Introduction
Spinal cord injury (SCI) is damaging to the axonal
tracts and cells around the site of injury and has devastating
consequences for patients' locomotor function and sensory function.
Unfortunately, the mechanical barriers caused by glial scars and
the inhibitory molecules secreted by astrocytes prevent axonal
regeneration following SCI. Numerous efforts have been made to
solve this problem, including the utilization of autografts and
suturing of the gaps (1).
However, the two approaches have limitations and have not proven to
be feasible (2). Tissue
engineering using a combination of biomaterial scaffolds and
transplanted seed cells is an inspiring and progressive method of
repairing and reconstructing the damaged spinal cord (3). Two important issues require
consideration when attempting to bridge the injury gap with tissue
engineering. One is the selection of appropriate cells for
transplantation, and the other is the identification of scaffolds
that are able to enhance cell survival and direct cell
differentiation into neurons.
It has been reported that neural stem cells (NSCs)
are promising seed cells for the treatment of SCI (4). NSCs differentiate into one of the
three major neural cell types that are able to repopulate the
damaged spinal cord (5,6). These cells can assist with locomotor
function and sensory function recovery by promoting cell survival
and the regeneration of host axons. The traditional method of
obtaining NSCs, differentiation from embryonic stem cells (ESCs)
(5), has ethical issues and
causes allogeneic immunological rejection. Fortunately, the
discovery of induced pluripotent stem cells (iPSCs) provides an
improved method of obtaining NSCs (7,8).
The derivation of iPSCs from patient-specific cells for use in
transplantation therapies avoids the problems associated with
immunological rejection and ethics. However, methods for the
generation of iPSCs typically use genome-integrating retroviral or
lentiviral vectors, which have safety issues due to insertional
mutations and residual transgene expression during iPSC derivation,
expansion and differentiation (9). To overcome these issues,
integration-free iPSCs with episomal vectors pCEP4-EO2S-ET2K and
pCEP4-miR-302-367 cluster have been generated. The use of the
pCEP4-EO2S-ET2K vector, encoding octamer-binding transcription
factor 4 (OCT4), sex determining region Y-box 2 (SOX2), Kruppel
like factor 4 and simian virus 40 large T antigen, has been
demonstrated to be a manufacturing practice-compliant method for
the generation of integration-free iPSCs (10). The NSCs induced from iPSCs exhibit
morphological features and NSC marker gene expression similar to
those of wild-type NSCs. Furthermore, these induced NSCs have the
potential to differentiate into neurons (11). Therefore, iPSC-derived NSCs are
regarded as excellent seed cells for tissue engineering.
Numerous bioengineered scaffolds have been reported
to improve neural regeneration in tissue engineering for the
treatment of SCI (3). Electrospun
fibers have been widely produced for neural tissue engineering
scaffolds due to their ability to provide a suitable
microenvironment for cell attachment, proliferation and migration
that is similar to the natural extracellular matrix (12,13).
Poly(L-lactic acid) (PLLA) is an ideal bioengineered
scaffold that has been approved by the United States Food and Drug
Administration. PLLA has been widely used as a drug delivery,
artificial catheter and tissue engineering scaffold material due to
its biocompatibility, tunable degradation rate and
non-immunogenicity (14,15). The aligned fibers with diameters
in the nanometer range obtained by the electrospinning of natural
polymers usually exhibit superior mechanical properties (16). The pore features of electrospun
fibers, such as fully interconnected pore structures and a
wide-open surface, facilitate the transport of water and nutrients
for cell growth (17). In
addition, the fibers may be aligned to provide directional guidance
for axonal growth. It has been shown that electrospun nanofibers
are capable of directing neurite outgrowth and glial cell migration
along the aligned orientation (14,18).
In the present study, an electrospun PLLA nanofiber
scaffold was synthesized as a three-dimensional (3D) system and its
biocompatibility with iPSC-derived NSCs was assessed, with the aim
of using the combined system to guide neurite outgrowth and build a
cell delivery platform for nerve tissue engineering purposes.
Materials and methods
Ethics statement
Female BALB/c nude immune deficient mice (n=5; age,
6–7 weeks old; weight, 18–22 g) were obtained from Beijing Vital
River Laboratory Animal Technology Co., Ltd. (Beijing, China) and
maintained at 22°C (room temperature), 50–60% relative humidity,
and a 12 h light/dark cycle in a specific pathogen-free facility.
All the mice were fed a sterilized commercial diet. Pregnant BL/6
mice (n=2) carrying the OCT4-EGFP (OG2) pluripotency reporter were
obtained from Guangzhou Institute of Biomedicine and Health
(Guangzhou, China). All experimental procedures and animal handling
were carried out in accordance with the guidelines and laws of the
Animal Care and Use Committee of Sun Yat-sen University (Guangzhou,
China; approval no. SYXK2012-0083), and conformed with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (8th edition, 2011).
Isolation of murine embryonic fibroblasts
and iPSC generation
Mouse embryonic fibroblasts (MEFs) and wild-type
NSCs were obtained from embryonic day 13.5–14.5 mouse embryos
following previously published protocols (19) and cultured in high-glucose
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 1 mM
non-essential amino acids (NEAA) and penicillin-streptomycin (MEF
medium). The oriP/EBNA1-based episomal vectors pEP4-EO2S-ET2K (7
µg; Addgene plasmid no. 20927; Addgene, Inc., Cambridge, MA,
USA) and pCEP4-miR-302-367 cluster (5 µg) mixture was
electroporated into 2×106 MEFs with a Nucleofector 2b
Device with an Amaxa Basic Nucleofector kit (Both Lonza Group,
Ltd., Basel, Switzerland) according to the manufacturer's protocol.
The program used was T-020. The 1×105 cells were seeded
onto plates covered with a MEF feeder layer following 2 days of
culture in MEF medium. For the induction to iPSCs, the culture
medium was switched to iPSC medium [knockout-DMEM supplemented with
15% knockout serum replacement (KSR; Gibco; Thermo Fisher
Scientific, Inc.), 1 mM β-mercaptoethanol, 1X NEAA, 1 mM GlutaMAX,
1X penicillin-streptomycin solution, 1,000 U/ml mouse leukemia
inhibitory factor (LIF; EMD Millipore, Billerica, MA, USA), 2i (0.5
µM PD0325901 and 3 µM CHIR99021), and 50 µg/ml
vitamin C]. The medium was refreshed every 2 days. The colonies
were counted 13–16 days after plating, and the colonies that
appeared similar to mouse ESCs were selected for further
cultivation. Reprogramming efficiency was calculated based on the
number of ESC-like alkaline phosphatase (AP)-positive colonies
normalized to the number of seeded cells that were
electroporated.
Differentiation of mouse iPSCs into NSCs
and neurons
iPSCs were dissociated with 0.125% trypsin-EDTA and
then transferred to low-attachment dishes (19). They were cultured in iPSC medium
without LIF and 2i to promote embryonic body (EB) formation. After
4 days, the EBs were treated with the same medium with additional 1
µM all-trans retinoic acid (RA; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). After another 4 days, EBs were collected
by centrifugation at 300 × g for 5 min in room temperature and
dissociated with Accutase (Gibco; Thermo Fisher Scientific, Inc.).
For differentiation toward a neuronal lineage, the EBs were
transferred to tissue culture dishes coated with 0.01%
poly-L-lysine (PLL; Sigma-Aldrich; Merck KGaA) and maintained in
NSC medium [DMEM/F12 supplemented with b27 and N2 (invitrogen;
Thermo Fisher Scientific, Inc.) supplements, 20 ng/ml basic
fibroblast growth factor (BFGF) and 20 ng/ml epidermal growth
factor (EGF) (both from Peprotech, Inc., Rocky Hill, NJ, USA)]. The
medium was refreshed every 2 days. After 7 days, differentiated
cells were dissociated with Accutase and cultured in low-attachment
dishes with NSC differentiation medium comprising DMEM/F12
supplemented with 20 ng/ml BFGF and 20 ng/ml EGF to form
neurospheres. For terminal differentiation into neurons and glial
cells, these cells were transferred to tissue culture dishes in NSC
differentiation medium (DMEM/F12 supplemented with 5% FBS, 1
µM RA, 1 mM GlutaMAX, 1 mM sodium pyruvate, 0.1 mM NEAA and
1X penicillin/streptomycin) for another 14 days with a medium
change every 2 days.
