Introduction
Pulmonary fibrosis (PF) is a chronic, progressive,
irreversible and lethal pulmonary disease, and currently, no
effective treatments are available (1,2).
There is evidence to indicate that epithelial-mesenchymal
transition (EMT) is closely associated with the process of fibrosis
(3). EMT involves profound
phenotypic changes, including the loss of cell-cell adhesion and
cell polarity, and endows cells with a migratory ability and
involves the synthesis of the extracellular matrix (4).
Transforming growth factor-β1 (TGF-β1) is a
multifunctional cytokine that regulates a wide range of biological
phenomena, including tissue morphogenesis, differentiation and
extracellular matrix remodeling (5). TGF-β1 can effectively induce the
conversion of alveolar type II epithelial cells into mesenchymal
cells. TGF-β1-mediated EMT in several cell types is primarily
dependent on the canonical TGF-β1/Smad signaling pathway (6,7).
Smad proteins are the key molecules in the process of TGF-β1 signal
transduction, and among these, Smad3 is a main substrate of TGF-β1
type I receptor kinase and mediates signal transduction in the cell
biology (8,9).
Bone marrow-derived mesenchymal stem cells (BMSCs)
are pluripotent stem cells derived from bone marrow, with the
potential of self-renewal and differentiation into various cell
types, such as adipocytes, cardiomyocytes (10), endotheliocytes and neurons
(11). BMSCs can perceive damage
signals and migrate into the damaged area. BMSCs also have a
stronger immune regulatory function, and can avoid immune rejection
when in allografts (12).
Moreover, it has been demonstrated that BMSCs have
anti-inflammatory and regenerative properties (13). In addition, certain studies have
indicated that BMSCs can modify the tissue microenvironment and
repair the damaged cells via secreting soluable factors. Such
soluble factors secreted from BMSCs play a considerable role in
terms of the regulation of the immune system, and are associated
with anti-inflammatory and anti-fibrotic effects (14–16). Since the transplantation of BMSCs
is associated with poor differentiation and survival rates, the
utilization of conditioned medium (CM) including trophic factors
from BMSCs has emerged as an alternative method (17,18). In a previous study, we
demonstrated that BMSCs exhibit anti-fibrotic properties by
alleviating the pathological state of silicosis, reducing the
inflammatory reaction and suppressing the deposition of collagen.
In addition, we also confirmed that BMSCs in lung tissues of rats
with silicosis inhibited the expression of tumor necrosis factor
(TNF)-α and TGF-β, and promoted the restoration of lung injury via
interleukin-1 receptor antagonist (IL-1RA) (19). However, whether BMSCs-CM can
mediate the EMT process, as well as the mechanism involved in this
process remain unknown.
In this study, to explore the effect of BMSCs-CM on
the development of EMT, we used A549 cells to confirm whether
BMSCs-CM can suppress the EMT process. Additionally, to elucidate
the possible molecular mechanisms responsible for the protective
effect of BMSCs-CM, we examined the levels of E-cadherin (E-calcium
mucins; E-cad), CK8, α-smooth muscle actin (α-SMA), vimentin,
p-Smad3, Snail1 and collagen I (COLI) and collagen III (COLIII).
This study provides some major theoretical and experimental
fundamental principles for BMSCs-CM as a novel therapeutic
agent.
Materials and methods
Culture of BMSCs
All experiments using rats were performed in
compliance with the guidelines of the national Institutes of Health
Guide for Care and use of Laboratory Animals. The experimental
procedures were approved by the north China university of Science
and Technology Experimental Ethics Committee. BMSCs were generated
from male Sprague-Dawley (SD) rats (n=15; 3–6 weeks old; weighing
100–120 g; obtained from Beijing Weitong Lihua, Beijing, China).
Rats were anesthetized with an intraperitoneal injection of chloral
hydrate (350–400 mg/kg) and were then sacrificed by cervical
dislocation. Fresh bone marrow cells were collected by flushing the
medullary cavity of rat femurs with Dulbecco's modified Eagle's
medium (DMEM; Gibco-BRL, Carlsbad, CA, USA). After filtering, the
cells were centrifuged at 1,000 rpm for 5 min. Purified cells were
dispersed into cell culture flasks (Corning Life Sciences,
Tewksbury, MA, USA), grown in DMEM supplemented with 10% fetal
bovine serum (FBS; Gibco-BRL), 100 U/ml penicillin and 100
μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and
then cultured at 37°C with 5% CO2. After 48 h,
non-adherent cells were removed and fresh medium was added. The
medium was replaced every 3 days. The adhered cells were allowed to
grow to approximately 90% confluency, and then trypsinized and
reseeded. Passage 3 (P3) BMSCs were used for this experiment.
Preparation of BMSCs-CM
After the 3rd generation of BMSCs had adhered to the
wall of the flasks for 24 h, they were washed with
phosphate-buffered saline (PBS) twice. The cells were then cultured
with serum-free DMEM, and the supernatant was collected after 48 h
(cells had reached a confluence of 80%). The collected supernatant
was then centrifuged at 1,000 rpm for 10 min. After the
supernatants were re-centrifuged at 3,000 rpm for 5 min, the CM was
collected with a clean filter to filter to eliminate any bacteria,
and then used in the later experiments.
Culture of A549 cells
The A549 cells were obtained from the Chinese
Academy of Science Cell Library (TCHu 150, Shanghai, China), and
were cultured in DMEM supplemented with 10% FBS (both from
Gibco-BRL) and 1% antibiotics (penicillin-streptomycin;
Sigma-Aldrich) in a humidified 5% CO2 at 37°C. The A549
cells were serum-starved for 24 h, and then stimulated with 5 ng/ml
TGF-β1 (Peprotech, Rocky Hill, NJ, USA) for 48 h.
Experiment groups
There were 4 experimental groups as follows: i) the
control group, in which cells were cultured in serum-free DMEM; ii)
the TGF-β1 stimulation group (TGF-β1 group), in which the cells
were cultured in serum-free DMEM and exposed to 5 ng/ml TGF-β1;
iii) the SIS3 inhibitor group (SIS3 group), in which the cells were
treated with 3 μM SIS3 (specific inhibitor of Smad3) which
was added 4 h prior to 5 ng/ml TGF-β1 exposure; iv) the BMSCs-CM
group (BMSCs-CM group), in which the BMSCs-CM was added prior to 5
ng/ml TGF-β1 exposure. Following incubation for 48 h, the
morphology of the cells undergoing EMT was observed under
phase-contrast microscope (CKX41; Olympus, Tokyo, Japan).
Observation of cell internal structure by
transmission electron microscopy (TEM)
The cells were digested with pancreatin and
centrifuged at 1,500 rpm for 5 min at 4°C. The cells were washed
with pre-cooled and sterile PBS, centrifuged under the same
conditions and the supernatant was discarded. The sediment was
fixed with 2.5% glutaraldehyde, and then subjected to electron
microscopy by first fixin gin 1% osmic acid, resin embedding and
sample preparation, and images were then acquired using a
transmission electron microscope (H-7650; Hitachi, Tokyo,
Japan).
Transwell migration assay
The migration ability of the cells was evaluated by
a Transwell migration chamber assay. The polycarbonate filters (8
μm pore size) were purchased from Corning Life Sciences. The
cells (1×104/insert) that were suspended in 100
μl serum-free DMEM were added to the upper chamber, while
600 μl of complete medium were added to the lower chamber at
37°C in a humidified atmosphere with 5% CO2. Following
incubation for 24 h, the cells that had passed through the
polycarbonate filter were stained with crystal violet and
photographed using an inverted phase contrast microscope. Each
migration assay was carried out in triplicate and repeated in 3
independent experiments.
Immunofluorescence analysis
The slides of the cells were fixed in 4%
paraformaldehyde at room temperature for 48 h, and washed 3 times
in PBS. The cell samples were permeabilized with 0.4% Triton X-100
for 30 min at room temperature, then washed with PBS. Following
incubation with blocking solution (5% normal goat serum) for 1 h,
the cells were incubated at 4°C overnight with 150 μl
diluted primary antibody mixture [E-cad (dilution, 1:50; 610181; BD
Transduction Laboratories, Franklin Lakes, NJ, USA), α-SMA
(dilution, 1:1,000; ab32575), p-Smad3 (dilution, 1:100; ab52903;
both from Abcam Ltd., Shanghai, China)]. The following day, after
being washed with PBS, secondary antibodies labeled with
tetramethyl rhodamine isothiocyanate (TRITC; Abcam, Cambridge, MA,
USA) and fluorescein isothiocyanate (FITC; BD Transduction
Laboratories) were added to each slide and the slides were
incubated at room temperature in a wet dark box for 2 h. Then
slides were then incubated with 4′,6-diamidino-2-phenylindole
(DAPI; Sigma-Aldrich) for 10 min. Fluorescent images were obtained
using a super resolution laser confocal microscope (Leica TCS SP8
STED 3X; serial no. 8100001247; Wetzlar, Germany), and 5 fields
from each slide were randomly selected for observation and
imaging.
