|
1
|
Gimenez LE, Kook S, Vishnivetskiy SA,
Ahmed MR, Gurevich EV and Gurevich VV: Role of receptor-attached
phosphates in binding of visual and non-visual arrestins to G
protein-coupled receptors. J Biol Chem. 287:9028–9040. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma D and Parameswaran N: Multifaceted
role of β-arrestins in inflammation and disease. Genes Immun.
16:5762015. View Article : Google Scholar
|
|
3
|
Smith JS and Rajagopal S: The β-arrestins:
Multifunctional regulators of G protein-coupled receptors. J Biol
Chem. 291:8969–8977. 2016. View Article : Google Scholar : PubMed/NCBI
|
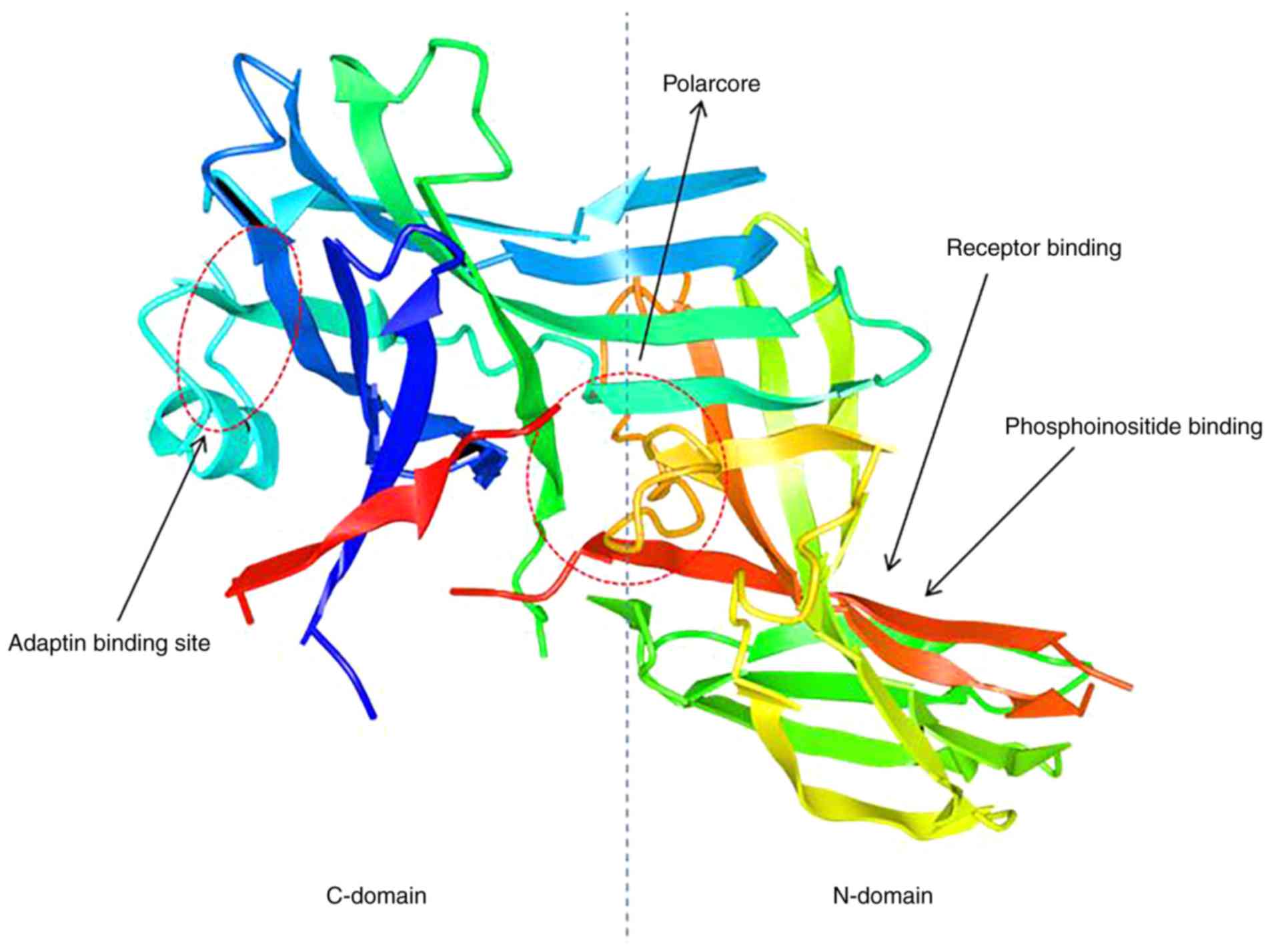
|
4
|
Hu S, Wang D, Wu J, Jin J, Wei W and Sun
W: Involvement of β-arrestins in cancer progression. Mol Biol Rep.
40:1065–1071. 2013. View Article : Google Scholar
|
|
5
|
Gurevich EV and Gurevich VV: Arrestins:
Ubiquitous regulators of cellular signaling pathways. Genome Biol.
