Introduction
A low level of radiation exists extensively in our
living and working environment, particularly in hospital-based
radio therapy facilities, inevitably increasing the risk of
constant exposure to low-dose ionizing radiation (LDIR). Unlike
high-dose ionizing radiation (HDIR), LDIR has been proven to have
different biological functions, such as hormesis (1,2)
and the adoptive effect (3). Our
previous studies indicated that LDIR stimulates the proliferation
of normal cells, including rat mesenchymal stem cells, mouse bone
marrow hematopoietic progenitor cells and several normal human cell
lines, such as MRC-5, HL-7702, 293T and 6550 HLEPiC. However, it
does not stimulate the proliferation of cancer cell lines, such as
K562, HL-60, NCI-H446, BEL7402, U251, HCT-8 and HeLa (4–6).
We also demonstrated that LDIR augments the expansion and
cytotoxicity of cultured human natural killer cells in vitro
(7).
Prostate cancer is the second most frequently
diagnosed cancer in men worldwide (8). Radiation therapy is one of the most
common methods for the treatment of prostate cancer in clinical
practice, usually at doses >70 Gy (9). However, the biological effect of
LDIR on prostate cancer is seldom investigated, even in
vitro. Since it was demonstrated that cells exposed to LDIR may
exhibit differential responses, it would be of interest to
investigate the molecular changes in prostate cancer cells
following LDIR.
Ataxia-telangiectasia mutated (ATM) is a crucial
factor involved in the processing of DNA damage, maintenance of
genome stability and control of cell cycle progression (10). According to Suzuki et al,
phosphorylated ATM foci were detected immediately after normal
human diploid cells were irradiated by LDIR (11). As a radiation sensor, the
activation of ATM is proven to be an early event and will interact
with several cell signaling pathways, such as Akt and
mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase (ERK) (12,13).
p53 is a key factor in the process of radiation
response, controlling the activation of DNA repair and cell
apoptosis pathways following acute radiation injury (14,15). Furthermore, the p53 gene is
the most frequent target for mutation and deletion in human
cancers, with over half of all tumors exhibiting p53 mutations
(16). Therefore, investigating
the behavior of p53null prostate tumor cells and
elucidating the role of p53 in normal prostate cells after LDIR,
may prove valuable for the radiation treatment of prostate
cancer.
Materials and methods
Cell cultivation and treatments
The human prostate cancer cell line PC-3 and the
normal prostate cell line RWPE-1 were purchased from American Type
Culture Collection (Manassas, VA, USA). PC-3 was maintained in
Dulbecco's modified Eagle's medium (DMEM; Life Technologies, Thermo
Fisher Scientific, Shanghai, China) supplemented with 10% fetal
bovine serum (HyClone, Beijing, China) and 1% antibiotics
(penicillin-streptomycin; Invitrogen, Thermo Fisher Scientific,
Carlsbad, CA, USA). RWPE-1 cells were maintained in
Keratinocyte-Serum Free Media (KSFM; Life Technologies, Thermo
Fisher Scientific, Grand Island, NY, USA) supplemented with 20
mg/ml bovine pituitary extract and 2 ng/ml epidermal growth factor
(both from Invitrogen, Thermo Fisher Scientific). All cells were
cultured at 37°C in a humidified incubator with a constant air flow
of 5% CO2.
In order to inhibit the function of p53 and ATM, 10
μM pifithrin-α (PFT-α; Beyotime Institute of Biotechnology,
Haimen, China) dissolved in dimethyl sulfoxide and 5 mM caffeine
(both from Sigma-Aldrich, Merck KGaA, Shanghai, China) dissolved in
distilled water were added to the cell culture media 24 and 2 h
prior to irradiation. Media containing the chemical inhibitors were
replaced with fresh medium immediately after the irradiation.
Irradiation strategy
Monolayer cells were irradiated at a dose rate of
12.5 mGy/min by X-RAD 320 (Precision X-Ray, North Branford, CT,
USA). The total dose was 20, 50, 75 or 100 mGy. After that, cell
culture media were replaced and cells were harvested immediately or
continually cultured until the next step experiment was performed.
Control groups were treated similarly, except for the
irradiation.
Cell proliferation assay
At 24 h prior to irradiation, 3×103 PC-3
and RWPE-1 cells were seeded in 96-well plates and then irradiated
with 20, 50, 75 or 100 mGy of X-rays. After the irradiation, the
cells were immediately transferred to the incubator and cultured
for a further 12, 24, 48 or 72 h. Cell proliferation assays were
determined using WST-1 Cell Proliferation reagent (Roche, Shanghai,
China). According to the manufacturer's instructions, 20 μl
WST-1 were added to 200 μl cell culture medium and then
incubated in the dark for 2 h. Subsequently, the absorbance at 450
and 630 nm was measured by a microplate reader (Synergy HT; BioTek,
Beijing, China). The final optical density (OD) was designated as
OD450-OD630-ODblank.
Cell cycle assay
Approximately 2×106 irradiated or
sham-irradiated cells were collected and washed with 1 ml cold
phosphate-buffered saline (PBS) twice to remove residual trypsin
and serum. The cells were pelleted and resuspended in 1 ml fixation
solution (300 μl PBS and 700 μl ethanol). Following
incubation at 4°C for 4 h, the cells were centrifuged at 250 × g
for 5 min and the fixation solution was removed. After washing
twice with 1 ml PBS, the cells were pelleted and suspended in 0.5
ml propidium iodide (PI; Sigma-Aldrich, Merck KGaA) staining
solution (50 μg/ml PI, 20 μg/ml RNase A and 0.2%
Triton X-100) and incubated in the dark at 37°C for 30 min. Cell
suspensions were filtered through a 400-mesh sieve prior to being
analyzed by a BD FACSCalibur flow cytometer (Becton-Dickinson,
Sparks, MD, USA).
