Introduction
Numerous chronic diseases are known to involve the
pathogenesis of inflammation, including atherosclerosis,
Alzheimer's disease and Parkinson's disease (1). The development of atherosclerosis
occurs as a response to injury of the vessel wall (2,3).
Endothelial inflammation involves the recruitment of circulating
monocytes by cells using a combination of chemokines and cell
surface adhesion molecules, such as monocyte chemotactic protein-1
and intercellular cell adhesion molecule-1 (ICAM-1) (3). The inflammatory cytokine tumor
necrosis factor (TNF)-α activates the nuclear factor-κB (NF-κB)
pathway, which serves a key role in the expression of cell adhesion
molecules in endothelial cells (ECs) (2,4).
Following stimulation with TNF-α, a kinase cascade induces
subsequent inhibitor of κB (IκB) phosphorylation, which rapidly
degrades, resulting in the released NF-κB translocating to the
nucleus to regulate gene transcription (5).
The prenylated stilbenoids arachidin-1 and
arachidin-3 are produced in germinated peanut roots (6). They are prenylated analogs of
piceatannol and resveratrol, respectively. Resveratrol and
piceatannol are considered to have a number of beneficial effects,
including anticancer, anti-atherogenic, anti-oxidative,
anti-inflammatory, antimicrobial and estrogenic activity (7). Their prenylated analogs are less
studied but display a wide spectrum of biological activity.
Arachidin-1 has been reported to possess various pharmacological
activities, including antioxidative, anti-inflammatory, anti-virus
infection and antitumorigenic effects (7–10).
However, to date it is not clear if arachidin-1 also has
anti-inflammatory and anti-oxidative effects in ECs. Since
arachidin-1 has been demonstrated to be pharmacologically active,
the detailed mechanism by which arachidin-1-modulates inflammation
in ECs requires further elucidation.
Numerous phytochemicals are electrophilic, a
property that has been reported to result in nuclear
factor-E2-related factor-2 (Nrf-2) activation and the induction of
phase II detoxifying enzyme expression (11,12). Results from in vitro and
in vivo studies have provided evidence that electrophilic
stress induces the complexation of Nrf-2 with Kelch-like
ECH-associated protein 1 (Keap1), enabling Nrf-2 to escape from
Keap1-mediated ubiquitination and degradation (13). As a result, Nrf-2 translocates to
the nucleus to form heterodimers with small Maf proteins and binds
to the antioxidant response element (ARE) in the promoter region of
phase II detoxifying enzymes and antioxidant enzymes, including
quinone oxidoreductase and heme oxygenase-1 (HO-1) (11,13). In addition, Nrf-2 has been shown
to control the expression of a number of thiol-regulating enzymes,
including glutathione S-transferase (GTP), glutamyl-cysteine
synthetase, thioredoxin and thioredoxin reductase (14). Increased levels of these
intracellular thiol-containing proteins may provide cytoprotective
effects under conditions of oxidative stress or inflammation. A
previous study conducted by the present research team demonstrated
that piceatannol, an analog of arachidin-1, induced the expression
of the Nrf-2- associated gene HO-1 (15). This phenomenon has broad potential
implications regarding the inhibitory effects of arachidin-1 upon
inflammation in association with Nrf-2-associated enzymes. For
these reasons, the present study investigated Nrf-2 associated
pathways in order to elucidate the mechanisms underlying the
cytoprotective effects of arachidin-1.
Materials and methods
Materials
The p3xARE/Luc vector was constructed as described
previously (16). Luciferase
assay kits were purchased from Promega Corporation (Madison, WI,
USA). Antibodies against HO-1 (SPA-896) and p65 (KAS-TF110) were
purchased from Stressgen Biotechnologies (San Diego, CA, USA).
ICAM-1 (sc-7891), IκBα (sc-847), Lamin B1 (sc-56143), α-tubulin
(sc-53646) and Nrf2 (sc-722) antibodies were obtained from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). TrxR1 antibody
(07-613) was purchased from Upstate (Charlottesville, VA, USA).
IκBα and Nrf-2 antibodies were obtained from Santa Cruz
Biotechnology, Inc. Bacterially derived TNF-α was purchased from
Calbiochem (EMD Millipore; Billerica, MA, USA). All other reagents
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany).
Purification of arachidin-1 and
arachidin-3 from peanut
Arachidin-1 and arachidin-3 were isolated using the
method originally described by Chang et al (17). Briefly, slices of Tainan 14 peanut
kernel (1.2 kg; Fengxiang Seed Co., Ltd., Chiayi, Taiwan) were
artificially aerated at 25°C and under 65l/min air flow rate for 16
h. Following pretreatment and freeze drying, the slices of peanut
kernel were pulverized into a powder and extracted with methanol.
The methanol extract was suspended in water and partitioned with an
equal volume of ethyl acetate. The ethyl acetate extract was passed
through Sephadex LH-20 and eluted with methanol. The fractions were
chromatographed using a LiChroprep RP-18 (2.5×52 cm) column eluted
with 0.05% trifluoroacetic acid-CH3CN (60:40) to obtain
arachidin-1 and arachidin-3. The yields of arachidin-1 and
arachidin-3 were 0.0045 and 0.0087% and the purities were 96.82 and
98.10%, respectively.
EC cultures
The human umbilical vein cell line EA.hy926 (ATCC
CRL-2922; ATCC, Manassas,VA, USA) was cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco-BRL, Gaithersburg, MD, USA)
supplemented with 10% fetal bovine serum (Gibco-BRL, Carlsbad, CA,
USA) at 37°C under 5% CO2 in air. When the ECs were
grown to confluence, the culture medium was replaced with
serum-free DMEM and the cells were incubated for 12 h prior to
experimental treatments.
Cell adhesion assay
The THP-1 cells (ATCC) were labeled with 1 µM
calcein-AM (Trevigen, Gaithersburg, MD, USA) in PBS for 40 min at
37°C, and then washed twice with PBS. After treatment, ECs were
washed with DMEM and co-cultured with calcein-labeled THP-1 cells
for 30 min. After washing twice with RPMI-1640 medium (Invitrogen,
Carlsbad, CA, USA), adherent cells were examined using an
enzyme-linked immunosorbent assay plate reader (FLx800; Bio-Tek
Instruments, Inc., Winooski, VT, USA) at 485 nm excitation and 538
nm emission wavelengths.
Cytotoxicity
The resazurin reduction test, an index of the
metabolic activity of living cells, was carried out using an
AlamarBlue® assay kit according to the manufacturer's
protocol (Serotec; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ECs were plated into 96-well microtiter plates (Falcon; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 20,000 cells/well and
incubated with AlamarBlue® reagent for 2 h at 37°C.
Fluorescence was then measured at excitation and emission
wavelengths of 570 and 600 nm, respectively.
