Introduction
The pineal gland is the most important producer of
melatonin. In 1959, Thillard (1)
demonstrated that pinealectomy in a chicken enabled construction of
a model of spinal deformity, and the association between melatonin
and spinal deformities has received considerable attention since.
It is well known that spinal deformity is often caused by the
abnormal proliferation of osteoblasts initially. It has been
confirmed that osteoblast proliferation is dependent on the
concentration of melatonin and the mechanism has been verified
(2,3). However, the ability of melatonin to
cause osteoblast apoptosis (plus the underlying molecular
mechanisms), and any potential protective factors is may possess
remains unclear.
Previous studies have demonstrated that there is a
potential connection between melatonin, endoplasmic reticulum
stress (ERS) and apoptosis. Furthermore, previous results have
indicated that melatonin is able to promote apoptosis (4,5);
however, other studies have suggested that melatonin is able to
inhibit ERS and/or apoptosis (6,7).
The traditional form of apoptosis is known as the caspase-dependent
pathway, consisting of the death receptor pathway and the
mitochondria-mediated pathway (8–10).
Cell apoptosis may be induced through the activation of caspase-3,
which triggers the caspase cascade (11). ERS is able to induce apoptosis
through the unfolded protein response (UPR), the ER overload
response and the sterol cascade (12–14). The UPR signal transduction pathway
is the most frequently used, and is mediated by three transmembrane
proteins involved in three signal transduction pathways; protein
kinase R-like endoplasmic reticulum kinase (PERK) pathway, the
inositol-requiring enzyme 1 (IRE1) pathway and the activating
transcription factor (ATF) 6α pathway (13,15–17). CCAAT/enhanced binding protein
homologous protein (CHOP) is an important downstream target of
ATF4, which is necessary in the ERS-induced apoptotic response
(18,19). c-Jun N-terminal kinase (JNK)
activates the death receptor pathway (20) (the JNK mediators belong to the
death receptor pathway). Collectively, these three pathways induce
apoptosis by activating or inhibiting different factors, including
TNF receptor-associated factor 2, apoptosis signal-regulating
kinase 1 and B-cell lymphoma 2 (Bcl-2) (21,22).
Periostin (POSTN) is a secreted extracellular matrix
protein, which was originally identified in cells of the
mesenchymal lineage, including osteoblasts, osteoblast-derived
cells, the periodontal ligament and periosteum. POSTN has been
associated with the epithelial-mesenchymal transition (EMT) in
cancer and also the differentiation of the mesenchyme in the
developing heart (23). This
protein belongs to the fasciclin I family and is expressed in
connective tissues, including the bone, skin, periodontal ligament
and tendon. In several types of cancer, POSTN can activate the
protein kinase B (Akt) and focal adhesion kinase-mediated signaling
pathways, leading to cell invasion, angiogenesis, metastasis and
the EMT (24). A previous study
also demonstrated that POSTN is expressed by osteoblasts and
osteoclasts (25).
Whether melatonin can induce or inhibit apoptosis in
the hFOB 1.19 human osteoblastic cell line through specific
pathways, and the role of POSTN in osteoblast apoptosis remains
unclear. Therefore the present study investigated the role of
periostin (POSTN) and high melatonin concentrations in the
apoptosis of hFOB 1.19 human normal fetal osteoblastic cells in
order to provide further clarification.
Materials and methods
Cell culture and reagents
The hFOB 1.19 normal human fetal osteoblastic cell
line was provided by the Department of Biochemistry and Molecular
Biology, Mayo Clinic (Rochester, MN, USA) (26) and was maintained in a 1:1 mixture
of Dulbecco's minimum essential (DME) and F12 medium without phenol
red (HyClone, Laboratories; GE Healthcare Life Sciences, Logan, UT,
USA), supplemented with 10% fetal bovine serum (FBS) and grown in
5% CO2 at 37°C. The medium was replaced every other day.
Cells that were passaged 8–11 times were used for all experiments.
Treatments with melatonin (2, 4 or 6 mM) dissolved in 0.2% dimethyl
sulfoxide (DMSO) or vehicle treatment with 0.2% DMSO (in culture
medium) were performed at 37°C with 10% FBS.
Melatonin was obtained from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany). Lipofectamine® 2000 was
purchased from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Primary monoclonal/polyclonal antibodies against the
indicated proteins were purchased from Abcam (Cambridge, MA, USA).
Goat anti-rabbit secondary antibodies were obtained from Zhongshan
Jinqiao Biotechnology Co., Ltd. (Beijing, China).
POSTN small interfering (si)RNA
transfection
Cells were cultured in DME and F12 medium
supplemented with 10% FBS (Clarks Bioscience, Seabrook, MD, USA) in
a humidified incubator at 37°C and 5% CO2. At 70–80%
confluence, cells were transfected with POSTN small interfering
(si)RNA (sense, 5′-GGAUCUAGAAGACGAUUAAGG-3′; antisense,
5′-UUAAUCGUCUUCUAGAUCCUU-3′; Shanghai GeneChem Co., Ltd., Shanghai,
China) using Lipofectamine® 2000 according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). Overall, there were 3 control groups: Blank control,
transfection reagent control and scramble siRNA control.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining and flow cytometric
analysis
An Annexin V FITC/PI staining kit was used to assess
apoptotic cell death according to manufacturer's protocol (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Briefly, the cells
were trypsinised, washed in PBS, resuspended in binding buffer,
stained with FITC-conjugated Annexin V and PI, analysed using the
BD LSRFortessa™ cell analyzer (BD Biosciences, Franklin Lakes, NJ,
USA) and evaluated using BD FACSDiva (version 6.2; BD Biosciences).
Apoptotic cells were defined as Annexin V-positive.
Western blot analysis
Following treatment, osteoblasts were extracted with
RIPA lysis buffer for 30 min at 4°C. The supernatants containing
total protein were harvested and proteins were quantified using the
bicinchoninic acid method. Samples with an average volume of 50
µg were resolved using SDS-PAGE (12% gel) and transferred
onto polyvinylidene fluoride membranes at 60 V for 2 h at 4°C.
Membranes were then immediately soaked in blocking buffer [5%
blocking buffer (0.5 liter) containing 25 mg bovine serum albumin
in TBS buffer to final volume of 0.5 l, maintained at 4°C] for 2 h
at room temperature. Subsequently, proteins were incubated with
primary antibodies against GRP78 (cat. no. ab21685), GRP94 (cat.
no. ab53075), ATF4 (cat. no. ab23760), ATF6α (cat. no. ab37149),
XBP1 (cat. no. ab37152), phosphorylated (p-)eIF2α (cat. no.
ab4837), POSTN (cat. no. ab79946), caspase-3 (ab90437) and p-JNK
(cat. no. ab124956). All primary antibodies were diluted at 1:5,000
and incubated overnight at 4°C, followed by incubation with goat
anti-rabbit peroxidase-conjugated secondary antibody (1:10,000;
cat. no. ab6721) for 2 h at room temperature. The DNR imaging
system (DNR Bio-Imaging Systems, Ltd., Jerusalem, Israel) was used
to visualize specific bands, and the optical density of each band
was measured using ImageJ software (version 1.51; NIH, Bethesda,
MD, USA). The expression ratio of the indicated target proteins to
β-actin (cat. no. ab8226) was calculated and presented graphically.
