Introduction
Diabetes mellitus is a common metabolic condition
with signature high blood glucose. It is associated with increased
risk of cardiovascular diseases (CVDs), and cardiovascular
complication accounts for >80% of diabetic-related mortalities
(1). High blood glucose is a
major contributor to the development of diabetic cardiovascular
complications (2). High glucose
(HG) has been indicated to injure vascular endothelial cells and
vascular smooth muscle cells (VSMCs), which are associated with the
development of CVDs (3–5). It is well established that
diabetes-induced vascular dysfunction and remodeling in multiple
vascular beds may be related to the activation of the endothelin-1
(ET-1) system (6–8).
ET-1 is a potent vasoconstrictor. The endothelin
type A (ETA) and endothelin type B (ETB)
receptors are two types of G protein-coupled receptors, and through
them, ET-1 induces strong and long-lasting vasoconstriction
(9). In healthy arteries, the
binding of ET-1 with ETA receptors on VSMCs mediates the
main part of the vasoconstriction, while the ETB
receptors are predominantly found on the endothelial cells and
mediate vasodilatation (9,10).
The ETB receptors located on endothelial cells are
termed as vasorelaxant ETB receptors. However, under
pathogenic conditions, the expression of ETB receptors
is upregulated via transcriptional mechanisms on VSMCs and induce
vasoconstriction instead, and these are termed vasoconstrictive
ETB receptors (11).
Previous study has demonstrated that ETB receptors were
highly expressed on the vascular smooth muscle layer in a diabetic
model (8).
Silent information regulator family protein 1
(Sirt1), a prominent member of a family of nicotinamide adenine
dinucleotide (NAD)-dependent deacetylases, has been reported to
affect a wide range of biological functions involving regulation of
metabolism, cell survival and organismal lifespan (12). Accumulating studies have
demonstrated that Sirt1 serves a protective role in CVDs (13,14), diabetes and its complications
(15,16). Diabetes in vivo or HG in
vitro may suppress the expression of Sirt1 and its activity in
VSMCs (17). Additionally,
reduced expression or activity of Sirt1 in VSMCs may contribute to
the development of vascular dysfunction and promote vascular aging
and CVDs (18). Currently, the
underlying molecular mechanisms of this remain unclear.
The present study was designed to determine whether
Sirt1 is involved in HG-mediated regulation of ETB
receptors in the superior mesenteric arteries (SMA) of rats. The
present study may identify novel targets for the mechanism of
diabetes-associated ischemic CVDs.
Materials and methods
Chemicals and drugs
Selective ETB receptor agonist
sarafotoxin 6c (S6c), inhibitor for extracellular signal-regulated
protein kinase 1 and 2 (ERK1/2; U0126) and resveratrol (Res;
activator of Sirt1), as well as glucose, were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Dulbecco's modified
Eagle's medium (DMEM) was obtained from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The inhibitor and Res were
dissolved in dimethyl sulfoxide (DMSO). The final concentration of
DMSO (vehicle) used in the experiments was 1 µl/ml, which
equals the volume of the inhibitor added to the organ culture. The
DMSO concentration was the same in all test conditions, and it was
used in the organ culture without the inhibitor to serve as a
control. S6c was dissolved in 0.9% saline with 0.1% bovine serum
albumin (Sigma-Aldrich; Merck KGaA). Glucose was diluted in DMEM
just prior to initiation of the experiments.
Tissue preparation and organ culture
procedure
A total of 80 male Sprague-Dawley rats (age, 8
weeks; weight, 300–350 g) were obtained from the Laboratory Animal
Center of Xi'an Jiaotong University (Xi'an, China). All rats were
housed in a temperature-controlled (20±2°C) and humidity-controlled
(40–70%) facility on a 12-h light/dark cycle. Rats had free access
to food and water. Following euthanasia with CO2, the
SMA was gently removed and freed from adhering tissue under a
dissecting microscope. The endothelium was denuded by perfusion
(4°C) of the vessel for 10 sec with Triton X-100 (0.1%, v/v)
followed by another 10 sec with a physiologic buffer solution (NaCl
119 mM, KCl 4.6 mM, NaHCO3 15 mM,
NaH2PO4 1.2 mM, MgCl2 1.2 mM,
CaCl2 1.5 mM and glucose 5.5 mM). The vessels were then
cut into 1–3-mm long cylindrical segments and incubated at 37°C in
a humidified atmosphere of 5% CO2 and 95% air in DMEM
[containing L-glutamine (584 mg/l) and normal glucose (5.5 mM)]
supplemented with penicillin (100 U/ml) and streptomycin (100
mg/ml) (Thermo Fisher Scientific, Inc.) (19,20). To mimic hyperglycemic conditions,
the cylindrical segments were exposed for up to 24 h to HG (15 or
25 mM) DMEM (normal DMEM supplemented with HG concentrations) in a
CO2 (5%) incubator at 37°C. Other experiments were
performed under similar conditions in the absence/presence of
inhibitor [U0126 (10 µM)] or Sirt1 activator [Res (10, 50 or
100 µM)], which were added to the medium prior to
incubation.
The animal experiments in the present investigation
were approved by the Laboratory Animal Administration Committee of
Xi'an Jiaotong University and conformed to the Guidelines for
Animal Experimentation of Xi'an Jiaotong University (20) and the Guide for the Care and Use
of Laboratory Animals published by the US National Institutes of
Health [NIH Publication no. 85–23, revised 2011 (21)].
In vitro pharmacology
The present experiments were performed according to
a previously published protocol (20). Briefly, fresh or incubated
cylindrical artery segments were immersed in temperature-controlled
(37°C) myograph individual baths (Organ Bath Model 700MO; J.P.
Trading, Aarhus, Denmark) containing 5 ml physiologic buffer
solution. The solution was continuously gassed with 5%
CO2 in O2, resulting in a pH of 7.4. The
cylindrical arterial segments were mounted for continuous recording
of isometric tension with LabChart 7 Pro software (ADInstruments,
Hastings, UK). A resting tone of 2 mN was applied to each segment,
and the segments were allowed to stabilize at this tension for at
least 1.5 h before exposure to a potassium-rich (60 mM
K+) buffer solution with the same composition as the
standard solution, except that NaCl was replaced by an equimolar
concentration of KCl. The potassium-induced contraction was used as
a reference for contractile capacity, and the segments were used
only if potassium elicited reproducible responses over 1.0 mN.