Protein extraction and western blot
analysis
Cell total protein was extracted with RIPA buffer
(Beyotime Institute of Biotechnology) supplemented with cocktail
protease inhibitor (Roche), and the protein concentration was
determined by bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology).
A total of 5–40 μg cell total protein was
separated by 4–8%, 5–10% or 5–15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and was then
electrophoretically transferred to polyvinylidene difluoride
membranes (0.45 μm; Millipore, Billerica, MA, USA) and
blocked at 37°C for 1 h with 5% skimmed milk in TBST [TBS (10
mmol/l Tris pH 7.5, 150 mmol/l NaCl) containing 0.1% Tween-20].
Subsequently, the membranes were incubated with the following
antibodies: mouse monoclonal antibody against p53 (DO7; sc-47698,
1:1,500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
rabbit monoclonal antibody against phospho-p53 (Ser15; D4S1H; cat.
no. 12571, 1:1,000; Cell Signaling Technology, Danvers, MA, USA),
rabbit polyclonal antibody against p21 (C-19; sc-397, 1:500; Santa
Cruz Biotechnology, Inc.), rabbit monoclonal antibody against ATM
(Y170; ab32420, 1:1,000; Abcam, Shanghai, China) and mouse
monoclonal antibody against β-actin (C-2; sc-8432, 1:3,000; Santa
Cruz Biotechnology, Inc.) at 4°C overnight. After washing for 3×5
min with TBST, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-mouse (ZB-2305) or goat anti-rabbit
(ZB-2301) secondary antibodies [IgG (H+L); 1:5,000; ZSGB-BIO,
Beijing, China] at 37°C for 1 h. After washing for another 3 times,
the immunocomplexes were detected with enhanced chemiluminescence
(Thermo Fisher Scientific, Beijing, China) and X-ray film (Kodak,
Beijing, China). Protein expression levels were determined
semiquantitatively by densitometric analysis with the Quantity One