All antibodies were purchased from Abcam.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assays
Total RNA was extracted from the osteoblasts using
the E.Z.N.A.® Total RNA Midi kit (Omega Bio-Tek, Inc.,
Norcross, GA, USA) according to manufacturer's protocol, and
quantified spectrophotometrically at 260 nm with acceptable 260/280
ratios being defined as between 1.8 and 2.0. The RNA quality was
also assessed using 1% agarose gel electrophoresis and staining
with 1 µg/ml ethidium bromide. The RT-qPCR analysis was
performed on a LightCycler® 480 High-Resolution Melting
Master (Roche Diagnostics, Indianapolis, IN, USA) using SYBR Premix
Ex Taq™ II (Takara Biotechnology Co., Ltd., Dalian, China). The DNA
polymerase, Takara Ex Taq™ HS, was also obtained from Takara
Biotechnology Co., Ltd. Amplifications were performed in a total
volume of 20 µl [10.0 µl of 2× SYBR Premix Ex Taq II
(Takara Biotechnology Co., Ltd.) 0.8 µl forward primer, 0.8
µl reverse primer, 2.0 µl DNA template, 6.4 µl
double distilled H2O], and cycled for 40 cycles
following initial denaturation (95°C for 30 sec) with the following
parameters: 95°C for 5 sec and 60°C for 30 sec. Primer sequences
are specified in Table I. β-actin
(forward, TCCTCCCTGGAGAAGAGCTA and reverse, TCAGGAGGAGCAATGATCTTG)
were used as internal controls. Analysis of the melting curves
supported the reliability of the results. The RT-qPCR data were
calculated using the 2−ΔΔCq method (27) (Applied Biosystems; Thermo Fisher
Scientific, Inc.).
 | Table IForward primer and reverse primer of
associated genes. |
Table I
Forward primer and reverse primer of
associated genes.
| Gene | Primers |
|---|
| XBP1 | F:
GCGCTGAGGAGGAAACTGAA |
| R:
GCGCTGTCTTAACTCCTGGT |
| ATF4 | F:
GTCCCTCCAACAACAGCAAG |
| R:
ACTTTCTGGGAGATGGCCAA |
| ATF6α | F:
CTGTTACCAGCTACCACCCA |
| R:
GGAGCCAAAGAAGGTGTTGG |
| POSTN | F:
GTGACAGAAGTGATCCACGGAG |
| R:
CTCTTGATCGCCTTCTAGACCC |
Statistical analysis
SPSS (version 20.0; IBM SPSS, Armonk, NY, USA) was
used to complete data processing. An independent samples t-test or
one-way analysis of variance, followed by the student Newman-Keuls
test, was used to evaluate the differences between groups with
various treatments. Results were expressed as mean ± standard error
of the mean. The N-fold gene expression values in gene expression
≤0.5 and >2 were considered to be significant, in accordance
with the values obtained from the control genes. P>0.05 was
considered to indicate a statistically significant difference.
Results
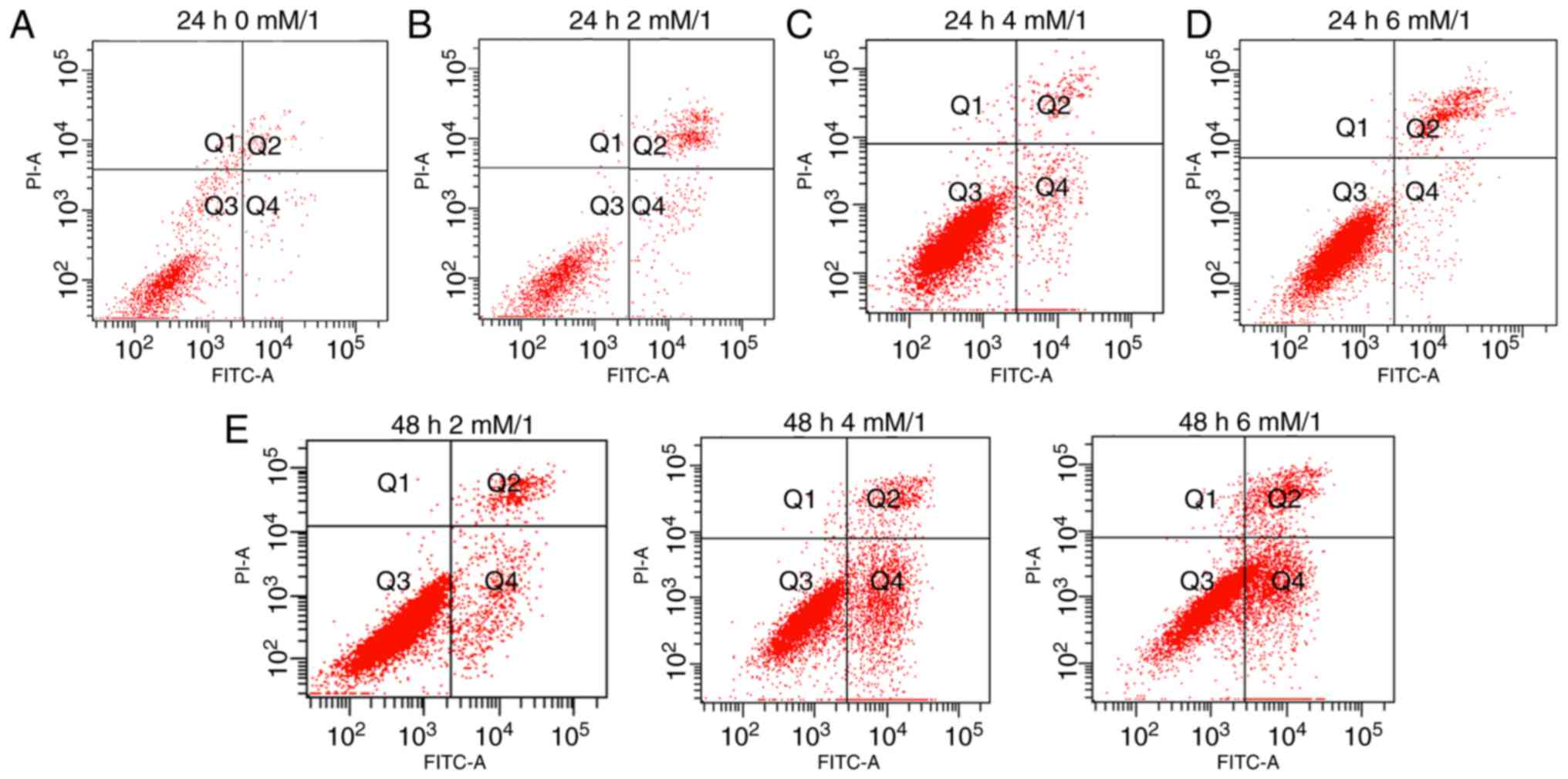
Detection of apoptosis
Apoptosis of the osteoblasts was detected using
Annexin V-FITC/PI staining. The number of early apoptotic cells was
assessed according to the number of scatters in the different
quadrants. The numbers of the early and late apoptotic cells were
observed in each group. Results demonstrate that the quantity of
early apoptotic cells in the Q4 (early apoptotic) region are
significantly different between groups, for example, Fig. 1A (0 mM/l melatonin, 24 h) and
Fig. 1B (2 mM/l melatonin, 24 h)
exhibit decrease levels of early apoptotic cells compared with
Fig. 1C (4 mM/l melatonin, 24 h).
However, the number of late apoptotic cells in the Q2 region of
Fig. 1B and D was increased
compared with Fig. 1C. Following
treatment with melatonin for 48 h, the number of late apoptotic
cells increased for all concentrations of melatonin, results also
demonstrated that the total quantity of apoptotic cells was
increased at 2 mM/l melatonin (Fig.
1E). Following comprehensive consideration, 4 mM melatonin
(dissolved in 0.2% DMSO) for 24 h was selected as the appropriate
experimental condition for subsequent experiments as this group
exhibited the greatest number of early apoptotic cells (Q4) and the
least number of late apoptotic cells (Q2).
