Introduction
Lower back pain affects 70–85% of the American
population daily (1), resulting
in annual government spending of >$100 billion on health care
(2). This pain may have
discogenic, neurogenic and ormyogenic causes, among others
(3). The degeneration of
intervertebral discs is a major cause of lower back pain as a
result of the upregulated expression of inflammatory cytokines
(4–7). Therefore, inflammatory cytokines
have received increasing attention from researchers; specifically,
interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are used
to establish models of intervertebral disc degeneration (8).
The degeneration of intervertebral discs is often
accompanied by the downregulated expression of type II collagen
(Col II) and aggrecan (9). Col II
and aggrecan are predominantly secreted by nucleus pulposus cells
(NPCs), and are used as phenotypic markers for NPCs. The
down-regulated expression of Col II and aggrecan reduces the
elastic modulus of intervertebral discs (10). Water loss and height reduction
cause the degeneration of intervertebral discs to occur (10,11).
Several studies have discussed the treatment of
intervertebral disc degeneration (12); these approaches include stem cell
transplantation, growth hormone injection and physiotherapy
(13). However, only a few of
these methods are widely accepted given the presence of wounds and
poor biosafety (14). No safe and
effective drug has been identified as an effective treatment for
intervertebral disc degeneration. Kartogenin (KGN) is a recently
discovered low-molecular weight molecule that induces the
differentiation of bone marrow mesenchymal stem cells (MSCs) into
chondrocytes via the core binding factor β (CBFβ)-runt related
transcription factor 1 (RUNX1) pathway (15). KGN also promotes the expression of
Col II and aggrecan in chondrocytes (16). KGN may be useful for treating
osteoarthritis. Recently, KGN was reported to effectively repair
articular cartilage damage (17).
However, its use for the treatment of the degeneration of
intervertebral discs has not been reported. In the present study,
KGN was used to address the difficulty of treating degeneration in
the intervertebral discs.
Materials and methods
Experimental subjects
Human NPCs (hNPCs) were purch ased from ScienCell
Research Laboratories, Inc. (San Diego, CA, USA; cat. no. 4800).
Laboratory animals used in the present study were 6-week-old male
B6 mice provided by the Model Animal Research Center of Nanjing
University (Nanjing, China), which has a certificate of conformity
(201500796). The present study followed the Ethics Codes for Labo
ratory Animals was approved by the Ethics Committee of the First
Affiliated Hospital of Nanjing Medical University (Nanjing,
China).
Culture of hNPCs and the organ culture of
mouse intervertebral discs
The hNPCs were passaged when 80% of the dish was
covered. The first three generations of these cells were used for
the experiments. For the organ culture of mouse intervertebral
discs, mice under carbon dioxide anesthesia were sacrificed by
cervical dislocation, and soaked in 75% alcohol for 5 min before
sterile operation on a clean bench. Segments were removed from the
lumbar spines of the mice with back-cuts. The intervertebral discs
L2-3 and L3-4 (including the upper and lower endplates) were
dissected and cultured on a 6-well plate, with 4 ml culture medium
added to each plate. The hNPCs and mouse intervertebral discs were
cultured in an incubator containing 5% CO2 and at 37̊C,
with the medium changed every other day. The culture media for the
hNPCs is the special media purchased from ScienCell Research
Laboratories (cat. no. 4801). The culture media for the mouse
intervertebral discs contained 10% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), 1% double-antibiotic
(streptomycin + penicillin; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and 1:1 Dulbecco's modified Eagle's medium
(DMEM)/F12 (Hyclone; GE Healthcare Life Sciences) in the volume
fraction.
Treatment groups
The hNPCs were divided into the following treatments
groups: Group 1, blank control; group 2, treatment with
inflammatory cytokines 10 ng/ml IL-1β and 25 ng/ml TNF-α (both from
PeproTech, Inc., Rocky Hill, NJ, USA); group 3, treated with 10
ng/ml IL-1β, 25 ng/ml TNF-α and 100 nmol/ml KGN [dissolved in
dimethyl sulfoxide (DMSO); Selleck Chemicals, Houston, TX, USA];
group 4, treated with 10 ng/ml IL-1β, 25 ng/ml TNF-α and 1
µmol/ml KGN. All hNPCs were cultured for 24 and 48 h. The
mouse intervertebral disc organ cultures were grouped as follows:
Group 1, blank control; group 2, treatment with inflammatory
cytokines 10 ng/ml IL-1β, 50 ng/ml TNF-α; group 3, treated with 10
ng/ml IL-1β, 25 ng/ml TNF-α and 100 nmol/ml KGN; group 4, treated
with 10 ng/ml IL-1β, 25 ng/ml TNF-α and 1 µmol/ml KGN. The
mouse intervertebral discs were cultured for 3 and 10 days. Each
group of hNPCs or mouse intervertebral discs received equal amounts
of the culture medium with DMSO (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany).
Immunofluorescence, histology and
immunohistochemistry
For the immunofluorescence assays, hNPCs were
cultured in a 24-well plate, rinsed three times with
phosphate-buffered saline (PBS; 3 min for each rinse), and fixed
for 30 min with 4% paraformaldehyde at room temperature (RT). The
excess paraformaldehyde was discarded, cells were rinsed with PBS
and incubated with 0.5% Triton X-100 (Sigma-Aldrich; Merck KGaA)
for 15 min at RT to permeablize the membranes. The cells were
blocked with goat serum (Beyotime Institute of Biotechnology,
Haimen, China) for 1 h at RT and rinsed with PBS before the primary
antibodies [anti-Collagen III (ab34712) and anti-aggrecan
(ab36861); diluted 1:100; Abcam, Cambridge, UK] was applied for
incubation at 4̊C overnight. Following rinsing with PBS, the
secondary antibodies [Cy3-conjugated anti-rabbitIgG (A0516) and
Alexa Fluor 488-labeled goat anti-rabbit IgG (A0423); diluted by
1:100; Beyotime Institute of Biotechnology] was applied and the
cells were incubated for 1 h in the dark at RT. Finally, the cells
were rinsed with PBS, and the nuclei were counterstained with DAPI
for 15 min at RT. The stained cells were imaged using a
fluorescence microscope.
For cell histology and immunohistochemistry,
intervertebral discs and the two endplates were fixed with 10%
paraformaldehyde for 24 h at RT, dehydrated with a graded ethanol
series (50% ethanol for 1 h; 50% ethanol for 1 h; 70% ethanol for 1
h; 80% ethanol for 1 h; 90% ethanol for 1 h; 95% ethanol for 1 h;
No.1 anhydrous ethanol for 1 h; No.2 anhydrous alcohol for 1 h;
anhydrous ethanol: xylene 1:1 for 1 h), and embedded in paraffin
after decalcification with 12.5% ethylenediaminetetraacetic acid
(pH 7.4) at RT for 28 days. The 5 µm-thick paraffin sections
were dewaxed, dehydrated, and stained with Alcian blue at RT for 15
min, as well as hematoxylin (at RT for 10 min) and eosin (at RT for
1 min) (H&E). In the results, the cells were considered
positive when the intervertebral discs were stained blue, and
negative when they were colorless.
