Introduction
Ionizing radiation (IR) carries energy strong enough
to ionize atoms and molecules, and break chemical bonds.
Radiotherapy is a widely used antitumor strategy and accounts for
25% of cancer therapy (1).
However, IR can break important biomolecules, such as DNA, damaging
or killing the affected cell (2).
Apart from its anti-proliferative and cell-killing effects in tumor
tissue, radiotherapy provokes severe damage to normal tissue
(3,4).
The mechanisms of IR-induced tissue damage are
complex; however, one of the main mechanisms is DNA damage-related,
and another is DNA damage-unrelated (4). Downstream pathways from those two
starts are cross-linked, forming an IR-induced signaling network
(5). This network involves two
major terminals: apoptosis and inflammation (6–8).
The p53-mediated apoptotic pathway is the most acclaimed mechanism
of radiation-induced damage (9).
Protein 53 (or tumor protein 53) is crucial in multicellular
organisms, functioning as a tumor suppressor involved in the
prevention of cancer (10,11).
B-cell lymphoma 2 (Bcl-2) is the founding member of the Bcl-2
family of regulator proteins that regulate cell death and apoptosis
(12–14). Human p53 can activate the
apoptotic effectors, BAX or BAK, resulting in mitochondrial
outer-membrane permeabilization and apoptosis, which can be opposed
by the anti-apoptotic protein, Bcl-2 (15,16).
AKT is a serine/threonine-specific protein kinase
that plays a key role in multiple cellular processes, such as
apoptosis and cell proliferation. AKT activation has been
recognized as a crucial player in IR-induced apoptosis (17,18). A previous study demonstrated that
mice with homozygous disruption of AKT exhibited growth retardation
and increased spontaneous apoptosis in tissues, such as the thymus
(19). Silent mating type
information regulator 2 homolog 1 (sirtuin 1), a histone
deacetylase, has various biological activities, including the
extension of lifespan (20). A
recent study demonstrated that sirtuin 1 plays an essential role in
the regulation of AKT activation (21). Furthermore, it has been reported
that the upregulation of sirtuin 1 is closely linked to AKT
activation (22).
Poly(ADP-ribose) polymerase (PARP) is a family of
proteins which play a role in a number of cellular processes
involving mainly DNA repair and programmed cell death (23). Activated PARP can deplete the ATP
of a cell in an attempt to repair the damaged DNA. ATP depletion in
a cell leads to lysis and cell death (necrosis) (24). In response to DNA damage caused by
IR, PARP binds to strand interruptions in DNA and undergoes rapid
auto-modification (25–28).
Simvastatin, a member of the statin (or HMG-CoA
reductase inhibitors) class, is a lipid-lowering drug used to
control elevated cholesterol, or hypercholesterolemia. It has been
reported that statins exert pleiotropic effects on cellular stress
responses, proliferation and apoptosis both in vitro and
in vivo (29,30). Our previous study found that
simvastatin significantly ameliorated IR-induced morphological
damage and apoptosis in the mouse jejunum and bone marrow (31). Notably, simvastatin also
significantly attenuated IR-induced apoptosis in the mouse thymus.
Individuals exposed to radiation exhibit a significant loss of
peripheral immune cells (7).
Since the thymus is a vital organ in the immune system, we selected
the thymus to be the focus of the present study.
In this study, we examined whether simvastatin
protects the mouse thymus from IR-induced damage in vivo and
in vitro, and explored the possible mechanisms underlying
the radioprotective effects of simvastatin.
Materials and methods
Mice and thymocytes
Male (n=40) C57BL/6J mice (Sino-British Sippr/BK
Laboratory, Shanghai, China), 5–7 weeks of age and weighing 18–22
g, were used for all animal experiments. The mice, housed in a
local animal housing facility under controlled conditions
(temperature, 21±2°C; lighting, 8:00–20:00), received a standard
mouse chow and tap water ad libitum. All animals received
humane care, and the experimental procedures were in compliance
with the institutional animal care guidelines. All the experiments
were performed in a random and blind manner. All the experiments
were approved by the local animal management and ethics committee.
Thymocytes were isolated from C57BL/6 mice and cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 6%
fetal bovine serum (FBS) and 25 mM HEPES (pH 7.2) and adjusted to a
density of 1×106/well for incubation at 37°C. The
relative amounts (approximately 85%) of CD4+
CD8+ thymocytes were determined at various times by FACS
analysis.
Experimental groups
Simvastatin was purchased from Sigma-Aldrich
(Shanghai, China). The mice were randomly separated into 4 groups
with 10 mice in each group as follows: the control (C), simvastatin
(S), radiation (R) and radiation + simvastatin (RS) group.
Simvastatin (20 mg/kg/day) was pre-administrated in gavage for 14
consecutive days in the S and RS groups, while mice in C and R
groups were administered the vehicle (0.5% CMC Na). The mice in the
R and RS groups endured 4 Gy 60Co γ-radiation. On 1, 3
and 7 days following radiation, the mice were sacrificed for the
analysis of IR-induced thymus damage and the expression of related
targets in the thymus. For cell analysis, the thymocytes were
isolated as previously described (9). Briefly, thymocytes were isolated
from a mouse aged 6 weeks in DMEM supplemented with 5% FBS and 25
mM HEPES (pH 7.2) and adjusted to a density of 1×106/ml.
Simvastatin at 20 mM was added to the thymocytes 3 h prior to
exposure to 8 Gy 60Co γ-radiation.
Morphological examination
Following sacrifice, the jejuna and femurs of the
mice were immersed in a 4% solution of paraformaldehyde in
phosphate-buffered saline (PBS) and were fixed in this solution for
24 h. The obtained jejunum segments were dehydrated in serial
alcohol solutions, while the femurs were decalcified using
Calci-Clear Rapid (SG HS-105; National Diagnostics, Atlanta, GA,
USA). Tissues were embedded in paraffin, cut into 5-µm-thick
sections, and stained with hematoxylin and eosin (H&E) for
light microscopic investigation (IX-71; Olympus, Tokyo, Japan).
These sections were subsequently used to determine villus length
(mm). The length was calculated as follows: the value measured
using a ruler was divided by the magnification of the image. One
slide per mouse was used and the average values were taken for a
minimum of 5 villi.
Weight measurement of thymi and
spleens
Following sacrifice, the thymi and spleens were
extracted from the mice. The weight of each organ was measured
using an analytic balance (ALB-224, Acculab) and recorded. The
relative organ weight was calculated as the absolute weight divided
by body weight of the corresponding mouse. The fold of control in
each group was then attained for statistical analysis.
Terminal
deoxynucleotidyltransferase-mediated dUTP nick-end labeling (TUNEL)
assay
Apoptosis in the thymus was analyzed by TUNEL
staining in accordance with the instructions of an in situ
cell death detection kit (Roche, Mannheim, Germany). In brief,
paraffin-embedded sections of the tissues were first deparaffinized
in gradient xylene/ethanol and digested for 15 min with 20
µg/ml proteinase K in 10 mM Tris-HCl buffer (pH 7.4). The
samples were then incubated with terminal deoxyribonucleotidyl
transferase enzyme (4810-30-05; R&D Systems Minneapolis, MN,
USA) followed by anti-digoxigenin conjugated to horseradish
peroxidase (NEF832001EA; PerkinElmer, Shanghai, China). Apoptotic
cells were recognized as those with brown-stained nuclei.
Transmission electron microscopy
(TEM)
For electron microscopy, the tissues were fixed as
described above and then post-fixed with osmium tetraoxide,
dehydrated in a graded ethanol series and embedded in epoxy resin.
Samples were sectioned (50 nm), counterstained with uranyl acetate
and lead citrate and observed under a Hitachi H-800 Transmission
Electron Microscope (Hitachi, Tokyo, Japan).
