Introduction
Hair follicles (HFs) are complicated organs composed
of multiple layers of epithelia of the outer root sheath (ORS)
keratinocytes, the matrix and its derivatives; the inner root
sheath and hair shaft; and mesenchymal cells called the dermal
papilla (DP) (1,2). The DP, which is surrounded by the
dermal sheath and the hair matrix, is considered to be essential to
hair induction because of secreted diffusible proteins that
regulate the growth and activity of the various cells in the
follicle (3,4). The ORS keratinocytes of the HF
surround the hair fiber and inner root sheath. The ORS
keratinocytes is distinct from other epidermal components, being
continuous with the surface epidermis. The ORS keratinocytes
consist of several layers of cells that can be identified by their
unique ultrastructural properties (1). Hair growth and the cycling of HFs
requires reciprocal interactions between the human dermal papilla
cells (hDPCs) and ORS keratinocytes (5).
Apoptosis can serve a role in follicular
miniaturization, but its association with androgenetic alopecia in
males is controversial (6–8).
Apoptosis is a complex process regulated by the Bcl-2 gene family
(9). The family members act as
anti- or pro-apoptotic regulators that are involved in a wide
variety of cellular activities. Bcl-2, an apoptosis inhibitor, and
Bax, an apoptosis promoter, show tightly regulated, hair
cycle-dependent expression patterns (10). Normal HFs also express high levels
of anti-apoptotic protein Bcl-2 (6,7).
Dickkopf-1 (DKK-1), which is a potent antagonist of
the Wnt/β-catenin signaling pathway, is inducible by
dihydrotestosterone (DHT) and promotes catagen progression and the
apoptotic cell death of HFs (11). Kwack et al (12,13) demonstrated that DKK-1 is secreted
from hDPCs in response to DHT and that it promotes the regression
of HFs by blocking Wnt/β-catenin signaling and by inhibiting the
growth of ORS keratinocytes and triggering apoptotic cell death.
The reports also identified that, although DKK-1 treatment rapidly
changed the anti-apoptotic protein Bcl-2, DKK-1 promoted the
pro-apoptotic protein Bax in a dose-dependent manner in ORS
keratinocytes.
Panax ginseng (PG) has a wide range of
pharmacological effects including anti-inflammatory (14,15), antioxidant (16), anticancer (17) and anti-aging (18–22) effects as well as the promotion of
hair growth (23,24). PG contains many other ingredients
such as sugars, proteins and lipids besides ginsenosides.
Ginsenosides are a unique component of ginseng that is found only
in ginseng, while sugars and proteins are common components of
other plants. Also, various studies have indicated that the
pharmacological effect of ginseng is derived from ginsenosides
(25,26). Recently, the authors reported that
PG extract, which is a ginsenoside-enriched PG extract made using
the repeated fractionalizing method, significantly enhanced the
proliferation of hDPCs, potassium channel-opening activity, and
human HF growth via a mechanism similar to that of minoxidil
(27). Usually, ginsenosides of
commercial PG extract are 3–6%, but a ginsenoside-enriched PG
extract are concentrated up to 20% using the preparation method
used. The major ginsenosides detected in the ginsenoside-enriched
PG extract were Rb1, Rb2, Rc, Rd, Re and Rg1. One of them,
ginsenoside Re showed the highest level among the six ginsenosides
and its content was approximately 6.23% (w/w) (27). In the current study, the authors
investigated the inhibitory effect of ginsenoside-enriched PG
extract on DKK-1-induced apoptosis in HFs in addition to the
underlying mechanism of action.
Materials and methods
The preparation of PG extract
The authors conducted experiments using the same
samples as PG extract, which had hair growth effect in our previous
studies (27). The root of PG was
obtained from Geumsan Ginseng Market (Geumsan-gun, Korea). The
dried and crushed roots of PG (300 g) were extracted with 70%
aqueous ethanol at 50°C for 8 h. The extracts were filtered and
concentrated under reduced pressure at 60°C. The residue was
dissolved with 100% ethanol and repeat filtration and vacuum
distillation.
Materials
Minoxidil, MTT and dimethyl sulfoxide were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Human DKK-1
was purchased from R&D Systems, Inc. (Minneapolis, MN,
USA).
Isolation and cultures of human ORS
keratinocytes
Non-balding scalp specimens were obtained from
patients undergoing hair transplantation surgery (IRB:DKUH
2013-08-012-001). The medical ethical committee of the Dankook
Medical College (Department of Dermatology, Cheonan, Korea)
approved all of the described studies, and informed written consent
was obtained from the patients. HFs were isolated and cultured by
the previously described method, with minor modifications (28). Cultured ORS keratinocytes of early
passage were used for the experiments and were maintained at 37°C
in a humidified atmosphere with 5% CO2.
MTT assay
Cell viability was determined using an MTT assay
that was performed by a slight modification of the method described
by Philpott et al (29).
Briefly, ORS keratinocytes were seeded at a density of
2×104 cells/well into 96-well plates and were cultured
for 24 h. Prior to treatment, the cells were cultured for 24 h in a
growth supplement-free medium. The cells were then treated with PG
extract and DKK-1 for 24 h. The samples were assessed by measuring
absorbance at 540 nm with a Synergy™ 2 Multi-Detection Microplate
Reader (BioTek Instruments, Inc., Winooski, VT, USA). The cell
viability rates were calculated from the optical density readings
and are represented as percentages of the control value (untreated
cells).
