Introduction
Melanoma is one of the most lethal forms of skin
tumors, responsible for >80% of all diagnosed skin
cancer-related mortalities (1).
Melanoma is a serious public health problem in all countries
throughout the world due to its unfavorable prognosis and the
limited treatments available (2).
Despite advances in melanoma treatment, with several novel targeted
therapies, as a result of the special characteristics and usual
resistance to standard chemotherapy, there is no systemic and
effective therapy that has a clear effect on the overall survival
of patients with malignant melanoma (3). Although our understanding of the
molecular biology of malignant melanoma has increased in recent
years, the molecular mechanism of melanomagenesis is not completely
understood.
Melanoma cells are highly resistant to traditional
chemotherapeutic and radiotherapeutic treatments (4). Immunotherapies (involving cancer
vaccines, adoptive immunotherapy, antibodies and cytokines) focus
on alternative therapies (5). The
challenge is to translate successful results from immune modulation
trials into clinical meaningful results in phase 2 or randomized
phase 3 drug trials (6).
Combining cancer vaccines with the adoptive transfer of T cells has
been shown to increase the levels of circulating tumor
antigen-specific regulatory T cells in patients, however, these
approaches produce only a few patient's clinical responses
(7). These observations in
melanoma support the fact that tumor resistance to immune
system-mediated destruction may be the main process in the tumor
microenvironment (7). To improve
the concepts of immune-based treatments, a better understanding of
the local and systemic tumor-resistance mechanisms is important in
melanoma patients.
The five members of the inhibitor of growth (ING)
gene family are ING1, ING2, ING3, ING4 and ING5. The ING family
have garnered attention due to their putative roles as tumor
suppressor genes (8,9). ING4 is a member of the ING family
that has been demonstrated to play important roles in numerous
cancer-related cellular processes including DNA damage, hypoxia,
cell proliferation, apoptosis, the cell cycle, migration and
angiogenesis (8). ING4 is located
in chromosome 12p13 and encodes a 249-amino acid protein containing
a conserved C-terminal plant homeodomain finger motif and two
nuclear localization signals (10). ING4 expression is reduced in
primary melanomas and metastatic melanomas (11). Upregulation of ING4 has been shown
to decrease the cell population in S phase, diminish the colony
forming efficiency and induce apoptosis in a p53-dependent manner
(10,11). However, the precise role of ING4
in melanoma angiogenesis is unclear.
The Fas death receptor/Fas ligand (Fas/FasL) system
is a key regulator of apoptosis (12). The Fas/FasL system, one of the
major extrinsic apoptosis signaling pathways, is a key regulator of
T-cell apoptosis (13). The
Fas/FasL-induced apoptotic pathway is active in early- and
intermediate-phase melanomas, but is often impaired in highly
metastatic melanomas (14). The
lack expression of Fas is correlated with the poor prognosis of
malignant melanomas (15). Recent
findings revealed that the Fas expression was downregulated and
FasL expression was upregulated during melanoma progression
(16).
The present study sought to investigate the impact
of ING4 on malignant melanoma cells. Firstly, lentivirus
(LV)-ING4-short hairpin (shRNA) or LV-ING4 were transfected into
human melanoma A375 cells. Next, the effects of ING4 overexpressed
or knockdown were determined on the apoptosis of A375 cells and
CD3+ T cells. The study aimed to reveal the potential
effects of ING4 in the regulation of the apoptosis pathway in A375
cells and CD3+ T cells. The study also aimed to
demonstrate that ING4 can serve as a target for gene therapy in
human melanoma cells.
Materials and methods
Cell and culture
The human melanoma A375 cell line, obtained from the
American Type Culture Collection (ATCC, Manassas, VA, USA), was
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum
(MinHai Bio-Engineering, Co., Ltd., Lanzhou, China) and 1%
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) in a humidified atmosphere at 37°C, in the presence of 5%
CO2 and 95% air. After selection of infected A375 cells,
the CD3+ T cells were co-cultured with A375 cells in WT,
LV-control, LV-ING4-shRNA and LV-ING4 groups separately.
Construction of overexpression or
knockdown ING4 vectors in A375 cells
pcDNA3.1-ING4 was constructed as described
previously (17). Lentiviral
vector (pLKO.1) constructs containing ING4 cDNA or shRNA sequences
for ING4 and an empty vector were purchased from Open Biosystems;
GE Healthcare Dharmacon, Inc. (Lafayette, CO, USA). Lentiviral
constructs were used to transiently transfect 293FT packaging cells
along with 3 µg VSV-G pseudoviral particles. At 16 h
post-transduction, the supernatant was replaced by Opti-MEM medium
supplemented with HEPES (Invitrogen; Thermo Fisher Scientific,
Inc.). Viral supernatant was harvested from the 293FT cells at 48 h
post-transfection and filtered to remove non-adherent cells.
Subconfluent A375 cells were infected by centrifugation at 300 × g
for 5 min at 4°C using virus-containing medium and 8 µg/ml
polybrene. Infected A375 cells were selected for using 2
µg/ml puromycin starting at 24 h after the initial
infection. The cells were identified at the protein levels using
western blot analysis.
Preparation of CD3+ T
cells
Peripheral blood mononuclear cells (PBMCs) were
isolated from the peripheral blood of healthy donors using Ficoll
density gradient centrifugation at 300 × g for 10 min at 20°C.
Activation and expansion of anti-CD3 T cells from PBMCs at 14 days
post-centrifugation was performed as previously described (18). CD3+ T-cell expansion
resulted in a product increase of 98.85±1.06% on day 15.
CD3+ T cells were cultured in RPMI-1640 containing 10%
fetal calf serum (Invitrogen; Thermo Fisher Scientific, Inc.). The
research protocol was approved by the Biomedical Research Ethics
Committee of the First Affiliated Hospital of Harbin Medical
University (2014-R-034).