Flow cytometric analysis
The effect of simvastatin on the apoptosis of
thymocytes was determined by Annexin V-FITC/PI assay in accordance
with the manufacturer's instructions provided with the Apoptosis
Detection kit (BD Biosciences, San Jose, CA, USA). In brief,
thymocytes were incubated with simvastatin (20 µM) or
vehicle (DMSO) for 3 h followed by exposure to 60Co
γ-radiation of 8 Gy. At 1, 6 and 24 h following exposure, the
thymocytes were harvested and washed twice with PBS. Following the
addition of FITC-Annexin V (5 µl) and PI (5 µl)
working solutions to the cells, the cell suspension were then
incubated at room temperature for 15 min in the dark and then
analyzed using a flow cytometer (FACSCalibur; BD Biosciences).
Protein extraction and western blot
analysis
Tissue/cell protein was extracted as previously
described (34). The protein
concentration was determined using a BCA Protein assay kit
(Beyotime Institute of Biotechnology, Shanghai, China). Samples of
approximately 30 µg were run on 10 or 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins
were electro-transferred onto nitrocellulose (NC) filter membranes.
After blocking, the membranes were incubated with the corresponding
primary antibodies for 2 h at room temperature. The anti-bodies
used were as follows: AKT (#9272), p53 (1C12 #2524), PARP (46D11
#9532) were obtained from Cell Signaling Technology; Bcl-2 (C-2
sc-7382) antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). p-p53 (Ser15 AP068), GAPDH (AG019) and
tubulin (AT819) antibodies were obtained from the Beyotime
Institute of Biotechnology. Sirtuin 1 (ab75435) antibody used was
purchased from Abcam (Cambridge, MA, USA). The membranes were then
incubated with IRDye 800CW-conjugated goat anti-rabbit/mouse
secondary antibody (1:5,000; Rockland, Gaithersburg, MD, USA) for 1
h at 25°C. The infrared fluorescence image was obtained using an
Odyssey infrared imaging system (Li-Cor Bioscience, Lincoln, NE,
USA) and the bands were quantified using Quantity One software