Immunofluorescence staining and AP
staining
For immu-nocytochemistry, cells were fixed with 4%
paraformaldehyde for 20 min in 4°C and then washed with
phosphate-buffered saline (PBS) three times. After treating with
0.2% Triton X-100 and blocking solution (6% FBS; Life Technologies,
Carlsbad, CA, USA) for 1 h in room temperature, the cells were
incubated with primary antibodies overnight at 4°C and incubated
with the respective secondary antibodies for 1 h at room
temperature. Nuclei were detected by Hoechst 33342 (Sigma-Aldrich;
Merck KGaA) staining in dark for 4 min at room temperature. Cells
were imaged with a fluorescence microscope and confocal laser
scanning microscopy (LSM 700; both Leica Microsystems, GmbH,
Wetzlar, Germany). Antibodies used targeted: SOX2 (1:400; Abcam,
Cambridge, MA, USA), OCT4 (1:400; EMD Millipore), homeobox protein
NANOG (NANOG; 1:1,000), stage-specific embryonic antigen 1 (SSEA1;
1:1,000) and NESTiN (1:200) (all from Abcam),
microtubule-associated protein 2 (MAP2; 1:800; EMD Millipore), β
iii tubulin (Tuj1; 1:400; Abcam), glial fibrillary acidic protein
(GFAP; 1:200), myelin basic protein (MBP; 1:200) and paired box 6
protein (PAX6; 1:200) (both from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Alexa 488- and Alexa 555-labeled secondary
antibodies were obtained from Invitrogen (Thermo Fisher Scientific,
Inc.). AP staining was performed using the Alkaline Phosphatase
Detection kit (EMD Millipore) according to the manufacturer's
protocol. The immunofluorescence was imaged under a confocal laser
scanning microscope (LSM 700; Leica Microsystems GmbH, Wetzlar,
Germany). Image processing and analysis were realized using ImageJ
(National Institutes of Health, Bethesda, MD, USA) and a plugin
called NeuronJ (20). In this
program, we used a computer mouse to select a starting point and an
ending point to trace the dendrite in order to get quantification
metrics. Data were organized using Excel (Microsoft, Redmond,
Washington, USA).
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted with TRizol (invitrogen;
Thermo Fisher Scientific, Inc.) and cDNA was synthesized using the
Reverse Transcription kit (Takara Biotechnology, Co., Ltd., Dalian,
China) following the manufacturer's protocol. RT-qPCR was conducted
using Maxima™ SYBR-Green/ROX qPCR Master Mix (Thermo Fisher
Scientific, Inc.) according to the instructions provided by the
manufacturer, with the Stratagene Mx3000P Real-time PCR system
(Agilent Technologies, Inc., Santa Clara, CA, USA). DNA was
extracted from the iPS cells with Qiagen DNeasy kit (Qiagen Co.,
Ltd., Shanghai, China). DNA fragments were amplified by PCR with
Taq DNA Polymerase (Thermo Fisher Scientific, Inc.). The PCR
reaction (50 µl) included 5 µl 10X Taq Buffer, 1.5 mM
MgCl2, 2 µmol bidirectional primers, 0.2 mM
deoxynucleoside triphosphates (dNTPs), 1.25 U TaqDNA polymerase, 1
µg cDNA and double-distilled water. The PCR reaction
conditions were as follows: denaturation at 94°C for 3 min;
denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec,
extension at 72°C for 45 sec, 30 cycles; extension at 72°C for 10
min. PCR products were separated on a 1.2% agarose gel (with
ethidium bromide staining). The resultant gel image was analyzed
using the AlphaImager gel analysis system. The primer sequences
used for the RT-qPCR and PCR analyses of iPSCs are listed in
Table I. The sequences of the
primer pairs used for the RT-qPCR analyses of NSCs are listed in
Table II. Relative mRNA
expression was calculated using the 2−ΔΔCT method and
GAPDH was used as an internal control (21).
 | Table IPrimers for the RT-qPCR and PCR
detection of iPSCs. |
Table I
Primers for the RT-qPCR and PCR
detection of iPSCs.
| Names | Primer
sequences |
|---|
| SOX2 | F:
5′-TAGAGCTAGACTCCGGGCGAT-3′ |
| R:
5′-TTGCCTTAAACAAGACCACGAAA-3′ |
| OCT4 | R:
5′-TTGCCTTAAACAAGACCACGAAA-3′ |
| R:
5′-TGCGGGCGGACATGGGGAGATCC-3′ |
| NANOG | F:
5′-CACAGTTTGCCTAGTTCTGAGG-3′ |
| R:
5′-GCAAGAATAGTTCTCGGGATGAA-3′ |
| EXO-SOX2 | F:
5′-ACCAGCTCGCAGACCTACAT-3′ |
| R:
5′-CCCCCTGAACCTGAAACATA-3′ |
| EXO-OCT4 | F:
5′-AGTGAGAGGCAACCTGGAGA-3′ |
| R:
5′-AGGAACTGCTTCCTTCACGA-3′ |
| miR-302-367 | R:
5′-CCCCCTGAACCTGAAACATA-3′ |
| R:
5′-CTCCCAAAGAGTCCTGTTCTGTCCT-3′ |
| EBNA | F:
5′-ATCGTCAAAGCTGCACACAG-3′ |
| R:
5′-CCCAGGAGTCCCAGTAGTCA-3′ |
| GAPDH | F:
5′-AGGTCGGTGTGAACGGATTTG-3′ |
| R:
5′-GGGGTCGTTGATGGCAACA-3′ |
 | Table IIPrimers for the RT-qPCR detection of
NSCs. |
Table II
Primers for the RT-qPCR detection of
NSCs.
| Names | Primer
sequences |
|---|
| NESTIN | F:
5′-CCCCTTGCCTAATACCCTTGA-3′ |
| R:
5′-GCCTCAGACATAGGTGGGATG-3′ |
| PAX6 | F:
5′-GTTGTGTGAGTAAAATTCTGGGC-3′ |
| R:
5′-GAGTCGCCACTCTTGGCTTA-3′ |
| BLBP | F:
5′-CGCAACCTGGAAGCTGACA-3′ |
| R:
5′-GCCCAGAGCTTTCATGTACTCA-3′ |
| ZFP42 | F:
5′-CCGGGATGAAAGTGAGATTAGC-3′ |
| R:
5′-TCACCTCGTATGATGCACTC-3′ |
| GAPDH | F:
5′-AGGTCGGTGTGAACGGATTTG-3′ |
| R:
5′-GGGGTCGTTGATGGCAACA-3′ |
Teratoma formation
To determine the pluripotency of the iPSCs in
vivo, the iPSCs were injected into immunocom-promised mice for
teratoma formation. The iPSC colonies were collected by 0.5 mM EDTA
treatment, and 2×106 iPSCs were subcutaneously injected
into 4-week-old BALB/c nude immune deficient mice. At 4 weeks after
the cell injection, the teratomas were collected and fixed in 10%
formalin at room temperature overnight. The teratomas were then
dehydrated through graded ethanol, embedded in paraffin and cut
into 5-µm-thick sections. After being dewaxed with ethanol,
the slices were soaked with hematoxylin at room temperature for 5
min. Following rinsing with water for 10 min, the slices were
stained with eosin for 3 min and dehydrated through graded ethanol.
The stained tissues, which were permeabilized with xylene and
mounted with resinene, could be observed under a microscope (TS100;
Nikon Corporation, Tokyo, Japan).
Patch clamp analysis
iPSC-derived neurons were tested on a microscopic
workbench with a patch clamp. Neurons were immersed in
extracellular fluid containing 95% O2 and 5%
CO2 during the whole process. Cells were visualized
using an Olympus patch clamp microscope (Olympus Corporation,
Tokyo, Japan). Medium-sized neurons with a bright cell margin and
smooth surface were selected for patch clamp analysis. Neurons were
inserted into the clamp and data were recorded using an Axon
MultiClamp 700B amplifier (Axon instruments; Molecular Devices,
LLC, Sunnyvale, CA, USA) and Igor 5.0 software (Wavemetrics, Inc.,
Lake Oswego, OR, USA).
Preparation of aligned and randomly
oriented nanofibrous scaffolds by electrospinning
Polymer solution was prepared by dissolving PLLA in
hexafluoroisopropanol at a concentration of 5% w/w. Electrospinning
was conducted with the application of a positive voltage (15 kV) to
the spinning solution with a distance between the needle tip and
the collecting plate surface of 15±2 cm. The solution was placed in
a syringe fitted with a 5-ml blunt-end needle and delivered through
a syringe pump to control the mass flow rate at 1 ml/h. For aligned
nanofibers, the collector was a motor-driven wheel. For random
nanofibers, the collector was a stationary plate. The spun
nanofibers were dried under vacuum at room temperature for 24
h.
Cell viability, adhesion and
proliferation on PLLA nanofibers scaffolds
To investigate the biocompatibility of the PLLA
nanofiber scaffolds, iNSCs were dissociated and then seeded on the
nanofiber scaffolds. As a control, iNSCs were also seeded and
cultured on tissue culture polystyrene (TCPs) coated with PLL,
which was used as a positive control for the NSC culture
experiments.
Cell survival on the PLLA fiber scaffolds and
PLL-coated TCPs was evaluated using a Cell Counting kit-8 (CCK-8;
Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) following the
manufacturer's protocol. Cells were cultured in 96-well plates for
2 days and then incubated with 100 µl CCK-8 solution per
well for 2 h at 37°C, and the absorbance was measured at 450 nm
(Elx800; BioTek instruments, Inc., Winooski, VT, USA). INSCs
(1×104/well) were seeded onto PLLA scaffolds in a
96-well plate and PLL-coated TCPs. Following incubation for 3, 6
and 9 h, the samples were washed with PBS three times to remove the
cells that did not adhere to the scaffolds. The remaining cells
were collected with 0.25% trypsin-EDTA and the number of cells was
counted under an inverted optical microscope (TS100; Nikon
Corporation). Cell proliferation was evaluated using an CCK-8
assay. After seeding the iNSCs (1×103/well) onto PLLA
and PLL-coated TCPs for 1, 3, 5, 7 and 9 days in the 96-well
plates, cells were incubated with CCK- 8 solution for 2 h at 37°C.
