Introduction
Vibrio vulnificus, a gram-negative bacterium,
causes severe to life-threatening infections in susceptible
individuals who have chronic liver disease following consumption of
V. vulnificus-containing seafood or acquisition of wound
infection. V. vulnificus infection can also cause several
diseases, including primary septicemia, gastroenteritis and
necrotizing fasciitis (1).
Dendritic cells (DCs), which function as professional antigen
presenting cells, are important in the link between innate and
adaptive immune responses. The cytokines secreted by activated DCs
are important factors determining the fate of CD4+ T
helper (Th) subsets. For example, interleukin-12 (IL-12), IL-4 and
IL-6, in addition to transforming growth factor-β (TGF-β), are
required for the maturation of Th1, Th2 and Th17 cells,
respectively (2). IL-12 is
released by Mycobacterium tuberculosis-infected DCs,
polarizing naïve CD4+ T cells to Th1 cells (3). In addition to the increase in Th1
cells by IL-12, the polarization of Th17 cells is induced by IL-6
derived from Streptococcus pyogenes-infected DCs (4).
Several effector Th cells are involved in inducing
immune responses against infection. For example, Th1 and Th2 cells
provide immune responses against intracellular bacterial infections
and parasitic pathogens, respectively (5). Th1 cells, associated with cellular
immune responses, are induced upon infection with several pathogens
(6). Th17 cells are critical in
extracellular bacterial and fungal pathogen infections (7,8).
Th17 cells are induced by extracellular bacteria, which adhere to
epithelial and mucosal barriers, including the intestine, lung and
skin, and are involved in host defense against infection (9). For example, Citrobacter
rodentium infection causes an increase in Th17 cells, inducing
the production of IL-22 in Peyer's patches and colonic epithelial
cells (10,11). Despite the importance of Th17
cells in protecting the host from bacterial infection, there are
several studies that dysregulated Th17 cell responses can cause
tissue damage, promoting the penetration of pathogens. For example,
in the case of infection with pathogens, including Borrelia
burgdorferi, Helicobacter pylori and Candida
albicans, increased Th17 cells promote inflammation, resulting
in tissue damage and exacerbated pathology (12–14). In addition, the significantly
elevated levels of proinflammatory cytokines associated with
excessive Th17 cells may contribute to sepsis, which is caused by
dysregulated host immune responses against infection (15,16). Despite extensive studies
investigating the induction of Th17 cells by certain extracellular
bacteria, whether V. vulnificus infection increases the Th17
cell population in mice and whether the increased Th17 cell
population is associated with sepsis remain to be elucidated.
The present study aimed to determine whether V.
vulnificus infection modulated the responses of Th17 cells
in vitro and in vivo. It was found that V.
vulnificus infection activated DCs, leading to the upregulation
of IL-17+ CD4+ cell populations through the
DC-derived secretion of IL-6. Additionally, the oral administration
of mice with V. vulnificus increased the numbers of Th17
cells in the lamina propria of the small intestine, suggesting that
V. vulnificus may induce Th17 cell responses in vitro
and in vivo.
Materials and methods
Mice
Female C57BL/6 mice were purchased from Orient Bio,
Inc. (Seoul, Korea). All animals were used for experiments at 7–9
weeks of age (weight, 17–20 g). All mice were provided with ad
libitum access to a standard laboratory chow diet (cat. no.
1314; Altromin Spezialfutter GmbH & Co. KG, Lage,
Nordrhein-Westfalen, Germany) and water. The animals were housed in
an SPF facility under a strict light cycle (lights on at 07:00 a.m.
and off at 07:00 p.m.) at 22±1°C and 52.5±2.5% relative humidity,
and all animal experiments were ethically performed in accordance
with the guidelines of the Korea University Institutional Animal
Care and Use Committee (Seoul, Korea; approval no.
KUIACUC-2015-244, 2016-170).
Bacterial strain and culture
conditions
The V. vulnificus MO6-24/O wild-type (WT)
used in the present study was obtained from Professor Sang Ho Choi
of Seoul National University (Seoul, Korea) as described previously
(17,18). For the infection experiments, the
bacteria were grown overnight at 30°C in Luria-Bertani medium
supplemented with 2.0% NaCl (LBS medium) and the grown bacteria
were incubated in fresh LBS medium at 30°C, diluted to
~1.8×108 colony forming units (CFUs)/ml in LBS,
centrifuged at room temperature for 3 min at 2,420 × g, and
resuspended in antibiotic-free growth medium prior to infection of
DCs, or in phosphate-buffered saline (PBS) prior to infection of
mice.
Preparation of murine bone-marrow-derived
dendritic cells (BMDCs)
The BMDCs were generated using a method originally
described by Inaba et al (19) with modification. For the
generation of DCs, bone-marrow was isolated from the femurs and
tibiae of mice, and flushed with RPMI-1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with fetal bovine
serum (FBS) (10%; Gibco; Thermo Fisher Scientific, Inc.), 2-ME (50
mM; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), HEPES (10
mM; WelGENE, Daegu, Korea), penicillin (100 U/ml) and streptomycin
(0.1 mg/ml) (both from Invitrogen; Thermo Fisher Scientific, Inc.),
using a syringe equipped with a 26-gauge needle. Cell clusters were
dissociated by gentle pipetting, and the cell suspension was
filtered through a 70-µm cell strainer to remove debris. The red
blood cells were lysed in a lysing solution containing 0.15 M
NH4Cl, 1 mM KHCO3 and 0.1 mM EDTA, and
5×105 cells/ml of the bone marrow cells were seeded into
Petri dishes containing 10 ml growth medium with GM-CSF (10 ng/ml;
ProSpec, Rehovort, Israel). After 3 and 5 days of culture at 37°C
in a 5% CO2 incubator, 5 ml of fresh medium containing
10 ng/ml of GM-CSF was added. On day 7, the loosely adherent DC
aggregates were harvested, and 1.5–2×106 cells/ml were
seeded in a 24-well plate for subsequent experiments.
In vitro infection protocol
The DCs were grown for 7 days in the growth medium
at 37°C in a 5% CO2 incubator. On day 7, the cells were
seeded onto a 24-well plate (1.5–2×106 cells/ml) in
antibiotic-free growth medium. Prior to infection, the bacteria
were centrifuged at room temperature for 3 min at 2,420 × g,
resuspended and adjusted to 1.5–2×108 CFU/ml in
antibiotic-free RPMI-1640 medium. The DCs were then treated with
LPS (500 ng/ml) for 3–60 min or infected with V. vulnificus
at various multiplicities of infection (MOI; the ratio of bacteria
number to the number of BMDCs) for various infection durations. The
timing and doses of V. vulnificus infection were determined
based on the method described in previous studies on infected
macrophages or epithelial cell lines, with minor modification to
MOI 0–10 for 0–120 min (20–22). Following infection, the cells were
washed twice with PBS and incubated for 20 h in
antibiotic-containing growth medium at 37°C under 5%
CO2.
In vitro polarization of CD4+
T cells with DCs
The DCs were infected with V. vulnificus and
post-incubated for 20 h. CD4+ T cells were isolated from
the lymph nodes of the C57BL/6 female mice by magnetic bead
purification (MACS; Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany). In a co-culture system, naive CD4+ T cells
were mixed with V. vulnificus-infected DCs at a 5:1 ratio in
the presence of anti-CD3ε (cat. no. 553057) and CD-28 (cat. no.
553294) monoclonal antibodies (mAbs) (BD Biosciences, San Diego,
CA, USA). The two Abs were used at 1:1,000 dilution, at 4°C for
anti-CD3ε and 37°C for anti-CD28. After 3 days, the cells were
re-stimulated with 50 ng/ml PMA and 1 µg/ml ionomycin for 6 h at
37°C and 5% CO2 in the presence of 1 µl/ml Golgiplug.
After 6 h, the cells were harvested and stained with fluorescent
antibodies for analysis using flow cytometry. In additional
experiments, neutralizing mAbs against IL-1β (cat. no. 16-7012) and
IL-6 (cat. no. 16-7061) (1 µg/ml; eBioscience, Inc., San Diego, CA,
USA) were added to the DC-CD4+ T cell co-cultures at
37°C under 5% CO2.