Reverse transcription-quantitative
polymerase chain reaction
The total RNA was isolated using TRIzol™ reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
2 µg RNA was reverse-transcribed into cDNA using
SuperScript® III Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.). Quantitative real-time TaqMan PCR technology
(TaqMan Universal PCR Master Mix, part no. 4304437) was used
(Applied Biosystems; Thermo Fisher Scientific, Inc., Santa Clara,
CA, USA). The cDNA samples were analyzed to determine the
expression of the following: Hs00608023_m1 (Bcl-2), Hs00180269_m1
(Bax), and Hs02758991_g1 (GAPDH). Commercially available these
probes were purchased and used in Thermo Fisher Scientific,
Inc.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick-end labelling
(TUNEL) assay
A TUNEL kit (In Situ Cell Death Detection
kit, Fluorescein, Roche Diagnostics GmbH, Mannheim, Germany) was
used according to the manufacturer's protocol to evaluate apoptotic
cells. Briefly, ORS keratinocytes at 2×104 cells/200
µl were seeded into eight-chamber slides (Nunc Lab-Tek;
Thermo Fisher Scientific, Inc., Roskilde, Denmark), were
serum-starved for 24 h, and were then treated with PG extract and
DKK-1 for 24 h. These cells were then fixed in 4% paraformaldehyde
for 10 min. After being washed with PBS, the cells were incubated
with 0.1% Triton X-100 in 0.1% sodium citrate for 1 h at room
temperature. After washing, the cells were treated with the TUNEL
reaction mixture and then were counterstained with
4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei.
Representative images were taken with a fluorescence microscope
(Olympus Corp., Tokyo, Japan) at ×100 magnification.
Immunocytochemistry assay
ORS keratinocytes at 2×104 cells/200
µl were seeded into eight-chamber slides, and then treated
with DKK-1 and PG extract for 24 h. These cells were then fixed in
4% paraformaldehyde for 10 min. After washing with Dulbecco's PBS,
the cells were permeabilized with 0.1% Triton X-100 in PBS for 10
min at room temperature and then blocked with 5% BSA in 0.05%
Triton X-100 for 30 min at room temperature. The samples were
incubated with Bcl-2 (1:200 dilution, sc-783; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and Bax antibody (1:200
dilution, sc-6236; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. They were then washed two times with PBS and four times
with distilled water followed by incubation with a Alexa Fluor™ 488
anti-rabbit (1:200 dilution, A-11034; Thermo Fisher Scientific,
Inc.) and Texas Red™-X anti-rabbit (1:200 dilution, T-6391; Thermo
Fisher Scientific, Inc.) secondary antibodies in 5% BSA blocking
solution for 2 h at room temperature. All samples were
counterstained with DAPI to visualize the nuclei. Representative
images were taken with a fluorescence microscope (Olympus Corp.) at
×100 magnification.
HF organ culture and assessment of hair
elongation
Anagen HFs from human scalp skin specimens were
obtained from patients undergoing hair transplantation surgery. The
medical ethical committee of the Dankook University Hospital
(Cheonan, Korea) approved all of the described studies. A total of
six HFs/well in 24-well plates were cultured in William's E medium
at 37°C in a humidified atmosphere with 5% CO2 in 500
µl basal medium supplemented with 10 µg/ml insulin,
10 ng/ml hydrocortisone, 2 mM L-glutamine, 0.1% Fungizone, 10
µg/ml streptomycin and 100 U/ml penicillin according to
Philpott's method, as previous described (30). Each experimental group contained
at least 30 anagen HFs derived from three different human
donors/volunteers. DKK-1 (50 ng/ml) was determined to optimally
induce catagen-like changes, shorten hair shaft length and hair
bulbs with minimal other histological alterations to the HFs.
Either the PG extract or MNX was added at the concentration,
respectively, of 20 ppm or 50 µM. The incubation medium was
renewed every 2 days. The HF elongation was measured directly at 2,
5 and 7 days of culture using a light stereo microscope (Olympus
Corp.).
Statistical analysis
The results are expressed as mean ± standard
deviation. The data was analyzed using a Student's t-test, and the
two-tailed value of P<0.05 was considered to indicate a
statistically significant difference. The data was processed by
SPSS software for Windows, version 22.0 (SPSS Inc., Chicago, IL,
USA).