Western blot analysis
The cells were washed with PBS 3 times. RIPA lysis
buffer (Aidlab Biotechnologies Co., Ltd., Beijing, China) was added
to cover the cell surface, on ice for 30 min. The cells were
scraped from the 6-well plates, and collected in an EP tube. The
cell suspension was centrifuged at 12,000 x g for 15 min at 4°C and
the supernatant was collected and stored at −80°C. The protein
concentration of the samples was determined using the BCA reagent
(Solarbio, Beijing, China). Proteins from each sample were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), and then electrotransferred onto
polyvinylidene fluoride (PVDF) nitrocellulose membranes (Roche
Diagnostics, Mannheim, Germany). The membranes were then incubated
with primary antibodies [CK8 (dilution, 1:200; ab59400; Abcam),
E-cad (dilution, 1:3,000; 610181; BD Transduction Laboratories),
vimentin (dilution, 1:200; ab92547; Epitomics, Burlingame, CA,
USA), α-SMA (dilution, 1:2,000; ab32575), p-Smad3 (dilution,
1:1,000; ab52903), Snail1 (dilution, 1:200; ab53519; Anbobio,
Shanghai, China), COLI (dilution, 1:500; sc-59772) and COLIII
(dilution, 1:500; sc-271249), β-actin (dilution, 1:500; sc-47778;
all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)],
overnight at 4°C. The following day, the membranes were incubated
with secondary antibodies [goat anti-rabbit IgG (dilution, 1:3,000;
SA1021) and goat anti-mouse IgG (dilution, 1:3,000; SA1022); both
from Wuhan Boster Biotechnology Ltd., Wuhan, China] for 2 h at
37°C. The immunoreactive bands were visualized by enhanced
chemiluminescence reagent (ECL; Bio-rad, Hercules, CA, USA). The
blots were scanned by densitometry, and the integrated density of
pixels was quantified using Image Quant 5.2 software (Molecular
Dynamics, Sunnyvale, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software. Data are presented as the means ± standard deviation
(SD). Each result represents data from at least 3 independent
experiments. One-way ANOVA was used to compare overall difference
in various groups. A P-value ≤0.05 was considered to indicate a
statistically significant difference.
Results
Morphological observation of BMSCs
The original generation of BMSCs cells gradually
adhered following inoculation for 24 h, and had completely adhered
within 48 h. The morphological characteristics of the primary cells
were circles and polygons, nuclear-centered and occasional
polygonal-shaped cells. Cell proliferated mainly in a clone mode.
BMSCs were purified by changing the medium several times and during
this process, hematopoietic stem cells and other non-adherent
growth cells were removed. After 10 days, primary cells which had
reached 95% confluence were passaged. Passaged cells overcame the
growth inhibition period and underwent accelerated growth.
Following passaging, cell morphology was more uniform and
consistent, forming mainly fibroblast-like flattened cells
(Fig. 1).
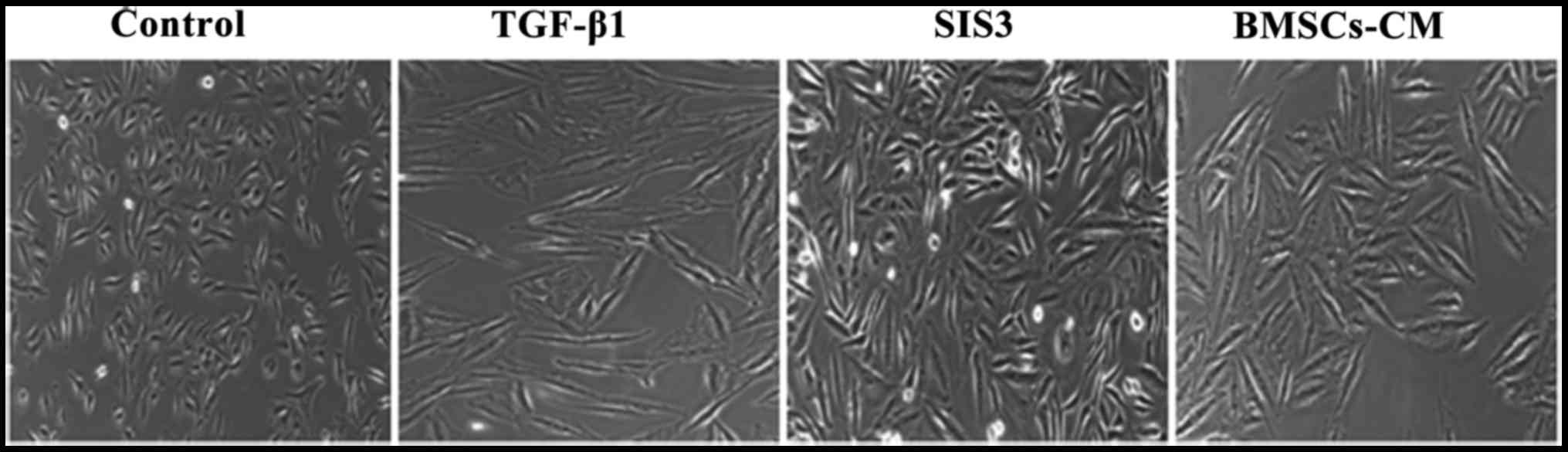
Changes in the morphological
characteristics of A549 cells
EMT was significantly promoted in the A549 cells
following exposure to 5 ng/ml TGF-β1 for 48 h. When the cells were
pretreated with the BMSCs-CM, EMT was attenuated, as shown in
Fig. 2. First, we investigated
the morphological changes in A549 cells following their exposure to
TGF-β1 for 48 h. Inverted phase contrast microscopy revealed that
the A549 cells has an epithelial cobblestone-like morphology, a
round or polygonal shape and exhibited very close cell-cell
proximity reminiscent of cellular tight-junctions in the control
group, while following exposure to TGF-β1, the cells displayed a
spindle-shaped elongated fibroblast-like morphology. These changes
became clearer with the passing of time. These morphological
changes became noticeably suppressed in the SIS3 group.
Furthermore, the cells in the BMSCs-CM group exhibited similar
morphological characteristics as those in the SIS3 grou (Fig. 2).
Changes in the lamellar corpuscle in A549
cells
TEM assay indicated that the characteristics of A549
cell osmiophilic multilamellar body had changed, as evidenced by
degeneration, swelling and the formatoin of vacuoles following
TGF-β1 exposure. However, these phenomena were attenuated in the
the SIS3 group and BMSCs-CM group (Fig. 3).
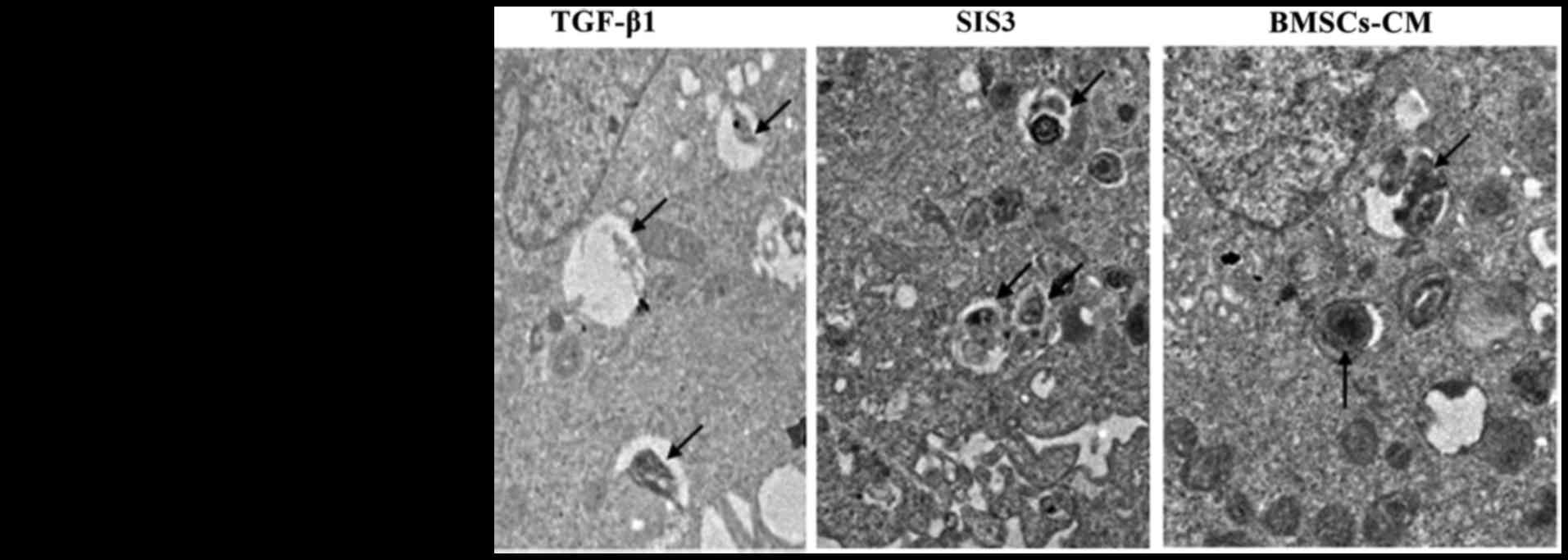 | Figure 3Ultrastructural evidence of EMT.