Concentration-response curves for S6c, a selective ETB
receptor agonist (10−11–10−7 M), were
obtained by cumulative administration of the reagent.
Western blotting
Arterial segment lysate preparation and western blot
analysis were performed as previously described (20). Briefly, following organ culture
for 24 h, the arterial segments were lysed on ice for 1 h in
radioimmuno-precipitation assay buffer [Tris-HCl (pH 8.0) 50 mM,
NaCl 150 mM, 1% Triton X-100 (v/v), 1% deoxycholic acid (w/v) and
0.1% sodium dodecyl sulfate] containing 0.5 mM phenylmethylsulfonyl
fluoride and protease inhibitors (Roche Diagnostics, Basel,
Switzerland). Protein concentration was measured with a BCA protein
assay kit (Thermo Fisher Scientific, Inc.). After being denatured
by boiling for 5 min in Laemmli loading buffer (Beyotime Institute
of Biotechnology, Haimen, China), equal amounts of protein (50
µg) were loaded and separated on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride membranes. To block
non-specific binding, the membranes were incubated with 5% bovine
serum albumin or non-fat dried milk for 1 h at 37°C. Subsequently,
the membranes were incubated with primary antibodies overnight at
4°C. The primary antibodies contained anti-phospho-p44/42 antibody
(1:1,000; 4370; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-p44/42 antibody (1:1,000; ab115799), anti-ETB
receptor antibody (1:1,000; ab65972), anti-Sirt1 antibody (1:1,000;
ab104833) and anti-β-actin antibody (1:1,000; ab8226) (Abcam,
Cambridge, MA, USA). After being washed with Tris-buffered saline
containing 0.1% Tween-20 (Beyotime Institute of Biotechnology), the
membranes were incubated with horseradish peroxidase-conjugated
goat anti-mouse or -rabbit immunoglobulin G (1:1,000; 31430 and
31460; Thermo Fisher Scientific, Inc.) for 1 h at 37°C, followed by
enhanced chemiluminescence using a SuperSignal West PicoSubstrate
kit (Pierce; Thermo Fisher Scientific, Inc.) and analyzed using the
ChemiDoc-it HR 410 imaging system (UVP, LLC, Phoenix, AZ, USA).
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. S6c-induced vasoconstriction data were presented as a
percentage of contraction induced by 60 mM K+. Two sets
of data were compared using the unpaired Student's t-test with
Welch's correction or two-way analysis of variance (ANOVA) with
Bonferroni post hoc test. One-way ANOVA with Dunnett's post hoc
test was applied for comparisons of more than two data sets. Data
analysis was performed using SPSS version 20.0 (IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of HG on ETB
receptor-mediated vasoconstriction and ETB receptor
protein expression in the SMA
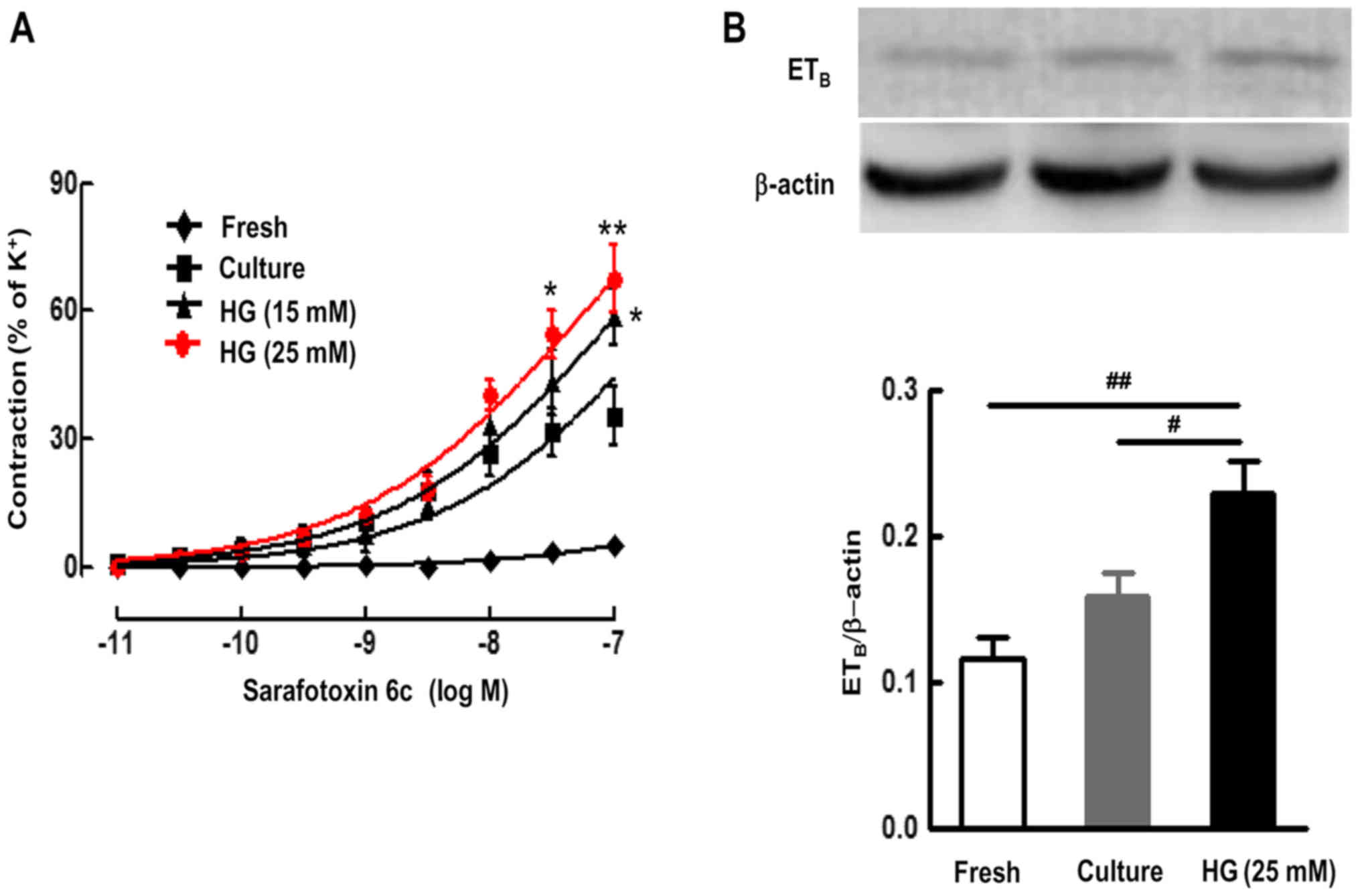
In the fresh SMA ring, S6c induced only negligible
contractions (Emax value, 5.12±1.05%). After organ culture alone
for 24 h, S6c induced strong contraction of SMA in a
concentration-dependent manner, with an Emax value of 35.38±6.79%
and a pEC50 value of 6.57±0.33. Culture with different doses of HG
(15 and 25 mM) further shifted the S6c-induced
concentration-contraction curve of organ culture artery toward the
left and significantly increased contractile responses to S6c, with
an Emax of 58.46±6.60% and pEC50 of 7.31±0.17 for 15 mM HG, and an
Emax of 67.58±8.04% and pEC50 of 7.51±0.15 for 25 mM HG,
respectively (P<0.05) (Fig.