Results
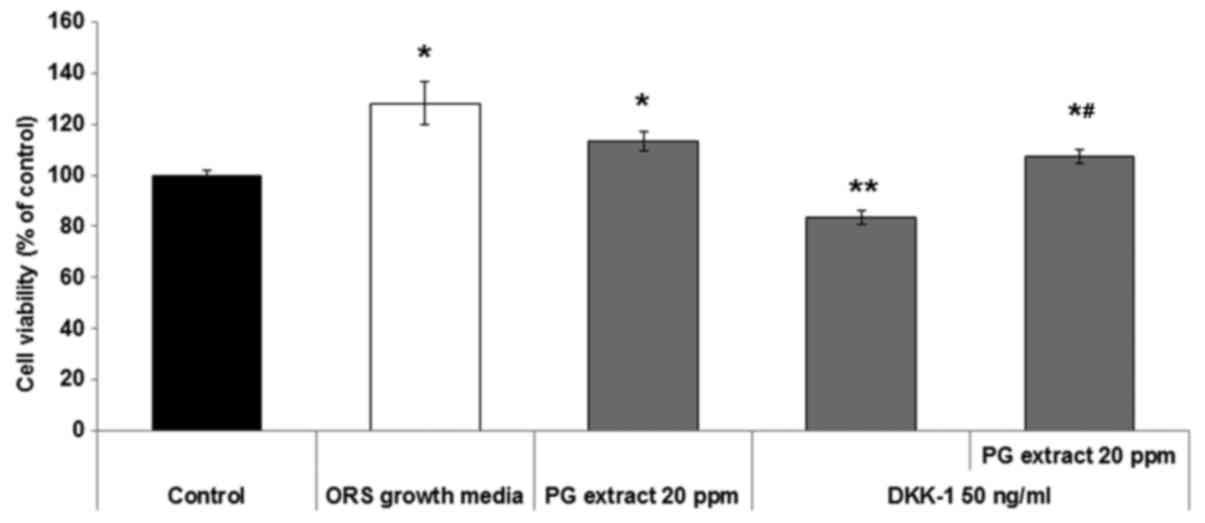
PG extract stimulates proliferation and
inhibits apoptosis in ORS keratinocytes
To investigate the potential role of PG extract on
the proliferation and inhibition of apoptosis in ORS keratinocytes,
the authors performed an MTT assay one day after treatment in the
presence or absence of DKK-1 and PG extract. The PG extract and
DKK-1 concentrations were determined by a previous study of the
authors (data not shown). The results indicated that the PG extract
enhanced the proliferation of ORS keratinocytes (Fig. 1) compared to untreated negative
controls and enhanced ORS growth cultured positive controls with
growth supplement medium. Treatment with DKK-1 (50 ng/ml)
significantly inhibited the viability of ORS keratinocytes compared
to the untreated negative control. PG extract significantly
counteracted the inhibitory effect of apoptosis by DKK-1 on ORS
keratinocyte (Fig. 1)
viability.
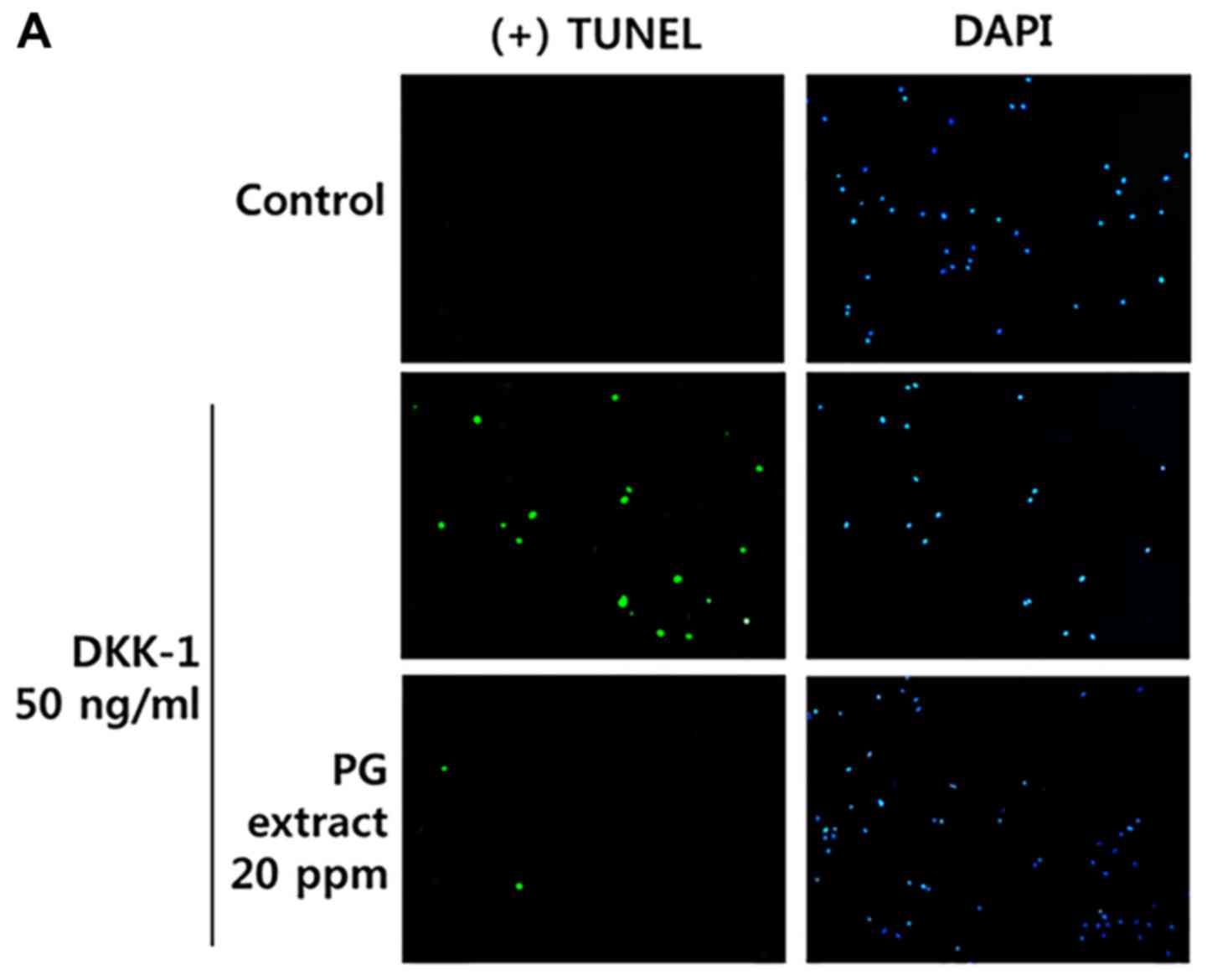
To confirm the inhibitory effect of apoptosis by the
PG extract in ORS keratinocytes, a TUNEL assay was performed.
