Introduction
According to the histomorphological characteristics,
lung morphogenesis can be divided into five stages in the rat
(1). First is the embryonic
period, from fertilization to E9.5 (embryonic day 9.5). During this
period of organogenesis, the lung primordium branches from the two
lung buds that lie on each side of the future esophagus (2). The segmental branching of the airway
is presented by 9.5 days of gestation, which results in the
formation of tubular lung, and the following stage is referred to
as the pseudoglandular period (E9.5–E16.5). At this stage, the
primary lung buds grow ventrally and caudally, and five secondary
buds are generated, which form the lobes of the mature lung.
Recurrent sprouting and bifurcations of the lung buds result in the
formation of pre-acinar airways arising from the process of
branching morphogenesis. At the late pseudoglandular stage, the
vascular development is quintessential, and all the numbers of
pre-acinar airways are completely formed. Structurally, the lung
development then enters the next phase, the canalicular period
(E16.5–E17.5). Extensive cellular differentiation occurs in the
late pseudoglandular and canalicular stages (3), the terminal branches of the
bronchial tree are taking shape, and the cuboidal epithelium
differentiates into type I and II cells, resulting in the formation
of a thin air-blood barrier, as well as the secretion of
surfactant. The cell differentiation continues, thereby forming the
terminal sacs. These terminal sacs, as the name suggests, leads to
the subsequent histological stage, which is known as the saccular
period, spanning E17.5 to postnatal day 5 (P5), comprising the
phase of rapid expansion (P1–P4). The major characteristics of this
stage are increased growth of lung parenchyma, further maturation
of the surfactant system, thinning of the interstitial tissue
between the airspaces and the production of the last generation of
airways by some future alveolar ducts and the outermost periphery
alveolar sacs. Together, these changes prepare the lung for
respiration after birth. The premature lung in the gestational age
<35 weeks remains in the canalicular or saccular phase (4), which are sophisticated metamorphosis
stages of lung development. The injury to the developing lung
during this period alters subsequent alveolar and vascular
development, resulting in simplified alveolar structures,
dysmorphic capillary configuration, variable interstitial
cellularity and fibroproliferation that are characteristic of the
'new' bronchopulmonary dysplasia (5). The alveolar period (P5–P30) is the
last stage in lung development that is responsible for the
accomplishment of alveolarization; it includes the phase of
alveolarization (P5–P13) and the equilibrium stage (P14–P30).
CCAAT/enhancer binding protein alpha (C/EBPα) is the
founding member of a family of basic region/leucine zipper
transcription factors. A previous study indicated that C/EBPα
participates in regulating the lung differentiation and pulmonary
maturation (6). The expression of
C/EBPα is initiated almost simultaneously to that of cellular
differentiation and the emergence of differentiation markers
(7). Furthermore, the studies in
lung epithelial cell lines have demonstrated that the promoters of
several differentiation-dependent genes harbor the C/EBP-binding
sites (8–11); the C/EBPα-mediated regulation of
these genes has also been proposed. Berg et al (12) reported that the abnormal
expression of C/EBPα disrupts the lung development. These results
indicated a role of C/EBPα in lung development; however, the
molecular mechanism is poorly understood.
The post-translational modification is a vital
regulatory mechanism underlying proteins exerting pleiotropic
effects, thereby improving the structure and function of target
proteins. Small ubiquitin-like modifier (SUMO) is a novel protein
that can modify the target proteins causing rapid changes in the
function and distribution of proteins, subcellular structures and
multiprotein complexes (13). The
pathway of sumoylation resembles that of ubiquitination, although
the enzymes involved in the conjugation of SUMO are different. The
SUMO peptide is first processed at the C-terminus by the
ATP-dependent heterodimeric SUMO-activating E1 enzyme (Aos1/Uba2).
Subsequently, it is transferred to the catalytic cysteine of the E2
conjugating enzyme, Ubc9 (14).
The final step involves the transfer of the SUMO moiety from E2 to
the specific substrate in the presence of an E3 ligase.
C/EBPα was previously reported to be
post-translationally modified by SUMO at a lysine residue (K159)
within the 'attenuator domain' of the protein that can negatively
affect the transcriptional activity (15–17). Hankey et al (18) demonstrated that changes in the
sumoylation status of C/EBPα might contribute towards a switch that
regulates its transcriptional activity during normal neutrophil
development. On the other hand, Sato et al (19) reported that the enhancement of
C/EBPα-mediated transactivation by BRG1, which is a core subunit of
the SWI/SNF chromatin remodeling complex, was inhibited by
sumoylation. Furthermore, sumoylation dramatically decreased the
expression of the liver-specific albumin gene that harbors the
C/EBPα binding site. Notably, the common endodermal origin and the
crucial role of C/EBPα in lung and liver suggest the potential
transcriptional regulation and that SUMO may have a role in both
organs. However, the role of SUMO-modification in the lung has not
yet been reported. The C/EBPα studies are primarily focused on the
mature lung. The mechanism through which C/EBPα regulates AEC-II
(alveolar epithelial cells type II) differentiation and its effect
on alveolar maturation in the premature lung have not yet
beenclarified. The studies on C/EBPα and AEC II
differentiation-related constituents, such as pulmonary surfactant
proteins, phosphatidylcholine (PC) and glycogen are poorly
reported.
In the present study, the authors investigated the
level and functional role of C/EBPα during rat lung development.
The correlation between the level of C/EBPα and the content of
glycogen during lung maturation established a role of C/EBPα in
lung differentiation. Furthermore, the changes in the status of
C/EBPα were shown to be associated with the secretion of pulmonary
surfactant. The SUMO modification of C/EBPα was also found to
participate in this phenomenon. These findings indicated that
C/EBPα serves a vital role in normal lung development, and provides
further insights into the involvement of SUMO.
Materials and methods
Animals
Sprague-Dawley rats (90–100-days old, weight 250–300
g) were purchased from the Animal Center of Jiangsu University. All
rats kept on a 12-h light/dark cycle at a room temperature of
23±2°C and a relative humidity of 50±5%, maintained on standard
laboratory food and water ad libitum throughout the
experiment. Rats were mated by 3:1 female: male ratio (15:5). The
next morning, the female rats were checked for fertility and
recorded as embryonic day 0.5 (E0.5). According to the different
stages during the development of rat lung, the authors chose
embryonic days 15.5, 17.5 and 19.5, and postnatal days 0.5, 4, 7
and 14 as the observation time-points. Embryos and lungs were
isolated from the embryonic and postnatal stages as previously
described, and a part of the samples was immediately fixed with 4%
paraformaldehyde; the remaining part of the samples was stored at
−80°C. The number of animals per group analyzed varies between 5
and 8. The protocols for animal studies were approved by the
Laboratory Animal Ethics Committee of the Affiliated Hospital of
Jiangsu University (Zhenjiang, China).
Histological analysis and periodic
acid-Schiff (PAS) staining
Tissues were fixed with 4% paraformaldehyde in
phosphate-buffered saline (PBS) for 24 h at 4°C, washed with PBS,
dehydrated by an alcohol gradient and embedded in paraffin.
Subsequently, 3 µm sections were sliced, followed by
deparaffinization and rehydration through xylene, ethanol and
water. The antigen retrieval was performed in 10 mM citrate buffer
(pH 6.0) at a constant pressure of 20 cm H2O. The tissue
sections were stained with either hematoxylin and eosin (H&E)
for histological analysis, or PAS reagent (G1281) from Beijing
Solarbio Science & Technology Co., Ltd., (Beijing, China) for
analyzing the content of glycogen; the content of glycogen in
alveolar epithelial cells reflect the maturity of AEC II (20). The sections were observed and
images acquired by microscopy at magnification, ×400. PAS staining
of the lung tissues revealed red- or purple-colored glycogen. The
images were analyzed by Image-Pro Plus III (Media Cybernetics,
Inc., Rockville, MD, USA) to obtain the mean gray value. A total of
3–5 samples/group were analyzed.
Antibodies
The primary antibodies used were anti-C/EBPα
(sc-9314), anti-SP-B (sc-7704) and anti-SP-C (sc-13979 from Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), anti-SP-A (LS-C357574)
from LifeSpan BioSciences, Inc. (Seattle, WA, USA), anti-SP-D
(bs-1583R) from BIOSS (Beijing, China), and anti-SUMO-1 (4930) and
anti-β-actin (#3700) from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Biotinylated secondary antibodies (FMS-MS01, FMS-Rb01,
FMS-Gt01) were procured from Fcmacs Biotech Co., Ltd. (Nanjing,
China). Donkey anti-rabbit IgG/fluorescein isothiocyanate
(bs-0295D-FITC) and donkey anti-goat IgG/phycoerythrin
(bs-0294D-PE) were obtained from BIOSS.
Western blot assay
Lung tissues were thawed on ice, washed with cold
PBS, sliced into small pieces, lysed in radioimmunoprecipitation
assay lysis buffer containing 1 mmol/l protease inhibitor PMSF
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and homogenized by
Dounce tissue grinders on ice. The extract was centrifuged at
12,000 × g and 4°C for 15 min; the supernatant was collected.
