Introduction
Stem cell therapy has been considered a potential
regenerative strategy for the treatment of various diseases,
including diabetes, Parkinson's disease, cancer and other chronic
long-standing conditions (1,2).
At present, various cell types have been used in stem cell therapy,
including embryonic stem cells, induced pluripotent stem cells,
hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs)
(3–6). Besides the application of HSCs for
the treatment of blood diseases, the majority of studies regarding
stem cell therapy have focused on MSCs. MSCs are adult stem cells
that can be isolated form bone marrow, adipose tissue, umbilical
cord, heart tissue and skin (7,8).
MSCs possess not only the ability to differentiate into numerous
mesenchymal lineages (9,10), but may also regulate the immune
response and repair injured tissues without ethical concerns
(11).
Compared with other sources of MSCs, adipose-derived
stem cells (ADSCs) are abundant and easier to obtain. Furthermore,
ADSCs have been reported to lack major histocompatibility
complex-II expression, and are more potent than bone marrow-derived
stem cells (BMSCs) in suppressing dendritic cell differentiation
(12,13). These findings suggested that ADSCs
possess better immunoregulatory abilities compared with BMSCs.
ADSCs-based therapy has been clinically used to treat perianal
fistula (14–18), postoperative enterocutaneous
fistula (19), osteoarthritis
(20), acute ischemic stroke
(21) and idiopathic pulmonary
fibrosis (22). Furthermore,
ADSCs-based therapy may be used to treat cardiovascular disease,
liver disease and neurological disorders in preclinical trails
(23–25).
Although ADSCs-based therapy has been widely used
for several diseases in preclinical and clinical investigations,
the long-term safety, particularly tumorigenicity, is a major
barrier for clinical translation of ADSCs-based therapy. Therefore,
the association between ADSCs and cancer has attracted extensive
interest in stem cell therapy. Numerous studies have indicated that
ADSCs may promote tumor progression. For example, Chu et al
reported that ADSCs could promote epithelial ovarian cancer growth
and metastasis (26). Muehlberg
et al revealed that ADSCs promoted breast cancer growth and
metastasis (27). Conversely,
other studies have suggested that ADSCs may suppress bladder tumor
growth and inhibit breast cancer metastasis (28,29). Therefore, the tumorigenicity of
ADSCs remains controversial.
It is widely recognized that various cytokines and
chemokines that are secreted from ADSCs may affect the
proliferation of tumor cells. However, the levels of cytokines and
chemokines secreted from ADSCs are closely associated with culture
conditions (30,31). For example, Bhang et al
demonstrated that ADSCs cultured under three-dimensional (3D)
culture conditions could produce higher concentrations of
angiogenic and/or anti-apoptotic factors, including vascular
endothelial growth factor, fibroblast growth factor-2, hepatocyte
growth factor and C-X-C motif chemokine ligand 12 compared with
cells cultured under two-dimensional (2D) conditions (32). In addition, Yang et al
demonstrated that the 3D culture method could enhance the activity
of ADSCs and increase the autophagic response upon hydrogen
peroxide (H2O2) treatment compared with the
2D culture method (33). Tian
et al revealed that human MSCs inhibited proliferation of
cancer cells in vitro, whereas they enhanced tumor formation
and growth in vivo (34),
thus indicating the dual effects of MSCs on the same tumor.
Therefore, whether ADSCs serve a protumorigenic or anti-tumorigenic
role in tumor growth depends on the in vivo or in
vitro growing conditions of ADSCs. Using an appropriate culture
method, which closely mimics in vivo conditions, may be of
great benefit to illustrate the association between cancer and
ADSCs. In order to better understand how ADSCs affect tumors, the
present study used different culture methods, including 2D culture
method, sphere culture method and AlgiMatrix™ 3D culture method, to
investigate whether cultured ADSCs may promote or inhibit the
growth of liver cancer cells, and to explore the underlying
mechanisms.
Materials and methods
Animals and ethics approval
A total of 5 adult male Sprague-Dawley (SD) rats
(weight, 180–200 g; age, 7–8 week old) were obtained from the
Center for Animal Experiments of Fujian Medical University (Fuzhou,
China; license no. SCXKmin2012-0002). The rats were housed at a
constant temperature (22±2°C), with 60% relative humidity, under a
12-h light/dark cycle. The rats had ad libitum access to
food and autoclaved water. The present study was approved by the
Animal Ethics Committee of Fuzhou General Hospital (Fuzhou,
China).
Cell culture
Rat ADSCs were derived from subcutaneous adipose
tissues according to the protocol described in our previous study
(35). Briefly, following
anesthetization of the male SD rats (n=5) using pentobarbital
sodium (40 mg/kg; Merck & Co., Inc., Whitehouse Station, NJ,
USA), adipose tissues (~3×1.5×0.5 cm) were scraped from the
subcutaneous inguinal region, cut into small pieces (~0.1×0.1×0.1
mm), and digested with 0.1% type I collagenase (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at 37°C for 60 min with gentle
agitation. Subsequently, the digested tissue was filtered through a
100-μm cell strainer, centrifuged at 400 × g for 5 min and
washed twice with PBS (HyClone; GE Healthcare Life Sciences, Logan,
UT, USA). The cell pellet was then suspended in expanding medium
comprising α-modified Eagle's medium (α-MEM; HyClone; GE Healthcare
Life Sciences) supplemented with 20% fetal bovine serum (FBS),
penicillin (100 U/ml) and streptomycin (100 μg/ml) (all from
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cell
suspension was transferred into a 6-well plate (Corning Inc.,
Corning, NY, USA) at a density of 1×106/ml and was
incubated at 37°C in an atmosphere containing 5% CO2.
After 24 h, the adherent cells were further expanded in complete
medium comprising α-MEM, 10% FBS, 100 U/ml penicillin and 100
μg/ml streptomycin. ADSCs from passage 3 or 4 were used in
the present study. Liver cancer cell lines, including the Hcclm3
human hepatocellular carcinoma (HCC) cell line and the HepG2 human
hepatoblastoma cell line (36),
were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China), and were cultured in complete medium
comprising Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin. All cells were
incubated in a humidified atmosphere containing 5% CO2
at 37°C.
Preparation of ADSCs-conditioned medium
(CM)
To collect the 2D-ADSCs-CM, sphere-ADSCs-CM and
3D-ADSCs-CM, rat ADSCs were seeded at a density of 2×105
cells/well into conventional 6-well plates, 6-well non-adherent
plates (Corning, Inc.) and AlgiMatrix™ 3D culture system 6-well
plates (Gibco; Thermo Fisher Scientific, Inc.), respectively. The
cells were cultured with StemPro® MSC SFM CTS™ medium
(MSC medium; Gibco; Thermo Fisher Scientific, Inc.). All cells were
incubated in a humidified atmosphere containing 5% CO2
at 37°C. After 48 h, the medium was collected, filtered through a
0.22 μm filter and stored at −80°C.
Cell viability assay
In order to observe the effects of ADSCs on tumor
cell proliferation, Hcclm3 and HepG2 cells were cultured in 96-well
plates at a density of 5×103 cells/well with 150
μl complete medium, at 37̊C in an atmosphere containing 5%
CO2 for 24 h. Subsequently, the cell culture medium of
each well was replaced with 100 μl ADSC-CM (5×104
ADSCs/ml MSC medium); cells incubated with 100 μl MSC medium
were used as the negative control. Following a 48 h incubation at
37°C, cell viability was determined using the Cell Counting kit-8
(CCK-8) cell proliferation kit (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) according to the manufacturer's protocol. A
microplate reader (SpectraMax M5; Molecular Devices, LLC,
Sunnyvale, CA, USA) was used to measure the absorbance of the
solution in each well at 450 nm. All experiments were performed in
triplicate.
Cell apoptosis assay
Flow cytometry was used to assess cell apoptosis.
Briefly, Hcclm3 and HepG2 cells were cultured in 6-well plates at a
density of 1×105 cells/well for 24 h. Subsequently, the
culture medium was discarded and replaced with 2 ml ADSC-CM
(5×104 ADSCs/ml MSC medium); cells incubated with 2 ml
MSC medium were used as the negative control. After 48 h, tumor
cells from all groups were further incubated with 1%
H2O2 for 10 min at room temperature, in order
to induce cell apoptosis of some tumor cells. Tumor cells were then
collected and cell apoptosis was analyzed using the Annexin
V-fluorescein isothiocyanate apoptosis assay kit (Dojindo Molecular
Technologies, Inc.) according to the manufacturer's protocol.
Finally, the labeled cells were assessed using a FACSVerse flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA), and all raw
data were analyzed with ModFit LT v2.0 software (Verity Software
House, Inc., Topsham, ME, USA). All experiments were performed in
triplicate.
