Introduction
Heatstroke is a life-threatening illness, which is
characterized by a core temperature (Tc) >40°C and central
nervous system dysfunction, and is associated with increased
morbidity and high mortality rates (1,2).
However, the potential mechanism underlying the high mortality
rates in heatstroke remains to be fully elucidated, and there is a
lack of targeted and effective treatment. As the gut contains a
large pool of bacteria and endotoxins, intestinal injury, and the
increased permeability-induced bacterial translocation and
endotoxemia have been implicated in the pathophysiological process
of heatstroke (3,4). However, the molecular changes
underlying small-intestine lesions during heat stress (HS) remain
to be fully characterized. Our previous study used 2D-gel
electrophoresis technology to identify 14 differentially expressed
proteins in the small intestines of mice subjected to HS. These 14
proteins may be involved in metabolism, chaperone functions, the
cytoskeleton, defense, signal transduction, and DNA repair and
recombination. Intestinal defensin-related cryptdin-2 (Cry-2), a
member of the α-defensin family of peptides, is upregulated in the
small intestinal tissue of heat-stressed mice (5).
Increasing evidence suggests that paneth cells,
which are found in the small intestines of mammals, are involved in
the mucosal barrier function (6)
by secreting apical granules containing antimicrobial peptides,
including α-defensins, which are termed cryptdins in mice (7). Cry-2 has potent antimicrobial
activity against certain microbes, including Escherichia
coli and Salmonella typhimurium (8,9).
Previous results suggest that luminal bacteria can increase the
expression of Cry-2, particularly in the ileum (10). In addition to their effects on
microbes, cryptdins also assist in regulating the innate
inflammatory response and cell death (11,12). However, the role of Cry-2 in
HS-induced mouse intestinal injury remains to be fully elucidated.
In order to confirm the possible association between intestinal
Cry-2 and the severity of intestinal injury, the present study
measured intestinal tissue injuries and levels of Cry-2 in the
ileal tissues of heat-stressed mice, and analyzed the correlations
between them.
Two biomarkers are traditionally used to assess
enterocytic damage and dysfunction, namely diamine oxidase (DAO)
and D-lactic acid (D-Lac). DAO is an enzyme of the catabolic
pathway and normally is present in intestinal mucosa and villi.
During ischemia, hypoxia, or sepsis, DAO can be released into the
circulation, and its serum concentration is positively correlated
with the integrity of the intestinal mucosa (13,14). D-Lac is produced by several types
of bacteria, and its increased concentration in serum indicates
that either the permeability of the intestinal wall is increased or
that elevated bacterial reproduction is occurring (15,16). In order to evaluate the severity
of HS-induced intestinal injury, the present study performed
intestinal histopathological analyses, measured pathological
scores, and measured serum and intestinal concentrations of DAO and
D-Lac.
Ulinastatin, a multivalent enzyme inhibitor, was
first identified and purified from human urine. Previous studies
have shown that ulinastatin can inhibit the inflammatory response
by decreasing associated mediators, including high-mobility group
box 1 and tumor necrosis factor-α (17,18). Pharmacological studies have shown
that this anti-inflammatory activity is the result of suppressing
the infiltration of neutrophils, and the production and secretion
of elastase and chemical mediators by macrophages and neutrophils
(19,20). In addition, ulinastatin can
attenuate oxidative damage (21).
Clinically, ulinastatin has been used for the treatment of patients
with pancreatitis and multiple organ dysfunction syndrome (MODS)
(22,23). Our previous study demonstrated
that ulinastatin reduced pulmonary edema and inflammatory exudation
in acute lung injury caused by heatstroke (24), possibly on the basis of its
anti-inflammatory effects. Therefore, the present study aimed to
investigate whether ulinastatin can reduce intestinal damage in
heatstroke; interventions with ulinastatin combined with cooling
treatments were also investigated to further elucidate the
association between Cry-2 and intestinal damage.
Materials and methods
Animals
A total of 60 the pathogen-free male BALB/c mice,
6–8-week-old and weighing 18–22 g, were purchased from the
Experimental Animal Center of Southern Medical University
(Guangzhou, China). The mice were housed in cages with a
temperature of 23°C and humidity of 55% (12-h light/dark cycle),
and they were given free access to standard food and water. All
animal procedures were approved by the Animal Care and Use
Committee of Southern Medical University, and the study was
performed according to the Guidelines for Animal Care of Southern
Medical University.
Heat-stress protocol and cooling
treatment
Heat-stress in the mice was established according to
our previously reported method (25). Briefly, the mice were fasted for
12 h prior to the experiment without limitation on the ingestion of
water. The animals in the HS group were placed in a prewarmed
incubator, which was maintained at 35.5±0.5°C with a relative
humidity of 60±5%, with the absence of food and water. The rectal
Tc was monitored every 30 min with a rectal thermometer. When the
Tc reached 40 or 42°C, respectively, the mice in these two groups
were sacrificed. The mice in another two groups, 40°C (40°C/6 h)
and 42°C (42°C/6 h), were allowed to cool at an ambient temperature
of 25±0.5°C and a humidity of 35±5% for 6 h subsequent to their Tc
reaching 40 or 42°C, prior to sacrifice. The animals in the sham
control group were heated to a temperature of 25±0.5°C and a
humidity of 35±5% for a time comparable to that of the HS groups
and the cooling treatment groups (37°C and 37°C/6 h, respectively).
All groups contained six animals.
