Galectins are a family of β-galactoside-binding
lectins with ≥1 evolutionary conserved carbohydrate-recognition
domain (CRD) (1). At present, 15
galectins have been identified in mammals, and are divided into
three types based on domain organization as follows: i) Prototype
galectins with one single CRD; ii) tandem-repeat galectins with two
CRDs; iii) chimera-type galectins with a single CRD connected to a
long, flexible N-terminal domain (1–3).
Note that Galectin-3 is the only chimera-type galectin. Human
galectin-3 is a 35-kDa protein that is coded by a single gene,
LGALS3, located on chromosome 14. The N-terminal domain of
galectin-3 is essential for its multimerization, sensitive to
proteolysis by matrix metalloproteinases and may participate in the
interaction with other intracellular proteins (3). Furthermore, the first 12 amino acids
of galectin-3 are necessary for its secretion and nuclear
translocation (4,5). The C-terminal CRD of galectin is
responsible for its interaction with glycoconjugates containing
N-acetyllactosamine. Thus, galectin-3 binds proteins in a
carbohydrate-dependent and -independent manner.
Galectin-3 is widely expressed in human tissues,
including all types of immune cell (macrophages, monocytes,
dendritic cells, eosinophils, mast cells, natural killer cells, and
activated T and B cells), epithelial cells, endothelial cells and
sensory neurons (3,6). The expression of galectin-3 in
tissues is developmentally regulated; it is more abundant during
embryogenesis and development compared with adult life (7). Furthermore, during the early stages
of embryogenesis, its expression pattern is more specific, located
predominantly in the epithelia, kidney, chondrocytes and liver
(8). However, galectin-3-knockout
mice are viable without obvious abnormalities with the exception of
premature senescence (9,10).
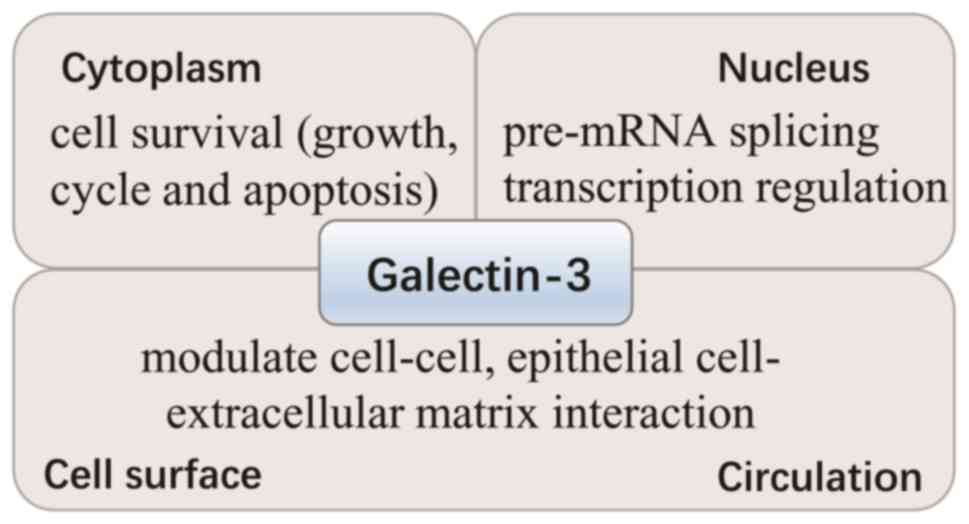
Galectin-3 is predominantly located in the cytoplasm
and shuttles into the nucleus (Fig.
1). In addition, it is secreted to the cell surface and into
biological fluids (3). The
different locations of galectin-3 contribute to its various
functions. In the cytoplasm, galectin-3 is important for cell
survival, due to its interaction with certain survival-associated
proteins, including B-cell lymphoma-2 (Bcl-2) and activated
guanosine-5′-triphosphate (GTP)-bound K-Ras. In the nucleus,
galectin-3 promotes pre-mRNA splicing and regulates gene
transcription, whereas extracellular galectin-3 modulates cell-cell
interactions, including between epithelial cells and the
extracellular matrix. Thus, it is involved in cell differentiation,
inflammation, fibrogenesis and the host defense (3,11).
Therefore, galectin-3 is pivotal in numerous biological activities
including cell growth, apoptosis, pre-mRNA splicing,
differentiation, transformation, angiogenesis, inflammation,
fibrosis and host defense. Previous evidence has indicated that
galectin-3 is involved in the pathogenesis of cardiovascular
remodeling, as well as in various autoimmune and inflammatory
processes (2,6,7,12–18).
A total of 97.5% of the galectin-3 reference
population was 27.5 ng/ml according to the product insert of an
ARCHITECT Galectin-3 assay (www.abbottdiagnostics.com). Both increased and
decreased expression levels of galectin-3 are observed in various
types of disease including heart, renal and liver disease, cancer
and infections. Furthermore, galectin-3 is a stable biomarker and
is not associated with age, body mass index or sex (19,20). Furthermore, galectin-3 does not
exhibit circadian variation and increases marginally following
exercise, returning to normal levels after 1–3 h (21). Therefore, galectin-3 may be used
in the diagnosis and prognosis of various types of disease, and
therefore may also serve as a therapeutic target for treating
disease. Despite several reviews commenting on these uses, numerous
studies have been published in the last 3 years and provided
certain novel ideas, particularly for the use of galectin-3 in
humans. Thus, the present review summarizes the current literature,
regarding biomarker characteristics and possible therapeutic
applications of galectin-3 in disease.
Elevated serum galectin-3 levels have been detected
in almost all types of cardiovascular disease and its prognostic
value for different clinical outcomes has been extensively
investigated in patients (Table
I).
Galectin-3 as a biomarker of fibrosis and
inflammation has been implicated in the development and progression
of HF, and may predict increased morbidity and mortality. Two
recent meta-analyses demonstrated that increased expression levels
of galectin-3 are associated with mortality in acute and chronic HF
(20,22), whereas another systematic review
indicated that galectin-3 is ineffective for predicting all-cause
mortality and cardiovascular mortality, particularly under the
influence of certain clinical factors including estimated
glomerular filtration rate (eGFR), left ventricular ejection
fraction (LVEF) and N-terminal pro-B-type natriuretic peptide
(NT-proBNP) (23).
A prospective cohort study with a 26-month follow-up
revealed that galectin-3 expression levels are independent
predictors of 26-month mortality in patients with chronic HF, and
that a galectin-3 level >21 ng/ml was associated with increased
mortality (24). In HF patients
with coronary heart disease, serum galectin-3 levels were
increased, and were an independent predictor of all-cause mortality
and re-hospitalization. Galectin-3 levels were markedly associated
with outcomes in HF patients with a preserved ejection fraction
(HFpEF) compared with HF patients with a reduced ejection fraction
(HFrEF) (25). Galectin-3 is also
associated with HF severity and exhibits dynamic changes during
mechanical unloading, and predicts survival rates following the use
of a left ventricular assist device (LVAD). Furthermore, galectin-3
is associated with the development of cardiac allograft
vasculopathy post-heart transplantation (HT). Galectin-3 may also
serve as a novel biomarker in patients with HF, during LVAD
support, and following HT (26).
Using patients hospitalized for HF pooled from three cohorts,
Meijers et al (27)
demonstrated that the plasma galectin-3 concentration is useful for
the prediction of near-term re-hospitalization (27). In patients with HF and functional
mitral regurgitation who underwent mitral valve repair, high
pre-operative serum galecin-3 was independently associated with the
absence of left ventricular (LV) reverse remodeling following
mitral valve repair (28).
Furthermore, the prognostic value of galectin in patients with HF
is not affected by HF therapeutic strategies (29–31) or age (32). However, the prognostic value of
galectin-3 in HF may differ among various ethnicities. Using a
sub-study of the Atherosclerosis Risk in Communities observational
cohort (1,375 white patients and 434 black patients) between 2004
and 2005, galectin-3 was identified to be independently associated
with a composite of HF or mortality among white patients; however,
not among black patients. Thus, galectin-3 may have limited
prognostic utility for predicting HF and mortality in black
patients (33).
Changes in galectin-3 over time may be a more
sensitive and accurate prognostic biomarker for HF. Galectin-3
expression levels are elevated in a substantial proportion of
patients with HF, particularly those with more severe HF and renal
dysfunction (34). Galectin-3
expression levels increase over time in these patients and the
increase is independently associated with a poorer clinical outcome
(34). In the Valsartan Heart
Failure Trial over a 4-month follow-up, for every 1 µg/l increase
in galectin-3, there was an associated increased risk of mortality,
primary morbid event and also hospitalization for HF (2.9, 2.1 and
2.2%, respectively) (34). In the
Controlled Rosuvastatin Multinational Trial in Heart Failure (over
a 3-month follow-up) and in the Coordinating Study Evaluating
Outcomes of Advising and Counseling Failure trial (over a 6-month
follow-up), increased galectin-3 expression levels were observed
(<17.8 to >17.8 ng/ml). This was associated with a
significant increase in hospitalization and mortality due to HF,
with an increase of >15% corresponding to a 50% increased in
relative hazard of adverse events, despite following extensive
clinical adjustments including age, sex, diabetes mellitus, LVEF,
renal function, medication (e.g. β-blockers, angiotensin converting
enzyme inhibitors and angiotensin receptor blockers) and NT-proBNP
(35). In patients with LV
systolic dysfunction with >10-month follow-up, an increase
<20 ng/ml was significantly associated with a lower rate of
adverse cardiovascular events and independently predicted fewer
adverse cardiovascular events following extensive clinical
adjustments. However, HF therapeutic strategies including
angiotensin-converting-enzyme inhibitors, angiotensin II receptor
blockers and thiazide diuretic exhibited no clear effects on
galectin-3 expression levels (29). In patients with stable chronic HF,
the change in galectin-3 expression levels over time, including
that at 6 months, are improved predictors of cardiovascular events
compared with baseline galectin-3 levels (36). In contrast, Miller et al
(37) identified that serial
galectin-3 monitoring in ambulatory HF patients provided no
additional prognostic benefit.
The association of galectin-3 with other cardiac
function parameters remains controversial. A previous study
demonstrated that in-patients with acute decompensated HF exhibited
no significant association between serum galectin-3 expression
levels and arterial stiffening markers, whereas increased
galectin-3 expression levels were associated with impaired
ventricular-arterial coupling, elevated pulmonary artery pressures
and severe systolic dysfunction (38). Increased galectin-3 expression
levels and arterial pulmonary pressure were identified to be
independent risk factors for all-cause mortality and readmission
(38). Galectin-3 expression
levels demonstrated no difference between patients with HFpEF or
HFrEF. However, it was associated with diastolic dysfunction
severity and LV stiffness in patients with HFpEF, and associated
with poor clinical outcome(s) independent of renal dysfunction and
other risk factors in patients with HFpEF (39). Furthermore, for patients with
compensated systolic HF undergoing treatment, galectin-3
demonstrated no association with eGFR, LVEF or functional capacity
(40). The serum levels of
galectin-3 are associated with changes in the LV structure and
function, indicating that galectin-3 may be involved in the process
of LV remodeling in chronic HF (41).
Galectin-3 reflects the inflammatory status and
fibrosis, and may be useful for evaluating cardiac and renal
function; as such, it may serve as a cardiorenal biomarker. In
outpatients with HF, increased serum levels of galectin-3 reflected
increased neurohumoral activity and reduced eGFR, however, not
myocardial function (42). In
another study enrolling a group of outpatients with chronic HF,
galectin-3 serum levels were significantly and independently
associated with micro-albuminuria (43). However, Zhang et al
(44) reported that for
inpatients with HF, galectin-3 only predicted mortality associated
with renal function for patients with an eGFR >60 ml/min/1.73
m2. Furthermore, for patients with HFpEF, galectin-3 was
associated with renal dysfunction severity, and was not
independently associated with the severity of pathophysiological
derangements (45). Another
cohort study confirmed that galectin-3 was markedly associated with
renal function in outpatients with HF (46). Collectively, these results
indicate that the adjustment for renal function may be required
when interpreting the significance of galectin-3 expression
levels.
