Introduction
Previous studies have suggested that somatic cells
from mice and humans may be reprogrammed to become induced
pluripotent stem cells (iPSCs) (1,2).
The reprogrammed cells were derived from a variety of different
tissues and organs, including fibroblasts, keratinocytes and urine
renal epithelial cells (3–7).
The human skin fibroblast (HSF)-iPSCs demonstrated clear
similarities with embryonic stem cells (ESCs) in their
proliferation, pluripotency, clonal morphology, growth
characteristics, surface markers, gene expression and epigenetics.
HSF-iPSCs provide a reliable source of skin seed cells for specific
patients, including individuals with large area burns or skin
defects (8,9). HSF-iPSCs do not have the same
ethical or immune rejection issues that are associated with ESCs
(8,9). iPSCs may also be used in drug
screening and experiments to aid the understanding of specific
disease mechanisms (10–12).
In previous studies, somatic cells were transduced
with vectors containing the human transcription factors NANOG,
octamer-binding transcription factor 4 (OCT4), sex-determining
region Y box 2 (SOX2), Kruppel-like factor 4 (Klf4) and c-Myc,
which subsequently reprogrammed them to become iPSCs (1,2).
Initially, lentivirus and retrovirus were used as vectors to
introduce key transcription factors into target cells and thus
induce reprogramming into iPSCs (13,14). However, lentivirus and retrovirus
are integrating viruses (15),
meaning that the virus gene may insert into the genome of the
target cells, potentially causing the reactivation of transgenes,
uncontrolled gene silencing and residual expression. These
alterations may lead to undesirable consequences, which makes them
unsuitable within a clinical setting (16). Using a non-integrating method is
considered more appropriate and may eliminate the problem of
insertional mutagenesis (17).
Previous research into epigenetics has confirmed
that iPSCs retain the memory of epigenetic signatures from their
original tissue and are more likely to differentiate toward
donor-associated cells (18).
Certain imprinted genes associated with growth, metabolism and
neurological development of iPSCs and initial somatic cells share
the same epigenetical and transcriptional statuses (19). The memory of the epigenetics may
limit the full differentiation potential of iPSCs (18). In the present study, a
non-integrating method was used to reprogram cells from the skin of
patients with burns to generate iPSCs. The results of the present
study may provide an experimental basis for the clinical use of
iPSCs as seed cells.
Materials and methods
Isolation of fibroblasts and cell
culture
Human dermal tissues were harvested from residual
skin used for skin grafts in patients with burns. All protocols
were approved by the Biomedical Ethics Committee of the Affiliated
Hospital of Nanchang University (Nanchang, China) and written
informed consent was obtained from all patients. Tissues were
washed 2–3 times with penicillin/streptomycin and
phosphate-buffered saline (PBS) (all from Beijing Solarbio Science
and Technology Co., Ltd., Beijing, China) within a vertical clean
bench. The tissues were subsequently cut into 2×10 mm sections. The
tissues were digested with 0.25% trypsin-ethylenediaminetetraacetic
acid (EDTA) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 4°C for ~5 h. High glucose Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS; both
from HyClone; GE Healthcare Life Sciences, Logan, UT, USA) was
added to terminate the digestion process. The epidermis and dermis
were separated using tweezers and dermal tissues were sliced into
sections (0.5–1 mm3). The distance between each section
was 0.3–0.5 cm, from which the fibroblasts came out. Culture dishes
containing the dermal tissues were inverted for drying for 4 h,
following which high glucose DMEM with 10% FBS was added and
cultured at 37°C for 24 h in an atmosphere containing 5%
CO2. The medium was replaced every 3 days. After 24 days
cultured, the fibroblasts were isolated from the dermal sections by
0.25% trypsin-EDTA digestion.
Passage of fibroblasts
To prevent cell density from inhibiting growth and
to obtain a larger number of proliferating cells, cells were
subcultured when they reached 80–90% confluence. The medium was
replaced and cells were detached with 0.5 ml 0.25% trypsin-EDTA
solution following washing with PBS. When the intercellular space
increased and cells appeared round under an inverted phase contrast
microscope (CTR6000; Leica Microsystems GmbH, Wetzlar, Germany),
high glucose DMEM with 10% FBS was added to terminate digestion.
The cells were centrifuged at 120 × g for 5 min at room
temperature. The supernatant was removed and the remaining cell
deposit was resuspended in 9 ml high glucose DMEM with 10% FBS and
cultured at 37°C with 5% CO2 following being subcultured
at a rate of 1:3. The medium was replaced every 3 days.
Transduction of HSFs and generation of
HSFs-iPSCs
At 2 days prior to transduction, HSFs at passage 3
were harvested and inoculated into 2 wells (1 well for counting and
the other for transduction; 2.5×105 cells/well) of a
6-well plate with high glucose DMEM and 10% FBS. The cells were
completely adhered and stretched on the day of transduction. When
cells reached 50–80% confluence thy were transduced using the
CytoTune™-iPS 2.0 Sendai reprogramming vectors (ID Pharma Co.,
Ltd., Tsukuba, Japan) containing the human transcription factors
NANOG, OCT4 and SOX2 (10 µl of each transcription tube).
Cells were incubated at 37°C in an atmosphere containing 5%
CO2 overnight and the medium was replaced on the first
day with fresh high glucose DMEM and 10% FBS to remove the Sendai
reprogramming vectors. Following this, the medium was replaced
every 2 days. At 6 days following transduction, the 6-well plates
were coated with 1% Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA). At day 7 post-transduction, the cells were detached with 0.5
ml 0.25% trypsin-EDTA solution following washing with PBS. When the
cells were observed as round under the inverted phase contrast
microscope, high glucose DMEM with 10% FBS was added to terminate
the digestion. Cells were centrifuged at 200 × g for 4 min at room
temperature, following which the cell pellet was resuspended in 1
ml high glucose DMEM with 10% FBS. The transduced cells were
subsequently cultured in high glucose DMEM with 10% FBS under
feeder-free conditions with 1% Matrigel-coated culture dish at a
density of 1–5×105 cells/100 mm at 37°C overnight in an
atmosphere containing 5% CO2. The medium was discarded
the next day and replaced with reprogramming culture medium
(ReproEasy; Beijing Cellapy Biotechnology Co., Ltd., Beijing,
China). The medium was replaced every day and the culture was
monitored until ESC-like colonies were observed under the inverted
phase contrast microscope. At 4 weeks following transduction, the
cell colonies were large and compacted enough to be picked out and
expanded. They covered the majority of the surface area of the
culture dish. The colonies were picked and transferred onto fresh
1% Matrigel-coated dishes with human PSCeasy medium (Beijing
Cellapy Biotechnology Co., Ltd.) for expansion.
