Introduction
Despite significant improvement in diagnosis and
treatment strategies in recent years, atherosclerosis and the
consequent diseases remain major contributors to mortality and
morbidity worldwide (1).
Pathophysiologically, atherosclerosis is recognized as an
inflammatory disease characterized by the activation and migration
of inflammatory cells into the subendothelial layer of the
arteries. Coronary heart disease (CHD), which is caused by
atherosclerotic lesions in the coronary arteries, has become the
most important public health problem in developed as well as
developing countries, and the incidence is continuously rising
worldwide with the acceleration of population aging (2,3).
In addition to conventional risk factors that have been associated
with atherosclerosis and CHD, overweight and obesity have been
linked to the pathogenesis of the above diseases (4,5).
With the increasing prevalence of obesity in the global population
(6), atherosclerosis-associated
diseases are expected to be even more prevalent in the future
(7). Therefore, the development
of effective therapies against atherosclerosis is of great clinical
significance.
Indeed, marked improvements have been made regarding
treatment options for atherosclerosis and associated diseases
(8,9), and stem cell-based therapies are
promising for patients with atherosclerosis, particularly CHD. With
their characteristics of extensive proliferation and multipotency,
stem cells have been suggested to be effective for repairing of
vascular atherosclerotic lesions (7,10–12). Mesenchymal stromal cells (MSCs),
which include bone marrow stromal cells (BMSCs) and adipose-tissue
derived mesenchymal stem cell (ADSC), are multipotent adult stem
cells that are most commonly applied in studies on stem-cell based
therapies for atherosclerotic diseases (13,14). In addition to the use of MSCs
themselves, bioengineering approaches based on gene therapy using
MSCs have also been explored in several preclinical studies
(15–17). The benefits of MSC-based therapies
in atherosclerosis have been suggested to involve numerous
potential mechanisms, including homing of MSCs to atherosclerotic
lesions, production of active cytokines, modulation of the immune
response, improved endothelial repair and attenuation of thrombosis
formation (18–20).
Although BMSCs are the most commonly used type of
stem cells in preclinical studies on cell-based therapies for
atherosclerosis, the relative rarity of these cells and the
invasive procedures required for their harvesting have limited
their use. As ADSCs are more readily accessible than BMSCs
(21), they are also considered
to be a potential cell source for the treatment of atherosclerotic
diseases. However, the differences in the biological
characteristics of ADSCs and BMSCs remain to be fully elucidated.
No significant differences in the morphology and immune phenotype
have been identified between BMSCs and ADSCs (22). However, the proliferative activity
and apoptotic tolerance of ADSCs were reported to be higher than
those of BMSCs (23–25). In addition, the cell population,
maximum lifespan and multipotency of BMSCs were found to decrease
more rapidly with increasing donor age compared with ADSCs
(26,27). MSCs have been demonstrated to be
capable of enhancing angiogenesis and improving cardiac function
in vivo. Kim et al (28) compared the therapeutic potential
of ADSCs and BMSCs by transplanting the same number of cells in a
nude mouse model of hind limb ischemia. The results indicated that
ADSCs are associated with better blood flow recovery than BMSCs. In
a rodent model injected with ADSCs to reconstruct abdominal wall
muscle defects, angiogenesis and muscle healing were significantly
improved compared with those in animals administered BMSCs
(29). In addition, an
experimental study demonstrated that ADSCs may induce a greater
improvement in infarct area and left ventricle infarct wall
thickness than BMSCs (30). The
above studies also indicated that application of ADSCs in
vivo in ischemic disease was associated with enhanced
angiogenesis and a greater improvement in heart function in terms
of efficacy and accessibility. The potential mechanisms underlying
these differences have not been comprehensively described, and
differences in the metabolic characteristics of the two stem cell
types may be involved. Therefore, the present study applied liquid
chromatography quadrupole time-of-flight mass spectrometry
(LC-QTOF-MS) to explore the differences in the metabolites of BMSCs
and ADSCs derived from elderly patients with CHD.
Materials and methods
Patients
A total of 30 elderly patients (age, ≥60 years) with
CHD and without hyperlipidemia and/or other metabolic abnormalities
who were hospitalized at The Second Affiliated Hospital of Harbin
Medical University (Harbin, China) from January, 2015 to October,
2016 were enrolled in the present study. The study protocol was
approved by the Ethics Committee of The Second Affiliated Hospital
of Harbin Medical University, and informed consent was obtained
from all patients.
Cell culture
Bone marrow was collected from 15 CHD patients. The
bone marrow was aspirated under local anaesthesia from the sternum
and collected in heparinized tubes. Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 3.7 g/l sodium bicarbonate, 1% penicillin and streptomycin,
and 10% fetal bovine serum (Biological Industries Israel
Beit-Haemek, Ltd., Kibbutz Beit-Haemek, Israel) was used for
culturing the isolated cells. After 72 h, unattached cells and
residual non-adherent red blood cells were removed by washing with
phosphate-buffered saline (PBS). ADSCs were derived from adipose
tissue of abdominal subcutaneous fat collected under anaesthesia
from the other 15 CHD patients as previously described (31). The adipose tissues were washed
with PBS containing 1% penicillin and streptomycin and subsequently
digested with collagenase type I (1 mg/ml; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 37°C for 45–60 min according to the
manufacturer's instructions for the collagenase with intermittent
shaking. Subsequently, the suspension was filtered using a
200-μm nylon mesh and the suspension was then centrifuged at
600 × g/min at 4°C for 10 min, to separate the floating adipocytes.
The cells were then cultured in a humidified atmosphere containing
5% CO2 at 37°C with the medium replaced every 3 days. At
passage 3, 105 cells in 2 ml cell culture medium were
seeded in 6-well plates. After 3 days, the supernatants were
collected and preserved at −80°C for subsequent analyses.
Sample preparation
Supernatant preparation for the analysis of BMSCs
and ADSCs was based on the following procedure: In brief, frozen
supernatant samples were thawed at 4°C for 50 min. After vortexing
for 10 sec, the solutions were centrifuged at 4,000 × g for 10 min
at 4°C. The upper aliquot solution (200 μl) was transferred
to a clean 2-ml centrifuge tube and then acetonitrile (1,000
μl) was added. After vortexing for 2 min, the samples were
centrifuged at 12,000 × g for 15 min at 4°C. The upper solution
(1,000 μl) was transferred to a clean 2-ml centrifuge tube
and then evaporated to dryness over a heat block at 35°C under
nitrogen gas. The residue was dissolved in 200 μl
acetonitrile/water (1:3, v/v) via vortexing for 1 min and
centrifugation at 12,000 × g for 15 min at 4°C. The supernatant
(200 μl) was transferred to an autosampler vial and injected
into the LC-QTOF-MS (6530 series; Agilent Technologies, Inc., Santa
Clara, CA, USA) apparatus for analysis. Equal amounts of
supernatant samples from 15 ADSC cultures and 15 BMSC cultures as
the samples were mixed for quality control (QC).