Analysis of apoptosis with Annexin
V/7-aminoactinomycin D (7-AAD) staining
Annexin V-fluorescein isothiocyanate (FITC) and
7-AAD (BD Biosciences, San Jose, CA, USA) were used for detection
of apoptotic cells in the melanoma A375 cells and CD3+ T
cells, according to the manufacturer's protocols, and then analyzed
by a dual-staining protocol with fluorescence-activated cell
sorting using FACScan (BD Biosciences, Franklin Lakes, NJ, USA).
Cell populations (1×106) were labeled with Annexin V and
7-AAD (BD Biosciences) following the provider's procedure, and
analyzed by flow cytometry.
In vivo model
A total of 20 BALB/c nude mice were purchased from
the Shanghai Laboratory Animal Center (Shanghai, China). Mice were
allowed free access to sterilized water and food, and were housed
in individual ventilated cages at 23±5°C under a 12-h light/dark
cycle. The A375 cells were resuspended in serum-free DMEM at a
concentration of 1×107 cells/ml. A total volume of 0.2
ml cell suspension (total 2×106 cells) was then injected
subcutaneously into the right anterior armpit of nude mice to
establish a xenograft model. The 20 injected mice were randomly
divided into 4 groups: WT, LV-control, LV-ING4-shRNA and LV-ING4
(n=5 per group). The experimental protocol was approved by the
Animal Care and Use Committee of the First Affiliated Hospital of
Harbin Medical University.
Western blot analysis
Melanoma A375 cells or CD3+ T cells were
lysed in buffer containing 150 mmol/l NaCl, 1% NP-40, 50 mmol/l
Tris (pH 8.0) and 20% glycerol, and normalized to total protein
concentration using Bio-Rad protein assay reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Whole cell proteins (30
µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to PVDF membranes using the Bio-Rad electro-transfer
system (Bio-Rad Laboratories, Inc.). The filters were hybridized
with ING4 (sc-135742), poly(ADP-ribose) polymerase (PARP;
sc-136208), caspase-8 (sc-6136), caspase-3 (sc-271759), Fas
(sc-4856) and FasL (sc-71096) (all 1:1,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature.
Anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1,000
dilution; sc-47724; Santa Cruz Biotechnology, Inc.) was used as a
loading control. The membrane was blocked by 5% fat-free milk for 1
h at room temperature and then incubated with the appropriate
primary antibody diluted in 3% BSA solution at 4°C overnight. After
incubation with DyLight dye-conjugated secondary antibodies (cat.
no. 35518; 1:10,000; Thermo Fisher Scientific, Inc.) for 1 h at
room temperature, blots were scanned by the Odyssey Western
Detection system (LI-COR Biosciences, Lincoln, NE USA), followed by
quantification with ImageStudio software (LI-COR Biosciences).
Immunohistochemistry
Immunohistochemistry (IHC) was performed as
described previously (19). Fine
sections (4–5 µm) were prepared from formalin-fixed tissue
sections on poly-L-lysine coated glass slides, and then stained
with anti-PARP, -caspase-8, -caspase-3, -Fas and -FasL by IHC.
Sections were evaluated and scored independently by an experienced
pathologist unaware of the treatment group. At least 5 fields per
slide were randomly chosen for analysis of IHC. The IHC was
evaluated according to the intensity of reactivity using a 4-tier
system: 0, no staining (-); 1, weak staining (+); 2, moderate
staining (++); and 3, strong staining (+++).
RNA extraction and RT-qPCR
Total RNA was extracted by using the TRIzol reagent
(Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. RNA was reverse transcribed into cDNA
using the PrimeScript II 1st strand cDNA Synthesis kit (Takara,
Shiga, Japan). qPCR was performed on an ABI Step-One plus machine
using SYBR-Green qPCR Master Mix (Biotool, Jupiter, FL, USA).
Primers used are as follows: ING4 forward, ATGACAGCTCTTCCAGCAA and
reverse, AGAAACTGTGTTGGAATCCAAG; GAPDH forward,
AATCCCATCACCATCTTCCA and reverse, TGGACTCCACGACGTACTCA. GAPDH was
used as endogenous control and 2−ΔΔCt method was used to
calculate the fold change.
Statistical analysis
All experiments were repeated at least three times,
and data are expressed as the mean ± standard error of the mean.