Picking out and transferring iPSCs
colonies
Using a 1 ml syringe, cell colonies were broken into
pieces and transferred onto a 1% Matrigel-coated 6-well plate
containing 1 ml human PSCeasy medium. Plates were incubated at 37°C
in a humidified atmosphere containing 5% CO2. At 48 h
post-transfer, colonies were attached to the culture plate and the
medium was replaced. Following this, the medium was replaced every
day. When the colonies covered 80–90% of the surface area of the
culture plate they were considered ready for passaging.
Alkaline phosphatase (AP) staining
AP staining was performed using the BCIP/NBT
Alkaline Phosphatase Color Development kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol. Briefly, iPSCs were washed with PBS 3 times for 3 min
each time. The iSPCs were fixed at room temperature in 4%
paraformaldehyde (Beijing Dingguo Changsheng Biotechnology Co.,
Ltd., Beijing, China) for 20 min and washed again in PBS. BCIP/NBT
solution was added to stain the iPSCs at room temperature for 20
min and the reaction was terminated by washing the cells twice with
distilled water. Images were captured under an inverted phase
contrast microscope (magnification, ×100).
Immunofluorescence staining
A round coverslip was coated with 1% Matrigel
overnight at 4°C. The following day it was picked out and iPSC
colonies were transferred onto it. The colonies were subsequently
cultured with human PSCeasy medium at 37°C for 48 h. When the
colonies covered the majority of the surface area, they were
considered ready for immunofluorescence staining. The iPSC colonies
were fixed with 4% paraformaldehyde at room temperature for 15 min
and subsequently washed 3 times with PBS. Colonies were treated
with 0.5% Triton X-100 for 15 min at room temperature and blocked
at room temperature using 3% bovine serum albumin (BSA;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 30 min.
Following removal of the blocking buffer, colonies were incubated
with primary antibodies directed against, OCT4 (sc-9081; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), TRA181 (MAB4381; EMD
Millipore, Billerica, MA, USA), NANOG (ab109250; Abcam, Cambridge,
UK), SSEA-4 (sc-21704; Santa Cruz Biotechnology, Inc.), SOX2
(630802; BioLegend, Inc., San Diego, CA, USA) and TRA-160
(sc-21705; Santa Cruz Biotechnology, Inc.) (all 1:100) at 4°C
overnight with a blocking buffer (3% BSA). Following washing with
PBS three times, the colonies were incubated with secondary
antibodies conjugated with Alexa Fluor 488 (A-11034) or Alexa Fluor
594 (A-11032) (1:100; both from Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 50 min with a 3% BSA blocking buffer.
The colonies were washed with PBS three times and a mounting medium
with DAPI (Shanghai Yeasen Biotechnology Co., Ltd., Shanghai,
China) was used to stain the cell nuclei. Images were captured
within 30 min using the inverted phase contrast microscope.
Karyotyping
When iPSCs cultured in 60 mm dishes reached 80–90%
confluence, they were treated with 50 ng/ml colcemid (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) for 7 h at 37°C before
being harvested using trypsin-EDTA solution. The iPSCs were
resuspended in 0.075 M KCl and incubated at 37°C for 20–40 min,
then fixed in 3:1 methanol: glacial acidic acid (both from
Sinopharm Chemical Reagent Co., Ltd.) at room temperature for 10
min. iPSCs were then centrifuged and fixed three times for (15 min
at room temperature, 15 min at room temperature and overnight at
4°C). The harvested cells were stained with Giemsa (Sinopharm
Chemical Reagent Co., Ltd.) at room temperature for 5 min.
VideoTesT-Karyo 3.1 software (Leica Microsystems GmbH) was used to
analyze the karyotype of iPSCs at passage 16.
Differentiation of iPSCs in vitro
When the iPSCs cultured in 6-well plates reached
80–90% confluence, they were harvested using EDTA solution (Beijing
Cellapy Biotechnology Co., Ltd.) and resuspended with a
differentiation medium [high glucose DMEM; 2 mM L-glutamine; 0.1 mM
nonessential amino acid (Invitrogen; Thermo Fisher Scientific,
Inc.); 0.1 mM b-mercaptoethanol (Sigma-Aldrich; Merck KGaA); 20%
FBS] at 37°C with 5% CO2. The medium was refreshed every
3 days and the iPSCs were cultured in suspension. Following 7 days
in suspension culture, embryoid bodies (EBs) had formed. The
following day the EBs were transferred to 1% Matrigel-coated 6-well
plates and cultured in the high glucose DMEM for 7 days at 37°C
with 5% CO2. The medium was refreshed every 2 days. The
cells were harvested and specific gene expression was measured by
polymerase chain reaction (PCR). This was done to demonstrate that
the cells underwent spontaneous differentiation.
PCR
Following 14 days of incubation as described above,
genomic DNA from iPSCs and EBs was extracted using a TIANamp
Genomic DNA kit (Tiangen Biotech Co., Ltd., Beijing, China)
according to the manufacturer's protocol. PCR was used to examine
the expression of genes representative of the endoderm, mesoderm
and ectoderm. The extracted genomic DNA of iPSCs and EBs was mixed
with primers and a TIANamp Genomic DNA kit (Tiangen Biotech Co.,
Ltd.) and the thermocycling conditions were as follows:
Pre-denaturation at 94°C for 4 min, denaturation at 94°C for 30
sec, annealing at 55°C for 30 sec and extension at 72°C for 30 sec
for 35 cycles, followed by a final elongation at 72°C for 2 min and
storage at 4°C. The amplified PCR products were resolved on 1.5%
agarose gels (Thermo Fisher Scientific, Inc.) and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as an internal control.