Chromatography
Each 10-μl aliquot of sample was injected
into a 2.1×100 mm (1.8 mm) ZORBAX SB-C18 column for subsequent
rapid resolution liquid chromatography (6530 series) (both from
Agilent Technologies, Inc.). A mixture of acetonitrile containing
0.1% formic acid (phase A) and water containing 0.1% formic acid
(phase B) were used as the mobile phase for electron spray
ionisation in positive mode (ESI+), while a mixture of
acetonitrile (phase A) and water (phase B) was used as the mobile
phase for ESI in negative mode (ESI−). The protocols for
the linear mobile phase gradient were as follows: 95% A held for 1
min; decreased to 2% A by 10 min; held at 2% A until 13 min;
increased to 95% A by 13.1 min; and held at 95% A until 20 min. The
flow rate of the mobile phase was 0.3 ml/min at 40°C.
MS
MS was performed using an Agilent 6530-QTOF MS
apparatus (6530 series; Agilent Technologies, Inc.) operating in
ESI+ or ESI− mode. The capillary voltage was
set as 4.0 kV for ESI+ and 3.5 kV for ESI−.
Nitrogen was applied as the desolvation gas at a flow rate of 10
l/min. The desolvation temperature was 350°C. The centroid data
were obtained with the full scan mode [mass-to-charge ratio (m/z) =
50–1,000].
Data pre-processing and annotation
The raw data were converted into mzData-format files
using MassHunter Qualitative Analysis Software (v. B.04.00; Agilent
Technologies, Inc.) and these files were further imported to the
XCMS package in R (v. 3.0.2) (r-project.org/) for pre-processing. The analyses
followed the default XCMS parameter settings, with the following
exceptions: xcms Set (fwhm, 10), group (minfrac, 0.5; bw, 30) and
rector (method, 'obiwarp'). The definitions are as follows: fwhm,
specifying the full width at half maximum of matched filtration
Gaussian model peak; minfrac, defining the minimum fraction of
samples in at least one sample group in which the peaks have to be
present to be considered as a peak group; and bw, defining the
bandwidth (standard deviation of the smoothing kernel) to be
used.
Subsequently, a data matrix was generated, including
results of retention time, m/z values and peak intensity. CAMERA in
R (v. 3.0.2) was used to annotate isotope peaks and generate
adducts and fragments in the peak lists (32). A total of 1,668 ions in
ESI+ mode and 829 ions in ESI− mode were
included for subsequent statistical analysis.
Statistical analysis
First, principal component analysis (PCA) was used
to detect the grouping trends and outliers (33). The Wilcoxon rank sum test was then
applied to determine the significance of each metabolite at
P<0.05. To identify the differences in metabolites between BMSCs
and ADSCs, a partial least squares discriminant analysis (PLS-DA)
was used (33). Permutation tests
with 100 iterations were included to validate the supervised model
and avoid overfitting (34).
Based on the PLS-DA model, parameters that described the variable
importance in the projection (VIP) for each metabolite were
calculated. With thresholds of P-values and VIP values of 0.05 and
1, respectively, the metabolic biomarkers were detected. The
Wilcoxon rank sum test was used on the R platform (v. 3.0.2). The
PCA and PLS-DA were performed using SIMCA-P (v. 11.5; Umetrics,
Malmö, Sweden).
Results
PCA score plots for discriminating BMSCs
and ADSCs
The baseline characteristics of the donors are
presented in Table I. There were
no significant differences between the groups of donors (BMSC
donors: 8 males and 7 females; median age, 64 years; age range,
61–73 years; median weight, 67 kg; weight range, 55–83 kg; mean
fasting glucose, 5.4 mmol/l; and fasting glucose range, 4.2–6.1
mmol/l. ADSC donors: 6 males and 9 females; median age, 65 years;
age range, 61–75 years; median weight, 65 kg; weight range, 50–85
kg; mean fasting glucose, 5.2 mmol/l; fasting glucose range,
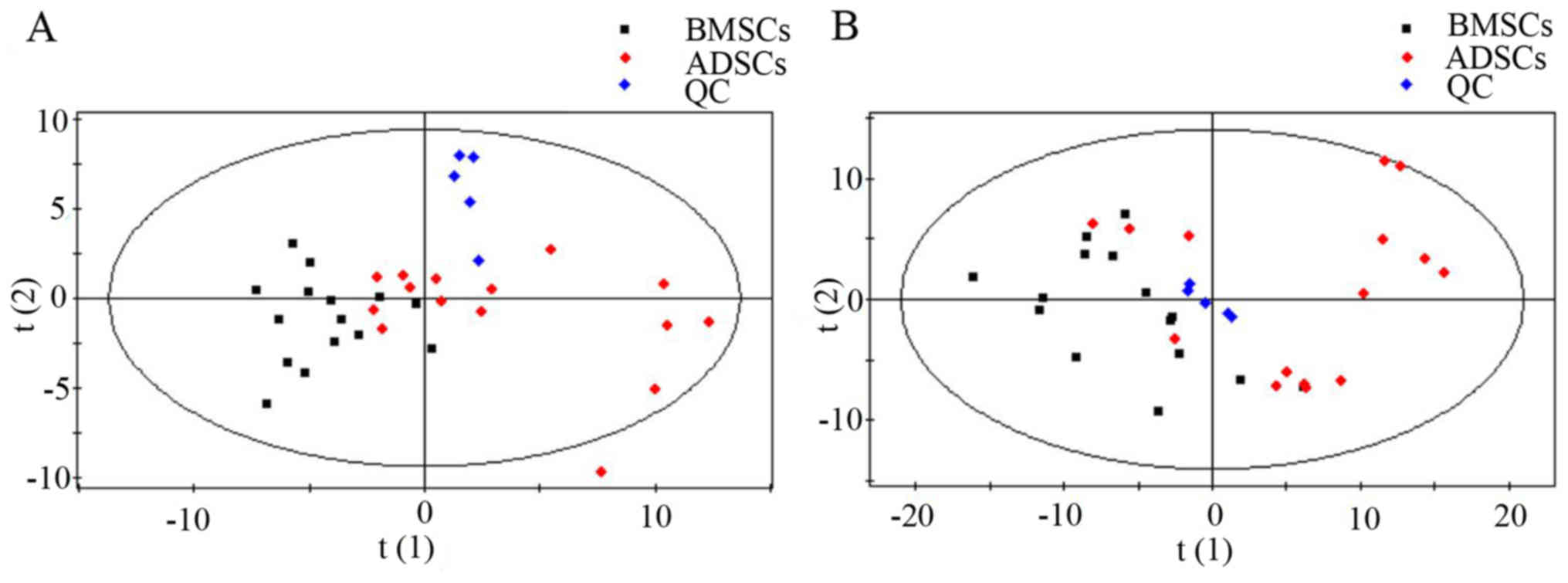
4.5–6.1 mmol/l). Metabolic analysis revealed numerous metabolic
differences between BMSCs and ADSCs. The results of the overall PCA
based on all the samples suggested that the QC samples were closely
clustered in plots of PCA scores, which demonstrated that the
results of the metabolic profiling platform were robust. In
addition, no outliers were present on the whole, and separation
trends were observed between BMSCs and ADSCs (Fig. 1).
 | Table IClinical characteristics of BMSC and
ADSC donors (n=15 per group). |
Table I
Clinical characteristics of BMSC and
ADSC donors (n=15 per group).
