Introduction
Inflammatory bowel disease (IBD) is one of the
chronic nonspecific inflammatory diseases of the intestine,
characterized by a leaky intestinal epithelial barrier (IEB).
Breakdown of the IEB is observed in both IBD models and patients
(1,2). IEB dysfunction is also found in some
healthy relatives of IBD patients, which suggests that IEB
dysfunction is not just the result of IBD, it may be the reason
(3–5).
The tight junction proteins (TJs) are important
components of IEB, forming seals between adjacent epithelial cells
near the apical surface, thus preventing paracellular diffusion of
microorganisms and other antigens across the epithelium. Most
scientists believe that the cytoskeletal structure alteration
followed by redistribution of TJs leading to breakdown of the IEB
(6,7). TJs are composed of multiple proteins
including transmembrane proteins (such as occludin, tricellulin,
claudins and junctional adhesion molecule) and intracellular
proteins [such as zonula occludens (ZO)-1, -2, -3 and cingulin]
(8). It has been reported that
tumor necrosis factor-α (TNF-α), nuclear factor-κB (NF-κB) and
myosin light chain kinase signaling pathway could regulate TJ
function (9,10).
Rho kinase (also known as ROCK) is an important
regulator of cytoskeleton, which was originally identified as an
effector of small GTPase Rho (11). Two isoforms of ROCK have been
identified in mammalian system: ROCK1 (also known as ROKβ or p160
ROCK) and ROCK2 (also known as ROKα). ROCK1 and ROCK2 share an
overall 65% homology at amino-acid level and 92% homology in kinase
domains (12). Despite their
similar kinase domain, ROCK1 and ROCK2 still serve different
functions. ROCK1 is specifically cleaved by caspase-3 (13,14), whereas ROCK2 is cleaved by
granzyme B or caspase-2 (15,16). ROCK1 is essential for the
formation of stress fibers (17),
whereas ROCK2 appears to be necessary for phagocytosis and cell
contraction (18). Until this
point, the differences of these two isoforms in the recognition of
downstream targets remain unclear.
Several studies have demonstrated that ROCK
participated in the process of intestinal inflammation and
epithelial barrier dysfunction. Ivanov et al (19) and Segain et al (20) reported RhoA activated in inflamed
intestinal mucosa in experimental colitis rats and in Crohn's
disease patients. Mihaescu et al (21) also found that Y-27632 (one of the
ROCK inhibitors) could relieve the IEB damage in radiation-induced
intestinal inflammation.
There are varies of downstream factors of ROCK, such
as myosin phosphatase-targeting subunit-1 (MYPT-1) of myosin light
chain phosphatase (MLCP) and MLC. ROCK may activate MLC directly or
in an indirect pattern by activating MYPT-1. Since MYPT-1 is a
negative regulator of MLCP, activating of MYPT-1 may reduce the
de-phosphorylation of MLC by MLCP, which finally lead to the
activation of MLC (11,12). Another downstream pathway of ROCK
is the NF-κB pathway (22,23).
After ROCK activation, IκBα is phosphorylated and rapidly degraded,
allowing NF-κB to transmigrate within the nucleus and induce
inflammatory cytokine gene transcription by binding to specific
promoter elements.
However, the precise molecular mechanisms of action
of ROCK in the intestinal inflammation and IEB dysfunction remain
unknown. In the present study, the authors used Y-27632 to evaluate
the role of ROCK inhibition in the intestinal inflammation and
barrier dysfunction in a 2,4,6-trinitrobenzene sulfonic acid
(TNBS)-induced colitis mouse model. The downstream molecules of
ROCK were also examined to clarify the alteration of the ROCK
signaling pathway during the experimental colitis. In order to
evaluate the differences of ROCK1 and ROCK2 in recognizing
downstream molecules, the authors used RNA interference technique
to silence ROCK1 and ROCK2 separately in human colon cancer cell
lines (Caco-2). The results suggested a significant role of ROCK
inhibitor in intestinal inflammation and barrier dysfunction.
Furthermore, the results suggested ROCK1 mainly modulates
phosphorylation of MYPT-1 and MLC, while ROCK2 regulates
phosphorylation of NF-κB.
Materials and methods
Induction of mouse colitis
Animal experiments were performed in accordance with
the guidelines on the protection of animals and were approved by
the Ethics Committee for Animal Experimentation of Fudan University
(Shanghai, China). A total of 60 male BALB/c mice, 4–5 weeks old
and weighing 18–22 g, were obtained from the Animal Center of Fudan
University (Shanghai, China), maintained in an environmentally
controlled room (23±2°C, 55±10% humidity) with a 12 h light/dark
cycle and free access to food and water. Colitis was induced by
intra-colonic administration of TNBS solution (150 mg/kg in 50%
ethanol) once daily for up to 7 days. Y-27632, dissolved in saline,
was administered by intraperitoneal injection at a dose of 10 mg/kg
body weight just following enteroclysis on a daily basis.
A total of 60 mice were randomly divided into 3
groups as follows: group 1 (n=10) receiving saline through
intracolonic and intraperitoneal injection; group 2 (n=30)
receiving intracolonic administration of TNBS/ethanol solution and
intraperitoneal injection of saline; group 3 (n=20) receiving
intracolonic administration of TNBS/ethanol solution and
intraperitoneal injection of Y-27632.
Diarrhea and bloody stool extents were recorded
every day, calculated according to Mayo Score as previously
described with modification (24). The final diarrhea score of each
group was expressed as: 1, normal; 2, 1–2 stools/day more than
normal; 3, 3–4 stools/day more than normal; 4, >4 stools/day
more than normal. The bloody stool score was recorded as: 1, none;
2, visible blood with stool less than half the time; 3, visible
blood with stool half of the time or more; 4, passing blood
alone.
Evaluation of colonic inflammation
The proximal ~1.0 cm of the colonic segment was used
for histological examination. The segment was fixed in 4%
formaldehyde and embedded in paraffin. A morphometric analysis was
performed in a blinded fashion by two investigators on haematoxylin
and eosin stained 4 µm transverse sections. The extent of
colonic inflammation was assessed using the scoring system
described by Ameho et al (25).
Measurement of intestinal
permeability
Mice (10 from each group) were anesthetized by an
intraperitoneal injection of 3.5% chloral hydrate (10 ml/kg body
weight) on day 7 before being sacrificed by cervical dislocation. A
10 cm distance of terminal ileum ligation was performed at ~5 cm
proximal to the ileocecal valve. Then a solution containing 20
mg/ml fluorescein isothiocyanate (FITC)-conjugated dextran
(FITC-dextran, molecular weight 4.4 kDa; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in a total volume of 0.5 ml phosphate-buffered
saline (PBS) was injected into the ligatured ileum. At 30 min
following injection, blood samples (1 ml) were obtained from the
peripheral vessel and centrifuged (3,000 × g at 4°C) for 1 min;
then 100 µl plasma was mixed with 0.9 ml PBS and added to a
96-well microplate. The concentration of FITC-dextran was then
determined by spectrophotofluorometry with an excitation wavelength
of 480 nm and an emission wavelength of 520 nm.
Transmission electron microscopy
(TEM)
Glutaraldehyde-fixed specimens were washed in PBS
and immersed in 1% OsO4. Following dehydration in graded
ethanol and immersion in the intermedium toluol, the specimens were
embedded in epoxy resin (Serva Electrophoresis Gmbh, Heidelberg,
Germany). Semithin (500 nm) and thin (50 nm) sections were cut with
an ultramicrotome. Semithin sections were stained with 1%
alkalinized toluidine blue, then cut into thin sections. Thin
sections were stained with uranyl acetate and lead citrate, and
examined by electron microscope Zeiss EM 10 CR (Zeiss AG,
Oberkochen, Germany).