Comparisons among multiple groups were determined by one-way
analysis of variance followed by Tukey's post-hoc test. Prism6
(GraphPad Software, Inc., La Jolla, CA, USA) was use to perform
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
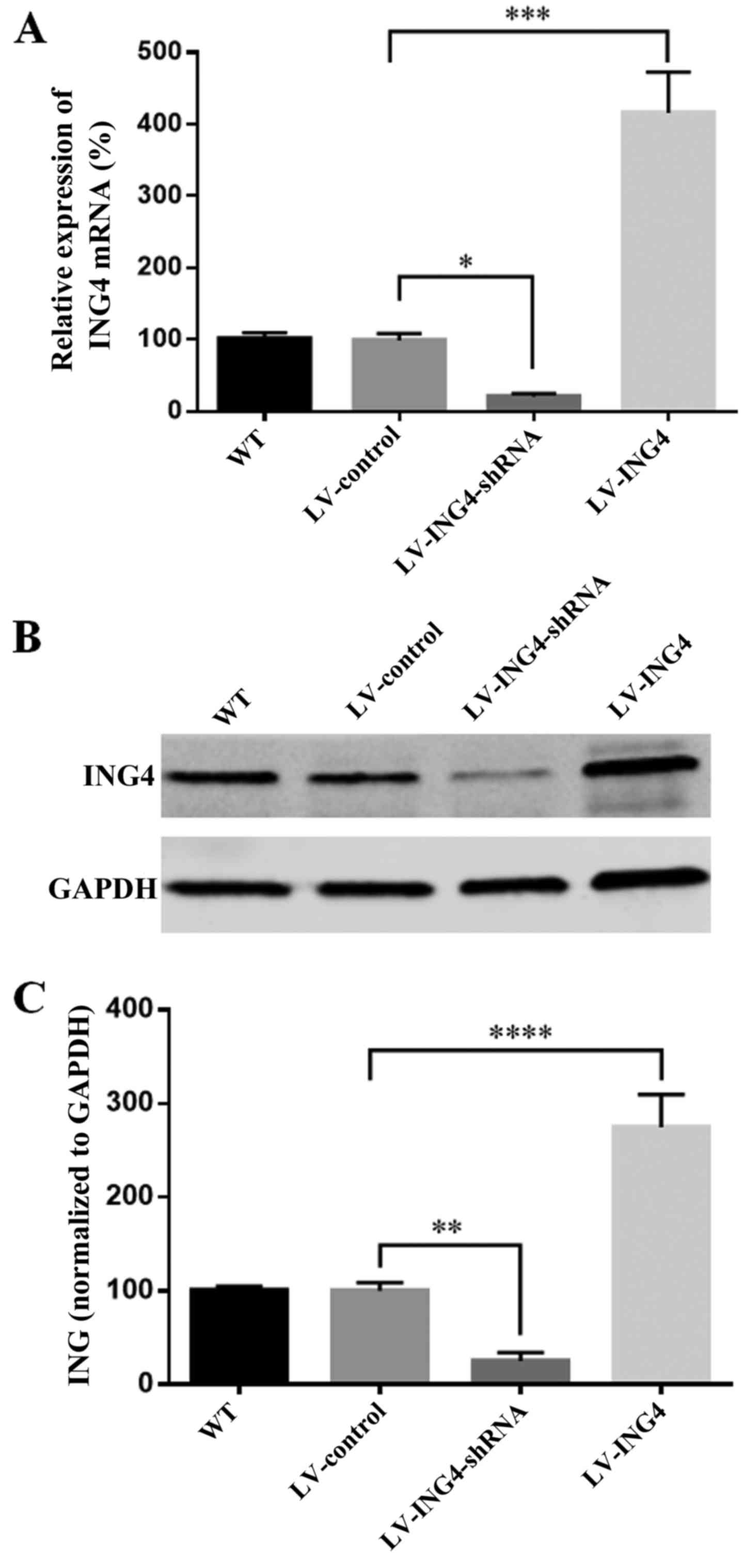
Expression of ING4 in melanoma A375 cell
transfectants
The expression of ING4 is 98% in dysplastic nevi,
and then significantly decreased to 67 or 53% in primary melanomas
and metastatic melanomas (11).
After the wild-type, non-targeting lentiviral, ING4 lentiviral
small hairpin RNA (LV-ING4-shRNA) and lentiviral pcDNA3.1-ING4
(LV-ING4) were transfected into A375 melanoma cell line,
respectively, the expression of ING4 was evaluated by RT-PCR and
western blot analysis. The overall knockdown efficiency of
LV-ING4-shRNA was 80% (Fig. 1A)
at the mRNA level and 70% at the protein levels (Fig. 1B and C). However, there was a
significant increase in ING4 mRNA of 415% and protein of 275% in
the A375/LV-ING4 group compared with the A375/LV-control (Fig. 1).
Overexpression of ING4 enhances apoptosis
of melanoma A375 cells and CD3+ T cells
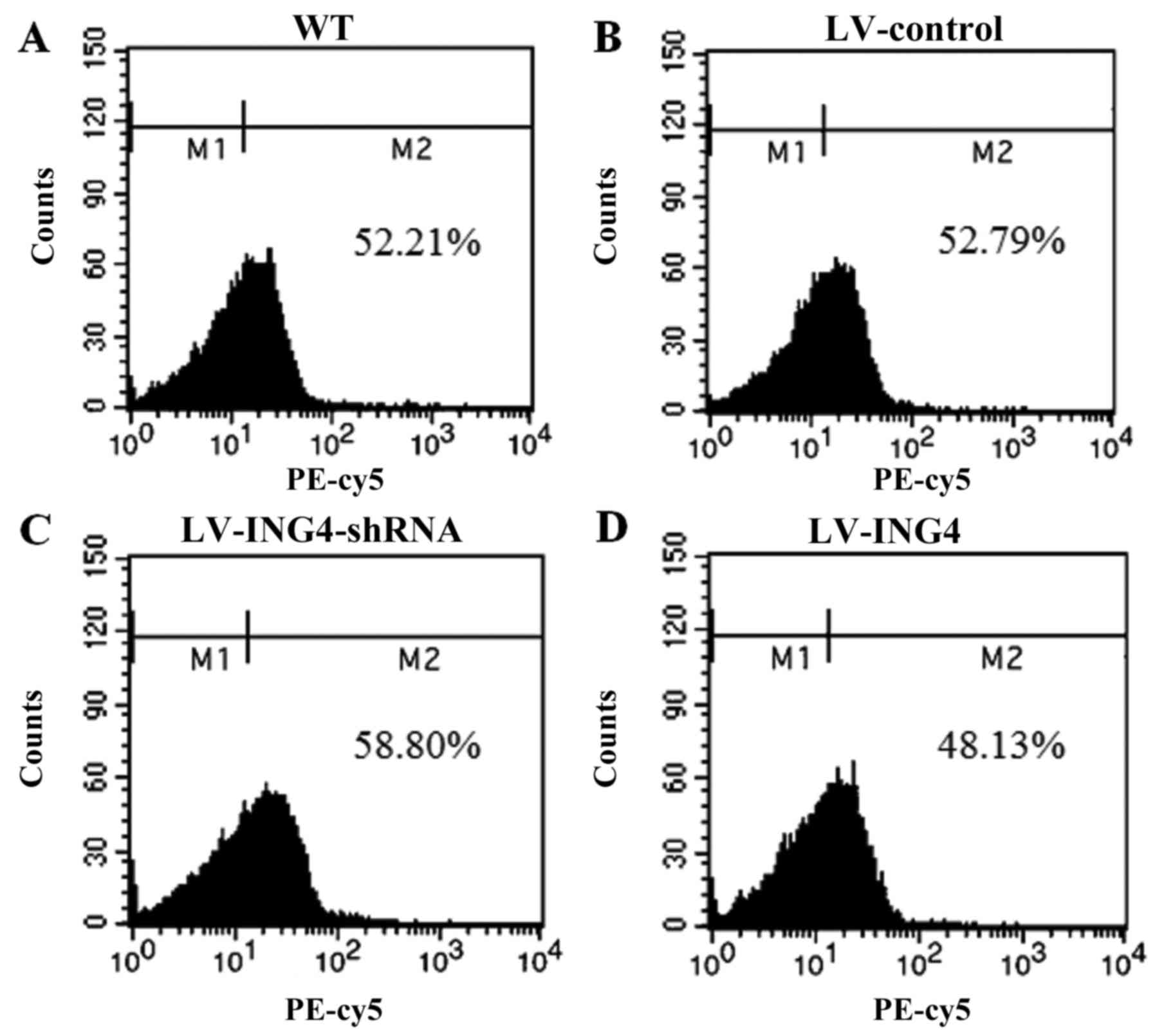
To examine the effect of ING4 on the A375 and T-cell
