Introduction
Myocardial infarction (MI) is among the main causes
of mortality worldwide (1).
Irreversible loss of cardiac myocytes and concomitant cicatrization
are induced by MI; therefore, patients exhibit poor cardiac pump
function and congestive heart failure. Cell-based therapy for MI
represents an emerging strategy in biological therapeutics
(2,3). As one of the most frequently
investigated cellular populations, bone marrow-derived mesenchymal
stem cells (MSCs) are particularly attractive therapeutic
candidates. MSC-based therapy relies on the self-renewal capability
of MSCs, and the ability of MSCs to differentiate into
cardiovascular cells and secrete multitudinous bioactive molecules.
These actions subsequently activate endogenous neovascularization,
immunomodulation and cardiac regeneration, thus resulting in
restoration of cardiac function (4). However, current evidence indicates
that poor viability of engrafted MSCs in the infarcted myocardium
is a primary limitation of the therapeutic efficacy of MSCs
(5,6). Reactive oxygen species (ROS),
including hydrogen peroxide (H2O2),
superoxide radicals and hydroxyl radicals, are produced during
infarction (7) or reperfusion of
ischemic hearts (8). ROS may lead
to impaired cell metabolism and decreased cell viability, thus
inhibiting transplanted MSCs from taking effect. Therefore,
protecting MSCs from apoptosis, together with enhancing their
ability to survive under oxidative stress, is crucial for
optimizing MSC-based therapy.
Polyphenols, or polyphenolic compounds, are widely
distributed in natural plants, and range from simple structures,
such as flavonoids, to highly complex polymeric substances,
including proanthocyanidins and ellagitannins (9). Due to their various biological
activities, polyphenols exhibit potential as effective therapeutic
drugs. In addition, they have been demonstrated to display numerous
pharmacological activities, including anticarcinogenic (10), antibacterial (11) and antidiabetic (12) effects. Furthermore, polyphenols
are strong antioxidants, due to their free radical-scavenging
activities (13). Geraniin is a
typical ellagitannin, which has been identified as the major active
compound extracted from Geranium sibiricum. A previous study
reported that geraniin possesses marked nitric oxide-scavenging,
superoxide radical-scavenging and β-carotene-linoleic
acid-bleaching properties due to its unique chemical structure
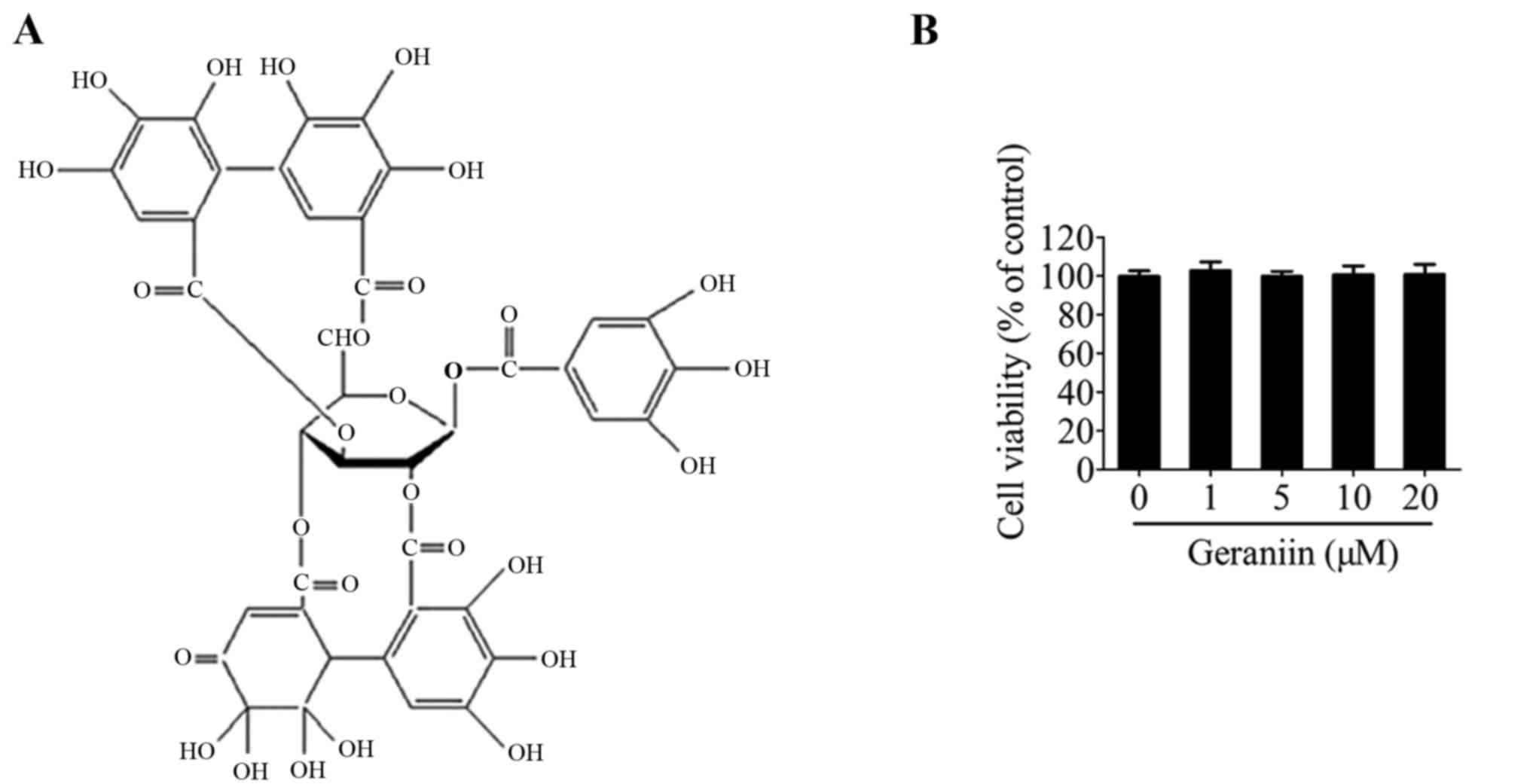
(Fig. 1A) (14). Furthermore, geraniin has been
confirmed to protect liver cells against ethanol-induced
cytotoxicity (15) and inhibit
apoptosis of pulmonary fibroblasts under γ-radiation conditions
(16). Our previous study
demonstrated that geraniin may exert strong ROS-scavenging
activities when preventing THP-1 macrophages from switching to an
M1 phenotype under lipopolysaccharide stimulation (17). Since H2O2 is
often used in vitro to simulate the oxidative stress
microenvironment detected in ischemic heart tissue, the present
study hypothesized that geraniin may defend MSCs against
H2O2-induced damage. The present study aimed
to investigate the cytoprotective effects of geraniin on MSCs
against H2O2-induced cellular injury, as well
as the underlying mechanism.