Scratch wound healing assay
Hcclm3 and HepG2 cells (1.5×105
cells/well) were seeded into 24-well plates and allowed to adhere
overnight. After reaching 80–90% confluence, a 'reference line' was
scratched at the bottom of the plate using a sterile 10 μl
pipette tip. Subsequently, cells were washed three times with PBS,
and were further incubated with 1 ml ADSC-CM (1.5×105
ADSCs/ml MSC medium) to observe the migration of liver cancer cells
into the cell-free area. Photomicrographs of cells migrating across
the 'reference line' were captured from different fields following
each treatment at 0 and 48 h with an inverted microscope (Zeiss
GmbH, Jena, Germany).
Cell adhesion assay
To observe the effects of ADSCs on liver cancer cell
adhesion, 96-well microplates were pretreated with 50 μl
fibronectin (20 μg/ml; BD Biosciences). Liver cancer cells
(4×103 cells/well) were susp ended in 100 μl
ADSCs-CM (4×104 ADSCs/ml MSC medium) and were added to
the pretreated 96-well plates. At the indicated time-point, the
attached cells were fixed in absolute methanol and stained with 10%
crystal violet at room temperature for 30 min. Subsequently, the
cells were washed with PBS and images were captured using an
inverted microscope (Zeiss GmbH). To further quantify the adhesive
capabilities of the cells, crystal violet-stained cells were
treated with 33% acetic acid (150 μl/well) in PBS for 5 min
at room temperature. A microplate reader (SpectraMax M5; Molecular
Devices, LLC) was used to measure the absorbance of the solution in
each well at 570 nm. All experiments were performed in
triplicate.
Cell migration and invasion assays
A cell migration assay was conducted using Transwell
units (no. 3422; Corning Inc.) and cell invasion was assessed using
Matrigel®-coated Transwell units (no. 354480; BD
Biosciences) on a 24-well plate. For tumor cell migration and
invasion assays, 1×105 liver cancer cells were cultured
in the upper compartment of the Transwell units with 200 μl
ADSCs-CM (5×105 ADSCs/ml MSC medium), whereas the lower
compartment of the units was filled with DMEM supplemented with 10%
FBS. After 48 h, the filters were fixed with 4% paraformaldehyde at
room temperature for 20 min and stained with 10% crystal violet at
room temperature for 30 min. Cells on the upper surface of the
Transwell units were removed using a cotton swab, and cells that
had passed through the lower surface of the filter were counted
under an inverted microscope (Zeiss GmbH) in five different fields
at a magnification of ×200.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Following incubation with ADSCs-CM for 48 h, total
RNA was isolated from liver cancer cells using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently,
mRNA was reverse transcribed to cDNA using a Transcriptor First
Strand cDNA Synthesis kit (Roche Applied Science, Mannheim,
Germany) according to the manufacturer's protocol. qPCR analysis
was performed using the ABI StepOnePlus Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with q-PCR
Master Mix (Toyobo Life Science, Osaka, Japan). Cycling conditions
were as follows: initial denaturation at 95°C for 2 min; 40 cycles
at 95°C for 15 sec, 60°C for 30 sec and 70°C for 30 sec. The PCR
primer sequences were as follows: E-cadherin, forward
CGAGAGCTACACGTTCACGG, reverse GGGTGTCGAGGGAAAAATAGG; N-cadherin,
forward AGCCAACCTTAACTGAGGAGT, reverse GGCAAGTTGATTGGAGGGATG;
vimentin, forward GACGCCATCAACACCGAGTT, reverse
CTTTGTCGTTGGTTAGCTGGT; zinc finger E-box-binding homeobox 1 (Zeb1),
forward CTACAACAACAAGACACTGCTGT, reverse TGTTCTTTCAGAGAGGTAAAGCG;
and β-actin, forward ATAGCACAGCCTGGATAGCAACGT AC and reverse
CACCTTCTACAATGAGCTGCGTGTG. Target gene expression was normalized to
that of β-actin. Relative gene expression was calculated using the
2−ΔΔCq method (37).
Experiments were independently repeated three times.
Western blotting
After incubating with ADSCs-CM for 48 h, liver
cancer cells were lysed in ice-cold radioimmunoprecipitation assay
buffer [0.5 M Tris-HCl (pH 7.4), 1.5 M NaCl, 2.5% deoxycholic acid,
10% NP-40 and 10 mM EDTA] with protease inhibitor cocktail (Roche
Diagnostics, Indianapolis, IN, USA). Protein quantification was
performed by bicinchoninic acid assay, and equal amounts of protein
lysate (40 μg) were separated by 12% SDS-PAGE. Proteins were
then transferred to nitrocellulose membranes in transfer buffer [12
mM Tris base, 96 mM glycine (pH 8.3) and 15% methanol].
Subsequently, the membranes were blocked at room temperature for 2
h in Tris-buffered saline containing 0.1% Tween (TBST) containing
5% bovine serum albumin (Sigma-Aldrich; Merck KGaA), and were
probed with E-cadherin (3195), vimentin (5741), N-cadherin (13116)
and Zeb1 (3396) antibodies (1:500 dilution; all from Cell Signaling
Technology, Inc., Danvers, MA, USA) and β-actin antibody (1:5,000
dilution; Beijing TransGen Biotech Co., Ltd., Beijing, China)
overnight at 4°C. The membranes were washed three times with TBST,
and were then incubated with the appropriate horseradish
peroxidase-conjugated secondary antibody (1:8,000 dilution;
sc-2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h
at room temperature. Finally, the protein expression levels were
detected by enhanced chemiluminescence using supersignal west pico
chemiluminescent substrate (Pierce; Thermo Fisher Scientific, Inc.)
and visualized by autoradiography using Image Lab™ 4.1 software
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
All quantitative data are expressed as the means ±
standard deviation. All statistical analyses were performed using
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Multiple comparisons were
analyzed by one-way analysis of variance, followed by LSD multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
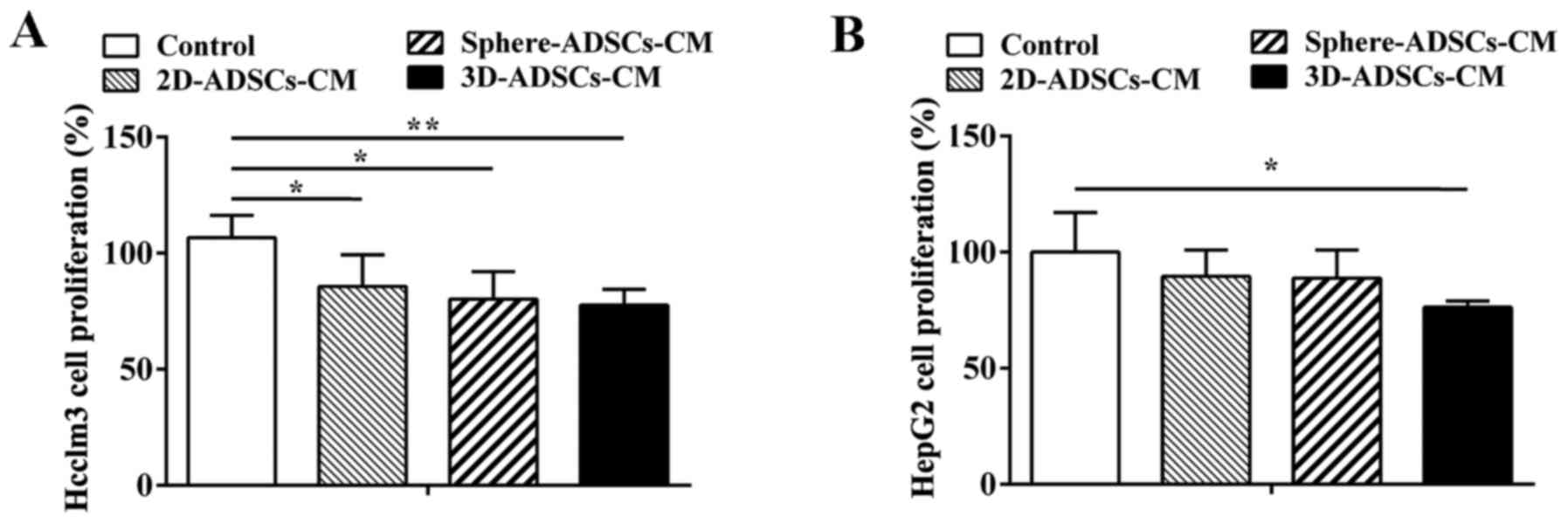
ADSCs-CM inhibits proliferation of liver
cancer cells
To investigate whether ADSCs affect the
proliferation of tumor cells, HepG2 and Hcclm3 cells were cultured
with three various types of ADSCs-CM: 2D-ADSCs-CM, sphere-ADSCs-CM
and 3D-ADSCs-CM for 48 h. Cell proliferation of treated tumor cells
was then evaluated using the CCK-8 assay. The results indicated
that the proliferation of Hcclm3 cells was inhibited following
culture with 2D-ADSCs-CM, sphere-ADSCs-CM and 3D-ADSCs-CM (Fig. 1A); however, despite a slight
decrease after culturing with 2D-ADSCs-CM or sphere-ADSCs-CM, the
proliferation of HepG2 cells was only significantly inhibited when
cultured with 3D-ADSCs-CM (Fig.