co-culture system, CD3+ T cells were isolated from the
blood samples of healthy donors. Flow cytometry analysis was
performed to confirm the purity of CD3+ T cells from the
A375 and CD3+ T-cell co-culture (Fig. 2).
Next, to determine whether the effect of ING4 in
A375 melanoma cells to enhance the apoptosis of A375 cells or
CD3+ T cells, the A375 cells were treated with
wild-type, LV-control, LV-ING4-shRNA or LV-ING4, and then
co-culture with CD3+ T cells at 48 h; double staining
with Annexin V-FITC/PI was used to detect the cell apoptosis
(Fig. 3). The percentage of early
apoptotic cells (Annexin V-positive/7-AAD-negative) was 6.24±0.75%
for the wild-type group, 6.02±1.52% for the LV-control group,
7.01±1.42% for the LV-ING4-shRNA group and 4.68±0.89% for the
LV-ING4 group. In addition, the percentage of late apoptotic cells
(Annexin V-positive/7-AAD -positive) was 5.76±1.37% for the
wild-type group, 8.41±1.47% for the LV-control group, 5.98±1.21%
for the LV-ING4-shRNA group and 54.37±6.13% for the LV-ING4 group
(Fig. 3A).
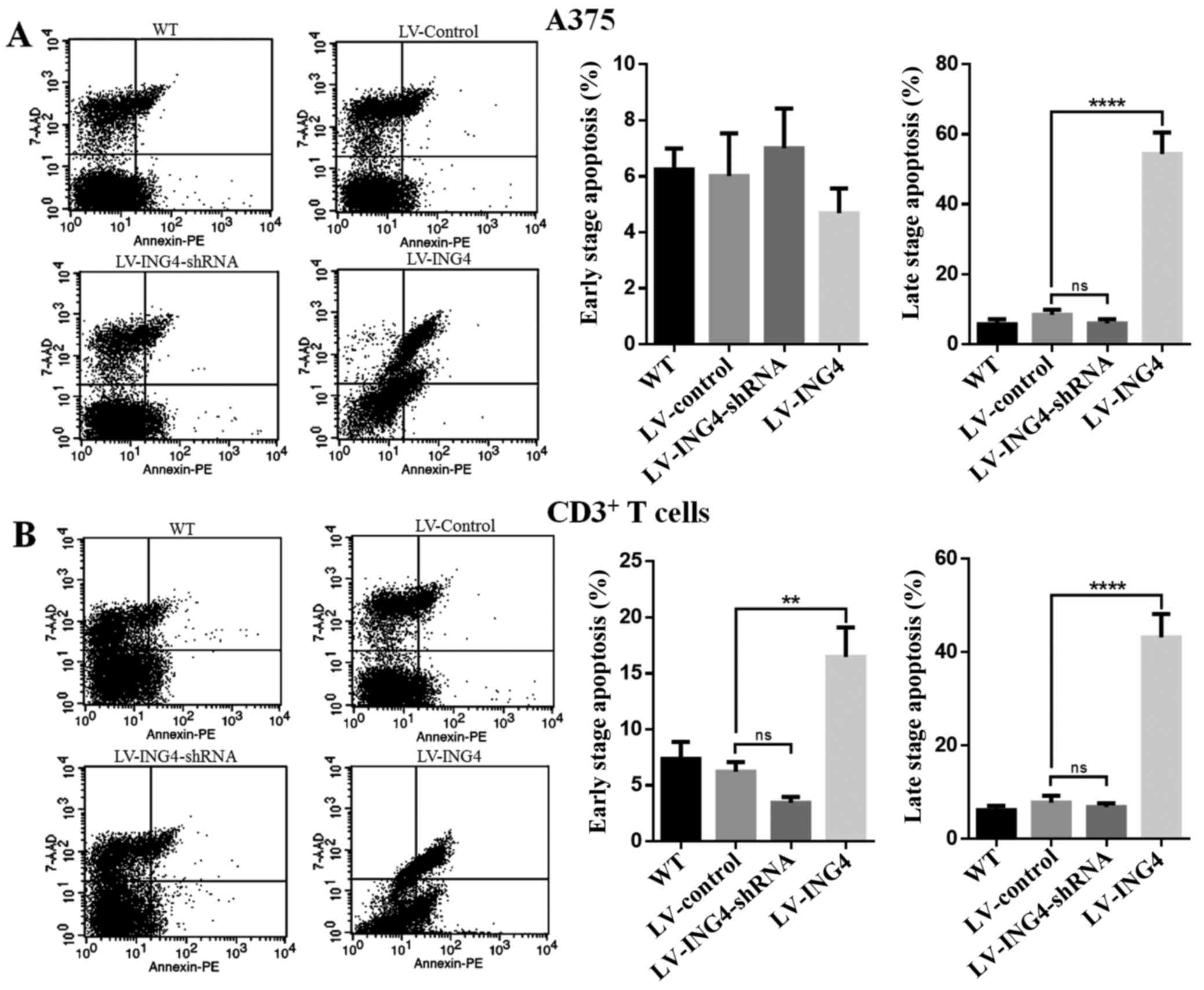 | Figure 3Overexpression of ING4 enhances
apoptosis of melanoma A375 cell lines and CD3+ T cells.