Materials and methods
Materials
Geraniin (purity ≥98%) was purchased from Shanghai
Yuanye Bio-Technology Co., Ltd. (Shanghai, China), and was
dissolved in dimethyl sulfoxide (DMSO) and stored at −80°C. The
stock concentration of geraniin was 10 mM. DMSO and
H2O2 were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Dulbecco's modified Eagle's medium
(DMEM)/F12 and fetal bovine serum (FBS) were obtained from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The conjugated
antibodies used to identify MSCs: Fluorescein isothiocyanate
(FITC)-labeled anti-CD29 (555005) and anti-CD44 (561859), and
phycoerythrin-labeled anti-CD45 (553091) and anti-CD90 (551401), as
well as the Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit, were all purchased from BD Biosciences (Franklin
Lakes, NJ, USA). Cell Counting kit-8 (CCK-8) was from Dojindo
Molecular Technologies, Inc. (Kumamoto, Japan). Hoechst 33342, JC-1
dye, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA)
fluorescent probe, glutathione (GSH) kit (S0053), malondialdehyde
(MDA) kit (S0131), ROS scavenger N-acetyl-L-cysteine (NAC), cell
mitochondrial protein isolation kit (C3601),
radioimmunoprecipitation assay (RIPA) lysis buffer, bicinchoninic
acid (BCA) protein assay kit and mouse polyclonal anti-β-actin
(AA128) were all purchased from Beyotime Institute of Biotechnology
(Beijing, China). Rabbit antibodies against phosphorylated-protein
kinase B [p-Akt (Ser473); 4060s], Akt (9272s), caspase-3 (9662s),
B-cell lymphoma 2 (Bcl-2; 2876s), Bcl-2-associated X protein (Bax;
2772s) and cytochrome c (Cyt C; 4272s), and LY294002
[phosphoinositide 3-kinase (PI3K) specific inhibitor], were all
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish peroxidase (HRP)-conjugated anti-rabbit (ZB-2301) and
anti-mouse (ZB-2305) secondary antibodies were obtained from
OriGene Technologies, Inc., (Beijing, China).
MSCs isolation and culture
MSCs were isolated and harvested from male
Sprague-Dawley rats (age, 3 weeks old; weight, 60–80 g), as
previously described with minor modifications (18). A total of 10 SPF Sprague-Dawley
rats were purchased from the Laboratory Animal Science Department
of the Second Affiliated Hospital of Harbin Medical University
(Harbin, China). They were kept under standard animal housing
conditions (temperature, 21±1°C; humidity, 55±5%), at a 12-h
dark/light cycle and had access to unlimited food and water. The
present study was approved by the Local Ethics Committee on Animal
Care and Use of Harbin Medical University. Briefly, total bone
marrow was flushed from the tibias and femurs of the rats with 10
ml DMEM/F12 using a sterile syringe. After centrifugation at 300 ×
g for 5 min, the remaining pellets were resuspended in 5 ml
DMEM/F12 supplemented with 10% FBS and 1% penicillin/streptomycin,
and were then seeded into cell culture flasks at 37°C in an
atmosphere containing 5% CO2. Following incubation for
48 h, the culture medium and non-adherent cells were discarded and
fresh medium was added. The medium was replaced every 72 h
thereafter. Cells were cultured until they reached 80% confluence,
after which they were passaged. Cells were split using 0.25%
trypsin and were expanded at a 1:2 or 1:3 dilution. Cells from
passages 3–5 were used in the subsequent experiments. The MSC
population was characterized according to positive (CD44, CD29 and
CD90) and negative (CD45) cell surface markers by flow cytometry,
as reported in our previous studies (18,19).
Cell treatments
All treatments were conducted at 37°C in the
incubator. MSCs were seeded into six-well plates or a 25
cm2 culture flask. Once cell density reached 60–70%,
H2O2 (100, 200, 300, 400 and 500 µM),
mixed with serum-free DMEM/ F12 for 4 h, was used to establish an
in vitro oxidative stress model. Geraniin, at 1, 5, 10 and
20 µM, was separately preincubated in DMEM/F12 for 24 h. The
inhibitor of PI3K, LY294002 (25 µM), or the ROS scavenger,
NAC (500 µM), was added 1 h prior to
H2O2 treatment without geraniin co-treatment.
Cells cultured in complete medium without any specific treatment
comprised the control group.
Cell viability assay
MSC viability was assessed using the CCK-8 assay.
Briefly, cells were plated into 96-well plates (3×103
cells/well). Following cell adhesion to the plates, appropriate
treatments were administered. Subsequently, the medium was removed
and replaced with 100 µl fresh DMEM/F12 and 10 µl
CCK-8 solution in each well. The plates were maintained at 37°C for
1 h. Finally, absorbance was detected at 450 nm using a Tecan
Infinite 200 PRO microplate reader (Tecan Austria GmbH, Grödig,
Austria).
Measurement of cell apoptosis
Apoptosis of MSCs was determined using the Annexin
V-FITC/PI staining method. Following treatment, the cells were
harvested, washed with ice-cold PBS and resuspended in 400
µl binding buffer. The cell suspension was then incubated
with 5 µl Annexin V solution for 15 min at room temperature
in the dark, followed by incubation with 5 µl PI for an
additional 5 min. The cells were immediately analyzed by flow
cytometry using BD FACSCanto II (BD Biosciences). Approximately
1×105 cells were detected in each sample. According to
the reaction principles, Annexin V−/PI−
staining signified viable cells, Annexin
V+/PI− indicated early apoptotic cells, and
Annexin V+/PI+ represented late apoptotic or
necrotic cells.
Assessment of morphological
alterations
MSCs were treated with geraniin and
H2O2 in six-well plates. Hoechst 33342 was
used to detect cell nuclear condensation and fragmentation. After
fixing in 4% paraformaldehyde for 15 min at room temperature, cells
were washed twice with PBS, stained with 5 µg/ml Hoechst
33342 for 5 min and washed a further two times with PBS. Finally,
cells were assessed by fluorescence microscopy. Apoptotic cells
were identified by condensed or fragmented nuclei.
ROS, GSH and MDA assays
Intracellular ROS levels were determined using a ROS
assay kit. Briefly, cells were incubated with the diluted
fluorescent probe, DCFH-DA, for 20 min at 37°C. Cells were then
washed three times with serum-free DMEM/F12, collected and analyzed
using a flow cytometer. Due to the important roles of GSH and MDA
in ROS-associated oxidative stress, their concentrations were also
measured using commercial kits according to the manufacturer's
protocols.
Detection of mitochondrial membrane
potential (Ψm)
JC-1 was used to measure alterations in Ψm. Briefly,
cells were washed with PBS, stained with 5 µM JC-1 and
maintained for 20 min at 37°C. Subsequently, cells were washed
twice with ice-cold JC-1 staining buffer and were then directly
observed under a fluorescence microscope, or were collected and
analyzed by flow cytometry.
Protein extraction and western blot
analysis
Cells were washed with ice-cold PBS, lysed with RIPA
lysis buffer and centrifuged at 12,000 × g for 15 min at 4°C. The
extraction of cytoplasmic and mitochondrial proteins was conducted
in accordance with the manufacturer's protocol. BCA protein assay
was used to quantify protein concentrations. Equal amounts of total
protein (50 µg/ lane) were separated by 8–12% SDS-PAGE and
were transferred onto polyvinylidene fluoride membranes. The
membranes were blocked with 5% skim milk diluted in Tris-buffered
saline containing 0.05% Tween-20 (TBST) at 37°C for 1 h, and were
then incubated with diluted primary antibodies against p-Akt (S473)
(1:1,000), total-Akt (1:1,000), cleaved caspase-3 (1:1,000), Bax
(1:1,000), Bcl-2 (1:1,000), Cyt C (1:1,000) and β-actin
(1:800) overnight at 4°C. After washing three times with TBST,
membranes were incubated with the corresponding HRP-conjugated
secondary antibodies (1:5,000) for 1 h at room temperature. The
images of the immune complexes were developed by ECL in the dark,
and images were captured using a Tanon-5200 (Tanon Science and
Technology Co., Ltd., Shanghai, China). Band density was determined
using ImageJ (1.48u; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All data are presented as the mean ± standard
deviation and were analyzed using SPSS 19.0 software (IBM Corp.,
Armonk, NY, USA). Differences between groups were analyzed by
one-way ANOVA with a Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of geraniin on MSC viability
Initially, the effects of geraniin on MSC viability
were examined using the CCK-8 assay. Treatment with geraniin for 24
h had little influence on cell viability compared with the 0
µM geraniin group (Fig.