Western blotting
A total of 106 cells were lysed on ice in
lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and a
protease inhibitor mixture). The total protein concentrations were
determined with the use of the Bio-Rad DC protein assay kit
(Bio-Rad Laboratories, Inc.). Equal amounts (10–20 µg) of
whole-cell extracts were boiled for 5 min prior to separation using
10% SDS-PAGE. The proteins were then transferred to a
nitrocellulose membrane (EMD Millipore) in Tris-glycine buffer (25
mM Tris-HCl, 192 mM Glycine, pH 8.3) at 10 V for 1.5 h. The
membranes were then blocked with PBS containing 5% non-fat milk for
2 h at 4°C and were subsequently probed with the appropriate
primary antibody overnight at 4°C, with gentle shaking. The next
day, following PBS washes, the membranes were incubated with
secondary antibodies [horseradish peroxidase-conjugated goat
anti-rabbit (34083) or anti-mouse (34081) antibody; Thermo Fisher
Scientific, Inc.] for 1 h at room temperature. The results were
visualized by chemiluminescence using ECL SuperSignal West Pico kit
(Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol.
Preparation of nuclear fractionation for
immunoblotting
ECs were collected by scraping and lysed with cell
lysis buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5
mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride and 0.3%
NP-40). The cell lysate was separated into nuclear fractions by
centrifugation at 500 × g for 5 min at 4°C. The nuclei were washed
using nuclei washing buffer (320 mM sucrose, 5 mM MgCl2,
10 mM HEPES at pH 7.4) and the nuclear protein was extracted using
a buffer containing 25% glycerol, 20 mM HEPES, 0.6 M KCl, 1.5 mM
MgCl2 and 0.2 mM EDTA for 15 min at 4°C.
Luciferase reporter assays
ECs were subcultured at a density of
1×106 cells in 60-mm dishes. ECs were subsequently
co-transfected with 100 ng p3xARE/Luc with pSV-β-galactosidase
using Lipofectamine-2000 (Invitrogen). Luciferase activity was
detected using a luciferase reporter assay system (Promega), and
the resulting luciferase activity was measured using a luminometer.
For each experiment, luciferase activity was determined in
triplicate and normalized with β-galactosidase activity.
RNA interference by small interfering RNA
(siRNA) of Nrf-2
The siRNA nucleotide sequence for human Nrf-2 was as
follows: 5'-UCCCGUUUGUAGAUGACAA-3' (18). The effects of this siRNA molecule
have been demonstrated in a previous study (16). A non-targeting siRNA,
5-GCAAGCUGACCC UGAAGUUCAU-3, was purchased from Ambion (Thermo
Fisher Scientific, Inc.). Cells were transfected with Nrf-2 siRNA
or non-targeting siRNA using Lipofectamine 2000 reagent according
to the manufacturer's protocol. Following 24 h of transfection, the
ECs were cultured in medium without serum for another 12 h prior to
treatment.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA isolated from the ECs using TRIzol was
reverse transcribed using SuperScript II reverse transcriptase
(both Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The cDNA was subjected to PCR
amplification using the following forward and reverse primer sets:
GAPDH, 5'-TAT CGT GGA AGG ACT CAT GACC-3' and 5'-TAC ATG GCA ACT
GTG AGG GG-3'; GST-Pi, 5'-CCC TCA CTG TTT CCC GTT GC-3' and 5'-TGA
ATG ACG GCG TGG AG-3'; thioredoxin-1 (Trx1), 5'-GGC ATG CAT TTG ACT
TCA-3' and 5'-ACG TGA TAT TCC TTG AAG TAG-3'; glutamate-cysteine
synthetase regulatory subunit (GCLM), 5'-CAG CGA GGA GCT TCA TGA
TTG-3' and 5'-TGA TCA CAG AAT CCA GCT GTGC-3'; glutamate-cysteine
synthetase catalytic subunit (GCLC), 5'-GTT CTT GAA ACT CTG CAA GAG
AAG-3' and 5'-ATG GAG ATG GTG TAT TCT TGT CC-3'; HO-1, 5'-GGT AAG
GAA GCC AGC CAA GAG-3' and 5'-GCC AGC AAC AAA GTG CAA GAT-3'. The
cDNA samples were amplified using Taq DNA polymerase (Thermo Fisher
Scientific, Inc.) for 17–25 cycles of 94°C (30 sec), 60°C (30 sec),
and 72°C (30 sec) using Mastercycler personal (Eppendorf, Hamburg,
Germany). The PCR products were separated on 1.5% agarose gels and
visualized by ethidium bromide staining.
Measurement of intracellular reactive
oxygen species (ROS)
The fluorescence probe
5-(and-6)-carboxy-2,7,dichlorodihydro fluorescein diacetate
(carboxy-H2DCFDA; Molecular Probes; Thermo Fisher
Scientific, Inc.) was used to detect the cellular production of
ROS. The final treatment concentration of
carboxy-H2DCFDA was 20 µM, and the cells were
incubated with this for 30 min in dark at 37°C. Following two
further washes with PBS, the cells were solubilized with 1% SDS and
5 mM Tris HCl (pH 7.4). H2DCF reacts with ROS to form
the green fluorescent compound DCF, which is detected by
spectrofluorophotometry (Rf-5301PC; Shimadzu Corporation, Kyoto,
Japan) with excitation and emission wavelengths of 475 and 525 nm,
respectively.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean of at least three independent experiments. Statistical
significance was assessed by one-way analysis of variance followed
by Tukey's test using SigmaPlot version 12 (Systat Software Inc.,
San Jose, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Arachidin-1 inhibits monocyte/EC adhesion
and ICAM-1 expression
The anti-inflammatory potential of two
peanut-derived prenylated stilbenoids, arachidin-1 and arachidin-3
(Fig. 1A and B), was evaluated
via assessment of their effects on the adhesion of monocytes to
ECs. The results demonstrated that TNF-α significantly increased
monocyte adhesion to the ECs and this adhesion was attenuated by
treatment of the ECs with arachidin-1 or arachidin-3 (Fig. 1C and D). In another experiment,
the cytotoxic effects of 24 h incubation with 5–50 µM
concentrations of arachidin-1 and arachidin-3 in ECs were assessed
(Fig. 1E and F). Arachidin-1
exhibited no toxic effects in ECs at dosages ≤25 µM.