1B). In addition, the proliferation of liver cancer cells was
more markedly reduced after culturing with 3D-ADSCs-CM compared
with sphere-ADSCs-CM or 2D-ADSCs-CM; however, there was no
significant difference detected in cell proliferation among the
three groups cultured with various types of ADSCs-CM (Fig. 1). These results suggested that
ADSCs-CM, particularly 3D-ADSCs-CM, could significantly inhibit
liver cancer cell proliferation, and the 3D culture method could
partly accelerate the inhibitory effects of ADSCs on liver cancer
cell proliferation.
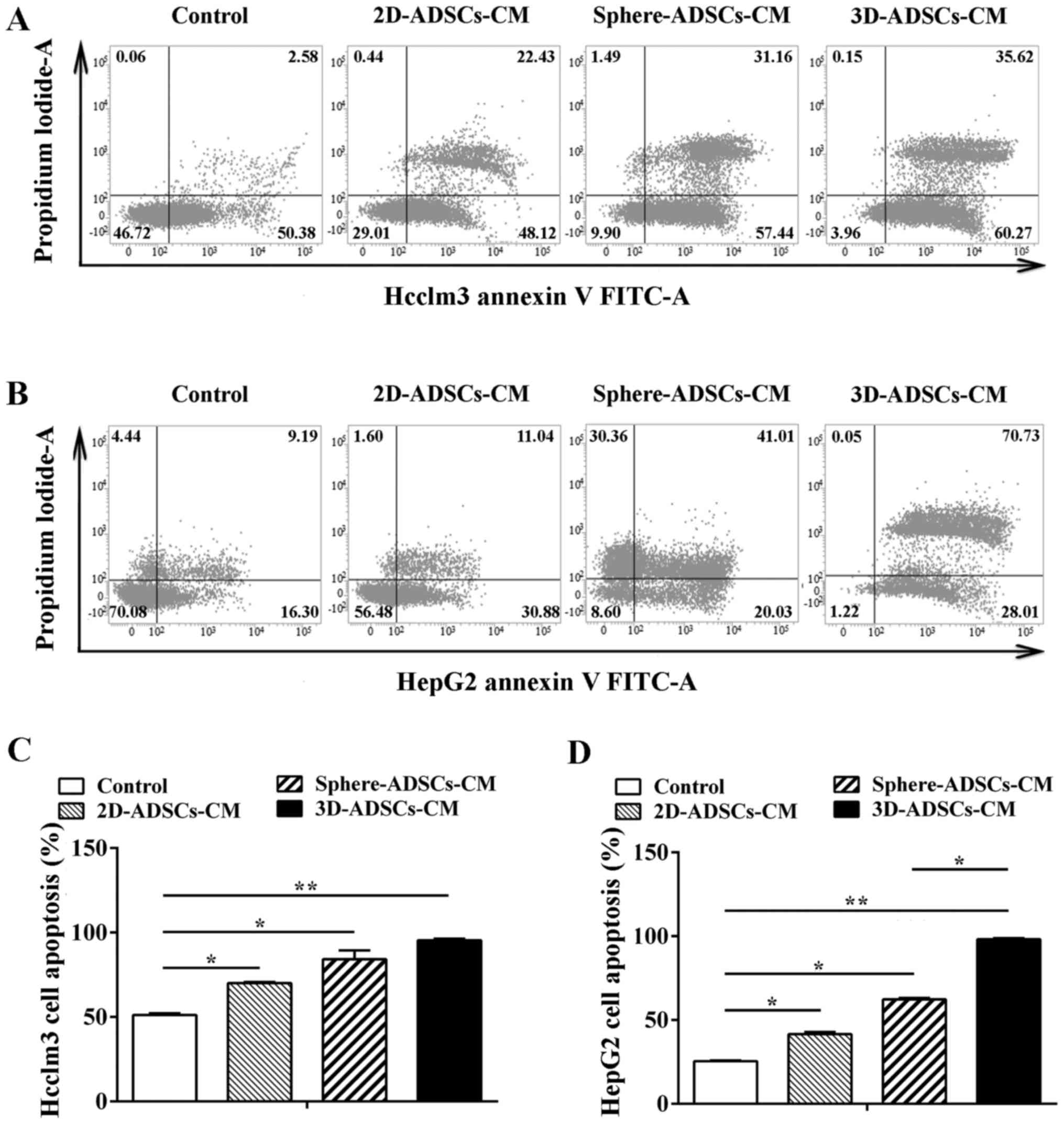
ADSCs-CM promotes apoptosis of liver
cancer cells
In order to evaluate the effects of ADSCs on the
apoptosis of Hcclm3 and HepG2 cells, the apoptotic rate of tumor
cells was determined following treatment with ADSCs-CM by flow
cytometry. Compared with the control liver cancer cells, the
proportion of apoptotic liver cancer cells was increased after
culturing with ADSCs-CM (Fig. 2A and
B). To further determine the apoptotic rate of liver cancer
cells, data were quantified by analyzing the percentage of Annexin
V-positive cells. According to these results, the liver cancer cell
apoptotic rate was significantly increased after culturing with
ADSCs-CM compared with the control group. In addition, the highest
cell apoptotic rate was observed in liver cancer cells treated with
sphere-ADSCs-CM or 3D-ADSCs-CM compared with those treated with
2D-ADSCs-CM (Fig. 2C and D).
These results clearly indicated that ADSCs-CM may significantly
promote the apoptosis of liver cancer cells, and apoptosis of liver
cancer cells could be enhanced by culturing with sphere-ADSCs-CM or
3D-ADSCs-CM. In addition, the present data demonstrated that more
significantly increased apoptosis of liver cancer cells could be
achieved after culturing with 3D-ADSCs-CM compared with
sphere-ADSCs-CM in HepG2 cells (Fig.
2C and D). These findings suggested that the 3D culture method
may result in the enhancement of ADSCs-induced HepG2 cell
apoptosis.
ADSCs-CM inhibits motility and adhesive
capacity of liver cancer cells
The present study used the wound healing assay to
evaluate motility of tumor cells after culturing with ADSCs-CM.
Compared with the control liver cancer cells, motility of liver
cancer cells was reduced after culturing with ADSCs-CM (Fig. 3A and B). To further determine
liver cancer cell motility, the distance of cell motility was
quantified. As shown in Fig. 3C and
D, it was confirmed that liver cancer cell motility was
decreased after culturing with ADSCs-CM; in particular, liver
cancer cells exhibited the lowest cell motility after culturing
with 3D-ADSCs-CM compared with 2D-ADSCs-CM or sphere-ADSCs-CM.
However, when liver cancer cells were cultured with
sphere-ADSCs-CM, there was no significant difference compared with
those cultured with 2D-ADSCs-CM. On the basis of these results, the
present study suggested that ADSCs-CM may significantly inhibit
liver cancer cell motility, and the 3D culture method could
accelerate the inhibitory effects of ADSCs on liver cancer cell
motility.
