Introduction
Transient receptor potential (TRP) proteins are
non-selective cation channels performing numerous roles as
versatile cellular sensors and effectors (1). The TRP superfamily consists of 33
channels, which are divided into seven families: TRPC, TRPM, TRPV,
TRPA, TRPP, TRPML and TRPN. Apart from the TRPN channels, all TRP
families are expressed in humans and are associated with diverse
conditions, such as neurological, cardiological, pulmonary, renal,
dermatological and urological diseases (1). TRPC1, the founding member of the
TRPC family, was the first cloned mammalian TRP protein, but its
molecular function as a channel in the development of kidney
disease, particularly podocytopathy, remains obscure.
Podocytopathy, including podocyte injury and loss,
is a major pathological mechanism underlying glomerular sclerosis
(2). Although numerous signaling
pathways have been implicated in podocyte damage (3,4),
it has been demonstrated that microRNAs are necessary for podocyte
homeostasis, with podocyte-selective deletion of dicer, an enzyme
that generates microRNAs (5),
promoting podocyte injury (6,7).
More recently, several microRNAs, including miR-193a (8), miR-29a (9), the miR-30 family (10,11) and the miR-135 family (12), have been implicated in podocyte
injury.
The miR-135 family is evolutionarily highly
conserved and comprises two members, miR-135a and miR-135b. It has
been reported that miR-135a functions as a tumor suppressor gene in
gastric (13), prostate (14) and renal cancer (15), and malignant glioma cells
(16). However, other studies
recently demonstrated that miR-135a acts as a tumor-promoting gene
involved in the development of several cancers, including the
pathogenesis of colorectal cancer (17), the promotion of paclitaxel
resistance in non-small-cell lung cancer (18), and the facilitation of growth and
invasion in colorectal cancer (19). In addition, it was demonstrated
that ectopic expression of miR-135 family members may induce actin
fiber disruption (12),
suggesting that miR-135a is involved in podocyte actin fiber and
cytoskeletal stability via an undetermined mechanism. Despite
promising findings, the precise function of the miR-135a remains
largely unknown, particularly in podocyte injury-associated renal
diseases.
The aim of the present study was to determine the
role and mechanisms of action of miR-135a and TRPC1 in podocyte
injury, and to elucidate the mechanisms underlying this type of
injury. miR-135a was found to be overexpressed in patients with
focal segmental glomerular sclerosis (FSGS) and in models of
podocyte injury; in addition, the ectopic expression of miR-135a
promoted podocyte injury by downregulating TRPC1 expression. Our
findings originally demonstrated that miR-135a and TRPC1 play an
important role in podocyte injury, and may provide novel insight
into the understanding of the molecular mechanisms underlying
podocyte injury, which may be crucial for the development of novel
therapeutic agents for the treatment of podocytopathy.
Materials and methods
Human samples
A total of 3 patients with FSGS were diagnosed by
renal biopsy at the Department of Nephrology of the First
Affiliated Hospital of Chongqing Medical University (Chongqing,
China). The control group comprised patients with kidney rupture
following incidents of violence or traffic accidents. The glomeruli
were collected from the renal tissues using the sieving technique,
as previously described (20),
placed in TRIzol reagent (Ambion, Austin, TX, USA) and stored at
−70°C for RNA extraction and molecular analysis. Human subject
research approval was granted by the Ethics Committee of the First
Affiliated Hospital of Chongqing Medical University. All the
participants enrolled in this study provided written informed
consent.
Animal experiments
The use of animals in the present study was approved
by the Ethics Committee of the First Affiliated Hospital of
Chongqing Medical University. BALB/c mice (6–7 weeks old, weighing
24–28 g, n=60) were used to create the adriamycin (ADR)-induced
podocyte injury model. The models were generated by administration
of an intravenous injection of ADR (10.5 mg/kg; Sigma-Aldrich;
Merck KGaA, St. Louis, MO, USA) as previously described (21,22). For the control group, the mice
received an equivalent volume of normal saline. The mice were
sacrificed by cervical vertebral dislocation on days 4, 7, 11, 15
and 20 following the administration of ADR or normal saline. In
total, there were 10 groups (5 ADR-treated groups and 5 normal
saline-treated groups) with 6 mice in each; one ADR-treated group
and one normal saline-treated group were sacrificed at the
indicated time points (4, 7, 11, 15 and 20 days). The glomeruli
were isolated from the kidneys using the sieving technique, as
previously described (20), and
were snap-frozen in liquid nitrogen for RNA and protein
extraction.
Cell culture and treatment
The mouse podocyte cell line 5 (MPC5) was used, and
the cells were cultured as previously described (23). In brief, the cells were cultured
at 33°C (10 U/ml of interferon-γ) with 5% CO2 and were
differentiated at 37°C (free interferon-γ) with 5% CO2
in RPMI Dutch-modified medium (Invitrogen; Thermo Fisher
Scientific, Carlsbad, CA, USA) supplemented with 10% v/v fetal calf
serum (Gibco-BRL; Thermo Fisher Scientific, Carlsbad, CA, USA).
293T cells were cultured in Dulbecco's modified Eagle's medium
(Invitrogen; Thermo Fisher Scientific) supplemented with 10% v/v
fetal bovine serum at 37°C with 5% CO2. Depending on the
experimental setup, the MPC5 cells were treated with different
doses of ADR and transforming growth factor (TGF)-β. For transient
transfection of miRNA mimics and plasmid DNA, Lipofectamine 3000
reagent (Invitrogen; Thermo Fisher Scientific, Grand Island, NY,
USA) was used, according to the manufacturer's instructions.
Bioinformatics analysis
Predicted miR-135a targets were obtained from
miRwalk (www.umm.uni-heidelberg.de/apps/zmf/mirwalk),
miRanda (www.microrna.org/microrna/home.do) and TargetScan
(www.targetscan.org/).
Plasmid construction
Genomic DNA fragments containing the 3′ untranslated
region (UTR) of TRPC1 were prepared by the amplification of genomic
DNA, cloned onto the pcDNA3.1-Luc reporter vector and verified by
sequencing. For the wild-type construct, the seed sequence was
wild-type. For the mutant construct, the seed sequence was mutated.
