Introduction
Stroke is the third cause of mortality worldwide and
is clinically characterized by a clear three-high (high incidence,
high morbidity and high mortality) phenomenon (1,2).
Ischemic stroke, which affects approximately 80% patients with
stroke, is currently a mainly leading cause of disability and
mortality in the aged population, due to limited medication and
therapy (3). Nowadays, the only
FDA-approved treatment for ischemic stroke is plasminogen
activator, which is administered within 4.5 h of stroke onset
(4). Therefore, novel therapeutic
strategies are urgently required.
Recently, several hypotheses have been put forward
to explain the pathophysiological processes of stroke. Increasing
evidence indicates that neuronal cell death is a main cellular
event in the pathogenesis of ischemic brain injury, which is often
in the form of apoptosis (5).
Apoptosis is characterized by cell shrinkage, chromatin
condensation, and the formation of cytoplasmic blebs and apoptotic
bodies, which lead to changes in cell permeability and cell
membrance damage (6).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, as well as lactate dehydrogenase (LDH) assay are usually
employed to evaluate cell viability. Succinate dehydrogenase in the
mitochondria of living cells can revert exogenous MTT into
formazan; however, it cannot do so in dead cells. LDH is a soluble
cytosolic enzyme present in most eukaryotic cells and can be
released into the culture medium upon cell death due to plasma
membrane damage (7). Thus, MTT
and LDH assays are regarded as the a valuable enzyme parameters in
apoptosis.
The mitochondrial pathway is a central pathway
leading to apoptosis in cerebral ischemia (8,9). A
number of genes and proteins can influence or instigate the
progression of apoptosis along the mitochondrial pathway (10). The most important genes associated
with apoptosis are proteins of the Bcl family and caspases
(11,12). Bcl-2/Bax family members are key
regulatory factors in the mitochondrial apoptotic pathway (13,14). They are divided into two groups,
anti-apoptotic (Bcl-2, Bcl-xL) and pro-apoptotic (Bax, Bad)
proteins. Upon stimulation with pro-apoptotic factors, Bax
translocates from the cytoplasm to inthe mitochondrial membrane,
which alters the permeability of the mitochondrial membrane and
promotes the release of cytochrome c (Cyt c) from the
mitochondria into the cytoplasm (11). It has been proven that the
activity of the Bcl-2 protein may be regulated through caspase
cleavage under various circumstances. The apoptotic cascade is
subsequently initiated, eventually leading to apoptosis (15).
Mitochondrial dysfunction leads to characteristic
morphological changes in neurons and results in abnormal upstream
and downstream protein expression in the mitochondrial pathway
(16).
In order to combat neuronal cell death, nowadays,
researchers are focusing on herbal extracts or their compounds due
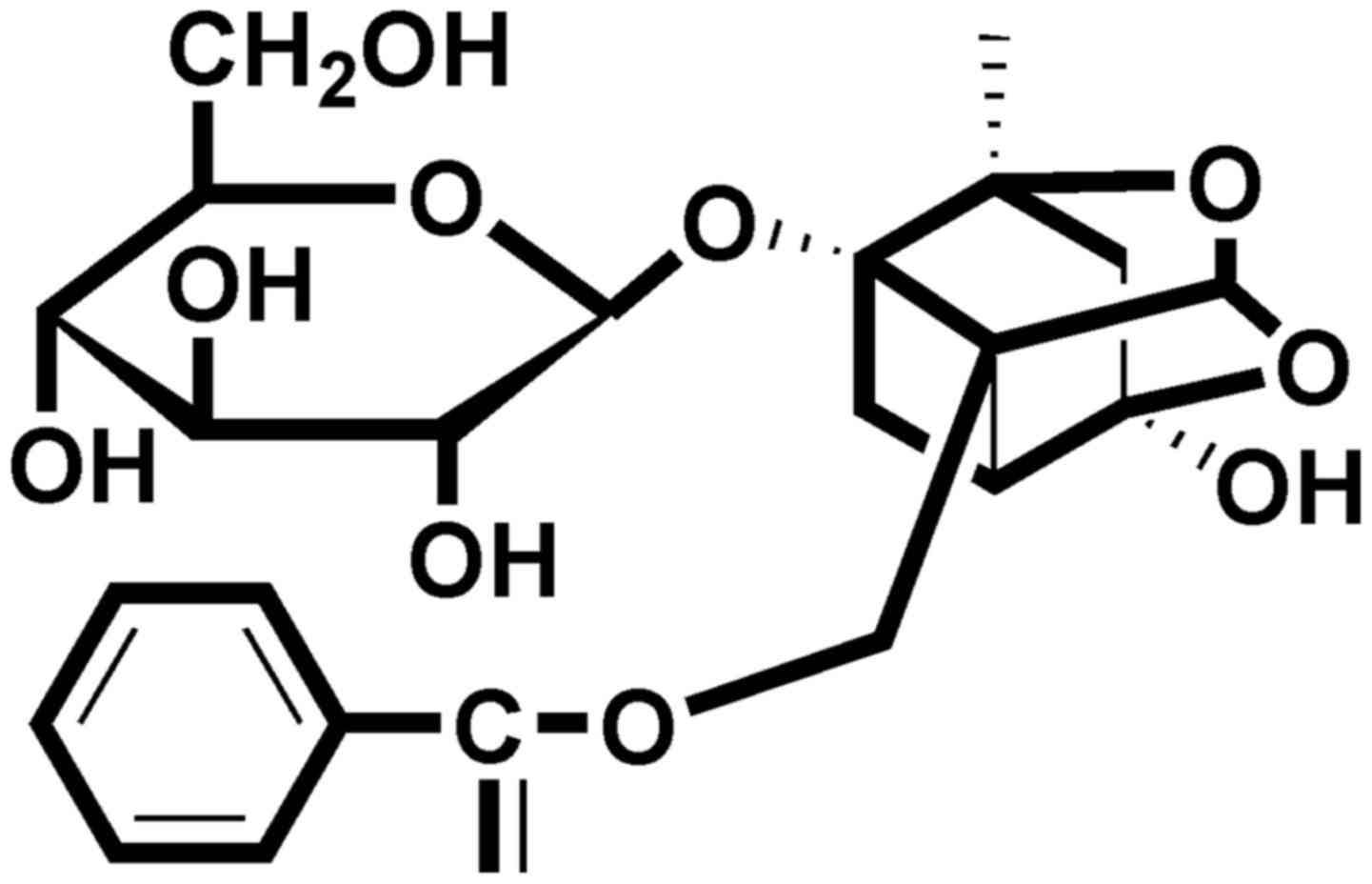
to their novel structures and few side-effects. Paeoniflorin (PF)
(Fig. 1), a monoterpene
glycoside, is a natural compound and the main active ingredient of
Radix Paeoniae (dried root of Paeonia lactiflora Pall.). It
has been reported that PF exerts neuroprotective effects in in
vivo models of cerebral ischemia (17–22) and in in vitro models of
cell injury induced by H2O2 (23), 1-methyl-4-phenylpyridinium
(MPP+) (24),
glutamate (25),
Aβ25–35 (26) and
lipopolysaccharide (27). The
effects of PF have been attributed to the involvement of multiple
modulatory pathways, such as anti-oxidative stress and
anti-inflammatory. Moreover, our recent study suggested that PF
exerted stable and potent neuroprotective effects against cerebral
ischemic injury and protected against N-methyl-D-aspartate
(NMDA)-induced cell apoptosis and neuronal loss (22). In a previous study of ours, we
also found that PF was one of the compounds found in the brain
tissue and cerebrospinal fluid of rats administerd PF after
suffering cerebral ischemia injury (28). Thus, as a continuation, the aim of
this study was to further investigate the neuroprotective effects
of PF against glutamate-induced PC12 cellular cytotoxicity and to
elucidate whether the mitochondrial apoptosis-associated pathway is
involved in these neuroprotective effects. Furthermoe, we
investigated whether the cellular permeability of PF is associated
with its protective effects.