For the Col II immunohistochemistry, the paraffin
sections were de-waxed, dehydrated, and incubated overnight at 4̊C
with anti-Col II (ab34712; diluted by 1:200; Abcam). After the
primary antibody was removed, the secondary antibody (Q-11401MP;
diluted by 1:100; Thermo Fisher Scientific, Inc.) was added for 1 h
at room temperature. The sections were washed three times with PBS,
with 3 min for each rinse. The stained cells were developed with
diaminobenzidine at RT for 5 min, counterstained with hematoxylin
at RT for 10 min, and mounted with a conventional medium when they
became transparent after dehydration. The results revealed that the
brownish yellow cells were Col II-positive, whereas the colorless
cells were negative.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was respectively extracted from hNPCs and
the nucleus pulposus tissues of the intervertebral discs of 3- and
10-day-old cultures with an RNA extraction kit (Bioteke
Corporation, Beijing, China). Following quantification, the RNA was
reverse transcribed into first strand cDNA with an iScript cDNA kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) with a 10 µl
reaction volume. qPCR was determined using SYBR-Green PCR Master
Mix (ABI, Foster City, CA, USA). The samples were treated with
recombinant DNase I (DNA-free DNA removal kit; Ambion, Austin, TX,
USA) to remove possible DNA contamination. β-actin was used as an
internal control. The PCR cycling conditions were as follows:
Initial pre-denaturation of 95°C for 1 min, denaturation for 15
sec, annealing of 55–65°C for 20 sec and extension of 72°C for 30
sec. A total of 50 cycles were performed. For RT-qPCR the following
primers were used: β-actin promoter forward (human),
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′; Col2a1 promoter forward (human),
5′-TGGACGATCAGGCGAAACC-3′ and reverse, 5′-GCTGCGGATGCTCTCAATCT-3′;
aggrecan promoter forward (human), 5′-TCAACTGCTGCAGAC CAGGAGGT-3′
and reverse, 5′-CCGATCCACTGGTAG TCTTGGGCA-3′; RUNX2 promoter
forward (human), 5′-CCGCCTCAGTGATTTAGGGC-3′ and reverse, 5′-GGG
TCTGTAATCTGACTCTGTCC-3′; β-actinpromoter forward (mouse),
5′-AACAGTCCGCCTAGAAGCAC-3′ and reverse, 5′-CGTTGACATCCGTAAAGACC-3′;
Col2a1 promoter forward (mouse), 5′-CAGGATGCCCGAAAATTAGGG-3′ and
reverse, 5′-ACCACGATCACCTCTGGGT-3′; aggrecan promoter forward
(mouse), 5′-GTGGAGCCGTGTTTCC AAG-3′ and reverse,
5′-AGATGCTGTTGACTCGAACCT-3′; RUNX2 promoter forward (mouse),
5′-GACTGTGGTTAC CGTCATGGC-3′ and reverse, 5′-ACTTGGTTTTTCATA
ACAGCGGA-3′. Based on the Cq value and relative standard curve of
the PCR product, the amount of RNA template contained in each
specimen was determined and compared with the amount of β-actin.
The specific value of the amount of RNA template to that of β-actin
was adopted as the final statistical data. The results were
processed using the ΔΔCq method (18).
Western blot analysis
The hNPCs after 24 and 48 h, and NP cultures of
mouse intervertebral discs after 3 and 10 days, for the different
treatment groups, were lysed (RIPA; Beyotime Institute of
Biotechnology) and centrifuged at 500 × g for 15 min. Following the
quantification of the supernatant (BCA method), cell proteins (30
µg per lane) were isolated by 5 and 6% polyacrylamide gel
electrophoresis at 110 V and electrophoretically transferred onto
PVDF membranes. The membranes were blocked for 2 h with 5% bovine
serum albumin reagent (Beyotime Institute of Biotechnology) and
incubated overnight at 4̊C with the primary antibodies (ab34712 and
ab36861, diluted by 1:1,000; Abcam) before incubation for 120 min
at room temperature with the secondary antibody (goat anti-rabbit
antibody IgG; Q-11401MP; 1:5,000; Thermo Fisher Scientific, Inc.)
and development for further analysis.
Statistical analysis
SPSS 19.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for all statistical analysis. The measurements
were presented as mean ± standard deviation, and the data between
groups were analyzed using one-way analysis of variance (ANOVA)
followed by a Bonferroni's posthoc test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of KGN on Col II and aggrecan
secretion
Immunohistochemistry and semi-quantitative western
blot analysis were performed to observe variations of Col II in the
intervertebral disc cultures. As shown by the immunofluorescence of
Col II in hNPCs (Fig. 1A and B),
the Col II expression was highest in the blank control group.
However, the expression of Col II was inhibited by adding IL-1β and
TNF-α. Col II expression was significantly elevated by the addition
of KGN, particularly at a high concentration. To observe variations
in the secretion of aggrecan by hNPCs, the expression of aggrecan
in hNPCs was determined by immunofluorescence. As Fig. 1 shows, the aggrecan expression in
the group under inflammatory cytokines was significantly inhibited
within 24 and 48 h (Fig. 1C and
D). However, by adding KGN, the expression was elevated,
particularly in the group treated with a high concentration of
KGN.