Occurrence of ERS
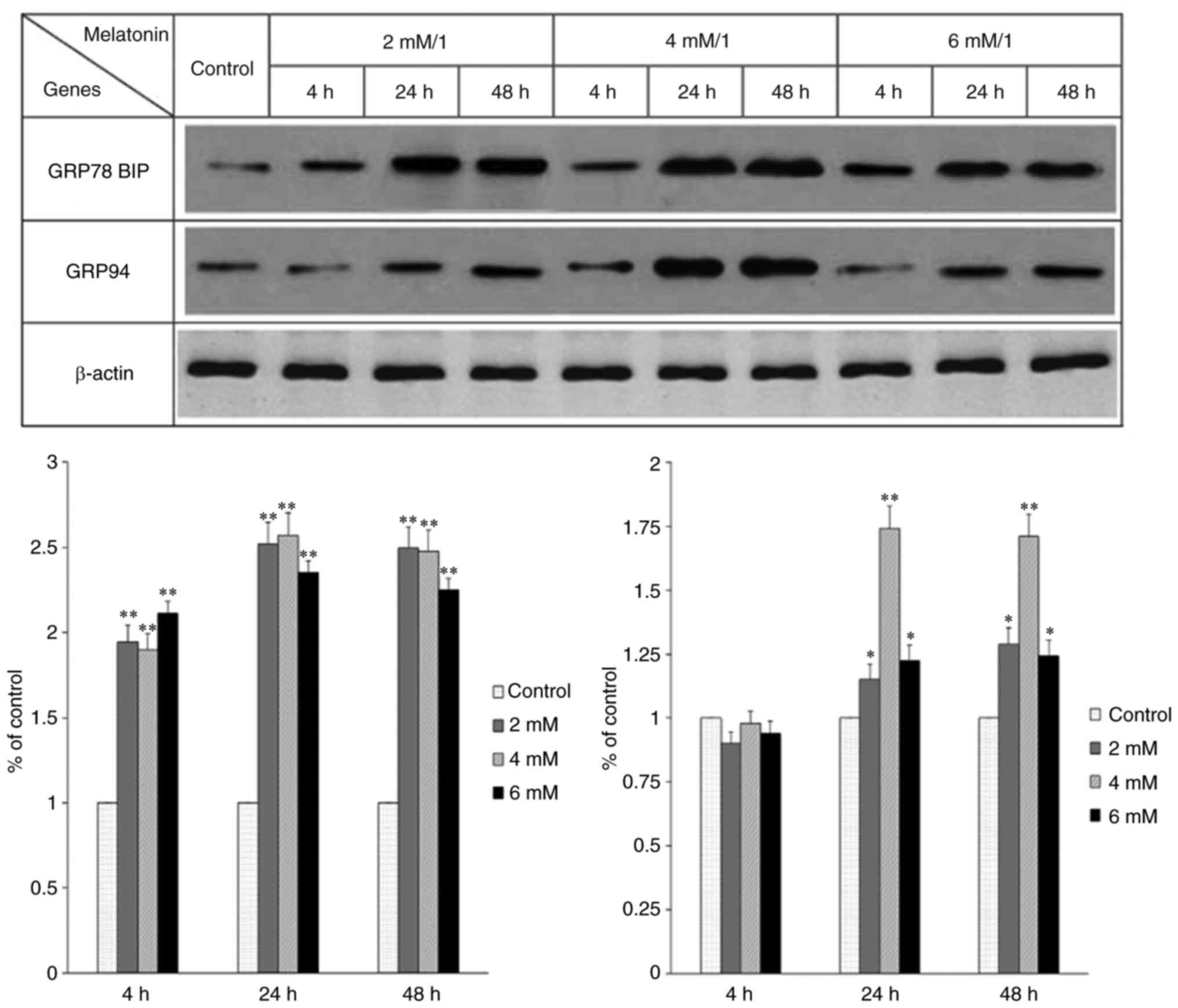
The occurrence of ERS was detected by assessing the
expression of GRP78 and GRP94. It was previously indicated that the
expression levels of these proteins are often increased
significantly when ERS occurs (28). In the present study, the
expression of these two proteins was observed and there were
significant differences among the groups treated with different
concentrations of melatonin for different durations (Fig. 2). The results showed that
treatment with a certain concentration of melatonin for a certain
length of time induced the occurrence of ERS in the hFOB 1.19 human
osteoblastic cell line.
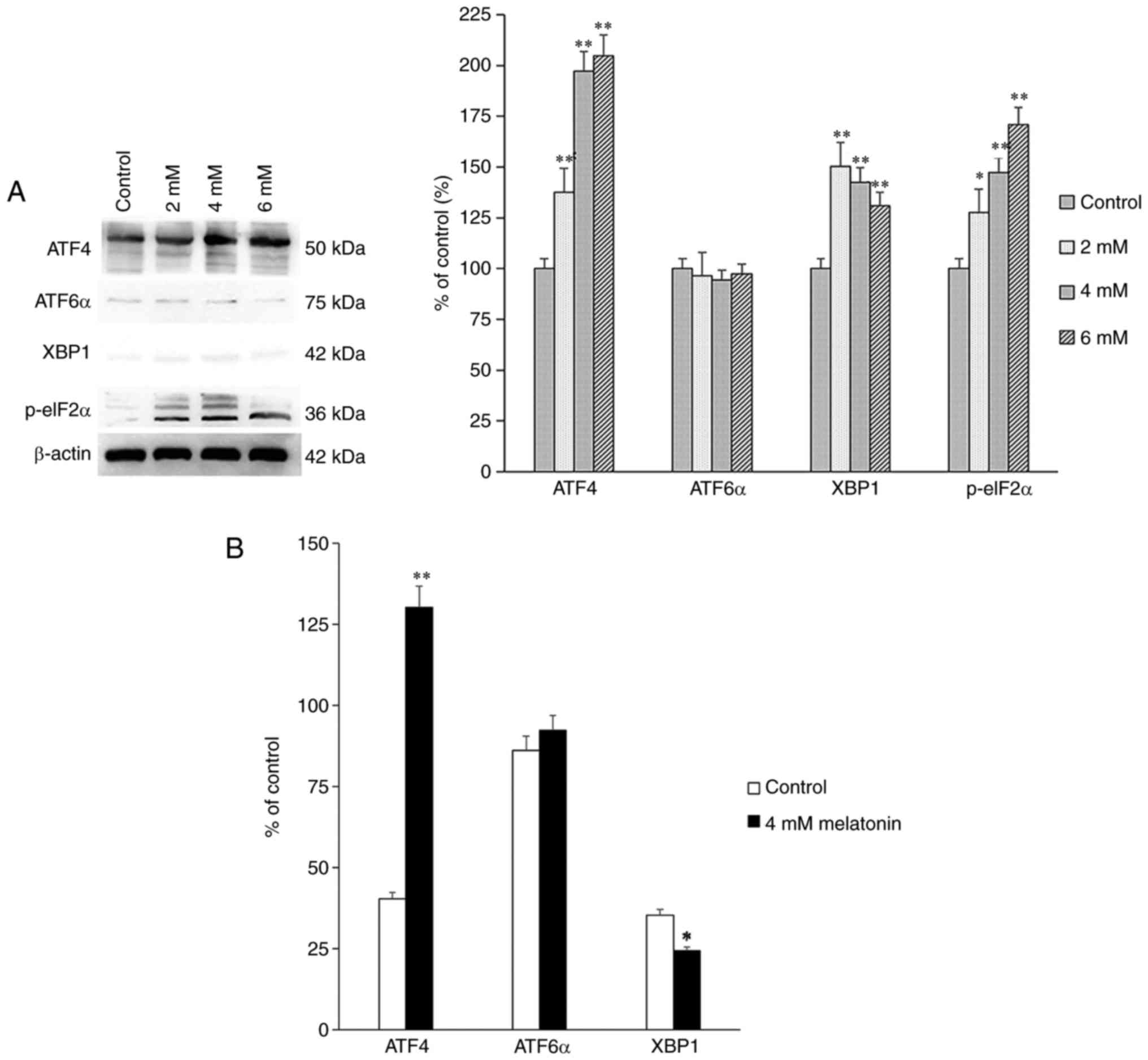
Signaling transduction pathway
expression
Proteins associated with the signaling transduction
pathways, including PERK, IRE1 and ATF6α, activated by UPR were
also assessed. The protein and mRNA expression levels of p-eIF2α,
ATF4, X-box binding protein 1 (XBP1) and ATF6α were measured using
western blot and RT-qPCR analyses, and the results showed that the
expression levels of p-eIF2α and ATF4 were increased significantly
among the groups (Fig. 3A and B).
The increased expression levels of the above proteins indicated
that melatonin induced apoptosis of the hFOB 1.19 human normal
osteoblastic cells through the PERK-eIF2α-ATF4 pathway, but not
through the IRE1 or ATF6α pathways.
