Introduction
Mesenchymal stem cells (MSCs) are multipotent
progenitor cells which are isolated from bone marrow (BM), adipose,
dental pulp, umbilical cord (UC), and UC blood (UCB) (1,2).
MSC administration provides a therapeutic benefit by their
recruitment to damaged organs where they are actively involved in
tissue repair (3–8). The beneficial effect is also
attributed to paracrine factors (9), such as cytoprotective effects
modulated by cytokines (10) and
pro-angiogenic and pro-arteriogenic effects (11). However, current MSC therapy has
limited therapeutic effectiveness, particularly due to poor in
vivo engraftment and survival of the transplanted cells
(12). Most (≥99%) intravenously
injected MSCs adhere to the lungs, and a mere 2–3% of them are
released into the circulation (13). Thus, understanding the precise
mechanism underlying the migration and engraftment of MSCs during
tissue repair is crucial.
Some chemokines [stromal cell-derived factor 1
(SDF-1)] and growth factors [e.g., vascular endothelial growth
factor (VEGF), platelet-derived growth factor (PDGF) or hepatocyte
growth factor (HGF)] play a crucial role in the mobilization and
engraftment of adult MSCs (14–19). Among them, SDF-1 and its receptor,
the CXCR4, is a pivotal factor in the homing/engraftment of stem
cells. Indeed, hematopoietic stem/progenitor cells (HSPCs) engraft
in the BM by following an SDF-1 gradient that is upregulated in the
BM after conditioning for transplantation (e.g., total body
irradiation and myeloablative chemotherapy) (14,18). The sensitivity/responsiveness of
HSPCs to a SDF-1 gradient is positively affected ('primed') by a
subset of molecules enriched in the damaged tissues including
bioactive lipids [e.g., sphingosine-1-phosphate (S1P) (20) and ceramide-1-phosphate (C1P)
(21,22), neutrophil-derived cationic peptide
cathelicidin (LL-37), β2-defensin, and soluble membrane attack
complex (sMAC) C5b-9 (21)].
We recently demonstrated that this priming
phenomenon observed in the process of HSPCs occurs similarly in
MSCs primed with S1P and C1P bioactive lipids and a cationic
peptide, LL-37 (3,23). In particular, the primed MSCs
exhibit cell migration, colony-forming activity, and
anti-inflammatory capacity in the cell culture condition, which
promote their therapeutic benefits to treat pulmonary arterial
hypertension (PAH). However, the primed MSCs still exhibit limited
in vivo engraftment into damaged tissues (3,23).
More importantly, even an extensive washing step prior to MSC
administration fails to completely remove the remaining priming
factors which can provoke an adverse inflammatory response
(3). Thus, development of priming
strategies at a low dosage is needed. The expression of CXCR4, a
main target for stem cell priming, is upregulated by the
DNA-demethylating agent, 5-azacytidine (5-Aza) (24), and histone deacetylase inhibitor,
valproic acid (VPA) (25). In the
present study, we investigated the role of these epigenetic
regulatory modulators in improving MSC priming strategies for
accelerating their therapeutic application.
Materials and methods
Culturing human umbilical cord-derived
MSCs
Human UC was obtained from healthy normal full-term
newborns after obtaining written informed parental consent in
accordance with the guidelines approved by the Ethics Committee on
the Use of Human Subjects at Asan Medical Center. Informed consent
was obtained from all pregnant mothers before UC collection.
UC-derived MSCs (UC-MSCs) used in the present study were grown in
low-glucose Dulbecco's modified Eagle's medium (DMEM) (HyClone,
Pittsburgh, PA, USA) supplemented with 2 mM L-glutamine, 20 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH
7.3), minimum essential medium (MEM) nonessential amino acid
solution, penicillin/streptomycin (Corning Cellgro; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 1 mg/ml ascorbic acid
(Sigma-Aldrich), 10% heat-inactivated fetal bovine serum (FBS)
(HyClone), 5 ng/ml human epidermal growth factor (Sigma-Aldrich,
St. Louis, MO, USA), 10 ng/ml basic fibroblast growth factor, and
50 mg/ml long-R3 insulin-like growth-factor 1 (ProSpec, Rehovot,
Israel) in a humidified atmosphere with 5% CO2 at 37°C.
UC-MSCs expanded less than five passages were used to ensure their
multipotency. The expression of surface proteins was analyzed as
described previously (3).
Cell migration assay
The 8 µm polycarbonate membranes were coated
with 50 µl 1.0% gelatin (Sigma-Aldrich) for 1 h. UC-MSCs
were primed with S1P (50 nM; Cayman Chemical, Ann Arbor, MI, USA),
VPA (0.5 mM), or 5-Aza (1 µM) (both from Sigma-Aldrich)
alone or in combination with 50 nM S1P for 1 day. The UC-MSCs
detached with TrypLE solution (Thermo Fisher Scientific, Pittsburgh
PA, USA) were washed and resuspended in DMEM containing 0.5% bovine
serum albumin (BSA), and seeded at a density of 3×104
cells/well into the upper chambers of Transwell inserts (Corning
Costar, Pittsburgh, PA, USA). The lower chambers were filled with
150 ng/ml SDF-1 (R&D Systems, Minneapolis, MN, USA) in 0.5% BSA
DMEM. After 1 day, the inserts were removed from the Transwell
plates. Cells remaining in the upper chambers were scraped off
using cotton wool, and cells that had transmigrated were fixed with
4% paraformaldehyde (PFA) solution in phosphate-buffered saline
(PBS) and stained with 0.5% crystal violet (Sigma-Aldrich). Stained
cells on the lower side of the membranes were quantified by digital
image analysis using the Image Pro 5.0 software (Media-Cybernetics,
Rockville, MD, USA).
Cell proliferation and colony forming
unit-fibroblast (CFU-F) assay
Cell proliferation after priming with the indicated
compounds for 24 h was determined by MTT assay (Sigma-Aldrich)
according to the manufacturer's protocol. Reduction of MTT reagent
was performed for 4 h and quantified by measuring the absorbance at
570 nm on a microplate spectrophotometer (Molecular Devices,
Sunnyvale, CA, USA). For the CFU-F assay, UC-MSCs were treated with
the indicated priming factors for 24 h, after which cells were
re-plated at a clonal density (600 cells/well) in 6-well culture
plates, and further maintained in culture media for 14 days. The
established colonies were washed twice with PBS, fixed, and stained
with 0.5% crystal violet (Sigma-Aldrich).
In vitro differentiation assay
In vitro differentiation into chondrogenic,
osteogenic or adipogenic lineages was performed as described
previously (26). Briefly,
UC-MSCs treated with the indicating priming factors were cultured
in StemPro chondrogenesis (Invitrogen, Carlsbad, CA, USA),
osteogenic (DMEM supplemented with 5% FBS, 50 µM
L-ascorbate-2-phosphate, 0.1 µM dexamethasone, and 10 mM
glycerophosphate), or adipogenic (DMEM supplemented with 5% FBS, 1
µM dexamethasone, 10 µM insulin, 200 µM
indomethacin, and 0.5 mM isobutylmethylxanthine) differentiation
medium. The chondrogenic differentiation was examined by Alcian
Blue staining (Sigma-Aldrich) and osteogenic differentiation was
noted by positive staining with Alizarin Red staining
(Sigma-Aldrich), which is specific for calcium. Adipogenic
differentiation characterized with intracellular lipid accumulation
was visualized by Oil Red O staining (Sigma-Aldrich).