Normal A549 cells can be identified by the presence of LBs in the
cytoplasm. In the TGF-β1 group, LBs were degeneration, swollen and
with vacuoles. Arrows indicate LBs; magnification, ×7,000. The cell
groups were as follows: control, cells cultured in serum-free DMEM;
TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml
TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specifc
inhibitor of Smad3), which was added 4 h prior to 5 ng/ml TGF-β1
exposure; BMSCs-CM, BMSCs-CM was added prior to 5 ng/ml TGF-β1
exposure. Nu, cell nucleus; LB, lamellar body; EMT,
epithelial-mesenchymal transition; TGF-β1, transforming growth
factor-β1. |
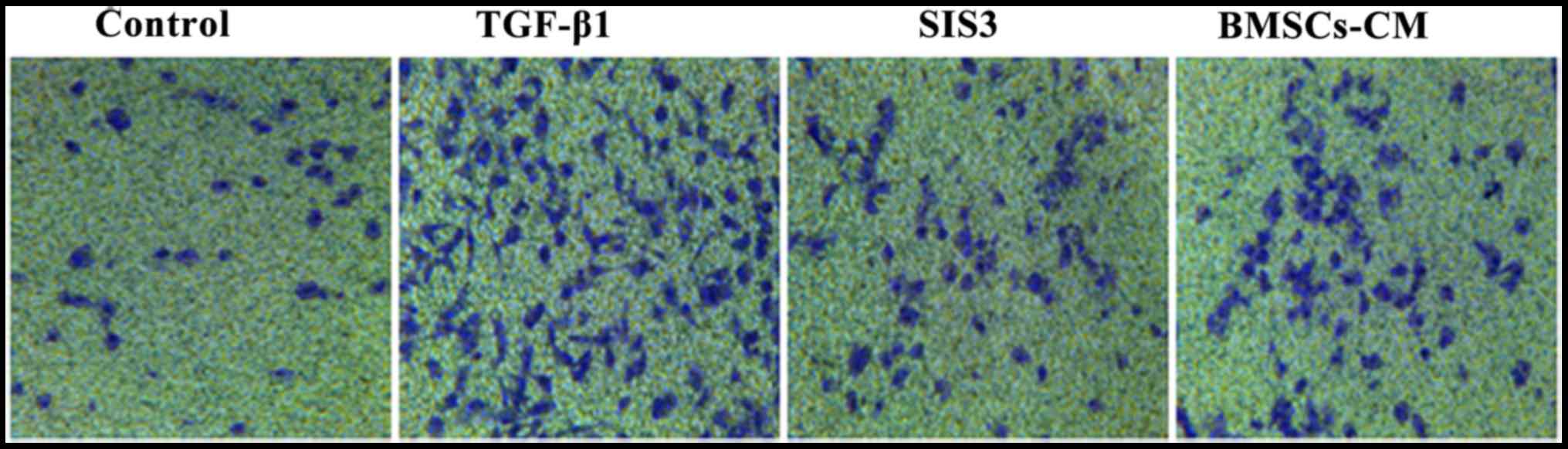
Effects of BMSCs-CM on the migratory
ability of A549 cells
As TGF-β1 has been reported to induce EMT (20–22), we used it as an experimental
system to evaluate the anti-metastatic potential of BMSCs-CM in
A549 cells. The migration assay revealed that the migratory ability
of the cells exposed to TGF-β1 was significantly enhanced compared
with that of the cells in the control group. However, the
TGF-β1-induced enhancement of the cell migratory ability was
substantially attenuated in the cells pre-treated with SIS3 or
BMSCs-CM compared with the TGF-β1 group. Moreover, the migratory
ability of the A549 cells in the BMSCs-CM group exhibited no
difference when compared with that of the cells in the SIS3 group
(Fig. 4).
Effects of BMSCs-CM on the expression of
epithelial cell markers and mesenchymal cell markers
To demonstrate the effect of BMSCs-CM on the EMT
process in A549 cells, we examined the expression levels of the
epithelial cell markers, E-cad and CK8, and the mesenchymal cell
markers, vimentin and α-SMA, by western blot analysis. As shown in
Fig. 5, the results revealed that
the expression levels of E-cad and CK8 were significantly
decreased, while the expression levels of α-SMA and vimentin were
increased following exposure to 5 ng/ml TGF-β1 compared with the
control group (P<0.05). By contrast, in the cells in the SIS3
group and the BMSCs-CM group the levels of vimentin and α-SMA were
decreased, while those of E-cad and CK8 were increased
(P<0.05).
