Introduction
Hyperoxia is an indispensable therapeutic measure in
the clinical intensive care of certain neonatal conditions;
however, long-term therapeutic hyperoxia may exert serious toxic
effects on several organs. Early exposure to hyperoxia results in
progressive lung disease in premature infants (1), and hyperoxia may induce
bronchopulmonary dysplasia, inhibit cell proliferation and decrease
cell viability (2).
Hyperoxia-induced epithelial disruption, such as weakening of tight
junctions and induction of a pro-inflammatory environment, is the
main cause of hyperoxia-related organ injury (3). Recently, the clinical use of
hyperoxia was reported to exacerbate organ injury by increasing the
production of reactive oxygen species (ROS) (4). ROS include superoxide radicals and
hydrogen peroxide (H2O2), as well as its
downstream products, such as peroxide and hydroxyl compounds. ROS
affect cell viability, proliferation, differentiation, aging,
apoptosis, and a number of physiological and pathological
processes. ROS, primarily oxygen ions and
H2O2, are produced in mitochondria (5). Under normal conditions in
vivo, the generation and removal of ROS are in dynamic balance,
and ROS are beneficial to the organism without causing harm.
However, excess generation of ROS in epithelial cells is harmful.
It has been reported that ROS coordinate the inflammatory response
of tissues (6), and that
H2O2 significantly reduces the activities of
superoxide dismutase, glutathione peroxidase, catalase and lipase
(7). When the intestinal mucosal
barrier is damaged, intestinal mucosal Th1 cytokines, such as tumor
necrosis factor (TNF)-α and interleukin (IL)-1, stimulate
epithelial cells to produce ROS. TNF-α is a central mediator of the
inflammatory response (8). ROS
are cytotoxic, but they may also act as second messengers in
intracellular signal transduction and control the action of several
signaling pathways, including mitogen-activated protein kinases
(MAPKs) (9). Apoptosis
signal-regulating kinase 1 (ASK1) is a MAPKKK of the c-Jun
N-terminal kinase (JNK) and p38 MAPK pathways (9). ASK1 is a member of the MAPK family,
and it plays an important role in the regulation of cellular
apoptotic processes.
Additionally, MAPKs are known to be important for
the transcriptional activation of nuclear factor (NF)-κB (10), which is a transcription factor
that has been shown to be a central regulator of inflammatory
response (11). The NF-κB family
of transcription factors comprises 5 subunits, designated RelA
(p65), RelB, c-Rel, p50 (NF-κB1) and p52 (NF-κB2).
Hypoxia-inducible factor-1α (HIF-1α) is a key regulatory factor in
the induction of the hypoxia gene and the repair of the cellular
oxygen environment. HIF-1α may also provide information on hypoxia
induction and oxidative stress (12). Long-term treatment with hyperoxia
may exert serious toxic effects on intestinal epithelial cells
in vitro and in vivo (2,13–16). Therefore, the aim of the prsent
study was to investigate the cause of intestinal injury during
hyperoxia, and determine whether ROS generation is a main factor in
intestinal injury under hyperoxic conditions.
Materials and methods
Cell culture
The human colon adenocarcinoma cell line Caco-2 was
obtained from the Cell Biological Institute of Shanghai, Chinese
Academy of Sciences (Shanghai, China). The cells were grown at 37°C
in air and 5% CO2 in sterile basal medium/DMEM-H
(Sigma-Aldrich, Merck KGaA, St. Louis, MO, USA) supplemented with
10% fetal bovine serum (Genview, Beijing, China), 1% L-glutamine,
100 U/ml penicillin, 100 mg/ml streptomycin and 0.25 mg/ml
amphotericin B. The culture medium was changed every 2–3 days.
Prior to treatment, the cells were plated with fresh medium
(1×106 cells/ml) and cultured. On the second day after
plating, Caco-2 cells were incubated with different concentrations
of H2O2 (100, 200 and 400 μM) and 85%
oxygen [cells were cultured in three gas incubators (CB160; Binder
GmbH, Tuttlingen, Germany), with 85% oxygen and 5% CO2]
for 24 h. A group of control cells received no treatment.
Subsequently, the cultured cells were harvested, and the RNA and
protein were extracted. All experiments were repeated 6–8
times.
Cell survival detected by MTT assay
Prior to treatment, cells were plated onto 96-well
microtiter plates with fresh medium and cultured at 37°C in air and
5% CO2 for 24 h. On the 2nd day after plating, the cells
were treated with either 100, 200 or 400 μM
H2O2, and 85% hyperoxia for 24 h. Cells
without any treatment were used as the control group. Subsequently,
the cells were treated with 20 μl MTT for 4 h at 37°C. The
reactions were stopped by adding DMSO and the absorbance of each
well at 450 nm was determined. Each sample was tested 6–8
times.
Determination of intracellular ROS
level
Cells were plated at the same cell density in a
culture flask. Cells without any treatment were used as the control
group. Cells were treated with H2O2 (100, 200
and 400 μM) or 85% hyperoxia exposure for 24 h. ROS kits
(E004; Nanjing Jiancheng Bioengineering Institute, Nanjing, China)
were used to measure ROS levels (including
O2− and H2O2),
according to the manufacturer's instructions.
Dichloro-dihydro-fluorescein diacetate (DCFH-DA; 10 μM) was
added to the cells and incubated for 30 min at 37°C. The cells were
then digested and suspended. The cell suspensions were centrifuged
at 1,000 × g for 10 min and washed twice with phosphate-buffered
saline (PBS). The cells were collected after centrifugation for
fluorescence detection. Flow cytometry (FACSCalibur;
Becton-Dickinson, San Jose, CA, USA) was used to measure
fluorescence intensity. The positive area of DCFH-DA was ROS
fluorescence intensity.