The absorbance of the solution in the 96-well plates was then
measured. Finally, a proliferation curve of the iNSCs on the
scaffolds was constructed.
Morphological examination using scanning
electron microscopy (SEM) and nuclear staining
Samples were washed with PBS and then fixed in 2.5%
glutaraldehyde overnight at 4°C. The following morning, samples
were washed three times prior to dehydration with a series of 30,
50, 70 and 100% ethanol (7 min each). After air-drying, the samples
were covered with gold using sputter coating (IB5 ion coater; Eiko
Engineering Co., Ltd., Hitachinaka, Japan) and the cell
morphologies were observed using SEM (Quanta 200; FEi; Thermo
Fisher Scientific, Inc.). For nuclear staining, nuclei were
detected by Hoechst 33342 staining (Sigma-Aldrich; Merck KGaA) as
described above.
Statistical analysis
Data are reported as the mean ± standard deviation.
Statistical analysis was conducted using Student's t-test for
comparing two groups of data and analysis of variance (ANOVA) with
Bonferroni comparison for three or more groups of data with SPSS
16.0 statistical software (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Generation of mouse iPSCs and analysis of
pluripotency markers
MEFs of low passage number (passage 3) were used for
the induction of iPSCs. The oriP/EBNA episomal vectors were
transfected into the MEFs through electroporation. ESC-like
colonies began to emerge at day 7 following plasmid transfection,
and by day 17, they were large enough to be selected for expansion.
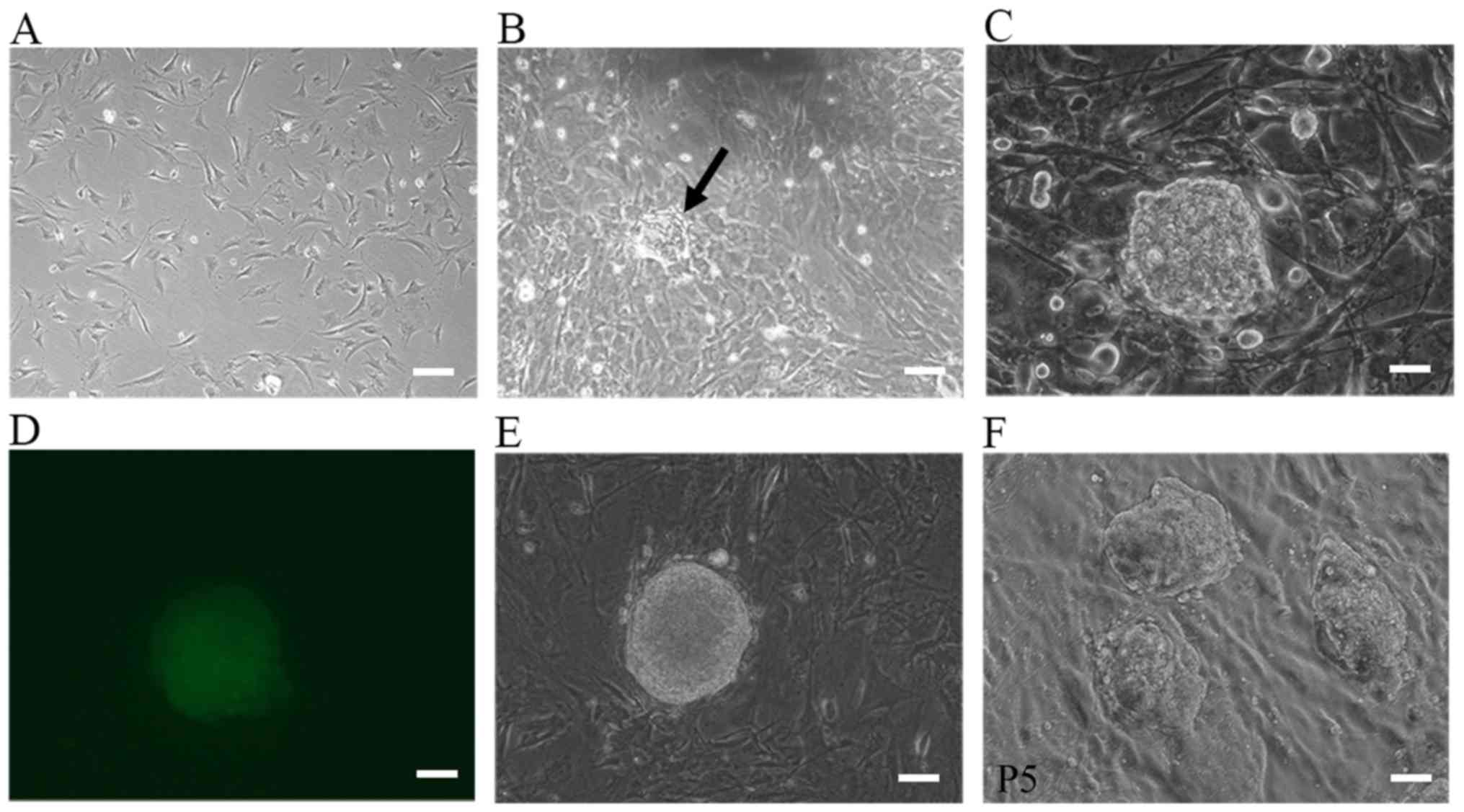
Fig. 1A–D shows the morphological
changes during the process of iPSC generation. For the
1×105 cells routinely used to start reprogramming, the
number of ESC-like colonies varied between 10 and 50 and the
reprogramming efficiency was 0.01–0.05%. Colonies were manually
selected and successfully expanded on MEF feeder cells. Some clones
were selected for subsequent studies (Fig. 1E and F). Immunocytochemical
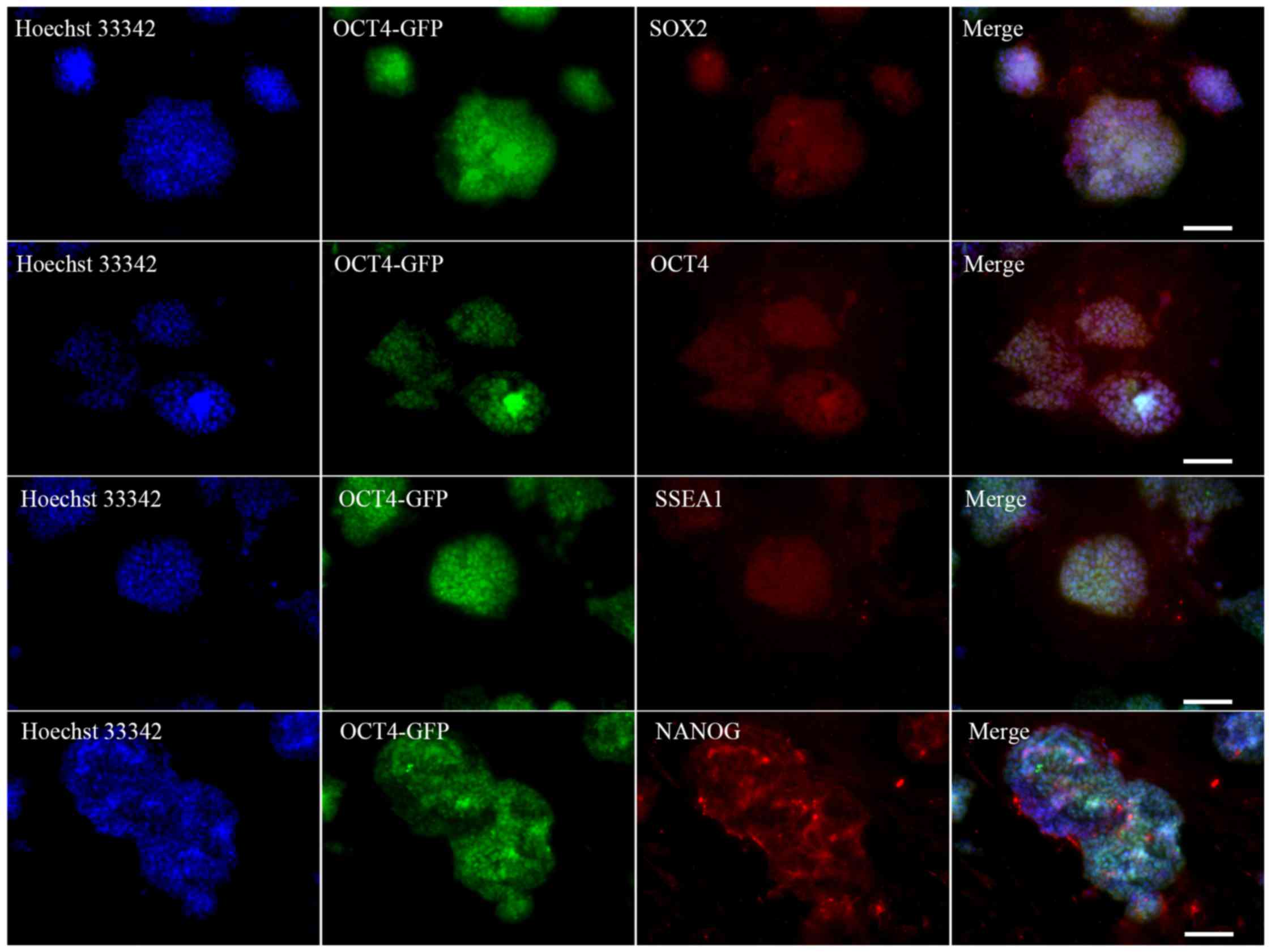
analysis on these morphologically ESC-like cells demonstrated that
they expressed the ESC markers SOX2, OCT4, SSEA1 and NANOG