TUNEL-positive cells undergoing apoptosis significantly increased
when ORS keratinocytes (Fig. 2A and
B) were treated with 50 ng/ml of DKK-1. The PG extract
decreased TUNEL-positive cells despite co-treatment with DKK-1.
Previously, it was found that there are many kinds
of ginsenosides in total extract by high-performance liquid
chromatography analysis (27). In
order to determine which of these ginsenosides is most effective,
we selected three representative ginsenosides of PG extract. (Rb1,
Re, Rg1) and conducted further experiments. As a result (Fig. 2C and Table I), it was demonstrated that each
ginsenoside inhibited apoptosis induced by DKK-1 to some extent.
However, each effect was found to be insufficient for total
extract.
 | Table IQuantification of apoptosis in ORS
keratinocytes by counting TUNEL-positive cells manually. |
Table I
Quantification of apoptosis in ORS
keratinocytes by counting TUNEL-positive cells manually.
| Sample | Apoptosis (%) |
|---|
| Control | 7.92±5.50 |
| DKK-1 (50
ng/ml) |
40.29±17.64a |
| PG extract (20 ppm)
+ DKK-1 (50 ng/ml) |
8.47±1.68b |
| Rb1 (1 µM) +
DKK-1 (50 ng/ml) |
33.15±10.34a |
| Re (1 µM) +
DKK-1 (50 ng/ml) | 32.30±13.01 |
| Rg1 (1 µM)+
DKK-1 (50 ng/ml) | 18.05±1.54 |
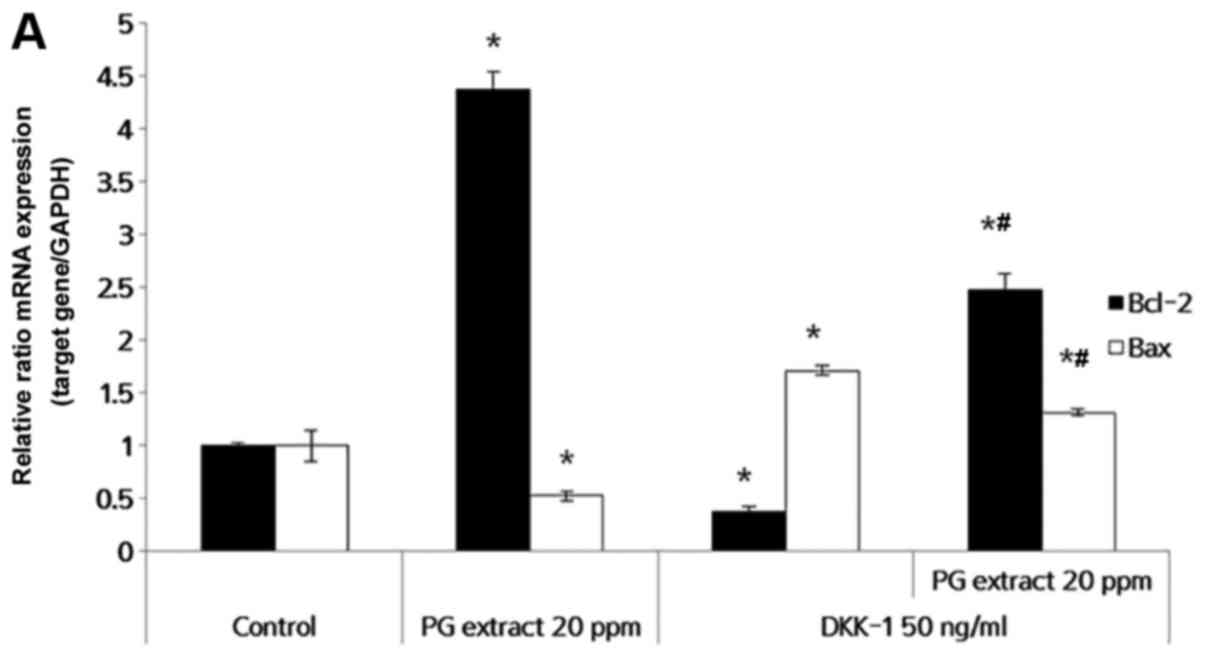
PG extract regulates the expression of
apoptosis-related genes in ORS keratinocytes
To further investigate the relevance of the
anti-apoptotic effects of PG extract on DKK-1, changes in the
expression of apoptosis-related genes were examined by RT-qPCR. ORS
keratinocytes were treated with PG extract and/or DKK-1 for 24 h.