1B). This result indicated that geraniin did not exert toxic
effects on MSCs.
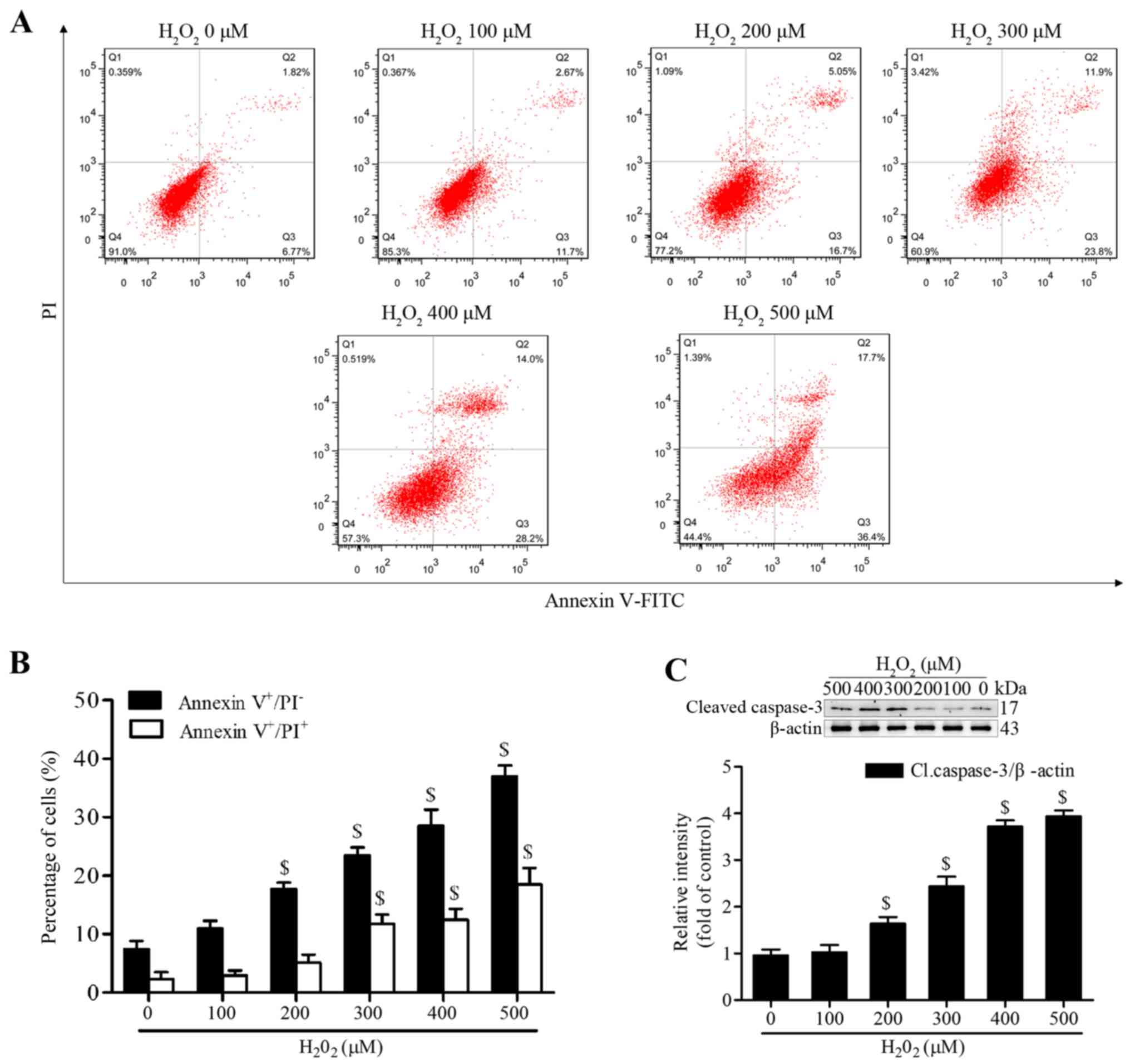
Geraniin significantly inhibits
H2O2-induced apoptosis of MSCs
H2O2 has been reported to
induce apoptosis of MSCs at various concentrations and time-points
(20,21). To establish a successful in
vitro oxidative stress model, the present study investigated
the proapoptotic effects of H2O2 on MSCs.
Following treatment with various concentrations of
H2O2 (100–500 µM) in serum-free medium
for 4 h, MSCs were stained with Annexin V-FITC/PI. The proportion
of early apoptotic cells (Annexin V+/PI−) was
significantly increased following treatment with ≥200 µM
H2O2, whereas the proportion of late
apoptotic or necrotic MSCs (Annexin V+/PI+)
was markedly increased following treatment with ≥300 µM
(Fig. 2A and B). In addition, the
expression levels of cleaved caspase-3 were significantly increased
at 200 µM and continued to increase to 500 µM
(Fig. 2C). We then selected
treatment with H2O2 at 300 µM for 4 h
as the condition to induce effective apoptosis, since this
concentration generated moderate apoptotic cells.