(Fig. 2). These colonies also
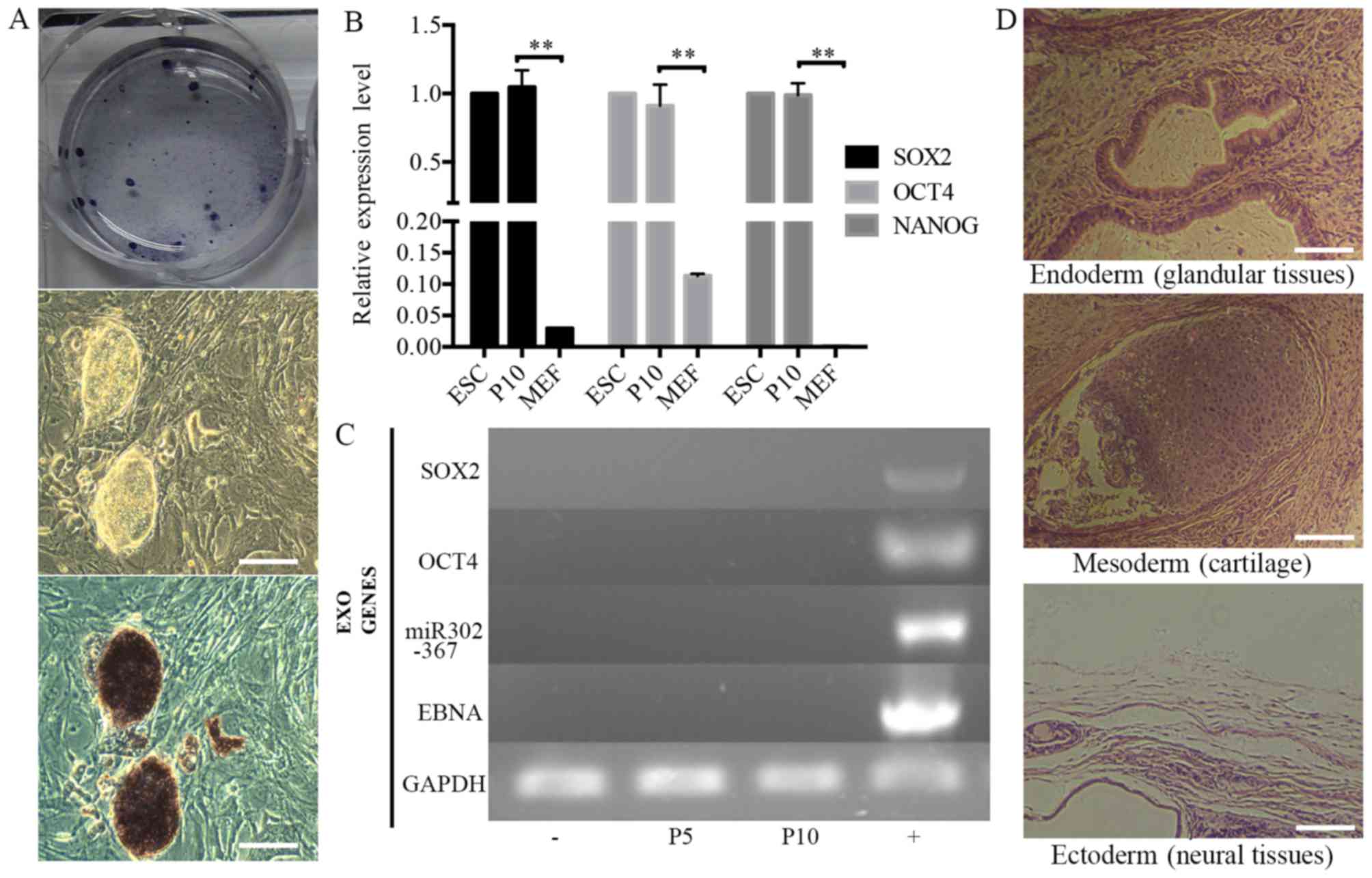
stained positive for AP (Fig.
3A). The source cells, MEFs, were negative for all the
aforementioned markers. The expression levels of the endogenous
pluripotency marker genes SOX2, OCT4 and NANOG were analyzed by
RT-qPCR and were comparable to those of mouse ESCs (Fig. 3B). Using PCR to specifically
amplify the transgenes used for reprogramming, it was confirmed
that the established iPSC colonies no longer harbored the exogenous
reprogramming factors and episomal backbones (Fig. 3C).
Differentiation potential of iPSCs
Teratoma formation assays were performed to examine
the differentiation potential of iPSCs in vivo. The iPSC
colonies were collected and injected into BALB/c nude mice. Tumor
formation efficiency was 100% and palpable tumor masses appeared in
all cases 1 week later. The tumors were allowed to develop for
another 3 weeks prior to harvesting. Histological examination was
performed by a qualified pathologist and the tumors were identified
as teratomas. The teratomas contained organized structures of three
germ layer cell derivatives, namely glandular tissues (endoderm),
cartilage (mesoderm) and neural tissue (ectoderm) (Fig. 3D).
In vitro differentiation into a neural
lineage
The ability of the iPSCs to differentiate into
functional neurons was investigated. The differentiation potential
was explored by performing immunocytochemical analysis and RT-qPCR.
Immunocytochemical analysis demonstrated that the induced NSC-like
cells expressed the NSC markers, NESTIN and paired box 6 (PAX6)
(Fig. 4A). For further
characterization, RT-qPCR revealed that the expression levels of
NESTIN, PAX6 and brain lipid-binding protein (BLBP), which are
typical NSC markers, were increased significantly in iNSCs compared
with MEFs (Fig. 4B) and iPSCs
(Fig. 4C) and the expression
levels of these markers in the iNSCs were similar to those in
wild-type NSCs (wt-NSCs; Fig.
4D). In addition, the iNSCs did not express significant levels
of the pluripotency-related genes OCT4, NANOG and zinc finger
protein 42 (ZFP42; Fig. 4E).