Immunohistochemistry
Sections (4 µm) of formalin fixed paraffin
embedded tissues were mounted on probe-on slides, deparaffinized in
xylene, and rehydrated in distilled H2O through graded
alcohol. Sections were blocked with normal mouse serum for 30 min
and incubated overnight at 4°C with occludin antibody (1:150;
13409-1-AP) and ZO-1 antibody (1:150; 21773-1-AP) (both from
ProteinTech Group, Inc., Chicago, IL, USA). The sections were
subsequently washed and incubated with SignalStain Boost
HRP-Polymer solution (8114; Cell Signaling Technology, Danvers, MA,
USA) for 30 min at room temperature, followed by incubation for
5–10 min with 3,3′-diaminobenzidine tetrachloride (D8001;
Sigma-Aldrich, St. Louis, MO, USA). Cells were considered as
positive once the dark-yellow granules were present either on the
membrane or in the cytoplasm. Five randomized microscopic views of
400-fold magnification of each section were observed in a blinded
fashion by one pathologist and a semi-quantitative scoring system
was based on both the staining intensity (0, negative; 1, weak; 2,
intermediate; 3, strong) and the percentage of positive cells (0,
0% positive cells; 1, 1–10% positive cells; 2, 11–50% positive
cells; 3, >50% positive cells). The final score of each sample
was obtained by multiplying the scores of staining intensity and
percentage of positive cells.
Cell culture
Caco-2 cells were purchased from the Shanghai Cell
Bank of Chinese Academy of Science (Shanghai, China) and cultured
in RPMI-1640 medium containing 15% fetal bovine serum. Cells were
seeded at 1×106 cells/well in 6-well plates and grown to
confluence. Caco-2 cells were then treated with either vehicle
(PBS) or 10 ng/ml recombinant human TNF-α (Sigma-Aldrich) for 24
h.
Small interfering RNA (siRNA)
transfection
The authors chose lentivirus pGLV-H1-GFP+Puro (LV3)
as a shuttle vector. The siRNAs targeting human ROCK1 and ROCK2
were as follows: ROCK1, GCATTTGGAGAAGTTCAATTG, GCAGAAGTAG
TTCTTGCATTG, GCACCAGTTGTACCCGATTTA and GGA TGAAGAGGGAAATCAAAG;
ROCK2, GGTTTATGCTAT GAAGCTTCT, GGAAGAAATCAGACAGCATCC, GCAGA
ACAGTATTTCTCAACC and GCTTCTCTTGAGGAAACT AAT. The siRNAs and
nonspecific control siRNA duplexes were synthesized, desalted and
purified by Shanghai GenePharma Co., Ltd. (Shanghai, China). Caco-2
cells (1×106/well) were plated in 6-well plates and were
cultured to confluence in RPMI-1640 medium. siRNA, pLV/helper-SL3,
pLV/helper-SL4 and pLV/helper-SL5 (4 µg each) and 40
µl Lipofectamine 2000 were mixed with 3 ml serum free
Opti-MEM I solution to form a transfection mixture. Following siRNA
transfection for 48 h, cells were treated with either vehicle (PBS)
or 10 ng/ml TNF-α for another 24 h. The knockdown efficiency was
assessed by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting after 72 h.
RT-qPCR
Total RNA of the colon and Caco-2 cell were isolated
using TRIzol reagent (Takara Bio, Inc., Ostu, Japan) according to
the manufacture's protocol. The primers were as follows: occludin
(mouse): (F) 5′-TTCAAACCCAATCATTATGCACC-3′ and (R)
5′-AAGAGTACGCTGGCTGAGAGAGC-3′; ZO-1 (mouse): (F)
5′-TTCCAGAACCGAAACCTGTGTATG-3′ and (R) 5′-GGCAGAGCACCATCAGAAGGG-3′;
ROCK1 (mouse and human): (F) 5′-TGATTCTGAGATGATTGGAGACCTTC-3′ and
(R) 5′-GAGTGATTAAGCATGTCTTGAGCCTC-3′; ROCK2 (mouse and human): (F)
5′-GAGACAACTGGATGAAACCAATGC-3′ and (R)
5′-GAATCTGTTTTGAACTTTCTGCCTG-3′; glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) (mouse): (F) 5′-CATGAGAAGTATGACAACAGCCT-3′
and (R) 5′-AGTCCTTCCACGATACCAAAGT-3′; occludin (human): (F)
5′-AGTGAATGACAAGCGGTTTTATCC-3′ and (R)
5′-CACAGGCGAAGTTAATGGAAGC-3′; ZO-1 (human): (F)
5′-GAGCACAGCAATGGAGGAAACAG-3′ and (R)
5′-AAATGAGGATTATCTCGTCCACCAG-3′; β-actin (human): (F)
5′-AGTGTGACGTTGACATCCGTA-3′ and (R) 5′-GCCAGAGCAGTAATCTCCTTCT-3′.
The First Strand cDNA of each sample was synthesized from 2
µg total RNA using SuperScript II according to the
manufacturer's instruction (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The reactions were as follows:
Pre-denaturation at 95°C for 3 min followed by 40 cycles of 95°C
for 12 sec (denaturation) and 62°C for 40 sec
(annealing/extension). The fluorescent products were detected by
LightCycle system (BioRad IQ5; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) before the completion of each cycle.
Western blot analysis
Both colon samples and Caco-2 cells were lysed and
homogenized in ice-cold radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). Proteins were
loaded onto each well of sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) ready gels (Bio-Rad Laboratories, Inc.)
for electrophoresis. Proteins were transferred onto a
nitrocellulose membrane (Bio-Rad Laboratories, Inc.) by electro
blotting. The membrane was washed and blocked with 5% milk in TBS
with 0.05% Tween-20, and incubated overnight with specific primary
antibodies at 4°C. Occludin antibody (1:500; 13409-1-AP), ZO-1
antibody (1:500; 21773-1-AP), GAPDH antibody (1:2,000; 10494-1-AP),
β-actin antibody (1:1,000; 20536-1-AP) (all from ProteinTech Group,
Inc.), NF-κB p65 rabbit mAb (1:1,000; 8242), phospho-NF-κB p65
(Ser536) antibody (1:1,000; 3031) (both from Cell Signaling
Technology, Inc.), ROCK1 antibody (1:200; 21850-1-AP),
ROCK2-Specific (C-Term) antibody (1:200; 20248-1-AP) (both from
ProteinTech Group, Inc.), phospho-MYPT-1 (Thr696) antibody
(1:1,000; 5163) and phospho-Myosin Light Chain 2 (Ser19) antibody
(1:1,000; 3671) (both from Cell Signaling Technology, Inc.) were
used as the primary antibodies. Goat anti-rabbit IgG-hRP (1:1,000;
SC-2004; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was
incubated as the secondary antibody at room temperature for 1 h.
Membranes were washed and the assessed proteins were detected using
an enhanced chemiluminescence reagent (GE Healthcare Life Sciences,
Chalfont, UK). Relative intensity of the bands was quantified using
Gel-Pro Analyzer 6.3 (Media Cybernetics LP, Silver Spring, MD,
USA).
