Introduction
Triple-negative breast cancer (TNBC), accounting for
approximately 15–25% of all breast cancer cases, is characterized
by the lack of estrogen receptor (ER−), progesterone
receptor (PR−) and HER2 amplification (HER2−)
(1,2). Although systematic therapeutic
approaches have reduced the mortality rate, TNBC is still
associated with high rates of cancer recurrence, frequent
metastasis to the brain and poor outcomes (2,3).
Therefore, an enhanced understanding of the molecular pathways
involved in the progression of TNBC may be helpful in the
prevention of metastasis and the design of effective therapeutic
strategies for this disease.
Progesterone plays an important role in mammary
epithelial cell proliferation and differentiation (4). Studies using human breast cancer
cell lines and patient tumor samples, as well as clinical studies
have indicated that progesterone is a risk factor for breast cancer
under certain conditions (5,6; and refs therein). Classically, the
effects of progesterone on cancer cells are attributed to the
binding of nuclear PR, the translocation of the progesterone/PR
complex into the nucleus and the subsequent activation of target
genes over the course of several hours (7,8).
Breast cancer is a heterogeneous disease and several distinct
subtypes exist, of which the triple-negative subtype has the most
severe clinical prognosis (3,9,10).
In TNBC, these mechanisms described above are not applicable due to
the lack of PR in these cancers. Thus, the role of progesterone in
the pathogenesis of TNBC remains controversial, and whether
progesterone is a promoter or inhibitor of TNBC has not yet been
fully elucidated.
During the past decade, the discovery of membrane
progesterone receptor α (mPRα), unrelated to the classical PR, in
fish and its subsequent identification in mammals, suggests a
potential mediator of non-traditional progestin actions (11,12), particularly in tissues in which PR
is absent. The broad distribution of mPRα mRNAs in reproductive and
non-reproductive tissues suggests they have diverse physiological
functions in vertebrates (13–17). Recently, changes have been
observed in the mRNA expression of mPRα in malignant human breast
tissues, and mPRα has been identified as an intermediary factor of
the progestin-induced intracellular signaling cascades in the
PR-breast cancer cell lines in vitro (15,18,19). However, the function and molecular
mechanisms of action of mPRα in mediating the effects of
progesterone on TNBC cells remain unknown.
In the present study, we demonstrate that mPRα is
expressed in TNBC tissues and that the expression level of mPRα is
negatively associated the TNM stage. Progesterone suppressed the
growth, migration and invasion of mPRα+ human TNBC
cells. Notably, the inhibitory effects of progesterone on
mPRα+ human TNBC cells were not mediated by PR or by PR
membrane component 1 (PGRMCl). Moreover, the knockdown of mPRα
expression impaired the inhibitory effects of progesterone on
mPRα+ tumor growth and metastasis in vivo. Our
data collectively indicate that progesterone suppresses TNBC growth
and metastasis to the brain via mPRα, which provides evidence of
the anti-neoplastic effects of the progesterone-mPRα pathway in the
treatment of human TNBC.
Materials and methods
Cell lines and cancer tissues
Two human TNBC cell lines [MDA-MB-231 (designated as
MB231) and MDA-MB-231-BR (brain-seeking cells; designated as
MB231br); Type Culture Collection of the Chinese Academy of
Sciences, Shanghai, China] were cultured in DMEM supplemented with
10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin, at
37°C with 5% CO2 in a humidified incubator. Primary TNBC
tissues and matched non-tumour tissues were collected from 55
patients with TNBC undergoing modified radical mastectomy between
2005–2010 at the Department of Oncology, Shanghai Seventh People's
Hospital, Shanghai, China. All the tissue samples were collected
after obtaining patient informed consent and ethics approval (this
study was approved by the Institutional Review Board of Shanghai
Seventh People's Hospital; approval no. 2015011602) and confirmed
by pathological examination.
Immunohistochemistry
Briefly, the tissue sections (4-µm-thick)
were deparaffinized in xylene and rehydrated in graded ethanol.
Following antigen retrieval, endogenous peroxidase quenching and
blocking with 10% normal goat serum, the samples were incubated
with primary anti-mPRα antibody (ab75508; Abcam, Cambridge, MA,
USA) at 4°C overnight and then incubated with a secondary antibody
(DakoCytomation, Glostrup, Denmark). The immunostained slides were
counterstained with hematoxylin. In each experiment, a negative
control was included in which the primary antibody was omitting.
Staining was scored by a trained research pathologist who was
blinded to patient clinical data.
Plasmid construction and
transfection
The full-length cDNA of mPRα was amplified using the
following primers: 5′-CATGGCGACGGTGGTGATG-3′ (forward) and
5′-GGCAGCAGAAGAAATAGGCG-3′ (reverse), and then subcloned into the
vector, pIRES2-EGFP (BD Biosciences Clontech). The sequence for the
construction of mPRα-shRNA was sense, 5′-GGAGCTGTAAGGTCTTCTTTA-3′;
and antisense, 5′-TAAAGAAGACCTTACAGCTCC-3′, and then subcloned into
the vector, pSUPER (GenerayBiotech Co., Ltd., Shanghai, China).
Sequence fidelity and reading frame accuracy of the mPRα expression
or mPRα-shRNA plasmid were achieved by DNA sequencing analysis. The
MB231 or MB231br cells were transfected using
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with the mPRα expression plasmid or mPRα-shRNA
plasmid, respectively. The pIRES2-EGFP vector or pSUPER vector was
also transfected into the MB231 or MB231br cells which served as
controls. The transfected cells were then cultured in medium
supplemented with G418 (Promega, Madison, WI, USA) or puromycin
(InvivoGen, San Diego, CA, USA) for the establishment of stably
transfected cell clones.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and reverse
transcribed using a reverse transcription kit (Promega) according
to the manufacturer's instructions. Quantitative PCR (qPCR) was
performed to determine the expression of mPRα using SYBR-Green PCR
master mix. The primers for mPRα were as follows: sense,
5′-GTGGTGATGGAGCAGATTGGT-3′ and antisense,
5′-TGCCAGGAGGACGATGAATAG-3′. The primers for GAPDH were as follows:
sense, 5′-TTGGCATCGTTGAGGGTCT-3′ and antisense,
5′-CAGTGGGAACACGGAAAGC-3′. The relative expression ratio of mPRα
was calculated using the 2−ΔΔCq method.
