Introduction
Chronic lower back pain is a worldwide problem that
may lead to loss of physical function, decreased quality of life
and psychological distress (1).
Lower back pain is a multifactorial condition for which
intervertebral disc (IVD) degeneration has been indicated as a
strong etiological factor (1).
Pathological changes associated with IVD degeneration include the
local accumulation of matrix metalloproteinases (MMPs) and the
decreased synthesis of extracellular matrix (ECM) (2–5). A
previous study by the present research team indicated that the
expression of stromal cell-derived factor-1 (SDF-1) and its
receptor, C-X-C chemokine receptor type 4 (CXCR4), is upregulated
in degenerated cartilage endplate (CEP) and nucleus pulposus (NP)
tissue (6). Cells in the CEP and
NP express CXCR4 protein, and there is a positive correlation
between the level of SDF-1 expression and the percentage of
CXCR4-positive cells (6).
Previous studies indicate that the induction of MMP-3, -9 and -13
expression occurs in joint chondrocytes via the combination of
SDF-1 and CXCR4 (7–9). However, whether the SDF-1/CXCR4
pathway induces MMP expression and ECM degradation in the CEP
remains unclear.
In most organs, the main sources of stem cells for
tissue regeneration that maintain a high self-repair capability are
the vascular system and bone marrow (10,11). However, since the IVD is
avascular, the migration of stem cells from blood vessels and bone
marrow to tissue defect sites is restricted by inaccessibility and
distance. Sakai et al (12) established a tail-looping disc
degeneration model in mice. They reported that only limited numbers
of bone marrow-derived mesenchymal stem cells (BMSCs) were
recruited into the IVD during disc degeneration, presumably because
of its avascular nature. Illien-Jünger et al (13) reported that the exposure of IVDs
to degenerative conditions induces the release of factors that
promote BMSC recruitment in ex vivo organ culture. The
migration of mesenchymal stem cells (MSCs) toward the NP mediated
through SDF-1 has been investigated using a disc explant culture
model (14). A hyaluronan-based
hydrogel release system containing SDF-1 was implanted into a
partially nucleotomized bovine disc, and the migration of exogenous
BMSCs through the CEP was significantly enhanced in discs implanted
with SDF-1 hydrogel (14).
As mentioned above, SDF-1 may be an inflammatory
cytokine involved in ECM degradation and also a factor for the
homing of endogenous stem cells into the IVD. Therefore, improved
knowledge concerning the inflammatory and homing effects of SDF-1
is likely to facilitate the development of stem cell therapy for
diseases caused by disc degeneration. The present study was
performed to determine whether the SDF-1/CXCR4 pathway induces MMP
expression in endplate chondrocytes and ECM degradation in CEP
explant culture, and whether SDF-1 implantation into the NP
improves the regeneration process in vivo.
Materials and methods
Materials
Unless otherwise specified, all materials were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Endplate chondrocyte isolation and
culture
Primary endplate chondrocytes were isolated from the
CEPs of young patients (18–29 years old; female to male proportion,
20:80) with fresh burst spinal fractures, for which magnetic
resonance imaging of the IVDs indicated Pfirrmann grade I to II
disc degeneration (15). Sample
collection was realized 1–2 days after fracture; the samples were
collected during surgery in the operation room. All patients
provided signed informed consent for their participation in the
study. The study was approved by the Ethics Committee of the Second
Affiliated Hospital of the School of Medicine of Zhejiang
University (Hangzhou, China). Briefly, the CEPs were harvested and
adjacent tissues stripped off. Following immersion in PBS
containing penicillin (100 U/ml) and streptomycin (100 mg/ml) for
10 min, the cartilage was cut into small pieces followed by
digestion with 0.25% (w/v) collagenase (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
atmosphere of 5% CO2 and 95% air. After 3 h, the
residual tissue was removed and the cell suspension was centrifuged
at 300 × g at room temperature for 5 min. The harvested cells were
maintained in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.)/F-12 containing 10% (v/v) fetal bovine
serum (FBS) and the medium was changed every 3 days. When the
cultured primary cells reached 80% confluence, they were detached
by treatment with 0.25% (w/v) trypsin and 0.1% (w/v)
ethylenediaminetetraacetic acid (Gibco; Thermo Fisher Scientific,
Inc.) and subcultured at a density of 1×104
cells/cm2. Cultured cells before passage 2 were used for
the experiments.
Cell treatment
Prior to use in the experiments, all cell samples
were starved of serum for 12 h. The cells were seeded in 6-well
plates at an initial density of 2×105/well for gene
expression analysis and in 60-mm dishes at an initial density of
5×105/well for protein experiments. When the
chondrocytes reached 70–80% confluency, they were incubated with
SDF-1 (50, 100 or 200 ng/ml; PeproTech, Inc., Rocky Hill, NJ, USA)
at 37°C in a humidifed atmosphere of 5% CO2 and 95% air
for 24 h. In order to examine the downstream signaling pathways
affected by the SDF-1 treatment, the endplate chondrocytes were
pretreated with 500 ng/ml AMD3100 (Selleck Chemicals, Houston, TX,
USA) at 37°C in a humidifed atmosphere of 5% CO2 and 95%
air for 30 min prior to the application of SDF-1.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc.) and qualified by measuring the absorbance
at 260 nm. cDNA was synthesized from 1 µg RNA using a Takara
RNA PCR kit (Takara Bio., Inc., Otsu, Japan) in accordance with the
manufacturer's protocol. qPCR was performed using SYBR-Green Master
mix (Takara Bio, Inc.) on an ABI StepOnePlus system (v2.3; Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primer sequences
used are listed in Table I.
Samples were examined in triplicate and the results were averaged.
The relative expression levels of genes were normalized to the
value of GAPDH using the ΔΔ cycle threshold (ΔΔCq) method (16), and differences in gene expression
were calculated using ABI software (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The thermocycling conditions used for
qPCR were as follows: stage 1, 95°C for 30 sec; stage 2, 95°C for 5
sec, 60°C for 30 sec (stage 2 was repeated for 40 times); stage 3,
95°C for 15 sec, 60°C for 1 min, 95°C for 15 sec.
 | Table IPrimer sequences used in this
study. |
Table I
Primer sequences used in this
study.
| Gene | Primer sequences |
|---|
| CXCR4 | F:
5′-CTCCTGCTGACTATTCCCGAC-3′ |
| R:
5′-GATAAGGCCAACCATGATGTGC-3′ |
| MMP-1 | F: 5′-CCA AAT GGG
CTT GAA GCT G-3′ |
| R: 5′-GGT ATC CGT
GTA GCA CAT TCT GTC-3′ |
| MMP-3 | F: 5′-TTT CCA GGG
ATTGAC TCA AAG A-3′ |
| R: 5′-AAG TGC
CCATAT TGT GCC TTC-3′ |
| MMP-13 | F:
5′-ATGCAGTCTTTCTTCGGCTTAG-3′ |
| R:
5′-ATGCCATCGTGAAGTCTGGT-3′ |
| GAPDH | F:
5′-GAAGGTGAAGGTCGGAGTC-3′ |
| R:
5′-GAAGATGGTGATGGGATTTC-3′ |
Western blot analysis
Cells were seeded at a density of 5×105
cells in 60-mm dishes and treated with serum-free medium for 12 h.