7:2362006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
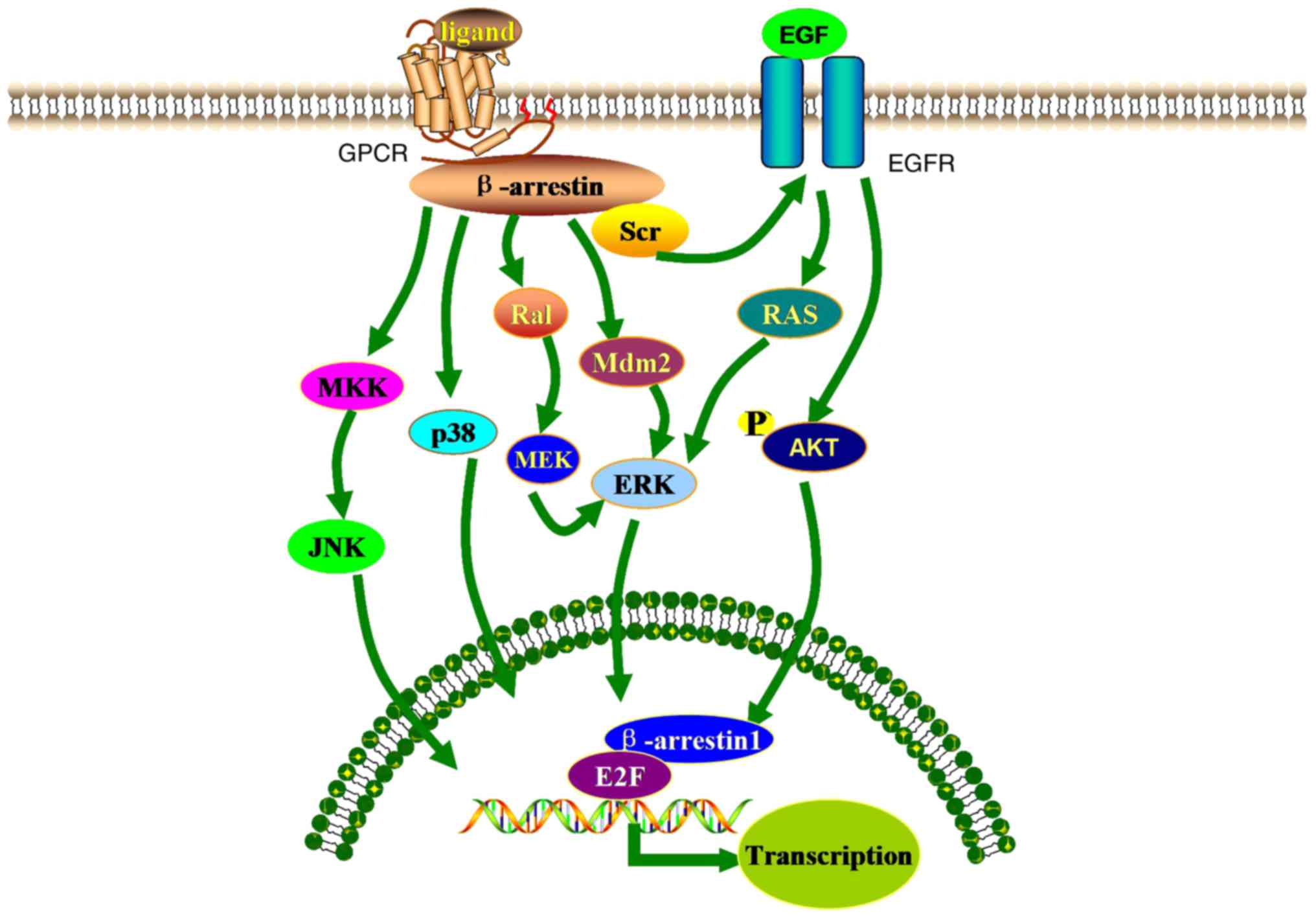
Ranjan R, Gupta P and Shukla AK: Gpcr
signaling: β-arrestins kiss and remember. Curr Biol. 26:R285–R288.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohout TA, Lin FS, Perry SJ, Conner DA and
Lefkowitz RJ: Beta-arrestin 1 and 2 differentially regulate
heptahelical receptor signaling and trafficking. Proc Natl Acad Sci
USA. 98:1601–1606. 2001.PubMed/NCBI
|
|
8
|
Enslen H, Lima-Fernandes E and Scott MG:
Arrestins as regulatory hubs in cancer signalling pathways. Handb
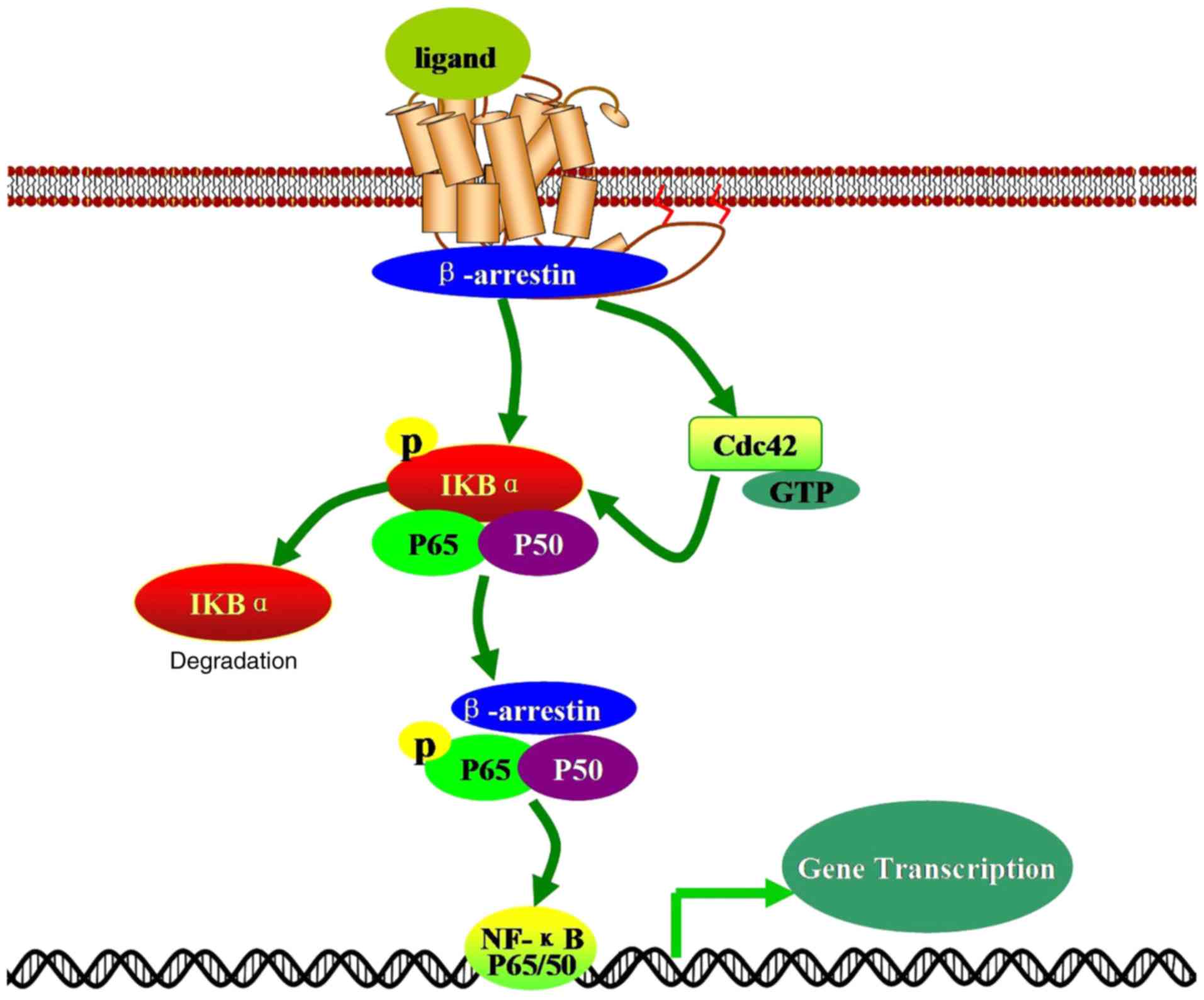