However, a cytotoxic effect was detectable following treatment with
5 µM arachidin-3. These data suggest that the reduction in
monocyte adhesion observed following treatment with arachidin-3 may
result from cell damage. These results also indicate that the
3'-hydroxyl group of the treatment agent is critical for
determining the levels of cytotoxicity. To further test whether
arachidin-1 is a modulator of ICAM-1 induction, ECs were stimulated
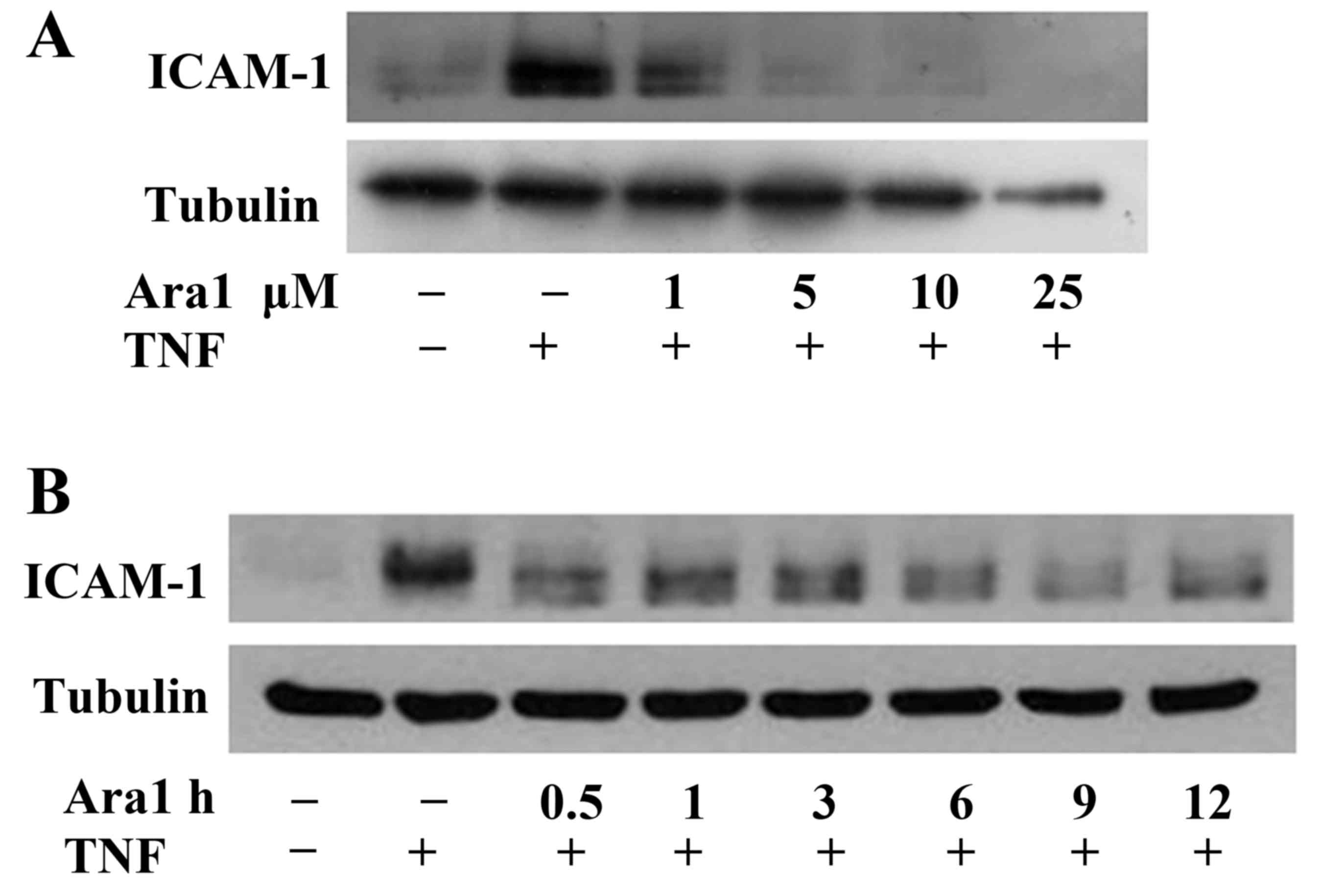
with TNF-α, with or without arachidin-1 pretreatment. Arachidin-1
appeared to have a dose-dependent inhibitory effect on the
TNF-α-induced ICAM-1 expression (Fig.
2A). The pretreatment of ECs with arachidin-1 also appeared to
inhibit TNF-α-induced ICAM-1 expression in a time-dependent manner
(Fig. 2B). This functional assay
implies that arachidin-1 exerts an anti-inflammatory effect via the
inhibition of adhesion molecule expression.
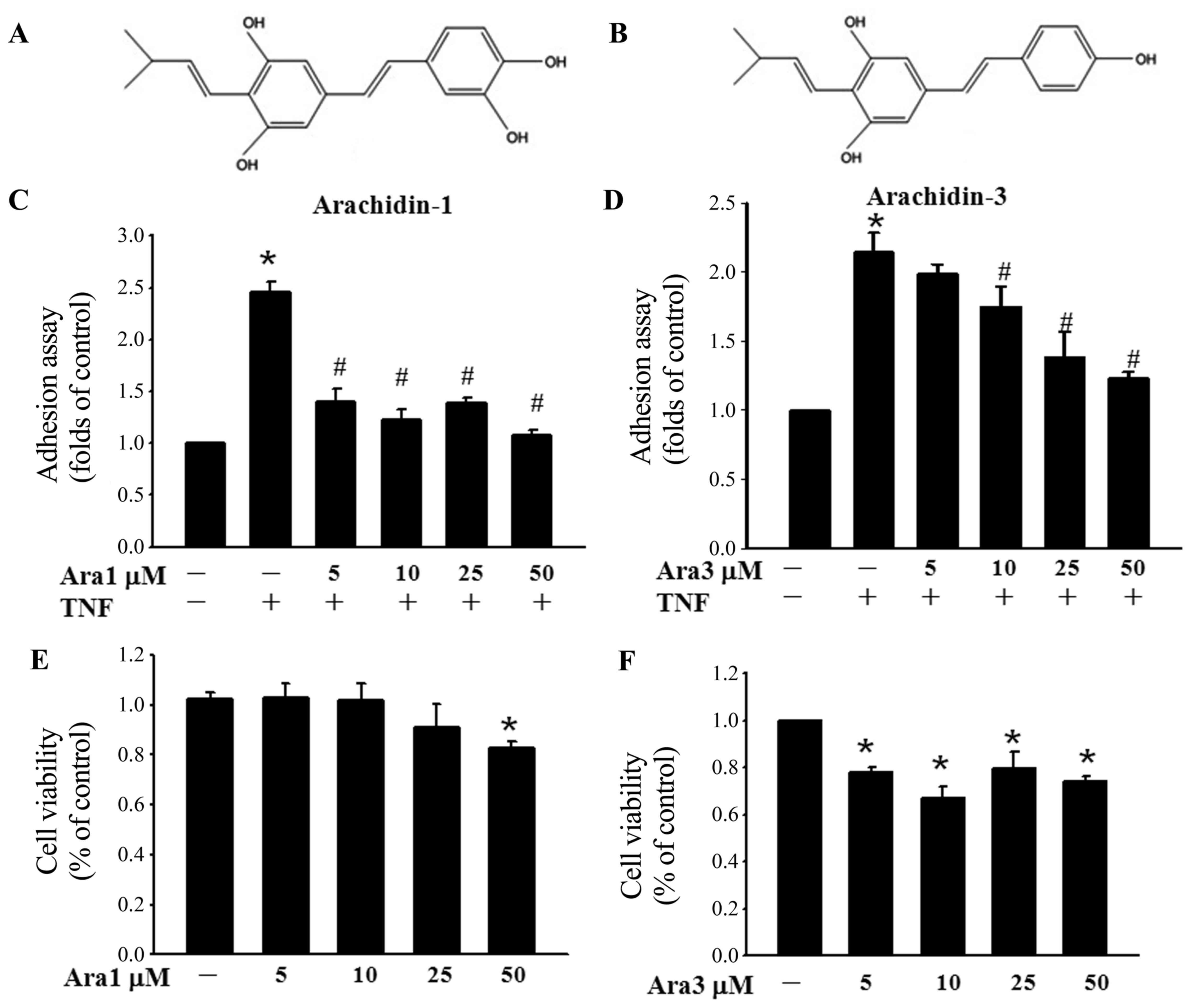 | Figure 1Arachidin-1 inhibits monocyte adhesion
to TNF-α-activated ECs, and ICAM-1 expression. (A) Arachidin-1 and
(B) arachidin-3 are naturally occurring prenylated analog of
piceatannol and resveratrol, respectively. A characteristic of the
structure of arachidin-1 is the 3'-hydroxyl group. ECs grown to
confluency in 96-well plate were pre-incubated with or without (C)
5-50 µM arachidin-1 or (D) arachidin-3 for 12 h, stimulated
with TNF-α (100 U/ml) for 6 h, and then added to 5×105
fluorescence-labeled THP-1 cells and allowed to adhere for 30 min.