The gels were run for 25 min at 100 V. Images were captured using a
Bio-Rad Gel document system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The primer sequences of all primers are listed in
Table I.
 | Table ISequences of the primers used in the
polymerase chain reactions. |
Table I
Sequences of the primers used in the
polymerase chain reactions.
| Gene | Primer sequence
(5′-3′)
| Size (bp) |
|---|
| Forward | Reverse |
|---|
| OCT4 |
CCTCACTTCACTGCACTGTA |
CAGGTTTTCTTTCCCTAGCT | 164 |
| GATA4 |
GACAATCTGGTTAGGGGAAGC |
GAGAGATGCAGTGTGCTCGT | 105 |
| MSX1 |
TGCCTCGCTCTACGGTGCCT |
GGCTGGAGGAATCGGCTGGC | 154 |
| SOX1 |
TTTCCCCTCGCTTTCTCA |
TGCAGGCTGAATTCGGTT | 104 |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG | 197 |
Teratoma formation
To evaluate the pluripotency of iPSCs in
vivo, the HSF-iPSCs were harvested and suspended with PBS in a
1.5 ml Eppendorf tube. A total of 1×107 cells were
injected subcutaneously into the hind legs of 4-week-old male
Non-obese diabetic-severe combine immune deficiency (SCID) mice
(n=7; 28–35 days old; 15–17 g; Charles River Systems, Inc.,
Burlington, MA, USA). Mice were housed at 22±2°C with 40–70%
humidity and a 12 h light/dark cycle with free access to food and
water. At 8 weeks following injection, the formed tumors were
dissected and harvested. The tumors were fixed in 4%
paraformaldehyde at 4°C for 2 days, embedded in paraffin blocks,
sliced into 3–4 µm sections and stained with hematoxylin and
eosin (H&E) staining for 5 min each at room temperature. Tissue
samples were observed using a light microscope at ×100
magnification. All animal experiments were approved by the Ethics
Committee of the First Affiliated Hospital of Nanchang
University.
Results
The morphological characteristics of
HSFs
HSFs were isolated from the skin tissues of patients
with burns and cultured in high glucose DMEM with 10% FBS.
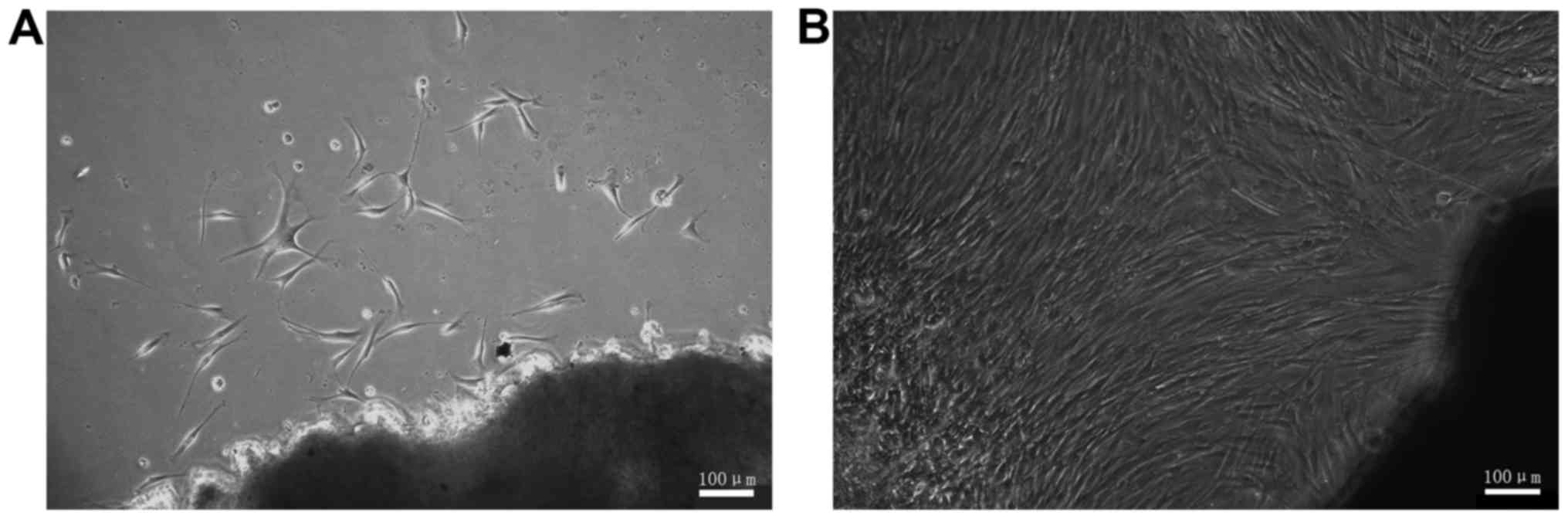
Following culturing for 11 days, it was possible to see fibroblast
cells that had broken off from the tissue segment (Fig. 1A). The cells exhibited branch- and
spindle-shaped morphology. Following culture for 24 days, the cells
covered the majority of the surface area of the culture dish
(Fig. 1B). Following passaging
culture the HSFs demonstrated a marked proliferation ability.