Immunohistochemistry analysis
The cells on coverslips that were treated with 100,
200 and 400 μM H2O2, or 85% hyperoxia
for 24 h were fixed with 4% paraformaldehyde. The cells were then
treated with 10% goat serum for 30 min and incubated with mouse
anti-human RelA (1:2,000; cat. no. SAB1404309) and rabbit
anti-human RelB (1:2,000; cat. no. SAB4300501) (both from
Sigma-Aldrich, Merck KGaA) (the antibody was diluted with PBS and
5% bovine serum albumin) overnight at 4°C, then incubated with a
secondary antibody [biotin-labeled goat anti-mouse IgG (cat. no.
SP-9002); biotin-labeled goat anti-rabbit IgG, (cat. no. SP-9001);
ZSGB-BIO, Beijing, China] for 40 min at 37°C. Finally, the cells
were stained by diaminobenzidine counterstained with hematoxylin.
The primary antibody was replaced with PBS as a negative control.
The median absorbance values of RelA and RelB were determined using
image analysis software (Prism; Shanghai, China) after
scanning.
Protein determination
The BCA Protein Assay kit (cat. no. P0013C; Beyotime
Institute of Biotechnology, Shanghai, China) was used to determine
protein concentrations in Caco-2 cells, according to the
manufacturer's instructions.
Western blot analysis
Proteins (40 ng) extracted from Caco-2 cells were
separated using sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride
membranes. The membranes were then incubated with Tris-buffered
saline-Tween-20 containing 5% skimmed milk for 2 h at room
temperature. The membranes were incubated overnight at 4°C with
mouse anti-human RelA (1:1,000; cat. no. SAB1404309) and rabbit
anti-human RelB (1:1,000; cat. no. SAB4300501) (both from
Sigma-Aldrich, Merck KGaA), rabbit anti-human HIF-1α (1:1,000; cat.
no. ab51608) and rabbit anti-human TNF-α (1:1,000; cat. no. ab6671)
(both from Abcam, Cambridge, UK), rabbit anti-human ASK1 (1:1,000;
cat. no. SAB1306399; Sigma-Aldrich, Merck KGaA), or GAPDH
(1:10,000; cat. no. KC-5G5; Kangcheng Bioengineering Co., Shanghai,
China), respectively. The membranes were then incubated with the
appropriate secondary antibodies (1:5,000; peroxidase-conjugated
goat anti-mouse IgG (cat. no. ZB-5305) and peroxidase-conjugated
goat anti-rabbit IgG (cat. no. ZB-5301); ZSGB-BIO). Finally, the
membranes were incubated with the SuperEnhanced chemiluminescence
(Applygen Technologies Inc., Beijing, China) and the images were
scanned by C300 (Azure Biosystems, Inc., Dublin, CA, USA).
Image-Pro Plus 6.0 software was used to analyze densitometry for
RelA, RelB, HIF-1α, TNF-α and ASK1 protein levels normalized to
GAPDH.
Quantitative polymerase chain reaction
(qPCR)analysis
Total RNA was extracted from Caco-2 cells using an
RNA Mini kit (RR047A; Takara Biotechnology Co., Ltd., Dalian,
China). cDNA was synthesized using 100 ng RNA (RR420A; Takara
Biotechnology Co., Ltd.). qPCR was performed using the
LightCycler® 480 Real-Time PCR system (Roche Applied
Science, Mannheim, Germany). The primers for RelA, RelB, HIF-1α,
TNF-α and ASK1 were as follows: RelA forward,
5′-GGAGCACAGATACCACCAAGA-3′ and reverse,
5′-CGGCAGTCCTTTCCTACAAG-3′; RelB forward,
5′-TGTGGTGAGGATCTGCTTCCAG-3′ and reverse,
5′-GGCCCGCTTTCCTTGTTAATTC-3′; HIF-1α forward,
5′-GCAGCAACGACACAGAAACT-3′ and reverse, 5′-AGCGGTGGGTAATGGAGAC-3′;
TNF-α forward, 5′-GGCGTGGAGCTGAGAGATAA-3′ and reverse,
5′-GTGTGGGTGAGGAGCACAT-3′; ASK1 forward,
5′-TTCACACAAAACGGATGTAACATT-3′ and reverse,
5′-CCTAAACAGTTATGGTCACATTTTGG-3′; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′.
The primers and fluorescent probes for RelA, RelB,
HIF-1α, TNF-α, ASK1 and the internal reference (GAPDH) were
purchased from Takara Biotechnology Co., Ltd. The PCR conditions
were as follows: A preliminary cycle at 95°C for 10 sec, followed
by 45 cycles at 95°C for 5 sec and 60°C for 20 sec, followed by 1
min at 60°C and 5 sec at 95°C. The efficiency of amplification for
each target gene (GAPDH) was confirmed to be 100% in the
exponential phase of PCR. The mRNA levels were normalized to GADPH
mRNA according to the following formula: Active levels of pIgR mRNA
= 2−(CtpIgR - CtGAPDH) ×100%. The levels of mRNA in the
Caco-2 cells exposed to hyperoxia were compared with those of the
control group.