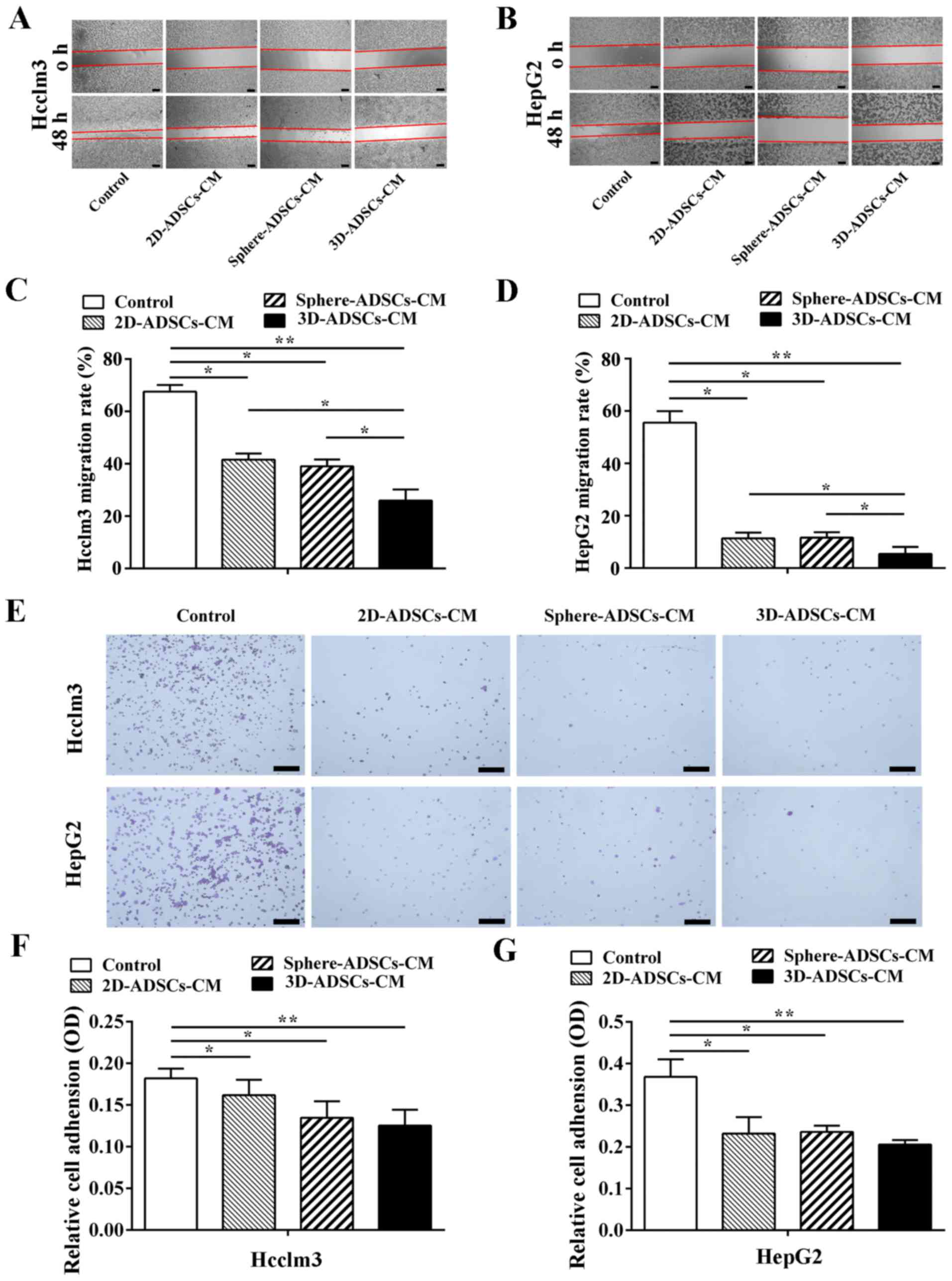 | Figure 3ADSCs-CM inhibits motility and
adhesive capacity of liver cancer cells. Motility of (A) Hcclm3 and
(B) HepG2 cells after culturing with ADSCs-CM (×100 magnification;
scale bar, 50 μm). Quantification of (C) Hcclm3 and (D)
HepG2 cell migration after culturing with ADSCs-CM. (E) Number of
adhesive cells after culturing with ADSCs-CM for 60 min (×50
magnification; scale bar, 200 μm). Quantification of (F)
Hcclm3 and (G) HepG2 cell adhesion after culturing with ADSCs-CM.
Control, untreated liver cancer cells; 2D-ADSCs-CM, liver cancer
cells treated with 2D-ADSCs-CM; sphere-ADSCs-CM, liver cancer cells
treated with sphere-ADSCs-CM; 3D-ADSCs-CM, liver cancer cells
treated with 3D-ADSCs-CM. For all groups, n=3.
*P<0.05 and **P<0.01. 2D,
two-dimensional; 3D, three-dimensional; ADSCs-CM, adipose
tissue-derived stem cells-conditioned medium; OD, optical
density. |
Due to the inhibitory effects of ADSCs on liver
cancer cell motility, the present study further evaluated the
effects of ADSCs on the adhesive capacity of liver cancer cells.
Hcclm3 and HepG2 cells were cultured with ADSCs-CM in fibro
nectincoated plates for 20, 40 and 60 min; adhesive cells were
examined by crystal violet staining. Although no significant
difference was observed between the control and ADSCs-CM-treated
liver cancer cells after 20 or 40 min (data not shown), the number
of adhesive cells was decreased following incubation with ADSCs-CM
for 60 min compared with the control liver cancer cells (Fig. 3E–G). In addition, a more obvious
inhibition of liver cancer cell adhesion was detected after
culturing with 3D-ADSCs-CM compared with sphere-ADSCs-CM or
2D-ADSCs-CM; however, no significant difference was detected among
the three ADSCs-CM groups. These data indicated that ADSCs could
inhibit liver cancer cell adhesion, and the 3D culture method could
partly enhance the inhibitory effects of ADSCs on liver cancer cell
adhesion.
ADSCs-CM suppresses migration and
invasion of liver cancer cells
To confirm the effects of ADSCs on migration of
tumor cells, liver cancer cells were cultured with ADSCs-CM in
Transwell units for 48 h; the liver cancer cells that penetrated
the membrane were examined by crystal violet staining. The results
indicated that the number of liver cancer cells that migrated
across the membrane was significantly decreased after culturing
with ADSCs-CM compared with the control liver cancer cells
(Fig. 4A–C), thus indicating that
ADSCs may suppress migration of liver cancer cells. The present
results also demonstrated that liver cancer cell migration was more
significantly decreased after culturing with 3D-ADSCs-CM or
sphere-ADSCs-CM compared with 2D-ADSCs-CM; in addition, the lowest
rate of liver cancer cell migration was observed after culturing
with 3D-ADSCs-CM compared with sphere-ADSCs-CM (Fig. 4B and C). These results indicated
that the sphere or 3D culture methods may enhance the inhibitory
effects of ADSCs on liver cancer cell migration, and the 3D culture
method is considered the best candidate to enhance the suppressive
effects of ADSCs on liver cancer cell migration.
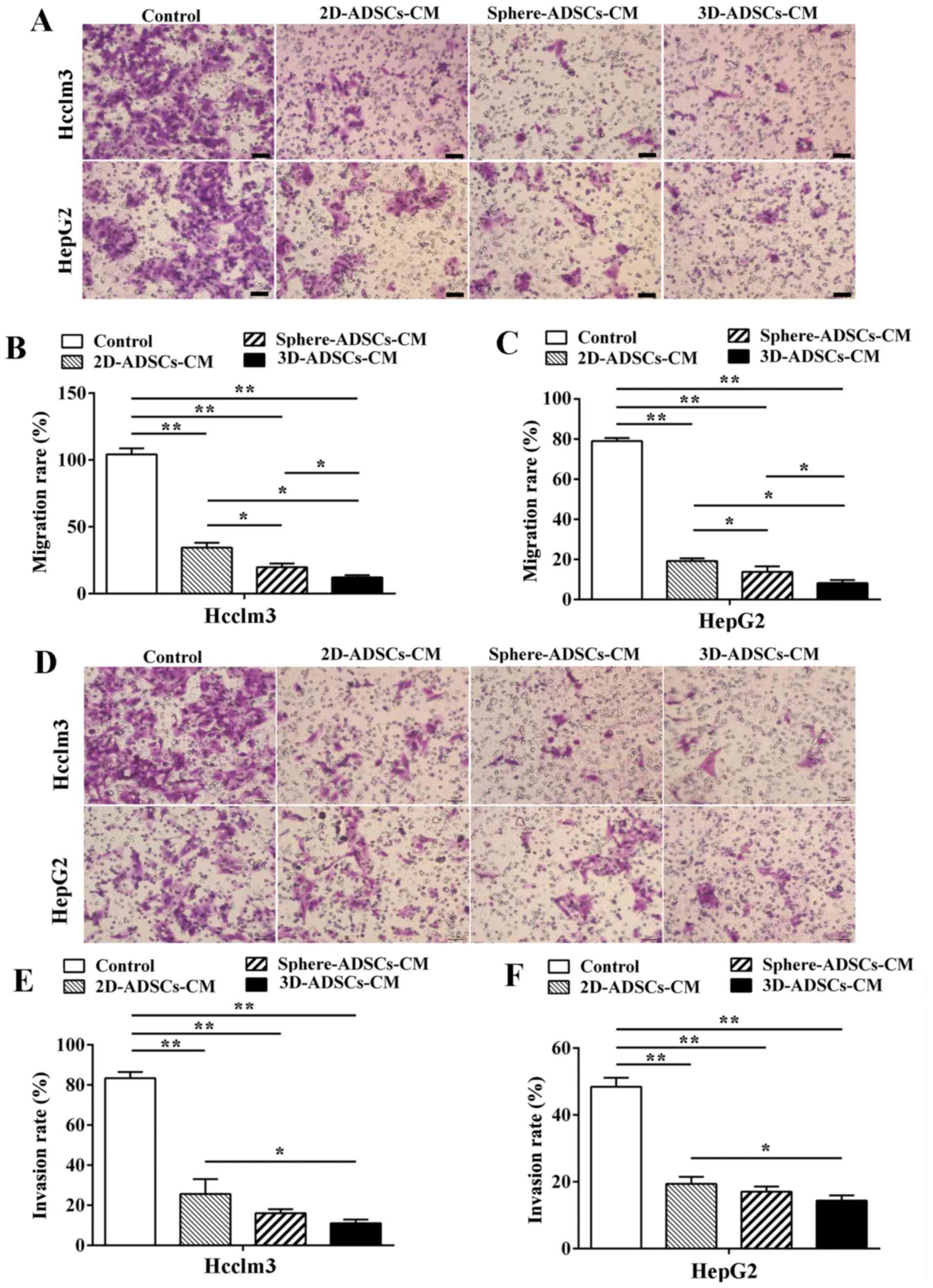 | Figure 4ADSCs-CM suppresses migration and
invasion of liver cancer cells. (A) Migration of liver cancer cells
after culturing with ADSCs-CM (×100 magnification; scale bar, 100
μm). Quantification of (B) Hcclm3 and (C) HepG2 cell
migration after culturing with ADSCs-CM. (D) Invasion of liver
cancer cells was observed after culturing with ADSCs-CM (×100
magnification; scale bar, 100 μm). Quantification of (E)
Hcclm3 and (F) HepG2 cell migration after culturing with
3D-ADSCs-CM. Control, untreated liver cancer cells; 2D-ADSCs-CM,
liver cancer cells treated with 2D-ADSCs-CM; sphere-ADSCs-CM, liver
cancer cells treated with sphere-ADSCs-CM; 3D-ADSCs-CM, liver
cancer cells treated with 3D-ADSCs-CM. For all groups, n=3.
