Introduction
Brain injuries range in scope from mild to severe.
Severe brain injuries result in permanent neurobiological damage
that can produce lifelong deficits to varying degrees (1,2).
There are many different types of treatments available for patients
with severe brain injury. Surgical treatment may be used to prevent
secondary injury by helping to maintain blood flow and oxygen to
the brain, and minimizing swelling and pressure (3–5).
Intracranial pressure can be decreased by lowering or maintaining
body temperature, elevating the head of the bed, keeping the person
still and comfortable, ensuring proper breathing and applying
hypertensive therapies (1,2,4,6).
Therapeutic or protective hypothermia is an active
treatment aimed at achieving and maintaining a specific body
temperature for a specific duration of time to improve health
outcomes during recovery following a period of suppressed blood
flow to the brain (7,8). Targeted temperature management is
thought to prevent brain injury through several mechanisms,
including decreasing the oxygen demand of the brain, reducing the
production of neurotransmitters, such as glutamate and reducing
free radicals that may damage the brain (7–10).
Recent studies have indicated that hypothermia increases the
survival of transplanted cells in a hypoxic environment (11,12); however, the detailed mechanisms
are poorly understood.
Small ubiquitin-like modifier (SUMO) proteins are a
family of small proteins that are covalently attached to and
detached from other proteins in cells to modify their function
(13). SUMOylation is a
post-translational modification involved in various cellular
processes, such as nuclear-cytosolic transport, transcriptional
regulation, apoptosis, maintaining protein stability, responding to
stress and cell cycle progression (14–19). Recent studies have demonstrated
that SUMO1/2/3 conjugation is markedly activated in the brain
during deep to moderate hypothermia, and that hypothermia induces
the nuclear trans-location of the SUMO-conjugating enzyme, Ubc9
(20–22). These functions may lead to the
development of novel strategies for preventive and therapeutic
interventions with an aim of making neurons more resistant to an
adverse environment.
Bone marrow-derived mesenchymal stem cells (BMSCs)
are multipotent stem cells capable of differentiating into numerous
cell types, including fibroblasts, as well as cartilage, bone,
muscle and brain cells (23–26). They secrete a large number of
growth factors and cytokines that are critical for the repair of
injured tissues (23,24,27). In this study, we determined
whether SUMOs and the SUMO cycle are involved in the effects of
hypothermia on BMSCs and their role in an adverse environment.
This study found that BMSCs subjected to 24 h of low
temperature conditions exhibited a low proliferative activity,
almost no differentiation and a higher tolerance to a hypoxic
environment. Similar to neurons exposed to low temperatures, the
level of SUMOylation was markedly increased upon exposure of the
BMSCs to hypothermic conditions. The knockdown of SUMO1/2/3 or the
inhibition of the SUMO-conjugating enzyme, Ubc9, markedly increased
the proportion of BMSCs differentiating into nerve cells in a
hypoxic environment. Moreover, the tolerance of the cells to the
adverse environment was significantly decreased. This study implies
that SUMOylation induced by hypothermia is essential to maintaining
the stemness and inhibiting the differentiation of BMSCs, and
enhances resistance against adverse conditions. This study may
provide the basis for a novel treatment option based on the SUMO
pathway, such as drugs that induce endogenous SUMO expression or
increase the supply of exogenous SUMO products.
Materials and methods
Animals and cell culture
To obtain BMSCs, 2 newborn male Sprague-Dawley rats
were purchased from the Animal Center of the Cancer Institute of
the Chinese Academy of Medical Sciences (Beijing, China). All
experimental procedures were carried out according to the
regulations and internal biosafety and bioethics guidelines of
Tianjin Medical University and the Tianjin Municipal Science and
Technology Commission. BMSCs were collected from the long bones of
hind legs as previously described (28). Briefly, the long bones were
dissected out, and bone marrow plugs were extracted from the bones
by flushing the bone marrow cavity with complete culture medium.
The cells were plated at 5×107 cells per
75-cm2 culture flask and incubated at 37°C in a
humidified atmosphere with 5% CO2. Non-adherent cells
were removed after 24 h, and the culture medium was replaced every
3 days. Adherent cells reached 90–95% confluence within 10–15 days
and were passaged with 0.25% trypsin (Invitrogen Life Technologies,
Carlsbad, CA, USA) at a ratio of 1:3. Cells at passage 4 were
characterized by flow cytometric analysis to detect the expression
of the surface antigens, CD34, CD45, CD71 and CD105 (Abcam Trading
Co. Ltd., Shanghai, China).
Exposure to hypothermia and hypoxia
To simulate hypothermic stress, the culture dishes
loaded with BMSCs were placed in incubators for 24 h at 37°C
(normal), 33°C (mild hypothermia), or 18°C (deep hypothermia) with
5% CO2. To evaluate the effects of hypothermia on the
tolerance of BMSCs to the adverse environment, culture medium
without glucose, L-aspartic acid, L-glutamic acid or sodium
pyruvate was equilibrated overnight in an anoxic chamber with 85%
N2, 10% H2 and 5% CO2. The
cultures were transferred to the anoxic chamber and washed 3 times
with anoxic medium. After 24 h of oxygen-glucose deprivation (OGD),
the cells were transferred back to the incubator at 37°C with 5%
CO2 for an additional 24 h.
Cell viability assay
Cell viability was determined by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich, St. Louis, MO, USA) according to the
manufacturer's instructions. Briefly, the BMSCs were seeded in
96-well plates at a density of 1×104 cells/well and
incubated as described above. Subsequently, 15 µl of MTT
solution (5 mg/ml in PBS) were added to each well. After 3 h of
incubation at 37°C, the culture medium was aspirated and 100
µl of dimethyl sulfoxide were added. The absorbance was
monitored using a spectrophotometer with a microplate reader at a
wavelength of 595 nm (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cell cycle analysis
The cells were collected by trypsinization, washed
in phosphate-buffered saline (PBS), and fixed in 70% ethanol for 30
min at 4°C. The cells were then treated with the DNA-binding dye
propidium iodide (50 µg/ml) and RNase (1 mg/ml) for 30 min
at 37°C in the dark. Finally, the cells were washed, and red
fluorescence was analyzed by a FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA) using a peak fluorescence gate to
discriminate aggregates.
Detection of lactate dehydrogenase (LDH)
activity
After harvesting the cells, the LD H content in the
conditioned medium was measured by an enzyme-linked immunosorbent
assay (ELI SA) using an LD H Activity Assay kit (cat. no.