software (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All data and results were calculated from at least
three replicate measurements and presented as means ± standard
deviation. The significance was determined by the Student's t-test
using SPSS 20.0 software (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered statistically significant (P<0.05 and P<0.01).
Results
LDIR induces differential cell growth in
prostate cancer PC-3 and normal RWPE-1 cells
In order to investigate the effect of LDIR on
prostate cancer PC-3 and normal RWPE-1 cells, four different doses
of LDIR were delivered, namely 20, 50, 75 and 100 mGy. Cell
proliferation was determined by WST-1 24 h post-LDIR. It was
observed that LDIR doses of 50, 75 and 100 mGy significantly
inhibited the growth of PC-3 cells compared with the
sham-irradiated group (P<0.01; Fig. 1A, left panel). Among these, the
75-mGy dose exerted the most prominent effect. By contrast, none of
the radiation doses affected the growth of RWPE-1 cells (P>0.05;
Fig. 1A, right panel).
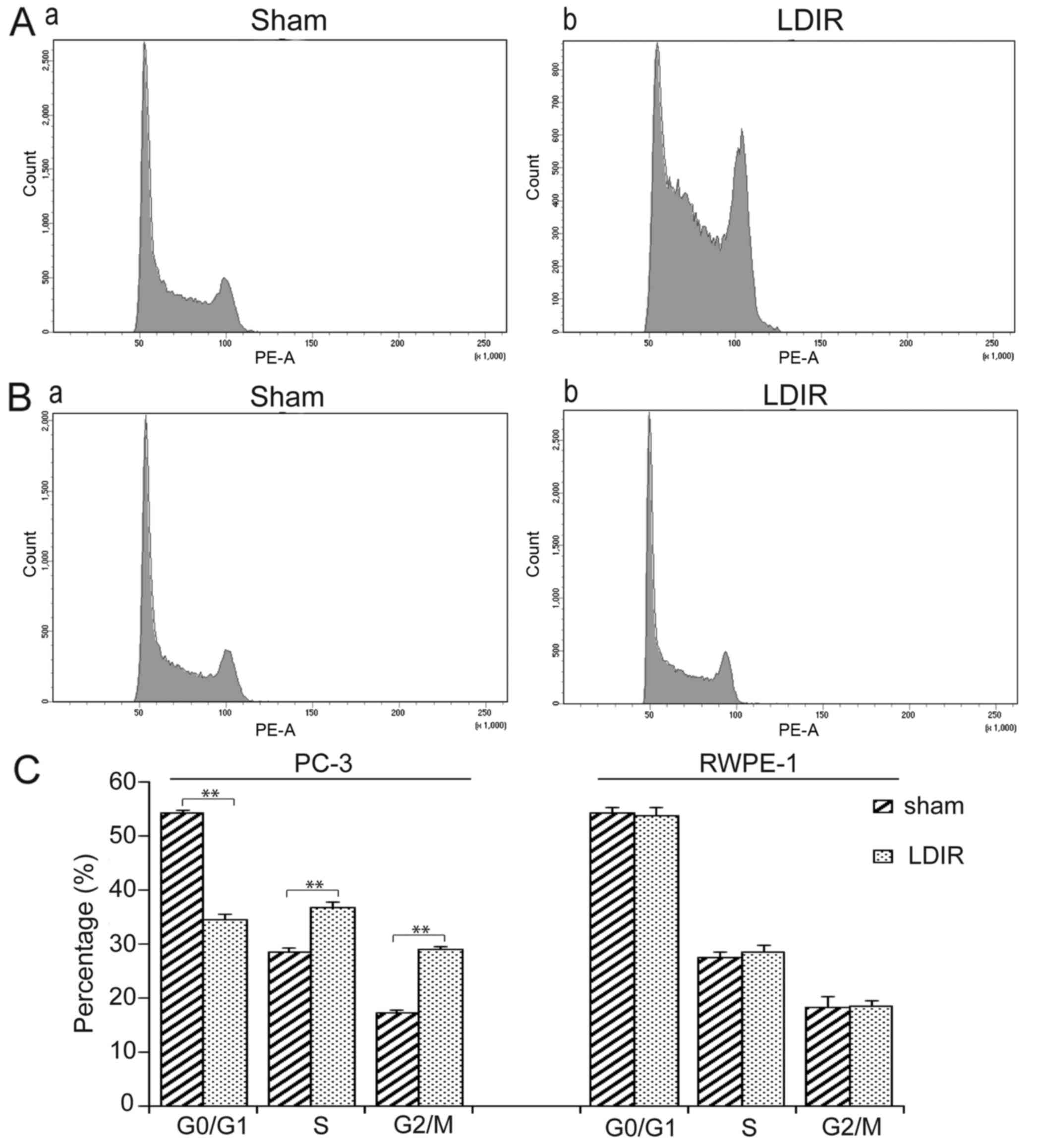
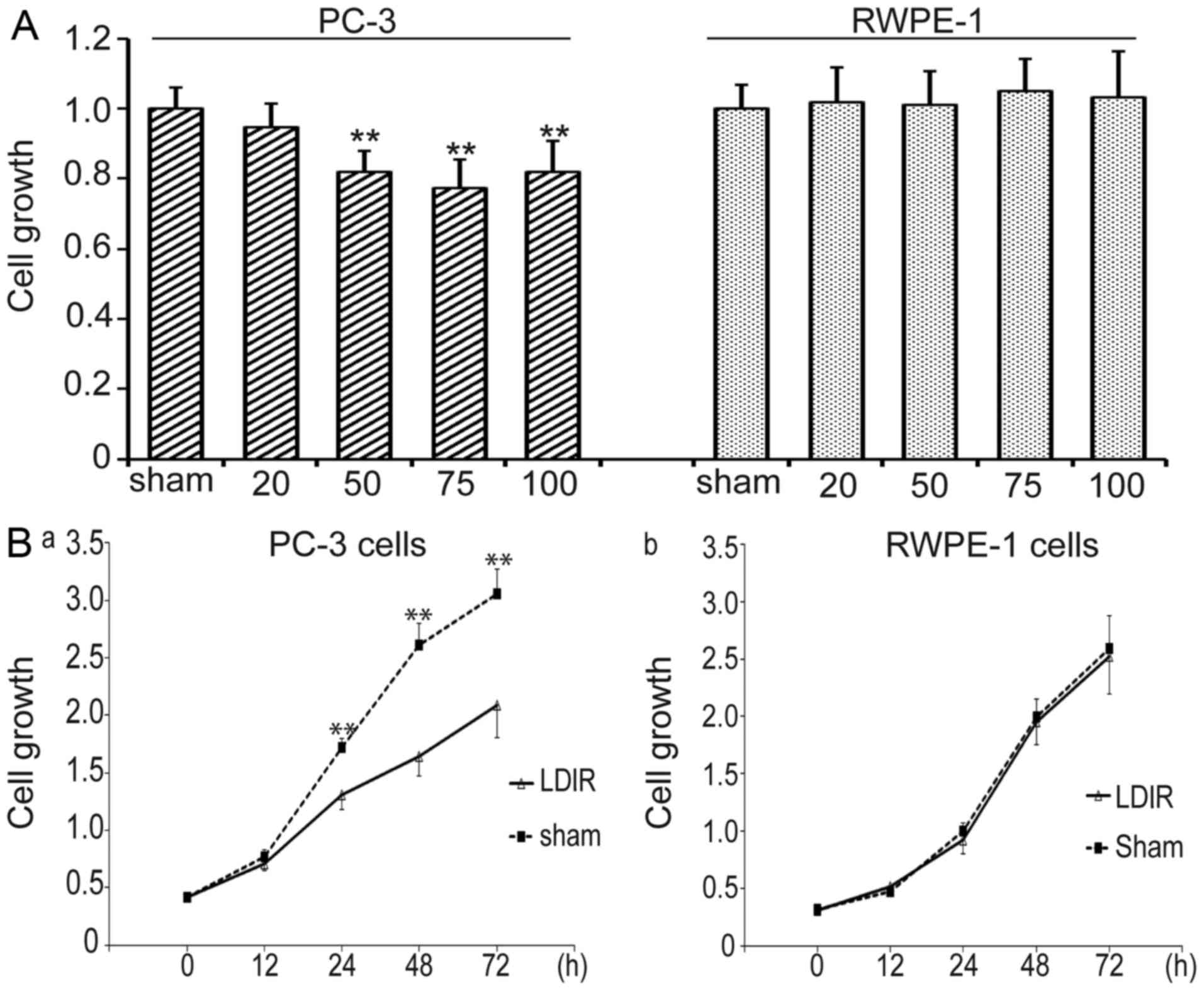 | Figure 1Low-dose ionizing radiation (LDIR)
induces differential responses in PC-3 and RWPE-1 cells. (A) The
cells were irradiated with 20, 50, 75 and 100 mGy LDIR, and cell
growth was determined by WST-1 at 24 h post-LDIR. PC-3 cells
exhibited decreased growth after 50, 75 and 100 mGy of LDIR,
whereas 75 mGy exerted the most prominent inhibitory effect;
however, none of the four doses of LDIR affected the growth of
normal prostate RWPE-1 cells. **P<0.01. (B) The
growth of PC-3 and RWPE-1 cells after 75 mGy LDIR was recorded, and
cell proliferation was determined by WST-1 at 12, 24, 48 and 72 h
post-LDIR. (a) PC-3 cells exhibited significantly decreased
proliferation at 24, 48 and 72 h post-LDIR. (b) RWPE-1 cells
exhibited no change in proliferation after 75 mGy LDIR.