Statistical analysis
Results were expressed as mean ± standard error of
mean. Data expressed as percentages were analyzed using Chi-square
test. Data with normal distribution were compared by one-way
analysis of variance and Student's t-test. Mann-Whitney U test was
performed in non-normal distribution data. Log-rank (Mantel-Cox)
test was used for survival curve. The statistical analysis was
performed using GraphPad Prism 5 (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
ROCK inhibitor attenuates TNBS-induced
colitis in mice
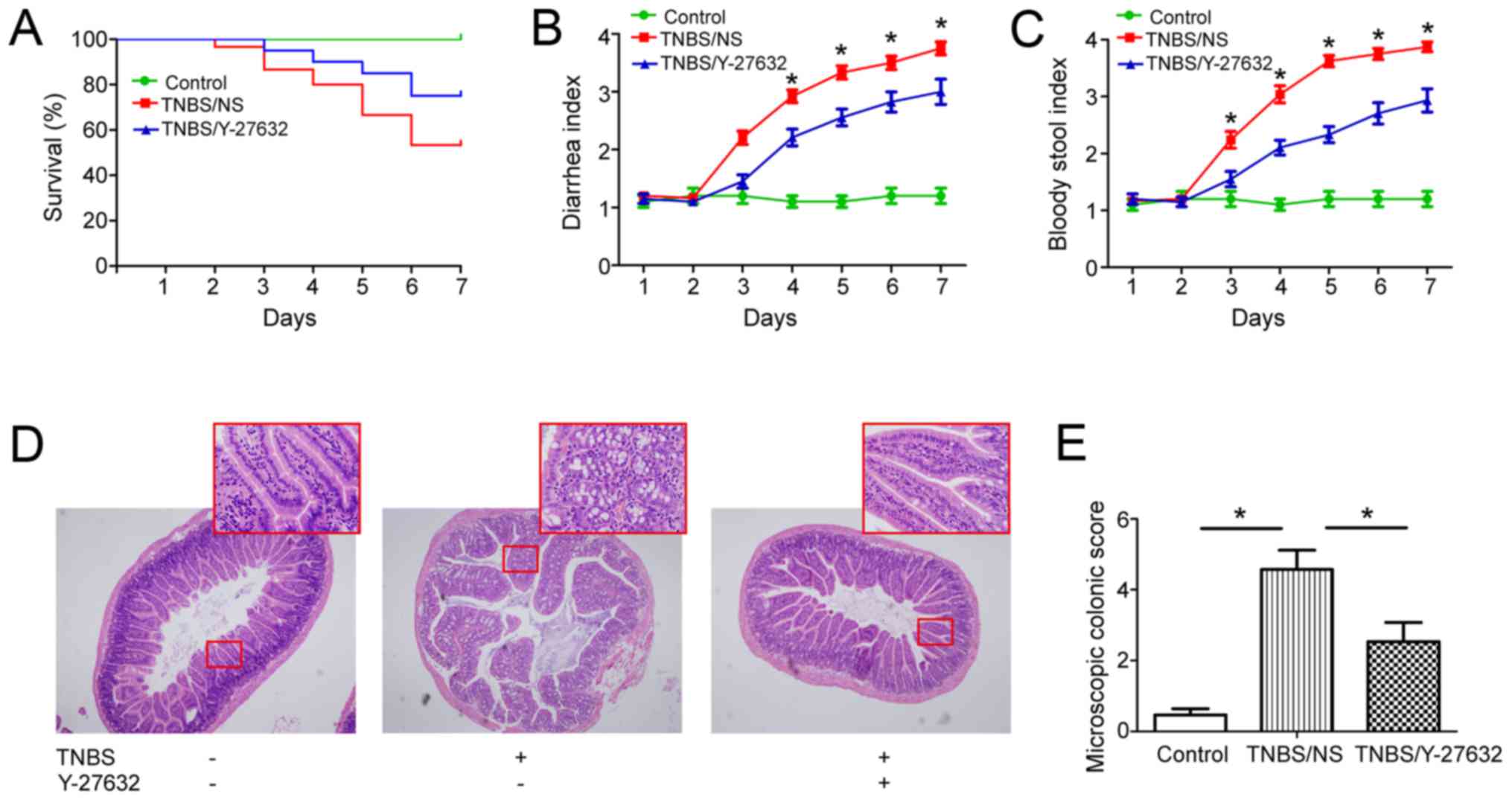
By the end of TNBS administration, 14 mice died in
group 2 (14/30, 46.7%) and 5 died in group 3 (5/20, 25%),
respectively. All mice remained alive in group 1. The survival
curve was analyzed using log-rank (Mantel-Cox) test (P<0.05)
(Fig. 1A). From day 3, mice in
group 2 began to present significant diarrhea, bloody stool, weight
loss and inactivity; while mice in group 3 presented slight
diarrhea and less bloody stool; no diarrhea and bloody stool was
found in group 1 (Fig. 1B and
C).
Moreover, the authors found that Y-27632 treatment
significantly ameliorated the colonic inflammation. In group 2,
inflammatory infiltration was observed throughout the whole colonic
wall, apparent folliculus lymphatics hyperplasia in the lamina
propria, some hemorrhagic focus in the submucosa when compared with
those in group 1, and they obviously alleviated in group 3
(Fig. 1D and E).
ROCK inhibitor regulates intestinal
permeability and IEB in TNBS-induced colitis in mice
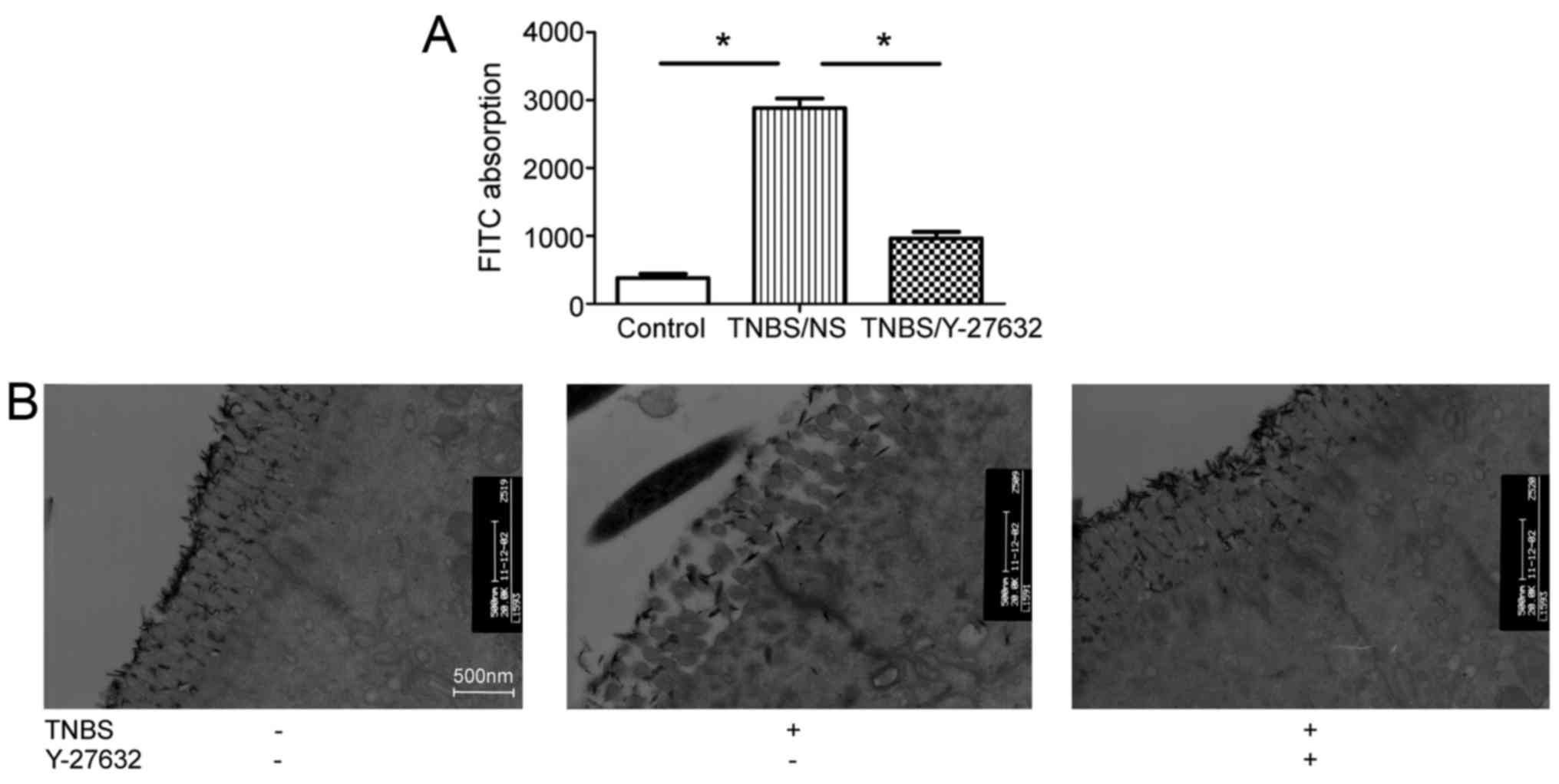
Intestinal permeability was evaluated by quantifying
the levels of FITC-dextran passing from the bowel lumen into the
blood circulation. Obviously, TNBS induced increased intestinal
permeability by ~8-fold compared with group 1 and inhibition of
ROCK decreased the intestinal permeability (Fig. 2A). The authors observed the
morphology of TJs, the primary components of IEB, through TEM. As
presented in Fig. 2B, TNBS
treatment caused colonic epithelial villus damage, intercellular
gap slightly widen, and tracer extravasation when compared to group
1. Y-27632 injection partially protected colonic epithelial villus
shape, decrease intercellular gap width and tracer extravasation in
group 3.