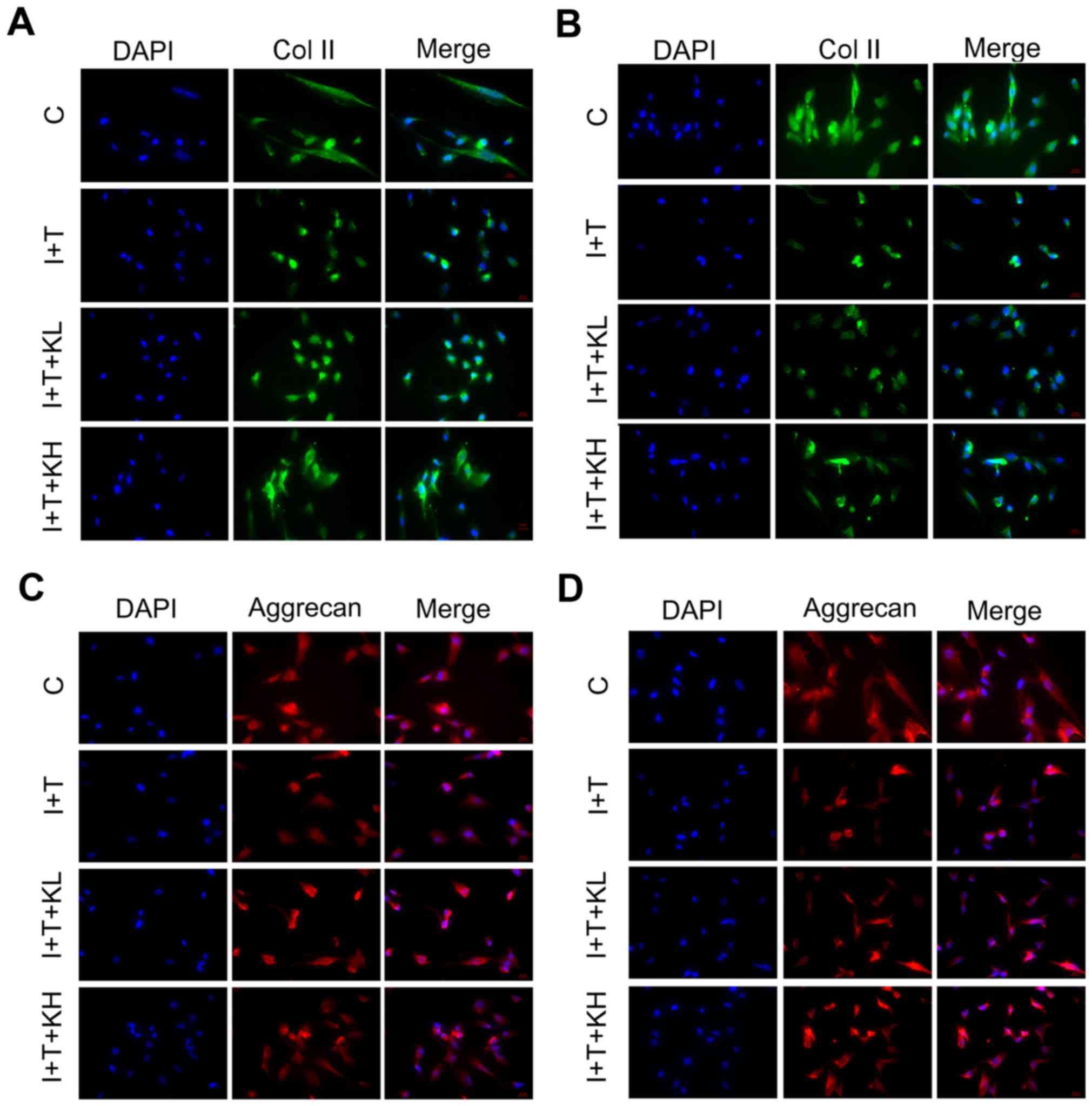 | Figure 1Col II and aggrecan expression were
elevated by KGN in hNPCs. Expression of Col II and aggrecan in
hNPCs after 24 and 48 h by immunofluorescence. (A) Col II, 24 h;
(B) Col II, 48 h; (C) aggrecan, 24 h; and (D) aggrecan, 48 h. The
expression of Col II and aggrecan were significantly inhibited in
the group under inflammatory cytokines alone. However, the
expression of Col II and aggrecan were elevated by KGN (original
magnification, ×200). Col II, type II collagen; KGN, kartogenin;
hNPC, human nucleus pulposus cells; IL-1β, interleukin-1β; TNF-α,
tumor necrosis factor-α; C, control; I+T, IL-1β + TNF-α; I+T+KL,
IL-1β + TNF-α + low concentration of KGN; I+T+KH, IL-1β + TNF-α +
high concentration of KGN. |
Similarly, from the immunohistochemistry analysis of
paraffin sections of the 3 and 10 day-old cultures of
intervertebral discs (Fig. 2A and
B) demonstrated that Col II expression in the group treated
with inflammatory cytokines was markedly reduced compared with the
blank control group. However, its expression was markedly increased
by treatment with KGN.
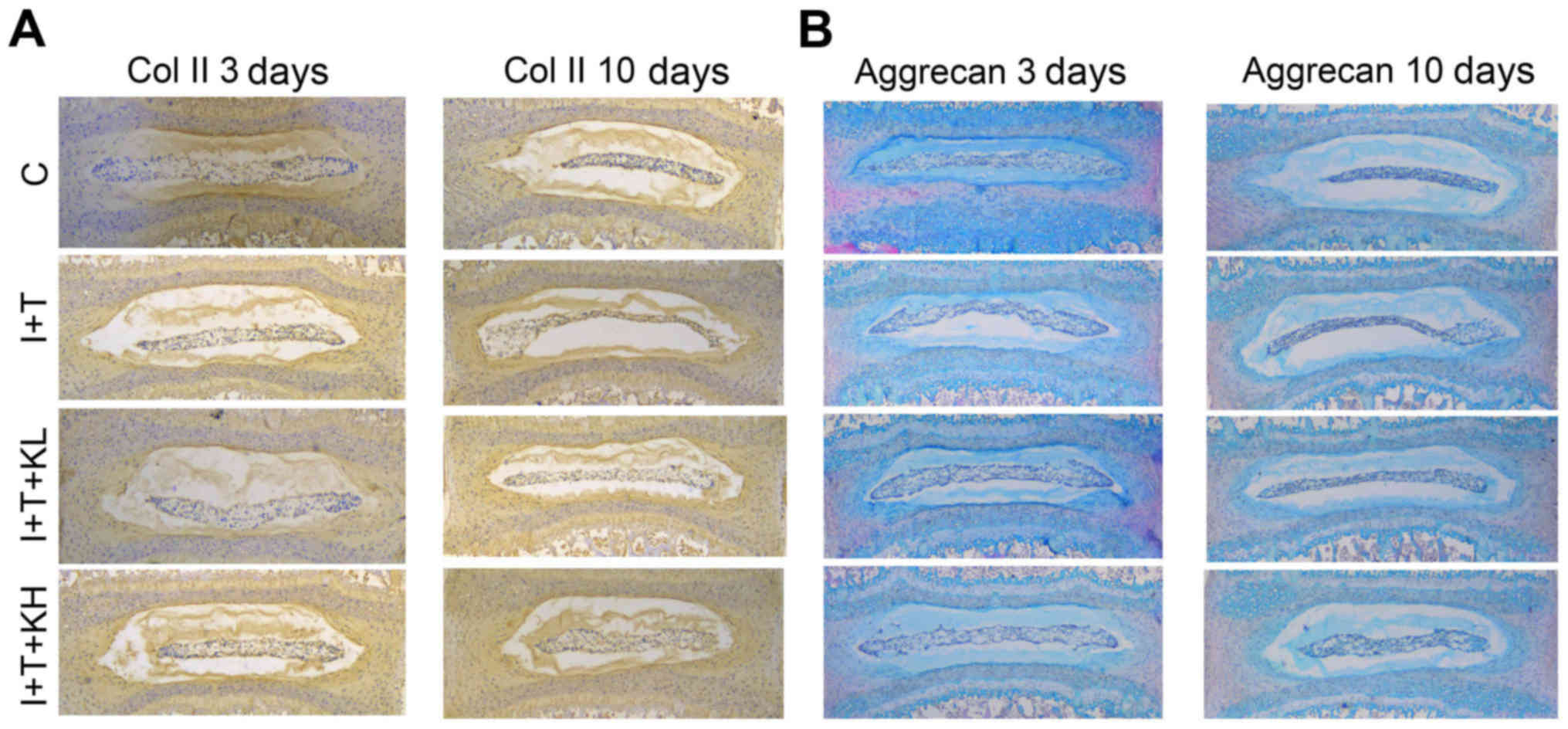 | Figure 2KGN promoted the expression of Col II
and aggrecan in mouse intervertebral discs. (A) Immunohistochemisty
of Col II and (B) aggrecan staining in paraffin sections of mouse
intervertebral discs (at 3 and 10 days). Col II expression in the