Generation of HSF-iPSCs
To generate iPSCs using a non-integrating method,
the HSFs were transduced with CytoTune-iPS 2.0 Sendai reprogramming
vectors containing the human transcription factors NANOG, OCT4 and
SOX2. The HSFs were cultured until they reached 50–80% confluence
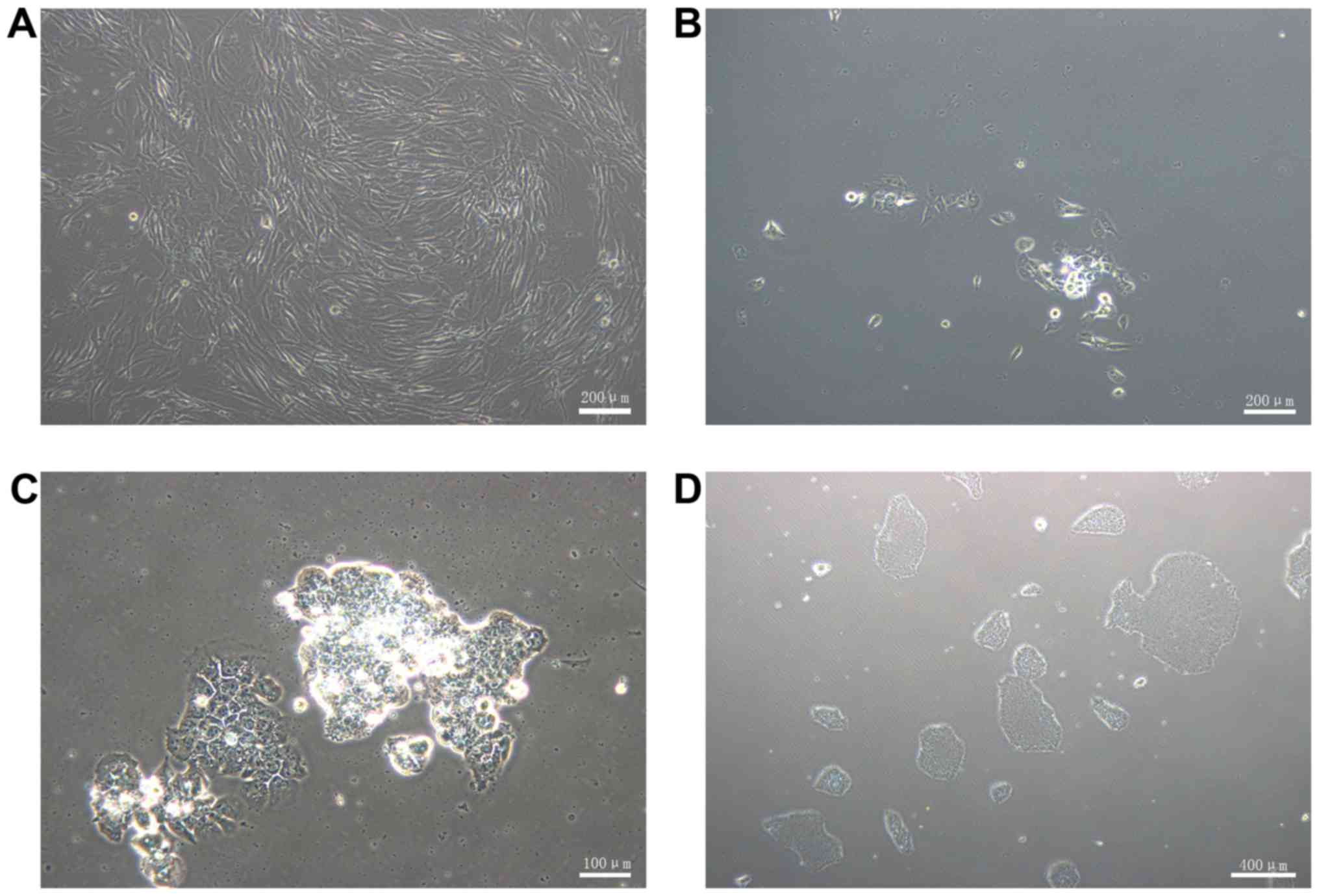
prior to transduction (Fig. 2A).
At 7 days post-transduction, the cells were detached and
transferred onto 1% Matrigel-coated 60 mm dishes. The following
day, several small ESC-like colonies were observed (Fig. 2B). At 4 weeks post-transduction,
the colonies exhibited representative human ESC-like morphology
(Fig. 2C), which includes a large
nucleoli and nucleus to cytoplasm ratio. The cells were packed
tightly and the border was distinct. The ESC-like morphology and
proliferation was maintained following passaging on 1%
Matrigel-coated dishes with complete PSCeasy medium (Fig. 2D). A total of 100 ESC-like
colonies per 1×105 HGFs was obtained, therefore the
reprogramming efficiency was ~0.1%.
Characteristics of HSF-iPSCs
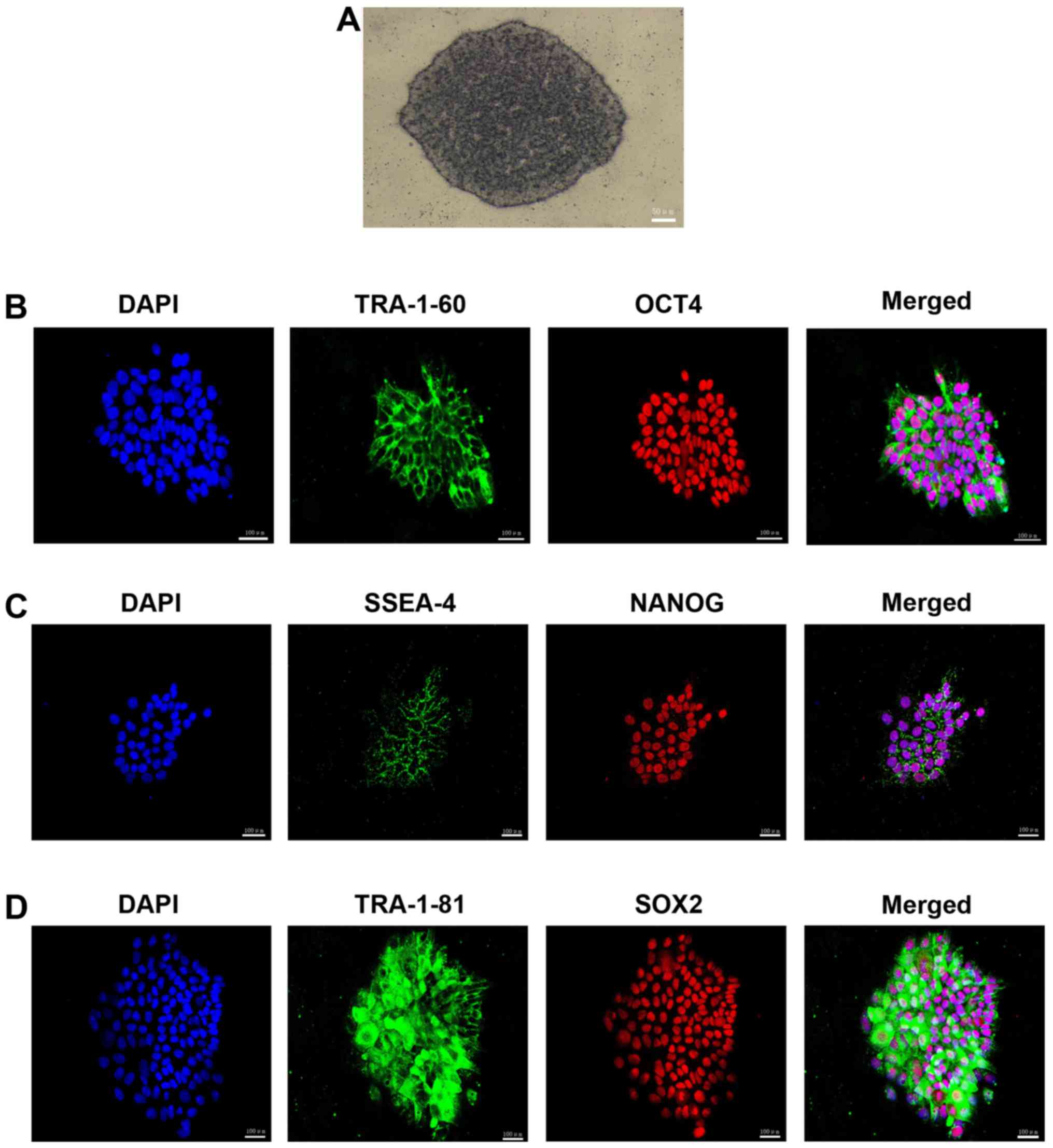
The colonies exhibited positive AP staining
(Fig. 3A), which indicated that
pluripotent stem cells had been successfully developed.
