Introduction
Inflammation is a defense response against
infection, tissue injury or noxious stimuli. In the inflammatory
process, inflammatory products such as nitric oxide (NO) and
pro-inflammatory cytokines serve important roles (1). In particular, NO is a crucial
mediator for the amplification of the inflammatory response. NO is
generated from L-arginine by inducible nitric oxide synthase
(iNOS). NO has various biological functions in mammalian cells,
including microbial immunity, vasodilation and the modulation of
cell signaling (2). However, the
excessive production of NO may cause damage to normal tissues and
exacerbate inflammatory diseases (3). The expression of iNOS and other
inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), is
regulated by several transcription factors, one of which is nuclear
factor-κB (NF-κB) (4). Therefore,
the regulation of NO production in an excessive inflammatory
response may facilitate the improvement of the inflammatory
symptoms.
Previous studies have shown that NF-κB is regulated
by several mechanisms, including histone acetylation and the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian
target of rapamycin (mTOR) signaling pathway (5,6).
The former mechanism, histone acetylation, is a
post-transcriptional modification process that is involved in the
regulation of gene transcription. This process is highly reversible
and widespread in eukaryotes. Lysine residues in histones are a
target of histone acetylation. Histone acetyltransferases and
histone deacetylases (HDACs) are enzymes that regulate histone
acetylation and deacetylation, respectively (7). One of the proteins that is regulated
by histone acetylation and deacetylation is heat shock protein 70
(HSP70) (8). It has been revealed
that the accumulation of HSP70 in the cytoplasm prevents the
translocation of cytosolic NF-κB into the nucleus (8). In the latter mechanism of NF-κB
regulation, PI3K/Akt/mTOR signal transduction. PI3K catalyzes the
phosphorylation of phosphatidylinositol (4,5)-bisphosphate to phosphatidylinositol
(3,4,5)-trisphosphate, which then
phosphorylates Akt. The phosphorylated Akt mediates the
phosphorylation of mTOR, and the phosphorylated mTOR encourages the
translocation of NF-κB into the nucleus (9,10).
Lovastatin is an inhibitor of
3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) used clinically for the
prevention of cardiovascular diseases via the lowering of
cholesterol levels. Commercially, lovastatin is obtained by the
fermentation of Aspergillus terreus, but several edible
mushrooms, such as Pleurotus ostreatus, are reported to
produce high quantities of lovastatin (11). Studies have demonstrated that
lovastatin also has anticancer and anti-inflammatory effects in
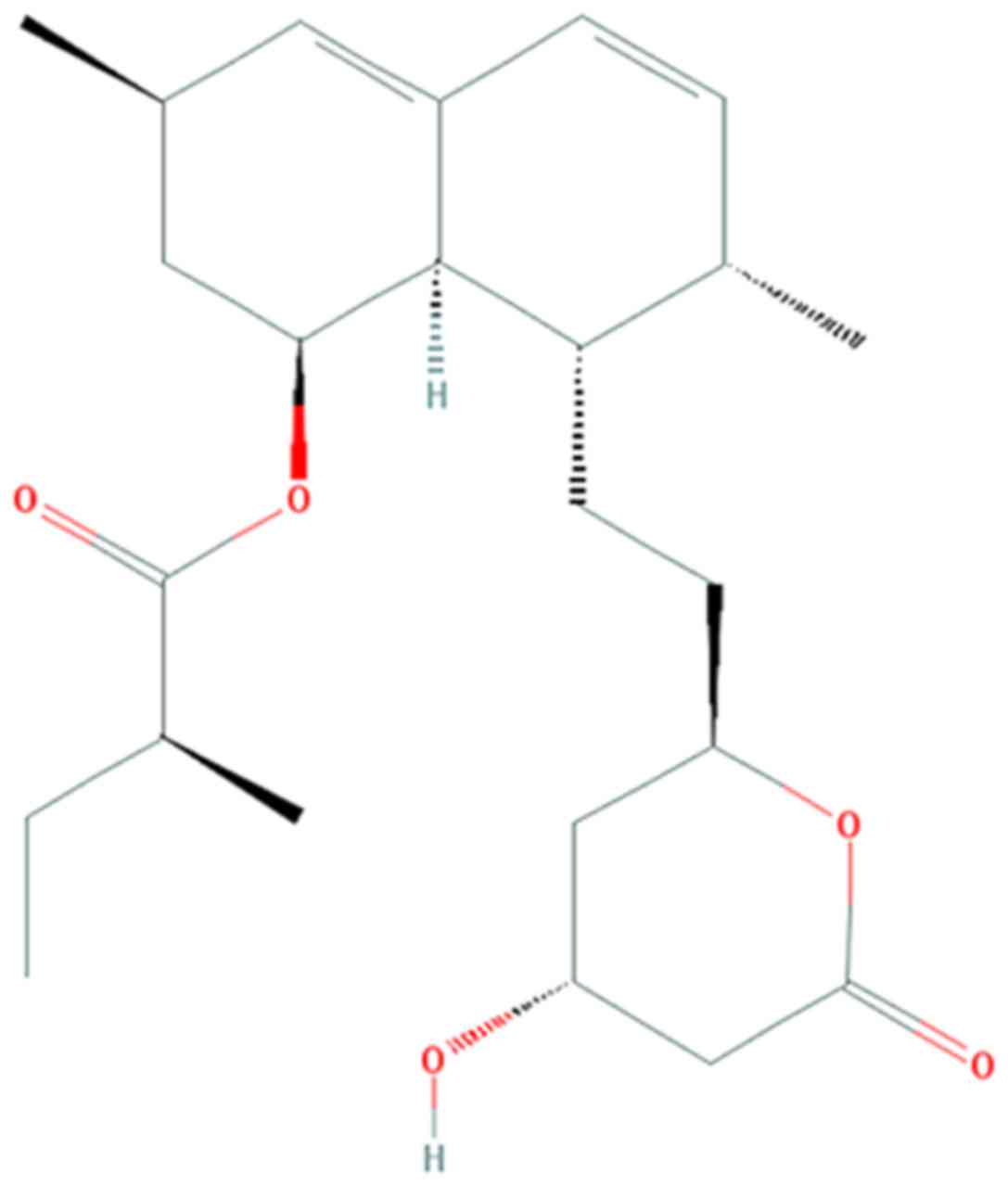
vitro and in vivo (12,13). The chemical structure of
lovastatin is presented in Fig. 1
(12).