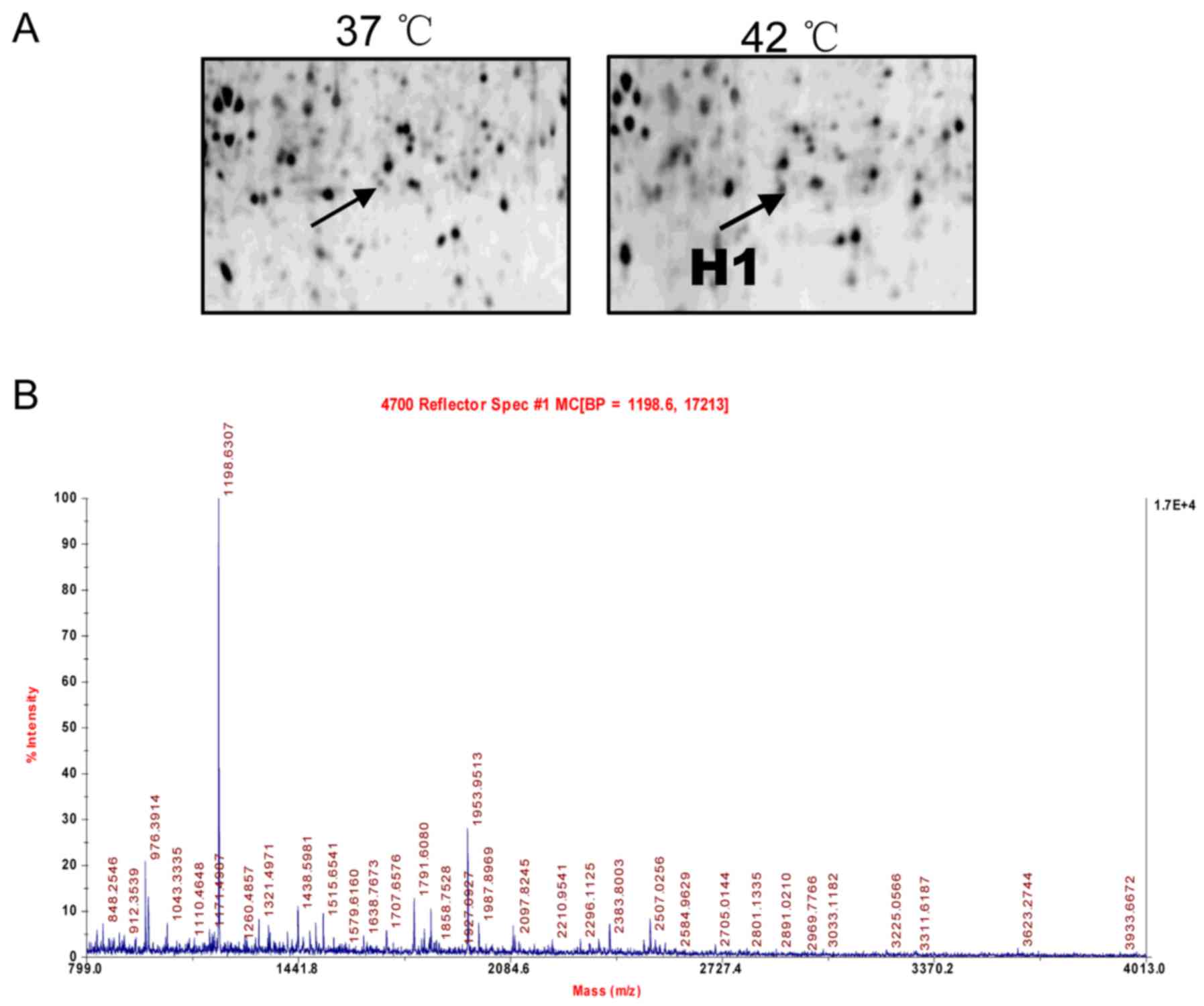
Identification of cryptdin-2 using 2D gel
electrophoresis, matrix-assisted laser
desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF
MS) and database analysis
The identification of cryptdin-2 by 2D gel
electrophoresis MALDI-TOF MS and database analysis were performed
according to our previously reported method (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) (5). Protein spots were analyzed using
ImageMaster 2D Elite (GE Healthcare Life Sciences, Pittsburgh, PA,
USA) and a comparative sequence search was performed in the Mascot
database (http://www.matrixscience.com). Enlarged images from
the 2D gels of spot H1 and its MALDI-TOF MS identification were
obtained.
Histopathological analysis of intestines
and intestinal crypts
The mice were anesthetized by intraperitoneal
injection of chloral hydrate. Ileal samples were separated and
fixed in 4% paraformaldehyde, followed by embedding in paraffin
blocks. Serial sections with dimensions of 5 mm were stained with
hematoxylin and eosin for microscopic evaluation at a magnification
of ×200 under a microscope (90I; Nikon, Tokyo, Japan). Morphologic
changes were assessed and graded on a scale of 0–5 using the
intestinal injury score developed by Chiu et al (26). Based on mucosal changes, the
grades were assessed between 0 and 5 as follows: Grade 0, normal
mucosal villi; grade 1, subepithelial space can be seen at the apex
of the villus; grade 2, sections with extension of the
subepithelial space and moderate lifting of the epithelial layer
from the lamina propria; grade 3, massive epithelial lifting down
the sides of villi; grade 4, sections with denuded villi and
dilated capillaries; and grade 5, sections with digestion and
disintegration of lamina propria. Morphological changes of the
intestinal crypts and the evaluation of defensin (red dye
particles) secreted by paneth cells were also detected
(magnification, ×400).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) identification of Cry-2
The mice were subjected to HS and were sacrificed
when their Tc reached 40 or 42°C, as described above. Ileal samples
were separated, and RT-qPCR analysis was performed to examine the
mRNA levels of Cry-2. Total RNA was isolated using a total RNA
purification kit (Promega Corp., Madison, WI, USA) and reverse
transcribed using ReverTra Ace (Toyobo Co., Ltd., Osaka, Japan).
PCR amplification (50 ng cDNA, 40 nM primers, cycling conditions:
95°C 20 sec and followed by 95°C 1 min, 60°C 20 sec for 40 cycles)
was performed and analysis according to the protocol of
fluorescence quantitative kit (SYBR Premix Ex Taq; Takara Holdings
Inc., Kyoto, Japan) and Agilent StrataGene Mx3005P qPCR system
(Agilent Technologies Inc., Santa Clara, CA, USA) on the resulting
cDNA samples using specific primers for Cry-2 (Cry2 forward,
5′-TCTCCTTTGGAGACCCAGAA-3′ and reverse, 5′-CAGGCGTTCTCTTCTTTTGC-3′)
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH forward,
5′-ATTGTCAGCAATGCATCCTG-3′ and reverse,
5′-ATGGACTGTGGTCATGAGCC-3′). The expression results of Cry-2
RT-qPCR were calculated by the 2−ΔΔCq method reported by
Livak and Schmittgen (27) and
shown as the ratio of Cry-2 over GAPDH, analyzed.
Ulinastatin treatment
The mice received ulinastatin by intraperitoneal
injection at a dose of 10WU/kg immediately following the Tc
reaching 40 or 42°C. The mice in the sham-heated group received the
same treatment at the same time. Tissue samples were extracted 6 h
following ulinastatin treatment.
Blood collection and determination of
serum levels of DAO, D-Lac and Cry-2
The mice were anesthetized with chloral hydrate by
intraperitonal injection, following which blood samples were
obtained from the eyes, placed into 1.5-ml heparinized
microcentrifuge tubes, and placed immediately on ice for 1 h. The
serum was separated by centrifugation at room temperature for 5 min
at 1,200 × g, and then stored at −80°C. The concentrations of DAO,
D-Lac, and Cry-2 in the serum were analyzed using DAO, D-Lac and
Cry-2 enzyme-linked immunosorbent assay (ELISA) assay kits (RD
mouse DAO, D-Lac and α-defensin-2 ELISA kits; R&D Systems China
Co., Ltd., Shanghai, China) according to the manufacturer's
protocol.
Detection of intestinal concentrations of
DAO, D-Lac, and Cry-2
The Ileal tissues were cut into smaller sections and
grinded, and total proteins were extracted in phosphate-buffered
saline (PBS) (containing 1 mM KH2PO4, 155 mM
NaCl, 3 mM Na2HPO4-7H2O), followed
by centrifugation at 4°C for 20 min at 13,400 × g. The protein
concentrations in the supernatant were quantified using a Micro BCA
protein assay (Pierce; Thermo Fisher Scientific, Inc.). The
concentrations of DAO, D-Lac and Cry-2 in the intestinal extracts
were analyzed using ELISA assay kits according to the
manufacturer's protocol.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
One-way analysis of variance was used for the comparison of
qualitative variables. The correlation between intestinal or serum
levels of Cry-2 and intestinal injury scores, and the
concentrations of intestinal and serum DAO and D-Lac, were
calculated using Spearman's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
2D gel electrophoresis and protein
identification
As described in our previous study (5), 2D gel electrophoresis was used to
separate proteins extracted from the small intestines of the
control and heat-stressed mice. Among all protein spots analyzed
using an ImageMaster 2D Elite (GE Healthcare Life Sciences) the H1
protein spots were found to be significantly altered between the
controls and heat-stressed mice. The protein was successfully
identified by MALDI-TOF MS and a subsequent comparative sequence
search was performed in the Mascot database. An enlarged image of
the H1 spot, subsequently identified as defensin-related Cry-2, on
the 2D gel and its MALDI-TOF MS identification are shown in
Fig. 1, respectively.
