Introduction
Colorectal cancer is one of leading causes of
cancer-associated mortality worldwide (1,2).
Despite advances in detection and therapy in previous years, the
incidence and mortality in patients with colorectal cancer is still
increasing throughout the world (3–6).
The major cause of mortality patients with colorectal cancer is
distant metastasis to other organs, such as the liver (7), lung (8), brain (9) and bone (10). Chronic inflammation has emerged as
a key contributor for development and metastasis of cancer,
including colorectal carcinoma (11,12). Inflammatory cytokines, such as
tumor necrosis factor-α (TNF-α), interleukins (ILs), including
IL-8, IL-6 and IL-1β, promote cancer progression (13). Among them, TNF-α contributes to
proliferation and metastasis of colorectal cancer cells and its
increased expression is often correlated with poor prognosis of
patients (14). Although TNF-α
expression has been considered to be a double-edged sword in
cancer, it has been suggested that targeting TNF-α may be a
successful strategy for cancer treatment (14,15). Activation of the TNF-α pathway
promotes nuclear translocation of Snail transcription factor
proteins (16). This
translocation promotes epithelial-mesenchymal transition (EMT), a
process that is considered to be a prerequisite for metastasis in
various types of cancer, including colorectal cancer (17–21). Furthermore, Snail overexpression
in patients with colorectal cancer has also been correlated with
aggressiveness and poor prognosis (22,23). Therefore, inhibition of
TNF-α-induced EMT is likely to be effective for treatment of
colorectal cancer.
Protein kinase B (AKT), one of the mitogen-activated
protein kinases (MAPKs), regulates proliferation, differentiation,
development, transformation and apoptosis (24). Disturbance of phosphoinositide
3-kinase/AKT signaling is associated with cancer progression and
poor prognosis (25). Deregulated
AKT phosphorylates glycogen synthase kinase-3β (GSK-3β), followed
by inactivation of GSK-3β (26).
Inactivated GSK-3β fails to tag Snail protein for ubiquitination
and subsequent degradation (26).
As Snail stabilization is tightly regulated by AKT/GSK-3β pathways,
targeting this pathway is a promising strategy for cancer
treatment. It has been reported that various anticancer agents
inhibit tumor growth via suppressing AKT/GSK-3β/Snail signaling
(27–31).
Traditional herbal medicines have previously been
researched for colorectal cancer treatment (32–37).
Danggui-Sayuk-Ga-Osuyu-Saenggang-Tang (DSGOST;
Danggui-Sini-Jia-Wuzhuyu-Shengjian-Tang in Chinese,
Tokishigyakukagoshuyushokyoto in Japanese) has long been used to
cure patients suffered from Raynaud's syndrome (38,39), dysmenorrhea (40), atopic dermatitis (41), but not cancer. Choi et al
(42) initially suggested a new
use for DSGOST in the treatment of cancer as DSGOST was shown to
inhibit pancreatic tumor growth and metastasis by suppressing
vascular endothelial growth factor (VEGF)/VEGF receptor 2-dependent
tumor angiogenesis. Furthermore, Nagata et al (43) demonstrated the inhibitory effect
of DSGOST on growth of gastric cancer cells. Taken together, we
hypothesize that DSGOST may be beneficial for cancer treatment.
In present study, the anticancer effect of DSOGST on
TNF-α-mediated metastatic phenotypes was examined in HCT116
colorectal cancer cells. A non-cytotoxic dose of DSGOST inhibited
TNF-α-induced migration and invasion of HCT116 cells. Furthermore,
TNF-α induced morphological changes with either downregulation of
E-cadherin or upregulation of N-cadherin whereas DSGOST reversed
TNF-α-mediated changes. Furthermore, DSGOST suppressed
TNF-α-induced nuclear translocation of Snail via inhibition of
AKT/GSK-3β pathways. Therefore, these results demonstrated for the
first time, the inhibitory effect of DSGOST on TNF-α-induced
migration and invasion in HCT116 cells.
Materials and methods
Preparation of DSGOST
DSGOST powder was prepared as described previously
(44). DSGOST consists of
following herbal drugs; 1 g Angelica radix, 1 g Cinnamomi cortex, 1
g Paeoniae root, 1 g Akebia root, 0.67 g of Asarum, 0.67 g
Glycyrrhiza, 1.67 g Zizyphus jujuba, 0.67 g Evodia fruit and
1.33 g ginger root. The dried mixture was dissolved in distilled
water and stored at −80°C until use.