| Characteristic | BMSC donors | ADSC donors | P-value |
|---|
| No. of
subjects | 15 | 15 | – |
| Age, years (median,
range) | 64, 61–73 | 65, 61–75 | 0.36 |
| Weight, kg (median,
range) | 67, 55–83 | 65, 50–85 | 0.48 |
| Sex | 8 M, 7 F | 6 M, 9 F | – |
| History of coronary
heart disease, years (median, range) | 18, 12–25 | 19, 13–26 | 0.44 |
| Fasting glucose,
mmol/l (median, range) | 5.4, 4.2–6.1 | 5.2, 4.5–6.1 | 0.47 |
PLS-DA plots and validation plots for
discriminating BMSCs and ADSCs
Via the application of the ESI+ and
ESI− modes, all of the statistically significant ions
were analysed (P<0.05 and VIP>1) (Fig. 2). Subsequently, a supervised
PLS-DA model was used to identify differences between BMSCs and
ADSCs. As presented in the PLS-DA score plot, an obvious separation
between BMSCs and ADSCs was present in the ESI+ mode
(Fig. 2A) and ESI−
mode (Fig. 2C). The PLS-DA models
contained two predictive components in ESI+ mode
[R2X= 0.409; R2Ycum=0.759; cumulative second
quartile (Q2cum)=0.429] and two components in
ESI− mode (R2X= 0.55; R2Ycum=
0.647; Q2cum=0.398). Permutation tests including 100
iterations and containing two predictive components were also
performed (35). The results
indicated that the permuted Q2cum values were lower than
the original values in almost all cases (Fig. 2B and D), which further confirmed
the validity of the supervised models. R2 identified the
outfit of the PLS model. Q2cum refers to the predicting
ability of the PLS model.
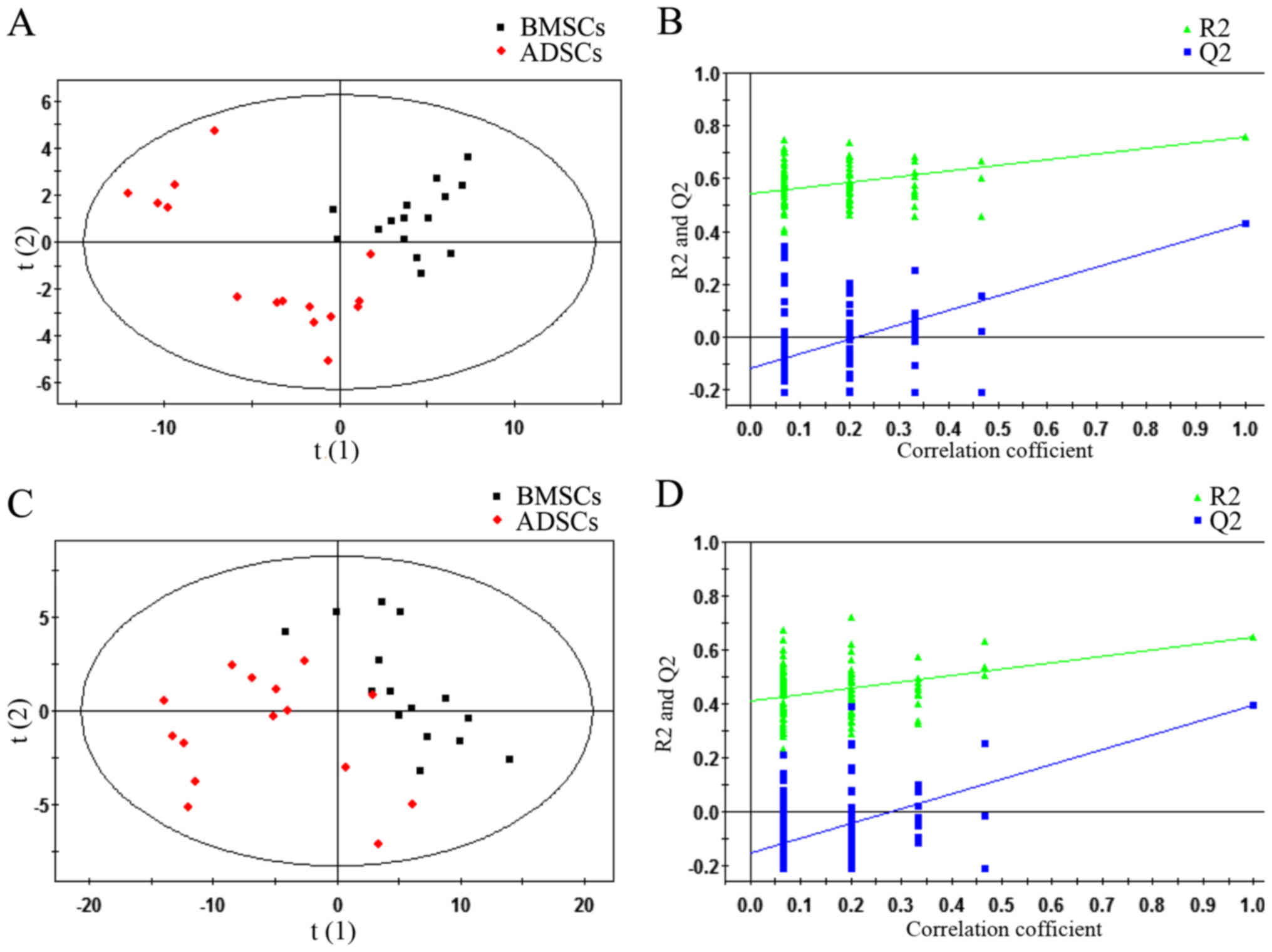 | Figure 2PLS-DA plots and validation plots for
discriminating ADSCs and BMSCs in ESI+ and
ESI− modes. (A) PLS-DA plot in ESI+ mode; (B)
validation plot in ESI+ mode; (C) PLS-DA plot in
ESI− mode; (D) validation plot in ESI− mode.