The TRPC1 coding sequence (CDS) was obtained by polymerase chain
reaction (PCR) and cloned into the pcDNA3.1(+) plasmid. The
sequences of the primers used are listed in Table I.
 | Table ISequences of the PCR primers for
construction. |
Table I
Sequences of the PCR primers for
construction.
| Name | Primer
sequences |
|---|
| TRPC1 3′UTR | |
| Sense |
5′-TGTATTTGCATACTTGCAA-3′ |
| Antisense |
5′-GAGGGAACACTTAAACCTG-3′ |
| TRPC1 3′UTR
(mutant) | |
| Sense |
5′-TGTATTTGCATACTTGCAA-3′ |
|
5′-CAATTACACATACGGCTTTCTT-3′ |
| Antisense |
5′-AAGAAAGCCGTATGTGTAATTG-3′ |
|
5′-GAGGGAACACTTAAACCTG-3′ |
| TRPC1 CDS | |
| Sense |
5′-ATGGGGGCCCCGCCTCCGTCT-3′ |
| Antisense |
5′-TTAATTTCTTGGATAAAACATAG-3′ |
Luciferase assays
miR-135a mimics and miRNA mimics negative control
were purchased from RiboBio Co., Ltd. (Guangzhou, China).
Luciferase assays were conducted as previously described (24). In brief, the 293T cells were
cultured in 24-well plates and transfected with 500 ng of the
reporter vector, 50 ng of pRL-CMV and 50 nM of miR-135a mimics or
the miRNA mimics negative control (RiboBio Co., Ltd.) using
Lipofectamine 3000 reagent (Invitrogen; Thermo Fisher Scientific).
According to the dual-Luciferase assay system manufacturer's
instructions (Promega, Madison, WI, USA), the Firefly and
Renilla Luciferase activities were measured at 36–48 h after
transfection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cultured cells and
renal tissue with TRIzol reagent (Ambion), according to the
manufacturer's instructions. cDNA was synthesized from 5 ng of
total RNA with the RevertAid First Strand cDNA synthesis kit
(Fermentas, Burlington, ON, Canada). RT-qPCR for the detection of
miR-135a was performed using miR-135a-specific PCR primers (RiboBio
Co., Ltd.) with the RevertAid First Strand cDNA Synthesis kit
(Fermentas) and SYBR Premix Ex Taq™ II (Takara, Dalian, China)
according to the manufacturers' instructions, with U6 as the
internal control. The sequences of the primers used in RT-qPCR are
presented in Table II. 18S was
used as the internal control.
 | Table IISequences of RT-PCR primers. |
Table II
Sequences of RT-PCR primers.
| Name | Primer
sequences | Product size
(bp) |
|---|
| TRPC1 | | |
| Sense |
5′-GGATTATTGGGATGATTTG-3′ | 143 |
| Antisense |
5′-GTGAGCCACCACTTTGAG-3′ | |
| 18S | | |
| Sense |
5′-GTAACCCGTTGAACCCCATT-3′ | 151 |
| Antisense |
5′-CCATCCAATCGGTAGTAGCG-3′ | |
Protein extraction and western blot
analysis
Total protein was extracted from the MPC5 cells and
isolated glomeruli using radioimmunoprecipitation assay lysis
buffer (Beyotime, Jiangsu, China) according to the manufacturer's
instructions. Western blot analysis was performed with a standard
method, as previously described (25). The antibodies used were as
follows: Rabbit anti-desmin (1:1,000; sc-14026), rabbit anti-snail
(1:1,000; sc-28199), rabbit anti-TRPC1 (1:1,000; sc-28199), rabbit
anti-Wilms tumor 1 (WT1; 1:500; sc-192), rabbit anti-E-cadherin
(1:1,000; sc-7870), mouse anti-β-tubulin (1:3,000; sc-80011), goat
anti-rabbit IgG-HRP (1:3,000; sc-2004), goat anti-mouse IgG-HRP
(1:3,000; sc-2005) (all from Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), and rabbit anti-nephrin (1:2,000; ab136894; Abcam,
Cambridge, MA, USA). Images were captured with a SPOT CCD camera
(Diagnostic, Sterling Heights, MI, USA). For the quantitative
analysis of the western blots, the protein band intensities were
quantified using ImageJ software and β-tubulin was used as the
internal control.
F-actin cytoskeleton staining and
immunofluorescence staining
Rhodamine-labeled phalloidin (diluted in
phosphate-buffered saline (PBS) containing 5% bovine serum albumin,
1:1,000; Sigma-Aldrich; Merck KGaA) was used to strain F-actin
according to the manufacturer's instructions. Images were captured
with a SPOT CCD camera (Diagnostic).
Flow cytometric analysis of apoptosis via
Annexin V staining
After treatment, the cultured podocytes were
collected and washed with ice-cold PBS twice, resuspended in 200
μl binding buffer, and then incubated with FITC-conjugated
Annexin V (final concentration, 0.5 μg/ml) for 15 min at
room temperature. Subsequently, the cells were again washed with
ice-cold PBS, centrifuged, and resuspended in 500 μl binding
buffer. Finally, 50 μg/ml propidium iodide was used to stain
the cells at room temperature for 5 min, followed by flow
cytometric analysis using a FACScan flow cytometer and CellQuest
software (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
All the data are presented as means ± standard
deviation. The two-tailed Student's t-test was employed to indicate
whether two sets of data (groups) differed significantly using
GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA). P-values <0.05 and <0.01 were considered statistically
significant and highly statistically significant, respectively. All
the experiments were performed at least 3 times.