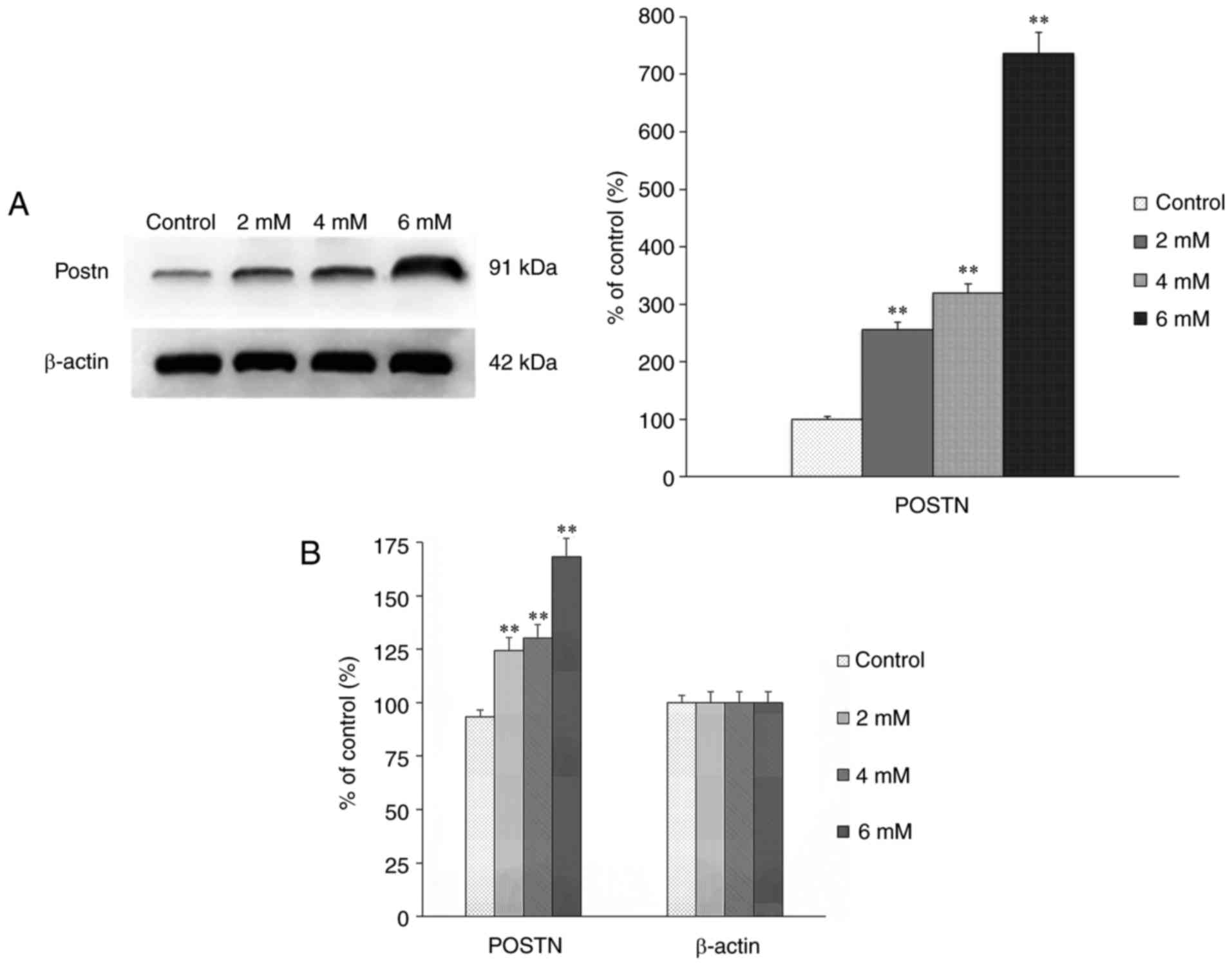
The expression of POSTN in the groups treated with
different concentrations of melatonin for 24 h was assessed using
western blot and RT-qPCR analyses. The results showed that the
expression of POSTN was positively correlated with the
concentration of melatonin (Fig. 4A
and B), which indicated that, with the increased intensity of
apoptosis, the upregulated expression of POSTN may be a mechanism
underlying the anti-apoptotic effect in osteoblasts.
To determine the transduction pathways through which
ERS mediate melatonin-induced apoptosis, the expression levels of
CHOP and caspase-3 were detected using western blot analysis. It
was observed that 4 mM melatonin significantly increased the
expression levels of ATF4, p-eIF2α, CHOP and caspase-3 following
treatment for 24 h (Fig. 5),
compared with those in the control groups. The levels of p-JNK were
also markedly increased (Fig. 5).
Therefore, melatonin-induced apoptosis may activate the JNK
pathway.
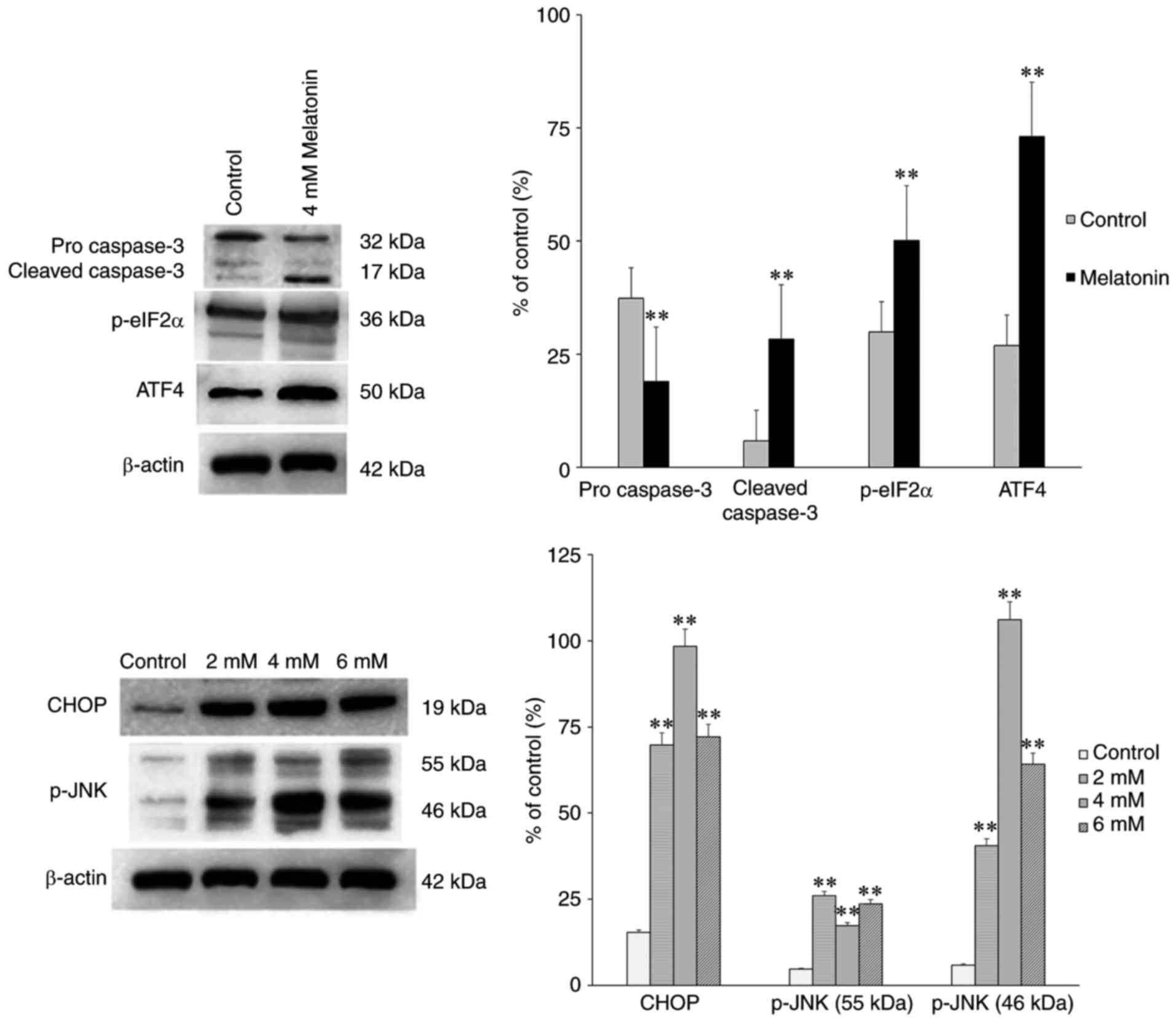 | Figure 5Effect of melatonin (2, 4 and 6 mM)
on the protein expression levels of pro caspase-3,
cleaved-caspase-3, p-eIF2α, CHOP and p-JNK in hFOB 1.19 human
osteoblastic cells for 24 h. Each bar represents the mean ±
standard error of the mean of three independent experiments.