Immunofluorescence staining was used to examine the presence of
pluripotency-associated proteins and revealed that the HSF-iPSCs
strongly expressed surface pluripotency markers, including TRA181,
SSEA-4 and TRA-160, as well as the intracellular pluripotency
markers OCT4, NANOG and SOX2 (Fig.
3B–D). These results suggest that HSFs may be reprogrammed to
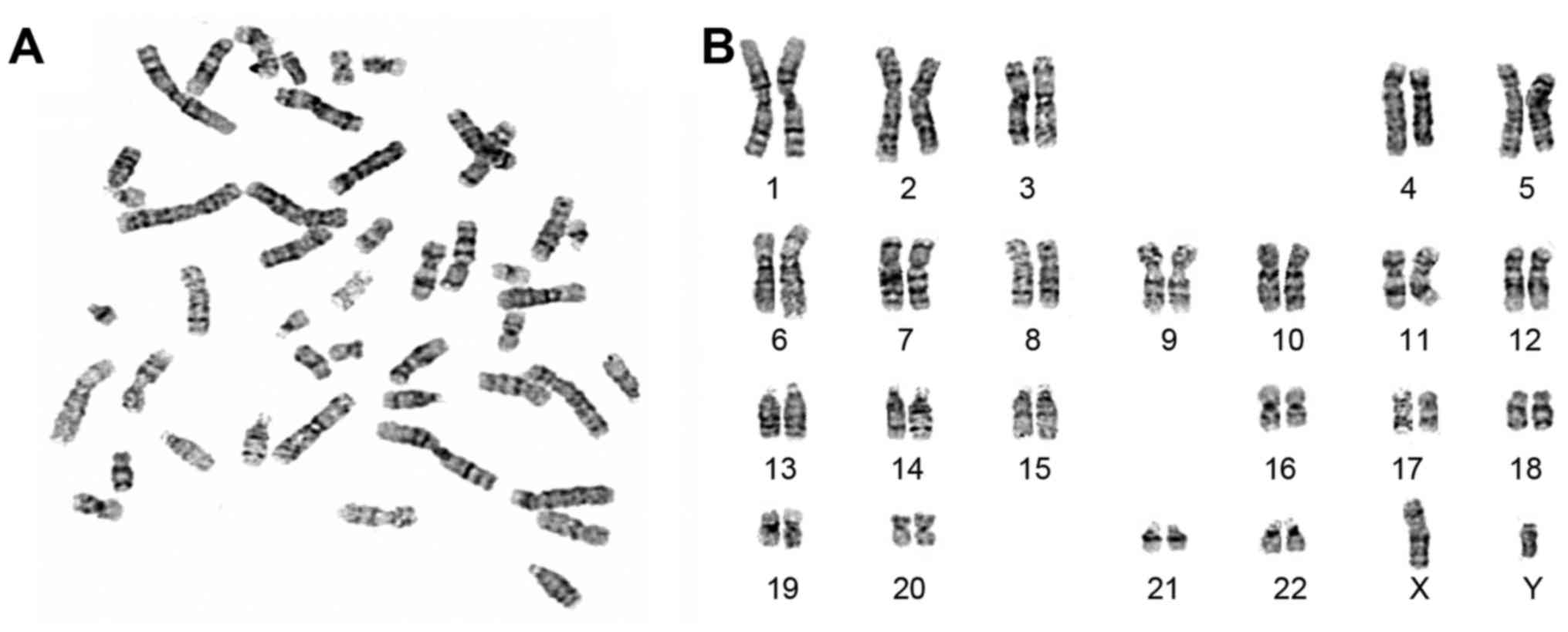
form pluripotent stem cells. The HSF-iPSCs were confirmed by
karyotype analysis (Fig. 4). They
exhibited a normal karyotype of 46 XY as confirmed by chromosomal
G-band analysis at passage 16.
