1991.PubMed/NCBI
|
|
39
|
Alonso A, Sasin J, Bottini N, Friedberg I,
Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J and Mustelin
T: Protein tyrosine phosphatases in the human genome. Cell.
117:699–711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tonks NK and Neel BG: Combinatorial
control of the specificity of protein tyrosine phosphatases. Curr
Opin Cell Biol. 13:182–195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wrighton KH, Willis D, Long J, Liu F, Lin
X and Feng XH: Small C-terminal domain phosphatases dephosphorylate
the regulatory linker regions of Smad2 and Smad3 to enhance
transforming growth factor-beta signaling. J Biol Chem.
281:38365–38375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Archambault J, Pan G, Dahmus GK, Cartier
M, Marshall N, Zhang S, Dahmus ME and Greenblatt J: FCP1, the
RAP74-interacting subunit of a human protein phosphatase that
dephosphorylates the carboxyl-terminal domain of RNA polymerase
IIO. J Biol Chem. 273:27593–27601. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salton SR: Teaching resources. Protein
phosphatases. Sci STKE. 2005:tr82005.PubMed/NCBI
|
|
44
|
Tootle TL, Silver SJ, Davies EL, Newman V,
Latek RR, Mills IA, Selengut JD, Parlikar BE and Rebay I: The
transcription factor eyes absent is a protein tyrosine phosphatase.
Nature. 426:299–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gentry MS, Dixon JE and Worby CA: Lafora
disease: insights into neurodegeneration from plant metabolism.
Trends Biochem Sci. 34:628–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gentry MS, Dowen RH III, Worby CA, Mattoo
S, Ecker JR and Dixon JE: The phosphatase laforin crosses
evolutionary boundaries and links carbohydrate metabolism to
neuronal disease. J Cell Biol. 178:477–488. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Niittylä T, Comparot-Moss S, Lue WL,
Messerli G, Trevisan M, Seymour MD, Gatehouse JA, Villadsen D,
Smith SM, Chen J, et al: Similar protein phosphatases control
starch metabolism in plants and glycogen metabolism in mammals. J
Biol Chem. 281:11815–11818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Case N, Thomas J, Sen B, Styner M, Xie Z,
Galior K and Rubin J: Mechanical regulation of glycogen synthase
kinase 3β (GSK3β) in mesenchymal stem cells is dependent on Akt
protein serine 473 phosphorylation via mTORC2 protein. J Biol Chem.
286:39450–39456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cole PA, Shen K, Qiao Y and Wang D:
Protein tyrosine kinases Src and Csk: a tail's tale. Curr Opin Chem
Biol. 7:580–585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nishi H, Hashimoto K and Panchenko AR:
Phosphorylation in protein-protein binding: effect on stability and
function. Structure. 19:1807–1815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nishi H, Fong JH, Chang C, Teichmann SA
and Panchenko AR: Regulation of protein-protein binding by coupling
between phosphorylation and intrinsic disorder: analysis of human
protein complexes. Mol Biosyst. 9:1620–1626. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Harita Y, Kurihara H, Kosako H, Tezuka T,
Sekine T, Igarashi T and Hattori S: Neph1, a component of the
kidney slit diaphragm, is tyrosine-phosphorylated by the Src family
tyrosine kinase and modulates intracellular signaling by binding to
Grb2. J Biol Chem. 283:9177–9186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kuwahara H, Nishizaki M and Kanazawa H:
Nuclear localization signal and phosphorylation of Serine350
specify intracellular localization of DRAK2. J Biochem.
143:349–358. 2008. View Article : Google Scholar
|
|
54
|
Shimazaki Y, Nishiki T, Omori A, Sekiguchi
M, Kamata Y, Kozaki S and Takahashi M: Phosphorylation of 25-kDa
synaptosome-associated protein. Possible involvement in protein
kinase C-mediated regulation of neurotransmitter release. J Biol
Chem. 271:14548–14553. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kataoka M, Kuwahara R, Iwasaki S,
Shoji-Kasai Y and Takahashi M: Nerve growth factor-induced
phosphorylation of SNAP-25 in PC12 cells: a possible involvement in
the regulation of SNAP-25 localization. J Neurochem. 74:2058–2066.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rosen OM and Erlichman J: Reversible
autophosphorylation of a cyclic 3′:5′-AMP-dependent protein kinase
from bovine cardiac muscle. J Biol Chem. 250:7788–7794.