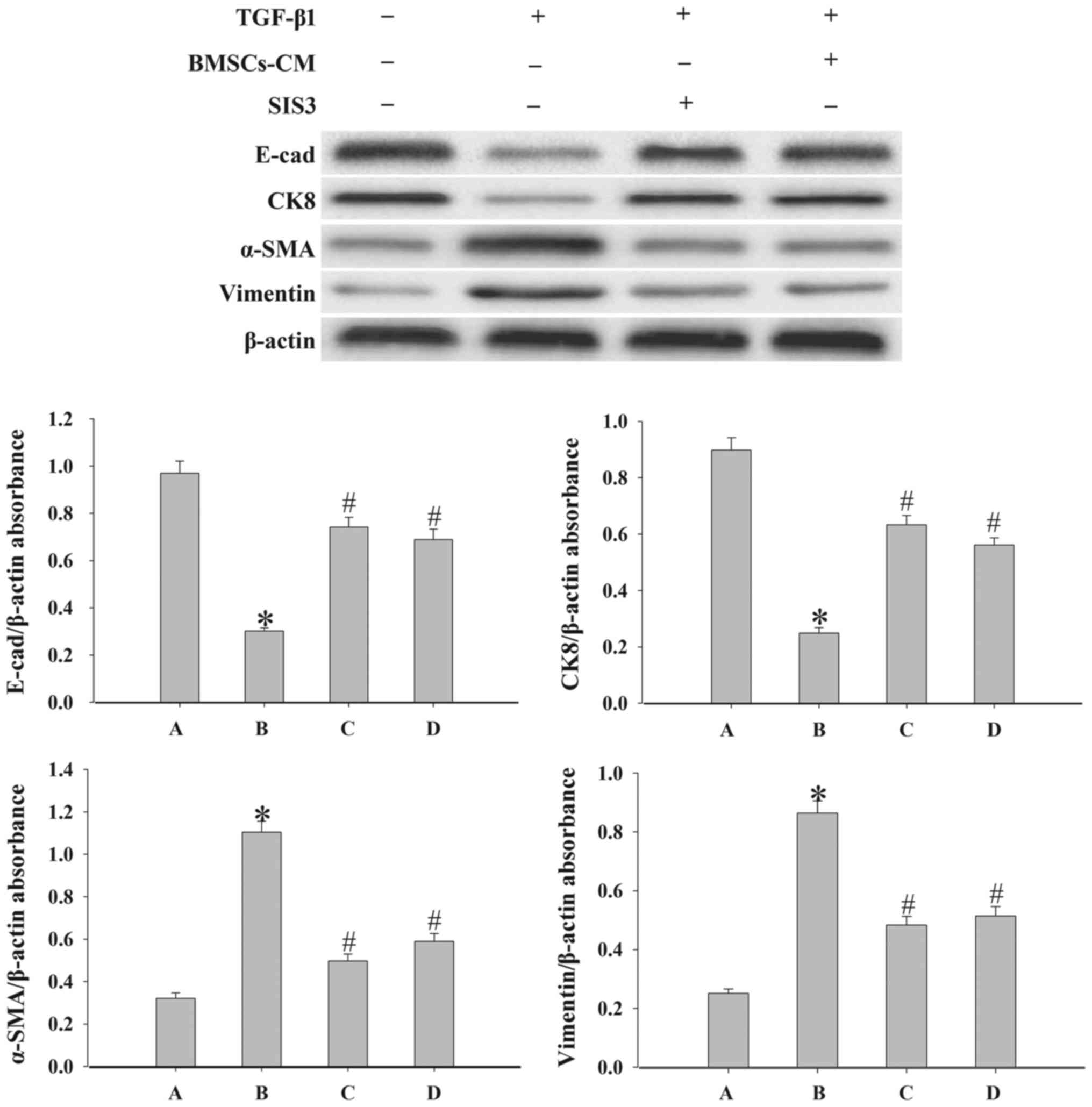 | Figure 5Evaluation of the epithelial cell
markers, E-cad and CK8, and the mesenchymal cell markers, α-SMA and
vimentin, expression in A549 cells. Western blot analysis was used
to examine the levels of E-cad, CK8, α-SMA and vimentin in the
cells. Densitometric analysis of protein expression with β-actin as
the control. The results demonstrated that treatment with BMSCs-CM
or SIS3 significantly increased the expression of E-cad and CK8,
and decreased α-SMA and vimentin protein expression following
exposure to TGF-β1 (*P<0.05 vs. control group;
#P<0.05 vs. TGF-β1 group). The bars are labeled as
follows: A, control group; B, TGF-β1 group; C, SIS3 group; and D,
BMSCs-CM group. The cell groups were as follows: control, cells
cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free
DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3
μM SIS3 (specific inhibitor of Smad3) which was added 4 h
prior to 5 ng/ml TGF-β1 exposure; BMSCs-CM, BMSCs-CM was added
prior to 5 ng/ml TGF-β1 exposure. BMSCs, bone marrow-derived
mesenchymal stem cells; CM, conditioned medium; TGF-β1,
transforming growth factor-β1; E-cad, E-calcium mucins; α-SMA,
α-smooth muscle actin. |
The results from immunofluorescence assay revealed
that E-cad fluorescence expression was decreased in the cell
membrane in the TGF-β1-exposed cells. By contrast, the expression
of E-cad in the cells in the BMSCs-CM group was inhibited to a
lesser extent compared with the cells in the TGF-β1 group. On the
other hand, α-SMA fluorescence expression was increased and
uniformly distributed in the cytoplasm in the TGF-β1-exposed cells.
However, the BMSCs-CM reduced the intensity of α-SMA fluorescence
expression and attenuated the spindle-like morphological changes.
The cells in the SIS3 group exhibited similar expression levels of
these markers as those in the BMSCs-CM group. These results
strongly suggest that BMSCs-CM promote the expression of E-cad and
suppress the expression of α-SMA (Fig. 6).
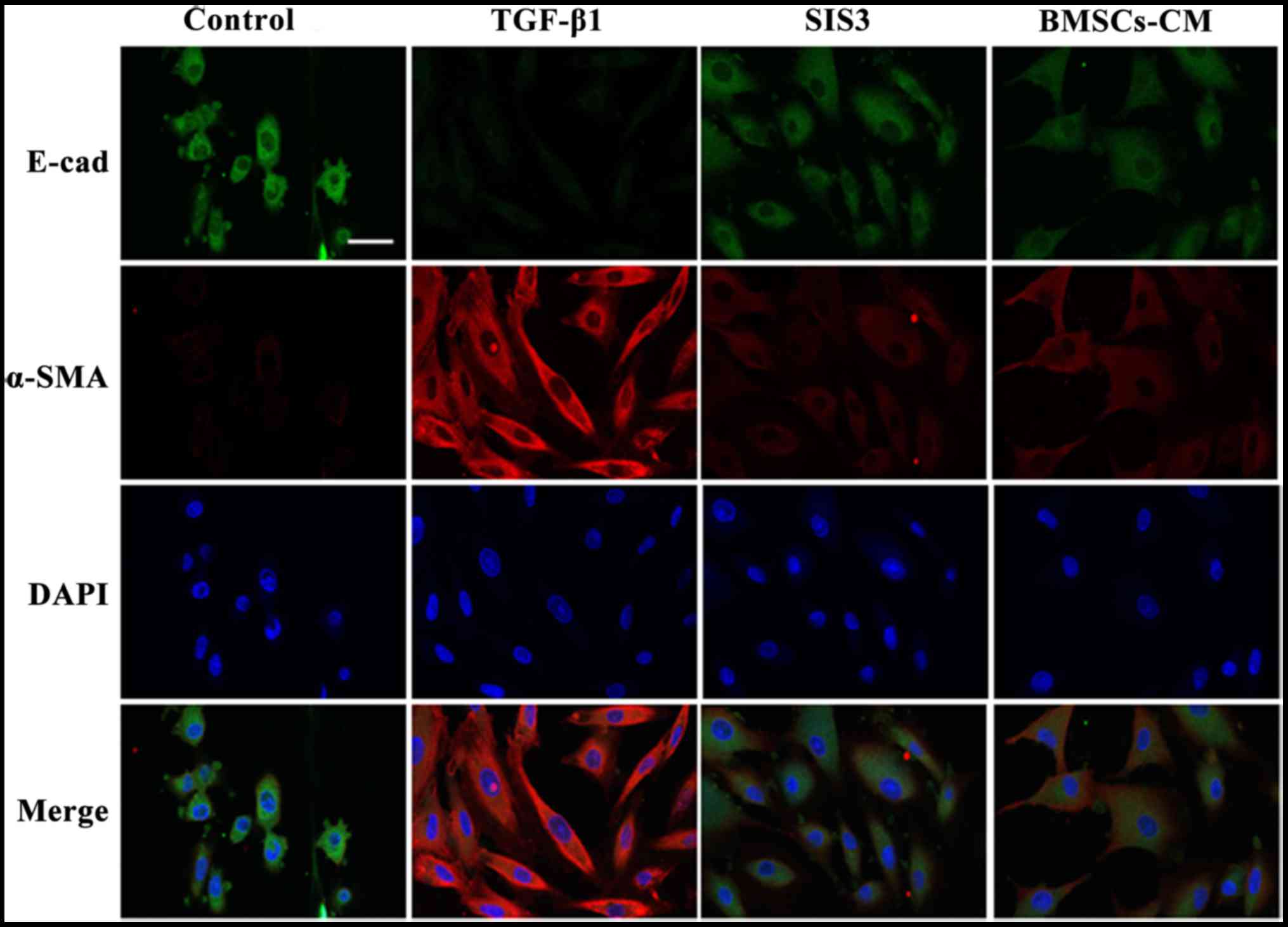 | Figure 6Co-localization of E-cad and α-SMA
occurs in A549 cells as determined by immunofluorescent staining.