The precise mechanisms associated with the
anti-inflammatory effects of lovastatin have not been fully
elucidated. Therefore, in the present study, the anti-inflammatory
effects of lovastatin were investigated and its molecular mechanism
was explored, focusing on HDAC1, HSP70 and the PI3K/Akt/mTOR
signaling pathway in lipopolysaccharide (LPS)-stimulated RAW264.7
macrophage cells. The results should indicate whether lovastatin
has potential as an inflammatory suppressor, and may support the
further clinical application of lovastatin.
Materials and methods
Cell culture and reagents
RAW264.7 murine macrophage cells were purchased from
the American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and 1% (v/v)
penicillin (100 U/ml)/streptomycin (100 µg/ml). The cells
were incubated under humidified conditions with 5% CO2
at 37°C. Lovastatin (Mevinolin), Escherichia coli LPS and
Griess reagent were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The DMEM, FBS and penicillin/streptomycin were
purchased from Mediatech (Corning Life Sciences; Manassas, VA,
USA). Monoclonal antibodies targeting iNOS (13120S), glyceraldehyde
3-phosphate dehydrogenase (GAPDH; 5174S), NF-κB (8242S), inhibitor
of NF-κBα (IκBα; 4814S), phosphor (p)-IκBα (ser32; 2859S), HDAC1
(5356S), acetyl-histone H3 (lys9; 9649S), histone H3 (9717S), PI3K
p110α (4249S), PI3K p110β (3011S), Akt (4691S), p-Akt (ser473;
4060S), mTOR (2983S), p-mTOR (ser2481; 2974S) and HSP70 (46477S)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Anti-rabbit IgG (H+L), F(ab′)2 fragment (Alexa
Fluor® 488 conjugate; 4412S) was purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell viability assay
For the cell viability assay, the cells
(1×104 cells/well) were seeded in 96-well microplates
and then treated with 1, 10, 50 or 100 µM lovastatin for 24
h. The cells were then treated with 1 µg/ml LPS. The medium
in each well was then discarded, and 100 µl DMEM containing
10% FBS was added to each well followed by 10 µl. EZ-cytox
Cell Viability Assay Solution WST-1® (Daeil Lab Service,
Gyenggi, Korea) and the cells were incubated for 3 h. Following
this, the optical density was measured at 460 nm using a microplate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Measurement of NO production
The cells were seeded in 24-well plates at a density
of 5×104 cells/well. The cells were pretreated with a
various concentration of lovastatin (1, 10 and 50 µM) for 1
h and then stimulated with LPS (1 µg/ml) for a further 24 h.
To measure the NO production, 100 µl culture supernatant and
the same volume of Griess reagent were reacted in a 96-well plate
for 10 min at room temperature in the dark, and the absorbance was
then measured at 540 nm using a microplate reader.
Western blot analysis
RAW264.7 cells were treated with LPS only or
following pretreatment with lovastatin as described above prior to
the western blotting of cell lysates. The cells were extracted
using cell lysis buffer [(50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1 mM
dithiothreitol, 0.5% NP-40, 1% Triton X-100, 1% deoxycholate and
0.1% sodium dodecyl sulfate (SDS)] and a cocktail of proteinase
inhibitors (phenylmethane sulfonyl fluoride, EDTA, aprotinin,
leupeptin and prostatin A; Intron Biotechnology, Inc., Seongnam,
Korea). For the preparation of nuclear extracts, the cultured cells
were harvested and lysed using NE-PER® Nuclear and
Cytoplasmic Extraction reagents (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The protein concentration
in the cell lysates was measured using Bradford reagent (Biosesang,
Inc., Seongnam, Korea). Equal volumes of the prepared proteins (30
µg per lane) were resolved by 12% SDS-polyacrylamide gel
electrophoresis and then transferred to a nitrocellulose membrane
(Pall Life Sciences, Port Washington, NY, USA). The membrane was
blocked with phosphate-buffered saline (PBS) with Tween-20 buffer
(135 mM NaCl, 2.4 mM KCl, 4.3 mM NaPO4, 1.4 mM
KH2PO4, and 0.5% Tween-20) containing 5%
skimmed milk for 1 h and then incubated with primary antibodies
[iNOS, GAPDH, NF-κB, IκBα, p-IκBα (ser32), Histone H3, HDAC1,
acetyl-histone H3 (lys9), PI3K p110α, PI3K p110β, Akt, p-Akt
(ser473), mTOR, p-mTOR (ser2481), and HSP70; dilution, 1:1,000;
temperature, 4°C; duration, overnight]. Following the incubation,
the membrane was incubated with anti-rabbit, anti-mouse or anti-rat
IgG antibodies conjugated with horseradish peroxidase (dilution,
1:1,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature. The labeled proteins were detected by reaction with an
enhanced chemiluminescent (ECL®) detection solution
(Pierce; Thermo Fisher Scientific, Inc.).
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from RAW264.7 cells using
2-mercaptoethanol (Sigma-Aldrich; Merck KGaA) and isolated using
RNeasy plus mini kit (Qiagen, Venlo, Netherlands). A nanodrop
(MECASYS, Daejeon, Korea) was used for quantifying the
concentrations of total RNA. RNA (1 µg) was synthesized to
cDNA with cDNA kit (Genetbio, Daejeon, Korea) and cDNA synthesis
was performed at 42°C for 1 h and then at 94°C for 5 min. Genes
encoded in 1 µg cDNA were amplified by PCR using specific
primers as follows: TNF-α forward, 5′-CCC CTC AGC AAA CCA CCA
AGT-3′ and reverse, 5′-CTT GGG CAG ATT GAC CTC AGC-3′; GAPDH
forward, 5′-AAC TTT GGC ATT GTG GAA GG-3′ and reverse, 5′-CAC ATT
GGG GGT AGG AAC AC-3′. PCR was performed under the following
conditions: denaturation (92°C, 1 min), annealing (57°C, 30 sec),
extension (72°C, 30 sec), and 23 cycles. Amplified PCR products
were observed on 2% agarose gel with ethidium bromide. The
intensity of bands was measured by Image J software (version 1.48;
National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence staining and confocal
microscopy of NF-κB
RAW264.7 cells were seeded on coverglass-bottom
dishes (SPL Life Sciences, Pocheon, Korea) and pretreated with 10
µM lovastatin for 1 h. The cells were then stimulated by
incubation with LPS (1 µg/ml) for 24 h. Following
incubation, the cells were stained using 1 µg/ml
4′,6-diamidino-2-phenylindole (DAPI; Roche Diagnostics GmbH,
Mannheim, Germany) for 15 min at 37°C, washed with PBS and fixed
with 4% formaldehyde (Junsei Chemical Co., Ltd., Tokyo, Japan) for
15 min at room temperature in the dark. The cells were blocked with
blocking solution (5% normal rabbit serum and 0.3% Triton X-100)
for 1 h in the dark at room temperature. The normal rabbit serum
was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA)
and Triton X-100 was purchased from Sigma-Aldrich (Merck KGaA). The
cells were incubated with the anti-NF-κB primary antibody
(dilution, 1:2,000; Cell Signaling Technology, Inc.) at 4°C
overnight. Following the reaction, the cells were washed with PBS
and then treated with anti-rabbit IgG (H+L), F(ab′)2 fragment
(Alexa Fluor® 488 conjugate; dilution, 1:2,000;) for 1 h
at room temperature. The cells were washed with PBS and Prolong
Gold Anti-fade Reagent® (Invitrogen; Thermo Fisher
Scientific, Inc.) was added to the slide at room temperature. The
cells were observed using a Carl Zeiss LSM 710 confocal laser
scanning microscope (Zeiss GmbH, Jena, Germany).