In vivo polarization of CD4+ T
cells with DCs
Naïve C57BL/6 mice were injected subcutaneously in
each footpad with 100 µg of ovalbumin (OVA; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) in 2 mg aluminum hydroxide dissolved
in 100 µl of PBS. Following 7 days of immunization, the mice were
injected subcutaneously with 1×106 of the OVA (10
µg/ml)-pretreated DCs, which were either uninfected or were
infected with V. vulnificus (MOI 5; 30 min). At day 7
post-injection, the mice were sacrificed and cells were isolated
from draining lymph nodes of mice (2×106 cells/ml),
which were cultured for 3 days in the presence of OVA (100 µg/ml)
at 37°C in a 5% CO2 incubator. After 3 days, the cells
were re-stimulated for 6 h with 50 ng/ml PMA and 1 µg/ml ionomycin
at 37°C and 5% CO2 in the presence of 1 µl/ml Golgiplug.
After 6 h, the cells were harvested and stained with fluorescent
antibodies for analysis using flow cytometry.
In vivo infection protocol and lamina
propria cell isolation
The mice were administered with 50 µg/ml rifampicin
for 24 h to ablate normal flora. Food was removed from the cages of
mice to empty their stomachs for 12 h prior to inoculation.
Subsequently, 50 µl of 8.5% (w/v) NaHCO3 was
administered orally, immediately followed by 100 µl of bacterial
suspension containing 1×107 CFU of V. vulnificus.
At day 2 post-infection, the mice were sacrificed and lamina
propria cells were isolated from the small intestine, as described
previously (23). Briefly, the
small intestines from the mice were washed in cold PBS for feces
clearance. Fat tissues and Peyer's patches were resected, and the
intestines were cut longitudinally, followed by washing in cold
PBS. The intestines were then cut into 2–3 cm segments and
incubated for 15 min RPMI medium containing 1 mM EDTA with gentle
stirring at 37°C, followed by washing with warm PBS. The incubation
was performed twice in medium containing EDTA. Subsequently, the
tissues were cut finely and incubated for 30 min in RPMI containing
collagenase D (0.1 mg/ml; Roche Diagnostics, Basel, Switzerland) at
37°C with gentle stirring. The incubated supernatants were then
collected and passed through a 70-µm cell strainer, and the
unfractionated cells were centrifuged at 4°C for 3 min at 400 × g.
The lamina propria lymphocytes were isolated by gradient
centrifugation with 40 and 85% Percoll gradient media (GE
Healthcare Life Sciences, Little Chalfont, UK).
Flow cytometric analysis
Antibodies were used at 1:250 dilution for both
surface and intracellular staining. The following mouse mAbs were
used for flow cytometry: CD4-FITC (cat. no. 553047), CD11c-FITC
(cat. no. 553801), interferon-γ (IFN-γ)-PE (cat. no. 554412),
IL-4-PE (cat. no. 554435), CD40-PE (cat. no. 553791), CD80-PE (cat.
no. 553769), I-Ab-PE (cat. no. 553552) (BD Biosciences), IL-17A-APC
(cat. no. 17-7177), Forkhead Box p3 (Foxp3)-APC (cat. no. 17-5773)
(eBioscience, Inc., San Diego, CA, USA) in pretitrated
concentrations. For the intracellular detection of IFN-γ, IL-17A,
IL-4 and Foxp3, the cells were re-stimulated with 50 ng/ml PMA and
1 µg/ml ionomycin for 6 h at 37°C and 5% CO2 in the
presence of 1 µl/ml Golgiplug. Subsequently, the cells were stained
intracellularly following permeabilization of the cells using
Cytofix/Cytoperm kits (BD Biosciences). The stained cells were
detected using a flow cytometer (FACSCalibur or BD Accuri C6 Plus;
BD Biosciences) gated on live CD11c+ or CD4+
cells. The data were analyzed using CellQuest software version
4.0.2 (BD Biosciences).
Semi quantitative reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was obtained from the cells using TRIzol
reagent and reverse transcribed into cDNA using a RocketScript™
Reverse Transcriptase kit (E-3162; Bioneer Corporation, Daejeon,
Korea). PCR was conducted using 1 µl of each 5′ and 3′ primer, 1 µl
of cDNA. dH2O was then added to a final volume of 20 µl.
PCR amplification of the cDNA was then performed using
AccuPower® PCR PreMix (K-2016; Bioneer Corporation) with
a thermal cycler (MJ Research, Inc., Watertown, MA, USA) or Bioneer
Corporation (Bioneer Corporation). The sequences of the PCR primers
used in the present study were as follows: Murine IL-1β forward,
5′-CTAAAGTATGGGCTGGACTG-3′ and reverse, 5′-AGCTTCAATGAAAGACCTCA-3′;
murine IL-6 forward, 5′-TGAACAACGATGATGCACTT-3′ and reverse,
5′-CGTAGAGAACAACATAAGTC-3′; murine IL-12p35 forward,
5′-TCAGCGTTCCAACAGCCTC-3′ and reverse, 5′-CGCAGAGTCTCGCCATTATG-3′;
murine IL-12p40 forward, 5′-TTATGCAAATTGTGAGCTTG-3′ and reverse,
5′-AGCTTCTTCATGTCTCCAAA-3′; murine IL-23p19 forward,
5′-AGCGGGACATATGAATCTAC-3′ and reverse, 5′-TAAGCTGTTGGCACTAAGGG-3′;
murine TGF-β forward, 5′-TATAGCAACAATTCCTGGCG-3′ and reverse,
5′-TCCTAAAGTCAATGTACAGC-3′; murine IFN-γ forward,
5′-TGCATCTTGGCTTTGCAGCTCTTCCTCATGGC-3′ and reverse,
5′-TGGACCTGTGGGTTGTTGACCTCAAACTTGGC-3′; murine IL-17A forward,
5′-CGCAAAAGTGAGCTCCAGAA-3′ and reverse, 5′-TGAAAGTGAAGGGGCAGCTC-3′;
murine β-actin forward, 5′-TGGAATCCTGTGGCATCCATGAAA-3′ and reverse,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. The temperature condition for PCR
amplification was 95°C for 5 min; followed by 28–36 cycles
consisting of 95°C for 30 sec, 55–61°C for 30 sec, and 72°C for 30
sec; plus a final cycle of 72°C for 5 min. Following amplification,
the products were separated on 1.5% (w/v) agarose gels and stained
with StainingSTAR (DyneBio, Gyeonggi-do, Korea).
Cytokine assays
The DCs were infected with V. vulnificus and
post-incubated in a 96-well plate (1×105 cells/ml) for
20 h. After 20 h, the cell supernatant was collected and the
secreted levels of cytokines were measured. The quantities of
IL-1β, IL-6, IL-12p40 and IL-12p70 in the culture supernatants were
determined using Mouse ELISA Ready-Set-Go! kits (IL-1β, IL-12p40
and IL-12p70; eBioscience, Inc.) and a mouse IL-6 ELISA kit (BD
Biosciences).
The cells isolated from the mesenteric lymph nodes,
Peyer's patches and lamina propria of the PBS- or V.
vulnificus-inoculated mice were seeded into a 96-well plate
(1×105 cells/ml) for 48 h in the presence of anti-CD3ε
(cat. no. 553057) and CD-28 (cat. no. 553294) mAbs (BD
Biosciences). Both Abs were used at 1:1,000 dilution, at 4°C for
anti-CD3ε and 37°C for anti-CD28. After 48 h, the cell supernatant
was collected and the secreted levels of cytokines were measured.
The quantities of IL-17A and IFN-γ in the culture supernatants were
determined using a Mouse ELISA Ready-Set-Go! kit (IL-17A;
eBioscience, Inc.) or sandwich ELISA with anti-mouse IFN-γ
monoclonal antibody (clone HB170, 1:1,000 dilution) for plate
coating and biotinylated secondary antibody (clone XMG1.2, 1:1,500
dilution). A standard curve was generated using recombinant IFN-γ
(BD Biosciences).