The data are presented as the relative adherent ratio as compared
with untreated ECs. The values represent mean ± standard error of
the mean (SEM). *P<0.05 vs. untreated ECs;
#P<0.05 vs. TNF-α alone. ECs were incubated with (E)
arachidin-1 or (F) arachidin-3 at concentrations of 5, 10, 25 and
50 µM for 24 h and cell viability was measured
spectrophotometrically using an Alamar blue assay, according to the
manufacturer's protocol. Data are expressed as the mean ± SEN.
*P<0.05 vs. control. Ara1, arachidin-1; Ara3,
arachidin-3; TNF, tumor necrosis factor; ECs, endothelial cells;
ICAM-1, intercellular adhesion molecule-1. |
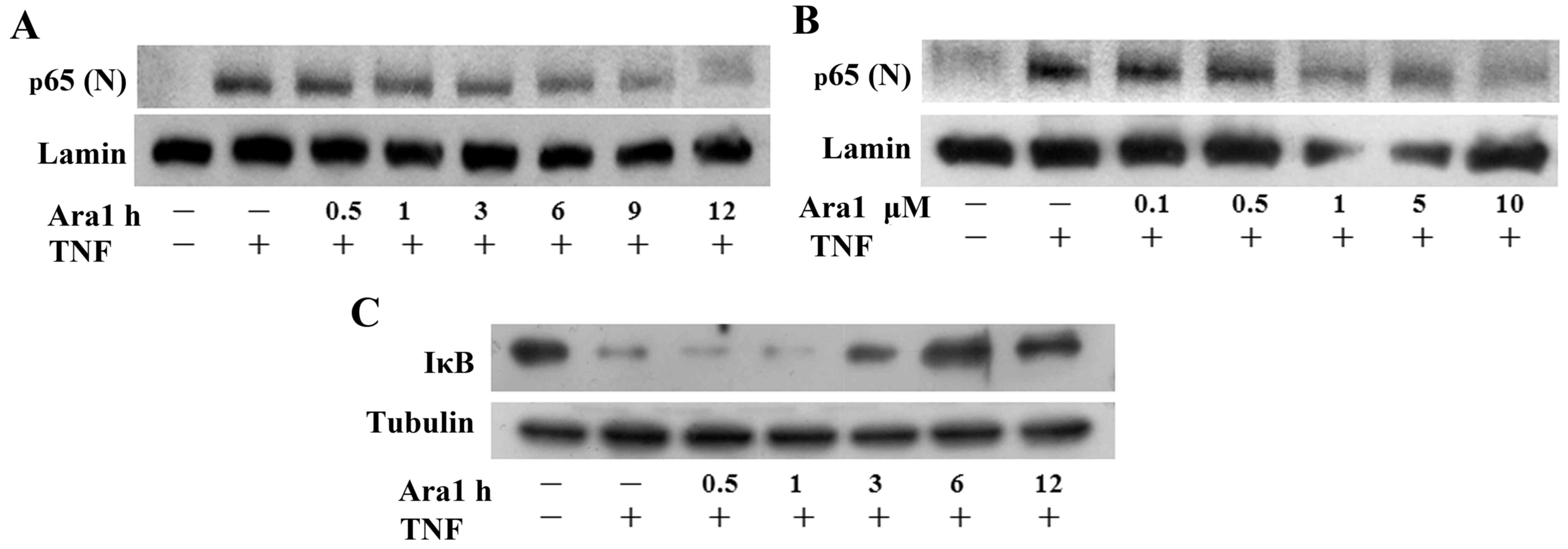
Arachidin-1 inhibits TNF-α-induced NF-κB
activation and blocks the degradation of IκBα
Since the NF-κB pathway is a well-known inflammatory
pathway (5), whether arachidin-1
regulates TNF-α-induced NF-κB activation was examined in the
present study. ECs were pretreated with arachidin-1 to examine
whether this agent regulates p65 nuclear translocation in
TNF-α-treated ECs. Western blotting demonstrated that TNF-α-induced
p65 nuclear translocation decreased following pretreatment with 5
µM arachidin-1 from 6 to 12 h (Fig. 3A). Following pretreatment at
concentrations of 0.1–10 µM for 12 h, it was observed that
TNF-α-induced p65 nuclear translocation was inhibited by ≥1
µM arachidin-1 pretreatment (Fig. 3B). To clarify the inhibitory
mechanisms of arachidin-1 in TNF-α-induced NF-κB activation, the
degradation of IκBα was determined over time. TNF-α alone induced a
marked degradation of IκBα following a 1-h treatment. Pretreatment
with 5 µM arachidin-1 for 6 and 12 h inhibited the
TNF-α-induced degradation of IκBα in ECs (Fig. 3C). These results indicate that
arachidin-1 inhibits NF-κB nuclear translocation and
transactivation in a time- and dose-dependent manner.