Statistical analysis
To determine the statistical significance of the
data, GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA,
USA) was used. The results are expressed as the mean ± standard
deviation. Every experiment was performed in triplicate. One-way
analysis of variance with post hoc Dunnett's multiple comparison
tests were used to assess differences among the untreated, LPS only
and lovastatin-treated groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
Viability of RAW264.7 cells following
lovastatin treatment
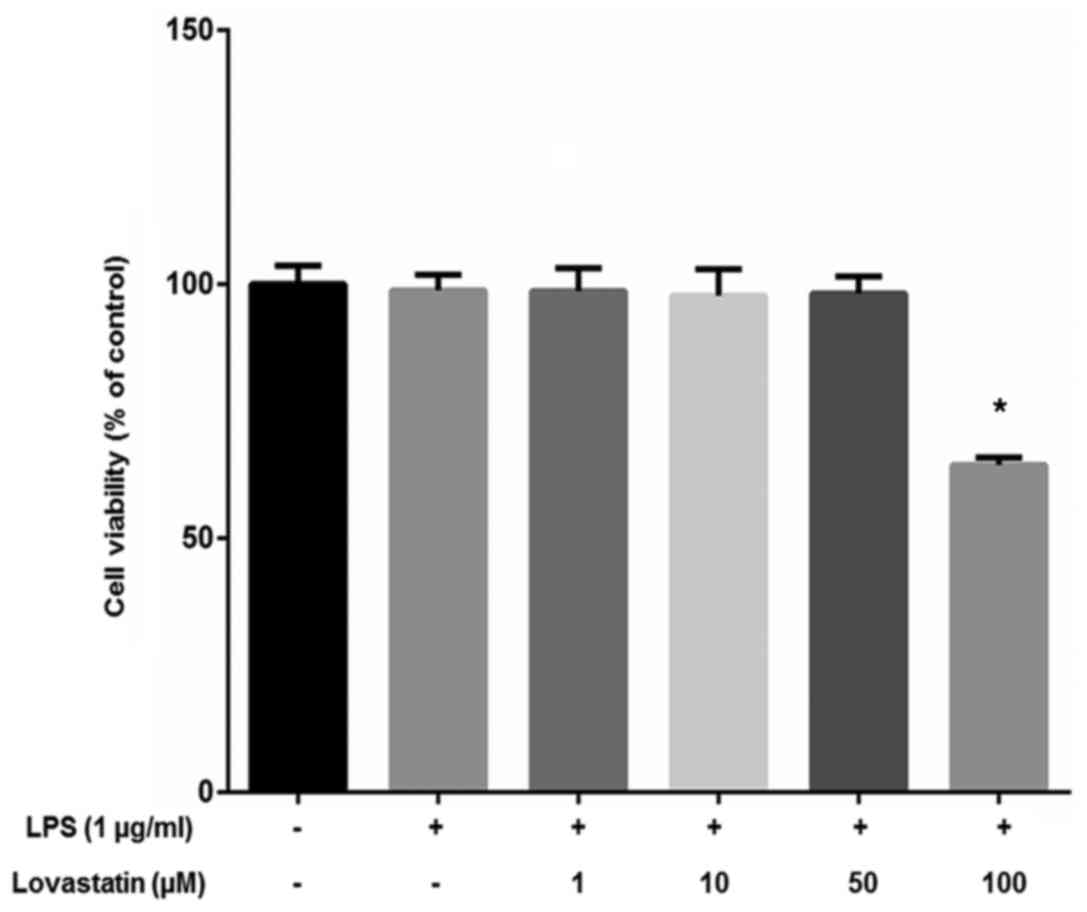
The viability of the RAW264.7 macrophage cells was
measured using the WST-1® assay. The cells were treated
with 1, 10, 50 or 100 µM lovastatin with 1 µg/ml LPS.
Lovastatin exhibited no significant effect on cell viability at
concentrations ≤50 µM, but 100 µM lovastatin
significantly inhibited the viability of the cells (Fig. 2). On the basis of the results of
the cell viability assay, ≤50 µM lovastatin was used in
further experiments to exclude the cytotoxic effects of lovastatin
on RAW264.7 cells.