Anti-inflammatory assay of MSCs
The in vitro anti-inflammatory potency of
MSCs was examined as described previously (3,23).
Briefly, MH-S, a murine alveolar macrophage cell line, was
maintained in DMEM-high-glucose supplemented with 10%
heat-inactivated FBS and penicillin/streptomycin. For the
inflammatory assay, 1×105 MH-S cells were seeded in
12-well culture plates, followed by stimulation with 0.1
µg/ml lipopolysaccharide (LPS) (Sigma-Aldrich) in the
absence or the presence of the conditioned medium (CM), which were
collected from IMR90 cells or UC-MSCs with or without priming.
After 5 h, medium conditioned by the MH-S macrophages was collected
and clarified by centrifugation at 500 × g for 10 min. A total of
50 µl of MH-S medium was used for murine tumor necrosis
factor-α (TNF-α) enzyme-linked immunosorbent assay (ELISA) kit
(Thermo Fisher Scientific).
Western blot analysis
UC-MSCs primed with 50 nM S1P and 0.5 mM VPA were
starved for 12 h in DMEM containing 0.5% BSA at 37°C, stimulated
with 150 ng/ml SDF-1 for 5, 10, 20 or 30 min, and the
phosphorylation of mitogen-activated protein kinases (MAPK),
phospho-MAPKp42/44 (#4376) or total
MAPKp42/44 (#9102) and phospho-AKT (#9271) or AKT
(Ser473; #9272) (all from Cell Signaling Technology, Danvers, MA,
USA) was analyzed by western blot analysis. Cell extracts were
prepared using cell lysis buffer (0.5% NP-40, 0.5% Triton X-100, 20
mM HEPES, 1.5 mM MgCl2, 2 mM DTT, 2 mM EDTA, 150 mM
NaCl) supplemented with protease and phosphatase inhibitor
cocktails (Roche Life Science, Basel, Switzerland). Protein lysate
was quantitated using BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.) and 30 µg cell extracts were separated by
10% SDS-PAGE and transferred onto nitrocellulose blotting membranes
(GE Healthcare, München, Germany). All primary antibodies were
diluted 1:1,000 and the peroxidase conjugated anti-rabbit secondary
antibody (#111-035-045) was diluted 1:2,000 (Jackson ImmunoResearch
Laboratories, West Grove, PA, USA).
RT-qPCR
For gene expression analysis, total RNA was prepared
and reverse-transcribed from the indicated cells using the RNeasy
Mini kit (Qiagen, Hilden, Germany) and TaqMan Reverse Transcription
Reagents (Applied Biosystems, Carlsbad, CA, USA), respectively. The
indicated transcripts were quantified by qPCR as previously
described (4,27). Primers were designated as follows:
CXCR4 forward, 5′-ACTACACCGAGGAAATGGGCT-3′ and reverse,
5′-CCCACAATGCCAGTTAAGAAGA-3′; MMP12 forward,
5′-GATCCAAAGGCCGTAATGTTCC-3′ and reverse,
5′-TGAATGCCACGTATGTCATCAG-3′; PDGFB forward,
5′-GGCAACACTGCTGTCCACAT-3′ and reverse, 5′-GTCCCACACCCACCTGGAA-3′;
PDGFRB forward, 5′-GATGCCGAGGAACTATTCATCT-3′ and reverse,
5′-TTTCTTCTCGTGCAGTGTCAC-3′; cMET forward,
5′-AGCGTCAACAGAGGGACCT-3′ and reverse,
5′-GCAGTGAACCTCCGACTGTATG-3′; VEGFB forward,
5′-TCAGGGATAGCCCAGTCAATACA-3′ and reverse,
5′-GCCACAGAAGGCTGTCTCCTT-3′; VEGFC forward,
5′-AGTTCCACCACCAAACATGCA-3′ and reverse,
5′-CACTATATGAAAATCCTGGCTCACA-3′; ANGPT1 forward,
5′-TGCTCACGTGGCTCGACTATA-3′ and reverse,
5′-AGCACAGCAAGCTCAGCAGTTT-3′; ANGPT2 forward,
5′-GGTTTGATGCATGTGGTCCTT-3′ and reverse,
5′-AATGCCGTTGAACTTATTTGTGTTC-3′; IDO1 forward,
5′-TCCGTGAGTTTGTCCTTTCAAA-3′ and reverse,
5′-CAGGGAGACCAGAGCTTTCACA-3′; IDO2 forward,
5′-GATTGATGCTCACCAGCTTCAAG-3′ and reverse,
5′-GCTCCCGGTGACCCTTCAG-3′; LIF forward,
5′-GAAAGCTTTGGTAGGTTCTTCGTT-3′ and reverse,
5′-TGCAGGTCCAGCCATCAGA-3′; GAPDH forward,
5′-TGAATGCCACGTATGTCATCAG-3′ and reverse,
5′-TGTTGCTGTAGCCAAATTCGTT-3′.
Statistical analysis
Data were analyzed using a non-parametric
Mann-Whitney test or a one-way ANOVA with the Bonferroni post-hoc
test to detect statistically significant differences. We used
GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA, USA)
to perform all analyses, and statistical significance was defined
as p<0.05, p<0.01 or p<0.001.
Results
Effect of 5-Aza on S1P-primed human
UC-MSCs
Treatment with 5-Aza and VPA at a low concentration
(1 µM and 0.5 mM, respectively) markedly increased the
expression of CXCR4 in the UC-MSCs (Fig. 1A). We next examined the effect of
these epigenetic regulators on the basic features of UC-MSCs. Both
5-Aza and VPA, independently of the priming with 50 nM S1P, had
less influence on the expression of surface marker proteins which
were positive for CD29, CD73 and CD90 but negative for CD34 and
CD45 (Figs. 1B and 2A). Furthermore, S1P priming together
with 5-Aza (5-Aza+S1P) or VPA (VPA+S1P) had little effect on the
multi-lineage differentiation capacity based on an in vitro
differentiation assay into the chondrogenic, osteogenic and
adipogenic lineages which were estimated by an increased level of
glycosaminoglycans (Alcian blue), mineral deposition (Alizarin Red
S), and lipid accumulation (Oil Red O staining), respectively
(Figs. 1C and 2B).