DKK-1-induced apoptosis was accompanied by Bcl-2/Bax expression in
many cells, including HF cells (12,13). In ORS keratinocytes, DKK-1
treatment significantly decreased anti-apoptotic factor Bcl-2
expression. PG extract alone increased Bcl-2 expression four times
more than the untreated control, and it reversed the DKK-1-induced
inhibition of Bcl-2 expression. DKK-1 also induced the expression
of the pro-apoptotic factor Bax. PG extract significantly inhibited
Bax expression in ORS keratinocytes (Fig. 3A) despite the presence of DKK-1.
In other words, PG extract promotes ORS keratinocyte survival and
increases the ratio of Bcl-2/Bax to further inhibit the cell death
(Fig. 3B). Increased protein
level of Bcl-2 and decreased protein of Bax were confirmed by
immunocytochemistry (Fig. 3C).
These results indicated that the effect of PG extract was mediated
through Bcl-2/Bax expression on ORS keratinocytes.
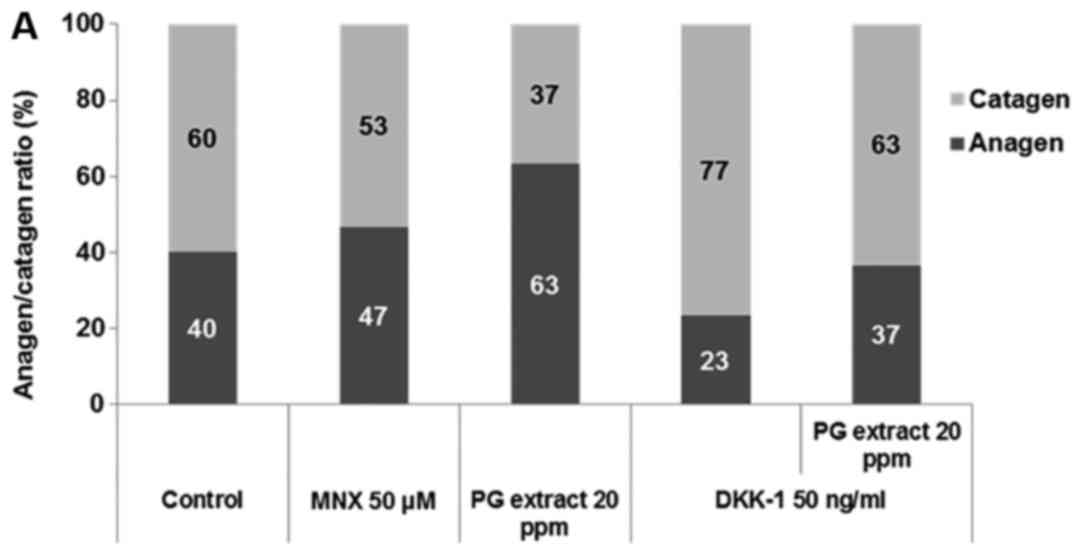
PG extract abrogates DKK-1 inhibition of
hair shaft elongation in human HF organ culture
In order to examine the effect of PG extract in the
presence or absence of DKK-1 at the organ level, the authors
performed an ex vivo culture of whole human scalp HFs.
Minoxidil (MNX) and vehicle served as positive and negative
controls, respectively. HFs treated with PG extract grew longer
than the negative control HFs at 5 days, which was similar to the
growth of HFs treated with MNX. This result is consistent with the
authors' previous study (27). A
low dose of DKK-1 (<50 ng/ml) produced no significant impairment
of hair shaft elongation compared to the vehicle (data not shown),
but a dose of 50 ng/ml DKK-1 significantly inhibited hair shaft
elongation. The authors observed a narrower hair bulb in HFs
treated with DKK-1 at the 50 ng/ml dose, which is reminiscent of
catagen-like regressive changes. They also measured the
anagen/catagen ratio (Fig. 4A),
and DKK-1 treatment resulted in anagen-to-catagen changes in the HF
organ culture. Thus, DKK-1 treatment at the dose of 50 ng/ml was
used to establish an ex vivo model of HF catagen induction.
With co-incubation of the PG extract and DKK-1, the PG extract
significantly abrogated DKK-1-induced growth inhibition of cultured
HFs ex vivo (Fig. 4B).