**P<0.01. |
Cell growth in the two cell lines was compared at
different timepoints following LDIR at 75 mGy. Cell proliferation
was determined by WST-1 at 0, 12, 24, 48 and 72 h post-LDIR. PC-3
cells exhibited decelerated proliferation between 12 and 72 h after
75 mGy LDIR (P<0.05 and P<0.01, respectively) (Fig. 1B). However, normal prostate RWPE-1
cells exhibited no obvious changes after 75-mGy irradiation
compared with the sham control group (P>0.05).
LDIR arrests the cell cycle at S and G2/M
in PC-3 cells
Flow cytometry was then used to analyze cell cycle
distribution at 24 h post-LDIR. It was observed that, after 75 mGy
LDIR, PC-3 cells exhibited a significant S and G2/M phase arrest
compared with the sham group (Fig.
2A). By contrast, there was no significant change in cell cycle
distribution in RWPE-1 cells following LDIR (Fig. 2B). Quantitation data revealed
that, in PC-3 cells, the S phase percentage increased 1.29-fold
(36.7 vs. 28.4%, P<0.01) and the G2/M phase percentage increased
1.69-fold (29 vs. 17.2%, P<0.01; Fig. 2C).
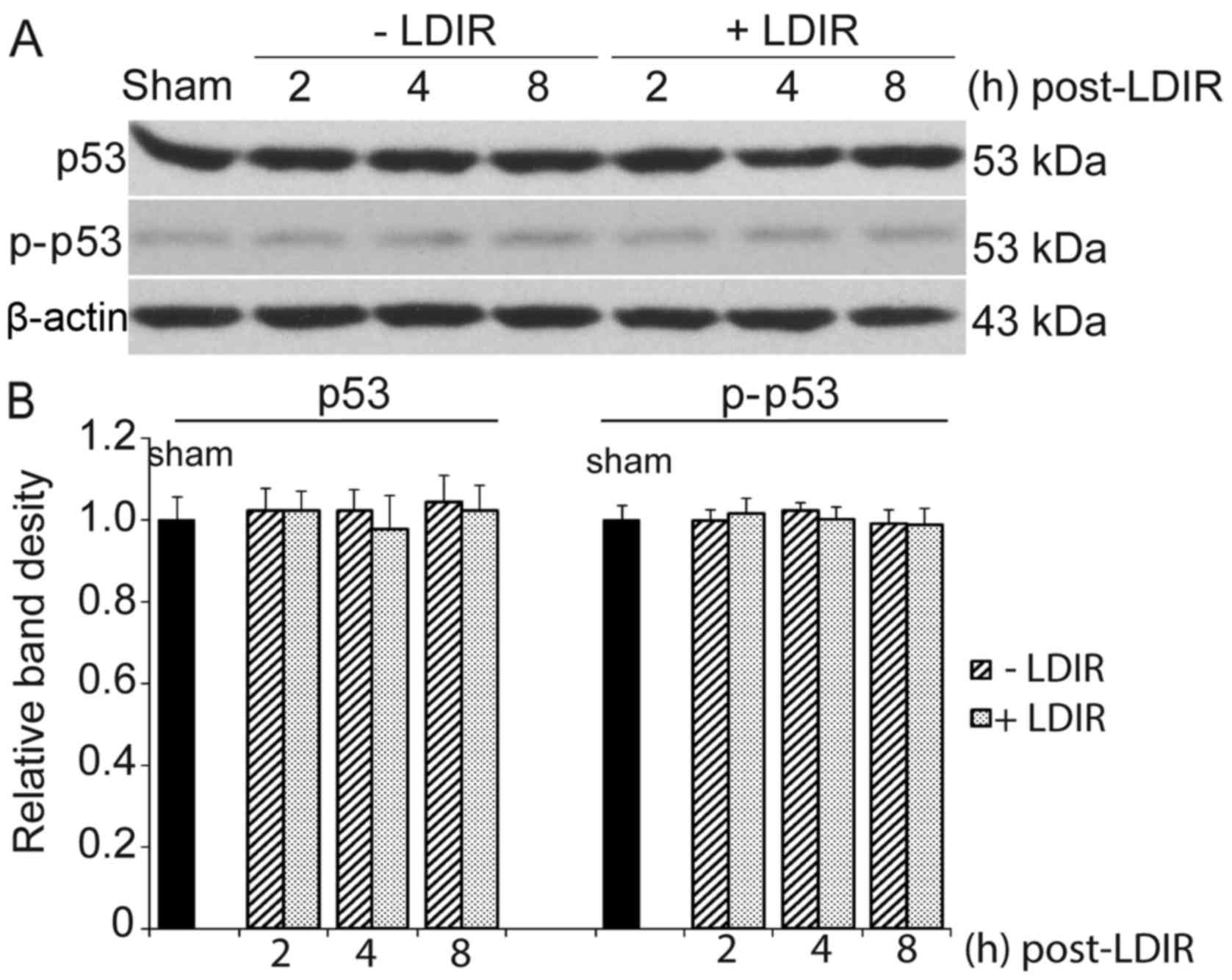
LDIR does not affect p53
Of note, 75 mGy LDIR only inhibited the
proliferation and cell cycle progression in PC-3 cells, but not in
normal prostate cells. PC-3 is a p53null cell
line. Since p53 is the most significant radiation-related protein,
the expression of p53 and phosphorylated p53 (Ser-15, p-p53) in
RWPE-1 cells was first examined. However, the western blotting
results revealed no significant changes in the expression of p53 or
p-p53 at 2, 4 and 8 h after 75 mGy LDIR, compared with that of the
sham-irradiation group (Fig.