Cell culture and cell viability
assay
HCT116 human colorectal cancer cell lines were
purchased from Korean Cell Line Bank (Seoul, Korea) and cultured in
RPMI-1640 medium (Welgene Inc., Daegu, Korea)supplemented with 10%
fetal bovine serum (FBS; JR Scientific, Woodland, CA, USA) and 1%
penicillin/streptomycin solution. For cell viability assay, HCT116
cells were seeded at the density of 4,000 cells/well in 96-wells
plate and then treated with DSGOST (0, 10, 50 and 100
µg/ml). After 72 h incubation, cell viability was determined
by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) colorimetric assay with an absorbance 570 nm.
Migration and invasion assay
For a cell migration assay, cells were cultured in
6-wells plates until 100% confluence and then the monolayer was
scratched the monolayer with a 200 µl pipette tip. Cells
were then pretreated with DSGOST for 2 h before incubation with
TNF-α (R&D Systems, Minneapolis, MN, USA) for 48 h. The
migrated area was analyzed by ImageJ software (64-bit Java
1.6.0_24; National Institutes of Health, Bethesda, MD, USA) and the
average was calculated from independent experiments. For the
invasion assay, Matrigel was mixed with coating buffer [0.01 M Tris
(pH 8.0), 0.7% NaCl] at 1:1 ratio and then added in upper side of
BD Transwell chamber in 24-well plates for overnight incubation.
HCT116 (2×105 cells) were pre-incubated with 50
µg/ml DSGOST for 2 h, followed by 20 ng/ml of TNF-α for 48 h
in the upper chamber. Culture medium with 10% FBS as
chemoattractant was added into the lower chamber. After 48 h
incubation, the cells were fixed with 4% paraformaldehyde for 5 min
and then permeabilized with 100% methanol for 20 min. Non-invading
cells were removed by cotton swab and the cells located at the
underside of chamber were stained with 0.05% crystal violet for 20
min at room temperature. The four areas were randomly selected and
cells were counted under a phase-contrast microscope.
Cellular fractionation
Cells were pretreated with DSGOST for 2 h, followed
by incubation with TNF-α for 6 h. Cell lysates were suspended in
500 µl hypotonic buffer (20 mM Tris-HCl, pH 7.4, 10 mM NaCl,
3 mM MgCl2) and incubated on ice for 15 min, followed by
centrifugation at 1,000 × g at 4°C. The supernatant (cytoplasmic
fraction) was stored at -80°C until use. The obtained pellets were
suspended in 50 µl cell extraction buffer containing 100 mM
Tris (pH 7.4), 2 mM Na3VO4, 100 mM NaCl, 1%
Triton X-100, 1 mM ethylenediaminetetraacetic acid (EDTA), 10%
glycerol, 1 mM EGTA, 0.1% sodium dodecyl sulfate (SDS), 1 mM NaF,
0.5% deoxycholate and 20 mM
Na4P2O7. After incubation on ice
for 15 min, the mixture was centrifuged at 14,000 × g at 4°C. The
supernatant (nuclear fraction) was stored at -80°C until use.
Western blotting
Cells were collected by scraping and then lysed with
RIPA buffer containing 50 mM Tris-HCl, (pH 7.5), 150 mM NaCl, 1%
Triton X-100, 2 mM EDTA, 0.1% SDS and 1% sodium deoxycholate. Equal
amount of proteins [20 µg per well; protein concentration
was determined using a Bio-Rad Bradford protein assay (Bio-Rad,
Hercules, CA, USA)] were separated by 10–12% SDS-PAGE, followed by
transfer to nitrocellulose membranes. After blocking with 2% skim
milk in PBS-Tween at room temperature for 1 h, membranes were
incubated with the appropriate antibodies at 4°C overnight.
Anti-E-cadherin (cat. no. 3195, 1:1,000), tight junction protein-1
(ZO-1; cat. no. 8193; 1:500), N-cadherin (cat. no. 4061; 1:1,000),
Snail (cat. no. 3879, 1:1,000), claudin-1 (cat. no. 13255; 1:500),
p-AKT (cat. no. 9271; 1:500) and p-GSK-3β (cat. no. 9336; 1:500)
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-lamin B (cat. no. sc6216; 1:500) and
β-actin (cat. no. sc47778; 1:2,000) antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Horseradish
peroxidase-conjugated secondary antibodies for mouse (cat. no.
7076; 1:1,000–3,000) and rabbit (cat. no. 7074; 1:1,000-2,000) were
purchased from Cell Signaling Technology, Inc. The membranes were
incubated with secondary antibodies for 1 h at room temperature. An
enhanced chemiluminescence kit (DoGen, Seoul, Korea) was used for
detection of HRP signal. ImageJ software (64-bit Java 1.6.0_24;
National Institutes of Health) was used for densitometry.