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
Values are presented as the means ± SEM. One-way
ANOVA was adopted for multiple comparisons involving more than 3
groups, and post hoc comparisons were performed using Neuman-Keuls
test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Simvastatin significantly inhibits
IR-induced apoptosis in the mouse intestine, bone marrow and
thymus
Our previous study reported that IR induced severe
damage in the intestines and bones of mice (31). In this study, we found that the
damage induced to the intestines and bones of mice by IR was
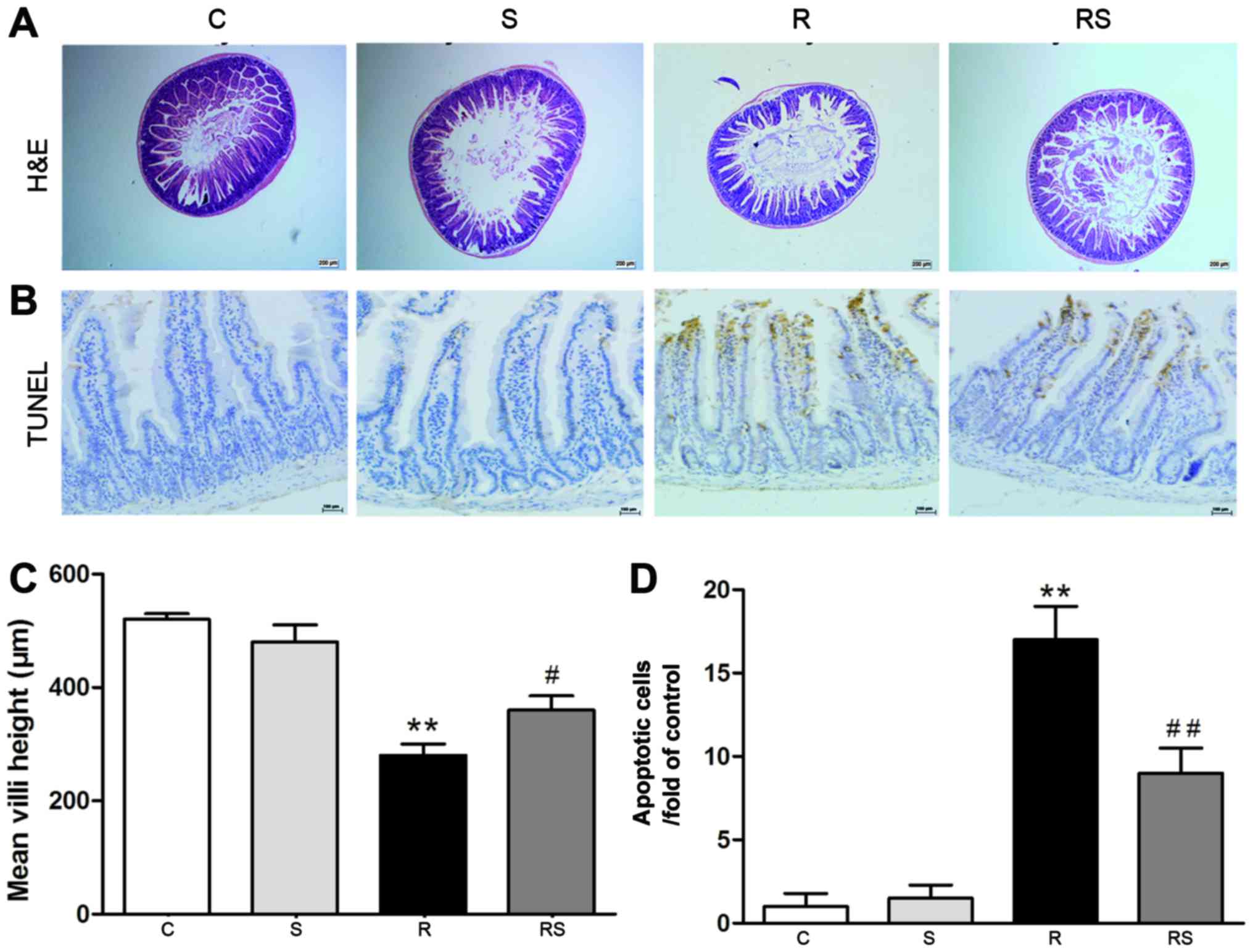
mitigated by simvastatin administration [mean height of villi: C,
520±10 µm; S, 480±30 µm; R, 280±20 µm; RS,
360±25 µm (Fig. 1A and C);
intestinal apoptotic cells/fold of control: C, 1.00±0.80; S,
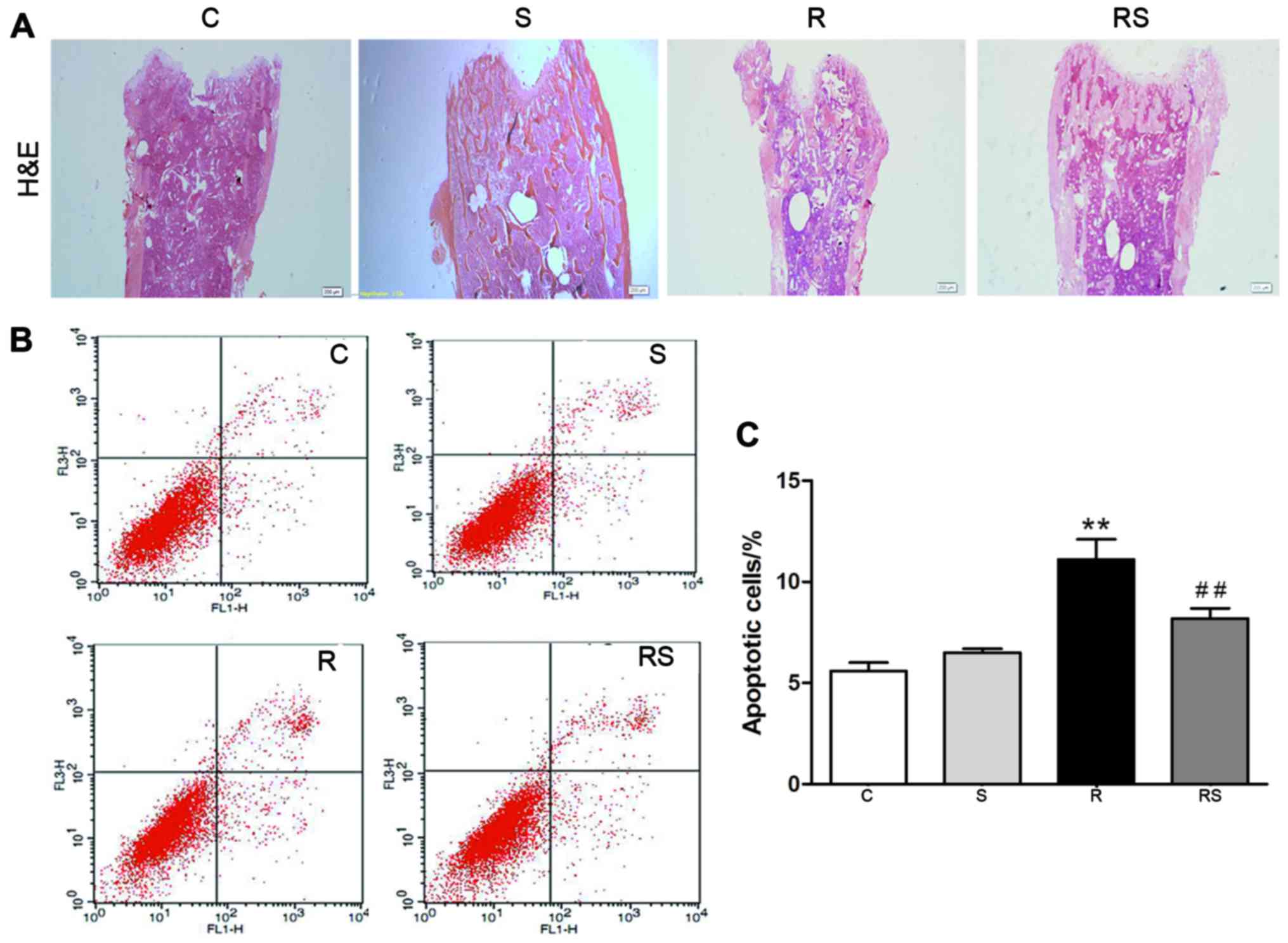
1.50±0.80; R, 17.00±2.00; RS, 9.00±1.50 (Fig. 1B and D); bone marrow apoptotic
cells/%: C, 5.60±0.40; S, 6.50±0.20; R, 11.10±1.00; RS, 8.20±0.50;
P<0.01 (Fig. 2)].
Cancer patients who undertake radiotherapy could
have a compromised function of the immune system (7). Thus, preserving thymus function may
be substantially beneficial. In this study, to evaluate the effects
of simvastatin on IR-induced damage in the immune system, we
examined the change in weight of the thymi and spleens, as well as
morphological changes and apoptosis in the mouse thymus 7 days
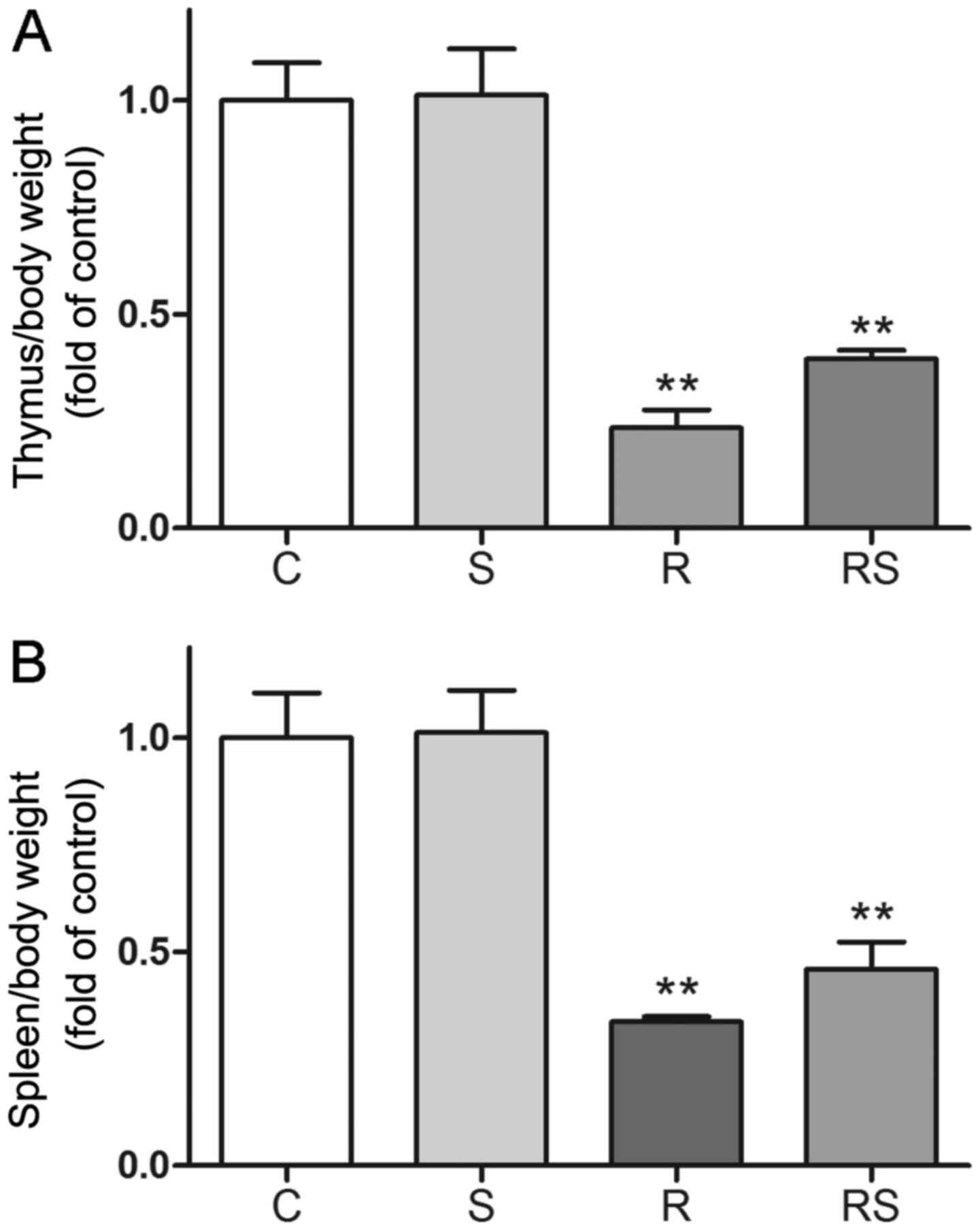
following exposure to IR. IR induced severe weight loss in the
spleens and thymi of the mice, as shown in Fig. 3. In addition, the administration
of simvastatin slightly alleviated the organ weight loss without
statistical significance compared to the controls [thymus/body
weight/fold of control: C, 1.00±0.08; S, 1.01±0.10; R, 0.23±0.04;
RS, 0.40±0.02; P<0.01 vs. control, P>0.05 vs. radiation;
spleen/body weight/fold of control: C, 1.00±0.10; S, 1.01±0.10; R,
0.34±0.01; RS, 0.46±0.06; P<0.01 vs. control, P>0.05 vs.