Western blot analysis
Cell lysates were prepared with RIPA (Beyotime
Institute of Biotechnology, Haimen, China) and separated by
SDS-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes. Following incubation with
blocking buffer (TBS + 0.1% Tween-20 + 5% not-fat milk), the
membranes were probed with the primary antibody (anti-mPRα
antibody; ab75508; Abcam) overnight at 4°C. The membranes were then
washed with TBST and incubated with an HRP-conjugated secondary
antibody (horseradish peroxidase-labeled goat anti-rabbit IgG;
A0208; Beyotime Institute of Biotechnology), and signals were
detected using ECL reagent (Millipore, Billerica, MA, USA). mPRα,
caspase-3, cleaved caspase-3 and GAPDH antibodies were from
Abcam.
Cell proliferation assay
Cell proliferation was determined using the Cell
Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies,
Inc.). Briefly, 5×105 cells were seeded in 24-well
plates and treated with various concentration of progesterone (20
ng/ml, 40 ng/ml and 80 ng/ml; Sigma-Aldrich, St. Louis, MO, USA),
RU486 (mifepristone; EMD Chemicals, Gibbstown, NJ, USA) or PGRMCl
neutralizing antibody (H-46; sc-98680, Santa Cruz Biotechnology,
Santa Cruz, CA, USA). CCK-8 solution was added to each well, and
the absorbance at 450 nm was measured using an Absorbance
Microplate Reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Cell migration and invasion assays
Cell migration assay was performed using Transwell
chambers (8 µm, 24-well insert; Corning Inc., Corning, NY,
USA). The cells were added to the upper chamber with serum-free
medium and the medium of the lower chamber contained 10% FBS.
Following incubation for 48 h, the migrated cells in the lower
chamber were stained with 0.1% crystal violet (C0121; Beyotime
Institute of Biotechnology). Invasion assays were completed under
the same conditions using Transwell membranes coated with Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA).
In vivo tumorigenesis and metastasis
assays
The cells were collected and injected into the left
ventricle of the hearts of 40 female athymic nude mice (SPF grade;
4–6 weeks old, weighing 18–22 g; Institute of Zoology, Chinese
Academy of Sciences, Shanghai, China) subjected to oophorectomy
