Introduction
Bone homeostasis is regulated by an appropriate
balance between resorption of old bone and formation of new bone,
which is known as bone remodeling. Osteoblasts (OBs), which are
responsible for bone formation, arise from mesenchymal stem cells
(MSCs) as OB progenitors, and OB differentiation and function are
positively regulated by several signaling pathways including
Wnt/β-catenin-mediated signaling which targets the expression of
osteogenic transcription factor Runx2 (1,2).
Additionally, OBs are associated with differentiation and
activation of osteoclasts (OCs) which are responsible for bone
resorption (3).
α2-antiplasmin (α2AP) is known to be synthesized in
various tissues, and functions as the principal inhibitor of
plasmin, a main component of the fibrinolytic system (4,5).
As a new function of α2AP, we previously found that α2AP is
associated with tissue remodeling, angiogenesis, extracellular
matrix (ECM) production, cell growth and cell differentiation
(6–11). α2AP is most phylogenetically
closely related to the non-inhibitory serine protease inhibitor,
pigment epithelium-derived factor (PEDF) (12), and they have very similar
structure (3 β-sheets and 9 β-helices) (13,14). Furthermore, Shiomi et al
recently reported that α2AP deficiency attenuated ovariectomy
(OVX)-induced bone loss, and α2AP is associated with osteoclast
formation (15). These
observations suggest that α2AP exhibits various functions not only
as a plasmin inhibitor, but also as a modulator of bone metabolism.
However, the mechanisms underlying α2AP-regulated bone metabolism
remain to be clarified at the cellular and molecular levels.
We herein investigated the roles of α2AP in bone
metabolism, particularly in regards to how α2AP affects OB
differentiation and bone formation.
Materials and methods
Animals
The α2AP-deficient (α2AP−/−) mice were
generated by homologous recombination using embryonic stem cells,
as previously described (16).
Wild-type (α2AP+/+) and α2AP−/− mice
littermates were housed in groups of 2–5 in filter-top cages with a
fixed 12-h light and 12-h dark cycle.
The animal experiments were approved by the Animal
Research Committee of Doshisha Women's College of Liberal Arts
(approval ID, Y15-024). All experiments were performed in
accordance with relevant guidelines and regulations.
In vivo calcein labeling
Calcein (Nacalai Tesque, Inc., Kyoto, Japan) in
saline was intraperitoneally injected into eight-week-old
α2AP+/+ and α2AP−/− mice (20 mg/kg). Two
injections were given 3 days apart. The undecalcified sections of
femurs from eight-week-old α2AP+/+ and
α2AP−/− mice were prepared by the Tohkai Cytopathology
Institute (Gifu, Japan). Bone formation was visualized using a
calcein incorporation assay as described by Naylor et al
(17). Briefly, the mineral
apposition rate (µm/day) in vivo was calculated by
identifying newly formed bone via calcein labeling. The data of
double-labeled regions were obtained by using fluorescence
microscopy, and the mineral apposition rate was calculated as the
distance of the double-labeled regions.
Immunohistochemical staining of
osteocalcin
Paraffin-embedded tissue of femurs in eight-week-old
α2AP+/+ and α2AP−/− mice was serially
sectioned at 4–7 µm distance. Then, the sections were labeled with
anti-rabbit osteocalcin antibody (cat. no. SC-30045; Santa Cruz
Biotechnology, Inc., Santa Cruz CA, USA), and then secondarily
labeled with Cy3-conjugated anti-rabbit IgG (cat. no. A10520;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The signals were
then detected using a laser scanning microscope. The stained images
obtained from separate fields on the specimens were analyzed using
ImageJ software.
Enzyme-linked immunosorbent assay
(ELISA)
The osteocalcin in the serum from eight-week-old
α2AP+/+ and α2AP−/− mice was then measured
using a mouse osteocalcin EIA kit (Biomedical Technologies,
Stoughton, MA, USA). The absorbance of the ELISA samples was
measured at 450 nm using Multiskan JX (Thermo LabSystems, Beverly,
MA, USA).
Measurement of alkaline phosphatase (ALP)
activity
We measured ALP activity in the serum and
osteoblasts from α2AP+/+ and α2AP−/− mice as
previously described (18). ALP
activity was determined using p-nitrophenyl phosphate
(Sigma-Aldrich, Steinheim, Germany) as a substrate. The absorbance
of the samples was measured at 405 nm using Multiskan JX (Thermo
LabSystems).