The (A) melanoma A375 cells and (B) CD3+ T cells were
stained with Annexin V-FITC and 7-AAD following transfection with
WT, LV-control, LV-ING4-shRNA or LV-ING4. Fluorescence-activated
cell sorting analysis of the cells was performed at 48 h
post-transfection with WT, LV-control, LV-ING4-shRNA or LV-ING4.
Percentages represent Annexin V-positive/7-AAD-negative (early
apoptotic) and Annexin V-positive/7-AAD-positive cells (late
apoptotic). Experiments were repeated three times. **P<0.01 and
****P<0.0001 vs LV-control. ING4, inhibitor of growth
4; FITC, fluorescein isothiocyanate; 7-AAD, 7-aminoactinomycin D;
WT, wild-type; shRNA, short hairpin RNA; CD, cluster of
differentiation; PE, phycoerythrin; LV, lentivirus; ns, not
significant. |
Similar results were obtained with CD3+ T
cells from the A375 and CD3+ T-cell co-culture system.
As shown in Fig. 3B, the
apoptosis of CD3+ T cells was significantly different in
the A375 cells transfected with LV-ING4 (the early apoptotic cells,
16.47±2.63%; the late apoptotic cells, 43.20±4.96%) compared with
that in the cells transfected with the LV-control (the early
apoptotic cells, 6.21±0.87%; the late apoptotic cells, 7.76±1.49%);
however, there was no significant difference compared with the
LV-ING4-shRNA group in the A375 and CD3+ T cell
co-culture system (Fig. 3B).
These results suggest that increased ING4 expression in melanoma
cells leads to increased lymphocyte apoptosis.
ING4 regulates the protein expression
involved in the apoptosis pathway in CD3+ and melanoma
A375 cells
In order to investigate the molecular mechanisms in
the apoptosis of CD3+ and A375 melanoma cells, the
effects of ING4 on the expression of target proteins involved in
the apoptosis of CD3+ and melanoma A375 cells was
investigated. The protein expression levels of PARP, caspase-8 and
caspase-3, which were involved in cell apoptosis according to
western blot analysis, were determined. The protein expression of
Fas and FasL was also examined. The results showed that increased
ING4 expression reduced the PARP, caspase-8 and caspase-3 levels,
but significantly increased the Fas and FasL protein expression in
the CD3+ T cells and the melanoma A375 cells (Fig. 4). The levels of PARP, caspase-8,
caspase-3, Fas and FasL were also analyzed in nude mice livers by
IHC. Higher expression of Fas and FasL, as well as lower expression
of PARP, caspase-8 and caspase-3 was observed in the livers of the
LV-ING4 group compared with other groups (Fig. 5).
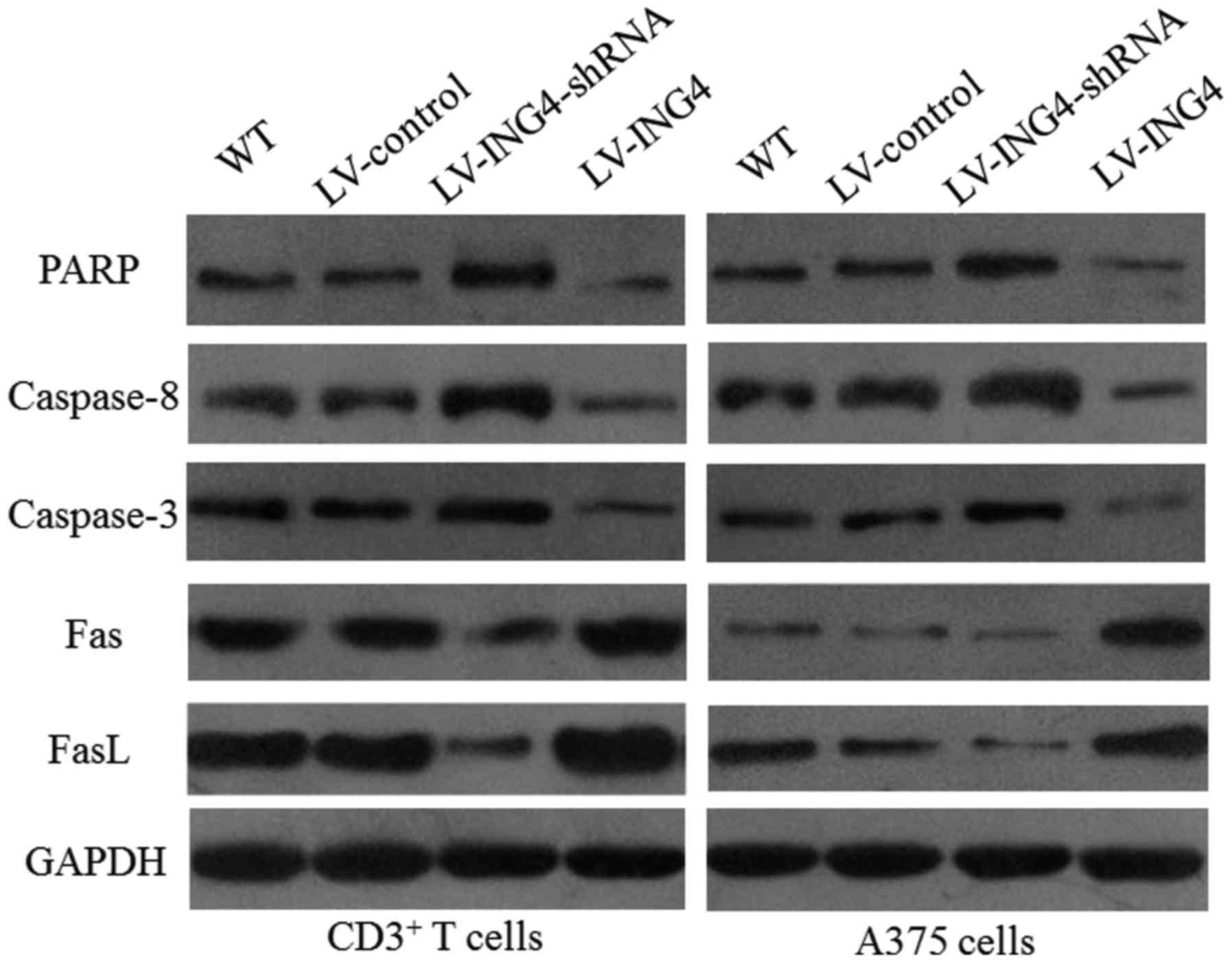 | Figure 4ING4 regulates the expression of
apoptotic proteins. Total proteins were extracted from differently
treated melanoma A375 cell lines and assessed by western blotting.
Upregulation of ING4 decreased PARP, caspase-8 and caspase-3
expression, and increased the expression of Fas and FasL.