BMSCs, bone marrow-derived mesenchymal stem cells; PLS-DA, partial
least squares discriminant analysis score; ESI+,
electron spray ionisation in positive; ESI−, electron
spray ionisation in negative; ADSCs, adipose tissue-derived
mesenchymal stem cells; ESI, electron spray ionization; Q2, second
quartile; R2, coefficient of determination; PLS-DA,
partial least squares discriminant analysis score; horizontal axis
t, principal component one; vertical axis t, principal component
two. |
Metabolite profiles of potential
biomarkers differing between BMSCs and ADSCs
Analysis of VIP values revealed discriminatory
metabolites that contributed to the differences between BMSCs and
ADSCs. Based on false discovery rate and VIP thresholds of 0.05 and
1, respectively, differential ions were selected as biomarker
candidates for subsequent metabolite identification. The
identification procedures were similar to strategies previously
published by our group (36,37). In total, 8 metabolites in
ESI+ mode and 8 metabolites in ESI− mode were
identified (Table II). D-lactic
acid, hydroxyindoleacetaldehyde, α-D-glucose, bovinic acid,
9,10-epoxyoctadecenoic acid, glyceraldehyde, phenylpyruvic acid,
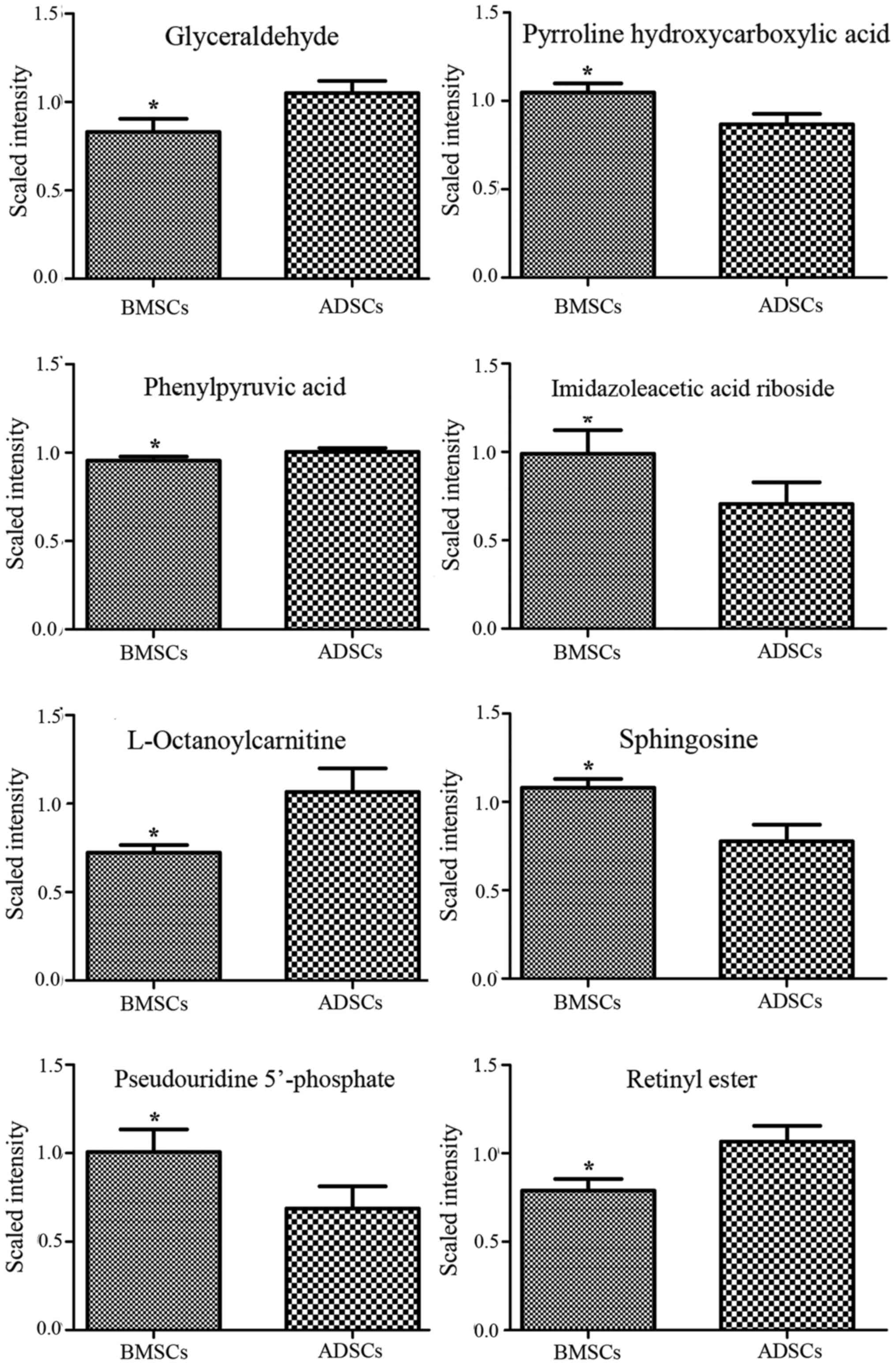
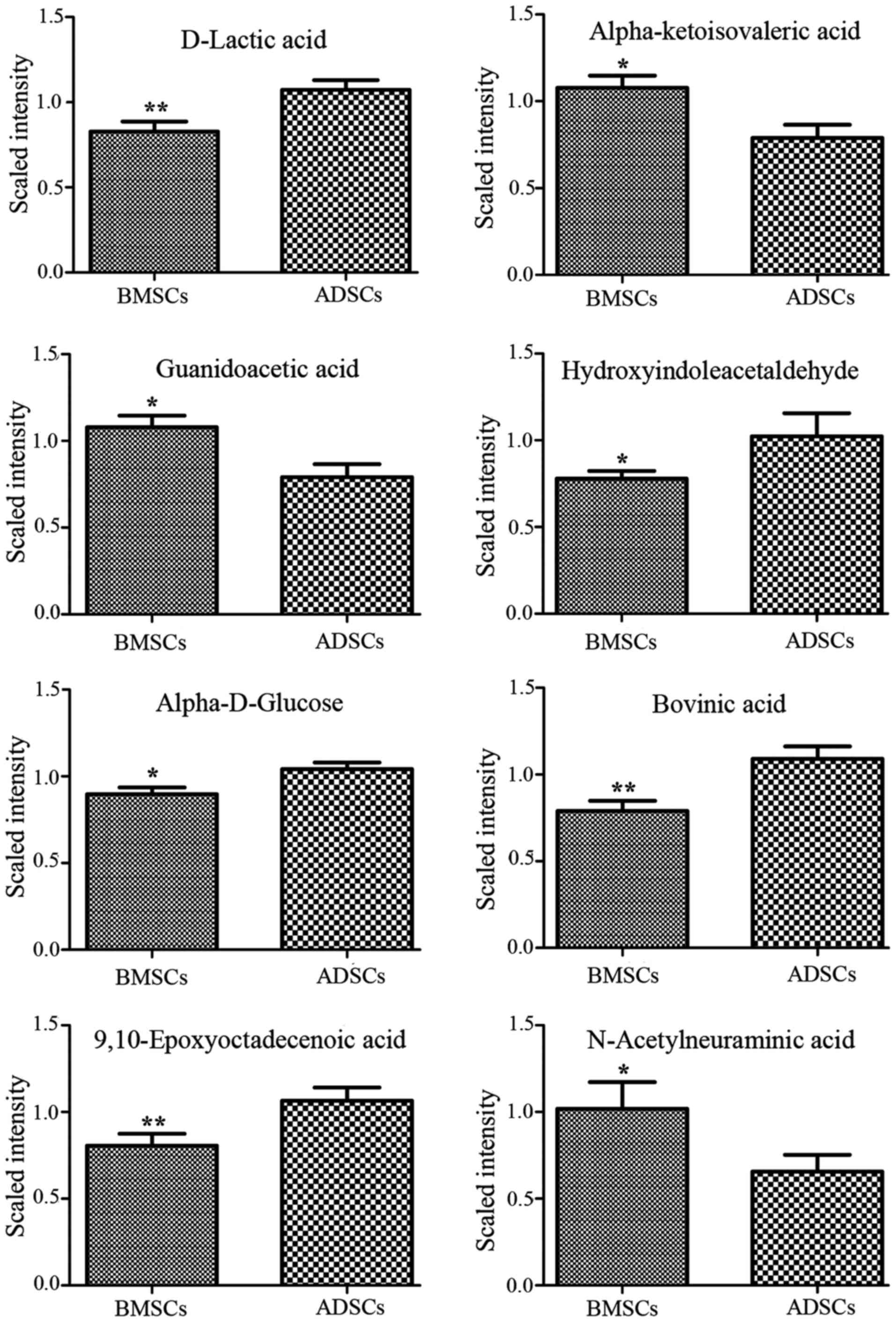
L-octanoylcarnitine and retinyl ester were observed to be elevated
in the supernatant of ADSCs compared with that of BMSCs (Figs. 3Figure 4–5). By contrast, α-ketoisovaleric acid,
guanidoacetic acid, N-acetylneuraminic acid, imidazoleacetic acid
riboside, sphingosine and pseudouridine 5′-phosphate levels were
lower in the supernatant of ADSCs compared with that of BMSCs
(Figs. 3Figure 4–5). The involved biochemical pathways
mapped in the Human Metabolome Database (HMDB) (38) and the Kyoto Encyclopaedia of Genes
and Genomes (KEGG) (39) included
the linoleic acid metabolic pathway, glycerolipid metabolism,
arginine and proline metabolism, mitochondrial β-oxidation of short
chain saturated fatty acids, pyrimidine metabolism, glycine and
serine metabolism, galactose metabolism and amino sugar
metabolism.