Immunocytochemistry
The cells (80% confluency) were cultured on
coverslip in 6-well plates with serum-free medium for 12 h and then
pretreated with or without DSGOST. After 2 h incubation with
DSGOST, cells were treated with TNF-α in medium with 1% FBS. Cells
were washed with cold-PBS, fixed with 4% paraformaldehyde for 30
min, permeabilized with 0.1% Triton X-100 for 15 min and blocked
with 2% bovine serum albumin (BSA) for 60 min at room temperature.
Cells were washed with 0.5% BSA and were incubated with antibodies
against E-cadherin (cat. no. 3195; 1:50), N-cadherin (cat. no.
4061; 1:50) and Snail (cat. no. 3879; 1:50) (Cell Signaling
Technology, Inc.) at 4°C overnight. Following incubation, cells
were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG
(cat. no. A-11008; 1:1,000; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) secondary antibodies for 1 h at room temperature.
Nuclei were stained with TO-PRO-3 iodide (Thermo Fisher
Scientific, Inc.). Confocal images were acquired with Zeiss
LSM5 PASCAL confocal laser scanning microscope system (Carl Zeiss
AG, Oberkochen, Germany). Fluorescence intensities were measured
under the same condition for all experiments.
Statistical analysis
Data are presented as the mean ± standard error or
standard deviation from at least three experiments with three
replicates per each experiment. The statistical differences of
means between the groups were analyzed by one-way analysis of
variance with post-hoc Tukey test using SPSS software version 22.0
(IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
DSGOST inhibits TNF‑α‑induced EMT in
HCT116 cells
To avoid interference resulting from the
anti-proliferative effect of DSGOST on HCT116 cells on HCT116
cells, the effect of DSGOST on proliferation of cells was examined
by an MTT assay. DSGOST (50 µg/ml) did not affect on the
proliferation of cells (Fig. 1A).
Thus, the effect of DSGOST (50 µg/ml) on TNF-α-mediated
changes in morphology of cells was examined. While the cells in the
TNF-α treatment group exhibited an invasive phenotype with a
mesenchymal morphology, DSGOST treatment abrogated this change
(Fig. 1B).
DSGOST reverses TNF‑α‑mediated EMT in
HCT116 cells
TNF-α-induced invasive phenotype is associated with
induction of EMT in colorectal cancer cells (16,45). Whether DSGOST prevents
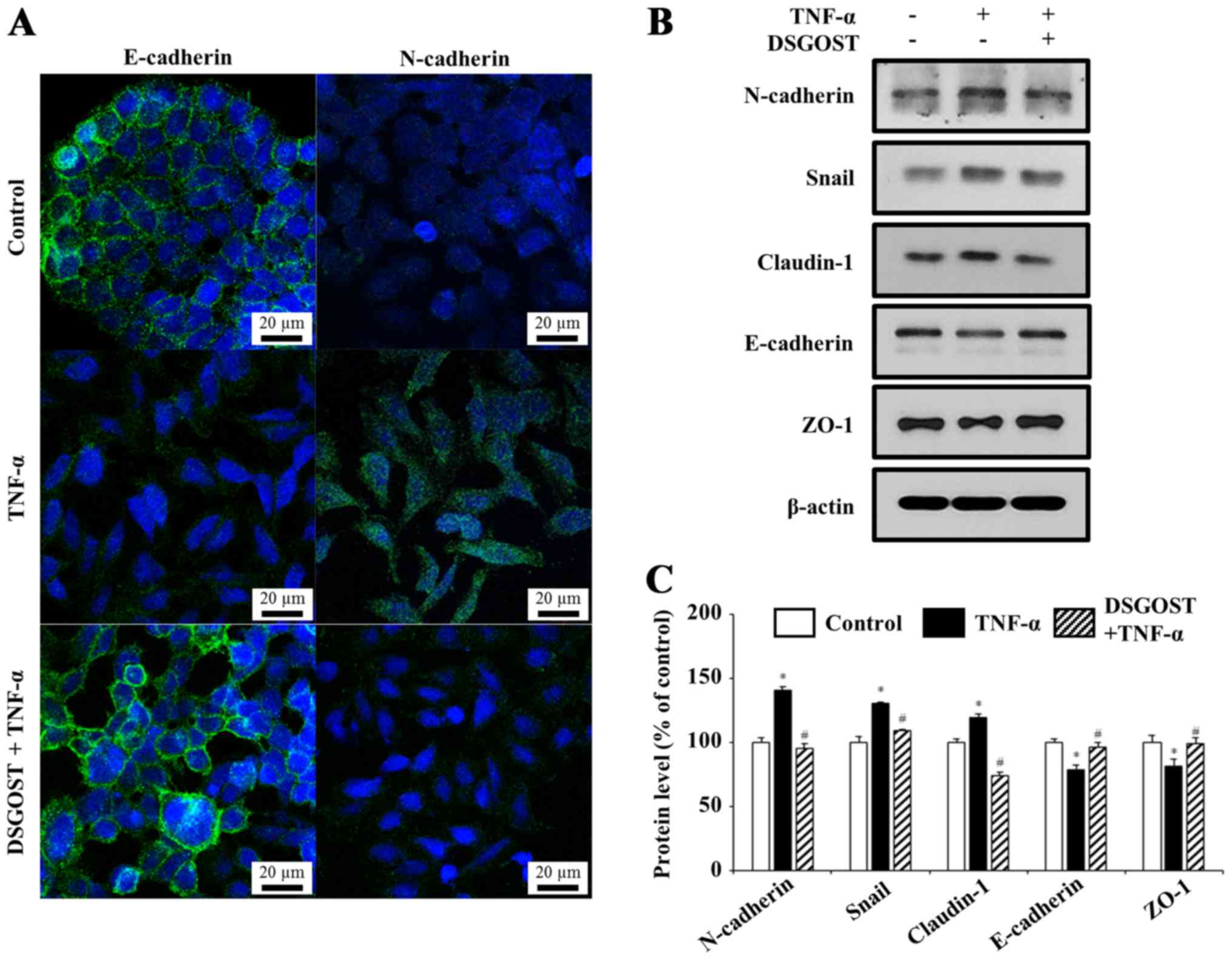
TNF-α-mediated changes in EMT markers was examined.