1975.PubMed/NCBI
|
|
57
|
Hunter T: The age of crosstalk:
phosphorylation, ubiquitination, and beyond. Mol Cell. 28:730–738.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xu X, Sarikas A, Dias-Santagata DC, Dolios
G, Lafontant PJ, Tsai SC, Zhu W, Nakajima H, Nakajima HO, Field LJ,
et al: The CUL7 E3 ubiquitin ligase targets insulin receptor
substrate 1 for ubiquitin-dependent degradation. Mol Cell.
30:403–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pende M, Um SH, Mieulet V, Sticker M, Goss
VL, Mestan J, Mueller M, Fumagalli S, Kozma SC and Thomas G:
S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and
rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation
and reveal a mitogen-activated protein kinase-dependent S6 kinase
pathway. Mol Cell Biol. 24:3112–3124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ferrer I, Blanco R, Carmona M, Puig B,
Domínguez I and Viñals F: Active, phosphorylation-dependent MAP
kinases, MAPK/ERK, SAPK/JNK and p38, and specific transcription
factor substrates are differentially expressed following systemic
administration of kainic acid to the adult rat. Acta Neuropathol.
103:391–407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Greenlund AC, Farrar MA, Viviano BL and
Schreiber RD: Ligand-induced IFN gamma receptor tyrosine
phosphorylation couples the receptor to its signal transduction
system (p91). EMBO J. 13:1591–1600. 1994.PubMed/NCBI
|
|
64
|
Igarashi K, Garotta G, Ozmen L, Ziemiecki
A, Wilks AF, Harpur AG, Larner AC and Finbloom DS: Interferon-gamma
induces tyrosine phosphorylation of interferon-gamma receptor and
regulated association of protein tyrosine kinases, Jak1 and Jak2,
with its receptor. J Biol Chem. 269:14333–14336. 1994.PubMed/NCBI
|
|
65
|
Decker T and Kovarik P: Serine
phosphorylation of STATs. Oncogene. 19:2628–2637. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu Z, Wang Y and Xue Y:
Phosphoproteomics-based network medicine. FEBS J. 280:5696–5704.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hirschi A, Cecchini M, Steinhardt RC,
Schamber MR, Dick FA and Rubin SM: An overlapping kinase and
phosphatase docking site regulates activity of the retinoblastoma
protein. Nat Struct Mol Biol. 17:1051–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Salazar C and Höfer T: Competition effects
shape the response sensitivity and kinetics of phosphorylation
cycles in cell signaling. Ann NY Acad Sci. 1091:517–530. 2006.
View Article : Google Scholar
|
|
69
|
Lienhard GE: Non-functional
phosphorylations? Trends Biochem Sci. 33:351–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Landry CR, Levy ED and Michnick SW: Weak
functional constraints on phosphoproteomes. Trends Genet.
25:193–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Levy ED, Michnick SW and Landry CR:
Protein abundance is key to distinguish promiscuous from functional
phosphorylation based on evolutionary information. Philos Trans R
Soc Lond B Biol Sci. 367:2594–2606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Olsen JV, Blagoev B, Gnad F, Macek B,
Kumar C, Mortensen P and Mann M: Global, in vivo, and site-specific
phosphorylation dynamics in signaling networks. Cell. 127:635–648.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rikova K, Guo A, Zeng Q, Possemato A, Yu
J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al: Global survey
of phosphotyrosine signaling identifies oncogenic kinases in lung
cancer. Cell. 131:1190–1203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Linding R, Jensen LJ, Ostheimer GJ, van
Vugt MA, Jørgensen C, Miron IM, Diella F, Colwill K, Taylor L,
Elder K, et al: Systematic discovery of in vivo phosphorylation
networks. Cell. 129:1415–1426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Newman RH, Hu J, Rho HS, Xie Z, Woodard C,
Neiswinger J, Cooper C, Shirley M, Clark HM, Hu S, et al:
Construction of human activity-based phosphorylation networks. Mol
Syst Biol. 9:6552013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Drake JM, Graham NA, Stoyanova T, Sedghi
A, Goldstein AS, Cai H, Smith DA, Zhang H, Komisopoulou E, Huang J,
et al: Oncogene-specific activation of tyrosine kinase networks
during prostate cancer progression. Proc Natl Acad Sci USA.