Results
Enhanced miR-135a expression in podocyte
injury
To investigate whether miR-135a is involved in the
pathogenesis of podocyte injury, glomeruli were first isolated from
3 patients with FSGS, whose podocytes were severely damaged, and
from 3 patients who had suffered kidney rupture due to incidents of
violence or traffic accidents. The ruptured kidney samples were
used as controls. miR-135a expression was examined by RT-qPCR in
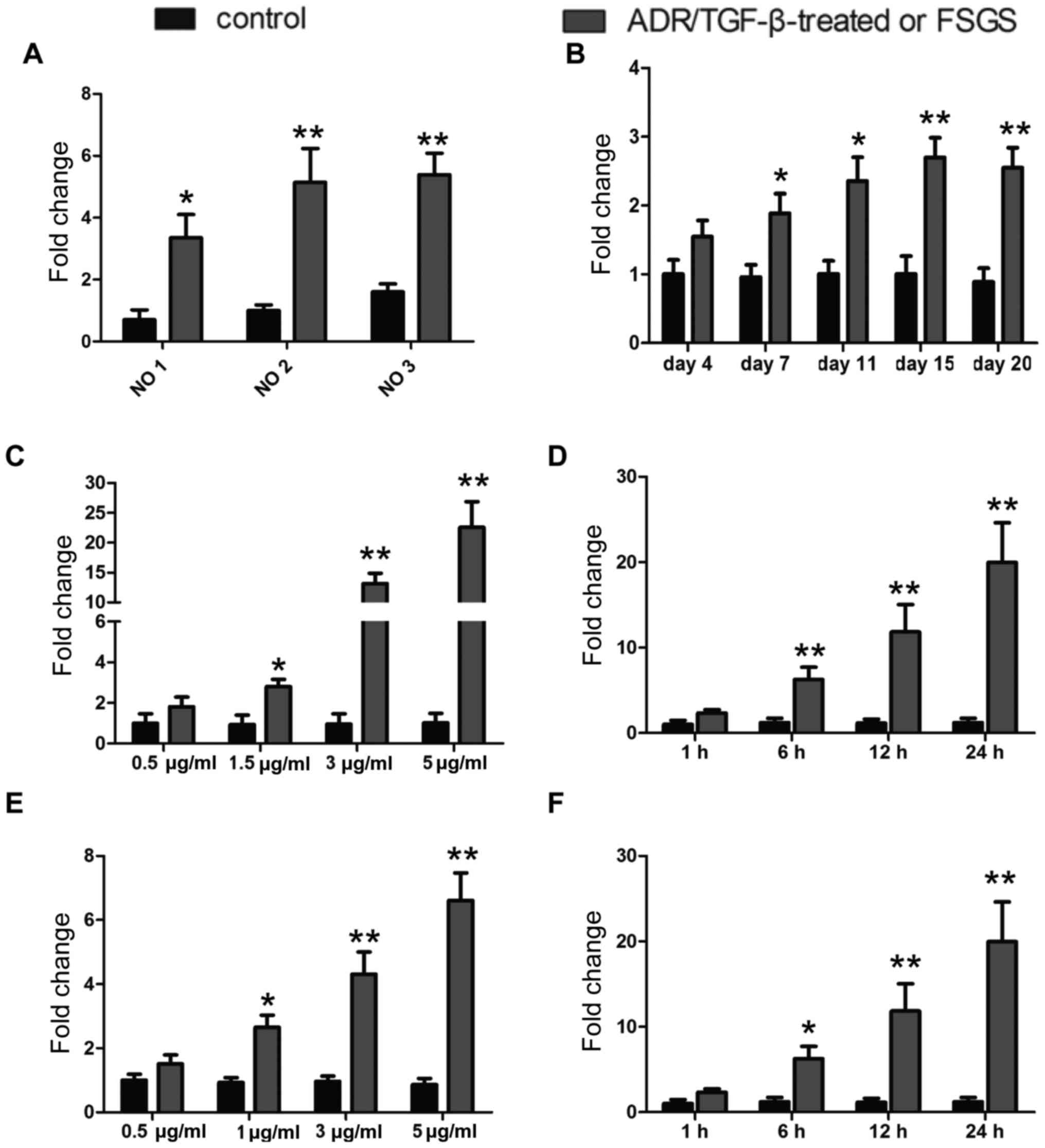
the glomeruli from these patients and, as indicated in Fig. 1A, miR-135a was highly expressed in
the glomeruli of FSGS patients compared with those of controls.
Furthermore, a mouse model was established by intravenous injection
of ADR (10.5 mg/kg), which is a widely recognized model of podocyte
injury (21,22), and the expression of miR-135a was
then detected in the isolated glomeruli. The expression level
increased significantly as early as day 4 following the injection
of ADR. On day 15 following treatment with ADR, the miR-135a
expression level improved by ~3-fold. Additionally, the expression
level of miR-135a was measured in in vitro models of
ADR-induced and TGF-β-induced podocyte injury. As illustrated
Fig. 1C–F, treatment with ADR
increased the expression of miR-135a in cultured MPC5 cells in a
dose-dependent manner [24 h after ADR treatment (Fig. 1C)], as well as in a time-dependent
manner [5 μg̸ml ADR treatment (Fig. 1D)]. Similarly, TGF-β enhanced the
expression level of miR-135a in a dose-dependent manner [24 h after
TGF-β treatment (Fig. 1E)], as
well as in a time-dependent manner [5 ng̸ml TGF-β treatment
(Fig. 1F)] in cultured MPC5
cells. Thus, these data clearly demonstrated that miR-135a was
upregulated in podocyte injury models and glomeruli isolated from
patients with FSGS.
miR-135a promotes podocyte injury and
apoptosis
As shown in Fig.