Statistical analysis
For each experiment, at least 6 generations of each
group were tested. The data from all groups were reported as the
means ± standard deviations. The t-test was used to determine
significant differences between treatment groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
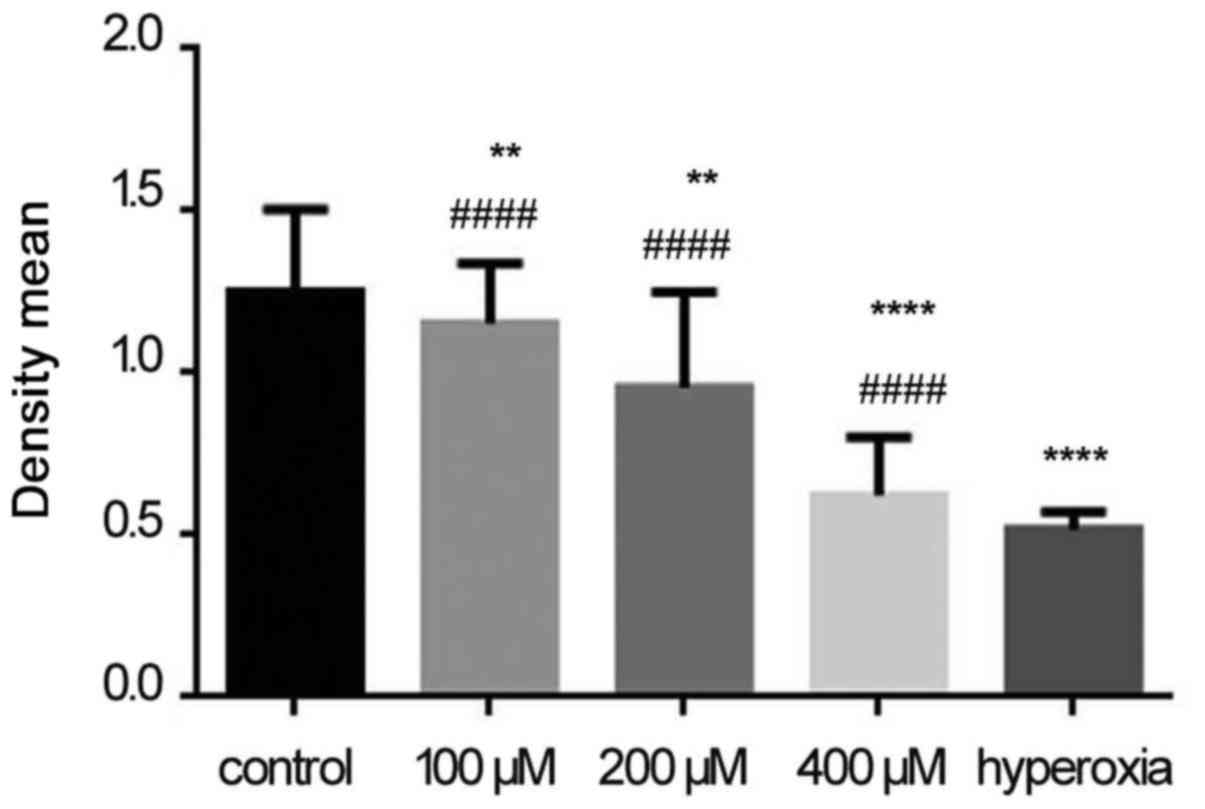
Cell survival
The cytotoxic effects of H2O2
are often attributed to ROS release. We investigated the
cytotoxicity of H2O2 in Caco-2 cells. As
shown in Fig. 1, the survival
rates of cells exposed to 100 μM H2O2
(P<0.01), 200 μM H2O2
(P<0.0001), 400 μM H2O2
(P<0.0001), and hyperoxia (P<0.0001), were significantly
lower compared with the control group. However, the survival rates
of the 100 μM H2O2 (P<0.0001), 200
μM H2O2 (P<0.0001), and 400
μM H2O2 (P<0.0001) groups were
higher compared with the hyperoxia group. These findings indicate
that ROS generation may be responsible for cell injury during
hyperoxia.
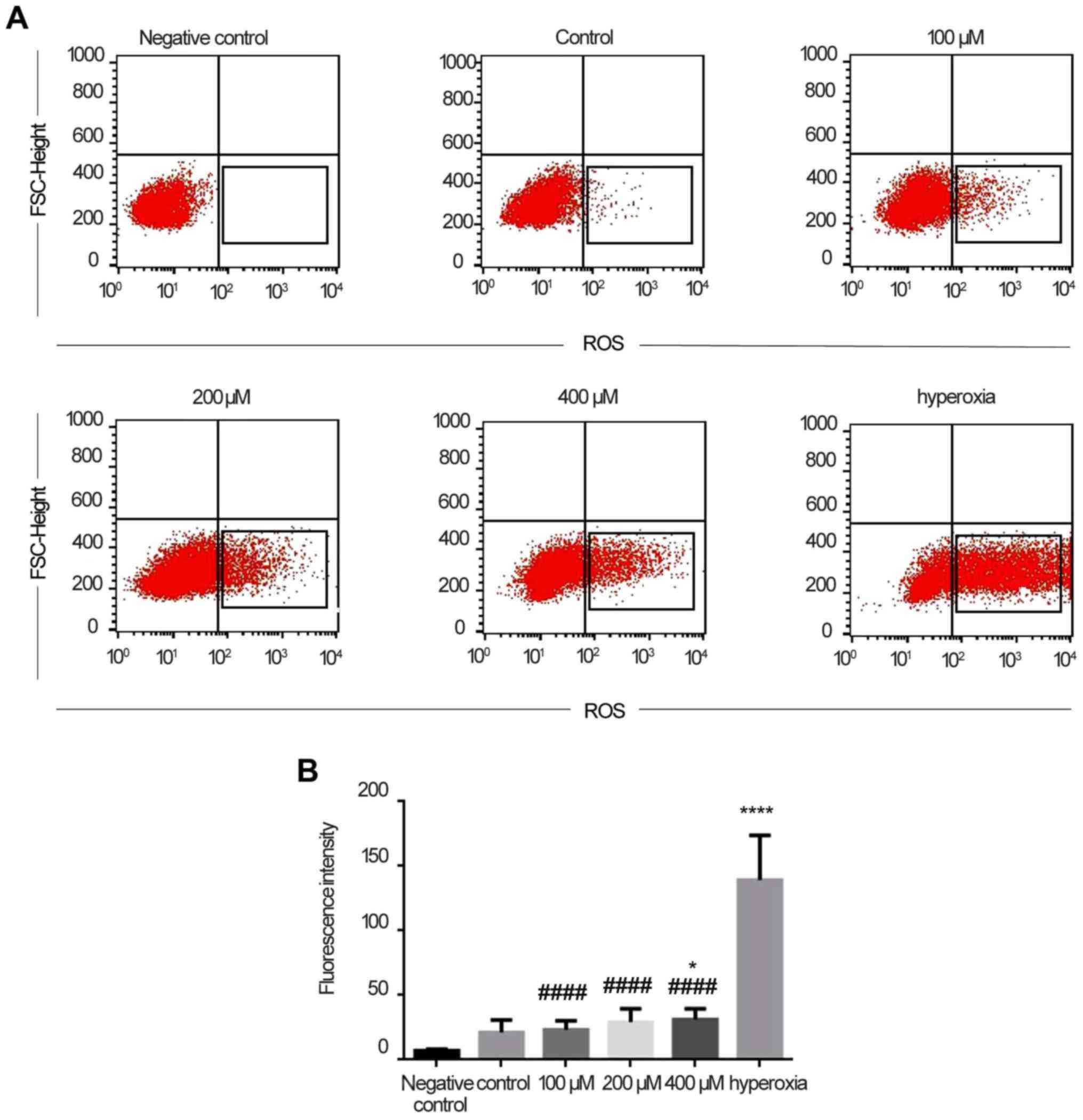
ROS detection in Caco-2 cells
As shown in Fig.