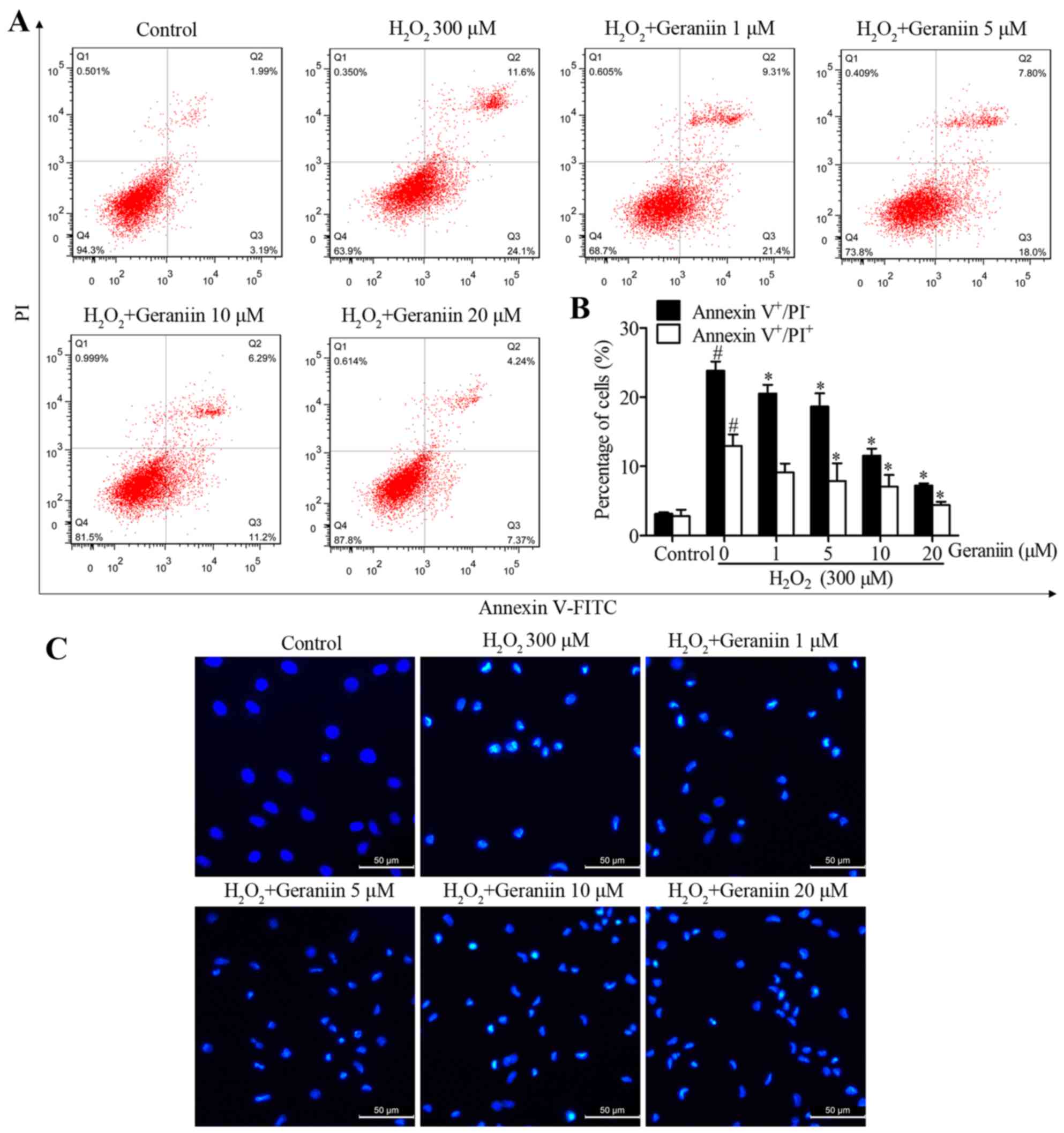
To determine whether geraniin rescues MSCs from
H2O2-induced apoptosis, MSCs were pretreated
with increasing concentrations of geraniin (1, 5, 10 and 20
µM) for 24 h. MSCs were then co-treated with geraniin and
H2O2. A significant reversal in the
percentage of H2O2-induced Annexin
V+/PI− cells, in response to geraniin, was
observed by flow cytometry (geraniin 1 µM, 20.53±1.25%; 5
µM, 18.67±1.89%; 10 µM, 11.57±1.01%; 20 µM,
7.23±0.31%; P<0.05 vs. H2O2, 23.83±1.32%)
(Fig. 3A and B). However, 1
µM geraniin had no effect on the proportion of Annexin
V+/PI+ cells, whereas the other
concentrations significantly reduced the percentage of late
apoptotic or necrotic cells (geraniin 5 µM, 7.88±2.58%; 10
µM, 7.05±1.72%; 20 µM, 4.40±0.48%; P<0.05 vs.
H2O2, 12.97±1.65%) (Fig. 3A and B). Furthermore, in apoptotic
cells, nuclei become condensed or fragmented, whereas in normal
cells, nuclei are circular or oval. Hoechst 33342 staining was used
to confirm the presence of nuclear morphological alterations. Cells
treated with H2O2 appeared to possess
shrunken and fragmented nuclei; however, those pretreated with
geraniin exhibited marked amelioration of
H2O2-induced nuclear impairment. These data
indicated that geraniin may effectively attenuate
H2O2-induced MSC apoptosis (Fig. 3C).
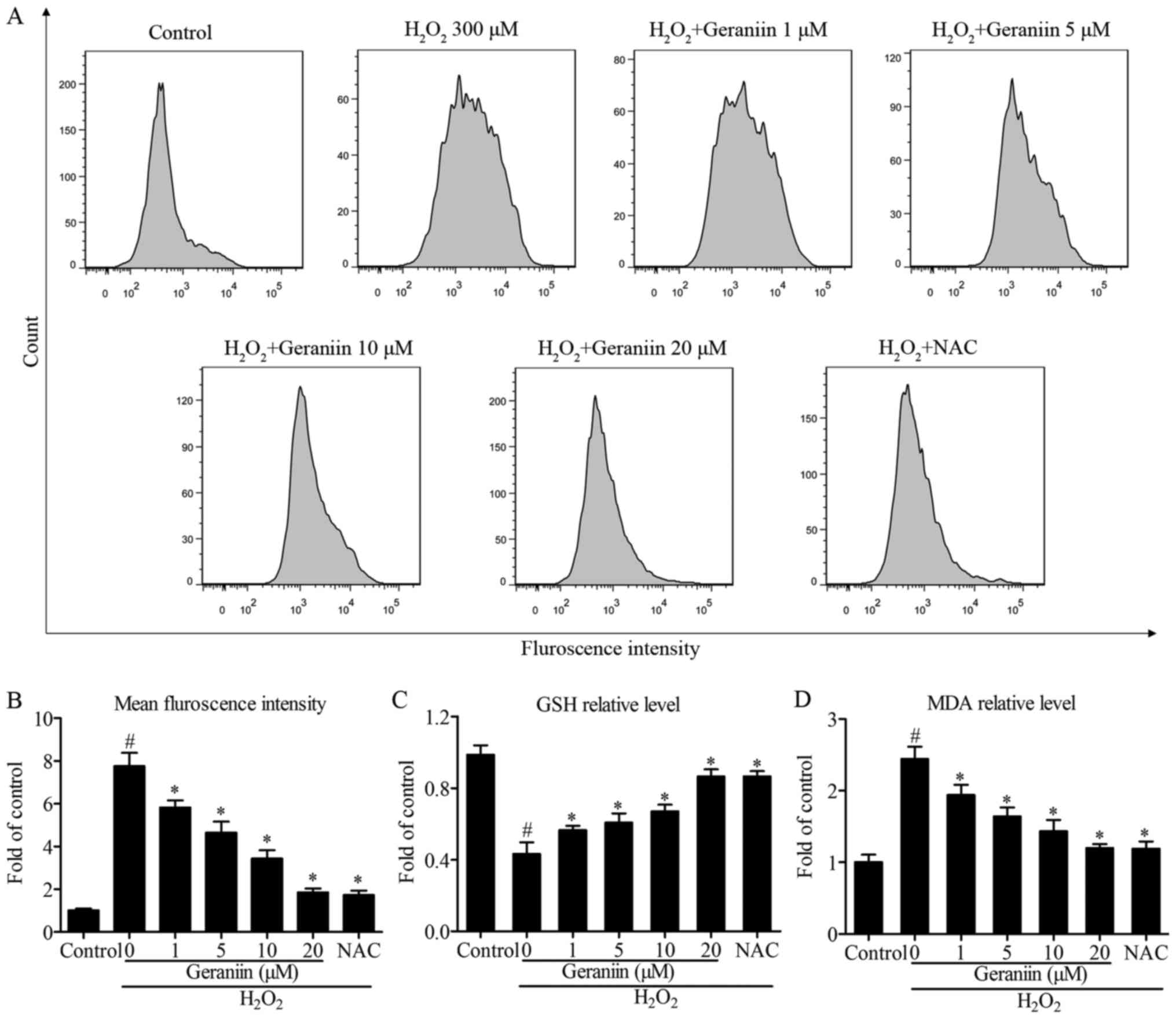
Geraniin exerts protective effects by
regulating ROS genera- tion
Since ROS serves a pivotal role in proapoptotic
signaling cascades (22), the
present study examined the effects of geraniin on ROS generation
using flow cytometry. The results demonstrated that
H2O2 induced a 7.6-fold increase in ROS
production compared with the control group. However, pretreatment
with geraniin markedly suppressed ROS generation in a
concentration-dependent manner (Fig.