Arachidin-1 induces Nrf-2 activation
It has previously been reported that Nrf-2-induced
phase II detoxifying and antioxidant enzymes provide cytoprotective
effects in ECs (13). In the
present study, ECs treated with arachidin-1 exhibited a continuous
increase of Nrf-2 nuclear accumulation (Fig. 4A). Nrf-2 regulates the ARE that
drives the expression of specific genes (11). The ability of arachidin-1 to
increase Nrf-2 transcriptional activity was demonstrated by
transfecting ECs with an ARE-luciferase reporter construct
(Fig. 4B). To investigate whether
Nrf-2 contributes to the protective effects of arachidin-1,
experiments using Nrf-2 siRNA were performed. Transfection with
Nrf-2 siRNA suppressed the inhibitory effects of arachidin-1 on
ICAM-1 expression (Fig. 4C),
NF-κB nuclear translocation (Fig.
4D) and IκBα degradation (Fig.
4E). These data indicate that arachidin-1 induces Nrf-2
activation and that this mechanism contributes to the
anti-inflammatory effects of this compound.
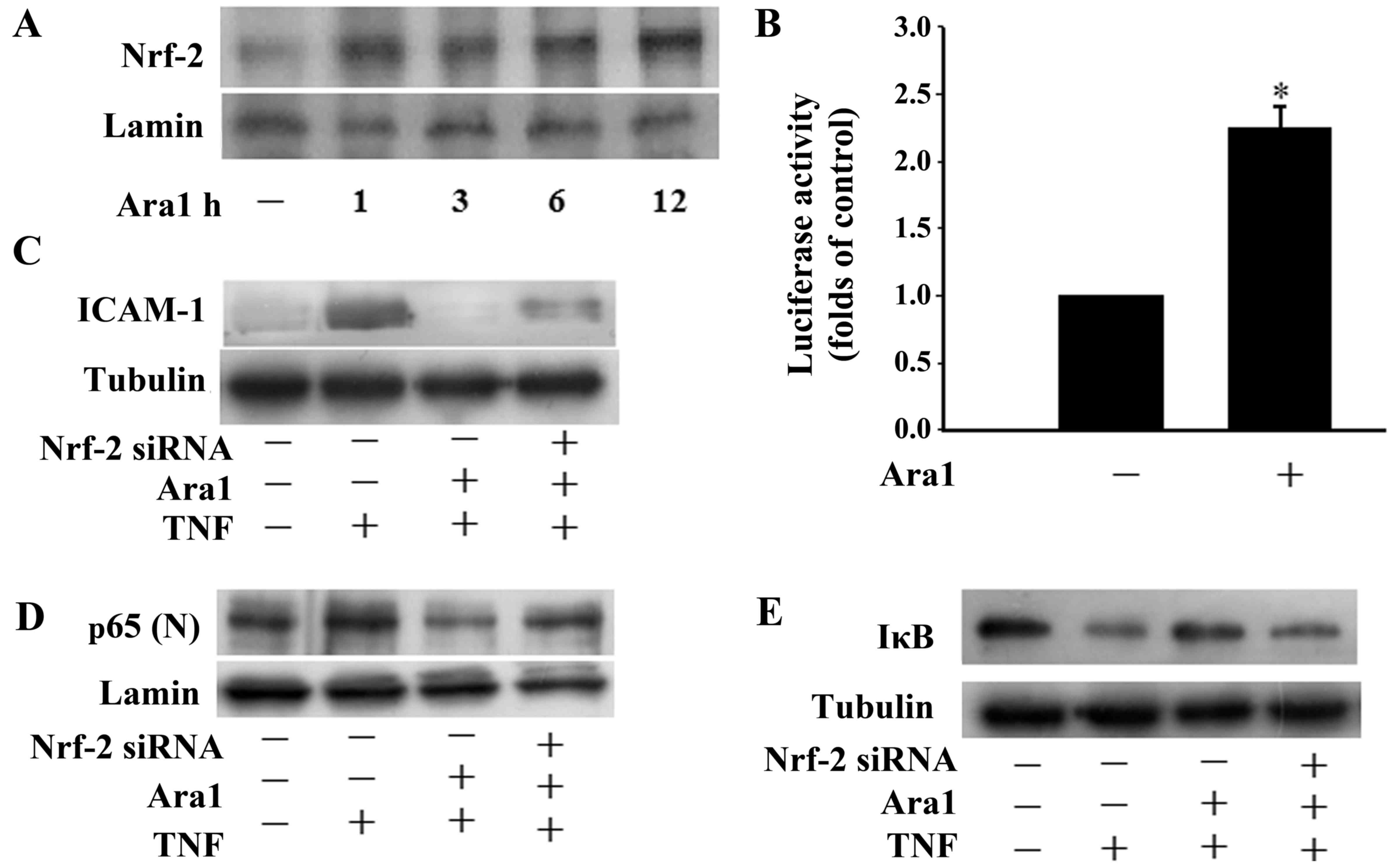 | Figure 4Arachidin-1 induces Nrf-2 activation.
(A) Nuclear extracts from ECs were prepared following treatment
with 5 µM arachidin-1 for the indicated time periods.
Immunoblots of the nuclear lysates were probed with Nrf-2-specific
antibodies. (B) The effects of 5 µM arachidin-1 on the
transcriptional induction of the ARE in ECs. Cells were transfected
with an ARE-luciferase construct. After 12 h, the cells were
treated with 5 µM arachidin-1. The cell lysates were
analyzed for luciferase activity as described in Materials and
methods. Luciferase activity was normalized to co-transfected
β-galactosidase activities. Induction is indicated relative to the
control. All values are presented as mean ± SEM.
*P<0.05. (C–E) Cells were transfected with control or
Nrf-2 siRNA for 36 h and then exposed to 5 µM arachidin-1
for 12 h. Cells were treated with TNF-α for 4 h, and cell lysates
or nuclear extracts were prepared and subjected to western blotting
with antibodies against (C) ICAM-1, (D) p65 (D) or (E) IκBα. Nrf-2,
nuclear factor-E2-related factor-2; ECs, endothelial cells; ARE,
antioxidant response element; siRNA, small interfering RNA; TNF,
tumor necrosis factor; ICAM-1, intercellular adhesion molecule-1;
IκB, inhibitor of κB; Ara1, arachidin-1. . |
Arachidin-1 induces Nrf-2-associated
phase II enzyme expression
The effect of arachidin-1 on phase II enzymes was
investigated using ECs in the current study. Cells were treated
with arachidin-1 at a concentration of 5 or 10 µM for 12 h.
RT-PCR analysis indicated that the mRNA expression levels of
GST-Pi, Trx1, HO-1, GCLC and GCLM were increased (Fig. 5A). The protein levels of HO-1 and
thioredoxin reductase-1 (TrxR-1) were evaluated by western blot
analysis (Fig. 5B). The results
suggest that arachidin-1 activates phase II enzymes through
increasing their expression at the mRNA and protein levels.