PG extract regulates the expression of
hair growth-related factors in HFs
There is evidence to suggest that several factors
such as cytokine, growth factor, and apoptosis-related factor are
involved in the hair growth cycle (31). In the above results, the authors
already confirmed that PG extract regulates hair growth-related
factors at the in vitro level. To confirm the inhibitory
effect of apoptosis by PG extract at the ex vivo level,
changes in the gene expression were examined by RT-qPCR using HFs
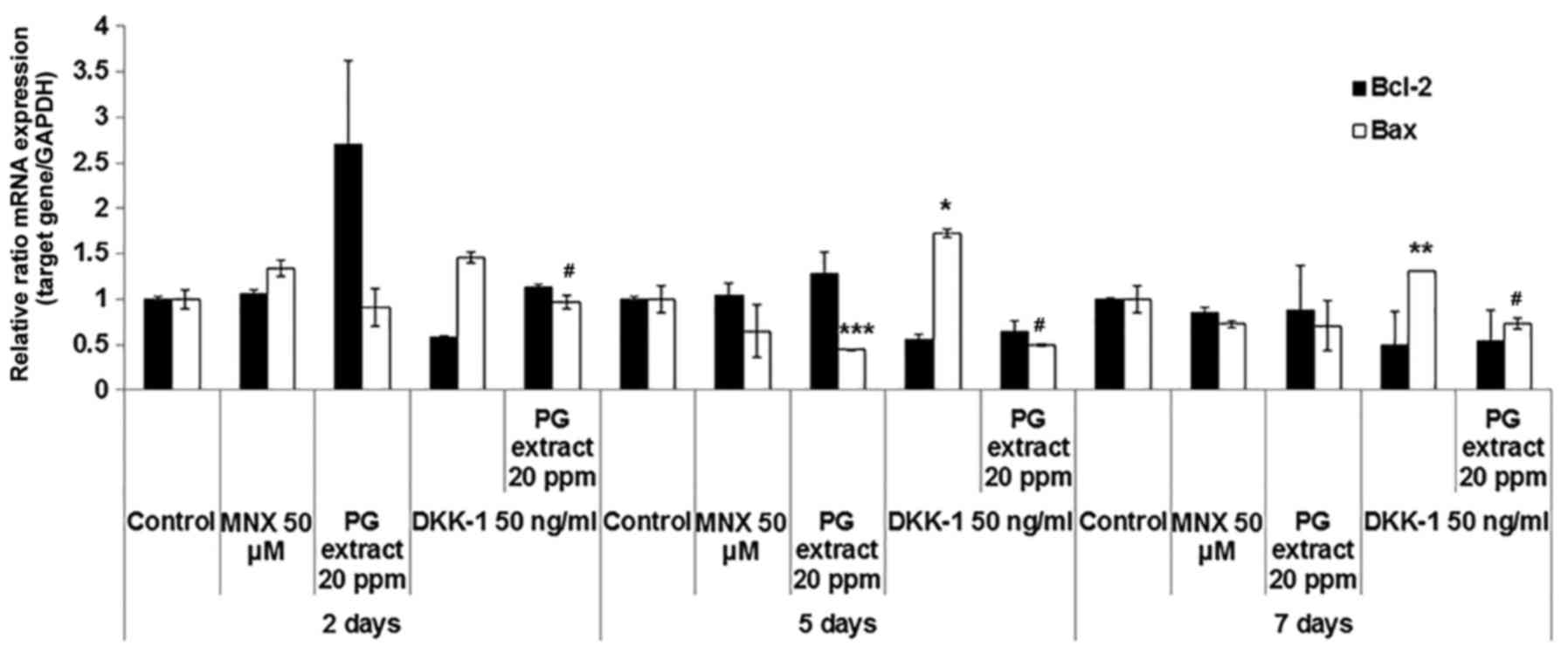
treated with PG extract and/or DKK-1 for 2, 5 and 7 days. The DKK-1
treatment significantly decreased anti-apoptotic factor Bcl-2
expression at 2 days. At the same time point, the PG extract
completely abolished the effect of DKK-1 on Bcl-2 expression in
HFs. On the other hand, DKK-1 treatment significantly increased Bax
expression in HFs, whereas the PG extract strongly inhibited this
induction of Bax for 5- and 7-day HFs (Fig. 5). Of note, the PG extract affected
only Bax expression and not Bcl-2 in longer ex vivo culture
experiments. These results suggested that PG extract antagonizes
DKK-1-induced catagen-like changes, in part, through the regulation
of apoptosis-related factor expression in HFs.
Discussion
The major finding of the current study is that PG
extract antagonizes DKK-1-induced HF changes, resulting in hair
loss. Previous studies (14–24,27) revealed that PG regulates a variety
of biological effects such as anti-inflammatory, antioxidant,
anticancer, and anti-aging effects, and of course, the promotion of
hair growth. Recently, the authors prepared a highly concentrated
ginseng extract with the repeated fractionalizing method and found
that the PG extract contained 194.8 mg/g (19.48% w/w) of
ginsenosides (27). Its
ginsenoside content was ~3 times higher than that of commercial
ginseng extracts for oral supplements in Korea and 14 times higher
than that of conventional ginseng root extract (32). This newly prepared PG extract for
treatment showed a significant hair growth effect with cultured
hDPCs and HFs, which was comparable to the growth from minoxidil
(27). Despite previous studies
of PG and its effects associated with hair growth (23,24), the mechanism underlying the
apoptosis response, particularly the induction of DKK-1, has not
been studied with respect to PG.
DKK-1 is well known as a WNT antagonist. It induces
apoptosis and inhibits the proliferation of cancer cells (33,34). DKK-1 is also inducible by
dihydrotestosterone (DHT), and the level of DKK-1 is increased in
the scalps of patients with male-pattern baldness compared to
normal levels (11), suggesting
that DKK-1 is involved in DHT-mediated balding in androgenic
alopecia. It was also found that DKK-1 is highly expressed during
the anagen-to-catagen transition. This implies that DKK-1 promotes
the regression of HFs by blocking Wnt/β-catenin signaling and by
inducing apoptosis in follicular keratinocytes (13). The authors supposed that DKK-1
might inhibit hair growth and cell proliferation via apoptosis. As
shown in these results, the viability of ORS keratinocytes was
decreased by DKK-1. A TUNEL assay demonstrated that the decreased
cell viability was mediated by apoptosis.