1, our results demonstrated that miR-135a expression was
upregulated in podocyte injury. We then determined whether the
increased expression of miR-135a was functionally significant in
podocyte damage. miR-135a mimics and miRNA mimics negative control
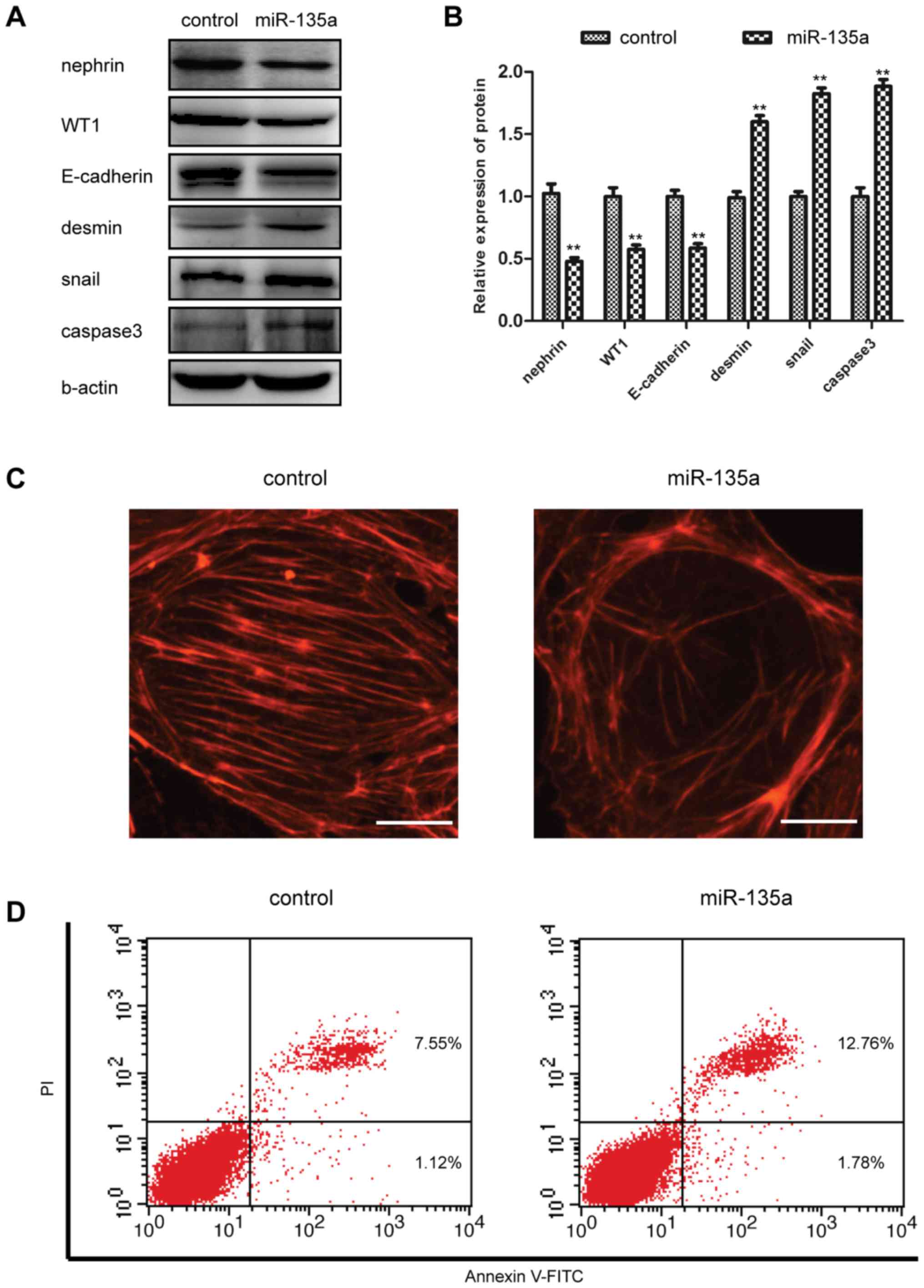
were transfected into the cultured MPC5 cells. As indicated in
Fig. 2A and B, the protein
expression levels of desmin and Snail, two markers associated with
podocyte injury, were significantly increased in the cells
transfected with miR-135a mimics compared with the cells
transfected with miRNA mimics negative control. However, the
expression of functional markers, such as nephrin, WT1 and
E-cadherin, was inhibited by miR-135a mimics (Fig. 2A and B). To further support the
hypothesis of the damaging effect of miR-135a on podocytes, at 48 h
following miR-135a mimics transfection into MPC5 cells, the
podocyte cytoskeleton was examined by means of
fluorescein-conjugated phalloidin staining, and it was observed
that the stress fibres in podocytes were severely reduced in number
and disordered (Fig. 2C). The
podocytes of cells transfected with miR-135a mimics also exhibited
significantly increased levels of apoptosis compared with miRNA
negative-transfected control cells (Fig. 2D). Furthermore, the ectopic
expression of miR-135a improved the protein expression level of
activated caspase-3 (Fig. 2A and
B). These date clearly indicated that miR-135a enhances
podocyte injury and promotes podocyte apoptosis.
The expression level of TRPC1 was
downregulated in podocyte injury
In order to further elucidate the molecular
mechanisms underlying miR-135a-induced podocyte injury, the targets
of miR-135a were predicted by online miRNA target prediction
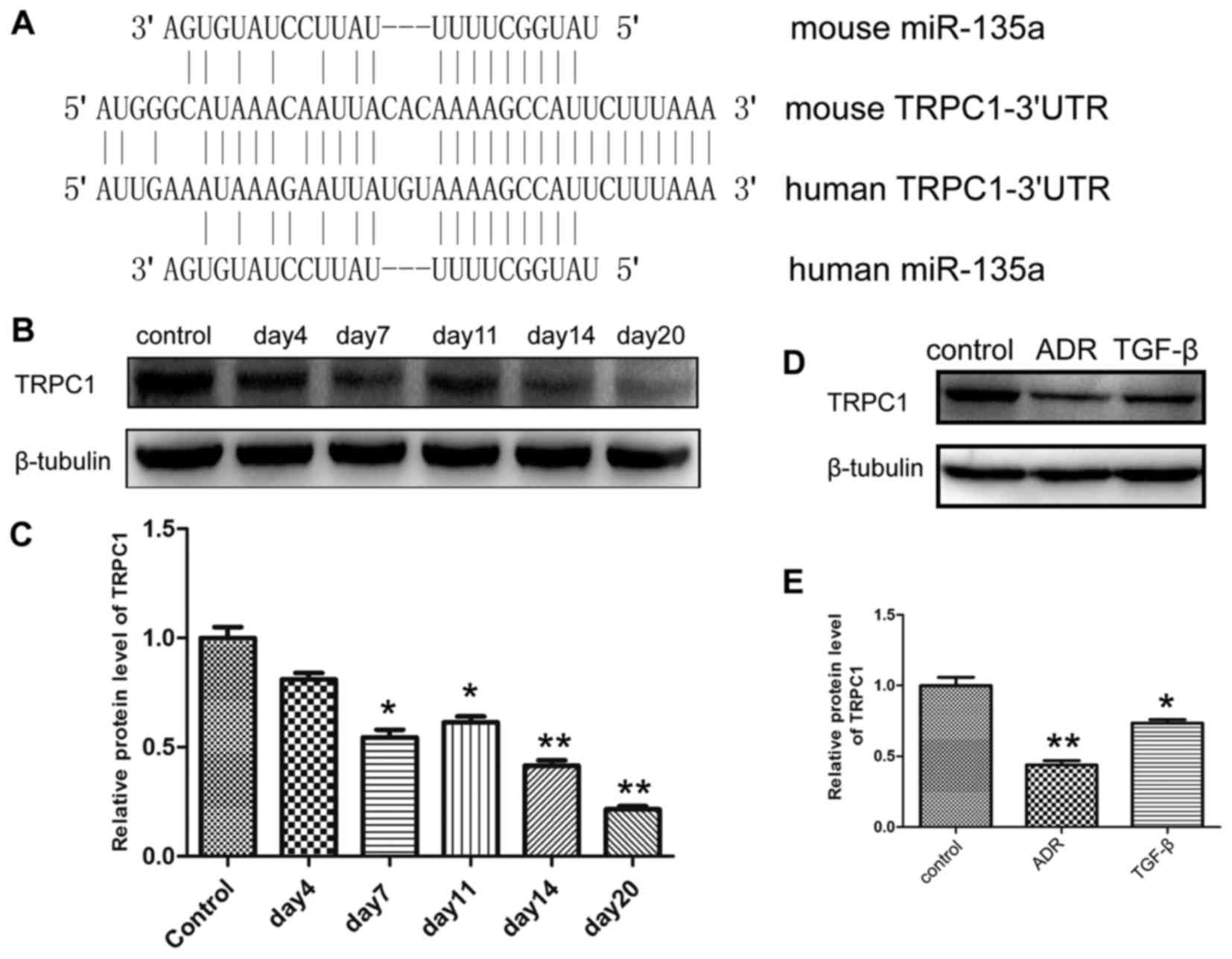