Furthermore, two main TJs, occludin and ZO-1, were
detected separately at mRNA and protein levels using RT-qPCR
(Fig. 3A), western blotting
(Fig. 3B and C) and
immunohistochemistry (Fig. 3D–F).
The results showed that the expressions of both occludin and ZO-1
were reduced apparently in group 2 compared with group 1. Y-27632
treatment may elevate occludin and ZO-1 levels.
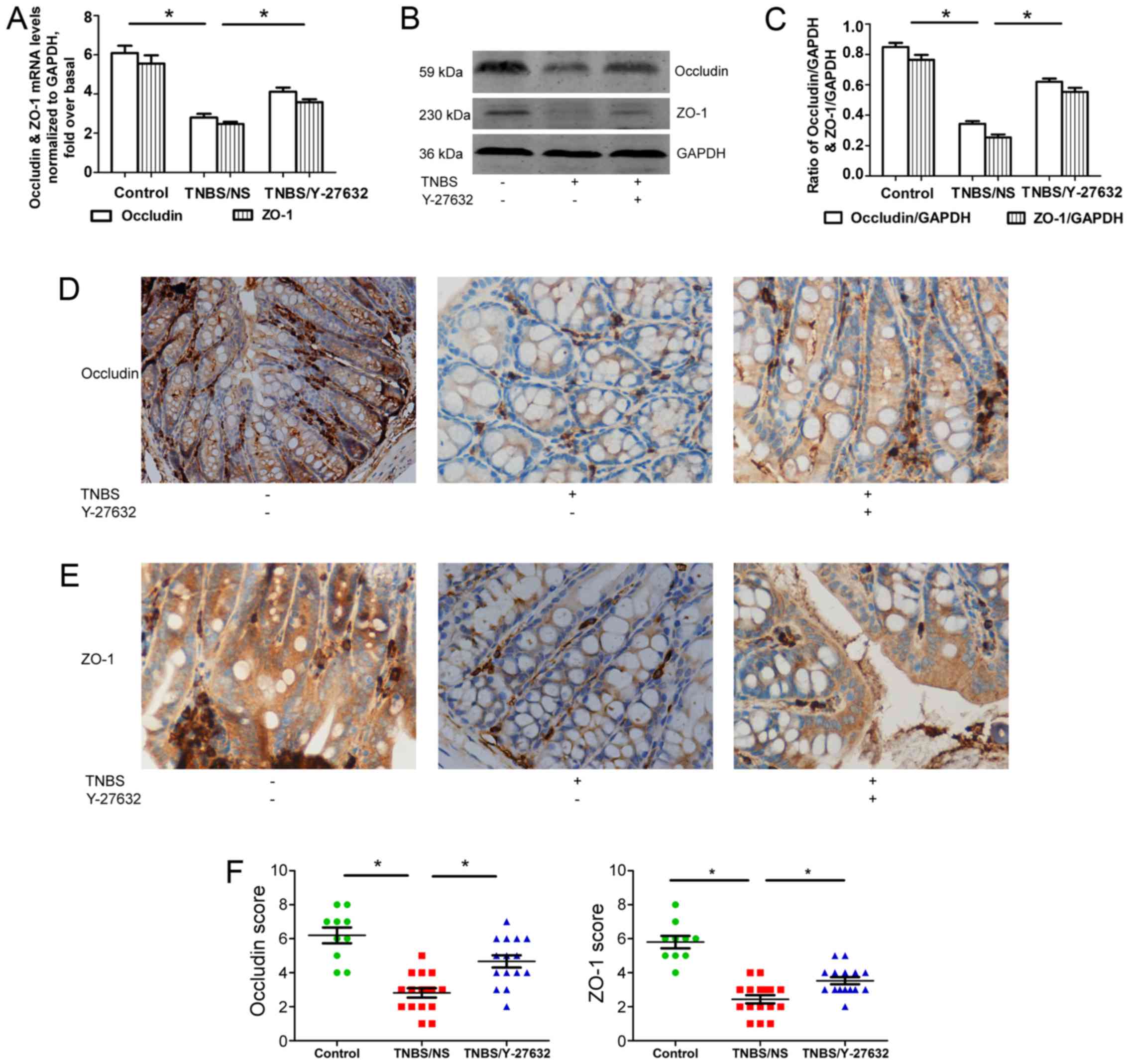 | Figure 3Increased occludin and ZO-1 in
TNBS-induced colitis mice by Y-27632. (A) The levels of occludin
and ZO-1 mRNA expression determined by reverse
transcription-quantitative polymerase chain reaction. Occludin and
ZO-1 levels were normalized with GAPDH expression in each sample.
(B) Western blotting for occludin and ZO-1 protein levels shown as
representative photos. (C) Bars representing the relative protein
quantification of occludin and ZO-1 on the basis of GAPDH. (D)
Typical patterns of occludin staining in Y-27632 and TNBS-treated
mouse colon (magnification, ×200). (E) Typical patterns of ZO-1
staining in Y-27632 and TNBS-treated mouse colon (magnification,
×200). (F) Scores of immunohistochemistry staining of occludin and
ZO-1 in three groups of mouse colon. Values are expressed as means
± standard error of the mean. *P<0.05 as indicated.
Control group, n=10, TNBS/NS group, n=16; TNBS/Y-27632 group, n=15.
ZO-1, zonula occludens-1; TNBS, 2,4,6-trinitrobenzene sulfonic
acid. NS, normal saline; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
ROCK1/ROCK2 and their downstream
molecules are inhibited by Y-27632 in mice with colitis
ROCK1 and ROCK2 mRNA and protein levels increased in
group 2 compared to group 1; while ROCK inhibitor obviously
downregulated both ROCK1 and ROCK2 levels in group 3 (Fig. 4A–C). Then, MYPT-1 and MLC, as the
main downstream molecules of the ROCK signaling pathway were tested
using western blotting. As shown in Fig. 4B and D, there was a significant
increase of phosphorylation of MLC in group 2 compared to that in
group 1; while Y-27632 inhibited the phosphorylation of MLC in
group 3. The phosphorylated MYPT-1 was similar in group 2 compared
with group 1; still Y-27632 treatment in group 3 inhibited the
phosphorylation of MYPT-1. In addition, the authors measured the
activation of the NF-κB pathway by detecting the expression of
total NF-κB p65 and phosphorylated NF-κB p65 (Ser536) using western
blotting. Both total and phosphorylated NF-κB p65 increased in the
group 2, and were downregulated after Y-27632 exposure in group 3
(Fig. 4B and E).