4A and B). Following treatment with NAC, a general ROS
scavenger, similar results were recorded compared with 20 µM
geraniin (mean fluorescence intensity: Geraniin 20 µM,
476.33±46.65; NAC, 443.80±53.15, P>0.05) (Fig. 4B). Furthermore, alterations in
intracellular GSH and MDA contents were investigated; cells
pretreated with geraniin had significantly increased GSH levels,
whereas MDA production was suppressed by geraniin (Fig. 4C and D). These findings indicated
that geraniin is able to enhance the cellular antioxidant system
and remove redundant ROS.
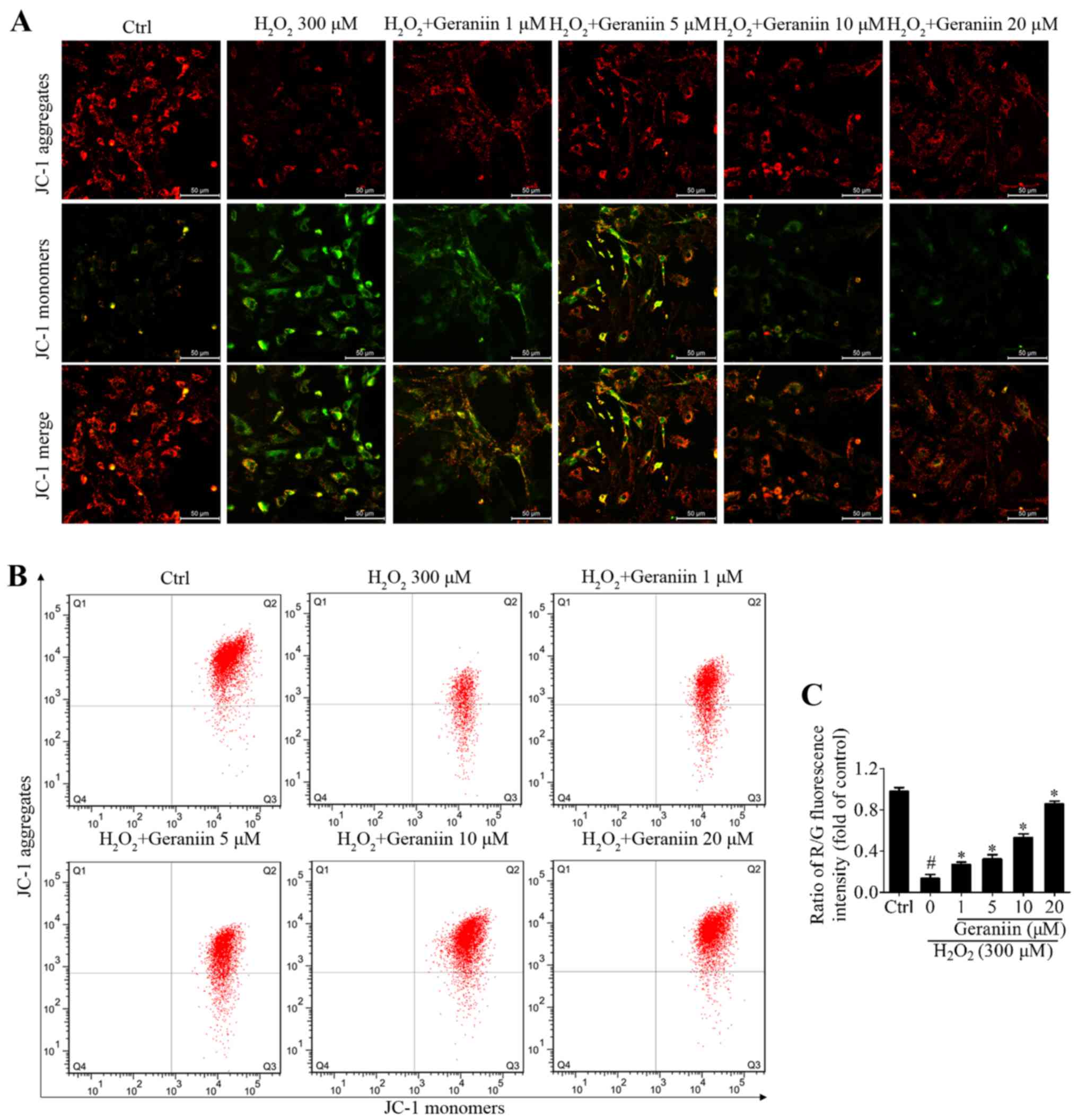
Geraniin protects MSCs against oxidative
stress through stabilizing the Ψm
Mitochondria in eukaryotic cells are the primary
components of respiration, and are critical in the defense against
oxidative stress-induced damage (23). Maintaining the Ψm is essential to
ensure the scavenging efficiency of ROS, and to prevent cell
apoptosis or other stress-associated events induced by excessive
ROS (24). JC-1 is a Ψm-sensitive
dye, which aggregates in the mitochondrial matrix and exhibits red
fluorescence in normal cells. However, when the Ψm is reduced, JC-1
is converted to its monomer state, which exhibits green
fluorescence. H2O2 resulted in a marked
reduction in Ψm within MSCs, whereas geraniin markedly upregulated
the Ψm, as identified by fluorescence microscopy (Fig. 5A). In addition, the ratio of
red/green fluorescence intensity was significantly downregulated
under H2O2 exposure compared with in the
control group; however, this effect was reversed by geraniin in a
concentration-dependent manner (Fig.
5B and C). These findings suggested that geraniin may exert
beneficial effects on mitochondrial function.
PI3K/Akt signaling is required for
geraniin to exert anti-apoptotic effects on MSCs
It has previously been reported that geraniin exerts
cytoprotective effects on HepG2 cells via activation of the
extracellular signal-regulated kinase 1/2 and PI3K/Akt pathways
(25). Due to the importance of
the PI3K/Akt pathway on classical survival signals (26), the present study aimed to
determine the association between geraniin and the PI3K/Akt pathway
in MSCs. Cells were treated with geraniin (20 µM) for the
indicated periods of time; the protein expression levels of p-Akt
(Ser473) were transiently upregulated at 15 min and peaked at 60
min, prior to subsequent downregulation (Fig. 6A). In addition, the effects of 1 h
treatment with various concentrations of geraniin on p-Akt (Ser473)
expression in MSCs was investigated; p-Akt expression was
upregulated in a dose-dependent manner. Conversely, p-Akt
expression was markedly inhibited following pretreatment with
LY294002, a PI3K-specific inhibitor (Fig. 6B). These findings indicated that
geraniin may activate the PI3K/Akt signaling pathway in a time- and
dose-dependent manner.