programmes, such as TargetScan (www.targetscan.org) PicTar (http://pictar.mdc-berlin.de) and miRanda (www.microrna.org), suggesting that miR-135a possessed
a highly conserved binding site in the 3′UTR of TRPC1 (Fig. 3A). Subsequently, the expression
level of TRPC1 was investigated. As shown in Fig. 3B and C, TRPC1 expression was
severely inhibited in the glomeruli isolated from ADR-induced
podocyte injury mouse models; on day 20 following ADR injection,
the expression level of TRPC1 had decreased by 75% compared with
the control group. In cultured MPC5 cells, the expression level of
TRPC1 was reduced by ADR and TGF-β (Fig. 3D and E).
miR-135a directly targets TRPC1 3′UTR and
inhibits TRPC1 expression
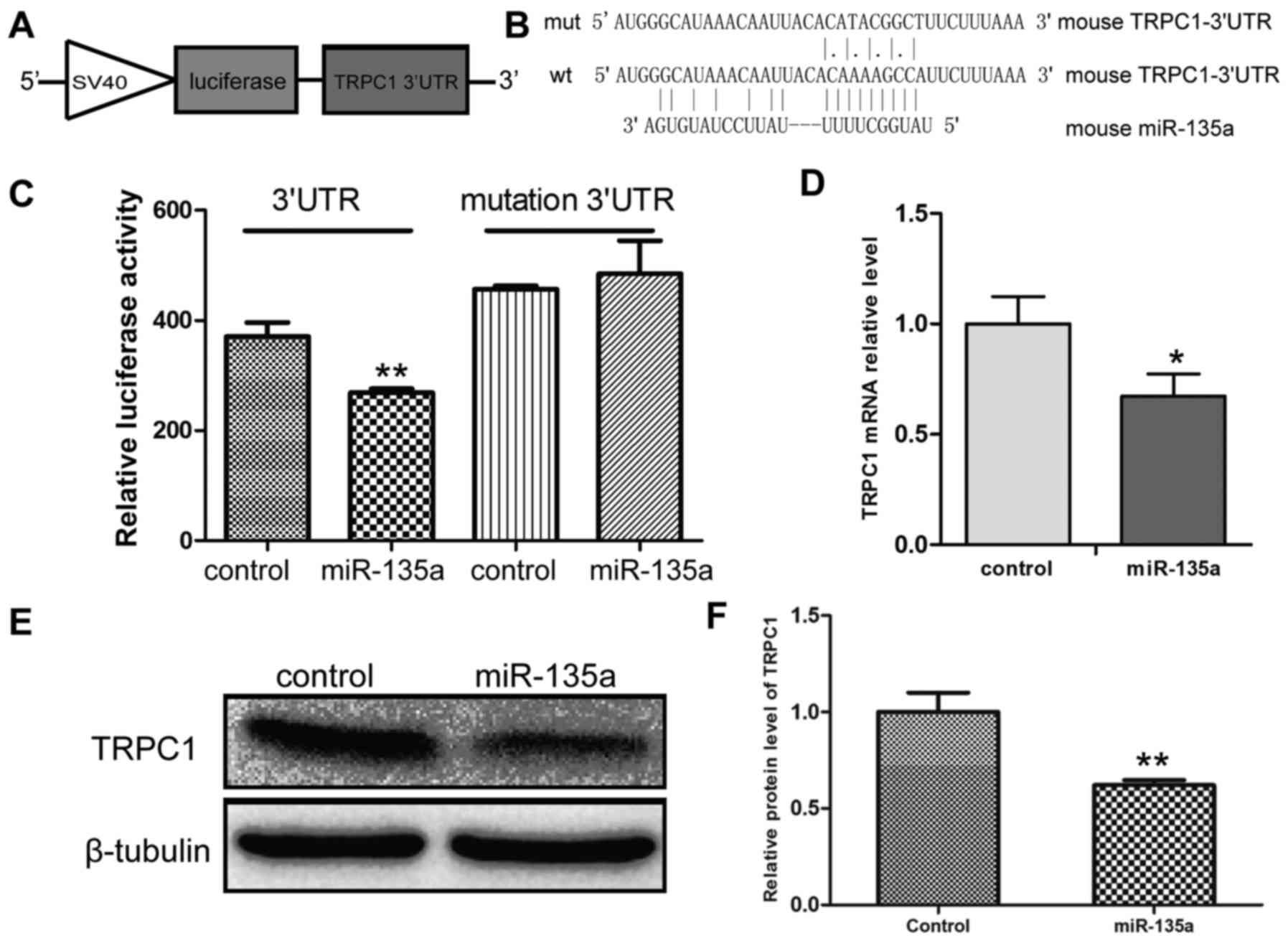
In order to further validate the results of
bioinformatics analysis, mouse TRPC1 3′UTR was cloned into the
pcDNA3.1-Luciferase reporter vector, generating a mutant reporter
vector in which the putative miR-135a binding site AAA GCC AU in
the TRPC1 3′UTR was mutated into ATA CGG CTU (Figs. 3A and 4A and B). Luciferase reporter assays
were conducted in HEK293T cells to determine whether miR-135a
significantly repressed TRPC1 transcript levels. The results
revealed that Luciferase expression was significantly suppressed in
the group transfected with the miR-135a mimics compared with miRNA
mimics negative control group, while mutations of the
miR-135a-binding sites reversed the suppression of Luciferase
expression (Fig. 4C). Moreover,
the effect of miR-135a on TRPC1 expression was also examined, and
the results indicated that miR-135a inhibited the expression of
TRPC1 at the protein as well as at the mRNA level (Fig. 4D–F). Taken together, these results
demonstrated that miR-135a directly binds to the 3′UTR of TRPC1 and
inhibits TRPC1 expression.