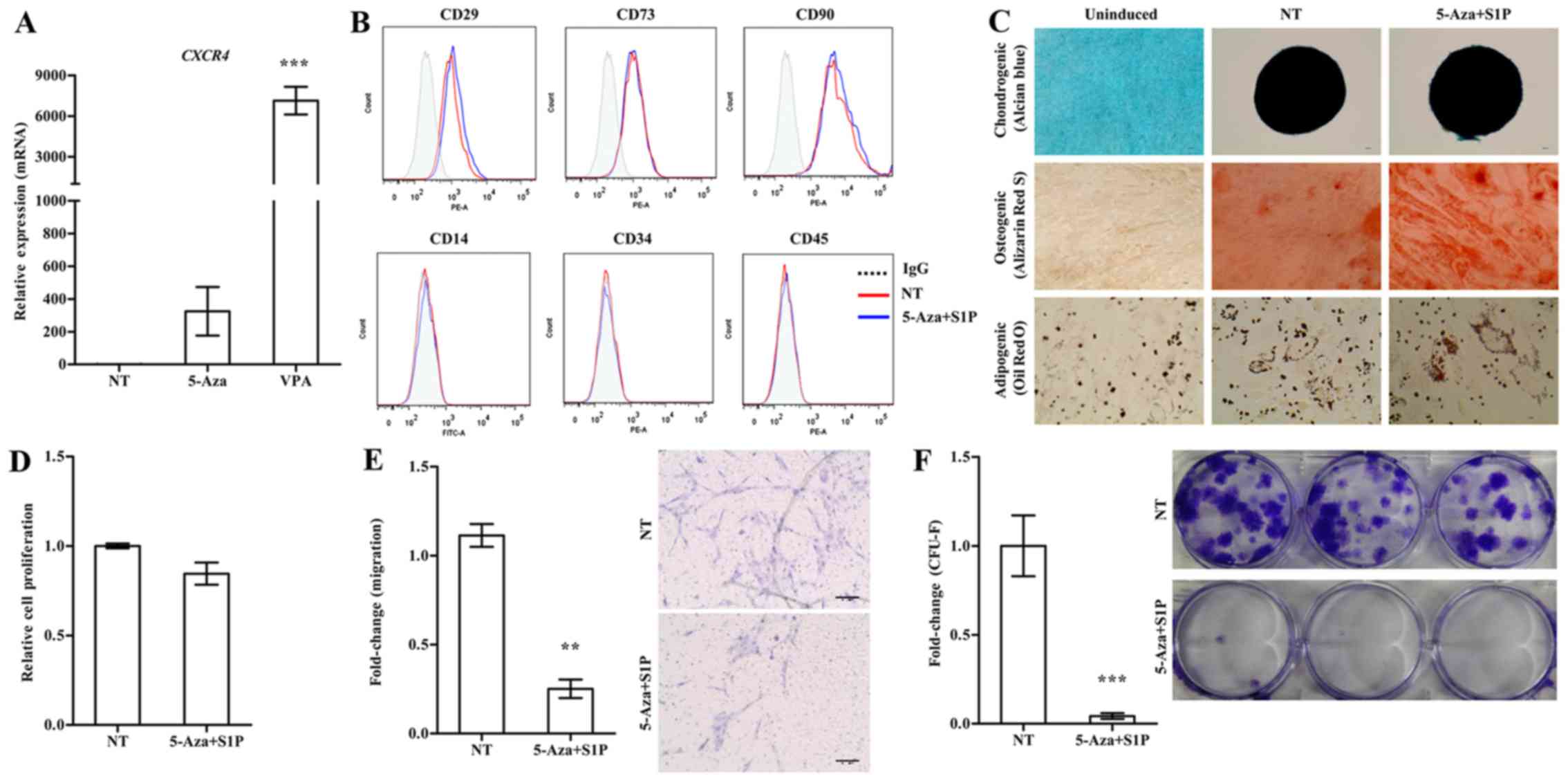 | Figure 1Adverse effect of 5-azacytidine
(5-Aza) on umbilical cord-derived mesenchymal stem cells (UC-MSCs)
primed with sphingosine-1-phosphate (S1P). (A) RT-qPCR analysis of
CXCR4 in human UC-MSCs treated with 1 µM 5-Aza or 0.5
mM valproic acid (VPA) for 24 h. The relative expression level of
the indicated genes is represented as the fold-change compared to
the value of non-treated MSCs (NT) and are shown as means ± SEM
(n=4), ***p<0.001, one-way ANOVA test. (B) Flow
cytometric analysis of the expression of the indicated MSC surface
(CD29, CD73 and CD90) and hematopoietic lineage (CD14, CD34 and
CD45) proteins in UC-MSCs primed with 1 µM 5-Aza together
with 50 nM S1P for 24 h. (C) Representative images of the
chondrogenic, osteogenic or adipogenic differentiation assay using
UC-MSCs primed with 5-Aza+S1P. The chondrogenesis, osteogenesis and
adipogenesis were determined using Alcian Blue, Alizarin Red S and
Oil Red O staining, respectively. (D) Cell proliferation (n=3), (E)
chemotaxis to stromal derived factor-1 (SDF-1) (n=6), and (F)
colony-forming unit-fibroblast (CFU-F) assay (n≥6) of UC-MSCs
primed with 5-Aza+S1P for 24 h. All data are presented as the mean
± SEM. **p<0.01 and ***p<0.001,
compared to non-primed cells (non-parametric Mann-Whitney test).
Representative images of the migrated cells (E) and stained
colonies (F) are shown in the right panels. |
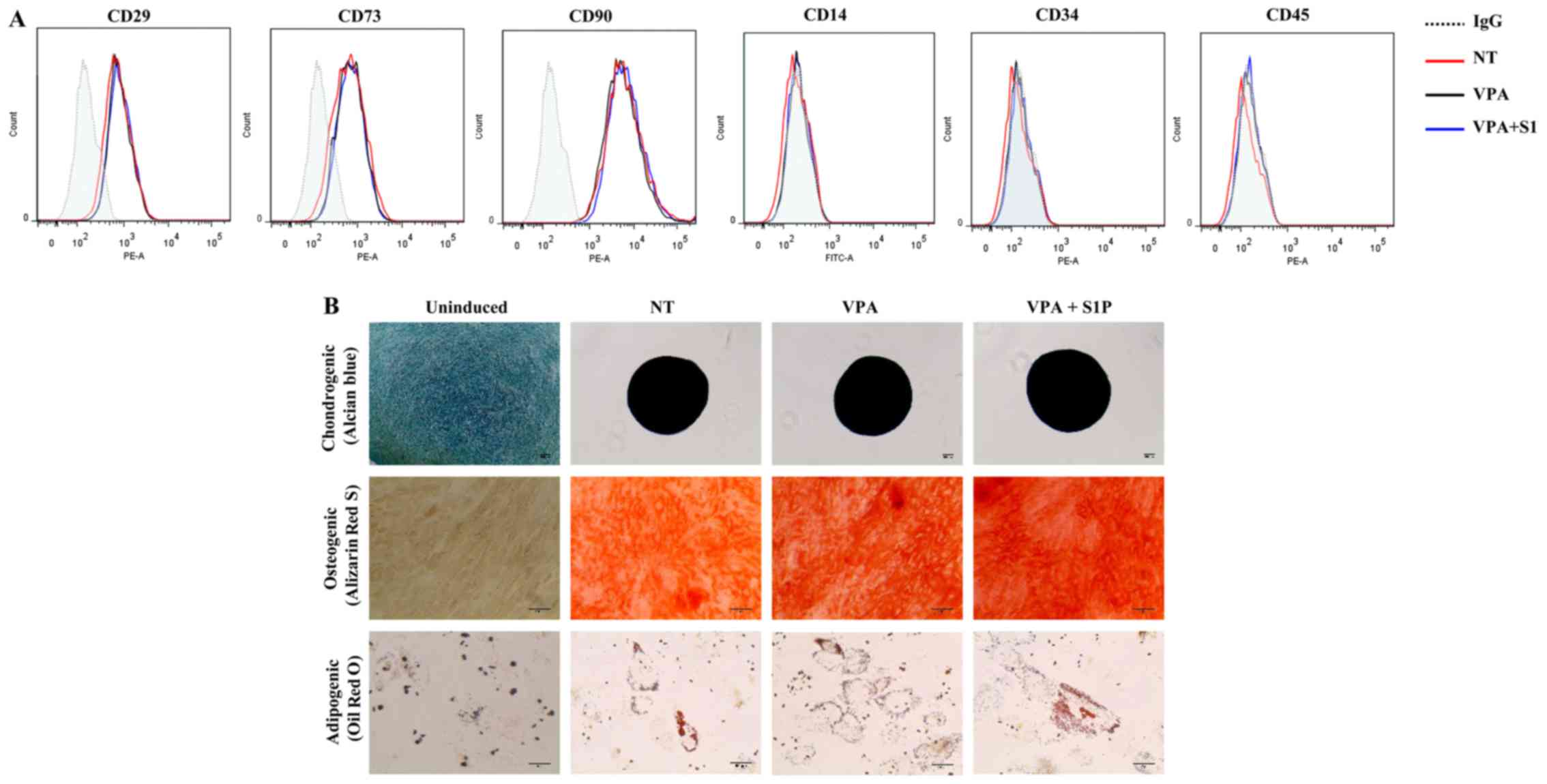 | Figure 2The effect of valproic acid (VPA) +
sphingosine-1-phosphate (S1P) priming on the basal features of
umbilical cord-derived mesenchymal stem cells (UC-MSCs). (A) Flow
cytometric analysis of surface antigens of the indicated MSC
surface (CD29, CD73 and CD90) and hematopoietic (CD14, CD34 and
CD45) lineage proteins in UC-MSCs primed with 0.5 mM VPA alone
(VPA) or in combination with 50 nM S1P (VPA+S1P) for 24 h. (B)
Representative images of the chondrogenic, osteogenic and
adipogenic differentiation assays in which each lineage
differentiation was determined using Alcian Blue (magnification,