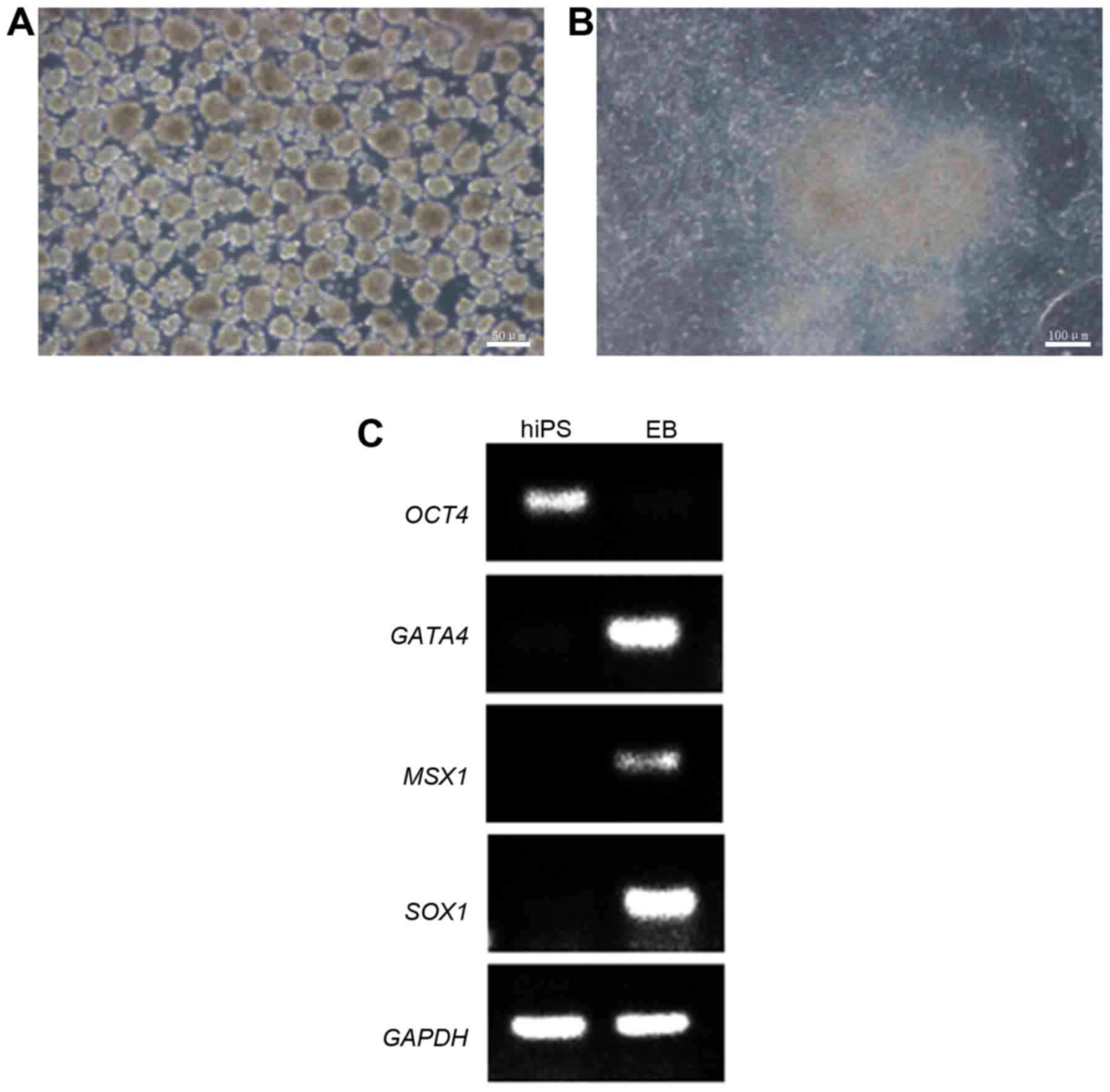
Differentiation of HSFs-iPSCs in vitro
and in vivo
To examine the differentiation potential of
HSF-iPSCs in vitro, an experiment was designed to culture
the HSF-iPSCs in suspension. The HSF-iPSCs differentiated to EBs
spontaneously following 7 days in a suspension culture (Fig. 5A). The EBs were harvested and
transferred to 1% Matrigel-coated 6-well plates and cultured for an
additional 7 days in differentiation medium (Fig. 5B). DNA was isolated from the EBs
and HSF-iPSCs and subsequently used for PCR to examine the
expression of genes specific to the three germ layers. Msh homeobox
1 (MSX1; endoderm), GATA4 (mesoderm) and SOX1 (ectoderm) were
revealed to be upregulated, whereas the ESC-specific gene OCT4 was
downregulated in the EBs (Fig.
5C). Conversely, OCT4 was upregulated in HSF-iPSCs and MSX1,
SOX1 and GATA4 were downregulated. The reference gene GAPDH was
upregulated in the EBs and HSF-iPSCs. These results suggest that
the HSF-iPSCs are capable of differentiating into various different
cell types in vitro.
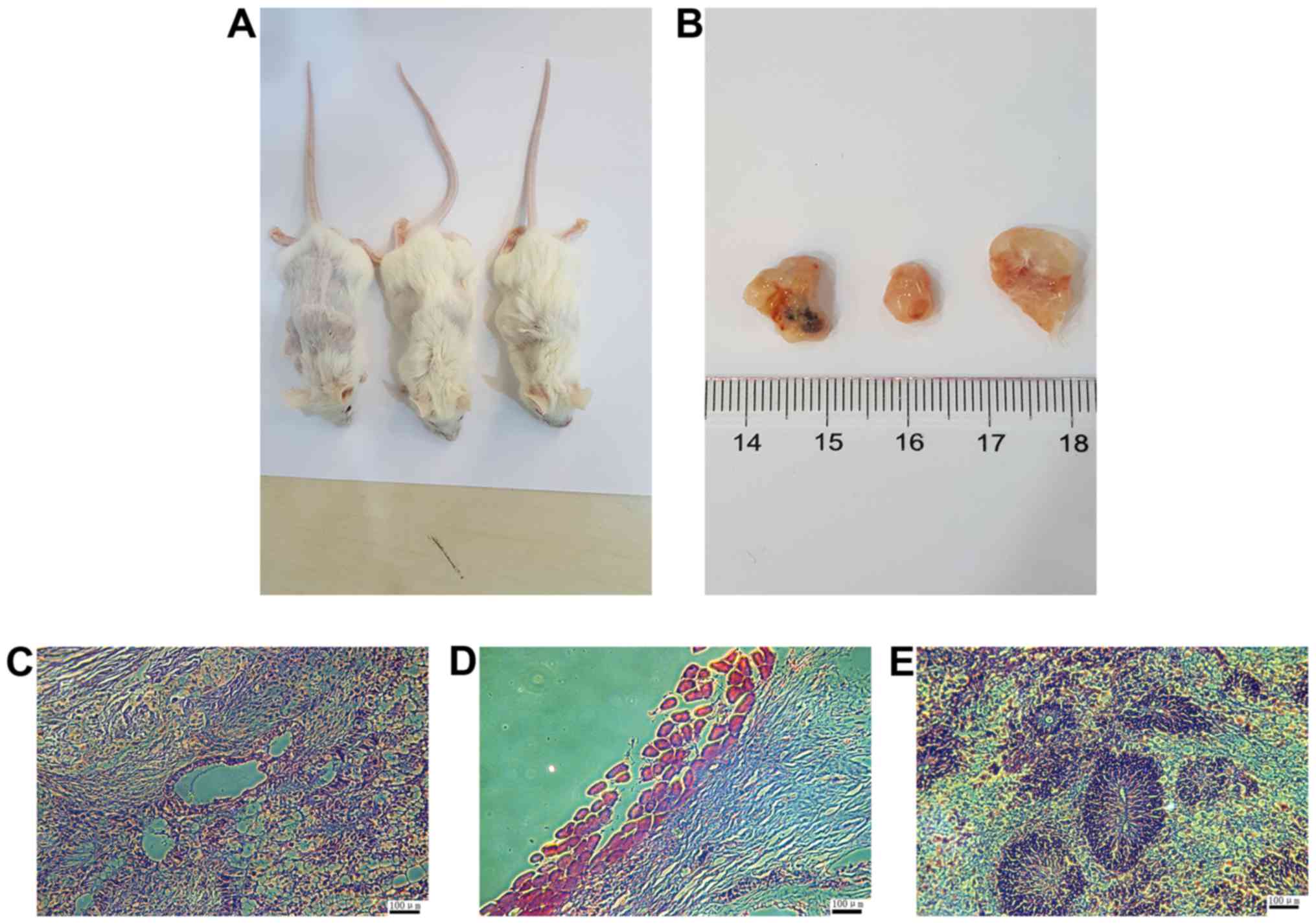
To examine the pluripotency of HSF-iPSCs in
vivo, they were injected into the hind legs of SCID mice. At 8
weeks later, visible teratomas had formed (Fig. 6A and B). HE staining confirmed
that the tumors contained derivatives of all three germ layers,
including glands (endoderm), muscles (mesoderm) and nerves
(ectoderm), as observed in Fig.