Histopathological changes in intestinal
crypts and RT-qPCR identification of Cry-2 in heat-stressed
mice
Male BALB/c mice were used to prepare the
heat-stressed mice, which were divided into three groups:
Sham-heated control mice (37°C) and mice heated to a Tc of 40 or
42°C, respectively. The ileal crypts were observed using a
microscope following hematoxylin and eosin staining (magnification,
×400). As shown in Fig. 2A, the
level of defensin (red dye particles, indicated by white arrows)
secreted by paneth cells in small intestinal crypts increased with
HS. RT-qPCR analysis was used to examine the mRNA levels of Cry-2,
the results of which were in accordance with the pathological
changes induced by HS, the expression of Cry-2 was higher at a
higher Tc (Fig. 2B). This finding
was confirmed by the results of the 2D-gel reported above.
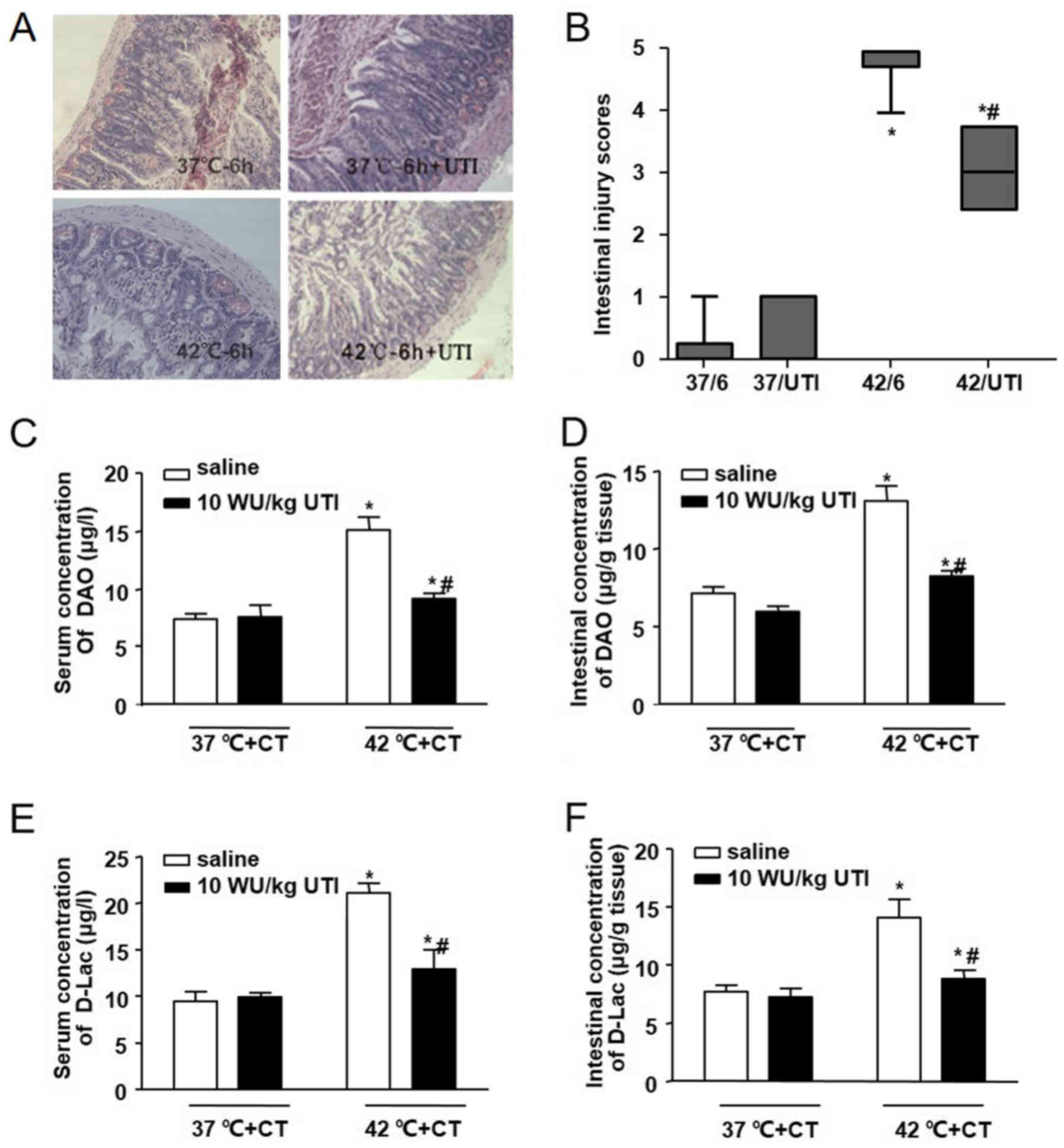
HS-induced intestinal pathologic
injury
The pathological changes in the intestines of mice
in each group are shown in Fig.
3A. In the 37°C group and the 37°C/6 h group, no significant
damage was observed. Intestinal injury gradually increased with the
increase in Tc, as shown by the aggravation of intestinal damage in
the 40°C group and the 42°C group. In the 40°C group, marked
epithelial necrosis was observed, with lesions exhibiting
epithelial necrosis and villi desquamation. As the Tc increased to
42°C, the damage to the villi increased. Intestinal damage was less
extensive in the 40°C/6 h group, compared with the damage in the
40°C group. A level of recovery among the villi was detected in the
40°C/6 h group. By contrast, intestinal damage persisted following
cooling in the heat-stressed mice whose Tc had reached 42°C during
HS. Epithelial loss and villi desquamation were observed in the
42°C/6 h group. Comparisons of intestinal injury scores showed
similar results in terms of the morphological changes (Fig. 3B).