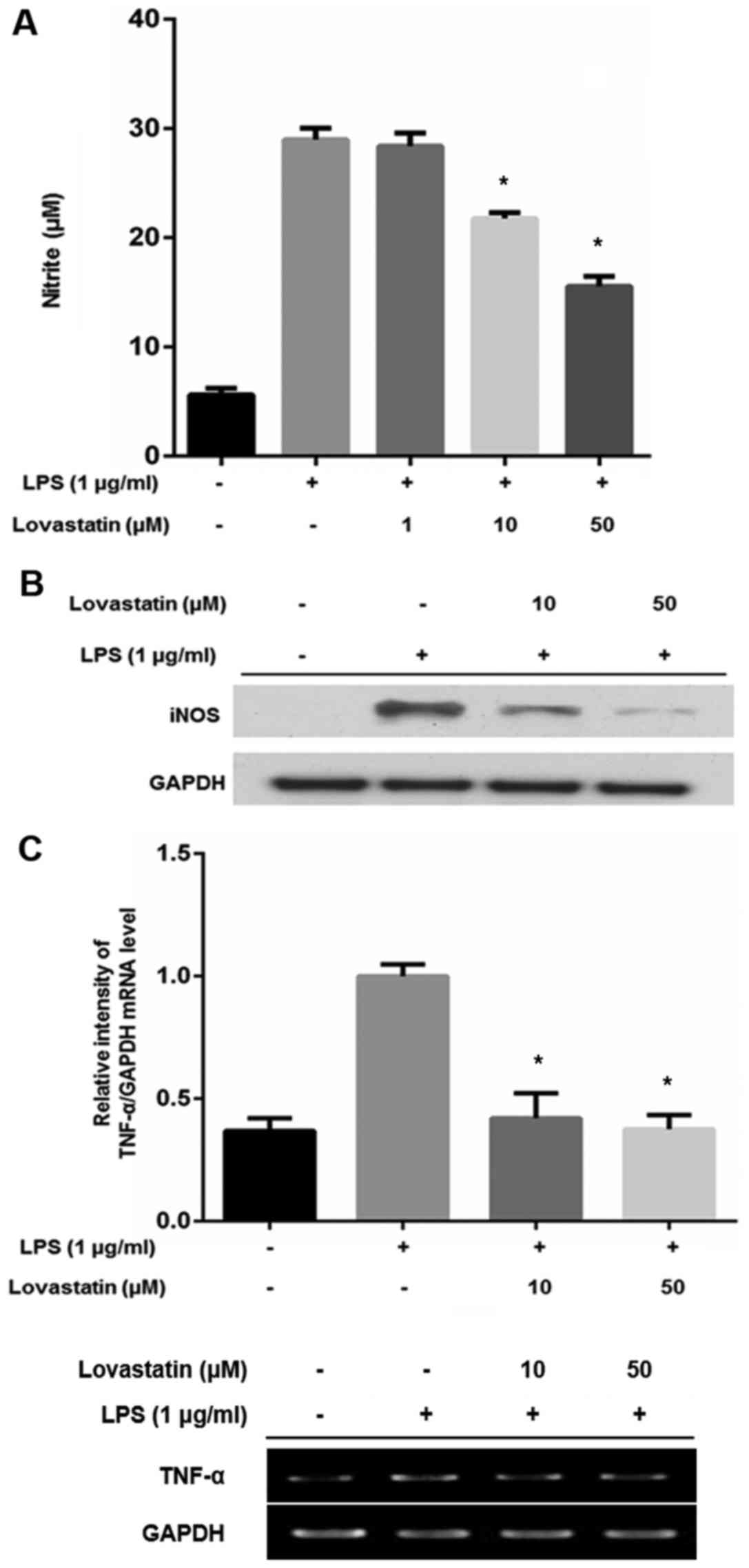
Lovastatin decreases NO production, iNOS
and TNF-α expression in RAW264.7 cells
NO production was determined using Griess reagent in
RAW264.7 cells treated with LPS alone, or LPS and lovastatin. When
cells were treated with LPS only, the level of NO production was
increased. However, when the RAW264.7 cells were also treated with
10 or 50 µM lovastatin, NO production was significantly
decreased compared with that of the LPS-only treated cells
(Fig. 3A). Since NO production
was downregulated by 10 and 50 µM lovastatin but not 10
µM, it was postulated that 10 and 50 µM lovastatin
may alter iNOS expression. Western blotting revealed that
lovastatin decreased the expression of iNOS in an apparently
dose-dependent manner, compared with that in the cells treated with
LPS only (Fig. 3B). The mRNA
level of TNF-α was also compared between the RAW264.7 cells treated
with LPS alone and those treated with LPS in combination with
lovastatin. The LPS treatment of RAW264.7 cells increased the TNF-α
transcript ~2.5-fold. However, 10 and 50 µM lovastatin
treatment significantly suppressed TNF-α expression at the
transcriptional level (Fig. 3C).
These results indicate that lovastatin has an inhibitory effect on
the production of NO and the expression of iNOS and TNF-α in
LPS-stimulated RAW264.7 cells.
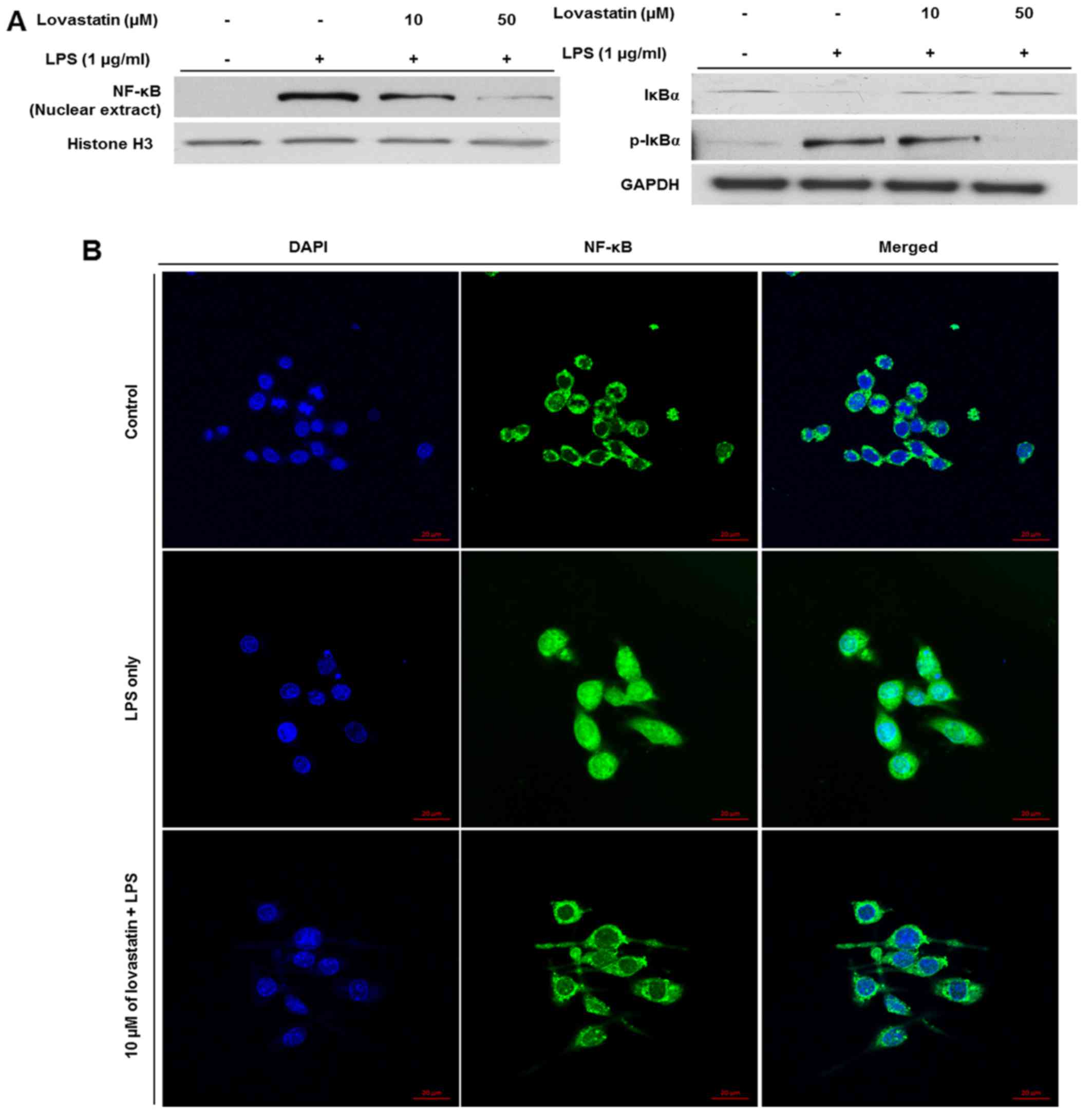
Lovastatin prevents the nuclear
translocation of NF-κB in LPS-stimulated RAW264.7 cells
As the aforementioned results show that iNOS and
TNF-α expression were reduced at the protein and mRNA levels,
respectively, it was hypothesized that lovastatin may affect the
activation of transcription factors. Among various transcription
factors, NF-κB is well-studied for its broad participation in the
transcription of inflammatory mediators, including iNOS and TNF-α
(14). To verify the regulatory
effect of lovastatin on NF-κB activation, the nuclear expression of
NF-κB was evaluated. The expression of NF-κB in nuclear extracts
from the LPS-stimulated RAW264.7 cells was decreased by lovastatin,
and the reduction appeared to be dose-dependent. Also, the
expression of IκBα, which inhibits the nuclear translocation of
NF-κB (15), was increased while
the phosphorylation of IκBα was decreased, and the reduction in
phosphorylation appeared to be a dose-dependent (Fig. 4A). When the cells were subjected
to immunofluorescence staining (Fig.