group treated with KGN was higher than that in the group under
inflammatory cytokines. The group under inflammatory cytokines had
a collapsed nucleus pulposus, thereby indicating the extracellular
matrix loss in the intervertebral disc. However, the group with KGN
did not exhibit such a phenomenon, with less loss of the
extracellular matrix from the nucleus pulposus tissues in the
intervertebral discs (original magnification, ×40). Col II, type II
collagen; KGN, kartogenin; IL-1β, interleukin-1β; TNF-α, tumor
necrosis factor-α; C, control; I+T, IL-1β + TNF-α; I+T+KL, IL-1β +
TNF-α + low concentration of KGN; I+T+KH, IL-1β + TNF-α + high
concentration of KGN. |
In Fig. 2C and D,
the paraffin sections of coronary regions of the mouse
intervertebral discs were stained with H&E + Alcian blue.
Aggrecan expression was inhibited by inflammatory cytokines
(Fig. 2C and D) compared with the
control group, but was obviously increased by adding KGN,
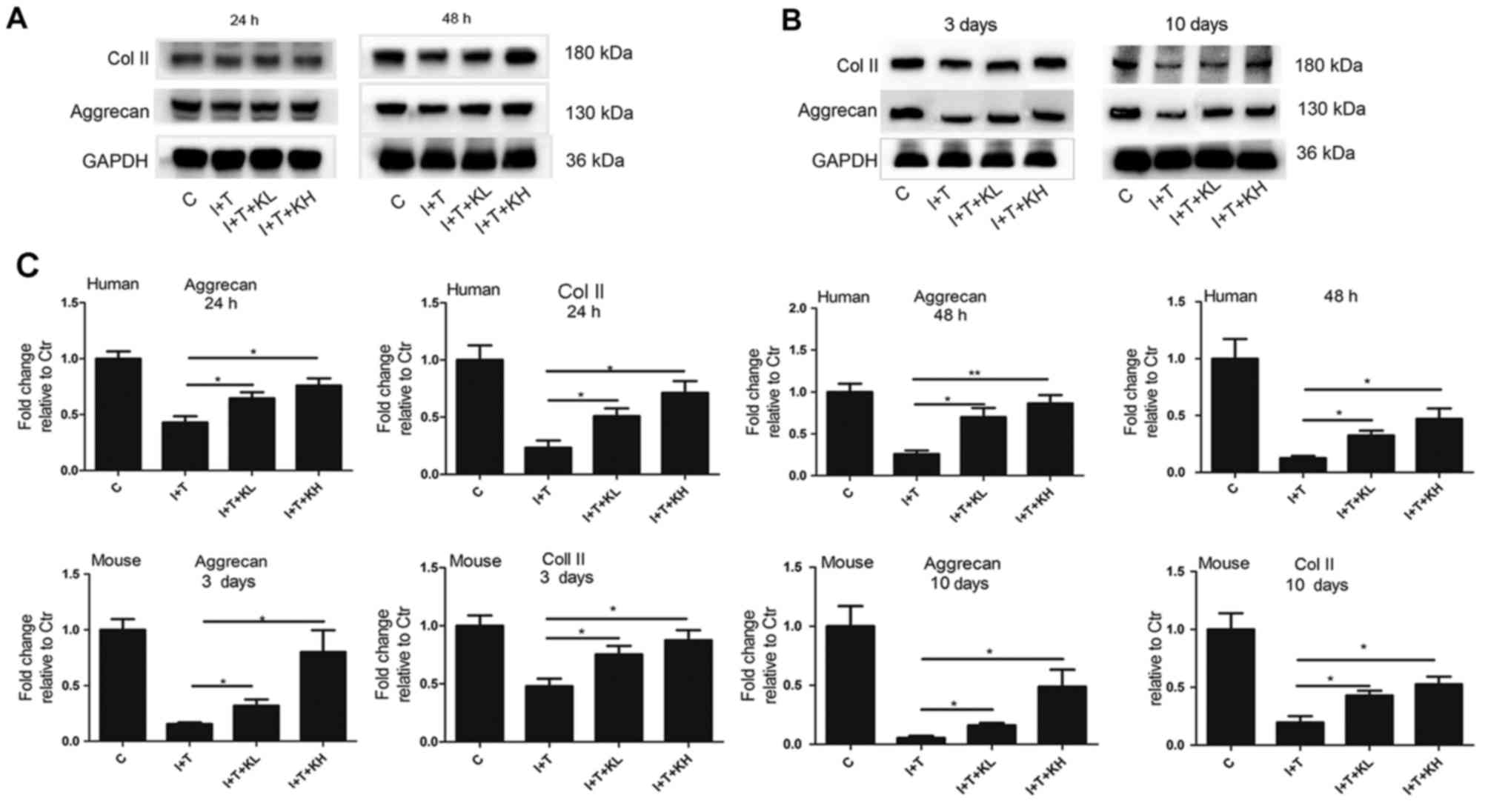
particularly at the high concentration. In Fig. 3A–C, western blot analysis showed
that the aggrecan expression in hNPCs and mouse intervertebral
discs was increased by adding KGN, especially at a high
concentration, and reduced by inflammatory cytokines.
The Col II expression in hNPCs and mouse
intervertebral discs was further determined by western blot
analysis. Results of the western blot analyses for the hNPCs and
mouse intervertebral discs in Fig.