Cell culture
Primary OBs derived from α2AP+/+ and
α2AP−/− mouse calvaria were obtained as previously
described (19). Primary OBs or
MC3T3-E1 cells were maintained in minimum essential medium (MEM)
(Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS) (Biowest, Nuaillé, France) and 1%
penicillin-streptomycin (Invitrogen Life Technologies) at 37° in a
humidified atmosphere of 5% CO2/95% air.
OB differentiation
OB differentiation in the primary OBs derived from
α2AP+/+ and α2AP−/− mouse calvaria and
MC3T3-E1 cells were induced as previously described (19). Briefly, primarily cultured OBs or
MC3T3-E1 cells were cultured for 14 days in the differentiation
media supplemented with 10 mM β-glycerophosphate and 10 nM
dexamethasone (both from Sigma-Aldrich), and 50 µg/ml ascorbic acid
(Wako Pure Chemical Industries, Ltd., Osaka, Japan) in 6-well
plates. After 14 days, the cells were then washed with
phosphate-buffered saline (PBS), and cell proteins were extracted
with a lysis buffer (10 mM Tris-HCl, pH 7.5, 0.1% Triton
X-100).
Reverse transcription-polymerase chain
reaction (RT-PCR)
We performed RT-PCR as previously described
(19). First-strand cDNA was
synthesized from total RNA using the High Fidelity RT-PCR kit
(Toyobo, Osaka, Japan). Quantitative RT-PCR (RT-qPCR) was performed
on the IQ5 real-time PCR detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) with SYBR-Green technology on cDNA
generated from the reverse transcription of purified RNA. The
2-step PCR reactions were performed as 92°C for 1 sec and 60°C for
10 sec. Runx2 mRNA expression was normalized against
GAPDH mRNA expression using the comparative cycle threshold
method. We used the following primer sequences: Runx2
forward, 5′-GAATGGCAGCACGCTATTAAATCC-3′ and reverse,
5′-GCCGCTAGAATTCAAAACAGTTGG-3′; GAPDH forward,
5′-TTCATTGACCTCAACTACATG-3′ and reverse,
5′-GTGGCAGTGATGGCATGGAC-3′.
Western blot analysis
Western blot analysis was performed as previously
described (20). Briefly, cells
were washed twice with cold PBS, harvested, and then sonicated in
lysis buffer containing 10 mM Tris-HCl buffer (pH 7.5), 1% SDS, 1%
Triton X-100, and a protease inhibitor cocktail (Roche, Mannheim,
Germany). The protein concentration in each lysate was measured
using a BCA protein assay kit (Pierce, Rockford, IL, USA). Proteins
in the supernatant were separated by electrophoresis on 10%
SDS-polyacrylamide gels and transferred to a PVDF membrane. We
detected β-catenin, phospho-lipoprotein receptor-related protein 6
(p-LRP6), LRP6 and GAPDH by incubation with anti-rabbit β-catenin
antibody (cat. no. Rb-1491; NeoMarkers, Fremont, CA, USA),
anti-rabbit phospho-LRP6 antibody (cat. no. bs-2905R) and
anti-rabbit LRP6 antibody (cat. no. bs-3253R) (both from Bioss
Inc., Woburn, MA, USA), and anti-rabbit GAPDH antibody (cat. no.
SAB2100894; Sigma-Aldrich) followed by incubation with horseradish
peroxidase-conjugated antibodies to rabbit IgG (cat. no. NA934-1ML;
Amersham Pharmacia Biotech, Uppsala, Sweden).
Spontaneous secretion of VEGF in primary
OBs
Spontaneous secretion of VEGF in primary OBs was
measured as previously described (6). The OBs were maintained in MEMα
containing 10% FBS. After 6 days, the medium was exchanged for
serum-free MEMα. After 24 h, the conditioned medium was collected,
and VEGF in the medium was then measured by VEGF ELISA kit (R&D
Systems, Minneapolis, MN, USA). The absorbance of the ELISA samples
was measured at 450 nm using Multiskan JX (Thermo LabSystems).