×100; scale bar, 100 µm), Alizarin Red S (magnification,
×200; scale bar, 100 µm), and Oil Red O staining
(magnification, ×200; scale bar, 50 µm), respectively. |
Contradictory to the upregulation of CXCR4
(Fig. 1A), UC-MSCs primed with
5-Aza+S1P had decreased migration activity in response to SDF-1 in
a Transwell migration assay; with ~30% less migration activity than
the unprimed cells (Fig. 1E). In
addition, 5-Aza+S1P priming severely impaired the capacity of
clonogenic CFU-F, which represents the number of self-renewing
clonogenic progenitor cells (Fig.
1F). The cellular proliferation of MSCs was only slightly
changed by the priming with 5-Aza+S1P (Fig. 1D). Therefore, these results
demonstrate that treatment with 5-Aza provides an adverse outcome
for the priming of UC-MSCs with S1P.
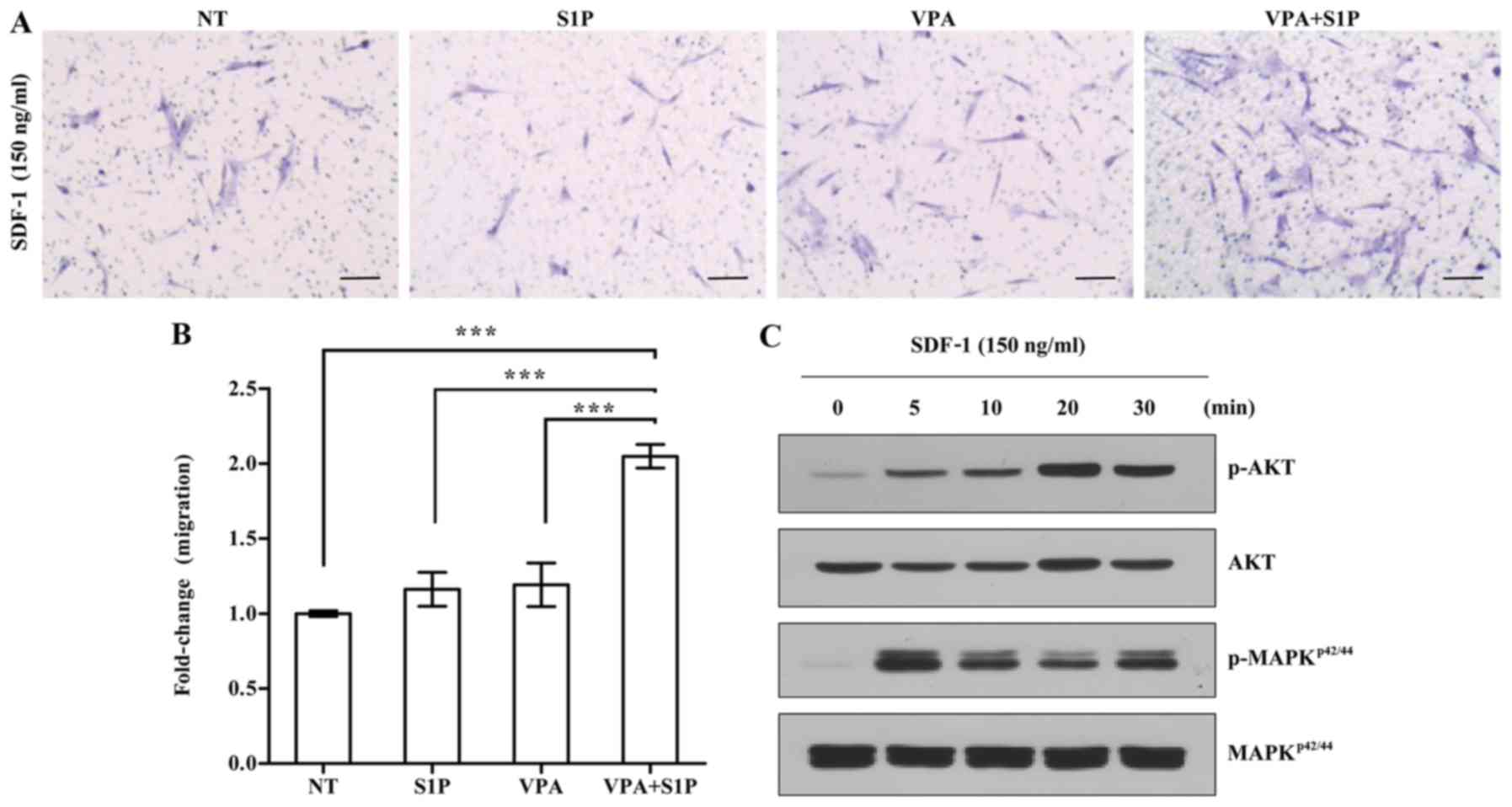
VPA enforces the priming of UC-MSCs with
a suboptimal dose of S1P
Next, we explored the effect of VPA on the priming
of UC-MSCs using a low dose of S1P. To address this issue, we
employed 50 nM S1P, which is 4-fold less than the optimal dose (200
nM) used for priming of both adipose- and UCB-derived MSCs
(3). In agreement, the priming
with a suboptimal dosage of S1P alone had little effect on the
responsiveness of UC-MSCs to the SDF-1 chemokine (Fig. 3A). In contrast, the priming of
UC-MSCs with VPA+S1P, but not VPA alone, significantly increased
the chemotactic activity to SDF-1 by 3-fold when compared with that
of the unprimed cells (Fig. 3A and
B). This enforced response to SDF-1 by VPA+S1P priming
completely differed from the case of 5-Aza+S1P priming. We next
explored the status of the signaling pathways implicated in the
migration of HSPCs (20,21,28,29). In line with the results of the
chemotaxis assay, UC-MSCs exposed to VPA+S1P had increased levels
of phosphorylated MAPKp42/44 and AKT proteins,
indicating activation of their signaling pathways (Fig. 3C). These results indicate that
VPA, and not 5-Aza, promote the priming of UC-MSCs at the
suboptimal dose of S1P by activating the MAPKp42/44 and
AKT signaling pathways.