1A). This suggests that organ culture enhanced the contraction
of the SMA induced by S6c. In addition, HG further enhanced the
ETB receptor-mediated contraction of the SMA induced by
S6c.
There were no significant differences in the
K+-induced contraction among the groups, and incubation
with control in the concentration used did not affect the
contractile response to S6c (data not shown).
The SMA segments were cultured for 24 h in the
presence or absence of HG (25 mM). ETB receptor protein
expression in vascular smooth muscles were assessed using western
blotting. The results demonstrated that there were low levels of
ETB receptor protein in fresh SMA segments. Organ
culture alone induced an increase in the ETB receptor
protein expression level compared to the fresh group; however, this
did not achieve statistical significance. Furthermore, HG (25 mM)
significantly elevated protein expression levels of ETB
receptors in cultured SMA segments, compared with the culture alone
group (P<0.05) (Fig. 1B).
Activating Sirt1 with Res blocks the
HG-increased protein expression of ETB receptors and
receptor-mediated vasoconstriction in the SMA
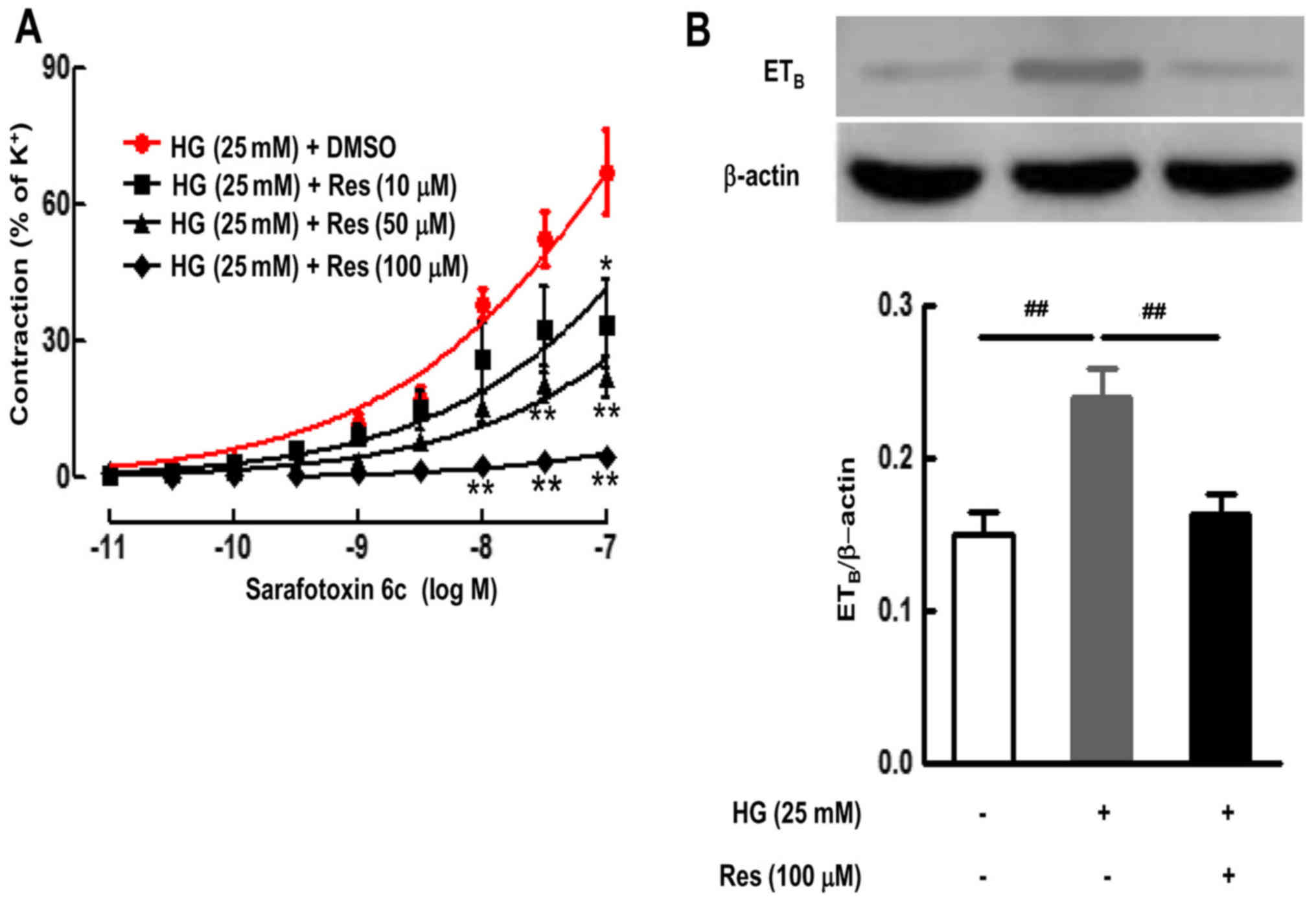
Culture with HG and different doses of Res (10, 50
or 100 µM) inhibited the HG-enhanced contractile responses
to S6c, and decreased the Emax from 67.03±9.27% in HG-cultured
artery to 33.56±9.87% (10 µM; P<0.05), 21.95±4.56% (50
µM; P<0.01) and 4.32±0.63% (100 µM; P<0.01)
(Fig. 2A).
