Introduction
Abnormal wound healing processes are present in
keloids, including excessive scarring, hyperproliferation of
fibroblasts and overabundant deposition of extracellular matrix
(ECM) components (1). Keloids
have been reported to occur after a certain degree or type of skin
wound (2). Although the
pathogenesis of keloids remains obscure, it has previously been
indicated that it involves aberrant cell activities and intricate
signaling pathways between various cellular populations (3). The clinical features of keloids are
as follows: i) Keloid scars exceed the original margins and invade
adjacent healthy tissue, thus behaving similarly to 'invasive'
benign skin tumors; ii) keloids seldom exhibit expected regression
with time; iii) keloids usually recur following regular treatment
(4). Butler et al
(5) presented four histological
features specific for keloids: i) Peculiar hyalinized and
eosinophilic collagen in keloids; ii) tongue-like advancing edge
underneath normal-appearing epidermis and papillary dermis; iii)
horizontal cellular fibrous bands in the upper reticular dermis;
iv) prominent fascia-like fibrous bands.
Keloids are often associated with pain and pruritus,
and are considered unsightly; therefore, they may affect patients'
mood and have an impact on quality of life. At present, there are
numerous multilevel therapies available for the treatment of
keloids, including silicon membrane, intralesional corticosteroid
or 5-fluorouracil injections, and cryosurgery or conventional
surgery with additional corticosteroids treatment or radiotherapy
(6). Although a wide range of
therapeutic options exists for keloid treatment, all of which have
their own strengths, a high risk of side-effects and frequent
recurrence remains (7).
Therefore, it is urgent and of great importance to identify
improved therapeutic approaches or drugs for the treatment of
keloids.
Ginsenoside Rg3 (Rg3) is a traditional Chinese
medicine, which is extracted from Panax ginseng. The
pharmacological components of ginseng include ginsenosides,
triterpene glycosides and secondary metabolites. There are two
optical isomers of Rg3, named 20R-Rg3 and 20S-Rg3, which result in
different hydroxyl positions at carbon-20. It has been suggested
that Rg3 exerts numerous biological activities, and has a wide
range of clinical and pharmacological effects (8). A previous study indicated that Rg3
may inhibit the proliferation of several types of tumor cell and
may induce apoptosis (9). The
anticarcinogenic effects of Rg3 have been demonstrated in
vitro and in vivo. He et al (10) reported that the proliferation of
colorectal cancer cells was inhibited by Rg3 via the Wnt/β-catenin
pathway. Wang et al (11)
revealed that Rg3 can induce the apoptosis of ovarian cancer cells
by inhibiting the phosphoinositide 3-kinase/protein kinase B
pathway. Previous studies have also reported that Rg3 may decrease
angiogenesis in tumors via the downregulation of the cytokine
vascular endothelial growth factor (VEGF) (12) or via a shortage of oxygen
(13).
Due to the tumor-like biological features of keloids
and the characteristics of Rg3, particularly the numerous antitumor
effects, it has been suggested that Rg3 may be a potential
therapeutic agent that targets keloids. Although Pazyar et
al (14) reported that
ginseng was effective against keloid scarring, this was an indirect
conclusion deduced from other studies, which did not directly
investigate the effects of ginseng on keloids, but investigated the
molecular mechanisms underlying keloid formation and the effects of
ginseng on other cell lines. Furthermore, no specific empirical
data was provided. The present study aimed to be the first, to the
best of our knowledge, to investigate the effects of Rg3 on human
keloid fibroblasts (KFs) in vitro, and to explore the
related molecular and cellular mechanisms.
Materials and methods
Materials and chemicals
Rg3 (purity, 98.6%) was purchased from Dalian
Fusheng Pharmaceutical, Ltd. (Dalian, China). Rg3 was dissolved in
dimethyl sulfoxide (dMSo) and filtered through a 0.22 μm
bacterial filter. The mixture was then diluted with dulbecco's
modified eagle's medium (dMeM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
HyClone; GE Healthcare life Sciences, logan, UT, USA) to form the
final concentrations (50 or 100 μg/ml). The final
concentrations of Rg3 were selected according to previous studies
and based on existing data regarding the effective dose (15,16). The final concentrations of DMSO in
the culture medium were <0.1%.
Subjects
A total of 15 Asian patients with keloids were
recruited to the present study. Keloid scar specimens were obtained
from these patients, which were aged between 22 and 35 years old,
without systemic diseases. All patient information is provided in
Table I. The lesions were
diagnosed as keloids according to clinical appearance, symptoms,
persistence for >1 year and extension beyond the original
margins. All of the patients' keloids were in the active stage and
none had undergone prior treatment. Prior to surgery, all patients
were informed of the purpose and procedure of the present study and
agreed to provide their resected lesion masses. The whole keloid
tissues were completely removed, following administration of local
anesthesia, from the skin of the neck, chest, abdomen and upper
limb, according to the standard surgical procedures. Prior written
informed consent was obtained from all participants and the present
study was approved by the Ethics Committee of Shanghai Ninth
People's Hospital affiliated Shanghai Jiao Tong University School
of Medicine (Shanghai, China).