4B), the expression of NF-κB (green) was detected in the
nucleus (blue) as well as the cytosol of cells when the cells were
treated with LPS only. However, when the RAW264.7 cells were
treated with LPS plus 10 µM lovastatin, the expression
levels of nuclear NF-κB (green) were decreased. The results
demonstrate that lovastatin hinders LPS-induced NF-κB translocation
from the cytosol into the nucleus, indicating that lovastatin may
thereby suppress the inflammatory mediators, including iNOS and
TNF-α, transcribed by NF-κB.
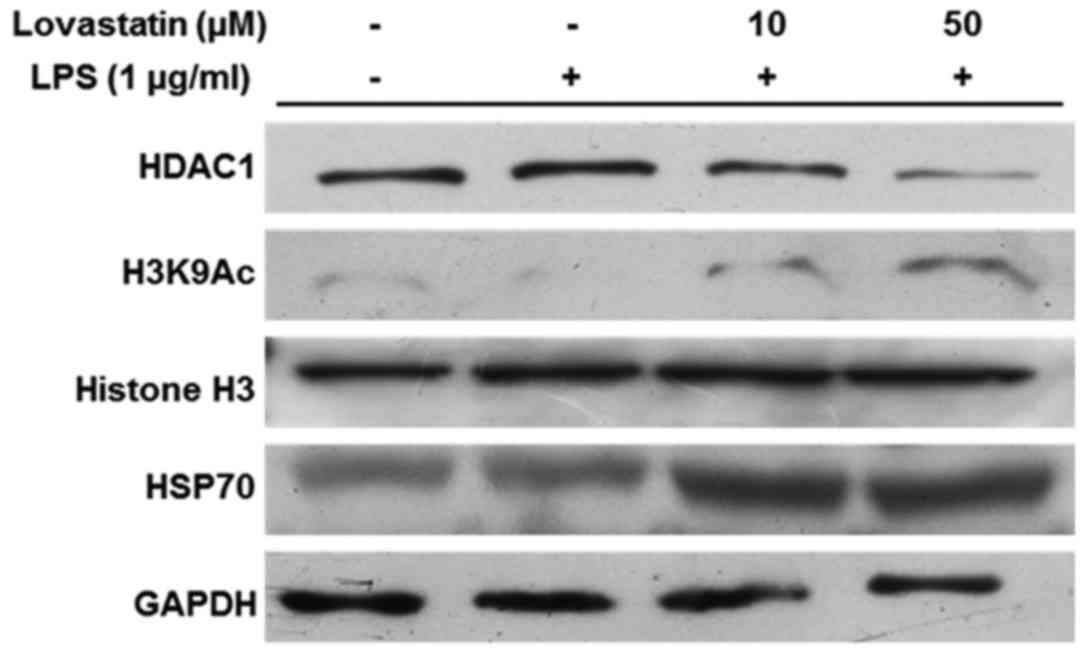
Lovastatin induces HSP70 accumulation via
HDAC1 inhibition
Previous studies have indicated that HDAC1 mediates
the exposure of the HSP70 promoter region through the acetylation
of histone H3, and that HSP70 upregulation is able to block NF-κB
translocation (16,17). In the present study, HDAC1
expression was markedly decreased by lovastatin, and the reduction
appeared to be dose-dependent (10 and 50 µM). However,
lovastatin treatment increased the acetylation of histone H3 at
lysine 9. The expression and accumulation of HSP70 was increased
when the RAW264.7 cells were treated with LPS in combination with
10 or 50 µM lovastatin (Fig.
5). Therefore, these results indicate that lovastatin regulates
the expression of HSP70 by inducing the downregulation of HDAC1,
which leads to the increased acetylation of histone H3 at the
lysine 9 residue.