using standard procedures. The mice were sacrificed after 5 weeks
and a CT scan was performed for the analysis of brain metastasis
formation. The brains were then collected, fixed and embedded in 4%
paraformaldehyde. Terminal deoxynucleo-tidyltransferase-mediated
dUTP nick-end labeling (TUNEL) assay was carried out following the
manufacturer's instructions of the DeadEnd™ Colorimetric TUNEL
system kit (Promega). All animal experiments were carried out
following the approval of the Institutional Review Board of
Shanghai Seventh People's Hospital; approval no. 2015011601)
Statistical analysis
Data analyses were carried out using SPSS v17.0
software with the Student's t-test. The results are presented as
the means ± SE. Statistical significance was determined at
P<0.05.
Results
mPRα is overexpressed in TNBC tissues and
is associated with clinicopathlogical characteristics
To evaluate the expression of mPRα in human TNBC
tissues, we first determined the expression level of mPRα by
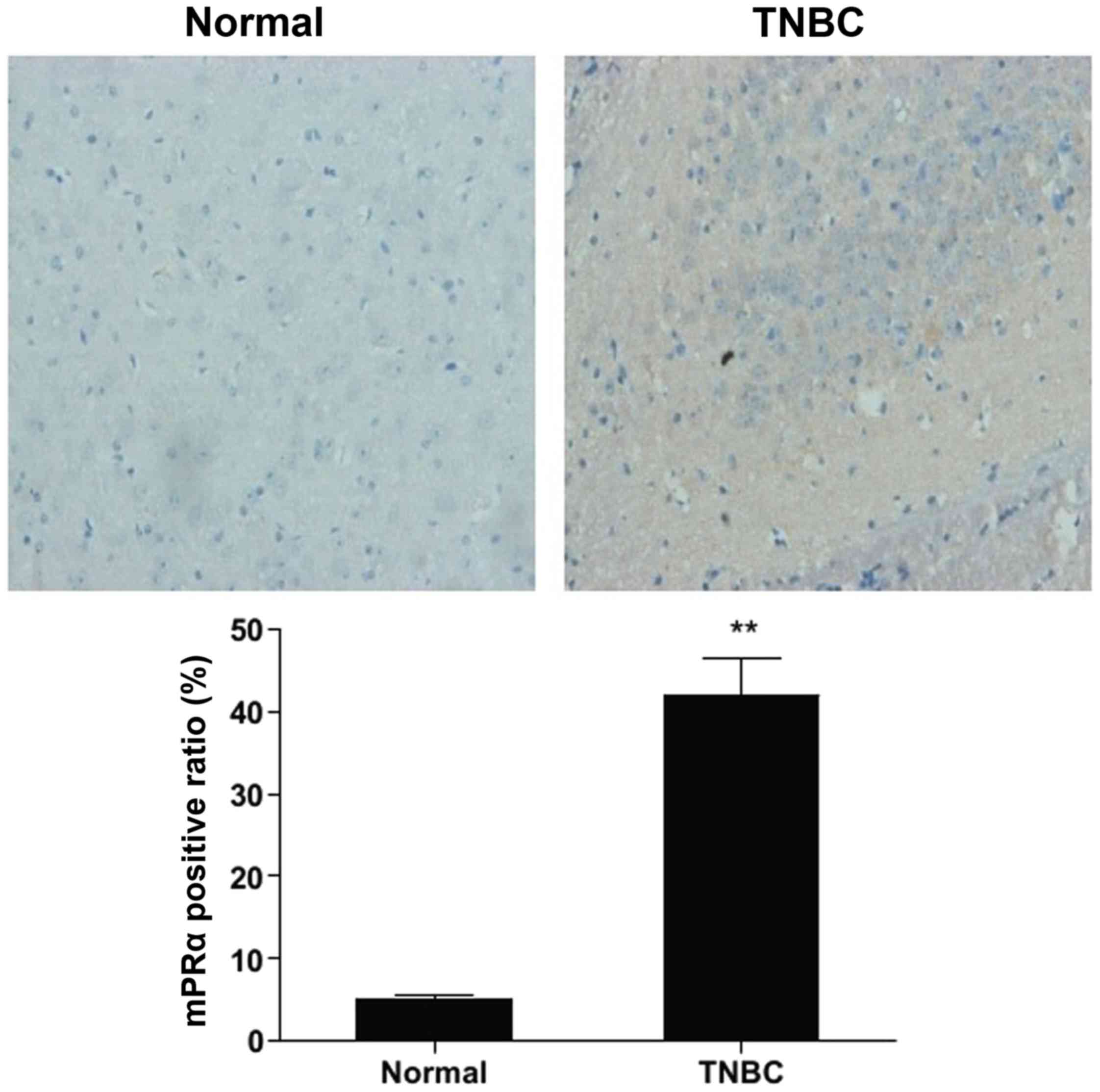
immunohistochemistry. Typical immunostaining of mPRα in normal and
TNBC specimens is shown in Fig.
1. The positive expression of mPRα was mainly observed in the
cytoplasm and/or cell membrane. In the normal breast tissues, mPRα
was detected at low levels; the ductal and alveolar epithelial
cells were shown to be negative or weakly positive and the
myoepithelial cells were shown to be moderately positive for mPRα.
By contrast, all 55 TNBC tissues were stained moderately to
strongly positive for mPRα antibody. The expression of mPRα tended
to decrease with the increasing TNM stage (P<0.05), while no
correlation was observed between mPRα expression and the patient
age, menopausal state or histological grade (Table I).
 | Table ICorrelation between the mPRα
expression level and clinicopathological parameters in the 55
patients with TNBC. |
Table I
Correlation between the mPRα
expression level and clinicopathological parameters in the 55
patients with TNBC.
| Characteristics | mPRα expression level
| P-value |
|---|
| Moderate | Strong |
|---|
| Age (years) |
| ≥50 | 12 | 13 | |
| <50 | 16 | 14 | >0.05 |
| Menopausal
status |