3).
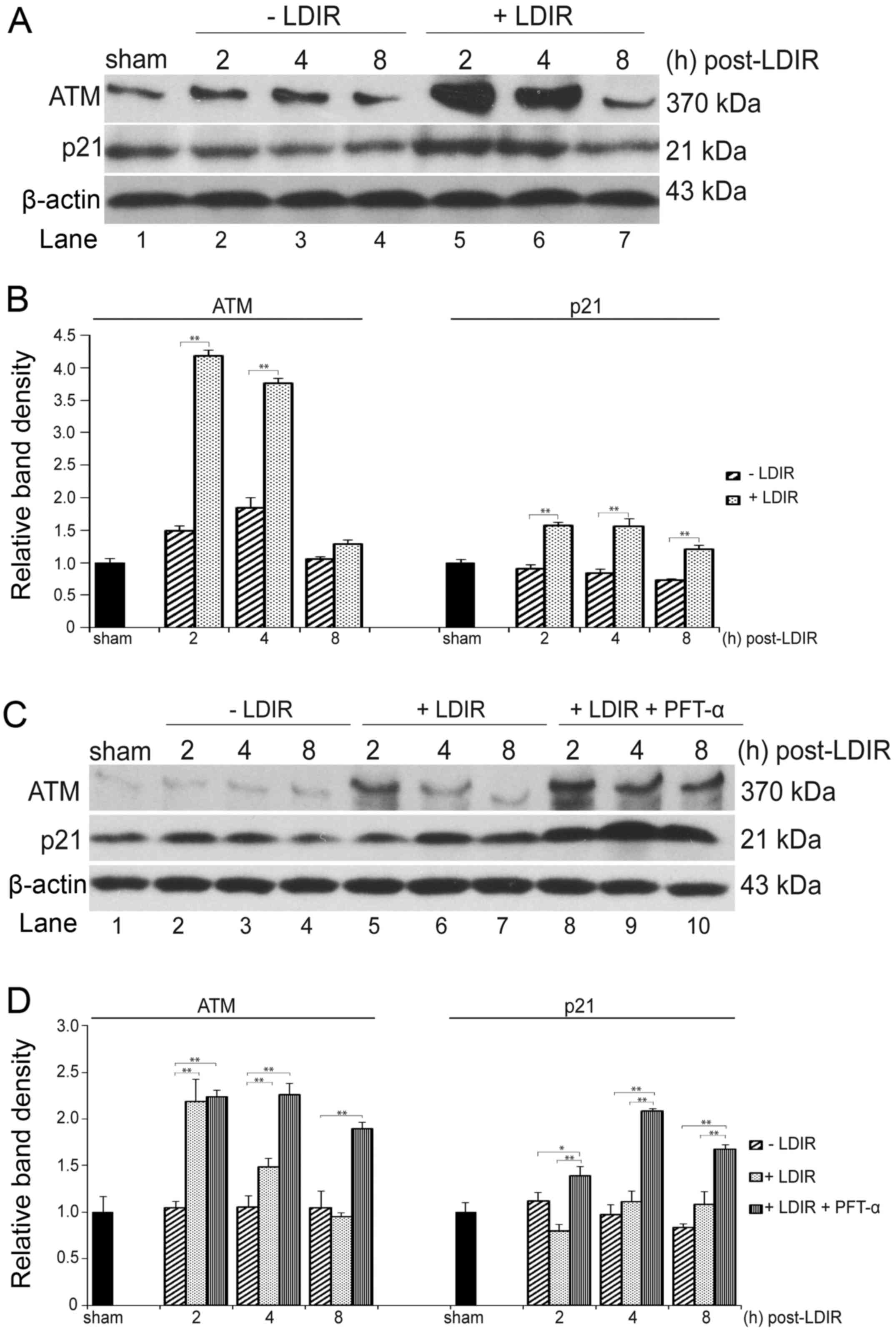
LDIR specifically activates the ATM/p21
pathway in p53-deficient cancer cells
The expression of ATM, which is also a well-known
critical DNA damage and radiation response sensor in mammalian
cells, was further examined (17). At 2, 4 and 8 h post-LDIR, the
total proteins of PC-3 and RWPE-1 cells were extracted and
separated by 4–8% SDS-PAGE, and the expression of ATM was detected
by western blotting. The expression of ATM was found to be
upregulated by 75 mGy LDIR in PC-3 as well as RWPE-1 cells at 2 and
4 h post-LDIR (Fig. 4A and C,
lanes 5 and 6). In PC-3 cells, the expression of ATM was
upregulated 2.8- and 2.04-fold, respectively (Fig. 4B, left panel; P<0.05). In
RWPE-1 cells, the ATM was activated 2.08- and 1.4-fold (Fig. 4D, left panel). At 8 h post-LDIR,
the expression of ATM in both cell lines recovered to a similar
level as in the sham-irradiated group (Fig. 4A and C, lane 7).
The expression of p21 was also quantitated in PC-3
and RWPE-1 cells by western blot analysis after 75 mGy LDIR. In
PC-3 cells, a significant upregulation of p21 was observed after
LDIR (Fig. 4A, lanes 5–7 and B,
right panel). However, the LDIR-induced p21 upregulation was absent
in normal RWPE-1 cells (Fig. 4C,
lanes 5–7 and D, right panel).
In order to mimic the p53 dysfunction in PC-3 cancer
cells, 10 μM PFT-α were used to inactivate p53 in normal
RWPE-1 cells prior to 75 mGy LDIR, and the expression of ATM and
p21 was measured by western blot analysis. Compared with the
non-irradiated group, the expression of ATM was found to be
significantly increased 2.13-, 2.14- and 1.8-fold at 2, 4 and 8 h
post-LDIR, respectively (Fig. 4C,
lanes 2–8 and 8-10 and D, left panel; P<0.05). Of note, RWPE-1
cells exhibited a significant upregulation of p21 (1.74-, 1.88- and
1.55-fold at 2, 4 and 8 h post-LDIR, respectively) compared with
cells that were only exposed to 75 mGy LDIR (Fig. 4C, lane 5–7 and 8–10 and D, right
panel) (P<0.05).