Overexpression of TRPC1 abrogates
miR-135a-induced podocyte injury
To investigate whether the miR-135a-induced podocyte
injury was TRPC1-dependent, mouse TRPC1 CDS was cloned into the
pcDNA3.1(+) vector (TRPC1 overexpression vector). Western blot
analyses confirmed that TRPC1 was significantly increased by
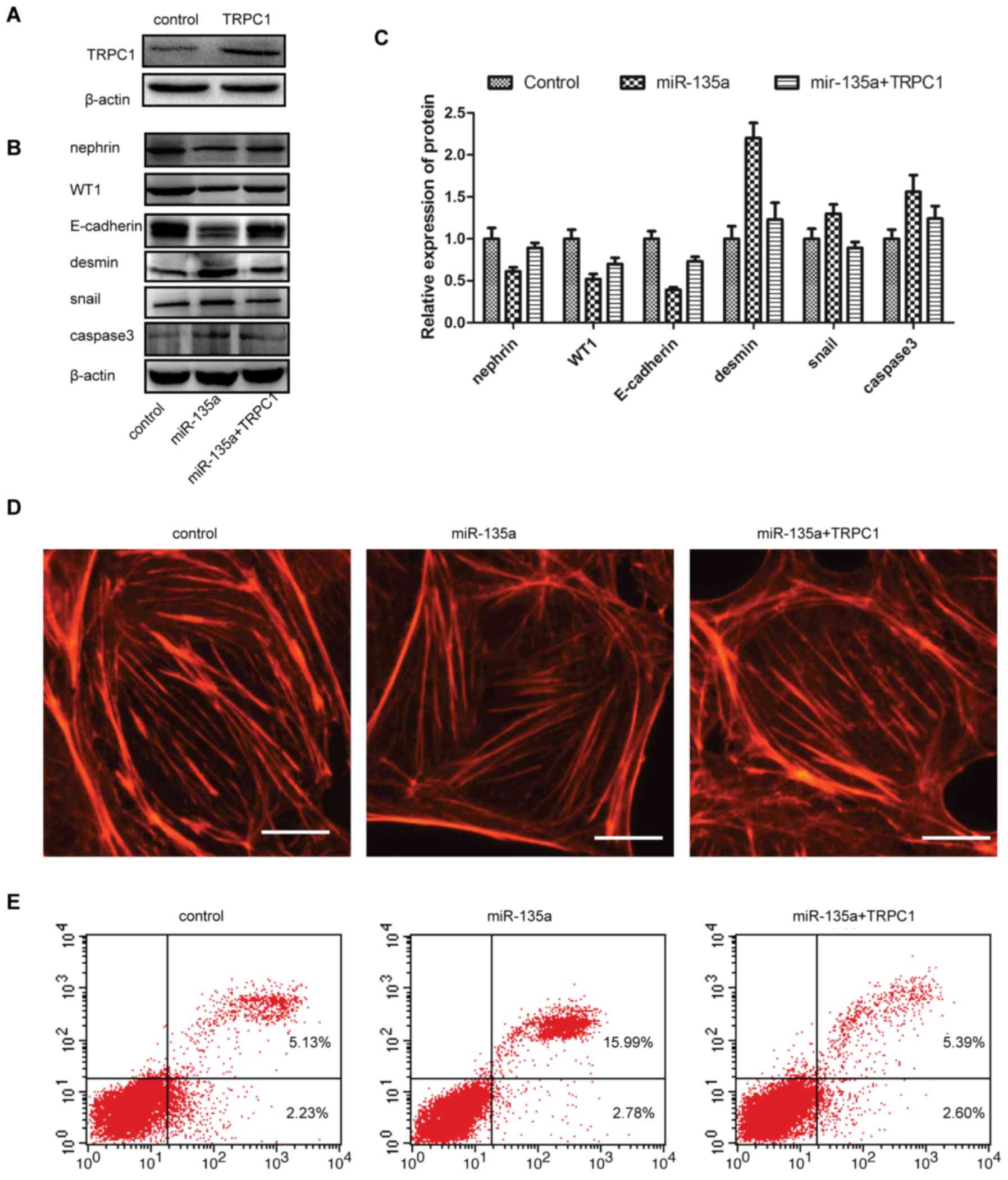
ectopic expression compare with control (Fig. 5A). The expression of podocyte
injury markers and functional markers was next determined, and the
results suggested that TRPC1 reversed the increased expression of
desmin̸Snail̸activated caspase-3, and inhibited the
miR-135a-induced expression of nephrin̸WT1̸E-cadherin (Fig. 5B and C). Furthermore, the effect
of miR-135a on the podocyte cytoskeleton was investigated, and the
results indicated that the miR-135a-induced stress fibre decrease
in number and disarray was reversed by overexpression of TRPC1.
Additionally, in order to further investigate the significance of
TRPC1 in podocyte injury, miR-135a mimics and the TRPC1
overexpression vector were co-transfected into cultured MPC5 cells,
and the apoptosis ratio was measured with flow cytometry; the
results indicated that miR-135a induced severe podocyte apoptosis,
which was reversed by ectopic expression of TRPC1 (Fig. 5E). Therefore, miR-135a induced
podocyte injury in a TRPC1-dependent manner.
Discussion
The findings of the present study, consistently with
those of previous studies (12),
indicated that miR-135a is an important signature microRNA in
podocyte injury, and its expression was significantly upregulated
in glomeruli isolated from FSGS patients and podocyte injury mouse
models, as well as in cultured cells treated with ADR and TGF-β.