6C–E, respectively. These results indicate that HSF-iPSCs are
able to differentiate into different cell types in vivo.
Discussion
Previous studies have successfully generated
patient-specific iPSCs to treat a variety of diseases, including
dystrophic epidermolysis bullosa, spinal muscular atrophy and
Huntington's disease (20–22).
In the present study, fibroblasts were isolated from the skin of
patients with burns and patient specific iPSCs were developed
following the reprogramming of fibroblasts. Human dermal tissues
were obtained from residual skin pieces following a skin graft on
the patient with burns. Fibroblasts were harvested using the tissue
block culture method and reprogrammed into iPSCs using the
non-integration method. This process may provide a source of seed
cells for patients with burns covering a large area, or individuals
with skin defects.
Harvested cells demonstrated typical fibroblast
morphology. In the present study, fibroblasts were transduced with
Sendai virus reprogramming vectors containing the human
transcription factors OCT4, SOX2 and NANOG, as opposed to OCT4,
SOX2, Klf4 and c-Myc, as previous research has revealed that Klf4
and c-Myc are proto oncogenes, which may increase the tumor
formation rate of iPSCs (23).
The principal reason why the Sendai virus was selected to transduce
the transcription factors was because it is a non-integrative virus
and has a minimal effect on the cell genome following transduction
(24–26).
Following transduction, the HSF-iPSCs morphology was
observed as similar to ESCs. The immunofluorescence staining of the
cells revealed the expression of pluripotency markers TRA181,
SSEA-4, TRA-160, OCT4, NANOG and SOX2. Subsequently, it was
demonstrated that cells were capable of differentiating into
different cell types from the three germ layers in vitro and
in vivo. These results suggest that HSF-iPSCs were
successfully obtained. The HSF-iPSCs exhibited a normal karyotype
of 46 XY as demonstrated using chromosomal G-band analysis. To
avoid the pollution of heterogeneous cells and improve the safety
of iPSCs, they were cultured on 1% Matrigel-coated dishes instead
of mouse embryonic fibroblast feeder-cells (27). In the present study, it was also
revealed that skin tissue in skin grafts was thinner than regular
skin tissue, as it did not contain subcutaneous tissue. The dermis
and epidermis were isolated following 4–6 h digestion, which is a
shorter time period than would be necessary for regular skin tissue
and reduced the damage of the digestive enzymes to the cells
(28).
The results of the present study demonstrate that
fibroblasts harvested from patients with burns may be reprogrammed
using Sendai virus vectors with OCT4, SOX2 and NANOG to form iPSCs
with non-exogenous genomic integration (17,29). HSF-iPSCs were demonstrated to be
pluripotent and remain in an undifferentiated state. Further study
is required to develop the differentiation of HSF-iPSCs into
specific cells or tissues of the skin, including fibroblasts,
keratinocytes, melanocytes or vascular tissue, lymphocytic tissue
and nerves (30–34). The results of the present study
provide an experimental basis for the development of functional
skin within a laboratory for use in a clinical setting. The
development of this novel treatment for disease or injury may be of
great significance to regenerative medicine and tissue
engineering.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460293), the
Science and Technology Planning Project of Jiangxi Province (grant
no. 20133BBG70026) and the Special Fund for Graduate Innovation
Project of Nanchang University (grant no. cx2016316).
References
|
1
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vodyanik Yu J, Smuga-Otto MA,
Antosiewicz-Bourget K, Frane J, Tian JL, Nie S, Jonsdottir J,
Ruotti GA, Stewart VR, et al: Induced pluripotent stem cell lines
derived from human somatic cells. Science. 318:1917–1920. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huangfu D, Osafune K, Maehr R, Guo W,
Eijkelenboom A, Chen S, Muhlestein W and Melton DA: Induction of
pluripotent stem cells from primary human fibroblasts with only
Oct4 and Sox2. Nat Biotechnol. 26:1269–1275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lowry WE, Richter L, Yachechko R, Pyle AD,
Tchieu J, Sridharan R, Clark AT and Plath K: Generation of human
induced pluripotent stem cells from dermal fibroblasts. Proc Natl
Acad Sci USA. 105:2883–2888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aasen T, Raya A, Barrero MJ, Garreta E,
Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia
G, et al: Efficient and rapid generation of induced pluripotent
stem cells from human keratinocytes. Nat Biotechnol. 26:1276–1284.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sochacki J, Devalle S, Reis M, Mattos P
and Rehen S: Generation of urine iPS cell lines from patients with
attention deficit hyperactivity disorder (ADHD) using a
non-integrative method. Stem Cell Res (Amst). 17:102–106. 2016.