*P<0.05 and **P<0.01. 2D,
two-dimensional; 3D, three-dimensional; ADSCs-CM, adipose
tissue-derived stem cells-conditioned medium |
Based on the observation that ASDCs-CM may suppress
liver cancer cell migration, the present study aimed to determine
whether ADSCs-CM could affect the invasion of liver cancer cells.
Hcclm3 and HepG2 cells were cultured with ADSCs-CM in
Matrigel®-coated Transwell units for 48 h, and liver
cancer cells that penetrated the membrane were examined by crystal
violet staining. Comp ared with the control liver cancer cells,
cell invasion was significantly inhibited after culturing with
ADSCs-CM (Fig. 4D–F), thus
suggesting that ADSCs may suppress the invasion of liver cancer
cells. In addition, the present results indicated that the lowest
rate of liver cancer cell invasion could be achieved after
culturing with 3D-ADSCs-CM compared with 2D-ADSCs-CM. Furthermore,
although the difference was not statistically significant, a slight
decrease in liver cancer cell invasion could be observed after
culturing with 3D-ADSCs-CM compared with sphere-ADSCs-CM. Liver
cancer cell invasion was also not significantly decreased after
culturing with sphere-ADSCs-CM compared with 2D-ADSCs-CM (Fig. 4D–F). Therefore, these results
suggested that the inhibitory effects of ADSCs on liver cancer cell
invasion could be enhanced by the 3D or sphere culture method, but
not by the sphere culture method.
ADSCs-CM suppresses
epithelial-mesenchymal transition (EMT) in liver cancer cells
In order to further explore the underlying
mechanisms by which ADSCs inhibit the migration and invasion of
liver cancer cells, the present study investigated whether ADSCs-CM
had an effect on EMT of liver cancer cells, since EMT serves an
important role in the progression of cancer invasion and metastasis
(38). Hcclm3 and HepG2 cells
were harvested after culturing with ADSCs-CM for 48 h, and EMT
markers were examined by RT-qPCR analysis. As shown in Fig. 5, ADSCs-CM significantly increased
the mRNA expression levels of E-cadherin, and downregulated the
mRNA expression levels of N-cadherin, vimentin and Zeb1 in Hcclm3
and HepG2 cells, thus indicating that ADSCs-CM could inhibit liver
cancer cell migration and invasion by downregulating EMT at the
transcriptional level.
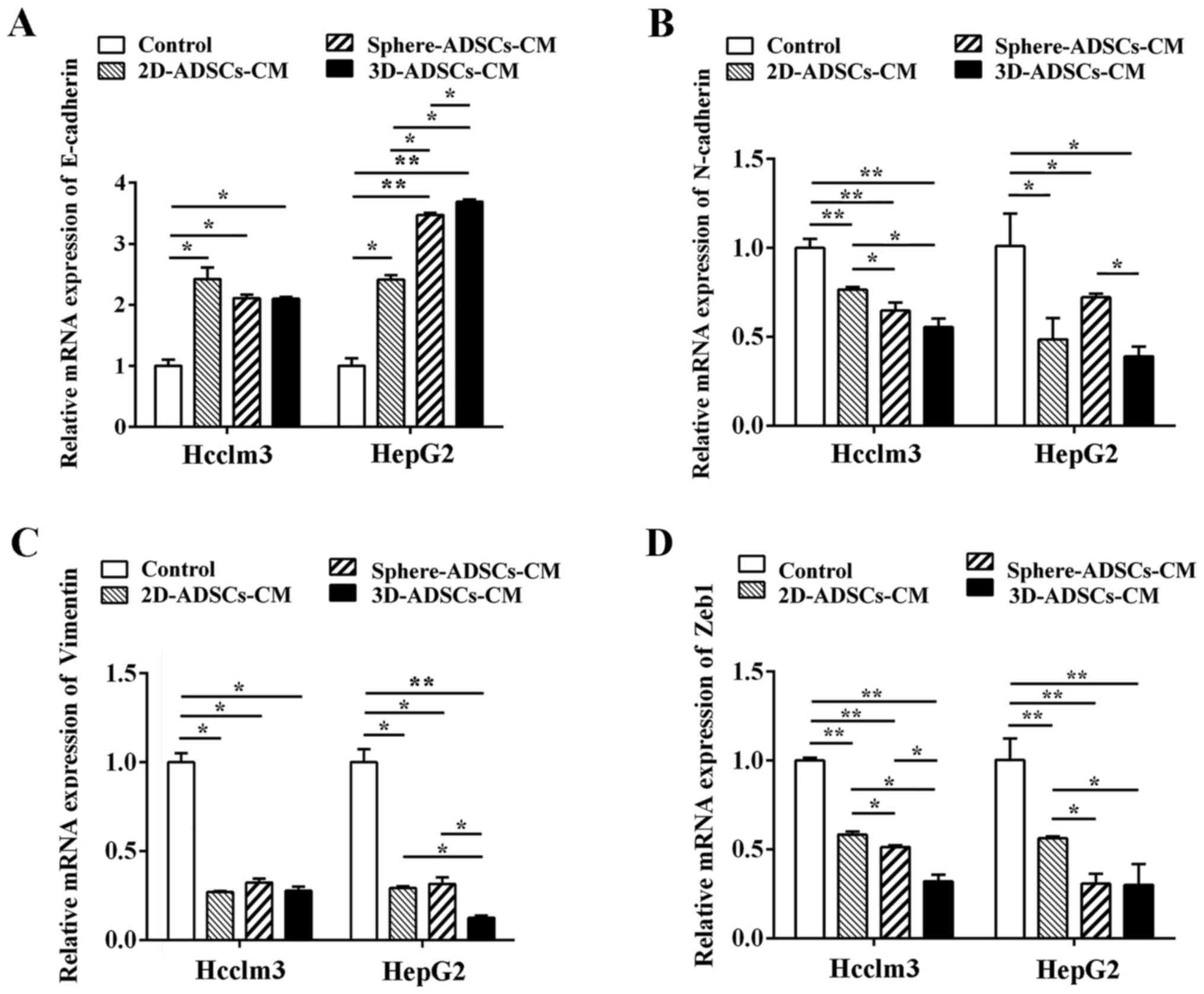 | Figure 5ADSCs-CM downregulates the mRNA
expression levels of EMT markers in liver cancer cells. mRNA
expression levels of (A) E-cadherin, (B) N-cadherin, (C) vimentin
and (D) Zeb1 in liver cancer cells after culturing with ADSCs-CM.
Control, untreated liver cancer cells; 2D-ADSCs-CM, liver cancer
cells treated with 2D-ADSCs-CM; sphere-ADSCs-CM, liver cancer cells
treated with sphere-ADSCs-CM; 3D-ADSCs-CM, liver cancer cells
treated with 3D-ADSCs-CM. For all groups, n=3.