 | Table IPatient and keloid specimen
information. |
Table I
Patient and keloid specimen
information.
| Sample no. | Gender | Ethnic group | Age (years) | Size of sample
(cm2) |
|---|
| S1 | M | Asian | 23 | 8×3 |
| S2 | M | Asian | 33 | 2×4 |
| S3 | M | Asian | 22 | 5×3 |
| S4 | F | Asian | 23 | 4×3 |
| S5 | M | Asian | 23 | 4×2 |
| S6 | M | Asian | 25 | 7×1 |
| S7 | F | Asian | 28 | 5×2 |
| S8 | F | Asian | 27 | 6×1 |
| S9 | M | Asian | 30 | 5.5×3 |
| S10 | M | Asian | 31 | 3.5×4 |
| S11 | M | Asian | 34 | 6×2 |
| S12 | F | Asian | 33 | 2×1 |
| S13 | F | Asian | 21 | 9×2 |
| S14 | F | Asian | 35 | 4.5×2.5 |
| S15 | M | Asian | 29 | 3×2 |
Culture of KFs
The adipose tissues and epidermis were removed from
the samples using sterilized scissors; the remaining dermis was cut
into 1×1 mm sections and digested in 0.25% collagenase for ~4 h at
37°C. Following centrifugation at 300 × g at room temperature for 5
min, the supernatant was discarded and the remaining precipitate,
including abundant KFs and several pieces of dermis tissue was
cultured in high glucose DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS, penicillin (100 U/ml) and
streptomycin (100 μg/ml) (Sigma-Aldrich; Merck KGaA,
darmstadt, Germany) in 100 mm dishes at 37°C in a humidified
incubator containing 5% Co2. The culture media were
replaced every 3 days. The fibroblasts gradually attached to the
dish or migrated out of the small pieces of dermis tissue within
7–10 days. KFs between passages 2 and 4 were used in the present
study.
Microscopic observation
The present study analyzed Rg3-induced cell death by
observing morphological alterations. KFs were seeded in 6-well
plates at a density of 8×104 cells/well and were
cultured at 37°C in an atmosphere containing 5% Co2.
Medium with DMSO (<0.1%) and with various concentrations of Rg3
(50 or 100 μg/ml) was added to the grouped wells. Following
a 72 h incubation at 37°C, KFs in the various groups were observed
and images were captured under an inverted microscope (Nikon IX70;
Nikon Corporation, Tokyo, Japan).
Cell viability assay
The viability of KFs treated with or without Rg3 was
determined using the Cell Counting kit-8 (CCK-8) assay (dojindo
Molecular Technologies, Inc., Kumamoto, Japan). The KFs were
cultured in serum-free medium for 24 h for synchronization.
Subsequently, the cells were incubated with DMEM containing 10% FBS
for the following experiments. KFs were seeded in 96-well plates at
a density of 1×104 cells/ml and were incubated overnight
for 24 h at 37°C in an atmosphere containing 5% CO2.
Medium with various Rg3 concentrations (50 or 100 μg/ml) was
then added to the wells. After treatment for 1, 2, 3, 4 and 5 days,
10 μl CCK-8 was added to each well, and the cells were
incubated at 37°C for 2.5 h according to the manufacturer's
protocol. Aliquots (100 μl) of incubated medium were
pipetted into a 96-well plate and colorimetric absorbance was
recorded at 450 nm using a microplate reader (Thermo Labsystems,
Helsinki, Finland).
Flow cytometry (FCM) analysis of Annexin
V-fluorescein isothiocyanate (FITC) staining
The present study applied the FCM method to measure
and analyze the rate of apoptosis according to the instructions
provided by the Annexin V-FITC kit (Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany). The KFs were incubated in 6-well plates with
medium containing various Rg3 concentrations (50 or 100
μg/ml). Following a 72 h incubation at 37°C, the KFs in each
well were collected by centrifugation at 300 × g at room
temperature for 5 min and washed twice with cold PBS. These cells
were then resuspended in 500 μl binding buffer, and
incubated with 10 μl Annexin V-FITC for 10 min at room
temperature in the dark. Subsequently, 10 μl propidium
iodide was added to the cells for 10 min at room temperature in the
dark; the reaction was terminated by chilling in an ice-bath.
Analysis of apoptotic rate was conducted using a flow cytometer (BD
Biosciences, San Jose, CA, USA); >10,000 cells from each well
were counted and the apoptotic percentage, as well as the
percentage of necrotic cell death, was quantitatively analyzed
using CellQuest software 5.1 (BD Biosciences).
RNA isolation and quantitative polymerase
chain reaction (qPCR)
KFs were added to a 10 cm culture dish at a density
of 5×104 cells/ml. After 48 h, the medium was changed
and the cells were cultured for 72 h in fresh medium with or
without various Rg3 concentrations (50 or 100 μg/ml).