Statistical analysis
All values are expressed as the mean ± standard
deviation of at least three independent experiments. Student's
t-test in SigmaPlot version 12.5 (Systat Software Inc., Chicago,
IL, USA) was used to compare experimental groups with control
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
V. vulnificus infection induces the
maturation and activation of BMDCs. To determine whether V.
vulnificus infection affected the maturation and activation of
DCs, the DCs were infected with V. vulnificus at an MOI of
0–10 for 30 min or at an MOI of 1 for 0–2 h, and the expression of
cell surface molecules, CD40, CD80 and MHC II, were evaluated using
flow cytometric analysis (Fig.
1). As shown in Fig. 1A, the
expression levels of CD40, CD80 and MHC II were significantly
increased in the V. vulnificus-infected DCs in an
MOI-dependent manner. As the infection duration increased, the
expression of cell surface markers also increased (Fig. 1B). These results showed that V.
vulnificus infection induced the maturation and activation of
DCs, and suggested that V. vulnificus-infected DCs may
possess the ability to present antigen to naïve CD4+ T
cells.
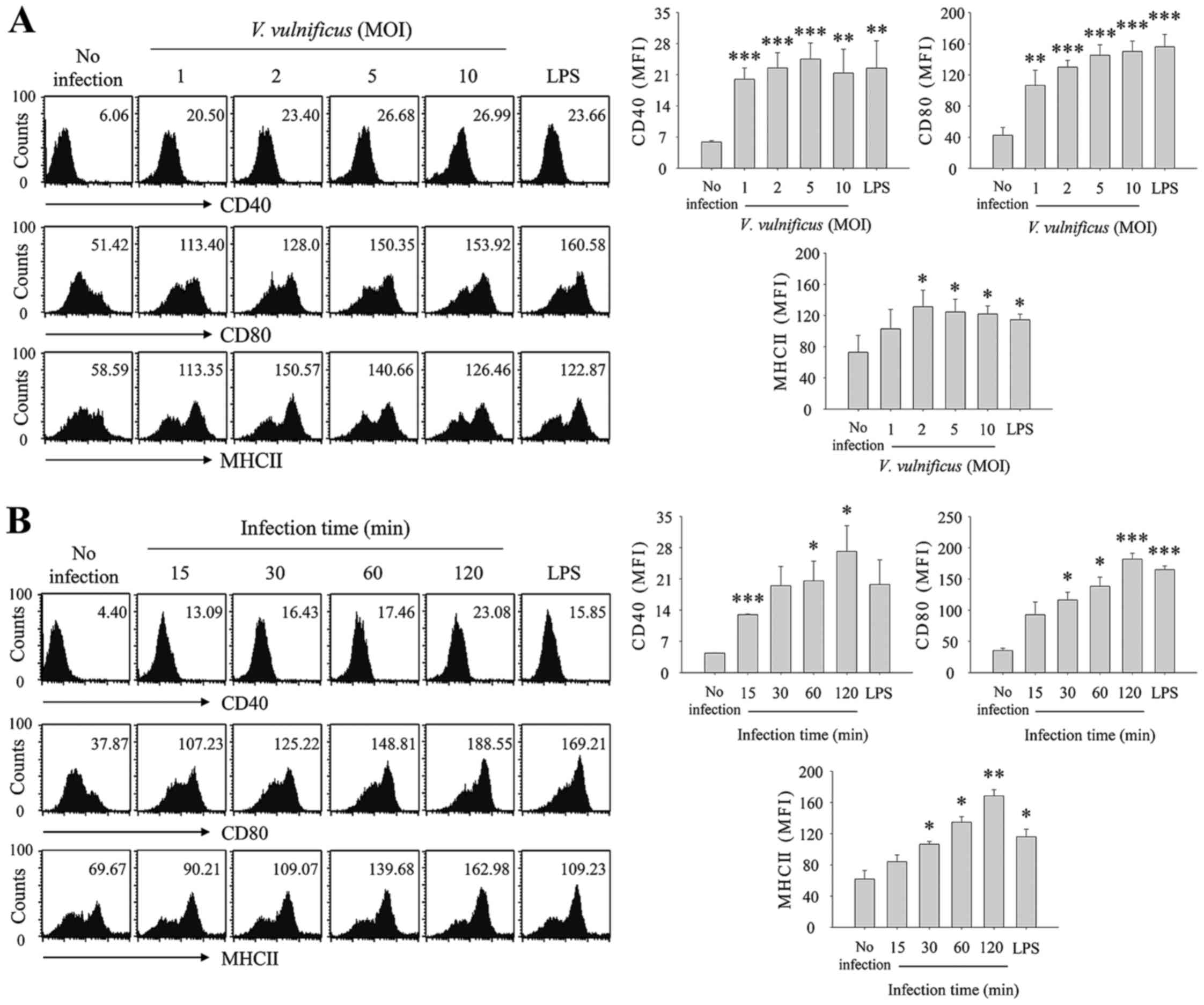 | Figure 1V. vulnificus infection
induces the maturation of bone marrow DCs. DCs were either infected
with V. vulnificus (A) at various MOIs (0, 1, 2, 5 and 10)
or were treated with LPS (500 ng/ml) for (A) 30 min, or were
infected with V. vulnificus (B) at an MOI of 1 for 0, 15,
30, 60 or 120 min or treated for 60 min with LPS (500 ng/ml). The
cells were stained with antibodies targeting CD40, CD80 and MHC II
for analysis using flow cytometry. The data shown are
representative of three independent experiments, and represent the
mean ± standard deviation of three independent experiments.
*P<0.05 vs. control (no infection);
**P<0.01 vs. control (no infection);
***P<0.005 vs. control (no infection). V.
vulnificus, Vibrio vulnificus; DCs, dendritic cells;
MOI, multiplicity of infection; LPS, lipopolysaccharide. |
V. vulnificus infection increases the
production of Th1/Th17-polarizing cytokines in DCs at mRNA and
protein levels. DCs are known to provide three signals to naïve
CD4+ T cells for differentiation into effector Th cells.
In particular, as the third signal, IL-12p70, IL-1β and IL-6 are
required for the polarization of Th1 and Th17 subsets. As shown in
Fig. 1, V. vulnificus
infection conferred DCs with the potential to differentiate naïve
CD4+ T cells. To investigate the ability of V.
vulnificus-infected DCs to provide CD4+ T cells with
differentiation signals, the expression of Th cell
polarization-associated cytokines in V. vulnificus-infected
DCs were measured at mRNA and secretion levels. As shown in
Fig. 2, infection of the DCs with
V. vulnificus for 30 min at various MOIs increased the
production of IL-1β, IL-6 and IL-12p35/40, which induced Th1 or
Th17 cell differentiation in an MOI-dependent manner (Fig. 2A). Consistent with these results,
the secretion levels of IL-1β, IL-6, IL-12p40 and IL-12p70, the
active form of IL-12, significantly increased as the MOI and
infection duration increased (Fig.
2B). These results suggested that the V.
vulnificus-infected DCs induced the polarization of naïve
CD4+ T cells into Th1 or Th17 cells by providing the
appropriate signals.