Exp Pharmacol. 219:405–425. 2014. View Article : Google Scholar
|
|
9
|
Rosanò L, Cianfrocca R, Masi S, Spinella
F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG and
Bagnato A: Beta-arrestin links endothelin a receptor to
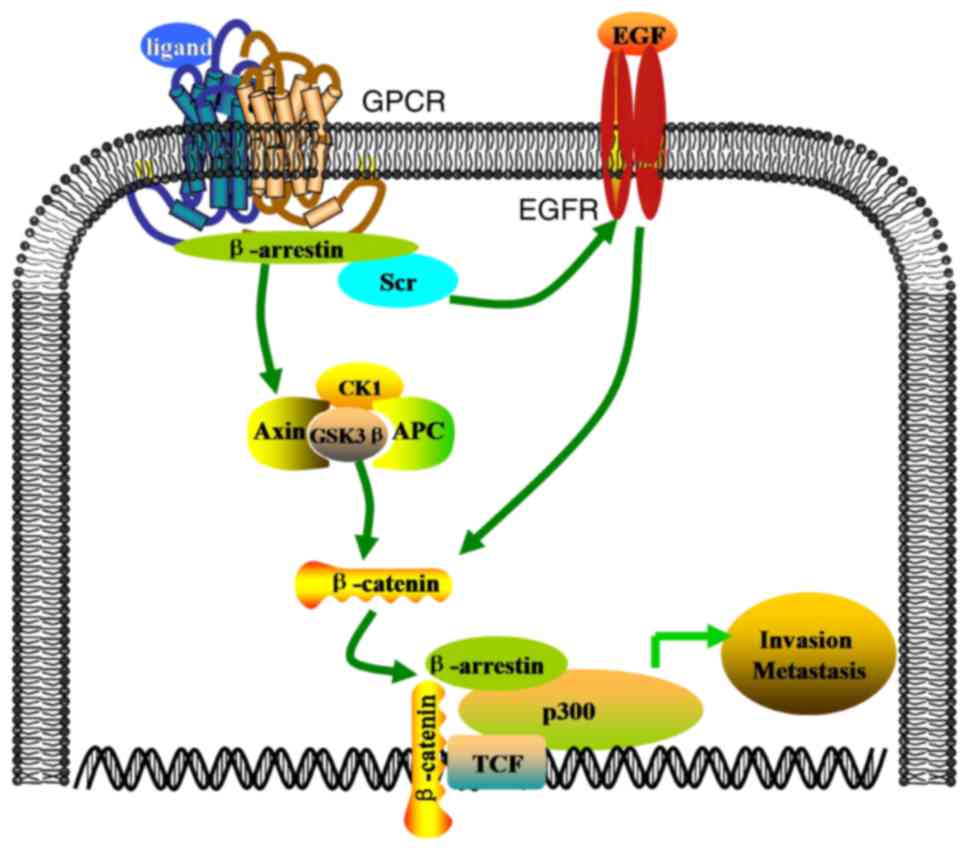
beta-catenin signaling to induce ovarian cancer cell invasion and
metastasis. Proc Natl Acad Sci USA. 106:2806–2811. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosanò L, Cianfrocca R, Tocci P, Spinella
F, Di Castro V, Caprara V, Semprucci E, Ferrandina G, Natali PG and
Bagnato A: Endothelin a receptor/β-arrestin signaling to the wnt
pathway renders ovarian cancer cells resistant to chemotherapy.