**P<0.01 vs. control cells. POSTN, periostin;
p-eIF2α, phosphorylated eukaryotic initiation factor 2α; ATF4,
activating transcription factor 4; CHOP, CCAAT/enhancer binding
protein homologous protein; JNK, c-Jun N-terminal kinase; p-,
phosphorylated. |
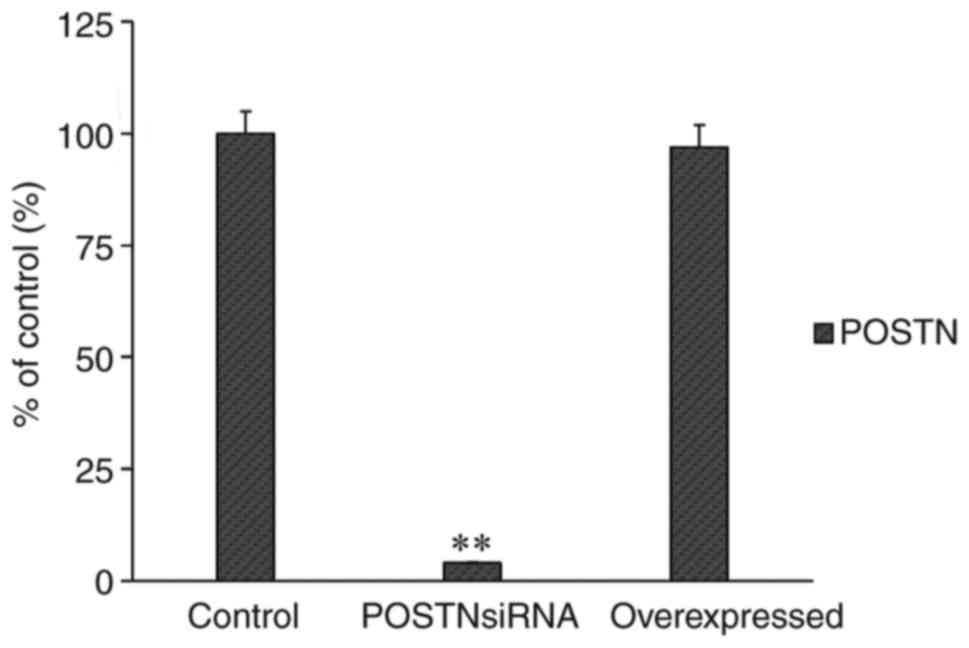
RT-qPCR analysis was performed to assess the mRNA
level of POSTN following transfection with the POSTN overexpression
plasmid or POSTN siRNA. The results showed that the transfections
were efficient (Fig. 6).
Effect of POSTN on melatonin-induced
apoptosis
Finally, either POSTN siRNA or control siRNA were
transfected into osteoblasts using Lipofectamine® 2000
according to the manufacturer's protocol. There were three control
groups: Blank control, transfection reagent control and scramble
siRNA control. A rescue experiment was also performed via
transfection with the POSTN overexpression plasmid (Genechem Co.,
Ltd.). Western blot analysis was then performed to assess the
protein levels of CHOP, ATF4, p-eIF2α, pro caspase-3, cleaved
caspase-3 and POSTN. The results demonstrated that the levels of
CHOP, ATF4, p-eIF2α and cleaved caspase-3 in the group treated with
melatonin in combination with POSTN siRNA were increased
significantly, compared with those in the control groups. Following
transfection with POSTN siRNA, the expression level of POSTN
decreased significantly. The POSTN overexpression plasmid was then
transfected in order to perform the rescue experiment. The results
demonstrated that following transfection with the POSTN
overexpression plasmid, the expression level of POSTN was increased
to a level similar to what was observed in the 4 mM
melatonin-treated group compared with the control group (Fig. 7). Collectively, it was deduced
that POSTN inhibited melatonin-induced cell apoptosis by
suppressing the PERK pathway.
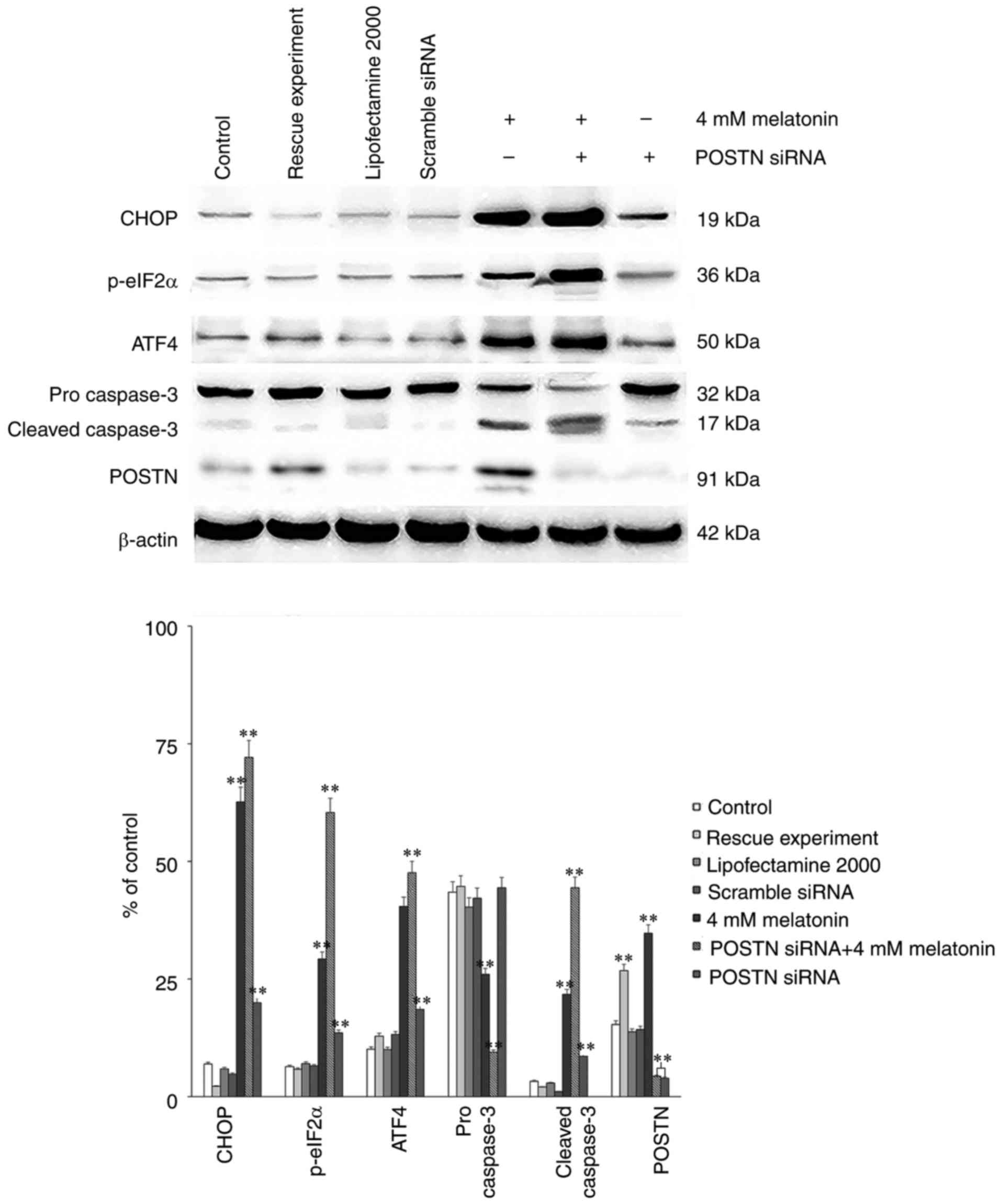 | Figure 7Protein levels from control groups
and rescue experiment of POSTN siRNA, and the effect of melatonin
(4 mM) alone or in combination with the POSTN-siRNA on the protein
expression levels of CHOP, p-eIF2α, ATF4, pro caspase-3, cleaved
caspase-3 and POSTN in hFOB 1.19 human osteoblastic cells. Each bar
represents the mean ± standard error of the mean of three
independent experiments. **P<0.01 vs.
melatonin-treated cells. p-eIF2α, phosphorylated eukaryotic
initiation factor 2α; CHOP, CCAAT/enhancer binding protein
homologous protein; ATF4, activating transcription factor 4; POSTN,
periostin; siRNA, small interfering RNA. |
Discussion
Melatonin may possess numerous functions in ageing,
tumor growth, reproduction and skeletal physiology, and has various
biological effects in human/animal cells (29–32). Several previous studies have
demonstrated that melatonin is vital in promoting bone formation by
strengthening osteoblast proliferation, differentiation and matrix
formation (33–36). Previous studies have confirmed
that melatonin has a concentration-dependent dual effect on
osteoblast proliferation (35,36); however, whether melatonin can
affect cell apoptosis remains unclear. In the present study, it was
initially demonstrated that melatonin induced cell apoptosis in the
hFOB 1.19 human osteoblastic cell line. When the duration of action
was prolonged to 24 h, apoptosis occurred in addition to the
ERS.