E-cad (green) was present in the cytoplasm, FITC-stained; α-SMA
(red) was present in the cytoplasm, TRITC-stained. Cell nuclei were
counterstained with DAPI (blue). Scale bar, 40 μm. The cell
groups were as follows: control, cells cultured in serum-free DMEM;
TGF-β1, cells cultured in serum-free DMEM and exposed to 5 ng/ml
TGF-β1; SIS3, cells were treated with 3 μM SIS3 (specific
inhibitor of Smad3) which was added 4 h prior to 5 ng/ml TGF-β1
exposure; BMSCs-CM, BMSCs-CM was added prior to 5 ng/ ml TGF-β1
exposure. E-cad, E-cadherin (E-calcium mucins); α-SMA, α-smooth
muscle actin; TRITC, tetramethyl rhodamine isothiocyanate; FITC,
fluorescein isothiocyanate; DAPI,
4′,6-diamidino-2-phenylindole. |
BMSCs-CM regulate the EMT process by
inhibiting the TGF-β1/Smad3 signaling pathway
In order to elucidate the mechanisms responsible for
the effects of BMSCs-CM on EMT, we examined the expression levels
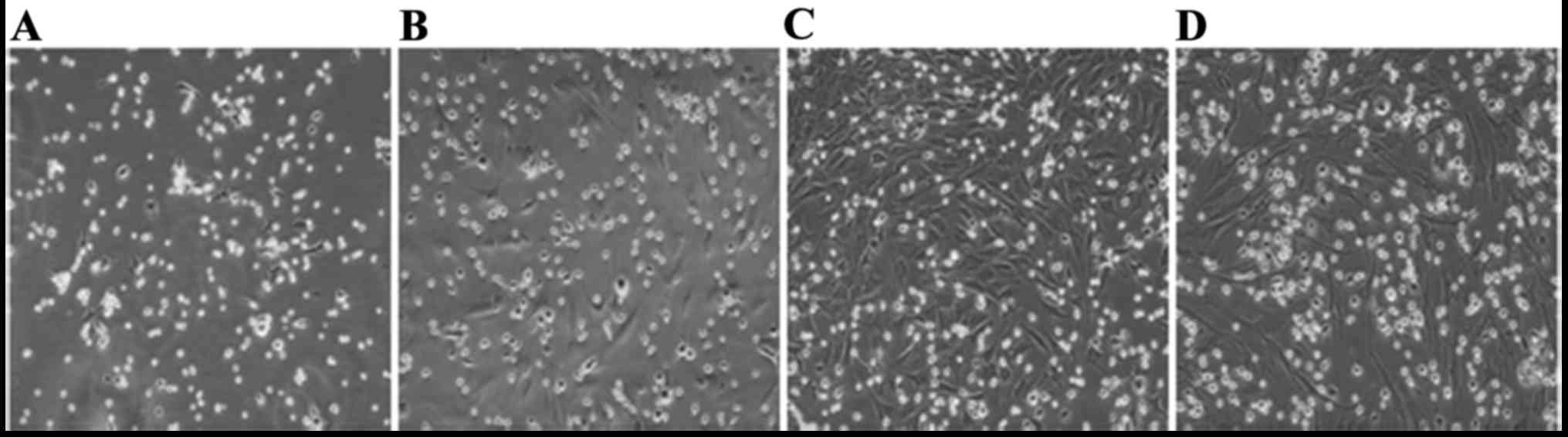
of p-Smad3, Snail1, COLI and COLIII by western blot analysis. SIS3
markedly decreased the expression of p-Smad3 that was induced by
TGF-β1 (P<0.05; Fig. 7).
However, SIS3 did not affect total Smad3 expression. In addition,
the results revealed that BMSCs-CM decreased the expression levels
of p-Smad3 and Snail1 (P<0.05; Fig. 7). In addition, the process of EMT
induced by TGF-β1 was accompanied by an increased expression of
COLI and COLIII (P<0.05; Fig.
8). However, when the cells were pre-treated with SIS3 or
BMSCs-CM, the expression levels of COLI, COLIII were decreased
(P<0.05).
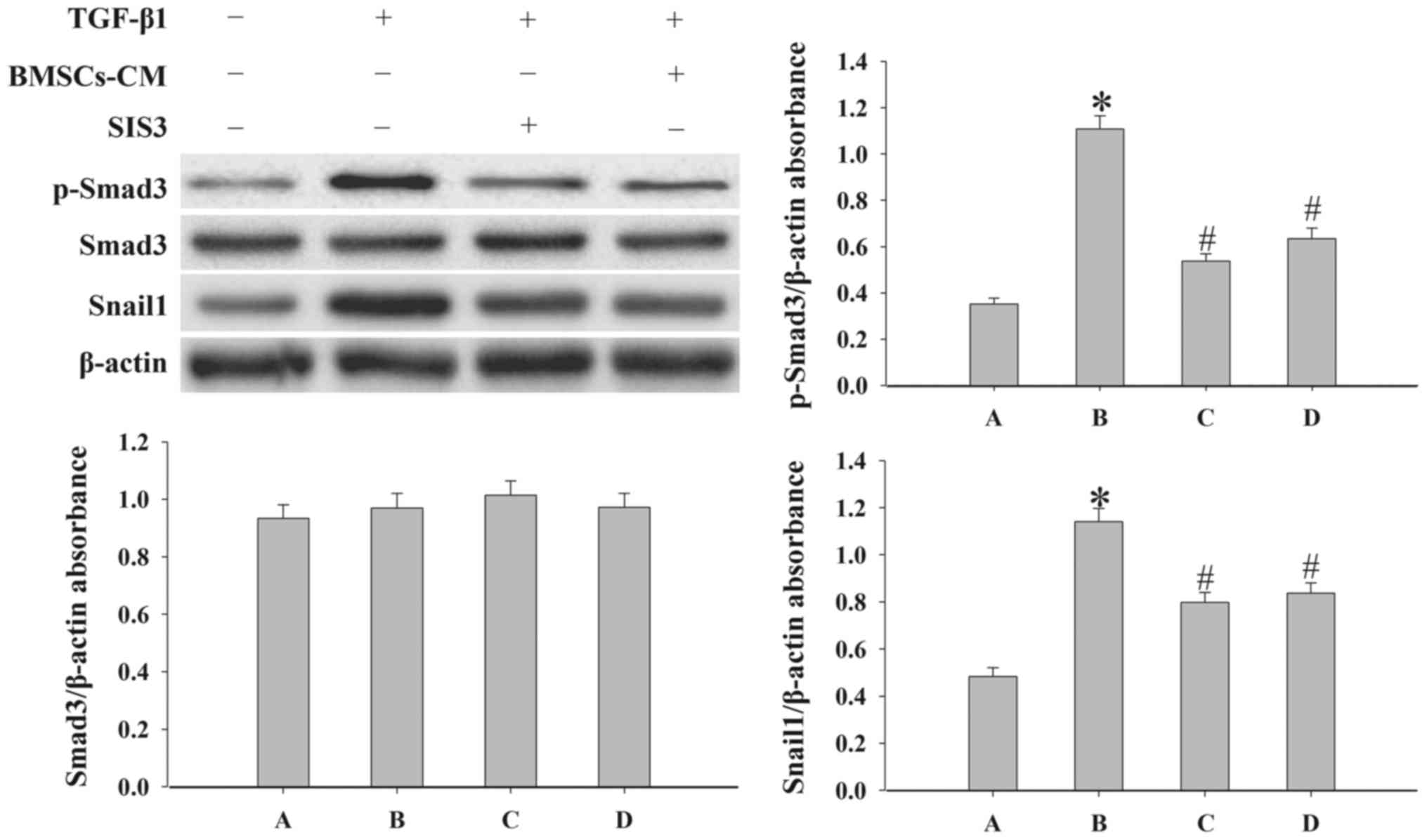 | Figure 7Evaluation of the expression levels
of p-Smad3, Smad3 and Snail1 in A549 cells. Western blot analysis
was used to examined the levels of p-Smad3, Smad3 and Snail1 in the
cells. Densitometric analysis of protein expression with β-actin as
the control. The results demonstrated that treatment with BMSCs-CM
or SIS3 significantly decreased the expression of p-Smad3 and
Snail1 following exposure to TGF-β1 (*P<0.05 vs.