This finding was associated with the development of kidney
diseases, particularly glomerulopathies; thus, miR-135a may be a
potential diagnostic biomarker for glomerular and podocyte injury.
More importantly, our study indicated that ectopic expression of
miR-135a promotes podocyte injury, in terms of down-regulating
functional markers, upregulating injury marker, reducing the number
of stress fibres and promoting apoptosis, which suggested that
targeting miR-135a may be a viable approach to preventing the
progression of podocyte injury.
Moreover, TRPC1 was identified as a target of
miR-135a during podocyte injury, and overexpression of TRPC1 was
able to reverse the damaging effects of miR-135a on podocytes.
These results are significant, as the expression of TRPC1 is
inhibited in several types of kidney disease and animal models of
kidney disease, such as nodular FSGS (26), as well as in animal models of
diabetes (26–28). Furthermore, it was recently
demonstrated that TRPC1 genetic polymorphisms are involved in
diabetic nephropathy in type 2 diabetes in Chinese patients, and
that TRPC1 is a strong positional and biological candidate for
diabetic nephropathy (26,28).
Therefore, we predicted that miR-135a-induced downregulation of
TRPC1 may be a major mechanism involved in podocyte injury. Of
note, it was demonstrated that overexpression of TRPC1 protects
against podocyte injury from miR-135a. Thus, TRPC1 may ameliorate
progression of podocyte damage.
Wu et al previously suggested that miR-135a
significantly downregulated Luciferase activity in wild-type as
well as mutant TRPC1 3′UTR, which suggested that miR-135a may not
directly target TRPC1 3′UTR to inhibit TRPC1 expression (16). Thus, it is worth considering
whether there is any association between the location of TRPC1
genetic variants and the binding site of miR-135a. Our results
confirmed the ability of miR-135a to reduce TRPC1 expression by
directly binding to the TRPC1 3′UTR. A novel mutant of TRPC1 3′UTR
was generated, which included the putative miR-135a-binding site,
and demonstrated that miR-135a significantly inhibited the
Luciferase activity of TRPC1 3′UTR, without affecting the mutant
TRPC1 3′UTR. Thus, our data indicated that TRPC1 is a direct target
of miR-135a.
However, the precise mechanism underlying
miR-135a-induced podocyte injury may also be associated with other
putative target genes of miR-135a. A recent study demonstrated that
miR-135a may activate the Wnt signaling pathway through inhibiting
GSK-β (12). In addition, studies
on miR-135a suggested that it modulates multiple target genes,
including MTSS1 (29), HOXA10
(30), SMAD5 (31) and JAK2 (32). Our data indicated that TGF-β1
induced the miR-135a-mediated reduction in TRPC1 expression,
suggesting that miR-135a may function downstream of TGF-β1
signaling, promoting the progression of podocyte injury in kidney
diseases. Additionally, TRP channels are expressed along the
nephron and play an important role in kidney function (33). Using online miRNA target
prediction programmes, such as TargetScan, PicTar and miRanda, we
predicted that the TRP superfamily members TRPC6, TRPM4 and TRPM7
may be potential targets of miR-135a. TRPC6, a glomerular slit
diaphragm protein, is necessary for normal renal function (34). TRPM4 and TRPM7 are Mg2+
transport channels and are ubiquitously expressed in the kidney
(33). Taken together, these
findings indicate that miR-135a may regulate TRPs members
collectively to affect podocyte physiological processes, and
abnormal regulation of miR-135a in TRPs members may contribute to
the development of podocyte injury.
In conclusion, the present study suggested that
miR-135a is a significant factor in podocyte injury. miR-135a
expression was found to be upregulated in podocyte injury, while
TRPC1 expression was downregulated. Ectopic expression of TRPC1
prevents podocyte injury by miR-135a. These findings provide novel
insights into the role of miR-135a in glomerulopathies and support
the development of a novel therapeutic strategy.
Acknowledgments
The authors wish to acknowledge the support of the
Sichuan Province Department of Education Program, China (grant no.
16ZB0275, awarded to X.Y.). The present study was also supported by
the fund research projects of Chengdu Medical College in Sichuan
Province, China (grant no. CYTD15-03, awarded to Y.X.).
References
|
1
|
Nilius B and Szallasi A: Transient
receptor potential channels as drug targets: From the science of
basic research to the art of medicine. Pharmacol Rev. 66:676–814.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barisoni L: Podocyte biology in segmental
sclerosis and progressive glomerular injury. Adv Chronic Kidney
Dis. 19:76–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Itoh M, Nakadate K, Horibata Y, Matsusaka
T, Xu J, Hunziker W and Sugimoto H: The structural and functional
organization of the podocyte filtration slits is regulated by
Tjp1/ZO-1. PLoS One. 9:e1066212014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shankland SJ: The podocyte's response to
injury: Role in proteinuria and glomerulosclerosis. Kidney Int.
69:2131–2147. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grishok A, Pasquinelli AE, Conte D, Li N,
Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G and Mello CC: Genes
and mechanisms related to RNA interference regulate expression of
the small temporal RNAs that control C. elegans developmental
timing. Cell. 106:23–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X and He JC: An update: The role of
Nephrin inside and outside the kidney. Sci China Life Sci.
58:649–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
May CJ, Saleem M and Welsh GI: Podocyte
dedifferentiation: A specialized process for a specialized cell.
Front Endocrinol (Lausanne). 5:1482014.
|
|
8
|
Gebeshuber CA, Kornauth C, Dong L, Sierig
R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R, et al:
Focal segmental glomerulosclerosis is induced by microRNA-193a and
its downregulation of WT1. Nat Med. 19:481–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CL, Lee PH, Hsu YC, Lei CC, Ko JY,
Chuang PC, Huang YT, Wang SY, Wu SL, Chen YS, et al: MicroRNA-29a
promotion of nephrin acetylation ameliorates hyperglycemia-induced
podocyte dysfunction. J Am Soc Nephrol. 25:1698–1709. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu J, Zheng C, Fan Y, Zeng C, Chen Z, Qin
W, Zhang C, Zhang W, Wang X, Zhu X, et al: Downregulation of
microRNA-30 facilitates podocyte injury and is prevented by
glucocorticoids. J Am Soc Nephrol. 25:92–104. 2014. View Article : Google Scholar :
|
|
11
|
Shi S, Yu L, Zhang T, Qi H, Xavier S, Ju W
and Bottinger E: Smad2-dependent downregulation of miR-30 is
required for TGF-β-induced apoptosis in podocytes. PLoS One.