K726-500; BioVision, Inc., Milpitas, CA, USA) in accordance
with the manufacturer's instructions.
Apoptosis assay
The BMSCs were seeded in 12-well plates and
incubated as described above. The cells were mounted in PBS,
immersed in permeabilization solution for 5 min, incubated with 25
µl TUNEL-labeling reaction mixture in a humidified box at
37°C for 60 min and then washed with PBS. Nuclei were
counterstained with Hoechst 33258 at a dilution of 1:1,000. Cell
apoptosis rate (number of apoptotic cells/total number of cells
×100%) was quantified.
Detection of cell differentiation
To induce BMSCs to differentiate into neural cells,
the BMSCs were cultured in 3% oxygen. Cell differentiation was then
detected by the immunocytochemical analysis of markers for
undifferentiated BMSCs (nestin) and differentiated BMSCs [glial
fibrillary acidic protein (GFAP) for astrocytes and β-tubulin III
for neurons]. Immunocytochemistry was performed as previously
described (29). Cells in 96-well
plates were fixed with 2% para-formaldehyde for 15 min at room
temperature, treated with 5% normal goat serum (Vector
Laboratories, Inc., Burlingame, CA, USA), and then stained with the
following antibodies: anti-nestin (mouse monoclonal IgG1, 1:200,
cat. no. ab11306), anti-β-tubulin III (mouse monoclonal IgG1,
1:500, cat. no. ab78078) and anti-GFAP (rabbit polyclonal, 1:200,
cat. no. ab16997) (all from Abcam, Cambridge, MA, USA). The primary
antibodies were detected with goat anti-mouse IgG-CruzFluor™ 488
(1:400; cat. no. sc-362257) or goat anti-mouse IgG-CruzFluor™ 594
(1:200; cat. no. sc-362277) or goat anti-rabbit IgG-CruzFluor™ 594
(1:200; cat. no. sc-362282) (all from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) secondary antibodies at 37°C for 1 h,
followed by mounting and observation under a fluorescence
microscope (Olympus DP70; Olympus Corp., Tokyo, Japan). The cells
were counterstained with 4,6-diamidino-2-phenylindole (Vector
Laboratories, Inc.). Images of 10 random fields were captured per
well. Image-Pro Plus version 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA) was used for image analysis.
Blockade of the SUMO pathway
Dominant negative mutant SUMO1/2/3 (siR-neg)
plasmids or the same DNA fragment carrying siR-SUMO1/2/3 were
subcloned into pCMV-Myc and then cloned into a lentiviral vector,
pCDH-CMV-MCS-EF1-copGFP (System Biosciences, Mountain View, CA,
USA). Transfection was performed using RNAifectin reagent (Applied
Biological Materials) following the manufacturer's instructions. In
brief, 1×105 BMSCs were seeded in 6-well plates and
incubated overnight. Approximately 100 pmol siRNAs per well was
used for transfection. In another group of BMSCs, 20 µM
spectomycin B1 (Nanjing Chemlin Chemical Co., Ltd., Nanjing, China)
was added to the culture medium to block the effects of Ubc9 for 24
h. The morphology of cells was observed under a high magnification
microscope. When cell morphology showed increased cell volume,
flattened, increased nucleus and nucleolar volume, it was regarded
as cellular aging.
Western blot analysis
Total protein was extracted by a cell lysis
solution. Western blot analysis was performed using 4–15% SDS-PAGE
gels (Bio-Rad Laboratories, Inc.). Following electrophoresis, the
proteins were transferred onto polyvinylidene fluoride membranes
(Bio-Rad Laboratories, Inc.). The membranes were blocked using
Tris/HCl-buffered salt solution containing 0.1% Tween-20 and 5%
skim milk powder, and then incubated with antibodies to anti-SUMO1
(1:2,000; cat. no. ab133352), anti-SUMO2/3 (1:5,000; cat. no.
ab3742) and anti-GFAP (1:2,000; cat. no. ab7260),
anti-proliferating cell nuclear antigen (PCNA; 1:1,000; cat. no.
ab18197), anti-octamer-binding transcription factor 4 (Oct4;
1:1,000; cat. no. ab18976) (all from Abcam), anti-p53 (1:2,000;
cat. no. sc-47698) or anti-hypo xia-inducible factor-1α (HIF-1α;
1:2,500; cat. no. sc-71247) (both from Santa Cruz Biotechnology,
Inc.) overnight at 4°C. The membranes were then washed 5 times in
0.1% TBST and incubated for 1 h with the secondary antibodies
chicken anti-rabbit IgG conjugated to horseradish peroxidase (HRP)
(1:2,000; cat. no. sc-516087) or chicken anti-goat IgG-HRP
(1:2,000; cat. no. sc-516086) (both from Santa Cruz Biotechnology,
Inc.). Labeled proteins were detected using a Super Signal protein
detection kit (Pierce, Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The membranes were then stripped and reprobed with a goat
anti-β-actin polyclonal primary antibody (1:1,000; cat. no.
sc-70319; Santa Cruz Biotechnology, Inc.). Changes in the levels of
SUMO-conjugated proteins were evaluated using ImageJ image analysis
software (NIH, Bethesda, MD, USA). The high molecular weight area
in each lane was cropped and analyzed.
Detection of nuclear translocation
To observe whether hypothermia can induce the
translocation of SUMO1 and SUMO2/3 complexes from the cytoplasm to
the nucleus in BMSCs, immunocytochemistry assay was performed.
Briefly, the BMSCs were seeded in 6-well plates and incubated at
37°C, 33°C, or 18°C with 5% CO2. After 24 h, the cells
were fixed with 2% paraformaldehyde for 15 min at room temperature,
treated with 5% normal goat serum (Vector Laboratories, Inc.), and
then stained with anti-SUMO1 (1:200, cat. no. ab133352) and
anti-SUMO2/3 (1:400, cat. no. ab81371) (both from Abcam). The SUMO1
antibodies were then detected with goat anti-rabbit IgG-CruzFluor™
594 (1:200; cat. no. sc-362282) and SUMO2/3 antibodies were
detected with goat anti-mouse IgG-CruzFluor™ 488 (1:400; cat. no.
sc-362257) (all from Santa Cruz Biotechnology Inc.) at 37°C for 1
h, followed by mounting and observation under a fluorescence
microscope (Olympus DP70; Olympus Corp.).