| Pre-menopause | 15 | 14 | |
| Menopause | 14 | 12 | >0.05 |
| Histological
grade |
| Grade I | 11 | 7 | |
| Grade II | 8 | 10 | >0.05 |
| Grade III | 9 | 10 | |
| TNM stage |
| I–II | 18 | 20 | |
| III–IV | 7 | 10 | <0.01 |
Progesterone suppresses the growth of
mPRα+ human TNBC cells
To determine whether progesterone affects the growth
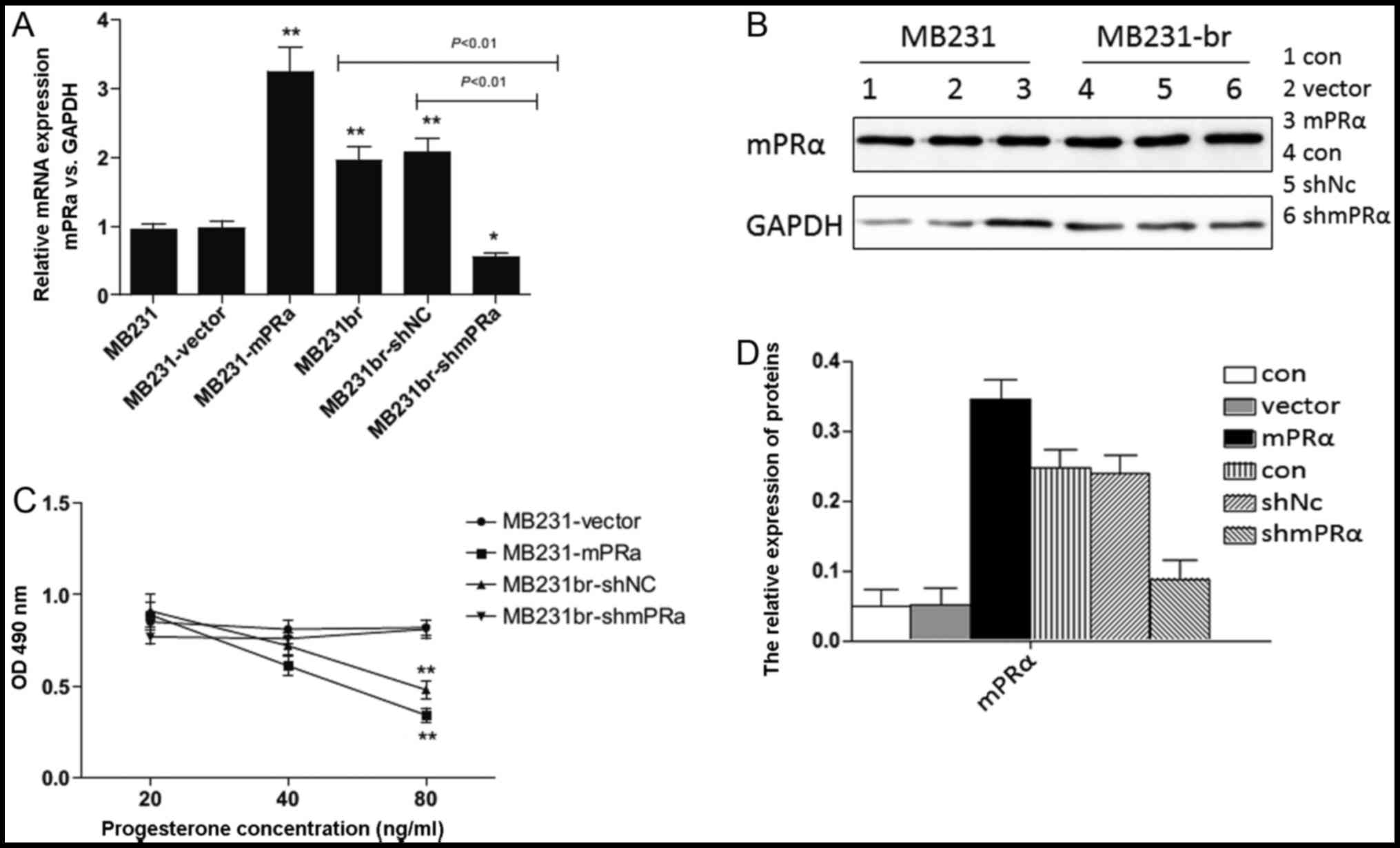
of TNBC cells with a different mPRα status, we first established
stably transfected TNBC cell lines with different mPRα expression
levels. The MB231 and MB231br cells are TNBC cells that do not
express ER, PR and HER2; mPRα expression was detected at higher
levels in the MB231br cells compared with the MB231 cells (Fig. 2A and B). Subsequently, mPRα
full-length expression vector or mPRα-shRNA were transfected into
the MB231 and MB231br cells, respectively. The overexpression or
knockdown of mPRα in the MB231 or MB231br cells was confirmed by
RT-qPCR (Fig. 2A) and western
blot analysis (Fig. 2B). Cell
proliferation was analyzed by CCK-8 assay in the presence of
various concentrations of progesterone (20 ng/ml, 40 ng/ml and 80
ng/ml). As shown in Fig. 2C, the
proliferation of the MB231br (MB231br-shNC) and MB231-mPRα (MB231
cells transfected with mPRα overexpression vector) cells, but not
that of the MB231br-shmPRα (MB231br cells transfected with shRNA
targeting mPRα) and MB231 (MB231-vector) cells was inhibited by
progesterone treatment in a dose-dependent manner. We then analyzed
cell apoptosis by examining the expression of cleaved caspase-3 by
western blot analysis following treatment with progesterone. As
shown in Fig. 2D, the expression
of cleaved caspase-3 was upregulated in both the MB231br and
MB231-mPRα cells, but not in the MB231br-shmPRα or MB231 cells
following treatment with progesterone. Thus, these results indicate
that progesterone suppresses the growth of mPRα+ TNBC
cells by inhibiting cell proliferation and inducing cell
apoptosis.
Progesterone suppresses the migration and
invasion of mPRα+ human TNBC cells
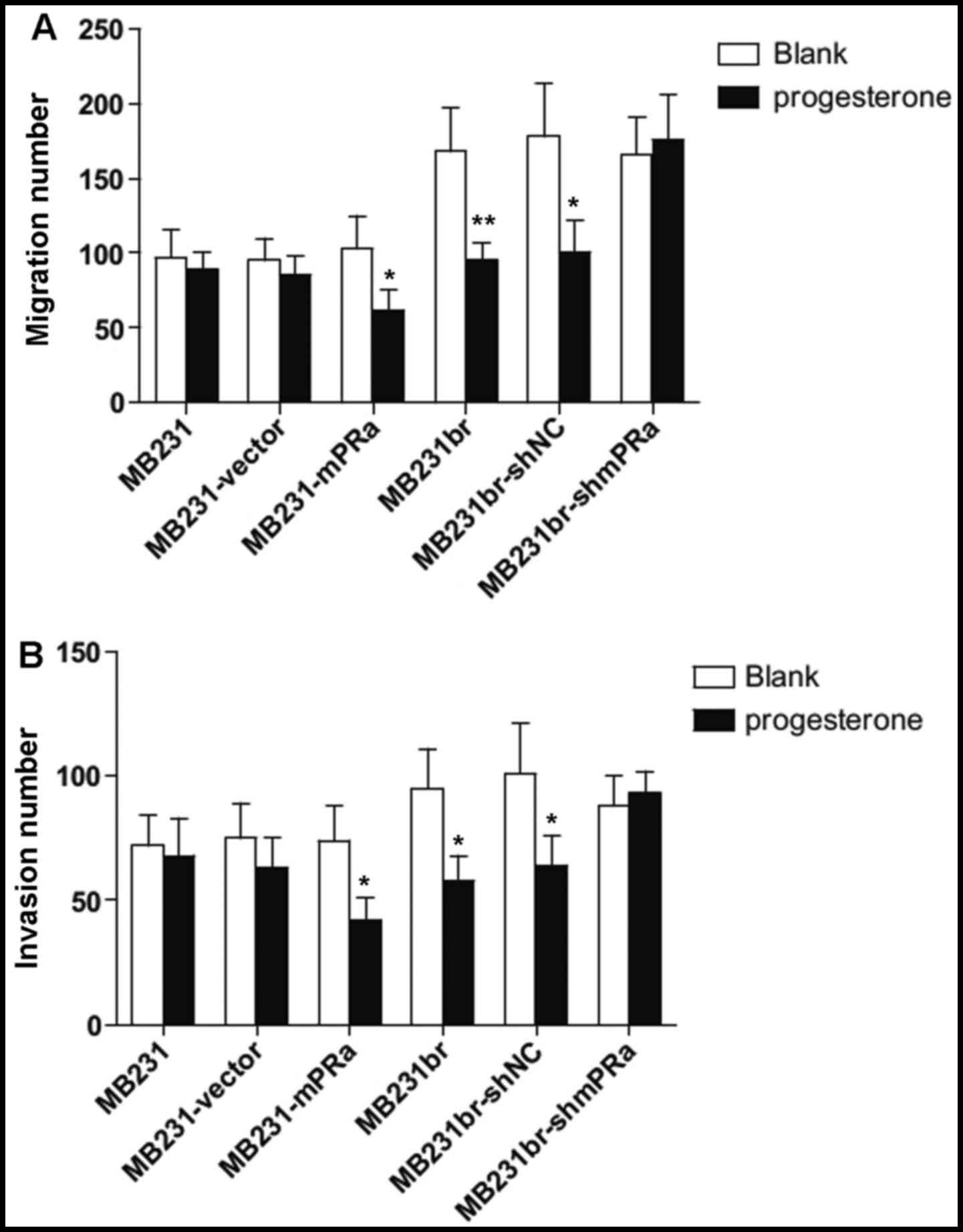
We then examined the effects of progesterone on the
migration and invasion of TNBC cells with a different mPRα
expression status. As shown in Fig.