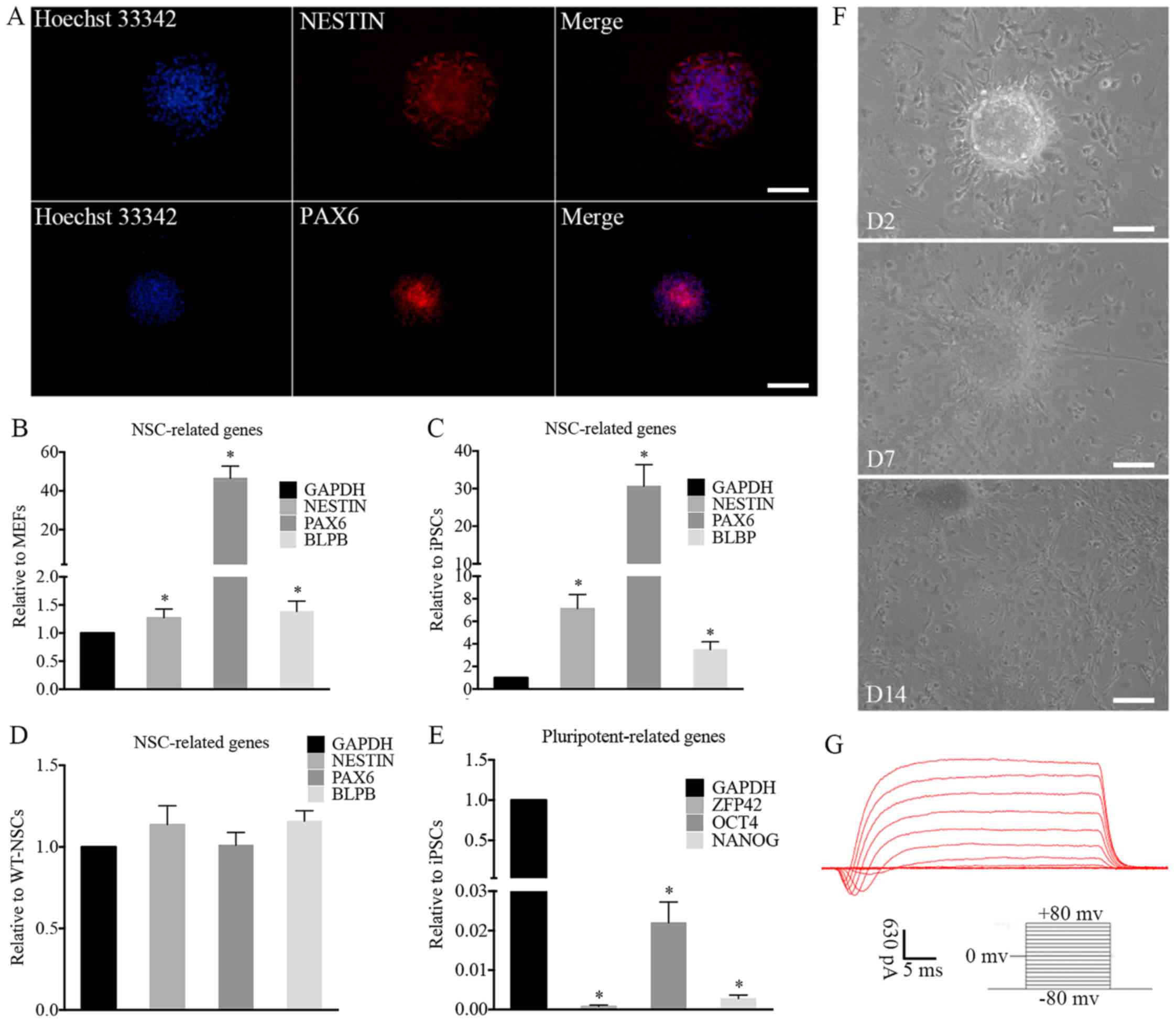 | Figure 4Characterization and differentiation
of iNSCs in vitro. (A) immunofluorescence of iNSCs. NSC
markers were assessed by immunofluorescence, including NESTiN and
PAX6. The expression of typical NSC-related genes on iNSCs
significantly increased compared with that on (B) MEFs and (C)
iPSCs (*P<0.05) with similar expression to (D)
wt-NSCs. (E) Compared with iPSCs, the expression of
pluripotency-related genes on iNSCs was negligible
(*P<0.05 vs. iPSCs). (F) Differentiated cells
migrated from neurospheres on D2 after adherence. Neurites and
differentiated cells were observed around the adherent NSCs on D7
and D14. (G) Electrophysiological function of differentiated
neurons. (A and F) Scale bars, 100 µm. iNSC, induced
pluripotent stem cell-derived neural stem cell; NSC, neural stem
cell; MEF, mouse embryonic fibroblast; iPSC, induced pluripotent
stem cell; wt, wild-type; PAX6, paired box 6; BLBP, brain
lipid-binding protein; ZFP42, zinc finger protein 42; OCT4,
octamer-binding transcription factor 4; D2, day 2; D7, day 7; D14,
day 14. |
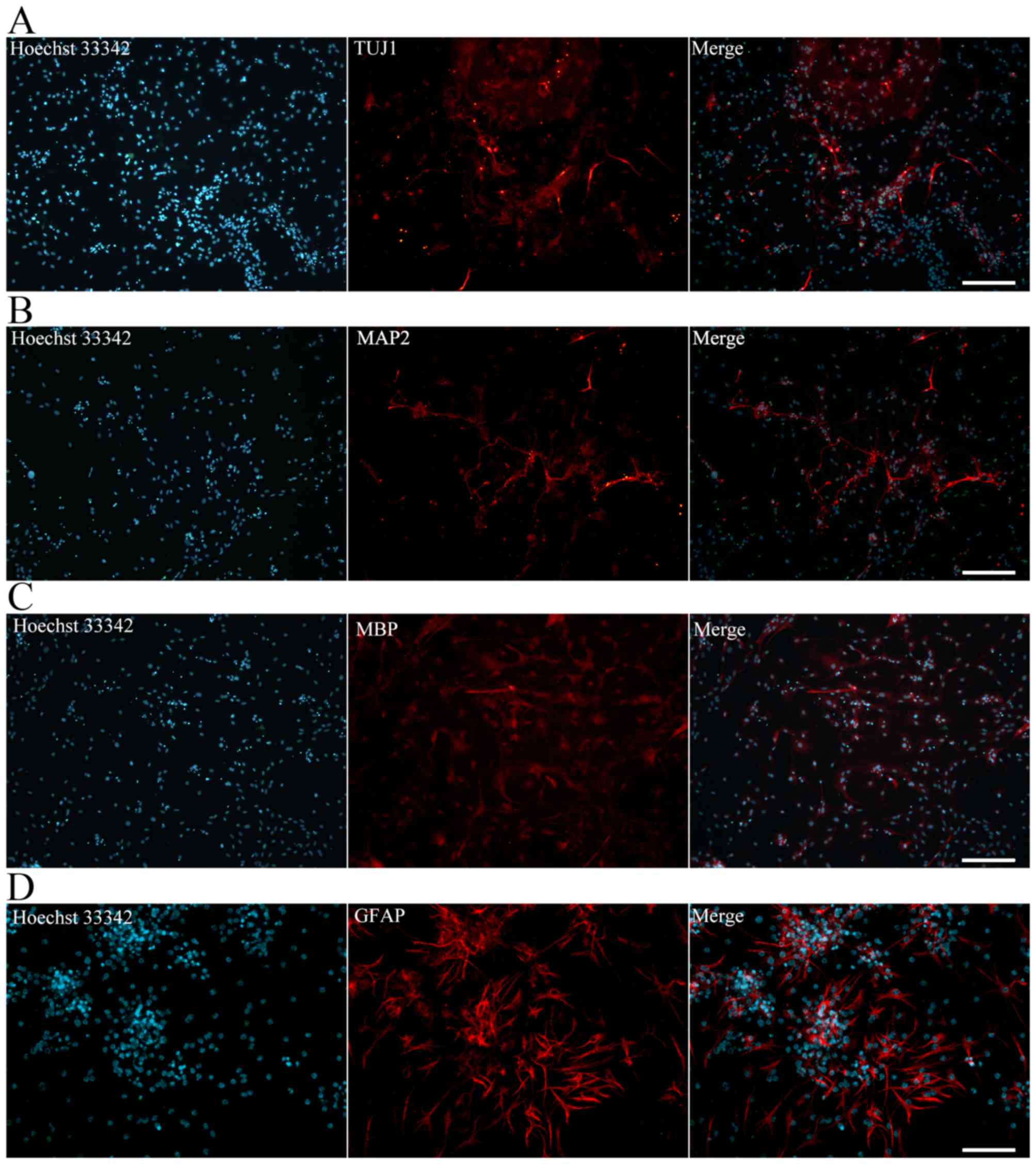
The iNSCs were further differentiated into neurons,
astrocytes and oligodendrocytes. The iNSCs cultured on PLL-coated
TCPs in NSC differentiation medium exhibited neural lineage
cell-like morphology (Fig. 4F).
Cells (63.2%) differentiated from iNSCs were detected to exhibit
inward Na+ and outward K+ currents, which are
the properties of mature, functional neurons (Fig. 4G). At 7 days after
differentiation, immunofluorescence staining revealed Tuj1-positive
neurons (Fig. 5A). MAP2-positive
mature neurons (Fig. 5B),
MBP-positive oligodendrocytes (Fig.
5C) and GFAP-positive astrocytes (Fig. 5D) were also observed after
culturing for more 7 days. Together, these data indicate that the
iNSCs were able to differentiate into neurons, astrocytes and
oligodendrocytes.