Immunocytochemistry data demonstrated that TNF-α treatment induced
mesenchymal morphology with decreased E-cadherin expression and
increased N-cadherin expression, whereas pretreatment of DSGOST
inhibited TNF-α-mediated responses (Fig. 2A). During EMT, epithelial cells
acquire a mesenchymal-like phenotype, including the loss of
E-cadherin-mediated cell-cell adhesion and the upregulation of
N-cadherin. In TNF-α-treated HCT116 cells, epithelial cell markers
E-cadherin and ZO-1 were decreased while mesenchymal markers Snail,
N-cadherin and claudin-1 were increased. Additionally, DSGOST
treatment reversed TNF-α-mediated protein expression (Fig. 2B and C). Thus, DSGOST is may
inhibit TNF-α-mediated EMT in HCT116 cells.
DSGOST suppresses TNF‑α‑promoted
migration and invasion of HCT116 cells
EMT process has been considered to be a prerequisite
for migration and invasion of various cell types (46). As DSGOST inhibited TNF-α-induced
EMT in HCT116 cells, it was also examined whether DSGOST suppresses
TNF-α-mediated migration and invasion using wound healing assay and
Transwell invasion assays, respectively. TNF-α increased migration
and invasion of HCT116 cells, and DSGOST treatment blocked
TNF-α-mediated migration and invasion (Figs. 3 and 4). Therefore, DSGOST is may suppress
TNF-α-induced migration and invasion of HCT116 cells.
DSGOST inhibits TNF‑α‑induced nuclear
translocation of Snail via inhibition of AKT/GSK‑3β pathways in
HCT116 cells
TNF-α activates either the expression or nuclear
trans-location of Snail protein, resulting in induction of EMT
(16). As presented in Figs. 5 and 6, TNF-α promoted nuclear trans-location
of Snail protein, whereas pretreatment with DSGOST prevented this
translocation. Thus, DSGOST inhibits TNF-α-mediated EMT by
suppressing Snail translocation into nucleus. As Snail is tightly
regulated by GSK-3β, which is phosphorylated and inactivated by AKT
(16,47), it was also further examined
whether DSGOST inhibits TNF-α-mediated AKT/GSK-3β signaling. While
TNF-α induced the phosphorylation of both AKT and GSK-3β, DSGOST
inhibited their phosphorylation (Fig.
6B). Therefore, data indicated that DSGOST inhibition of
TNF-α-induced Snail nuclear translocation is mediated by blocking
the AKT/GSK-3β pathways.
Discussion
While DSGOST has been researched for the treatment
of various disorders, recent studies have suggested DSGOST as a
novel candidate for treatment of cancer (42,43). Thus, we hypothesized that DSGOST
would be an effective treatment for colorectal cancer. Since TNF-α
activates the EMT process in colorectal cancer cells, resulting in
tumor metastasis, the inhibitory effect of DSGOST treatment on
TNF-α-mediated migration and invasion of HCT116 cells was
investigated in the current study. The findings showed that DSGOST
suppressed TNF-α-mediated migration and invasion. Furthermore,
DSGOST also blocked TNF-α-mediated EMT via inhibiting
AKT/GSK-3β/Snail signaling.