3A, following treatment with progesterone, the MB231br cells
(untreated group, 168±29 cells/field vs. treated group 95±12
cells/field, P<0.05), but not the MB231 cells (untreated group,
97±19 cells/field vs. treated group 89±12 cells/field) exhibited
decreased migration. This inhibition was blocked by the knockdown
of mPRα expression in the MB231br cells (untreated group, 166±25
cells/field vs. treated MB231br-shmPRα group, 176±30 cells/field)
(Fig. 3A). Moreover, the
introduction of exogenous mPRα cDNA into the MB231 cells enhanced
the responsiveness of the cells to progesterone treatment,
decreasing migration (untreated group, 103±22 cells/field vs.
treated MB231-mPRα group 62±14 cells/field) (Fig. 3A). Similarly, in the presence of
progesterone, the MB231br cells (untreated group, 95±16 cells/field
vs. treated group 58±10 cells/field, P<0.05) and MB231-mPRα
cells (untreated group, 74±14 cells/field vs. treated group 42±9
cells/field, P<0.05), but not the MB231 (untreated group, 72±12
cells/field vs. treated group 68±15 cells/field) or MB231br-shmPRα
cells (untreated group, 88±12 cells/field vs. treated group 93±9
cells/field) exhibited a decreased invasion (Fig. 3B). Thus, these results indicate
that progesterone suppresses the migration and invasion of
mPRα+ TNBC cells.
The inhibitory effects of progesterone in
mPRPα+ human TNBC cells are not mediated by either PR or
PGRMCl
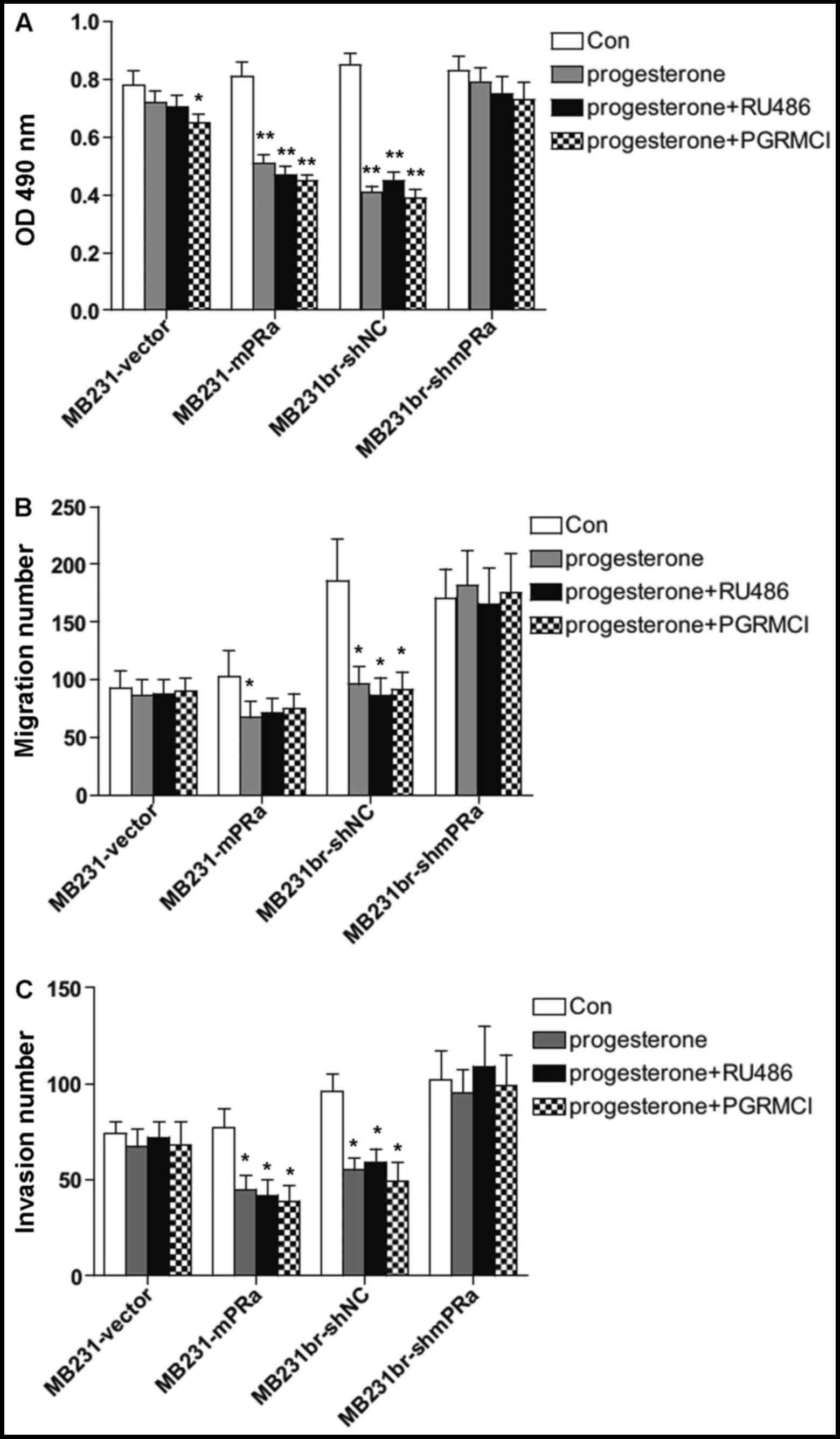
Although the MB231br and MB231 cells are basically
negative for nuclear PR expression, it has been reported that
cancer cells may repress PR in response to sex hormone treatments
(20). In this study, in order to
exclude the role of PR in the above-mentioned effects of
progesterone on TNBC cells, the MB231br-shNC, MB231br-shmPRα,
MB231-vector and MB231-mPRα cells were co-incubated with
progesterone plus RU486 (mifepristone), a PR-specific blocker. As
expected, RU486 had no effects on the inhibitory effects of
progesterone on cell proliferation, migration and invasion
(Fig. 4A, B and C). PGRMCl is the
other type of progesterone membrane receptor that mediates
non-classical progestins actions (21). In this study, in order to exclude
the possible role of PGRMCl in the inhibitory effects of
progesterone on TNBC cells, the MB231br cells and MB231-mPRα cells
were then co-incubated with progesterone plus PGRMCl neutralizing
antibody. As shown in Fig. 4,
PGRMCl neutralizing antibody had no effects on the inhibitory
effects of progesterone on cell proliferation, migration and
invasion. Thus, these results suggested that the inhibitory effects
of progesterone on mPRα+ human TNBC cells are not
mediated by either PR or PGRMCl.
Knockdown of mPRa expression impairs the
inhibitory effects of progesterone on mPRα+ tumor growth
and metastasis in vivo