The ER is a site for processing proteins and storing
calcium ions (37,38). Cell dysfunction caused by
alterations in the cellular environment may lead to ERS. ERS is a
recognized as a stress response of subcellular organelles, which
can activate three signal transduction pathways to relieve stress
(10–12) and maintain cell homeostasis. Due
to results presented in Fig. 2,
it may be assumed that when the induction of ERS is enhanced, and
when the duration of induction is prolonged (which overloads the
anti-stress capacity of ERS), unfolded proteins continue to form
and no longer degrade, and apoptosis may occur. Hamamura and Yokota
(39) demonstrated that different
intensities of ERS produced bidirectional regulation in mouse
osteoblasts. A low level of ERS had protective effects on
osteoblasts by increasing the expression of Runt-related
transcription factor 2 (Runx2) and other bone morphogenetic
factors, which promotes bone formation and remodeling.
Contrastingly, increased levels of ERS increased the expression of
ATF4, which subsequently increased the expression of downstream
CHOP transcription factors. Furthermore, the increased expression
of CHOP may cause apoptosis (40). Yu and Hong (41) also identified that Runx2 promotes
bone formation, inhibited the transformation of LC3II and inhibited
autophagy.
ERS occurred following treatment with 4 mM melatonin
for 24 h. It was demonstrated that melatonin induced ERS in the
hFOB 1.19 human osteoblastic cell line. By assessing the expression
of GRP78, it was demonstrated that melatonin induced ERS in the
human hFOB 1.19 osteoblastic cells. GRP78 binds tightly to the
three transmembrane signal proteins, PERK, IRE1α and ATF6α, which
are inactive under normal circumstances (42,43); when ERS occurs, GRP78 separates
from one or all of the specified proteins, leading to an increase
in protein expression causing a series of signaling cascades. Once
these three proteins are activated, they may induce apoptosis by
activating or inhibiting various factors.
Melatonin is able to clear reactive oxygen species
and activate antioxidant systems (44). However, studies have demonstrated
that increased concentrations of melatonin have pro-oxidant effects
(43) and that oxidative stress
contributes to activation of the UPR, which may lead to ERS
(45). These two opposing effects
may be independent of cell types (43), namely, a pro-apoptotic effect in
cancer cells vs. an anti-apoptotic effect in normal cells under
normal conditions (46). The
results of the present study do not conform to the above
conclusions. Melatonin used at high concentrations (4 mM) induced
ERS and induced apoptosis in the hFOB 1.19 human osteoblastic cell
line, which complicates the biological effects of melatonin.
According to the different concentrations of
melatonin and durations of action, the present study assessed
apoptosis, ERS-associated proteins and other indicators. The
results revealed that ERS had a protective effect. Results
presented in Fig. 1 indicated
that, following treatment with 4 mM melatonin for 24 h, the
intensity of melatonin-induced apoptosis reached its peak (Figs. 1 and 2). Consistent with the duration of
action of melatonin, ERS no longer protected osteoblasts but
instead induced apoptosis, and the results of the 24 and 48 h
groups also exhibited an apoptotic response (Fig. 1). Following comprehensive
consideration, 4 mM melatonin for 24 h was selected as the
appropriate experimental conditions for subsequent experiments.
The present study assessed the expression levels of
pro caspase-3 and cleaved caspase-3 as markers of apoptosis. The
expression levels of ATF4, CHOP and cleaved caspase-3 increased
(Figs. 3 and 5B) indicated that the melatonin
concentration and duration of action may have surpassed the
threshold under which the UPR was able to maintain cell
homeostasis, and may have led to subsequent cell apoptosis. The
three transmembrane proteins mentioned above separate from binding
immunoglobulin protein and are involved in three signal
transduction pathways (16,17), namely, the PERK-eIF2α-ATF4
pathway, the IRE1α-XBP1 pathway and the ATF6α pathway. eIF2α is
immediately phosphorylated by PERK in response to ERS, which
prevents global protein synthesis, but selectively induces the
translation of ATF4 (47). CHOP
is an important downstream target of ATF4, and the presence of CHOP
is an essential factor for the apoptotic response to ERS (48). The present study demonstrated
that, when apoptosis occurs, the levels of p-eIF2α and ATF4
increased sequentially, whereas no significant changes were
observed in the expression levels of XBP1 and ATF6α (Fig. 3A). These results indicated that
melatonin-induced ERS led to the phosphorylation of eIF2α and
activated the translation of ATF4, finally causing the activation
of CHOP. Previous studies have demonstrated that melatonin can
increase the levels of CHOP and decrease the Bcl-2/Bcl-2-associated
X protein (Bax) ratio in human hepatoma cells (5). Furthermore, the activation of CHOP
leads to changes in gene expression, which favors apoptosis by
decreasing the expression of Bcl-2 and increasing the expression of
Bax (21,49). Following ERS, three pathways are
involved in inducing apoptosis, namely, the mitochondria-mediated
pathway (10), death receptor
pathway and the ER pathway (caspase pathway). In the present study,
the expression of p-eIF2α, ATF4 and cleaved-caspase-3 increased
(Fig. 5), which demonstrated that
the melatonin-induced apoptosis was associated with the ER pathway
and the eIFα-ATF4 pathway. The JNKs are one of the three
well-defined subgroups of mitogen-activated protein kinases
(MAPKs); the other two subgroups comprise the ERKs and p38 MAPKs.
All three of the above-mentioned subgroups can be activated by a
cascade of phosphorylation events, and are involved in gene
expression, differentiation, cell survival and cell death (50). In the present study, the level of
p-JNK was significantly increased (Fig. 5), which indicated that the JNK
subgroup of MAPKs was significantly upregulated in the
melatonin-treated groups, compared with that in the control group.
This suggested that the JNK pathways may be associated with
apoptosis of the hFOB 1.19 human osteoblastic cell line and that
melatonin takes effect through this pathway. CHOP is involved in
another major pathway through which apoptotic responses to ERS
occur, namely, the ER pathway (51). Previous studies have indicated
that CHOP may be involved in the death receptor pathway,
particularly the death receptor 5 pathway (8,9).
POSTN is an extracellular matrix protein, which is
secreted by osteoblasts (50).
This protein is expressed in bone tissues, although its function
remains unclear. In several types of cancer, POSTN can activate the
Akt and focal adhesion kinase-mediated signaling pathways,
subsequently leading to cell invasion, angiogenesis, metastasis and
epithelial-mesenchymal transition (52). POSTN is involved in several cancer
biological processes, including proliferation (53,54), migration (55), invasion/metastasis (56,57) and angiogenesis (57,58). Studies have demonstrated that the
upregulation of POSTN is able to prevent cell apoptosis in
different cell types (59–61).
In the present study, it was indicated that the inhibition of POSTN
increased the intensity of melatonin-induced cell apoptosis in the
hFOB 1.19 human osteoblastic cell line. The present study assessed
the apoptosis and ERS-associated proteins CHOP, ATF4, p-eIF2α,
p-JNK, procaspase-3 and cleaved caspase-3 in the control group,
compared wit the POSTN-inhibited group, and the two groups were
treated with identical concentrations of melatonin for identical
durations. The results demonstrated that in the POSTN-inhibited
group, expression of the above-mentioned proteins increased
following treatment with 4 mM melatonin for 24 h, which suggested
that when the protein level of POSTN decreased, the intensity of
melatonin-induced apoptosis increased. These two parameters were
negatively associated. These results indicated that POSTN may have
a protective effect in melatonin-induced apoptosis of the hFOB 1.19
human osteoblastic cell line.