Materials and methods
Reagents
PF (>98% purity) was purchased from the National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China). Dimethyl sulfoxide (DMSO), glutamate,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and Hoechst 33342 were all purchased from Sigma-Aldrich (St. Louis,
MO, USA). Trypsin, RPMI-1640 medium, fetal bovine serum (FBS) and
penicillin-streptomycin were all purchased from Hyclone (Logan, UT,
USA). The LDH assay kit was from Nanjing Jiancheng Biochemical
Reagent Co., Ltd. (Nanjing, China). The Annexin V/propidium iodide
(PI) apoptosis assay kit was obtained from Roche Diagnostics
(Indianapolis, IN, USA). Antibodies to caspase-3 (#9665), caspase-9
(#9508), Bcl-xL (#2764), Bcl-2 (#3498), p-21 (#2947), p-53 (#2524)
and cleaved PARP (#9545) were all purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Antibodies to p-Bad and Bax
were both obtained from Sangon Biological Engineering Co., Ltd.
(Shanghai, China). The secondary antibodies were from Xiamen Lulong
Biotech Development Co., Ltd. (Xiamen, China). Polyvinylidene
fluoride membranes were from Merck KGaA (Darmstadt, Germany). All
other reagents were from the Beyotime Institute of Biotechnology
(Nanjing, China) unless otherwise stated.
Cell culture and treatment
PC12 cells (North Carolina Chuanglian Biotechnology
Research Institute, Beijing, China) were maintained in RPMI-1640
medium supplemented with 10% FBS and 1% penicillin-streptomycin
mixed solution at 37°C in a 5% CO2 incubator. After
seeding onto 96-, 24- or 6-well plates for 24 h, the cells were
cultured in medium without serum and incubated in the presence or
absence of various concentrations of PF for 24 h followed by
exposure to glutamate for 24 h. The control cells were not treated
with PF or glutamate as the vehicle control. The glutamate-exposed
cells were treated with glutamate for 24 h alone.
Determination of cell viability
Cell viability was measured by MTT assay, as well as
by LDH assay. Briefly, for the MTT assay, following treatment, 10
μl MTT solution (5 mg/ml) were added to each well for an
additional 4 h of incubation. The MTT reagent was then replaced
with DMSO (100 μl/well) carefully to dissolve the formazan
crystals. The absorbance at 570 nm was measured using a microplate
reader (Infinite M200 Pro; Tecan, Männedorf, Switzerland). For the
LDH assay, following treatment, the culture medium of each well was
collected for LDH determination. The optical absorbance at 450 nm
was measured using a microplate reader. Results were expressed as
the percentage of the absorbance of the control cells, which was
considered as 100%.
Analysis of morphological changes
The PC12 cells were cultured and treated in a
similar manner as described above. Following treatment,
morphological changes were observed under a phase-contrast
microscope (TS-100F; Nikon, Tokyo, Japan) and images of the cells
were acquired using a digital camera(Olympus, Tokyo, Japan).
Cell apoptosis
Cell apoptosis was determined using two different
methods, as follows:
Fluorescence staining. Following treatment as
described above, the PC12 cells were stained with Hoechst 33342 (5
μg/ml) after removing the culture medium, and the PC12 cells
were then observed under a fluorescence microscope (Advanced
Microscopy Group, Bothell, WA, USA) at ×200 magnification in order
to observe the apoptotic cells.
Flow cytometric analysis. Following
treatment, the PC12 cells were collected and quantified according
to the manufacturer's instructions. Briefly, the PC12 cells were
resuspended in binding buffer and stained with Annexin V/PI for 15
min. The samples were then analyzed using a flow cytometer with an
excitation wavelength of 488 nm and an emission wavelength of 530
nm (Becton-Dickinson, Bedford, MA, USA). Apoptotic cells were
expressed as a percentage of the total number of cells.
Cellular permeability analysis by
high-performance liquid chromatography (HPLC)
In this study, we found that PF at 100 μM
exerted a significant neuroprotective effect; thus, we selected
this concentration to explore its cell permeability. Briefly, the
cells were treated with 100 μM PF for 4, 8 and 24 h to
examine the cellular permeability of PF. Following treatment, the
cells were washed with Tris-buffered saline and collected in a
microcentrifuge tube with ice-cold lysis phosphate-buffered saline
(PBS) buffer. The collections were vortexed after being sonicated.
Intracellular PF was extracted by centrifugation at 12,000 × g for
15 min at 4°C. The supernatant was then collected and filtered
through a 0.22 μm PTFE membrane (Millipore, Milford, MA,
USA) prior to injecting into the HPLC autosampler (Shimadzu Co.,
Kyoto, Japan). The samples were injected into the HPLC system
(Shimadzu Co.) equipped with an diamonsil C18 reversed-phase column
(I.D., 4.6 mm x 250 mm, 5 μm) at a flow rate of 1.0 ml/min
using 30% water (containing 0.1% methanoic acid) and 70%
acetonitrile as the mobile phase with a detection wavelength of UV
230 nm.
Western blot analysis
Following treatment, the cells were harvested and
lysed using RIPA lysis buffer containing protease inhibitor
cocktail (PMSF) on ice, and were subsequently centrifuged at 12,000
× g for 15 min at 4°C. The supernatant was collected and the
protein concentration was determined by the BCA method. The protein
expression levels was then analyzed as described in a previous
study (29). Briefly, protein was
electrophoresed on 12% density SDS acrylamide gels, transferred
from the gel to PVDF membranes using an electric transfer system,
and incubated with antibodies to caspase-3 (1:500), caspase-9
(1:500), p-Bad (1:500), Bcl-xL (1:1,000), Bax (1:1,000), Bcl-2
(1:1,000), cleaved PARP (1:1,000) and β-actin (1:1,000) overnight
at 4°C. Subsequently, the membranes were incubated for 2 h at room
temperature with a secondary antibody (1:7,000). Finally, they were
evaluated using the ECL western detection reagents on Image Lab
analysis software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
β-actin was used as a loading control. Three repeats of the
experiments were performed.
Real-time PCR analysis
Following treatment, the cells were harvested and
total RNA was extracted using TRizol reagent (Invitrogen,
Barcelona, Spain). mRNA was reverse transcribed into cDNA according
to the manufacturer's instructions of the PrimeScript®
RT reagent kit (Takara Bio, Inc., Otsu, Japan). cDNA was used as
template for the Real-Time PCR assays with Power
SYBR®-Green PCR Master mix (Thermo Fisher Scientific,
Inc., Shanghai, China). The PCR analysis was performed in a 7900
Real-Time PCR system (Applied Biosystems, Inc., Foster City, CA,
USA) and the results were analyzed with software provided by the
7900 Real-Time PCR system. The results were expressed as the ratio
between glutamate and control cells. Primer sequences used in the
reactions were as follows: Bax reverse primer, 5′-GAT CAG CTC GGG
CAC TTT AG-3′ and forward primer, 5′-TGC AGA GGA TGA TTG CTG AC-3′;
Bcl-2 reverse primer, 5′-ATG CCG GGT CAG GTA CTC AG-3′ and forward
primer, 5′-GGT GGT GGA GGA ACT CTT CA-3′.
Statistical analysis
Data are presented as the means ± standard deviation
(SD). One-way analysis of variance (ANOVA) [SPSS 20.0 statistical
software (SPSS, Inc., Chicago, IL, USA)] followed by a post hoc LSD
test were used to evaluate multiple group differences. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of PF on PC12 cell viability
The effect of PF itself on the basal growth of PC12
cells was examined. The results (Fig.
2A) revealed that compared with the control group, the
viability of the PC12 cells treated with PF alone at various
concentrations was not significantly increased or decreased (in
cells treated wth PF at 100, 200 and 300 μm, viability was
102.11±6.2, 100.11±6.5 and 99.81±6.6% of control,
respectively).