 | Table IIDetailed information on 16
supernatant metabolites. |
Table II
Detailed information on 16
supernatant metabolites.
| A, ESI+
mode |
|---|
|
|---|
| ID | Metabolite | m/z | RT (min) | ppm | FCa | P-value | VIP | Pathway |
|---|
| P1 | Glyceraldehyde | 113.0197 | 56.58 | 10 | 0.79 | 0.036203 | 1.299 | Glycerolipid
metabolism |
| P2 | Pyrroline
hydroxycarboxylic acid | 130.0505 | 56.52 | 5 | 1.21 | 0.012093 | 1.8038 | Arginine and
proline metabolism |
| P3 | Phenylpyruvic
acid | 165.0547 | 56.58 | 0 | 0.95 | 0.044253 | 1.0174 | Phenylalanine and
tyrosine metabolism |
| P4 | Imidazoleacetic
acid riboside | 281.0754 | 56.1 | 3 | 1.40 | 0.019103 | 1.4403 | Histidine
metabolism |
| P5 |
L-octanoylcarnitine | 288.217 | 505.38 | 0 | 0.68 | 0.023787 | 1.4542 | Mitochondrial
β-oxidation of short chain saturated fatty acids |
| P6 | Sphingosine | 322.2682 | 840.22 | 10 | 1.39 | 0.019103 | 1.6411 | Sphingolipid
metabolism |
| P7 | Pseudouridine
5′-phosphate | 325.0374 | 56.3 | 17 | 1.47 | 0.048815 | 1.4483 | Pyrimidine
metabolism |
| P8 | Retinyl ester | 325.2118 | 870.735 | 5 | 0.74 | 0.040057 | 1.7041 | Retinol
metabolism |
|
| B, ESI−
mode |
|
| N1 | D-Lactic acid | 89.02636 | 54.65 | 21 | 0.77 | 0.009531 | 1.4873 | Pyruvate
metabolism |
| N2 | α-ketoisovaleric
acid | 115.0404 | 59.73 | 2 | 1.37 | 0.015247 | 1.4307 | Pantothenate and
CoA biosynthesis |
| N3 | Guanidoacetic
acid | 116.044 | 59.7 | 9 | 1.36 | 0.015247 | 1.4643 | Glycine and serine
metabolism |
| N4 |
Hydroxyindoleacetaldehyde | 174.0551 | 465.62 | 5 | 0.76 | 0.040057 | 1.0804 | Tryptophan
metabolism |
| N5 | α-D-glucose | 179.0563 | 51.34 | 0 | 0.86 | 0.026482 | 1.3418 | Galactose
metabolism |
| N6 | Bovinic acid | 279.2292 | 887.24 | 13 | 0.72 | 0.004494 | 1.641 | Linoleic acid
metabolic pathway |
| N7 |
9,10-Epoxyoctadecenoic acid | 295.2244 | 722.755 | 11 | 0.76 | 0.002637 | 1.349 | Linoleic acid
metabolic pathway |
| N8 | N-acetylneuraminic
acid | 308.0994 | 51.19 | 2 | 1.55 | 0.044253 | 1.1037 | Amino sugar
metabolism |
| N9 | 11,13-EpOME | 295.2244 | 722.755 | 11 | 0.76 | 0.002637 | 1.349 | Linoleic acid
metabolic pathway |
Discussion
The results of the Scandinavian Simvastatin Survival
Study were published in The Lancet 20 years ago (40). At present, dyslipidemia is major
cause of atherosclerotic vascular disease. Recent studies further
underlined the significance of dyslipidemia in cardiovascular
disease through performing research on lipids [high-density
lipoprotein cholesterol (HDL-C), low-density lipoprotein
cholesterol (LDL-C) and triglycerides] and cardiovascular disease
(41–43). Autologous MSC transplantation has
emerged as a novel treatment for atherosclerosis-associated
diseases, and pilot studies have demonstrated a promising clinical
effect for this treatment strategy. However, the relative
efficacies of BMSC- and ADSC-based cellular therapies for
atherosclerosis-associated diseases have remained largely elusive.
Establishing the metabolic signatures of these cell types will be
helpful for understanding differences between them and be of
significance for the development of clinical treatments. The
present results regarding unknown and annotated analytes indicated
that the supernatant of ADSCs contained significantly different
levels of metabolites compared with BMSCs. Of note, the metabolites
accounting for the differences between the supernatants of ADSCs
and BMSCs were matched with known human metabolites in the HMDB
(hmdb.ca/) or KEGG (kegg.jp/kegg/pathway.html), and these results were
further confirmed by a manual search for similarities between the
annotated and the library spectra for each metabolite.
Overall, the results of the present metabolite
pathway enrichment analysis retrieved 15 potential pathways that
were considered to be different between ADSCs and BMSCs. Two
annotated metabolites included bovinic acid and
9,10-epoxyoctadecenoic acid, which are components of the linoleic
acid pathway. The linoleic acid pathway contains 15 metabolites and
participates in protecting the body against disease states such as
atherosclerosis, thrombosis, diabetes, high blood pressure, skin
inflammation, aging and cancer. Bovinic acid is a predominant
conjugated linoleic acid (CLA) in human adipose tissue, comprising
a group of fatty acids with 18 carbon atoms, and has
anti-atherogenic and anticarcinogenic activities (44,45). As the pathophysiological process
of atherosclerosis is complex and involves numerous cellular
pathways, reversal of particular pathways may not be sufficient for
the prevention of the disease. However, studies suggested that
administration of CLA may be associated with the regression of
atherosclerosis in rabbits (46)
and other animal models (47).
Evidence from a patient study has demonstrated that CLA has
anti-inflammatory effects via the reduction of oxidative stress
(48).