3A–C demonstrated that Col II expression in the group treated
with inflammatory cytokines was significantly reduced compared with
the control group. However, its expression was increased by varying
degrees by the addition of KGN, particularly at the high
concentration (Fig. 3A–C).
Effects of KGN on aggrecan and Col II
gene expression
The Col II and aggrecan mRNA expression in hNPCs and
mouse NPCs was determined to identify whether KGN can increase the
gene expression of Col II and aggrecan (Fig. 4A–H). In Fig. 4, the mRNA expression of Col II and
aggrecan was reduced in the groups treated with inflammatory
cytokines compared with the control group. However, their
expression was increased following treatment with KGN, particularly
at the high concentration.
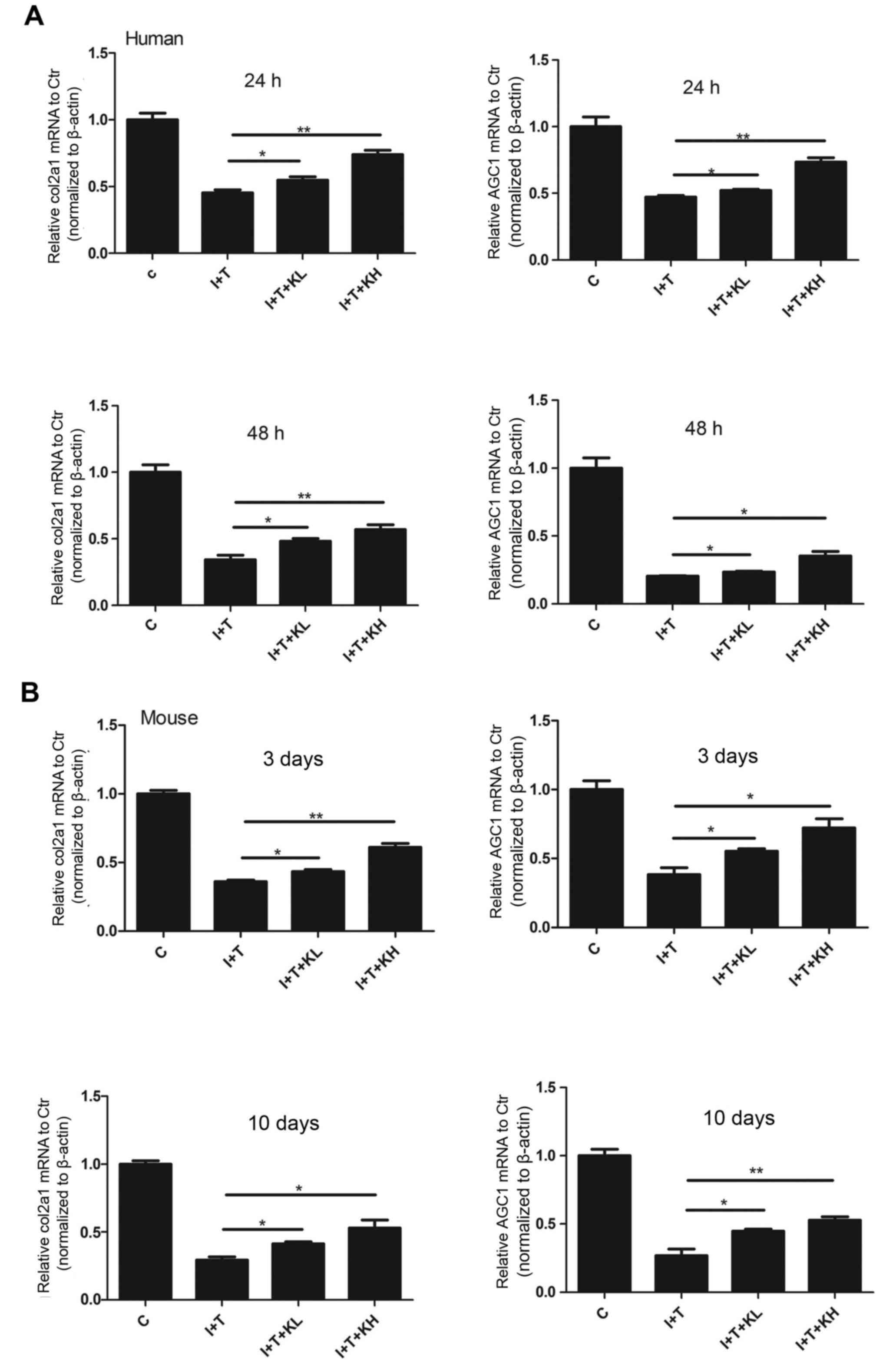 | Figure 4KGN increased the mRNA expression of
Col II and aggrecan. Reverse transcription-quantitative polymerase
chain reaction of genes associated with Col II and aggrecan in (A)
human nucleus pulposus cells and (B) mouse intervertebral discs.
The inflammatory cytokines significantly inhibited the expression
of genes associated with Col II and aggrecan. Their expression was
elevated by adding KGN; although their expression levels did not
reach that of the blank control group, the difference was still
statistically significant compared with the group under
inflammatory cytokines (*P<0.05 and
**P<0.001). Col II, type II collagen; col2a1,
collagen type II α 1 chain; AGC1, aggrecan; KGN, kartogenin; IL-1β,
interleukin-1β; TNF-α, tumor necrosis factor-α; C, control; I+T,
IL-1β + TNF-α; I+T+KL, IL-1β + TNF-α + low concentration of KGN;
I+T+KH, IL-1β + TNF-α + high concentration of KGN. |
Effects of KGN on the gene expression of
RUNX2
To investigate the mechanism of KGN in secretion of
Col II and aggrecan expression, RUNX2 mRNA expression in NPCs was
determined. The expression of RUNX2 mRNA was reduced by the
cytokines; whereas, it was significantly increased by co-treatment
with the KGN and the cytokines, compared with cytokine treatment
alone (Fig. 5).