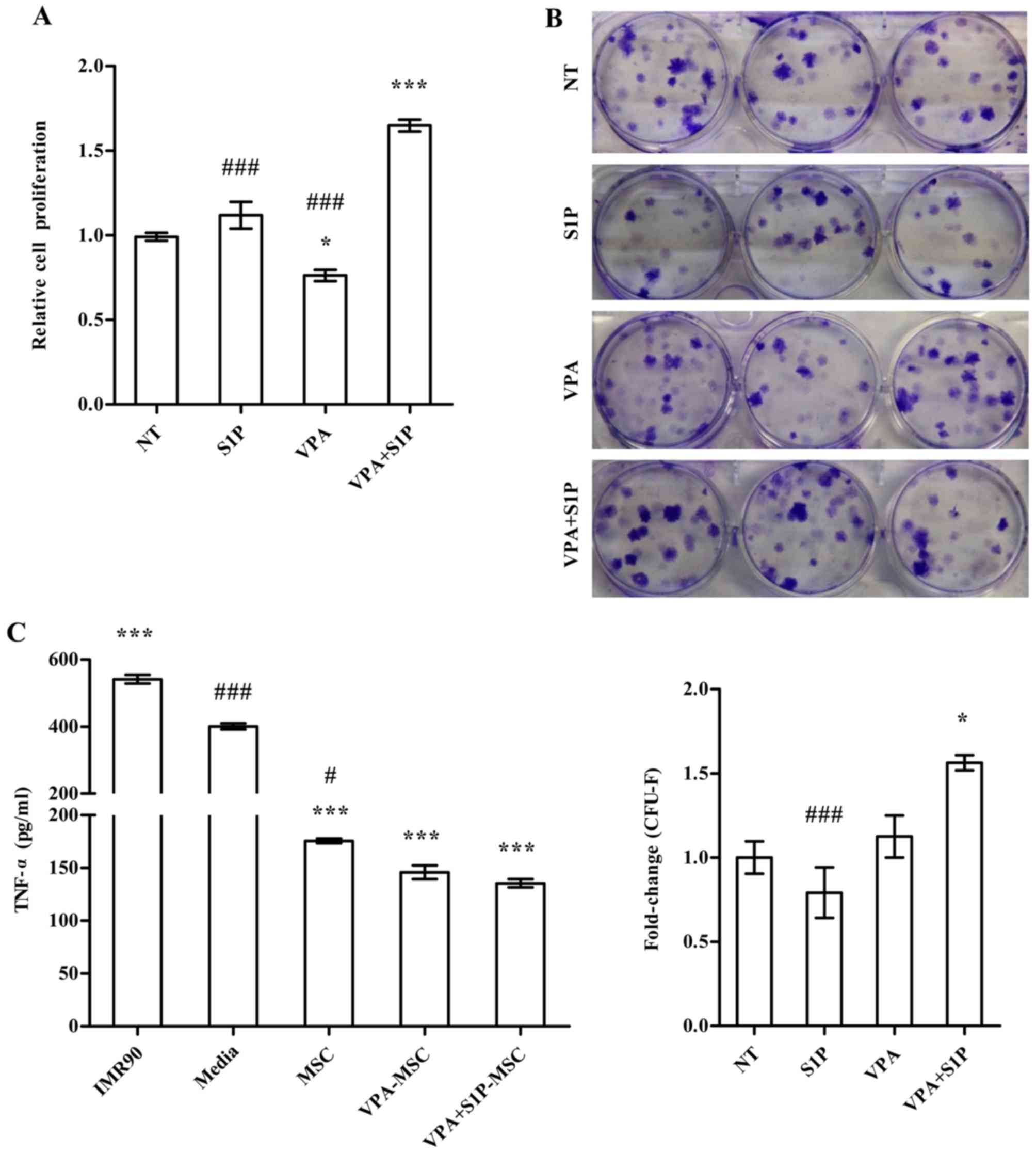
Enhanced therapeutic capacities of
UC-MSCs primed with VPA+S1P
We further examined the effect of VPA+S1P priming on
other cellular activities which are related to the therapeutic
effect of MSCs. Unlike 5-Aza+S1P (Fig. 1), UC-MSCs primed with VPA+S1P had
enhanced capacity for both cell proliferation (Fig. 4A) and clonogenic CFU-F abilities
(Fig. 4B). These beneficial
effects were not observed in the UCB-MSCs primed by VPA or S1P
alone (Fig. 4A and B). Since MSCs
exert anti-inflammatory and immunomodulatory properties (30–32), we next explored whether VPA+S1P
priming can influence the anti-inflammatory effect of UC-MSCs. To
address this issue, we prepared the CM collected from UC-MSCs
unprimed, primed by VPA or S1P alone, and primed by VPA+S1P and
examined whether CM could suppress the secretion of TNF-α from MH-S
cells, an alveolar macrophage cell line, by stimulation with LPS
(3). CM from UC-MSCs was
effective to reduce the secretion of TNF-α from LPS-stimulated MH-S
cells (Fig. 4C). In particular,
CM derived from the VPA+S1P-primed UC-MSCs further inhibited TNF-α
secretion to a greater extent than UC-MSCs unprimed or primed with
VPA or S1P alone, although a significant difference was observed
only between naive and VPA+S1P-primed cells. When we used CM from
IMR90, a type of human lung fibroblast as a control, a further
increase in TNF-α secretion was observed in our in vitro
anti-inflammation assays (Fig.
4C).