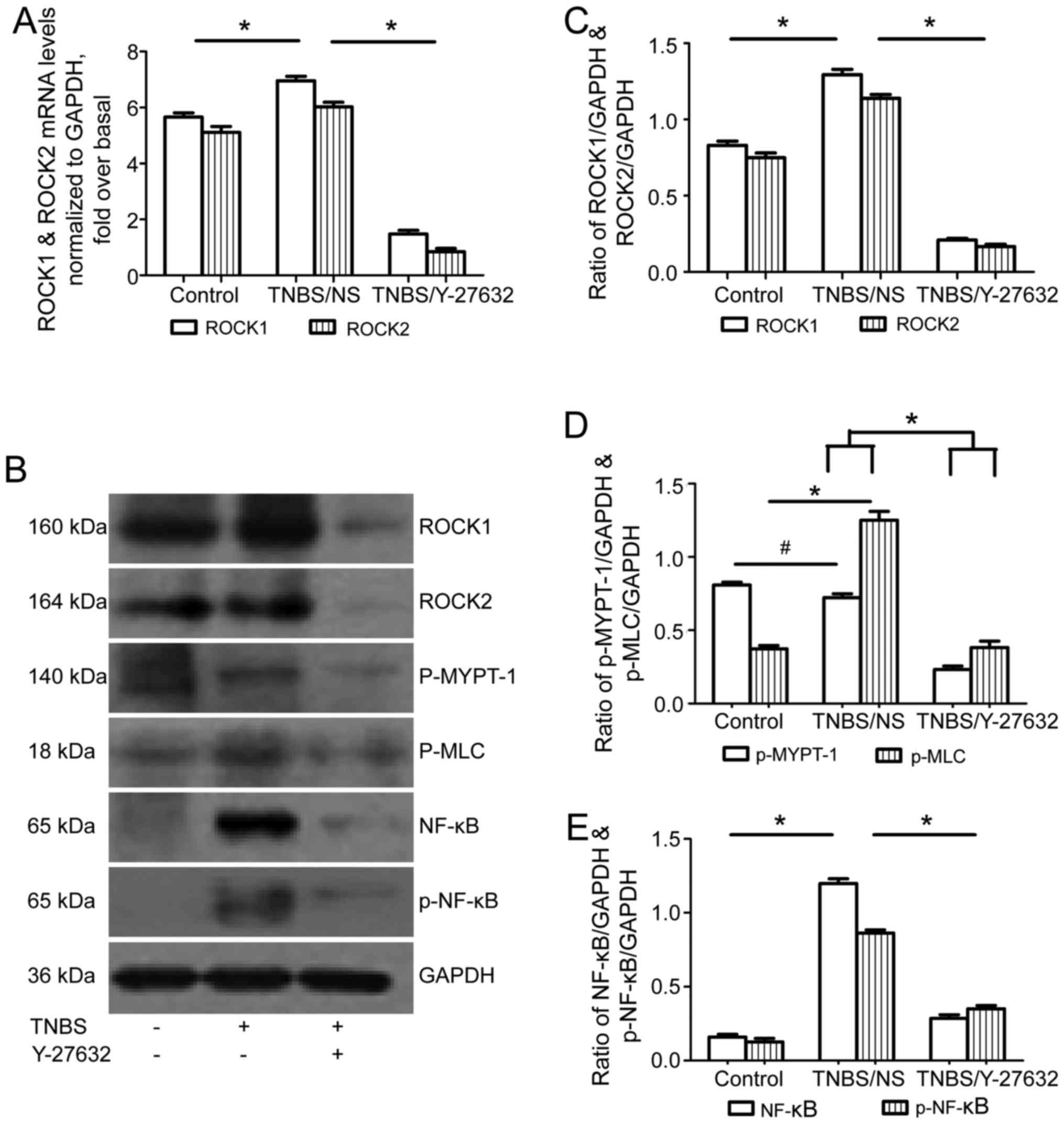 | Figure 4Effect of Y-27632 on ROCK expression
levels and its downstream signaling pathway in TNBS-induced colitis
mice. (A) The levels of ROCK1 and ROCK2 mRNA expression determined
by reverse transcription-quantitative polymerase chain reaction.
ROCK1 and ROCK2 mRNA levels were normalized with GAPDH expression
in each sample. *P<0.05 as indicated. (B) Western
blotting for ROCK1, ROCK2, p-MYPT-1, p-MLC, NF-κB p65 and p-NF-κB
p65 protein levels shown as representative images. (C) Bars
representing the relative protein quantification of ROCK1 and ROCK2
on the basis of GAPDH. (D) Bars representing the relative protein
quantification of p-MYPT-1 and p-MLC on the basis of GAPDH. (E)
Bars representing the relative protein quantification of NF-κB p65
and p-NF-κB p65 on the basis of GAPDH. *P<0.05 as
indicated; #P>0.05 as indicated. Values are expressed
as means ± standard error of the mean. Control group, n=10; TNBS/NS
group, n=16; TNBS/Y-27632 group, n=15. ROCK, Rho kinase; TNBS,
2,4,6-trinitrobenzene sulfonic acid; NS, normal saline; NF-κB,
nuclear factor-κB; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
MYPT-1, myosin phosphatase-targeting subunit-1; MLC, myosin light
chain. |
ROCK1 and/or ROCK2 blockage has no
influence on TJs in Caco-2 cells activated by TNF-α
Following 24 h of stimulation with TNF-α, ROCK1 and
ROCK2 in Caco-2 cells were significantly upregulated both in mRNA
and protein levels. ROCK1 and/or ROCK2 interference blocked the
corresponding expression, which was confirmed by RT-qPCR and
western blotting (Fig. 5A, C and
D). Then, the authors explored the contribution of each ROCK
isoform toward TNF-α-mediated activation of occludin and ZO-1. As
presented in Fig. 5C and E, the
expressions of occludin and ZO-1 were similar regardless of the
ROCK RNAi interference and TNF-α treatment. RT-qPCR showed that the
gene expressions of occludin and ZO-1 were in accordance with the
results of western blot analysis (Fig. 5B).