During catagen, HFs undergo apoptosis, and there is
a decline in the apoptotic protein Bcl-2 and an increase in the
pro-apoptotic protein Bax (35).
The ratio of these factors is important in regulating the hair
cycle. In previous studies, DKK-1 treatment rapidly changed the
anti-apoptotic protein Bcl-2. DKK-1 promoted the pro-apoptotic
protein Bax in a dose-dependent manner (12,13). Therefore, the present study
investigated the effect of PG extract in ORS keratinocytes; the
extract induces apoptosis by DKK-1 in these cells. The PG extract
alone significantly increased cellular proliferation. It was
correlated with the mRNA level of Bcl-2 expression increase, and it
also inhibited Bax gene expression (Fig. 3A and B). Furthermore, when the PG
extract was co-treated with DKK-1, the effect of the DKK-1 was
inhibited. This suggested that PG extract may abolish the apoptotic
signal stimulation of DKK-1 and help ORS keratinocytes to
survive.
HF organ culture is now considered a useful tool for
evaluating the effect of hair growth ex vivo. To further
confirm the results shown in the above data, the authors
investigated the effect of PG extract on DKK-1-induced catagen-like
changes in cultured human HFs. It was shown that catagen-like
morphological change was induced in DKK-1-treated HFs. Hair growth
was also inhibited by DKK-1 during the incubation period. The PG
extract significantly stimulated hair elongation, overcoming the
inhibitory effect of DKK-1-induced hair growth, and finally, the
hair was significantly more elongated than the untreated
control.
In summary, PG extract has the potential to protect
apoptosis in HFs. These findings suggested that PG extract may
enhance ORS and hDPC stimulation of HF growth despite the presence
of DKK-1, a strong catagen inducer via apoptosis.
Abbreviations:
|
PG extract
|
Panax ginseng extract
|
|
ORS
|
outer root sheath
|
|
HFs
|
hair follicles
|
|
DKK-1
|
Dickkopf-1
|
Acknowledgments
The study was supported by grants from Amorepacific
R&D Center (R17E117001).
References
|
1
|
Inui S, Fukuzato Y, Nakajima T, Yoshikawa
K and Itami S: Androgen-inducible TGF-beta1 from balding dermal
papilla cells inhibits epithelial cell growth: A clue to understand
paradoxical effects of androgen on human hair growth. FASEB J.
16:1967–1969. 2002.PubMed/NCBI
|
|
2
|
Li L, Mignone J, Yang M, Matic M, Penman
S, Enikolopov G and Hoffman RM: Nestin expression in hair follicle
sheath progenitor cells. Proc Natl Acad Sci USA. 100:9958–9961.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reynolds AJ and Jahoda CA: Cultured dermal
papilla cells induce follicle formation and hair growth by
transdifferentiation of an adult epidermis. Development.
115:587–593. 1992.PubMed/NCBI
|
|
4
|
Millar SE: Molecular mechanisms regulating
hair follicle development. J Invest Dermatol. 118:216–225. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Botchkarev VA and Paus R: Molecular
biology of hair morphogenesis: Development and cycling. J Exp
Zoolog B Mol Dev Evol. 298:164–180. 2003. View Article : Google Scholar
|
|
6
|
Botchkareva NV, Ahluwalia G and Shander D:
Apoptosis in the hair follicle. J Invest Dermatol. 126:258–264.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawaya ME, Blume-Peytavi U, Mullins DL,
Nusbaum BP, Whiting D, Nicholson DW, Lotocki G and Keane RW:
Effects of finasteride on apoptosis and regulation of the human
hair cycle. J Cutan Med Surg. 6:1–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Domyati M, Attia S, Saleh F, Bassyouni
M, Barakat M and Abdel-Wahab H: Evaluation of apoptosis regulatory
markers in androgenetic alopecia. J Cosmet Dermatol. 9:267–275.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Müller-Röver S, Rossiter H, Lindner G,
Peters EM, Kupper TS and Paus R: Hair follicle apoptosis and Bcl-2.
J Investig Dermatol Symp Proc. 4:272–277. 1999. View Article : Google Scholar
|
|
11
|
Kwack MH, Ahn JS, Kim MK, Kim JC and Sung
YK: Preventable effect of L-threonate, an ascorbate metabolite, on
androgen-driven balding via repression of
dihydrotestosterone-induced dickkopf-1 expression in human hair
dermal papilla cells. BMB Rep. 43:688–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwack MH, Sung YK, Chung EJ, Im SU, Ahn
JS, Kim MK and Kim JC: Dihydrotestosterone-inducible dickkopf 1
from balding dermal papilla cells causes apoptosis in follicular
keratinocytes. J Invest Dermatol. 128:262–269. 2008. View Article : Google Scholar
|
|
13
|
Kwack MH, Kim MK, Kim JC and Sung YK:
Dickkopf 1 promotes regression of hair follicles. J Invest
Dermatol. 132:1554–1560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu S, Concha C, Lin F and Persson Waller
K: Adjuvant effect of ginseng extracts on the immune responses to
immunisation against Staphylococcus aureus in dairy cattle. Vet
Immunol Immunopathol. 91:29–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Na HS, Lim YJ, Yun YS, Kweon MN and Lee
HC: Ginsan enhances humoral antibody response to orally delivered
antigen. Immune Netw. 10:5–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong CE and Lyu SY: Anti-inflammatory and
anti-oxidative effects of korean red ginseng extract in human
keratinocytes. Immune Netw. 11:42–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yun TK and Choi SY: Preventive effect of
ginseng intake against various human cancers: A case-control study
on 1987 pairs. Cancer Epidemiol Biomarkers Prev. 4:401–408.