Statistical analysis
All experiments were repeated at least 3 times. The
results were analyzed using the Student's t-test or two-way
analysis of variance (ANOVA). Data are expressed as the means ±
standard error of the mean. All tests were two-tailed, and the
level of statistical significance was set at p≤0.05. GraphPad Prism
6 software (GraphPad Software, Inc., La Jolla, CA, USA) was used
for all statistical tests.
Results
Hypothermia inhibits the proliferation
and differentiation of BMSCs, and increases their tolerance to
hypoxia
To verify that the cells used in the following
experiments were BMSCs, 4 proteins to identify BMSCs were detected
by flow cytometry. The cells exhibited a high expression of the
BMSC-specific proteins, CD71 and CD105, but did not express the
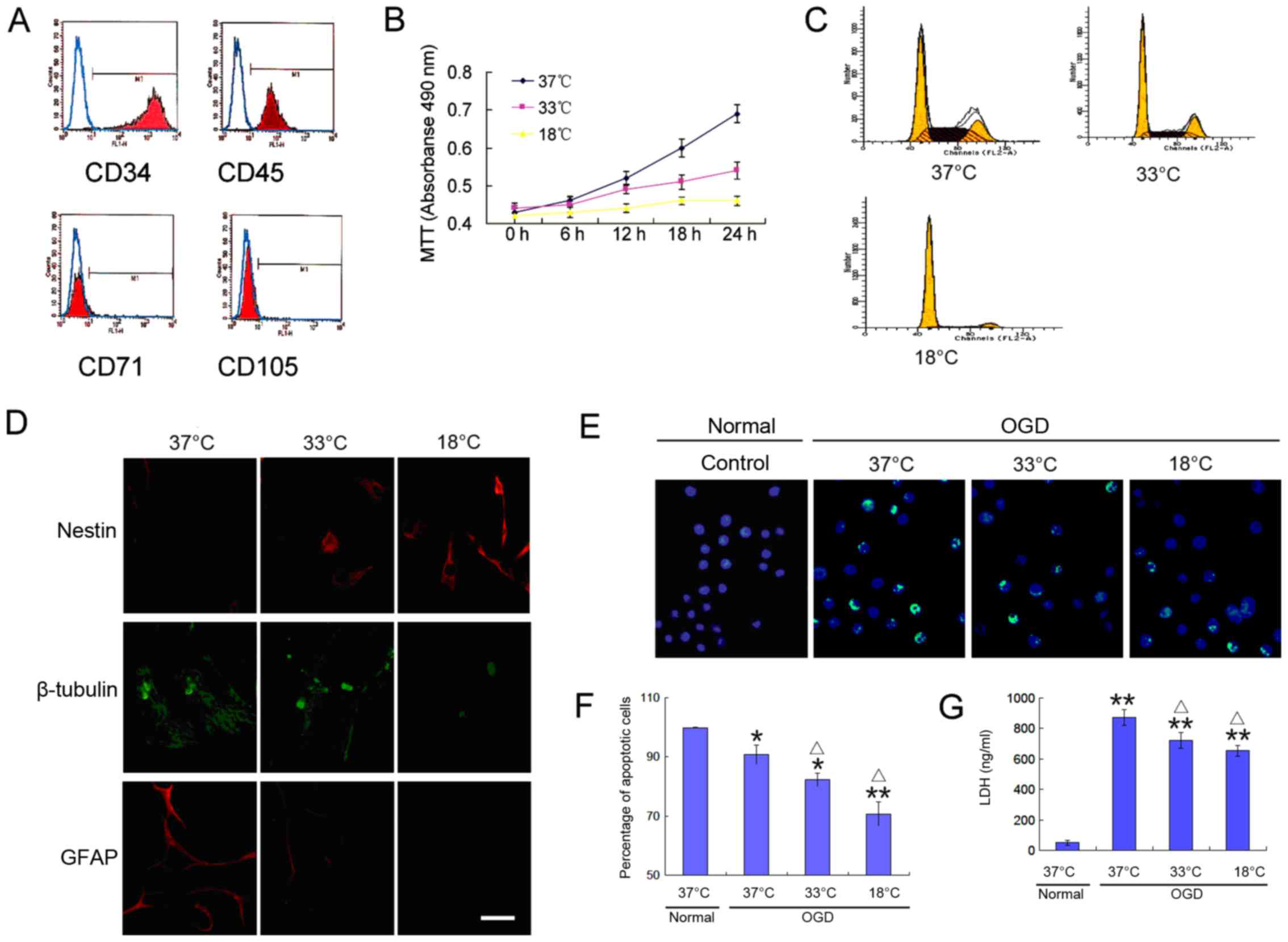
hematopoietic stem cell-specific proteins, CD34 and CD45 (Fig. 1A). To observe the growth
characteristics of BMSCs at a low temperature, we examined their
proliferative activity, their ability to differentiate into neural
cells and their tolerance to hypoxic environments. The results
revealed the slow growth of BMSCs at a low temperature (Fig. 1B and C), and a decreased ability
to differentiate into neural cells (Fig. 1D). These trends increased with the
decreasing temperature. When the BMSCs were cultured under
oxygen-deprived conditions for 24 h, a large number of apoptotic
cells was detected (Fig. 1E and
F), which was accompanied by high levels of lactate
dehydrogenase (LDH) release (Fig.
1G). Of note, the cell apoptotic rate and LDH release were
significantly reduced at a low temperature (Fig. 1E–G). These results indicate that a
low temperature environment reduces the proliferative activity of
BMSCs, decreases their differentiation potential, and increases
their tolerance to an adverse environment.