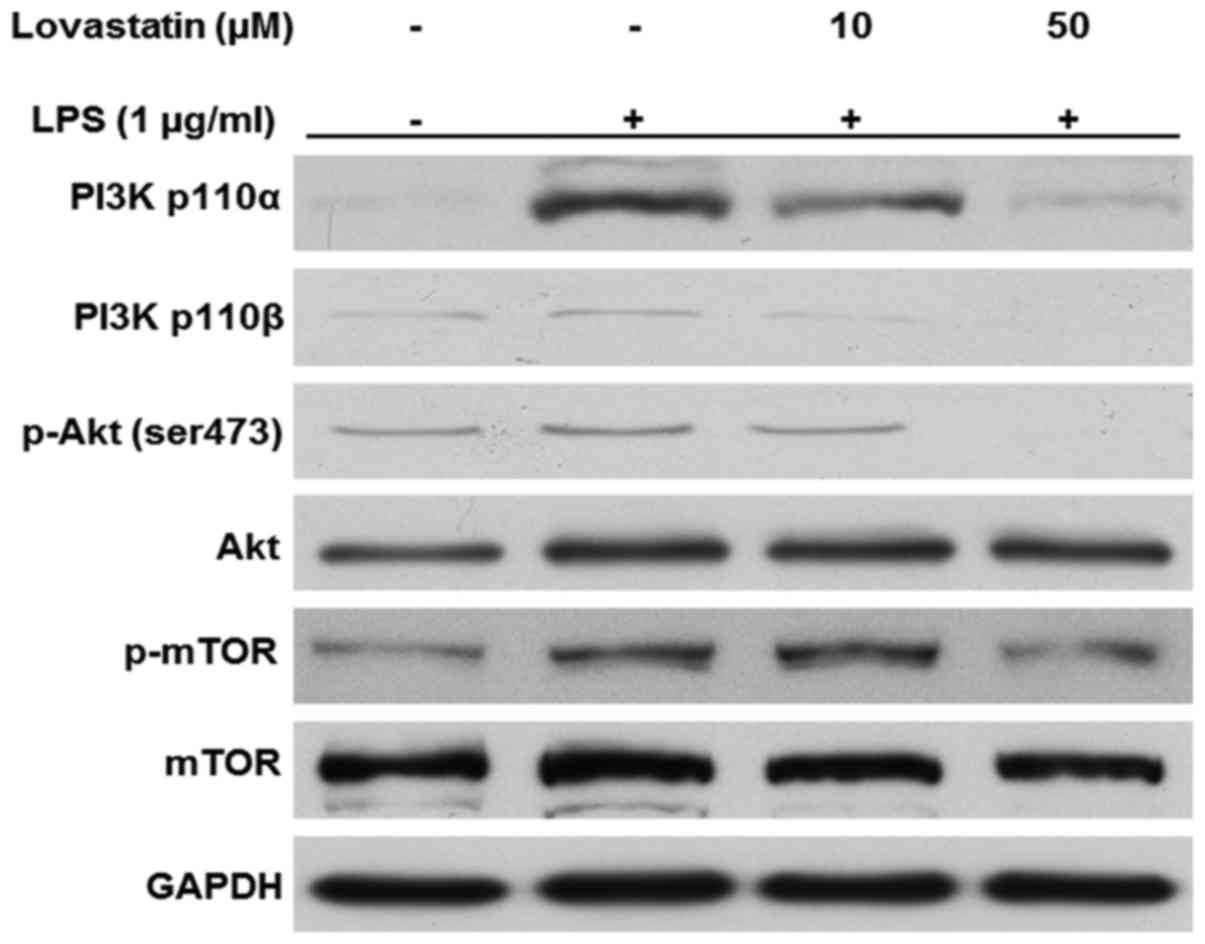
Lovastatin inhibits the PI3K/Akt/mTOR
signaling pathway
In the present study, the PI3K/Akt/mTOR signaling
pathway in LPS-stimulated macrophages appeared to be inhibited by
the presence of lovastatin. The protein levels of the PI3K 110α and
p110β catalytic subunits were increased when the cells were treated
with LPS only; however, when the cells were treated with 10 or 50
µM lovastatin prior to LPS, the expression of the two PI3K
subunits was decreased. Likewise, the phosphorylation of Akt at
serine 473 residue and mTOR at serine 2481 residue were decreased
by lovastatin in a comparable manner. However, lovastatin did not
alter the expression of Akt and mTOR in the whole-cell lysates
(Fig. 6). Therefore, these
results suggest that lovastatin exerts regulatory effects on the
PI3K/Akt/mTOR signaling pathway in LPS-stimulated RAW264.7
cells.
Discussion
Inflammation is an important innate immune response
that protects the host from antigens. However, the overexpression
of pro-inflammatory mediators, such as NO and pro-inflammatory
cytokines, may occur as an aberrant inflammatory response (18,19). Macrophages serve pivotal roles in
inflammation through the production of pro-inflammatory mediators
and the regulation of inflammatory cells (18,20).
Lovastatin has been prescribed as a drug for the
treatment of cardiovascular diseases for many years, and it has
also been reported that lovastatin is able to inhibit tumor growth
and inflammation (11,13,21). Previous studies have demonstrated
that the inhibition of HDAC affects inflammation via various
signaling pathways (22,23). In addition, many studies have
revealed that the PI3K/Akt signaling pathway is associated with the
inflammatory response. PI3K/Akt signal transduction acts upstream
of NF-κB activation (24-26). Specifically, the inhibition of
PI3K p110α and p110β subunits is reported to have a therapeutic
function in the treatment of chronic inflammatory diseases
(27). Therefore, in the present
study, whether lovastatin reduces inflammation by the
downregulation of HDAC and PI3K/Akt in LPS-stimulated RAW264.7
macrophage cells was investigated.
NO serves a key role in inflammation and it is used
for the evaluation of macrophage activation (28). In the present study, lovastatin at
the concentrations 10 and 50 µM decreased NO production
without cytotoxicity in LPS-stimulated RAW264.7 cells. This result
is consistent with the downregulation of iNOS expression that was
also observed in response to lovastatin treatment in the present
study, since iNOS promotes the production of NO (29). Another well-known target of
anti-inflammatory treatment is TNF-α (30), the expression of which was
decreased at the transcriptional level by lovastatin in the present
study. Therefore, these data suggest that lovastatin regulates
inflammation by decreasing NO production and TNF-α expression.
NF-κB is associated with the transcription of
numerous pro-inflammatory factors, including iNOS and TNF-α
(31). When activated, NF-κB is
phosphorylated and translocated into the nucleus (32). However, the present study
demonstrated through western blot analysis and immunofluorescence
staining that the translocation of NF-κB was attenuated by
lovastatin in LPS-stimulated RAW264.7 cells. Previously, studies
have shown that NF-κB participates in the expression of iNOS and
TNF-α (33-35). IκBα binds to NF-κB and suppresses
its translocation; when IκBα is phosphorylated and degraded by
proteasomes, NF-κB is activated and translocates into the nucleus
(36). The western blotting
results in the present study indicate that the expression of IκBα
was increased and its phosphorylation was decreased in the presence
of lovastatin. Thus, the lovastatin-induced reduction of iNOS and
TNF-α expression may result from the inhibition of NF-κB
translocation into the nucleus.