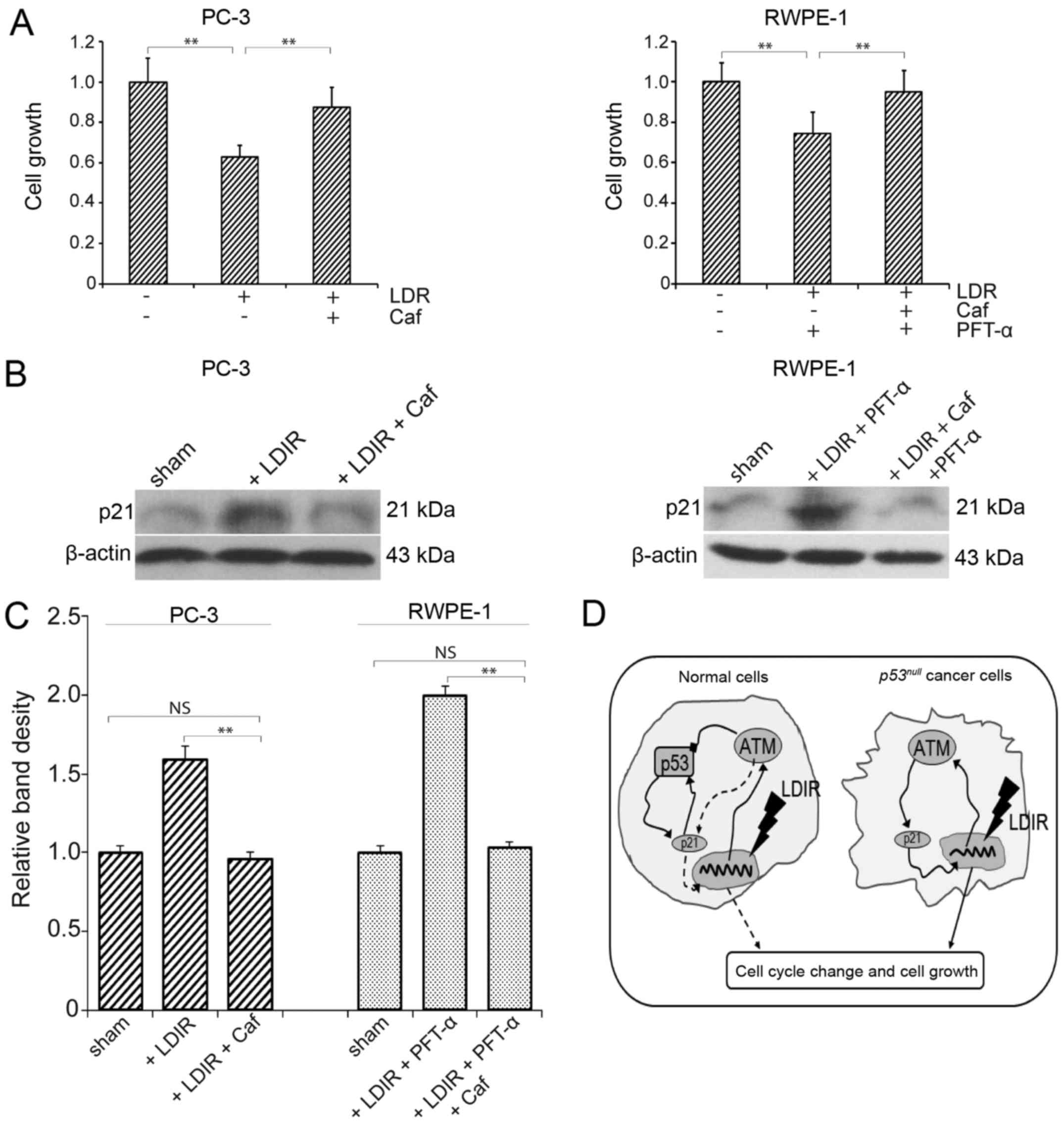
ATM inhibitors restore proliferation of
PC-3 cells
To further investigate the role of the ATM/p21
pathway in LDIR-induced changes in cell proliferation, caffeine was
used to block the function of ATM. Following inhibition of ATM with
caffeine, the proliferation of PC-3 cells and PFT-α-treated RWPE-1
cells was measured at 24 h post-LDIR. We found that, after caffeine
treatment, the proliferation inhibition in PC-3 cells as well as in
p53-inactivated RWPE-1 cells was abolished (P<0.05; Fig. 5A), and the expression of p21 was
restored to a level similar to that in the sham irradiation group
(Fig. 5B and C). Taken together,
these data suggest the involvement of the ATM/p21 pathway in the
differential LDIR-induced biological effect in normal and
p53null prostate cancer cells (Fig. 5D).
Discussion
Different doses of radiation may exert distinct
biological effects on normal and cancer cells. HDIR induces cell
cycle arrest and cell apoptosis, and it is commonly utilized in
clinical practice for killing tumor cells. However, LDIR has a
different biological function compared with HDIR. Doses of 75 and
50 mGy are the LDIR doses most frequently used in our laboratory to
demonstrate the proliferation characteristics of several normal and
tumor cell lines (5,6,18).
The majority of these tested normal cell lines exhibit increased
cell proliferation and S phase cell population, while this response
is not observed in cancer cell lines. However, as is well-known,
tumor cells are heterogeneous, and commonly harbor gene mutations.