Following normalization of protein concentrations between the
samples by BCA kit (Beyotime Institute of Biotechnology, Shanghai,
China), 10 ml of the lung lysates were resolved by 12% sodium
dodecyl sulfate-polyacrylamide-gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride membranes. The membranes
were blocked in 5% milk-TBST containing 0.1% Tween-20 at 37°C for 2
h, and probed overnight with primary antibodies at 4°C. The
following primary antibodies were used: anti-C/EBPα (1:200),
anti-SP-A (1:500), anti-SP-B (1:200), anti-SP-C (1:500), anti-SP-D
(1:200), anti-SUMO-1 (1:200) and anti-β-actin (1:1,000). The
membranes were washed with TBST containing 0.1% Tween-20 and
incubated with biotinylated secondary antibodies (1:5,000) for 1 h
at 37°C. The immunoreactive bands were visualized by FluorChem FC3
chemiluminescence (ProteinSimple, San Jose, CA, USA) according to
the manufacturer's recommendations. The protein expressions were
quantified by densitometric analysis using LANE 1D software (Sage,
Beijing, China).
PC assay
PC assay kit (ab83377, from Abcam, Cambridge, UK)
was used to measure the levels of PC in various samples. Lung
tissues were thawed on ice, washed with cold PBS, resuspended in
the assay buffer provided by the kit, and homogenized with a Dounce
homogenizer on ice. Then, the samples were centrifuged for 5 min at
4°C at 12,000 × g to exclude the insoluble material and collect the
supernatant. An equivalent of 2.5 µg of the samples was
loaded per well; PC reaction mixture was added, followed by
development mixture according to the protocol of the kit. The
colorimetric reaction was measured at 570 nm. The PC assay was
carried out three times, following which, the concentration of PC
was estimated.
Immunofluorescence
Double immunofluorescence staining was used to
detect the localization of sumoylated C/EBPα. The sections were
deparaffinized and rehydrated by xylene, ethanol and water. Antigen
retrieval was performed in 10 mM citrate buffer (pH 6.0) at a
constant pressure of 20 cm H2O. Subsequently, the
sections were blocked with 5% serum in PBS for 20 min at room
temperature, followed by incubation with the mixture of anti-C/EBPα
(1:100) and anti-SUMO-1 (1:100) antibodies overnight at 4°C.
Anti-C/EBPα was detected using a secondary PE-conjugated antibody
(1:500) and anti-SUMO-1 was detected by a secondary FITC-conjugated
antibody (1:500). The nucleus was counterstained with DAPI for 5
min at room temperature. All washes and antibody dilutions were
made in PBS.
Immunoprecipitation
The immunoprecipitation protocol by Sato et
al (19) was utilized for the
detection of sumoylated C/EBPα. In order to detect the
electrophoretic mobility of sumoylated C/EBPα, immunoprecipitation
assay was carried out. A total of 20 mg lung tissues were thawed on
ice, washed with cold PBS, sliced into small pieces and lysed with
a buffer containing 1% Triton X-100, 150 mM NaCl, 20 mM Tris (pH
7.5) supplemented with protease inhibitors, followed by grinding
using Dounce tissue grinders on ice. The extract was centrifuged at
12,000 × g and 4°C for 15 min; the supernatant was collected. The
chromatin was preincubated with 2 µg anti-C/EBPα antibody
for 30 min on ice. Then, 20 µl Protein A/G PLUS-Agarose
beads (Santa Cruz Biotechnology) were added, and the mixture was
incubated overnight at 4°C with gentle agitation. The
antibody-coated beads were washed with lysis buffer and the
immunoprecipitated proteins eluted by heat denaturation for 5 min
in 5X Laemmli buffer containing 100 mM β-dithiothreitol.
Immunoblotting, to detect the SUMO-1 protein, was performed as
previously described.
Statistical analysis
The results are expressed as the mean ± standard
error of the mean of at least three independent experiments. All
data were analyzed by the SPSS statistical software (version 17.0;
SPSS, Inc., Chicago, IL, USA). Pearson's correlation analysis was
used to assess the relationship between indicators. P<0.05 was
considered to indicate a statistically significant difference.
Results
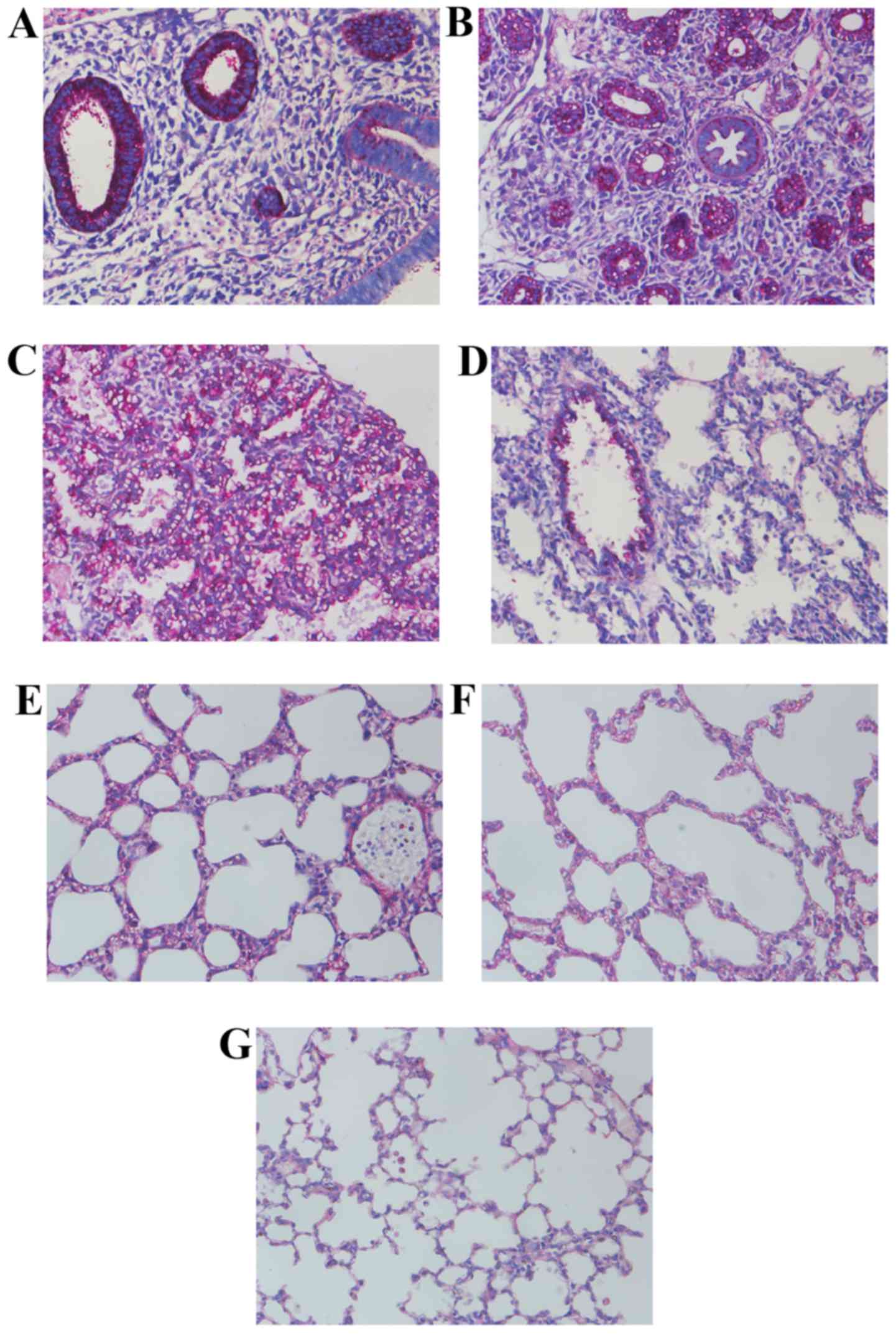
Histomorphological variations of lung
morphogenesis in different stages
As presented in Fig.
1, the ring-like pre-acinar airways could be observed at the
late pseudoglandular stage at E15.5, lined by high columnar
epithelium, surrounded by dense connective tissues. At E17.5, the
primitive lung buds were separated completely. A large number of
acinar luminae appeared, lined with simple cuboidal epithelium. In
addition, the authors also observed the alveolar type I epithelial
cells (AEC-I) at this stage. The primary alveolar was shaped at the
early canalicular stage at E19.5, surrounded by thin connective
tissues arranged in streak-like conformation. At P0.5, the number
of primary alveoli was increased with irregularity in the
structures. An increasing number of alveolar septa were formed at
the late canalicular stage at P4 with some ridges protruding into
the alveolar space. At P7, the size of air sacs was predisposed
towards uniformity, and the interstitial tissues thinned out. By
P14, the basic unit in the lung was mature alveoli, which appeared
uniformly sized and separated by a razor-thin septum.