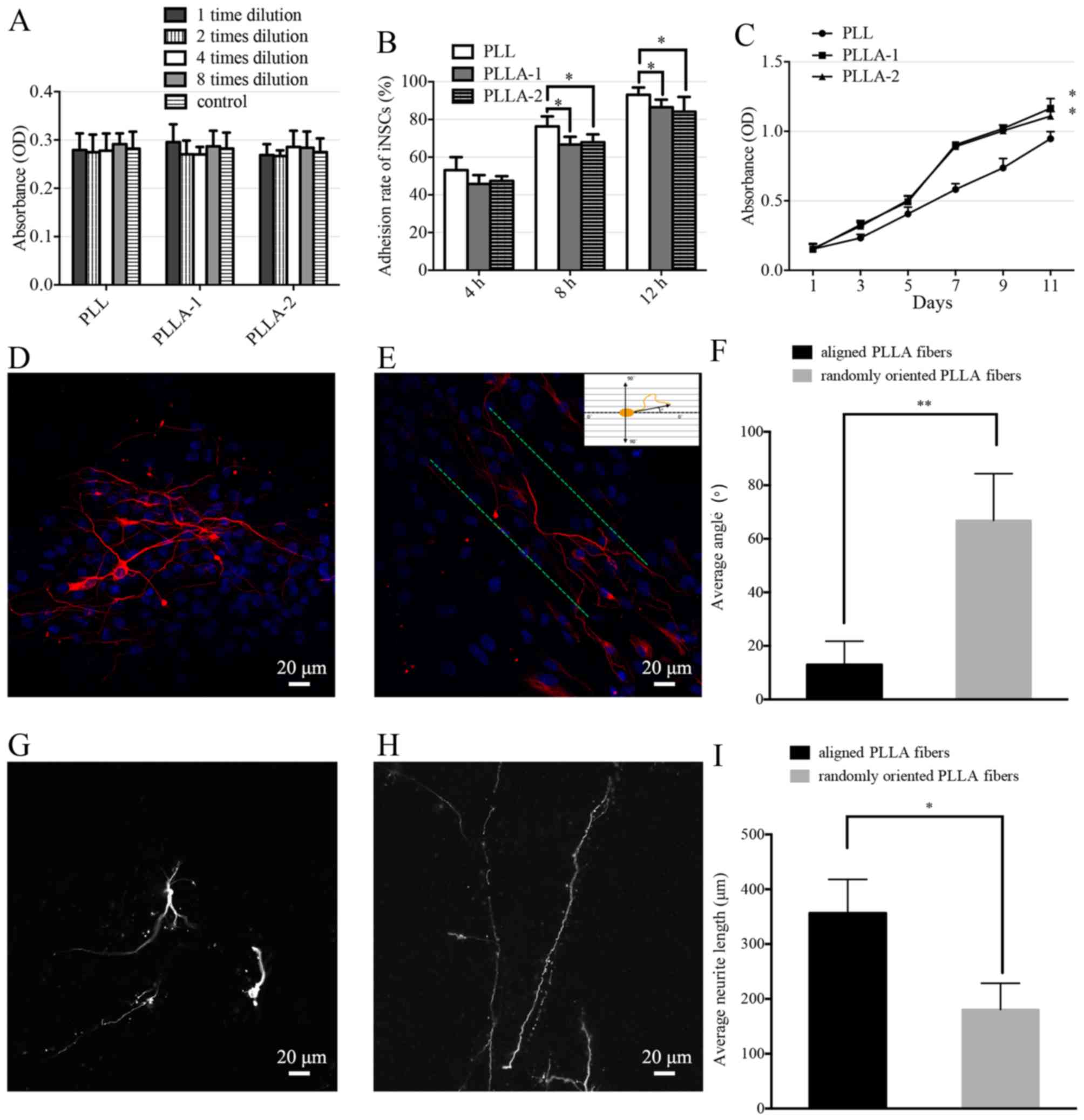
iNSC survival, adhesion and process
extension on PLLA nanofiber scaffolds
The cell viability of the iNSCs on the PLL-coated
TCPs and PLLA nanofiber scaffolds did not differ significantly
(Fig. 6A). The adhesion rates of
iNSCs on PLL-coated TCPs were significantly higher than those of
iNSCs on PLLA nanofibers at each time-point (Fig. 6B). The proliferation of iNSCs on
PLLA nanofibers and PLL-coated TCPs was evaluated (Fig. 6C). The number of the cells in the
PLL-coated TCPs and PLLA scaffolds increased steadily from the
first day of incubation; however, the iNSCs proliferated
significantly more strongly on the PLLA scaffolds compared with
those on PLL-coated TCPs.
Analysis of neurite growth on PLLA
nanofiber scaffolds as a 3D culture system
Following 14 days of iNSC culture with
differentiation medium, the neurites exhibited randomly oriented
growth patt erns on the randomly oriented PLLA nanofibers (Fig. 6D), and parallel growth on aligned
PLLA nanofibers (Fig. 6E).
Fig. 6E shows how the measurement
of the neurite orientation was calculated. The average neurite
angle on the randomly oriented PLLA nanofibers was calculated as
66.9±17.5°. The average neurite angle on the aligned PLLA
nanofibers was 13.0±8.8°, which was significantly smaller than that
on the randomly oriented nanofibers (Fig. 6F). The neurite length was measured
as a linear distance between the tip of the neurite and the cell
junction. The average neurite length on the randomly oriented PLLA
nanofibers (Fig. 6G) was calcu
lated to be 180.7±47.7 µm. The average neurite length on the
aligned PLLA nanofibers (Fig. 6H)
was 356.7±61.2 µm, which was significantly longer than that
of the neurites on the randomly oriented nanofibers (Fig. 6I).
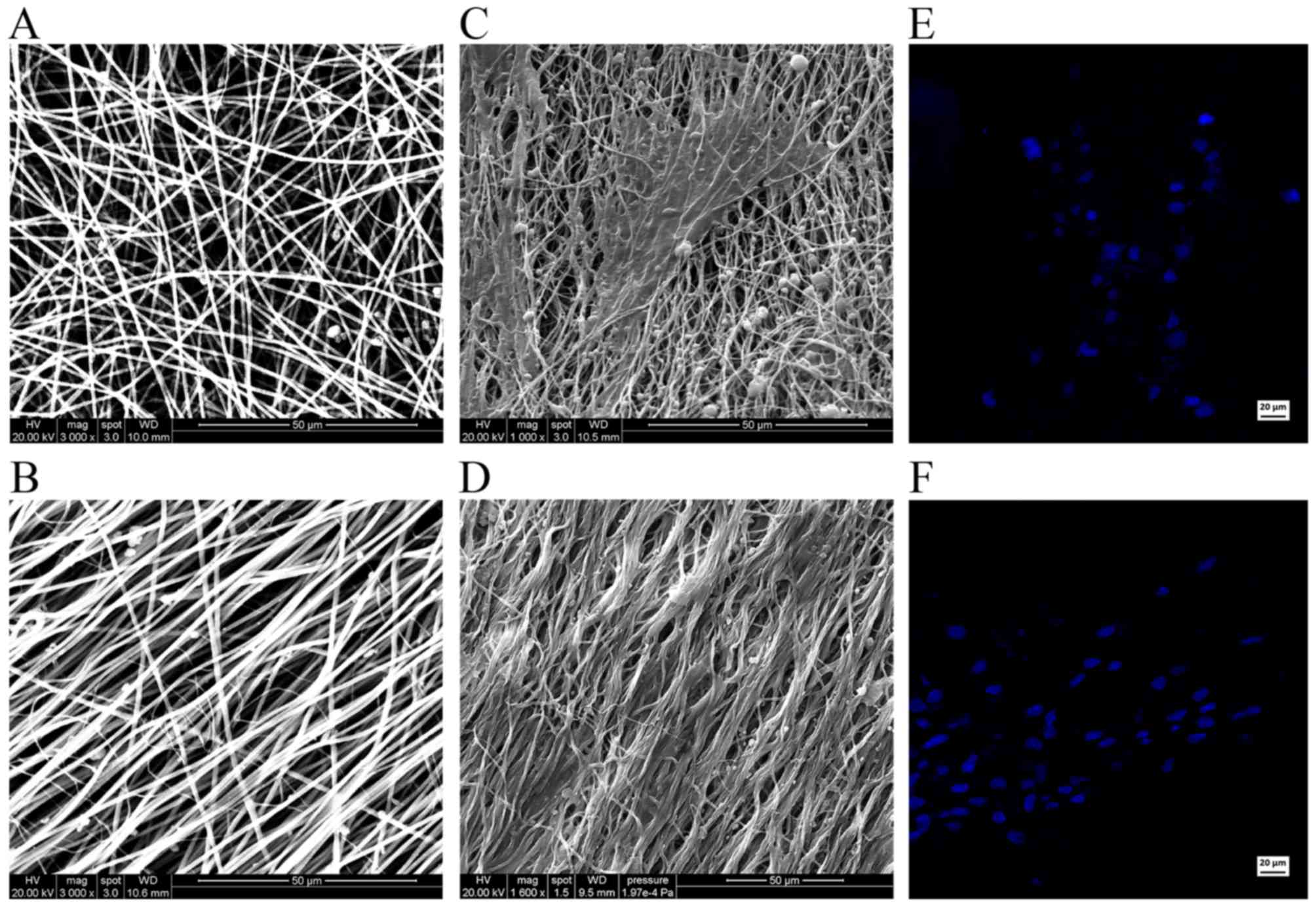
SEM images reveal the relationship between the iNSCs
and the supporting nanofibers. Scanning electron micrographs
demonstrated the randomly oriented PLLA fibers (Fig. 7A) and aligned PLLA fibers
(Fig. 7B). On the randomly
oriented PLLA nanofibers, the cells projected neurites that were
randomly oriented (Fig. 7C).
However, on the aligned PLLA nanofibers, the neurites were observed
to be parallel to the nanofibers (Fig. 7D). Hoechst staining demonstrated
the normal morphology and quality of the nuclei in the two groups
(Fig. 7E and F).
Discussion
Following SCI, nervous system damage causes severe
physical and sensory loss that may have severe adverse effects on
the quality of life. SCI often causes the death of glial cells and
nerve cells local to the injury, which was considered to be
unrenewable until 1981 when David and Aguayo reported the injured
axons could be regenerated in the appropriate circumstances
(22). Numerous studies have
attempted to supplement neurons and glial cells with the aim of
reestablishing the neural connections and achieving functional
recovery (23). NSCs are able to
differentiate into neurons, astrocytes and oligodendrocytes. The
ability of transplanted NSCs to integrate into host tissue and
thereby recover the function of damaged neural tissue by improving
neurogenesis and axonal regrowth has been reported (24).
However, due to the multifactorial and multiphasic
pathophysiology of the brain and SCI, NSC transplantation alone is
likely to cause glial scar and tissue collapse, ultimately
affecting the recovery of spinal cord function.
In one cell transplantation approach, a cell
suspension is directly injected via fine needles or glass
capillaries. Using such an approach, it has been observed that the
cell suspension quickly diffuses following injection and does not
distribute into the spine cord gap (25). Furthermore, with the lack of an
effective support, the surrounding tissues usually collapse. One
available strategy to address these issues is to combine the
seeding cells with scaffolds to support the reconstruction. The
ideal scaffolds should inhibit glial scar formation and provide
guidance for neurite outgrowth. This encouraged the present
research team to prepare an ideal scaffold for nerve tissue
engineering and investigate its biocompatibility with seeding cells
to promote reconstruction of the injured spinal cord.