The ETB receptor protein expression in
the SMA following organ culture with Res (100 µM) was
examined using western blotting. Compared with the control group
(culture alone), HG significantly upregulated the levels of
ETB receptors (P<0.01) (Fig. 2B). However, Res (100 µM)
significantly inhibited the HG-induced elevation of the
ETB receptor protein levels (P<0.01) (Fig. 2B).
These findings suggest that HG-increased protein
expression of ETB receptors and receptor-mediated
contractile function were related to Sirt1 signaling pathways.
Sirt1 activator may effectively inhibit HG-induced upregulation of
ETB receptor protein expression and receptor-mediated
contractile function.
ERK1/2 signaling pathway is involved in
the upregulation of ETB receptor protein expression and
receptor-mediated vasoconstriction induced by HG in the organ
culture SMA
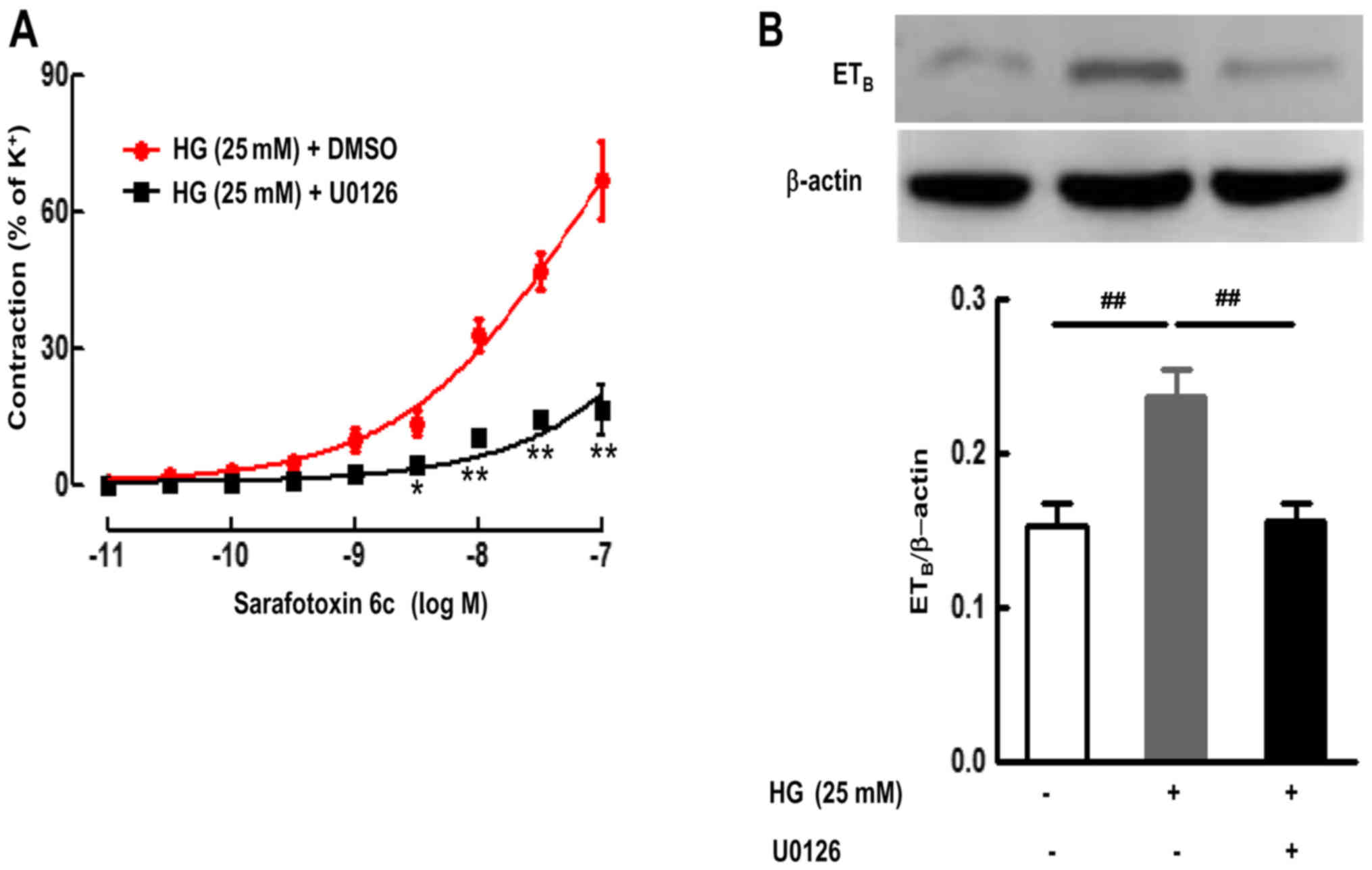
As demonstrated in Fig. 3, culture with HG and the inhibitor
for ERK1/2 (U0126) significantly attenuated the HG-enhanced
contractile responses to S6c, and decreased the Emax from
66.89±8.36% in HG-cultured artery to 16.44±5.51% (P<0.01)
(Fig. 3A).