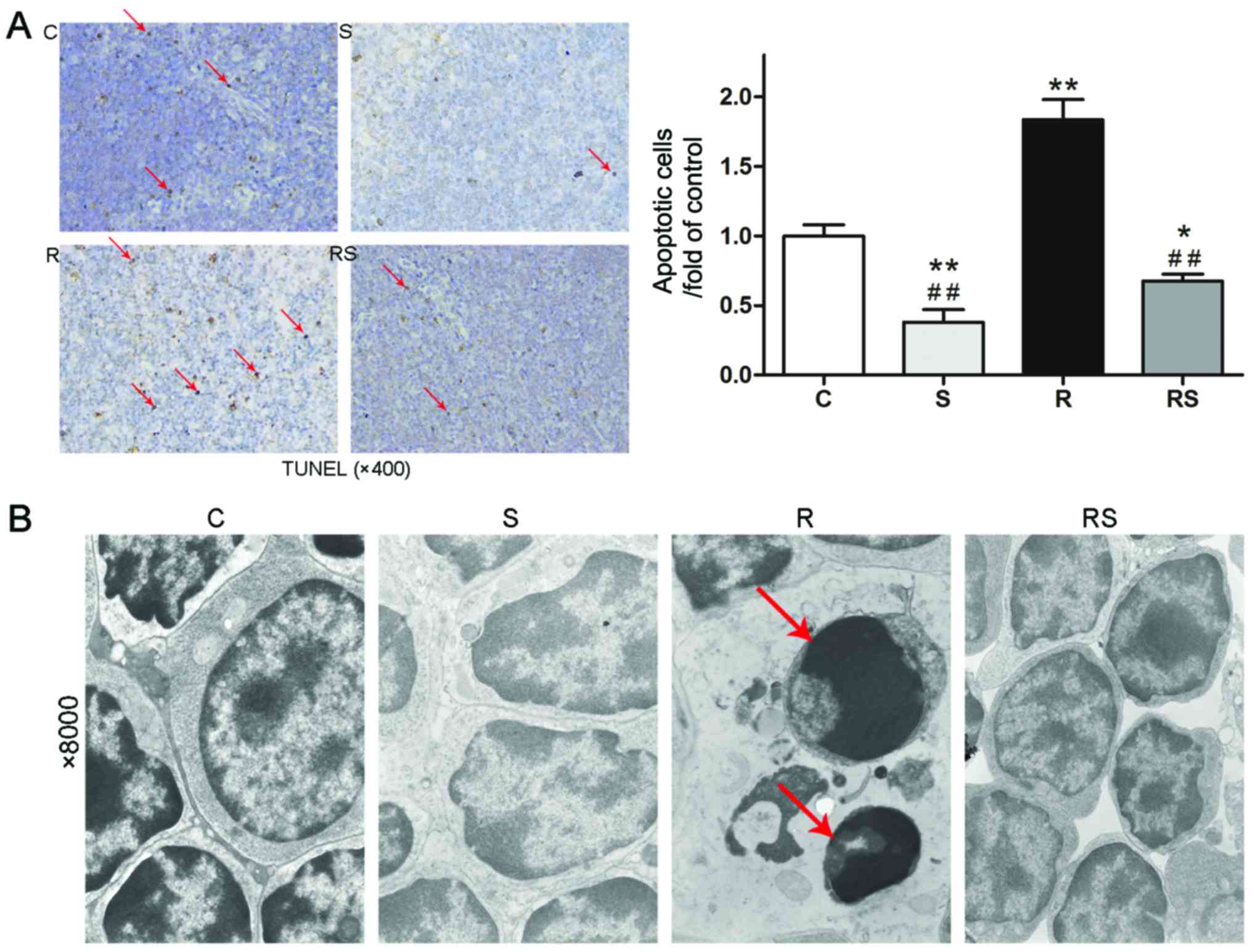
radiation (Fig. 3)]. However,
TUNEL staining and TEM examination of the mouse thymus indicated
that IR induced severe cell apoptosis in the mouse thymus, which
was significantly attenuated by pre-treatment with simvastatin
[apoptotic cells/fold of control: C, 1.00±0.19; S, 0.38±0.22; R,
1.84±0.36; RS, 0.68±0.12; P<0.01 (Fig. 4A)].
Radioprotective effects of simvastatin in
vivo are related to the AKT/sirtuin 1, and p53/Bcl-2 and PARP
pathways
To elucidate the mechanisms through which
simvastatin protects the mouse thymus from IR-induced apoptosis, we
examined the expression of p53/Bcl-2, AKT/sirtuin 1 and PARP. It
was found that simvastatin alone did not affect the expression of
p53/Bcl-2, AKT/sirtuin 1 and PARP compared with the controls. Of
note, 3 days following radiation exposure, p53 expression and
phosphorylation significantly increased, and the expression levels
of Bcl-2, AKT, sirtuin 1 and PARP were significantly decreased.
Importantly, simvastatin treatment significantly inhibited p53
expression and phosphorylation, and increased Bcl-2, AKT, sirtuin 1
and PARP expression in the mice exposed to IR [AKT expression 3
days after radiation/fold of control: C, 1.00±0.15; S, 0.95±0.10;
R, 0.30±0.11; RS, 0.53±0.13; p53 expression 3 days after
radiation/fold of control: C, 1.00±0.29; S, 1.15±0.12; R,
2.19±0.13; RS, 1.43±0.13; PARP expression 3 days after
radiation/fold of control: C, 1.00±0.31; S, 0.97±0.23; R,
0.04±0.01; RS, 0.41±0.21; P<0.01; quantitative data of protein
expression at day 1 and day 7 are not shown (Fig. 5)].