Hypothermia induces SUMOylation and SUMO
nuclear transfer in BMSCs
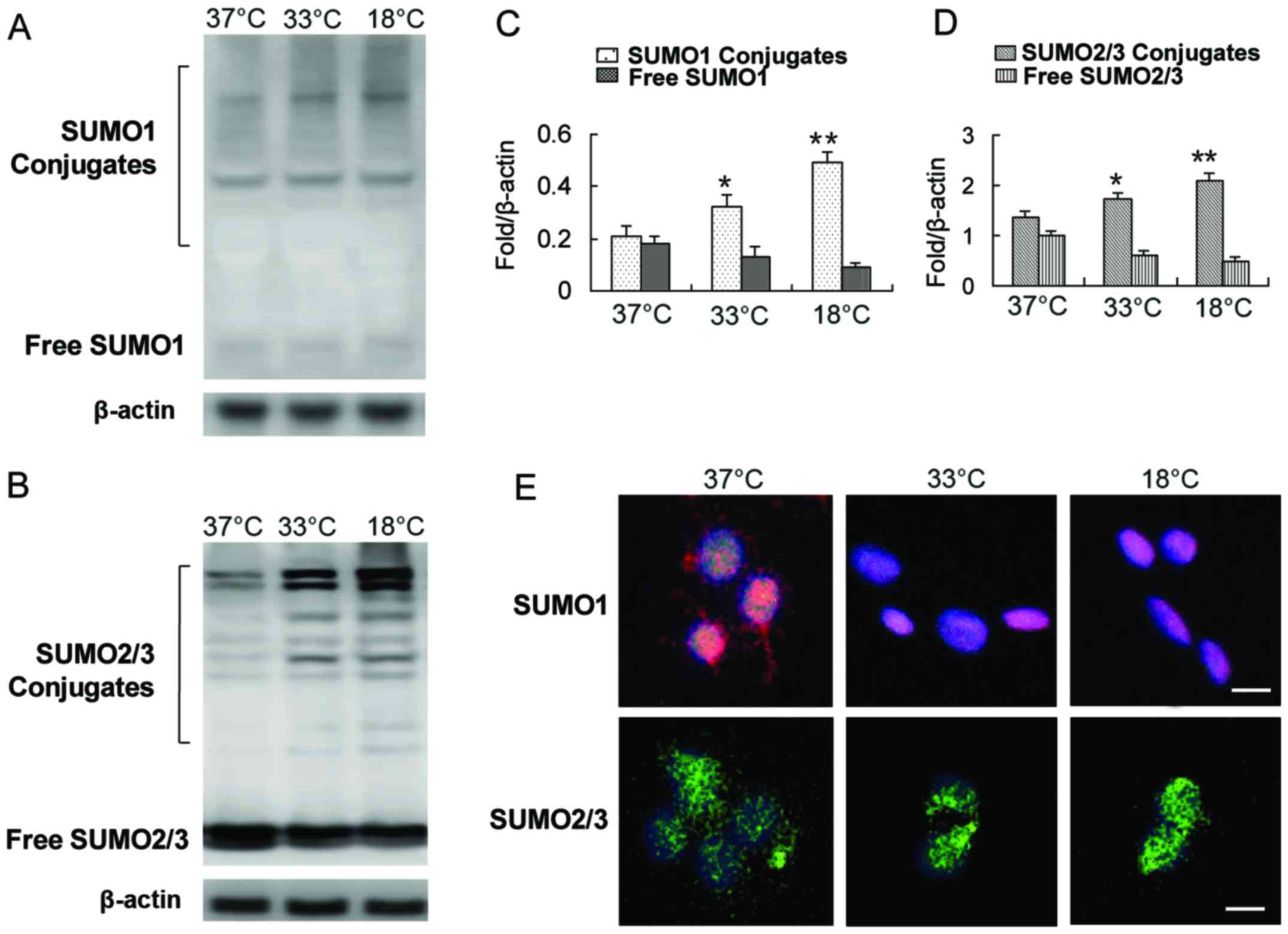
Some studies have shown that SUMO1/2/3 conjugation
is markedly activated in neurons at low temperatures (20,21). Whether BMSCs have a similar
response to low temperature stress as neurons is unknown. Our
results revealed that exposure of the cells to a low temperature
induced the modification of numerous target proteins by SUMOs,
including SUMO1 and SUMO2/3 (Fig.
2A–D). The levels of SUMO1 and SUMO2/3 in the covalent binding
state were increased significantly, while the levels of free SUMO1
and SUMO2/3 were decreased (Fig.
2A–D). One of the concomitant phenomena of protein SUMOylation
caused by exogenous stress is the translocation of the SUMO complex
from the cytoplasm to the nucleus (14,17,20–22). We also observed a shift of SUMO1
and the SUMO2/3 complex from the cytoplasm to the nucleus with a
decrease in incubation temperature (Fig. 2E).
SUMOs are essential for BMSC
proliferation and stemness maintenance
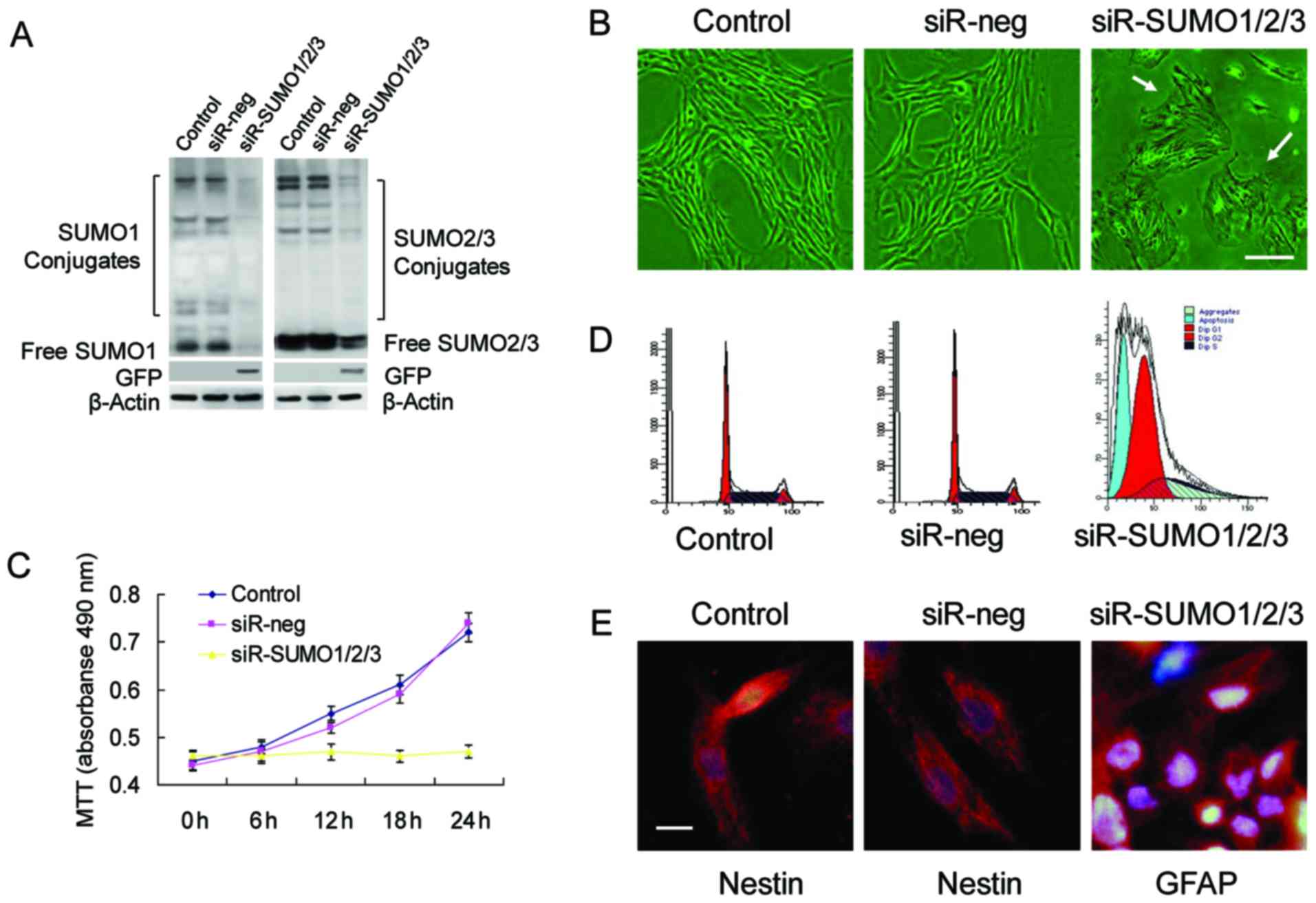
To determine whether SUMOs are required for the
proliferation and stemness maintenance of BMSCs, we used RNA
interference to successfully knockdown the expression of SUMO1/2/3
in BMSCs (Fig. 3A). Upon the
knockdown of SUMO1/2/3, the BMSCs almost stopped proliferating and
most of the cells exhibited marked cell aging, evidenced by
enlarged and flattened cell bodies, and an increased nucleus and
nucleolar volume (Fig. 3B–D).