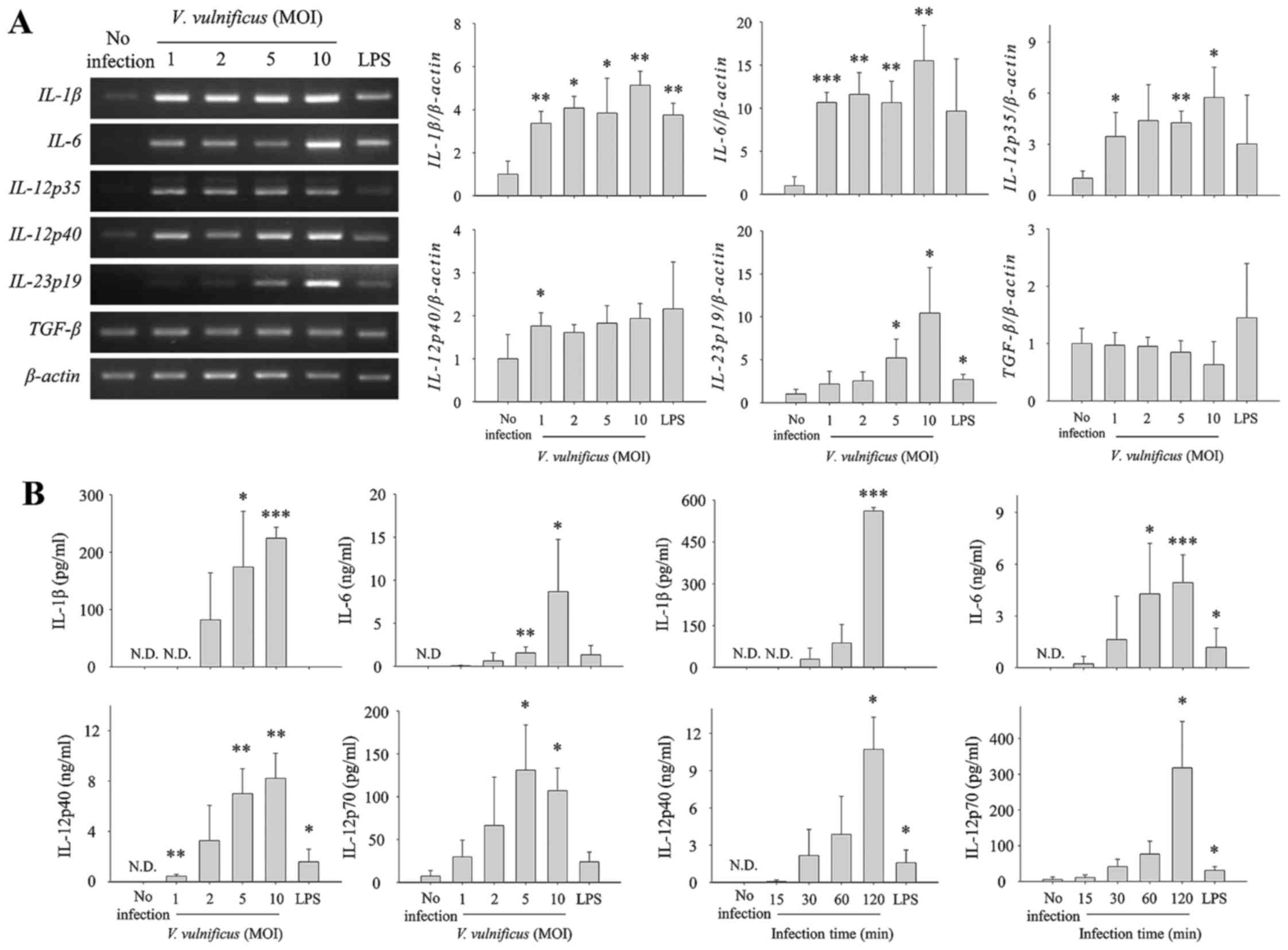 | Figure 2V. vulnificus infection
induces the expression and secretion of Th1/Th17-polarizing
cytokines in DCs. Total RNA was extracted from DCs infected with
V. vulnificus at various MOIs for 30 min. (A) Expression
levels of mRNAs of several Th-polarizing cytokines were determined
using semi-quantitative reverse transcription-polymerase chain
reaction analysis. The data shown are representative of three
independent experiments and represent the mean ± standard deviation
of three independent experiments. (B) DCs were infected for 30 min
with V. vulnificus at various MOIs, or at an MOI of 1 for
various incubation durations, or were treated for 60 min with LPS
(500 ng/ml). The supernatants were collected, and the protein
levels of IL-1β, IL-6, IL-12p40 and IL-12p70 were determined using
ELISA kits. The data shown in (B) represent the means ± SD of three
independent experiments. *P<0.05 vs. control (no
infection); **P<0.01 vs. control (no infection);
***P<0.005 vs. control (no infection). V.
vulnificus, Vibrio vulnificus; DCs, dendritic cells; Th,
T helper; MOI, multiplicity of infection; LPS, lipopolysaccharide;
IL, interleukin; TGF, transforming growth factor; ND, not
detected. |
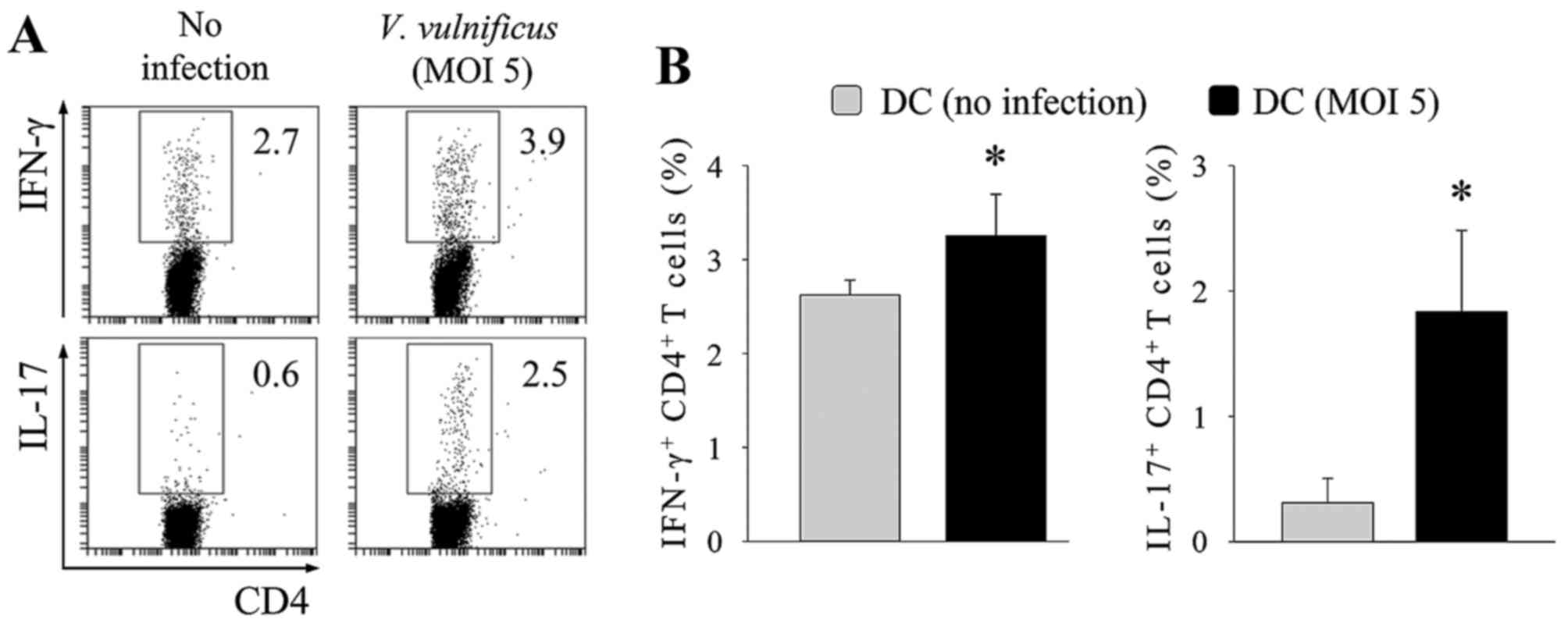
V. vulnificus-infected DCs induce Th17
polarization predominantly through the secretion of IL-6. The above
results demonstrated that the V. vulnificus-infected DCs
acquired the ability to efficiently differentiate naïve
CD4+ T cells into Th1 or Th17 cells. To determine
whether V. vulnificus infection enabled DCs to induce the
polarization of naive CD4+ T cells into Th1 or Th17
cells, the V. vulnificus-infected DCs were cocultured with
lymph node-derived naïve CD4+ T cells at a ratio of 1:5
under T-cell receptor stimulation (1 µg/ml anti-CD3ε and 1 µg/ml
CD28) for 3 days to assess the polarizing ability of DCs (Fig. 3). On day 3 of coculture, the
populations of IFN-γ+ or IL-17+
CD4+ cells were measured using flow cytometry. As shown
in Fig. 3, the V.
vulnificus-infected DCs induced higher frequencies of
IL-17+ CD4+ cells, compared with those of the
uninfected DCs, in an MOI-dependent manner. By contrast, the
population of IFN-γ+ CD4+ cells decreased
upon V. vulnificus infection (Fig. 3), whereas the percentage of
IL-4+ and Foxp3+ CD4+ cells
remained unchanged upon V. vulnificus infection (data not
shown).