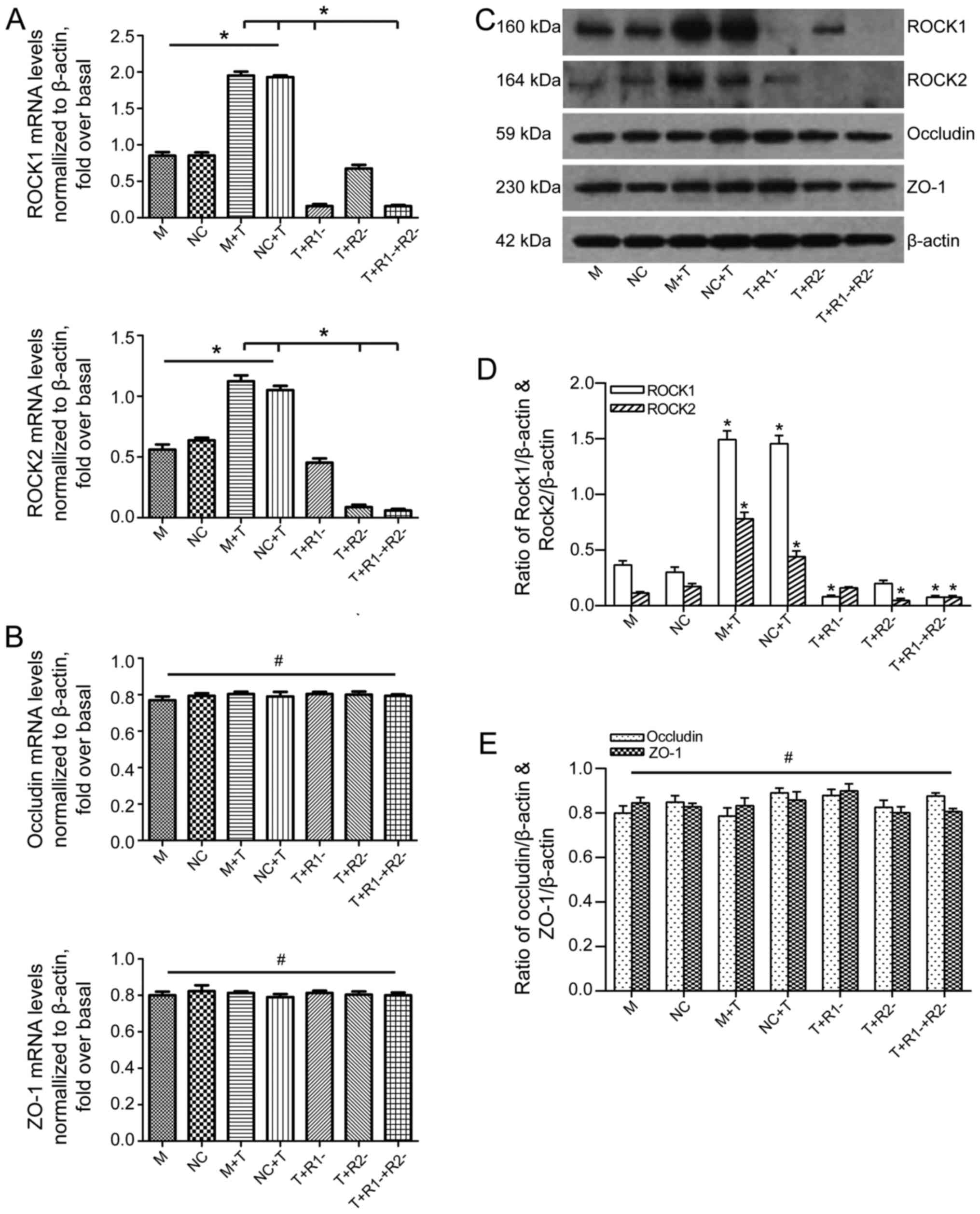 | Figure 5The effect of ROCK1 and/or ROCK2
blockage on the expression of occludin and ZO-1 after TNF-α
activation in Caco-2 cells. (A) The levels of ROCK1 and ROCK2 mRNA
expression determined by RT-qPCR. ROCK1 and ROCK2 levels were
normalized with β-actin expression in each sample. (B) The levels
of occludin and ZO-1 mRNA expression determined by RT-qPCR.
Occludin and ZO-1 levels were normalized with β-actin expression in
each sample. (C) Western blotting for ROCK1, ROCK2, occludin and
ZO-1 protein levels showed as typical patterns. (D) Bars
representing the relative protein quantification of ROCK1 and ROCK2
on the basis of β-actin. (E) Bars representing the relative protein
quantification of occludin and ZO-1 on the basis of β-actin. Values
are expressed as means ± standard error of the mean.
*P<0.05 as indicated; #P>0.05 as
indicated. M, mock; NC, negative control, treated with blank
vehicles; M+T, mock+TNF-α; NC+T, negative control+TNF-α;
T+R1−, TNF-α+ROCK1 RNAi transfection; T+R2−,
TNF-α+ROCK2 RNAi transfection; T+R1−+R2−,
TNF-α+ROCK1 RNAi and ROCK2 RNAi transfection. ROCK, Rho kinase;
ZO-1, zonula occludens-1; TNF-α, tumor necrosis factor-α; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; RNAi,
RNA interference. |
ROCK1 and ROCK2 RNAi inhibit different
downstream molecules in TNF-α treated Caco-2 cells
TNF-α treatment caused an increase of
phosphorylation of MYPT-1. ROCK1 blockage suppressed the
phosphorylation of MYPT-1, whereas merely ROCK2 blockage had no
obvious inhibition to phosphorylation of MYPT-1 (Fig. 6A and B). The phosphorylation of
MLC was upregulated following TNF-α treatment, and it seemed that
blockage of ROCK1 could inhibit MLC phosphorylation, while ROCK2
blockage only had a slight inhibition effect on phosphorylation of
MLC (Fig. 6A and B).
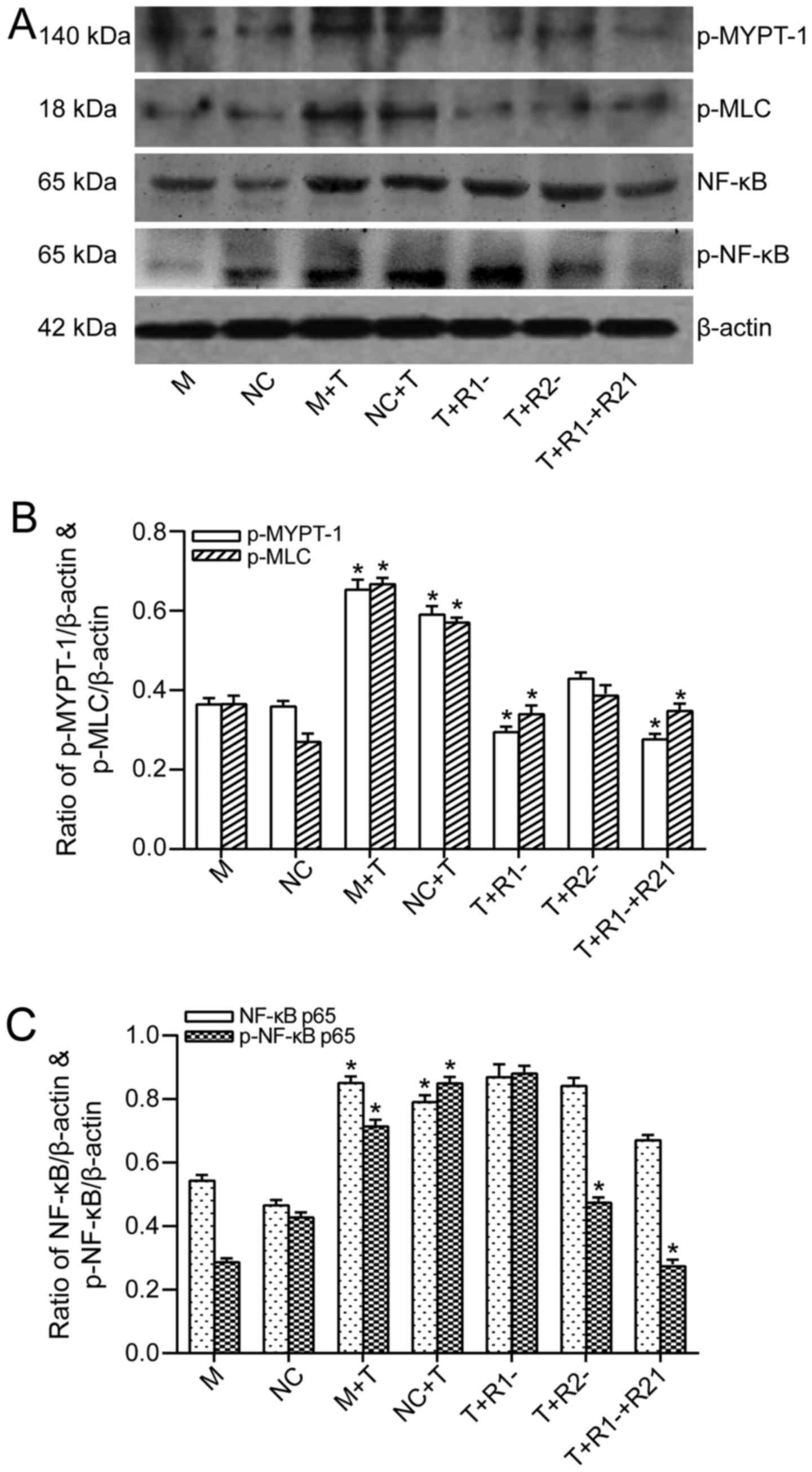 | Figure 6The effect of ROCK1 and/or ROCK2
blockage on the expression of downstream molecular of ROCK signal
pathway after TNF-α activation in Caco-2 cells. (A) Western blot
analysis for p-MYPT-1, p-MLC, NF-κB p65 and p-NF-κB p65 protein
levels showed as typical patterns. (B) Bars representing the
relative protein quantification of p-MYPT-1 and p-MLC on the basis
of β-actin. *P<0.05 for T group vs. M or NC group,
T+R1− group vs. T group, and
T+R1−+R2− groups vs. T group. (C) Bars
representing the relative protein quantification of NF-κB p65 and
p-NF-κB p65 on the basis of β-actin. *P<0.05 for T
group vs. M or NC group, T+R2− group vs. T group, and
T+R1−+R2− groups vs. T group. Values are
expressed as means ± standard error of the mean. M, mock; NC,
negative control, treated with blank vehicles; M+T, mock+TNF-α;
NC+T, negative control+TNF-α; T+R1−, TNF-α+ROCK1 RNAi
transfection; T+R2−, TNF-α+ROCK2 RNAi transfection;
T+R1−+R2−, TNF-α+ROCK1 RNAi and ROCK2 RNAi
transfection. ROCK, Rho kinase; NF-κB, nuclear factor-κB; RNAi, RNA
interference; MYPT-1, myosin phosphatase-targeting subunit-1; MLC,
myosin light chain. |
Following this, the authors focused on the NF-κB
signaling pathway. TNF-α exposure also stimulated the expressions
of both total NF-κB p65 and phosphorylated NF-κB p65. The blockage
of either of the two ROCK isoforms had no impact on the suppression
of the expression of total NF-κB p65. Knockdown of ROCK1 had no
impact on TNF-α-mediated NF-κB p65 phosphorylation, whereas ROCK2
knockdown had an inhibitive impact on NF-κB p65 phosphorylation
(Fig. 6A and C).