Cancer Res. 74:7453–7464. 2014. View Article : Google Scholar
|
|
11
|
Spinella F, Caprara V, Di Castro V, Rosanò
L, Cianfrocca R, Natali PG and Bagnato A: Endothelin-1 induces the
transactivation of vascular endothelial growth factor receptor-3
and modulates cell migration and vasculogenic mimicry in melanoma
cells. J Mol Med (Berl). 91:395–405. 2013. View Article : Google Scholar
|
|
12
|
Eichel K, Jullié D and von Zastrow M:
β-arrestin drives map kinase signalling from clathrin-coated
structures after GPCR dissociation. Nature Cell Biol. 18:303–310.
2016. View
Article : Google Scholar
|
|
13
|
Bourquard T, Landomiel F, Reiter E,
Crépieux P, Ritchie DW, Azé J and Poupon A: Unraveling the
molecular architecture of a G protein-coupled
receptor/β-arrestin/erk module complex. Sci Rep. 5:107602015.
View Article : Google Scholar
|
|
14
|
Sun WY, Hu SS, Wu JJ, Huang Q, Ma Y, Wang
QT, Chen JY and Wei W: Down-regulation of β-arrestin2 promotes
tumour invasion and indicates poor prognosis of hepatocellular
carcinoma. Sci Rep. 6:356092016. View Article : Google Scholar
|
|
15
|
Kim M, Suh YA, Oh JH, Lee BR, Kim J and
Jang SJ: Corrigendum: KIF3A binds to β-arrestin for suppressing
wnt/β-catenin signalling independently of primary cilia in lung
cancer. Sci Rep. 7:467732017. View Article : Google Scholar
|
|
16
|
Lee SU, Ahn KS, Sung MH, Park JW, Ryu HW,
Lee HJ, Hong ST and Oh SR: Indacaterol inhibits tumor cell
invasiveness and mmp-9 expression by suppressing IKK/NF-κB
activation. Mol Cells. 37:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conner DA, Mathier MA, Mortensen RM,
Christe M, Vatner SF, Seidman CE and Seidman JG: Beta-arrestin1
knockout mice appear normal but demonstrate altered cardiac
responses to beta-adrenergic stimulation. Circ Res. 81:1021–1026.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bohn LM, Lefkowitz RJ, Gainetdinov RR,
Peppel K, Caron MG and Lin FT: Enhanced morphine analgesia in mice
lacking beta-arrestin 2. Science. 286:2495–2498. 1999. View Article : Google Scholar
|
|
19
|
Gu YJ, Sun WY, Zhang S, Wu JJ and Wei W:
The emerging roles of β-arrestins in fibrotic diseases. Acta
Pharmacol Sin. 36:1277–1287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Philipp M, Evron T and Caron MG: The role
of arrestins in development. Prog Mol Biol Transl Sci. 118:225–242.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bayburt TH, Vishnivetskiy SA, McLean MA,
Morizumi T, Huang CC, Tesmer JJ, Ernst OP, Sligar SG and Gurevich
VV: Monomeric rhodopsin is sufficient for normal rhodopsin kinase
(grk1) phosphorylation and arrestin-1 binding. J Biol Chem.
286:1420–1428. 2011. View Article : Google Scholar :
|
|
22
|
Hamdan FF, Rochdi MD, Breton B, Fessart D,
Michaud DE, Charest PG, Laporte SA and Bouvier M: Unraveling G
protein-coupled receptor endocytosis pathways using real-time
monitoring of agonist-promoted interaction between beta-arrestins
and AP-2. J Biol Chem. 282:29089–29100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han M, Gurevich VV, Vishnivetskiy SA,
Sigler PB and Schubert C: Crystal structure of beta-arrestin at 1.9
A: Possible mechanism of receptor binding and membrane
translocation. Structure. 9:869–880. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan H, Liao Y, Tang Q, Liang L and Chen
XY: Role of β-arrestins in the pathogenesis of inflammatory bowel
disease. World Chinese J Digestol. 18:3114–3120. 2010. View Article : Google Scholar
|
|
25
|
Nobles KN, Guan Z, Xiao K, Oas TG and
Lefkowitz RJ: The active conformation of beta-arrestin1: Direct
evidence for the phosphate sensor in the n-domain and
conformational differences in the active states of beta-arrestins1
and -2. J Biol Chem. 282:21370–21381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo J, Tsakem EL, Breitman M and Gurevich
VV: Identification of arrestin-3-specific residues necessary for
JNK3 kinase activation. J Biol Chem. 286:27894–27901. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin FT, Miller WE, Luttrell LM and
Lefkowitz RJ: Feedback regulation of beta-arrestin1 function by
extracellular signal-regulated kinases. J Biol Chem.