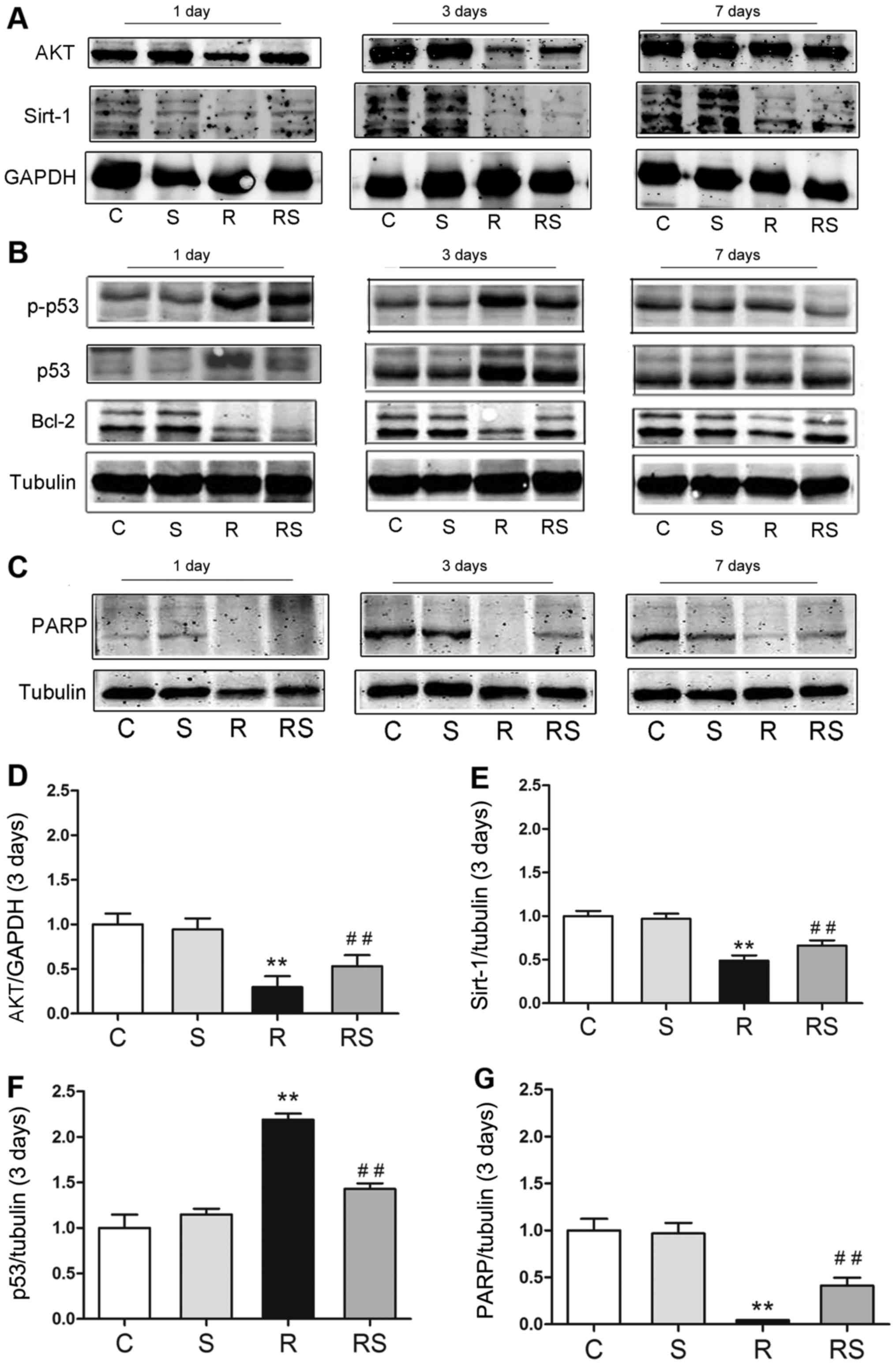 | Figure 5Radioprotective effects of
simvastatin on mouse thymi after radiation are related to p53
expression/phosphorylation (B) and AKT/sirtuin 1 (A),
poly(ADP-ribose) polymerase (PARP) expression (C) in vivo.
(D–G) The quantitative results of AKT, sirtuin 1, p53 and PARP
expression 3 days after radiation are shown. At 3 days after
irradiation, the expression of (D) AKT, (E) sirtuin 1 and (G) PARP
was significantly decreased in the radiated mouse thymus, while
simvastatin significantly augmented their expression. (F) Compared
to the radiated group, p53 expression was significantly suppressed
by simvastatin (F). Values are the means ± SEM. C, control; S,
simvastatin; R, ionizing radiation; radiation + simvastatin (RS),
IR + simvastatin; n=6, **P<0.01 vs. C group;
##P<0.01 vs. R group (One-way ANOVA, Newman-Keuls
multiple comparison test). |
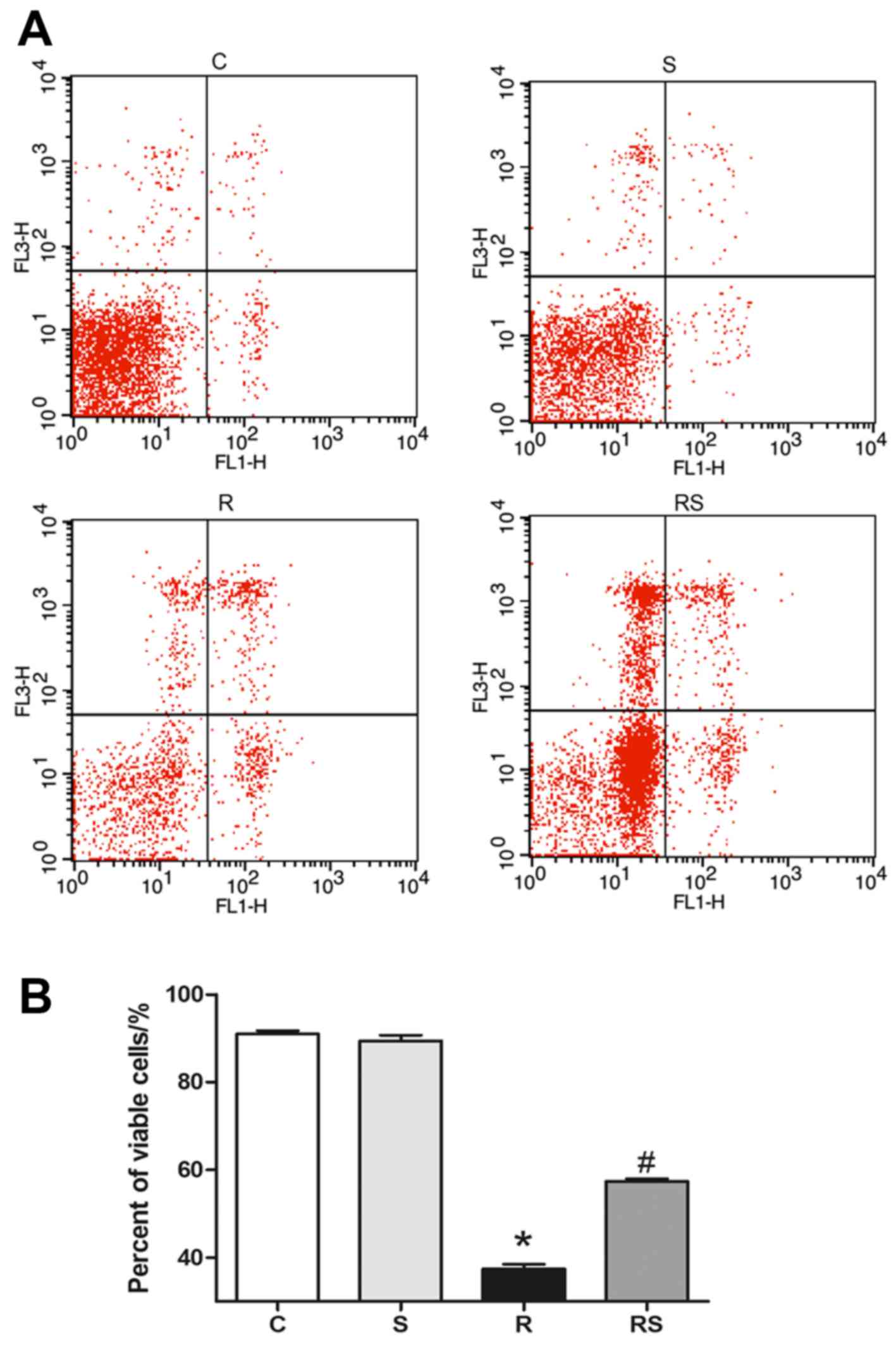
Simvastatin significantly inhibits
IR-induced apoptosis of thymocytes in vitro
We adopted Annexin V/PI flow cytometry assay to
evaluate thymocyte apoptosis following exposure to IR. It was found
that the percentage of viable thymocytes significantly decreased 24
h after radiation compared to the control, and simvastatin
treatment significantly ameliorated radiation-induced apoptosis in
thymocytes cultured in vitro [percentage of viable cells: C,
91.00±1.77; S, 89.44±3.04; R, 37.45±2.57; RS, 57.37±1.49; P<0.01
(Fig. 6)].