Statistical analysis
All data are expressed as the means ± SEM. The
significance of the effect of each treatment (P<0.05) was
determined by analysis of variance (ANOVA) followed by the least
significant difference test.
Results
Effect of α2AP deficiency on bone
formation in mice
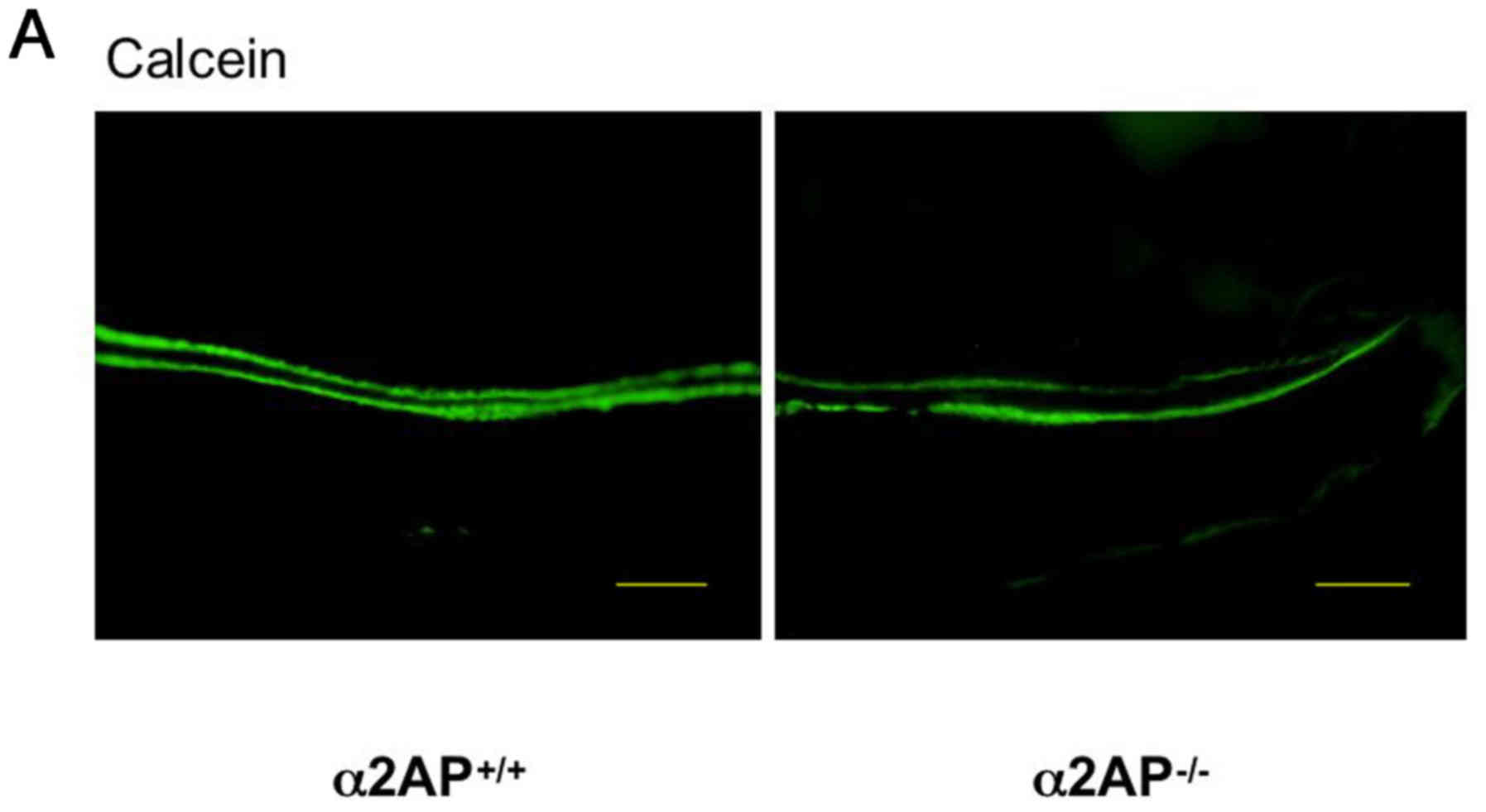
We investigated the effect of α2AP deficiency on
bone formation by examining calcein incorporation after its
injection in the α2AP+/+ and α2AP−/− mice.