Several studies have also demonstrated that
consumption of CLA reduced the fat mass or the percentage of body
fat in healthy and in obese/overweight adults (49–51). As such, the conclusions from
meta-analyses of previous patient studies were that intake of CLA
reduced body weight and body-fat mass (52). The potential mechanisms of action
of CLA may involve metabolic effects of inhibiting lipogenesis and
accelerating lipolysis (53). Via
interactions with the peroxisome proliferator-activated receptors
(PPARs), CLA has been proven to initiate the transcription of genes
associated with the differentiation of adipocytes, which involve
lipolysis (β-oxidation) and mitochondrial biogenesis (54). Of note, the activation of PPARγ
was associated with delayed progression of atherosclerosis and
dyslipidemia. In addition, a recent study confirmed that the
effects of CLA against inflammation were mainly mediated via the
inhibition of nuclear factor-κB and mitogen-activated protein
kinase signalling pathways (55).
Furthermore, clinical studies have reported that CLA
may provide a great benefit for human health. An inverse
association between cis-9, trans-11 CLA and the risk
of myocardial infarction has been detected among Costa Rican
subjects (56). Another human
study drew a similar conclusion, namely that intake of CLA
increased HDL-C and reduced the LDL-C/HDL-C ratio in type 2
diabetic patients (57). In
addition, CLA was also reported to improve insulin sensitivity in
young patients, which was correlated with decreased fasting insulin
levels (58). A clinical trial
indicated an effect of CLA on Crohn's disease, where intake of 6 g
CLA/day for 12 weeks improved inflammatory bowel disease
questionnaire responses and decreased the Crohn's disease activity
index (59). In the Swedish
Mammography Cohort study, intake of CLA was demonstrated to reduce
the risk of colorectal cancer by 13% and the risk of distal colon
cancer by 34% (60). In a study
on breast cancer patients, CLA inhibited tumour metastasis in
premenopausal women (61,62). In South African children, the
potential preventive effects of CLA on laryngeal papillomatosis
have been reported, which may cause airway obstruction in young
children (63). The
abovementioned patient studies indicated the potential application
of CLA in cardiovascular diseases, metabolic syndrome, immune
system diseases and cancer, either alone or complementary to
present treatments.
BMSCs have been proposed as a cell source for
atherosclerosis therapy. However, ADSCs have emerged as a novel
cell source with easy accessibility, and they may be collected from
elderly patients with less injury than bone marrow. In addition, in
elderly patients, BMSCs reside in the bone marrow stroma in smaller
quantities compared with those in young patients, whereas the
amount of ADSCs is often greater due to the dramatic increase in
the incidence of obesity worldwide. Both cell types are well
tolerated by humans. However, the relative efficacies of BMSC- and
ADSC-based stem cell therapies for patients with
atherosclerosis-associated diseases, such as CHD, remain to be
determined. A recent study suggested that ADSC transfusion was
associated with a repressed increase in body weight and improved
dyslipidemia in obese mice (64).
In addition, CLA has been proven to stimulate lipolysis in human
adipocytes and diminish the synthesis of fatty acids, although the
specific mechanisms remain to be determined (65). Furthermore, ADSCs have been
suggested to be more immunosuppressive than BMSCs, as ADSCs are
associated with a more marked inhibition of the expression of
functionally important co-stimulatory molecules on the surface of
monocyte-derived dendritic cells (66). The results of the present study
suggested that ADSCs may possibly act upon adipose tissue via the
production of CLA and participate in the linoleic acid pathway,
which may provide additional treatment effects as compared with
BMSCs.
Nevertheless, there are some limitations of the
present study. The study enrolled 30 patients, all of which were
elderly, and used the BMSCs from 15 of them and the ADSCs from the
other 15 patients. However, it may have been appropriate to assess
the ADSCs and BMSCs from the same patient and then determine the
differences in metabolites. Therefore, based on the study design,
it cannot be excluded that the differences in metabolites between
ADSCs and BMSCs may have been due to them being taken from two
different populations/groups. Furthermore, no control group was
used, such as a group of younger patients for comparison.
In conclusion, the results of the present study
revealed a marked difference regarding the metabolic
characteristics of ADSCs and BMSCs. ADSCs exhibited differences
regarding components of the linoleic acid pathway, including
bovinic acid, 12,13-EpOME, 13-hydroxyoctadecadienoic acid and
9,10-epoxyoctadecenoic acid as compared with BMSCs. These results
enhanced the current understanding of the metabolic differences
between ADSCs and BMSCs and may represent the underlying mechanisms
responsible for the different efficacies of ADSC- and BMSC-based
stem cell therapies for atherosclerosis-associated diseases.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81471805), Chinese
Postdoctoral Science Foundation (grant no. 2014M551272),
Postdoctoral Science Foundation of Heilongjiang Province (grant no.
LBH-Z14135), Scientific Research Project of Educational Department
of Heilongjiang Province (grant no. 12541434), Merit Aid Program
for Returnees of Human Resource Department of Heilongjiang Province
(2014; grant no. 454), Harbin Municipal Science and Technology
Research Fund of Innovative Talents Project (grant no.
RC2016QN004036), 'Yu Weihan' Outstanding Young Investigator Award
(2014) and Earl Bakken Scholarship (2016) to K.K. The authors would
like to thank Ms. Li Ruiting (Key Laboratory of Drug Quality
Control and Pharmacovigilance, China Pharmaceutical University,
Ministry of Education, Nanjing, China) and Ms. Sun Meng (Key
Laboratory of Education of the Ministry for Myocardial Ischemia,
The Second Affiliated Hospital of Harbin Medical University,
Harbin, China) for technical and scientific advice.
Abbreviations:
|
CHD
|
coronary heart disease
|
|
MSCs
|
mesenchymal stromal cells
|
|
ADSCs
|
adipose tissue-derived mesenchymal
stem cells
|
|
BMSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
QC
|
quality control
|
|
PCA
|
principal component analysis
|
|
PLS-DA
|
partial least squares discriminant
analysis
|
|
m/z
|
measured mass to charge ratio
|
|
ESI
|
electron spray ionization
|
|
VIP
|
variable importance in the
projection
|
|
HMDB
|
Human Metabolome Database
|
|
KEGG
|
Kyoto Encyclopaedia of Genes and
Genomes
|
|
CLA
|
conjugated linoleic acid
|
|
PPARs
|
peroxisome proliferator-activated
receptors
|
References
|
1
|
Mozaffarian Go AS, Roger D, Benjamin VL,
Berry EJ, Blaha JD, Dai MJ, Ford S, Fox ES, Franco CSS, et al:
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Executive summary: heart disease and
stroke statistics - 2014 update: a report from the American Heart
Association. Circulation. 129:399–410. 2014. View Article : Google Scholar
|
|
2
|
Kelly BB, Narula J and Fuster V:
Recognizing global burden of cardiovascular disease and related
chronic diseases. Mt Sinai J Med. 79:632–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muka T, Imo D, Jaspers L, Colpani V,
Chaker L, van der Lee SJ, Mendis S, Chowdhury R, Bramer WM, Falla
A, et al: The global impact of non-communicable diseases on
healthcare spending and national income: A systematic review. Eur J
Epidemiol. 30:251–277. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guilherme A, Virbasius JV, Puri V and
Czech MP: Adipocyte dysfunctions linking obesity to insulin
resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 9:367–377.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al: Chronic
inflammation in fat plays a crucial role in the development of
obesity-related insulin resistance. J Clin Invest. 112:1821–1830.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ng M, Fleming T, Robinson M, Thomson B,
Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF,
et al: Global, regional, and national prevalence of overweight and
obesity in children and adults during 1980–2013: A systematic
analysis for the Global Burden of Disease Study 2013. Lancet.