We further examined whether the knockdown of mPRα
expression in TNBC cells can reverse the inhibitory effects of
progesterone on tumor growth and metastasis in vivo. The
MB231br-NC cells and MB231br-shmPRα cells were injected into the
left ventricle of the hearts of athymic nude mice subjected to
oophorectomy. Five weeks later, the mice were euthanized, and
H&E staining and a CT scan were then performed for the analysis
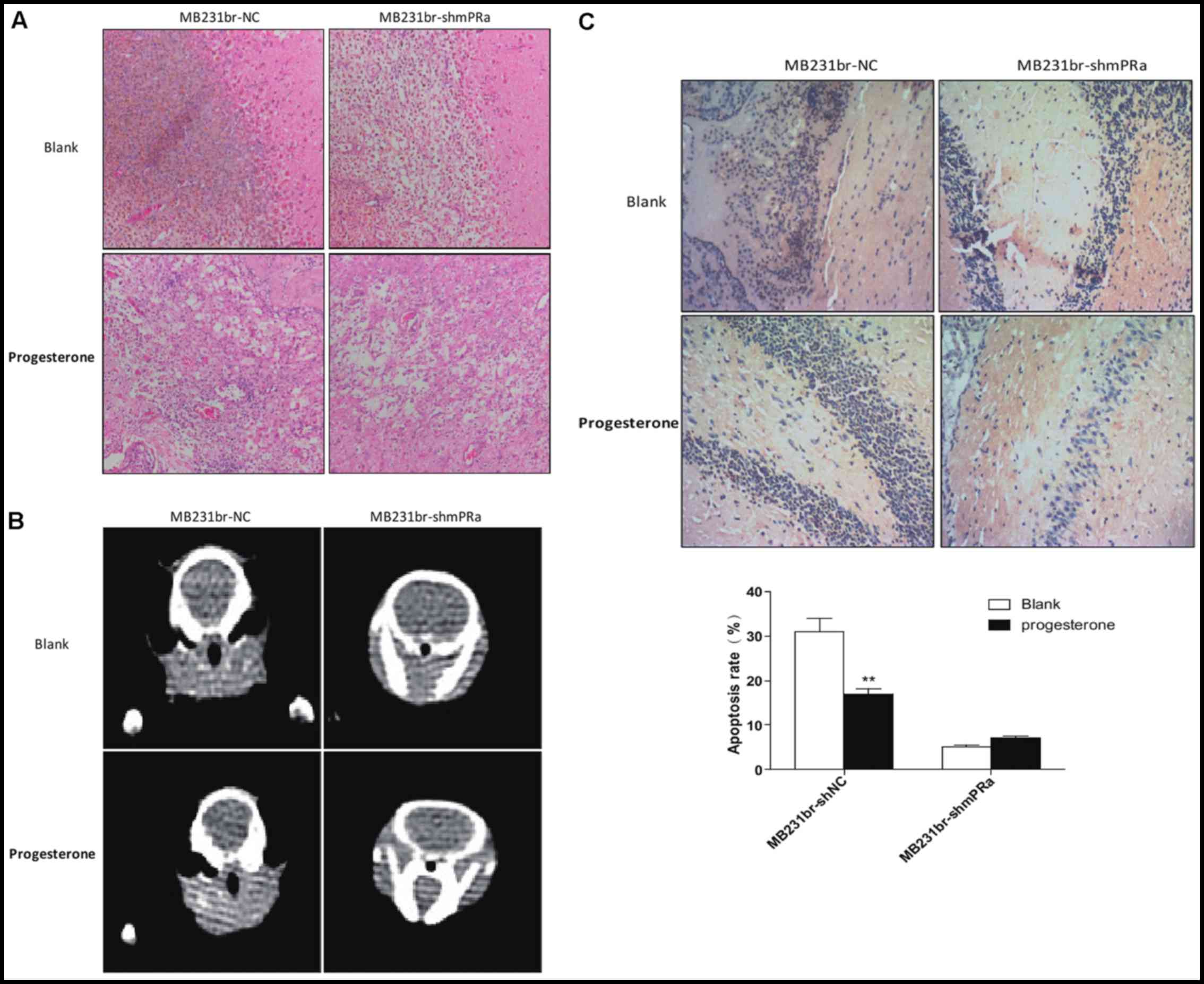
of brain metastasis formation. As shown in Fig. 5A and B, the MB231br-NC cells and
MB231br-shmPRα cells formed brain metastases in the nude mice with
oophorectomy. However, following treatment with progesterone, the
MB231br-NC cells, but not the MB231br-shmPRα cells, did not form
brain metastases in the nude mice with oophorectomy. In addition,
TUNEL assays of the brain tissues were performed. As shown in
Fig. 5C, the MB231br-shmPRα cells
exhibited less tumor cell-positive staining and a significantly
lower apoptotic index than the MB231br-NC cells (P<0.01). These
results suggested that the knockdown of mPRa expression impairs the
inhibitory effects of progesterone on mPRα+ tumor growth
and metastasis in vivo.
Discussion
Progesterone plays an important role in mammary
gland development in females and also appears to be involved in the
development of breast cancer (5,6).
There is evidence to indicate that progesterone promotes rodent
mammary carcinogenesis under certain conditions, in which PR is
necessary for murine mammary gland tumorigenesis (7,8).
Breast cancer is a heterogeneous disease and several distinct
subtypes exist, of which the triple-negative subtype has the worst
clinical prognosis (9,10). However, the role of progesterone
as either a promoter or inhibitor of TNBC that lacks the expression
of PR has not yet been fully elucidated. In this study, we
demonstrated that mPRα was overexpressed in human TNBC tissues and
the expression level of mPRα was negatively associated with the TNM
stage. We found that mPRα mediates the inhibitory effects of
progesterone on TNBC cell growth, migration and invasion in
vitro, as well as growth and metastasis in vivo. These
results provide evidence of a novel mechanism mediated by the
progesterone-mPRα axis in the development and progression of
TNBC.
The mPRα receptor has been associated with
manyphysiologic functions in vertebrates. It induces oocyte
maturation, stimulates sperm hypermotility, modulates immune
function, downregulates gonadotropin-releasing hormone (GnRH)
secretion and adjusts human myometrial cell contractility (11,17,21–23). mPRα was first identified in human
breast cancer biopsies and epithelial-derived breast cancer cell
lines by Dressing et al (18). Recently, it has been identified as
an intermediary factor of the progestin-induced intracellular