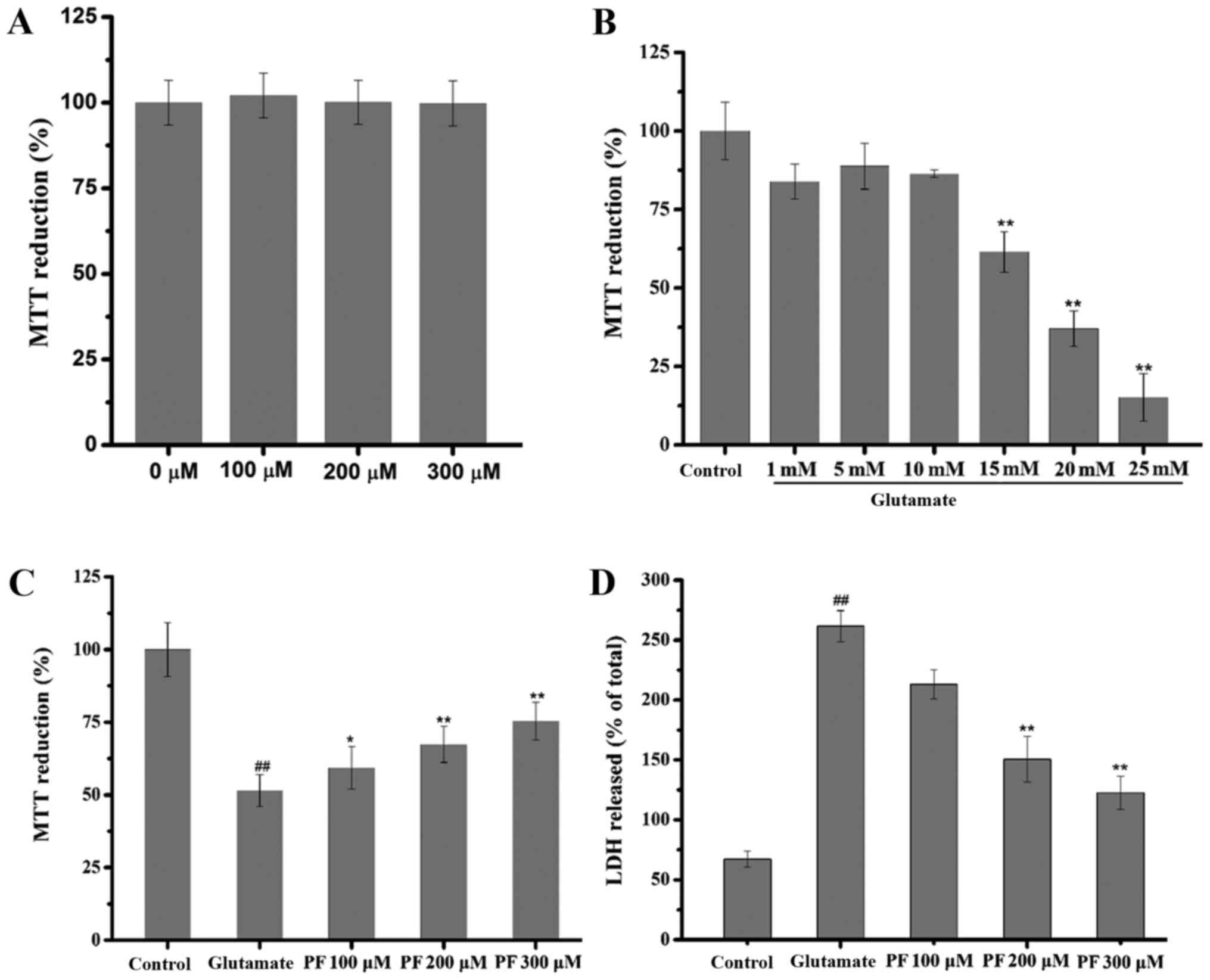 | Figure 2Effect of paeoniflorin (PF) and
glutamate on PC12 cells. (A) Effect of PF itself on basal growth of
PC12 cells. PC12 cells were treated with various concentrations of
PF (100, 200 and 300 μM) for 24 h, and cell viability was
then assessed by MTT assay. Data are represented as the means ± SD,
n=6 wells for each group. (B) Effect of glutamate on the viability
of PC12 cells. PC12 cells were exposed to various concentrations of
glutamate (1, 5, 10, 15, 20 and 25 mM) for 24 h, and cell viability
was then assessed by MTT assay. Data are represented as the means ±
SD, n=6 wells for each group. **p<0.01 vs. control
group. (C) Effect of PF on cell viability in glutamate-exposed
cells and (D) on lactate dehydrogenase (LDH) leakage. Following
treatment of the cells with various concentrations of PF (100, 200
and 300 μM) for 24 h and exposure to 15 mM of glutamate for
24 h, cell viability was assessed by (C) MTT assay and (D) LDH
assay was determined using a microplate reader at 570 and 450 nm,
respectively. Data are represented as the means ± SD, n=6 wells for
each group. ##p<0.01 vs. control group;
*p<0.05, **p<0.01 vs. glutamate group.
All data were analyzed by one-way analysis of variance (ANOVA)
(SPSS 20.0 statistical software) followed by a post hoc LSD test to
evaluate multiple group differences. |
Cytotoxic effects of glutamate on PC12
cells
The concentration-dependent response of
glutamate-induced cytotoxicity was determined by MTT assay. The
results revealed that cell viability was inhibited by glutamate
with an IC50 value of 17.181 mM (Fig. 2B); thus, glutamate at the
concentration of 15 mM was selected for use in the subsequent
experiments.
Effect of PF on the glutamate-induced
decrease in PC12 cell viability
As shown in Fig.
2C, cell viability decreased to 51.5% of control following
exposure to (15 mM) for 24 h. However, cell viability markedly
increased to 59.4, 67.4 and 75.4% of the control following
treatment with various concentrations of PF (100, 200 and 300
μM) prior to exposure to. glutamate
Effect of PF on LDH release
The effect of PF on the release of LDH in the
glutamate-exposed PC12 cells is shown in Fig. 2D. Exposure to glutamate (15 mM)
for 24 h resulted in an increase in LDH release into the medium,
which was significantly increased (261.92%) as compared with the
control group. However, pre-treatment with various concentrations
of PF (100, 200 and 300 μM) for 24 h decreased LDH leakage
to 213.58, 150.51 and 122.02%, respectively.
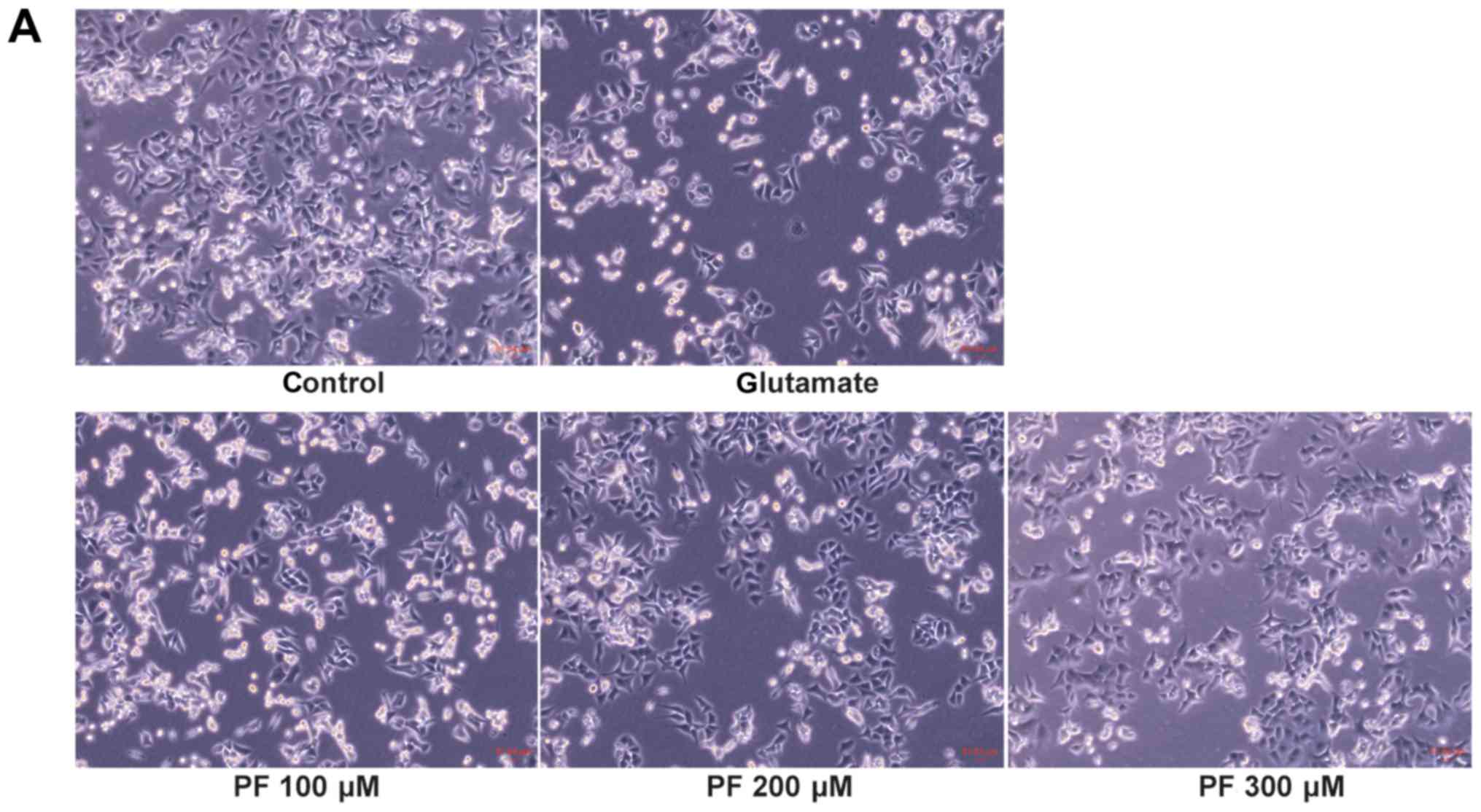
Effect of PF on the apoptosis of PC12
cells
As shown in Fig.