The samples were lysed in radioimmunoprecipitation assay lysis
buffer containing a protease inhibitor cocktail (Sigma-Aldrich;
Merck KGaA) for 30 min on ice. Cell lysates were centrifuged at
12,000 × g for 15 min, and the supernatant was collected. Protein
content was quantified using a BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Equal amounts of protein (40 µg) were separated by
10% SDS-PAGE at 100 V for 1.5 h. The proteins were then transferred
onto polyvinylidene difluoride membranes at 250 mA for 2 h. The
membranes were blocked with 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) for 1 h at room temperature and then incubated with
primary antibody at 4°C overnight. The primary antibodies targeted
human CXCR4 (60042-1-Ig), MMP-1 (10371-2-AP), MMP-3 (66338-1-Ig)
and MMP-13 (18165-1-AP) (dilution, 1:1,000; all from ProteinTech
Group, Inc., Chicago, IL, USA) and GAPDH (dilution, 1:1,000;
ab8245; Abcam, Cambridge, MA, USA). After washing five times with
TBS and Tween-20, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (dilution, 1:1,000;
ab205719) for 1 h at room temperature. The target proteins were
visualized using an enhanced chemiluminescence detection system
(ChemiDoc™ XRS+ imaging system; EMD Millipore,
Billerica, MA, USA) and hyper-enhanced chemiluminescence film. Band
density analysis was performed using Quantity One software (version
4.6.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Zymography
The chondrocyte culture medium was collected,
centrifuged at 1,000 × g for 30 min at 4°C, and concentrated
100-fold with a Centriprep centrifugal filter concentrator (EMD
Millipore). The sample was loaded into an SDS-PAGE gel containing
1-mg/ml gelatin (for the detection of MMP-2 and -9) or collagen
(for the detection of MMP-1, -3 and -13) and subjected to
electrophoresis at a constant voltage. The gels were washed with
2.5% Triton X-100 to remove SDS, rinsed with 50 mM Tris-HCl (pH
7.5), and then incubated overnight at room temperature with
developing buffer (50 mM Tris-HCl, pH 7.5, 5 mM CaCl2, 1
µM ZnCl2, 0.02% thimerosal and 1% Triton X-100).
The zymographic activities were visualized by staining with 1%
Coomassie Blue. Zymography band density analysis was performed
using Quantity One software.
CEP explant culture
According to the method described by Chen et
al (17), the CEPs harvested
from young patients were initially cultured at 37°C with 5%
CO2 in 10% FBS (v/v), antibiotics [penicillin (100 U/ml)
and streptomycin (100 mg/ml)] and 2 mM L-glutamine (Gibco; Thermo
Fisher Scientific, Inc.). After 2 days, the explants were washed in
serum-free medium and placed in 24-well culture dishes with fresh
serum-free medium containing different concentrations of SDF-1 (50,
100 or 200 ng/ml) or 500 ng/ml AMD3100 (with or without 100 ng/ml
SDF-1) and cultured for a further 2 days. In the combined treatment
group, in order to examine the downstream signaling pathways
underlying the effects of SDF-1 treatment, the CEPs were pretreated
with AMD3100 for 30 min prior to SDF-1 administration.
Animal model
A total of 20 female Sprague-Dawley rats (200–250 g;
8 weeks old; Shanghai Laboratory Animal Center of Chinese Academy
of Sciences) were used. The principles of laboratory animal care
(18) were followed. The animals
were housed in animal-holding units at 24°C in a 12/12-h light/dark
cycle. The experiments were approved by the Ethics Committee of the
Second Affiliated Hospital of the School of Medicine of Zhejiang
University. The animals were anesthetized by the intraperitoneal
injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). Coccygeal
intervertebral level 2–3 was selected for the study. A rat tail
disc degeneration model was induced by percutaneous needle puncture
as described by Mao et al (19). A 30-gauge needle was inserted at
coccygeal intervertebral level 2–3 at the level of the annulus
fibrosus (AF), crossing the NP up to the contralateral AF. When
full penetration was achieved, the needle was rotated 360° twice
and held in place for 30 sec. The rats were randomly divided into
four groups (n=5) and injected with 3 µl PBS (control
group), or 3 µl PBS containing 200 ng/ml SDF-1, 500 ng/ml
AMD3100, or 200 ng/ml SDF-1 plus 500 ng/ml AMD3100 using a Hamilton
micro-syringe. The animals were sacrificed by CO2
inhalation 30 days after disc puncture.
Histological examination
The samples (the coccygeal intervertebral discs cut
from the sacrificed animals) were fixed at room temperature in 4%
paraformaldehyde for 4 days and decalcified with 10% buffered
formic acid for 2 months. Decalcified specimens (at room
temperature) were dehydrated in alcohol, embedded in paraffin
blocks, and cut into 5-µm sections. The sections were
stained with Safranin-O (for 8 min)/Fast Green (for 5 min) at room
temperature. Photographic images of the stained sections were
captured using a DP70 CCD camera coupled to an AX-70 microscope
(both from Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation
from experiments performed at least in triplicate. Data were
analyzed using one way anova followed by Tukey's Multiple
Comparison test. All statistical analyses were performed using SPSS
software (version 6.0; SPSS, Inc., Chicago, IL, USA). P-values were
two-tailed, and P<0.05 was considered to indicate a
statistically significant result.
Results
SDF-1 upregulates CXCR4 expression in
human endplate chondrocytes
RT-qPCR demonstrated that SDF-1 increased CXCR4 mRNA
expression in human endplate chondrocytes, and this effect appeared
to be dose-dependent. In subconfluent monolayer culture, the
stimulatory effect of SDF-1 on CXCR4 mRNA expression was detected
at levels as low as 50 ng/ml. When used at a dose of 200 ng/ml,
SDF-1 induced a 6-fold increase in CXCR4 mRNA expression (Fig. 1). To confirm the effects of SDF-1
on CXCR4 protein expression, western blot analysis was performed
following the treatment of endplate chondrocytes with SDF-1 for 24
h. The results demonstrated that SDF-1 increased the level of CXCR4
protein, and the increase appeared to be dose-dependent (Figs. 2 and 3). Furthermore, the results of RT-qPCR
and western blotting indicated that AMD3100 (500 ng/ml), a
CXCR4-specific chemical inhibitor, significantly increased the mRNA
and cell-surface expression of CXCR4.