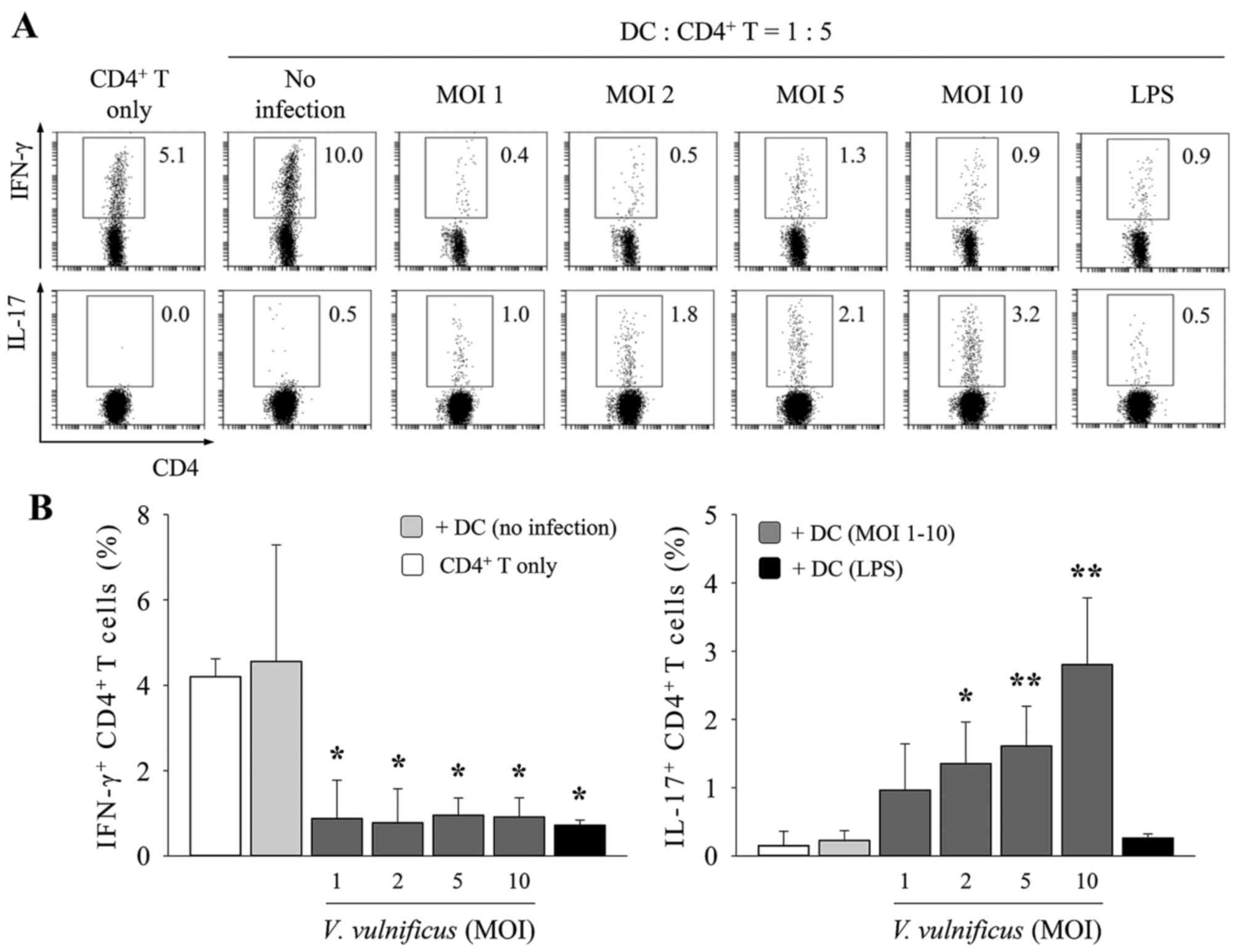 | Figure 3V. vulnificus-infected DCs
induce T helper cell 17 polarization in vitro. DCs were
infected with V. vulnificus at 0, 1, 2, 5 or 10 MOI or were
treated with LPS (500 ng/ml) for 60 min. The DCs were then
cocultured with naïve CD4+ T cells isolated from lymph
nodes for 3 days in the presence of anti-CD3ε and anti-CD28
monoclonal antibodies prior to (A) flow cytometric analysis (data
representative of three independent experiments) and (B)
determination of the expression of CD4, IFN-γ and IL-17 (data
presented as the mean ± standard deviation of three independent
experiments). *P<0.05 vs. control (no infection);
**P<0.01 vs. control (no infection). V.
vulnificus, Vibrio vulnificus; DCs, dendritic cells;
MOI, multiplicity of infection; LPS, lipopolysaccharide; IL-17,
interleukin-17; IFN-γ, interferon-γ. |
To investigate the mechanism by which V.
vulnificus infection mediates Th17 responses, IL-1β- and
IL-6-neutralizing antibodies were added to cocultures of V.
vulnificus-infected DCs and naïve CD4+ T cells. The
results showed that the addition of IL-1β-neutralizing antibodies
marginally reduced the frequencies of IL-17+
CD4+ cells, whereas the addition of IL-6-neutralizing
antibodies markedly inhibited the increase in IL-17+
CD4+ cells (Fig. 4).
As shown in Fig. 4C, the effects
of neutralizing antibodies increased as the concentration
increased. The addition of IL-6-neutralizing antibodies also
increased the population of IFN-γ+ CD4+ cells
in a concentration-dependent manner (data not shown). These results
indicated that IL-6 secreted by the V. vulnificus-infected
DCs was a critical factor, which significantly promoted the
induction of IL-17-producing CD4+ T cells, and that IL-6
in particular may inhibit the differentiation of IFN-γ+
CD4+ cells, consistent with previous findings (24).
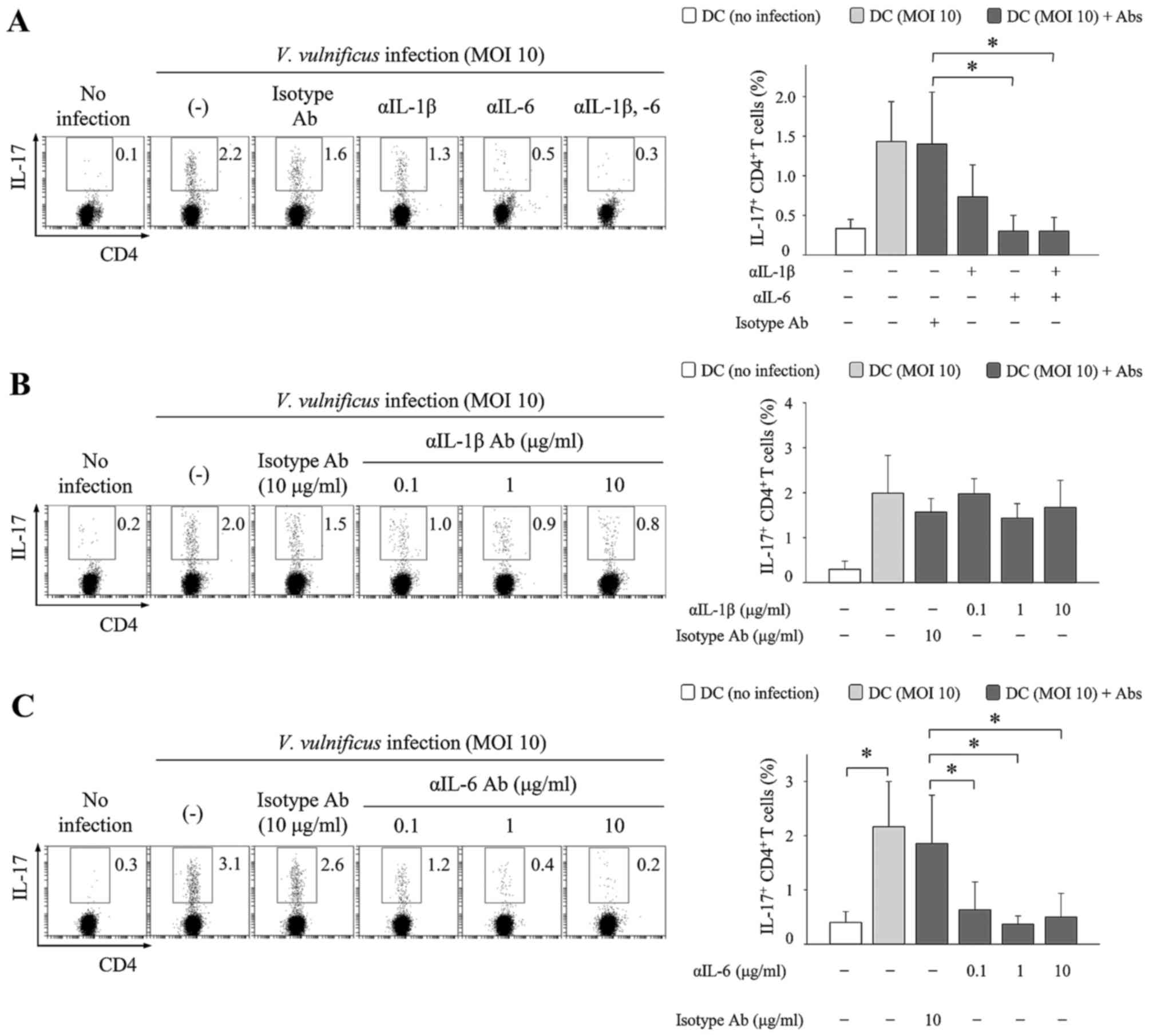 | Figure 4IL-6 is essential in V.