2A, there was no fluorescence in the negative control, whereas
the control group exhibited a small amount of fluorescence. The
fluorescence intensity increased with increasing concentrations of
H2O2. In the hyperoxia group, the
fluorescence intensity was stronger, and significantly higher
compared with that in the H2O2 (P<0.0001)
and control (P<0.0001) groups (Fig. 2B). Therefore, it was initially
concluded that Caco-2 cells produced more ROS under hyperoxic
conditions rather than in a normal environment.
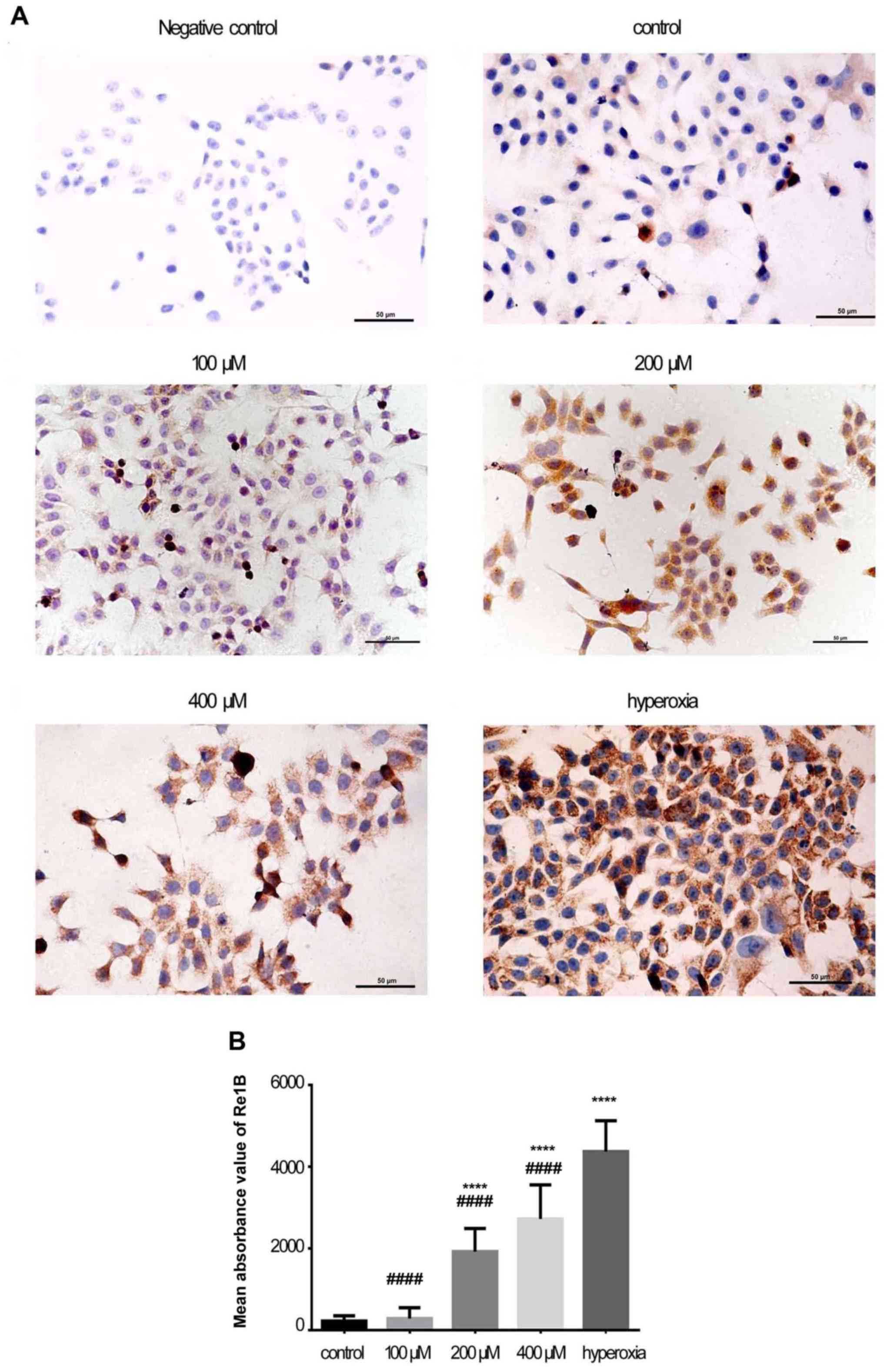
Immunohistochemical staining of RelA and
RelB
As shown in Figs.