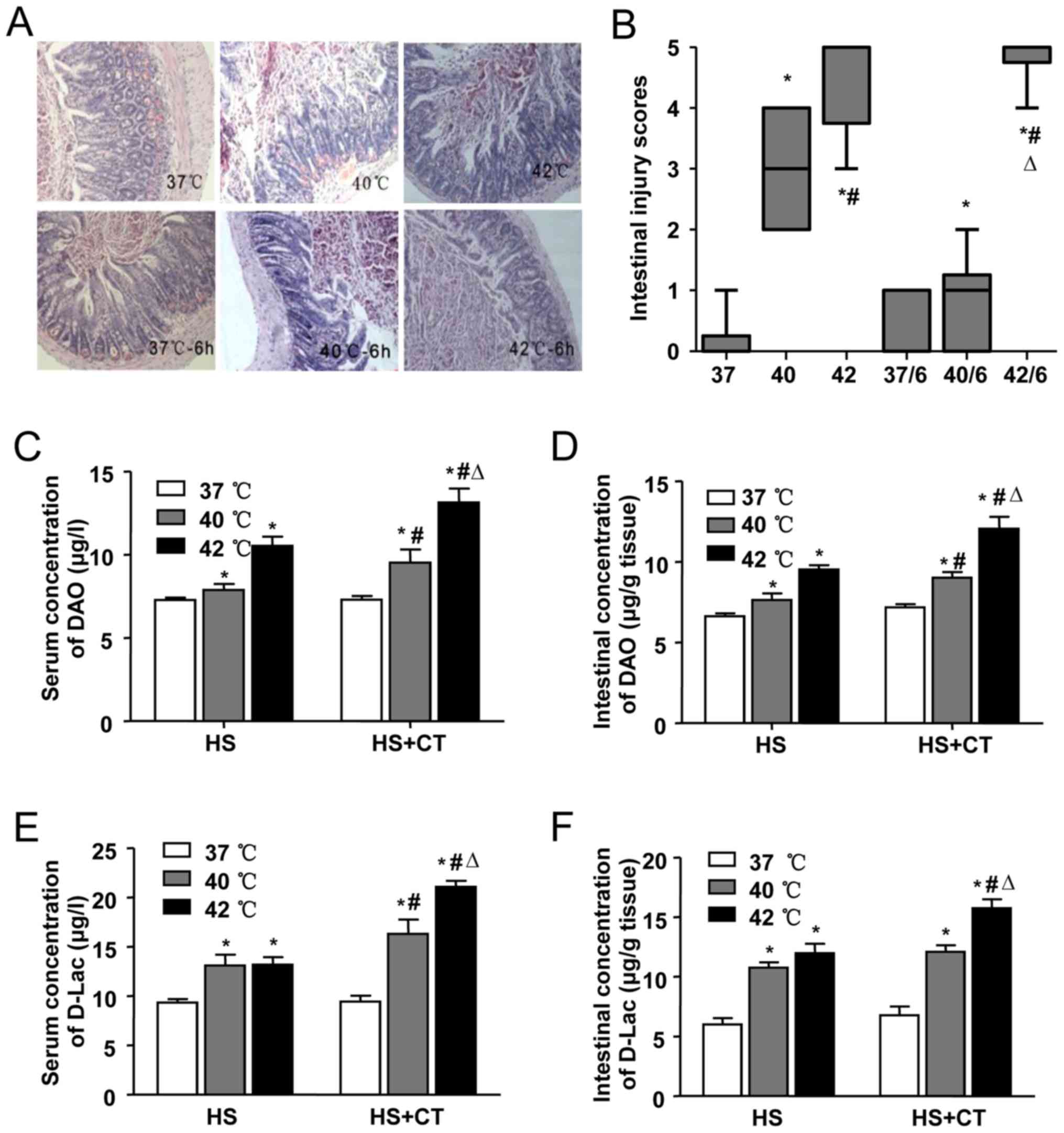 | Figure 3Effects of heat stress and cooling
treatment on intestinal injury. Male Balb/c mice were used to
establish heat-stressed mice, which were then divided into six
groups: Sham-heated control mice (37°C), mice heated with Tc at
40°C or 42°C, and groups in which the animals were removed from the
incubator and allowed to cool at an ambient temperature of 25±0.5°C
for 6 h following core temperature reaching 40°C (40/6) or 42°C
(42/6), or the sham control temperature (37/6). (A) Representative
images of hematoxylin and eosin-stained ileal tissues
(magnification, ×200). (B) Morphological changes in mice ileal
tissues were assessed and graded in a blinded-manner by two
certified veterinary pathologists using the Chiu intestinal injury
score. Concentrations of DAO in (C) serum and (D) ileal tissues,
and those of D-LAC in the (E) serum and (F) ileal tissues were
analyzed using an ELISA. Data are presented as the mean ± standard
deviation. One-way analysis of variance followed by the
Newman-Keuls test was performed. *P<0.05 vs. 37°C
group; #P<0.05 vs. 40°C group; △P<0.05
vs. 42°C group (n=6). DAO, diamine oxidase; D-Lac, D-lactic acid;
HS, heat stress; CT, cooling treatment; ELISA, enzyme-linked
immunosorbent assay. |
In addition to pathological changes, chemical
markers of intestinal injury induced by HS were also detected. In
this series, intestinal and serum concentrations of DAO were
significantly increased in the 40°C group and the 42°C group, and
increased further even following cooling treatment for 6 h
(Fig. 3C and D), suggesting
severe damage to the intestinal mucosa. In accordance with the
changes in DAO, the levels of D-Lac increased in the serum and the
intestines of the mice in the 40°C group and the 42°C group.
Following cooling treatment, the concentrations of D-Lac also
increased, compared with the concentrations in the 40°C group and
the 42°C group (Fig. 3E and F),
which suggested that the permeability of the intestinal wall
increased during HS and cooling treatment.
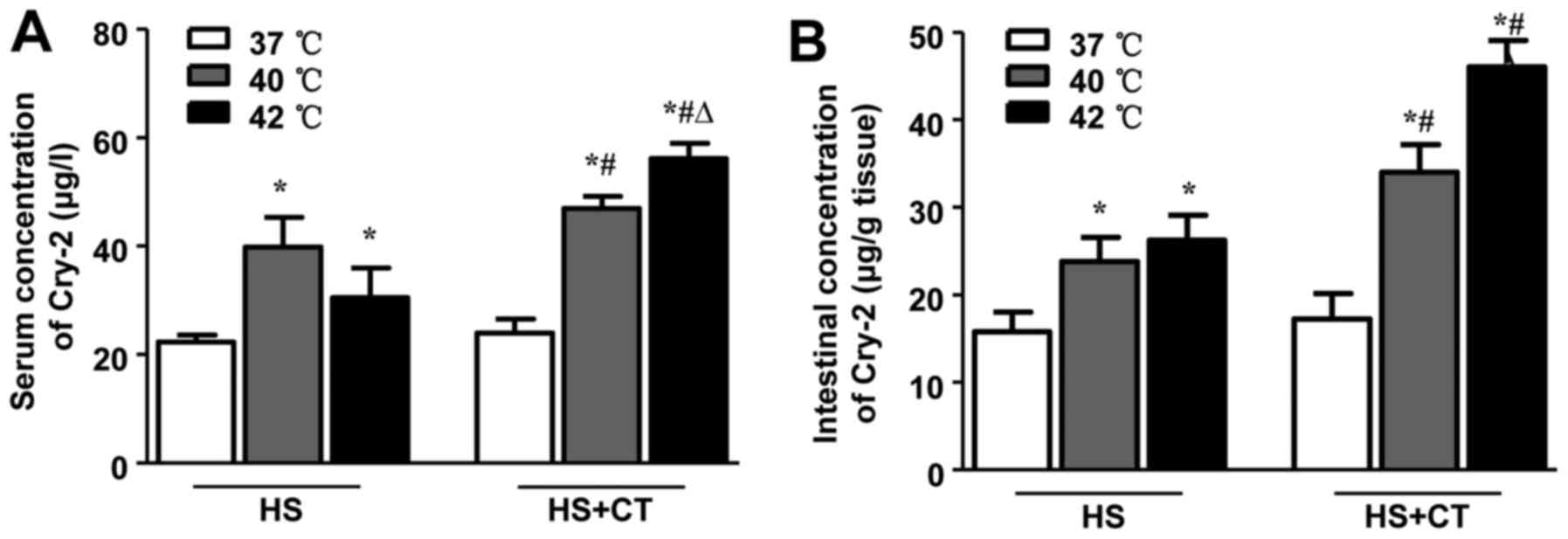
HS increases concentrations of Cry-2 in
serum and intestinal tissue in association with intestinal
injury
Previous studies have shown that members of the
defensin family, including Cry-2, are induced during several
pathological conditions, including chronic colitis (28). As shown in Fig. 4, the results of the present study
demonstrated that HS induced an increase in concentrations of Cry-2
in the serum and intestines of the mice. Following cooling
treatment, the levels of Cry-2 in the 40°C/6 h group and the 42°C/6
h group were significantly higher, compared with the concentrations
in the 40°C group and 42°C group. These findings are consistent
with the changes in intestinal injury, not only in terms of the
pathological scores but also in terms of the chemical
biomarkers.