*P<0.05 and **P<0.01. 2D,
two-dimensional; 3D, three-dimensional; ADSCs-CM, adipose
tissue-derived stem cells-conditioned medium; EMT,
epithelial-mesenchymal transition; Zeb1, zinc finger E-box-binding
homeobox 1. |
The present results also indicated that increased
expression of E-cadherin mRNA (in HepG2 cells), and reduced mRNA
expression of N-cadherin (in Hcclm3 cells) and Zeb1 (in Hcclm3 and
HepG2 cells), was observed after culturing with sphere-ADSCs-CM or
3D-ADSCs-CM compared with 2D-ADSCs-CM; the E-cadherin mRNA level
was also significantly increased in HepG2 cells after culturing
with 3D-ADSCs-CM compared with sphere-ADSCs-CM. In addition,
vimentin mRNA expr ession (in HepG2 cells) was significantly lower
after culturing with 3D-ADSCs-CM compared with 2D-ADSCs-CM or
sphere-ADSCs-CM. Reduced mRNA expression of N-cadherin (in HepG2
cells) and Zeb1 (in Hcclm3 cells) was observed after culturing with
3D-ADSCs-CM compared with sphere-ADSCs-CM; however, the mRNA
expression levels of the other EMT markers were not significantly
different among the cells cultured with the various types of
ADSCs-CM (Fig. 5). On the basis
of these results, it may be suggested that the sphere or 3D culture
methods partly enhance the effects of ADSCs on down-regulation of
the mRNA expression of EMT markers in liver cancer cells. The 3D
culture method may be more effective at enhancing the suppressive
effects of ADSCs on the mRNA expression levels of EMT markers in
liver cancer cells.
To further confirm the effects of ADSCs on
translational regulation of EMT, western blotting was used to
evaluate the protein expression levels of ETM markers in liver
cancer cells. In accordance with the results of RT-qPCR, ADSCs-CM
significantly increased E-cadherin protein expression, and
decreased the protein expression levels of N-cadherin, vimentin and
Zeb1 in liver cancer cells (Fig.
6), which further confirmed the downregulation of EMT in liver
cancer cells after culturing with ADSCs-CM.
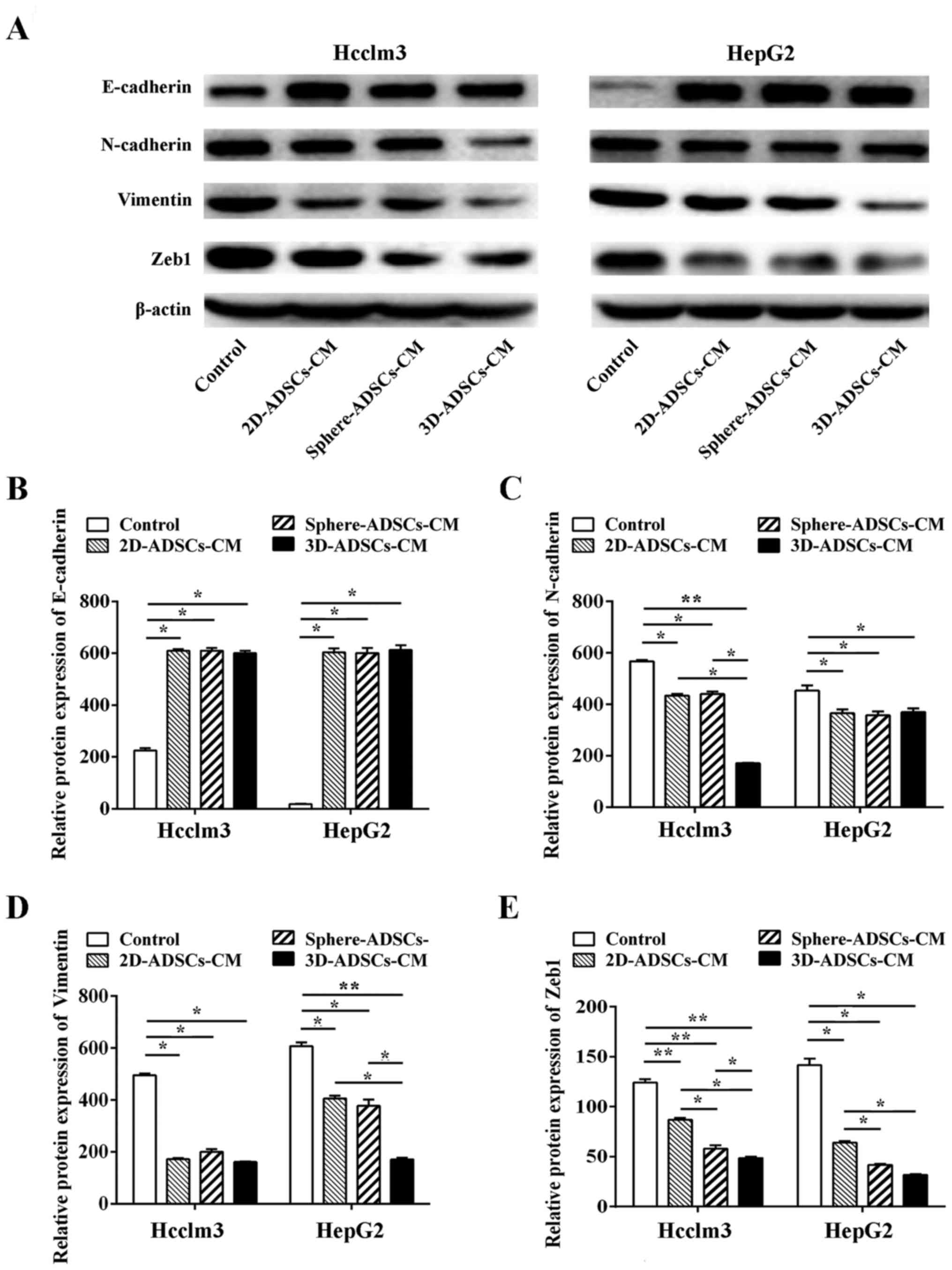 | Figure 6ADSCs-CM downregulates the protein
expression levels of EMT markers in liver cancer cells. (A) Western
blotting of EMT markers in liver cancer cells after culturing with
ADSCs-conditioned medium. Semi-quantitative analysis of (B)
E-cadherin, (C) N-cadherin, (D) vimentin and (E) Zeb1 expression in
liver cancer cells after culturing with ADSCs-CM. Control,
untreated liver cancer cells; 2D-ADSCs-CM, liver cancer cells
treated with 2D-ADSCs-CM; sphere-ADSCs-CM, liver cancer cells
treated with sphere-ADSCs-CM; 3D-ADSCs-CM, liver cancer cells
treated with 3D-ADSCs-CM. For all groups, n=3.
*P<0.05 and **P<0.01. 2D,
two-dimensional; 3D, three-dimensional; ADSCs-CM, adipose
tissue-derived stem cells-conditioned medium; EMT,
epithelial-mesenchymal transition; Zeb1, zinc finger E-box-binding
homeobox 1. |
The results of western blotting indicated that
sphere-ADSCs-CM could enhance the downregulation of Zeb1 expression
in HepG2 cells compared with 2D-ADSCs-CM; in addition, 3D-ADSCs-CM
could further enhance the downregulation of N-cadherin (in Hcclm3
cells), vimentin (in HepG2 cells) and Zeb1 (in Hcclm3 cells)
compared with 2D-ADSCs-CM or sphere-ADSCs-CM. However, the
expression of other EMT markers was not significantly different
among cells cultured with various types of ADSCs-CM (Fig. 6B–E). Therefore, these data
confirmed that the sphere or 3D culture methods could partly
enhance the effects of ADSCs on downregulation of EMT in liver
cancer cells, and the 3D culture method may be more effective at
enhancing the suppressive effects of ADSCs on EMT in liver cancer
cells.
Discussion
Autologous MSCs represent an attractive source for
cell-based regenerative medicine, since these cells are present in
peripheral blood, bone marrow and nearly all adult tissues or
organs, including adipose tissue, dermis, muscle, liver, spleen and
lung (39). In particular, ADSCs
have been suggested as a novel promising strategy for the treatment
of various major diseases, including cardiovascular diseases, liver
diseases and cerebral diseases, due to their many advantages, such
as increased abundance and easy acquisition compared with other
types of MSC (23–25,40). However, the clinical application
of ADSCs-based therapy has yet to be implemented, due to numerous
aspects. Firstly, the quality control of ADSCs preparation should
be confirmed, since various methods of cell isolation and
preparation may lead to the transplantation of subtly different
cell populations (41). Secondly,
the clinical use of ADSCs may be hampered by the lack of
understanding of cell behavior following transplantation and the
mechanisms by which ADSCs provide therapeutic effects (41,42). Notably, there remain several
risks, including adverse events, ectopic tissue formation and in
vivo tumorigenic safety after cell transplantation (43). Among these barriers to the
clinical application of ADSCs, safety is the prerequisite for
ADSCs-based therapy; therefore, the safety of ADSCs has attracted a
great deal of interest in cell-based regenerative medicine.
Although it has been clinically proven that
autologous ADSCs exhibit short-lived safety for patients (44,45), the long-term safety, particularly
tumorigenic safety, remains controversial. It has previously been
reported that ADSCs may promote tumor growth due to properties of
regeneration and vascularization, which are closely associated with
tumor initiation and metastasis (46); however, other studies indicated
that ADSCs may inhibit tumor progression, due to their attributes,
including tumor-homing instinct, immunological characterization,
and their capacity for self-renewal and potential for
differentiation (28,29). It is generally accepted that
molecules secreted from ADSCs may influence the effects of ADSCs on
tumor growth. Therefore, the culture conditions of ADSCs may have
an important role in determining the association between ADSCs and
tumor cells, since various culture conditions could affect the
secretion of molecules from ADSCs (32,33). Notably, Tian et al reported
that human MSCs may inhibit proliferation of cancer cells in
vitro and enhance tumor growth in vivo (34), thus suggesting that MSCs exert a
dual effect on the same tumor under various growing conditions.