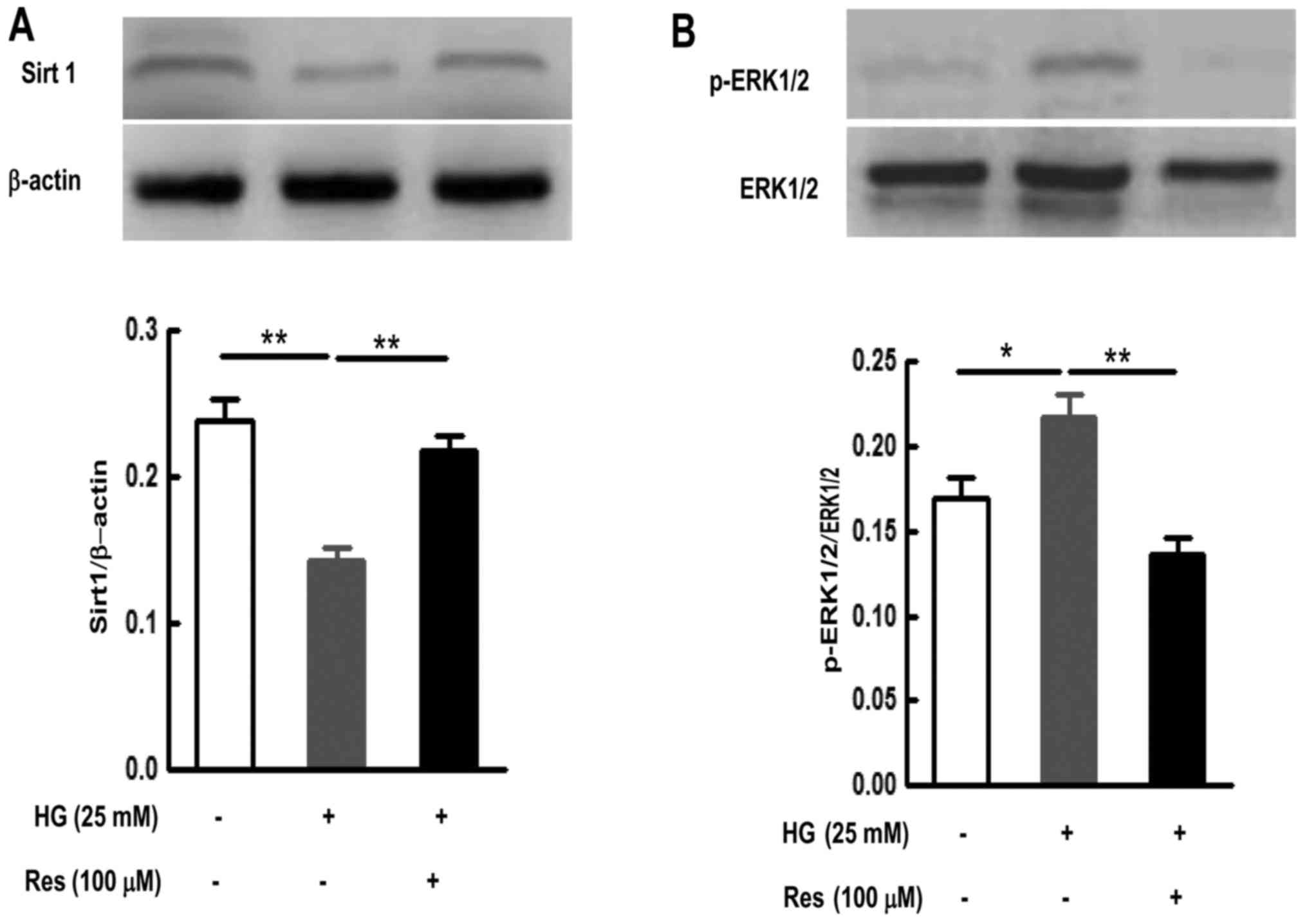
The level of phosphorylated ERK1/2 (p-ERK1/2) and
ETB receptor protein expression in the SMA were examined
following culture with or without HG (25 mM) in the presence of or
absence of U0126. As demonstrated in Fig. 4, the level of p-ERK1/2 was low in
the control group following organ culture for 24 h. Compared with
the control group, HG (25 mM) significantly upregulated the levels
of p-ERK1/2 (P<0.05) (Fig.
4B). However, the inhibitor for ERK1/2 (U0126) significantly
reduced the increases in ETB receptor protein expression
induced by HG (P<0.01) (Fig.
3B). Additionally, U0126 almost completely abolished the
HG-induced upregulation of ETB receptor protein
expression in SMA segments (Fig.
3B). These results suggest that HG activated the ERK1/2
signaling pathway, which resulted in upregulation of ETB
receptor expression and receptor-mediated contractile function.
Activating Sirt1 with Res inhibits the
HG-induced activation of ERK1/2 signaling pathways
In order to further study the relationship between
Sirt1 and ERK1/2 signaling pathways in HG-cultured rat SMA, Res
(100 µM) was used for activating Sirt1, and then the level
of p-ERK1/2 was detected using western blotting. The results
demonstrated that the level of Sirt1 was relatively high in SMA
segments following organ culture for 24 h. However, culture with HG
significantly decreased Sirt1 protein expression compared with the
level in culture alone (P<0.01) (Fig. 4A). Res significantly reversed the
decrease in Sirt1 protein expression caused by HG (P<0.01)
(Fig. 4A). In addition, Res also
inhibited the increase in the level of p-ERK1/2 induced by HG in
SMA segments (P<0.01) (Fig.
4B). These results indicate that the activation of Sirt1 could
inhibit the HG-induced activation of ERK1/2 signaling pathways.