Expression of GAPDH was used as an internal control. ING4,
inhibitor of growth 4; PARP, poly(ADP-ribose) polymerase; LV,
lentivirus; shRNA, short hairpin RNA; WT, wild-type; Fas, Fas death
receptor; FasL, Fas ligand; GAPFH, glyceraldehyde 3-phosphate
dehydrogenase; CD, cluster of differentiation. |
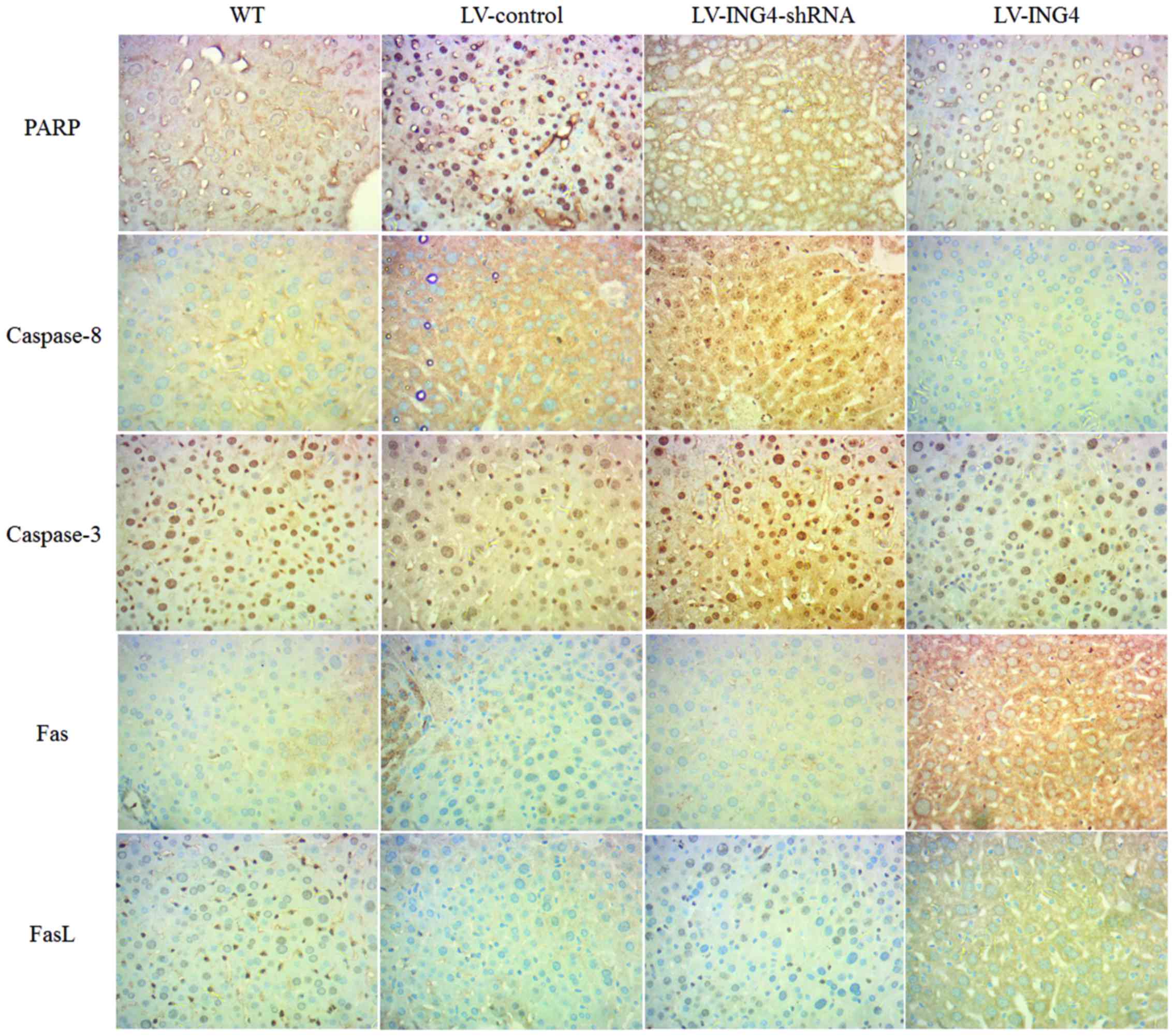 | Figure 5Expression of PARP, caspase-8,
caspase-3, Fas and FasL in nude mouse livers assessed by
immunohistochemistry in the WT, LV-control, LV-ING4-shRNA and
LV-ING4 groups. ING4, inhibitor of growth 4; PARP, poly(ADP-ribose)
polymerase; LV, lentivirus; shRNA, short hairpin RNA; WT,
wild-type; Fas, Fas death receptor; FasL, Fas ligand. |
Discussion
ING4, a novel member of the conserved ING family,
has been identified as a critical tumor suppressor in various
cancer types. Previous studies suggested that knockdown of ING4
gene expression or ING4 gene deletion was associated with the
progression or poor prognosis of high-grade tumors, such as those
in lung cancer (20,21), brain tumors (19,22,23) and those in ovarian cancer
(24). In addition, previous
findings have shown that ING4 expression is significantly reduced
in human malignant melanomas, and that through regulating different
signaling pathways, ING4 level is closely associated with the
proliferation, apoptosis, invasion, metastasis and survival of
malignant melanomas cells (11).
Meanwhile, a previous study showed that ING4 could significantly
reduce tumor cell growth via the regulation of cell cycle
progression according to experiments where exogenous ING4 was
transfected into a lung cancer cell line (17). We hypothesized that ING4 could
play an inhibitory role by inducing cell apoptosis in human
malignant melanomas. The present study was designed to elucidate
the possible mechanisms of the ING4 expression involved in the
induction of A375 cell apoptosis.
Apoptosis or programmed cell death consists of the
ordered disassembly of the cell from within, as opposed to necrosis
or accidental cell death (25).
Cellular immunity plays an important role in the antitumor immune
response. CD3+ T cells recognize peptides of exogenous
antigens, which are presented by major histocompatibility complex
class II molecules. Through the release of cytokines,
CD3+ T cells activate natural killer cells, enhance the
cytotoxicity of effector cells and increasing the sensitivity of
cytotoxic T lymphocytes to target cells, and subsequently lyse
tumor cells with perforin or by inducing cell apoptosis (26). To reveal the apoptotic effect of
ING4 in melanoma A375 cells and CD3+ T cells, dual
staining with Annexin V-FITC/7-AAD was used to measure cell
apoptosis. It was found that ING4 overexpression could induce
melanoma A375 cells and CD3+ T-cell apoptosis
significantly (Fig. 3). Apoptosis