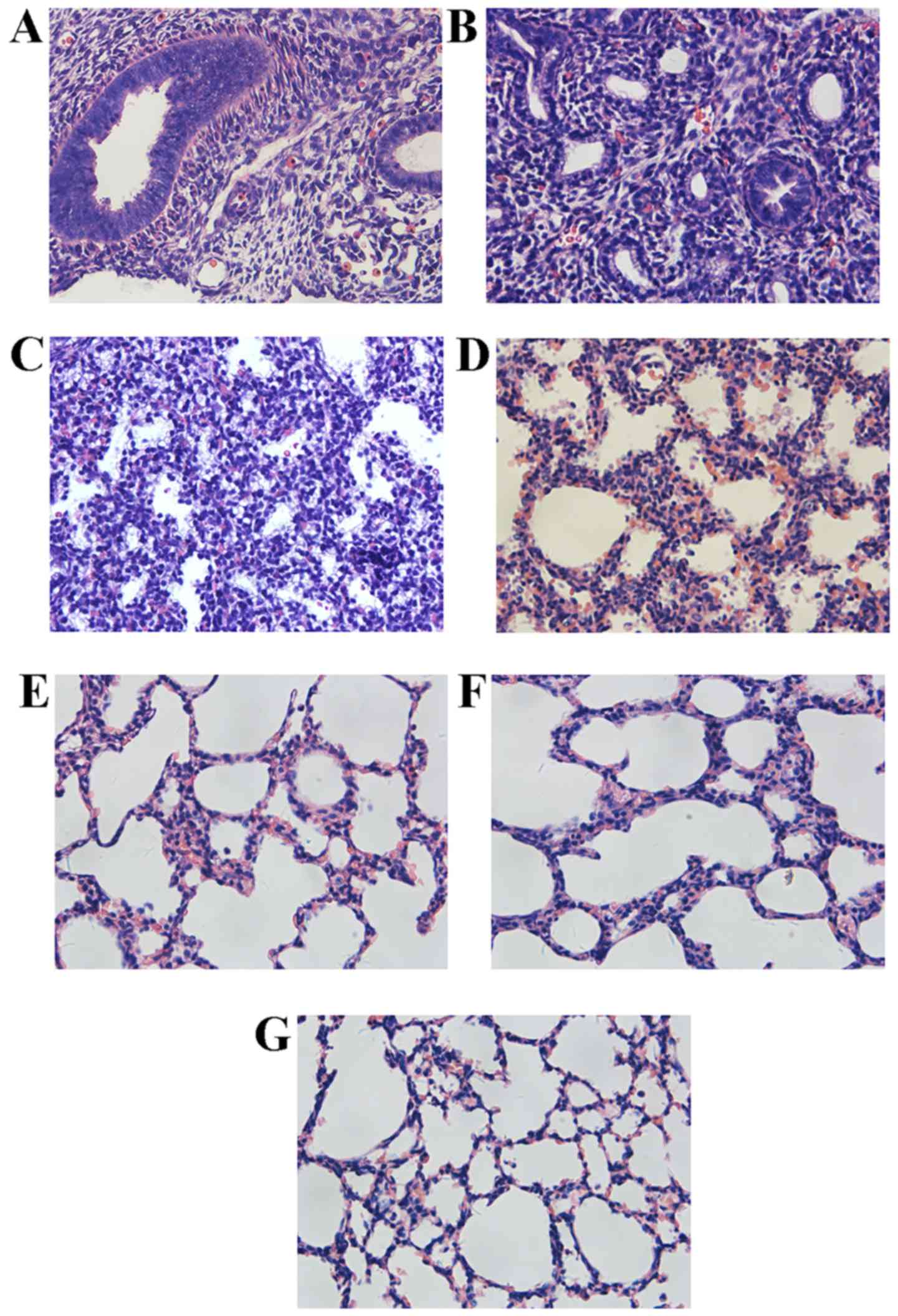 | Figure 1Histology of transverse sections of
isolated lungs from several stages of development, fixed in 4%
paraformaldehyde, embedded in paraffin and stained with hematoxylin
and eosin (H&E). (A) Lung from E15.5, the ring-like pre-acinar
airways lined by high columnar epithelium, and surrounded by dense
connected tissues. (B) Lung from E17.5, more acinar luminae
appeared in this stage, lined with simple cuboidal epithelium. (C)
Lung from E19.5, primary alveolar had taken shape and was
surrounded by thinner connective tissues that arranged in a streak.
(D) Lung from P0.5, a number of primary alveoli increased, with
irregular structures. (E) Lung from P4, several ridges protruding
into the alveolar space. (F) Lung from P7, air sacs tend to be
uniform, and the interstitial tissues thin out. (G) Lung from P14,
the basic unit in lung is mature alveoli, with uniform size and
separated by razor-thin septum. Magnification, ×400. |
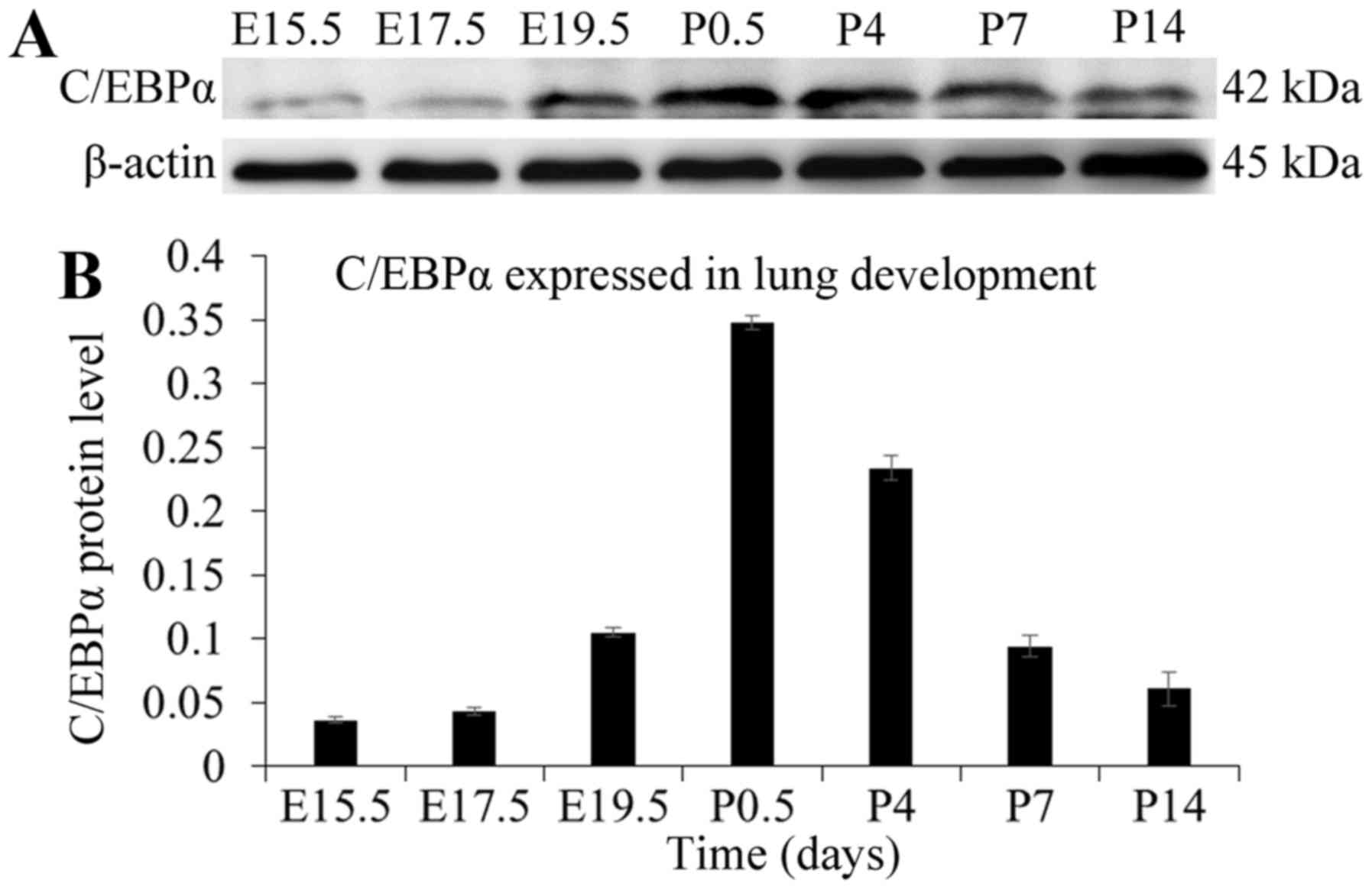
Dynamic expression pattern of C/EBPα
protein during lung development
Rat lungs from different developmental stages were
investigated for the expression pattern of C/EBPα using the western
blot assay. As presented in Fig.
2, little expression of C/EBPα protein could be observed in
lung tissues of the late pseudoglandular stage at E15.5. However,
after the pseudoglandular-canalicular transition, the amount of
C/EBPα protein increased gradually, and the trend was extremely
obvious at E19.5 and P0.5. The C/EBPα protein reached its peak
level at P0.5, following which, the expression weakened and finally
stabilized at P14, resembling the expression pattern in the adult
animal (12). In summary, C/EBPα
displayed a trend of initial increase followed by a decrease during
the lung development. C/EBPα was originally expressed in the late
pseudoglandular phase characterized by growth and branching of the
lung, which coincided with the cell differentiation and time of
emergence of differentiation markers. After the
pseudoglandular-canalicular transition, the expression increases
and exhibits a widespread pattern, correlating with the extensive
cellular differentiation occurring during this period (7).