The prognostic value of galectin-3 has also been
compared with that of other available biomarkers. The Penn Heart
Failure study assessed an ambulatory HF cohort, which was comprised
of patients with reduced, preserved or recovered LVEF. When this
was compared with the established biomarkers for HF [including
soluble suppression of tumorigenicity 2 (sST2), troponin I and
B-type natriuretic peptide (BNP)], gelectin-3 and BNP were revealed
to be the most accurate risk discriminators for patients with
preserved and recovered LVEF through a 5 year follow-up,
respectively (47). In patients
with HFpEF, galectin-3 was a significantly more sensitive, but less
specific as a biomarker when compared with BNP (48), whereas another study demonstrated
the opposite conclusion, that the specificity of galectin-3 for
predicting chronic HF was increased compared with that of
N-terminal pro-BNP (NT-proBNP; however, its sensitivity was not
comparatively increased (49).
When patients with HF were stratified using eGFR values and the
predictive value of fibroblast growth factor-23, galectin-3 and
sST2 was compared, sST2 was the best predictor of mortality in
patients with the lowest eGFR values, fibroblast growth factor-23
was the most relevant biomarker for patients with HF and
intermediate eGFR values, and galectin-3 was the best biomarker for
patients with HF and eGFR values >73 ml/min/1.73 m2
(50). However, galectin-3 has
decreased prognostic value compared with matrix metalloproteinase-2
regarding clinical outcome prediction in patients with chronic
systolic HF (51). In addition,
galectin-3 has less prognostic value than sST2 for long-term risk
stratification of ambulatory HF patients (52). One study speculated that sST2 may
be more useful for monitoring long-term HF and that galectin-3 may
be more useful for the diagnosis of HF remodeling (53).
The combination of galectin-3 with BNP provided an
improved predictive value in discharged patients with HF following
an acute decompensated HF episode compared to BNP alone (54). Galectin-3 alone as a risk
predictor was not effective enough to assess sudden or in-hospital
mortality, whereas combining galectin-3 and NT-proBNP levels
significantly improved discrimination and reclassification when
predicting all-cause and cardiovascular-associated mortality
(55). The combination of
galectin-3 and ST2 may be used to identify high systemic fibrosis
in patients with acute HF, thus providing a powerful risk
stratification value (56).
Therefore, galectin-3 is not recommended for use as a single
prognostic biomarker for patients with HF; however, it is
recommended in combination with other established biomarkers
(23).
Galection-3 may also be used for the diagnosis of
HF. In a previous study, the areas under the receiver operating
characteristic curve for serum and salivary galectin-3 were 0.86
and 0.73, respectively (57). In
patients with HFpEF, the area under the curve for serum galectin-3
was 0.98 (58). These results
indicated the potential utility of using galectin-3 to diagnose
HF.
In patients with aortic valve stenosis, galectin-3
expression levels in serum and the myocardium were positively
associated with levels of fibrosis and relative wall thickness,
which are crucial indicators of geometric remodeling (59). Furthermore, galectin-3 in valvular
interstitial cells obtained from patient's aortic stenosis induced
the expression of inflammatory, fibrotic and osteogenic markers. In
addition, its inhibitor, modified citrus pectin, decreased the
expression levels of inflammatory, fibrotic and osteogenic markers
in valvular interstitial cells undergoing osteoblastic
differentiation, indicating its potential function in calcification
in aortic stenosis (60).
However, a prospective study which enrolled patients with at least
mild degenerative aortic stenosis from two ongoing cohort studies
demonstrated that galectin-3 was not associated with aortic
stenosis severity or functional status, and did not provide
prognostic information on the occurrence of aortic
stenosis-associated events (61).
In a prospective cohort study with long-term
follow-up (median, 7.2 years) in patients with CHD, galectin-3
presented as a strong independent predictor of cardiovascular
mortality (62). In patients with
chronic CHD, increased serum galectin-3 levels were associated with
a greater incidence of cardiovascular events (63). In contrast, another large cohort
study with a 13-year follow-up of patients with stable CHD,
following adjustment for markers of hemodynamic stress, myocardial
injury, inflammation and renal dysfunction, galectin-3 was not able
to independently predict recurrent cardiovascular events (64). In patients with coronary artery
disease, serum galectin-3 levels exhibited a significantly positive
association with coronary artery disease severity, as determined by
the Gensini score and number of diseased vessels (65), and increased serum levels of
galectin-3 reflected the increased degree of myocardial fibrosis
(66). Contrastingly, in patients
with acute coronary syndrome, serum galectin-3 levels demonstrated
a significantly positive association with the Gensini score;
however, not with the number of diseased vessels (67). In addition, acute coronary
syndrome patients with higher galectin-3 expression levels
exhibited a decreased LVEF and eGFR (68).
In patients with a first anterior ST-elevated
myocardial infarction (STEMI) and left anterior descending artery
occlusion treated by primary percutaneous coronary intervention
(pPCI), increased galectin-3 serum levels were measured during
hospitalization and were associated with an increased risk of LV
remodeling (69). Furthermore,
elevated galectin-3 expression levels were associated with a higher
rate of new-onset atrial fibrillation (AF) and diuretic treatment
during hospitalization, and were the most effective independent
predictor of the combined 30-day major adverse clinical outcome in
patients with first MI without prior HF treated with pPCI (70,71). Galectin-3 levels were also
associated with reinfarction following initial MI (72). Galectin-3 measured acutely
following STEMI was demonstrated as an independent predictor of
increased extracellular volume during a 6-month follow-up, which
may be useful for long-term risk stratification (73), and may be used to predict LVEF and
infarction size after 4 months when measured immediately following
a MI (74). In addition, serum
galectin-3 levels varied significantly following a STEMI over a
short time period and were associated with the timing of
reperfusion (75). In acute MI,
galectin-3 expression levels were positively and significantly
associated with certain inflammatory factors (76), whereas another study demonstrated
that galectin-3 expression levels did not change during acute MI
and had no association between galectin-3 levels and acute ischemic
myocardial injury (77). In acute
MI, galectin-3 was positively and significantly associated with
certain biomarkers including matrix metalloproteinase 3, monocyte
chemoattractant protein-1, and interleukin (IL)-8 involved in
extracellular matrix turnover, but not with LV remodeling (78). Furthermore, galectin-3 was
positively associated with MI size and LV remodeling in patients
with a history of complicated MI (77).
Galectin-3 expression levels were increased in
patients with AF, particularly in persistent AF (79,80). Furthermore, galectin-3 expression
independently predicted atrial tachyarrhythmia recurrences
following a single ablation procedure (79,81,82). In addition, galectin-3 was
independently associated with new-onset AF (83), with atrial remodeling (84) and with left atrial volume index in
AF patients with preserved LV function (80). However, the rhythm outcome of
catheter ablation cannot be predicted using galectin-3 levels
(85).
In patients with hypertension, galectin-3 was
independently associated with LV remodeling and, therefore, may be
a valuable biomarker for the detection of early cardiac remodeling
in hypertension (86). In
addition, it was associated with ambulatory microvolt T-wave
alternans positivity, decreased eGFR and increased LV myocardial
index in hypertensive patients (87). Increased galectin-3 expression
levels were observed in patients with pulmonary arterial
hypertension (88). Increased
galectin-3 was associated with multiple indices of right ventricle
function and morphology (88),
and was predictive of impaired right ventricle function (89).
In patients with hypertrophic cardiomyopathy,
galectin-3 expression levels were increased and associated with the
increased degree of LV hypertrophy; however, it was not associated
with decreased myocardial LV diastolic and systolic functions
(90). Furthermore, galectin-3
was associated with late gadolinium enhancement-assessed myocardial
replacement fibrosis in patients with non-ischemic dilated
cardiomyopathy (91).
Galectin-3 may serve as a biomarker for post-HT
outcomes. Galectin-3 analyzed 10 days following HT displayed an
association with heart function assessed 1 year after HT (92). Furthermore, galectin-3 fluctuated
in patients followed up for 12 months after HT, and a novel process
was indicated when galectin-3 increased by double or decreased by
one-half compared to the baseline level (93). Contrastingly, Grupper et al
(94) reported that although the
galectin-3 expression level increased despite HT, and was
associated with renal dysfunction, it was not associated with the
presence of myocyte hypertrophy and interstitial fibrosis post HT
(94).
Patients exhibiting peripheral artery disease also
demonstrated increased galectin-3 levels, and galectin-3 was
positively associated with homeostatic model assessment, but not
arterial elasticity and microalbuminuria following adjustment for
age and sex (95). A prospective
observational study indicated that elevated plasma galectin-3
levels were markedly associated with inflammation, severity and
poor prognosis following intracerebral hemorrhage (96). In adults with single-ventricle
Fontan circulation, elevated galectin-3 was associated with an
increased risk of non-elective cardiovascular hospitalization or
mortality (97). Contrastingly, a
low galectin-3 intra-plaque concentration appears to be associated
with clinically and ultrasonically defined unstable human carotid
plaques in patients with high-grade carotid stenosis (98).
An increased plasma galectin-3 level was associated
with increased myocardial fibrosis in patients with
aldosterone-producing adenoma (99). In addition, increased plasma
levels of galectin-3 facilitate with predicting the occurrence of
postoperative strokes among female patients who undergo carotid
endarterectomy (100).
Galectin-3 expression levels were also associated with the severity
and a poor prognosis following aneurysmal subarachnoid hemorrhage
(101). However, in the general
population, galectin-3 did not predict incident cardiometabolic
disease following adjustment for cardiometabolic risk factors
(102).
In a rat model of ischemia/reperfusion and folic
acid-induced acute renal failure (ARF), galectin-3 mRNA expression
levels began to increase 2 h after injury, and the increased levels
continued until 28 days after injury in ischemic ARF and 7 days
after injury in toxic ARF. Furthermore, in ischemic ARF, the level
of galectin-3 mRNA expression was significantly negatively
associated with serum reciprocal creatinine levels at 48 h after
injury. In addition, galectin-3 was initially localized in tubules
and subsequently in macrophages during the stages of regeneration.
These results indicated that galectin-3 may be important in acute
tubular injury and the subsequent regeneration stage (103).
In addition to its role in ARF, galectin-3 functions
in chronic kidney disease (CKD). It may be used in the diagnosis of
CKD, prediction of clinical outcomes in patients with CKD, and
prediction of renal function in the general population. Patients
with CKD demonstrated reduced eGFR and increased serum galectin-3
expression, and galectin-3 exhibited an area under the receiver
operating characteristic curve of 0.89, indicating its potential
role in the diagnosis of CKD. Furthermore, six-year kidney survival
rates of a low (≤6 ng/ml) and a high (>6 ng/ml) galectin-3 group
were 47.3 and 22.8%, respectively (104). In the Framingham Offspring Study
with 2,450 participants who attended cycle 6 (1995–1998) and had
follow-up data at cycle 8 (2005–2008), increased serum galectin-3
levels at baseline were associated with increased risk of rapid
eGFR decline and incident CKD (despite adjustments for known
clinical predictors of CKD); however, it was not associated with
the risk of incident albuminuria (105). These results indicated that
galectin-3 predicts tubulointerstitial fibrosis, but not glomerular
injury (105). However, in the
Cardiovascular Health Study with a community-based multicenter
cohort of 2,763 older adults with no history of HF, increased serum
galectin-3 levels were not associated with a 30% decline in eGFR or
the development of incident eGFR following adjustments for
potential confounders and other cardiac biomarkers (106).
The association between galectin-3, renal function
and adverse outcomes was investigated in two large cohorts of
patients over broad ranges of renal function from the German
Diabetes Mellitus Dialysis (4D) study (1,168 dialysis patients with
type 2 diabetes mellitus; 4-year follow-up) and the Ludwigshafen
Risk and Cardiovascular Health study (2,579 patients with coronary
angiograms; 10-year follow-up). Patients were divided into three
groups according to their renal function (stratified by eGFR).
Baseline serum galectin-3 levels increased with declined renal
function and were independently associated with clinical endpoints
of cardiovascular endpoints, infection and all-cause mortality in
patients with impaired renal function; however, not in participants
with normal kidney function (107).
In patients undergoing maintenance hemodialysis
(HD), serum galectin-3 levels were correlated with early
velocities/early diastolic mitral annular velocities (E'), left
atrial volume index, and E'. Further analysis demonstrated that
galectin-3 may be used in the diagnosis of LV diastolic function
with a sensitivity and specificity of 67.6 and 84.6%, respectively
(108). Serum galectin-3 levels
were also revealed to be associated with LV hypertrophy independent
of left ventricle geometry in patients with HD, as well as in the
general population (109). A
multicenter, prospective, observational study identified that an
increased galectin-3 level was a prognostic determinant of
all-cause mortality and cardiac mortality in patients with HD
(110). Another study confirmed
this finding, and the authors identified that >23.73 ng/ml
gelectin-3 was an independent predictor of mortality in patients
with HD (111).