In the late 1970s, TNF-α was considered to be an
anticancer agent by suppressing tumor cell proliferation (48,49). On the other hand, several in
vitro studies have reported the tumor promoting effect of TNF-α
(16,50,51). Furthermore, clinical studies have
reported that TNF-α expression levels in serum and tissues were
elevated in patients with tumors (52–55), suggesting that TNF-α may be a
prognostic marker in patients with cancer. While the role of TNF-α
in tumor malignancy is still controversial, the effect of
recombinant TNF-α on EMT in HCT116 cells was investigated in the
current study. The data showed that TNF-α treatment induced EMT
phenotype. Furthermore, TNF-α also promoted migration and invasion.
Although the cell type, TNF-α concentration and incubation period
of TNF-α in the present study were limited, and the precise
mechanisms of the effects remain to be demonstrated, the data
provide evidence that TNF-α may contribute to tumor metastasis by
inducing EMT.
The role of Snail in EMT has been validated in
several cancer types (18–20,22).
Poor clinical outcomes of patients with colorectal cancer are
associated with Snail overexpression (22,23). Wang et al (16) reported that TNF-α-induced EMT is
regulated by Snail protein in at least two types of colorectal
cancer cells, HCT116 and Caco-2. Furthermore, the same study
clearly showed that AKT/GSK-3β signaling is responsible for
TNF-α-mediated Snail stabilization in those cells, while inhibitors
of nuclear factor-κB (NF-κB), MAPK and p38 failed to abrogate
TNF-α-induced Snail expression (16). Since Snail stabilization is
tightly regulated by AKT/GSK-3β signaling pathways, DSGOST
inhibition of AKT/GSK-3β signaling indicates that one of the
potential biological mechanisms of DSGOST inhibition of
TNF-α-mediated EMT in HCT116 colorectal cancer cells is via
blocking of AKT/GSK-3β/Snail signaling.
The potential effectiveness of multi-target
approaches in cancer therapy has been emphasized previously
(56). Phytochemicals and herbal
mixtures, which may act on multiple targets, have anticancer
activity (57–66). Genistein, a dietary component with
an anticancer effects against breast and prostate cancer, has been
shown to regulate apoptosis, cell cycle, and NF-κB and AKT pathways
(58). Proanthocyanidins have
been reported to exhibit anticancer effects via antioxidative and
anti-inflammatory properties (59). Ginsenoside, from ginseng plants,
modulates the immune system, apoptosis and metastasis (57). Traditional Chinese medicine
theory-based multi-herb prescriptions have benefits in the
treatment of diseases including breast (60) and lung (61) cancer. SH003, a herbal prescription
composed of Astragalus membranaceus, Angelica gigas
and Trichosanthes kirilowii Maximowicz, exerts inhibitory
effects against tumor growth by targeting apoptosis, autophagy and
angiogenesis in several cancer types (62,64–66). A previous study reported the
antitumor and anti-angiogenesis effects of DSGOST (42). The present study also demonstrated
that DSGOST inhibits TNF-α-mediated invasion and migration of
HCT116 cells. Since both invasive phenotype and tumor angiogenesis
contribute to tumor metastasis and poor prognosis of patients with
cancer, we hypothesize that the multi-target properties of DSGOST
may be useful for improving the prognosis of patients with cancer
by dual inhibition of invasion and tumor angiogenesis. However,
further in vitro and in vivo studies are required to
demonstrate the anticancer property of DSGOST with a clear
biological mechanism in several cancer types.
In conclusion, the data of the current study
demonstrates, that DSGOST inhibits TNF-α-mediated EMT via
suppressing AKT/GSK-3β/Snail pathways. Although the precise
mechanism of DSGOST remains unclear, the results of the present
study suggests that DSGOST is potentially beneficial for treating
colorectal cancer. Furthermore, taken together with previous
results indicating DSGOST inhibition of tumor angiogenesis, this
suggests that DSGOST may be a novel multi-target herbal medicine
for inhibition of tumor metastasis.
Acknowledgments
This study was supported by a grant from Korean
Medicine R&D Project of the Ministry of Health and Welfare
(grant no. B110043).
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pourhoseingholi MA: Increased burden of
colorectal cancer in Asia. World J Gastrointest Oncol. 4:68–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar
|
|
5
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adam R, Avisar E, Ariche A, Giachetti S,
Azoulay D, Castaing D, Kunstlinger F, Levi F and Bismuth F:
Five-year survival following hepatic resection after neoadjuvant
therapy for nonresectable colorectal. Ann Surg Oncol. 8:347–353.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamilton TD, Leugner D, Kopciuk K, Dixon
E, Sutherland FR and Bathe OF: Identification of prognostic
inflammatory factors in colorectal liver metastases. BMC Cancer.