109:1643–1648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Harsha HC and Pandey A: Phosphoproteomics
in cancer. Mol Oncol. 4:482–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hynes NE and MacDonald G: ErbB receptors
and signaling pathways in cancer. Curr Opin Cell Biol. 21:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sharma A, Tan TH, Cheetham G, Scott HS and
Brown MP: Rare and novel epidermal growth factor receptor mutations
in non-small-cell lung cancer and lack of clinical response to
gefitinib in two cases. J Thorac Oncol. 7:941–942. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Petricoin EF, Zoon KC, Kohn EC, Barrett JC
and Liotta LA: Clinical proteomics: translating benchside promise
into bedside reality. Nat Rev Drug Discov. 1:683–695. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
84
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tarrant MK and Cole PA: The chemical
biology of protein phosphorylation. Annu Rev Biochem. 78:797–825.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Paul MK and Mukhopadhyay AK: Tyrosine
kinase - role and significance in cancer. Int J Med Sci. 1:101–115.
2004. View Article : Google Scholar
|
|
87
|
Murphree AL and Benedict WF:
Retinoblastoma: clues to human oncogenesis. Science. 223:1028–1033.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Stehelin D, Guntaka RV, Varmus HE and
Bishop JM: Purification of DNA complementary to nucleotide
sequences required for neoplastic transformation of fibroblasts by
avian sarcoma viruses. J Mol Biol. 101:349–365. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hunter T and Cooper JA: Protein-tyrosine
kinases. Annu Rev Biochem. 54:897–930. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Sefton BM and Hunter T: From c-src to
v-src, or the case of the missing C terminus. Cancer Surv.
5:159–172. 1986.PubMed/NCBI
|
|
91
|
Sefton BM, Hunter T and Raschke WC:
Evidence that the Abelson virus protein functions in vivo as a
protein kinase that phosphorylates tyrosine. Proc Natl Acad Sci
USA. 78:1552–1556. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sefton BM, Hunter T, Beemon K and Eckhart
W: Evidence that the phosphorylation of tyrosine is essential for
cellular transformation by Rous sarcoma virus. Cell. 20:807–816.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu W, Doshi A, Lei M, Eck MJ and Harrison
SC: Crystal structures of c-Src reveal features of its
autoinhibitory mechanism. Mol Cell. 3:629–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Young MA, Gonfloni S, Superti-Furga G,
Roux B and Kuriyan J: Dynamic coupling between the SH2 and SH3
domains of c-Src and Hck underlies their inactivation by C-terminal
tyrosine phosphorylation. Cell. 105:115–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shawver LK, Slamon D and Ullrich A: Smart
drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell.
1:117–123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lee CH, Syu SH, Liu KJ, Chu PY, Yang WC,
Lin P and Shieh WY: Interleukin-1 beta transactivates epidermal
growth factor receptor via the CXCL1-CXCR2 axis in oral cancer.
Oncotarget. 6:38866–38880. 2015.PubMed/NCBI
|
|
98
|
Corless CL, Fletcher JA and Heinrich MC:
Biology of gastrointestinal stromal tumors. J Clin Oncol.
22:3813–3825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Javidi-Sharifi N, Traer E, Martinez J,
Gupta A, Taguchi T, Dunlap J, Heinrich MC, Corless CL, Rubin BP,
Druker BJ, et al: Crosstalk between KIT and FGFR3 promotes
gastrointestinal stromal tumor cell growth and drug resistance.
Cancer Res. 75:880–891. 2015. View Article : Google Scholar :
|
|
100
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zhu N, Xiao H, Wang LM, Fu S, Zhao C and
Huang H: Mutations in tyrosine kinase and tyrosine phosphatase and
their relevance to the target therapy in hematologic malignancies.