Therefore, determination of the differential LDIR response among
diverse types of tumor cells may be of interest, and may be proven
useful for individualizing radiotherapy. Since p53 gene
mutations or deletions are common in tumor cells, in the present
study a p53null type prostate tumor cell line
(PC-3) and a normal prostate cell line (RWPE-1) were selected as
the investigation model. First, changes in cell proliferation after
LDIR were investigated. PC-3 cells exhibited radiation sensitivity
to 20–100 mGy LDIR at a dose rate of 12.5 mGy/min, but RWPE-1 cells
did not respond to this dose. This is the first time it was
demonstrated that 75 mGy LDIR inhibits tumor cell proliferation in
our laboratory. It remains unknown whether this is a general
characteristic for all p53null type tumor cells,
and more cell types must be investigated for confirmation.
The molecular mechanisms leading to different
biological responses between normal and cancer cells are of
particular interest. ATM acts upstream of several signal
transduction pathways initiated by ionizing radiation, such as the
Akt (12), ERK (13) and p53 pathways (19). Xie et al recently
demonstrated that infiltrating mast cells may alter the sensitivity
of prostate cancer to chemotherapy and radiotherapy via modulating
p38̸p53/p21 signaling and ATM phosphorylation (20). Huang et al reported that
extremely low-frequency electromagnetic fields inhibit HaCat cell
proliferation and lead to a G1 phase arrest through the activation
of the ATM-Chk2-p21 pathway (21). In the present study, we also
demonstrated an intrinsic correlation between the LDIR-activated
ATM-p53-p21 pathway and inhibition of cell proliferation. We found
that, although 75 mGy LDIR activated ATM in
p53null type PC-3 cells as well as in normal
RWPE-1 cells, it only activated p21 and inhibited cell
proliferation in PC-3 cells. We hypothesized that p53 blocks
LDIR-induced cell signaling, as p53 acts as a DNA damage repairer
and cell cycle arrester (22).
Therefore, the expression of p53 and p-p53 was investigated in
RWPE-1 cells. No change in p53 and p-p53 expression was observed in
RWPE-1 cells at 2, 4 and 8 h post-LDIR. However, the possibility
that p53 is not involved in this process could not be excluded.
Using inhibitors to inactivate p53 in RWPE-1 cells, we observed
that LDIR induced p21 upregulation and cell proliferation
inhibition, although the ATM upregulation remained unchanged. This
is in consistent with the findings in PC-3 cells. When the function
of ATM was blocked, the LDIR-induced p21 overexpression and cell
proliferation inhibition were abolished in both PC-3 and RWPE-1
cells.
p21 is a transcriptional target of p53, which
induces cell cycle arrest through its function as a
cyclin-dependent kinase inhibitor (23,24). However, in our LDIR model,
following p53 inactivation, the expression of p21 increased
significantly. Therefore, we hypothesized that there is a
p53-independent ATM/p21 pathway in p53null
prostate cancer cells, which may be activated by LDIR and directly
lead to cell cycle arrest and proliferation inhibition (Fig. 5D, right model). By contrast, in
normal cells with wild-type p53, the classical ATM-p53-p21 pathway
is a dominant irradiation response pathway (25). The difference in our model was
that the radiation dose was insufficient for stimulating
overexpression and phosphorylation of p53. However, although the
p53/p-p53 ratio did not change, they play a key role in the
feedback regulation of p21 (26),
as well as the maintenance of the stability of mitosis. After p53
is blocked in normal cells, the ATM/p21 pathway may be activated by
LDIR, to a similar extent as in PC-3 cells (Fig. 5D, left model). The effect of HDIR
on the ATM/p21 pathway was also investigated. When the irradiation
dose was increased to 2 Gy or higher, the expression of p53 in
RWPE-1 cells markedly increased (data not shown). Unlike the
interaction between ATM and p21 in LDIR, when cells were exposed to
HDIR, the classical ATM, p53 and p21 crosstalk was proven to be the
dominant response pathway when cells were exposed to HDIR, as
previously reported (25,27). Of note, it remains unknown whether
ATM directly activates p21 in this LDIR model, or whether there are
other intermediate factors, such as Chk2 (21,28); further investigation is required
to address this issue.
Acknowledgments
This study was supported by grants from the National
Science Foundation of China (no. 81302380 to D.-H.Y. and no.
81302379 to X.-Y.L.) and the Jilin Provincial Science and
Technology Department (no. 20140520017JH).
References
|
1
|
Luckey TD: Physiological benefits from low
levels of ionizing radiation. Health Phys. 43:771–789. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feinendegen LE: Evidence for beneficial
low level radiation effects and radiation hormesis. Br J Radiol.
78:3–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olivieri G, Bodycote J and Wolff S:
Adaptive response of human lymphocytes to low concentrations of
radioactive thymidine. Science. 223:594–597. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Wang G, Cui J, Xue L and Cai L:
Low-dose radiation (LDR) induces hematopoietic hormesis:
LDR-induced mobilization of hematopoietic progenitor cells into
peripheral blood circulation. Exp Hematol. 32:1088–1096. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang X, So YH, Cui J, Ma K, Xu X, Zhao Y,
Cai L and Li W: The low-dose ionizing radiation stimulates cell
proliferation via activation of the MAPK/ERK pathway in rat
cultured mesenchymal stem cells. J Radiat Res (Tokyo). 52:380–386.