14:5422014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weiss L, Grundmann E, Torhorst J, Hartveit
F, Moberg I, Eder M, Fenoglio-Preiser CM, Napier J, Horne CH, Lopez
MJ, et al: Haematogenous metastatic patterns in colonic carcinoma:
An analysis of 1541 necropsies. J Pathol. 150:195–203. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noura S, Ohue M, Shingai T, Fujiwara A,
Imada S, Sueda T, Yamada T, Fujiwara Y, Ohigashi H, Yano M, et al:
Brain metastasis from colorectal cancer: Prognostic factors and
survival. J Surg Oncol. 106:144–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jimi S, Yasui T, Hotokezaka M, Shimada K,
Shinagawa Y, Shiozaki H, Tsutsumi N and Takeda S: Clinical features
and prognostic factors of bone metastases from colorectal cancer.
Surg Today. 43:751–756. 2013. View Article : Google Scholar
|
|
11
|
Terzic J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology. 138:2101–2114
e2105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balkwill FR and Mantovani A:
Cancer-related inflammation: Common themes and therapeutic
opportunities. Semin Cancer Biol. 22:33–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klampfer L: Cytokines, inflammation and
colon cancer. Curr Cancer Drug Targets. 11:451–464. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balkwill F: TNF-alpha in promotion and
progression of cancer. Cancer Metastasis Rev. 25:409–416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Wang HS, Zhou BH, Li CL, Zhang F,
Wang XF, Zhang G, Bu XZ, Cai SH and Du J: Epithelial-mesenchymal
transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated
stabilization of snail in colorectal cancer. PLoS One.
8:e566642013. View Article : Google Scholar
|
|
17
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong C, Wu Y, Yao J, Wang Y, Yu Y,
Rychahou PG, Evers BM and Zhou BP: G9a interacts with Snail and is
critical for Snail-mediated E-cadherin repression in human breast
cancer. J Clin Invest. 122:1469–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pon YL, Zhou HY, Cheung AN, Ngan HY and
Wong AS: p70 S6 kinase promotes epithelial to mesenchymal
transition through snail induction in ovarian cancer cells. Cancer
Res. 68:6524–6532. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Zhu X, Hu J, He G, Li X, Wu P, Ren
X, Wang F, Liao W, Liang L, et al: The positive feedback between
Snail and DAB2IP regulates EMT, invasion and metastasis in
colorectal cancer. Oncotarget. 6:27427–27439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bates RC and Mercurio AM: The
epithelial-mesenchymal transition (EMT) and colorectal cancer
progression. Cancer Biol Ther. 4:365–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kroepil F, Fluegen G, Vallböhmer D, Baldus
SE, Dizdar L, Raffel AM, Hafner D, Stoecklein NH and Knoefel WT:
Snail1 expression in colorectal cancer and its correlation with
clinical and pathological parameters. BMC Cancer. 13:1452013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwon CH, Park HJ, Choi JH, Lee JR, Kim HK,
Jo HJ, Kim HS, Oh N, Song GA and Park DY: Snail and serpinA1
promote tumor progression and predict prognosis in colorectal
cancer. Oncotarget. 6:20312–20326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McCubrey JA, Steelman LS, Bertrand FE,
Davis NM, Sokolosky M, Abrams SL, Montalto G, D'Assoro AB, Libra M,
Nicoletti F, et al: GSK-3 as potential target for therapeutic
intervention in cancer. Oncotarget. 5:2881–2911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pappalardo F, Russo G, Candido S, Pennisi
M, Cavalieri S, Motta S, McCubrey JA, Nicoletti F and Libra M:
Computational modeling of PI3K/AKT and MAPK signaling pathways in
melanoma cancer. PLoS One. 11:e01521042016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciuffreda L, McCubrey JA and Milella M:
Signaling intermediates (PI3K/PTEN/AKT/mTOR and RAF/MEK/ERK
pathways) as therapeutic targets for anticancer and
anti-angiogenesis treatments. Curr Signal Transduct Ther.
4:130–143. 2009. View Article : Google Scholar
|
|
29
|
El Touny LH and Banerjee PP: Akt GSK-3
pathway as a target in genistein-induced inhibition of TRAMP
prostate cancer progression toward a poorly differentiated
phenotype. Carcinogenesis. 28:1710–1717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bocca C, Bozzo F, Bassignana A and
Miglietta A: Antiproliferative effects of COX-2 inhibitor celecoxib
on human breast cancer cell lines. Mol Cell Biochem. 350:59–70.