274:15971–15974. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morrison DK: Map kinase pathways. Cold
Spring Harb Perspect Bio. 4(pii): a0112542012.
|
|
30
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen-activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou H, Li XM, Meinkoth J and Pittman RN:
Akt regulates cell survival and apoptosis at a postmitochondrial
level. J Cell Biol. 151:483–494. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okada T, Sinha S, Esposito I, Schiavon G,
López-Lago MA, Su W, Pratilas CA, Abele C, Hernandez JM, Ohara M,
et al: The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT
by restraining RAS-MAPK signalling. Nat Cell Biol. 17:81–94. 2015.
View Article : Google Scholar
|
|
33
|
Gu Y, Wang Q, Guo K, Qin W, Liao W, Wang
S, Ding Y and Lin J: TUSC3 promotes colorectal cancer progression
and epithelial-mesenchymal transition (EMT) through WNT/β-catenin
and MAPK signalling. J Pathol. 239:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaufhold S and Bonavida B: Central role of
snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer Res. 72:1878–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou G, Peng F, Zhong Y, Chen Y, Tang M
and Li D: Rhein suppresses matrix metalloproteinase production by
regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian
carcinoma cells. Int J Onco. 50:933–941. 2017. View Article : Google Scholar
|
|
37
|
Sangpairoj K, Vivithanaporn P,
Apisawetakan S, Chongthammakun S, Sobhon P and Chaithirayanon K:
RUNX1 regulates migration, invasion, and angiogenesis via 38 MAPK
pathway in human glioblastoma. Cell Mol Neurobiol. 2016.Epub ahead
of print.
|
|
38
|
Cepeda MA, Evered CL, Pelling JJH and
Damjanovski S: Inhibition of MT1-MMP proteolytic function and
ERK1/2 signalling influences cell migration and invasion through
changes in MMP-2 and MMP-9 levels. J Cell Commun Signal.
11:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Suyama K, Shapiro I, Guttman M and Hazan
RB: A signaling pathway leading to metastasis is controlled by
N-cadherin and the FGF receptor. Cancer Cell. 2:301–314. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luttrell LM, Roudabush FL, Choy EW, Miller
WE, Field ME, Pierce KL and Lefkowitz RJ: Activation and targeting
of extracellular signal-regulated kinases by beta-arrestin
scaffolds. Proc Natl Acad Sci USA. 98:2449–2454. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fong AM, Premont RT, Richardson RM, Yu YR,
Lefkowitz RJ and Patel DD: Defective lymphocyte chemotaxis in
beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA.
99:7478–7483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Décaillot FM, Kazmi MA, Lin Y, Ray-Saha S,
Sakmar TP and Sachdev P: Cxcr7/cxcr4 heterodimer constitutively
recruits beta-arrestin to enhance cell migration. J Biol Chem.
286:32188–32197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu D, Li R, Wu J, Jiang L and Zhong HA:
Drug design targeting the cxcr4/cxcr7/cxcl12 pathway. Curr Top Med
Chem. 16:1441–1451. 2016. View Article : Google Scholar
|
|
44
|
Coggins L, Trakimas D, Chang SL, Ehrlich
A, Ray P, Luker KE, Linderman JJ and Luker GD: Cxcr7 controls
competition for recruitment of β-arrestin 2 in cells expressing
both cxcr4 and cxcr7. PLoS On. 9:e983282014. View Article : Google Scholar
|
|
45
|
Zhang P, He X, Tan J, Zhou X and Zou L:
β-arrestin2 mediates β-2 adrenergic receptor signaling inducing
prostate cancer cell progression. Oncol Rep. 26:1471–1477.
2011.PubMed/NCBI
|
|
46
|
Buchanan FG, Gorden DL, Matta P, Shi Q,
Matrisian LM and DuBois RN: Role of beta-arrestin 1 in the
metastatic progression of colorectal cancer. Proc Natl Acad Sci
USA. 103:1492–1497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lan T, Wang H, Zhang Z, Zhang M, Qu Y,
Zhao Z, Fan X, Zhan Q, Song Y and Yu C: Downregulation of
β-arrestin 1 suppresses glioblastoma cell malignant progression vis
inhibition of src signaling. Exp Cell Res. 357:51–58. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ge L, Shenoy SK, Lefkowitz RJ and DeFea K:
Constitutive protease-activated receptor-2-mediated migration of
MDA MB-231 breast cancer cells requires both beta-arrestin-1 and
-2. J Biol Chem. 279:55419–55424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Parisis N, Metodieva G and Metodiev MV:
Pseudopodial and β-arrestin-interacting proteomes from migrating
breast cancer cells upon AR2 activation. J Proteomics. 80:91–106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Girnita L, Shenoy SK, Sehat B, Vasilcanu
R, Vasilcanu D, Girnita A, Lefkowitz RJ and Larsson O:
Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK
activation and cell cycle progression. J Biol Chem.
282:11329–11338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schaal C and Chellappan SP:
Nicotine-mediated cell proliferation and tumor progression in
smoking-related cancers. Mol Cancer Res. 12:14–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu H, Zhang Q, Li K, Gong Z, Liu Z, Xu Y,
Swaney MH, Xiao K and Chen Y: Prognostic significance of USP33 in
advanced colorectal cancer patients: New insights into
β-arrestin-dependent ERK signaling. Oncotarget. 7:81223–81240.