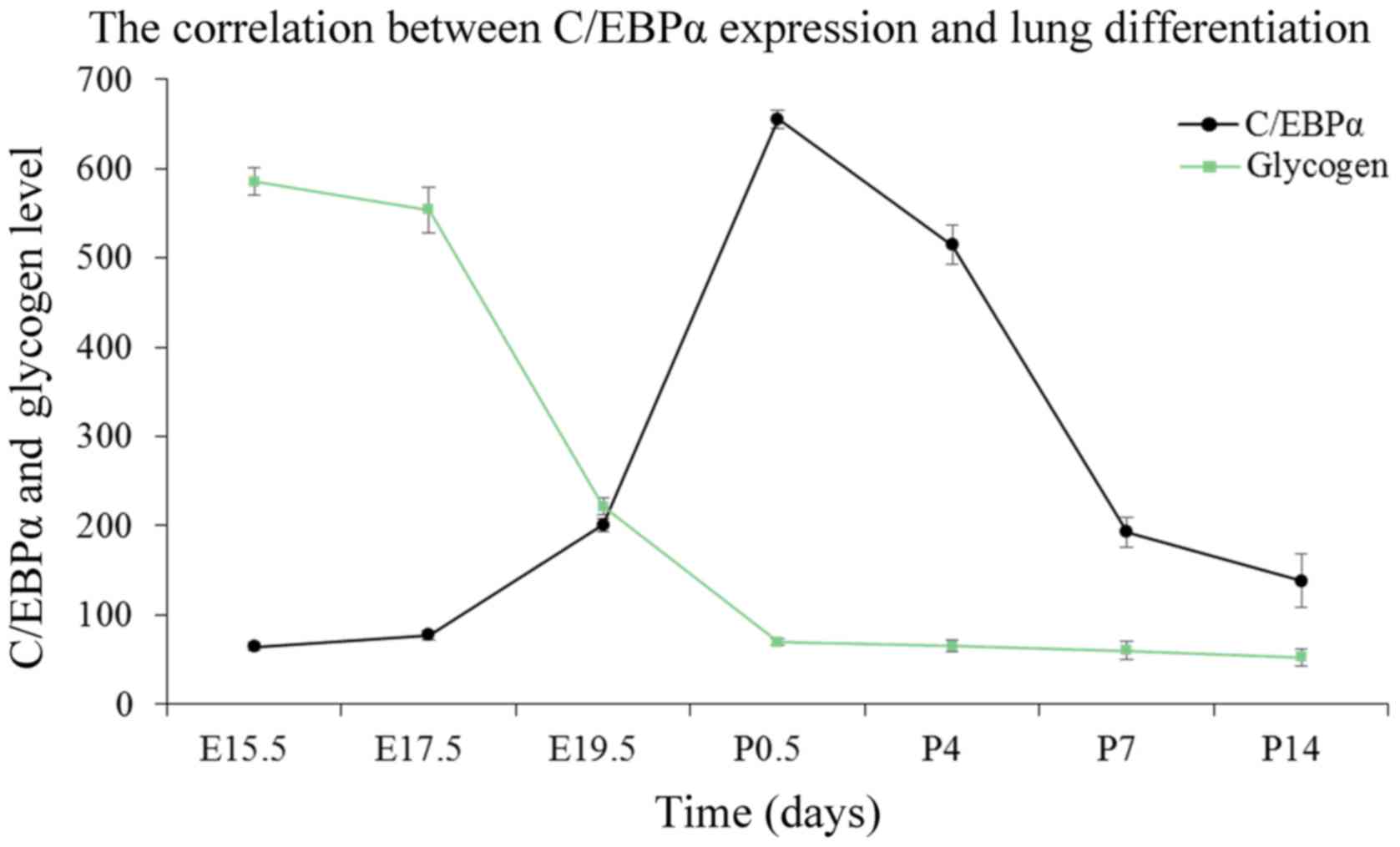
Correlation between glycogen content and
C/EBPα protein expression
The epithelial content of glycogen reflects the
maturity of AEC-II (20). The
intracellular glycogen is transformed into the pulmonary surfactant
phospholipids; with AEC-II differentiation, the content is reduced
gradually. As indicated by PAS staining of lung tissues in Fig. 3, the glycogen was identified by
red or purple color. Glycogen occurs in the cytoplasm; the content
was high at the late pseudoglandular stage at E15.5, followed by a
decrease as the lung develops. This tendency is noticeable,
especially at the canalicular and early saccular stages, which
correspond to a wide range of cell differentiations during this
stage. Finally, the glycogen content stabilized at the alveolar
stage. To investigate the functional role of C/EBPα in AEC-II
differentiation, the authors analyzed the link between the changes
in the status of C/EBPα and the content of glycogen during lung
maturation. As observed in Fig.
4, the level of glycogen exhibited a pattern approximately
opposite to the expression of C/EBPα protein at the embryo and
early postnatal stages, establishing a positive correlation between
the C/EBPα expression and lung differentiation. At the late
development period of the lung, primary and mature alveolar became
the basic structural unit of lung tissue, and the pulmonary
function improved gradually. The reduced glycogen consumption
corresponds to the relatively low level of C/EBPα. This suggested
that C/EBPα might facilitate cell differentiation throughout the
lung development phase.
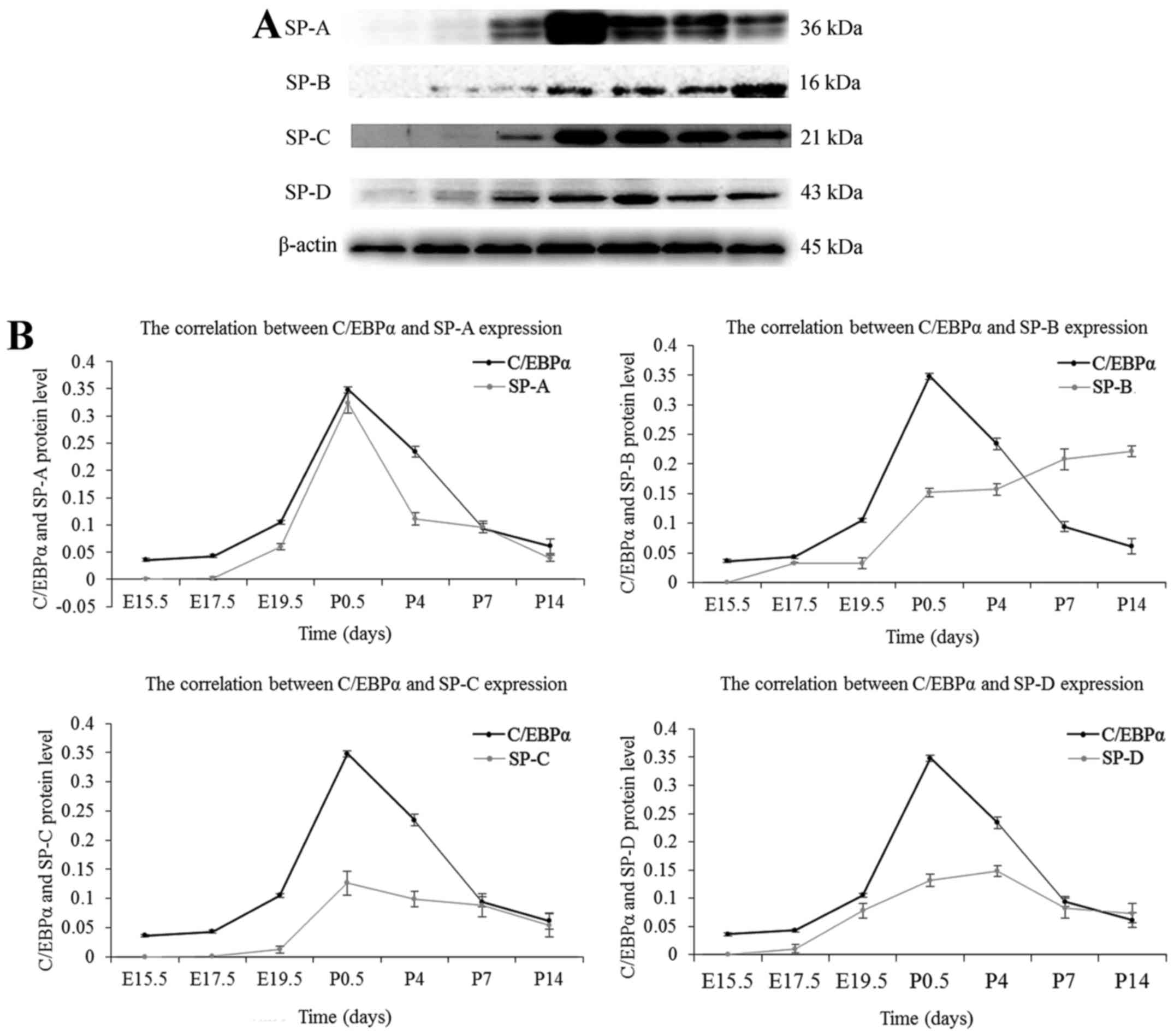
Correlation between pulmonary surfactant
proteins and C/EBPα expression
Lung surfactant contains four associated proteins,
surfactant protein (SP)-A, SP-B, SP-C and SP-D. In the present
study, the authors investigated the secretion of pulmonary
surfactant proteins during the whole development process of lung,
using the western blot assay. As presented in Fig. 5, little expression of SP-A could
be seen at the late pseudoglandular stage at E15.5, whereas SP-B,
SP-C, and SP-D were first detected at E17.5; the expression of SPs
was increased gradually with the development of fetal lung. The
secretion of SP-A, SP-C and SP-D was maximal at the postnatal age
between 12 h and 4 days, then decreased gradually and stabilized at
P14. The expression of SP-B continued to rise after birth, and the
increase was superior to the embryonic period. SP-B secretion
increased slowly at P14 and stabilized gradually. The expression
patterns of SPs were approximately similar to that of the C/EBPα
protein except for SP-B. The results suggested a positive
correlation between C/EBPα expression and the secretion of SPs; SPs
are differentiation-dependent genes, and these results were
consistent with the finding that C/EBPα promotes lung
differentiation. However, the function of C/EBPα on SP-B during the
postnatal days was not clarified.