2011. View Article : Google Scholar
|
|
31
|
Firdous AB, Sharmila G, Balakrishnan S,
RajaSingh P, Suganya S, Srinivasan N and Arunakaran J: Quercetin, a
natural dietary flavonoid, acts as a chemopreventive agent against
prostate cancer in an in vivo model by inhibiting the EGFR
signaling pathway. Food Funct. 5:2632–2645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yim NH, Jung YP, Kim A, Ma CJ, Cho WK and
Ma JY: Oyaksungisan, a traditional herbal formula, inhibits cell
proliferation by induction of autophagy via JNK activation in human
colon cancer cells. Evid Based Complement Alternat Med.
2013:2318742013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou F, Li W, Shi Q, Li H, Liu S, Zong S,
Ren J, Chai J and Xu J: Yi Ai Fang, a traditional Chinese herbal
formula, impacts the vasculogenic mimicry formation of human
colorectal cancer through HIF-1α and epithelial mesenchymal
transition. BMC Complement Altern Med. 16:4282016. View Article : Google Scholar
|
|
34
|
Ohnishi Y, Fujii H, Hayakawa Y, Sakukawa
R, Yamaura T, Sakamoto T, Tsukada K, Fujimaki M, Nunome S, Komatsu
Y, et al: Oral administration of a Kampo (Japanese herbal) medicine
Juzen-taiho-to inhibits liver metastasis of colon 26-L5 carcinoma
cells. Jpn J Cancer Res. 89:206–213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leung WK, Wu JC, Liang SM, Chan LS, Chan
FK, Xie H, Fung SS, Hui AJ, Wong VW, Che CT, et al: Treatment of
diarrhea-predominant irritable bowel syndrome with traditional
Chinese herbal medicine: A randomized placebo-controlled trial. Am
J Gastroenterol. 101:1574–1580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hosokawa A, Ogawa K, Ando T, Suzuki N,
Ueda A, Kajiura S, Kobayashi Y, Tsukioka Y, Horikawa N, Yabushita
K, et al: Preventive effect of traditional Japanese medicine on
neurotoxicity of FOLFOX for metastatic colorectal cancer: A
multicenter retrospective study. Anticancer Res. 32:2545–2550.
2012.PubMed/NCBI
|
|
37
|
Yoshikawa K, Shimada M, Nishioka M, Kurita
N, Iwata T, Morimoto S, Miyatani T, Komatsu M, Kashihara H and
Mikami C: The effects of the Kampo medicine (Japanese herbal
medicine) 'Daikenchuto' on the surgical inflammatory response
following laparoscopic colorectal resection. Surg Today.
42:646–651. 2012. View Article : Google Scholar
|
|
38
|
Tsukada R, Yamaguchi T, Hang L, Iseki M,
Kobayashi H and Inada E: Effect of a traditional Japanese medicine
goshajinkigan, tokishigyakukagoshuyushokyoto on the warm and cold
sense threshold and peripheral blood flow. Health (NY). 6:757–763.
2014. View Article : Google Scholar
|
|
39
|
Yoko K, Akira T and Hiroshi S: Efficacy of
Kampo formula Tokishigyakukagoshuyushokyoto for cold syndrome
evaluated with a novel clinical method using a patient-based
questionnaire database. Kampo Med. 63:299–304. 2012. View Article : Google Scholar
|
|
40
|
Oya A, Oikawa T, Nakai A, Takeshita T and
Hanawa T: Clinical efficacy of Kampo medicine (Japanese traditional
herbal medicine) in the treatment of primary dysmenorrhea. J Obstet
Gynaecol Res. 34:898–908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hiromi K, Hisashi T, Shigeto Y, Takeshi N,
Nobuyuki M, Daisuke T and Masamitsu I: Usefulness of Kampo formulas
in the treatment of atopic dermatitis. J Tradit Medicines.
29:93–96. 2012.
|
|
42
|
Choi HS, Lee K, Kim MK, Lee KM, Shin YC,
Cho SG and Ko SG: DSGOST inhibits tumor growth by blocking
VEGF/VEGFR2-activated angiogenesis. Oncotarget. 7:21775–21785.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nagata T, Toume K, Long LX, Hirano K,
Watanabe T, Sekine S, Okumura T, Komatsu K and Tsukada K:
Anticancer effect of a Kampo preparation Daikenchuto. J Nat Med.
70:627–633. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee K, Cho SG, Woo SM, Kim AJ, Lee KM, Go
HY, Sun SH, Kim TH, Jung KY, Choi YK, et al: Danggui Sayuk Ga Osuyu
Senggang Tang ameliorates cold induced vasoconstriction in vitro
and in vivo. Mol Med Rep. 14:4723–4728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bates RC and Mercurio AM: Tumor necrosis
factor-alpha stimulates the epithelial-to-mesenchymal transition of
human colonic organoids. Mol Biol Cell. 14:1790–1800. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by GSK-3
beta-mediated phosphorylation in control of epithelial-mesenchymal
transition. Nat Cell Biol. 6:931–940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Green S, Dobrjansky A and Chiasson MA:
Murine tumor necrosis-inducing factor: Purification and effects on
myelomonocytic leukemia cells. J Natl Cancer Inst. 68:997–1003.