Stem cells, such as ESCs, mesenchymal stem cells and
NSCs, have been investigated in nervous system treatment (5,26).
Each of these stem cells displays different degrees of regeneration
and functional restoration. Among them, NSCs have been reported to
be a suitable source of therapeutic cells in neural lesions with
the ability to promote the survival of injured neurons and
functional recovery (27).
Furthermore, transplanted NSCs do not cause obvious graft rejection
due to their low immunogenicity (28). These advantages indicate that NSCs
are the ideal seeding cells for tissue engineering to repair
SCI.
There are a number of methods by which SCs may be
obtained. The primitive method is to isolate them from the
hippocampus, which is not a practical approach for transplantation
(29). In addition, NSCs can be
differentiated from ESCs (30)and
iPSCs(31). However, the ethical
issues which hamper the further development of ESCs remain to be
solved. In a revolutionary study, Takahashi and Yamanaka
reprogrammed adult somatic cells to become ESC-like cells, termed
iPSCs, which paved the way for a new era in regenerative medicine
and tissue engineering (32). The
iPSC line was observed to behave similarly to ESCs, and conformed
to the major aspects of pluripotency, such as unlimited
self-renewal, multi-lineage differentiation potential. A number of
studies have demonstrated that iPSCs are able to effectively
differentiate into functional neurons in vitro and in
vivo (33). The generation of
patient-specific iPSCs reduces the risk of immune rejection
following transplantation and provides the most suitable seeding
cells for regenerative medicine. However, initial attempts to
generate iPSCs typically used genome-integrating retroviral or
lentiviral vectors, which limits their clinical application
(9). The genomic integration of
transgenes creates insertional mutagenesis and the continued
expression of oncogenic proteins, which increases the risk of tumor
formation (34).
To overcome these obstacles, several non-integrating
approaches had been reported to generate mouse and human iPSCs,
including Sendai virus (35), the
piggyBac system (36), episomal
vectors (37) and direct protein
delivery (38). The majority of
these reprogramming approaches are inefficient or laborious. The
direct delivery of proteins, RNA or modifying Sendai virus vectors
is technically demanding, and requires the repeated delivery of the
reprogramming factors (34).
In the present study, electroporation of episomal
vector (pCEP4-EO2S-ET2K) was conducted to deliver the reprogramming
factors into MEFs and obtain non-integrating iPSCs. In addition,
pCEP4-miR-302-367 cluster (39),
which greatly enhances reprogramming efficiency, was added to the
transfection system.
A reprogramming efficiency of up to 0.05% was
achieved, which was lower than that of the retroviral or the
lentiviral infection approaches (0.1–1%) (40), but higher than that of standard
episomal vectors (~0.005%) (37).
Furthermore, exogenous reprogramming factors were not detectable in
the reprogrammed iPSCs at passages 5 and 10, which is an important
safety advantage for clinical application. In addition, plasmid
vectors can be manufactured and qualified for good manufacturing
practice with a relatively low cost. The capacity of the
reprogramed iPSCs to differentiate into neural lineage cells was
then investigated. The iNSCs exhibited the expression of the
hallmark NSC markers NESTIN, PAX6 and BLBP, with similar expression
levels to those in wt-NSCs. In addition, the expression of
pluripotent-related genes in these cells was extremely low compared
with that of iPSCs. The iNSCs were cultured for further induction
in vitro and transplanted in PLLA scaffolds. Cells were
observed to survive for prolonged periods and differentiate into
mature neurons with the expected electrophysiological properties
and glial cells.
Despite surgical interventions and entubulation, the
functional recovery of SCI remains very challenging in clinical
practice (23). The misdirection
of regenerating neurons and the gaps between the injured spinal
cord are the main issues of concern (41).
Recently, the development of tissue engineering
approaches using functional cells combined with biodegradable
scaffolds has shown considerable promise (3,42).
The ideal scaffold is able to provide mechanical support as well as
a suitable environment, similar to the natural extracellular
matrix, that is able to improve cell adhesion and growth. Due to
its topographic features and physical properties, PLLA has been
widely studied in many fields, particularly tissue engineering
(15). Furthermore, it has been
reported that this scaffold construct provides a microenvironment
for seeding cells that maintains the morphology and functional
phenotype of the cells without causing immunological rejection
(14). Aligned PLLA nanofibers
have been demonstrated to promote the differentiation of
pheochromocytoma 12 cells, guide neurite outgrowth and support
Schwann cell migration along the aligned orientation (14,18). Furthermore, nanofibrous scaffolds
have the capacity to be rolled and packed into a cylindrical shape
mimicking the normal spinal cord. This may not only provide ample
substrate for cell transplantation, but also support the structure
to avoid collapse of the surrounding tissues (43). In addition, the ability of aligned
PLLA nanofibers to conduct neonatal nerve growth along the fibrous
orientation and thereby promote nerve functional recovery in
vivo has been demonstrated (44).
In the present study, electrospinning was used to
synthesize aligned electrospun PLLA nanofibers and randomly
oriented electrospun PLLA nanofibers as a delivery platform. The
biocompatibility of these nanofibers with iNSCs was then tested
in vitro. The viability of the iNSCs, measured by CCK-8
assay, was similar on the two PLLA nanofiber scaffolds and on the
PLL-coated TCPs. This indicates that PLLA nanofibers have no
cytotoxic or inhibitory effects on iNSC survival. Even though cell
adhesion rates did not exhibit an apparent improvement on PLLA
nanofibrous scaffolds compared with those on PLL-coated TCPs at
each time-point, the majority of the cells adhered to the PLLA as
time passed. This may be due to the hydrophobic surface of the PLLA
scaffolds providing less favorable adhesion conditions than the
hydrophilic surface of the PLL-coated TCPs. The proliferation of
iNSCs did not differ significantly on day 1 according to whether
they were supported on the two PLLA nanofiber scaffolds or the
PLL-coated TCPs. However, from day 3, the iNSCs proliferated more
strongly on the PLLA nanofibers scaffolds compared with those on
the PLL-coated TCPs. Therefore, it may be concluded that the PLLA
nanofibrous scaffolds greatly improved iNSCs proliferation.
The aligned PLLA nanofibers appear to be better
suited for neurite outgrowth than were the randomly oriented PLLA
nanofibers, because the alignment provided guidance cues to direct
axonal outgrowth. In the present study, the neurite length and
angle between neurite outgrowth and PLLA nanofibers were
calculated. The average length of the neurites in the
differentiated iNSCs cultured on the aligned PLLA nanofibers was
significantly greater than those on the randomly oriented PLLA
nanofibers. The majority of the neurites from differentiated iNSCs
on the aligned PLLA nanofibers were oriented within 13.0±8.8°,
whereas those on those on the randomly oriented PLLA nanofibers
exhibited random orientation (66.9±17.5°). Therefore, the aligned
PLLA nanofibrous scaffolds exhibit a potent ability to direct
differentiated iNSCs and elongate neurite growth length. SEM
results also demonstrated that differentiated iNSCs tightly
attached and aligned with the nanofibers. The above results
demonstrate that aligned PLLA nanofibers are highly effective for
neurite guidance and the extension of differentiated iNSCs.
In conclusion, iPSC-derived NSCs are promising cell
candidates for the treatment of SCI as they can be generated from a
wide range of somatic cells without causing immunology issues and
ethical issues. Also, they can effectively develop into a neural
lineage. In the present study, integration-free iPSCs were
successfully generated with the episomal vectors pEP4-EO2S-ET2K and
pCEP4-miR-302-367 cluster. The integration-free iPSCs were
differentiated into NSCs and their cytocompatibility and neurite
growth on PLLA nanofibrous scaffolds in vitro were
investigated. The results indicated that PLLA nanofibers had no
cytotoxic or adverse effects on iNSC survival and proliferation,
and the aligned PLLA nanofibers were able to direct and elongate
neurite outgrowth, supporting their potential usefulness for nerve
guidance when implanted at the SCI site. The combination of
iPSC-derived NSCs with PLLA scaffolds as a cell-scaffold strategy
has great potential for applications in nerve tissue engineering.
Studies are ongoing to investigate their feasibility for such
applications in vivo.
Acknowledgments
This study was supported by the Natural Science
Foundation of China (grant nos. 81772349, 31470949, 31170947 and
81472122), the Guangdong Natural Sciences Foundation of China
(S2012020011099 and S2013010016413) and the Guangzhou Science and
Technology Planning Project of China (2013J4100062). The authors
thank Mr. Baojian Liao for the plasmids, Mr. Bin Jiang for patch
clamp analysis, and Mr. Dongbo Qiu and members of the Cell-gene
Therapy Translational Medicine Research Center for help and
discussion.
References
|
1
|
Nichols CM, Brenner MJ, Fox IK, Tung TH,
Hunter DA, Rickman SR and Mackinnon SE: Effects of motor versus
sensory nerve grafts on peripheral nerve regeneration. Exp Neurol.