384:766–781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whitman SC: A practical approach to using
mice in atherosclerosis research. Clin Biochem Rev. 25:81–93.
2004.
|
|
8
|
Cannon CP, Harrington RA, James S,
Ardissino D, Becker RC, Emanuelsson H, Husted S, Katus H, Keltai M,
Khurmi NS, et al: PLATelet inhibition and patient outcomes
investigators: Comparison of ticagrelor with clopidogrel in
patients with a planned invasive strategy for acute coronary
syndromes (PLATO): A randomised double-blind study. Lancet.
375:283–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meier P and Timmis A: Almanac 2012:
interventional cardiology: the national society journals present
selected research that has driven recent advances in clinical
cardiology. Heart. 98:1701–1709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gómez-Gaviro MV, Lovell-Badge R,
Fernández-Avilés F and Lara-Pezzi E: The vascular stem cell niche.
J Cardiovasc Transl Res. 5:618–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klein D, Weisshardt P, Kleff V, Jastrow H,
Jakob HG and Ergün S: Vascular wall-resident CD44+
multipotent stem cells give rise to pericytes and smooth muscle
cells and contribute to new vessel maturation. PLoS One.
6:e205402011. View Article : Google Scholar
|
|
12
|
Zengin E, Chalajour F, Gehling UM, Ito WD,
Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N and Ergün S:
Vascular wall resident progenitor cells: A source for postnatal
vasculogenesis. Development. 133:1543–1551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caplan AI: Mesenchymal stem cells. Stem
Cells Transl Med. 6:1445–1451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krampera M, Glennie S, Dyson J, Scott D,
Laylor R, Simpson E and Dazzi F: Bone marrow mesenchymal stem cells
inhibit the response of naive and memory antigen-specific T cells
to their cognate peptide. Blood. 101:3722–3729. 2003. View Article : Google Scholar
|
|
15
|
Caplan AI: Adult mesenchymal stem cells
for tissue engineering versus regenerative medicine. J Cell
Physiol. 213:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kode JA, Mukherjee S, Joglekar MV and
Hardikar AA: Mesenchymal stem cells: Immunobiology and role in
immunomodulation and tissue regeneration. Cytotherapy. 11:377–391.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prockop DJ, Brenner M, Fibbe WE, Horwitz
E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L and Keating A:
Defining the risks of mesenchymal stromal cell therapy.
Cytotherapy. 12:576–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tolar J, Le Blanc K, Keating A and Blazar
BR: Concise review: Hitting the right spot with mesenchymal stromal
cells. Stem Cells. 28:1446–1455. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mirza A, Hyvelin JM, Rochefort GY,
Lermusiaux P, Antier D, Awede B, Bonnet P, Domenech J and Eder V:
Undifferentiated mesenchymal stem cells seeded on a vascular
prosthesis contribute to the restoration of a physiologic vascular
wall. J Vasc Surg. 47:1313–1321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao
BS, Wang K, Chu B and Li S: Antithrombogenic property of bone
marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc
Natl Acad Sci USA. 104:11915–11920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gimble JM, Guilak F and Bunnell BA:
Clinical and preclinical translation of cell-based therapies using
adipose tissue-derived cells. Stem Cell Res Ther. 1:192010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Ugarte DA, Morizono K, Elbarbary A,
Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim
P, et al: Comparison of multi-lineage cells from human adipose
tissue and bone marrow. Cells Tissues Organs. 174:101–109. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh
K, Bae YC and Jung JS: Characterization and expression analysis of
mesenchymal stem cells from human bone marrow and adipose tissue.
Cell Physiol Biochem. 14:311–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen
P, Ma K and Zhou C: Comparative analysis of mesenchymal stem cells
from bone marrow, cartilage, and adipose tissue. Stem Cells Dev.
17:761–773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Khan D, Delling J and Tobiasch E:
Mechanisms underlying the osteo- and adipo-differentiation of human
mesenchymal stem cells. Sci World J. 793823:2012. View Article : Google Scholar
|
|
26
|
Mueller SM and Glowacki J: Age-related
decline in the osteogenic potential of human bone marrow cells
cultured in three-dimensional collagen sponges. J Cell Biochem.
82:583–590. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stenderup K, Justesen J, Clausen C and
Kassem M: Aging is associated with decreased maximal life span and
accelerated senescence of bone marrow stromal cells. Bone.
33:919–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim Y, Kim H, Cho H, Bae Y, Suh K and Jung
J: Direct comparison of human mesenchymal stem cells derived from
adipose tissues and bone marrow in mediating neovascularization in
response to vascular ischemia. Cell Physiol Biochem. 20:867–876.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Steenberghe M, Schubert T, Guiot Y,
Goebbels RM and Gianello P: Improvement of mesh recolonization in
abdominal wall reconstruction with adipose vs. bone marrow
mesenchymal stem cells in a rodent model. J Pediatr Surg.
52:1355–1362. 2017. View Article : Google Scholar
|
|
30
|
Rasmussen JG, Frøbert O, Holst-Hansen C,
Kastrup J, Baandrup U, Zachar V, Fink T and Simonsen U: Comparison
of human adipose-derived stem cells and bone marrow-derived stem
cells in a myocardial infarction model. Cell Transplant.
23:195–206. 2014. View Article : Google Scholar
|
|
31
|
Razavi S, Zarkesh-Esfahani H, Morshed M,
Vaezifar S, Karbasi S and Golozar MA: Nanobiocomposite of
poly(lactide-co-glycolide)/chitosan electrospun scaffold can
promote proliferation and transdifferentiation of Schwann-like
cells from human adipose-derived stem cells. J Biomed Mater Res A.
103:2628–2634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuhl C, Tautenhahn R, Böttcher C, Larson
TR and Neumann S: CAMERA: An integrated strategy for compound
spectra extraction and annotation of liquid chromatography/mass
spectrometry data sets. Anal Chem. 84:283–289. 2012. View Article : Google Scholar
|
|
33
|
Trygg J, Holmes E and Lundstedt T:
Chemometrics in metabonomics. J Proteome Res. 6:469–479. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Velzen EJ, Westerhuis JA, van
Duynhoven JP, van Dorsten FA, Hoefsloot HC, Jacobs DM, Smit S,
Draijer R, Kroner CI and Smilde AK: Multilevel data analysis of a
crossover designed human nutritional intervention study. J Proteome
Res. 7:4483–4491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fong MY, McDunn J and Kakar SS:
Identification of metabolites in the normal ovary and their
transformation in primary and metastatic ovarian cancer. PLoS One.
6:e199632011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ke C, Hou Y, Zhang H, Yang K, Wang J, Guo
B, Zhang F, Li H, Zhou X, Li Y, et al: Plasma Metabolic Profiles in
Women are Menopause Dependent. PLoS One. 10:e01417432015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang T, Wu X, Yin M, Fan L, Zhang H, Zhao
F, Zhang W, Ke C, Zhang G, Hou Y, et al: Discrimination between
malignant and benign ovarian tumors by plasma metabolomic profiling
using ultra performance liquid chromatography/mass spectrometry.