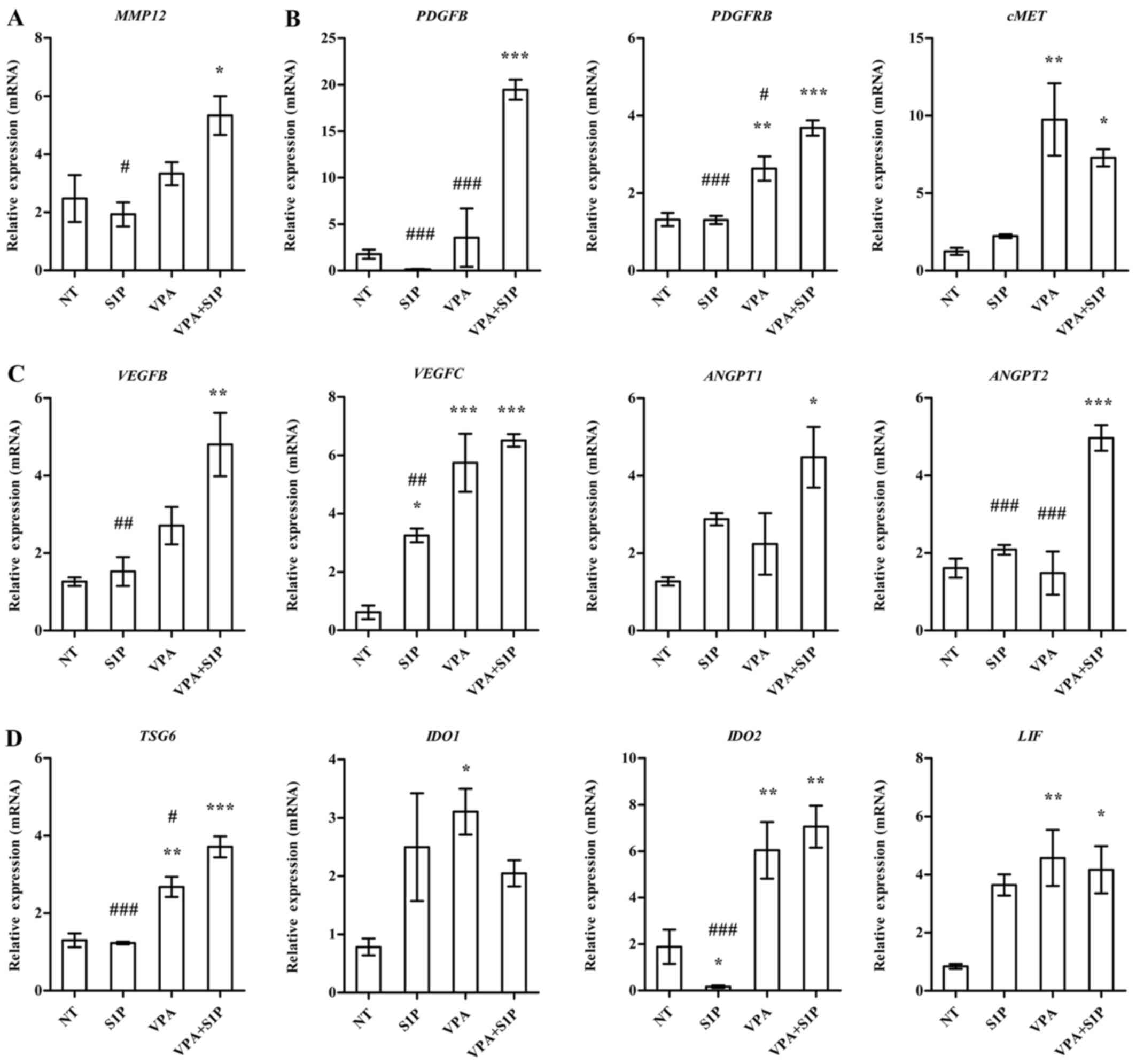
To obtain a mechanistic insight into the effect of
VPA+S1P priming, we examined the expression of growth factors,
pro-inflammation cytokines and anti-inflammatory factors secreted
by MSCs (3,23). In line with the aforementioned
data, UC-MSCs primed with VPA+S1P had significantly upregulated
expression of a subset of growth factors and their receptors
(PDGFB, PDGFRB and cMET) (Fig.
5B), pro-angiogenesis factors (VEGFB, VEGFC, ANGPT1 and ANGPT2)
(Fig. 5C), anti-inflammatory
factors (LIF, TSG6 and IDO2) (Fig.
5D) and a stem cell migration-related protein (MMP12) (Fig. 5A). Most of the aforementioned
genes affected by VPA+S1P priming were downregulated by 5-Aza+S1P
priming (data not shown) and little change was noted following
priming with VPA or S1P alone (Fig.
5). Collectively, these results indicate that priming of MSCs
with a minimal dose of VPA and S1P can effectively promote the
migration, proliferation, self-renewal and anti-inflammatory
capacity of MSCs, which are crucial for their therapeutic
potency.
Discussion
Our present study highlights that the priming of
UC-MSCs combined with VPA and S1P enhanced migration, self-renewal,
and anti-inflammatory potency of MSCs using a minimal dosage of
these compounds. Priming molecules, such as bioactive lipids, are
enriched in damaged tissue and can function as strong
chemo-attractants for HSPCs and MSCs. However, the priming of MSCs
with a high dose of these molecules could pose a risk of
stimulating the inappropriate inflammatory response when these
factors left remaining inside the primed cells are released after
MSC therapy. Thus, the present study provides crucial experimental
clues for the optimization of priming of MSCs with a minimal amount
of priming factors by enforcing CXCR4 signaling, which can assist
in developing more practical and safe protocols for clinical
applications.
Previously, we reported that bioactive lipids (S1P
and C1P) or cationic peptide (LL-37) stimulate the migratory
potential of several tissue (UCB, adipose and BM)-derived MSCs
(3,23). However, compared with HSPCs, the
priming of MSCs still exhibits a limited effect, particularly in
vivo engraftment of infused stem cells. Unlike HSPCs, only a
small proportion of MSCs express functionally active CXCR4 receptor
on their surfaces, and this expression diminishes with passage
(33). CXCR4 is a key to
mediating specific migration of these cells (14) and its upregulation could overcome
the limited in vivo engraftment ability of primed MSCs
(33). By employing epigenetic
regulatory compounds, we found that a DNA-demethylation agent
(5-Aza) and a histone deacetylase inhibitor (VPA) significantly
increased the expression of CXCR4 in UC-MSCs (Fig. 1A). Importantly, the priming
combined with VPA and S1P increased the responsiveness to SDF-1,
and the CXCR4 signaling cascade was activated by a suboptimal dose
(Fig. 3). Unexpectedly, the
treatment of 5-Aza severely impaired the chemotactic activity to
SDF-1 (Fig. 1E) and the
clonogenic ability of CFU-F (Fig.
1F), although the influence on cellular proliferation was less
(Fig. 1D). These results indicate
that the expression level of CXCR4 alone may not be sufficient to
enhance the effects of MSC priming; thus further in-depth
investigation of the molecular nature of MSC priming is
required.
Mechanistically, priming molecules such as bioactive
lipids (S1P) or cationic peptide (LL-37) enhance the mobilization
or engraftment of adult SCs including HSPCs and MSCs by the
incorporation of CXCR4 into lipid rafts which is better connected
with downstream signaling proteins, and in turn, enables the primed
cells to better respond to the SDF-1α gradient (34). Of importance, the priming of MSCs
also potentiates several important cellular properties of MSCs,
such as self-renewal, anti-inflammation and pro-angiogenesis which
are pivotal to the beneficial outcomes of MSC therapy (3,23).
In line with previous studies, we confirmed that the optimized
priming strategies combined with VPA and S1P with a suboptimal dose
also enhanced these beneficial outcomes of UC-MSCs in in
vitro cell culture assays (Fig.