Correlation between the secretion of
pulmonary surfactant phospholipids and C/EBPα expression
The pulmonary surfactant phospholipids consist
primarily of PC, synthesized in AEC-II, stored in the lamellar
body, and excreted to the alveolar surface, thereby reducing the
surface tension, increasing the compliance and preventing the
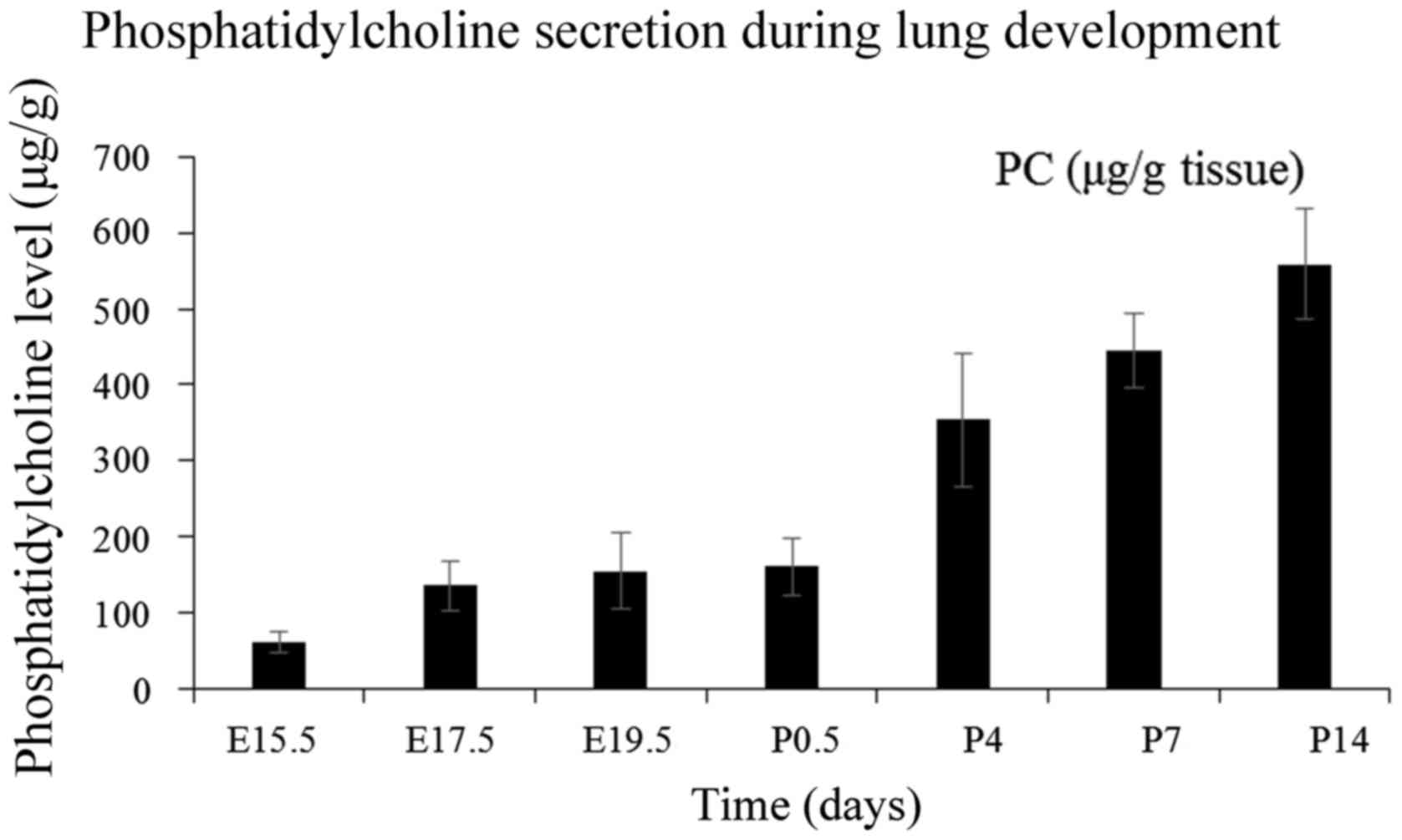
alveolar collapse. The authors investigated the secretion of PC
through lung tissues from various developmental stages. The
augmented production of PC by fetal lungs does not commence until
the gestation is ~80% complete (21). As presented in Fig. 6, the amount of PC at the late
pseudoglandular stage is low. After a slow increasing period before
birth, the content seems to increase rapidly at postnatal days and
almost stabilizes during the alveolar period. The secretion pattern
of PC was in accordance with the expression profile of C/EBPα in
utero; however, the after-birth tendency was opposite without a
significant correlation. Chen et al (22) reported that C/EBPα exerts a
positive effect on adipocyte differentiation and restrains the
accumulation of lipid. A slow increase of PC was observed at the
embryonic period with a high expression of C/EBPα. Conversely,
C/EBPα expression was reduced after birth, but the content of PC
increased rapidly. Thus, it is possible that C/EBPα accelerates the
secretion of AEC-II and metabolism of PC and other extracellular
lipids concurrently. The secretion and cyclic utilization of PC are
primarily regulated by SPs (23).
As previously noted, C/EBPα exerts a positive effect on the
expression of SP genes in the embryonic period, and thus, it may be
speculated that C/EBPα can indirectly promote the synthesis of
PC.
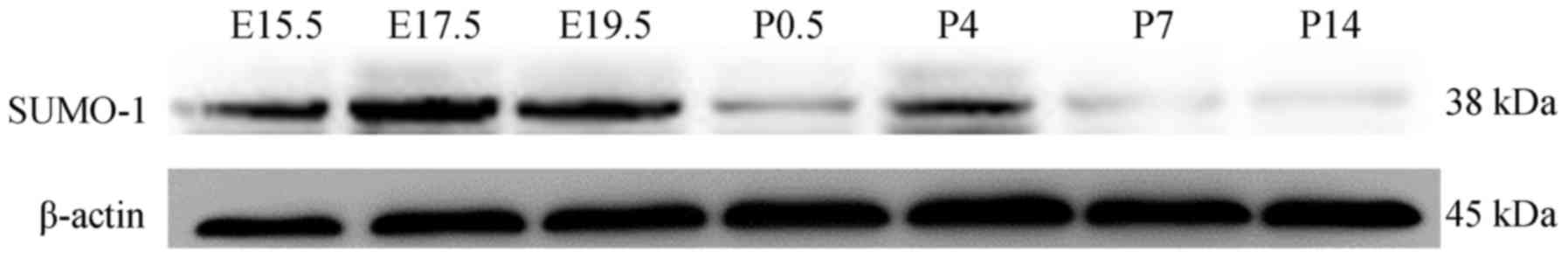
SUMO-modification of C/EBPα occurs during
lung development
C/EBPα is considered to serve a vital role in lung
morphogenesis and cytodifferentiation (12). The modification of C/EBPα by SUMO
post-translationally can alter the protein function (24). However, explicit data on the
sumoylated C/EBPα in lung development is absent, and therefore, rat
lung tissues were used to study the sumoylation of C/EBPα. As
presented in Fig. 7, SUMO-1
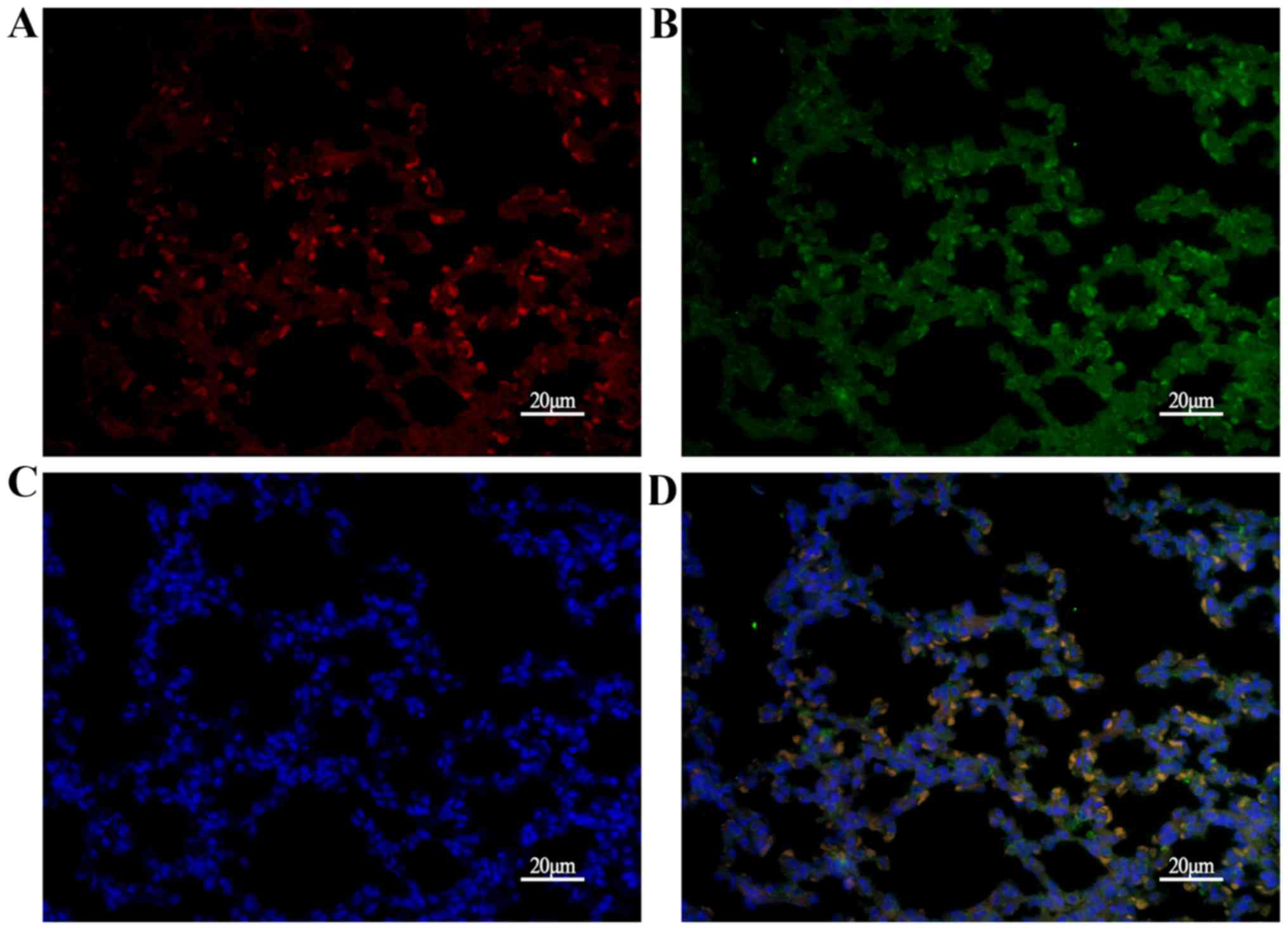
protein expressed during lung maturation. Next, C/EBPα and SUMO-1
were analyzed using immunofluorescence. As presented in Fig. 8, C/EBPα was partially colocalized