Serum galectin levels were reported to be a
predictor of therapeutic response to renal denervation. In patients
with resistant hypertension undergoing renal sympathetic
denervation, baseline serum galectin levels were significantly
increased in responders compared with non-responders, and no
significant changes in galectin-3 levels were observed during the
follow-up period. These results indicated that galectin-3 may
assist in identifying patients suitable for renal denervation
(112).
Expression levels of galectin-3 were identified to
be upregulated in serum and tumor tissues of patients with RCC
(113–117). Males were significantly
associated with increased mRNA expression levels of galectin-1 and
-3 in clear cell RCC, indicating that galectins may be involved in
the pathogenesis of the higher prevalence of RCC in men (115). A marked overexpression of
galectin-3 was predominantly identified in renal tumors with
oncocytic features, including oncocytomas and chromophobe RCC with
positive immunohistochemical rates of 100 and 89%, respectively,
whereas significantly decreased expression levels of galecin-3 were
observed in clear cell and papillary RCCs (113). Furthermore, the combined use of
galectin-1 and -3 for RCC diagnosis demonstrated specificity and
sensitivity of 98 and 47%, respectively (114). These results indicated the
potential role of galectin-3 in the diagnosis of different types of
RCC (Table I).
Differentiating malignant lesions from benign
thyroid lesions is clinically challenging. Even the most accurate
method, thyroid fine needle aspiration biopsy, has only 70–90%
accuracy (118). Thus, as a
pro-angiogenic marker, the value of galectin-3 in the diagnosis of
thyroid carcinoma has been widely investigated (Table I). The latest meta-analysis
indicated that galectin-3 may be a potentially useful immuno-marker
to distinguish between patients with papillary thyroid carcinoma
(PTC) and patients without PTC. In addition, PTC patients with
positive expression of galectin-3 were prone to lymph node
metastasis (118). However,
there are controversial results regarding other types of thyroid
carcinoma.
Previous studies support the potential diagnostic
value of galectin-3 for malignant lesions. Manivannan et al
(119) demonstrated that
galectin-3 expression levels in tumor tissues differentiate benign
from malignant follicular neoplasms. Focal and diffuse positivity
for galectin-3 was associated with malignant thyroid follicular
neoplasms (119). Mataraci et
al (120) also identified
that the percentage and intensity of staining for galectin-3 were
increased in malignant lesions, particularly in papillary
carcinomas (120). Galectin
expression levels were significantly increased in malignant thyroid
neoplasms when compared with benign neoplasms. However, no
significant differences were identified when comparing galectin-3
expression levels in PTC and other malignant lesions. Previous
studies have demonstrated that there was no marked staining
intensity for intracytoplasmatic or intranuclear expression of
galectin-3 in benign thyroid neoplasms. Furthermore, there was a
lack of weak intensity for intracytoplasmatic or intranuclear
expression of galectin-3 in malignant neoplasms, therefore, diffuse
and strong staining for galectin-3 differentiates malignant from
benign thyroid neoplasms (121,122). The increased expression levels
of galectin-3 on Thin-Prep fine needle aspiration cytology were
associated with hypertrophy and/or cellular hyperproliferation,
pathophysiological situations that are common to adenomas and
thyroid carcinomas (123).
Overexpression of galectin-3 protein was observed in papillary
thyroid carcinoma with lymph node metastases (124). A previous study compared
glypican-3 (a member of the glypican family of heparan-sulfate
proteoglycans bound to the plasma membrane) with galectin-3 and
demonstrated that galectin-3 was more sensitive in diagnosing
thyroid carcinoma; however, it was less specific in discriminating
follicular-patterned neoplasm (125). Besides tissue expression, the
preoperative serum galectin-3 level had diagnostic value, as it was
significantly higher in the cancer patients than in the control
subjects (126).
Galectin-3 is also used in combination with other
biomarkers for a differential diagnosis of thyroid lesions. The
most commonly combined biomarkers are Hector Battifora mesothelial
epitope-1 (HBME-1) and cytokeratin-19 (127–130). Galectin-3 and HBME-1 may be used
as single discriminators between follicular thyroid adenoma and
carcinoma. Significant differences in galectin-3 and HBME-1 were
identified between benign and malignant lesions, and also between
the subgroups of benign and malignant lesions (127–130). Galectin-3 and HBME-1 have an
excellent sensitivity and specificity for malignant thyroid lesions
(100 and 89.1%, respectively) (129). Despite core needle biopsies
leading to the diagnosis of the majority of thyroid nodules, the
accuracy is increased by also observing the galectin-3,
cytokeratin-19 and HBME-1 panels, indicating their additional
diagnostic value when combined with routine histology (127–130). It was also reported that
galectin 3, cluster of differentiation (CD)44 and, to an extent,
HBME-1, are useful immunocytochemical parameters with the potential
to support the fine needle aspiration cytology diagnosis of PTC,
particularly in situations where the differential diagnoses is
complicated (131). The levels
of parafibromin and galectin-3 expression were significantly
increased among patients with parathyroid adenoma, atypical
parathyroid adenomas and parathyroid carcinoma, whereas HBME-1
expression levels were not (132). The level of parafibromin
expression was increased, whereas galectin-3 expression was
decreased in arathyroid adenoma (132). Parafibromin expression,
galectin-3 negativity, and a Ki-67 proliferation index <1% were
identified to be beneficial in the differential diagnosis of
parathyroid tumors (132).
Another study reported that the loss of parafibromin and
overexpression of galectin-3 and Ki-67 may assist in distinguishing
parathyroid carcinoma from other types of parathyroid tumor
(133). The combination of two
or three of these markers may produce improved sensitivity and/or
specificity for the diagnosis of parathyroid carcinoma (133). Galectin-3 and Bcl-2 exhibited a
similar trend of downregulation from high levels in PTC to low
levels in anaplastic thyroid carcinoma. During thyroid tumor
progression from PTC to anaplastic thyroid carcinoma,
downregulation of galectin-3 and Bcl-2 (antiapoptotic molecules)
and a stepwise increase in survivin (inhibitor of apoptosis) were
observed (134).
Contrastingly, certain studies indicated that
galectin-3 is not a potential diagnostic or prognostic biomarker
(135–138). Studies demonstrated that
galectin-3 expression levels in tumor tissues were not
significantly associated with age, sex, extrathyroidal extension,
lymph node metastasis, or total metastasis, age, completeness of
resection, invasion or size score category and cannot be used for
prognostic prediction (135). In
thyroid tumors of uncertain malignant potential, sytokeratin-19,
galectin-3, HBME-1, and CD56 stained negatively in the majority of
cases (90.3, 83.9, 87.1 and 61%, respectively), and no
statistically significant differences were observed when compared
with the immunoprofile of benign thyroid lesions (136). In another study assessing the
efficacy of cytokeratin 19, thyroperoxidase, HBME-1 and galectin-3
in the evaluation of the aggressiveness of PTC, increased levels of
cytokeratin 19 expression and negative thyroperoxidase expression
indicated the total tumor diameter of PTC; however, the expression
levels of cytokeratin 19, thyroperoxidase, HBME-1, and galectin-3
did not contribute to the identification of PTCs with other
potentially aggressive behaviors (137). In papillary microcarcinoma, the
level of galectin-3 expression exhibited no significant association
with prognostic factors, including extrathyroidal extension and
lymph node metastasis (138).
Galectin-3 promotes tumor progression in HCC. In
mice bearing HCC, the galectin-3 expression level in tumor tissue
was significantly increased, while serum galectin-3 levels also
demonstrated obvious changes (139). In an
N-diethylnitrosamine-induced HCC mouse model, galectin-3 knockout
mice developed a significantly smaller tumor burden with a less
invasive phenotype compared with the wild-type animals. Galectin-3
was upregulated in the wild-type HCC tumor tissue; however, not in
the surrounding parenchyma. In vitro studies demonstrated
that the migration of hepatoma cells was significantly decreased
and the reorganization of the actin cytoskeleton, RhoA GTPase
activity and phosphorylation of myosin light chain 2 were decreased
in the galectin-3 small interfering RNA-transfected cells.
Furthermore, in vitro and in vivo evidence
demonstrated that galectin-3 deficiency reduced hepatoma cell
proliferation and increased apoptosis among these cells. These
results indicated that galectin-3 promotes hepatoma cell motility
and invasion via an autocrine signaling pathway (140). Serum galectin-3 levels were
decreased in chronic hepatitis B or C patients when compared with
patients with HCC and cirrhosis; however, there were no significant
differences identified between patients with HCC and patients with
cirrhosis (141). Another study
confirmed that there was no difference in serum galectin-3 levels
in HCC and cirrhotic patients (142). HCC patients with metastatic
spread and poor prognosis suffering from portal vein invasion
exhibited elevated serum galectin-3 levels (142). Expression levels of galectin-3
in HCC tissues were significantly increased and were associated
with a poor prognosis. Furthermore, galectin-3 expression levels in
tumor cells stimulated angiogenesis (143). Collectively, these results
indicate that galectin-3 may be used for prognosis, but not for the
diagnosis of patients with HCC.
At present, results regarding the serum levels and
tissue expression of galectin-3 in patients with prostate cancer
are controversial. In patients with metastatic prostate cancer,
serum galectin-3 levels were uniformly higher compared with those
in control subjects without cancer (144). Furthermore, serum galectin-3
levels were positively associated with prostate specific antigen in
prostate cancer patients, particularly at early clinical time
course (145). Galectin-3
expression levels in prostate tissue were increased in benign
prostatic hyperplasia compared with normal tissue samples and
markedly lower in adenocarcinoma (146). Galectin-3 demonstrated nuclear
and cytoplasmic localization in benign, adjacent-benign and tumor
tissues, with a decreasing gradient of galectin-3 expression levels
observed in benign, adjacent-benign and tumor tissue samples
(147).
Tissue expression and serum levels of galectin-3
were significantly higher in pancreatic carcinoma tissues or
patients with pancreatic carcinoma when compared with the adjacent
non-tumorous tissues or in benign pancreatic diseases and healthy
individuals (148). Higher
galectin-3 expression levels in tissues were associated with poor
differentiation tissues (148).
Serum galectin-3 was not associated with carcinoembryonic antigen
and CA19-9; however, a combination of these three markers may
increase the diagnostic sensitivity of pancreatic carcinoma
diagnosis to 97.5% (148).
Furthermore, galectin-3 was differentially expressed in different
pancreatic carcinoma tissues (149). Furthermore, there was an
increased expression in pancreatic ductal adenocarcinoma; however,
not in pancreatic neuroendocrine neoplasms and gastrointestinal
stromal tumors. Thus, galectin-3 may be used to help diagnose
pancreatic ductal adenocarcinoma and rule out pancreatic
neuroendocrine neoplasms and gastrointestinal stromal tumors
(149).
The serum level of galectin-3 was increased
11.3-fold in patients with colorectal cancer and markedly increased
31-fold in those with metastases (150). Simultaneous determination of
serum galectin-3 and -4 levels demonstrated an increased
specificity and sensitivity in distinguishing patients with
colorectal cancer without metastases from those with liver
metastases. Furthermore, increased serum galectin-3/-4 expression
levels at the time of primary tumor removal in patients without
clinically detectable metastases were associated with poorer
survival rates over the next 10 years (151). Increased serum galectin-3
expression levels were observed in patients with colon cancer when
compared in patients with rectal cancer (152). No association was identified
between tissue galectin-3 expression levels and clinicopathological
parameters of patients with colorectal cancer, whereas the level of
tissue galectin-3 expression was positively associated with serum
IL-17 and IL-23. Serum galectin-3 levels were significantly
associated with IL-17, but not IL-23 in patients with colorectal
cancer (153). Shimura et
al (152) also confirmed the
serum galectin-3 levels was associated with IL-17 production.
Furthermore, it was also inversely associated with the production
of IL-10 and IL-12 (152). These
results indicated that galectin-3 may be a key factor in the
regulation of tumor-associated inflammatory processes.