may be induced through the extrinsic pathway (activation of cell
surface death receptors) or the intrinsic pathway (alterations in
the integrity of the mitochondrial membrane that induce the release
of cytochrome c). These pathways converge at the level of
the effector caspases (caspase-3, -6, -7 and -8) (27). Once activated, these effector
caspases cleave cytoskeletal and nuclear proteins, including PARP,
thereby initiating cellular disassembly (27). The present study demonstrated that
PARP, caspase-8 and caspase-3 were decreased in response to
overexpression of ING4 in melanoma cells (Figs. 4 and 5), thereby supporting the premise that
cell death occurs in a manner consistent with apoptosis.
Cell death induced by Fas/FasL interaction results
in apoptotic signaling in numerous different cell types (28). Fas, FasL and caspase-8 play
important roles in the regulation of apoptosis induction (29). In the present study, the data
showed that overexpression of ING4 markedly activated Fas and FasL
proteins in melanoma cells and CD3+ T cells (Figs. 4 and 5).
Furthermore, the results suggested that
overexpression of ING4 level enhances the apoptosis of A375 cells,
which may contribute to a close association with Fas/FasL pathway
activation (Figs. 4 and 5). To the best of our knowledge, this
study shows that ING4 functions as a tumor suppressor protein in
human melanomas. It validates the theory that ING4 can serve as a
prognostic marker and a therapeutic target in human melanomas.
In conclusion, in the present study, vectors LV-ING4
and LV-ING4-shRNA were generated and the effect of overexpression
of ING4 on the apoptosis of human melanoma A375 cells and
CD3+ T cells was investigated. The results demonstrated
that the high expression level of ING4 could significantly increase
melanomas cell apoptosis via the Fas/FasL pathway activation, and
also induce the apoptosis of CD3+ T cells. This
indicates that ING4 gene therapy could be considered as a novel
approach to treat human melanomas.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81072234).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubin KM and Lawrence DP: Your patient
with melanoma: Staging, prognosis, and treatment. Oncology
(Williston Park). 23(Suppl 8): 13–21. 2009.
|
|
3
|
Grossman D and Altieri DC: Drug resistance
in melanoma: Mechanisms, apoptosis, and new potential therapeutic
targets. Cancer Metastasis Rev. 20:3–11. 2001. View Article : Google Scholar
|
|
4
|
La Porta CA: Mechanism of drug sensitivity
and resistance in melanoma. Curr Cancer Drug Targets. 9:391–397.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alexandrescu DT, Ichim TE, Riordan NH,
Marincola FM, Di Nardo A, Kabigting FD and Dasanu CA: Immunotherapy
for melanoma: current status and perspectives. J Immunother.