Analysis of cell cycle changes by flow cytometry confirmed that a
large number of cells was arrested at the G0/G1 phase, which was
accompanied by a certain percentage of apoptotic cells (Fig. 3D). Cell differentiation
experiments revealed that the BMSCs in which the SUMO1/2/3 gene was
knocked down no longer maintained their stemness and differentiated
toward neural cells (Fig.
3E).
Inhibition of Ubc9 blocks SUMO protein
modification and reduces the tolerance of BMSCs to adverse
environmental conditions
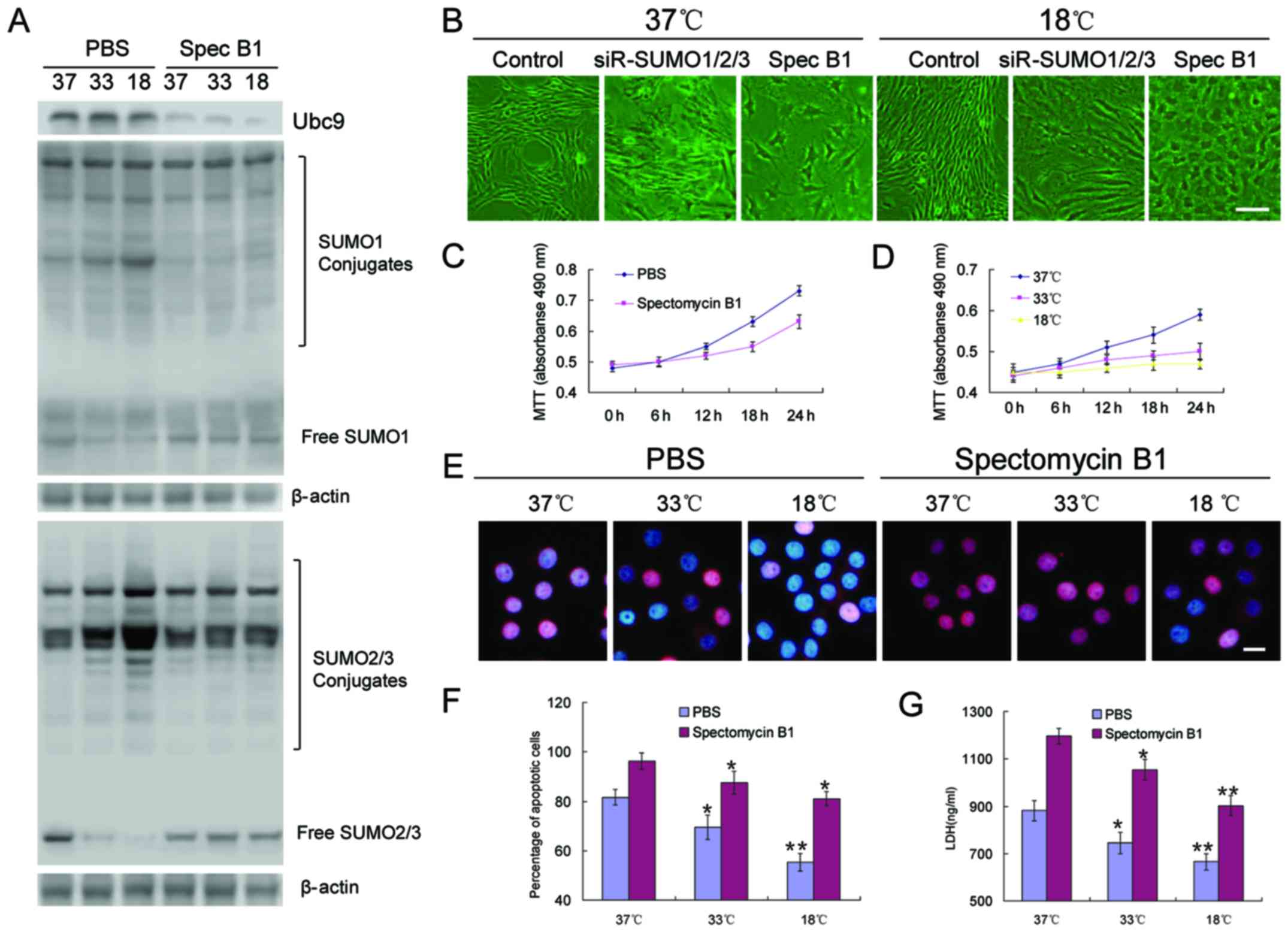
To clarify the role of SUMOylation in BMSC
proliferation, differentiation and tolerance to adverse conditions,
spectomycin B1, a reported Ubc9 inhibitor, was employed to prevent
the binding of SUMOs to SUMO substrates. The results revealed that,
unlike the effects of SUMO1/2/3 knockdown in BMSCs, the inhibition
of Ubc9 did not cause the rapid aging of the cells, but slowed the
proliferation rate, and low temperatures appeared to further
inhibit these processes (Fig.