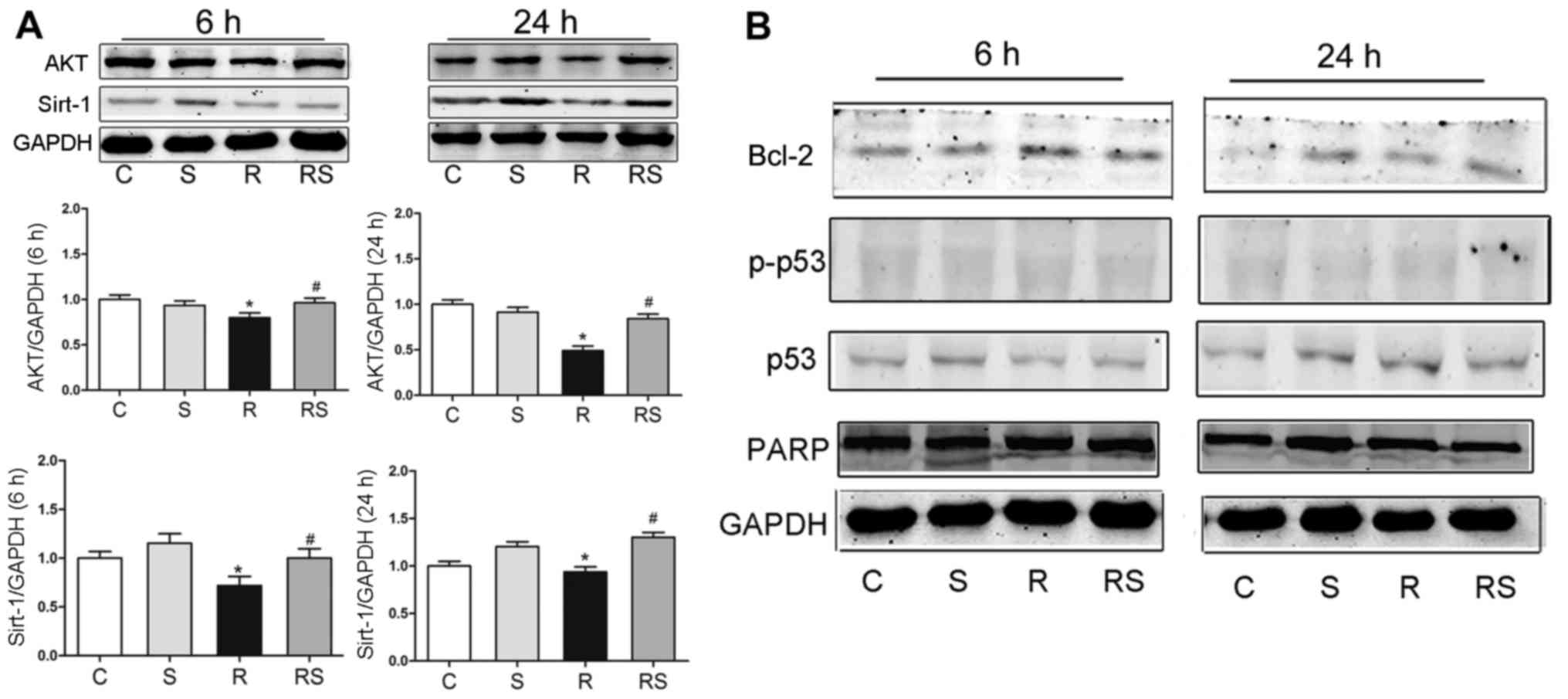
Radioprotective effects of simvastatin on
thymocytes are related to the AKT/sirtuin 1 pathway in vitro
To elucidate the mechanisms through which
simvastatin protected the thymocytes from radiation damage in
vitro, we examined the expression of p53/Bcl-2, AKT/sirtuin 1
and PARP at 6 and 24 h following expo-sure to IR by western blot
analysis. It was found that at 6 and 24 h after radiation, AKT
expression significantly decreased compared to the control, and
simvastatin significantly elevated the AKT level compared to the
radiation group. The Sirtuin 1 level was significantly lower at 6
and 24 h when compared to the control, and simvastatin
significantly augmented sirtuin 1 expression when compared to the
radiation group [AKT expression at 6 h after radiation: C,
1.00±0.04; S, 0.94±0.05; R, 0.80±0.03; RS, 0.96±0.05; AKT
expression 24 h after radiation: C, 1.00±0.07; S, 0.92±0.09; R,
0.49±0.03; RS 0.84±0.06; sirtuin 1 expression 6 h after radiation:
C, 1.00±0.70; S, 1.15±0.10; R, 0.72±0.11; RS, 1.00±0.13; sirtuin 1
expression 24 h after radiation: C, 1.00±0.04; S, 1.20±0.07; R,
0.94±0.09; RS, 1.30±0.11; P<0.01 (Fig. 7)]. However, simvastatin treatment
did not significantly alter the expression of Bcl-2, p53,
phosphorylated p53 and PARP after radiation exposure (quantitative
data not shown).
Discussion
In the present study, the radioprotective effects of
pre-treatment with simvastatin were investigated in the mouse
thymus and isolated thymocytes cultured in vitro. The main
finding was that pre-treatment with simvastatin ameliorated
radiation-induced damage to the thymus in vivo and in
vitro, which was possibly related to the activation of the
AKT/sirtuin 1 pathway.
Statins have been reported to exert pleiotropic
effects apart from lowering cholesterol. In particular, statins
have been shown to be effective in protecting normal tissue from
radiation-induced damage (1,31–34). Ostrau et al reported that
lovastatin attenuated IR-induced normal tissue damage in
vivo (35). We previously
reported that simvastatin attenuated radiation-induced tissue
damage in mice (31). Mathew
et al demonstrated that simvastatin attenuated
radiation-induced murine lung injury and dysregulated lung gene
expression (32).
Lowe et al demonstrated that p53 was required
for the radiation-induced apoptosis of mouse thymocytes (9). The activation of p53 facilitates
apoptosis, autophagy etc. in response to radiation (36–38). The present study showed that the
levels of both p53 and p-p53 were significantly increased in mice
following exposure to radiation. Radiation increases cell death,
DNA fragmentation, downregulates Bcl-2 and upregulates
Bcl-2-associated X protein (Bax) (39, 40). It has been reported that
radiation-induced DNA double-strand breaks can promote p53
activation, and Bcl-2-overexpressing cells have a higher survival
rate (41). In neoplasms,
mutation of the p53 tumor suppressor or overexpression of
pro-survival Bcl-2, is a key step toward malignant transformation
and therapeutic resistance (42).
It has been demonstrated that reducing the affinity of p53 to the
anti-apoptotic protein, Bcl-2, reduces the radiosensitivity of
normal tissue (43). The present
study found that simvastatin not only inhibited p53 expression and
activation, but also increased the Bcl-2 level. These findings
demonstrate that the radioprotective effects of simvastatin are at
least partially related to the p53/Bcl-2 pathway in mice.
AKT signaling regulates cellular processes,
including proliferation, invasion and apoptosis (44). AKT suppresses DNA damage
processing and checkpoint activation in late the G2 phase after
radiation (45). Edwards et
al demonstrated that AKT in the tumor vascular endothelium
plays an important role in enhancing the efficacy of IR (18). An in vitro study indicated
that the activation of AKT results in reduced Bax translocation to
the mitochondria, inhibiting apoptosis (46). Sirtuin 1 is an important regulator
of radiation-induced cellular senescence (47). It has been demonstrated that
resveratrol, an activator of sirtuin 1, inhibits apoptosis induced
by radiation by effectively antagonizing oxidation (48). In addition, recent studies
demonstrate that targeted silencing of sirtuin 1 expression may be
beneficial by promoting p53-induced apoptosis in cancer cells, and
by sensitizing cancerous cells to radiation therapy (49). In this study, we found that the
expression of AKT and sirtuin 1 was decreased by radiation and
augmented by simvastatin pre-treatment in the mouse thymus and in
cultured thymocytes. In addition, AKT has been reported to be
related to sirtuin 1. Gao et al reported that sirtuin 1 was
upregulated through the AKT signaling pathway in the proliferation
and migration of endothelial cells (50). It has also been found that the
PI3K-AKT-GSK3β signaling pathway is required for sirtuin 1
induction by endoplasmic reticulum stress (51). However, Ge et al showed
that sirtuin 1 knockdown attenuated PPAR-γ dependent AKT activation
in HepG2 cells (52). In summary,
it is suggested that AKT/sirtuin 1 may be involved in the
radioprotective effects of simvastatin.