2016.PubMed/NCBI
|
|
53
|
Li XX, Zheng HT, Huang LY, Shi DB, Peng
JJ, Liang L and Cai SJ: Silencing of CXCR7 gene represses growth
and invasion and induces apoptosis in colorectal cancer through ERK
and β-arrestin pathways. Int J Oncol. 45:1649–1657. 2016.
View Article : Google Scholar
|
|
54
|
Goertzen CG, Dragan M, Turley E, Babwah AV
and Bhattacharya M: KISS1R signaling promotes invadopodia formation
in human breast cancer cell via β-arrestin2/ERK. Cell Signal.
28:165–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dasgupta P, Rizwani W, Pillai S, Davis R,
Banerjee S, Hug K, Lloyd M, Coppola D, Haura E and Chellappan SP:
Arrb1-mediated regulation of E2F target genes in nicotine-induced
growth of lung tumors. J Natl Cancer Inst. 103:317–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Korinek V, Barker N, Willert K, Molenaar
M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O and Clevers
H: Two members of the tcf family implicated in wnt/beta-catenin
signaling during embryogenesis in the mouse. Mol Cell Biol.
18:1248–1256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mythreye K and Blobe GC: The type iii
TGF-beta receptor regulates epithelial and cancer cell migration
through beta-arrestin2-mediated activation of Cdc42. Proc Natl Acad
Sci USA. 106:8221–8226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim GH, Her JH and Han JK: Ryk cooperates
with frizzled 7 to promote wnt11-mediated endocytosis and is
essential for xenopus laevis convergent extension movements. J Cell
Biol. 182:1073–1082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Habas R, Dawid IB and He X: Coactivation
of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate
gastrulation. Genes Dev. 17:295–309. 2008. View Article : Google Scholar
|
|
60
|
Kypta RM and Waxman J: Wnt/β-catenin
signalling in prostate cancer. Nat Rev Urol. 9:418–428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Meng X, Zhu D, Yang S, Wang X, Xiong Z,
Zhang Y, Brachova P and Leslie KK: Cytoplasmic metadherin (MTDH)
provides survival advantage under conditions of stress by acting as
RNA-binding protein. J Biol Chem. 287:4485–4491. 2012. View Article : Google Scholar :
|
|
62
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu Q, Krause M, Samoylenko A and Vainio S:
Wnt signaling in renal cell carcinoma. Cancers (Basel). 8(pii):
E572016. View Article : Google Scholar
|
|
64
|
Chen Z, He X, Jia M, Liu Y, Qu D, Wu D, Wu
P, Ni C, Zhang Z, Ye J, et al: β-catenin overexpression in the
nucleus predicts progress disease and unfavourable survival in
colorectal cancer: A meta-analysis. PLoS One. 8:e638542013.
View Article : Google Scholar
|
|
65
|
Aminuddin A and Ng PY: Promising druggable
target in head and neck squamous cell carcinoma: Wnt signaling.
Front Pharmacol. 7:2442016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liang S, Zhang S, Wang P, Yang C, Shang C,
Yang J and Wang J: Lncrna, TUG1 regulates the oral squamous cell
carcinoma progression possibly via interacting with
Wnt/beta-catenin signaling. Gene. 608:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liang J, Liang L, Ouyang K, Li Z and Yi X:
MALAT1 induces tongue cancer cells' EMT and inhibits apoptosis
through wnt/β-catenin signaling pathway. J Oral Pathol Med.
46:98–105. 2017. View Article : Google Scholar
|
|
68
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Howard S, Deroo T, Fujita Y and Itasaki N:
A positive role of cadherin in Wnt/β-catenin signalling during
epithelial-mesenchymal transition. PLoS On. 6:e238992011.
View Article : Google Scholar
|
|
70
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Felipe Lima J, Nofech-Mozes S, Bayani J
and Bartlett JM: Emt in breast carcinoma-a review. J Clin Me.
5(pii): E652016. View Article : Google Scholar
|
|
72
|
Grant CM and Kyprianou N: Epithelial
mesenchymal transition (EMT) in prostate growth and tumor
progression. Transl Androl Urol. 2:202–211. 2003.
|
|
73
|
Ko CJ, Huang CC, Lin HY, Juan CP, Lan SW,
Shyu HY, Wu SR, Hsiao PW, Huang HP, Shun CT and Lee MS:
Androgen-induced TMPRSS2 activates matriptase and promotes
extracellular matrix degradation, prostate cancer cell invasion,
tumor growth, and metastasis. Cancer Res. 75:2949–2960. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liao X, Thrasher JB, Pelling J,
Holzbeierlein J, Sang QX and Li B: Androgen stimulates matrix
metalloproteinase-2 expression in human prostate cancer.
Endocrinology. 144:1656–1663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang Y, Jiao L, Hou J, Xu C, Wang L, Yu Y,
Li Y, Yang C, Wang X and Sun Y: Dishevelled-2 silencing reduces
androgen-dependent prostate tumor cell proliferation and migration
and expression of Wnt-3a and matrix metalloproteinases. Mol Biol
Rep. 40:4241–4250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sun L, Liu T, Zhang S, Guo K and Liu Y:
Oct4 induces EMT through LEF1/β-catenin dependent WNT signaling
pathway in hepatocellular carcinoma. Oncol Lett. 13:2599–2606.