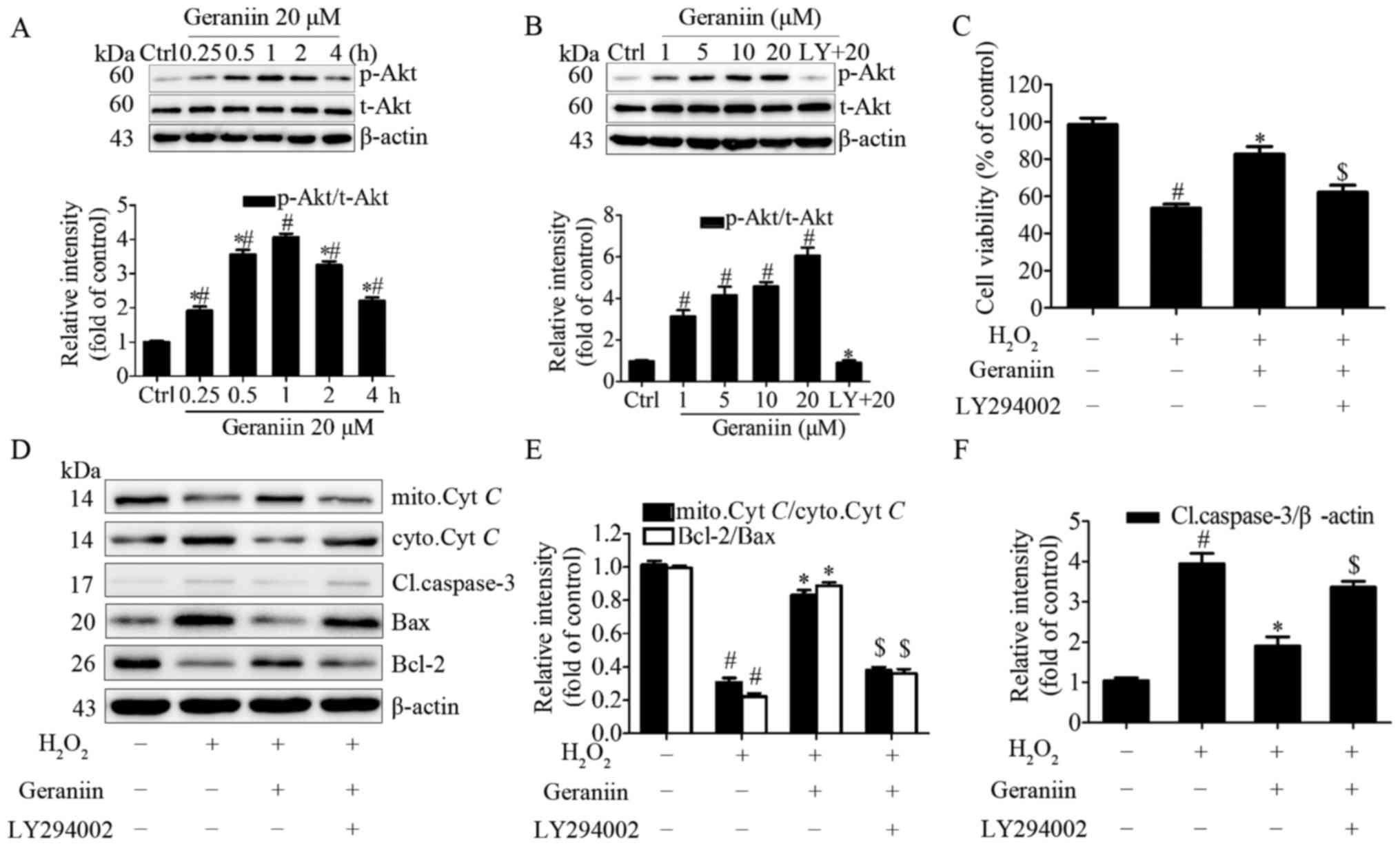 | Figure 6PI3K/Akt signaling is involved in the
anti-apoptotic effects of geraniin on MSCs. (A) Cells were treated
with geraniin (20 µM) for the indicated time-periods or (B)
with the indicated concentrations of geraniin for 1 h. The
expression levels of p-Akt were evaluated by western blotting. (C)
Cells were pretreated with LY294002, and were then incubated with
geraniin and H2O2; cell viability was
measured using the Cell Counting kit-8 assay. Expression levels of
proteins associated with the mitochondrial apoptosis pathway were
assessed (D) by western blotting and (E and F) data were
semi-quantified. Data are presented as the mean ± standard
deviation of three independent experiments. #P<0.05
compared with the control group; *P<0.05 compared
with the group treated with 20 µM geraniin for 1 h (A and
B); *P<0.05 compared with the
H2O2 group (C-F); $P<0.05
compared with the geraniin + H2O2 group. Akt,
protein kinase B; Bax, Bcl-2-associated X protein; Bcl-2, B-cell
lymphoma 2; Cl.caspase-3, cleaved caspase-3; ctrl, control;
cyto.Cyt C, cytoplasmic cytochrome c;
H2O2, hydrogen peroxide; LY, LY294002;
mito.Cyt C, mitochondrial cytochrome c; p-,
phosphorylated; t-, total. |
To gain further insight into the role of the
PI3K/Akt pathway in the protective effects of geraniin on MSCs,
cells were preconditioned with LY294002 and were then exposed to 20
µM geraniin and H2O2. PI3K inhibition
significantly attenuated the anti-apoptotic effects of geraniin on
MSCs under H2O2 treatment, as evidenced by a
decrease in cell survival rate using CCK-8 assay (Fig. 6C). Since reductions in the Ψm and
increased cleaved caspase-3 expression are initiating and
amplifying factors of the mitochondrial apoptosis pathway, it may
be suggested that H2O2 induces apoptosis of
MSCs through regulating the mitochondrial apoptosis pathway.
Therefore, the expression levels of Cyt C, cleaved
caspase-3, Bax and Bcl-2 were investigated. Treatment with geraniin
induced a marked increase in the expression levels of mitochondrial
Cyt C and Bcl-2, and a decrease in the expression levels of
cytoplasmic Cyt C, cleaved caspase-3 and Bax (Fig. 6D–F). These effects were reversed
by LY294002. These data indicated that the PI3K/Akt signaling
pathway may contribute to the prosurvival role of geraniin in MSCs
under H2O2 treatment.
Discussion
The present study demonstrated that geraniin could
attenuate H2O2-induced cell damage through
promoting MSC survival, reducing cellular ROS production and
maintaining mitochondrial function. Furthermore, the effects of
geraniin were mediated by activating the PI3K/Akt signaling
pathway. Conversely, inhibition of the PI3K/Akt pathway weakened
the protective effects of geraniin. To the best of our knowledge,
the present study is the first to report the cytoprotective effects
of geraniin on MSCs and reveal the underlying mechanism.
MSCs are easily isolated and expanded, and can be
transplanted in the heart; therefore, they are considered leading
candidates for cellular therapy (27). Substantial data from preclinical
and clinical studies support the cardioprotective effects of MSCs.
Amado et al demonstrated that, following MSC implantation
into swine, reappearance of myocardial tissue and restoration of
cardiac contractility could be detected using serial computed
tomography imaging (28).