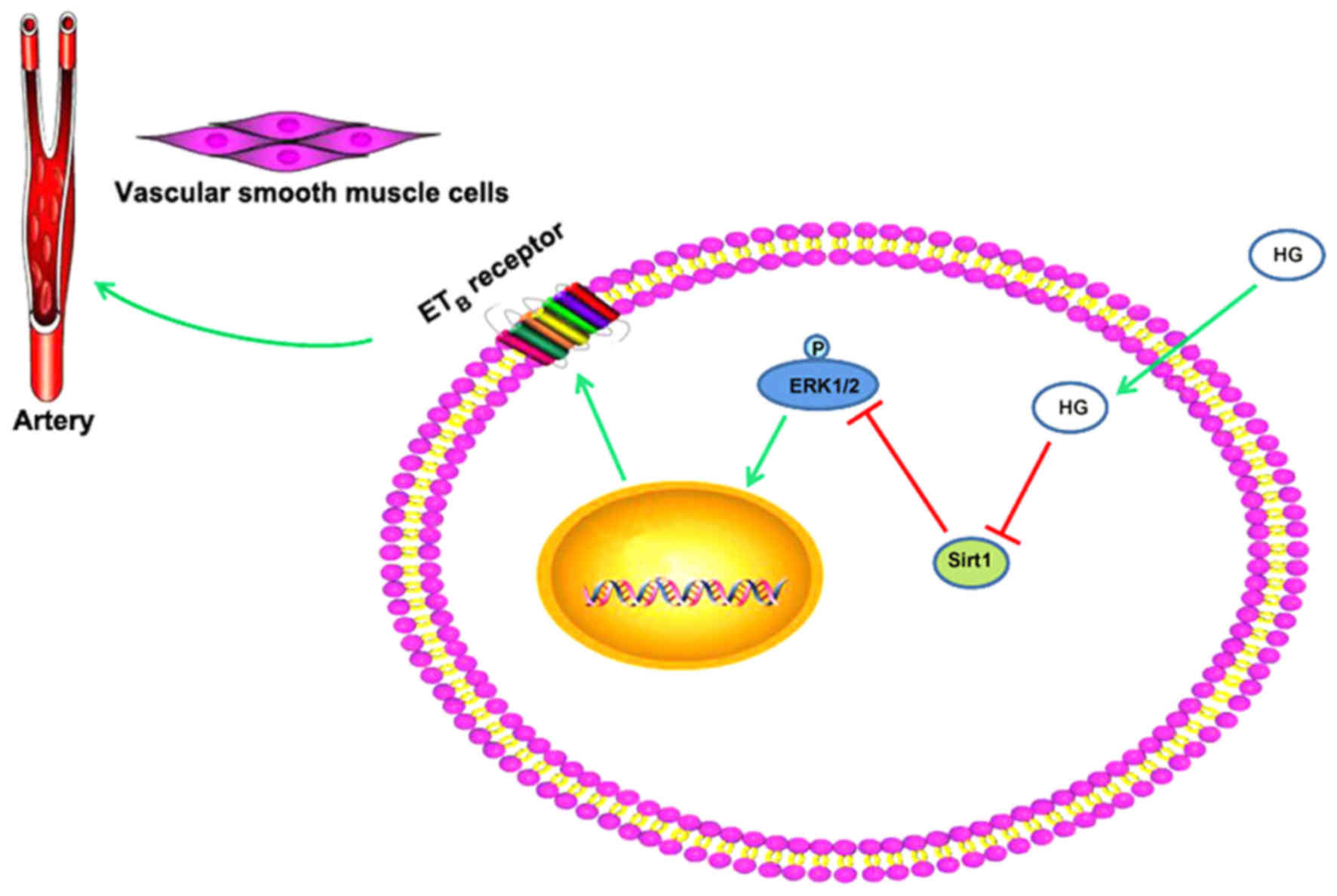
Therefore, in the present study, HG upregulated ETB
receptor expression and the receptor-mediated contractile response
by downregulating the level of Sirt1 and activating the ERK1/2
signaling pathway in SMA (Fig.
5).
Discussion
The present study investigated the effect of HG on
ETB receptor protein expression in VSMCs and its
mechanisms. The results suggested that HG enhanced
receptor-mediated contractile responses to S6c and increased the
ETB receptor protein expression in rat SMA. Meanwhile,
the level of Sirt1 was downregulated and the ERK1/2 signaling
pathway was activated in the HG-cultured rat SMA. Additionally, the
activation of ERK1/2 signaling pathways could be inhibited by Sirt1
activator. This suggested that HG decreased the level of Sirt1
protein and activated the ERK1/2 signaling pathway, which resulted
in upregulation of ETB receptor protein expression and
receptor-mediated vasoconstriction in rat SMA.
Phenotypic modification of VSMCs may occur in
physiological and pathophysiological settings, which is the basis
of VSMC vasomotion and proliferation (22). Study has demonstrated that
ETB receptors were involved in phenotypic modification
of VSMCs (11). Organ culture
provides a model for exploring the mechanisms involved in
upregulation of vascular smooth muscle ETB receptors
(11). In the present study,
organ culture of the SMA was also used as a model to investigate
the effect of HG on ETB receptor expression in rat SMA.
The present results demonstrated that HG significantly upregulated
ETB receptor protein expression and the
receptor-mediated vasoconstriction induced by S6c in the organ
culture SMA. In endothelial cells, ETB receptors mediate
vasodilatation via release of nitric oxide and prostacyclin
(23). However, ETB
receptors induce vasoconstriction in VSMCs, which may be related to
the phosphatidylinositol 4,5-bisphosphate system (24). In addition, in the present study,
the level of Sirt1 in HG-cultured rat SMA was significantly
decreased. This suggested that Sirt1 may be involved in the process
of HG increasing ETB receptor expression and the
receptor-mediated vasoconstriction in the organ culture SMA.
Sirt1 has been implicated in the process of aging,
metabolism and tolerance to oxidative stress (25). In recent years, Sirt1 has been
implicated in the pathogenesis of CVDs (26). Additionally, studies have recently
identified that Sirt1 could improve VSMC functions (27) and serve a protective role in CVDs
(13,14). Sirt1 deficiency in VSMCs could
lead to vascular dysfunction and promote CVDs (18). A study by Badi et al
(28) reported that
downregulation of Sirt1 by miR-34a in VSMCs promotes senescence and
inflammation. Other researchers also agreed that the loss of
endogenous Sirt1 protein in human VSMCs directly contributes to the
induction of cellular senescence and deficits of cellular function,
including an impaired stress response, and reduced capacity for
cell migration and proliferation (29). However, diabetes induction in
vivo and HG concentrations in vitro significantly
downregulated the level of Sirt1 protein in rat VSMCs (17). This finding was also demonstrated
in the present study. Sirt1 is a NAD-dependent deacetylase, and it
requires NAD for its enzymatic activity (30). Previous study demonstrated that
inhibition of NAD biosynthesis may be one of the major mechanisms
involved in the downregulation of Sirt1 induced by HG or
hyperglycemia (17). In the
present study, activating Sirt1 with Res significantly inhibited
HG-induced upregulation of ETB receptor expression and
receptor-mediated vasoconstriction in the organ culture SMA. This
suggested that the enhancement of receptor-mediated
vasoconstriction and the increase in ETB receptor
protein expression was related to the downregulation of Sirt1 in
HG-cultured rat SMA.