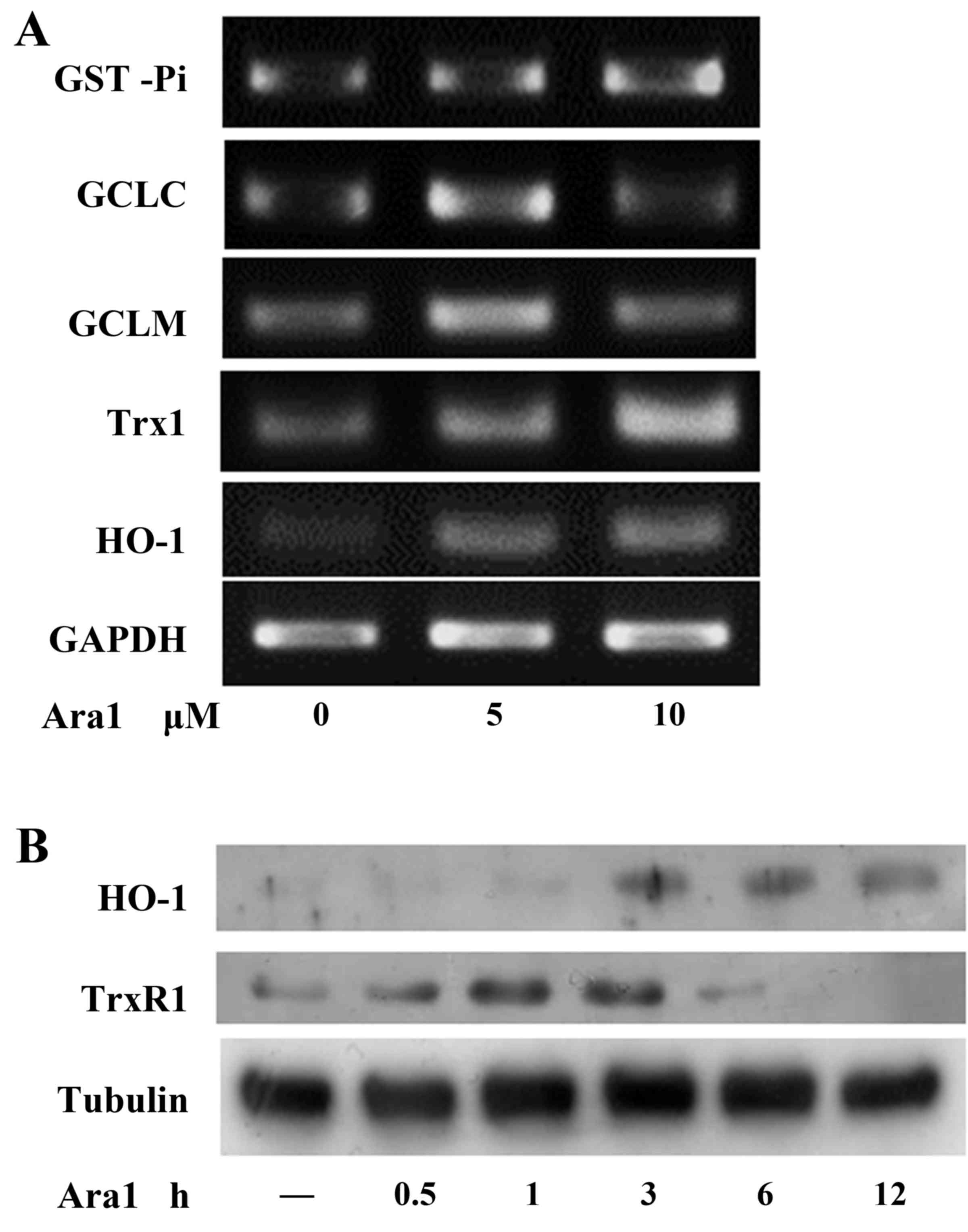 | Figure 5Arachidin-1 induces Nrf-2-associated
phase II enzyme expression. (A) ECs were treated with 5 or 10
µM arachidin-1 at 12 h and then subjected to reverse
transcription-polymerase chain reaction analysis of GST-Pi, GCLM,
GCLC, Trx-1, HO-1 and GAPDH. (B) EC cultures were incubated with 5
µM arachidin-1 for the indicated time periods. Western blot
analysis was then performed with antibodies against HO-1, TrxR-1 or
tubulin as indicated. Nrf-2, nuclear factor-E2-related factor-2;
ECs, endothelial cells; GST, glutathione S-transferase; GCLM,
glutamate-cysteine synthetase regulatory subunit; GCLC,
glutamate-cysteine synthetase catalytic subunit; Trx-1,
thio-redoxin-1; HO-1, heme oxygenase-1; TrxR-1, thioredoxin
reductase-1; Ara1, arachidin-1. |
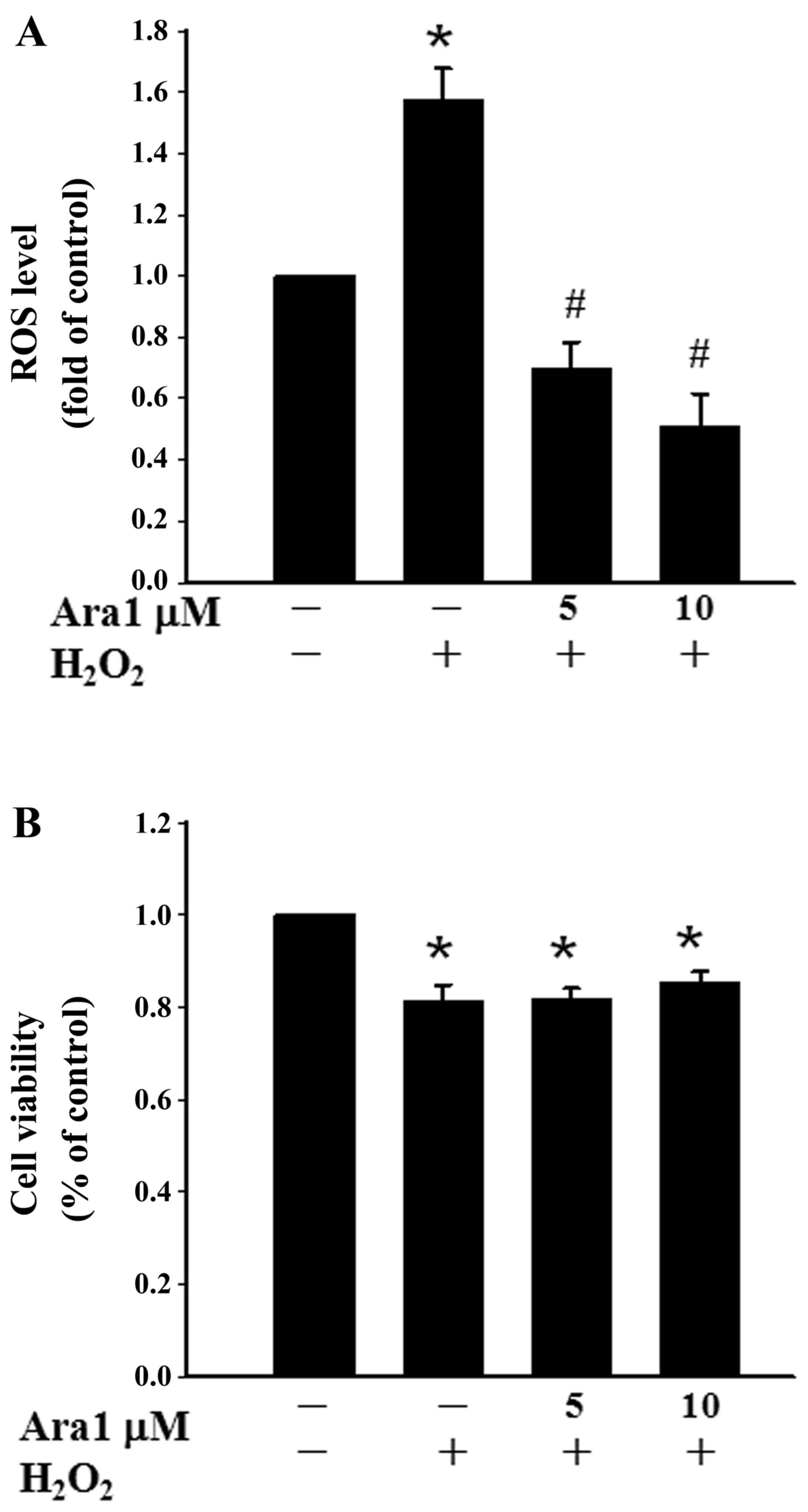
Arachidin-1 reduces
H2O2-induced ROS levels
To evaluate the antioxidant property of arachidin-1,
the effects of arachidin-1 in H2O2-treated
cells were examined. It was observed that the exposure of cells to
0.5 mM H2O2 for 1 h increased the ROS level.