Therefore, the present study used various culture methods,
including 2D culture, sphere culture and AlgiMatrix™ 3D culture, to
determine the effects of ADSCs on liver cancer cell growth. The
results indicated that ADSCs-CM could inhibit the cell
proliferation, motility and adhesive capacity, as well as migration
and invasion of liver cancer cells, and could also promote
apoptosis of liver cancer cells, thus clearly suggesting that ADSCs
may inhibit liver cancer cell growth. It has previously been
reported that 2D-ADSCs-CM may inhibit HCC cell (SMMC7721) growth
and promote cell death via downregulation of protein kinase B
signaling (47). In addition,
MSCs have previously effectively inhibited cell growth and promoted
apoptosis of HepG2 cells (48).
In concordance with these previous results, the present study
revealed that ADSCs-CM inhibited cell growth of HCC-derived Hcclm3
cells and hepatoblastoma-derived HepG2 cells.
It has been reported that sphere or 3D culture
methods may promote the secretion of cytokines and chemo kines from
ADSCs (32,33); consequently, sphere or 3D culture
conditions may theoretically enhance the effects of ADSCs on tumor
cells. As predicted, the present study demonstrated that sphere or
3D culture methods could enhance the inhibitory effects of ADSCs on
liver cancer cell migration, and could accelerate the effects of
ADSCs on liver cancer cell apoptosis. Furthermore, more obvious
inhibitory effects of ADSCs on liver cancer cell migration could be
achieved using the 3D culture method compared with the sphere
culture method. Therefore, the present study indicated that the
culture methods could alter the effects of ADSCs on liver cancer
cell growth, and the 3D culture method may be considered the best
candidate to enhance the effects of ADSCs on the inhibition of
liver cancer cell growth. It has previously been reported that
sphere or 3D culture methods could more closely mimic the ADSCs
in vivo environment compared with the traditional 2D culture
method (49–51). Accordingly, the present study
confirmed that ADSCs could inhibit liver cancer cell growth;
therefore, ADSCs may exert therapeutic effects in the treatment of
patients with liver cancer. However, further in vivo studies
are required prior to its clinical use, and further studies
regarding the ADSCs-derived antitumor molecules are required, in
order to investigate the underlying antitumor mechanisms.
Numerous studies have demonstrated that ADSCs may
repair injured tissues by inhibiting inflammation (24,52), and may also inhibit transforming
growth factor (TGF)-β1 signaling in animal models of experimental
peritoneal fibrosis (52).
Notably, inflammatory cytokines, such as TGF-β and epidermal growth
factor, may stimulate EMT of tumor cells (53). Therefore, the present study aimed
to determine whether ADSCs could suppress the migration and
invasion of liver cancer cells via the inhibition of EMT using
RT-qPCR and western blotting. The results indicated that culturing
with three types of ADSCs-CM upregulated E-cadherin expression, and
downregulated N-cadherin, vimentin and Zeb1 expression, thus
suggesting that ADSCs may suppress liver cancer cell migration and
invasion via the downregulation of EMT signaling. Therefore, ADSCs
may be used to prevent the recurrence and metastasis of liver
cancer.
In conclusion, the present study demonstrated that
ADSCs may promote liver cancer cell apoptosis, and inhibit liver
cancer cell proliferation, motility and adhesion, as well as cell
migration and invasion via downregulation of EMT signaling. ADSCs
may provide a novel promising therapeutic approach for the
treatment of patients with liver cancer. In addition, the 3D
culture method could enhance the inhibitory effects of ADSCs on
liver cancer cell growth, and may provide a novel approach to
explore the association between ADSCs and tumor.
Acknowledgments
The present study was supported by the specialized
Science and Technology Key Project of Fujian Province (grant no.
2013YZ0002-3), the Science and Technology Infrastructure
Construction Program of Fujian Province (grant no. 2014Y2005), the
Youth Scientific Research Project of Fujian Provincial Health and
Family Planning Commission (grant no. 2017-1-85), the Scientific
Innovation Project of Fujian Provincial Health and Family Planning
Commission (grant no. 2014-CXB-24), the Scientific Foundation of
Fuzhou Health Department (grant nos. 2013-S-wq15, 2013-S-wp1,
2014-S-wq-17, 2015-S-wq13 and 2014-S-wq20), and the Project of
Fuzhou Science and Technology Department (grant nos. 2014-S-139-3,
2016-s-124-9 and 2016-s-124-4). Parts of this work were presented
as an Abstract at the 26th Annual Conference of APASL (February
15-19, 2017, Shanghai, China).
References
|
1
|
Hashemi M and Kalalinia F: Application of
encapsulation technology in stem cell therapy. Life Sci.
143:139–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ojeh N, Pastar I, Tomic-Canic M and
Stojadinovic O: Stem cells in skin regeneration, wound healing, and
their clinical applications. Int J Mol Sci. 16:25476–25501. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daughtry B and Mitalipov S: Concise
review: parthenote stem cells for regenerative medicine: genetic,
epigenetic, and developmental features. Stem Cells Transl Med.
3:290–298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao T, Zhang ZN, Rong Z and Xu Y:
Immunogenicity of induced pluripotent stem cells. Nature.
474:212–215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Panchalingam KM, Jung S, Rosenberg L and
Behie LA: Bioprocessing strategies for the large-scale production
of human mesenchymal stem cells: a review. Stem Cell Res Ther.
6:2252015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serafini M, Dylla SJ, Oki M, Heremans Y,
Tolar J, Jiang Y, Buckley SM, Pelacho B, Burns TC, Frommer S, et
al: Hema topoietic reconstitution by multipotent adult progenitor
cells: precursors to long-term hematopoietic stem cells. J Exp Med.
204:129–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Nbaheen M, Vishnubalaji R, Ali D,
Bouslimi A, Al-Jassir F, Megges M, Prigione A, Adjaye J, Kassem M
and Aldahmash A: Human stromal (mesenchymal) stem cells from bone
marrow, adipose tissue and skin exhibit differences in molecular
phenotype and differentiation potential. Stem Cell Rev. 9:32–43.
2013. View Article : Google Scholar :
|
|
8
|
Sarugaser R, Lickorish D, Baksh D,
Hosseini MM and Davies JE: Human umbilical cord perivascular
(HUCPV) cells: a source of mesenchymal progenitors. Stem Cells.
23:220–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salem HK and Thiemermann C: Mesenchymal
stromal cells: current understanding and clinical status. Stem
Cells. 28:585–596. 2010.
|
|
12
|
Cui L, Yin S, Liu W, Li N, Zhang W and Cao
Y: Expanded adipose-derived stem cells suppress mixed lymphocyte
reaction by secretion of prostaglandin E2. Tissue Eng.
13:1185–1195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ivanova-Todorova E, Bochev I, Mourdjeva M,
Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I and
Kyurkchiev DS: Adipose tissue-derived mesenchymal stem cells are
more potent suppressors of dendritic cells differentiation compared
to bone marrow-derived mesenchymal stem cells. Immunol Lett.
126:37–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park KJ, Ryoo SB, Kim JS, Kim TI, Baik SH,
Kim HJ, Lee KY, Kim M and Kim WH: Allogeneic adipose-derived stem
cells for the treatment of perianal fistula in Crohn's disease: a
pilot clinical trial. Colorectal Dis. 18:468–476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de la Portilla F, Alba F, García-Olmo D,
Herrerías JM, González FX and Galindo A: Expanded allogeneic
adipose-derived stem cells (eASCs) for the treatment of complex
perianal fistula in Crohn's disease: results from a multicenter
phase I/IIa clinical trial. Int J Colorectal Dis. 28:313–323. 2013.