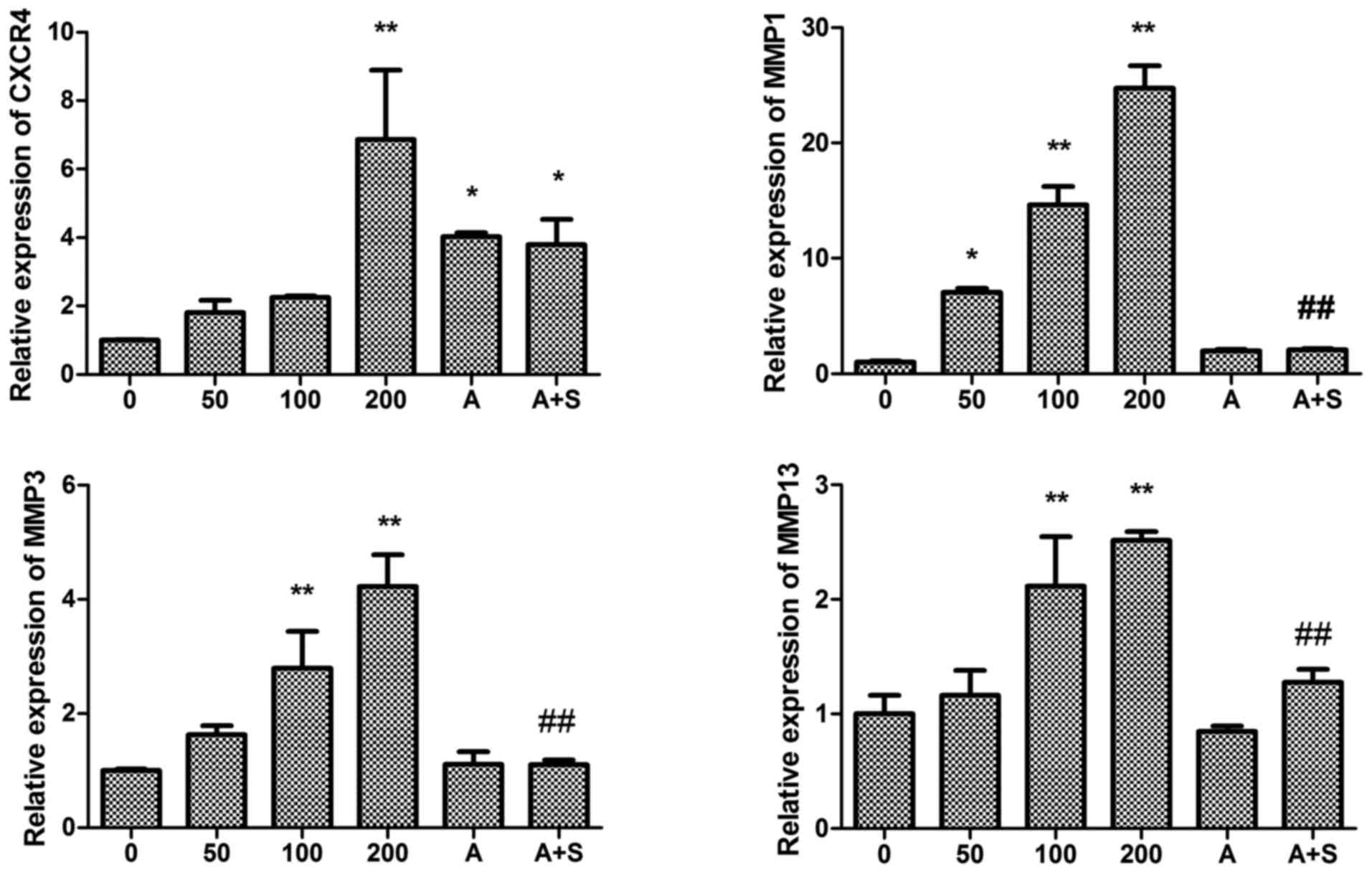 | Figure 1Effect of SDF-1 and the CXCR4-specific
chemical inhibitor AMD3100 on CXCR4 and MMP-1, -3 and -13 mRNA
expression in endplate chon-drocytes. SDF-1 significantly increased
CXCR4 and MMP-1, -3 and -13 mRNA expression. AMD3100 significantly
decreased MMP-1, -3 and -13 mRNA expression. Human endplate
chondrocytes were incubated with various concentrations of SDF-1
(0, 50, 100 or 200 ng/ml), AMD3100 (500 ng/ml), or SDF-1 (100
ng/ml) + AMD3100 (pretreated for 30 min prior to SDF-1
administration) for 24 h. The cell lysates were collected, and mRNA
levels were determined by reverse transcription-quantitative
polymerase chain reaction. The expression levels of (A) CXCR4, (B)
MMP-1, (C) MMP-3, and(D) MMP-13 were determined compared with those
of GAPDH. *P<0.05 and **P<0.01 vs. 0
ng/ml; #P<0.05 and ##P<0.01 vs. 100
ng/ml. SDF-1, stromal cell-derived factor-1; A, AMD3100; A + S,
AMD3100 and SDF-1; CXCR4, C-X-C chemokine receptor type 4; MMP,
matrix metalloproteinase. |
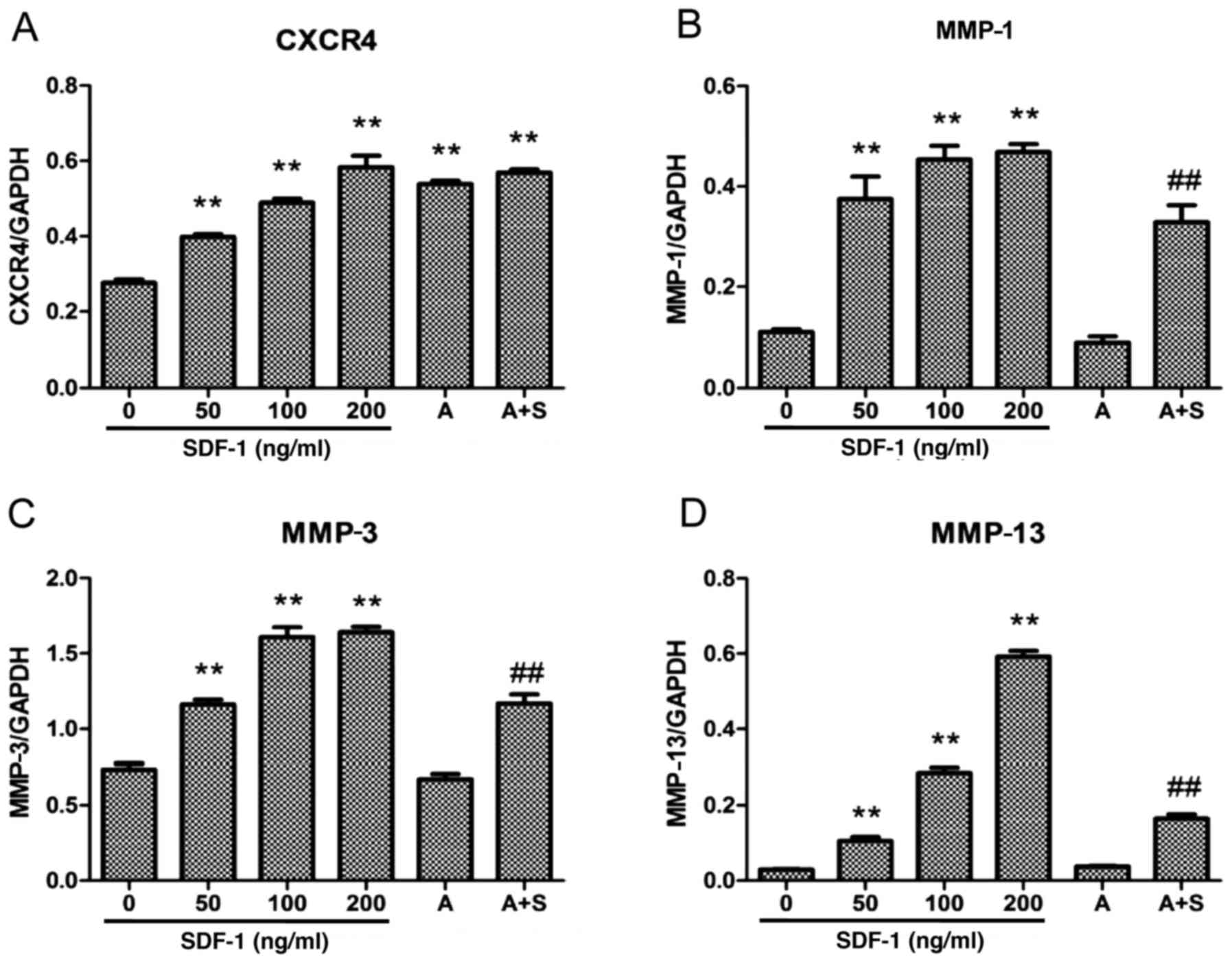 | Figure 3Effect of SDF-1 and the CXCR4-specific
chemical inhibitor AMD3100 on CXCR4 and MMP-1, -3 and -13 protein
expression in endplate chon-drocytes. SDF-1 significantly increased
CXCR4 and MMP-1, -3 and -13 protein expression. AMD3100
significantly decreased MMP-1, -3 and -13 protein expression. Human
endplate chondrocytes were incubated with various concentrations of
SDF-1 (0, 50, 100 or 200 ng/ml), AMD3100 (500 ng/ml), or SDF-1 (100
ng/ml) + AMD3100 (pretreated for 30 min prior to SDF-1