In the present study, the POSTN siRNA was
transfected into the hFOB 1.19 human osteoblastic cell line and the
efficiency of transfection was assessed using RT-qPCR analysis
(Fig. 6). According to the
results of the western blot analysis, the effect of 4 mM melatonin
alone or in combination with POSTN siRNA demonstrated significant
differences. The expression levels of the ERS and apoptosis-related
proteins peaked in the melatonin + POSTN siRNA group, and the
expression levels were lowest in the group treated with POSTN siRNA
alone. These results indicated that POSTN may mediate this process
by suppressing the expression levels of p-eIF2α and ATF4 (Fig. 7). The expression of POSTN has been
demonstrated to be inducible by stress or pressure overloading
(62). In the present study,
melatonin was used to investigate the effects of ERS and apoptosis
on the expression of POSTN in human osteoblast cells. Following the
induction of ERS and apoptosis, the expression of POSTN was
detected and the results demonstrated that, with the increasing
concentrations and durations of melatonin treatment, the expression
of POSTN exhibited concentration-and time-dependent increases
(Fig. 4A).
In conclusion, melatonin induced cell apoptosis by
activating the PERK-p-eIF2α-ATF signal transduction pathway, and
ERS mediated this process by triggering the cascade reactions of
CHOP, caspase-3 and p-JNK. The upregulated expression of POSTN had
a protective effect against apoptosis of the hFOB 1.19 human
osteoblastic cell line. The expression level of POSTN in this
process was positively associated with the concentration of
melatonin, which suggested that the existence of POSTN may be a
protective mechanism in this biological process. POSTN inhibited
apoptosis by affecting the expression levels of the pathway-related
proteins and protecting the hFOB 1.19 human osteoblastic cell line.
Previous studies have confirmed that POSTN had a stimulatory effect
in the proliferation of hFOB 1.19 human osteoblastic cells, which
was in accordance with the conclusion of the present study. In
addition, the preferential localisation of these cells to the
periosteum, which is highly sensitive to mechanical stimulation,
suggested that POSTN and transforming growth factor β-induced
protein may be important to the maintenance of bone strength.
Therefore, the development of specific and sensitive assays for
serum measurements of these proteins is critical for investigating
their potential as biomarkers of adaptive bone remodeling.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81472044). The
authors would like to thank Dr M. Subramaniam of the Department of
Biochemistry and Molecular Biology, Mayo Clinic, Rochester MN, USA
for providing osteoblasts.
References
|
1
|
Thillard MJ: Deformations de la colonne
vertebrale consecutives à l'épiphysectomie chez le poussin. Extrait
C R Assoc Anat. 46:22–26. 1959.In French.
|
|
2
|
Liu L, Zhu Y, Xu Y and Reiter RJ:
Melatonin delays cell proliferation by inducing G1 and G2/M phase
arrest in a human osteoblastic cell line hFOB 1.19. J Pineal Res.
50:222–231. 2011.
|
|
3
|
Liu L, Zhu Y, Xu Y and Reiter RJ:
Prevention of ERK activation involves melatonin-induced G(1) and
G(2)/M phase arrest in the human osteoblastic cell line hFOB 1.19.
J Pineal Res. 53:60–66. 2012. View Article : Google Scholar
|
|
4
|
Moreira AJ, Ordoñez R, Cerski CT, Picada
JN, García-Palomo A, Marroni NP, Mauriz JL and González-Gallego J:
Melatonin activates endoplasmic reticulum stress and apoptosis in
rats with diethylnitrosamine-induced hepatocarcinogenesis. PLoS
One. 10:e01445172015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zha L, Fan L, Sun G, Wang H, Ma T, Zhong F
and Wei W: Melatonin sensitizes human hepatoma cells to endoplasmic
reticulum stress-induced apoptosis. J Pineal Res. 52:322–331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wongprayoon P and Govitrapong P: Melatonin
protects sh-sy5y neuronal cells against methamphetamine-induced
endoplasmic reticulum stress and apoptotic cell death. Neurotox
Res. 31:1–10. 2017. View Article : Google Scholar
|
|
7
|
Espino J, Bejarano I, Paredes SD, Barriga
C, Reiter RJ, Pariente JA and Rodríguez AB: Melatonin is able to
delay endoplasmic reticulum stress-induced apoptosis in leukocytes
from elderly humans. AGE (Dordr). 33:497–507. 2011. View Article : Google Scholar
|
|
8
|
Yi L, Zongyuan Y, Cheng G, Lingyun Z,
Guilian Y and Wei G: Quercetin enhances apoptotic effect of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) in
ovarian cancer cells through reactive oxygen species (ROS) mediated
CCAAT enhancer-binding protein homologous protein (CHOP)-death
receptor 5 pathway. Cancer Sci. 105:520–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He L, Jang JH, Choi HG, Lee SM, Nan MH,
Jeong SJ, Dong Z, Kwon YT, Lee KS, Lee KW, et al: Oligomycin a
enhances apoptotic effect of TRAIL through CHOP-mediated death
receptor 5 expression. Mol Carcinog. 52:85–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong SB, Mu TY, Wang GW and Jiang X:
Mitochondria-mediated apoptosis in ma mMals. Protein Cell.
5:737–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Faitova J, Krekac D, Hrstka R and Vojtesek
B: Endoplasmic reticulum stress and apoptosis. Cell Mol Biol Lett.
11:488–505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phal HL: Signal transduction from the
endoplasmic reticulum to the cell nucleus. Physiol Rev. 79:683–701.
1999. View Article : Google Scholar
|
|
14
|
Espenshade PJ: SREBPs: Sterol-regulated
transcription factors. J Cell Sci. 15:973–976. 2006. View Article : Google Scholar
|
|
15
|
Davenport EL, Morgan GJ and Davies FE:
Untangling the unfolded protein response. Cell Cycle. 7:865–869.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malhotra JD and Kaufman RJ: The
endoplasmic reticulum and the unfolded protein response. Semin Cell
DevBiol. 18:716–731. 2007. View Article : Google Scholar
|
|
17
|
Gupta S, Deepti A, Deegan S, Lisbona F,
Hetz C and Samali A: HSP72 protects cells from ER stress-induced
apoptosis via enhancement of IRE1alpha-XBP1 signaling through a
physical interaction. PLoS Biol. 8:e10004102010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Long ZS: Expression and significance of
CHOP related ERS after acute spinal cord injury in rats. Chin J
Spine Spinal Cord. 19:458–463. 2009.In Chinese.
|
|
19
|
Szegezdi E, Duffy A, O'Mahoney ME, Logue
SE, Mylotte LA, O'brien T and Samali A: ER stress contributes to
ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res
Commun. 349:1406–1411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takeda K, Shimozono R, Noguchi T, Umeda T,
Morimoto Y, Naguro I, Tobiume K, Saitoh M, Matsuzawa A and Ichijo
H: Apoptosis signal-regulation kinase (ASK) 2 functions as a
mitogen-activated protein kinase in a heteromeric complex with
ASK1. J Biol Chem. 282:7522–7531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li F, Guo Y, Sun S, Jiang X, Tang B, Wang
Q and Wang L: Free cholesterol-induced macrophage apoptosis is
mediated by inositol-requiring enzyme 1 alpha-regulated activation
of Jun N-terminal kinase. Acta Biochim Biophys Sin Shanghai.
40:226–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuzawa A, Saegusa K, Noguchi T,
Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K
and Ichijo H: Ros-dependent activation of the TRAF6-ASK1-p38
pathway is selectively required for TLR4-mediated innate immunity.