with SUMO-1 in the AECs at P14, suggesting that sumoylation of
C/EBPα occurred in the lung. Then, the expression of sumoylated
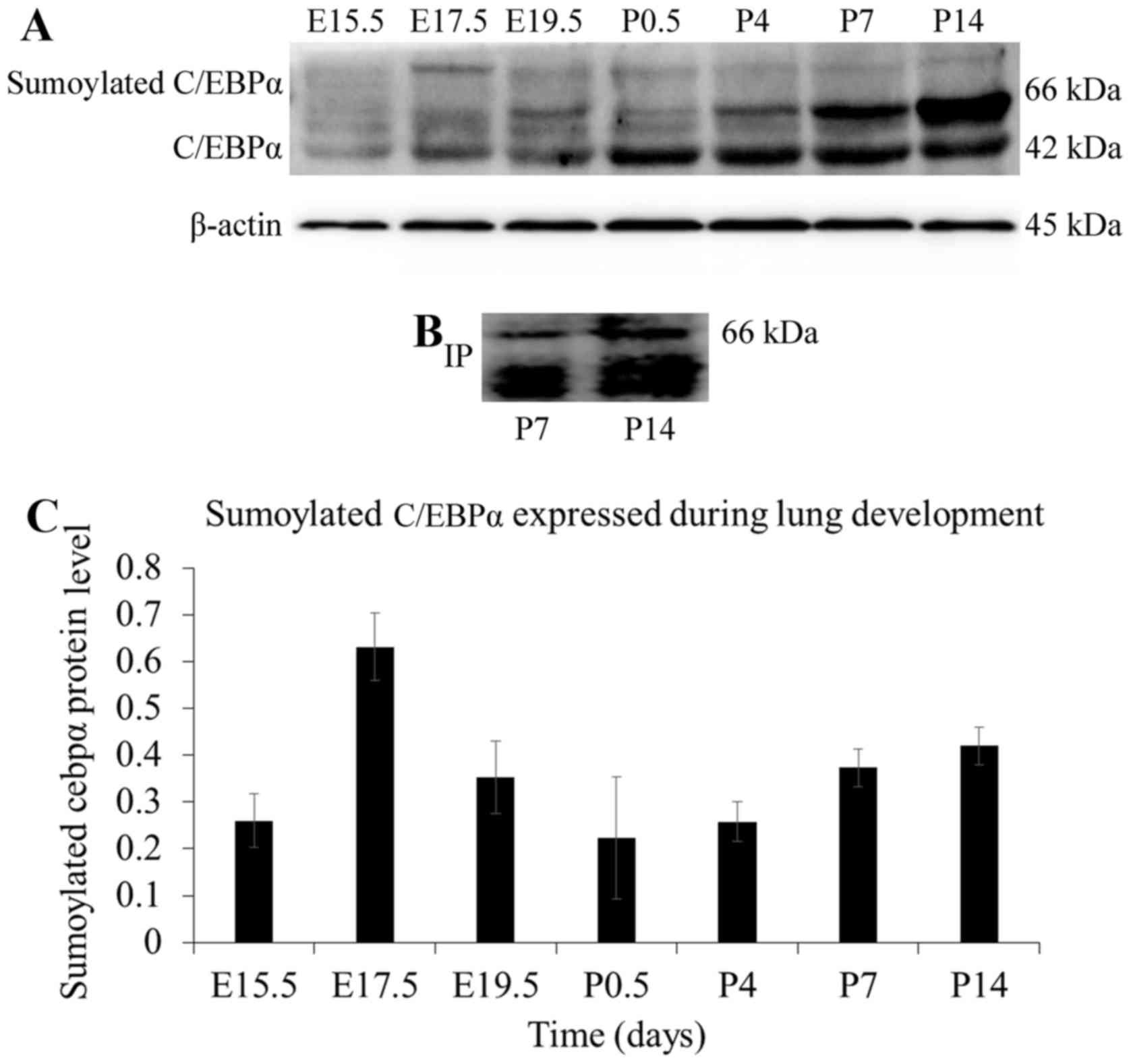
C/EBPα during lung development was assessed. As shown in Fig. 9A, a shifted band attributable to
modified C/EBPα was detected at different time-points during the
development of rat lung. The shifted band seemed to be sumoylated
C/EBPα, as its mobility was in accordance with that of the
confirmed sumoylated C/EBPα in the hepatocytes (19). To confirm whether this shifted
band was indeed SUMO-1-modified C/EBPα, the immunoprecipitation
assay was carried out. As shown in Fig. 9B, the sumoylated C/EBPα was
precipitated with the anti-C/EBPα antibody and immunoreacted with
the anti-SUMO-1 antibody. The mobility of the SUMO-1-modified
C/EBPα was found to be identical with that of the shifted band
described above. Therefore, it was confirmed that this shifted
C/EBPα band was generated by sumoylation.
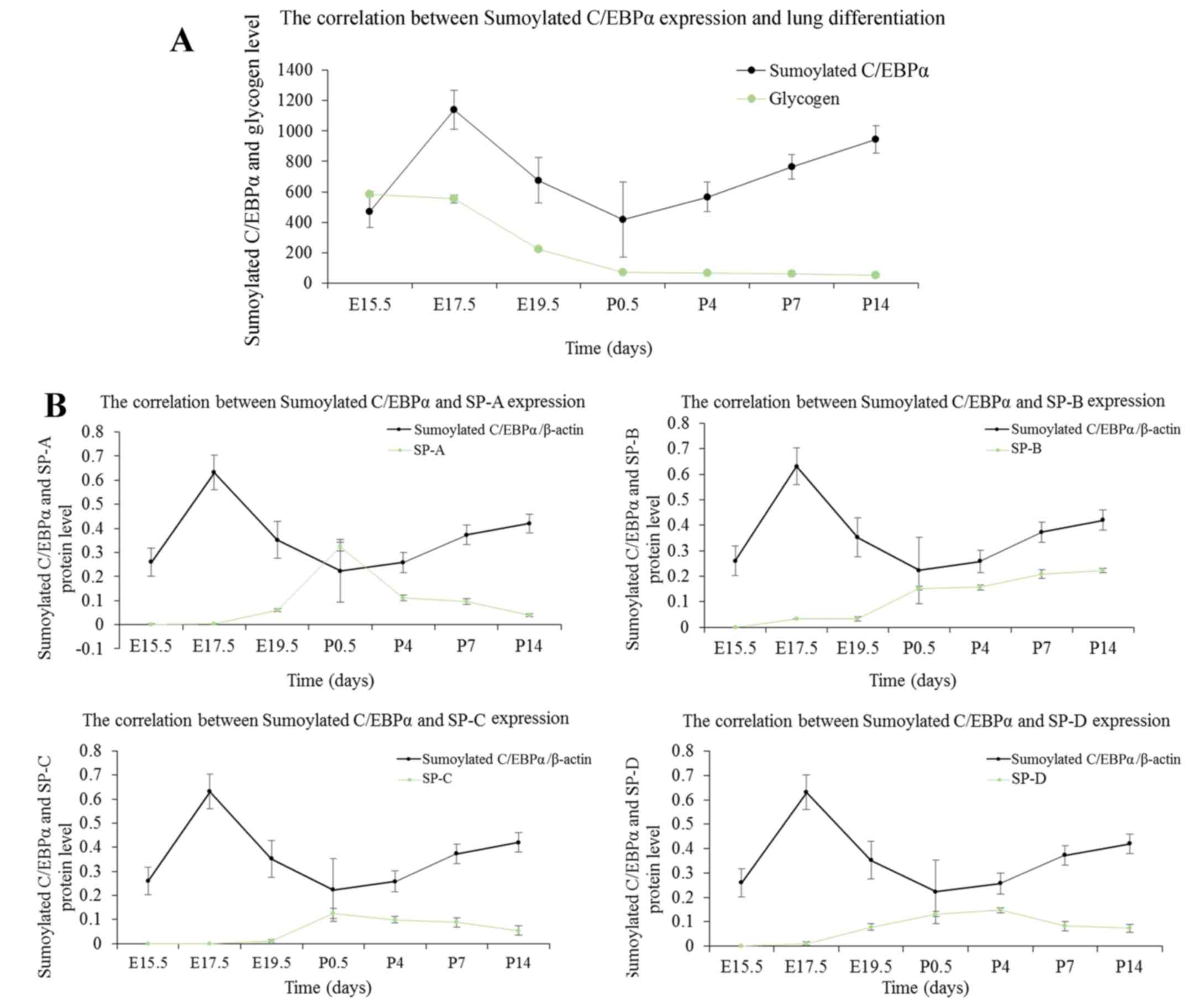
Correlation between sumoylated C/EBPα and
lung development
The above experiments suggested that the C/EBPα
protein was sumoylated in the lung. C/EBPα is an established key
regulator of lung development (25,26), and thus, the function of the
sumoylated C/EBPα protein needs to be addressed. As shown in
Fig. 9, sumoylated C/EBPα
exhibits a developmental expression pattern during lung
development, which was scarcely detected at the late
pseudoglandular stage at E15.5; the expression increased and was
maximal at E17.5, following which, the sumoylated C/EBPα decreased
until the saccular period. At the alveolar stage, it increased
slightly and finally stabilized. The decreased sumoylation of
C/EBPα occurred at the lung development stage, which was
characterized by extensive cell differentiation and secretion of
pulmonary surfactant. The results demonstrated that the amount of
glycogen exhibited a pattern approximately similar to the
expression of sumoylated C/EBPα protein at the embryo and early
postnatal stages. A negative correlation between sumoylation status
of C/EBPα and lung differentiation was observed in Fig. 10A. In addition, as shown in
Fig. 10B, the correlations of
pulmonary surfactant secretion with C/EBPα and sumoylated C/EBPα
were inverse. These data suggested that sumoylation may exert a
suppressive effect on C/EBPα-mediated AEC-II differentiation and
secretion.