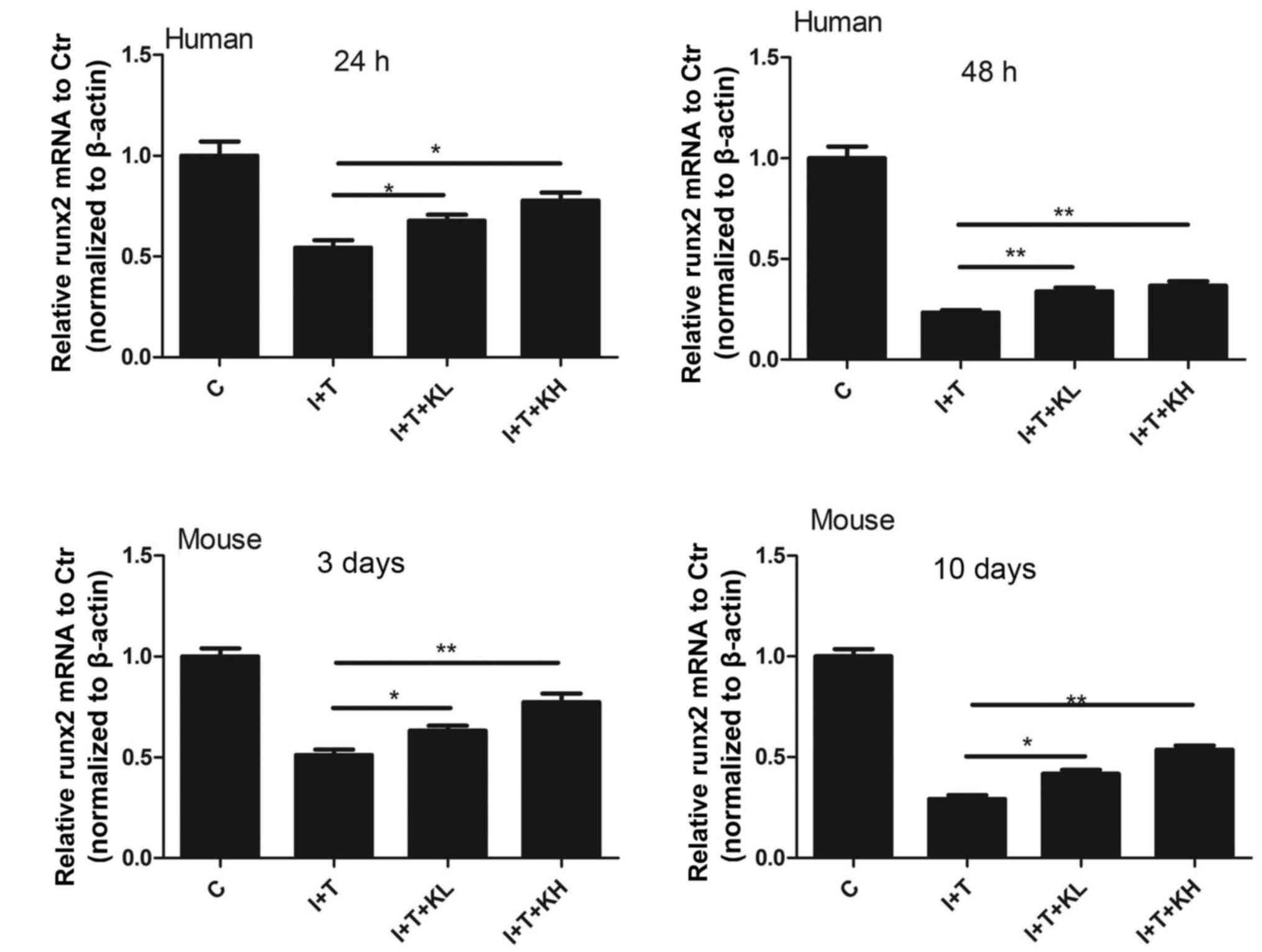 | Figure 5RUNX2 expression was elevated by KGN.
RUNX2 expression in human nucleus pulposus cells and mouse
intervertebral discs were inhibited by inflammatory cytokines.
However, it was elevated after the treatment of KGN, especially at
a high concentration (*P<0.05 and
**P<0.001). RUNX2, runt related transcription factor
2; KGN, kartogenin; IL-1β, interleukin-1β; TNF-α, tumor necrosis
factor-α; C, control; I+T, IL-1β + TNF-α; I+T+KL, IL-1β + TNF-α +
low concentration of KGN; I+T+KH, IL-1β + TNF-α + high
concentration of KGN. |
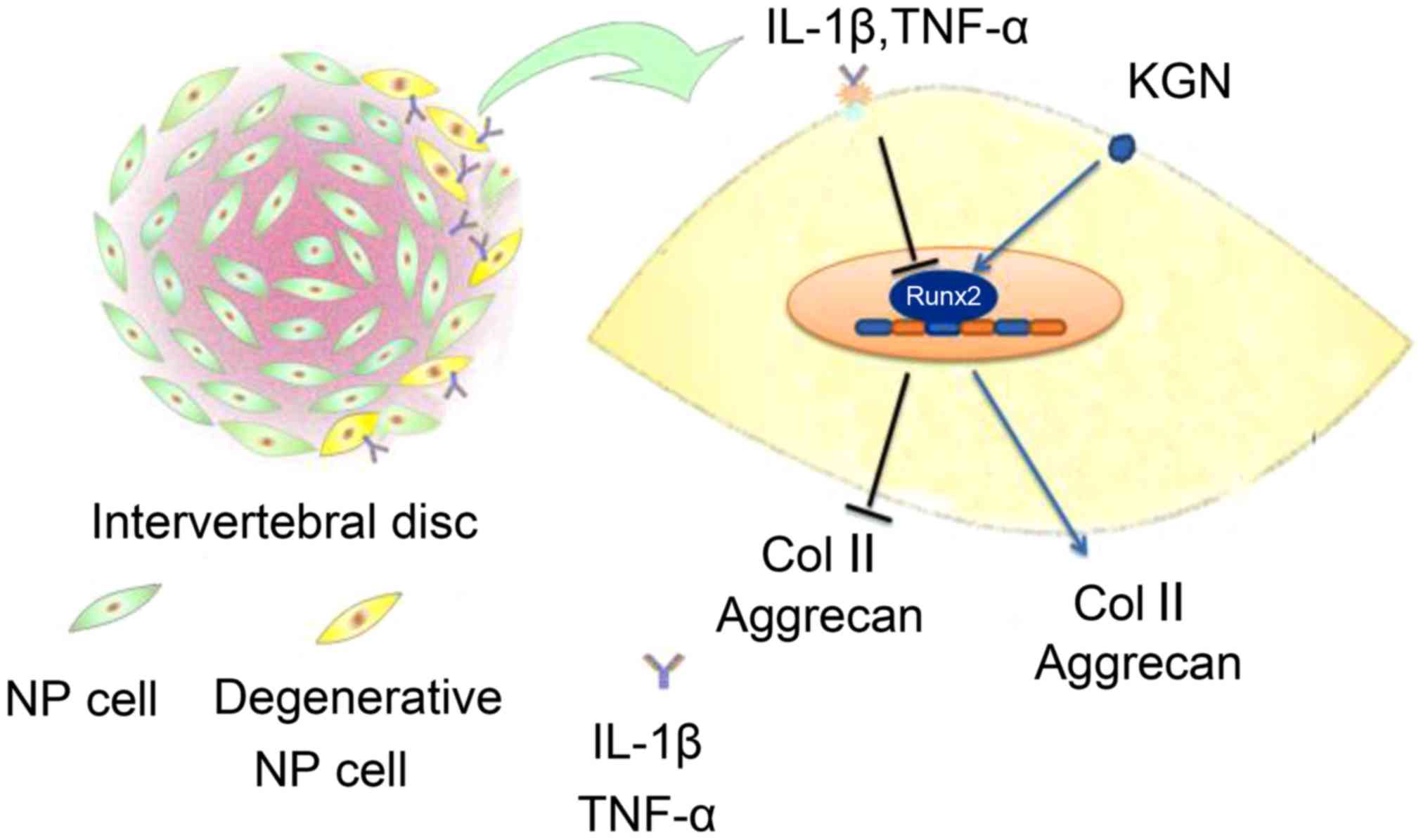
IL-1β and TNF-α inhibited the secretion of Col II
and aggrecan in NPCs and suppressed RUNX2 expression, which is the
critical transcription factor required for Col II and aggrecan gene
expression. KGN effectively increased the expression of RUNX2 and
reversed the degeneration of NPCs induced by IL-1β and TNF-α
(Fig. 6).