Expression levels of galectin-3 protein were
significantly greater in the breast tumor tissues compared with the
paracancerous tissue, and galectin-3 was markedly expressed in
triple-negative breast cancers when compared with other types of
breast cancer (154,155). Furthermore, the expression
levels of galectin-3 were not identified as an independent
prognostic factor for breast cancer, but were associated with
chemotherapeutic resistance (154). In addition, the serum galectin-3
level was significantly increased in breast cancer patients
compared with healthy control subjects (150). However, two previous studies
demonstrated that low galectin-3 expression levels in breast cancer
were significantly associated with increased tumor vascular
invasion, and reduced disease-free survival and long-term overall
survival (156,157). Furthermore, in vitro
breast cancer stem cell models demonstrated that galectin-3
knockdown led to epithelial-mesenchymal transition, increased
sphere-formation ability, drug-resistance and increased aldefluor
activity. In addition, in vivo orthotopic mouse models
demonstrated that galectin-3-negative breast cancer stem cells were
associated with enhanced tumorigenicity. These results indicated
that loss of galectin-3 may be associated with
epithelial-mesenchymal transition and cancer stemness-associated
traits, and therefore, may predict poor response to chemotherapy
and poor prognosis (157).
The serum and tissue expression levels of galectin-3
were statistically increased in patients with bladder cancer
compared with control and cystitis groups. Serum galectin-3 levels
were increased in patients with transitional cell carcinoma
compared with squamous cell carcinoma. Patients with high-grade
transitional cell carcinoma had a significantly increased serum
galectin level compared with those with low-grade tumors, as did
those with muscle-invasive transitional cell carcinoma compared
with papillary non-invasive tumors (158,159). These results indicated that
galectin-3 may be a potential diagnostic biomarker for bladder
cancer.
Serum levels of galectin-3 in patients with gastric
cancer were significantly increased compared with those in benign
disease patients and healthy control subjects, and the serum
galectin-3 level was associated with lymph node metastasis and
distant metastasis (160).
Tissue galectin-3 expression was reduced by 1.5-fold in 50% of
gastric tumors. The reduced galectin-3 expression level was
associated with the presence of distant metastasis, and with a
higher invasive phenotype in vitro (161). In contrast, it was previously
reported that baseline serum galectin-3 levels exhibited no
significant difference between patients with gastric cancer and
healthy control subjects. Serum galectin-3 levels were not
associated with the stage of disease or chemotherapy responsiveness
(162). However, these studies
confirmed that the galectin-3 expression level had no association
with overall survival rates, and was not a reliable biomarker for
determining prognosis in gastric adenocarcinoma (160,162,163).
Serum galectin-3 levels were significantly higher in
patients with non-acute promyelocytic leukemia compared with the
control group subjects. Patients with higher galectin-3 expression
levels had lower complete remission rates and a significantly
shorter overall survival. An increased galectin-3 expression level
was an independent poor prognostic marker (164). Furthermore, serum galectin-3
levels in patients with non-Hodgkin's lymphoma were associated with
cardiovascular events and were significantly increased in patients
with cardiovascular events compared with patients without a
cardiovascular event. Overall, increased serum galectin was
associated with increased 12-month cumulative cardiovascular events
(165).
High expression levels of galectin-3 in endometrial
tumor tissues were independently associated with tumor depth and
histological grade, and were associated with shorter survival rates
(166). The serum and tissue
expression levels of galectin-3 were significantly increased in
patients with osteosarcoma compared with control subjects.
Increased galectin-3 expression levels in serum or tumors was
associated with the Enneking stage of cancer (167). Furthermore, an increased tumor
expression level was associated with the occurrence of metastasis
(167). In oral squamous cell
carcinoma, serum and tissue expression levels of galectin-3 were
significantly increased compared with those in control subjects.
Patients with an increased tumor load exhibited an increased
expression of galectin-3 compared with those with a decreased tumor
load. Furthermore, exogenous galectin-1 treatment significantly
increased survival, proliferation and angiogenesis in oral squamous
cell carcinoma cell lines (168). Galectin-3 was expressed in
prolactin and adrenocorticotropic hormone-producing pituitary
adenomas tumors with high positivity (91.6 and 90.9%,
respectively), and an increased expression level was a strong
predictive marker for recurrence/tumor progression (169). In the gallbladder, the positive
expression rates of galectin-3 were increased in adenocarcinoma
compared with peritumoral tissues, polyps and chronic
cholecystitis. An increased expression level of galectin-3 was
associated with decreased overall survival and was an independent
prognostic marker in gallbladder adenocarcinoma (170). These results indicate that
expression levels of galectin-3 and the Sambucus nigra
agglutinin binding site may have important effects on the
carcinogenesis, progression and biological behaviors of gallbladder
cancer (170).
Results regarding abnormal expression levels of
galectin-3 in cancer have been summarized in Table II. Collectively, these studies
reveal that galectin-3 is expressed abnormally in many types of
cancer, which indicates that galectin-3 is not a tumor-specific
biomarker. Thus, galectin-3 will be effective when applied in
combination with other specific biomarkers.
As galecin-3 has been demonstrated to perform
numerous functions in the pathogenesis of diseases outlined
previously, and may serve as a therapeutic target for these
diseases, its potential clinical applications have been evaluated
using cell and animal models (Table
III). RN1, a polysaccharide that binds to galectin-3 and
suppresses its expression, significantly inhibited growth of
pancreatic ductal adenocarcinoma cells in vitro, in
vivo and in patient-derived xenografts (171). Galectin-3 targeted
N-(2-hydroxypropyl) methacrylamide
copolymer-(G3-C12)-5-fluorouracil conjugates significantly improved
the anti-tumor activity of fluorouracil in nude mice bearing PC-3,
a prostate tumor xenograft (172). TFD100, a glycopeptide from cod
binds galectin-3 with picomolar affinity and blocks
galectin-3-mediated angiogenesis, tumor-endothelial cell
interactions and metastasis of prostate cancer cells in mice
(173). Galectin-3C, a
truncated, dominant negative form of galectin-3, hypothesized to
act by blocking endogenous galectin-3, significantly reduced the
growth, motility, invasion, and angiogenic potential of cultured
ovarian cancer cell lines and primary cells established from
ovarian cancer patients (174).
Modified citrus pectin, a galectin inhibitor, blocked
aldosterone-induced cardiac and renal fibrosis, and improved cardio
renal dysfunction in experimental hyperaldosteronism (175). In addition, modified citrus
pectin prevented isoproterenol-induced LV dysfunction and fibrosis
in mice with HF and cardiac-specific hyperaldosteronism, inhibited
adipose tissue inflammation and decreased the differentiation
degree of adipocytes in obese mice, as well as reducing plaque
progression in an atherosclerosis mouse model (176–178). Furthermore, N-acetyllactosamine,
a galectin-3 inhibitor, reduced proteinuria, improved renal
function and decreased renal damage in mice with hypertensive
nephropathy and HF (177).
Galectin-3-antagonists and a selective galactose-coumarin-derived
galectin-3 inhibitor attenuated bleomycin-induced pulmonary
fibrosis in mouse models (179,180). GR-MD-02, a complex
carbohydrate-based drug that binds to galectin-3, improved liver
histology with significant reductions in non-alcoholic
steatohepatitis (NASH) activity and collagen deposition, and
reduced fibrosis in NASH mice with fibrosis (181). Furthermore, GR-MD-02 has been
evaluated in a phase I clinical trial in participants with NASH and
advanced fibrosis to determine its safety, pharmacokinetics and
exploratory pharmacodynamic markers. GR-MD-02 doses were in the
upper range (8 mg/kg) of the targeted therapeutic dose determined
from pre-clinical data, and were safe and well tolerated with
evidence of a pharmacodynamic effect, which therefore provided
support for a phase II clinical trial (182).
Galectin-3 is a multifunctional protein involved in
various types of disease. Its prognostic value in predicting the
outcomes of HF and its diagnostic value in thyroid carcinoma
diagnosis have been extensively investigated, and indicate its
potential application for HF prognosis and thyroid carcinoma
diagnosis (Table I). The
development of detection methods of galectin-3 is required to
improve the sensitivity, accuracy and consensus between different
laboratories, and to provide supports for its clinical utility. In
addition, normal reference ranges need to be established.
The functions of galectin-3 in fibrosis and
immunity have also been extensively investigated, indicating its
possible therapeutic utility in certain types of fibrotic disease
and infection. In all cases, fibrotic disease progress to severe
disease, including HF, renal failure and cirrhosis. Galectin-3 may
represent a therapeutic approach to delay the progression of these
diseases. Further animal studies and clinical trials are required
to develop novel drugs targeting galectin-3.
The preset study was supported by the National
Natural Science Foundation of China (grant nos. 81300517 and
81560668), Xizang Minzu University Fund (grant no. xzmyzp02) and
the Shanghai Rising-Star Program (A type; grant no. 15QA1400800).
Andrew Soh and Yijie Zheng are employees of Abbott Diagnostics. The
other authors declare no conflicts of interest.
|
1
|
Argüeso P and Panjwani N: Focus on
molecules: Galectin-3. Exp Eye Res. 92:2–3. 2011. View Article : Google Scholar :
|
|
2
|
Saccon F, Gatto M, Ghirardello A,
Iaccarino L, Punzi L and Doria A: Role of galectin-3 in autoimmune
and non-autoimmune nephropathies. Autoimmun Rev. 16:34–47. 2017.
View Article : Google Scholar
|
|
3
|
Newlaczyl AU and Yu LG: Galectin-3-a
jack-of-all-trades in cancer. Cancer Lett. 313:123–128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gong HC, Honjo Y, Nangia-Makker P, Hogan
V, Mazurak N, Bresalier RS and Raz A: The NH2 terminus of
galectin-3 governs cellular compartmentalization and functions in
cancer cells. Cancer Res. 59:6239–6245. 1999.
|
|
5
|
Menon RP and Hughes RC: Determinants in
the N-terminal domains of galectin-3 for secretion by a novel
pathway circumventing the endoplasmic reticulum-Golgi complex. Eur
J Biochem. 264:569–576. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Oliveira FL, Gatto M, Bassi N, Luisetto
R, Ghirardello A, Punzi L and Doria A: Galectin-3 in autoimmunity
and autoimmune diseases. Exp Biol Med (Maywood). 240:1019–1028.
2015. View Article : Google Scholar
|
|
7
|
Pugliese G, Iacobini C, Ricci C, Blasetti
Fantauzzi C and Menini S: Galectin-3 in diabetic patients. Clin
Chem Lab Med. 52:1413–1423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dumic J, Dabelic S and Flögel M:
Galectin-3: An open-ended story. Biochim Biophys Acta.
1760:616–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colnot C, Fowlis D, Ripoche MA, Bouchaert
I and Poirier F: Embryonic implantation in galectin 1/galectin 3
double mutant mice. Dev Dyn. 211:306–313. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SJ, Lee HW, Gu Kang H, La SH, Choi IJ,
Ro JY, Bresalier RS, Song J and Chun KH: Ablation of galectin-3
induces 27 (KIP1)-dependent premature senescence without oncogenic
stress. Cell Death Differ. 21:1769–1779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen SC and Kuo PL: The role of galectin-3
in the kidneys. Int J Mol Sci. 17:5652016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ruvolo PP: Galectin 3 as a guardian of the
tumor microenvironment. Biochim Biophys Acta. 1863:427–437. 2016.
View Article : Google Scholar
|
|
13
|
Meijers WC, Lopez-Andrés N and de Boer RA:
Galectin-3, cardiac function, and fibrosis. Am J Pathol.
186:2232–2234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Y, Yéléhé-Okouma M, Ea HK, Jouzeau JY
and Reboul P: Galectin-3: A key player in arthritis. Joint Bone
Spine. 84:15–20. 2017. View Article : Google Scholar
|
|
15
|
Meijers WC, van der Velde AR,
Pascual-Figal DA and de Boer RA: Galectin-3 and post-myocardial
infarction cardiac remodeling. Eur J Pharmacol. 763:115–121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lala RI, Puschita M, Darabantiu D and
Pilat L: Galectin-3 in heart failure pathology-'another brick in
the wall'. Acta Cardiol. 70:323–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Funasaka T, Raz A and Nangia-Makker P:
Galectin-3 in angiogenesis and metastasis. Glycobiology.
24:886–891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shin T: The pleiotropic effects of
galectin-3 in neuroinflammation: A review. Acta Histochem.
115:407–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meeusen JW, Johnson JN, Gray A, Wendt P,
Jefferies JL, Jaffe AS, Donato LJ and Saenger AK: Soluble ST2 and
galectin-3 in pediatric patients without heart failure. Clin
Biochem. 48:1337–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schindler EI, Szymanski JJ, Hock KG,
Geltman EM and Scott MG: Short- and long-term biologic variability
of galectin-3 and other cardiac biomarkers in patients with Stable
heart failure and healthy adults. Clin Chem. 62:360–366. 2016.