Nat Immunol. 6:587–592. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoersch S and Andrade-Navarro MA:
Periostin shows increased evolutionary plasticity in its
alternatively spliced region. BMC Evol Biol. 10:302010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morra L and Moch H: Periostin expression
and epithelial-mesenchymal transition in cancer: A review and an
update. Virchows Arch. 459:465–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Merle B, Bouet G, Rousseau JC, Bertholon C
and Garnero P: Periostin and transforming growth factor β-induced
protein (TGF βIp) are both expressed by osteoblasts and
osteoclasts. Cell Biol Int. 38:398–404. 2014. View Article : Google Scholar
|
|
26
|
Subramaniam M, Jalal SM, Rickard DJ,
Harris SA, Bolander ME and Spelsberg TC: Further characterization
of human fetal osteoblastic hFOB 1.19 and hFOB/ER alpha cells: Bone
formation in vivo and karyotype analysis using multicolor
fluorescent in situ hybridization. J Cell Biochem. 87:9–15. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Pizarro JG, Yeste-Velasco M, Esparza JL,
Verdaguer E, Pallàs M, Camins A and Folch J: The antiproliferative
activity of melatonin in B65 rat dopaminergic neuroblastoma cells
is related to the downregulation of cell cycle-related genes. J
Pineal Res. 45:8–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reiter RJ, Tan DX and Fuentes-Broto L:
Melatonin: A multi-tasking molecule. Prog Brain Res. 181:127–151.
2010. View Article : Google Scholar
|
|
30
|
Akbarzadeh M, Rahbarghazi R, Nabat E,
Movassaghpour AA, Shanehbandi D, Faramarzian Azimi Maragheh B,
Matluobi D, Barazvan B, Kazemi M, Samadi N and Nouri M: The impact
of different extracellular matrices on melatonin effect in
proliferation and stemness properties of ovarian cancer cells.
Biomed Pharmacother. 87:288–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bavithra S, Selvakumar K, Sundareswaran L
and Arunakaran J: Neuroprotective effect of melatonin against pcbs
induced behavioural, molecular and histological changes in cerebral
cortex of adult male wistar rats. Neurochem Res. 42:428–438. 2017.
View Article : Google Scholar
|
|
32
|
Khan S, Adhikari JS, Rizvi MA and
Chaudhury NK: Melatonin attenuates 60 Co γ-ray-induced
hematopoietic, immunological and gastrointestinal injuries in
C57BL/6 male mice. Environ Toxicol. 32:501–518. 2017. View Article : Google Scholar
|
|
33
|
Satomura K, Tobiume S, Tokuyama R,
Yamasaki Y, Kudoh K, Maeda E and Nagayama M: Melatonin at
pharmacological doses enhances human osteoblastic differentiation
in vitro and promotes mouse cortical bone formation in vivo. J
Pineal Res. 42:231–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Man GC, Wang WW, Yeung BH, Lee SK, Ng BK,
Hung WY, Wong JH, Ng TB, Qiu Y and Cheng JC: Abnormal proliferation
and differentiation of osteoblasts from girls with adolescent
idiopathic scoliosis to melatonin. J Pineal Res. 49:69–77.
2010.PubMed/NCBI
|
|
35
|
Nakade O, Koyama H, Ariji H, Yajima A and
Kaku T: Melatonin stimulates proliferation and type I collagen
synthesis in human bone cells in vitro. J Pineal Res. 27:106–110.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sanchez-Hidalgo M, Lu Z, Tan DX, Maldonado
MD, Reiter RJ and Gregerman RI: Melatonin inhibits fatty
acid-induced triglyceride accumulation in ROS17/2.8 cells:
Implications for osteoblast differentiation and osteoporosis. Am J
Physiol Regul Integr Comp Physiol. 292:R2208–R2215. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anelli T and Sitia R: Protein quality
control in the early secretory pathway. EMBO J. 27:315–327. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pizzo P and Pozzan T:
Mitochondria-endoplasmic reticulum choreography: Structure and
signaling dynamics. Trends Cell Biol. 17:511–517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hamamura K and Yokota H: Stress to
endoplasmic reticulum of mouse osteoblasts induces apoptosis and
transcriptional activation for bone remodeling. FEBS Lett.
581:1769–1774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wysokinski D, Pawlowska E and Blasiak J:
RUNX2: A master bone growth regulator that may be involved in the
DNA damage response. DNA Cell Biol. 34:305–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu SH and Hong A: Runx2 promotes
osteogenic differentiating C2C12 cells through inhibiting
macroautophagy. Chin J Pathophysiol. 29:481–487. 2013.In
Chinese.
|
|
42
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fernández A, Ordóñez R, Reiter RJ,
González-Gallego J and Mauriz JL: Melatonin and endoplasmic
reticulum stress: Relation to autophagy and apoptosis. J Pineal
Res. 59:292–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang HM and Zhang Y: Melatonin: A
well-documented antioxidant with conditional pro-oxidant actions. J
Pineal Res. 57:131–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brown MK and Naidoo N: The UPR and the
anti-oxidant response: Relevance to sleep and sleep loss. Mol
Neurobiol. 42:103–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bizzarri M, Proietti S, Cucina A and
Reiter RJ: Molecular mechanisms of the pro-apoptotic actions of
melatonin in cancer: A review. Expert Opin Ther Targets.
17:1483–1496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin. 46:629–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fu HY, Okada K, Liao Y, Tsukamoto O,
Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, et al:
Ablation of C/EBP homologous protein attenuates endoplasmic
reticulum-mediated apoptosis and cardiac dysfunction induced by
pressure overload. Circulation. 122:361–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hoersch S and Andrade-Navarro MA:
Periostin shows increased evolutionary plasticity in its
alternatively spliced region. BMC Evol Biol. 10:302010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lai E, Teodoro T and Volchuk A:
Endoplasmic reticulum stress: Signaling the unfolded protein
response. Physiology (Bethesda). 22:193–201. 2007.
|
|
52
|
Morra L and Moch H: Periostin expression
and epithelial-mesenchymal transition in cancer: A review and an
update. Virchows Arch. 459:465–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Erkan M, Kleeff J, Gorbachevski A, Reiser
C, Mitkus T, Esposito I, Giese T, Büchler MW, Giese NA and Friess
H: Periostin creates a tumor-supportive microenvironment in the
pancreas by sustaining fibrogenic stellate cell activity.
Gastroenterology. 132:1447–1464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tai IT, Dai M and Chen LB: Periostin
induction in tumor cell line explants and inhibition of in vitro
cell growth by anti-periostin antibodies. Carcinogenesis.
26:908–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gillan L, Matei D, Fishman DA, Gerbin CS,
Karlan BY and Chang DD: Periostin secreted by epithelial ovarian
carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5)
integrins and promotes cell motility. Cancer Res. 62:5358–5364.
2002.PubMed/NCBI
|
|
56
|
Kudo Y, Ogawa I, Kitajima S, Kitagawa M,
Kawai H, Gaffney PM, Miyauchi M and Takata T: Periostin promotes
invasion and anchorage-independent growth in the metastatic process
of head and neck cancer. Cancer Res. 66:6928–6935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Siriwardena BS, Kudo Y, Ogawa I, Kitagawa
M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M and Takata T:
Periostin is frequently overexpressed and enhances invasion and
angiogenesis in oral cancer. Br J Cancer. 95:1396–1403. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shao R, Bao S, Bai X, Blanchette C,
Anderson RM, Dang T, Gishizky ML, Marks JR and Wang XF: Acquired
expression of periostin by human breast cancers promotes tumor
angiogenesis through up-regulation of vascular endothelial growth
factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li B, Wang L and Chi B: Upregulation of
periostin prevents p53-mediated apoptosis in SGC-7901 gastric
cancer cells. Mol Biol Rep. 40:1677–1683. 2013. View Article : Google Scholar
|
|
60
|
Aukkarasongsup P, Haruyama N, Matsumoto T,
Shiga M and Moriyama K: Periostin inhibits hypoxia-induced
apoptosis in human periodontal ligament cells via TGF-β signaling.
Biochem Biophys Res Commun. 441:126–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Luo JH, Zhou J and Gao Y: Correlation
between periostin and SNCG and esophageal cancer invasion,
infiltration and apoptosis. Asian Pac J Trop Med. 6:516–519. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Stansfield WE, Andersen NM, Tang RH and
Selzman CH: Periostin is a novel factor in cardiac remodeling after
experimental and clinical unloading of the failing heart. Ann
Thorac Surg. 88:1916–1921. 2009. View Article : Google Scholar : PubMed/NCBI
|