Discussion
The current study aimed to determine whether KGN can
slow the degeneration of intervertebral discs. In degenerative
intervertebral discs, the expression of several other members of
the IL family is elevated, including IL-1, IL-2, IL-6, IL-8, IL-12
and IL-17. In addition, upregulated expression of interferon γ,
nitric oxide (NO) and prostaglandin E2 (PGE2)
released TNF-α, inducible NO, and PG, respectively, as detected in
degenerative intervertebral discs. The expression of IL-1β and
TNF-α is considered particularly important in intervertebral disc
degeneration (19). IL-1β
promotes the release of matrix metalloproteinase, which can degrade
Col II and aggrecan (20), and
affect the metabolism of cartilage matrix (21). TNF-α is associated with NPC
apoptosis, disc protrusion and nerve root irritation (22).
Models have been established for the degeneration in
intervertebral discs, such as the disc puncture model and the
annulus fibrosus cutting model for animals standing upright.
However, these models do not fully simulate the degeneration of
intervertebral discs (23). In
this study, a novel model for the degeneration in intervertebral
discs was adopted. The in vitro organ culture of
intervertebral discs effectively simulated the slow degeneration of
intervertebral discs; a single intervertebral disc with the upper
and lower endplates was dissected from mice and maintained in a
culture medium containing IL-1β and TNF-α (24).
Johnson et al (16) used high-throughput screening and
identified that KGN has a low molecular weight (<1,000), as well
as a simple structure, specific targets, and fewer side effects,
thereby making it superior to macromolecular polypeptide drugs. KGN
binds to fibroin A, thus disrupting it's binding to CBFβ. Genes of
the Runt domain protein family are collectively referred to as
'RUNXX'. The transcription factor of the RUNXX family can bind to
CBFβ, thus producing heterodimers and conferring a stronger ability
to bind to DNA. CBFβ can bind to RUNX1, 2 and 3 and activate the
transcription of Col II and aggrecan (15,17). Takeda et al (25) reported that RUNX2 may promote the
maturation of chondrocytes. The expression of RUNX2 is initially
high in chondrocytes and the hypertrophic zone, thus inducing the
hypertrophy of chondrocytes (26). Therefore, the expression of Col II
and aggrecan are sustained in hypertrophic chondrocytes.
Additionally, KGN may also induce the Col II secretion in dermal
fibroblasts via the Smad4/5 pathway (27).
Notably, KGN may facilitate MSC differentiation into
chondrocytes, maintain chondrocyte functions and promote the
secretion of chondrocyte-associated proteins. Kang et al
(17) recently reported that
injection of KGN into the articular cavity regulates the
regeneration of chondrocytes and promotes the expression of Col II
and aggrecan. Xu et al (28) reported that KGN may effectively
repair articular cartilage damage and elevate the expression of Col
II and aggrecan. NPCs are cartilage-like cells with features
similar to those of chondrocytes. Therefore, KGN may slow the
degeneration of these cells.
Previous studies suggested that the degeneration of
NPCs is a major cause of intervertebral disc degeneration. The
increase or reduction of Col II and aggrecan secreted by NPCs may
lead to the degeneration of intervertebral discs. Degeneration
models using hNPCs and mouse intervertebral discs exposed to
inflammatory cytokines have been established in the current study.
The expression of Col II and aggrecan were evaluated using
immunofluorescence, immunohistochemistry and western blot analysis.
Particularly at high concentration used, KGN increased the
expression of Col II and aggrecan to varying degrees. The mRNA
expression of Col II and aggrecan was also altered by the
inflammatory cytokines.
In addition, KGN treatment increased RUNX2 mRNA
levels, which is the key transcription factor required for Col II
and aggrecan expression in NPCs (15,17).
The intervertebral disc is a specialized tissue that
is relatively closed in vivo; all substances are slowly
transported via small blood vessels in the two endplates and
annulus fibrosus. However, the transport of drugs to the
intervertebral discs following injection via the abdominal cavity
of mice is extremely difficult (29). None of the in vivo mouse
models of intervertebral disc degeneration can effectively simulate
the slow degeneration of normal intervertebral discs. Thus, a
limitation of the present study is that the effects of KGN on the
degeneration of intervertebral discs under inflammatory cytokines
may not be possible by direct injection in vivo. In
addition, the ex vivo model used in the present study did
not involve the two endplates, although KGN has the ability to
regulate the two endplates. Unfortunately, the intervertebral disc
has a special form. Specific in vivo biological reactions
occur between the endplates and nucleus pulposus, and between the
annulus fibrosus and nucleus pulposus. Studies confined to
chondrocytes or NPCs cannot effectively simulate the presence of a
nucleus pulposus with high permeability, low pH and hypoxemia in
vivo. The present study used ex vivo organ cultures,
where the end plates are retained following removal of the upper
and lower vertebrae, thus effectively simulating the in vivo
state of NPCs and the slow degeneration of intervertebral
discs.
In conclusion, combined with previous studies on the
effects of KGN on hNPCs, a potential method has been developed to
slow the degeneration of intervertebral discs. However, a safe or
effective drug has not yet been identified to eliminate
intervertebral disc degeneration. To the best of our knowledge, the
present study is the first to report that KGN can effectively slow
the degeneration of intervertebral discs exposed to inflammatory
cytokines. Therefore, in addition to being a safe and effective
drug for treating osteoarthritis, KGN presents a novel approach to
eliminate degeneration in intervertebral discs and produces new
possibilities for investigating intervertebral disc degeneration
under the actions of inflammatory cytokines.
Acknowledgments
This study was supported by grants from the NSFC
(no. 81520108018).