vulnificus infection-induced T helper cell 17 polarization. DCs
were infected with V. vulnificus at MOI of 1 for 60 min,
following which the DCs were cocultured with naïve CD4+
T cells isolated from lymph nodes for 3 days in the presence of
anti-CD3ε and anti-CD28 monoclonal antibodies, and (A) anti-IL-1β
(10 µg/ml), anti-IL-6 (10 µg/ml), or isotype antibodies (10 µg/ml),
or (B) anti-IL-1β and isotype-specific antibodies (0.1, 1 or 10
µg/ml), or (C) anti-IL-6 and isotype-specific antibodies (0.1, 1 or
10 µg/ml) (C). Data shown are representative of three independent
experiments and are presented as the mean ± standard deviation of
three independent experiments. *P<0.05. V.
vulnificus, Vibrio vulnificus; DCs dendritic cells; IL,
interleukin; MOI, multiplicity of infection; Ab, antibody. |
V. vulnificus-infected DCs induce the
polarization of Th17 cells in vivo. As shown in Figs. 1Figure 2Figure 3–4, it was demonstrated that the V.
vulnificus-infected DCs had the ability to polarize naïve
CD4+ T cells into IL-17-producing cells in vitro.
To confirm that V. vulnificus infection enables DCs to
induce the polarization of Th17 cells, V.
vulnificus-infected DCs were subcutaneously injected into
OVA-immunized mice, and the IL-17+ CD4+ cell
population was analyzed in isolated lymph node cells using flow
cytometry. As shown in Fig. 5,
the percentages of IL-17+ CD4+ cells
significantly increased in the mice injected with V.
vulnificus-infected DCs, whereas few IL-17+
CD4+ cells were detected in the mice injected with
immature DCs. These results indicated that the V.
vulnificus-infected DCs were capable of inducing Th17 responses
in vivo.
Oral administration of V. vulnificus in mice
increases the population of intestinal Th17 cells. The results of
the present study showed that V. vulnificus infection
promoted Th17 differentiation in vitro through antigen
presentation and the secretion of Th17-polarizing cytokines by
V. vulnificus-infected DCs. Therefore, to examine whether
V. vulnificus infection directed the fate of CD4+
T cells toward Th17 cells in vivo, mice were orally
administered with 107 CFUs of V. vulnificus. At 2
days post-infection, the mice were sacrificed and organs were
retrieved for the analysis of Th cell populations. As shown in
Fig. 6A, the mRNA expression of
IFN-γ was higher in the Peyer's patches and the lamina propria of
the V. vulnificus-infected mice, compared with that in the
uninfected mice. The levels of IL-17 were also increased in the
mesenteric lymph nodes, Peyer's patches and lamina propria of the
V. vulnificus-infected mice. Flow cytometric analysis of the
CD4+ T cells isolated from the small intestinal lamina
propria revealed that IL-17+ CD4+ cells were also
increased in V. vulnificus-infected mice, compared with
uninfected mice (Fig. 6B).
Elevated levels of IFN-γ and IL-17A in the V.
vulnificus-infected mice were also confirmed by ELISA (Fig. 6C), indicating that infection
through the ingestion of V. vulnificus-contaminated foods
may induce an increase in Th17 cells.
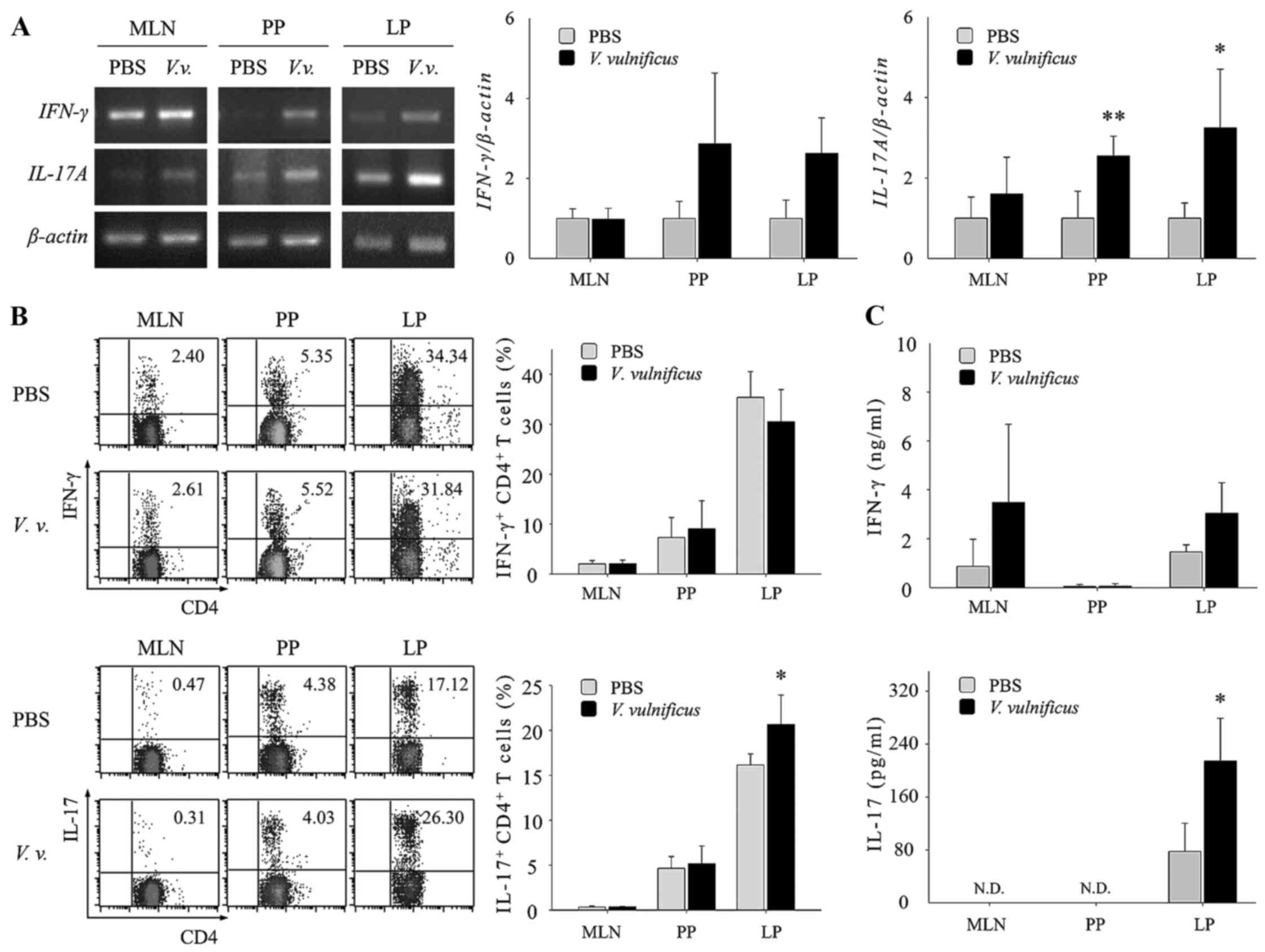 | Figure 6V. vulnificus infection in
mice increases the population of the Th17 subset in the small
intestines. (A) Mice were orally administered with V.
vulnificus (1×107 CFU/mouse). After 2 days, the mice
were sacrificed and total RNA was extracted from the MLN, PP and LP
of uninfected mice or the mice infected with V. vulnificus.