4B–D). OGD induced the significant apoptosis of BMSCs. However,
the inhibition of Ubc9 further induced apoptosis, resulting in a
higher percentage of apoptotic cells (Fig. 4E and F), and higher levels of LDH
(Fig. 4G).
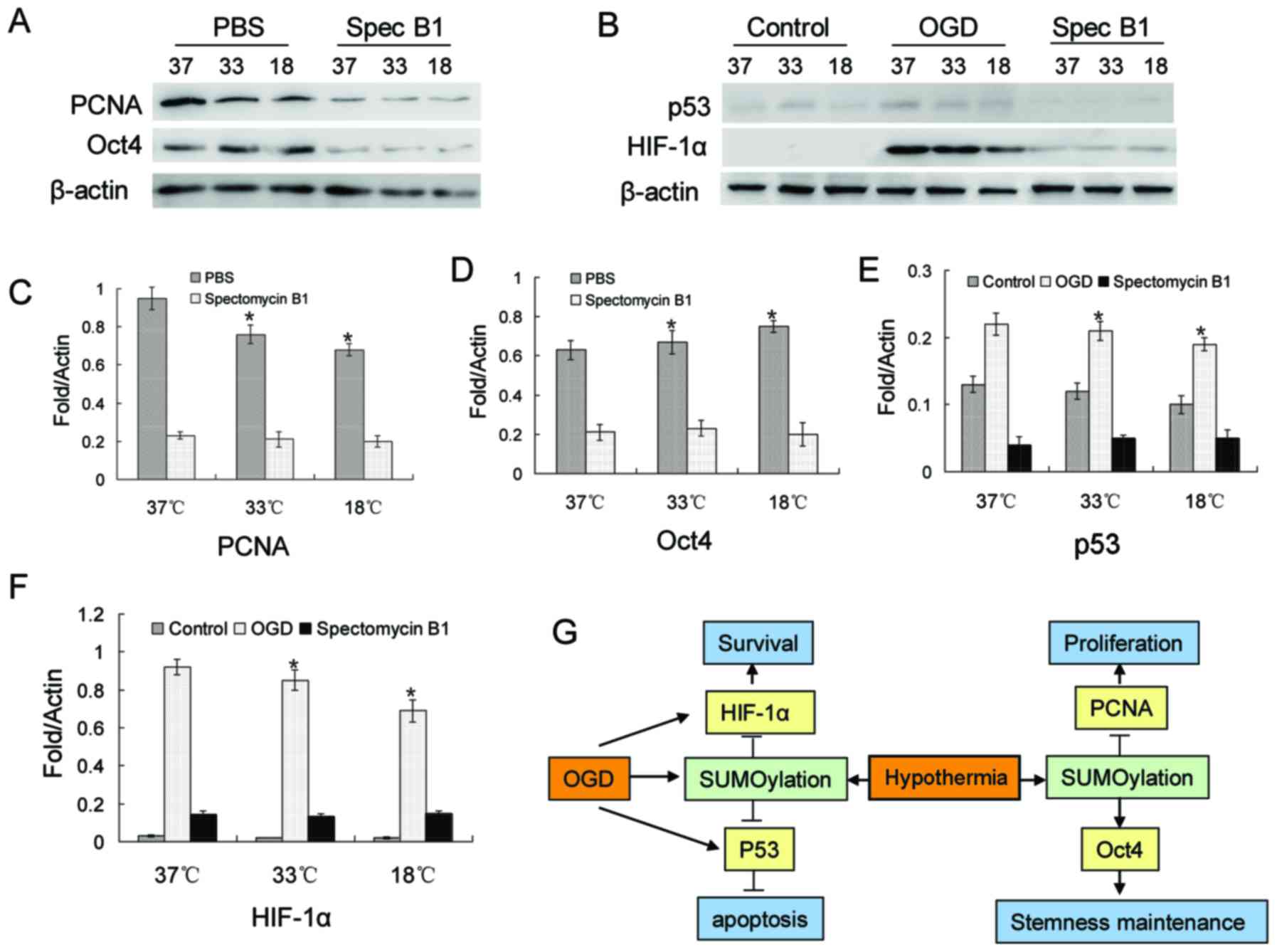
PCNA, Oct4, p53 and HIF-1α SUMOylation is
regulated by low temperatures, and participates in BMSC
proliferation, stemness maintenance and resistance to
apoptosis
Based on previous studies (30–33), 4 proteins were investigated in
this study. The relults of western blot revealed a decrease in the
protein level of PCNA, while Oct4 expression increased with the
decrease in temperature (Fig. 5A, C
and D). However, when Ubc9 was suppressed, the protein levels
of both PCNA and Oct4 were decreased significantly (Fig. 5A, C and D). In BMSCs, there was a
small amount of p53 and almost no HIF-1α expression under normal
culture conditions (Fig. 5B, E and
F). However, the protein expression levels of p53 and HIF-1α
were significantly increased in the OGD environment (Fig. 5B, E and F). This increasing trend
was blocked by spectomycin B1 through the blocking of the binding
of SUMOs to their target proteins (Fig. 5B, E and F).
Discussion
Therapeutic hypothermia is a potential treatment for
traumatic brain injury (8,10).
Previous studies have suggest that, by slowing destructive cellular
processes, and reducing edema and intracranial pressure,
hypothermia may improve neurological outcomes in patients with
traumatic brain injury or ischemic stroke (34,35). Furthermore, in patients with
severe brain injury, treatment with mild hypothermia increases the
survival rate of transplanted stem cells in the injured brain area
(11,12). However, the exact mechanisms
involved remain unclear. In this study, we first investigated the
effects of low temperature on the increase of BMSC tolerance to
hypoxia in terms of SUMOylation, as it has been indicated that
therapeutic hypothermia increases the SUMOylation of numerous
proteins, which enhances the resistance of neurons to an adverse
environment (36). By translation
into clinical practice, such a strategy will further broaden the
clinical treatment options and increase the confidence of doctors
and patients in terms of treatment of brain injury.
First, we found that hypothermia increased the
survival of BMSCs in a hypoxic environment. Furthermore,
hypothermia was accompanied by very low cell proliferation and a
decreased ability to differentiate into neural cells. To
investigate whether the proliferative characteristics of BMSCs in a
low temperature environment are dependent on SUMO protein
modifications, an RNA interference plasmid, which silenced the
expression of SUMO1/2/3 proteins, was transfected into the BMSCs.