190:347–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
IJkema-Paassen J, Jansen K, Gramsbergen A
and Meek MF: Transection of peripheral nerves, bridging strategies
and effect evaluation. Biomaterials. 25:1583–1592. 2004. View Article : Google Scholar
|
|
3
|
Evans NR, Davies EM, Dare CJ and Oreffo
RO: Tissue engineering strategies in spinal arthrodesis: the
clinical imperative and challenges to clinical translation. Regen
Med. 8:49–64. 2013. View Article : Google Scholar
|
|
4
|
Ziv Y, Avidan H, Pluchino S, Martino G and
Schwartz M: Synergy between immune cells and adult neural
stem/progenitor cells promotes functional recovery from spinal cord
injury. Proc Natl Acad Sci USA. 103:13174–13179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu Y and Wang MY: Neural stem cell grafts
for complete spinal cord injury. Neurosurgery. 71:N13–N15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piltti KM, Salazar DL, Uchida N, Cummings
BJ and Anderson AJ: Safety of human neural stem cell
transplantation in chronic spinal cord injury. Stem Cells Transl
Med. 2:961–974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi HW, Kim JS, Choi S, Hong YJ, Kim MJ,
Seo HG and Do JT: Neural stem cells differentiated from iPS cells
spontaneously regain pluripotency. Stem Cells. 32:2596–2604. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Gu Q, Wang X, Ma Q, Tang H, Yan X,
Guo X, Yan H, Hao J and Zeng F: Directed neuronal differentiation
of mouse embryonic and induced pluripotent stem cells and their
gene expression profiles. Int J Mol Med. 32:25–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okita K, Ichisaka T and Yamanaka S:
Generation of germ-line-competent induced pluripotent stem cells.
Nature. 448:313–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Briggs JA, Sun J, Shepherd J, Ovchinnikov
DA, Chung TL, Nayler SP, Kao LP, Morrow CA, Thakar NY, Soo SY, et
al: Integration-free induced pluripotent stem cells model genetic
and neural developmental features of down syndrome etiology. Stem
Cells. 31:467–478. 2013. View Article : Google Scholar
|
|
11
|
Hoveizi E, Ebrahimi-Barough S, Tavakol S
and Sanamiri K: In vitro differentiation of human iPS cells into
neural like cells on a biomimetic polyurea. Mol Neurobiol.
54:601–607. 2017. View Article : Google Scholar
|
|
12
|
Pham QP, Sharma U and Mikos AG:
Electrospinning of polymeric nanofibers for tissue engineering
applications: a review. Tissue Eng. 12:1197–1211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ebrahimi-Barough S, Hoveizi E, Norouzi
Javidan A and Ai J: Investigating the neuroglial differentiation
effect of neuroblastoma conditioned medium in human endometrial
stem cells cultured on 3D nanofibrous scaffold. J Biomed Mater Res
A. 103:2621–2627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HB, Mullins ME, Cregg JM, McCarthy CW
and Gilbert RJ: Varying the diameter of aligned electrospun fibers
alters neurite outgrowth and Schwann cell migration. Acta Biomater.
6:2970–2978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kabiri M, Oraee-Yazdani S, Shafiee A,
Hanaee-Ahvaz H, Dodel M, Vaseei M and Soleimani M:
Neuroregenerative effects of olfactory ensheathing cells
transplanted in a multi-layered conductive nanofibrous conduit in
peripheral nerve repair in rats. J Biomed Sci. 22:352015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lim SH and Mao HQ: Electrospun scaffolds
for stem cell engineering. Adv Drug Deliv Rev. 61:1084–1096. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghasemi-Mobarakeh L, Prabhakaran MP,
Morshed M, Nasr-Esfahani MH and Ramakrishna S: Electrospun
poly(epsilon-caprolactone)/gelatin nanofibrous scaffolds for nerve
tissue engineering. Biomaterials. 29:4532–4539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Lü X and Ding F: influence of
poly(L-lactic acid) aligned nanofibers on C12 differentiation. J
Biomed Nanotechnol. 11:816–827. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu C, Huang Y, Pang M, Yang Y, Li S, Liu
L, Shu T, Zhou W, Wang X, Rong L, et al: Tissue-engineered
regeneration of completely transected spinal cord using induced
neural stem cells and gelatin-electrospun poly
(lactide-co-glycolide)/polyethylene glycol scaffolds. PLoS One.
10:pp. e01177092015, View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meijering E, Jacob M, Sarria JC, Steiner
P, Hirling H and Unser M: Design and validation of a tool for
neurite tracing and analysis in fluorescence microscopy images.
Cytometry A. 58:167–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
22(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
22
|
David S and Aguayo AJ: Axonal elongation
into peripheral nervous system 'bridges' after central nervous
system injury in adult rats. Science. 214:931–933. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson EO, Zoubos AB and Soucacos PN:
Regeneration and repair of peripheral nerves. Injury. 36(Suppl 4):
S24–S29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Darsalia V, Kallur T and Kokaia Z:
Survival, migration and neuronal differentiation of human fetal
striatal and cortical neural stem cells grafted in stroke-damaged
rat striatum. Eur J Neurosci. 26:605–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paul C, Samdani AF, Betz RR, Fischer I and
Neuhuber B: Grafting of human bone marrow stromal cells into spinal
cord injury: a comparison of delivery methods. Spine. 34:328–334.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu G, Cheng Y, Guo S, Feng Y, Li Q, Jia
H, Wang Y, Tong L and Tong X: Transplantation of adipose-derived
stem cells for peripheral nerve repair. Int J Mol Med. 28:565–572.
2011.PubMed/NCBI
|
|
27
|
Park Ki, Teng YD and Snyder EY: The
injured brain interacts reciprocally with neural stem cells
supported by scaffolds to reconstitute lost tissue. Nat Biotechnol.
20:1111–1117. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ring KL, Tong LM, Balestra ME, Javier R,
Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC and Huang
Y: Direct reprogramming of mouse and human fibroblasts into
multi-potent neural stem cells with a single factor. Cell Stem
Cell. 11:100–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Elkabetz Y and Studer L: Human ESC-derived
neural rosettes and neural stem cell progression. Cold Spring Harb
Symp Quant Biol. 73:377–387. 2008. View Article : Google Scholar
|
|
31
|
Kobayashi Y, Okada Y, Itakura G, Iwai H,
Nishimura S, Yasuda A, Nori S, Hikishima K, Konomi T, Fujiyoshi K,
et al: Pre-evaluated safe human iPSC-derived neural stem cells
promote functional recovery after spinal cord injury in common
marmoset without tumorigenicity. PloS one. 7:e527872012. View Article : Google Scholar
|
|
32
|
Takahashi K and Yamanaka S: induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aboody K, Capela A, Niazi N, Stern JH and
Temple S: Translating stem cell studies to the clinic for CNS
repair: current state of the art and the need for a Rosetta stone.
Neuron. 70:597–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okita K, Matsumura Y, Sato Y, Okada A,
Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, et
al: A more efficient method to generate integration-free human iPS
cells. Nat Methods. 8:409–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fusaki N, Ban H, Nishiyama A, Saeki K and
Hasegawa M: Efficient induction of transgene-free human pluripotent
stem cells using a vector based on Sendai virus, an RNA virus that
does not integrate into the host genome. Proc Jpn Acad Ser B Phys
Biol Sci. 85:348–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Talluri TR, Kumar D, Glage S, Garrels W,
Ivics Z, Debowski K, Behr R and Kues WA: Non-viral reprogramming of
fibroblasts into induced pluripotent stem cells by Sleeping Beauty
and piggyBac transposons. Biochem Biophys Res Commun. 450:581–587.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jia F, Wilson KD, Sun N, Gupta DM, Huang
M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, et al: A nonviral
minicircle vector for deriving human iPS cells. Nat Methods.
7:197–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim D, Kim CH, Moon Ji, Chung YG, Chang
MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al: Generation of
human induced pluripotent stem cells by direct delivery of
reprogramming proteins. Cell Stem Cell. 4:472–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu S, Wilson KD, Ghosh Z, Han L, Wang Y,
Lan F, Ransohoff KJ, Burridge P and Wu JC: MicroRNA-302 increases
repro gramming efficiency via repression of NR2F2. Stem Cells.
31:259–268. 2013. View Article : Google Scholar :
|
|
40
|
Yu J, Hu K, Smuga-Otto K, Tian S, Stewart
R, Slukvin II and Thomson JA: Human induced pluripotent stem cells
free of vector and transgene sequences. Science. 324:797–801. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wrobel MR and Sundararaghavan HG: Directed
migration in neural tissue engineering. Tissue Eng Part B Rev.
20:93–105. 2014. View Article : Google Scholar
|
|
42
|
Hoveizi E, Tavakol S and Ebrahimi-Barough
S: Neuroprotective effect of transplanted neural precursors
embedded on PLA/CS scaffold in an animal model of multiple
sclerosis. Mol Neurobiol. 51:1334–1342. 2015. View Article : Google Scholar
|
|
43
|
Li J and Shi R: Fabrication of patterned
multi-walled poly-l-lactic acid conduits for nerve regeneration. J
Neurosci Methods. 165:257–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hurtado A, Cregg JM, Wang HB, Wendell DF,
Oudega M, Gilbert RJ and McDonald JW: Robust CNS regeneration after
complete spinal cord transection using aligned poly-L-lactic acid
microfibers. Biomaterials. 32:6068–6079. 2011. View Article : Google Scholar : PubMed/NCBI
|