Discussion
An intact monolayer of intestinal epithelial cells
protects the body from pathogens and other toxic luminal
substances. To keep the IEB intact, it is important for prevention
and treatment of IBD (26). The
TJs are important components of IEB, which are composed of multiple
proteins including trans-membrane proteins and intracellular
proteins, such as occludin and ZO-1 (27). RhoA is a member of small GTPases
that is involved in numerous cellular functions, such as regulation
of actin filament reorganization and cell shape. ROCK, as a
downstream effector of RhoA, is an important regulator of
cytoskeleton. Several studies have shown that ROCK participated in
the process of intestinal inflammation and epithelial barrier
dysfunction (28–30). However, the effect of ROCK in the
intestinal inflammation and IEB dysfunction remains unknown.
In the present study, the authors proved that the
intestinal permeability increased in TNBS-induced colitis, and ROCK
inhibitor Y-27632 could alleviate the epithelial leakage. Although
no difference was observed in the TJ shapes by TEM, there was a
wider gap between the intestinal villa cells, tracer extravasation
in colitis mice, while Y-27632 reversed these effects. That meant
that the ROCK inhibitor could improve intestinal permeability
through improving and enhancing TJ function, not shape.
Furthermore, the authors evaluated the expression
levels of intestinal TJs with or without ROCK inhibitor. The
decrease of occludin and ZO-1 in TNBS colitis mice is accordance
with the results of Ji et al (31). In addition, the authors confirmed
ROCK inhibitor could upregulate the two substances expression. It
is reported that Y-27623 could ameliorate ethanol induced ZO-1
decrease and redistribution (32). The results furthermore confirmed
Y-27632 changed the ZO-1 expression both in mRNA and in protein
levels. Occludin was thought of one of the downstream molecules of
ROCK and could be phosphorylated by ROCK activation. However, there
was no report hitherto that ROCK could increase the expressions of
either the gene or protein of occludin (33). The upregulation of occludin mRNA
and protein levels in the present study was probably due to the
multiple interference factors in systemic inflammation.
In addition, the authors also demonstrated that two
ROCK signal pathways were activated during TNBS-induced colitis,
including the MLC pathway and the NF-κB pathway. There was an
upregulation of phosphorylation of MLC but not MYPT-1, which
indicated that ROCK may affect the cytoskeleton structure via
activating MLC. ROCK inhibitor caused a significant decrease of
both p-MYPT-1 and p-MLC, suggesting that besides inhibiting the
phosphorylation of MLC directly, Y-27632 could activate MLCP
through suppressing MYPT-1 activation, thus inhibiting MLC
indirectly. Since MLC is one of the most important proteins of the
cytoskeleton structure, the authors believe that ROCK inhibitor
improving the TJ function via inhibiting MLC activation may be one
of the mechanisms.
The NF-κB signaling pathway is also activated in
TNBS-induced colitis, which is in accordance with Segain et
al (20). The phosphorylation
at the site Ser536 detected by western blotting confirmed the NF-κB
activation. As NF-κB is one of the key factors regulating the
transcription of multiple inflammatory molecules, the authors
speculated that ROCK activation may promote the generation of
inflammatory molecules through activating NF-κB and thus lead to an
acceleration of intestinal epithelial damage.
In Caco-2 cells, it turned out that there was no
difference of occludin and ZO-1 expressions between TNF-α and ROCK
RNAi treated groups. That confirmed the speculation that there was
no direct relationship between the expression of occludin/ZO-1 and
ROCK, with or without TNF-α treatment. The variation of TJs levels
may ascribe to the activation of multiple signaling pathways and
the production of large amount of inflammatory factors.
The authors further evaluated the different roles of
ROCK1 and ROCK2 in recognizing the downstream molecules in Caco-2
cell lines separately. Following TNF-α treatment, the dual
ROCK1/ROCK2 blockage significantly inhibited phosphorylation of the
ROCK substrates such as MLC, MYPT-1 and NF-κB. However, individual
knockdown of ROCK1 could significantly suppress phosphorylation of
MYPT-1 and MLC. While sole ROCK2 knockdown only had a slight
inhibitory effect on phosphorylation of MYPT-1 and MLC. That meant
the activation of MYPT-1 an MLC depended more on ROCK1 than on
ROCK2.
The authors then turned their attention to the NF-κB
pathway. The total expression of NF-κB was upregulated following
TNF-α treatment, while the inhibition of ROCK1 and/or ROCK2 did not
alter the expression of NF-κB. However, the phosphorylation of
NF-κB was inhibited more significantly when ROCK2 were blocked,
suggesting that ROCK2 is more important in the activation of the
NF-κB pathway. The results are in accordance to the studies of
Shimada and Rajagopalan (34)
using a human endothelial cell line HUVECs. In the present study,
we did not completely clarify the exact action of the two ROCK
isoform differences in TNF-α-treated Caco-2 cells. More complex
signal transduction pathways may be involved in the pathogenesis of
intestinal inflammation.
In conclusion, the present study confirmed that ROCK
inhibitor could alleviate colitis and IEB dysfunction; ROCK
downstream signaling pathways (MLC and NF-κB) were activated in
colitis mice and inhibited by Y-27632, which improved IEB
dysfunction. ROCK1 and ROCK2 knockout separately played different
roles in recognizing the downstream molecules. Thus, ROCK inhibitor
may be a potential therapeutic method in IBD and further studies
are required to further clarify the exact mechanism of ROCK and its
pathway in regulating TJ function in IEB.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81400628 and
81500460) and the Science and Technology Commission of Shanghai
Municipality (grant no. 16411952300) and the Shanghai Municipal
Health Bureau (grant no. 201440392).
References
|
1
|
Zeissig S, Bojarski C, Buergel N, Mankertz
J, Zeitz M, Fromm M and Schulzke JD: Downregulation of epithelial
apoptosis and barrier repair in active Crohn's disease by tumour
necrosis factor alpha antibody treatment. Gut. 53:1295–1302. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turck D, Ythier H, Maquet E, Deveaux M,
Marchandise X, Farriaux JP and Fontaine G: Intestinal permeability
to [51Cr] EDTA in children with Crohn's disease and celiac disease.