View Article : Google Scholar
|
|
21
|
Issa SF, Christensen AF, Lottenburger T,
Junker K, Lindegaard H, Hørslev-Petersen K and Junker P: Within-day
variation and influence of physical exercise on circulating
Galectin-3 in patients with rheumatoid arthritis and healthy
individuals. Scand J Immunol. 82:70–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen A, Hou W, Zhang Y, Chen Y and He B:
Prognostic value of serum galectin-3 in patients with heart
failure: A meta-analysis. Int J Cardiol. 182:168–170. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Srivatsan V, George M and Shanmugam E:
Utility of galectin-3 as a prognostic biomarker in heart failure:
Where do we stand. Eur J Prev Cardiol. 22:1096–1110. 2015.
View Article : Google Scholar
|
|
24
|
Medvedeva EA, Berezin II, Surkova EA,
Yaranov DM and Shchukin YV: Galectin-3 in patients with chronic
heart failure: Association with oxidative stress, inflammation,
renal dysfunction and prognosis. Minerva Cardioangiol. 64:595–602.
2016.PubMed/NCBI
|
|
25
|
Yu X, Sun Y, Zhao Y, Zhang W, Yang Z, Gao
Y, Cai H, Li Y, Wang Q, Bian B and Nie J: Prognostic value of
plasma galectin-3 levels in patients with coronary heart disease
and chronic heart failure. Int Heart J. 56:314–318. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coromilas E, Que-Xu EC, Moore D, Kato TS,
Wu C, Ji R, Givens R, Jorde UP, Takayama H, Naka Y, et al: Dynamics
and prognostic role of galectin-3 in patients with advanced heart
failure, during left ventricular assist device support and
following heart transplantation. BM. Cardiovasc Disord. 16:1382016.
View Article : Google Scholar
|
|
27
|
Meijers WC, Januzzi JL, deFilippi C,
Adourian AS, Shah SJ, van Veldhuisen DJ and de Boer RA: Elevated
plasma galectin-3 is associated with near-term rehospitalization in
heart failure: A pooled analysis of 3 clinical trials. Am Heart J.
167:853–860.e4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kortekaas KA, Hoogslag GE, de Boer RA,
Dokter MM, Versteegh MI, Braun J, Marsan NA, Verwey HF, Delgado V,
Schalij MJ and Klautz RJ: Galectin-3 and left ventricular reverse
remodelling after surgical mitral valve repair. Eur J Heart Fail.
15:1011–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Motiwala SR, Szymonifka J, Belcher A,
Weiner RB, Baggish AL, Sluss P, Gaggin HK, Bhardwaj A and Januzzi
JL: Serial measurement of galectin-3 in patients with chronic heart
failure: Results from the ProBNP outpatient tailored chronic heart
failure therapy (PROTECT) study. Eur J Heart Fail. 15:1157–1163.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koukoui F, Desmoulin F, Galinier M,
Barutaut M, Caubère C, Evaristi MF, Murat G, De Boer R, Berry M,
Smih F and Rouet P: The prognostic value of plasma galectin-3 in
chronic heart failure patients is maintained when treated with
mineralocorticoid receptor antagonists. PLoS One. 10:e01191602015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Edelmann F, Holzendorf V, Wachter R, Nolte
K, Schmidt AG, Kraigher-Krainer E, Duvinage A, Unkelbach I, Düngen
HD, Tschöpe C, et al: Galectin-3 in patients with heart failure
with preserved ejection fraction: Results from the Aldo-DHF trial.
Eur J Heart Fail. 17:214–223. 2015. View Article : Google Scholar
|
|
32
|
Teixeira A, Arrigo M, Vergaro G,
Cohen-Solal A and Mebazaa A: Clinical benefits of natriuretic
peptides and galectin-3 are maintained in old dyspnoeic patients.
Arch Gerontol Geriatr. 68:33–38. 2017. View Article : Google Scholar
|
|
33
|
McEvoy JW, Chen Y, Halushka MK,
Christenson E, Ballantyne CM, Blumenthal RS, Christenson RH and
Selvin E: Galectin-3 and risk of heart failure and death in blacks
and whites. J Am Heart Assoc. 5(pii): e0030792016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anand IS, Rector TS, Kuskowski M, Adourian
A, Muntendam P and Cohn JN: Baseline and serial measurements of
galectin-3 in patients with heart failure: Relationship to
prognosis and effect of treatment with valsartan in the Val-HeFT.
Eur J Heart Fail. 15:511–518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van der Velde AR, Gullestad L, Ueland T,
Aukrust P, Guo Y, Adourian A, Muntendam P, van Veldhuisen DJ and de
Boer RA: Prognostic value of changes in galectin-3 levels over time
in patients with heart failure: Data from CORONA and COACH. Circ
Heart Fail. 6:219–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piper SE, de Courcey J, Sherwood RA,
Amin-Youssef GF and McDonagh TA: Serial galectin-3 for the
monitoring of optimally treated stable chronic heart failure: A
pilot study. Int J Cardiol. 207:279–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miller WL, Saenger AK, Grill DE, Slusser
JP, Bayes-Genis A and Jaffe AS: Prognostic value of serial
measurements of soluble suppression of tumorigenicity 2 and
Galectin-3 in ambulatory patients with chronic heart failure. J
Card Fail. 22:249–255. 2016. View Article : Google Scholar
|
|
38
|
Lala RI, Darabantiu D, Pilat L and
Puschita M: Galectin-3: A link between myocardial and arterial
stiffening in patients with acute decompensated heart failure. Arq
Bras Cardiol. 106:121–129. 2016.In English, Portuguese. PubMed/NCBI
|
|
39
|
Beltrami M, Ruocco G, Dastidar AG, Franci
B, Lucani B, Aloia E, Nuti R and Palazzuoli A: Additional value of
Galectin-3 to BNP in acute heart failure patients with preserved
ejection fraction. Clin Chim Acta. 457:99–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Atabakhshian R, Kazerouni F, Raygan F,
Amirrasouli H, Rahimipour A and Shakeri N: Assessment of the
relationship between galectin-3 and ejection fraction and
functional capacity in the patients with compensated systolic heart
failure. Int Cardiovasc Res J. 8:143–147. 2014.
|
|
41
|
Carrasco-Sánchez FJ, Aramburu-Bodas O,
Salamanca-Bautista P, Morales-Rull JL, Galisteo-Almeda L,
Páez-Rubio MI, Arias-Jiménez JL, Aguayo-Canela M and Pérez-Calvo
JI: Predictive value of serum galectin-3 levels in patients with
acute heart failure with preserved ejection fraction. Int J
Cardiol. 169:177–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stoltze Gaborit F, Bosselmann H, Kistorp
C, Iversen K, Kumler T, Gustafsson F, Goetze JP, Sölétormos G,
Tønder N and Schou M: Galectin 3: Association to neurohumoral
activity, echocardiographic parameters and renal function in
outpatients with heart failure. BMC Cardiovasc Disord. 16:1172016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Iacoviello M, Aspromonte N, Leone M,
Paradies V, Antoncecchi V, Valle R, Caldarola P, Ciccone MM,
Gesualdo L and Serio FD: Galectin-3 Serum levels are independently
associated with microalbuminuria in chronic heart failure
outpatients. Res Cardiovasc Med. 5:e289522015. View Article : Google Scholar
|
|
44
|
Zhang R, Zhang Y, An T, Guo X, Yin S, Wang
Y, Januzzi JL, Cappola TP and Zhang J: Prognostic value of sST2 and
galectin-3 for death relative to renal function in patients
hospitalized for heart failure. Biomark Med. 9:433–441. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
AbouEzzeddine OF, Haines P, Stevens S,
Nativi-Nicolau J, Felker GM, Borlaug BA, Chen HH, Tracy RP,
Braunwald E and Redfield MM: Galectin-3 in heart failure with
preserved ejection fraction. A RELAX trial substudy
(Phosphodiesterase-5 inhibition to improve clinical status and
exercise capacity in diastolic heart failure). JACC Heart Fail.
3:245–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zamora E, Lupón J, de Antonio M, Galán A,
Domingo M, Urrutia A, Troya M and Bayes-Genis A: Renal function
largely influences Galectin-3 prognostic value in heart failure.
Int J Cardiol. 177:171–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
French B, Wang L, Ky B, Brandimarto J,
Basuray A, Fang JC, Sweitzer NK and Cappola TP: Prognostic value of
galectin-3 for adverse outcomes in chronic heart failure. J Card
Fail. 22:256–262. 2016. View Article : Google Scholar :
|
|
48
|
Yin QS, Shi B, Dong L and Bi L:
Comparative study of galectin-3 and B-type natriuretic peptide as
biomarkers for the diagnosis of heart failure. J Geriatr Cardiol.
11:79–82. 2014.PubMed/NCBI
|
|
49
|
Chen K, Jiang RJ, Wang CQ, Yin ZF, Fan YQ,
Cao JT, Han ZH, Wang Y and Song DQ: Predictive value of plasma
galectin-3 in patients with chronic heart failure. Eur Rev Med
Pharmacol Sci. 17:1005–1011. 2013.PubMed/NCBI
|
|
50
|
Gruson D, Ferracin B, Ahn SA and Rousseau
MF: Comparison of fibroblast growth factor 23, soluble ST2 and
Galectin-3 for prognostication of cardiovascular death in heart
failure patients. Int J Cardiol. 189:185–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chang YY, Chen A, Wu XM, Hsu TP, Liu LY,
Chen YH, Wu YW, Lin HJ, Hsu RB, Lee CM, et al: Comparison the
prognostic value of galectin-3 and serum markers of cardiac
extracellular matrix turnover in patients with chronic systolic
heart failure. Int J Med Sci. 11:1098–1106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bayes-Genis A, de Antonio M, Vila J,
Peñafiel J, Galán A, Barallat J, Zamora E, Urrutia A and Lupón J:
Head-to-head comparison of 2 myocardial fibrosis biomarkers for
long-term heart failure risk stratification: ST2 versus galectin-3.
J Am Coll Cardiol. 63:158–166. 2014. View Article : Google Scholar
|
|
53
|
Wu AH, Wians F and Jaffe A: Biological
variation of galectin-3 and soluble ST2 for chronic heart failure:
Implication on interpretation of test results. Am Heart J.
165:995–999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Feola M, Testa M, Leto L, Cardone M, Sola
M and Rosso GL: Role of galectin-3 and plasma B type-natriuretic
peptide in predicting prognosis in discharged chronic heart failure
patients. Medicine (Baltimore). 95:e40142016. View Article : Google Scholar
|
|
55
|
Zhang Y, Zhang R, An T, Huang Y, Guo X,
Yin S, Wang Y, Ji S, Lv R, Zhang J and Maisel A: The utility of
galectin-3 for predicting cause-specific death in hospitalized
patients with heart failure. J Card Fail. 21:51–59. 2015.
View Article : Google Scholar
|
|
56
|
Wang CH, Yang I, Liu MH, Hsu KH and Kuo
LT: Estimating systemic fibrosis by combining galectin-3 and ST2
provides powerful risk stratification value for patients after
acute decompensated heart failure. Cardiol J. 23:563–572.
2016.PubMed/NCBI
|
|
57
|
Zhang X, Wan Y, Chata R, Brazzale A,
Atherton JJ, Kostner K, Dimeski G and Punyadeera C: A pilot study
to demonstrate diagnostic potential of galectin-3 levels in saliva.
J Clin Pathol. 69:1100–1104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Polat V, Bozcali E, Uygun T, Opan S and
Karakaya O: Diagnostic significance of serum galectin-3 levels in
heart failure with preserved ejection fraction. Acta Cardiol.
71:191–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhou K, Zhou Y, Zhao Y, Tan C, Yuan Z, Li
J, Liao X, Gu L and Zhou X: The relationship between Galectin-3 and
different patterns of ventricular geometry remodelling in aortic
valve stenosis. Heart Lung Circ. 25:371–377. 2016. View Article : Google Scholar
|
|
60
|
Sadaba JR, Martinez-Martinez E, Arrieta V,
Álvarez V, Fernández-Celis A, Ibarrola J, Melero A, Rossignol P,
Cachofeiro V and López-Andrés N: Role for Galectin-3 in calcific
aortic valve stenosis. J Am Heart Assoc. 5:pp. e0043602016,
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Arangalage D, Nguyen V, Robert T,
Melissopoulou M, Mathieu T, Estellat C, Codogno I, Huart V, Duval
X, Cimadevilla C, et al: Determinants and prognostic value of
Galectin-3 in patients with aortic valve stenosis. Heart.