Furthermore, the POSEIDON randomized trial demonstrated that
intravenous administration of allogeneic MSCs within 7 days of
acute MI markedly attenuated cardiac hypertrophy, reduced
ventricular arrhythmia, improved heart function and decreased
rehospitalization for cardiac complications (27). However, the practical applications
of MSCs are restricted by their poor survival rate. A previous
study indicated that after 4 days, only 0.44% of engrafted MSCs
survived and resided in the myocardium (29). This low survival rate is mainly
due to the hostile microenvironment in injured heart tissue, and
ROS burst in the infarcted region is the major risk factor
(30). As one of the most stable
ROS, H2O2 is often used to establish an in
vitro oxidative stress model for studies regarding
apoptosis-associated mechanisms (31). In the present study, treatment
with 300 µM H2O2 for 4 h markedly
increased apoptosis of MSCs, thus indicating that exogenous ROS
burst results in the critical inducement of transplanted MSC
apoptosis. In addition, this result reflects the necessity to
identify novel methods to enhance MSC survival upon oxidative
stress injury.
Pharmacological studies have confirmed that
polyphenols are efficacious in treating ischemic heart diseases.
Polyphenols extracted from grapes and wine have been reported to
increase coronary flow in vivo (32). Epigallocatechin gallate, which is
the major polyphenolic compound in green tea, has been revealed to
prevent oxidative stress-induced cardiomyocyte apoptosis in
vitro (33). In addition,
emerging evidence demonstrated that the mechanism of action of
polyphenols is predominantly ascribed to their strong free
radical-scavenging activity. Polyphenols are rich in hydroxyl
groups, which can capture free radicals and inactivate ROS
(34). There are numerous
hydroxyl groups within geraniin, indicating its strong antioxidant
activity. Therefore, the present study detected the antioxidative
effects of geraniin on MSCs exposed to H2O2;
the results confirmed that geraniin pretreatment may reduce
excessive cellular ROS levels and preserve mitochondrial function,
which may contribute to inhibiting
H2O2-induced apoptosis. Furthermore, cells
can defend themselves against oxidative stress through specific
scavenging mechanisms, in which GSH and MDA are vital participants.
GSH is an important intracellular antioxidative mediator that
interacts with redundant ROS and balances cellular oxidation
status. MDA is the end product of cellular lipid peroxidation in
response to ROS damage. The severity of oxidative injury is, to
some degree, reflected by cellular GSH and MDA levels. In the
present study, geraniin was able to restore GSH levels, and
decrease the amount of MDA, thus suggesting that geraniin functions
through enhancing the intrinsic antioxidant repair system to
inhibit ROS production and prevent accumulation of intracellular
oxidative damage. Furthermore, reduced Ψm indicates severe
mitochondrial respiratory chain dysfunction and increased
generation of ROS, which enhances oxidative stress and activates
caspase family-mediated mitochondrial apoptosis (35). The present study indicated that
geraniin treatment increases Bcl-2 expression, and decreases the
expression levels of Bax and cleaved caspase-3, thus suggesting
that geraniin may act on Ψm stabilization. In addition, in normal
cells, Cyt C is located in the mitochondrial inner membrane
and works as an electron transmitter. Numerous proapoptotic
stimuli, including ROS, increase permeability of the outer membrane
and promote mobilization of Cyt C from the mitochondria to
the cytosol. In the cytosol, Cyt C mediates maturation of
the caspase family, including caspase-3, which ultimately induce
cell death (36). In the present
study, geraniin treatment inhibited the release of Cyt C
from the mitochondria to the cytosol, which indirectly indicated
that geraniin preserved mitochondrial membrane integrity. Taken
together, these results revealed that geraniin improved MSC
viability under oxidative stress by enhancing the ability of the
antioxidant defense system to remove excessive ROS, maintaining
mitochondrial function and inhibiting the mitochondrial apoptosis
pathway.
The PI3K/Akt pathway has been reported to serve an
important role in the biological behavior of MSCs in vitro
and in MSC engraftment for ischemic treatment in vivo. A
previous study confirmed that cell survival, proliferation and
migration were enhanced when the PI3K/Akt pathway was activated in
MSCs (21). In addition,
transplantation of MSCs overexpressing Akt preserved normal cardiac
metabolism and pH post-MI (37).
In the present study, the results verified that geraniin could
activate the PI3K/Akt signaling pathway, whereas PI3K inhibition
abolished the effects of geraniin on MSCs. However, inhibiting PI3K
using LY294002 could not absolutely abrogate the protective effects
of geraniin, thus suggesting that other pathways may be involved in
the actions of geraniin. Numerous studies have indicated that
geraniin activates the PI3K/Akt and mitogen-activated protein
kinase (MAPK) pathways (25,38). Whether MAPK or other pathways also
contribute to the beneficial effects of geraniin requires further
study. Furthermore, the stromal cell-derived factor 1/C-X-C
chemokine receptor type 4 (CXCR4) axis serves a vital role in
mobilization and recruitment of MSCs to the infarcted area
(39). PI3K/Akt activation has
also been demonstrated to induce an increase in CXCR4 expression in
MSCs (40). Whether geraniin may
promote CXCR4 expression via the PI3K/Akt pathway remains unknown
and requires further research.
In conclusion, the present study provided
preliminary evidence to suggest that geraniin exerts prosurvival
effects on MSCs against H2O2-induced cellular
oxidative stress, predominantly by activating the PI3K/Akt
signaling pathway. These findings indicated that geraniin may be
considered a potential drug to enhance MSC-based therapeutic
efficacy for the future treatment of MI.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 30800233 to
Y.Z.; grant no. 81330033 to B.Y.).
References
|
1
|
Murray CJ, Vos T, Lozano R, Naghavi M,
Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S,
et al: Disability-adjusted life years (DALYs) for 291 diseases and
injuries in 21 regions, 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2197–2223. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ripa RS, Haack-Sørensen M, Wang Y,
Jørgensen E, Mortensen S, Bindslev L, Friis T and Kastrup J: Bone
marrow derived mesenchymal cell mobilization by granulocyte-colony
stimulating factor after acute myocardial infarction: Results from
the Stem Cells in Myocardial Infarction (STEMMI) trial. Circulatio.
116(Suppl 11): I24–I30. 2007. View Article : Google Scholar
|
|
3
|
Zhang Z, Liang D, Gao X, Zhao C, Qin X, Xu
Y, Su T, Sun D, Li W, Wang H, et al: Selective inhibition of
inositol hexakisphosphate kinases (IP6Ks) enhances mesenchymal stem
cell engraftment and improves therapeutic efficacy for myocardial
infarction. Basic Res Cardiol. 109:4172014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karantalis V and Hare JM: Use of
mesenchymal stem cells for therapy of cardiac disease. Circ Res.
116:1413–1430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisher SA, Zhang H, Doree C, Mathur A and
Martin-Rendon E: Stem cell treatment for acute myocardial
infarction. Cochrane Database Syst Rev. 9:CD0065362015.
|
|
6
|
Geng YJ: Molecular mechanisms for
cardiovascular stem cell apoptosis and growth in the hearts with
atherosclerotic coronary disease and ischemic heart failure. Ann NY
Acad Sci. 1010:687–697. 2003. View Article : Google Scholar
|
|
7
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar
|
|
8
|
Logue SE, Gustafsson AB, Samali A and
Gottlieb RA: Ischemia/ reperfusion injury at the intersection with
cell death. J Mol Cell Cardiol. 38:21–33. 2005. View Article : Google Scholar
|
|
9
|
Quideau S, Deffieux D, Douat-Casassus C
and Pouységu L: Plant polyphenols: Chemical properties, biological
activities, and synthesis. Angew Chem Int Ed Engl. 50:586–621.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang NJ, Shin SH, Lee HJ and Lee KW:
Polyphenols as small molecular inhibitors of signaling cascades in
carcinogenesis. Pharmacol Ther. 130:310–324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marín L, Miguélez EM, Villar CJ and Lombó
F: Bioavailability of dietary polyphenols and gut microbiota
metabolism: Antimicrobial properties. BioMed Res Int. 905215:2015.