The double calcein-labeled regions in the femurs from the
eight-week-old α2AP+/+ and α2AP−/− mice are
shown in Fig. 1A. The mineral
apposition rate was calculated as the distance of the double
calcein-labeled regions. The distance between the double
calcein-labels in the femurs from the α2AP−/− mice was
larger than that from the α2AP+/+ mice (Fig. 1B). Additionally, we examined the
expression of osteocalcin in the femurs from the α2AP+/+
and α2AP−/− mice. The level of osteocalcin expression in
the femurs from the α2AP−/− mice was significantly
higher than that of the α2AP+/+ mice at the protein
level (Fig. 1C and D).
Furthermore, we examined the levels of osteocalcin and ALP activity
in the serum of the α2AP+/+ and α2AP−/− mice.
The levels of osteocalcin and ALP activity in the serum of the
α2AP−/− mice were significantly higher than those of the
α2AP+/+ mice (Fig. 1E and
F, respectively).
Effect of α2AP deficiency on OB
differentiation and function
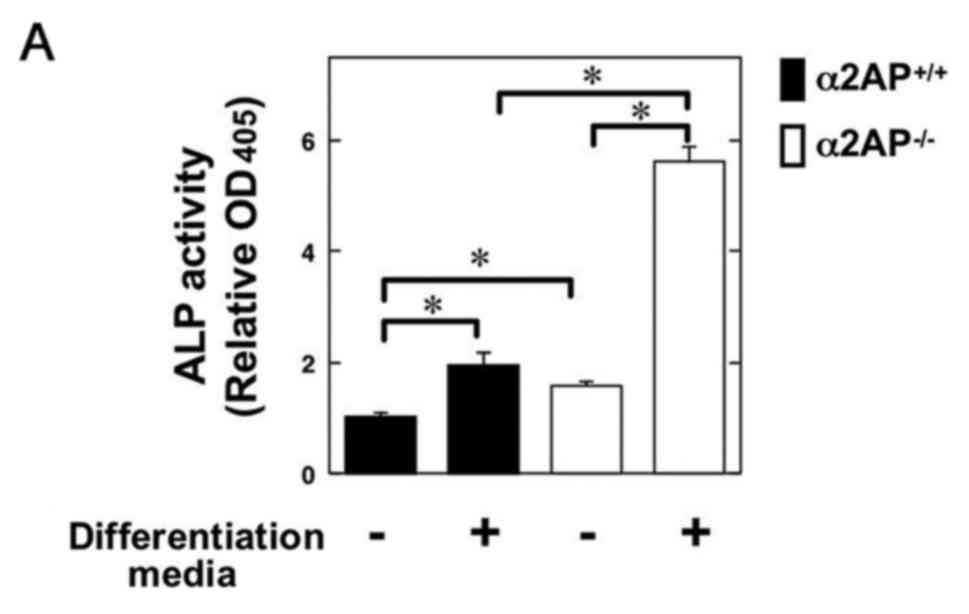
Next, to clarify the role of α2AP in OB
differentiation, we examined the ALP activity in primary calvarial
OBs from the α2AP+/+ and α2AP−/− mice in the
absence or presence of OB differentiation media. Intriguingly, the
α2AP deficiency resulted in upregulation of ALP activity in OBs
(Fig. 2A). Additionally, we
examined the expression level of Runx2, which is an
essential transcription factor for OB differentiation, in OBs from
the α2AP+/+ and α2AP−/− mice in the presence
of the OB differentiation media. The level of Runx2 mRNA
expression in the α2AP−/− OBs was significantly higher
than that in the WT OBs (Fig.
2B). It has been reported that PEDF, which is most
phylogenetically closely related to α2AP, inhibits the
Wnt/β-catenin pathway by blocking LRP6 (21). Therefore, to clarify whether or
not α2AP is associated with the Wnt/β-catenin pathway, we examined
the expression of β-catenin in OBs from the α2AP+/+ and
α2AP−/− mice. The expression of β-catenin in the
α2AP−/− OBs was significantly higher than that in the
α2AP+/+ OBs at the protein level (Fig. 2C).