administration) for 24 h. The protein levels of CXCR4 and MMPs were
determined by western blot analysis. The expression levels of (A)
CXCR4, (B) MMP-1, (C) MMP-3 and (D) MMP-13 were determined relative
to those of GAPDH: **P<0.01 vs. 0 ng/ml;
##P<0.01 vs. 100 ng/ml. SDF-1, stromal cell-derived
factor-1; A, AMD3100; A + S, AMD3100 and SDF-1; CXCR4, C-X-C
chemokine receptor type 4; MMP, matrix metalloproteinase. |
SDF-1/CXCR4 interaction is responsible
for MMP-1, -2, -3, -9 and -13 expression in human endplate
chondrocytes
The results of RT-qPCR, western blotting and
zymography indicate that SDF-1 increased the mRNA and protein
expression levels of MMP-1, -3 and -13 in human endplate
chondrocytes, and the increases appeared to be dose-dependent
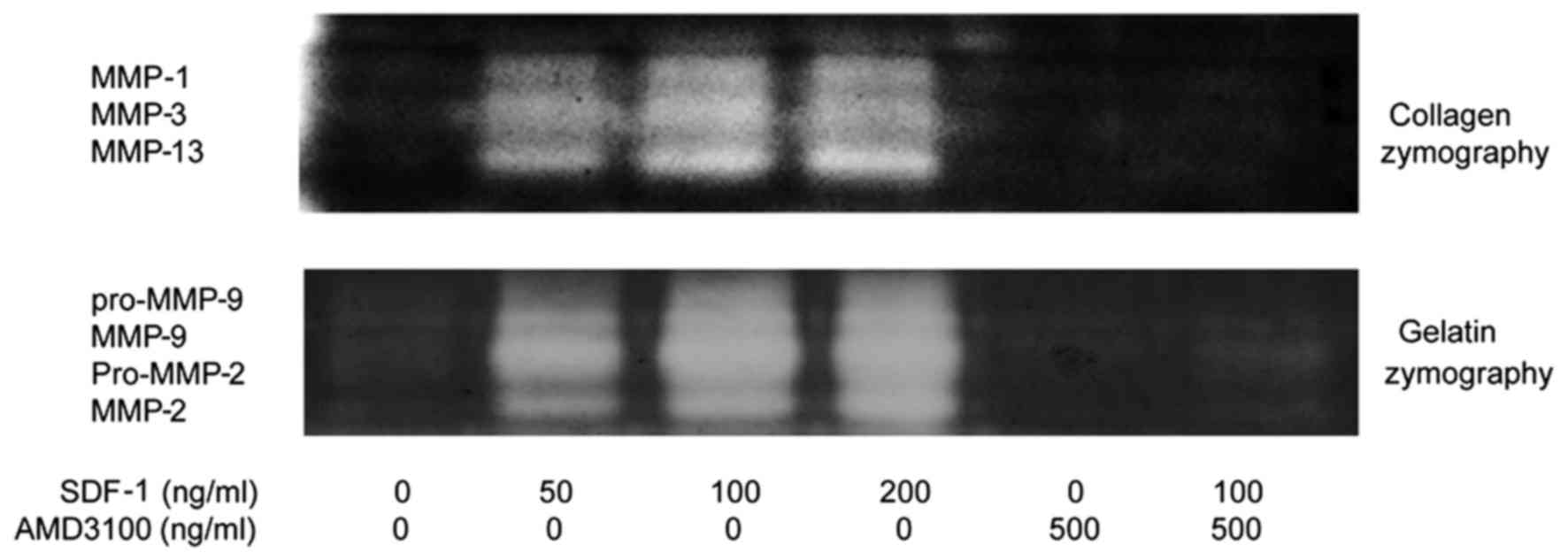
(Figs. 1Figure 2Figure 3Figure 4–5). The zymography results also suggest
that SDF-1 increased MMP-2 and -9 protein expression in a
dose-dependent manner (Figs. 4
and 5). The effects of SDF-1 on
MMPs were observed at a dose of 50 ng/ml, and these effects
appeared to be greater when the dose was increased. To determine
whether the SDF-1/CXCR4 interaction induced increases in MMP mRNA
and protein levels, the CXCR4-specific chemical inhibitor AMD3100
was used to inhibit this interaction. The results demonstrate that
AMD3100 significantly antagonized the SDF-1-induced MMP-1, -2, -3,
-9 and -13 expression. These results suggest that the SDF-1-induced
MMP mRNA and protein expression resulted from the interaction
between SDF-1 and CXCR4.