PARP is also closely linked to apoptosis. Jeffrey
et al demonstrated that the inhibition of PARP enhanced the
efficacy of radiation (26).
Bryant et al and others have also reviewed the potential of
PARP inhibitors as antitumor agents (28,53). PARP-1 activity is essential in the
upstream regulation of radiation-induced NF-κB activation (27). Consistent with this, the present
study demonstrated that simvastatin upregulated PARP expression in
the thymi of mice exposed to radiation.
Our in vitro experiments also found that
simvastatin treatment significantly increased the survival of
thymocytes exposed to radiation. In this study, we did not find
that simvastatin treatment altered p53/Bcl-2 and PARP expression in
cultured thymocytes. IR induces complex physiological signaling
modifications. However, the above-mentioned reaction was dependent
of the whole-function intactness of the organism exposed to
irradiation. The results of the present study showed that IR
significantly altered AKT/sirtuin 1 expression, but not that of
p53, Bcl-2 and PARP, implying that the AKT/sirtuin 1 signaling
pathway is dominant in the in vitro post-irradiation
reaction. Yu and Little from the Harvard School of Public Health
also proved that in three human lymphoblast cell lines, the
p53/Bcl-2 pathway was not required for IR-induced apoptosis
(54), which coincided with our
findings.
In conclusion, the present study demonstrated that
simvastatin pre-treatment attenuated radiation-induced damage to
the thymus in mice possibly by activating the AKT/sirtuin 1
pathway.
Acknowledgments
The present study was supported by the National
Major Special Foundation of China (no. 2013ZX09J13110-07B).
References
|
1
|
Wang J, Boerma M, Fu Q, Kulkarni A, Fink
LM and Hauer-Jensen M: Simvastatin ameliorates radiation
enteropathy development after localized, fractionated irradiation
by a protein C-independent mechanism. Int J Radiat Oncol Biol Phys.
68:1483–1490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Christiansen H, Saile B, Neubauer-Saile K,
Tippelt S, Rave-Fränk M, Hermann RM, Dudas J, Hess CF, Schmidberger
H and Ramadori G: Irradiation leads to susceptibility of
hepatocytes to TNF-alpha mediated apoptosis. Radiother Oncol.
72:291–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stewart FA and Dörr W: Milestones in
normal tissue radiation biology over the past 50 years: From
clonogenic cell survival to cytokine networks and back to stem cell
recovery. Int J Radiat Biol. 85:574–586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolesnick R and Fuks Z: Radiation and
ceramide-induced apoptosis. Oncogene. 22:5897–5906. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okunieff P, Chen Y, Maguire DJ and Huser
AK: Molecular markers of radiation-related normal tissue toxicity.
Cancer Metastasis Rev. 27:363–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim Y and He YY: Ultraviolet
radiation-induced non-melanoma skin cancer: Regulation of DNA
damage repair and inflammation. Genes Dis. 1:188–198. 2014.
View Article : Google Scholar
|
|
7
|
Najafi M, Fardid R, Hadadi G and Fardid M:
The mechanisms of radiation-induced bystander effect. J Biomed Phys
Eng. 4:163–172. 2014.
|
|
8
|
Stoecklein VM, Osuka A, Ishikawa S,
Lederer MR, Wanke-Jellinek L and Lederer JA: Radiation exposure
induces inflammasome pathway activation in immune cells. J Immunol.
194:1178–1189. 2015. View Article : Google Scholar :
|
|
9
|
Lowe SW, Schmitt EM, Smith SW, Osborne BA
and Jacks T: p53 is required for radiation-induced apoptosis in
mouse thymocytes. Nature. 362:847–849. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
May P and May E: Twenty years of p53
research: Structural and functional aspects of the p53 protein.
Oncogene. 18:7621–7636. 1999. View Article : Google Scholar
|
|
11
|
McBride OW, Merry D and Givol D: The gene
for human p53 cellular tumor antigen is located on chromosome 17
short arm (17p13). Proc Natl Acad Sci USA. 83:130–134. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tjalma WA, Weyler JJ, Bogers JJ,
Pollefliet C, Baay M, Goovaerts GC, Vermorken JB, van Dam PA, van
Marck EA and Buytaert PM: The importance of biological factors
(Bcl-2, bax, p53 PCNA, MI, HPV and angiogenesis) in invasive
cervical cancer. Eur J Obstet Gynecol Reprod Biol. 97:223–230.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cleary ML, Smith SD and Sklar J: Cloning
and structural analysis of cDNAs for Bcl-2 and a hybrid
Bcl-2/immunoglobulin transcript resulting from the t(14;18)
translocation. Cell. 47:19–28. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsujimoto Y, Finger LR, Yunis J, Nowell PC
and Croce CM: Cloning of the chromosome breakpoint of neoplastic B
cells with the t(14;18) chromosome translocation. Science.
226:1097–1099. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adán N, Guzmán-Morales J, Ledesma-Colunga
MG, Perales-Canales SI, Quintanar-Stéphano A, López-Barrera F,
Méndez I, Moreno-Carranza B, Triebel J, Binart N, et al: Prolactin
promotes cartilage survival and attenuates inflammation in
inflammatory arthritis. J Clin Invest. 123:3902–3913. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Follis AV, Llambi F, Ou L, Baran K, Green
DR and Kriwacki RW: The DNA-binding domain mediates both nuclear
and cytosolic functions of p53. Nat Struct Mol Biol. 21:535–543.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li HF, Kim JS and Waldman T:
Radiation-induced Akt activation modulates radioresistance in human
glioblastoma cells. Radiat Oncol. 4:432009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edwards E, Geng L, Tan J, Onishko H,
Donnelly E and Hallahan DE: Phosphatidylinositol 3-kinase/Akt
signaling in the response of vascular endothelium to ionizing
radiation. Cancer Res. 62:4671–4677. 2002.PubMed/NCBI
|
|
19
|
Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol
K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al: Growth
retardation and increased apoptosis in mice with homozygous
disruption of the Akt1 gene. Genes Dev. 15:2203–2208. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Zhang KY, Zhang P, Chen LX, Wang L,
Xie M, Wang CY and Tang XQ: Hydrogen sulfide inhibits
formaldehyde-induced endoplasmic reticulum stress in PC12 cells by
upregulation of SIRT-1. PLoS One. 9:e898562014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pillai VB, Sundaresan NR and Gupta MP:
Regulation of Akt signaling by sirtuins: Its implication in cardiac
hypertrophy and aging. Circ Res. 114:368–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Q, Sun X, Li X, Dong X, Li P and Zhao
L: Resveratrol attenuates intermittent hypoxia-induced insulin
resistance in rats: Involvement of Sirtuin 1 and the
phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT pathway. Mol Med
Rep. 11:151–158. 2015.
|
|
23
|
Isabelle M, Moreel X, Gagné JP, Rouleau M,
Ethier C, Gagné P, Hendzel MJ and Poirier GG: Investigation of
PARP-1, PARP-2, and PARG interactomes by affinity-purification mass
spectrometry. Proteome Sci. 8:222010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu SW, Andrabi SA, Wang H, Kim NS, Poirier
GG, Dawson TM and Dawson VL: Apoptosis-inducing factor mediates
poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad
Sci USA. 103:18314–18319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lindahl T, Satoh MS, Poirier GG and
Klungland A: Post-translational modification of poly(ADP-ribose)
polymerase induced by DNA strand breaks. Trends Biochem Sci.