Future Oncol. 11:659–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kraus J, Kraus M, Liu N, Besse L, Bader J,
Geurink PP, de Bruin G, Kisselev AF, Overkleeft H and Driessen C:
The novel β2-selective proteasome inhibitor LU-102 decreases
phosphorylation of I kappa B and induces highly synergistic
cytotoxicity in combination with ibrutinib in multiple myeloma
cells. Cancer Chemother Pharmacol. 76:383–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Jagarlamudi KK, Hansson LO and Eriksson S:
Breast and prostate cancer patients differ significantly in their
serum thymidine kinase 1 (TK1) specific activities compared with
those hematological malignancies and blood donors: implications of
using serum TK1 as a biomarker. BMC Cancer. 15:662015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hou S, Isaji T, Hang Q, Im S, Fukuda T and
Gu J: Distinct effects of β1 integrin on cell proliferation and
cellular signaling in MDA-MB-231 breast cancer cells. Sci Rep.
6:184302016. View Article : Google Scholar
|
|
105
|
Paladino D, Yue P, Furuya H, Acoba J,
Rosser CJ and Turkson J: A novel nuclear Src and p300 signaling
axis controls migratory and invasive behavior in pancreatic cancer.
Oncotarget. 7:7253–7267. 2016.
|
|
106
|
Li Z, Lin P, Gao C, Peng C, Liu S, Gao H,
Wang B, Wang J, Niu J and Niu W: Integrin β6 acts as an unfavorable
prognostic indicator and promotes cellular malignant behaviors via
ERK-ETS1 pathway in pancreatic ductal adenocarcinoma (PDAC). Tumour
Biol. 37:5117–5131. 2016. View Article : Google Scholar
|
|
107
|
Meh raein-Ghom i F, Chu rch DR, Sch reiber
CL, Weichmann AM, Basu HS and Wilding G: Inhibitor of p52 NF-κB
subunit and androgen receptor (AR) interaction reduces growth of
human prostate cancer cells by abrogating nuclear translocation of
p52 and phosphorylated AR(ser81). Genes Cancer. 6:428–444.
2015.
|
|
108
|
Barber TD, Vogelstein B, Kinzler KW and
Velculescu VE: Somatic mutations of EGFR in colorectal cancers and
glioblastomas. N Engl J Med. 351:28832004. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Parsons DW, Wang TL, Samuels Y, Bardelli
A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK,
et al: Colorectal cancer: mutations in a signalling pathway.
Nature. 436:7922005. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Stephens P, Edkins S, Davies H, Greenman
C, Cox C, Hunter C, Bignell G, Teague J, Smith R, Stevens C, et al:
A screen of the complete protein kinase gene family identifies
diverse patterns of somatic mutations in human breast cancer. Nat
Genet. 37:590–592. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ludwig JA and Weinstein JN: Biomarkers in
cancer staging, prognosis and treatment selection. Nat Rev Cancer.
5:845–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rubio-Viqueira B and Hidalgo M: Targeting
mTOR for cancer treatment. Adv Exp Med Biol. 587:309–327. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hudson CC, Liu M, Chiang GG, Otterness DM,
Loomis DC, Kaper F, Giaccia AJ and Abraham RT: Regulation of
hypoxia-inducible factor 1alpha expression and function by the
mammalian target of rapamycin. Mol Cell Biol. 22:7004–7014. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dancey JE: Therapeutic targets: MTOR and
related pathways. Cancer Biol Ther. 5:1065–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Thomas GV, Tran C, Mellinghoff IK, Welsbie
DS, Chan E, Fueger B, Czernin J and Sawyers CL: Hypoxia-inducible
factor determines sensitivity to inhibitors of mTOR in kidney
cancer. Nat Med. 12:122–127. 2006. View
Article : Google Scholar
|
|
117
|
Ahmadian MR: Prospects for anti-ras drugs.