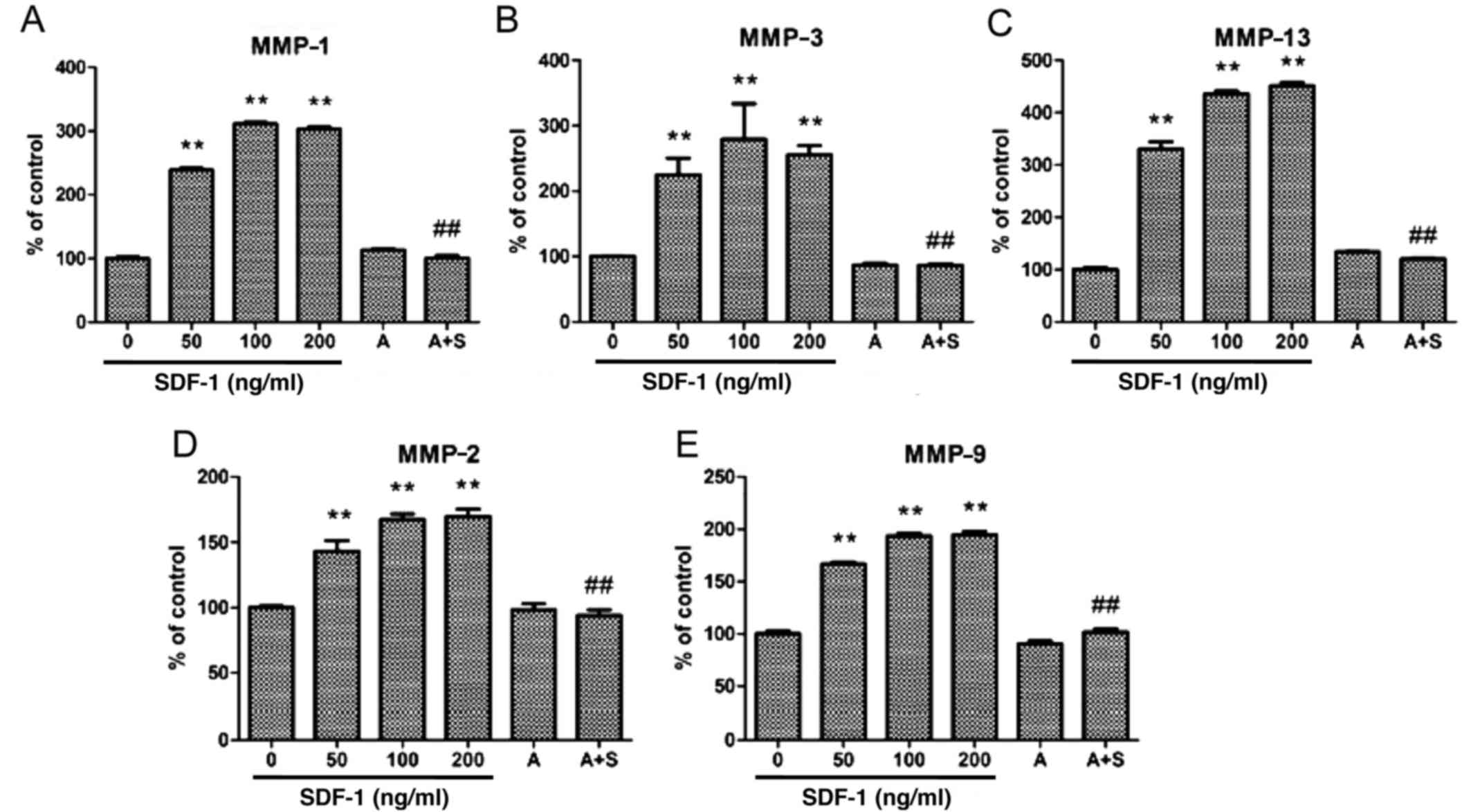 | Figure 5Zymographic analysis revealed that
SDF-1 increased MMP-1, -2, -9, -3 and -13 proteolytic activity.