102:862–868. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Maiolino G, Rossitto G, Pedon L, Cesari M,
Frigo AC, Azzolini M, Plebani M and Rossi GP: Galectin-3 predicts
long-term cardiovascular death in high-risk patients with coronary
artery disease. Arterioscler Thromb Vasc Biol. 35:725–732. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tuñón J, Blanco-Colio L, Cristóbal C,
Tarín N, Higueras J, Huelmos A, Alonso J, Egido J, Asensio D,
Lorenzo Ó, et al: Usefulness of a combination of monocyte
chemoattractant protein-1, galectin-3, and N-terminal probrain
natriuretic peptide to predict cardiovascular events in patients
with coronary artery disease. Am J Cardiol. 113:434–440. 2014.
View Article : Google Scholar
|
|
64
|
Jansen H, Koenig W, Jaensch A, Mons U,
Breitling LP, Scharnagl H, Stojakovic T, Schunkert H, Brenner H and
Rothenbacher D: Prognostic Utility of Galectin-3 for recurrent
cardiovascular events during Long-term follow-up in patients with
stable coronary heart disease: Results of the KAROLA study. Clin
Chem. 62:1372–1379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Aksan G, Gedikli Ö, Keskin K, Nar G, İnci
S, Yıldız SS, Kaplan Ö, Soylu K, Kılıçkesmez KO and Şahin M: Is
galectin-3 a biomarker, a player-or both-in the presence of
coronary atherosclerosis. J Investig Med. 64:764–770. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lepojarvi ES, Piira OP, Pääkkö E,
Lammentausta E, Risteli J, Miettinen JA, Perkiömäki JS, Huikuri HV
and Junttila MJ: Serum PINP, PIIINP, galectin-3, and ST2 as
surrogates of myocardial fibrosis and echocardiographic left
venticular diastolic filling properties. Front Physiol. 6:2002015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gucuk Ipek E, Akin Suljevic S, Kafes H,
Basyigit F, Karalok N, Guray Y, Dinc Asarcikli L, Acar B and
Demirel H: Evaluation of galectin-3 levels in acute coronary
syndrome. Ann Cardiol Angeiol (Paris). 65:26–30. 2016. View Article : Google Scholar
|
|
68
|
George M, Shanmugam E, Srivatsan V,
Vasanth K, Ramraj B, Rajaram M, Jena A, Sridhar A, Chaudhury M and
Kaliappan I: Value of pentraxin-3 and galectin-3 in acute coronary
syndrome: A short-term prospective cohort study. Ther Adv
Cardiovasc Dis. 9:275–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Di Tano G, Caretta G, De Maria R, Parolini
M, Bassi L, Testa S and Pirelli S: Galectin-3 predicts left
ventricular remodelling after anterior-wall myocardial infarction
treated by primary percutaneous coronary intervention. Heart.
103:71–77. 2017. View Article : Google Scholar
|
|
70
|
Szadkowska I, Wlazeł RN, Migała M,
Szadkowski K, Zielińska M, Paradowski M and Pawlicki L: The
association between galectin-3 and clinical parameters in patients
with first acute myocardial infarction treated with primary
percutaneous coronary angioplasty. Cardiol J. 20:577–582. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tsai TH, Sung PH, Chang LT, Sun CK, Yeh
KH, Chung SY, Chua S, Chen YL, Wu CJ, Chang HW, et al: Value and
level of galectin-3 in acute myocardial infarction patients
undergoing primary percutaneous coronary intervention. J
Atheroscler Thromb. 19:1073–1082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Szadkowska I, Wlazel RN, Migala M,
Bajon-Laskowska K, Szadkowski K, Zielińska M, Paradowski M and
Pawlicki L: The association between galectin-3 and occurrence of
reinfarction early after first myocardial infarction treated
invasively. Biomarkers. 18:655–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Perea RJ, Morales-Ruiz M, Ortiz-Perez JT,
Bosch X, Andreu D, Borras R, Acosta J, Penela D, Prat-González S,
de Caralt TM, et al: Utility of galectin-3 in predicting
post-infarct remodeling after acute myocardial infarction based on
extracellular volume fraction mapping. Int J Cardiol. 223:458–464.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
van der Velde AR, Lexis CP, Meijers WC,
van der Horst IC, Lipsic E, Dokter MM, van Veldhuisen DJ, van der
Harst P and de Boer RA: Galectin-3 and sST2 in prediction of left
ventricular ejection fraction after myocardial infarction. Clin
Chim Acta. 452:50–57. 2016. View Article : Google Scholar
|
|
75
|
Milner TD, Viner AC, MacKinnon AC, Sethi T
and Flapan AD: Temporal expression of galectin-3 following
myocardial infarction. Acta Cardiol. 69:595–602. 2014. View Article : Google Scholar
|
|
76
|
Alturfan AA, Basar I, Emekli-Alturfan E,
Ayan F, Koldas L and Emekli N: Galectin-3 and plasma cytokines in
patients with acute myocardial infarction. Lab Med. 45:336–341.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Singsaas EG, Manhenke CA, Dickstein K and
Orn S: Circulating Galectin-3 levels are increased in patients with
ischemic heart disease, but are not influenced by acute myocardial
infarction. Cardiology. 134:398–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Weir RA, Petrie CJ, Murphy CA, Clements S,
Steedman T, Miller AM, McInnes IB, Squire IB, Ng LL, Dargie HJ and
McMurray JJ: Galectin-3 and cardiac function in survivors of acute
myocardial infarction. Circ Heart Fail. 6:492–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Takemoto Y, Ramirez RJ, Yokokawa M, Kaur
K, Ponce-Balbuena D, Sinno MC, Willis BC, Ghanbari H, Ennis SR,
Guerrero-Serna G, et al: Galectin-3 regulates atrial fibrillation
remodeling and predicts catheter ablation outcomes. JACC Basic
Transl Sci. 1:143–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gurses KM, Yalcin MU, Kocyigit D, Canpinar
H, Evranos B, Yorgun H, Sahiner ML, Kaya EB, Ozer N, Tokgozoglu L,
et al: Effects of persistent atrial fibrillation on serum
galectin-3 levels. Am J Cardiol. 115:647–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Clementy N, Benhenda N, Piver E, Pierre B,
Bernard A, Fauchier L, Pages JC and Babuty D: Serum galectin-3
levels predict recurrences after ablation of atrial fibrillation.
Sci Rep. 6:343572016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li Wu XY, Wen SN, Nie SN, Deng JG, Bai WN,
Liu R, Tang N, Zhang RB, Du TX, et al: Plasma galectin-3 predicts
clinical outcomes after catheter ablation in persistent atrial
fibrillation patients without structural heart disease. Europace.
17:1541–1547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen D, Procter N, Goh V, Liu S, Chua SJ,
Assadi-Khansari B, Stewart S, Horowitz JD, Sverdlov AL and Ngo DT:
New onset atrial fibrillation is associated with elevated
galectin-3 levels. Int J Cardiol. 223:48–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yalcin MU, Gurses KM, Kocyigit D, Canpinar
H, Canpolat U, Evranos B, Yorgun H, Sahiner ML, Kaya EB, Hazirolan
T, et al: The association of serum galectin-3 levels with atrial
electrical and structural remodeling. J Cardiovasc Electrophysiol.
26:635–640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kornej J, Schmidl J, Ueberham L, John S,
Daneschnejad S, Dinov B, Hindricks G, Adams V, Husser D and
Bollmann A: Galectin-3 in patients with atrial fibrillation
undergoing radiofrequency catheter ablation. PLoS One. 10:pp.
e01235742015, View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yao Y, Shen D, Chen R, Ying C, Wang C, Guo
J and Zhang G: Galectin-3 predicts left ventricular remodeling of
hypertension. J Clin Hypertens (Greenwich. 18:506–511. 2016.
View Article : Google Scholar
|
|
87
|
Pusuroglu H, Akgul O, Erturk M, Bolat I,
Tasbulak O, Ornek V, Gul M, Ozyilmaz SO, Yildirim HA, Kokturk U, et
al: Assessment of relationship between galectin-3 and ambulatory
ECG-based microvolt T-wave alternans in sustained
systolic-diastolic hypertension patients. Blood Press Monit.
21:265–270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Calvier L, Legchenko E, Grimm L, Sallmon
H, Hatch A, Plouffe BD, Schroeder C, Bauersachs J, Murthy SK and
Hansmann G: Galectin-3 and aldosterone as potential tandem
biomarkers in pulmonary arterial hypertension. Heart. 102:390–396.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Agoston-Coldea L, Lupu S, Petrovai D,
Mocan T and Mousseaux E: Correlations between echocardiographic
parameters of right ventricular dysfunction and Galectin-3 in
patients with chronic obstructive pulmonary disease and pulmonary
hypertension. Med Ultrason. 17:487–495. 2015.PubMed/NCBI
|
|
90
|
Yakar Tülüce S, Tülüce K, Cil Z, Emren SV,
Akyildiz ZI and Ergene O: Galectin-3 levels in patients with
hypertrophic cardiomyopathy and its relationship with left
ventricular mass index and function. Anatol J Cardiol. 16:344–348.
2016.
|
|
91
|
Vergaro G, Del Franco A, Giannoni A,
Prontera C, Ripoli A, Barison A, Masci PG, Aquaro GD, Cohen Solal
A, Padeletti L, et al: Galectin-3 and myocardial fibrosis in
nonischemic dilated cardiomyopathy. Int J Cardiol. 184:96–100.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Franeková J, Hošková L, Sečník P Jr,
Pazderník M, Kotrbatá M, Kubíček Z and Jabor A: The role of timely
measurement of galectin-3, NT-proBNP, cystatin C and hsTnT in
predicting prognosis and heart function after heart
transplantation. Clin Chem Lab Med. 54:339–344. 2016. View Article : Google Scholar
|
|
93
|
Franeková J, Kubíček Z, Hošková L,
Kotrbatá M, Sečník P, Kautzner J and Jabor A: Long-term biological
variability of galectin-3 after heart transplantation. Clin Chem
Lab Med. 53:119–123. 2015. View Article : Google Scholar
|
|
94
|
Grupper A, Nativi-Nicolau J, Maleszewski
JJ, Geske JR, Kremers WK, Edwards BS, Kushwaha SS and Pereira L:
Circulating galectin-3 levels are persistently elevated after heart
transplantation and are associated with renal dysfunction. JACC
Heart Fail. 4:847–856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Casanegra AI, Stoner JA, Tafur AJ, Pereira
HA, Rathbun SW and Gardner AW: Differences in galectin-3, a
biomarker of fibrosis, between participants with peripheral artery
disease and participants with normal ankle-brachial index. Vasc
Med. 21:437–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yan XJ, Yu GF, Jie YQ, Fan XF, Huang Q and
Dai WM: Role of galectin-3 in plasma as a predictive biomarker of
outcome after acute intracerebral hemorrhage. J Neurol Sci.
368:121–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Opotowsky AR, Baraona F, Owumi J, Loukas
B, Singh MN, Valente AM, Wu F, Cheng S, Veldtman G, Rimm EB and
Landzberg MJ: Galectin-3 is elevated and associated with adverse
outcomes in patients with single-ventricle fontan circulation. J Am
Heart Asso. 5:e0027062016. View Article : Google Scholar
|
|
98
|
Kadoglou NP, Sfyroeras GS, Spathis A,
Gkekas C, Gastounioti A, Mantas G, Nikita KS, Karakitsos P and
Liapis CD: Galectin-3, carotid plaque vulnerability, and potential
effects of statin therapy. Eur J Vasc Endovasc Surg. 49:4–9. 2015.