control group; #P<0.05 vs. TGF-β1 group). The bars
are labeled as follows: A, control group; B, TGF-β1 group; C, SIS3
group; and D, BMSCs-CM group. The cell groups were as follows:
control, cells cultured in serum-free DMEM; TGF-β1, cells cultured
in serum-free DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were
treated with 3 μM SIS3 (specific inhibitor of Smad3) which
was added 4 h prior to 5 ng/ml TGF-β1 exposure. BMSCs-CM, BMSCs-CM
was added prior to 5 ng/ml TGF-β1 exposure. BMSCs, bone
marrow-derived mesenchymal stem cells; CM, conditioned medium;
TGF-β1, transforming growth factor-β1. |
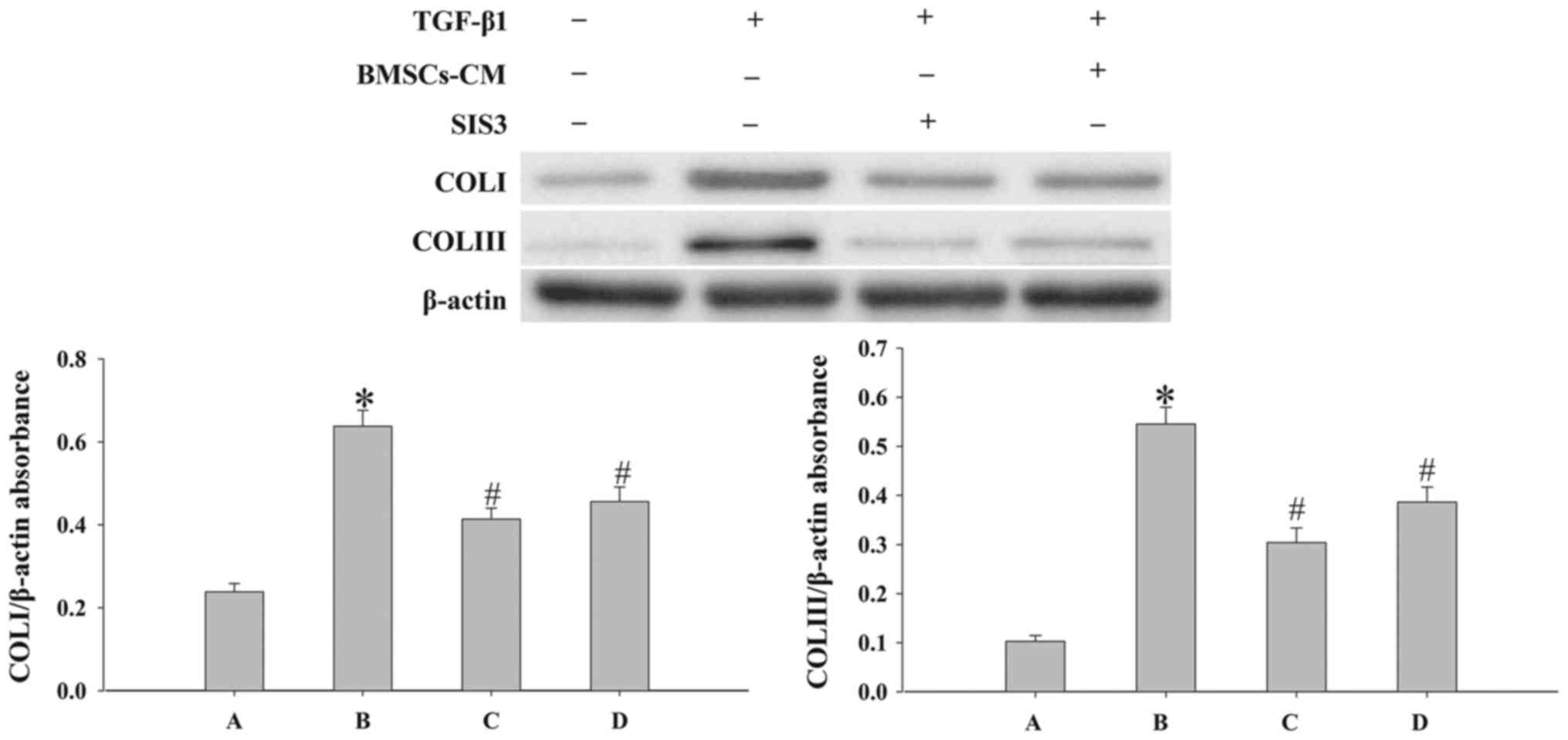 | Figure 8Evaluation of the expression levels
of COLI and COLIII in cells. Western blot analysis was used to
examine the levels of COLI and COLIII in the cells. Densitometric
analysis of protein expression with β-actin as the control. The
results demonstrated that treatment with BMSCs-CM or SIS3
significantly decreased the expression of COLI and COLIII following
exposure to TGF-β1 (*P<0.05 vs. control group;
#P<0.05 vs. TGF-β1 group). The bars are labeled as
follows: A, control group; B, TGF-β1 group; C, SIS3 group; and D,
BMSCs-CM group. The cell groups were as follows: control, cells
cultured in serum-free DMEM; TGF-β1, cells cultured in serum-free
DMEM and exposed to 5 ng/ml TGF-β1; SIS3, cells were treated with 3
μM SIS3 (specific inhibitor of Smad3) which was added 4 h
prior to 5 ng/ml TGF-β1 exposure. BMSCs, bone marrow-derived
mesenchymal stem cells; CM, conditioned medium; TGF-β1,
transforming growth factor-beta 1; COLI, collagen I. |
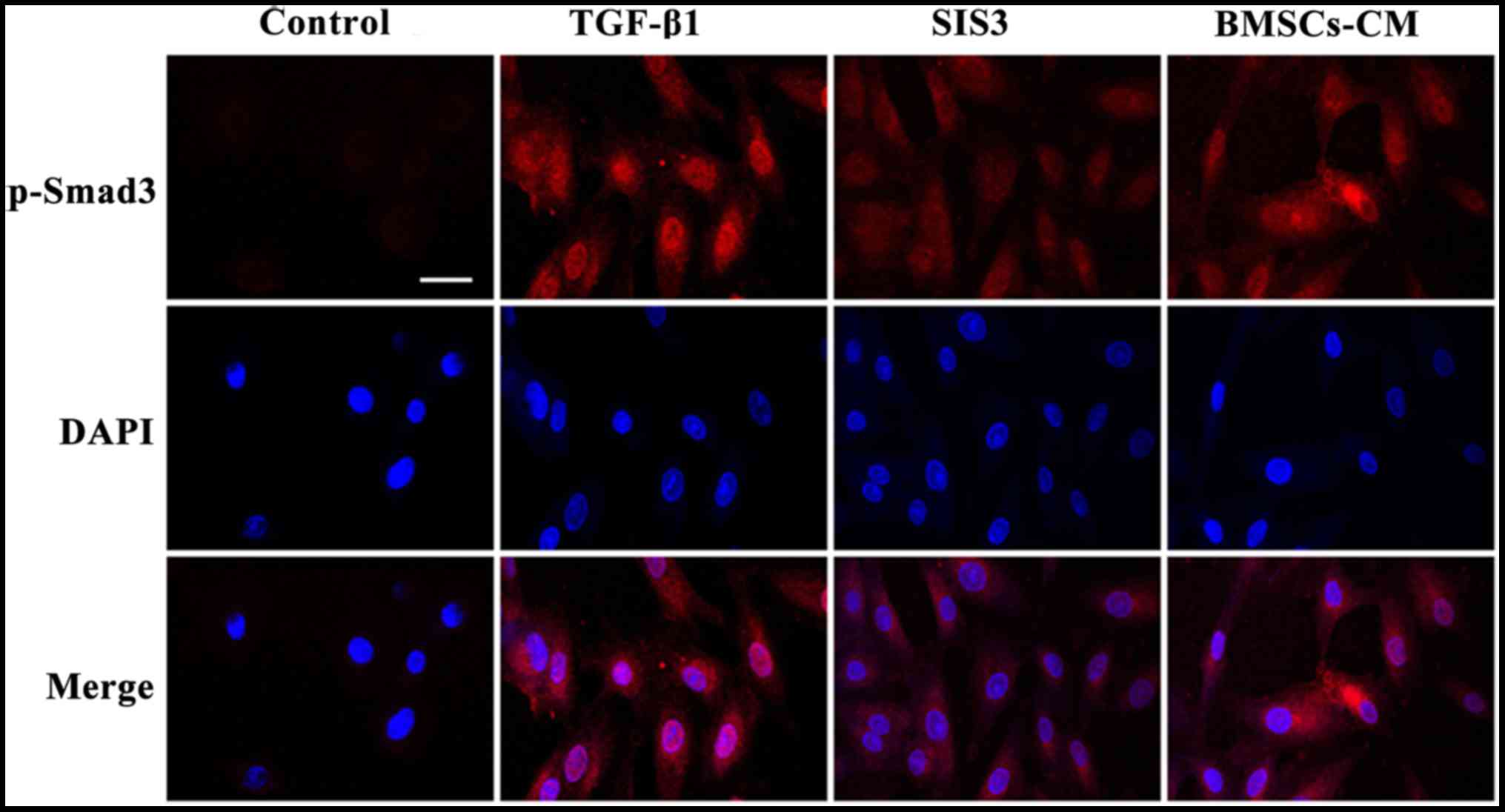
The results from immunofluorescence assay also
indicated that the A549 cells had an obvious nuclear expression of
p-Smad3 accompanied by a spindle-shaped-like morphology following
TGF-β1 exposure. At the same time, the fluorescent expression of
p-Smad3 was observed in the cytoplasm. Following pre-treatment with
SIS3, the TGF-β1-induced morphological changes were not evident and
the fluorescence intensity of p-Smad3 was weakened mainly in the
nucleus. Furthermore, no significant difference in fluorescence
expressions was observed between the BMSCs-CM group and the SIS3
group (Fig. 9).