Discussion
In the present study, the authors demonstrated that
C/EBPα exhibits a dynamic expression pattern during lung
development. To investigate the functional roles of C/EBPα in the
lung, the authors analyzed its correlation to the differentiation
and secretion of AEC-II, the accepted stem cell of the pulmonary
epithelium (27). It was
identified that the changes in the status of C/EBPα were associated
with AEC-II differentiation as reflected by the content of glycogen
during lung maturation. The expression pattern of C/EBPα protein is
almost identical to that of SPs, and exerts a positive effect on
AEC-II differentiation and secretion. In addition, SUMO was
demonstrated to modify C/EBPα in the lung tissue, and sumoylation
may have a negative effect on C/EBPα-mediated lung maturation.
During previous years, the physiological roles of
C/EBPα have begun to be identified. C/EBPα is relatively highly
expressed in the lung; is a key regulator of lung differentiation
(25,26) and directly activates the
transcription of several lung-specific and
differentiation-dependent genes (10,11). C/EBPα expression is initiated in
close temporal proximity to the emergence of AEC-II and the
original formation of pulmonary surfactant system. The result has
shown a positive correlation between the C/EBPα expression and
AEC-II differentiation and secretion. The expression patterns of
SPs, except SP-B, are almost similar to that of the C/EBPα protein.
The promoter of SP-A and SP-D gene harbors the C/EBP binding sites
(8,9). The current observations are in
agreement with those described previously; C/EBPα was positively
correlated with the expression trend of the two hydrophilic
proteins, SP-A and SP-D. SP-B and SP-C are small hydrophobic
proteins; their promoter contains the TTF-1 binding sites (28). TTF-1 is the synergy transcription
factor of C/EBPα. C/EBPα, SP-A and SP-D occur primarily in AEC-II,
and to a lesser extent in bronchioalveolar epithelial (Clara) cells
(7,29,30). On the other hand, the expression
of the SP-C gene is restricted to AEC-II (31), whereas that of SP-B occurs in both
AEC-II and bronchiolar epithelial cells (29). As a result, a part of SP-B is not
regulated by C/EBPα. In summary, C/EBPα exhibits a dynamic
expression pattern during lung development; the expression of
C/EBPα is related to the glycogen content and the secretion of SPs.
Altogether, these results suggested that the role of C/EBPα in lung
maturation is exercised by the regulation of pulmonary
surfactant.
The authors demonstrated that C/EBPα could be
modified by SUMO-1 protein in the lung; the sumoylated C/EBPα
displays a negative correlation with AEC-II differentiation and
secretion. This suggests that SUMO-modification may be involved in
the regulation of C/EBPα-induced lung maturation. SUMO-1 modifies
C/EBPα at lysine residue 159 within a conserved domain that
negatively modulates the transcriptional activity (17). Sumoylation confers new
protein-protein interaction properties of the transcription
factors, which in turn can significantly alter the transcriptional
activity. Histone deacetylases (HDACs) are typically correlated
with the repression of transcription and can be recruited to
sumoylated transcription factor complexes. C/EBPα promotes the
transcription of several genes expressed in a tissue-specific and
differentiation-dependent manner (32,33); HDAC3 interacts with sumoylated
C/EBPα to negatively regulate the liver X receptor alpha expression
(34). C/EBPα associates with the
SWI/SNF complex and p21 can increase its stability and
transcription activity; however, the sumoylation of C/EBPα inhibits
the binding with these factors (19,35,36). Taken together, the presented
results suggested that sumoylation might suppress the
C/EBPα-mediated transactivation of SP genes and restrain PC
synthesis indirectly. SPs are differentiation-dependent genes, and
these results were consistent with the finding in our study that
the sumoylation of C/EBPα expression is converse to lung
differentiation. Therefore, additional studies on the effect of
ectopic sumoylation of C/EBPα in lung are essential.
Furthermore, the results showed that the level of
C/EBPα sumoylation was low in the developing lung, less than half
of the total level of C/EBPα, similar to that reported for other
sumoylated proteins (37–40). However, the physiological effects
of sumoylation were abstruse. Intriguingly, the descending
expression of sumoylated C/EBPα corresponded to the increasing
C/EBPα at canalicular and saccular stages, the sophisticated
metamorphosis period during the lung development. Injury during
this phase will inhibit the expression of C/EBPα, resulting in
abnormal lung differentiation and pulmonary surfactant secretion
(41). In addition, a transient
increase in the expression of SUMO-1 was observed at P4. The lung
tissue was in a period of expansion during the postnatal days 1–3,
which led to the improvement of pulmonary morphology and functions.
At P4, the differentiation of AEC-II approached equilibrium, and
the proliferation served as the major hallmark of expression. Sato
et al (19) found that
sumoylation of C/EBPα blocked its inhibitory effect on cell
proliferation by disrupting the formation of a
proliferation-inhibition complex owing to the failure of
interaction between sumoylated C/EBPα and other proteins. This
phenomenon has not yet been reported in the lung. However, the
common endodermal origin and the crucial role of C/EBPα in the lung
and liver suggested a common role of SUMO in both organs. This
feature may provide a valuable insight into further investigations.
The current results found that SUMO-modification participates in
normal C/EBPα-mediated lung development, and offers a possibility
that it might continue to partake in abnormal ways; thus,
sumoylated C/EBPα may be a putative therapeutic target in lung
injury.
Two isoforms were generated by the C/EBPα gene, with
the resultant p42 kDa (full length) and p30 kDa (truncated) C/EBPα
proteins, and the N-terminus is abbreviated in the p30 kDa protein.
The heterodimerization between the two isoforms can restrain the
ability of p42 C/EBPα to transactivate the target genes (24). The p30 C/EBPα can be sumoylated,
although it is less sensitive to the changes in sumoylation than
the p42 isoform (18). p30 C/EBPα
enhances the sumoylation of p42 C/EBPα via the upregulation of
Ubc9, which results in decreased transcriptional activity of the
latter (15). Changes in the
ratio of p42:p30 have been confirmed to contribute to tipping the
scales from normal myelopoiesis to pre-leukemic or even leukemic
myelopoiesis (42). As indicated
in the results, there is a shifted band with electrophoretic
mobility just lower to that of the sumoylated C/EBPα. We also
observed a band at the consistent location by the
immunoprecipitation assay. The shifted band could be speculated as
the sumoylated p30 C/EBPα. Since similar investigations in the lung
are absent, further studies are essential.
In conclusion, the authors found that C/EBPα
exhibits a dynamic expression pattern during lung development and
exhibits a positive effect on AEC-II differentiation and secretion.
SUMO post-translationally modifies C/EBPα that occurs in the lung
tissue; its expression during development corresponded to that
during lung differentiation and several differentiation-dependent
genes expression. Furthermore, these results suggested that
sumoylation may act as a negative regulator of the C/EBPα-mediated
transactivation in the lung.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81370746), the
National Natural Science Foundation of Jiangsu Province (grant no.
BK20161356) and the Social Development Foundation of Zhenjiang,
China (grant no. SH2015071).