4), paralleled with upregulation of several trophic,
immunosuppressive, anti-inflammatory and proangiogenic factors
(Fig. 5). Thus, the in
vivo efficacy of UC-MSCs primed with VPA+S1P should be further
examined.
The priming factors for HSPCs and MSCs are generally
enriched in damaged tissues as part of the repair process; thus,
they are reported to increase tissue inflammation and remodeling
(21,28,29). Thus, priming of stem cells with a
high concentration of these factors abnormally increases their
content inside the cells. In our previous study of UCB-MSCs primed
with S1P, cells under normal culture contained a low level of S1P;
however, the priming of S1P increased the S1P content significantly
(3). In particular, extensive
washing of cells with normal saline solution before administration
of S1P-primed MSCs cannot completely remove the remaining S1P.
Thus, the optimization of priming strategies with a lower dosage
could be crucial to prevent the possibility of residual priming
factors provoking an inappropriate inflammatory response and
vascular remodeling. The present study provides a strong candidate
to ensure the safe application of the MSC priming method. Notably,
a high dose of VPA (~10 mM) was found to decrease the proliferation
potential and multi-lineage differentiation capability of human
MSCs by activating the transcription of p21CIP1/WAF1,
which eventually arrested the cell cycle at the G2⁄M phase
(35). In addition, aberrant
epigenetic regulation can lead to tumorigenesis and premature stem
cell aging (36). Thus, to
translate the MSC priming to human clinical trials, it will be
important to optimize types or combinations of priming factors and
also to carefully consider their safety. To address issues of
safety, long-term monitoring of the hypothetical risks for tumor
development from primed MSCs should be thoroughly examined.
In conclusion, VPA as a combination factor ensures
the priming effect of S1P with a suboptimal dose, which can provide
the optimized and safe application of MSC therapy in the clinic.
Our findings suggest that combination strategies for MSC priming
would overcome the poor in vivo engraftment of the infused
MSCs, and finally improve the therapeutic potency of MSCs.
Acknowledgments
The present study was co-supported by the Global
High-Tech Biomedicine Technology Development Program of the
National Research Foundation (NRF) and Korea Health Industry
Development Institute (KHIDI) (MSIP&MOHW) (no.
2015M3D6A1065364), by Basic Science Research Program through the
NRF (no. 2015R1A2A1A15054754), and by the Korea Korean Health
Technology R&D Project, Ministry of Health & Welfare of the
Republic of Korea (no. HI14C3339).
Glossary
Abbreviations
Abbreviations:
|
5-Aza
|
5-azacytidine
|
|
BM
|
bone-marrow
|
|
C1P
|
ceramide-1-phosphate
|
|
CD
|
cluster of differentiation
|
|
CFU-F
|
colony-forming unit-fibroblast
|
|
CM
|
conditioned medium
|
|
HGF
|
hepatocyte growth factor
|
|
HSPC
|
hematopoietic stem/progenitor cell
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MSCs
|
mesenchymal stem cells
|
|
PAH
|
pulmonary artery hypertension
|
|
PDGF
|
platelet-derived growth factor
|
|
S1P
|
sphingosine-1-phosphate
|
|
SDF-1
|
stromal cell-derived factor 1
|
|
sMAC
|
soluble membrane attack complex
|
|
TNF-α
|
tumor necrosis factor-α
|
|
UC-MSCs
|
umbilical cord-derived MSCs
|
|
UCB
|
umbilical cord blood
|
|
VPA
|
valproic acid
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Knaän-Shanzer S: Concise review: The
immune status of mesenchymal stem cells and its relevance for
therapeutic application. Stem Cells. 32:603–608. 2014. View Article : Google Scholar
|
|
2
|
Bianco P, Cao X, Frenette PS, Mao JJ,
Robey PG, Simmons PJ and Wang CY: The meaning, the sense and the
significance: Translating the science of mesenchymal stem cells
into medicine. Nat Med. 19:35–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kang H, Kim K-H, Lim J, Kim YS, Heo J,
Choi J, Jeong J, Kim Y, Kim SW, Oh YM, et al: The therapeutic
effects of human mesenchymal stem cells primed with sphingosine-1
phosphate on pulmonary artery hypertension. Stem Cells Dev.
24:1658–1671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin HJ, Lee HJ, Heo J, Lim J, Kim M, Kim
MK, Nam HY, Hong GH, Cho YS, Choi SJ, et al: Senescence associated
MCP-1 secretion is dependent on a decline in BMI1 in human
mesenchymal stromal cells. Antioxid Redox Signal. 24:471–485. 2015.
View Article : Google Scholar
|
|
5
|
Connick P, Kolappan M, Crawley C, Webber
DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ,
et al: Autologous mesenchymal stem cells for the treatment of
secondary progressive multiple sclerosis: An open-label phase 2a
proof-of-concept study. Lancet Neurol. 11:150–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang D, Li J, Zhang Y, Zhang M, Chen J, Li
X, Hu X, Jiang S, Shi S and Sun L: Umbilical cord mesenchymal stem
cell transplantation in active and refractory systemic lupus
erythematosus: A multicenter clinical study. Arthritis Res Ther.
16:R792014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song M, Lim J, Yu HY, Park J, Chun JY,
Jeong J, Heo J, Kang H, Kim Y, Cho YM, et al: Mesenchymal stem cell
therapyalleviates interstitial cystitis by activating Wnt signaling
pathway. Stem Cells Dev. 24:1648–1657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim A, Yu HY, Heo J, Song M, Shin JH, Lim
J, Yoon SJ, Kim Y, Lee S, Kim SW, et al: Mesenchymal stem cells
protect against the tissue fibrosis of ketamine-induced cystitis in
rat bladder. Sci Rep. 6:308812016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song M, Heo J, Chun JY, Bae HS, Kang JW,
Kang H, Cho YM, Kim SW, Shin DM and Choo MS: The paracrine effects
of mesenchymal stem cells stimulate the regeneration capacity of
endogenous stem cells in the repair of a
bladder-outlet-obstruction-induced overactive bladder. Stem Cells
Dev. 23:654–663. 2014. View Article : Google Scholar
|
|
10
|
Gnecchi M, Zhang Z, Ni A and Dzau VJ:
Paracrine mechanisms in adult stem cell signaling and therapy. Circ
Res. 103:1204–1219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gnecchi M, He H, Noiseux N, Liang OD,
Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, et al:
Evidence supporting paracrine hypothesis for Akt-modified
mesenchymal stem cell-mediated cardiac protection and functional
improvement. FASEB J. 20:661–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaquero J, Zurita M, Oya S and Santos M:
Cell therapy using bone marrow stromal cells in chronic paraplegic
rats: Systemic or local administration? Neurosci Lett. 398:129–134.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee RH, Pulin AA, Seo MJ, Kota DJ,
Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P and Prockop
DJ: Intravenous hMSCs improve myocardial infarction in mice because
cells embolized in lung are activated to secrete the
anti-inflammatory protein TSG-6. Cell Stem Cell. 5:54–63. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca
R, Wojakowski W and Ratajczak J: The pleiotropic effects of the
SDF-1CXCR4 axis in organogenesis, regeneration and tumorigenesis.