3A, compared with the control group, the morphology of the PC12
cells in the glutamate group was markedly altered; the cells shrank
and became round. However, cell morphology was protected when the
cells were pre-treated with various concentrations of PF prior to
exposure to glutamate.
Fluorescence staining was employed to investigate
the nuclear of apoptosis cell. As shown in the Fig. 3B, apoptotic cells became thinner
with pyknotic nuclei and exhibited light blue fluorescence
following exposure to glutamate for 24 h; in addition, more nuclear
fragmentation was observed than in the control cells. However,
pre-treatment with various concentrations of PF (100, 200 and 300
μM) attenuated these changes and reduced the number of
apoptotic cells.
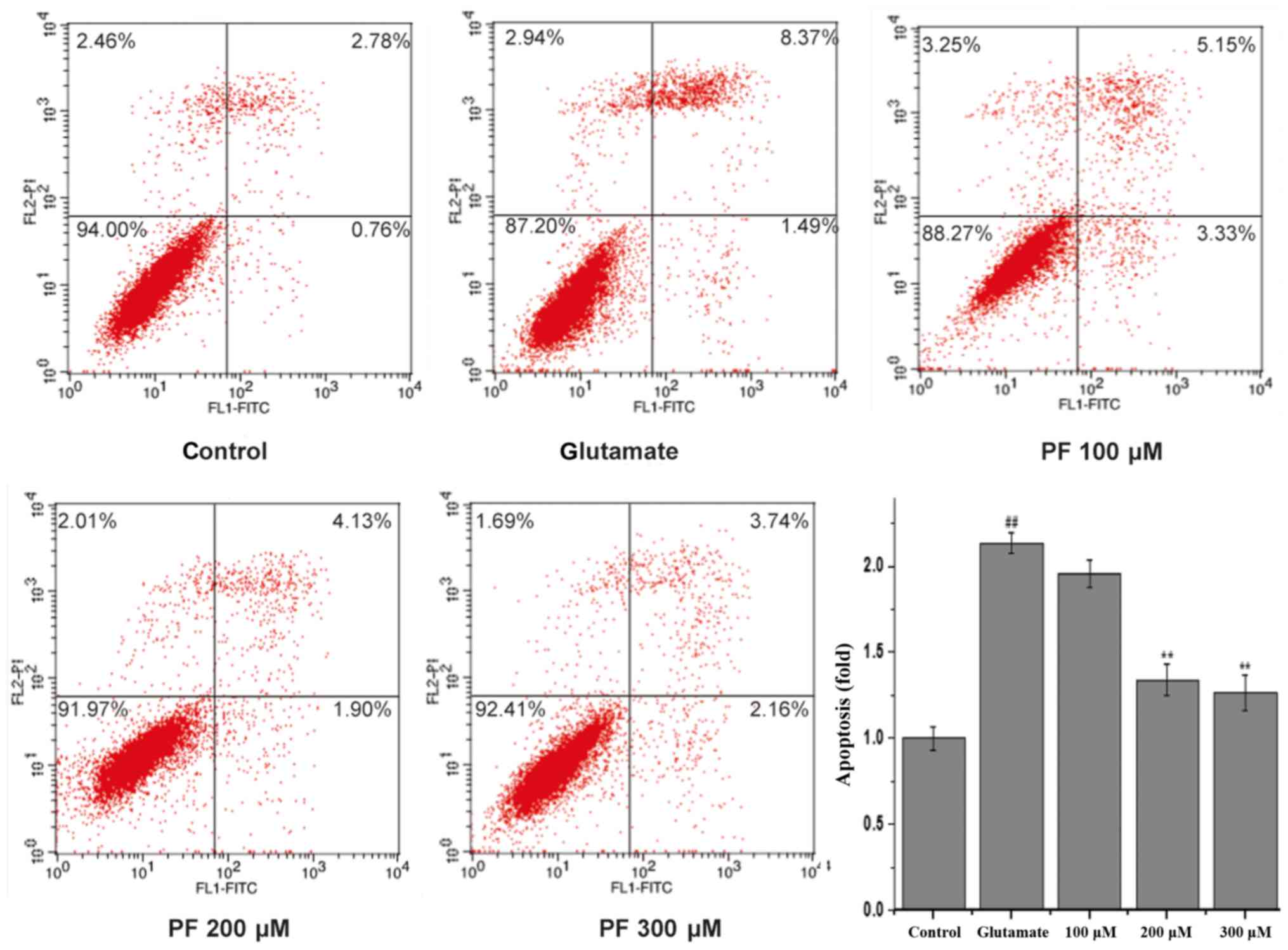
To quantitatively demonstrate the effect of PF on
glutamate-induced apoptosis, Annexin V/PI staining was evaluated by
flow cytometric analysis. As shown in Fig. 4, 3.54% of the total cells were
apoptotic in the control group. However, the apoptotic rate was
markedly increased to 9.86% vs. the control group following
incubation with glutamate. Treatment with PF (100, 200 and 300
μM) markedly reduced the apoptotic ratio of the cells (cell
apoptotic rate was 8.48, 6.03 and 5.9%, respectively).