8:e755722013. View Article : Google Scholar
|
|
12
|
Yang X, Wang X, Nie F, Liu T, Yu X, Wang
H, Li Q, Peng R, Mao Z, Zhou Q, et al: miR-135 family members
mediate podocyte injury through the activation of Wnt/β-catenin
signaling. Int J Mol Med. 36:669–677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Chen X, Chen X, Wang X, Ji A,
Jiang L, Sang F and Li F: miR-135a acts as a tumor suppressor in
gastric cancer in part by targeting KIFC1. Onco Targets Ther.
9:3555–3563. 2016.PubMed/NCBI
|
|
14
|
Wan X, Pu H, Huang W, Yang S, Zhang Y,
Kong Z, Yang Z, Zhao P, Li A, Li T, et al: Androgen-induced
miR-135a acts as a tumor suppressor through downregulating RBAK and
MMP11, and mediates resistance to androgen deprivation therapy.
Oncotarget. 7:51284–51300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada Y, Hidaka H, Seki N, Yoshino H,
Yamasaki T, Itesako T, Nakagawa M and Enokida H: Tumor-suppressive
microRNA-135a inhibits cancer cell proliferation by targeting the
c-MYC oncogene in renal cell carcinoma. Cancer Sci. 104:304–312.
2013. View Article : Google Scholar
|
|
16
|
Wu S, Lin Y, Xu D, Chen J, Shu M, Zhou Y,
Zhu W, Su X, Zhou Y, Qiu P, et al: MiR-135a functions as a
selective killer of malignant glioma. Oncogene. 31:3866–3874. 2012.
View Article : Google Scholar
|
|
17
|
Nagel R, le Sage C, Diosdado B, van der
Waal M, Oude Vrielink JA, Bolijn A, Meijer GA and Agami R:
Regulation of the adenomatous polyposis coli gene by the miR-135
family in colorectal cancer. Cancer Res. 68:5795–5802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Holleman A, Chung I, Olsen RR, Kwak B,
Mizokami A, Saijo N, Parissenti A, Duan Z, Voest EE and Zetter BR:
miR-135a contributes to paclitaxel resistance in tumor cells both
in vitro and in vivo. Oncogene. 30:4386–4398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou W, Li X, Liu F, Xiao Z, He M, Shen S
and Liu S: MiR-135a promotes growth and invasion of colorectal
cancer via metastasis suppressor 1 in vitro. Acta Biochim Biophys
Sin (Shanghai). 44:838–846. 2012. View Article : Google Scholar
|
|
20
|
Ni L, Saleem M and Mathieson PW: Podocyte
culture: Tricks of the trade. Nephrology (Carlton). 17:525–531.
2012. View Article : Google Scholar
|
|
21
|
Fogo AB: Animal models of FSGS: Lessons
for pathogenesis and treatment. Semin Nephrol. 23:161–171. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Wang YP, Tay YC and Harris DC:
Progressive adriamycin nephropathy in mice: Sequence of histologic
and immunohistochemical events. Kidney Int. 58:1797–1804. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mundel P, Reiser J, Zúñiga Mejía Borja A,
Pavenstädt H, Davidson GR, Kriz W and Zeller R: Rearrangements of
the cytoskeleton and cell contacts induce process formation during
differentiation of conditionally immortalized mouse podocyte cell
lines. Exp Cell Res. 236:248–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kolfschoten IG, van Leeuwen B, Berns K,
Mullenders J, Beijersbergen RL, Bernards R, Voorhoeve PM and Agami
R: A genetic screen identifies PITX1 as a suppressor of RAS
activity and tumorigenicity. Cell. 121:849–858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bae GU, Lee JR, Kim BG, Han JW, Leem YE,
Lee HJ, Ho SM, Hahn MJ and Kang JS: Cdo interacts with APPL1 and
activates Akt in myoblast differentiation. Mol Biol Cell.
21:2399–2411. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niehof M and Borlak J: HNF4 alpha and the
Ca-channel TRPC1 are novel disease candidate genes in diabetic
nephropathy. Diabetes. 57:1069–1077. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen K, Jin X, Li Q, Wang W, Wang Y and
Zhang J: Association of TRPC1 gene polymorphisms with type 2
diabetes and diabetic nephropathy in Han Chinese population. Endocr
Res. 38:59–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang D, Freedman BI, Flekac M, Santos E,
Hicks PJ, Bowden DW, Efendic S, Brismar K and Gu HF: Evaluation of
genetic association and expression reduction of TRPC1 in the
development of diabetic nephropathy. Am J Nephrol. 29:244–251.
2009. View Article : Google Scholar :
|
|
29
|
Liu S, Guo W, Shi J, Li N, Yu X, Xue J, Fu
X, Chu K, Lu C, Zhao J, et al: MicroRNA-135a contributes to the
development of portal vein tumor thrombus by promoting metastasis
in hepato-cellular carcinoma. J Hepatol. 56:389–396. 2012.
View Article : Google Scholar
|
|
30
|
Chen Y, Zhang J, Wang H, Zhao J, Xu C, Du
Y, Luo X, Zheng F, Liu R, Zhang H, et al: miRNA-135a promotes
breast cancer cell migration and invasion by targeting HOXA10. BMC
Cancer. 12:1112012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Q, Wang Y, Minto AW, Wang J, Shi Q,
Li X and Quigg RJ: MicroRNA-377 is up-regulated and can lead to
increased fibronectin production in diabetic nephropathy. FASEB J.
22:4126–4135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Navarro A, Diaz T, Martinez A, Gaya A,
Pons A, Gel B, Codony C, Ferrer G, Martinez C, Montserrat E, et al:
Regulation of JAK2 by miR-135a: Prognostic impact in classic
Hodgkin lymphoma. Blood. 114:2945–2951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Woudenberg-Vrenken TE, Bindels RJ and
Hoenderop JG: The role of transient receptor potential channels in
kidney disease. Nat Rev Nephrol. 5:441–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reiser J, Polu KR, Möller CC, Kenlan P,
Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C,
et al: TRPC6 is a glomerular slit diaphragm-associated channel
required for normal renal function. Nat Genet. 37:739–744. 2005.
View Article : Google Scholar : PubMed/NCBI
|