signaling cascades in PR− breast cancer cell lines in
vitro (15,18,19). mPRα mediates
epithelial-mesenchymal transition (EMT) through the activation of
the PI3K/Akt pathway (19). In
this study, the expression of mPRα was detected in both normal and
malignant breast tissues and its expression level was negatively
associated with the TNM stage of TNBC, which is consistent with
previous results (18,19,24). However, knowledge of the aberrant
expression and potential role of mPRα in TNBC remains largely
unknown. In this study, we demonstrated that progesterone
suppressed the growth, migration and invasion of human TNBC cells
via mPRα in vitro and in vivo. Importantly, these
effects induced by progesterone treatment were significantly
blocked by transfection with mPRα-specific shRNA. Therefore, mPRα
may contribute to cancer development, proliferation and metastasis
in TNBC.
Classically, progesterone exerts its effects through
the binding of nuclear PR and subsequently activates downstream
pathways (7,8). A previous study demonstrated that
cancer progenitor cells may proliferate and express PR in response
to sex hormone treatments (6);
thus, the classical nuclear PR was first considered as a molecular
mediator of the progesterone's inhibitory effects on TNBC cells
even though they are basically negative for nuclear PR expression
in normal culture condition. PGRMCl is the other type of
progesterone membrane receptor that mediated the non-classical
progestins actions (21). To
exclude the possible role of PR or PGRMCl in the inhibitory effects
of progesterone on TNBC cells, we introduced RU486 or PGRMCl
neutralizing antibody into the culture system; neither of these had
an effect on the inhibitory effects of progesterone. Thus, our data
demonstrate that the status of mPRα in TNBC cells play an essential
role in determining the cell biological behavior of TNBC in
responding to progesterone treatment. However, the detailed
molecular mechanisms need to be further explored in the future.
In conclusion, our study has provided experimental
evidence indicating a role for progesterone in inhibiting TNBC
development and progression. Although the detailed mechanisms
require further investigation, a strong link between mPRα
expression and the effects of progesterone may provide a possible
explanation as to how progesterone suppresses TNBC progression.
Progesterone may play a duel role in breast cancer; therefore, a
better understanding of the role of the progesterone-mPRα axis in
various subtypes of breast cancer may help to provide insight into
its complex function. In conclusion, our study indicates that
progesterone suppresses TNBC cell growth and metastasis to the
brain via mPRα, and may therefore serve as a potential target in
the treatment of TNBC.