AMD3100 significantly decreased MMP-1, -2, -9, -3 and -13
proteolytic activity. Human endplate chondrocytes were incubated
with various concentrations of SDF-1 (0, 50, 100 and 200 ng/ml),
AMD3100 (500 ng/ml), or SDF-1 (100 ng/ml) + AMD3100 (pretreated for
30 min before SDF-1 administration) for 24 h. The activity of (A)
MMP-1, (B) MMP-2, (C) MMP-3, (D) MMP-9 and (E) MMP-13 was
determined compared with the control group: **P<0.01
vs. 0 ng/ml; ##P<0.01 vs. 100 ng/ml. SDF-1, stromal
cell-derived factor-1; A, AMD3100; A + S, AMD3100 and SDF-1; MMP,
matrix metalloproteinase. |
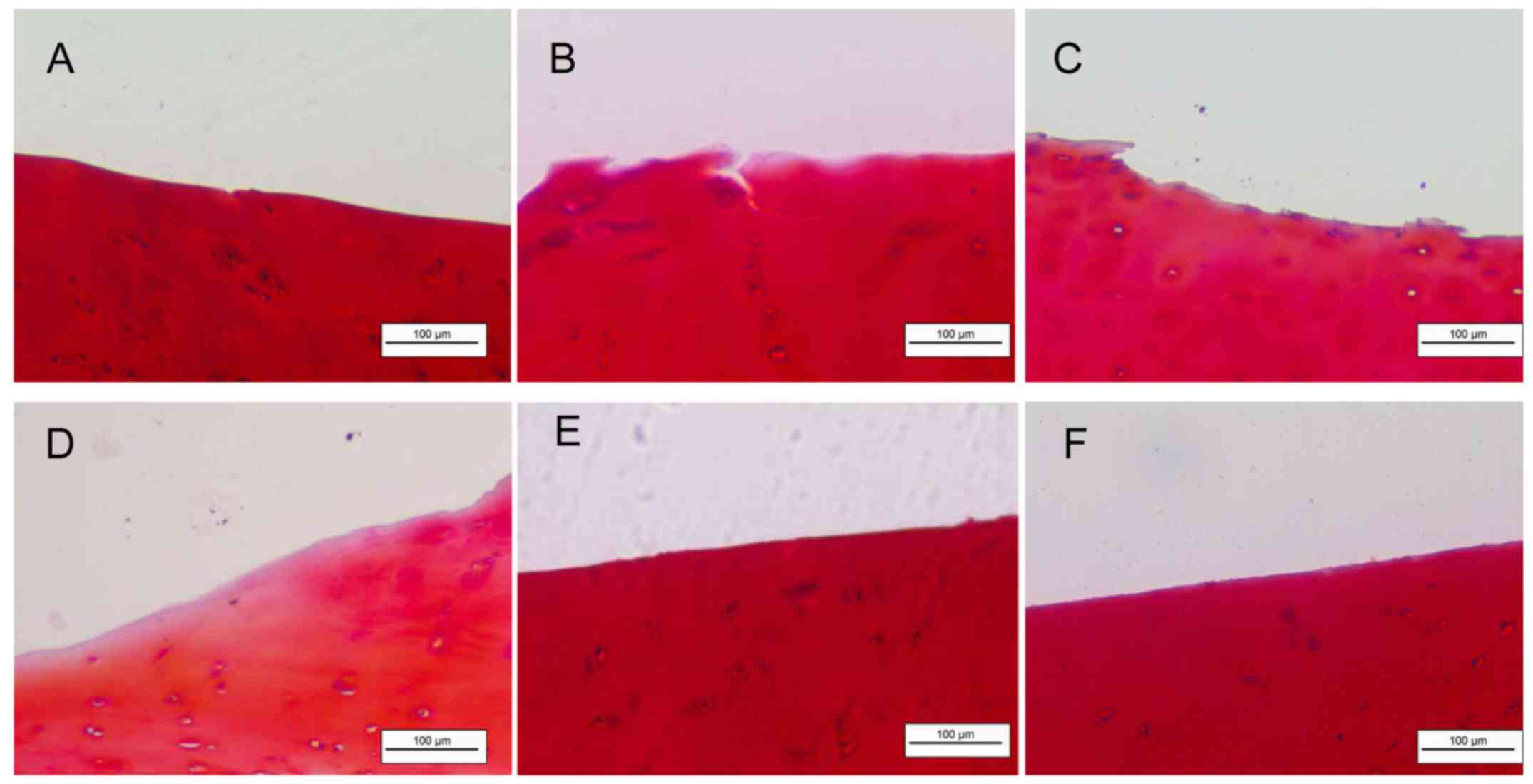
SDF-1 accelerates degradation of the ECM
in human CEP explants
Fig. 6 presents
aggrecan degradation in the cartilage explant culture model treated
with SDF-1 and/or the CXCR4 inhibitor AMD3100, and sham-treated
controls. Proteoglycan staining with Safranin-O revealed the
reduction of the ECM to the deep zones of the cartilage layers in
the 200 ng/ml SDF-1 group (Fig.
6D). The AMD3100 with and without SDF-1 groups clearly
exhibited less cartilage cleavage compared with that in the groups
treated with SDF-1 alone.
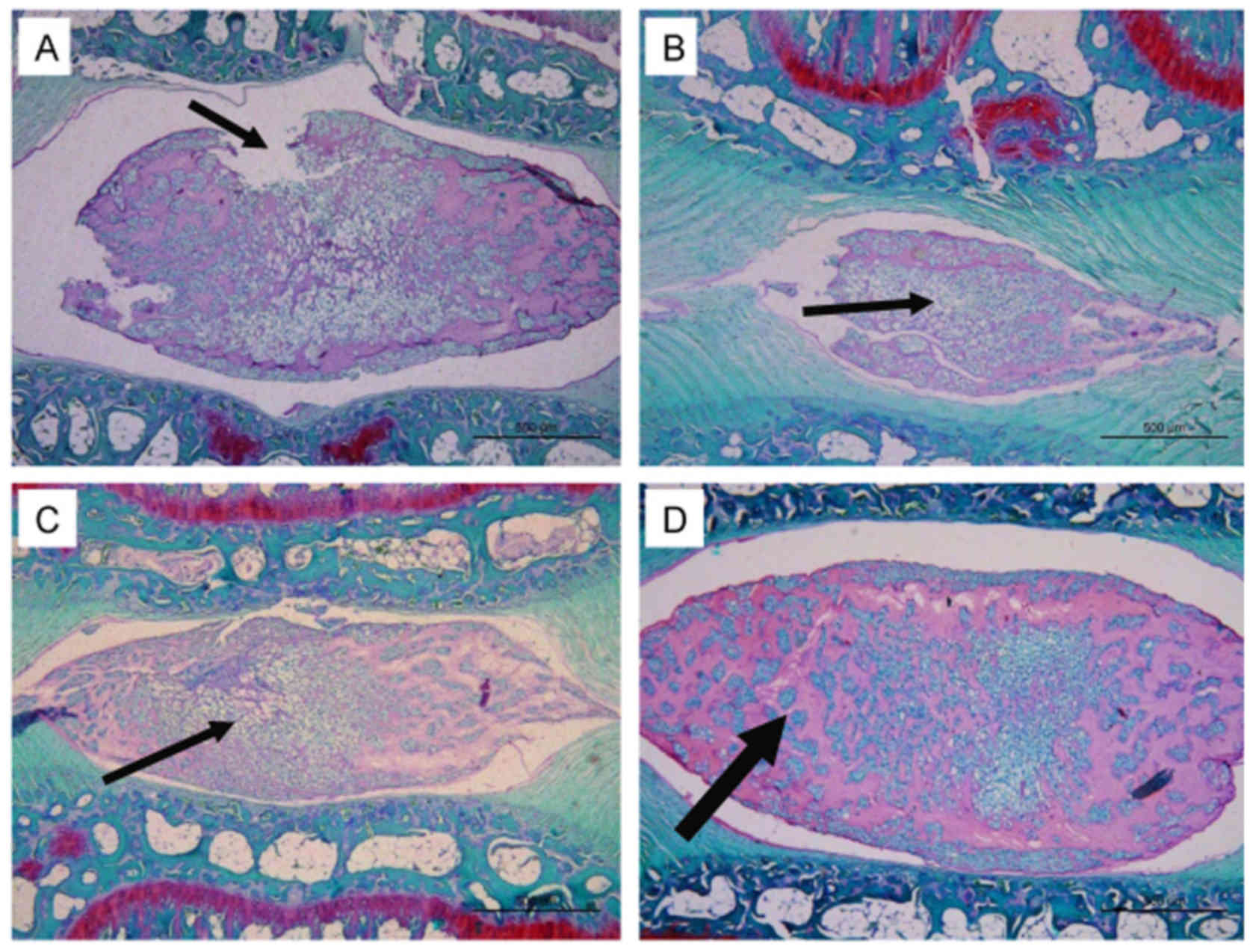
SDF-1 ameliorates NP degeneration in
vivo
In the rat tail disc degeneration model, 3 µl
PBS containing SDF-1 and/or AMD3100 was injected into the NP.