View Article : Google Scholar
|
|
16
|
Herreros MD, Garcia-Arranz M, Guadalajara
H and De-La-Quintana P: Autologous expanded adipose-derived stem
cells for the treatment of complex cryptoglandular perianal
fistulas: a phase III randomized clinical trial (FATT 1: fistula
Advanced Therapy Trial 1) and long-term evaluation. Dis Colon
Rectum. 55:762–772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garcia-Olmo D, Herreros D, Pascual I,
Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P,
Garcia-Arranz M and Pascual M: Expanded adipose-derived stem cells
for the treatment of complex perianal fistula: a phase II clinical
trial. Dis Colon Rectum. 52:79–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
García-Olmo D, García-Arranz M, Herreros
D, Pascual I, Peiro C and Rodríguez-Montes JA: A phase I clinical
trial of the treatment of Crohn's fistula by adipose mesenchymal
stem cell transplantation. Dis Colon Rectum. 48:1416–1423. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizushima T, Takahashi H, Takeyama H,
Naito A, Haraguchi N, Uemura M, Nishimura J, Hata T, Takemasa I,
Yamamoto H, et al: A clinical trial of autologous adipose-derived
regenerative cell transplantation for a postoperative
enterocutaneous fistula. Surg Today. 46:835–842. 2016. View Article : Google Scholar
|
|
20
|
Jo CH, Lee YG, Shin WH, Kim H, Chai JW,
Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, et al: Intra-articular
injection of mesenchymal stem cells for the treatment of
osteoarthritis of the knee: a proof-of-concept clinical trial. Stem
Cells. 32:1254–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Díez-Tejedor E, Gutiérrez-Fernández M,
Martínez-Sánchez P, Rodríguez-Frutos B, Ruiz-Ares G, Lara ML and
Gimeno BF: Reparative therapy for acute ischemic stroke with
allogeneic mesenchymal stem cells from adipose tissue: a safety
assessment: a phase II randomized, double-blind,
placebo-controlled, single-center, pilot clinical trial. J Stroke
Cerebrovasc Dis. 23:2694–2700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tzouvelekis A, Paspaliaris V, Koliakos G,
Ntolios P, Bouros E, Oikonomou A, Zissimopoulos A, Boussios N,
Dardzinski B, Gritzalis D, et al: A prospective, non-randomized, no
placebo-controlled, phase Ib clinical trial to study the safety of
the adipose derived stromal cells-stromal vascular fraction in
idiopathic pulmonary fibrosis. J Transl Med. 11:1712013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Ma X, Zhao L, Zhou X, Ma Y, Sun H,
Yang Y and Chen B: Injection of basic fibroblast growth factor
together with adipose-derived stem cell transplantation: improved
cardiac remodeling and function in myocardial infarction. Clin Exp
Med. 16:539–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seki A, Sakai Y, Komura T, Nasti A,
Yoshida K, Higashimoto M, Honda M, Usui S, Takamura M, Takamura T,
et al: Adipose tissue-derived stem cells as a regenerative therapy
for a mouse steatohepatitis-induced cirrhosis model. Hepatology.
58:1133–1142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You D, Jang MJ, Kim BH, Song G, Lee C, Suh
N, Jeong IG, Ahn TY and Kim CS: Comparative study of autologous
stromal vascular fraction and adipose-derived stem cells for
erectile function recovery in a rat model of cavernous nerve
injury. Stem Cells Transl Med. 4:351–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chu Y, Tang H, Guo Y, Guo J, Huang B, Fang
F, Cai J and Wang Z: Adipose-derived mesenchymal stem cells promote
cell proliferation and invasion of epithelial ovarian cancer. Exp
Cell Res. 337:16–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muehlberg FL, Song YH, Krohn A, Pinilla
SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM,
Devarajan E, et al: Tissue-resident stem cells promote breast
cancer growth and metastasis. Carcinogenesis. 30:589–597. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu X, Su B, Ge P, Wang Z, Li S, Huang B,
Gong Y and Lin J: Human adipose derived stem cells induced cell
apoptosis and S phase arrest in bladder tumor. Stem Cells Int 2015.
619290:2015. View Article : Google Scholar
|
|
29
|
Sun B, Roh KH, Park JR, Lee SR, Park SB,
Jung JW, Kang SK, Lee YS and Kang KS: Therapeutic potential of
mesenchymal stromal cells in a mouse breast cancer metastasis
model. Cytotherapy. 11:289–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rettinger CL, Fourcaudot AB, Hong SJ,
Mustoe TA, Hale RG and Leung KP: In vitro characterization of
scaffold-free three-dimensional mesenchymal stem cell aggregates.
Cell Tissue Res. 358:395–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ballotta V, Smits AI, Driessen-Mol A,
Bouten CV and Baaijens FP: Synergistic protein secretion by
mesenchymal stromal cells seeded in 3D scaffolds and circulating
leukocytes in physiological flow. Biomaterials. 35:9100–9113. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhang SH, Lee S, Shin JY, Lee TJ, Jang HK
and Kim BS: Efficacious and clinically relevant conditioned medium
of human adipose-derived stem cells for therapeutic angiogenesis.
Mol Ther. 22:862–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang CM, Huang YJ and Hsu SH: Enhanced
autophagy of adipose-derived stem cells grown on chitosan
substrates. Biores Open Access. 4:89–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian LL, Yue W, Zhu F, Li S and Li W:
Human mesenchymal stem cells play a dual role on tumor cell growth
in vitro and in vivo. J Cell Physiol. 226:1860–1867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan F, Liao N, Zheng Y, Wang Y, Gao Y,
Wang S, Jiang Y and Liu X: Intrahepatic transplantation of
adipose-derived stem cells attenuates the progression of
non-alcoholic fatty liver disease in rats. Mol Med Rep.
12:3725–3733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lopez-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
38
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
39
|
Tobita M, Tajima S and Mizuno H: Adipose
tissue-derived mesenchymal stem cells and platelet-rich plasma:
stem cell transplantation methods that enhance stemness. Stem Cell
Res Ther. 6:2152015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bura A, Planat-Benard V, Bourin P,
Silvestre JS, Gross F, Grolleau JL, Saint-Lebese B, Peyrafitte JA,
Fleury S, Gadelorge M, et al: Phase I trial: the use of autologous
cultured adipose-derived stroma/stem cells to treat patients with
non-revascularizable critical limb ischemia. Cytotherapy.
16:245–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feisst V, Meidinger S and Locke MB: From
bench to bedside: use of human adipose-derived stem cells. Stem
Cells Cloning. 8:149–162. 2015.PubMed/NCBI
|
|
42
|
Srijaya TC, Ramasamy TS and Kasim NH:
Advancing stem cell therapy from bench to bedside: lessons from
drug therapies. J Transl Med. 12:2432014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vériter S, André W, Aouassar N, Poirel HA,
Lafosse A, Docquier PL and Dufrane D: Human adipose-derived
mesenchymal stem cells in cell therapy: safety and feasibility in
different 'Hospital Exemption' clinical applications. PLoS One.
10:e01395662015. View Article : Google Scholar
|
|
44
|
Fodor PB and Paulseth SG: Adipose derived
stromal cell (ADSC) injections for pain management of
osteoarthritis in the human knee joint. Aesthet Surg J. 36:229–236.
2016. View Article : Google Scholar
|
|
45
|
Pak J, Chang JJ, Lee JH and Lee SH: Safety
reporting on implantation of autologous adipose tissue-derived stem
cells with platelet-rich plasma into human articular joints. BMC
Musculoskelet Disord. 14:3372013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ritter A, Friemel A, Fornoff F, Adjan M,
Solbach C, Yuan J and Louwen F: Characterization of adipose-derived
stem cells from subcutaneous and visceral adipose tissues and their
function in breast cancer cells. Oncotarget. 6:34475–34493. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao W, Ren G, Zhang L, Zhang Z, Liu J,
Kuang P, Yin Z and Wang X: Efficacy of mesenchymal stem cells
derived from human adipose tissue in inhibition of hepatocellular
carcinoma cells in vitro. Cancer Biother Radiopharm. 27:606–613.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J,
Hu H, Guan W and Ma Y: Inhibitory effect and mechanism of
mesenchymal stem cells on liver cancer cells. Tumour Biol.
35:1239–1250. 2014. View Article : Google Scholar
|
|
49
|
Ylostalo JH, Bartosh TJ, Tiblow A and
Prockop DJ: Unique characteristics of human mesenchymal
stromal/progenitor cells pre-activated in 3-dimensional cultures
under different conditions. Cytotherapy. 16:1486–1500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cesarz Z and Tamama K: Spheroid culture of
mesenchymal stem cells. Stem Cells Int 2016. 9176357:2016.
View Article : Google Scholar
|
|
51
|
Khodabandeh Z, Vojdani Z, Talaei-Khozani
T, Jaberipour M, Hosseini A and Bahmanpour S: Comparison of the
expression of hepatic genes by human Wharton's jelly mesenchymal
stem cells cultured in 2D and 3D collagen culture systems. Iran J
Med Sci. 41:28–36. 2016.PubMed/NCBI
|
|
52
|
Wakabayashi K, Hamada C, Kanda R, Nakano
T, Io H, Horikoshi S and Tomino Y: Adipose-derived mesenchymal stem
cells transplantation facilitate experimental peritoneal fibrosis
repair by suppressing epithelial-mesenchymal transition. J Nephrol.
27:507–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lv N, Gao Y, Guan H, Wu D, Ding S, Teng W
and Shan Z: Inflammatory mediators, tumor necrosis factor-α and
interferon-γ, induce EMT in human PTC cell lines. Oncol Lett.
10:2591–2597. 2015. View Article : Google Scholar : PubMed/NCBI
|