To further confirm the correlation between
intestinal or serum levels of Cry-2 and intestinal injury scores,
the intestinal and serum concentrations of DAO and D-Lac were
analyzed using Spearman's correlation analysis. As shown in
Table I, the serum and intestinal
levels of Cry-2 were positively correlated with the Chiu scores
(r=0.612; P=0.0001; r=0.541; P=0.0006, respectively). The findings
also suggested that a significantly positive correlation existed
between the levels of Cry-2 and D-Lac (r=0.778 in serum; r=0.850 in
intestines). Similarly, correlations were observed between the
levels of Cry-2 and DAO in the serum and the intestines (r=0.567;
P=0097; r=0.528; P=0.0074, respectively). These results indicated
that Cry-2 may be a biomarker of HS-induced intestinal injury.
 | Table ICorrelation analysis between Cry-2
and intestinal injury. |
Table I
Correlation analysis between Cry-2
and intestinal injury.
| Cry-2
expression | Variable | Spearman's rank
|
|---|
| n | Correlation
coefficient | Significance
(two-tailed) |
|---|
| Serum | Chiu score | 36 | 0.612a | 0.0001 |
| DAO | 36 | 0.567a | 0.0097 |
| D-Lac | 36 | 0.778a | <0.0001 |
| Intestine | Chiu score | 36 | 0.541a | 0.0006 |
| DAO | 36 | 0.528a | 0.0074 |
| D-Lac | 36 | 0.850a | <0.0001 |
Ulinastatin protects against HS-induced
intestinal damage
To determine the possible protective role of
ulinastatin on intestinal injury in the heat-stressed mice, 100,000
U/kg ulinastatin was administered by intraperitoneal injection as
soon as the Tc reached 42°C. Cooling treatments for 6 h also were
performed, and intestinal pathologic changes, Chiu scores, and
serum and intestinal biomarkers (DAO and D-lac) were determined
(Fig. 5). Compared with the
42°C/6 h group, pathological sections from the 42°C/6 h+UTI group
showed that villi damage was mitigated by ulinastatin treatment.
This was confirmed by changes in the villi length. Villi swelling
and vascular congestion were also moderated in the 42°C/6 h+UTI
group. Similar changes were observed in the serum and intestinal
levels of DAO and D-Lac. These results demonstrated that
ulinastatin treatment may be beneficial in mitigating HS-induced
intestinal damage.
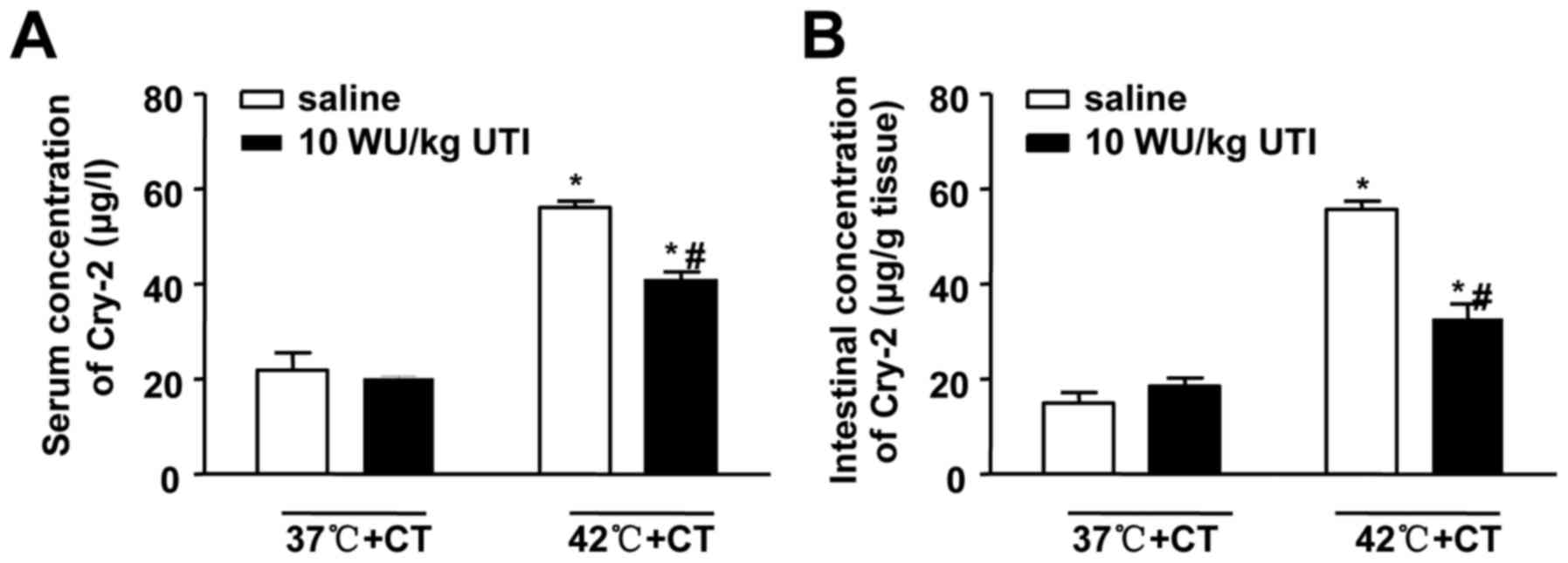
Ulinastatin decreases serum and
intestinal concentrations of Cry-2 in correlation with intestinal
injury
To investigate the effects of ulinastatin on Cry-2,
the present study measured serum and intestinal levels of Cry-2
following the treatments involving ulinastatin and cooling. The
results showed that ulinastatin decreased the HS-induced serum and
intestinal concentrations of Cry-2 (Fig. 6). Spearman's correlation analysis
was also performed, and, as shown in Table II, the serum and intestinal
levels Cry-2 were positively correlated with Chiu scores (r= 0.728;
P<0.001; r= 0.651; P= 0.001, respectively). The results also
suggested significant positive correlations between the levels of
Cry-2 and D-Lac (r=0.838 in serum; r=0.877 in intestine). Similar
correlations were also observed between the levels of Cry-2 and DAO
in the serum and the intestines (r=0.694; P=001; r=0.780;
P<0.001, respectively). These results further established the
correlation between the levels of Cry-2 and the severity of
intestinal damage, even following ulinastatin treatment. In
addition, the results provided further confirmation that Cry-2 may
be a novel biomarker of HS-induced intestinal injury.