20:405–411. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Albert JM, Cao C, Kim KW, Willey CD, Geng
L, Xiao D, Wang H, Sandler A, Johnson DH, Colevas AD, et al:
Inhibition of poly(ADP-ribose) polymerase enhances cell death and
improves tumor growth delay in irradiated lung cancer models. Clin
Cancer Res. 13:3033–3042. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Veuger SJ, Hunter JE and Durkacz BW:
Ionizing radiation-induced NF-kappaB activation requires PARP-1
function to confer radioresistance. Oncogene. 28:832–842. 2009.
View Article : Google Scholar
|
|
28
|
Bryant HE and Helleday T: Poly(ADP-ribose)
polymerase inhibitors as potential chemotherapeutic agents. Biochem
Soc Trans. 32:959–961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lopez-Pedrera C, Ruiz-Limon P,
Valverde-Estepa A, Barbarroja N and Rodriguez-Ariza A: To
cardiovascular disease and beyond: New therapeutic perspectives of
statins in autoimmune diseases and cancer. Curr Drug Targets.
13:829–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fritz G, Henninger C and Huelsenbeck J:
Potential use of HMG-CoA reductase inhibitors (statins) as
radioprotective agents. Br Med Bull. 97:17–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao X, Yang H, Jiang G, Ni M, Deng Y, Cai
J, Li Z, Shen F and Tao X: Simvastatin attenuates radiation-induced
tissue damage in mice. J Radiat Res (Tokyo). 55:257–264. 2014.
View Article : Google Scholar
|
|
32
|
Mathew B, Huang Y, Jacobson JR, Berdyshev
E, Gerhold LM, Wang T, Moreno-Vinasco L, Lang G, Zhao Y, Chen CT,
et al: Simvastatin attenuates radiation-induced murine lung injury
and dysregulated lung gene expression. Am J Respir Cell Mol Biol.
44:415–422. 2011. View Article : Google Scholar :
|
|
33
|
Lacerda L, Reddy JP, Liu D, Larson R, Li
L, Masuda H, Brewer T, Debeb BG, Xu W, Hortobágyi GN, et al:
Simvastatin radiosensitizes differentiated and stem-like breast
cancer cell lines and is associated with improved local control in
inflammatory breast cancer patients treated with postmastectomy
radiation. Stem Cells Transl Med. 3:849–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oh DS, Koontz B, Freedland SJ, Gerber L,
Patel P, Lewis S, Yoo DS, Oleson J and Salama JK: Statin use is
associated with decreased prostate cancer recurrence in men treated
with brachytherapy. World J Urol. 33:93–97. 2015. View Article : Google Scholar
|
|
35
|
Ostrau C, Hülsenbeck J, Herzog M, Schad A,
Torzewski M, Lackner KJ and Fritz G: Lovastatin attenuates ionizing
radiation-induced normal tissue damage in vivo. Radiother Oncol.
92:492–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi DW, Na W, Kabir MH, Yi E, Kwon S,
Yeom J, Ahn JW, Choi HH, Lee Y, Seo KW, et al: WIP1, a homeostatic
regulator of the DNA damage response, is targeted by HIPK2 for
phosphorylation and degradation. Mol Cell. 51:374–385. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Contreras AU, Mebratu Y, Delgado M,
Montano G, Hu CA, Ryter SW, Choi AM, Lin Y, Xiang J, Chand H, et
al: Deacetylation of p53 induces autophagy by suppressing Bmf
expression. J Cell Biol. 201:427–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie L, Pi X, Mishra A, Fong G, Peng J and
Patterson C: PHD3-dependent hydroxylation of HCLK2 promotes the DNA
damage response. J Clin Invest. 122:2827–2836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang XY, Ma ZC, Wang YG, Tan HL, Xiao CR,
Liang QD, Tang XL, Cheng Y and Gao Y: Tetramethylpyrazine protects
lymphocytes from radiation-induced apoptosis through nuclear
factor-κB. Chin J Nat Med. 12:730–737. 2014.PubMed/NCBI
|
|
41
|
Milyavsky M, Gan OI, Trottier M, Komosa M,
Tabach O, Notta F, Lechman E, Hermans KG, Eppert K, Konovalova Z,
et al: A distinctive DNA damage response in human hematopoietic
stem cells reveals an apoptosis-independent role for p53 in
self-renewal. Cell Stem Cell. 7:186–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sidi S, Sanda T, Kennedy RD, Hagen AT,
Jette CA, Hoffmans R, Pascual J, Imamura S, Kishi S, Amatruda JF,
et al: Chk1 suppresses a caspase-2 apoptotic response to DNA damage
that bypasses p53 Bcl-2, and caspase-3. Cell. 133:864–877. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Strom E, Sathe S, Komarov PG, Chernova OB,
Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM,
Skaliter R, et al: Small-molecule inhibitor of p53 binding to
mitochondria protects mice from gamma radiation. Nat Chem Biol.
2:474–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu N, Hegarat N, Black EJ, Scott MT,
Hochegger H and Gillespie DA: Akt/PKB suppresses DNA damage
processing and checkpoint activation in late G2. J Cell Biol.
190:297–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tessner TG, Muhale F, Riehl TE, Anant S
and Stenson WF: Prostaglandin E2 reduces radiation-induced
epithelial apoptosis through a mechanism involving AKT activation
and bax translocation. J Clin Invest. 114:1676–1685. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hong EH, Lee SJ, Kim JS, Lee KH, Um HD,
Kim JH, Kim SJ, Kim JI and Hwang SG: Ionizing radiation induces
cellular senescence of articular chondrocytes via negative
regulation of SIRT1 by p38 kinase. J Biol Chem. 285:1283–1295.
2010. View Article : Google Scholar :
|
|
48
|
Li J, Feng L, Xing Y, Wang Y, Du L, Xu C,
Cao J, Wang Q, Fan S, Liu Q, et al: Radioprotective and antioxidant
effect of resveratrol in hippocampus by activating Sirt1. Int J Mol
Sci. 15:5928–5939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nerurkar PV and Nerurkar VR: Can Sir(2)
regulate cancer? Cellscience. 4:50–56. 2008.PubMed/NCBI
|
|
50
|
Gao Z, Wang H, Xiao FJ, Shi XF, Zhang YK,
Xu QQ, Zhang XY, Ha XQ and Wang LS: SIRT1 mediates
Sphk1/S1P-induced proliferation and migration of endothelial cells.
Int J Biochem Cell Biol. 74:152–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Koga T, Suico MA, Shimasaki S, Watanabe E,
Kai Y, Koyama K, Omachi K, Morino-Koga S, Sato T, Shuto T, et al:
Endoplasmic Reticulum (ER) Stress Induces Sirtuin 1 (SIRT1)
Expression via the PI3K-Akt-GSK3β Signaling Pathway and Promotes
Hepatocellular Injury. J Biol Chem. 290:30366–30374. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ge Z, Zhang P, Hong T, Tang S, Meng R, Bi
Y and Zhu D: Erythropoietin alleviates hepatic insulin resistance
via PPARγ-dependent AKT activation. Sci Rep. 5:178782015.
View Article : Google Scholar
|
|
53
|
Kotsopoulos IC, Kucukmetin A, Mukhopadhyay
A, Lunec J and Curtin NJ: Poly(ADP-Ribose) polymerase in cervical
cancer pathogenesis: Mechanism and potential role for PARP
inhibitors. Int J Gynecol Cancer. 26:763–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu Y and Little JB: p53 is involved in but
not required for ionizing radiation-induced caspase-3 activation
and apoptosis in human lymphoblast cell lines. Cancer Res.
58:4277–4281. 1998.PubMed/NCBI
|