Leukemia. 20:1915–1924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuzuki Y, Fukumura D, Oosthuyse B, Koike
C, Carmeliet P and Jain RK: Vascular endothelial growth factor
(VEGF) modulation by targeting hypoxia-inducible factor-1alpha→
hypoxia response element→ VEGF cascade differentially regulates
vascular response and growth rate in tumors. Cancer Res.
60:6248–6252. 2000.PubMed/NCBI
|
|
16
|
Morikawa S, Mabuchi Y, Kubota Y, Nagai Y,
Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori
T, et al: Prospective identification, isolation, and systemic
transplantation of multipotent mesenchymal stem cells in murine
bone marrow. J Exp Med. 206:2483–2496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Corpechot C, Barbu V, Wendum D, Chignard
N, Housset C, Poupon R and Rosmorduc O: Hepatocyte growth factor
and c-Met inhibition by hepatic cell hypoxia: A potential mechanism
for liver regeneration failure in experimental cirrhosis. Am J
Pathol. 160:613–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kucia M, Zhang YP, Reca R, Wysoczynski M,
Machalinski B, Majka M, Ildstad ST, Ratajczak J, Shields CB and
Ratajczak MZ: Cells enriched in markers of neural tissue-committed
stem cells reside in the bone marrow and are mobilized into the
peripheral blood following stroke. Leukemia. 20:18–28. 2006.
View Article : Google Scholar
|
|
19
|
Li J, Guo W, Xiong M, Han H, Chen J, Mao
D, Tang B, Yu H and Zeng Y: Effect of SDF-1/CXCR4 axis on the
migration of transplanted bone mesenchymal stem cells mobilized by
erythropoietin toward lesion sites following spinal cord injury.
Int J Mol Med. 36:1205–1214. 2015.PubMed/NCBI
|
|
20
|
Ratajczak MZ, Lee H, Wysoczynski M, Wan W,
Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A and Ratajczak
J: Novel insight into stem cell mobilization-plasma
sphingosine-1-phosphate is a major chemoattractant that directs the
egress of hematopoietic stem progenitor cells from the bone marrow
and its level in peripheral blood increases during mobilization due
to activation of complement cascade/membrane attack complex.
Leukemia. 24:976–985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim CH, Wu W, Wysoczynski M, Abdel-Latif
A, Sunkara M, Morris A, Kucia M, Ratajczak J and Ratajczak MZ:
Conditioning for hematopoietic transplantation activates the
complement cascade and induces a proteolytic environment in bone
marrow: A novel role for bioactive lipids and soluble C5b-C9 as
homing factors. Leukemia. 26:106–116. 2012. View Article : Google Scholar
|
|
22
|
Kim C, Schneider G, Abdel-Latif A,
Mierzejewska K, Sunkara M, Borkowska S, Ratajczak J, Morris AJ,
Kucia M and Ratajczak MZ: Ceramide-1-phosphate regulates migration
of multipotent stromal cells and endothelial progenitor
cells–implications for tissue regeneration. Stem Cells. 31:500–510.
2013. View Article : Google Scholar :
|
|
23
|
Lim J, Kim Y, Heo J, Kim KH, Lee S, Lee
SW, Kim K, Kim IG and Shin DM: Priming with ceramide-1 phosphate
promotes the therapeutic effect of mesenchymal stem/stromal cells
on pulmonary artery hypertension. Biochem Biophys Res Commun.
473:35–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee S, Kim H-S, Roh K-H, Lee BC, Shin TH,
Yoo JM, Kim YL, Yu KR, Kang KS and Seo KW: DNA methyltransferase
inhibition accelerates the immunomodulation and migration of human
mesenchymal stem cells. Sci Rep. 5:80202015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen L, Cui X, Wu Z, Jia L, Yu Y, Zhou Q,
Hu X, Xu W, Luo D, Liu J, et al: Transplantation of bone marrow
mesenchymal stem cells pretreated with valproic acid in rats with
an acute spinal cord injury. Biosci Trends. 8:111–119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu KR, Yang SR, Jung JW, Kim H, Ko K, Han
DW, Park SB, Choi SW, Kang SK, Schöler H, et al: CD49f enhances
multi-potency and maintains stemness through the direct regulation
of OCT4 and SOX2. Stem Cells. 30:876–887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heo J, Lim J, Lee S, Jeong J, Kang H, Kim
Y, Kang JW, Yu HY, Jeong EM, Kim K, et al: Sirt1 regulates DNA
methylation and differentiation potential of embryonic stem cells
by antagonizing Dnmt3l. Cell Rep. 18:1930–1945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu W, Kim CH, Liu R, Kucia M, Marlicz W,
Greco N, Ratajczak J, Laughlin MJ and Ratajczak MZ: The bone
marrow-expressed antimicrobial cationic peptide LL-37 enhances the
responsiveness of hematopoietic stem progenitor cells to an SDF-1
gradient and accelerates their engraftment after transplantation.
Leukemia. 26:736–745. 2012. View Article : Google Scholar
|
|
29
|
Lee HM, Wu W, Wysoczynski M, Liu R,
Zuba-Surma EK, Kucia M, Ratajczak J and Ratajczak MZ: Impaired
mobilization of hematopoietic stem/progenitor cells in C5-deficient
mice supports the pivotal involvement of innate immunity in this
process and reveals novel promobilization effects of granulocytes.
Leukemia. 23:2052–2062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim N and Cho SG: New strategies for
overcoming limitations of mesenchymal stem cell-based immune
modulation. Int J Stem Cells. 8:54–68. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li D, Han Y, Zhuang Y, Fu J, Liu H, Shi Q
and Ju X: Overexpression of COX-2 but not indoleamine
2,3-dioxygenase-1 enhances the immunosuppressive ability of human
umbilical cord-derived mesenchymal stem cells. Int J Mol Med.
35:1309–1316. 2015.PubMed/NCBI
|
|
32
|
Yang W, Yang Y, Yang JY, Liang M and Song
J: Treatment with bone marrow mesenchymal stem cells combined with
plumbagin alleviates spinal cord injury by affecting oxidative
stress, inflammation, apoptotis and the activation of the Nrf2
pathway. Int J Mol Med. 37:1075–1082. 2016.PubMed/NCBI
|
|
33
|
Jones GN, Moschidou D, Lay K, Abdulrazzak
H, Vanleene M, Shefelbine SJ, Polak J, de Coppi P, Fisk NM and
Guillot PV: Upregulating CXCR4 in human fetal mesenchymal stem
cells enhances engraftment and bone mechanics in a mouse model of
osteogenesis imperfecta. Stem Cells Transl Med. 1:70–78. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ratajczak MZ, Kim CH, Wojakowski W,
Janowska-Wieczorek A, Kucia M and Ratajczak J: Innate immunity as
orchestrator of stem cell mobilization. Leukemia. 24:1667–1675.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee S, Park JR, Seo MS, Roh KH, Park SB,
Hwang JW, Sun B, Seo K, Lee YS, Kang SK, et al: Histone deacetylase
inhibitors decrease proliferation potential and multilineage
differentiation capability of human mesenchymal stem cells. Cell
Prolif. 42:711–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wagner W, Weidner CI and Lin Q: Do
age-associated DNA methylation changes increase the risk of
malignant transformation? BioEssays. 37:20–24. 2015. View Article : Google Scholar
|