 | Table IICorrelation analysis between Cry-2
and intestinal injury following ulinastatin treatment. |
Table II
Correlation analysis between Cry-2
and intestinal injury following ulinastatin treatment.
| Cry-2
expression | Variable | Spearman's rank
|
|---|
| n | Correlation
coefficient | Significance
(two-tailed) |
|---|
| Serum | Chiu score | 24 | 0.728a | <0.001 |
| DAO | 24 | 0.694a | 0.001 |
| D-Lac | 24 | 0.838a | <0.001 |
| Intestine | Chiu score | 24 | 0.651a | 0.001 |
| DAO | 24 | 0.780a | <0.001 |
| D-Lac | 24 | 0.877a | <0.001 |
Discussion
In our previous study, 2D gel electrophoresis was
used to identify various HS-induced intestinal proteins. The
present study confirmed for the first time, to the best of our
knowledge, that HS upregulated the expression of Cry-2. The
concentrations of Cry-2 in the serum and intestines were positively
correlated with the severity of HS-induced intestinal damage. In
addition, ulinastatin protected the intestines from HS-induced
injury and downregulated the expression of Cry-2. This reduced
expression of Cry-2 was also correlated with changes in the degree
of intestinal injury. Therefore, Cry-2 may be involved in and may
be a novel predictor of HS-induced intestinal injury.
Severe HS, including that in heatstroke, has been
reported to have a direct cytotoxic effect and to lead to organ
damage. HS also causes gastrointestinal dysfunction, which is
important in the pathophysiological process of heatstroke (4). The results of the present study
demonstrated that HS can lead to intestinal damage, as shown by the
pathological changes and the increased concentrations of DAO and
D-Lac. Physically, the intestinal barrier can prevent colonization
by bacteria entering the systemic circulation. The dysfunction of
this barrier is important in promoting heat-induced MODS, as is the
pathophysiologic process in sepsis (29). The basal levels of DAO and D-Lac
are usually low in the systemic circulation, and are usually
observed only in the intestines. In the present study, the
increased concentrations of DAO and D-Lac suggested that the
permeability of the intestinal wall was increased following HS.
Pathological sections in the present study revealed decreased
length of villi, with the presence of denuded villi in severe
cases. Damage to the intestinal mucosa allows endotoxins to escape
the intestinal lumen, leading to secondary bacteremia.
The mechanism of HS-induced intestinal damage
remains to be fully elucidated. Current views focus mainly on
direct thermal stresses (30),
followed by insults from inflammatory and ischemia-induced reactive
oxygen species.
In vitro experiments have shown that direct
HS can induce intestinal cell apoptosis (30) and inflammation via disturbance of
the B-cell lymphoma-2 (Bcl-2)-associated X protein/Bcl-2 balance
and activation of the nuclear factor-κB (NF-κB) signaling pathway
(31). HS can downregulate tight
junction proteins, resulting in increased intestinal permeability.
The heat stroke-induced systemic inflammatory cascade is also
important in the pathology. Our previous study demonstrated that
levels of pro-inflammatory cytokines were increased in the
intestines and positively correlated with intestinal injury scores
(25). Enterocytes are sensitive
to oxygen restriction, and heat-induced splanchnic vessel
contraction leads to the production of reactive oxygen species and
stress in the endoplasmic reticulum (32,33) particularly at the tips of the
villi. This ischemic insult aggravates intestinal damage and
dysfunction of the intestinal barrier. As reported in previous
studies (25,34), the results of the present study
showed that intestinal damage was aggravated following cooling
treatment. This may result from gut reperfusion during the cooling
treatment and the sustained systemic inflammatory cascade (35,36). The inflammatory responses are not
only stimulated by thermal stress but also are activated by gut
endotoxins released due to dysfunction of the intestinal
barrier.
Defensin-related Cry-2, an intestinal α-defensin,
was previously found to be upregulated in the small intestines of
mice with heatstroke (5). The
enteric surface barrier is crucial in preventing the translocation
of macromolecules and bacteria from the colonized mouse gut lumen.
In the gut lumen, paneth cells are key members and the main
producers of antimicrobial peptides, primarily α-defensins, which
are termed cryptdins in mice (37). Cryptdins, which have potent
microbiacidal activity, are secreted into the lumen in response to
bacterial stimuli (38). The
results of the present study confirmed this change by
histopathological detection and RT-qPCR analysis. In addition, HS
increased the serum and intestinal concentrations of Cry-2, and was
correlated with changes in Chiu scores, and the serum and
intestinal concentrations of D-Lac and DAO. Therefore, the
concentration of Cry-2 may indicate intestinal damage, and the
level of Cry-2 may vary according to the severity of injury.
The results of the present study confirmed that the
concentration of Cry-2 was increased in heat-stressed mice and that
the concentrations of cryptdin were positively correlated with the
extent of lesions. However, whether increased cryptdin results from
pathological factors or whether cryptdin induces injury remains to
be elucidated. It appears that both are involved. A certain
concentration of cryptdin is necessary to induce antimicrobial
activity. During infection and inflammation, the translocation of
multitude microbes from the enteric cavity to the circulation is
increased. According to the Wehkamp et al raised 'on-demand'
mechanism (39), the increased
defensin may be protective to the host. Large numbers of copies of
cryptdin genes are present in paneth cells, even under resting
conditions (40), which may
explain the rapid response of cryptdins to infection.
Cryptdins mediate enteric innate immunity, and their
absence may contribute to enhanced susceptibility to infection
(7,40). Cry-2 can combat infections and
augment the activity of conventional antibiotics in vitro
and in vivo (8,9). α-defensins may suppress inflammation
by controlling the production of interleukin-1β (IL-1β) (11). By contrast, defensin is cytotoxic
and pro-inflammatory. This cytotoxicity is based on the interaction
of positively charged peptides with negatively charged phospholipid
bilayers of cell membranes, leading to the disorganization of the
cytomembrane (41). Under resting
conditions, defensins have no effect on normal cells, and it is
unclear whether alteration of the defensin recognition site or
cytomembrane potential occurs during the pathologic process of
heatstroke. In addition, defensin has effects on the regulation of
inflammation, including the upregulation of IL-8 and inducing the
chemotactic activity of T lymphocytes (12,41). Ulinastatin has multiple functions,
including reducing inflammation, regulating immune responses and
alleviating ischemia-reperfusion injury; it has been used to treat
pancreatitis, sepsis, shock, and cardiac and renal ischemic
reperfusion (41–43). The present study showed that
ulinastatin effectively alleviated heat-induced intestinal damage,
even during cooling treatment, resulting in decreased pathological
injury and reduced concentrations of DAO and D-Lac. A previous
study showed that ulinastatin pretreatment reduced the inflammatory
exudation in heat-induced acute lung injury (24). Ulinastatin's anti-inflammatory
effects include inactivating the elastase secreted by neutrophils,
decreasing inflammatory mediators and downregulating inflammatory
transcription factors, including NF-κB (19,22,45). Under inflammatory conditions,
ulinastatin can attenuate dysfunctions of the endothelial barrier
by upregulating the expression of vascular endothelial-cadherin; it
also prevents endothelial apoptosis (21,46,47). These effects against
hyperpermeability may account for the protective effect of
ulinastatin against heat-induced damage.