Semi-quantitative reverse transcription-polymerase chain reaction
analysis was performed to determine the mRNA expression levels of
Th1/Th17-associated genes. (B) Flow cytometric analysis of the
expression of CD4, IFN-γ and IL-17 in the MLN, PP and LP of
uninfected mice or mice infected with V. vulnificus. The
data shown are presented as the mean ± standard deviation of three
independent experiments. (C) Cells isolated from the MLN, PP and LP
were cultured for 48 h, and supernatants were collected. The
protein levels of IFN-γ and IL-17A were determined using ELISA
kits. *P<0.05 vs. control (PBS group);
**P<0.01 vs. control (PBS group). Th, T helper cell;
IL-17, interleukin-17; IFN-γ, interferon-γ; V.v, Vibrio
vulnificus; MLN, mesenteric lymph nodes; PP, Peyer's patches;
LP lamina propria; ND, not detected. |
Discussion
The present study demonstrated for the first time,
to the best of our knowledge, that V. vulnificus infection
induced Th17 responses by stimulating the maturation of DCs and the
expression of Th17-polarizing cytokines in DCs in vitro.
Additionally, the population of Th17 cells increased in the lamina
propria of the small intestines in V. vulnificus-inoculated
mice, compared with that in uninfected mice. V. vulnificus
is a gram-negative bacterium, which causes several diseases,
including gastroenteritis and sepsis, via the ingestion of
contaminated food or wound infection (1). Although the innate immune response
against V. vulnificus infection has been investigated, few
studies have described the adaptive immune response against V.
vulnificus infection. Therefore, in the present study, whether
the DC-mediated Th cell response occurs upon V. vulnificus
infection was investigated.
The mechanism by which V. vulnificus
infection increases Th17 cell responses remains to be fully
elucidated. However, the findings of the present study suggested
that the population of Th17 cells was increased by the secretion of
IL-1β and IL-6 from V. vulnificus-infected DCs. V.
vulnificus infection was shown to significantly induce the
maturation and activation of DCs, which secreted the
Th17-polarizing cytokines IL-1β and IL-6. Inhibiting IL-6 with
neutralizing anti-IL-6 mAb significantly decreased the Th17 cell
responses induced by V. vulnificus infection. In addition,
the oral administration of V. vulnificus increased Th17 cell
populations in mice, indicating that V. vulnificus induced
Th17 cell responses, predominantly via DC-derived IL-6.
The effects of V. vulnificus infection on Th1
responses in vitro and in vivo are likely to be
different. In a coculture system of V. vulnificus-infected
DCs and CD4+ T cells, the Th1 cell population was
significantly decreased upon V. vulnificus infection,
despite the observation that V. vulnificus increased the
expression of IL-12, a Th1-polarizing cytokine, in the DCs. The
decrease in the Th1 cell population by V. vulnificus
infection was recovered to the level observed in the uninfected
control by inhibiting IL-6 with neutralizing anti-IL-6 monoclonal
antibodies (data not shown), indicating that the V.
vulnificus-mediated inhibition of the Th1 cell population may
be due to the secretion of IL-6 from DCs. A previous study showed
that Th1 differentiation is negatively regulated by IL-6, a
Th17-polarizing cytokine, in the presence of anti-CD3ε/28
stimulation (24). Of note, the
oral administration of V. vulnificus in mice did not
decrease Th1 cell responses. This discrepancy between V.
vulnificus-induced Th1 effects in vitro and in
vivo may be due to the different environments and conditions
encountered during exposure of V. vulnificus to host cells.
In a coculture system of V. vulnificus-infected DCs with
CD4+ T cells, the infected DCs was in contact with
CD4+ T cells only, and the secreted factors were
restricted to the small space within the coculture wells; by
contrast, in the whole body, V. vulnificus is likely to come
into contact with several types of immune cell, including DCs, with
secreted factors rapidly dispersed throughout the body.
Several studies have demonstrated that Th17 cells
can be induced by a variety of bacterial infections. Induced Th17
cells have a positive or a negative effect on the host defense
mechanism against infection. In terms of the positive effects of
Th17 cells, increased Th17 cells provide protection to the host by
assisting in the clearance of pathogens. For example, in the
Peyer's patches of C. rodentium-infected mice, the
population of Th17 cells is increased, promoting the production of
IL-22, which is crucial in directly inducing the Reg family of
antimicrobial peptides, including RegIIIβ and RegIIIγ, in colonic
epithelial cells (10,11). However, if Th17 cell responses are
inappropriately regulated in infections, dysregulated Th17 cell
responses promote tissue damage, exacerbate inflammation and lead
to disease progression (13,14,25). Similarly, excessive Th17 cells
responses induced by pathogen infection, which are initially
designed to be critical in the clearance of pathogens, are known to
be important in sepsis. The neutralization of IL-17 improves sepsis
by suppressing plasma proinflammatory cytokines, including tumor
necrosis factor-α, IL-1β and IL-6. In addition, in LPS-stimulated
peripheral blood mononuclear cells from patients with sepsis, the
absence of IL-17 results in increased production of IL-10 and
decreased production of IL-12 (16,26). Whether the role of Th17 cells in
infections is protective or detrimental may depend on the type of
pathogen and the condition of infection.
Adaptive immune responses against infection with
other Vibrio species, including V. cholera, have also
been examined. V. cholera O395 is known to secrete outer
membrane vesicles (OMVs), which are internalized into intestinal
epithelial cells. These epithelial cells are stimulated by the
internalized OMVs and then activate DCs, resulting in upregulation
of the expression of costimulatory molecules and promoting the
release of Th17-polarizing cytokines; these activated DCs promote
the polarization of CD4+ T cells into inflammatory
Th2/Th17 cells (27). Similarly,
in the present study, the numbers of Th17 cells were increased upon
V. vulnificus infection. However, whether structural
components of V. vulnificus and/or secreted factors from
V. vulnificus infection are involved in the induction of
Th17 cell responses remains to be elucidated.
According to several studies, the upregulation of
certain virulence factor-associated genes depends on the contact of
host cells with pathogens (22,28). The expression of several virulence
factors of V. vulnificus is significantly increased upon
contact with host cells (29–31). Furthermore, V. vulnificus
has been reported to activate the host innate immune response by
the binding of lipoprotein and flagellin to toll-like receptor 2 or
5 (32,33). Therefore, induced virulence
factors and the detrimental function of Th17 cells may be involved
in aggravating the pathogenesis caused by V. vulnificus by
impeding the defensive role of Th17 cells.
In conclusion, the present study demonstrated that
DCs were activated upon V. vulnificus infection, resulting
in an induction of the Th17 response by producing Th17-polarizing
cytokines, including IL-1β and IL-6. The oral administration of
V. vulnificus in mice also induced an increase in the
numbers of intestinal Th17 cells. Taken together, the results
suggested that an understanding of the adaptive immune responses
against V. vulnificus infection may facilitate the
development of prophylactic and therapeutic agents against diseases
caused by V. vulnificus. In addition to the results obtained
in the present study, elucidating the roles of V. vulnificus
virulence factors in the pathogenesis of V. vulnificus and
the host immune response is crucial in future investigations on
V. vulnificus.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (grant no. NRF-2017R1A2B2009442), the Creative
Materials Discovery Program (grant no. 2016M3D1A1021387), the Korea
Drug Development Fund (grant no. KDDF-201612-09) and the
Agriculture, Food and Rural Affairs Research Center Support
Program, Ministry of Agriculture, Food and Rural Affairs, Korea
(grant no. 710002-07-7-HD120).