Clin Chim Acta. 413:861–868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wishart DS, Jewison T, Guo AC, Wilson M,
Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, et al: HMDB
3.0 - The Human Metabolome Database in 2013. Nucleic Acids Res.
41:D801–D807. 2013. View Article : Google Scholar
|
|
39
|
Kanehisa M, Goto S, Sato Y, Kawashima M,
Furumichi M and Tanabe M: Data, information, knowledge and
principle: Back to metabolism in KEGG. Nucleic Acids Res.
42:D199–D205. 2014. View Article : Google Scholar :
|
|
40
|
No authors listed. Randomised trial of
cholesterol lowering in 4444 patients with coronary heart disease:
The Scandinavian Simvastatin Survival Study (4S). Lancet.
344:1383–1389. 1994.PubMed/NCBI
|
|
41
|
Nordestgaard BG and Varbo A: Triglycerides
and cardiovascular disease. Lancet. 384:626–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rader DJ and Hovingh GK: HDL and
cardiovascular disease. Lancet. 384:618–625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ridker PM: LDL cholesterol: Controversies
and future therapeutic directions. Lancet. 384:607–617. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Campbell B and Kreider RB: Conjugated
linoleic acids. Curr Sports Med Rep. 7:237–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Loscher CE, Draper E, Leavy O, Kelleher D,
Mills KH and Roche HM: Conjugated linoleic acid suppresses NF-kappa
B activation and IL-12 production in dendritic cells through
ERK-mediated IL-10 induction. J Immunol. 175:4990–4998. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kritchevsky D, Tepper SA, Wright S,
Czarnecki SK, Wilson TA and Nicolosi RJ: Conjugated linoleic acid
isomer effects in atherosclerosis: Growth and regression of
lesions. Lipids. 39:611–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Toomey S, Harhen B, Roche HM, Fitzgerald D
and Belton O: Profound resolution of early atherosclerosis with
conjugated linoleic acid. Atherosclerosis. 187:40–49. 2006.
View Article : Google Scholar
|
|
48
|
Hassan Eftekhari M, Aliasghari F,
Babaei-Beigi MA and Hasanzadeh J: Effect of conjugated linoleic
acid and omega-3 fatty acid supplementation on inflammatory and
oxidative stress markers in atherosclerotic patients. ARYA
Atheroscler. 9:311–318. 2013.
|
|
49
|
Dilzer A and Park Y: Implication of
conjugated linoleic acid (CLA) in human health. Crit Rev Food Sci
Nutr. 52:488–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McCrorie TA, Keaveney EM, Wallace JM,
Binns N and Livingstone MB: Human health effects of conjugated
linoleic acid from milk and supplements. Nutr Res Rev. 24:206–227.
2011. View Article : Google Scholar
|
|
51
|
Onakpoya IJ, Posadzki PP, Watson LK,
Davies LA and Ernst E: The efficacy of long-term conjugated
linoleic acid (CLA) supplementation on body composition in
overweight and obese individuals: A systematic review and
meta-analysis of randomized clinical trials. Eur J Nutr.
51:127–134. 2012. View Article : Google Scholar
|
|
52
|
Risérus U, Berglund L and Vessby B:
Conjugated linoleic acid (CLA) reduced abdominal adipose tissue in
obese middle-aged men with signs of the metabolic syndrome: a
randomised controlled trial. Int J Obes Relat Metab Disord.
25:1129–1135. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Churruca I, Fernández-Quintela A and
Portillo MP: Conjugated linoleic acid isomers: Differences in
metabolism and biological effects. Biofactors. 35:105–111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abduljabbar R, Al-Kaabi MM, Negm OH,
Jerjees D, Muftah AA, Mukherjee A, Lai CF, Buluwela L, Ali S, Tighe
PJ, et al: Prognostic and biological significance of peroxisome
proliferator-activated receptor-gamma in luminal breast cancer.
Breast Cancer Res Treat. 150:511–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang WC, Tu RS, Chen YL, Tsai YY, Lin CF
and Liou CJ: Conjugated linoleic acids suppress inflammatory
response and ICAM-1 expression through inhibition of NF-κB and MAPK
signaling in human bronchial epithelial cells. Food Funct.
7:2025–2033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Smit LA, Baylin A and Campos H: Conjugated
linoleic acid in adipose tissue and risk of myocardial infarction.
Am J Clin Nutr. 92:34–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Moloney F, Yeow TP, Mullen A, Nolan JJ and
Roche HM: Conjugated linoleic acid supplementation, insulin
sensitivity, and lipoprotein metabolism in patients with type 2
diabetes mellitus. Am J Clin Nutr. 80:887–895. 2004.PubMed/NCBI
|
|
58
|
Eyjolfson V, Spriet LL and Dyck DJ:
Conjugated linoleic acid improves insulin sensitivity in young,
sedentary humans. Med Sci Sports Exerc. 36:814–820. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bassaganya-Riera J, Hontecillas R, Horne
WT, Sandridge M, Herfarth HH, Bloomfeld R and Isaacs KL: Conjugated
linoleic acid modulates immune responses in patients with mild to
moderately active Crohn's disease. Clin Nutr. 31:721–727. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Larsson SC, Bergkvist L and Wolk A:
High-fat dairy food and conjugated linoleic acid intakes in
relation to colorectal cancer incidence in the Swedish Mammography
Cohort. Am J Clin Nutr. 82:894–900. 2005.PubMed/NCBI
|
|
61
|
McCann SE, Ip C, Ip MM, McGuire MK, Muti
P, Edge SB, Trevisan M and Freudenheim JL: Dietary intake of
conjugated linoleic acids and risk of premenopausal and
postmenopausal breast cancer, Western New York Exposures and Breast
Cancer Study (WEB Study). Cancer Epidemiol Biomarkers Prev.
13:1480–1484. 2004.PubMed/NCBI
|
|
62
|
Moon HS: Biological effects of conjugated
linoleic acid on obesity-related cancers. Chem Biol Interact.
224:189–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Louw L: Effects of conjugated linoleic
acid and high oleic acid safflower oil in the treatment of children
with HPV-induced laryngeal papillomatosis: A randomized,
double-blinded and crossover preliminary study. Lipids Health Dis.
11:1362012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu GY, Liu J, Wang YL, Liu Y, Shao Y, Han
Y, Qin YR, Xiao FJ, Li PF, Zhao LJ, et al: Adipose-derived
mesenchymal stem cells ameliorate lipid metabolic disturbance in
mice. Stem Cells Transl Med. 5:1162–1170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Martins SV, Madeira A, Lopes PA, Pires VM,
Alfaia CM, Prates JA, Moura T and Soveral G: Adipocyte membrane
glycerol permeability is involved in the anti-adipogenic effect of
conjugated linoleic acid. Biochem Biophys Res Commun. 458:356–361.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ivanova-Todorova E, Bochev I, Mourdjeva M,
Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I and
Kyurkchiev DS: Adipose tissue-derived mesenchymal stem cells are
more potent suppressors of dendritic cells differentiation compared
to bone marrow-derived mesenchymal stem cells. Immunol Lett.
126:37–42. 2009. View Article : Google Scholar : PubMed/NCBI
|