3A and 4A, RelA and RelB
staining was mostly localized in the cytoplasm in the control
group. In the H2O2 and hyperoxia groups, RelA
and RelB staining was observed in the cytoplasm and nucleus. The
expression of RelA was higher in the 100 μM
H2O2 (P<0.0001), 200 μM
H2O2 (P<0.0001), and 400 μM
H2O2 (P<0.0001) groups compared with the
control group (Fig. 3B). The
expression of RelB was higher only in the 200 μM
H2O2 (P<0.0001) and 400 μM
H2O2 (P<0.0001) groups (Fig. 4B). In the hyperoxia group, the
morphology of the Caco-2 cells changed and the expression of RelA
and RelB was higher (P<0.0001) compared with that in the control
and H2O2 groups.
Protein expression of RelA, RelB, HIF-1α,
TNF-α and ASK1
The protein expressions of RelA (60 kDa), RelB (70
kDa), HIF-1α (100 kDa), TNF-α (20 kDa) and ASK1 (135 kDa) were
measured (Fig. 5A). The protein
expressions of RelA, RelB, HIF-1α, TNF-α and ASK1 increased with
increasing concentrations of H2O2; their
levels in the hyperoxia group were higher compared with those in
the H2O2 and control groups. Densitometry
analysis (RelA, RelB, HIF-1α, TNF-α and ASK1 densitomety/GAPDH
densitomety) revealed that the intensity of all these proteins in
the hyperoxia group was significantly higher compared with that in
the control and H2O2 groups (P<0.05;
Fig. 5B).
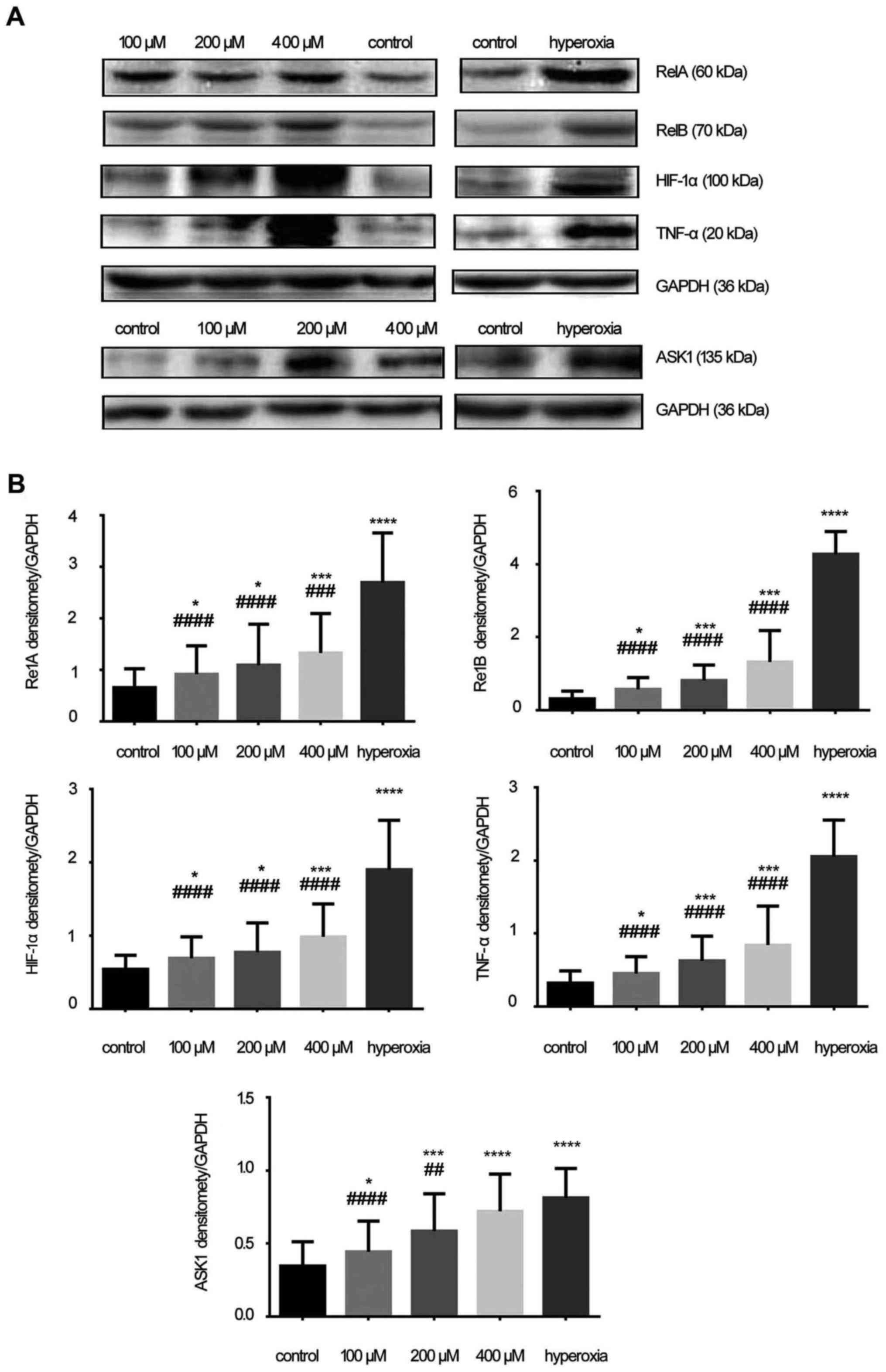 | Figure 5Expression of RelA, RelB,
hypoxia-inducible factor-1α (HIF-1α), tumor necrosis factor-α
(TNF-α) and apoptosis signal-regulating kinase 1 (ASK1) proteins.