Our results revealed that BMSCs lacking SUMO1/2/3 expression did
not proliferate and underwent rapid cell aging. This result
indicates that SUMO1/2/3 are essential for the proliferation and
survival of BMSCs. Furthermore, a reported Ubc9 inhibitor,
spectomycin B1 (37), was
employed to inhibit the binding of SUMO1/2/3 to SUMO substrates.
Unlike the effects of SUMO1/2/3 knockdown in BMSCs, the inhibition
of Ubc9 did not cause the rapid aging of the cells, but decreased
the cell proliferation rate. This indicates that spectomycin B1 is
more suitable for follow-up studies. Unlike known SUMOylation
inhibitors, such as ginkgolic acid, spectomycin B1 binds directly
to E2 (Ubc9) and selectively blocks the formation of the E2-SUMO
intermediate (37). Our results
revealed that spectomycin B1 effectively inhibited Ubc9. Moreover,
the inhibition of Ubc9 effectively offset the increase in
SUMOylation induced by treatment with hypothermia in the BMSCs.
Upon he loss of the protective effect of SUMOylation on proteins,
BMSC stemness maintenance was poor and they had a low tolerance
against the same adverse environment. These results indicate that
protein SUMOylation is essential for BMSC proliferation, stemness
maintenance and enhanced tolerance to adverse conditions.
To delineate the underlying molecular mechanisms, we
investigated 4 reported SUMO target proteins, PCNA, Oct4, p53 and
HIF-1α (30–33). PCNA, the DNA clamp for replicative
polymerases, plays a central role in tje regulation of the cell
cycle and their modification by ubiquitin (Ub) and SUMOs (38). In eukaryotic cells, PCNA is
modified by Ub or Poly-Ub at lysine 164 (Lys164), and
post-translational modifications of PCNA control the processing of
replication intermediates (39).
We found that hypothermia reduced the expression level of PCNA, and
the inhibition of Ubc9 further decreased PCNA expression. This
result is consistent with the very slow proliferation rate of
BMSCs. The transcription factor, Oct4, is a master regulator that
affects the fate of pluripotent stem cells and germ cell precursors
(40). Oct4 expression is tightly
regulated, and small changes in its expression level can have
dramatic effects on differentiation and stemness maintenance
(41). Oct4 is a target for
SUMO-1 modification, and SUMOylation of Oct4 occurs at a single
lysine, Lys118, located at the end of the amino-terminal
transactivation domain and next to the Pit1-Oct-Unc86 (POU)
DNA-binding domain (40). The
SUMOylation of Oct4 significantly increases Oct4 stability and
increases DNA binding (40). Our
results revealed that, with the decrease in temperature, the
expression level of Oct4 showed a gradually increasing trend.
However, the inhibition of Ubc9 reduced the Oct4 expression level.
These results suggest that Oct4 may be involved in the stemness
maintenance of BMSCs. p53 is an anti-proliferative transcription
factor that increases the transcription rate of various genes
involved in mitosis and apoptosis, which undergoes modification by
SUMOylation (42). In this study,
we found that a hypoxic environment induced the expression of p53
in BMSCs, but there was little effect on the BMSCs upon the
inhibiton of Ubc9. Finally, we found that OGD conditions
significantly induced the expression of HIF-1α, another protein
that undergoes SUMOylation (43),
and spectomycin B1 was able to offset this change. The
above-mentioned results suggest a similar phenomenon in which SUMOs
respond to various stresses by controlling multiple target proteins
simultaneously to promote the proliferation and survival of BMSCs
in adverse environments (Fig.
5G).
In conclusion, our results indicate the involvement
of SUMOs and the SUMO pathway in the tolerance of BMSCs against
adverse environments, and suggest a novel protective mechanism
against hypothermia for BMSC survival. The results may lead to the
future development of effective methods to improve the success rate
of BMSC transplantation in adverse environments, such as brain
injury and cerebral ischemia, to ultimately improve patient
rehabilitation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81471175), and the Tianjin Health
Bureau Science and Technology Projects (no. 2014KY23).
References
|
1
|
Russo MV and McGavern DB: Inflammatory
neuroprotection following traumatic brain injury. Science.
353:783–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masoudi MS, Rezaee E, Hakiminejad H,
Tavakoli M and Sadeghpoor T: Cisternostomy for management of
intracranial hypertension in severe traumatic brain injury; case
report and literature review. Bull Emerg Trauma. 4:161–164.
2016.PubMed/NCBI
|
|
3
|
Limpastan K, Norasetthada T,
Watcharasaksilp W and Vaniyapong T: Factors influencing the outcome
of decompressive craniectomy used in the treatment of severe
traumatic brain injury. J Med Assoc Thai. 96:678–682.
2013.PubMed/NCBI
|
|
4
|
Shi J, Han P and Kuniyoshi SM: Cognitive
impairment in neurological diseases: lessons from apolipoprotein E.
J Alzheimers Dis. 38:1–9. 2014.
|
|
5
|
Benarroch EE: Microglia: multiple roles in
surveillance, circuit shaping, and response to injury. Neurology.
81:1079–1088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang EH, Adorjan I, Mundim MV, Sun B,
Dizon ML and Szele FG: Traumatic brain injury activation of the
adult subventricular zone neurogenic niche. Front Neurosci.
10:3322016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pietrini D, Piastra M, Luca E, Mancino A,
Conti G, Cavaliere F and De Luca D: Neuroprotection and hypothermia
in infants and children. Curr Drug Targets. 13:925–935. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed AI, Bullock MR and Dietrich WD:
Hypothermia in traumatic brain injury. Neurosurg Clin N Am.
27:489–497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lazaridis C and Robertson CS: Hypothermia
for increased intra-cranial pressure: is it dead? Curr Neurol
Neurosci Rep. 16:782016. View Article : Google Scholar
|
|
10
|
Leshnower BG, Myung RJ, Thourani VH,
Halkos ME, Kilgo PD, Puskas JD and Chen EP: Hemiarch replacement at
28°C: an analysis of mild and moderate hypothermia in 500 patients.