View Article : Google Scholar
|
|
7
|
Xue Y, Cai X, Wang L, Liao B, Zhang H,
Shan Y, Chen Q, Zhou T, Li X, Hou J, et al: Generating a
non-integrating human induced pluripotent stem cell bank from
urine-derived cells. PLoS One. 8:e705732013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamanaka S: Induced pluripotent stem
cells: Past, present, and future. Cell Stem Cell. 10:678–684. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guha P, Morgan JW, Mostoslavsky G,
Rodrigues NP and Boyd AS: Lack of immune response to differentiated
cells derived from syngeneic induced pluripotent stem cells. Cell
Stem Cell. 12:407–412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishida Y, Kawakami H, Kitajima H,
Nishiyama A, Sasai Y, Inoue H and Muguruma K: Vulnerability of
purkinje cells generated from spinocerebellar ataxia type 6
patient-derived iPSCs. Cell Rep. 17:1482–1490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Son MY, Kim YD, Seol B, Lee MO, Na HJ, Yoo
B, Chang JS and Cho YS: Biomarker discovery by modeling Behçet's
disease with patient-specific human induced pluripotent stem cells.
Stem Cells Dev. 26:133–145. 2017. View Article : Google Scholar
|
|
12
|
Ye L, Chang JC, Lin C, Sun X, Yu J and Kan
YW: Induced pluripotent stem cells offer new approach to therapy in
thalassemia and sickle cell anemia and option in prenatal diagnosis
in genetic diseases. Proc Natl Acad Sci USA. 106:9826–9830. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilmut I: The first direct reprogramming
of adult human fibroblasts. Cell Stem Cell. 1:593–594. 2007.
View Article : Google Scholar
|
|
15
|
Okita K, Nakagawa M, Hyenjong H, Ichisaka
T and Yamanaka S: Generation of mouse induced pluripotent stem
cells without viral vectors. Science. 322:949–953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mack AA, Kroboth S, Rajesh D and Wang WB:
Generation of induced pluripotent stem cells from CD34+
cells across blood drawn from multiple donors with non-integrating
episomal vectors. PLoS One. 6:e279562011. View Article : Google Scholar
|
|
17
|
Fusaki N, Ban H, Nishiyama A, Saeki K and
Hasegawa M: Efficient induction of transgene-free human pluripotent
stem cells using a vector based on Sendai virus, an RNA virus that
does not integrate into the host genome. Proc Jpn Acad Ser B Phys
Biol Sci. 85:348–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan
P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al: Epigenetic memory in
induced pluripotent stem cells. Nature. 467:285–290. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stadtfeld M, Apostolou E, Akutsu H, Fukuda
A, Follett P, Natesan S, Kono T, Shioda T and Hochedlinger K:
Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse
induced pluripotent stem cells. Nature. 465:175–181. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Itoh M, Kiuru M, Cairo MS and Christiano
AM: Generation of keratinocytes from normal and recessive
dystrophic epidermolysis bullosa-induced pluripotent stem cells.
Proc Natl Acad Sci USA. 108:8797–8802. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebert AD, Yu J, Rose FF Jr, Mattis VB,
Lorson CL, Thomson JA and Svendsen CN: Induced pluripotent stem
cells from a spinal muscular atrophy patient. Nature. 457:277–280.
2009. View Article : Google Scholar :
|
|
22
|
Tousley A and Kegel-Gleason KB: Induced
pluripotent stem cells in Huntington's disease research: Progress
and opportunity. J Huntingtons Dis. 5:99–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borooah S, Phillips MJ, Bilican B, Wright
AF, Wilmut I, Chandran S, Gamm D and Dhillon B: Using human induced
pluripotent stem cells to treat retinal disease. Prog Retin Eye
Res. 37:163–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishimura K, Sano M, Ohtaka M, Furuta B,
Umemura Y, Nakajima Y, Ikehara Y, Kobayashi T, Segawa H, Takayasu
S, et al: Development of defective and persistent Sendai virus
vector: A unique gene delivery/expression system ideal for cell
reprogramming. J Biol Chem. 286:4760–4771. 2011. View Article : Google Scholar :
|
|
25
|
Lieu PT, Fontes A, Vemuri MC and Macarthur
CC: Generation of induced pluripotent stem cells with CytoTune, a
non-integrating Sendai virus. Methods Mol Biol. 997:45–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Churko JM, Burridge PW and Wu JC:
Generation of human iPSCs from human peripheral blood mononuclear
cells using non-integrative Sendai virus in chemically defined
conditions. Methods Mol Biol. 1036:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun N, Panetta NJ, Gupta DM, Wilson KD,
Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, et al:
Feeder-free derivation of induced pluripotent stem cells from adult
human adipose stem cells. Proc Natl Acad Sci USA. 106:15720–15725.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song Y, Ding J, Jin R, Jung J, Li S, Yang
J, Wang A and Li Z: Expression and purification of FGF21 in Pichia
pastoris and its effect on fibroblast-cell migration. Mol Med Rep.
13:3619–3626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao HX, Li Y, Jin HF, Xie L, Liu C, Jiang
F, Luo YN, Yin GW, Li Y, Wang J, et al: Rapid and efficient
reprogramming of human amnion-derived cells into pluripotency by
three factors OCT4/SOX2/NANOG. Differentiation. 80:123–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kogut I, Roop DR and Bilousova G:
Differentiation of human induced pluripotent stem cells into a
keratinocyte lineage. Methods Mol Biol. 1195:1–12. 2014.PubMed/NCBI
|
|
31
|
Ohta S, Imaizumi Y, Okada Y, Akamatsu W,
Kuwahara R, Ohyama M, Amagai M, Matsuzaki Y, Yamanaka S, Okano H,
et al: Generation of human melanocytes from induced pluripotent
stem cells. PLoS One. 6:e161822011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zanotelli MR, Ardalani H, Zhang J, Hou Z,
Nguyen EH, Swanson S, Nguyen BK, Bolin J, Elwell A, Bischel LL, et
al: Stable engineered vascular networks from human induced
pluripotent stem cell-derived endothelial cells cultured in
synthetic hydrogels. Acta Biomater. 35:32–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khayyatan F, Nemati S, Kiani S, Hojjati
Emami S and Baharvand H: Behaviour of human induced pluripotent
stem cell-derived neural progenitors on collagen scaffolds varied
in freezing temperature and laminin concentration. Cell J.
16:53–62. 2014.PubMed/NCBI
|
|
34
|
Ando M, Nishimura T, Yamazaki S, Yamaguchi
T, Kawana-Tachikawa A, Hayama T, Nakauchi Y, Ando J, Ota Y,
Takahashi S, et al: A Safeguard system for induced pluripotent stem
cell-derived rejuvenated T cell therapy. Stem Cell Reports.
5:597–608. 2015. View Article : Google Scholar : PubMed/NCBI
|