(A) The expression of RelA (60 kDa), RelB (70 kDa), HIF-1α (100
kDa), TNF-α (20 kDa) and ASK1 (135 kDa). (B) Densitometric analysis
of RelA, RelB, HIF-1α, TNF-α and ASK1. In the hydrogen peroxide
(H2O2) group, expression of these proteins
was higher compared with the control group, while in the hyperoxia
group the proteins expression of RelA, RelB, HIF-1α, TNF-α and ASK1
were significantly increased. The results were presented as mean ±
standard deviation. *P<0.05, ***P <0.001 and
****P<0.0001 compared with the control group;
###P<0.001 and ####P<0.0001 compared
with the hyperoxia group, unpaired Student's t-test, n≥6. |
mRNA levels of RelA, RelB, HIF-1α, TNF-α
and ASK1
In the hyperoxia group, the mRNA levels of RelA,
RelB, HIF-1α, TNF-α and ASK1 were significantly increased than in
the control and H2O2 groups (P<0.001;
Fig. 6). The mRNA levels of RelA,
RelB, HIF-1α, TNF-α and ASK1 also increased with increasing
concentrations of H2O2 (P<0.01; Fig. 6).
 | Figure 6RelA, RelB, hypoxia-inducible
factor-1α (HIF-1α), tumor necrosis factor-α (TNF-α) and apoptosis
signal-regulating kinase 1 (ASK1) mRNA levels. The levels of RelA,
RelB, HIF-1α, TNF-α and ASK1 mRNAs from the hydrogen peroxide
(H2O2) groups and hyperoxia group were
significantly increased compared with the control group. The
results are presented as mean ± standard deviation.
*P<0.05, ***P<0.001 and
****P<0.0001 compared with the control group;
#P<0.05, ##P<0.01 and
####P<0.0001 compared with the hyperoxia group,
unpaired Student's t-test, n≥6. |
Discussion
Cells are continuously exposed to ROS, which are
generated by aerobic metabolism. Excessive generation of ROS causes
severe damage to cells in the form of oxidative stress (17). ROS are byproducts of oxygen
metabolism, which plays a crucial role in cell signaling and in
maintaining the balance of the organism (18). In complex biological systems,
physiologically produced ROS act as second messenger signals that
affect cell proliferation and differentiation (19). ROS play an important role as
signaling intermediates that induce a variety of cellular
responses, such as proliferation, differentiation, senescence and
apoptosis (17). As the levels of
ROS increase, the rate of apoptosis gradually increases (20). ROS include superoxide radicals,
H2O2, hydroperoxyl radicals and hydroxyl
radicals. H2O2 significantly increases free
radicals in the cells, resulting in critical DNA damage (7), and
H2O2-induced oxidative stress increases cell
apoptosis (21). During normal
metabolism, ROS signal cells to stimulate proliferation or to
induce cellular damage, depending on their concentration (22).
In the present study, the results of the MTT assay
demonstrated that the amount of apoptotic cells significantly
increased during hyperoxia and at higher doses of
H2O2. The results of flow cytometry revealed
that the levels of ROS under conditions of hyperoxia were
significantly higher compared with those under normal conditions.
Initially, on the basis of these findings, it was concluded that
hyperoxia may stimulate organs to produce more ROS (23). Hyperoxia may also cause oxidative
damage and induce production of ROS in the mitochondria and
expression of antioxidant proteins (24), and may also increase the
antioxidant response to ROS (25). Therefore, increasing levels of ROS
is the first step in the series of reactions that constitute
intestinal inflammation (18).
Hyperoxia-induced epithelial disruption is associated with tight
junction weakening and the development of a pro-inflammatory
environment (3).
ROS induce the production of cytokines during
inflammation (26). Subsequently,
TNF-α may lead to cell damage by inducing the production of
intracellular mitochondrial ROS, thereby increasing intestinal
inflammation and damage (27).
High expression of TNF-α in intestinal epithelial cells suggests
that TNF-α levels may be indicative of intestinal damage (27). Therefore, TNF-α expression was
detected in the intestinal epithelium. The results revealed that
the damage to the intestinal epithelial cells that was induced by
H2O2 or hyperoxia increased the expression of
TNF-α at the protein and gene levels. TNF-α activates MLK3 and
leads to JNK activation in vivo (28). As a member of the MAPK family,
ASK1 is present in several physiological and pathological
processes. ASK1 is required for apoptosis induced by oxidative
stress and TNF (29). In normal
cells, activation of ASK1 is strictly controlled by phosphorylation
and dephosphorylation of serine/threonine. Since it may be
activated by several stress and inflammatory factors, the
overexpression of ASK1 may induce cell apoptosis through the MAPK
signaling pathway, and ASK1 is activated in cells treated with
TNF-α (30,31). ROS also directly or indirectly
affect signaling molecules, such as protein kinases (MAPK), which
cause oxidative damage to organs (1,32).
During oxidative stress, H2O2 catalyzes the
phosphorylation of ASK1, and then activates the downstream JNK/p38
pathway, promoting cell apoptosis (33). Therefore, the role of ASK1 in the
MAPK family was specifically investigated. Our results demonstrated
that, with the increased oxidative stress induced by hyperoxia, the
expression of ASK1 significantly increased at the protein and gene
levels.
TNF-α mediates activation of the NF-κB survival
pathway in the cytoplasm (34).
The NF-κB pathway regulates genes encoding acute reactive proteins,
cytokines, cell adhesion molecules, and immune regulatory molecules
in inflammation. NF-κB is an important signaling molecule, and it
is involved in physiological responses induced by hyperoxia in
different cell types and tissues (35). NF-κB may also regulate
inflammatory response and epithelial cell damage or death (36). It has also been reported that the
activation of oxidative stress and NF-κB plays an important role in
the pathogenesis of inflammatory bowel disease (IBD) (37). In the present study, RelA and RelB
expression in the NF-κB signaling pathway were significantly
increased at both the protein and gene levels during hyperoxia and
at higher doses of H2O2. This proves that
intestinal inflammation may occur in hyperoxia and that ROS play an
important role in inflammation induced by hyperoxia.