33:570–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korn EL, Liu P-Y, Lee SJ, Chapman JA,
Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer
EA, et al: Meta-analysis of phase II cooperative group trials in
metastatic stage IV melanoma to determine progression-free and
overall survival benchmarks for future phase II trials. J Clin
Oncol. 26:527–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gajewski TF: Identifying and overcoming
immune resistance mechanisms in the melanoma tumor
microenvironment. Clin Cancer Res. 12:2326s–2330s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coles AH and Jones SN: The ING gene family
in the regulation of cell growth and tumorigenesis. J Cell Physiol.
218:45–57. 2009. View Article : Google Scholar
|
|
9
|
Gunduz M, Gunduz E, Rivera RS and
Nagatsuka H: The inhibitor of growth (ING) gene family: Potential
role in cancer therapy. Curr Cancer Drug Targets. 8:275–284. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiseki M, Nagashima M, Pedeux RM,
Kitahama-Shiseki M, Miura K, Okamura S, Onogi H, Higashimoto Y,
Appella E, Yokota J, et al: p29ING4 and p28ING5 bind to p53 and
p300, and enhance p53 activity. Cancer Res. 63:2373–2378.
2003.PubMed/NCBI
|
|
11
|
Li J, Martinka M and Li G: Role of ING4 in
human melanoma cell migration, invasion and patient survival.
Carcinogenesis. 29:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friesen C, Herr I, Krammer PH and Debatin
K-M: Involvement of the CD95 (APO-1/FAS) receptor/ligand system in
drug-induced apoptosis in leukemia cells. Nat Med. 2:574–577. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hahne M, Rimoldi D, Schröter M, Romero P,
Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard
D, et al: Melanoma cell expression of Fas(Apo-1/CD95) ligand:
Implications for tumor immune escape. Science. 274:1363–1366. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bullani RR, Wehrli P, Viard-Leveugle I,
Rimoldi D, Cerottini JC, Saurat JH, Tschopp J and French LE:
Frequent downregulation of Fas (CD95) expression and function in
melanoma. Melanoma Res. 12:263–270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Helmbach H, Rossmann E, Kern MA and
Schadendorf D: Drug-resistance in human melanoma. Int J Cancer.
93:617–622. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ekmekcioglu S, Okcu MF, Colome-Grimmer MI,
Owen-Schaub L, Buzaid AC and Grimm EA: Differential increase of Fas
ligand expression on metastatic and thin or thick primary melanoma
cells compared with interleukin-10. Melanoma Res. 9:261–272. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Cai L, Liang M, Wang Y, Yang J and
Zhao Y: ING4 induces cell growth inhibition in human lung
adenocarcinoma A549 cells by means of Wnt-1/β-catenin signaling
pathway. Anat Rec (Hoboken). 291:593–600. 2008. View Article : Google Scholar
|
|
18
|
Clay TM, Custer MC, Sachs J, Hwu P,
Rosenberg SA and Nishimura MI: Efficient transfer of a tumor
antigen-reactive TCR to human peripheral blood lymphocytes confers
anti-tumor reactivity. J Immunol. 163:507–513. 1999.PubMed/NCBI
|
|
19
|
Li X, Cai L, Chen H, Zhang Q, Zhang S,
Wang Y, Dong Y, Cheng H and Qi J: Inhibitor of growth 4 induces
growth suppression and apoptosis in glioma U87MG. Pathobiology.
76:181–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang QS, Li M, Zhang LY, Jin Y, Tong DD,
Yu Y, Bai J, Huang Q, Liu FL, Liu A, et al: Downregulation of ING4
is associated with initiation and progression of lung cancer.
Histopathology. 57:271–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Zhang Q, Cai L, Wang Y, Wang Q,
Huang X, Fu S, Bai J, Liu J, Zhang G, et al: Inhibitor of growth 4
induces apoptosis in human lung adenocarcinoma cell line A549 via
Bcl-2 family proteins and mitochondria apoptosis pathway. J Cancer
Res Clin Oncol. 135:829–835. 2009. View Article : Google Scholar
|
|
22
|
Garkavtsev I, Kozin SV, Chernova O, Xu L,
Winkler F, Brown E, Barnett GH and Jain RK: The candidate tumour
suppressor protein ING4 regulates brain tumour growth and
angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen JC, Unoki M, Ythier D, Duperray A,
Varticovski L, Kumamoto K, Pedeux R and Harris CC: Inhibitor of
growth 4 suppresses cell spreading and cell migration by
interacting with a novel binding partner, liprin α1. Cancer Res.
67:2552–2558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Yu L, Wang Y, Zhang Y, Wang Y and
Zhang G: Expression of tumor suppressor gene ING4 in ovarian
carcinoma is correlated with microvessel density. J Cancer Res Clin
Oncol. 138:647–655. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan FK-M, Luz NF and Moriwaki K:
Programmed necrosis in the cross talk of cell death and
inflammation. Annu Rev Immunol. 33:79–106. 2015. View Article : Google Scholar :
|
|
26
|
Chen G and Emens LA: Chemoimmunotherapy:
Reengineering tumor immunity. Cancer Immunol Immunother.
62:203–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hohlbaum AM, Moe S and Marshak-Rothstein
A: Opposing effects of transmembrane and soluble Fas ligand
expression on inflammation and tumor cell survival. J Exp Med.
191:1209–1220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagata S and Golstein P: The Fas death
factor. Science. 267:1449–1456. 1995. View Article : Google Scholar : PubMed/NCBI
|