Additionally, the present study demonstrated that
the level of p-ERK1/2 was significantly increased in HG-cultured
rat SMA segments. ERK1/2 is one of three main signaling pathways of
mitogen-activated protein kinases, and it has received attention
for its involvement in the regulation of ETB receptors
in VSMCs (20). Risk factors for
CVDs, including cigarette smoke particles (31), minimally modified low-density
lipoprotein (32,33), low-density lipoprotein (19) or homocysteine (20), could induce ETB
receptors to be highly expressed on VSMCs through the ERK1/2
signaling pathway. Notably, when a specific ERK1/2 inhibitor
(U0126) was used to block activation of ERK1/2 in the present
study, the HG-induced upregulation of ETB receptor
protein expression and receptor-mediated vasoconstriction in the
organ culture SMA were blocked distinctly. This suggested that HG
upregulated receptor-mediated vasoconstriction and ETB
receptor expression by activating ERK1/2 signaling pathways.
A previous study has demonstrated that Sirt1
overexpression in VSMCs could significantly inhibit angiotensin
II-induced VSMC hypertrophy by suppressing phosphorylation of
ERK1/2 (34). This suggested that
Sirt1 could regulate the activity of the ERK1/2 pathway. In the
present study, activating Sirt1 with Res significantly blocked the
upregulation of p-ERK induced by HG in the organ culture SMA. This
result was in accordance with a previous study, which demonstrated
that Sirt1 activator (Res) inhibited ERK1/2 phosphorylation in
VSMCs (35). In other cells,
Sirt1 activator partly reversed the ultraviolet B-induced damage on
human retinal pigment epithelial cells by inhibiting AKT and ERK
phosphorylation (36).
Conversely, researchers reported that Sirt1 deletion led to
enhanced pro-inflammatory signaling as demonstrated by increased
signal transducer and activator of transcription and ERK
phosphorylation (37).
In conclusion, the present results indicate that HG
upregulated ETB receptor expression through the
Sirt1-ERK1/2 signaling pathways in SMA. The upregulation of
ETB receptor expression could enhance receptor-mediated
vascular contractile responses and result in CVDs. The present
study may provide novel therapeutic targets for the prevention and
treatment of vasospasm and diabetes-associated CVDs.
Acknowledgments
The present study was supported in part by the
Natural Science Foundation of Shaanxi Province (grant no.
2014PT013) and the Science and Technological Project of Shaanxi
Province (grant no. 2016JQ8043).
Abbreviations:
|
CVDs
|
cardiovascular diseases
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
ERK1/2
|
extracellular signal-regulated protein
kinase 1/2
|
|
ET-1
|
endothelin-1
|
|
ETA
|
endothelin type A
|
|
ETB
|
endothelin type B
|
|
HG
|
high glucose
|
|
NAD
|
nicotinamide adenine dinucleotide
|
|
Res
|
resveratrol
|
|
S6c
|
sarafotoxin 6c
|
|
Sirt1
|
silent information regulator family
protein 1
|
|
SMA
|
superior mesenteric arteries
|
|
VSMCs
|
vascular smooth muscle cells
|
References
|
1
|
Amos AF, McCarty DJ and Zimmet P: The
rising global burden of diabetes and its complications: Estimates
and projections to the year 2010. Diabet Med. 14(Suppl 5): S1–S85.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massi-Benedetti M and Federici MO:
Cardiovascular risk factors in type 2 diabetes: The role of
hyperglycaemia. Exp Clin Endocrinol Diabetes. 107(Suppl 4):
S120–S123. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu ZX, Cai WH, Wang T, Ye HB, Zhu YT, Chi
LS, Duan YM, Sun CC, Xuan YH and Jin LT: bFGF-regulating MAPKs are
Involved in high glucose-mediated ROS production and delay of
vascular endothelial cell migration. PLoS One. 10:e01444952015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu L, Sun G, Zhang H, Zhang Y, Chen X,
Jiang X, Jiang X, Krauss S, Zhang J, Xiang Y, et al: PGC-1alpha is
a key regulator of glucose-induced proliferation and migration in
vascular smooth muscle cells. PLoS One. 4:e41822009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan JY, Zhou Q, Yu HM, Hou ML and Lu LH:
High glucose promotes vascular smooth muscle cell calcification by
activating WNT signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao.
35:29–33. 2015.In Chinese. PubMed/NCBI
|
|
6
|
Abdelsaid M, Kaczmarek J, Coucha M and
Ergul A: Dual endothelin receptor antagonism with bosentan reverses
established vascular remodeling and dysfunctional angiogenesis in
diabetic rats: Relevance to glycemic control. Life Sci.
118:268–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan ZA and Chakrabarti S: Endothelins in
chronic diabetic complications. Can J Physiol Pharmacol.
81:622–634. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelly-Cobbs AI, Harris AK, Elgebaly MM, Li
W, Sachidanandam K, Portik-Dobos V, Johnson M and Ergul A:
Endothelial endothelin B receptor-mediated prevention of
cerebrovascular remodeling is attenuated in diabetes because of
up-regulation of smooth muscle endothelin receptors. J Pharmacol
Exp Ther. 337:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davenport AP, Hyndman KA, Dhaun N, Southan
C, Kohan DE, Pollock JS, Pollock DM, Webb DJ and Maguire JJ:
Endothelin. Pharmacol Rev. 68:357–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneider MP, Boesen EI and Pollock DM:
Contrasting actions of endothelin ETA and ETB
receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol.