Ulinastatin may also protect the intestines from
ischemia induced by splanchnic vessel contraction following heat
stimuli. Kato et al found that ulinastatin has lysosomal
membrane-stabilizing properties (48), which can prevent the rupture of
lysosomes under pathological conditions. In the reperfusion state,
ulinastatin maintains energy production by reducing the severity of
mitochondrial dysfunction (44).
The present study confirmed that post-treatment ulinastatin had a
protective effect in heat-stressed mice, and the protection may
result from its anti-inflammatory and anti-ischemic functions.
These findings indicated that ulinastatin may be used in the
treatment of patients with heatstroke.
Defensins are induced via cell receptors, which
recognize pathogen-associated molecular patterns, including
Toll-like receptors (TLRs), TLR2 and TLR4 (49). The results of the present study
showed that ulinastatin decreased the level of cryptdin, and this
effect may be associated with the suppressive effect of on the
activity of TLR4 (50).
Nucleotide-binding oligomerization domain protein 2 is another
crucial sensor protein involved in the production of defensin via
the NF-κB pathway (49,51). Therefore, ulinastatin may
downregulate the expression of Cry-2 by inhibiting NF-κB (22). Ulinastatin exerts a protective
effect on cells via inhibiting the release of cytochrome c and
activation of caspase-3, which are beneficial to vascular
permeability (46). The decreased
concentration of Cry-2 may be the result of reduced permeability
following ulinastatin treatment.
In conclusion, the present study confirmed for the
first time, to the best of our knowledge, that HS in a mouse model
upregulated the expression of Cry-2, and that the serum and
intestinal concentrations of Cry-2 were positively correlated with
the severity of HS-induced intestinal damage. It was also found
that ulinastatin protected the intestines from HS-induced injury
and concurrently downregulated the expression of Cry-2. The latter
was also correlated with changes in intestinal injury. Therefore,
ulinastatin administration may be beneficial for patients with
heatstroke, and Cry-2 may be a novel predictor of HS-induced
intestinal injury.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81571940
and 81101467), the Project Team of Natural Science Foundation of
Guangdong Province (grant no. s2013030013217), the Project of
Medical Research of PLA (grant no. BWS12J108), the Guangzhou
Science and Technology Planning Project of China (grant no.
201504281714528) and the Natural Science Foundation of Guangdong
Province (grant no. 10151001002000001).
References
|
1
|
Leon LR and Bouchama A: Heat stroke. Compr
Physiol. 5:611–647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sucholeiki R: Heatstroke. Semin Neurol.
25:307–314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lambert GP, Gisolfi CV, Berg DJ, Moseley
PL, Oberley LW and Kregel KC: Selected contribution:
Hyperthermia-induced intestinal permeability and the role of
oxidative and nitrosative stress. J Appl Physiol. 1985.
92:1750–1761; discussion 1749. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang P-C, He S-H and Zheng P-Y:
Investigation into the signal transduction pathway via which heat
stress impairs intestinal epithelial barrier function. J
Gastroenterol Hepatol. 22:1823–1831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Liu JH, Liu Y, Tang Y, Meng F, Sun
X, Tang J, Wang JH and Su L: Proteomic analysis and identification
of intestinal FBP as a predictor of gut dysfunction during
heatstroke in mice. J Surg Res. 173:332–340. 2012. View Article : Google Scholar
|
|
6
|
Ouellette AJ, Hsieh MM, Nosek MT,
Cano-Gauci DF, Huttner KM, Buick RN and Selsted ME: Mouse Paneth
cell defensins: Primary structures and antibacterial activities of
numerous cryptdin isoforms. Infect Immun. 62:5040–5047.
1994.PubMed/NCBI
|
|
7
|
Ouellette AJ, Satchell DP, Hsieh MM, Hagen
SJ and Selsted ME: Characterization of luminal paneth cell
alpha-defensins in mouse small intestine. Attenuated antimicrobial
activities of peptides with truncated amino termini. J Biol Chem.
275:33969–33973. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Preet S, Verma I and Rishi P: Cryptdin-2:
A novel therapeutic agent for experimental Salmonella typhimurium
infection. J Antimicrob Chemother. 65:991–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh AP, Prabha V and Rishi P: Efficacy
of cryptdin-2 as an adjunct to antibiotics from various generations
against Salmonella. Indian J Microbiol. 54:323–328. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inoue R, Tsuruta T, Nojima I, Nakayama K,
Tsukahara T and Yajima T: Postnatal changes in the expression of
genes for cryptdins 1-6 and the role of luminal bacteria in
cryptdin gene expression in mouse small intestine. FEMS Immunol Med
Microbiol. 52:407–416. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi J, Aono S, Lu W, Ouellette AJ, Hu X,
Ji Y, Wang L, Lenz S, van Ginkel FW, Liles M, et al: A novel role
for defensins in intestinal homeostasis: Regulation of IL-1beta
secretion. J Immunol. 179:1245–1253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HT, Kim M, Kim JY, Brown KM, Ham A,
D'Agati VD and Mori-Akiyama Y: Critical role of interleukin-17A in
murine intestinal ischemia-reperfusion injury. Am J Physiol
Gastrointest Liver Physiol. 304:G12–G25. 2013. View Article : Google Scholar
|
|
13
|
Miyoshi J, Miyamoto H, Goji T, Taniguchi
T, Tomonari T, Sogabe M, Kimura T, Kitamura S, Okamoto K, Fujino Y,
et al: Serum diamine oxidase activity as a predictor of
gastrointestinal toxicity and malnutrition due to anticancer drugs.
J Gastroenterol Hepatol. 30:1582–1590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan S, Yokoyama Y, Dickens E, Cash TG,
Freeman BA and Parks DA: Xanthine oxidase activity in the
circulation of rats following hemorrhagic shock. Free Radic Biol
Med. 15:407–414. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Demircan M, Cetin S, Uguralp S, Sezgin N,
Karaman A and Gozukara EM: Plasma D-lactic acid level: A useful
marker to distinguish perforated from acute simple appendicitis.
Asian J Surg. 27:303–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith SM, Eng RH and Buccini F: Use of
D-lactic acid measurements in the diagnosis of bacterial
infections. J Infect Dis. 154:658–664. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Wang Y, Luo H, Luo Z, Liu L, Xu W,
Zhang T, Yang N, Long X, Zhu N, et al: Ulinastatin reduces urinary
sepsis related inflammation by upregulating IL-10 and
downregulating TNF-α levels. Mol Med Rep. 8:29–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang SY, Li ZJ, Wang X, Li WF and Lin ZF:
Effect of ulinastatin on HMGB1 expression in rats with acute lung
injury induced by sepsis. Genet Mol Res. 14:4344–4353. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakatani K, Takeshita S, Tsujimoto H,
Kawamura Y and Sekine I: Inhibitory effect of serine protease
inhibitors on neutrophil-mediated endothelial cell injury. J Leukoc
Biol. 69:241–247. 2001.PubMed/NCBI
|
|
20
|
Xu CE, Zhang MY, Zou CW and Guo L:
Evaluation of the pharmacological function of ulinastatin in
experimental animals. Molecules. 17:9070–9080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Ma D, Chen M, Zhang L, Zhang L,
Zhang J, Qu X and Wang C: Ulinastatin attenuates LPS-induced human
endothelial cells oxidative damage through suppressing JNK/c-Jun
signaling pathway. Biochem Biophys Res Commun. 474:572–578. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng C, Li B, Wang LL, Chen LI, Zhou X, Lv
FQ and Li TS: Effect of peritoneal lavage with ulinastatin on the
expression of NF-κB and TNF-α in multiple organs of rats with
severe acute pancreatitis. Exp Ther Med. 10:2029–2034. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Atal SS and Atal S: Ulinastatin - a newer
potential therapeutic option for multiple organ dysfunction
syndrome. J Basic Clin Physiol Pharmacol. 27:91–99. 2016.