View Article : Google Scholar
|
|
99
|
Liao CW, Lin YT, Wu XM, Chang YY, Hung CS,
Wu VC, Wu KD, Lin YH and TAIPAI Study Group: The relation among
aldosterone, galectin-3, and myocardial fibrosis: A prospective
clinical pilot follow-up study. J Investig Med. 64:1109–1113. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Edsfeldt A, Bengtsson E, Asciutto G, Dunér
P, Björkbacka H, Fredrikson GN, Nilsson J and Goncalves I: High
plasma levels of Galectin-3 are associated with increased risk for
stroke after carotid endarterectomy. Cerebrovasc Dis. 41:199–203.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu H, Liu Y, Zhao J, Liu H and He S:
Prognostic value of plasma galectin-3 levels after aneurysmal
subarachnoid hemorrhage. Brain Beha. 6:e005432016. View Article : Google Scholar
|
|
102
|
Nayor M, Wang N, Larson MG, Vasan RS, Levy
D and Ho JE: Circulating Galectin-3 is associated with
cardiometabolic disease in the community. J Am Heart Asso. 5(pii):
e0023472015. View Article : Google Scholar
|
|
103
|
Nishiyama J, Kobayashi S, Ishida A,
Nakabayashi I, Tajima O, Miura S, Katayama M and Nogami H:
Up-regulation of galectin-3 in acute renal failure of the rat. Am J
Pathol. 157:815–823. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ji F, Zhang S, Jiang X, Xu Y, Chen Z, Fan
Y and Wang W: Diagnostic and prognostic value of galectin-3, serum
creatinine, and cystatin C in chronic kidney diseases. J Clin Lab
Ana. Oct 11–2017.Epub ahead of print. View Article : Google Scholar
|
|
105
|
O'Seaghdha CM, Hwang SJ, Ho JE, Vasan RS,
Levy D and Fox CS: Elevated galectin-3 precedes the development of
CKD. J Am Soc Nephrol. 24:1470–1477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bansal N, Katz R, Seliger S, DeFilippi C,
Sarnak MJ, Delaney JA, Christenson R, de Boer IH, Kestenbaum B,
Robinson-Cohen C, et al: Galectin-3 and Soluble ST2 and kidney
function decline in older adults: The cardiovascular health study
(CHS). Am J Kidney Dis. 67:994–996. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Drechsler C, Delgado G, Wanner C, Blouin
K, Pilz S, Tomaschitz A, Kleber ME, Dressel A, Willmes C, Krane V,
et al: Galectin-3, renal function, and clinical outcomes: Results
from the LURIC and 4D studies. J Am Soc Nephrol. 26:2213–2221.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Gurel OM, Yilmaz H, Celik TH, Cakmak M,
Namuslu M, Bilgiç AM, Bavbek N, Akcay A and Eryonucu B: Galectin-3
as a new biomarker of diastolic dysfunction in hemodialysis
patients. Herz. 40:788–794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yilmaz H, Gurel OM, Celik HT, Bozkurt A,
Yildirim ME, Bilgic I, Bilgic MA, Bavbek N and Akcay A:
Relationship of galectin-3 to left ventricular geometry and
hypertrophy in chronic hemodialysis patients. Herz. 40:702–708.
2015. View Article : Google Scholar
|
|
110
|
Ozkan G, Ulusoy S, Mentese A, Guvercin B,
Karahan SC, Yavuz A, Altay DU and Ocal M: Can be galectin-3 a novel
marker in determining mortality in hemodialysis patients. Clin
Biochem. 48:768–773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hogas S, Schiller A, Voroneanu L,
Constantinescu D, Timar R, Cianga P, Siriopol D, Bob F, Cianga C,
Onofriescu M, et al: Predictive value for Galectin 3 and
cardiotrophin 1 in hemodialysis patients. Angiology. 67:854–859.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Schwerg M, Eilers B, Wienecke A, Baumann
G, Laule M, Knebel F, Stangl K and Stangl V: Galectin-3 and
prediction of therapeutic response to renal sympathetic
denervation. Clin Exp Hypertens. 38:399–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bing Z, Lal P, Lu S, Ziober A and
Tomaszewski JE: Role of carbonic anhydrase IX, α-methylacyl
coenzyme a racemase, cytokeratin 7, and galectin-3 in the
evaluation of renal neoplasms: A tissue microarray
immunohistochemical study. Ann Diagn Pathol. 17:58–62. 2013.
View Article : Google Scholar
|
|
114
|
Kaneko N, Gotoh A, Okamura N, Matsuo E,
Terao S, Watanabe M, Yamada Y, Hamami G, Nakamura T, Ikekita M, et
al: Potential tumor markers of renal cell carcinoma: α-enolase for
postoperative follow up, and galectin-1 and galectin-3 for primary
detection. Int J Urol. 20:530–535. 2013. View Article : Google Scholar
|
|
115
|
von Klot CA, Kramer MW, Peters I,
Hennenlotter J, Abbas M, Scherer R, Herrmann TR, Stenzl A, Kuczyk
MA, Serth J and Merseburger AS: Galectin-1 and Galectin-3 mRNA
expression in renal cell carcinoma. BMC Clin Pathol. 14:152014.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Straube T, Elli AF, Greb C, Hegele A,
Elsässer HP, Delacour D and Jacob R: Changes in the expression and
subcellular distribution of galectin-3 in clear cell renal cell
carcinoma. J Exp Clin Cancer Res. 30:892011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Sakaki M, Fukumori T, Fukawa T, Elsamman
E, Shiirevnyamba A, Nakatsuji H and Kanayama HO: Clinical
significance of Galectin-3 in clear cell renal cell carcinoma. J
Med Invest. 57:152–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Tang W, Huang C, Tang C, Xu J and Wang H:
Galectin-3 may serve as a potential marker for diagnosis and
prognosis in papillary thyroid carcinoma: A meta-analysis. Onco
Targets Ther. 9:455–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Manivannan P, Siddaraju N, Jatiya L and
Verma SK: Role of pro-angiogenic marker galectin-3 in follicular
neoplasms of thyroid. Indian. J Biochem Biophys. 49:392–394.
2012.
|
|
120
|
Mataraci EA, Ozgüven BY and Kabukcuoglu F:
Expression of cytokeratin 19, HBME-1 and galectin-3 in neoplastic
and nonneoplastic thyroid lesions. Pol J Pathol. 63:58–64.
2012.PubMed/NCBI
|
|
121
|
Matesa-Anić D, Moslavac S, Matesa N, Cupić
H and Kusić Z: Intensity and distribution of immunohistochemical
expression of galectin-3 in thyroid neoplasms. Acta Clin Croat.
51:237–241. 2012.
|
|
122
|
Sumana BS, Shashidhar S and Shivarudrappa
AS: Galectin-3 immunohistochemical expression in thyroid neoplasms.
J Clin Diagn Res. 9:EC07–EC11. 2015.PubMed/NCBI
|
|
123
|
Papale F, Cafiero G, Grimaldi A, Marino G,
Rosso F, Mian C, Barollo S, Pennelli G, Sorrenti S, De Antoni E and
Barbarisi A: Galectin-3 expression in thyroid fine needle cytology
(t-FNAC) uncertain cases: Validation of molecular markers and
technology innovation. J Cell Physiol. 228:968–974. 2013.
View Article : Google Scholar
|
|
124
|
Salajegheh A, Dolan-Evans E, Sullivan E,
Irani S, Rahman MA, Vosgha H, Gopalan V, Smith RA and Lam AK: The
expression profiles of the galectin gene family in primary and
metastatic papillary thyroid carcinoma with particular emphasis on
galectin-1 and galectin-3 expression. Exp Mol Pathol. 96:212–218.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Al-Sharaky DR and Younes SF: Sensitivity
and specificity of Galectin-3 and Glypican-3 in
Follicular-patterned and other thyroid neoplasms. J Clin Diagn Res.
10:EC06–EC10. 2016.PubMed/NCBI
|
|
126
|
Yilmaz E, Karsidag T, Tatar C and Tüzün S:
Serum Galectin-3: Diagnostic value for papillary thyroid carcinoma.
Ulus Cerrahi Derg. 31:192–196. 2015.PubMed/NCBI
|
|
127
|
Paunovic I, Isic T, Havelka M, Tatic S,
Cvejic D and Savin S: Combined immunohistochemistry for thyroid
peroxidase, galectin-3, CK19 and HBME-1 in differential diagnosis
of thyroid tumors. APMIS. 120:368–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Abd-El Raouf SM and Ibrahim TR:
Immunohistochemical expression of HBME-1 and galectin-3 in the
differential diagnosis of follicular-derived thyroid nodules.
Pathol Res Pract. 210:971–978. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Trimboli P, Guidobaldi L, Amendola S,
Nasrollah N, Romanelli F, Attanasio D, Ramacciato G, Saggiorato E,
Valabrega S and Crescenzi A: Galectin-3 and HBME-1 improve the
accuracy of core biopsy in indeterminate thyroid nodules.
Endocrine. 52:39–45. 2016. View Article : Google Scholar
|
|
130
|
Durry MF, Miskad UA, Leiwakabessy WN and
Cangara MH: Diagnostic value of galectin-3 and hector battifora
mesothelial epitope (hbme)-1 as a marker for malignancy in the
diagnosis of thyroid lesions. Patholog. 48(Suppl 1): S122–S123.
2016.
|
|
131
|
Das DK, Al-Waheeb SK, George SS, Haji BI
and Mallik MK: Contribution of immunocytochemical stainings for
galectin-3, CD44, and HBME1 to fine-needle aspiration cytology
diagnosis of papillary thyroid carcinoma. Diagn Cytopathol.
42:498–505. 2014. View Article : Google Scholar
|
|
132
|
Karaarslan S, Yurum FN, Kumbaraci BS, Pala
EE, Sivrikoz ON, Akyildiz M and Bugdayci MH: The role of
parafibromin, Galectin-3, HBME-1, and Ki-67 in the differential
diagnosis of parathyroid tumors. Oman Med J. 30:421–427. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wang O, Wang CY, Shi J, Nie M, Xia WB, Li
M, Jiang Y, Guan H, Meng XW and Xing XP: Expression of Ki-67,
galectin-3, fragile histidine triad, and parafibromin in malignant
and benign parathyroid tumors. Chin Med J (Engl. 125:2895–2901.
2012.
|
|
134
|
Selemetjev SA, Savin SB, Paunovic IR,
Tatic SB and Cvejic D: Changes in the expression pattern of
apoptotic molecules (galectin-3, Bcl-2, Bax, survivin) during
progression of thyroid malignancy and their clinical significance.
Wien Klin Wochenschr. 127:337–344. 2015. View Article : Google Scholar
|
|
135
|
Lee YM and Lee JB: Prognostic value of
epidermal growth factor receptor, 53 and galectin-3 expression in
papillary thyroid carcinoma. J Int Med Res. 41:825–834. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Nechifor-Boilă A, Cătană R, Loghin A, Radu
TG and Borda A: Diagnostic value of HBME-1, CD56, Galectin-3 and
Cytokeratin-19 in papillary thyroid carcinomas and thyroid tumors
of uncertain malignant potential. Rom J Morphol Embryol. 55:49–56.
2014.
|
|
137
|
Liu Z, Li X, Shi L, Maimaiti Y, Chen T, Li
Z, Wang S, Xiong Y, Guo H, He W, et al: Cytokeratin 19,
thyroperoxidase, HBME-1 and galectin-3 in evaluation of aggressive
behavior of papillary thyroid carcinoma. Int J Clin Exp Med.
7:2304–2308. 2014.PubMed/NCBI
|
|
138
|
Gweon HM, Kim JA, Youk JH, Hong SW, Lim
BJ, Yoon SO, Park YM and Son EJ: Can galectin-3 be a useful marker
for conventional papillary thyroid microcarcinoma. Diagn
Cytopathol. 44:103–107. 2016. View Article : Google Scholar
|
|
139
|
Wang X, Yuegao, Bai L, Ibrahim MM, Ma W,
Zhang J, Huang Y, Wang B, Song L and Tang J: Evaluation of annexin
A7, Galectin-3 and Gelsolin as possible biomarkers of
hepatocarcinoma lymphatic metastasis. Biomed Pharmacother.
68:259–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Serizawa N, Tian J, Fukada H, Baghy K,
Scott F, Chen X, Kiss Z, Olson K, Hsu D, Liu FT, et al: Galectin 3
regulates HCC cell invasion by RhoA and MLCK activation. Lab
Invest. 95:1145–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Ulu M, Alacacioglu A, Yuksel E, Pamukk BO,
Bozkaya G, Ari A, Yuksel A, Sop G and Alacacioglu I: Prognostic
significance of serum galectin-3 levels in patients with
hepatocellular cancer and chronic viral hepatitis. Saudi J
Gastroenterol. 21:47–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Eisa NH, Ebrahim MA, Ragab M, Eissa LA and
El-Gayar AM: Galectin-3 and matrix metalloproteinase-9: Perspective
in management of hepatocellular carcinoma. J Oncol Pharm Pract.