47:731–759. 2007. View Article : Google Scholar
|
|
11
|
Xu CB, Sun Y and Edvinsson L:
Cardiovascular risk factors regulate the expression of vascular
endothelin receptors. Pharmacol Ther. 127:148–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki M and Bartlett JD: Sirtuin1 and
autophagy protect cells from fluoride-induced cell stress. Biochim
Biophys Acta. 1842:245–255. 2014. View Article : Google Scholar :
|
|
13
|
Ma L and Li Y: SIRT1: Role in
cardiovascular biology. Clin Chim Acta. 440:8–15. 2015. View Article : Google Scholar
|
|
14
|
Winnik S, Auwerx J, Sinclair DA and Matter
CM: Protective effects of sirtuins in cardiovascular diseases: From
bench to bedside. Eur Heart J. 36:3404–3412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kitada M and Koya D: SIRT1 in type 2
diabetes: Mechanisms and therapeutic potential. Diabetes Metab J.
37:315–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toniolo A, Warden EA, Nassi A, Cignarella
A and Bolego C: Regulation of SIRT1 in vascular smooth muscle cells
from streptozotocin-diabetic rats. PLoS One. 8:e656662013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitada M, Ogura Y and Koya D: The
protective role of Sirt1 in vascular tissue: Its relationship to
vascular aging and atherosclerosis. Aging (Albany NY). 8:2290–2307.
2016. View Article : Google Scholar
|
|
19
|
Xu CB, Zheng JP, Zhang W, Liu E, Edvinsson
L and Zhang Y: Low density lipoprotein induces upregulation of
vasoconstrictive endothelin type B receptor expression. Vascul
Pharmacol. 60:42–48. 2014. View Article : Google Scholar
|
|
20
|
Chen Y, Zhang H, Liu E, Xu CB and Zhang Y:
Homocysteine regulates endothelin type B receptors in vascular
smooth muscle cells. Vascul Pharmacol. 87:100–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Zhao S, Wang Y, Chen Y, Lin Y, Zhu
N, Zheng H, Wu M, Cheng D, Li Y, et al: Urotensin II promotes
atherosclerosis in cholesterol-fed rabbits. PLoS One. 9:e950892014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Majesky MW: Developmental basis of
vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol.
27:1248–1258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Orléans-Juste P, Labonté J, Bkaily G,
Choufani S, Plante M and Honoré JC: Function of the endothelin(B)
receptor in cardiovascular physiology and pathophysiology.
Pharmacol Ther. 95:221–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ivey ME, Osman N and Little PJ:
Endothelin-1 signalling in vascular smooth muscle: Pathways
controlling cellular functions associated with atherosclerosis.
Atherosclerosis. 199:237–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feige JN and Auwerx J: Transcriptional
targets of sirtuins in the coordination of mammalian physiology.
Curr Opin Cell Biol. 20:303–309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsushima S and Sadoshima J: The role of
sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol.
309:H1375–H1389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang MJ, Zhou Y, Chen L, Wang X, Long CY,
Pi Y, Gao CY, Li JC and Zhang LL: SIRT1 improves VSMC functions in
atherosclerosis. Prog Biophys Mol Biol. 121:11–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Badi I, Burba I, Ruggeri C, Zeni F,
Bertolotti M, Scopece A, Pompilio G and Raucci A: MicroRNA-34a
induces vascular smooth muscle cells senescence by SIRT1
downregulation and promotes the expression of age-associated
pro-inflammatory secretory factors. J Gerontol A Biol Sci Med Sci.
70:1304–1311. 2015. View Article : Google Scholar
|
|
29
|
Thompson AM, Wagner R and Rzucidlo EM:
Age-related loss of SirT1 expression results in dysregulated human
vascular smooth muscle cell function. Am J Physiol Heart Circ
Physiol. 307:H533–H541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imai S, Armstrong CM, Kaeberlein M and
Guarente L: Transcriptional silencing and longevity protein Sir2 is
an NAD-dependent histone deacetylase. Nature. 403:795–800. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu CB, Zheng JP, Zhang W, Zhang Y and
Edvinsson L: Lipid-soluble smoke particles upregulate vascular
smooth muscle ETB receptors via activation of
mitogen-activating protein kinases and NF-kappaB pathways. Toxicol
Sci. 106:546–555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jie L, Yong-Xiao C, Zu-Yi Y and Cang-Bao
X: Minimally modified LDL upregulates endothelin type B receptors
in rat coronary artery via ERK1/2 MAPK and NF-κB pathways. Biochim
Biophys Acta. 1821:582–589. 2012. View Article : Google Scholar
|
|
33
|
Li J, Cao YX, Liu Y and Xu CB: Minimally
modified LDL upregulates endothelin type B receptors in rat basilar
artery. Microvasc Res. 83:178–184. 2012. View Article : Google Scholar
|
|
34
|
Li L, Gao P, Zhang H, Chen H, Zheng W, Lv
X, Xu T, Wei Y, Liu D and Liang C: SIRT1 inhibits angiotensin
II-induced vascular smooth muscle cell hypertrophy. Acta Biochim
Biophys Sin (Shanghai). 43:103–109. 2011. View Article : Google Scholar
|
|
35
|
Miyazaki R, Ichiki T, Hashimoto T, Inanaga
K, Imayama I, Sadoshima J and Sunagawa K: SIRT1, a longevity gene,
downregulates angiotensin II type 1 receptor expression in vascular
smooth muscle cells. Arterioscler Thromb Vasc Biol. 28:1263–1269.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chou WW, Chen KC, Wang YS, Wang JY, Liang
CL and Juo SH: The role of SIRT1/AKT/ERK pathway in ultraviolet B
induced damage on human retinal pigment epithelial cells. Toxicol
In Vitro. 27:1728–1736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao R, Chen J, Hu Y, Li Z, Wang S, Shetty
S and Fu J: Sirt1 deletion leads to enhanced inflammation and
aggravates endotoxin-induced acute kidney injury. PLoS One.
9:e989092014. View Article : Google Scholar : PubMed/NCBI
|