View Article : Google Scholar
|
|
24
|
Zhou G, Xu Q, Liu Y, Wang Z, Su L and Guo
X: Protective effects of ulinastatin against acute lung injury
induced by heatstroke in mice. Nan Fang Yi Ke Da Xue Xue Bao.
35:1277–1282. 2015.In Chinese. PubMed/NCBI
|
|
25
|
Liu Z, Sun X, Tang J, Tang Y, Tong H, Wen
Q, Liu Y and Su L: Intestinal inflammation and tissue injury in
response to heat stress and cooling treatment in mice. Mol Med Rep.
4:437–443. 2011.PubMed/NCBI
|
|
26
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I. A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Rahman A, Fahlgren A, Sundstedt C,
Hammarström S, Danielsson A and Hammarström ML: Chronic colitis
induces expression of β-defensins in murine intestinal epithelial
cells. Clin Exp Immunol. 163:123–130. 2011. View Article : Google Scholar :
|
|
29
|
Gathiram P, Wells MT, Raidoo D, Brock-Utne
JG and Gaffin SL: Portal and systemic plasma lipopolysaccharide
concentrations in heat-stressed primates. Circ Shock. 25:223–230.
1988.PubMed/NCBI
|
|
30
|
Tang J, Jiang Y, Tang Y, Chen B, Sun X, Su
L and Liu Z: Effects of propofol on damage of rat intestinal
epithelial cells induced by heat stress and lipopolysaccharides.
Braz J Med Biol Res. 46:507–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Y, Zhou G, Wang Z, Guo X, Xu Q, Huang
Q and Su L: NF-κB signaling is essential for resistance to heat
stress-induced early stage apoptosis in human umbilical vein
endothelial cells. Sci Rep. 5:135472015. View Article : Google Scholar
|
|
32
|
Hall DM, Baumgardner KR, Oberley TD and
Gisolfi CV: Splanchnic tissues undergo hypoxic stress during whole
body hyperthermia. Am J Physiol. 276:G1195–G1203. 1999.PubMed/NCBI
|
|
33
|
Yin P, Xu J, He S, Liu F, Yin J, Wan C,
Mei C, Yin Y, Xu X and Xia Z: Endoplasmic reticulum stress in heat-
and shake-induced injury in the rat small intestine. PLoS One.
10:e01439222015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johnson JS, Sapkota A and Lay DC Jr: Rapid
cooling after acute hyperthermia alters intestinal morphology and
increases the systemic inflammatory response in pigs. J Appl
Physiol. 120:1249–1259. 2016. View Article : Google Scholar
|
|
35
|
Starkie RL, Hargreaves M, Rolland J and
Febbraio MA: Heat stress, cytokines, and the immune response to
exercise. Brain Behav Immun. 19:404–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Welc SS, Judge AR and Clanton TL: Skeletal
muscle interleukin-6 regulation in hyperthermia. Am J Physiol Cell
Physiol. 305:C406–C413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dupont A, Heinbockel L, Brandenburg K and
Hornef MW: Antimicrobial peptides and the enteric mucus layer act
in concert to protect the intestinal mucosa. Gut Microbes.
5:761–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ayabe T, Satchell DP, Wilson CL, Parks WC,
Selsted ME and Ouellette AJ: Secretion of microbicidal
alpha-defensins by intestinal Paneth cells in response to bacteria.
Nat Immunol. 1:113–118. 2000. View
Article : Google Scholar
|
|
39
|
Wehkamp J, Schmid M and Stange EF:
Defensins and other antimicrobial peptides in inflammatory bowel
disease. Curr Opin Gastroenterol. 23:370–378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mastroianni JR and Ouellette AJ:
Alpha-defensins in enteric innate immunity: Functional Paneth cell
alpha-defensins in mouse colonic lumen. J Biol Chem.
284:27848–27856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mattar EH, Almehdar HA, Yacoub HA, Uversky
VN and Redwan EM: Antimicrobial potentials and structural disorder
of human and animal defensins. Cytokine Growth Factor Rev.
28:95–111. 2016. View Article : Google Scholar
|
|
42
|
Zhang C, Wang Y, Fu W, Zhang W, Wang T and
Qin H: A meta-analysis on the effect of ulinastatin on serum levels
of C-reactive protein, interleukin 6, and tumor necrosis factor
alpha in Asian patients with acute pancreatitis. Genet Test Mol
Biomarkers. 20:118–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang Y, Xie K, Zhang J, Dang Y and Qiong
Z: Prospective clinical and experimental studies on the
cardioprotective effect of ulinastatin following severe burns.
Burns. 34:674–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Masuda T, Sato K, Noda C, Ikeda KM,
Matsunaga A, Ogura MN, Shimizu K, Nagasawa H, Matsuyama N and Izumi
T: Protective effect of urinary trypsin inhibitor on myocardial
mitochondria during hemorrhagic shock and reperfusion. Crit Care
Med. 31:1987–1992. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang LZ, Luo MY, Zhang JS, Ge FG, Chen JL
and Zheng CQ: Effect of ulinastatin on serum inflammatory factors
in Asian patients with acute pancreatitis before and after
treatment: A meta-analysis. Int J Clin Pharmacol Ther. 54:890–898.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang L, Huang X, Kong G, Xu H, Li J, Hao
D, Wang T, Han S, Han C, Sun Y, et al: Ulinastatin attenuates
pulmonary endothelial glycocalyx damage and inhibits endothelial
heparanase activity in LPS-induced ARDS. Biochem Biophys Res
Commun. 478:669–675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin B, Liu Y, Li T, Zeng K, Cai S, Zeng Z,
Lin C, Chen Z and Gao Y: Ulinastatin mediates protection against
vascular hyperpermeability following hemorrhagic shock. Int J Clin
Exp Pathol. 8:7685–7693. 2015.PubMed/NCBI
|
|
48
|
Kato Y, Kudo M, Shinkawa T, Mochizuki H,
Isaji M, Shiromizu I and Hoshida K: Role of O-linked carbohydrate
of human urinary trypsin inhibitor on its lysosomal
membrane-stabilizing property. Biochem Biophys Res Commun.
243:377–383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lehrer RI: Multispecific myeloid
defensins. Curr Opin Hematol. 14:16–21. 2007. View Article : Google Scholar
|
|
50
|
Gao C, Li R and Wang S: Ulinastatin
protects pulmonary tissues from lipopolysaccharide-induced injury
as an immunomodulator. J Trauma Acute Care Surg. 72:169–176.
2012.
|
|
51
|
Kobayashi KS, Chamaillard M, Ogura Y,
Henegariu O, Inohara N, Nuñez G and Flavell RA: Nod2-dependent
regulation of innate and adaptive immunity in the intestinal tract.
Science. 307:731–734. 2005. View Article : Google Scholar : PubMed/NCBI
|