21:323–330. 2015. View Article : Google Scholar
|
|
143
|
Jiang SS, Weng DS, Wang QJ, Pan K, Zhang
YJ, Li YQ, Li JJ, Zhao JJ, He J, Lv L, et al: Galectin-3 is
associated with a poor prognosis in primary hepatocellular
carcinoma. J Transl Med. 12:2732014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Balan V, Wang Y, Nangia-Makker P, Kho D,
Bajaj M, Smith D, Heilbrun L, Raz A and Heath E: Galectin-3: A
possible complementary marker to the PSA blood test. Oncotarget.
4:542–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Nakajima K, Heilbrun LK, Hogan V, Smith D,
Heath E and Raz A: Positive associations between galectin-3 and PSA
levels in prostate cancer patients: A prospective clinical study-I.
Oncotarget. 7:82266–82272. 2016.PubMed/NCBI
|
|
146
|
Araujo-Filho JL, Melo-Junior MR, Beltrão
EI, de Lima LR, Antunes CB and de Carvalho LB Jr:
Immunochemiluminescent detection of galectin-3 in tumoral tissue
from prostate. Int J Clin Exp Pathol. 6:1861–1867. 2013.PubMed/NCBI
|
|
147
|
Knapp JS, Lokeshwar SD, Vogel U,
Hennenlotter J, Schwentner C, Kramer MW, Stenzl A and Merseburger
AS: Galectin-3 expression in prostate cancer and benign prostate
tissues: Correlation with biochemical recurrence. World J Urol.
31:351–358. 2013. View Article : Google Scholar
|
|
148
|
Xie L, Ni WK, Chen XD, Xiao MB, Chen BY,
He S, Lu CH, Li XY, Jiang F and Ni RZ: The expressions and clinical
significances of tissue and serum galectin-3 in pancreatic
carcinoma. J Cancer Res Clin Oncol. 138:1035–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Jiang K, Lawson D, Cohen C and Siddiqui
MT: Galectin-3 and PTEN expression in pancreatic ductal
adenocarcinoma, pancreatic neuroendocrine neoplasms and
gastrointestinal tumors on fine-needle aspiration cytology. Acta
Cytol. 58:281–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Barrow H, Guo X, Wandall HH, Pedersen JW,
Fu B, Zhao Q, Chen C, Rhodes JM and Yu LG: Serum galectin-2, -4,
and -8 are greatly increased in colon and breast cancer patients
and promote cancer cell adhesion to blood vascular endothelium.
Clin Cancer Res. 17:7035–7046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Barrow H, Rhodes JM and Yu LG:
Simultaneous determination of serum galectin-3 and -4 levels
detects metastases in colorectal cancer patients. Cell Oncol
(Dordr). 36:9–13. 2013. View Article : Google Scholar
|
|
152
|
Shimura T, Shibata M, Gonda K, Nakajima T,
Chida S, Noda M, Suzuki S, Nakamura I, Ohki S and Takenoshita S:
Association between circulating galectin-3 levels and the
immunological, inflammatory and nutritional parameters in patients
with colorectal cancer. Biomed Rep. 5:203–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Petrovic S, Radosavljevic GD, Pantic J,
Jovanovic I, Jankovic N and Arsenijevic N: Circulating and tissue
galectin-1 and galectin-3 in colorectal carcinoma: Association with
clinicopathological parameters, serum CEA, IL-17 and IL23. J BUON.
21:941–949. 2016.PubMed/NCBI
|
|
154
|
Zhang H, Luo M, Liang X, Wang D, Gu X,
Duan C, Gu H, Chen G, Zhao X, Zhao Z and Liu C: Galectin-3 as a
marker and potential therapeutic target in breast cancer. PLoS One.
9:e1034822014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Koo JS and Jung W: Clinicopathlogic and
immunohistochemical characteristics of triple negative invasive
lobular carcinoma. Yonsei Med J. 52:89–97. 2011. View Article : Google Scholar :
|
|
156
|
Yamaki S, Fujii T, Yajima R, Hirakata T,
Yamaguchi S, Fujisawa T, Tsutsumi S, Asao T, Yanagita Y, Iijima M
and Kuwano H: Clinicopathological significance of decreased
galectin-3 expression and the long-term prognosis in patients with
breast cancer. Surg Today. 43:901–905. 2013. View Article : Google Scholar
|
|
157
|
Ilmer M, Mazurek N, Gilcrease MZ, Byrd JC,
Woodward WA, Buchholz TA, Acklin K, Ramirez K, Hafley M, Alt E, et
al: Low expression of galectin-3 is associated with poor survival
in node-positive breast cancers and mesenchymal phenotype in breast
cancer stem cells. Breast Cancer Res. 18:972016. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
El Gendy H, Madkour B, Abdelaty S, Essawy
F, Khattab D, Hammam O, El Kholy A and Nour HH: Galectin 3 for the
diagnosis of bladder cancer. Arab J Urol. 12:178–181. 2014.
View Article : Google Scholar
|
|
159
|
Gendy HE, Madkour B, Abdelaty S, Essawy F,
Khattab D, Hammam O and Nour HH: Diagnostic and prognostic
significance of serum and tissue Galectin 3 expression in patients
with carcinoma of the bladder. Curr Urol. 7:185–190. 2014.
View Article : Google Scholar
|
|
160
|
Cheng D, Liang B and Li Y: Serum
galectin-3 as a potential marker for gastric cancer. Med Sci Monit.
21:755–760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Leal MF, Calcagno DQ, Chung J, de Freitas
VM, Demachki S, Assumpção PP, Chammas R, Burbano RR and Smith MC:
Deregulated expression of annexin-A2 and galectin-3 is associated
with metastasis in gastric cancer patients. Clin Exp Med.
15:415–420. 2015. View Article : Google Scholar
|
|
162
|
Tas F, Bilgin E, Tastekin D, Erturk K and
Duranyildiz D: Clinical significance of serum Galectin-3 levels in
gastric cancer patients. J Gastrointest Cancer. 47:182–186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Gomes TS, Oshima CT, Forones NM, De
Oliveira Lima F and Ribeiro DA: Expression of galectin-3 in gastric
adenocarcinoma. Indian J Med Res. 140:69–76. 2014.PubMed/NCBI
|
|
164
|
Gao N, Yu WZ, Guo NJ, Wang XX and Sun JR:
Clinical significance of galectin-3 in patients with adult acute
myeloid leukemia: A retrospective cohort study with long-term
follow-up and formulation of risk scoring system. Leuk Lymphoma.
58:1394–1402. 2017. View Article : Google Scholar
|
|
165
|
Samura B: Galectin-3 as a prognostic
biomarker in patients with Non-Hodgkin lymphoma. Georgian Med News.
7–11. 2015.PubMed/NCBI
|
|
166
|
Lambropoulou M, Deftereou TE, Kynigopoulos
S, Patsias A, Anagnostopoulos C, Alexiadis G, Kotini A, Tsaroucha
A, Nikolaidou C, Kiziridou A, et al: Co-expression of galectin-3
and CRIP-1 in endometrial cancer: Prognostic value and patient
survival. Med Oncol. 33:82016. View Article : Google Scholar
|
|
167
|
Zhou X, Jing J, Peng J, Mao W, Zheng Y,
Wang D, Wang X, Liu Z and Zhang X: Expression and clinical
significance of galectin-3 in osteosarcoma. Gene. 546:403–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Aggarwal S, Sharma SC and Das SN:
Galectin-1 and galectin-3: Plausible tumour markers for oral
squamous cell carcinoma and suitable targets for screening
high-risk population. Clin Chim Acta. 442:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Righi A, Morandi L, Leonardi E, Farnedi A,
Marucci G, Sisto A, Frank G, Faustini-Fustini M, Zoli M, Mazzatenta
D, et al: Galectin-3 expression in pituitary adenomas as a marker
of aggressive behavior. Hum Pathol. 44:2400–2409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Yang LP, Jiang S, Liu JQ, Miao XY and Yang
ZL: Up-regulation of galectin-3 and Sambucus nigra agglutinin
binding site is associated with invasion, metastasis and
poor-progression of the gallbladder adenocarcinoma.
Hepatogastroenterology. 59:2089–2094. 2012.PubMed/NCBI
|
|
171
|
Zhang L, Wang P, Qin Y, Cong Q, Shao C, Du
Z, Ni X, Li P and Ding K: RN1, a novel galectin-3 inhibitor,
inhibits pancreatic cancer cell growth in vitro and in vivo via
blocking galectin-3 associated signaling pathways. Oncogene.
36:1297–1308. 2017. View Article : Google Scholar
|
|
172
|
Yang Y, Zhou Z, He S, Fan T, Jin Y, Zhu X,
Chen C, Zhang ZR and Huang Y: Treatment of prostate carcinoma with
(galectin-3)-targeted HPMA copolymer-(G3-C12)-5-Fluorouracil
conjugates. Biomaterials. 33:2260–2271. 2012. View Article : Google Scholar
|
|
173
|
Guha P, Kaptan E, Bandyopadhyaya G,
Kaczanowska S, Davila E, Thompson K, Martin SS, Kalvakolanu DV,
Vasta GR and Ahmed H: Cod glycopeptide with picomolar affinity to
galectin-3 suppresses T-cell apoptosis and prostate cancer
metastasis. Proc Natl Acad Sci USA. 110:5052–5057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Mirandola L, Yu Y, Cannon MJ, Jenkins MR,
Rahman RL, Nguyen DD, Grizzi F, Cobos E, Figueroa JA and
Chiriva-Internati M: Galectin-3 inhibition suppresses drug
resistance, motility, invasion and angiogenic potential in ovarian
cancer. Gynecol Oncol. 135:573–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Calvier L, Martinez-Martinez E, Miana M,
Cachofeiro V, Rousseau E, Sádaba JR, Zannad F, Rossignol P and
López-Andrés N: The impact of galectin-3 inhibition on
aldosterone-induced cardiac and renal injuries. JACC Heart Fail.
3:59–67. 2015. View Article : Google Scholar
|
|
176
|
Vergaro G, Prud'homme M, Fazal L, Merval
R, Passino C, Emdin M, Samuel JL, Cohen Solal A and Delcayre C:
Inhibition of Galectin-3 pathway prevents isoproterenol-induced
left ventricular dysfunction and fibrosis in mice. Hypertension.
67:606–612. 2016.PubMed/NCBI
|
|
177
|
Martinez-Martinez E, Calvier L, Rossignol
P, Rousseau E, Fernández-Celis A, Jurado-López R, Laville M,
Cachofeiro V and López-Andrés N: Galectin-3 inhibition prevents
adipose tissue remodelling in obesity. Int J Obes (Lond).
40:1034–1038. 2016. View Article : Google Scholar
|
|
178
|
MacKinnon AC, Liu X, Hadoke PW, Miller MR,
Newby DE and Sethi T: Inhibition of galectin-3 reduces
atherosclerosis in apolipoprotein E-deficient mice. Glycobiology.
23:654–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Delaine T, Collins P, MacKinnon A, Sharma
G, Stegmayr J, Rajput VK, Mandal S, Cumpstey I, Larumbe A, Salameh
BA, et al: Galectin-3-binding glycomimetics that strongly reduce
bleomycin-induced lung fibrosis and modulate intracellular glycan
recognition. Chembiochem. 17:1759–1770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Rajput VK, MacKinnon A, Mandal S, Collins
P, Blanchard H, Leffler H, Sethi T, Schambye H, Mukhopadhyay B and
Nilsson UJ: A Selective Galactose-coumarin-derived Galectin-3
inhibitor demonstrates involvement of Galectin-3-glycan
interactions in a pulmonary fibrosis model. J Med Chem.
59:8141–8147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Traber PG and Zomer E: Therapy of
experimental NASH and fibrosis with galectin inhibitors. PLoS One.
8:e834812013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Harrison SA, Marri SR, Chalasani N, Kohli
R, Aronstein W, Thompson GA, Irish W, Miles MV, Xanthakos SA,
Lawitz E, et al: Randomised clinical study: GR-MD-02, a galectin-3
inhibitor, vs. placebo in patients having non-alcoholic
steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther.
44:1183–1198. 2016. View Article : Google Scholar : PubMed/NCBI
|