Pretreating cells with 5 or 10 µM arachidin-1 for 12 h
significantly reduced the intracellular ROS level (Fig. 6A). In addition, the protective
effects of arachidin-1 under oxidative stress were examined by
subjecting the cells to treatment with 0.5 mM
H2O2 for 1 h. The results demonstrate that
H2O2 increases cytotoxicity, and arachidin-1
did not prevent this oxidative stress-induced cell death (Fig. 6B).
Discussion
Arachidin-1 is an electrophilic phytochemical
present in germinated peanut roots and the present study provides
the first evidence that this compound, at non-cytotoxic
concentrations, enhances the suppression of TNF-α-induced monocyte
adhesion to ECs by downregulating the expression of ICAM-1. The
present data also demonstrate that the inhibitory effects of
arachidin-1 on the activation of the inflammatory transcriptional
factor, NF-κB, are mediated via blocking the degradation of IκBα,
and exerted through the induction of Nrf-2-associated phase II
detoxifying and antioxidant enzymes. The Nrf-2-associated
protective mechanisms may be the major effecter of the
anti-inflammatory and anti-oxidative effects arachidin-1.
Resveratrol and piceatannol are dietary polyphenols
that are recognized to have numerous health-promoting effects,
including anti-oxidative, anti-inflammatory and anticancer
properties (19). Although
arachidin-1 and arachidin-3 are naturally occurring prenylated
analogs of piceatannol and resveratrol, respectively, the
biological activity of these prenylated stilbenoids is less
studied. The addition of a prenyl group to these compounds
increases their hydrophobicity, which may facilitate their
attachment to cell membranes or translocation into cells.
Theoretically, prenylated stilbenoids should have much more potent
biological activity and bioavailability than stilbenoids. Dietary
resveratrol has been shown to have a low bioavailability; a
previous study revealed that for a dietary 25-mg oral dose of
resveratrol, only <5 ng/ml amounts of unchanged resveratrol were
detected in plasma (20).
Therefore, arachidin-1 may have greater potential applicability
than resveratrol. A previous study by the present research team
demonstrated that piceatannol induced Nrf-2-related HO-1 expression
in ECs, which peaked at 6 h with 25 µM piceatannol treatment
(15). Other researchers
demonstrated that 30 µM piceatannol treatment significantly
upregulated the expression of HO-1 at 6 h in human breast
epithelial cells (21). In the
present study, it was demonstrated that prenylated piceatannol,
arachidin-1, induced HO-1 expression when applied to ECs at a
concentration of 5 µM for 3–12 h. A comparison of these
findings indicates that the prenylated stilbenoid has a greater
efficiency in cell protection than the unprenylated molecule.
The present study provides the first evidence in ECs
that arachidin-1 is anti-inflammatory at non-cytotoxic
concentrations. The results show that the inhibitory effect
differed between arachidin-1 and arachidin-3, indicating that even
subtle changes of the chemical structure significantly affect the
potency. Notably, the only structural difference between these two
prenylated stilbenoids is the 3'-hydroxyl group present in
arachidin-1 (Fig. 1A and B). It
is notable that arachidin-3 was cytotoxic. Thus, the reduction of
cytotoxicity is a crucial factor in the modification of the
structure of prenylated stilbenoids.
The redox-sensitive transcription factor Nrf-2
serves a pivotal role in the induced expression of a number of
cytoprotective enzymes (13). The
present study findings demonstrate that arachidin-1 induces Nrf-2
translocation and activates ARE-luciferase promoter activity,
indicating that it directly induces Nrf-2 via its ARE (Fig. 4A). They also indicate that the
anti-inflammatory effects of arachidin-1 exerted through protection
against ICAM-1 expression and IκBα degradation were reduced by the
transfection of Nrf-2 siRNA (Fig. 4C
and D). Furthermore, arachidin-1 was found to induce
Nrf-2-associated phase II enzyme expression. These data suggest
that arachidin-1 induces Nrf-2-activated phase II enzymes involved
in the anti-inflammatory effect in ECs. Although the exact
mechanisms by which arachidin-1 activates the induction of
Nrf-2-associated genes remain unknown, arachidin-1 may contribute
to the activation of Nrf-2 through its catechol moiety (22). Although arachidin-1 possesses
reducing properties, it may also act as an electrophile. The
catechol moiety of arachidin-1 can be oxidized to the electrophilic
o-quinone form (23). The
electrophilicity of the quinone may lead to the activation of the
Nrf-2/Keap1 pathway due to the modification of specific cysteine
residues of Keap1, which allows Nrf-2 to translocate to the nucleus
and bind to ARE, leading to an increased expression of
Nrf-2-associated genes. The characteristic catechol moiety of
arachidin-1 appears to be critical for the induction of Nrf-2
activation and subsequent upregulation of phase II enzymes.