2011. View Article : Google Scholar
|
|
6
|
Jiang H, Xu Y, Li W, Ma K, Cai L and Wang
G: Low-dose radiation does not induce proliferation in tumor cells
in vitro and in vivo. Radiat Res. 170:477–487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang G, Kong Q, Wang G, Jin H, Zhou L, Yu
D, Niu C, Han W, Li W and Cui J: Low-dose ionizing radiation
induces direct activation of natural killer cells and provides a
novel approach for adoptive cellular immunotherapy. Cancer Biother
Radiopharm. 29:428–434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho E, Mostaghel EA, Russell KJ, Liao JJ,
Konodi MA, Kurland BF, Marck BT, Matsumoto AM, Dalkin BL and
Montgomery RB: External beam radiation therapy and abiraterone in
men with localized prostate cancer: Safety and effect on tissue
androgens. Int J Radiat Oncol Biol Phys. 92:236–243. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Savitsky K, Sfez S, Tagle DA, Ziv Y,
Sartiel A, Collins FS, Shiloh Y and Rotman G: The complete sequence
of the coding region of the ATM gene reveals similarity to cell
cycle regulators in different species. Hum Mol Genet. 4:2025–2032.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki K, Okada H, Yamauchi M, Oka Y,
Kodama S and Watanabe M: Qualitative and quantitative analysis of
phosphorylated ATM foci induced by low-dose ionizing radiation.
Radiat Res. 165:499–504. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Viniegra JG, Martínez N, Modirassari P,
Hernández Losa J, Parada Cobo C, Sánchez-Arévalo Lobo VJ, Aceves
Luquero CI, Alvarez-Vallina L, Ramón Y, Cajal S, Rojas JM, et al:
Full activation of PKB/Akt in response to insulin or ionizing
radiation is mediated through ATM. J Biol Chem. 280:4029–4036.
2005. View Article : Google Scholar
|
|
13
|
Ahmed KM, Nantajit D, Fan M, Murley JS,
Grdina DJ and Li JJ: Coactivation of ATM/ERK/NF-kappaB in the
low-dose radiation-induced radioadaptive response in human skin
keratinocytes. Free Radic Biol Med. 46:1543–1550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CL, Blum JM and Kirsch DG: Role of p53
in regulating tissue response to radiation by mechanisms
independent of apoptosis. Transl Cancer Res. 2:412–421. 2013.
|
|
15
|
Menon V and Povirk L: Involvement of p53
in the repair of DNA double strand breaks: Multifaceted Roles of
p53 in homologous recombination repair (HRR) and non-homologous end
joining (NHEJ). Subcell Biochem. 85:321–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Freed-Pastor WA and Prives C: Mutant p53:
One name, many proteins. Genes Dev. 26:1268–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim GD, Choi YH, Dimtchev A, Jeong SJ,
Dritschilo A and Jung M: Sensing of ionizing radiation-induced DNA
damage by ATM through interaction with histone deacetylase. J Biol
Chem. 274:31127–31130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang H, Li W, Li X, Cai L and Wang G:
Low-dose radiation induces adaptive response in normal cells, but
not in tumor cells: In vitro and in vivo studies. J Radiat Res
(Tokyo). 49:219–230. 2008. View Article : Google Scholar
|
|
19
|
Canman CE, Lim DS, Cimprich KA, Taya Y,
Tamai K, Sakaguchi K, Appella E, Kastan MB and Siliciano JD:
Activation of the ATM kinase by ionizing radiation and
phosphorylation of p53. Science. 281:1677–1679. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie H, Li C, Dang Q, Chang LS and Li L:
Infiltrating mast cells increase prostate cancer chemotherapy and
radiotherapy resistances via modulation of p38/p53/p21 and ATM
signals. Oncotarget. 7:1341–1353. 2016. View Article : Google Scholar :
|
|
21
|
Huang CY, Chang CW, Chen CR, Chuang CY,
Chiang CS, Shu WY, Fan TC and Hsu IC: Extremely low-frequency
electromagnetic fields cause G1 phase arrest through the activation
of the ATM-Chk2-p21 pathway. PLoS One. 9:e1047322014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roos WP, Thomas AD and Kaina B: DNA damage
and the balance between survival and death in cancer biology. Nat
Rev Cancer. 16:20–33. 2016. View Article : Google Scholar
|
|
23
|
Lin K, Adamson J, Johnson GG, Carter A,
Oates M, Wade R, Richards S, Gonzalez D, Matutes E, Dearden C, et
al: Functional analysis of the ATM-p53-p21 pathway in the LRF CLL4
trial: blockade at the level of p21 is associated with short
response duration. Clin Cancer Res. 18:4191–4200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dotto GP: p21(WAF1/Cip1): More than a
break to the cell cycle? Biochim Biophys Acta. 1471:M43–M56.
2000.PubMed/NCBI
|
|
25
|
Cao L, Kawai H, Sasatani M, Iizuka D,
Masuda Y, Inaba T, Suzuki K, Ootsuyama A, Umata T, Kamiya K, et al:
A novel ATM/TP53/p21-mediated checkpoint only activated by chronic
γ-irradiation. PLoS One. 9:e1042792014. View Article : Google Scholar
|
|
26
|
Pang LY, Scott M, Hayward RL, Mohammed H,
Whitelaw CB, Smith GC and Hupp TR: p21(WAF1) is component of a
positive feedback loop that maintains the p53 transcriptional
program. Cell Cycle. 10:932–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo JL, Cao JP, Zhu W, Feng S, Sheng FJ,
Zhu CY and Zheng SY: Interaction between ATM and
radiation-activated phosphorylation of P53 and P21. Ai Zheng.
24:1059–1063. 2005.In Chinese. PubMed/NCBI
|
|
28
|
Aliouat-Denis CM, Dendouga N, Van den
Wyngaert I, Goehlmann H, Steller U, van de Weyer I, Van Slycken N,
Andries L, Kass S, Luyten W, et al: p53-independent regulation of
p21Waf1/Cip1 expression and senescence by Chk2. Mol Cancer Res.
3:627–634. 2005. View Article : Google Scholar : PubMed/NCBI
|