Effect of α2AP on OB differentiation and
function
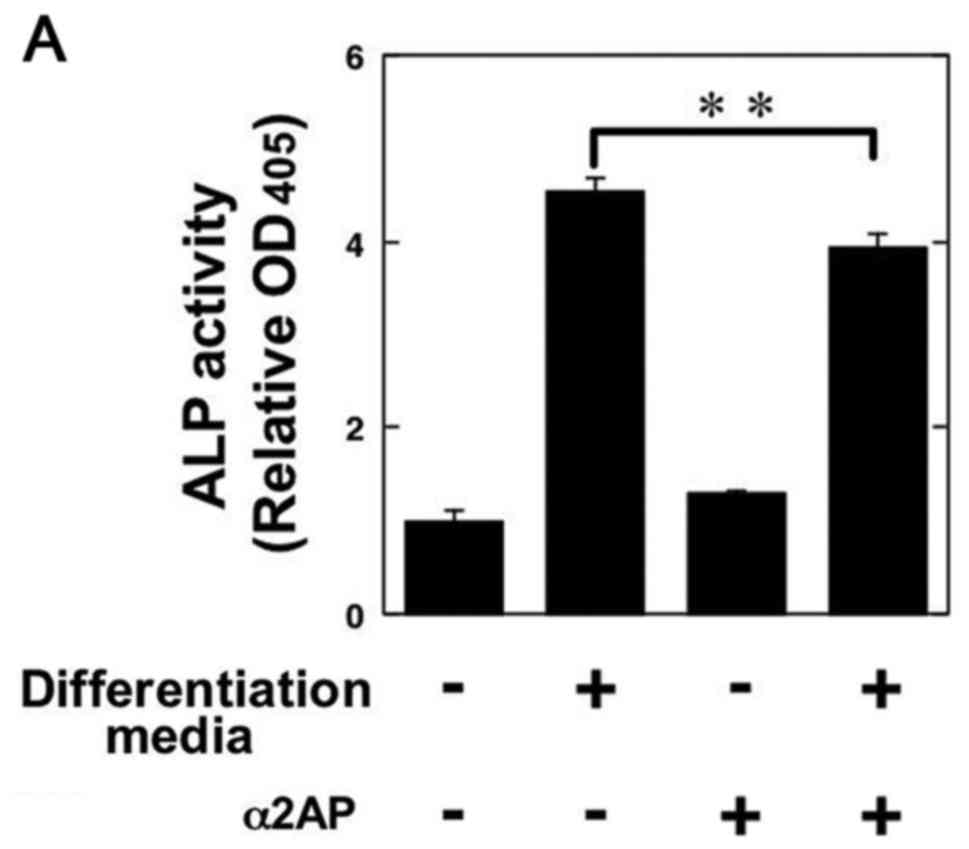
In order to clarify the roles of α2AP in the
functions of OBs, we examined ALP activity in the α2AP-treated
mouse osteoblastic MC3T3-E1 cells. α2AP treatment attenuated the OB
differentiation media-induced ALP activation in the MC3T3-E1 cells
(Fig. 3A). We also showed that
α2AP treatment significantly attenuated the Runx2 mRNA
expression in the MC3T3-E1 cells in the OB differentiation media
(Fig. 3B). Furthermore, we
demonstrated that α2AP attenuated the Wnt-3a-induced β-catenin
expression and LRP6 phosphorylation at the protein level (Fig. 3C).
Discussion
In the present study, we investigated the roles of
α2AP in the stat uses of differentiation and function of OBs that
regulate bone formation. We found that the bone formation rate and
osteocalcin expression in the femur and serum ALP activity were
significantly elevated in the α2AP-deficient mice compared with
these parameters in the WT mice (Fig.
1). Additionally, α2AP deficiency promoted osteogenic
transcription factor expression and ALP activity in OBs (Fig. 2A and B). In contrast, the α2AP
treatment attenuated them (Fig. 3A
and B). These data strongly suggest that α2AP negatively
regulates OB differentiation and function.
Although α2AP is known to be a plasmin inhibitor, we
previously found that α2AP regulates ECM production, cell growth,
and cell differentiation in the absence of plasmin (7–10).
We also showed that plasminogen deficiency did not affect OB
differentiation (19). We herein
showed that α2AP attenuated OB differentiation in the absence of
plasmin (Fig. 3A and B). These
observations suggest that α2AP-mediated OB differentiation is not
carried out by its action as a plasmin inhibitor.