View Article : Google Scholar
|
|
12
|
Habtemariam S and Varghese GK: The
antidiabetic therapeutic potential of dietary polyphenols. Curr
Pharm Biotechnol. 15:391–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collins AR: Assays for oxidative stress
and antioxidant status: Applications to research into the
biological effectiveness of polyphenols. Am J Clin Nut. 81(Suppl
1): 261S–267S. 2005.
|
|
14
|
Wu N, Zu Y, Fu Y, Kong Y, Zhao J, Li X, Li
J, Wink M and Efferth T: Antioxidant activities and xanthine
oxidase inhibitory effects of extracts and main polyphenolic
compounds obtained from Geranium sibiricum L. J Agric Food Chem.
58:4737–4743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Londhe JS, Devasagayam TP, Foo LY, Shastry
P and Ghaskadbi SS: Geraniin and amariin, ellagitannins from
Phyllanthus amarus, protect liver cells against ethanol induced
cytotoxicity. Fitoterapia. 83:1562–1568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang KA, Lee IK, Zhang R, Piao MJ, Kim KC,
Kim SY, Shin T, Kim BJ, Lee NH and Hyun JW: Radioprotective effect
of geraniin via the inhibition of apoptosis triggered by
γ-radiation-induced oxidative stress. Cell Biol Toxicol. 27:83–94.
2011. View Article : Google Scholar
|
|
17
|
Liu X, Li J, Peng X, Lv B, Wang P, Zhao X
and Yu B: Geraniin inhibits LPS-induced THP-1 macrophages switching
to M1 phenotype via SOCS1/NF-κB pathway. Inflammation.
39:1421–1433. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang F, Cui J, Lv B and Yu B: Nicorandil
protects mesenchymal stem cells against hypoxia and serum
deprivation-induced apoptosis. Int J Mol Med. 36:415–423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang LL, Liu JJ, Liu F, Liu WH, Wang YS,
Zhu B and Yu B: MiR-499 induces cardiac differentiation of rat
mesenchymal stem cells through wnt/β-catenin signaling pathway.
Biochem Biophys Res Commun. 420:875–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang XY, Fan XS, Cai L, Liu S, Cong XF and
Chen X: Lysophosphatidic acid rescues bone mesenchymal stem cells
from hydrogen peroxide-induced apoptosis. Apoptosis. 20:273–284.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, Li D, Shi C, Xin T, Yang J, Zhou
Y, Hu S, Tian F, Wang J and Chen Y: Effects of exendin-4 on bone
marrow mesenchymal stem cell proliferation, migration and apoptosis
in vitro. Sci Rep. 5:128982015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maryanovich M and Gross A: A ROS rheostat
for cell fate regulation. Trends Cell Biol. 23:129–134. 2013.
View Article : Google Scholar
|
|
23
|
Johnson DT, Harris RA, Blair PV and
Balaban RS: Functional consequences of mitochondrial proteome
heterogeneity. Am J Physiol Cell Physiol. 292:C698–C707. 2007.
View Article : Google Scholar
|
|
24
|
Brand MD, Buckingham JA, Esteves TC, Green
K, Lambert AJ, Miwa S, Murphy MP, Pakay JL, Talbot DA and Echtay
KS: Mitochondrial superoxide and aging: Uncoupling-protein activity
and superoxide production. Biochem Soc Symp. 71:203–213. 2004.
View Article : Google Scholar
|
|
25
|
Wang P, Peng X, Wei ZF, Wei FY, Wang W, Ma
WD, Yao LP, Fu YJ and Zu YG: Geraniin exerts cytoprotective effect
against cellular oxidative stress by upregulation of Nrf2-mediated
antioxidant enzyme expression via I3K/AKT and ERK1/2 pathway.
Biochim Biophys Acta. 1850:1751–1761. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gnecchi M, He H, Liang OD, Melo LG,
Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, et al:
Paracrine action accounts for marked protection of ischemic heart
by Akt-modified mesenchymal stem cells. Nat Med. 11:367–368. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hare JM, Fishman JE, Gerstenblith G,
DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E,
Johnston PV, Brinker JA, et al: Comparison of allogeneic vs
autologous bone marrow-derived mesenchymal stem cells delivered by
transendocardial injection in patients with ischemic
cardiomyopathy: The POSEIDON randomized trial. JAMA. 308:2369–2379.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amado LC, Schuleri KH, Saliaris AP, Boyle
AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, et
al: Multimodality noninvasive imaging demonstrates in vivo cardiac
regeneration after mesenchymal stem cell therapy. J Am Coll
Cardiol. 48:2116–2124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toma C, Pittenger MF, Cahill KS, Byrne BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Urao N and Ushio-Fukai M: Redox regulation
of stem/progenitor cells and bone marrow niche. Free Radic Biol
Med. 54:26–39. 2013. View Article : Google Scholar :
|
|
31
|
Gechev TS and Hille J: Hydrogen peroxide
as a signal controlling plant programmed cell death. J Cell Biol.
168:17–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fitzpatrick DF, Hirschfield SL and Coffey
RG: Endothelium-dependent vasorelaxing activity of wine and other
grape products. Am J Physiol. 265:H774–H778. 1993.PubMed/NCBI
|
|
33
|
Sheng R, Gu ZL, Xie ML, Zhou WX and Guo
CY: EGCG inhibits cardiomyocyte apoptosis in pressure
overload-induced cardiac hypertrophy and protects cardiomyocytes
from oxidative stress in rats. Acta Pharmacol Sin. 28:191–201.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sangeetha P, Das UN, Koratkar R and
Suryaprabha P: Increase in free radical generation and lipid
peroxidation following chemotherapy in patients with cancer. Free
Radic Biol Med. 8:15–19. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Andón FT and Fadeel B: Programmed cell
death: Molecular mechanisms and implications for safety assessment
of nanomaterials. Acc Chem Res. 46:733–742. 2013. View Article : Google Scholar
|
|
36
|
Garrido C, Galluzzi L, Brunet M, Puig PE,
Didelot C and Kroemer G: Mechanisms of cytochrome c release from
mitochondria. Cell Death Differ. 13:1423–1433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gnecchi M, He H, Melo LG, Noiseaux N,
Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ and Ingwall JS:
Early beneficial effects of bone marrow-derived mesenchymal stem
cells overexpressing Akt on cardiac metabolism after myocardial
infarction. Stem Cells. 27:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhai JW, Gao C, Ma WD, Wang W, Yao LP, Xia
XX, Luo M, Zu YG and Fu YJ: Geraniin induces apoptosis of human
breast cancer cells MCF-7 via ROS-mediated stimulation of 38 MAPK.
Toxicol Mech Methods. 26:311–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zaruba MM, Theiss HD, Vallaster M, Mehl U,
Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, et al:
Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac
function after acute myocardial infarction. Cell Stem Cell.
4:313–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC,
Jiang T, Lin MC, Chen JH, Wang B, Zhang R, et al: The chemokine
CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated
VEGF production and tumour angiogenesis via I3K/AKT signalling. J
Pathol. 224:344–354. 2011. View Article : Google Scholar : PubMed/NCBI
|