NF-κB activation is also regulated by HDAC1
expression. When HDAC1 expression is decreased, histone H3
acetylation at the lysine 9 residue is facilitated. The promoter
region of the HSP70 gene is then exposed, which promotes HSP70
transcription (8). The
accumulation of HSP70 in the cytoplasm leads to inhibition of the
translocation of NF-κB from the cytoplasm into the nucleus
(17). On the basis of previous
studies, it was hypothesized that reduction of NF-κB translocation
into the nucleus is associated with decreased HDAC1 expression and
increased HSP70 expression. The western blotting results in the
present study reveal that HDAC1 expression was downregulated by
lovastatin in LPS-stimulated RAW264.7 cells. Also, histone H3
acetylation at lysine 9 and HSP70 expression was upregulated by
lovastatin (10 and 50 µM) in the LPS-stimulated RAW264.7
cells. These results support the hypothesis that the inhibitory
effect of lovastatin on HDAC1 disturbs the translocation of
cytosolic NF-κB into the nucleus in LPS-stimulated RAW264.7
macrophages.
Although lovastatin may reduce NF-κB nuclear
translocation via the induction of HSP70, the possibility that
lovastatin influences other signal transduction pathways to
regulate NF-κB activation in the cytoplasm was also considered. It
has been reported that PI3K/Akt/mTOR signal transduction
contributes to NF-κB translocation in the inflammatory response;
PI3K phosphorylates Akt at serine 473, which induces NF-κB
translocation (9). This
regulatory function of the PI3K/Akt signaling pathway on NF-κB
translation occurs through the phosphorylation of mTOR, downstream
of Akt, at the serine 2481 residue (37). In the present study, western
blotting revealed that the expression of PI3K p110α and p110β
catalytic subunits in LPS-stimulated RAW264.7 cells was decreased
by 10 and 50 µM lovastatin. In addition, the phosphorylation
of PI3K and Akt was downregulated by lovastatin, resulting in
reduced levels of mTOR expression in the LPS-stimulated RAW264.7
cells. This is likely to prevent the translocation of cytosolic
NF-κB into the nucleus in the LPS-stimulated RAW264.7 cells.
In conclusion, the results of the present study
indicate that the downregulation of HDAC1 expression and inhibition
of the PI3K/Akt/mTOR signaling pathway by lovastatin results in the
inhibition of cytosolic NF-κB translocation into the nucleus in
LPS-stimulated RAW264.7 macrophages. Furthermore, the inhibitory
effect of lovastatin on NF-κB activation induces an
anti-inflammatory response in LPS-stimulated RAW264.7 macrophage
cells through suppression of the expression of iNOS and TNF-α.
These observations suggest that lovastatin is a potential
therapeutic agent for the amelioration of inflammatory diseases via
the regulation of macrophages.
Acknowledgments
The present study was supported by the Golden Seed
Project (Center for Horticultural Seed Development; grant no.
213003-04-2-SBI10), Ministry of Agriculture, Food and Rural Affairs
(MAFRA), Ministry of Oceans and Fisheries (MOF), Rural Development
Administration (RDA) and Korea Forest Service (KFS).
References
|
1
|
Umamaheswari S and Sangeetha KS:
Anti-Inflammatory Effect of Selected Dihydroxyflavones. J Clin
Diagn Res. 9:FF05–FF07. 2015.PubMed/NCBI
|
|
2
|
Paradise WA, Vesper BJ, Goel A, Waltonen
JD, Altman KW, Haines GK and Radosevich JA: Nitric oxide:
Perspectives and emerging studies of a well known cytotoxin. Int J
Mol Sci. 11:2715–2745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng XX, Zhang SH, Wang XL, Ye TJ, Li H,
Yan XF, Wei L, Wu ZP, Hu J, Zou CP, et al: Panax Notoginseng flower
saponins (PNFS) inhibit LPS-stimulated NO overproduction and iNOS
gene overexpression via the suppression of TLR4-mediated
MAPK/NF-kappa B signaling pathways in RAW264.7 macrophages. Chin
Med. 10:152015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medicherla K, Sahu BD, Kuncha M, Kumar JM,
Sudhakar G and Sistla R: Oral administration of geraniol
ameliorates acute experimental murine colitis by inhibiting
pro-inflammatory cytokines and NF-κB signaling. Food Funct.
6:2984–2995. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salminen A, Paimela T, Suuronen T and
Kaarniranta K: Innate immunity meets with cellular stress at the
IKK complex: Regulation of the IKK complex by HSP70 and HSP90.
Immunol Lett. 117:9–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hao NB, Tang B, Wang GZ, Xie R, Hu CJ,
Wang SM, Wu YY, Liu E, Xie X and Yang SM: Hepatocyte growth factor
(HGF) upregulates heparanase expression via the PI3K/Akt/NF-κB
signaling pathway for gastric cancer metastasis. Cancer Lett.
361:57–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito K, Charron CE and Adcock IM: Impact of
protein acetylation in inflammatory lung diseases. Pharmacol Ther.
116:249–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marinova Z, Ren M, Wendland JR, Leng Y,
Liang MH, Yasuda S, Leeds P and Chuang DM: Valproic acid induces
functional heat-shock protein 70 via Class I histone deacetylase
inhibition in cortical neurons: A potential role of Sp1
acetylation. J Neurochem. 111:976–987. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Zhang C, Huang Y, Yu Y, Li R, Li
M, Liu N, Liu P and Qiao J: Up-regulated expression of WNT5a
increases inflammation and oxidative stress via PI3K/AKT/NF-κB
signaling in the granulosa cells of PCOS patients. J Clin
Endocrinol Metab. 100:201–211. 2015. View Article : Google Scholar
|
|
10
|
Pan H, Xu LH, Ouyang DY, Wang Y, Zha QB,
Hou XF and He XH: The second-generation mTOR kinase inhibitor
INK128 exhibits anti-inflammatory activity in
lipopolysaccharide-activated RAW264.7 cells. Inflammation.
37:756–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alarcón J, Aguila S, Arancibia-Avila P,
Fuentes O, Zamorano-Ponce E and Hernández M: Production and
purification of statins from Pleurotus ostreatus (Basidiomycetes)
strains. Z Naturforsch C. 58:62–64. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin YC, Lin JH, Chou CW, Chang YF, Yeh SH
and Chen CC: Statins increase p21 through inhibition of histone
deacetylase activity and release of promoter-associated HDAC1/2.
Cancer Res. 68:2375–2383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo W, Liu H, Li L, Yang M and Du A:
Regulation of lovastatin on a key inflammation-related microRNA in
myocardial cells. Chin Med J (Engl). 127:2977–2981. 2014.
|
|
14
|
van Loo G and Beyaert R: Negative
regulation of NF-κB and its involvement in rheumatoid arthritis.