2017.PubMed/NCBI
|
|
77
|
Zhang Y: Ganodermalucidum (Reishi)
suppresses proliferation and migration of breast cancer cells via
inhibiting Wnt/β-catenin signaling. Biochem Biophys Res Commun.
488:679–684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rosanò L, Cianfrocca R, Tocci P, Spinella
F, Di Castro V, Spadaro F, Salvati E, Biroccio AM, Natali PG and
Bagnato A: β-arrestin-1 is a nuclear transcriptional regulator of
endothelin-1-induced β-catenin signaling. Oncogene. 32:5066–5077.
2013. View Article : Google Scholar
|
|
79
|
Turm H, Maoz M, Katz V, Yin YJ, Offermanns
S and Bar-Shavit R: Protease-activated receptor-1 (AR1) acts via a
novel galpha13-dishevelled axis to stabilize beta-catenin levels. J
Biol Chem. 285:15137–15148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bonnans C, Flaceliere M, Grillet F, Dantec
C, Desvignes JP, Pannequin J, Severac D, Dubois E, Bibeau F,
Escriou V, et al: Essential requirement for β-arrestin2 in mouse
intestinal tumors with elevated wnt signaling. Proc Natl Acad Sci
USA. 109:3047–3052. 2012. View Article : Google Scholar
|
|
81
|
Duan X, Zhang T, Kong Z, Mai X, Lan C,
Chen D, Liu Y, Zeng Z, Cai C, Deng T, et al: β-arrestin 1 promotes
epithelial-mesenchymal transition via modulating GSK-3β/β-catenin
pathway in prostate cancer cells. Biochem Biophys Res Commun.
479:204–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Witherow DS, Garrison TR, Miller WE and
Lefkowitz RJ: Beta-arrestin inhibits NF-kappaB activity by means of
its interaction with the Nf-KappaB inhibitor IkappaBalpha. Proc
Natl Acad Sci USA. 101:8603–8607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kim YR, Kim IJ, Kang TW, Choi C, Kim KK,
Kim MS, Nam KI and Jung C: HOXB13 downregulates intracellular zinc
and increases NF-κB signaling to promote prostate cancer
metastasis. Oncogene. 33:4558–4567. 2014. View Article : Google Scholar
|
|
84
|
Jiang L, Lin C, Song L, Wu J, Chen B, Ying
Z, Fang L, Yan X, He M, Li J and Li M: Microrna-30e* promotes human
glioma cell invasiveness in an orthotopic xenotransplantation model
by disrupting the NF-κB/IκBα negative feedback loop. J Clin Invest.
122:33–47. 2012. View Article : Google Scholar
|
|
85
|
Karin M: Nuclear factor-kappab in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Karin M, Cao Y, Greten FR and Li ZW:
Nf-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu B, Han M and Wen JK:
Acetylbritannilactone inhibits neointimal hyperplasia after balloon
injury of rat artery by suppressing nuclear factor-{kappa}B
activation. J Pharmacol Exp Ther. 324:292–298. 2008. View Article : Google Scholar
|
|
88
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kong D, Li Y, Wang Z, Banerjee S and
Sarkar FH: Inhibition of angiogenesis and invasion by
3.3′-diindolylmethane is mediated by the nuclear factor-kappaB
downstream target genes MMP-9 and uPA that regulated
bioavailability of vascular endothelial growth factor in prostate
cancer. Cancer Res. 67:3310–3319. 2002. View Article : Google Scholar
|
|
90
|
Liao D, Zhong L, Duan T, Zhang RH, Wang X,
Wang G, Hu K, Lv X and Kang T: Aspirin suppresses the growth and
metastasis of osteosarcoma through the NF-κB pathway. Clin Cancer
Res. 21:5349–5359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cianfrocca R, Tocci P, Semprucci E,
Spinella F, Di Castro V, Bagnato A and Rosanò L: β-arrestin 1 is
required for endo-thelin-1-induced NF-κB activation in ovarian
cancer cells. Life Sci. 118:179–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Raghuwanshi SK, Nasser MW, Chen X,
Strieter RM and Richardson RM: Depletion of beta-arrestin-2
promotes tumor growth and angiogenesis in a murine model of lung
cancer. J Immunol. 180:5699–5706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B
and Pei G: Identification of beta-arrestin2 as a G protein-coupled
receptor-stimulated regulator of NF-kappaB pathways. Mol Cell.
14:303–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang Y, Tang Y, Teng L, Wu Y, Zhao X and
Pei G: Association of beta-arrestin and TRAF6 negatively regulates
toll-like receptor-interleukin 1 receptor signaling. Nat Immunol.
7:139–147. 2006. View
Article : Google Scholar
|
|
95
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:11–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bedini A, Baiula M, Vincelli G, Formaggio
F, Lombardi S, Caprini M and Spampinato S: Nociceptin/orphanin FQ
antagonizes lipopolysaccharide-stimulated proliferation, migration
and inflammatory signaling in human glioblastoma U87 cells. Biochem
Pharmacol. 140:89–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lino MM and Merlo A: I3K inase signaling
in glioblastoma. J Neurooncol. 103:417–427. 2011. View Article : Google Scholar
|
|
98
|
Chalhoub N and Baker SJ: PTEN and the
I3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2017.