1982.PubMed/NCBI
|
|
49
|
Carswell EA, Old LJ, Kassel RL, Green S,
Fiore N and Williamson B: An endotoxin-induced serum factor that
causes necrosis of tumors. Proc Natl Acad Sci USA. 72:3666–3670.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zins K, Abraham D, Sioud M and Aharinejad
S: Colon cancer cell-derived tumor necrosis factor-alpha mediates
the tumor growth-promoting response in macrophages by upregulating
the colony-stimulating factor-1 pathway. Cancer Res. 67:1038–1045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ferrajoli A, Keating MJ, Manshouri T,
Giles FJ, Dey A, Estrov Z, Koller CA, Kurzrock R, Thomas DA, Faderl
S, et al: The clinical significance of tumor necrosis factor-alpha
plasma level in patients having chronic lymphocytic leukemia.
Blood. 100:1215–1219. 2002.PubMed/NCBI
|
|
53
|
Csiszár A, Szentes T, Haraszti B, Balázs
A, Petrányi GG and Pócsik E: The pattern of cytokine gene
expression in human colorectal carcinoma. Pathol Oncol Res.
10:109–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Grimm M, Lazariotou M, Kircher S,
Höfelmayr A, Germer CT, von Rahden BH, Waaga-Gasser AM and Gasser
M: Tumor necrosis factor-α is associated with positive lymph node
status in patients with recurrence of colorectal cancer-indications
for anti-TNF-α agents in cancer treatment. Cell Oncol (Dordr).
34:315–326. 2011. View Article : Google Scholar
|
|
55
|
Al Obeed OA, Alkhayal KA, Al Sheikh A,
Zubaidi AM, Vaali-Mohammed MA, Boushey R, Mckerrow JH and Abdulla
MH: Increased expression of tumor necrosis factor-α is associated
with advanced colorectal cancer stages. World J Gastroenterol.
20:18390–18396. 2014. View Article : Google Scholar
|
|
56
|
Petrelli A and Giordano S: From single- to
multi-target drugs in cancer therapy: When aspecificity becomes an
advantage. Curr Med Chem. 15:422–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: Multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Banerjee S, Li Y, Wang Z and Sarkar FH:
Multi-targeted therapy of cancer by genistein. Cancer Lett.
269:226–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nandakumar V, Singh T and Katiyar SK:
Multi-targeted prevention and therapy of cancer by
proanthocyanidins. Cancer Lett. 269:378–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gao JL, He TC, Li YB and Wang YT: A
traditional Chinese medicine formulation consisting of Rhizoma
Corydalis and Rhizoma Curcumae exerts synergistic antitumor
activity. Oncol Rep. 22:1077–1083. 2009.PubMed/NCBI
|
|
61
|
Di Maio M, Costanzo R, Giordano P,
Piccirillo MC, Sandomenico C, Montanino A, Carillio G, Muto P,
Jones DR, Daniele G, et al: Integrated therapeutic approaches in
the treatment of locally advanced non-small cell lung cancer.
Anticancer Agents Med Chem. 13:844–851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Choi YJ, Choi YK, Lee KM, Cho SG, Kang SY
and Ko SG: SH003 induces apoptosis of DU145 prostate cancer cells
by inhibiting ERK-involved pathway. BMC Complement Altern Med.
16:5072016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Choi EK, Kim SM, Hong SW, Moon JH, Shin
JS, Kim JH, Hwang IY, Jung SA, Lee DH, Lee EY, et al: SH003
selectively induces p73 dependent apoptosis in triple negative
breast cancer cells. Mol Med Rep. 14:3955–3960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Choi YK, Cho SG, Choi YJ, Yun YJ, Lee KM,
Lee K, Yoo HH, Shin YC and Ko SG: SH003 suppresses breast cancer
growth by accumulating p62 in autolysosomes. Oncotarget. Aug
19–2016.Epub ahead of print.
|
|
65
|
Choi HS, Kim MK, Lee K, Lee KM, Choi YK,
Shin YC, Cho SG and Ko SG: SH003 represses tumor angiogenesis by
blocking VEGF binding to VEGFR2. Oncotarget. 7:32969–32979. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Choi YK, Cho SG, Woo SM, Yun YJ, Park S,
Shin YC and Ko SG: Herbal extract SH003 suppresses tumor growth and
metastasis of MDA-MB-231 breast cancer cells by inhibiting
STAT3-IL-6 signaling. Mediators Inflamm. 2014:4921732014.
View Article : Google Scholar : PubMed/NCBI
|