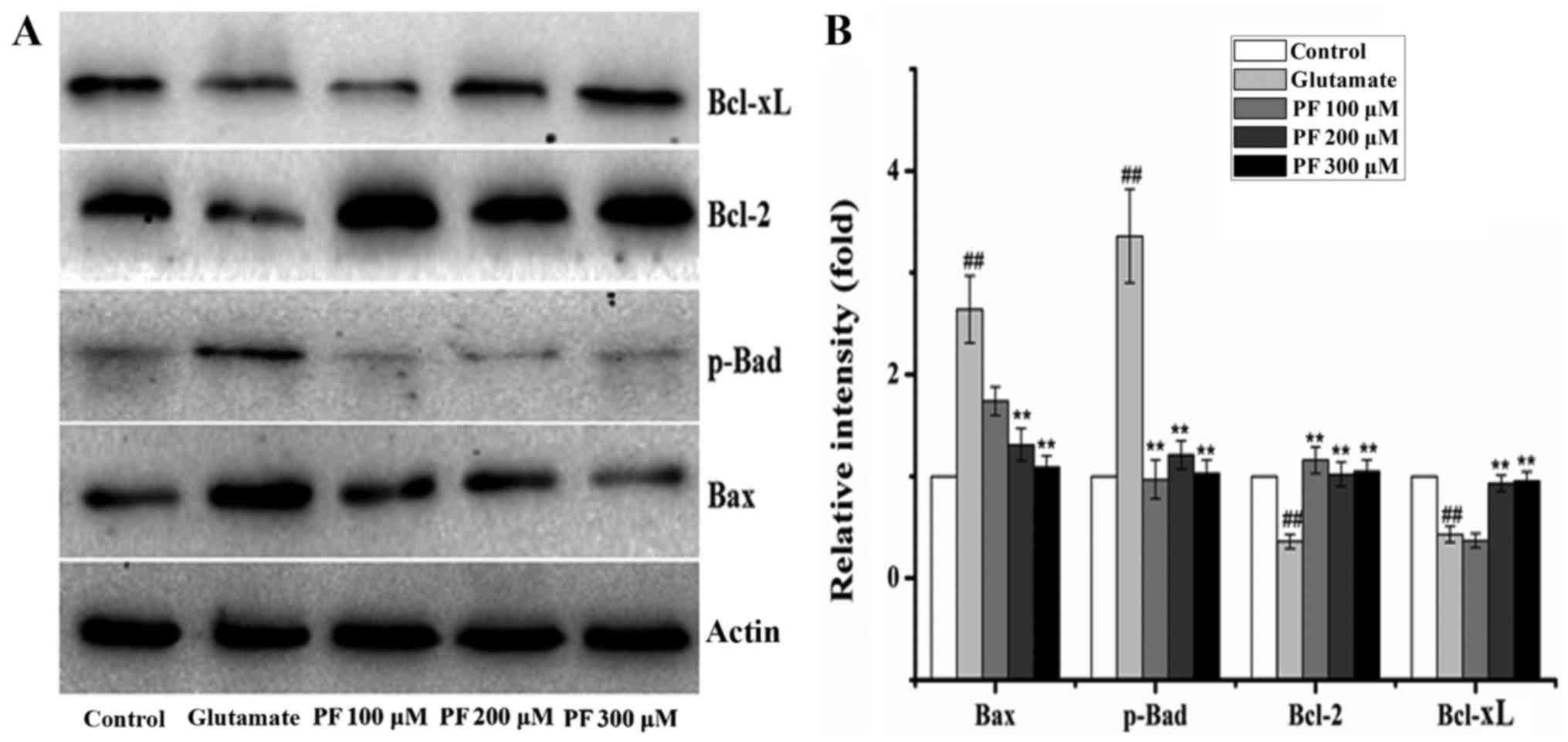
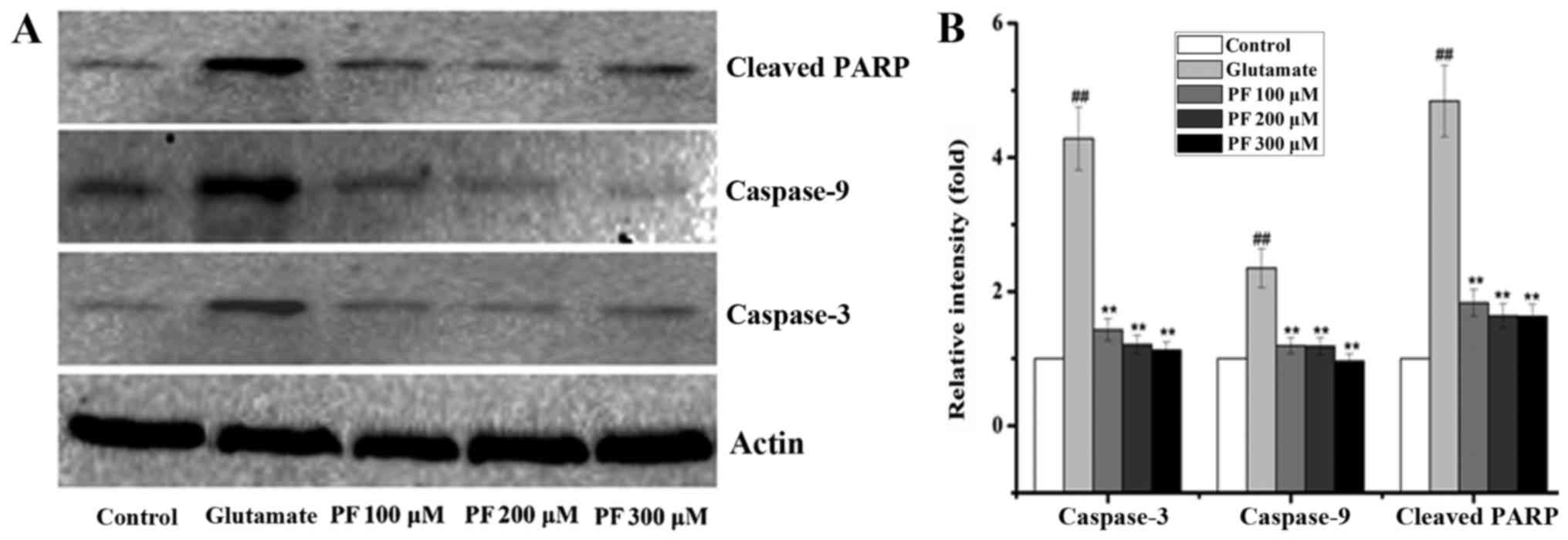
Effects of PF on the expression of
apoptosis-related proteins in PC12 cells
The Bcl-2, Bax, Bcl-xL and p-Bad proteins belong to
the Bcl-2 family and play an important role in cell apoptosis.
Thus, we examined the expression of these proteins. The results
(Fig. 5) revealed that compared
with the control group, the protein expression levels of Bax and
p-Bad were significantly increased following exposure to 15 mM
glutamate for 24 h, whereas the levels of Bcl-2 and Bcl-xL were
significantly decreased. However, after the cells were incubated
with various concentrations of PF (100, 200 and 300 μM) for
24 h prior to expose to glutamate, the protein expression levels of
Bax and p-Bad were decreased to 1.7- and 0.9-fold, 1.3- and
1.2-fold, and 1.1- and 1.1-fold of the glutamate group value,
respectively, and the levels of Bcl-2 and Bcl-xL increased to 1.2-
and 0.4-fold, 1.0- and 0.9-fold, 1.1- and 0.9-fold of the glutamate
group value.
The Bcl-2 family exerts its pro- or anti-apoptotic
effects and activates caspase-9 and caspase-3, leading to
apoptosis. Thus, we also examined the levels of caspase-9 and
caspase-3. As shown in Fig. 6,
glutamate elevated the activity of caspase-3 and caspase-9 in the
PC12 cells, while the levels of caspase-3 and caspase-9 were
significantly decreased by PF. Moreover, PARP is a family of
proteins involved in a number of cellular processes involving
mainly DNA repair and programmed cell death. It can be activated in
cells experiencing stress and/or DNA damage and is inactivated by
caspase cleavage. We also thus exmained the expression of PARP. As
shown in Fig. 6, exposure to
glutamate for 24 h significantly increased the protein level of
cleaved PARP, whereas this was markedly reversed by PF
pre-treatment.