NF-κB is involved in stimulating HIF-1α mRNA
expression. There is significant evidence on the need for an intact
NF-κB pathway for proper oxygen- and ligand-induced HIF activity
(38). HIF-1α is key to the
pathogenesis of IBD, and the role of HIF-1α in immune cells is
becoming increasingly important (39). Therefore, the protein and mRNA
levels of HIF-1α were measured. The results demonstrated that 85%
hyperoxia and high concentrations of H2O2
exerted similar effects in terms of activating the HIF signaling
pathway. This proves that ROS play an important role during
hyperoxia.
In conclusion, intestinal epithelial cells were
destroyed and the levels of ROS increased during hyperoxia. Thus,
ROS may play an important role in intestinal injury in a hyperoxic
environment. In the future, further research focusing on the
intestinal mechanisms of hyperoxic injury may reveal an even more
important role of ROS. Novel strategies for treating
hyperoxia-induced intestinal injury must also be investigated.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 30871158 and 81170604) and
the Outstanding Scientific Fund of Shengjing Hospital.
Glossary
Abbreviations
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
H2O2
|
hydrogen peroxide
|
|
TNF-α
|
tumor necrosis factor-α
|
|
ASK1
|
apoptosis signal-regulating kinase
1
|
|
MAP
|
mitogen-activated protein
|
|
JNK
|
c-Jun N-terminal kinase
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
References
|
1
|
Zhao HW, Ali SS and Haddad GG: Does
hyperoxia selection cause adaptive alterations of mitochondrial
electron transport chain activity leading to a reduction of
superoxide production. Antioxid Redox Signal. 16:1071–1076. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torbati D, Tan GH, Smith S, Frazier KS,
Gelvez J, Fakioglu H and Totapally BR: Multiple-organ effect of
normobaric hyperoxia in neonatal rats. J Crit Care. 21:85–93. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Shmgani HS, Moate RM, Macnaughton PD,
Sneyd JR and Moody AJ: Effects of hyperoxia on the permeability of
16HBE14o- cell monolayers - the protective role of antioxidant
vitamins E and C. FEBS J. 280:4512–4521. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong Y, Sun LI, Sun R, Chen H, Yu Y and
Xie K: Combination therapy of molecular hydrogen and hyperoxia
improves survival rate and organ damage in a zymosan-induced
generalized inflammation model. Exp Ther Med. 11:2590–2596. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carnesecchi S, Deffert C, Pagano A,
Garrido-Urbani S, Métrailler-Ruchonnet I, Schäppi M, Donati Y,
Matthay MA, Krause KH and Barazzone Argiroffo C: NADPH oxidase-1
plays a crucial role in hyperoxia-induced acute lung injury in
mice. Am J Respir Crit Care Med. 180:972–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niethammer P, Grabher C, Look AT and
Mitchison TJ: A tissue-scale gradient of hydrogen peroxide mediates
rapid wound detection in zebrafish. Nature. 459:996–999. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai X, Chen X, Wang X, Xu C, Guo Q, Zhu L,
Zhu S and Xu J: Pre-protective effect of lipoic acid on injury
induced by H2O2 in IPEC-J2 cells. Mol Cell
Biochem. 378:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oncel MY, Yurttutan S, Alyamac Dizdar E,
Gokce IK, Gonul II, Topal T, Canpolat FE and Dilmen U: Beneficial
effect of etanercept on hyperoxic lung injury model in neonatal
rats. J Invest Surg. 29:1–5. 2016. View Article : Google Scholar
|
|
9
|
Matsuzawa A and Ichijo H: Redox control of
cell fate by MAP kinase: physiological roles of ASK1-MAP kinase
pathway in stress signaling. Biochim Biophys Acta. 1780:1325–1336.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vermeulen L, De Wilde G, Van Damme P,
Vanden Berghe W and Haegeman G: Transcriptional activation of the
NF-kappaB p65 subunit by mitogen- and stress-activated protein
kinase-1 (MSK1). EMBO J. 22:1313–1324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhatia HS, Baron J, Hagl S, Eckert GP and
Fiebich BL: Rice bran derivatives alleviate microglia activation:
possible involvement of MAPK pathway. J Neuroinflammation.
13:1482016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bentovim L, Amarilio R and Zelzer E: HIF1α
is a central regulator of collagen hydroxylation and secretion
under hypoxia during bone development. Development. 139:4473–4483.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baylor AE, Diebel LN, Liberati DM,
Dulchavsky SA, Diglio CA and Brown WJ: The effects of varying
oxygen conditions and immunoglobulin A on barrier defense to
bacterial invasion. Am Surg. 69:231–237. 2003.PubMed/NCBI
|
|
14
|
Diebel LN, Liberati DM, Brown WJ,
Dulchavsky SA, Painter TM, Diglio CA and Montgomery PC: Secretory
immunoglobulin A blocks hypoxia-augmented bacterial passage across
Madin-Darby canine kidney cell monolayers. J Trauma. 43:759–763.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diebel LN, Liberati DM, Dulchavsky SA,
Diglio CA and Brown WJ: Synergistic effect of hyperoxia and immuno
globulin A on mucosal barrier defense. J Trauma. 46:374–378. 1999.
View Article : Google Scholar
|
|
16
|
Liu DY and Li JJ: Effect of hyperoxia on
the intestinal IgA secretory component in neonatal rats and on
intestinal epithelial cells in vitro. Braz J Med Biol Res.