View Article : Google Scholar
|
|
99
|
Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y,
Zhang Y, Hua S, Fu Q, Zhao M, et al: Downregulation of FAP
suppresses cell proliferation and metastasis through PTEN/I3K/AKT
and RAS-ERK signaling in oral squamous cell carcinoma. Cell Death
Di. 5:e11552014. View Article : Google Scholar
|
|
100
|
Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y,
Zhao L, Qu H, Fan Y and Wu C: Antagonism of miR-21 reverses
epithelial-mesenchymal transition and cancer stem cell phenotype
through AKT/ERK1/2 inactivation by targeting PTEN. PLoS On.
7:e395202012. View Article : Google Scholar
|
|
101
|
Jensen RL: Brain tumor hypoxia:
Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a
therapeutic target. J Neurooncol. 92:317–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen B, Zeng X, He Y, Wang X, Liang Z, Liu
J, Zhang P, Zhu H, Xu N and Liang S: STC2 promotes the
epithelial-mesenchymal transition of colorectal cancer cells
through AKT-ERK signaling pathways. Oncotarget. 7:71400–71416.
2016.PubMed/NCBI
|
|
103
|
Wang Z, Qu L, Deng B, Sun X, Wu S, Liao J,
Fan J and Peng Z: Styk1 promotes epithelial-mesenchymal transition
and tumor metastasis in human hepatocellular carcinoma through
Mek/Erk and I3K/AKT signaling. Sci Rep. 6:332052016. View Article : Google Scholar
|
|
104
|
Zhang Y, Yang CQ, Gao Y, Wang C, Zhang CL
and Zhou XH: Knockdown of CXCR7 inhibits proliferation and invasion
of osteosarcoma cells through inhibition of the I3K/AKT and
β-arrestin pathways. Oncol Rep. 32:965–972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zou L, Yang R, Chai J and Pei G: Rapid
xenograft tumor progression in beta-arrestin1 transgenic mice due
to enhanced tumor angiogenesis. FASEB J. 22:355–364. 2008.
View Article : Google Scholar
|
|
106
|
Alvarez CJ, Lodeiro M, Theodoropoulou M,
Camiña JP, Casanueva FF and Pazos Y: Obestatin stimulates
aktsignalling in gastric cancer cells through
beta-arrestin-mediated epidermal growth factor receptor
transactivation. Endocr Relat Cancer. 16:599–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nawaz Z, Patil V, Paul Y, Hegde AS,
Arivazhagan A, Santosh V and Somasundaram K: Pi3 kinase pathway
regulated mirnome in glioblastoma: Identification of mir-326 as a
tumour suppressor miRNA. Mol Cance. 15:742016. View Article : Google Scholar
|
|
108
|
Lima-Fernandes E, Enslen H, Camand E,
Kotelevets L, Boularan C, Achour L, Benmerah A, Gibson LC, Baillie
GS, Pitcher JA, et al: Distinct functional outputs of PTEN
signalling are controlled by dynamic association with β-arrestins.
EMBO J. 30:2557–2568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li Y, Guo G, Song J, Cai Z, Yang J, Chen
Z, Wang Y, Huang Y and Gao Q: B7-H3 promotes the migration and
invasion of human bladder cancer cells via the I3K/AKT/STAT3
signaling pathway. J Cancer. 8:816–824. 2017. View Article : Google Scholar :
|
|
110
|
Tayeh M, Nilwarangoon S, Mahabusarakum W
and Watanapokasin R: Anti-metastatic effect of rhodomyrtone from
rhodomyrtus tomentosa on human skin cancer cells. Int J Oncol.
50:1035–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Scott MG, Le Rouzic E, Périanin A,
Pierotti V, Enslen H, Benichou S, Marullo S and Benmerah A:
Differential nucleo-cytoplasmic shuttling of beta-arrestins.
Characterization of a leucine-rich nuclear export signal in
beta-arrestin2. J Biol Chem. 277:37693–37701. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M,
Zhang M, Bao G, Wang F, Zhang X, et al: A nuclear function of
beta-arrestin1 in GPCR signaling: Regulation of histone acetylation
and gene transcription. Cell. 123:833–847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kim JI, Lakshmikanthan V, Frilot N and
Daaka Y: Prostaglandin E2 promotes lung cancer cell migration via
EP4-betaArrestin1-c-src signalsome. Mol Cancer Res. 8:569–577.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lin EW, Karakasheva TA, Hicks PD, Bass AJ
and Rustgi AK: The tumor microenvironment in esophageal cancer.
Oncogene. 35:5337–5349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Clark AG and Vignjevic DM: Modes of cancer
cell invasion and the role of the microenvironment. Curr Opin Cell
Biol. 36:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ji RC: Hypoxia and lymphangiogenesis in
tumor microenvironment and metastasis. Cancer Lett. 346:6–16. 2014.
View Article : Google Scholar
|
|
117
|
Whalen EJ, Rajagopal S and Lefkowitz RJ:
Therapeutic potential of β-arrestin- and G protein-biased agonists.
Trends Mol Med. 17:126–139. 2011. View Article : Google Scholar
|
|
118
|
Bologna Z, Teoh JP, Bayoumi AS, Tang Y and
Kim IM: Biased G protein-coupled receptor signaling: New player in
modulating physiology and pathology. Biomol Ther (Seoul). 25:12–25.
2017. View Article : Google Scholar
|