Ann Thorac Surg. 93:1910–1916. 2012. View Article : Google Scholar
|
|
11
|
McAdams RM and Juul SE: Neonatal
encephalopathy: update on therapeutic hypothermia and other novel
therapeutics. Clin Perinatol. 43:485–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dailey T, Mosley Y, Pabon M, Acosta S,
Tajiri N, van Loveren H, Kaneko Y and Borlongan CV: Advancing
critical care medicine with stem cell therapy and hypothermia for
cerebral palsy. Neuroreport. 24:1067–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wimmer P, Schreiner S and Dobner T: Human
pathogens and the host cell SUMOylation system. J Virol.
86:642–654. 2012. View Article : Google Scholar :
|
|
14
|
Kumar A and Zhang KY: Advances in the
development of SUMO specific protease (SENP) inhibitors. Comput
Struct Biotechnol J. 13:204–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilson VG: Sumoylation at the
host-pathogen interface. Biomolecules. 2:203–227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Swatek KN and Komander D: Ubiquitin
modifications. Cell Res. 26:399–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ulrich HD: Ubiquitin and SUMO in DNA
repair at a glance. J Cell Sci. 125:249–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Craig TJ and Henley JM: Protein
SUMOylation in spine structure and function. Curr Opin Neurobiol.
22:480–487. 2012. View Article : Google Scholar :
|
|
19
|
Chang E and Abe J: Kinase-SUMO networks in
diabetes-mediated cardiovascular disease. Metabolism. 65:623–633.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang W, Ma Q, Mackensen GB and Paschen W:
Deep hypothermia markedly activates the small ubiquitin-like
modifier conjugation pathway; implications for the fate of cells
exposed to transient deep hypothermic cardiopulmonary bypass. J
Cereb Blood Flow Metab. 29:886–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang W and Paschen W: SUMO proteomics to
decipher the SUMO-modified proteome regulated by various diseases.
Proteomics. 15:1181–1191. 2015. View Article : Google Scholar :
|
|
22
|
Wang L, Ma Q, Yang W, Mackensen GB and
Paschen W: Moderate hypothermia induces marked increase in levels
and nuclear accumulation of SUMO2/3-conjugated proteins in neurons.
J Neurochem. 123:349–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamasaki S, Mera H, Itokazu M, Hashimoto Y
and Wakitani S: Cartilage repair with autologous bone marrow
mesenchymal stem cell transplantation: review of preclinical and
clinical studies. Cartilage. 5:196–202. 2014. View Article : Google Scholar
|
|
24
|
Mezey É and Nemeth K: Mesenchymal stem
cells and infectious diseases: smarter than drugs. Immunol Lett.
168:208–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross CL, Siriwardane M, Almeida-Porada G,
Porada CD, Brink P, Christ GJ and Harrison BS: The effect of
low-frequency electromagnetic field on human bone marrow
stem/progenitor cell differentiation. Stem Cell Res (Amst).
15:96–108. 2015. View Article : Google Scholar
|
|
26
|
Lynch K and Pei M: Age associated
communication between cells and matrix: a potential impact on stem
cell-based tissue regeneration strategies. Organogenesis.
10:289–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Błogowski W, Bodnarczuk T and Starzyńska
T: Concise review: pancreatic cancer and bone marrow-derived stem
cells. Stem Cells Transl Med. 5:938–945. 2016. View Article : Google Scholar :
|
|
28
|
Yang Z, Zhu L, Li F, Wang J, Wan H and Pan
Y: Bone marrow stromal cells as a therapeutic treatment for
ischemic stroke. Neurosci Bull. 30:524–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Z, Jiang Z, Huang J, Huang S, Li Y, Yu
S, Yu S and Liu X: miR-7 inhibits glioblastoma growth by
simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK
pathways. Int J Oncol. 44:1571–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Parker JL and Ulrich HD: A
SUMO-interacting motif activates budding yeast ubiquitin ligase
Rad18 towards SUMO-modified PCNA. Nucleic Acids Res.
40:11380–11388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tahmasebi S, Ghorbani M, Savage P,
Gocevski G and Yang XJ: The SUMO conjugating enzyme Ubc9 is
required for inducing and maintaining stem cell pluripotency. Stem
Cells. 32:1012–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stehmeier P and Muller S: Regulation of
p53 family members by the ubiquitin-like SUMO system. DNA Repair
(Amst). 8:491–498. 2009. View Article : Google Scholar
|
|
33
|
Lee YJ, Bernstock JD, Nagaraja N, Ko B and
Hallenbeck JM: Global SUMOylation facilitates the multimodal
neuroprotection afforded by quercetin against the deleterious
effects of oxygen/glucose deprivation and the restoration of
oxygen/glucose. J Neurochem. 138:101–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sadaka F and Veremakis C: Therapeutic
hypothermia for the management of intracranial hypertension in
severe traumatic brain injury: a systematic review. Brain Inj.
26:899–908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shankaran S: Current status of hypothermia
for hypoxemic ischemia of the newborn. Indian J Pediatr.
81:578–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YJ and Hallenbeck JM: SUMO and
ischemic tolerance. Neuromolecular Med. 15:771–781. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hirohama M, Kumar A, Fukuda I, Matsuoka S,
Igarashi Y, Saitoh H, Takagi M, Shin-ya K, Honda K, Kondoh Y, et
al: Spectomycin B1 as a novel SUMOylation inhibitor that directly
binds to SUMO E2. ACS Chem Biol. 8:2635–2642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dieckman LM, Freudenthal BD and Washington
MT: PCNA structure and function: insights from structures of PCNA
complexes and post-translationally modified PCNA. Subcell Biochem.
62:281–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parker JL and Ulrich HD: In vitro PCNA
modification assays. Methods Mol Biol. 920:569–589. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei F, Schöler HR and Atchison ML:
Sumoylation of Oct4 enhances its stability, DNA binding, and
transactivation. J Biol Chem. 282:21551–21560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang F, Yao Y, Jiang Y, Lu L, Ma Y and Dai
W: Sumoylation is important for stability, subcellular
localization, and transcriptional activity of SALL4, an essential
stem cell transcription factor. J Biol Chem. 287:38600–38608. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Laura MV, de la Cruz-Herrera CF, Ferreirós
A, Baz-Martínez M, Lang V, Vidal A, Muñoz-Fontela C, Rodríguez MS,
Collado M and Rivas C: KSHV latent protein LANA2 inhibits sumo2
modification of p53. Cell Cycle. 14:277–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang F, Cai F, Shi R, Wei JN and Wu XT:
Hypoxia regulates sumoylation pathways in intervertebral disc
cells: implications for hypoxic adaptations. Osteoarthritis
Cartilage. 24:1113–1124. 2016. View Article : Google Scholar : PubMed/NCBI
|