The induction of Nrf-2-associated enzymes has been
recog-nized as a mechanism underlying the antioxidant properties of
phytochemicals (24). A previous
study demonstrated that piceatannol protects cells from
glutamate-mediated oxidative injury by upregulating cellular
Nrf-2-associated HO-1 expression (25). In the present study, arachidin-1
was revealed to induce the expression of HO-1, GST and other
thiol-containing enzymes in ECs, indicating a protective effect of
arachidin-1 against oxidative stress. Although arachidin-1 did not
prevent H2O2-induced cell damage, it
significantly reduced the H2O2-induced
increase in ROS levels (Fig. 6).
These findings suggest that the anti-inflammatory effect of
arachidin-1 was in accordance with its Nrf-2-associated
anti-oxidative property. However, whether the reduction in
oxidative stress was dependent on phase II enzyme induction
requires further investigation.
In conclusion, the present study demonstrates that
an Nrf-2-regulated antioxidant response serves a pivotal role in
the anti-inflammatory mechanism underlying the inhibition of NF-κB
activation in arachidin-1-treated ECs. A clearer understanding of
the mechanisms of action of arachidin-1 in the future may
contribute to a potential therapeutic application for the treatment
of pathological inflammation.
Acknowledgments
The present study was supported by the Ministry of
Economic Affairs of Taiwan, R.O.C (grant no.
104-EC-17-A-18-S1-226). The authors thank Ms. Wan-Yu Chen and Mr.
Yu-Ting Huang for assistance in selected experiments.
References
|
1
|
Fernandes A, Miller-Fleming L and Pais TF:
Microglia and inflammation: Conspiracy, controversy or control?
Cell Mol Life Sci. 71:3969–3985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glass CK and Witztum JL: Atherosclerosis.
the road ahead. Cell. 104:503–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ledebur HC and Parks TP: Transcriptional
regulation of the intercellular adhesion molecule-1 gene by
inflammatory cytokines in human endothelial cells. Essential roles
of a variant NF-kappa B site and p65 homodimers. J Biol Chem.
270:933–943. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Condori J, Sivakumar G, Hubstenberger J,
Dolan MC, Sobolev VS and Medina-Bolivar F: Induced biosynthesis of
resveratrol and the prenylated stilbenoids arachidin-1 and
arachidin-3 in hairy root cultures of peanut: Effects of culture
medium and growth stage. Plant Physiol Biochem. 48:310–318. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piotrowska H, Kucinska M and Murias M:
Biological activity of piceatannol: Leaving the shadow of
resveratrol. Mutat Res. 750:60–82. 2012. View Article : Google Scholar
|
|
8
|
Huang CP, Au LC, Chiou RY, Chung PC, Chen
SY, Tang WC, Chang CL, Fang WH and Lin SB: Arachidin-1, a peanut
stilbenoid, induces programmed cell death in human leukemia HL-60
cells. J Agric Food Chem. 58:12123–12129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ko JC, Chen HJ, Huang YC, Tseng SC, Weng
SH, Wo TY, Huang YJ, Chiu HC, Tsai MS, Chiou RY, et al: HSP90
inhibition induces cytotoxicity via downregulation of Rad51
expression and DNA repair capacity in non-small cell lung cancer
cells. Regul Toxicol Pharmacol. 64:415–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ball JM, Medina-Bolivar F, Defrates K,
Hambleton E, Hurlburt ME, Fang L, Yang T, Nopo-Olazabal L, Atwill
RL, Ghai P, et al: Investigation of stilbenoids as potential
therapeutic agents for rotavirus gastroenteritis. Adv Virol.
2015:2935242015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang KW, Lee SJ and Kim SG: Molecular
mechanism of nrf2 activation by oxidative stress. Antioxid Redox
Signal. 7:1664–1673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH
and Wung BS: Upregulation of heme oxygenase-1 by
Epigallocatechin-3-gallate via the phosphatidylinositol
3-kinase/Akt and ERK pathways. Life Sci. 78:2889–2897. 2006.
View Article : Google Scholar
|
|
13
|
Chen B, Lu Y, Chen Y and Cheng J: The role
of Nrf2 in oxidative stress-induced endothelial injuries. J
Endocrinol. 225:R83–R99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen T, Yang CS and Pickett CB: The
pathways and molecular mechanisms regulating Nrf2 activation in
response to chemical stress. Free Radic Biol Med. 37:433–441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wung BS, Hsu MC, Wu CC and Hsieh CW:
Piceatannol upregulates endothelial heme oxygenase-1 expression via
novel protein kinase C and tyrosine kinase pathways. Pharmacol Res.
53:113–122. 2006. View Article : Google Scholar
|
|
16
|
Liao BC, Hsieh CW, Liu YC, Tzeng TT, Sun
YW and Wung BS: Cinnamaldehyde inhibits the tumor necrosis
factor-alpha-induced expression of cell adhesion molecules in
endothelial cells by suppressing NF-kappaB activation: Effects upon
IkappaB and Nrf2. Toxicol Appl Pharmacol. 229:161–171. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang JC, Lai YH, Djoko B, Wu PL, Liu CD,
Liu YW and Chiou RY: Biosynthesis enhancement and antioxidant and
anti-inflammatory activities of peanut (Arachis hypogaea L.)
arachidin-1, arachidin-3, and isopentadienylresveratrol. J Agric
Food Chem. 54:10281–10287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh A, Boldin-Adamsky S, Thimmulappa RK,
Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F,
Watson WH, et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kasiotis KM, Pratsinis H, Kletsas D and
Haroutounian SA: Resveratrol and related stilbenes: Their
anti-aging and anti-angiogenic properties. Food Chem Toxicol.
61:112–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr
and Walle UK: High absorption but very low bioavailability of oral
resveratrol in humans. Drug Metab Dispos. 32:1377–1382. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HH, Park SA, Almazari I, Kim EH, Na HK
and Surh YJ: Piceatannol induces heme oxygenase-1 expression in
human mammary epithelial cells through activation of ARE-driven
Nrf2 signaling. Arch Biochem Biophys. 501:142–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin D, Dai F, Sun LD and Zhou B: Toward an
understanding of the role of a catechol moiety in cancer
chemoprevention: The case of copper- and o-quinone-dependent Nrf2
activation by a catechol-type resveratrol analog. Mol Nutr Food
Res. 59:2395–2406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sirota R, Gibson D and Kohen R: The role
of the catecholic and the electrophilic moieties of caffeic acid in
Nrf2/Keap1 pathway activation in ovarian carcinoma cell lines.
Redox Biol. 4:48–59. 2015. View Article : Google Scholar :
|
|
24
|
Surh YJ, Kundu JK and Na HK: Nrf2 as a
master redox switch in turning on the cellular signaling involved
in the induction of cytoprotective genes by some chemopreventive
phytochemicals. Planta Med. 74:1526–1539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Son Y, Byun SJ and Pae HO: Involvement of
heme oxygenase-1 expression in neuroprotection by piceatannol, a
natural analog and a metabolite of resveratrol, against
glutamate-mediated oxidative injury in HT22 neuronal cells. Amino
Acids. 45:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|