References
|
1
|
Cardoso WV: Lung morphogenesis revisited:
Old facts, current ideas. Dev Dyn. 219:121–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakai T, Larsen M and Yamada KM:
Fibronectin requirement in branching morphogenesis. Nature.
423:876–881. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prodhan P and Kinane TB: Developmental
paradigms in terminal lung development. BioEssays. 24:1052–1059.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mullassery D and Smith NP: Lung
development. Semin Pediatr Surg. 24:152–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merritt TA, Deming DD and Boynton BR: The
'new' bronchopulmonary dysplasia: Challenges and commentary. Semin
Fetal Neonatal Med. 14:345–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mendelson CR: Role of transcription
factors in fetal lung development and surfactant protein gene
expression. Annu Rev Physiol. 62:875–915. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cassel TN and Nord M: C/EBP transcription
factors in the lung epithelium. Am J Physiol Lung Cell Mol Physiol.
285:L773–L781. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He Y and Crouch E: Surfactant protein D
gene regulation. Interactions among the conserved
CCAAT/enhancer-binding protein elements. J Biol Chem.
277:19530–19537. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenberg E, Li F, Reisher SR, Wang M,
Gonzales LW, Ewing JR, Malek S, Ballard PL, Notarfrancesco K,
Shuman H, et al: Members of the C/EBP transcription factor family
stimulate expression of the human and rat surfactant protein A
(SP-A) genes. Biochim Biophys Acta. 1575:82–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nord M, Låg M, Cassel TN, Randmark M,
Becher R, Barnes HJ, Schwarze PE, Gustafsson JA and Lund J:
Regulation of CCSP (PCB-BP/uteroglobin) expression in primary
cultures of lung cells: Involvement of C/EBP. DNA Cell Biol.
17:481–492. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cassel TN, Gustafsson JA and Nord M:
CYP2B1 is regulated by C/EBP alpha and C/EBP delta inlung
epithelial cells. Mol Cell Biol Res Commun. 3:42–47. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berg T, Didon L and Nord M: Ectopic
expression of C/EBPalpha in the lung epithelium disrupts late lung
development. Am J Physiol Lung Cell Mol Physiol. 291:L683–L693.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verger A, Perdomo J and Crossley M:
Modification with SUMO. A role in transcriptional regulation. EMBO
Rep. 4:137–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tatham MH, Jaffray E, Vaughan OA, Desterro
JM, Botting CH, Naismith JH and Hay RT: Polymeric chains of SUMO-2
and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and
Ubc9. J Biol Chem. 276:35368–35374. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geletu M, Balkhi MY, Peer Zada AA,
Christopeit M, Pulikkan JA, Trivedi AK, Tenen DG and Behre G:
Target proteins of C/EBPalphap30 in AML: C/EBPalphap30 enhances
sumoylation of C/EBPalphap42 via up-regulation of Ubc9. Blood.
110:3301–3309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Subramanian L, Benson MD and Iñiguez-Lluhí
JA: A synergy control motif within the attenuator domain of
CCAAT/enhancer-binding protein alpha inhibits transcriptional
synergy through its PIASy-enhanced modification by SUMO-1 or
SUMO-3. J Biol Chem. 278:9134–9141. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim J, Cantwell CA, Johnson PF, Pfarr CM
and Williams SC: Transcriptional activity of CCAAT/enhancer-binding
proteins is controlled by a conserved inhibitory domain that is a
target for sumoylation. J Biol Chem. 277:38037–38044. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hankey W, Silver M, Sun BS, Zibello T,
Berliner N and Khanna-Gupta A: Differential effects of sumoylation
on the activities of CCAAT enhancer binding protein alpha (C/EBPα)
p42 versus p30 may contribute in part, to aberrant C/EBPα activity
in acute leukemias. Hematol Rep. 3:e52011. View Article : Google Scholar
|
|
19
|
Sato Y, Miyake K, Kaneoka H and Iijima S:
Sumoylation of CCAAT/enhancer-binding protein alpha and its
functional roles in hepatocyte differentiation. J Biol Chem.
281:21629–21639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ridsdale R and Post M: Surfactant lipid
synthesis and lamellar body formation in glycogen-laden type II
cells. Am J Physiol Lung Cell Mol Physiol. 287:L743–L751. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quirk JG, Bleasdale JE, MacDonald PC and
Johnston JM: A role for cytidine monophosphate in the regulation of
the glycerophospholipid composition of surfactant in developing
lung. Biochem Biophys Res Commun. 95:985–992. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen SF, Sun J, Zheng L, Zhang ZL and Sun
YS: Expressions of peroxisome proliferator-activated receptor gamma
and CCAAT/enhancer binding protein alpha during the differentiation
process of mouse 3T3-L1 preadipocytes. J Clin Rehabilitative Tissue
Eng Res. 37:206–212. 2010.
|
|
23
|
Zhang XQ, Zhang P, Yang Y, Qiu J, Kan Q,
Liang HL, Zhou XY and Zhou XG: Regulation of pulmonary surfactant
synthesis in fetal rat type II alveolar epithelial cells by
microRNA-26a. Pediatr Pulmonol. 49:863–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khanna-Gupta A: Sumoylation and the
function of CCAAT enhancer binding protein alpha (C/EBP alpha).
Blood Cells Mol Dis. 41:77–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Didon L, Roos AB, Elmberger GP, Gonzalez
FJ and Nord M: Lung-specific inactivation of CCAAT/enhancer binding
protein alpha causes a pathological pattern characteristic of COPD.
Eur Respir J. 35:186–197. 2010. View Article : Google Scholar
|
|
26
|
Miglino N, Roth M, Tamm M and Borger P:
Asthma and COPD - The C/EBP connection. Open Respir Med J. 6:1–13.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bishop AE: Pulmonary epithelial stem
cells. Cell Prolif. 37:89–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang MC, Guo Y, Liu CC, Weissler JC and
Yang YS: The TTF-1/TAP26 complex differentially modulates
surfactant protein-B (SP-B) and -C (SP-C) promoters in lung cells.
Biochem Biophys Res Commun. 344:484–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wohlford-Lenane CL and Snyder JM:
Localization of surfactant-associated proteins SP-A and SP-B mRNA
in rabbit fetal lung tissue by in situ hybridization. Am J Respir
Cell Mol Biol. 7:335–343. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crouch EC: Structure, biologic properties,
and expression of surfactant protein D (SP-D). Biochim Biophys
Acta. 1408:278–289. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wohlford-Lenane CL, Durham PL and Snyder
JM: Localization of surfactant-associated protein C (SP-C) mRNA in
fetal rabbit lung tissue by in situ hybridization. Am J Respir Cell
Mol Biol. 6:225–234. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Birkenmeier EH, Gwynn B, Howard S, Jerry
J, Gordon JI, Landschulz WH and McKnight SL: Tissue-specific
expression, developmental regulation, and genetic mapping of the
gene encoding CCAAT/enhancer binding protein. Genes Dev.
3:1146–1156. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsukada J, Yoshida Y, Kominato Y and Auron
PE: The CCAAT/enhancer (C/EBP) family of basic-leucine zipper
(bZIP) transcription factors is a multifaceted highly-regulated
system for gene regulation. Cytokine. 54:6–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren J, Li D, Li Y, Lan X, Zheng J, Wang X,
Ma J and Lu S: HDAC3 interacts with sumoylated C/EBPα to negatively
regulate the LXRα expression in rat hepatocytes. Mol Cell
Endocrinol. 374:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harris TE, Albrecht JH, Nakanishi M and
Darlington GJ: CCAAT/enhancer-binding protein-alpha cooperates with
p21 to inhibit cyclin-dependent kinase-2 activity and induces
growth arrest independent of DNA binding. J Biol Chem.
276:29200–29209. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Timchenko NA, Wilde M and Darlington GJ:
C/EBPalpha regulates formation of S-phase-specific E2F-p107
complexes in livers of newborn mice. Mol Cell Biol. 19:2936–2945.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Le Drean Y, Mincheneau N, Le Goff P and
Michel D: Potentiation of glucocorticoid receptor transcriptional
activity by sumoylation. Endocrinology. 143:3482–3489. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hirano Y, Murata S, Tanaka K, Shimizu M
and Sato R: Sterol regulatory element-binding proteins are
negatively regulated through SUMO-1 modification independent of the
ubiquitin/26 S proteasome pathway. J Biol Chem. 278:16809–16819.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Degerny C, Monte D, Beaudoin C, Jaffray E,
Portois L, Hay RT, de Launoit Y and Baert JL: SUMO modification of
the Ets-related transcription factor ERM inhibits its
transcriptional activity. J Biol Chem. 280:24330–24338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Desterro JM, Keegan LP, Jaffray E, Hay RT,
O'Connell MA and Carmo-Fonseca M: SUMO-1 modification alters ADAR1
editing activity. Mol Biol Cell. 16:5115–5126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bassères DS, Levantini E, Ji H, Monti S,
Elf S, Dayaram T, Fenyus M, Kocher O, Golub T, Wong KK, et al:
Respiratory failure due to differentiation arrest and expansion of
alveolar cells following lung-specific loss of the transcription
factor C/EBPalpha in mice. Mol Cell Biol. 26:1109–1123. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pabst T, Mueller BU, Zhang P, Radomska HS,
Narravula S, Schnittger S, Behre G, Hiddemann W and Tenen DG:
Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer
binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat
Genet. 27:263–270. 2001. View
Article : Google Scholar : PubMed/NCBI
|