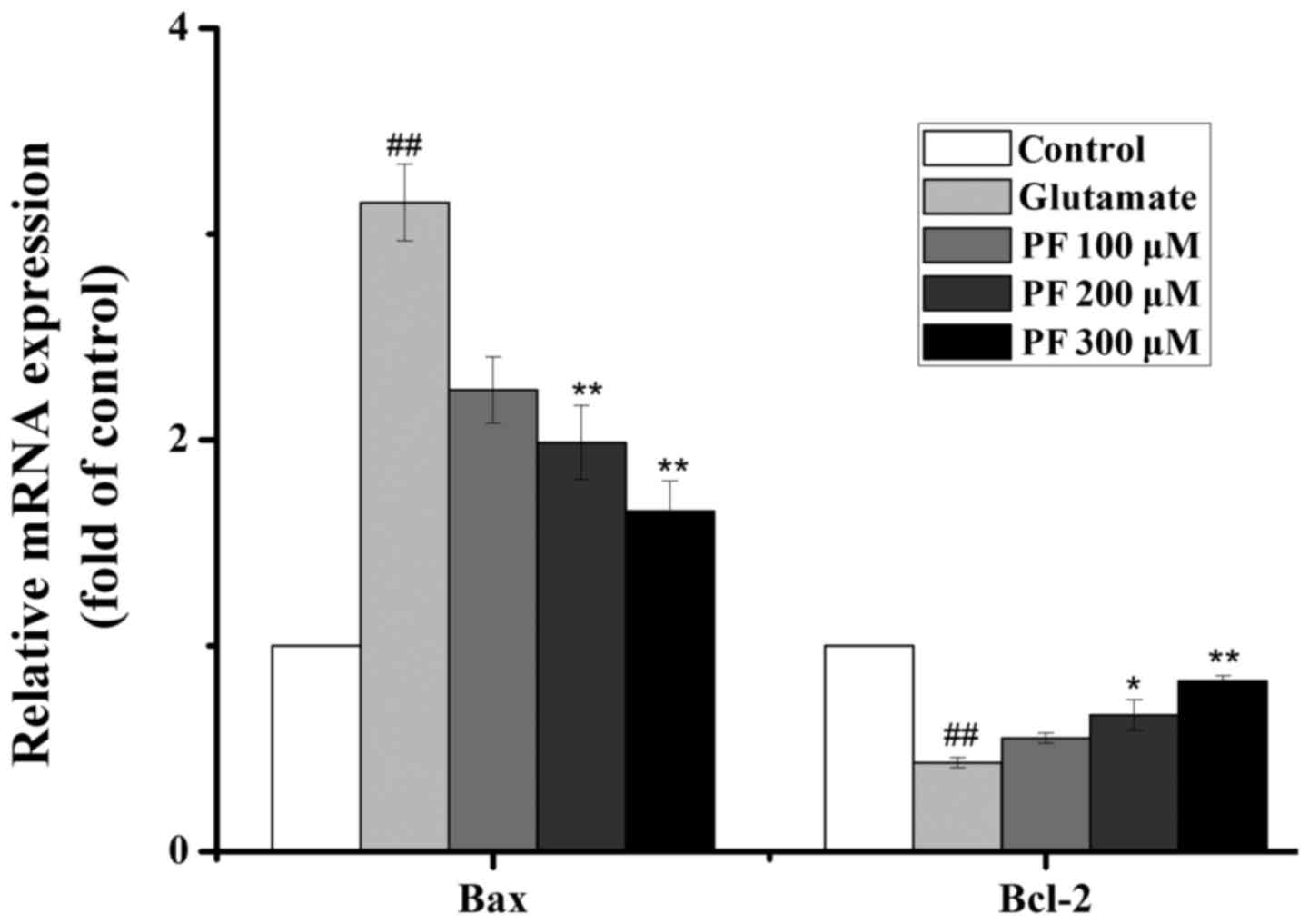
Effects of PF on the mRNA expression of
Bcl-2 and Bax
As presented in Fig.
7, the real-time PCR analysis revealed that exposure to
glutamate increased the mRNA level of Bax and decreased the mRNA
level of Bcl-2. By contrast, PF treatment profoundly downregulated
the mRNA level of Bax and upregulated the mRNA level of Bcl-2.
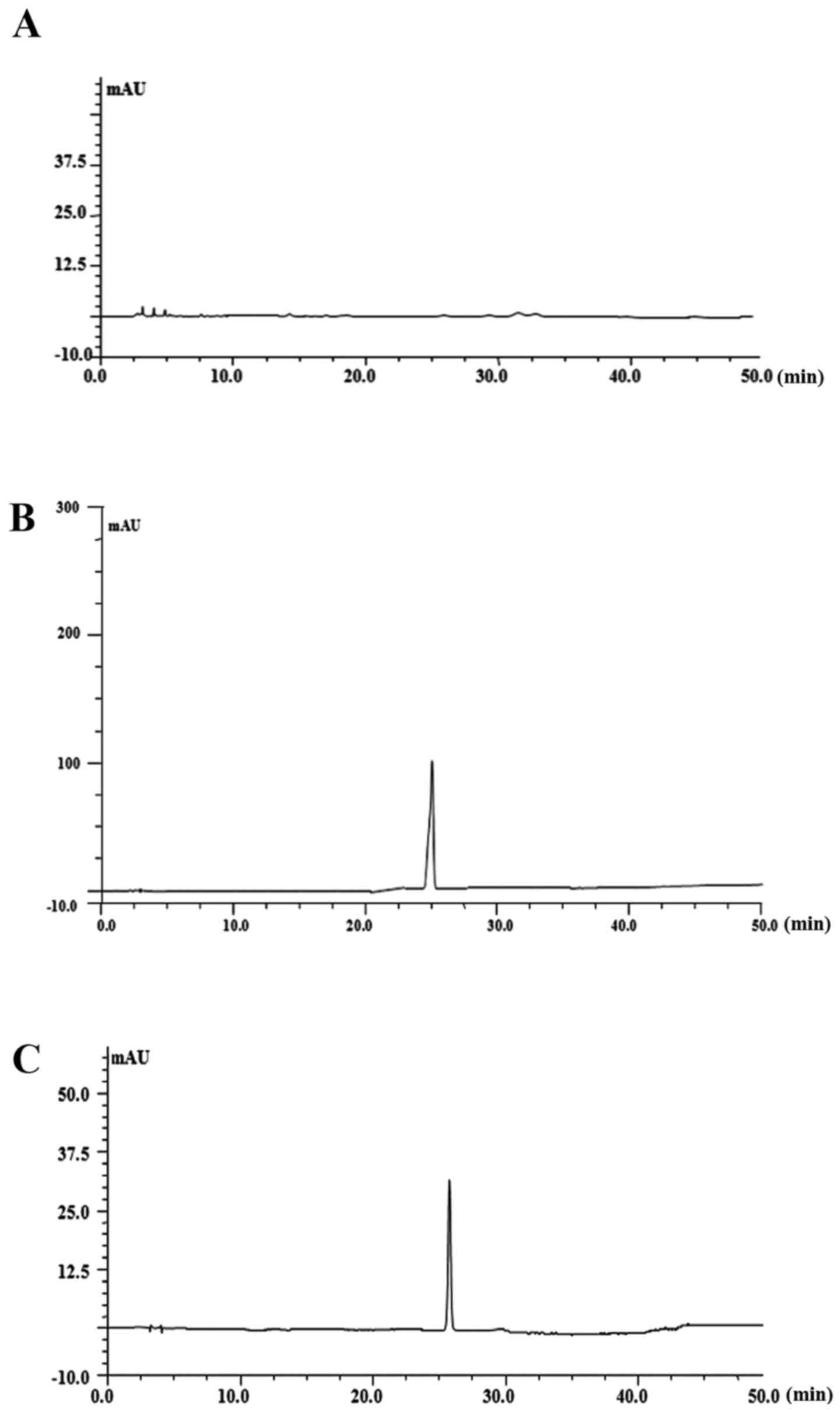
Effect of PF on cell permeability
The permeability of PF across the membrane barrier
in PC12 cells was evaluated by HPLC with the index of the
intracellular content of PF. As shown in Fig. 8, treatment with 100 μM PF
for 8, 12 and 24 h resulted in an increase of PF incorporation to
0.54, 13.4 and 24 μM, respectively.
Discussion
Ischemic stroke is third leading cause of mortality
worldwide and is associated with a high incidence of long-term
disability in surviving individuals (30). It triggers a complex cascade of
pathophysiological processes, including excitotoxicity, oxidative
stress, calcium overload, inflammation and apoptosis (31,32). Excitotoxicity is a major event
that induces neuronal death in ischemic stroke (33). Glutamate is an excitatory
neurotransmitter, which exists in abundance in the hippocampus, the
brain cortex and other body parts of the central nervous system.
Under pathological conditions, the content of glutamate is
increased, and causes the influx of Ca2+, leading to the
apoptosis and necrosis of neurons through various mechanisms. As
such, glutamate is widely used as an excitotoxicity-inducing agent
to study the molecular mechanisms and to develop drugs for ischemic
stroke therapy (34–36).