α2AP is most phylogenetically closely related to
PEDF (12), and they have very
similar structure (3 β-sheets and 9 α-hel ices) (13,14). It has been reported that PEDF
inhibits the Wnt/β-catenin pathway by blocking LRP6, which is
coreceptor for Wnts (21). The
Wnt-3a-induced LRP6 activation results in inhibition of β-catenin
degradation (22), and the
Wnt/LRP6/β-catenin axis plays an important role in OB
differentiation (23–25). We herein showed that the
expression status of β-catenin was elevated in the OBs from the
α2AP-deficient mice than in that from WT mice (Fig. 2C). In addition, the α2AP treatment
attenuated Wnt-3a-induced β-catenin expression and LRP6 activation
(Fig. 3C). These data strongly
suggest that α2AP negatively modulates OB differentiation by
inhibiting the Wnt/LRP6/β-catenin axis.
In a previous study, we showed that α2AP deficiency
enhanced VEGF expression in fibroblasts (6). α2AP deficiency also enhanced VEGF
expression in primary osteoblasts (data not shown). It has been
reported that osteoblast-derived VEGF positively regulates OB
differentiation and bone formation activity of OBs in autocrine or
paracrine manners (26).
Additionally, activation of the Wnt/β-catenin pathway induces VEGF
production (27). The
α2AP-mediated Wnt/β-catenin pathway may also regulate the
production of VEGF production, and the α2AP-regulated VEGF
production may be associated with bone homeostasis.
In conclusion, α2AP affects bone metabolism by
negatively regulating OB differentiation and function. These
findings provide a basis for therapeutic strategies for various
bone disorders.
References
|
1
|
Buttery LD, Bourne S, Xynos JD, Wood H,
Hughes FJ, Hughes SP, Episkopou V and Polak JM: Differentiation of
osteoblasts and in vitro bone formation from murine embryonic stem
cells. Tissue Eng. 7:89–99. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yavropoulou MP and Yovos JG: The role of
the Wnt signaling pathway in osteoblast commitment and
differentiation. Hormones (Athens). 6:279–294. 2007. View Article : Google Scholar
|
|
3
|
Long F: Building strong bones: molecular
regulation of the osteoblast lineage. Nat Rev Mol Cell Biol.
13:27–38. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collen D: Identification and some
properties of a new fast-reacting plasmin inhibitor in human
plasma. Eur J Biochem. 69:209–216. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Menoud PA, Sappino N, Boudal-Khoshbeen M,
Vassalli JD and Sappino AP: The kidney is a major site of
alpha(2)-antiplasmin production. J Clin Invest. 97:2478–2484. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanno Y, Hirade K, Ishisaki A, Nakajima K,
Suga H, Into T, Matsushita K, Okada K, Matsuo O and Matsuno H: Lack
of alpha2-antiplasmin improves cutaneous wound healing via
over-released vascular endothelial growth factor-induced
angiogenesis in wound lesions. J Thromb Haemost. 4:1602–1610. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanno Y, Kuroki A, Okada K, Tomogane K,
Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin is involved
in the production of transforming growth factor beta1 and fibrosis.
J Thromb Haemost. 5:2266–2273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanno Y, Kawashita E, Minamida M, Kaneiwa
A, Okada K, Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin
is associated with the progression of fibrosis. Am J Pathol.
176:238–245. 2010. View Article : Google Scholar :
|
|
9
|
Kanno Y, Kawashita E, Kokado A, Kuretake
H, Ikeda K, Okada K, Seishima M, Ueshima S, Matsuo O and Matsuno H:
α2AP mediated myofibroblast formation and the development of renal
fibrosis in unilateral ureteral obstruction. Sci Rep. 4:59672014.
View Article : Google Scholar
|
|
10
|
Kawashita E, Kanno Y, Asayama H, Okada K,
Ueshima S, Matsuo O and Matsuno H: Involvement of α2-antiplasmin in
dendritic growth of hippocampal neurons. J Neurochem. 126:58–69.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanno Y, Kawashita E, Kokado A, Okada K,
Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin regulates the
development of dermal fibrosis in mice by prostaglandin F(2α)
synthesis through adipose triglyceride lipase/calcium-independent
phospholipase A(2). Arthritis Rheum. 65:492–502. 2013. View Article : Google Scholar
|
|
12
|
Irving JA, Pike RN, Lesk AM and Whisstock
JC: Phylogeny of the serpin superfamily: implications of patterns
of amino acid conservation for structure and function. Genome Res.