Abbreviations:
|
DC
|
dendritic cell
|
|
BMDC
|
bone-marrow-derived dendritic
cell
|
|
MOI
|
multiplicity of infection
|
References
|
1
|
Jones MK and Oliver JD: Vibrio vulnificus:
Disease and pathogenesis. Infect Immun. 77:1723–1733. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu J, Yamane H and Paul WE:
Differentiation of effector CD4 T cell populations (*). Annu Rev
Immunol. 28:445–489. 2010. View Article : Google Scholar
|
|
3
|
Hickman SP, Chan J and Salgame P:
Mycobacterium tuberculosis induces differential cytokine production
from dendritic cells and macrophages with divergent effects on
naive T cell polarization. J Immunol. 168:4636–4642. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Linehan JL, Dileepan T, Kashem SW, Kaplan
DH, Cleary P and Jenkins MK: Generation of Th17 cells in response
to intranasal infection requires TGF-β1 from dendritic cells and
IL-6 from CD301b+ dendritic cells. Proc Natl Acad Sci
USA. 112:12782–12787. 2015. View Article : Google Scholar
|
|
5
|
Kapsenberg ML: Dendritic-cell control of
pathogen-driven T-cell polarization. Nat Rev Immunol. 3:984–993.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizuno Y, Takada H, Nomura A, Jin CH,
Hattori H, Ihara K, Aoki T, Eguchi K and Hara T: Th1 and
Th1-inducing cytokines in Salmonella infection. Clin Exp Immunol.
131:111–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwakura Y, Nakae S, Saijo S and Ishigame
H: The roles of IL-17A in inflammatory immune responses and host
defense against pathogens. Immunol Rev. 226:57–79. 2008. View Article : Google Scholar
|
|
8
|
Kolls JK and Khader SA: The role of Th17
cytokines in primary mucosal immunity. Cytokine Growth Factor Rev.
21:443–448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atarashi K, Tanoue T, Ando M, Kamada N,
Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, et
al: Th17 cell induction by adhesion of microbes to intestinal
epithelial cells. Cell. 163:367–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Shi QG, Lin F, Liang YG, Sun LJ, Mu
JS, Wang YG, Su HB, Xu B, Ji CC, et al: Cytokine IL-6 is required
in Citrobacter rodentium infection-induced intestinal Th17
responses and promotes IL-22 expression in inflammatory bowel
disease. Mol Med Rep. 9:831–836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa
SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, et al:
Interleukin-22 mediates early host defense against attaching and
effacing bacterial pathogens. Nat Med. 14:282–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Codolo G, Amedei A, Steere AC, Papinutto
E, Cappon A, Polenghi A, Benagiano M, Paccani SR, Sambri V, Del
Prete G, et al: Borrelia burgdorferi NapA-driven Th17 cell
inflammation in lyme arthritis. Arthritis Rheum. 58:3609–3617.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Liu XF, Zhuang Y, Zhang JY, Liu T,
Yin Z, Wu C, Mao XH, Jia KR, Wang FJ, et al: Helicobacter
pylori-induced Th17 responses modulate Th1 cell responses, benefit
bacterial growth, and contribute to pathology in mice. J Immunol.
184:5121–5129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zelante T, De Luca A, Bonifazi P,
Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C,
Mosci P, et al: IL-23 and the Th17 pathway promote inflammation and
impair anti-fungal immune resistance. Eur J Immunol. 37:2695–2706.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flierl MA, Rittirsch D, Gao H, Hoesel LM,
Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL,
et al: Adverse functions of IL-17A in experimental sepsis. FASEB J.
22:2198–2205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JH, Cho YJ, Chun J, Seok YJ, Lee JK,
Kim KS, Lee KH, Park SJ and Choi SH: Complete genome sequence of
Vibrio vulnificus MO6-24/O. J Bacteriol. 193:2062–2063. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wright AC, Simpson LM, Oliver JD and
Morris JG Jr: Phenotypic evaluation of acapsular transposon mutants
of Vibrio vulnificus. Infect Immun. 58:1769–1773. 1990.PubMed/NCBI
|
|
19
|
Inaba K, Inaba M, Romani N, Aya H, Deguchi
M, Ikehara S, Muramatsu S and Steinman RM: Generation of large
numbers of dendritic cells from mouse bone marrow cultures
supplemented with granulocyte/macrophage colony-stimulating factor.
J Exp Med. 176:1693–1702. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toma C, Higa N, Koizumi Y, Nakasone N,
Ogura Y, McCoy AJ, Franchi L, Uematsu S, Sagara J, Taniguchi S, et
al: Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins
and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa
B signaling. J Immunol. 184:5287–5297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chuang CC, Chuang YC, Chang WT, Chen CC,
Hor LI, Huang AM, Choi PC, Wang CY, Tseng PC and Lin CF: Macrophage
migration inhibitory factor regulates interleukin-6 production by
facilitating nuclear factor-kappa B activation during Vibrio
vulnificus infection. BMC Immunol. 11:502010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee BC, Lee JH, Kim MW, Kim BS, Oh MH, Kim
KS, Kim TS and Choi SH: Vibrio vulnificus rtxE is important for
virulence, and its expression is induced by exposure to host cells.
Infect Immun. 76:1509–1517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weigmann B, Tubbe I, Seidel D, Nicolaev A,
Becker C and Neurath MF: Isolation and subsequent analysis of
murine lamina propria mononuclear cells from colonic tissue. Nat
Protoc. 2:2307–2311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diehl S, Anguita J, Hoffmeyer A, Zapton T,
Ihle JN, Fikrig E and Rincón M: Inhibition of Th1 differentiation
by IL-6 is mediated by SOCS1. Immunity. 13:805–815. 2000.
View Article : Google Scholar
|
|
25
|
Jin W and Dong C: IL-17 cytokines in
immunity and inflammation. Emerg Microbes Infect. 2:e602013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu HP, Shih CC, Chu CM, Huang CY, Hua CC,
Liu YC and Chuang DY: Effect of interleukin-17 on in vitro cytokine
production in healthy controls and patients with severe sepsis. J
Formos Med Assoc. 114:1250–1257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chatterjee D and Chaudhuri K: Vibrio
cholerae O395 outer membrane vesicles modulate intestinal
epithelial cells in a NOD1 protein-dependent manner and induce
dendritic cell-mediated Th2/Th17 cell responses. J Biol Chem.
288:4299–4309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dey AK, Bhagat A and Chowdhury R: Host
cell contact induces expression of virulence factors and VieA, a
cyclic di-GMP phosphodiesterase, in Vibrio cholerae. J Bacteriol.
195:2004–2010. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim SM, Park JH, Lee HS, Kim WB, Ryu JM,
Han HJ and Choi SH: LuxR homologue SmcR is essential for Vibrio
vulnificus pathogenesis and biofilm detachment, and its expression
is induced by host cells. Infect Immun. 81:3721–3730. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lim JG, Choi SH and Camilli A: IscR is a
global regulator essential for pathogenesis of Vibrio vulnificus
and induced by host cells. Infect Immun. 82:569–578. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oh MH, Jeong HG and Choi SH: Proteomic
identification and characterization of Vibrio vulnificus proteins
induced upon exposure to INT-407 intestinal epithelial cells. J
Microbiol Biotechnol. 18:968–974. 2008.PubMed/NCBI
|
|
32
|
Goo SY, Han YS, Kim WH, Lee KH and Park
SJ: Vibrio vulnificus IlpA-induced cytokine production is mediated
by Toll-like receptor 2. J Biol Chem. 282:27647–27658. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ,
Ahn OS, Lee JJ, Song HC, Kim JM, Choy HE, et al: A bacterial
flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant
activity to induce protective immunity. Infect Immun. 74:694–702.
2006. View Article : Google Scholar :
|