The exogenous addition of glutamate into cultured
cells induces cells to undergo a series of processes, such as cell
excitotoxicity, DNA fragmentation and cell apoptosis. PC12 cells
are derived from Rattus norvegicus pheochromocytoma tumors,
and it has been proven that their cell morphology and physiological
function are very similr to those of neurons, and the NMDA receptor
with strong affinity for glutamate, is expressed in abundance in
PC12 cell membranes (37,38). Therefore, the PC12 cell line has
been used as an in vitro experimental model of cerebral
ischemia. Previous studies in PC12 cells have found that glutamate
exposure significantly induces apparent cell morphological changes,
a decrease in cell viability and apoptosis (39,40). In this study, the exposure of the
PC12 cells to glutamate significantly altered the cell
morphological characteristics and decreased cell viability; similar
results were obtained from Hoechst 33342 staining and Annexin V/PI
staining. Glutatamate also increased the LDH release from PC12
cells. These findings were consistent with those of previous
studies (39,40). Of note, pre-treatment of the PC12
cells with PF markedly increased cell viability and decreased the
LDH release, which implied that PF protected the PC12 cells agaist
glutamate-induced cytotoxicity, at least partially, due to its
anti-apoptotic effects.
To the best of our knowledge, the apoptosis of
neurons plays an important role in the cerebral ischemia
induced-cascade response (41,42). A number of genes and proteins can
influence the progression of apoptosis along the mitochondrial
pathway (10). The most important
genes of apoptosis are proteins of the Bcl family and caspases
(11,12). There are two groups,
anti-apoptotic (Bcl-2, Bcl-xL) and pro-apoptotic (Bax, Bad)
proteins in the Bcl-2 family (42). The abnormal expression levels of
pro-apoptotic proteins and anti-apoptotic proteins are considered
to determine cell death or survival by controlling apoptosis
(43,44). Previous studies have indicated
that the Bcl-2 family plays a vital regulatory role in cell
apoptosis (15,45). The Bcl-2 and Bcl-xL proteins are
the major anti-apoptotic factors. In the present study, treatment
with PF prior to exposure to glutamate for 24 h significantly
increased the Bcl-2 and Bcl-xL expression level and decreased the
Bax and Bad expression level. Moreover, Bcl-2 mRNA expression was
increased and Bax mRNA expression was decreased. This result
implied that the neuroprotective effects of PF are likely connected
with the inhibition of Bax and Bad expression and the increase of
Bcl-2 and Bcl-xL expression.
Apoptogenic factors release and activate the
executioners of apoptosis, the caspases (46). It has been found that caspases
play an important role in the apoptotic process; the regulation of
caspases has a shown promising effect in attenuating apoptosis
(47). Caspase-3 is the executive
factor of apoptosis, as it activates DNA fragmentation, which in
turn activates endonucleases to cleave nuclear DNA and as a result,
leads to cell death (48,49). Caspase-9 is an initiator caspase
and is activated during programmed cell death (apoptosis). Once
initiated, caspase-9 then cleaves pro-caspase-3, which cleaves
several cellular targets. In the present study, glutamate markedly
increased caspase-3 and caspase-9 protein expression. However,
pre-incubation with PF decreased their expression. Combined with
results of cell viability, the results of Annexin V/PI staining and
the analysis of the upstream and downstream mitochondrial
apoptosis-associated proteins (Bcl-2, Bcl-xL, Bax, Bad, caspase-3
and -9), indicated that PF inhibited the apoptosis of PC12 cells
induced by glutamate, which was consistent with the study of Sun
et al (50). Their study
proved that PF was able to protect PC12 cells from
glutamate-induced cytotoxicity and apoptosis by investigating cell
apoptosis, as well as mitochondrial membrane potential (MMP) by
flow cytometric analysis and the expression profiles of Bcl-2 and
Bax by western blot analysis. In this study, we demonstrated these
effects by analyzing the protein expression profiles of Bcl-2,
Bcl-xL, Bax, Bad, cleaved PARP, caspase-3 and caspase-9, as well as
the mRNA expression of Bcl-2 and Bax. It is known that PF is a
water-soluble compound, and not liposoluble compounds or materials
are not easy to penetrate cell membranes (51). Thus, we investigated whether it
can pass through the cytomembrane when PC12 cells were incubated
with PF in vitro. The intracellular concentrations of PF in
PC12 cells were determined by HPLC analysis. The results
illustrated that PF had difficulty passing through cytomembrane,
even after 24 h of incubation as only small amounts had passed
through. This suggested that the protective effect of PF on
glutamate-induced PC12 cell cytotoxicity was mainly provided with a
small quantity of PF incorporating into the PC12 cells. Therefore,
it was legitimately concluded that the elevation in the
penetrability of PF could obviously produce a stronger protective
effect than our present results in glutamate-exposed PC12 cells.
Although PF has been proven to exert potent neuroprotective
effects, and in this study we used PF to testify the intercellular
peak, and we found that PF was detected in cells, we presumed that
PF may be the active substance. PF may be metabolized in PC12 cells
and perhaps its metabolite is also the active substance. This
requires further investigation in future studies.
In conclusion, the results of this study indicated
that PF is able to protect PC12 cells from glutamate-induced
cytotoxicity and apoptosis by upregulating Bcl-2, Bcl-xL,
downregulating Bax, Bad, cleaved PARP and ultimately inhibiting
caspase-3 and caspase-9 activity. Therefore, PF may be a potential
neuroprotective compound which may be used to protect against
neuronal injury induced by glutamate. However, further studies are
required in order to further elucidate the underlying molecular
mechanisms.
Acknowledgments
This study was carried out at the State Key
Laboratory of Chinese Pharmacies of Fujian Provincial Department of
Science and Technology, the Collaborative Innovation Center for
Rehabilitation Technology, and the TCM Rehabilitation Research
Center of SATCM. This study was supported by grants from the
Project of Fujian Province Colleges and Universities in the New
Century Excellent Talents, the National Natural Science Foundation
of China (nos. 81503204 and 81674046), the 2015 Strategic Emerging
Industries Project of Fujian Province (no. 2015Y0060).
References
|
1
|
Simerabet M, Robin E, Aristi I, Adamczyk
S, Tavernier B, Vallet B, Bordet R and Lebuffe G: Preconditioning
by an in situ administration of hydrogen peroxide: involvement of
reactive oxygen species and mitochondrial ATP-dependent potassium
channel in a cerebral ischemia-reperfusion model. Brain Res.
1240:177–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ,
Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et
al: American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics -2011
update: a report from the American Heart Association. Circulation.
123:e18–e209. 2011. View Article : Google Scholar
|
|
4
|
Peplow PV: Neuroimmunomodulatory effects
of transcranial laser therapy combined with intravenous tPA
administration for acute cerebral ischemic injury. Neural Regen
Res. 10:1186–1190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang F, Yin W and Chen J: Apoptosis in
cerebral ischemia: executional and regulatory signaling mechanisms.
Neurol Res. 26:835–845. 2004. View Article : Google Scholar
|
|
6
|
Reyhane H and Homa M: A comprehensive
review on anticancer mechanisms of the main carotenoid of saffron,
crocin. J Pharm Pharmacol. Jul 3–2017.Epub ahead of print.
|
|
7
|
Liu Y, Peterson DA, Kimura H and Schubert
D: Mechanism of cellular
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reduction. J Neurochem. 69:581–593. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atif F, Yousuf S and Agrawal SK: S-Allyl
L-cysteine diminishes cerebral ischemia-induced mitochondrial
dysfunctions in hippocampus. Brain Res. 1265:128–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu B: Mitochondrial dynamics and
neurodegeneration. Curr Neurol Neurosci Rep. 9:212–219. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Z, Lu W, Li Y and Tang B: Alpinetin
promotes Bax translocation, induces apoptosis through the
mitochondrial pathway and arrests human gastric cancer cells at the
G2/M phase. Mol Med Rep. 7:915–920. 2013.
|
|
11
|
Adams JM and Cory S: Apoptosomes: engines
for caspase activation. Curr Opin Cell Biol. 14:715–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, -6, and -7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar
|
|
13
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szabò I, Soddemann M, Leanza L, Zoratti M
and Gulbins E: Single-point mutations of a lysine residue change
function of Bax and Bcl-xL expressed in Bax- and Bak-less mouse
embryonic fibroblasts: novel insights into the molecular mechanisms
of Bax-induced apoptosis. Cell Death Differ. 18:427–438. 2011.