References
|
1
|
Andersson GB: Epidemiological features of
chronic low-back pain. Lancet. 354:581–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katz JN: Lumbar disc disorders and
low-back pain: socioeconomic factors and consequences. J Bone Joint
Surg A. 88(Suppl 2): 21–24. 2006.
|
|
3
|
Jenkins H: Classification of low back
pain. Australas Chiropr Osteopathy. 10:91–97. 2002.
|
|
4
|
Le Maitre CL, Freemont AJ and Hoyland JA:
The role of interleukin-1 in the pathogenesis of human
intervertebral disc degeneration. Arthritis Res Ther. 7:R732–R745.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Séguin CA, Pilliar RM, Roughley PJ and
Kandel RA: Tumor necrosis factor-alpha modulates matrix production
and catabolism in nucleus pulposus tissue. Spine. 30:1940–1948.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le Maitre CL, Hoyland JA and Freemont AJ:
Catabolic cytokine expression in degenerate and herniated human
intervertebral discs: IL-1beta and TNFalpha expression profile.
Arthritis Res Ther. 9:R772007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian Y, Yuan W, Fujita N, Wang J, Wang H,
Shapiro IM and Risbud MV: Inflammatory cytokines associated with
degenerative disc disease control aggrecanase-1 (ADAMTS-4)
expression in nucleus pulposus cells through MAPK and NF-κB. Am J
Pathol. 182:2310–2321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Tian Y, Wang J, Phillips KL, Binch
AL, Dunn S, Cross A, Chiverton N, Zheng Z, Shapiro IM, et al:
Inflammatory cytokines induce NOTCH signaling in nucleus pulposus
cells: implications in intervertebral disc degeneration. J Biol
Chem. 288:16761–16774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Pan H, Yang H, Wang J, Zhang K, Li
X, Wang H, Ding W, Li B and Zheng Z: LIM mineralization protein-1
suppresses TNF-alpha induced intervertebral disc degeneration by
maintaining nucleus pulposus extracellular matrix production and
inhibiting matrix metalloproteinases expression. J Orthop Res.
33:294–303. 2015. View Article : Google Scholar
|
|
10
|
Yang X and Li X: Nucleus pulposus tissue
engineering: a brief review. Eur Spine J. 18:1564–1572. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang SZ, Rui YF, Lu J and Wang C: Cell and
molecular biology of intervertebral disc degeneration: current
understanding and implications for potential therapeutic
strategies. Cell Prolif. 47:381–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vasiliadis ES, Pneumaticos SG,
Evangelopoulos DS and Papavassiliou AG: Biologic treatment of mild
and moderate intervertebral disc degeneration. Mol Med. 20:400–409.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakai D and Grad S: Advancing the cellular
and molecular therapy for intervertebral disc disease. Adv Drug
Deliv Rev. 84:159–171. 2015. View Article : Google Scholar
|
|
14
|
Masuda K: Biological repair of the
degenerated intervertebral disc by the injection of growth factors.
Eur Spine. 17(Suppl 4): 441–451. 2008. View Article : Google Scholar
|
|
15
|
Marini JC and Forlino A: Replenishing
cartilage from endogenous stem cells. N Engl J Med. 366:2522–2524.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson K, Zhu S, Tremblay MS, Payette JN,
Wang J, Bouchez LC, Meeusen S, Althage A, Cho CY, Wu X, et al: A
stem cell-based approach to cartilage repair. Science. 336:717–721.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang ML, Ko JY, Kim JE and Im GI:
Intra-articular delivery of kartogenin-conjugated chitosan
nano/microparticles for cartilage regeneration. Biomaterials.
35:9984–9994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Johnson ZI, Schoepflin ZR, Choi H, Shapiro
IM and Risbud MV: Disc in flames: roles of TNF-α and IL-1β in
intervertebral disc degeneration. Eur Cell Mater. 30:104–116. 2015.
View Article : Google Scholar
|
|
20
|
Patel KP, Sandy JD, Akeda K, Miyamoto K,
Chujo T, An HS and Masuda K: Aggrecanases and aggrecanase-generated
fragments in the human intervertebral disc at early and advanced
stages of disc degeneration. Spine. 32:2596–2603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Videman T, Saarela J, Kaprio J, Näkki A,
Levälahti E, Gill K, Peltonen L and Battié MC: Associations of 25
structural, degra-dative, and inflammatory candidate genes with
lumbar disc desiccation, bulging, and height narrowing. Arthritis
Rheum. 60:470–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Igarashi T, Kikuchi S, Shubayev V and
Myers RR: 2000 Volvo Award winner in basic science studies:
exogenous tumor necrosis factor-alpha mimics nucleus
pulposus-induced neuropathology. Molecular, histologic, and
behavioral comparisons in rats. Spine. 25:2975–2980. 2000.
View Article : Google Scholar
|
|
23
|
Beierfuss A, Dietrich H, Kremser C,
Hunjadi M, Ritsch A, Rulicke T, Thome C and Mern DS: Knockout of
Apolipoprotein E in rabbit promotes premature intervertebral disc
degeneration: A new in vivo model for therapeutic approaches of
spinal disc disorders. PloS One. 12:pp. e01875642017, View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pelle DW, Peacock JD, Schmidt CL,
Kampfschulte K, Scholten DJ II, Russo SS, Easton KJ and Steensma
MR: Genetic and functional studies of the intervertebral disc: a
novel murine intervertebral disc model. PLoS One. 9:pp.
e1124542014, View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takeda S, Bonnamy JP, Owen MJ, Ducy P and
Karsenty G: Continuous expression of Cbfa1 in nonhypertrophic
chondrocytes uncovers its ability to induce hypertrophic
chondrocyte differentiation and partially rescues Cbfa1-deficient
mice. Genes Dev. 15:467–481. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Studer D, Millan C, Öztürk E,
Maniura-Weber K and Zenobi-Wong M: Molecular and biophysical
mechanisms regulating hypertrophic differentiation in chondrocytes
and mesenchymal stem cells. Eur Cell Mater. 24:118–135. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Zhou J, Zhang N, Zhang X and Li Q:
A heterocyclic molecule kartogenin induces collagen synthesis of
human dermal fibroblasts by activating the smad4/smad5 pathway.
Biochem Biophys Res Commun. 450:568–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu X, Shi D, Shen Y, Xu Z, Dai J, Chen D,
Teng H and Jiang Q: Full-thickness cartilage defects are repaired
via a microfracture technique and intraarticular injection of the
small-molecule compound kartogenin. Arthritis Res Ther. 17:202015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kepler CK, Ponnappan RK, Tannoury CA,
Risbud MV and Anderson DG: The molecular basis of intervertebral
disc degeneration. Spine J. 13:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|