Discussion
PF is a chronic, progressive, irreversible and
lethal pulmonary disease, with a poor prognosis and complex
pathogenesis. At present, the main aim of PF treatment is to
relieve symptoms as much as possible. EMT is a biological process
through which epithelial cells undergo a phenotypic transition into
mesenchymal cells. This process was initially identified in normal
tissue development, such as during embryonic development and
organogenesis (23). Previous
studies have suggested that EMT plays an important role in the
formation and development of organ fibrosis (24,25). Following TGF-β1 exposure in
vitro, the morphology of pleural mesothelial cells (MET-5A) has
been shown to change from a flat paved stone-like appearance into a
spindle-shaped appearance, and this is accompanied by a high α-SMA
expression and decreased E-cad expression (26). Individual cell motility has been
shown to be enhanced in this process, which is a feature of
TGF-β1-induced EMT (27). In this
study, we demonstrated that polygonal A549 cells acquired a
spindle-shaped morphology following exposure to TGF-β1 for 48 h.
Immunofluorescence analysis revealed that the expression of
α-SMA-positive filaments was increased and the levels of E-cad
protein in the cytoplasm were decreased following exposure to
TGF-β1. Similar results were observed by western blot analysis.
Taken together, all these results suggest that TGF-β1 successfully
induced A549 cell transformation into myofibroblasts.
TGF-β1 can be secreted by macrophages, lung
epithelial cells and fibroblasts, and is one of the most potent EMT
mediators (28–30). The present study demonstrated that
TGF-β1-mediated EMT was primarily dependent on canonical
TGF-β1/Smad signaling; and canonical TGF-β1 signaling has been
demonstrated in several cell types (31,32). The signaling of Smad3 is modulated
via phosphorylation and cytosol-nucleus translocation (33). Smad3 regulates the lung phenotype
in pulmonary fibrosis, and the inhibition of p-Smad3 can reduce
TGF-β1-induced EMT and fibrosis (34,35). A number of studies have reported
that Smad2/3 are involved in the EMT process (36–39). For example, in hepatic fibrosis, a
number of fibrogenic genes (collagens) and markers (α-SMA and
E-cad) are Smad3-dependent, and the deletion of Smad3 inhibits COLI
expression and blocks EMT (40).
In addition, increased p-Smad2/3 expression was detected during EMT
in retinal pigment epithelium cells, which indicated an association
between increased p-Smad2/3 expression and the EMT process
(41). Our experimental results
revealed that the expression of p-Smad3 was elevated following
exposure to TGF-β1. This phenomenon was accompanied by a reduction
in the levels of epithelial cell markers, while the levels of
mesenchymal cell markers and COLI, COLIII were increased. This
further supports a role for p-Smad3 in the regulation of EMT.
Snail has been known as an essential regulator in
TGF-β1-induced EMT (42). TGF-β1
has been shown to induce Snai1 expression either through a
Smad3-mediated mechanism or through interaction with other
signaling pathways, including Ras, Wnt and Notch (43,44). In this study, the resutls of
western blot analysis indicated that the expression levels of E-cad
were increased, while those of Snail1, α-SMA were decreased
significantly in the SIS3 group and the BMSCs-CM group. This
further confirmed that p-Smad3 and Snail1 play an important role in
the EMT process.
Studies in vivo have demonstrated the
anti-fibrotic properties of BMSCs in the liver (45), kidneys (46) and heart (47), and the application of BMSCs in the
treatment of pulmonary fibrosis has gained increasing attention.
In vitro, BMSCs can inhibit collagen synthesis and suppress
the transdifferentiation of hepatocytes into myofibroblasts
(48). Our group has also shown
that BMSCs can inhibit silica-induced pulmonary fibrosis and
collagen synthesis in vivo (49). However, whether BMSCs-CM is
capable of regulating EMT by regulating the TGF-β1/p-Smad3
signaling pathways has not yet been reported, at least to the best
of our knowledge. Our present results revealed that treatment of
the cells with BMSCs-CM prior to TGF-β1 exposure, downregulated the
expression of p-Smad3, Snail1, α-SMA and vimentin, and upregulated
the expression levels of E-cad and CK8. This confirms that BMSCs-CM
suppresses TGF- β1-induced EMT via the modulation of p-Smad3,
Snail1, COLI and COLIII expression. On the other hand, the
migratory ability of the A549 cells was weakened. Similar findings
were observed in cells pre-treated with SIS3. Taken together, our
results suggest that BMSCs-CM inhibits EMT by modulating p-Smad3
and Snail1 expression, thus suppressing pulmonary fibrosis.
In conclusion, the results of this study demonstrate
that TGF-β1/Smad3 signaling is a key pathway in TGF-β1-induced EMT.
Furthermore, BMSCs-CM can prevent the EMT process, and ameliorate
pulmonary fibrosis, which may provided a novel multitarget
treatment agent for pulmonary fibrosis.
Acknowledgments
This study was supported by grants from the Science
and Technology Support Key Funding Project of Hebei Province (no.
09276191D); Hebei Province Occupational Disease Prevention and
Control research (no. 13277709D); the Natural Science Foundation of
Hebei Province (grant no. H2015209309).
Abbreviations:
|
PF
|
pulmonary fibrosis
|
|
TGF-β1
|
transforming growth factor-β1
|
|
BMSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
EMT
|
epithelial-mesenchymal transition
|
|
E-cad
|
E-cadherin (E-calcium mucin)
|
|
α-SMA
|
α-smooth muscle actin
|
|
CM
|
conditioned medium
|
|
COLI
|
collagen I, COLIII, collagen III
|
|
IL-1RA
|
interleukin-1 receptor antagonist
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
TEM
|
transmission electron microscopy
|
|
PBS
|
phosphate-buffered saline
|
|
TRITC
|
tetramethyl rhodamine
isothiocyanate
|
|
FITC
|
fluorescein isothiocyanate
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
PVDF
|
polyvinylidene fluoride
|
|
ECL
|
chemiluminescence
|
References
|
1
|
Ley B, Collard HR and King TE Jr: Clinical
course and prediction of survival in idiopathic pulmonary fibrosis.
Am J Respir Crit Care Med. 183:431–440. 2011. View Article : Google Scholar
|
|
2
|
Richeldi L, Costabel U, Selman M, Kim DS,
Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G,
et al: Efficacy of a tyrosine kinase inhibitor in idiopathic
pulmonary fibrosis. N Engl J Med. 365:1079–1087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelialmesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arias AM: Epithelial mesenchymal
interactions in cancer and development. Cell. 105:425–431. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng XM, Nikolic-Paterson DJ and Lan HY:
TGF-β: The master regulator of fibrosis. Nat Rev Nephrol.
12:325–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pirozzi G, Tirino V, Camerlingo R, Franco
R, La Rocca A, Liguori E, Martucci N, Paino F, Normanno N and Rocco
G: Epithelial to mesenchymal transition by TGFβ-1 induction
increases stemness characteristics in primary non small cell lung
cancer cell line. PLoS On. 6:e215482011. View Article : Google Scholar
|
|
7
|
Yang G, Liang Y, Zheng T, Song R, Wang J,
Shi H, Sun B, Xie C, Li Y, Han J, et al: FCN2 inhibits
epithelial-mesenchymal transition-induced metastasis of
hepatocellular carcinoma via TGF-β/Smad signaling. Cancer Lett.
378:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flanders KC: Smad3 as a mediator of the
fibrotic response. Int J Exp Pathol. 85:47–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu F, Liu C, Zhou D and Zhang L:
TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J
Histochem Cytochem. 64:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Wang C, Huang Q, Wu D, Cao J, Xu
X, Yang C and Li X: Caveolin-1 plays an important role in the
differentiation of bone marrow-derived mesenchymal stem cells into
cardiomyocytes. Cardiology. 136:40–48. 2017. View Article : Google Scholar
|
|
11
|
Nagaya N, Kangawa K, Itoh T, Iwase T,
Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M,
et al: Transplantation of mesenchymal stem cells improves cardiac
function in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mezey E: The therapeutic potential of bone
marrow-derived stromal cells. J Cell Biochem. 112:2683–2687. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee
WR and Lee JH: The anti-inflammatory and anti-angiogenic role of
mesenchymal stem cells in corneal wound healing following chemical
injury. Stem Cells. 26:1047–1055. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi M, Liu ZW and Wang FS:
Immunomodulatory properties and therapeutic application of
mesenchymal stem cells. Clin Exp Immunol. 164:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiesa S, Morbelli S, Morando S, Massollo
M, Marini C, Bertoni A, Frassoni F, Bartolomé ST, Sambuceti G,
Traggiai E, et al: Mesenchymal stem cells impair in vivo T-cell
priming by dendritic cells. Proc Natl Acad Sci USA.