Representative histological sections of the degenerated discs are
shown in Fig. 7. The histological
appearance of the tail disc indicated that injection with PBS
solution induced NP degeneration, including an interrupted border
between the AF and the NP, ECM degradation and morphological
shrinkage of the NP. However, the injection of SDF-1 without
AMD3100 ameliorated the NP degeneration compared with that in the
other groups. In particular, the majority of the ECM was retained
and NP cells were grouped into clusters and separated by dense
areas of proteoglycan matrix in the SDF-1 group (Fig. 7D). The other three groups
exhibited greater reduction of the ECM and fewer cells in the NP
(Fig. 7A–C).
Discussion
In a previous study, the present research team
demonstrated the upregulation of SDF-1 and its receptor CXCR4 in
degenerated human and rat IVDs (6). The results of the present study
suggest that SDF-1 upregulates CXCR4 expression at the mRNA and
protein levels in human endplate chondrocytes isolated from the
human lumbar spine. Previous studies have demonstrated that SDF-1
induces the expression of MMP-3, -9 and -13 in the cartilage cells
of patients with osteoarthritis and rheumatoid arthritis (7–9).
The present study reveals similar findings in human endplate
chondrocytes. Furthermore, AMD3100, a CXCR4 inhibitor, resulted in
a significant reduction in SDF-1-mediated MMP expression. These
observations indicate that the SDF-1/CXCR4 pathway is involved in
the expression of MMPs in endplate cartilage. The expression of
MMPs, as matrix-destructive enzymes, at high levels in degenerative
endplate cartilage is likely to results in the breakdown of ECM
proteins.
Cartilage explant culture systems have been
established to investigate the reactions of chondrocytes maintained
in an organized structure to growth or inflammatory factors. The
integrity of the tissue and chondrocytes is maintained in these
ex vivo culture systems, allowing the cells to communicate
in a manner similar to that in vivo, at least for a limited
period of time (20). The effects
of growth factor treatment may be investigated during culture; the
addition of growth factors and other agents to the culture medium
as single components or in combination with inhibitors enables the
investigation of their specific roles during chondrocyte
differentiation and the observation of alterations in cartilaginous
structures (17,21,22). In a previous study by Song et
al (22), human joint
cartilage explants were efficiently transfected with small
interfering RNA that specifically decreased the expression of a
disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS)-4 and -5. The results suggested that the suppression of
ADAMTS-4 and -5, individually or in combination, ameliorated the
degradation of aggrecan in cytokine-stimulated joint cartilage. In
the present study, endplate cartilage explants were cultured under
similar conditions to joint cartilage explants. SDF-1 was added
alone or in combination with the inhibitor AMD3100 to the culture
medium to investigate the degradative effect of SDF-1 on aggrecan
in the CEP. Proteoglycan staining using Safranin-O revealed a
marked reduction of aggrecan in the SDF-1 group compared with the
control group and the SDF-1 plus inhibitor group.
In addition to its role in ECM degradation, which
involves the upregulation of MMP expression by chondrocytes, SDF-1
is known as a homing factor that induces the homing of
stem/progenitor cells to the site of release, and therefore has
therapeutic potential (23–25). The present study suggests that
SDF-1 implantation has the ability to promote the regeneration of
NP tissue during the degeneration process in vivo, despite
SDF-1 inducing aggrecan degradation in endplate explant culture.
Barkho et al (26)
demonstrated that, in response to SDF-1, adult neural
stem/progenitor cells differentiated into migratory cells with
increased levels of MMP-3 and -9 expression, and that blocking the
expression of MMP-3 or -9 in adult neural stem/progenitor cells
significantly reduced chemokine-induced cell migration. Son et
al (24) observed that MSCs
homed to sites of tissue injury following signaling cues regulated
by a gradient of SDF-1 in an MMP-dependent manner. Therefore, the
degeneration or remodeling of ECM in endplate cartilage induced by
SDF-1-associated inflammation may enhance the migration of
exogenous stem cells toward the NP. In bone and cardiovascular
tissues, the control of inflammation has been shown to be critical
in shifting the degeneration/regeneration toward regeneration
(27–29). It has been suggested that novel
therapies for IVD degeneration should aim to restore the
homeostatic inflammatory conditions in the disc, rather than to
completely inhibit inflammation, thus enabling endogenous repair
mechanisms (30).
In a previous study, a recombinant adenoviral vector
carrying the SDF-1 transgene was constructed and applied to
transduce a novel scaffold-free living hyaline cartilage graft
(SDF-t-LhCG) (31). The results
indicated that the SDF-1-induced activation and recruitment of
endogenous stem cells was augmented in SDF-t-LhCG implants. Due to
the increased supply of endogenous stem cells recruited by SDF-1-,
enhanced chondrogenesis was observed in the SDF-t-LhCG implants
in situ. In another study, full-thickness bovine chondral
defects were filled with hydrogel containing recombinant human
SDF-1α, and increased cell migration followed by chondrogenic
induction occurred (32). These
results demonstrated that rhSDF-1α markedly improved the
recruitment of migratory chondrogenic progenitor cell to defects.
Furthermore, cartilage generated in rhSDF-1α-containing defects
exhibited significantly greater inter-facial strength than
controls, and acquired mechanical properties comparable with those
of native cartilage. Chen et al (33) reported that cartilage regeneration
with SDF-1 effectively promoted BMSC migration and the repair of
cartilage defects.
Thus, previous studies have indicated that SDF-1 is
a promising therapeutic molecule that may make a significant
contribution to MSC migration, and the results of the present study
raise the possibility of using SDF-1 implants in the NP, and its
homing action as an MSC chemoattractant, to promote IVD
regeneration.
Acknowledgments
The present study was financially supported by the
National Natural Science Foundation of China (NSFC; grant no.
81672246).
References
|
1
|
Chan WC, Sze KL, Samartzis D, Leung VY and
Chan D: Structure and biology of the intervertebral disk in health
and disease. Orthop Clin North Am. 42:447–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Peroglio M, Alini M and Grad S:
Potential and limitations of intervertebral disc endogenous repair.