J Pediatr Gastroenterol Nutr. 6:535–537. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
May GR, Sutherland LR and Meddings JB: Is
small intestinal permeability really increased in relatives of
patients with Crohn's disease? Gastroenterology. 104:1627–1632.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clayburgh DR, Shen L and Turner JR: A
porous defense: The leaky epithelial barrier in intestinal disease.
Lab Invest. 84:282–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katz KD, Hollander D, Vadheim CM, McElree
C, Delahunty T, Dadufalza VD, Krugliak P and Rotter JI: Intestinal
permeability in patients with Crohn's disease and their healthy
relatives. Gastroenterology. 97:927–931. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edelblum KL and Turner JR: The tight
junction in inflammatory disease: Communication breakdown. Curr
Opin Pharmacol. 9:715–720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen L, Weber CR and Turner JR: The tight
junction protein complex undergoes rapid and continuous molecular
remodeling at steady state. J Cell Biol. 181:683–695. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hering NA and Schulzke JD: Therapeutic
options to modulate barrier defects in inflammatory bowel disease.
Dig Dis. 27:450–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steed E, Balda MS and Matter K: Dynamics
and functions of tight junctions. Trends Cell Biol. 20:142–149.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ivanov AI: Structure and regulation of
intestinal epithelial tight junctions: Current concepts and
unanswered questions. Adv Exp Med Biol. 763:132–148. 2012.
|
|
11
|
Sebbagh M, Renvoizé C, Hamelin J, Riché N,
Bertoglio J and Bréard J: Caspase-3-mediated cleavage of ROCK I
induces MLC phosphorylation and apoptotic membrane blebbing. Nat
Cell Biol. 3:346–352. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaturvedi LS, Marsh HM and Basson MD:
Role of RhoA and its effectors ROCK and mDia1 in the modulation of
deformation-induced FAK, ERK, p38, and MLC motogenic signals in
human Caco-2 intestinal epithelial cells. Am J Physiol Cell
Physiol. 301:C1224–C1238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sapet C, Simoncini S, Loriod B, Puthier D,
Sampol J, Nguyen C, Dignat-George F and Anfosso F: Thrombin-induced
endothelial microparticle generation: Identification of a novel
pathway involving ROCK-II activation by caspase-2. Blood.
108:1868–1876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turner MS, Fen-Fen-Lin, Trauger JW,
Stephens J and LoGrasso P: Characterization and purification of
truncated human Rho-kinase II expressed in Sf-21 cells. Arch
Biochem Biophys. 405:13–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen XQ, Tan I, Ng CH, Hall C, Lim L and
Leung T: Characterization of RhoA-binding kinase ROKalpha
implication of the pleckstrin homology domain in ROKalpha function
using region-specific antibodies. J Biol Chem. 277:12680–12688.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsui T, Amano M, Yamamoto T, Chihara K,
Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A and Kaibuchi K:
Rho-associated kinase, a novel serine/threonine kinase, as a
putative target for small GTP binding protein Rho. EMBO J.
15:2208–2216. 1996.PubMed/NCBI
|
|
17
|
Ward Y, Yap SF, Ravichandran V, Matsumura
F, Ito M, Spinelli B and Kelly K: The GTP binding proteins Gem and
Rad are negative regulators of the Rho-Rho kinase pathway. J Cell
Biol. 157:291–302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leung T, Chen XQ, Manser E and Lim L: The
p160 RhoA-binding kinase ROK alpha is a member of a kinase family
and is involved in the reorganization of the cytoskeleton. Mol Cell
Biol. 16:5313–5327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ivanov AI, Parkos CA and Nusrat A:
Cytoskeletal regulation of epithelial barrier function during
inflammation. Am J Pathol. 177:512–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Segain JP, Raingeard de la Blétière D,
Sauzeau V, Bourreille A, Hilaret G, Cario-Toumaniantz C, Pacaud P,
Galmiche JP and Loirand G: Rho kinase blockade prevents
inflammation via nuclear factor kappa B inhibition: Evidence in
Crohn's disease and experimental colitis. Gastroenterology.
124:1180–1187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mihaescu A, Santén S, Jeppsson B and
Thorlacius H: Rho kinase signalling mediates radiation-induced
inflammation and intestinal barrier dysfunction. Br J Surg.
98:124–131. 2011. View
Article : Google Scholar
|
|
22
|
Anwar KN, Fazal F, Malik AB and Rahman A:
RhoA/Rho-associated kinase pathway selectively regulates
thrombin-induced intercellular adhesion molecule-1 expression in
endothelial cells via activation of I kappa B kinase beta and
phosphorylation of RelA/p65. J Immunol. 173:6965–6972. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodriguez PL, Sahay S, Olabisi OO and
Whitehead IP: ROCK I-mediated activation of NF-kappaB by RhoB. Cell
Signal. 19:2361–2369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voiosu T, Benguş A, Dinu R, Voiosu AM,
Bălănescu P, Băicuş C, Diculescu M, Voiosu R and Mateescu B: Rapid
fecal calprotectin level assessment and the SIBDQ score can
accurately detect active mucosal inflammation in IBD patients in
clinical remission: A prospective study. J Gastrointestin Liver
Dis. 23:273–278. 2014.PubMed/NCBI
|
|
25
|
Ameho CK, Adjei AA, Harrison EK, Takeshita
K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A,
et al: Prophylactic effect of dietary glutamine supplementation on
interleukin-8 and tumour necrosis factor alpha production in
trinitrobenzene sulphonic acid induced colitis. Gut. 41:487–493.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong X, Liu Z, Lan D, Niu J, Miao J, Yang
G, Zhang F, Sun Y, Wang K and Miao Y: Critical role of Keratin 1 in
maintaining epithelial barrier and correlation of its
down-regulation with the progression of inflammatory bowel disease.
Gene. 608:13–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turner JR, Buschmann MM, Romero-Calvo I,
Sailer A and Shen L: The role of molecular remodeling in
differential regulation of tight junction permeability. Semin Cell
Dev Biol. 36:204–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moyer RA, Wendt MK, Johanesen PA, Turner
JR and Dwinell MB: Rho activation regulates CXCL12 chemokine
stimulated actin rearrangement and restitution in model intestinal
epithelia. Lab Invest. 87:807–817. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Le Dréan G, haure-Mirande V, Ferrier L,
Bonnet C, hulin P, de Coppet P and Segain JP: Visceral adipose
tissue and leptin increase colonic epithelial tight junction
permeability via a RhoA-ROCK-dependent pathway. FASEB J.
28:1059–1070. 2014. View Article : Google Scholar
|
|
30
|
Elamin E, Masclee A, Dekker J and Jonkers
D: Ethanol disrupts intestinal epithelial tight junction integrity
through intracellular calcium-mediated Rho/ROCK activation. Am J
Physiol Gastrointest Liver Physiol. 306:G677–G685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji R, Wang A, Shang H, Chen L, Bao C, Wu
L, Wu H and Shi Y: Herb-partitioned moxibustion upregulated the
expression of colonic epithelial tight junction-related proteins in
Crohn's disease model rats. Chin Med. 11:202016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tong J, Wang Y, Chang B, Zhang D and Wang
B: Y-27632 inhibits ethanol-induced increase in intestinal
epithelial barrier permeability. Mol Med Rep. 9:2357–2361. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Furuse M, hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S and Tsukita S: Occludin: A novel integral
membrane protein localizing at tight junctions. J Cell Biol.
123:1777–1788. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimada H and Rajagopalan LE: Rho kinase-2
activation in human endothelial cells drives lysophosphatidic
acid-mediated expression of cell adhesion molecules via NF-kappaB.
65J Biol Chem. 285:12536–12542. 2010. View Article : Google Scholar
|