Acknowledgments
This study was supported by the Shanghai Natural
Science Foun dation (grant no. 12ZR1422800), the Foundation of
Shanghai TCM Oncology of TCM clinical key subject (ZYXK2012010) and
the Foundation of Pudong New Area tumor key subject groups
(PWZxq2014-12).
References
|
1
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Ruijter TC, Veeck J, de Hoon JP, van
Engeland M and Tjan-Heijnen VC: Characteristics of triple-negative
breast cancer. J Cancer Res Clin Oncol. 137:183–192. 2011.
View Article : Google Scholar :
|
|
3
|
Rakha EA and Chan S: Metastatic
triple-negative breast cancer. Clin Oncol (R Coll Radiol).
23:587–600. 2011. View Article : Google Scholar
|
|
4
|
Macias H and Hinck L: Mammary gland
development. Wiley Interdiscip Rev Dev Biol. 1:533–557. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuhl H and Schneider HP: Progesterone -
promoter or inhibitor of breast cancer. Climacteric. 16(Suppl 1):
54–68. 2013. View Article : Google Scholar
|
|
6
|
Axlund SD and Sartorius CA: Progesterone
regulation of stem and progenitor cells in normal and malignant
breast. Mol Cell Endocrinol. 357:71–79. 2012. View Article : Google Scholar :
|
|
7
|
Lydon JP, Ge G, Kittrell FS, Medina D and
O'Malley BW: Murine mammary gland carcinogenesis is critically
dependent on progesterone receptor function. Cancer Res.
59:4276–4284. 1999.PubMed/NCBI
|
|
8
|
Obr AE and Edwards DP: The biology of
progesterone receptor in the normal mammary gland and in breast
cancer. Mol Cell Endocrinol. 357:4–17. 2012. View Article : Google Scholar :
|
|
9
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: prognostic and therapeutic implications.
World J Clin Oncol. 5:412–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah R, Rosso K and Nathanson SD:
Pathogenesis, prevention, diagnosis and treatment of breast cancer.
World J Clin Oncol. 5:283–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu Y, Rice CD, Pang Y, Pace M and Thomas
P: Cloning, expression, and characterization of a membrane
progestin receptor and evidence it is an intermediary in meiotic
maturation of fish oocytes. Proc Natl Acad Sci USA. 100:2231–2236.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Y, Bond J and Thomas P:
Identification, classification, and partial characterization of
genes in humans and other vertebrates homologous to a fish membrane
progestin receptor. Proc Natl Acad Sci USA. 100:2237–2242. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chapman NR, Kennelly MM, Harper KA,
Europe-Finner GN and Robson SC: Examining the spatio-temporal
expression of mRNA encoding the membrane-bound progesterone
receptor-alpha isoform in human cervix and myometrium during
pregnancy and labour. Mol Hum Reprod. 12:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dosiou C, Hamilton AE, Pang Y, Overgaard
MT, Tulac S, Dong J, Thomas P and Giudice LC: Expression of
membrane progesterone receptors on human T lymphocytes and Jurkat
cells and activation of G-proteins by progesterone. J Endocrinol.
196:67–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dressing GE and Thomas P: Identification
of membrane progestin receptors in human breast cancer cell lines
and biopsies and their potential involvement in breast cancer.
Steroids. 72:111–116. 2007. View Article : Google Scholar
|
|
16
|
Fernandes MS, Pierron V, Michalovich D,
Astle S, Thornton S, Peltoketo H, Lam EW, Gellersen B, Huhtaniemi
I, Allen J, et al: Regulated expression of putative membrane
progestin receptor homologues in human endometrium and gestational
tissues. J Endocrinol. 187:89–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karteris E, Zervou S, Pang Y, Dong J,
Hillhouse EW, Randeva HS and Thomas P: Progesterone signaling in
human myometrium through two novel membrane G protein-coupled
receptors: potential role in functional progesterone withdrawal at
term. Mol Endocrinol. 20:1519–1534. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dressing GE, Alyea R, Pang Y and Thomas P:
Membrane progesterone receptors (mPRs) mediate progestin induced
antimorbidity in breast cancer cells and are expressed in human
breast tumors. Horm Cancer. 3:101–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zuo L, Li W and You S: Progesterone
reverses the mesenchymal phenotypes of basal phenotype breast
cancer cells via a membrane progesterone receptor mediated pathway.
Breast Cancer Res. 12:R342010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai D, Wolf DM, Litman ES, White MJ and
Leslie KK: Progesterone inhibits human endometrial cancer cell
growth and invasiveness: down-regulation of cellular adhesion
molecules through progesterone B receptors. Cancer Res. 62:881–886.
2002.PubMed/NCBI
|
|
21
|
Thomas P: Characteristics of membrane
progestin receptor alpha (mPRalpha) and progesterone membrane
receptor component 1 (PGMRC1) and their roles in mediating rapid
progestin actions. Front Neuroendocrinol. 29:292–312. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sleiter N, Pang Y, Park C, Horton TH, Dong
J, Thomas P and Levine JE: Progesterone receptor A (PRA) and
PRB-independent effects of progesterone on gonadotropin-releasing
hormone release. Endocrinology. 150:3833–3844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tubbs C and Thomas P: Progestin signaling
through an olfactory G protein and membrane progestin
receptor-alpha in atlantic croaker sperm: potential role in
induction of sperm hypermotility. Endocrinology. 150:473–484. 2009.
View Article : Google Scholar
|
|
24
|
Xie M, Zhu X, Liu Z, Shrubsole M, Varma V,
Mayer IA, Dai Q, Chen Q and You S: Membrane progesterone receptor
alpha as a potential prognostic biomarker for breast cancer
survival: a retrospective study. PLoS One. 7:e351982012. View Article : Google Scholar : PubMed/NCBI
|