10:1845–1864. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Law RH, Sofian T, Kan WT, Horvath AJ,
Hitchen CR, Langendorf CG, Buckle AM, Whisstock JC and Coughlin PB:
X-ray crystal structure of the fibrinolysis inhibitor
alpha2-anti-plasmin. Blood. 111:2049–2052. 2008. View Article : Google Scholar
|
|
14
|
Tombran-Tink J, Aparicio S, Xu X, Tink AR,
Lara N, Sawant S, Barnstable CJ and Zhang SS: PEDF and the serpins:
phylogeny, sequence conservation, and functional domains. J Struct
Biol. 151:130–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shiomi A, Kawao N, Yano M, Okada K, Tamura
Y, Okumoto K, Matsuo O, Akagi M and Kaji H: α2-Antiplasmin is
involved in bone loss induced by ovariectomy in mice. Bone.
79:233–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada K, Lijnen HR, Dewerchin M, Belayew
A, Matsuo O, Collen D and Bernaerts R: Characterization and
targeting of the murine alpha2-antiplasmin gene. Thromb Haemost.
78:1104–1110. 1997.PubMed/NCBI
|
|
17
|
Naylor AJ, Azzam E, Smith S, Croft A,
Poyser C, Duffield JS, Huso DL, Gay S, Ospelt C, Cooper MS, et al:
The mesenchymal stem cell marker CD248 (endosialin) is a negative
regulator of bone formation in mice. Arthritis Rheum. 64:3334–3343.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanno Y, Into T, Lowenstein CJ and
Matsushita K: Nitric oxide regulates vascular calcification by
interfering with TGF-signalling. Cardiovasc Res. 77:221–230. 2008.
View Article : Google Scholar
|
|
19
|
Kanno Y, Ishisaki A, Kawashita E, Chosa N,
Nakajima K, Nishihara T, Toyoshima K, Okada K, Ueshima S,
Matsushita K, et al: Plasminogen/plasmin modulates bone metabolism
by regulating the osteoblast and osteoclast function. J Biol Chem.
286:8952–8960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanno Y, Shu E, Kanoh H and Seishima M:
The antifibrotic effect of α2AP neutralization in systemic
sclerosis dermal fibroblasts and mouse models of systemic
sclerosis. J Invest Dermatol. 136:762–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park K, Lee K, Zhang B, Zhou T, He X, Gao
G, Murray AR and Ma JX: Identification of a novel inhibitor of the
canonical Wnt pathway. Mol Cell Biol. 31:3038–3051. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tamai K, Zeng X, Liu C, Zhang X, Harada Y,
Chang Z and He X: A mechanism for Wnt coreceptor activation. Mol
Cell. 13:149–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Guan X, Guo F, Zhou J, Chang A,
Sun B, Cai Y, Ma Z, Dai C, Li X, et al: miR-30e reciprocally
regulates the differentiation of adipocytes and osteoblasts by
directly targeting low-density lipoprotein receptor-related protein
6. Cell Death Dis. 4:e8452013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fei Y, Xiao L, Doetschman T, Coffin DJ and
Hurley MM: Fibroblast growth factor 2 stimulation of osteoblast
differentiation and bone formation is mediated by modulation of the
Wnt signaling pathway. J Biol Chem. 286:40575–40583. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Honda T, Yamamoto H, Ishii A and Inui M:
PDZRN3 negatively regulates BMP-2-induced osteoblast
differentiation through inhibition of Wnt signaling. Mol Biol Cell.
21:3269–3277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu K and Olsen BR: Osteoblast-derived VEGF
regulates osteoblast differentiation and bone formation during bone
repair. J Clin Invest. 126:509–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Sang A, Zhu M, Zhang G, Guan H, Ji
M and Chen H: Tissue factor induces VEGF expression via activation
of the Wnt/β-catenin signaling pathway in ARPE-19 cells. Mol Vis.
22:886–897. 2016.
|