1995.PubMed/NCBI
|
|
18
|
Cho S, Won CH, Lee DH, Lee MJ, Lee S, So
SH, Lee SK, Koo BS, Kim NM and Chung JH: Red ginseng root extract
mixed with Torilus fructus and Corni fructus improves facial
wrinkles and increases type I procollagen synthesis in human skin:
A randomized, double-blind, placebo-controlled study. J Med Food.
12:1252–1259. 2009. View Article : Google Scholar
|
|
19
|
Kang TH, Park HM, Kim YB, Kim H, Kim N, Do
JH, Kang C, Cho Y and Kim SY: Effects of red ginseng extract on UVB
irradiation-induced skin aging in hairless mice. J Ethnopharmacol.
123:446–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim S, Kang BY, Cho SY, Sung DS, Chang HK,
Yeom MH, Kim DH, Sim YC and Lee YS: Compound K induces expression
of hyaluronan synthase 2 gene in transformed human keratinocytes
and increases hyaluronan in hairless mouse skin. Biochem Biophys
Res Commun. 316:348–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwok HH, Yue PY, Mak NK and Wong RN:
Ginsenoside Rb(1) induces type I collagen expression through
peroxisome proliferator-activated receptor-delta. Biochem
Pharmacol. 84:532–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee J, Jung E, Lee J, Huh S, Kim J, Park
M, So J, Ham Y, Jung K, Hyun CG, et al: Panax ginseng induces human
Type I collagen synthesis through activation of Smad signaling. J
Ethnopharmacol. 109:29–34. 2007. View Article : Google Scholar
|
|
23
|
Matsuda H, Yamazaki M, Asanuma Y and Kubo
M: Promotion of hair growth by ginseng radix on cultured mouse
vibrissal hair follicles. Phytother Res. 17:797–800. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SH, Jeong KS, Ryu SY and Kim TH: Panax
ginseng prevents apoptosis in hair follicles and accelerates
recovery of hair medullary cells in irradiated mice. In Vivo.
12:219–222. 1998.PubMed/NCBI
|
|
25
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: Multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leung KW and Wong AS: Pharmacology of
ginsenosides: A literature review. Chin Med. 5:202010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SN, Kim S, Hong YD, Park H, Shin SH,
Kim AR, Park BC, Shin SS, Park JS, Park M, et al: The ginsenosides
of Panax ginseng promote hair growth via similar mechanism of
minoxidil. J Dermatol Sci. 77:132–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Magerl M, Kauser S, Paus R and Tobin DJ:
Simple and rapid method to isolate and culture follicular papillae
from human scalp hair follicles. Exp Dermatol. 11:381–385. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Philpott MP, Sanders DA and Kealey T:
Effects of insulin and insulin-like growth factors on cultured
human hair follicles: IGF-I at physiologic concentrations is an
important regulator of hair follicle growth in vitro. J Invest
Dermatol. 102:857–861. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Philpott MP, Green MR and Kealey T: Human
hair growth in vitro. J Cell Sci. 97:463–471. 1990.PubMed/NCBI
|
|
31
|
Philpott MP, Sanders D, Westgate GE and
Kealey T: Human hair growth in vitro: A model for the study of hair
follicle biology. J Dermatol Sci. 7(Suppl): S55–S72. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SN, Ha YW, Shin H, Son SH, Wu SJ and
Kim YS: Simultaneous quantification of 14 ginsenosides in Panax
ginseng C.A. Meyer (Korean red ginseng) by HPLC-ELSD and its
application to quality control. J Pharm Biomed Anal. 45:164–170.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hall CL, Zhang H, Baile S, Ljungman M,
Kuhstoss S and Keller ET: P21CIP-1/WAF-1 induction is required to
inhibit prostate cancer growth elicited by deficient expression of
the Wnt inhibitor Dickkopf-1. Cancer Res. 70:9916–9926. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirata H, Hinoda Y, Nakajima K, Kawamoto
K, Kikuno N, Ueno K, Yamamura S, Zaman MS, Khatri G, Chen Y, et al:
Wnt antagonist DKK1 acts as a tumor suppressor gene that induces
apoptosis and inhibits proliferation in human renal cell carcinoma.
Int J Cancer. 128:1793–1803. 2011. View Article : Google Scholar
|
|
35
|
Lindner G, Botchkarev VA, Botchkareva NV,
Ling G, van der Veen C and Paus R: Analysis of apoptosis during
hair follicle regression (catagen). Am J Pathol. 151:1601–1617.
1997.PubMed/NCBI
|