108:17384–17389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suganuma S, Tada K, Hayashi K, Takeuchi A,
Sugimoto N, Ikeda K and Tsuchiya H: Uncultured adipose-derived
regenerative cells promote peripheral nerve regeneration. J Orthop
Sci. 18:145–151. 2013. View Article : Google Scholar
|
|
17
|
Chen L, Tredget EE, Wu PY and Wu Y:
Paracrine factors of mesenchymal stem cells recruit macrophages and
endothelial lineage cells and enhance wound healing. PLoS On.
3:e18862008. View Article : Google Scholar
|
|
18
|
Li X, Luo Q, Sun J and Song G: Conditioned
medium from mesenchymal stem cells enhances the migration of
hepatoma cells through CXCR4 upregulation and F-actin remodeling.
Biotechnol Lett. 37:511–521. 2015. View Article : Google Scholar
|
|
19
|
Zhao MM, Cui JZ, Cui Y, Li R, Tian YX,
Song SX, Zhang J and Gao JL: Therapeutic effect of exogenous bone
marrow derived mesenchymal stem cell transplantation on silicosis
via paracrine mechanisms in rats. Mol Med Rep. 8:741–746. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe-Takano H, Takano K, Hatano M,
Tokuhisa T and Endo T: DA-Raf-mediated suppression of the Ras-ERK
pathway is essential for TGF-β1-induced epithelial-mesenchymal
transition in alveolar epithelial type 2 cells. PloS On.
10:e01278882015. View Article : Google Scholar
|
|
21
|
Yeh YC, Wei WC, Wang YK, Lin SC, Sung JM
and Tang MJ: Transforming growth factor-{beta}1 induces
Smad3-dependent {beta}1 integrin gene expression in
epithelial-to-mesenchymal transition during chronic
tubulointerstitial fibrosis. Am J Pathol. 177:1743–1754. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Willis BC and Borok Z: TGF-beta-induced
EMT: mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiery JP: Epithelial-mesenchymal
transitions in cancer onset and progression. Bull Acad Natl Med.
193:1969–1979. 2009.In French.
|
|
24
|
Carew RM, Wang B and Kantharidis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116. 2012.
View Article : Google Scholar
|
|
25
|
Hosper NA, van den Berg PP, de Rond S,
Popa ER, Wilmer MJ, Masereeuw R and Bank RA:
Epithelial-to-mesenchymal transition in fibrosis: Collagen type I
expression is highly upregulated after EMT, but does not contribute
to collagen deposition. Exp Cell Res. 319:3000–3009. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wettstein G, Bellaye PS, Kolb M, Hammann
A, Crestani B, Soler P, Marchal-Somme J, Hazoume A, Gauldie J,
Gunther A, et al: Inhibition of HSP27 blocks fibrosis development
and EMT features by promoting Snail degradation. FASEB J.
27:1549–1560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perez RE, Navarro A, Rezaiekhaligh MH,
Mabry SM and Ekekezie II: TRIP-1 regulates TGF-β1-induced
epithelialmesenchymal transition of human lung epithelial cell line
A549. Am J Physiol Lung Cell Mol Physiol. 300:L799–L807. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He X, Young SH, Schwegler-Berry D,
Chisholm WP, Fernback JE and Ma Q: Multiwalled carbon nanotubes
induce a fibrogenic response by stimulating reactive oxygen species
production, activating NF-κB signaling, and promoting
fibroblast-to-myofibroblast transformation. Chem Res Toxicol.
24:2237–2248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aschner Y and Downey GP: Transforming
growth factor-β: Master regulator of the respiratory system in
health and disease. Am J Respir Cell Mol Biol. 54:647–655. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamminen JA, Myllärniemi M, Hyytiäinen M,
Keski-Oja J and Koli K: Asbestos exposure induces alveolar
epithelial cell plasticity through MAPK/Erk signaling. J Cell
Biochem. 113:2234–2247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang YE: Non-Smad pathways in TGF-β
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar :
|
|
32
|
Reyes-Reyes EM, Ramos IN, Tavera-Garcia MA
and Ramos KS: The aryl hydrocarbon receptor agonist benzo(a)pyrene
reactivates LINE-1 in HepG2 cells through canonical TGF-β1
signaling: Implications in hepatocellular carcinogenesis. Am J
Cancer Res. 6:1066–1077. 2016.
|
|
33
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-β family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang C, Song X, Li Y, Han F, Gao S, Wang
X, Xie S and Lv C: Low-dose paclitaxel ameliorates pulmonary
fibrosis by suppressing TGF-β1/Smad3 pathway via miR-140
upregulation. PLoS One. 8:e707252013. View Article : Google Scholar
|
|
35
|
Xie L, Zhou D, Xiong J, You J, Zeng Y and
Peng L: Paraquat induce pulmonary epithelial-mesenchymal transition
through transforming growth factor-β1-dependent mechanism. Exp
Toxicol Pathol. 68:69–76. 2016. View Article : Google Scholar
|
|
36
|
Liu L, Wang Y, Yan R, Li S, Shi M, Xiao Y
and Guo B: Oxymatrine inhibits renal tubular EMT induced by high
glucose via upregulation of SnoN and inhibition of TGF-β1/Smad
signaling pathway. PLoS On. 11:e01519862016. View Article : Google Scholar
|
|
37
|
Dong Z, Tai W, Lei W, Wang Y, Li Z and
Zhang T: IL-27 inhibits the TGF-β1-induced epithelial-mesenchymal
transition in alveolar epithelial cells. BMC Cell Biol. 17:72016.
View Article : Google Scholar
|
|
38
|
Zhang L, Cheng X, Gao Y, Zhang C, Bao J,
Guan H, Yu H, Lu R, Xu Q and Sun Y: Curcumin inhibits metastasis in
human papillary thyroid carcinoma BCPAP cells via downregulation of
the TGF-β/Smad2/3 signaling pathway. Exp Cell Res. 341:157–165.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kong D, Zhang F, Shao J, Wu L, Zhang X,
Chen L, Lu Y and Zheng S: Curcumin inhibits cobalt chloride-induced
epithelial- to-mesenchymal transition associated with interference
with TGF-β/Smad signaling in hepatocytes. Lab Invest. 95:1234–1245.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masszi A and Kapus A: Smaddening
complexity: The role of Smad3 in epithelial-myofibroblast
transition. Cells Tissues Organs. 193:41–52. 2011. View Article : Google Scholar
|
|
41
|
Chen Z, Mei Y, Lei H, Tian R, Ni N, Han F,
Gan S and Sun S: LYTAK1, a TAK1 inhibitor, suppresses proliferation
and epithelial mesenchymal transition in retinal pigment epithelium
cells. Mol Med Rep. 14:145–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Smith KA, Zhou B, Avdulov S, Benyumov A,
Peterson M, Liu Y, Okon A, Hergert P, Braziunas J, Wagner CR, et
al: Transforming growth factor-1 induced epithelial mesenchymal
transition is blocked by a chemical antagonist of translation
factor eIF4E. Sci Rep. 5:182332015. View Article : Google Scholar
|
|
43
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zavadil J, Cermak L, Soto-Nieves N and
Böttinger EP: Integration of TGF-beta/Smad and Jagged1/Notch
signalling in epithelial-to-mesenchymal transition. EMBO J.
23:1155–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang ZC, Yang S, Huang JJ, Chen SL, Li QQ
and Li Y: Effect of bone marrow mesenchymal stem cells on the Smad
expression of hepatic fibrosis rats. Asian Pac J Trop Med.
7:321–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lang H and Dai C: Effects of bone marrow
mesenchymal stem cells on plasminogen activator inhibitor-1 and
renal fibrosis in rats with diabetic nephropathy. Arch Med Res.
47:71–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cai B, Tan X, Zhang Y, Li X, Wang X, Zhu
J, Wang Y, Yang F, Wang B, Liu Y, et al: Mesenchymal stem cells and
cardiomyocytes interplay to prevent myocardial hypertrophy. Stem
Cells Transl Med. 4:1425–1435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jang YO, Kim MY, Cho MY, Baik SK, Cho YZ
and Kwon SO: Effect of bone marrow-derived mesenchymal stem cells
on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model.
BMC Gastroenterol. 14:1982014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao MM, Cui JZ, Li R, Du LJ, Tian YX, Kan
Q, Zhang J and Gao JL: Effects of bone mesenchymal stem cell
transplantation on silicosis fibrosis in rats. Chin J Pathophysiol.
28:2205–2210. 2012.
|