Arthritis Res Ther. 13:2212011. View
Article : Google Scholar
|
|
15
|
Gveric D, Kaltschmidt C, Cuzner ML and
Newcombe J: Transcription factor NF-kappaB and inhibitor I
kappaBalpha are localized in macrophages in active multiple
sclerosis lesions. J Neuropathol Exp Neurol. 57:168–178. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trujillo-Gonzalez I, Cervantes-Roldan R,
Gonzalez-Noriega A, Michalak C, Reyes-Carmona S, Barrios-Garcia T,
Meneses-Morales I and Leon-Del-Rio A: Holocarboxylase synthetase
acts as a biotin-independent transcriptional repressor interacting
with HDAC1, HDAC2 and HDAC7. Mol Genet Metab. 111:321–330. 2014.
View Article : Google Scholar
|
|
17
|
de Jong PR, Schadenberg AW, Jansen NJ and
Prakken BJ: Hsp70 and cardiac surgery: Molecular chaperone and
inflammatory regulator with compartmentalized effects. Cell Stress
Chaperones. 14:117–131. 2009. View Article : Google Scholar :
|
|
18
|
Jeong EJ, Seo H, Yang H, Kim J, Sung SH
and Kim YC: Anti-inflammatory phenolics isolated from Juniperus
rigida leaves and twigs in lipopolysaccharide-stimulated RAW264.7
macrophage cells. J Enzyme Inhib Med Chem. 27:875–879. 2012.
View Article : Google Scholar
|
|
19
|
Piwowarski JP, Kiss AK, Granica S and
Moeslinger T: Urolithins, gut microbiota-derived metabolites of
ellagitannins, inhibit LPS-induced inflammation in RAW 264.7 murine
macrophages. Mol Nutr Food Res. 59:2168–2177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu PJ, Jin H, Zhang JY, Wang GF, Li JR,
Zhu ZG, Tian YX, Wu SY, Xu W, Zhang JJ, et al: Pyranocoumarins
isolated from Peucedanum praeruptorum Dunn suppress
lipopolysaccharide-induced inflammatory response in murine
macrophages through inhibition of NF-κB and STAT3 activation.
Inflammation. 35:967–977. 2012. View Article : Google Scholar
|
|
21
|
Mulder KC, Mulinari F, Franco OL, Soares
MS, Magalhães BS and Parachin NS: Lovastatin production: From
molecular basis to industrial process optimization. Biotechnol Adv.
33:648–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gillenwater AM, Zhong M and Lotan R:
Histone deacetylase inhibitor suberoylanilide hydroxamic acid
induces apoptosis through both mitochondrial and Fas (Cd95)
signaling in head and neck squamous carcinoma cells. Mol Cancer
Ther. 6:2967–2975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cantley MD, Fairlie DP, Bartold PM, Marino
V, Gupta PK and Haynes DR: Inhibiting histone deacetylase 1
suppresses both inflammation and bone loss in arthritis.
Rheumatology (Oxford). 54:1713–1723. 2015. View Article : Google Scholar
|
|
24
|
Jayasooriya RG, Lee KT, Kang CH, Dilshara
MG, Lee HJ, Choi YH, Choi IW and Kim GY: Isobutyrylshikonin
inhibits lipopolysaccharide-induced nitric oxide and prostaglandin
E2 production in BV2 microglial cells by suppressing the
PI3K/Akt-mediated nuclear transcription factor-κB pathway. Nutr
Res. 34:1111–1119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stark A, Sriskantharajah S, Hessel EM and
Okkenhaug K: PI3K inhibitors in inflammation, autoimmunity and
cancer. Curr Opin Pharmacol. 23:82–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schabbauer G, Tencati M, Pedersen B,
Pawlinski R and Mackman N: PI3K-Akt pathway suppresses coagulation
and inflammation in endotoxemic mice. Arterioscler Thromb Vasc
Biol. 24:1963–1969. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hawkins PT and Stephens LR: PI3K
signalling in inflammation. Biochim Biophys Acta. 1851:882–897.
2015. View Article : Google Scholar
|
|
28
|
Predonzani A, Calì B, Agnellini AH and
Molon B: Spotlights on immunological effects of reactive nitrogen
species: When inflammation says nitric oxide. World J Exp Med.
5:64–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu PW, Chen MF, Tsai AP and Lee TJ: STAT1
mediates oroxylin a inhibition of iNOS and pro-inflammatory
cytokines expression in microglial BV-2 cells. PLoS One.
7:e503632012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu J, Zhang Y, Wu G, Xiao Z, Zhou H and
Yu X: Inhibitory effects of oligochitosan on TNF-α, IL-1β and
nitric oxide production in lipopolysaccharide-induced RAW264.7
cells. Mol Med Rep. 11:729–733. 2015. View Article : Google Scholar
|
|
31
|
Jung YC, Kim ME, Yoon JH, Park PR, Youn
HY, Lee HW and Lee JS: Anti-inflammatory effects of galangin on
lipopolysaccharide-activated macrophages via ERK and NF-κB pathway
regulation. Immunopharmacol Immunotoxicol. 36:426–432. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mo XM and Sun HX: The Anti-inflammatory
effect of the CXCR4 antagonist-N15P peptide and its modulation on
inflammation-associated mediators in LPS-induced PBMC.
Inflammation. 38:1374–1383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chun JM, Nho KJ, Kim HS, Lee AY, Moon BC
and Kim HK: An ethyl acetate fraction derived from Houttuynia
cordata extract inhibits the production of inflammatory markers by
suppressing NF-κB and MAPK activation in
lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complement
Altern Med. 14:2342014. View Article : Google Scholar
|
|
34
|
Kim J, Han AR, Park EY, Kim JY, Cho W, Lee
J, Seo EK and Lee KT: Inhibition of LPS-induced iNOS, COX-2 and
cytokines expression by poncirin through the NF-kappaB inactivation
in RAW 264.7 macrophage cells. Biol Pharm Bull. 30:2345–2351. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aktan F: iNOS-mediated nitric oxide
production and its regulation. Life Sci. 75:639–653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Magnani M, Crinelli R, Bianchi M and
Antonelli A: The ubiquitin-dependent proteolytic system and other
potential targets for the modulation of nuclear factor-κB (NF-κB).
Curr Drug Targets. 1:387–399. 2000. View Article : Google Scholar
|
|
37
|
Hou YC, Chiu WC, Yeh CL and Yeh SL:
Glutamine modulates lipopolysaccharide-induced activation of NF-κB
via the Akt/mTOR pathway in lung epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 302:L174–L183. 2012. View Article : Google Scholar
|