View Article : Google Scholar :
|
|
15
|
Clem RJ, Cheng EH, Karp CL, Kirsch DG,
Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona
MA, et al: Modulation of cell death by Bcl-xL through caspase
interaction. Proc Natl Acad Sci USA. 95:554–559. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin MY and Sheng ZH: Regulation of
mitochondrial transport in neurons. Exp Cell Res. 334:35–44. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YF, Wu KJ and Wood WG: Paeonia
lactiflora extract attenuating cerebral ischemia and arterial
intimal hyperplasia is mediated by paeoniflorin via modulation of
VSMC migration and Ras/MEK/ERK signaling pathway. Evid Based
Complement Alternat Med. 2013:4824282013.PubMed/NCBI
|
|
18
|
Xiao L, Wang YZ, Liu J, Luo XT, Ye Y and
Zhu XZ: Effects of paeoniflorin on the cerebral infarction,
behavioral and cognitive impairments at the chronic stage of
transient middle cerebral artery occlusion in rats. Life Sci.
78:413–420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang NY, Liu CH, Hsieh CT and Hsieh CL:
The anti-inflammatory effect of paeoniflorin on cerebral infarction
induced by ischemia-reperfusion injury in Sprague-Dawley rats. Am J
Chin Med. 38:51–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoni-florin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar
|
|
21
|
Liu DZ, Xie KQ, Ji XQ, Ye Y, Jiang CL and
Zhu XZ: Neuro-protective effect of paeoniflorin on cerebral
ischemic rat by activating adenosine A1 receptor in a manner
different from its classical agonists. Br J Pharmacol. 146:604–611.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Li H, Huang M, Huang M, Chu K, Xu
W, Zhang S, Que J and Chen L: Paeoniflorin, a monoterpene
glycoside, protects the brain from cerebral ischemic injury via
inhibition of apoptosis. Am J Chin Med. 43:543–557. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu YM, Jin R, Yang L, Zhang J, Yang Q, Guo
YY, Li XB, Liu SB, Luo XX and Zhao MG: Phosphatidylinositol 3
kinase/protein kinase B is responsible for the protection of
paeoniflorin upon H2O2-induced neural
progenitor cell injury. Neuroscience. 240:54–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao BY, Yang YP, Luo WF, Mao CJ, Han R,
Sun X, Cheng J and Liu CF: Paeoniflorin, a potent natural compound,
protects PC12 cells from MPP+ and acidic damage via autophagic
pathway. J Ethnopharmacol. 131:122–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao QQ, Zhong XM, Feng CR, Pan AJ, Li ZY
and Huang Z: Protective effects of paeoniflorin against
glutamate-induced neurotoxicity in PC12 cells via antioxidant
mechanisms and Ca(2+) antagonism. Cell Mol Neurobiol. 30:1059–1066.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang K, Zhu L, Zhu X, Zhang K, Huang B,
Zhang J, Zhang Y, Zhu L, Zhou B and Zhou F: Protective effect of
paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by
preventing mitochondrial dysfunction. Cell Mol Neurobiol.
34:227–234. 2014. View Article : Google Scholar
|
|
27
|
Nam KN, Yae CG, Hong JW, Cho DH, Lee JH
and Lee EH: Paeoniflorin, a monoterpene glycoside, attenuates
lipopolysaccharide-induced neuronal injury and brain microglial
inflammatory response. Biotechnol Lett. 35:1183–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Ye M, Zhang Y, Huang M, Xu W, Chu K,
Chen L and Que J: Blood-brain barrier permeability of Gualou Guizhi
granules and neuroprotective effects in ischemia/reperfusion
injury. Mol Med Rep. 12:1272–1278. 2015.PubMed/NCBI
|
|
29
|
Que J, Ye M, Zhang Y, Xu W, Li H, Xu W and
Chu K: Bryonolic acid, a triterpenoid, protect against
N-methyl-d-aspartate-induced neurotoxicity in PC12 cells.
Molecules. 21:4182016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
World Health Statistics 2011. World Health
Organization; Geneva, Switzerland: pp. 1702011
|
|
31
|
Coyle JT and Puttfarcken P: Oxidative
stress, glutamate, and neurodegenerative disorders. Science.
262:689–695. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dirnagl U, Iadecola C and Moskowitz MA:
Pathobiology of ischaemic stroke: an integrated view. Trends
Neurosci. 22:391–397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takagi N, Besshoh S, Marunouchi T, Takeo S
and Tanonaka K: Effects of metabotropic glutamate mGlu5 receptor
antagonist on tyrosine phosphorylation of NMDA receptor subunits
and cell death in the hippocampus after brain ischemia in rats.
Neurosci Lett. 530:91–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hudspith MJ: Glutamate: a role in normal
brain function, anaesthesia, analgesia and CNS injury. Br J
Anaesth. 78:731–747. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao LL, Du GH and Wang MW: The effect of
salidroside on cell damage induced by glutamate and intracellular
free calcium in PC12 cells. J Asian Nat Prod Res. 8:159–165. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu L, Wang N, Zhang Y, Wang Y, Li J, Wu Q
and Liu Y: Neuroprotective effect of muscone on glutamate-induced
apoptosis in PC12 cells via antioxidant and Ca(2+) antagonism.
Neurochem Int. 70:10–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawakami Z, Kanno H, Ikarashi Y and Kase
Y: Yokukansan, a kampo medicine, protects against glutamate
cytotoxicity due to oxidative stress in PC12 cells. J
Ethnopharmacol. 134:74–81. 2011. View Article : Google Scholar
|
|
38
|
Ma S, Liu H, Jiao H, Wang L, Chen L, Liang
J, Zhao M and Zhang X: Neuroprotective effect of ginkgolide K on
glutamate-induced cytotoxicity in PC12 cells via inhibition of ROS
generation and Ca(2+) influx. Neurotoxicology. 33:59–69. 2012.
View Article : Google Scholar
|
|
39
|
Wang X and Zhu G: Study on protective
effect of salvianolic acid B on glutamate-induced excitotoxicity in
pheochromocytoma PC12 cells. Zhongguo Zhong Yao Za Zhi. 37:353–357.
2012.In Chinese. PubMed/NCBI
|
|
40
|
Song MX, Yang JC and Wang WP: A study of
PC12 cell damage induced by glutamate. J Univer Sci Techno of
Suzhou. 23:62–65. 2006.
|
|
41
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ouyang YB and Giffard RG: MicroRNAs affect
BCL-2 family proteins in the setting of cerebral ischemia.
Neurochem Int. 77:2–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Autret A and Martin SJ: Bcl-2 family
proteins and mitochondrial fission/fusion dynamics. Cell Mol Life
Sci. 67:1599–1606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu X, Xu K, Yan M, Wang Y and Zheng X:
Protective effects of galantamine against Aβ-induced PC12 cell
apoptosis by preventing mitochondrial dysfunction and endoplasmic
reticulum stress. Neurochem Int. 57:588–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chornyy S, Parkhomenko J and Chorna N:
Thiamine deficiency caused by thiamine antagonists triggers
upregulation of apoptosis inducing factor gene expression and leads
to caspase 3-mediated apoptosis in neuronally differentiated rat
PC-12 cells. Acta Biochim Pol. 54:315–322. 2007.PubMed/NCBI
|
|
49
|
Zhang Y, Goodyer C and LeBlanc A:
Selective and protracted apoptosis in human primary neurons
microinjected with active caspase-3, -6, -7, and -8. J Neurosci.
20:8384–8389. 2000.PubMed/NCBI
|
|
50
|
Sun R, Wang K, Wu D, Li X and Ou Y:
Protective effect of paeoniflorin against glutamate-induced
neurotoxicity in PC12 cells via Bcl-2/Bax signal pathway. Folia
Neuropathol. 50:270–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tanaka T, Kataoka M, Tsuboi N and Kouno I:
New monoterpene glycoside esters and phenolic constituents of
Paeoniae radix, and increase of water solubility of
proanthocyanidins in the presence of paeoniflorin. Chem Pharm Bull
(Tokyo). 48:201–207. 2000. View Article : Google Scholar
|