43:1034–1041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujisawa T, Takeda K and Ichijo H: ASK
family proteins in stress response and disease. Mol Biotechnol.
37:13–18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruh J, Vogel F, Schmidt E, Werner M, Klar
E, Secchi A, Gebhard MM, Glaser F and Herfarth C: Effects of
hydrogen peroxide scavenger Catalase on villous microcirculation in
the rat small intestine in a model of inflammatory bowel disease.
Microvasc Res. 59:329–337. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jones RM, Luo L, Ardita CS, Richardson AN,
Kwon YM, Mercante JW, Alam A, Gates CL, Wu H, Swanson PA, et al:
Symbiotic lactobacilli stimulate gut epithelial proliferation via
Nox-mediated generation of reactive oxygen species. EMBO J.
32:3017–3028. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Z, Moruzzi N, Catrina SB, Grill V and
Björklund A: Hyperoxia inhibits glucose-induced insulin secretion
and mitochondrial metabolism in rat pancreatic islets. Biochem
Biophys Res Commun. 443:223–228. 2014. View Article : Google Scholar
|
|
21
|
Jin S, Ray RM and Johnson LR:
TNF-alpha/cycloheximi-de-induced apoptosis in intestinal epithelial
cells requires Rac1-regulated reactive oxygen species. Am J Physiol
Gastrointest Liver Physiol. 294:G928–G937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kajino-Sakamoto R, Omori E, Nighot PK,
Blikslager AT, Matsumoto K and Ninomiya-Tsuji J: TGF-beta-activated
kinase 1 signaling maintains intestinal integrity by preventing
accumulation of reactive oxygen species in the intestinal
epithelium. J Immunol. 185:4729–4737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Camporesi EM and Bosco G: Hyperbaric
oxygen pretreatment and preconditioning. Undersea Hyperb Med.
41:259–263. 2014.PubMed/NCBI
|
|
24
|
Klimova TA, Bell EL, Shroff EH, Weinberg
FD, Snyder CM, Dimri GP, Schumacker PT, Budinger GR and Chandel NS:
Hyperoxia-induced premature senescence requires p53 and pRb, but
not mitochondrial matrix ROS. FASEB J. 23:783–794. 2009. View Article : Google Scholar :
|
|
25
|
Steer JH, Mann TS, Lo SZ, Inglis JJ, Yap
HS, Henry PJ and Joyce DA: Early induction of uncoupling protein-2
in pulmonary macrophages in hyperoxia-associated lung injury. Inhal
Toxicol. 25:544–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kina S, Nakasone T, Takemoto H, Matayoshi
A, Makishi S, Sunagawa N, Liang F, Phonaphonh T and Sunakawa H:
Regulation of chemokine production via oxidative pathway in HeLa
cells. Mediators Inflamm. 2009:1837602009. View Article : Google Scholar
|
|
27
|
Baregamian N, Song J, Bailey CE,
Papaconstantinou J, Evers BM and Chung DH: Tumor necrosis
factor-alpha and apoptosis signal-regulating kinase 1 control
reactive oxygen species release, mitochondrial autophagy, and c-Jun
N-terminal kinase/p38 phosphorylation during necrotizing
enterocolitis. Oxid Med Cell Longev. 2:297–306. 2009. View Article : Google Scholar
|
|
28
|
Sathyanarayana P, Barthwal MK, Kundu CN,
Lane ME, Bergmann A, Tzivion G and Rana A: Activation of the
Drosophila MLK by ceramide reveals TNF-alpha and ceramide as
agonists of mammalian MLK3. Mol Cell. 10:1527–1533. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takeda K, Matsuzawa A, Nishitoh H and
Ichijo H: Roles of MAPKKK ASK1 in stress-induced cell death. Cell
Struct Funct. 28:23–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakagawa H and Maeda S: Inflammation- and
stress-related signaling pathways in hepatocarcinogenesis. World J
Gastroenterol. 18:4071–4081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gore A, Muralidhar M, Espey MG, Degenhardt
K and Mantell LL: Hyperoxia sensing: from molecular mechanisms to
significance in disease. J Immunotoxicol. 7:239–254. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Subramanian RR, Zhang H, Wang H, Ichijo H,
Miyashita T and Fu H: Interaction of apoptosis signal-regulating
kinase 1 with isoforms of 14-3-3 proteins. Exp Cell Res.
294:581–591. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chun JN, Choi B, Lee KW, Lee DJ, Kang DH,
Lee JY, Song IS, Kim HI, Lee SH, Kim HS, et al: Cytosolic Hsp60 is
involved in the NF-kappaB-dependent survival of cancer cells via
IKK regulation. PLoS One. 5:e94222010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zara S, De Colli M, Rapino M, Di Valerio
V, Marconi GD, Cataldi A, Macchi V, De Caro R and Porzionato A:
NF-κB involvement in hyperoxia-induced myocardial damage in newborn
rat hearts. Histochem Cell Biol. 140:575–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zaher TE, Miller EJ, Morrow DM, Javdan M
and Mantell LL: Hyperoxia-induced signal transduction pathways in
pulmonary epithelial cells. Free Radic Biol Med. 42:897–908. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Babu D, Lee JS, Park SY, Thapa D, Choi MK,
Kim AR, Park YJ and Kim JA: Involvement of NF-kappaB in the
inhibitory actions of Platycarya strobilacea on the
TNF-alpha-induced monocyte adhesion to colon epithelial cells and
chemokine expression. Arch Pharm Res. 31:727–735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cummins EP, Keogh CE, Crean D and Taylor
CT: The role of HIF in immunity and inflammation. Mol Aspects Med.
47–48:24–34. 2016. View Article : Google Scholar
|
|
39
|
Flück K and Fandrey J: Oxygen sensing in
intestinal mucosal inflammation. Pflugers Arch. 468:77–84. 2016.
View Article : Google Scholar
|