Curr Stem Cell Res Ther. 10:329–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roughley PJ: Biology of intervertebral
disc aging and degeneration: Involvement of the extracellular
matrix. Spine. 29:2691–2699. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goupille P, Jayson MI, Valat JP and
Freemont AJ: Matrix metalloproteinases: The clue to intervertebral
disc degeneration? Spine. 23:1612–1626. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pockert AJ, Richardson SM, Le Maitre CL,
Lyon M, Deakin JA, Buttle DJ, Freemont AJ and Hoyland JA: Modified
expression of the ADAMTS enzymes and tissue inhibitor of
metalloproteinases 3 during human intervertebral disc degeneration.
Arthritis Rheum. 60:482–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Zhang L, Chen L, Li W, Li F and
Chen Q: Stromal cell-derived factor-1 and its receptor CXCR4 are
upregulated expression in degenerated intervertebral discs. Int J
Med Sci. 11:240–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanbe K, Takagishi K and Chen Q:
Stimulation of matrix metalloprotease 3 release from human
chondrocytes by the interaction of stromal cell-derived factor 1
and CXC chemokine receptor 4. Arthritis Rheum. 46:130–137. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiu YC, Yang RS, Hsieh KH, Fong YC, Way
TD, Lee TS, Wu HC, Fu WM and Tang CH: Stromal cell-derived factor-1
induces matrix metalloprotease-13 expression in human chondrocytes.
Mol Pharmacol. 72:695–703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanbe K, Takemura T, Takeuchi K, Chen Q,
Takagishi K and Inoue K: Synovectomy reduces stromal-cell-derived
factor-1 (SDF-1) which is involved in the destruction of cartilage
in osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br.
86:296–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seta N and Kuwana M: Human circulating
monocytes as multi-potential progenitors. Keio J Med. 56:41–47.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krause DS: Plasticity of marrow-derived
stem cells. Gene Ther. 9:754–758. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakai D, Nishimura K, Tanaka M, Nakajima
D, Grad S, Alini M, Kawada H, Ando K and Mochida J: Migration of
bone marrow-derived cells for endogenous repair in a new
tail-looping disc degeneration model in the mouse: A pilot study.
Spine J. 15:1356–1365. 2015. View Article : Google Scholar
|
|
13
|
Illien-Jünger S, Pattappa G, Peroglio M,
Benneker LM, Stoddart MJ, Sakai D, Mochida J, Grad S and Alini M:
Homing of mesenchymal stem cells in induced degenerative
intervertebral discs in a whole organ culture system. Spine.
37:1865–1873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pereira CL, Gonçalves RM, Peroglio M,
Pattappa G, D'Este M, Eglin D, Barbosa MA, Alini M and Grad S: The
effect of hyaluronan-based delivery of stromal cell-derived
factor-1 on the recruitment of MSCs in degenerating intervertebral
discs. Biomaterials. 35:8144–8153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine. 26:1873–1878. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Chen P, Zhu S, Wang Y, Mu Q, Wu Y, Xia Q,
Zhang X, Sun H, Tao J, Hu H, et al: The amelioration of cartilage
degeneration by ADAMTS-5 inhibitor delivered in a hyaluronic acid
hydrogel. Biomaterials. 35:2827–2836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guide for the Care and Use of Laboratory
Animals. (NIH Publication No. 85-23). Revised 1985.
|
|
19
|
Mao HJ, Chen QX, Han B, Li FC, Feng J, Shi
ZL, Lin M and Wang J: The effect of injection volume on disc
degeneration in a rat tail model. Spine. 36:E1062–E1069. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wuelling M and Vortkamp A: Cartilage
explant cultures. Methods Mol Biol. 1130:89–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Minina E, Wenzel HM, Kreschel C, Karp S,
Gaffield W, McMahon AP and Vortkamp A: BMP and Ihh/PTHrP signaling
interact to coordinate chondrocyte proliferation and
differentiation. Development. 128:4523–4534. 2001.PubMed/NCBI
|
|
22
|
Song RH, Tortorella MD, Malfait AM, Alston
JT, Yang Z, Arner EC and Griggs DW: Aggrecan degradation in human
articular cartilage explants is mediated by both ADAMTS-4 and
ADAMTS-5. Arthritis Rheum. 56:575–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grunewald M, Avraham I, Dor Y,
Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L,
Abramovitch R and Keshet E: VEGF-induced adult neovascularization:
Recruitment, retention, and role of accessory cells. Cell.
124:175–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Son BR, Marquez-Curtis LA, Kucia M,
Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ and
Janowska-Wieczorek A: Migration of bone marrow and cord blood
mesenchymal stem cells in vitro is regulated by stromal-derived
factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves
matrix metalloproteinases. Stem Cells. 24:1254–1264. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wynn RF, Hart CA, Corradi-Perini C,
O'Neill L, Evans CA, Wraith JE, Fairbairn LJ and Bellantuono I: A
small proportion of mesenchymal stem cells strongly expresses
functionally active CXCR4 receptor capable of promoting migration
to bone marrow. Blood. 104:2643–2645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barkho BZ, Munoz AE, Li X, Li L,
Cunningham LA and Zhao X: Endogenous matrix metalloproteinase
(MMP)-3 and MMP-9 promote the differentiation and migration of
adult neural progenitor cells in response to chemokines. Stem
Cells. 26:3139–3149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Claes L, Recknagel S and Ignatius A:
Fracture healing under healthy and inflammatory conditions. Nat Rev
Rheumatol. 8:133–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mountziaris PM, Spicer PP, Kasper FK and
Mikos AG: Harnessing and modulating inflammation in strategies for
bone regeneration. Tissue Eng Part B Rev. 17:393–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boccafoschi F, Mosca C and Cannas M:
Cardiovascular biomaterials: When the inflammatory response helps
to efficiently restore tissue functionality? J Tissue Eng Regen
Med. 8:253–267. 2014. View Article : Google Scholar
|
|
30
|
Molinos M, Almeida CR, Caldeira J, Cunha
C, Gonçalves RM and Barbosa MA: Inflammation in intervertebral disc
degeneration and regeneration. J R Soc Interface. 12:201411912015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang F, Leong W, Su K, Fang Y and Wang
DA: A transduced living hyaline cartilage graft releasing
transgenic stromal cell-derived factor-1 inducing endogenous stem
cell homing in vivo. Tissue Eng Part A. 19:1091–1099. 2013.
View Article : Google Scholar :
|
|
32
|
Yu Y, Brouillette MJ, Seol D, Zheng H,
Buckwalter JA and Martin JA: Functional full-thickness articular
cartilage repair by rhSDF-1α loaded fibrin/HA hydrogel network via
chondrogenic progenitor cells homing. Arthritis Rheumatol.
67:1274–1285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen P, Tao J, Zhu S, Cai Y, Mao Q, Yu D,
Dai J and Ouyang H: Radially oriented collagen scaffold with SDF-1
promotes osteochondral repair by facilitating cell homing.
Biomaterials. 39:114–123. 2015. View Article : Google Scholar
|