Br J Haematol. 116:511–518. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Goodsell DS: The molecular perspective:
the ras oncogene. Oncologist. 4:263–264. 1999.PubMed/NCBI
|
|
119
|
Sawyers CL: Shifting paradigms: the seeds
of oncogene addiction. Nat Med. 15:1158–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hainaut P and Plymoth A: Targeting the
hallmarks of cancer: towards a rational approach to next-generation
cancer therapy. Curr Opin Oncol. 25:50–51. 2013. View Article : Google Scholar
|
|
121
|
Gonzalez de Castro D, Clarke PA,
Al-Lazikani B and Workman P: Personalized cancer medicine:
molecular diagnostics, predictive biomarkers, and drug resistance.
Clin Pharmacol Ther. 93:252–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Druker BJ: Imatinib mesylate in the
treatment of chronic myeloid leukaemia. Expert Opin Pharmacother.
4:963–971. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Stegmeier F, Warmuth M, Sellers WR and
Dorsch M: Targeted cancer therapies in the twenty-first century:
lessons from imatinib. Clin Pharmacol Ther. 87:543–552. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Bachman KE, Argani P, Samuels Y, Silliman
N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, et al:
The IK3CA gene is mutated with high frequency in human breast
cancers. Cancer Biol Ther. 3:772–775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Serra V, Markman B, Scaltriti M, Eichhorn
PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S,
et al: NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K
signaling and inhibits the growth of cancer cells with activating
PI3K mutations. Cancer Res. 68:8022–8030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Brose MS, Volpe P, Feldman M, Kumar M,
Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, et
al: BRAF and RAS mutations in human lung cancer and melanoma.
Cancer Res. 62:6997–7000. 2002.PubMed/NCBI
|
|
127
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al BRIM-3 Study Group: Improved survival with vemurafenib in
melanoma with BRAF V600E mutation. N Engl J Med. 364:2507–2516.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Young K, Minchom A and Larkin J: BRIM-1,
-2 and -3 trials: improved survival with vemurafenib in metastatic
melanoma patients with a BRAF(V600E) mutation. Future Oncol.
8:499–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al RECORD-1 Study Group: Efficacy of everolimus in
advanced renal cell carcinoma: a double-blind, randomised,
placebo-controlled phase III trial. Lancet. 372:449–456. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Abdurahman A, Anwar J, Turghun A, Niyaz M,
Zhang L and Awut I: Epidermal growth factor receptor gene mutation
status and its association with clinical characteristics and tumor
markers in non-small-cell lung cancer patients in Northwest China.
Mol Clin Oncol. 3:847–850. 2015.PubMed/NCBI
|
|
133
|
Ulivi P, Chiadini E, Dazzi C, Dubini A,
Costantini M, Medri L, Puccetti M, Capelli L, Calistri D, Verlicchi
A, et al: Nonsquamous, non-small-cell lung cancer patients who
carry a double mutation of EGFR, EML4-ALK or KRAS: frequency,
clinical-pathological characteristics, and response to therapy.
Clin Lung Cancer. 17:384–390. 2016. View Article : Google Scholar
|
|
134
|
Larkin J, Ascierto PA, Dréno B, Atkinson
V, Liszkay G, Maio M, Mandalà M, Demidov L, Stroyakovskiy D, Thomas
L, et al: Combined vemurafenib and cobimetinib in BRAF-mutated
melanoma. N Engl J Med. 371:1867–1876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Carvajal-Hausdorf DE, Schalper KA, Pusztai
L, Psyrri A, Kalogeras KT, Kotoula V, Fountzilas G and Rimm DL:
Measurement of domain-specific HER2 (ERBB2) expression may classify
benefit from trastuzumab in breast cancer. J Natl Cancer Inst.
107:djv1362015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Hasskarl J: Sorafenib: targeting multiple
tyrosine kinases in cancer. Recent Results Cancer Res. 201:145–164.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Aita Y, Ishii KA, Saito Y, Ikeda T,
Kawakami Y, Shimano H, Hara H and Takekoshi K: Sunitinib inhibits
catecholamine synthesis and secretion in pheochromocytoma tumor
cells by blocking VEGF receptor 2 via PLC-γ-related pathways. Am J
Physiol Endocrinol Metab. 303:E1006–E1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Demetri GD, van Oosterom AT, Garrett CR,
Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich
MC, Morgan JA, et al: Efficacy and safety of sunitinib in patients
with advanced gastrointestinal stromal tumour after failure of
imatinib: a randomised controlled trial. Lancet. 368:1329–1338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Czarnecka AM, Solarek W, Kornakiewicz A
and Szczylik C: Tyrosine kinase inhibitors target cancer stem cells
in renal cell cancer. Oncol Rep. 35:1433–1442. 2016.
|
|
140
|
Lankheet NA, Huitema AD, Mallo H,
Adriaansz S, Haanen JB, Schellens JH, Beijnen JH and Blank CU: The
effect of seasonal variation and secretion of sunitinib in sweat on
the development of hand-foot syndrome. Eur J Clin Pharmacol.
69:2065–2072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Axelsson J, Rippe A and Rippe B: mTOR
inhibition with temsirolimus causes acute increases in glomerular
permeability, but inhibits the dynamic permeability actions of
puromycin aminonucleoside. Am J Physiol Renal Physiol.
308:F1056–F1064. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wan X, Shen N, Mendoza A, Khanna C and
Helman LJ: CCI-779 inhibits rhabdomyosarcoma xenograft growth by an
antiangiogenic mechanism linked to the targeting of
mTOR/Hif-1alpha/VEGF signaling. Neoplasia. 8:394–401. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, et al: Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol. 20:719–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Cutillas PR: Role of phosphoproteomics in
the development of personalized cancer therapies. Proteomics Clin
Appl. 9:383–395. 2015. View Article : Google Scholar
|
|
145
|
Klempner SJ, Myers AP and Cantley LC: What
a tangled web we weave: emerging resistance mechanisms to
inhibition of the phosphoinositide 3-kinase pathway. Cancer Discov.
3:1345–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Robin X, Creixell P, Radetskaya O, Santini
CC, Longden J and Linding R: Personalized network-based treatments
in oncology. Clin Pharmacol Ther. 94:646–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Elkabets M, Vora S, Juric D, Morse N,
Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH,
et al: mTORC1 inhibition is required for sensitivity to PI3K110α
inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med.
5:196ra992013. View Article : Google Scholar
|
|
148
|
Straussman R, Morikawa T, Shee K,
Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J,
Frederick DT, et al: Tumour micro-environment elicits innate
resistance to RAF inhibitors through HGF secretion. Nature.
487:500–504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Murray BW and Miller N: Durability of
kinase-directed therapies - a network perspective on response and
resistance. Mol Cancer Ther. 14:1975–1984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Daub H, Specht K and Ullrich A: Strategies
to overcome resistance to targeted protein kinase inhibitors. Nat
Rev Drug Discov. 3:1001–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yoshida T, Zhang G and Haura EB: Targeting
epidermal growth factor receptor: central signaling kinase in lung
cancer. Biochem Pharmacol. 80:613–623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Balius TE and Rizzo RC: Quantitative
prediction of fold resistance for inhibitors of EGFR. Biochemistry.
48:8435–8448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Dixit A and Verkhivker GM: Hierarchical
modeling of activation mechanisms in the ABL and EGFR kinase
domains: thermodynamic and mechanistic catalysts of kinase
activation by cancer mutations. PLOS Comput Biol. 5:e10004872009.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Yun CH, Mengwasser KE, Toms AV, Woo MS,
Greulich H, Wong KK, Meyerson M and Eck MJ: The T790M mutation in
EGFR kinase causes drug resistance by increasing the affinity for
ATP. Proc Natl Acad Sci USA. 105:2070–2075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Solit DB, Garraway LA, Pratilas CA, Sawai
A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, et al: BRAF
mutation predicts sensitivity to MEK inhibition. Nature.
439:358–362. 2006. View Article : Google Scholar
|
|
156
|
Denis MG, Vallée A and Théoleyre S: EGFR
T790M resistance mutation in non small-cell lung carcinoma. Clin
Chim Acta. 444:81–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Niederst MJ and Engelman JA: Bypass
mechanisms of resistance to receptor tyrosine kinase inhibition in
lung cancer. Sci Signal. 6:re62013. View Article : Google Scholar : PubMed/NCBI
|