Subsequently, KFs were harvested and total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Reverse transcription was then performed; briefly, cdNA was
synthesized from 2 μg total RNA using oligo(dT) and AMV
reverse transcriptase (Promega Corporation, Madison, WI, USA). The
reverse transcription was conducted according to manufacturer's
protocol. RNA integrity and the success of reverse transcription
were monitored by qPCR amplification of GAPdH transcripts. qPCR was
conducted using the Power SYBR-Green PCR master mix (2X) (Applied
Biosystems; Thermo Fisher Scientific, Inc.) on a real-time thermal
cycler (Mx3000P™ qPCR system; Agilent Technologies, Inc., Santa
Clara, CA, USA). The mixture was incubated at 95°C for 10 min,
followed by 40 cycles (30 sec at 95°C, 30 sec at annealing
temperature listed in Table II),
and was finally incubated at 72°C for 5 min. The amplified products
were normalized against the internal reference gene (GAPDH). GAPGH
was amplified as an internal control, and relative gene expression
analysis was performed using the 2−ΔΔCq method (17). The primers for qPCR analysis are
listed in Table II.
 | Table IIPrimer pairs used for quantitative
polymerase chain reaction analysis. |
Table II
Primer pairs used for quantitative
polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) | Annealing
temperature (°C) | Product size
(bp) |
|---|
| Type I
collagen | Forward:
GGCGGCCAGGGCTCCGACCC | | |
| Reverse:
AATTCCTGGTCTGGGGCACC | 60 | 319 |
| Type III
collagen | Forward:
TGGTGTTGGAGCCGCTGCCA | | |
| Reverse:
CTCAGCACTAGAATCTGTCC | 60 | 346 |
| Fibronectin | Forward:
GCCACTGGAGTCTTTACCACA | | |
| Reverse:
CCTCGGTGTTGTAAGGTGGA | 58 | 61 |
| α-SMA | Forward:
CATCATGCGTCTGGATCTGG | | |
| Reverse:
GGACAATCTCACGCTCAGCA | 60 | 107 |
| CTGF | Forward:
ACAAGGGCCTCTTCTGTGACTT | | |
| Reverse:
GGTACACCGTACCACCGAAGAT | 60 | 102 |
| IFN-γ | Forward:
TGCAGGTCATTCAGATGTAGCGGA | | |
| Reverse:
TGTCTTCCTTGATGGTCTCCACACTC | 60 | 182 |
| TGF-β1 | Forward:
GAAGTGGATCCACGAGCCCAAG | | |
| Reverse:
GCTGCACTTGCAGGAGCGCAC | 60 | 227 |
| TGF-β3 | Forward:
GGTTTTCCGCTTCAATGTGT | | |
| Reverse:
GCTCGATCCTCTGCTCATTC | 60 | 119 |
| VEGF | Forward:
ACGAAGTGGTGAAGTTCATGGAA | | |
| Reverse:
AAGATGTCCACCAAGGTCTCGAT | 60 | 73 |
| PAI-1 | Forward:
TCATCATCAATGACTGGGTGAAGAC | | |
| Reverse:
TTCCACTGGCCGTTGAAGTAGAG | 60 | 127 |
| Smad-7 | Forward:
GTGGCATACTGGGAGGAGAA | | |
| Reverse:
GATGGAGAAACCAGGGAACA | 60 | 309 |
| MMP-1 | Forward:
GGAGCTGTAGATGTCCTTGGGGT | | |
| Reverse:
GCCACAACTGCCAAATGGGCTT | 60 | 139 |
| MMP-3 | Forward:
AGGACAAAGCAGGATCACAGTTG | | |
| Reverse:
CCTGGTACCCACGGAACCT | 58 | 68 |
| GAPDH | Forward:
TCACCATCTTCCAGGAGCG | | |
| Reverse:
CTGCTTCACCACCTTCTTGA | 60 | 572 |
Scratch wound assay
The scratch wound assay was used to evaluate the
migration of KFs (18). Briefly,
KFs (2×105 cells/well) were plated into 6-well culture
plates and were incubated until they reached ~100% confluence. A
scratch wound was generated on the cell monolayer using a sterile
200 μl pipette tip, in order to form a cell-free 'wound'
~0.83±0.05 mm in width. The cell cultures were incubated with fresh
medium containing various Rg3 concentrations (50 or 100
μg/ml). Digital images of each wound were captured under a
Nikon Eclipse E200 microscope (Nikon Corporation) immediately (0
h), and 24 and 48 h after scratch generation. Cell migration was
analyzed using the commercial software Image Pro-Plus version 6.0
(Media Cybernetics, Inc., Rockville, MD, USA). Data (means ±
standard deviation, n=3) are expressed as the percentage of the
scratched cell-free zone filled with KFs. For each sample, images
were captured from three random views to obtain the mean value. The
final mean percentages and standard deviation were determined from
three KF samples.
Immunofluorescence staining for type I
collagen, α-smooth muscle actin (α-SMA) and Ki-67 expression
KFs were grown in 6-well plates at a density of
2×104 cells/ml. Following incubation for 24-36 h at 37°C
in an atmosphere containing 5% CO2, medium with or
without various Rg3 concentrations (50 or 100 μg/ml) was
added to the wells. After 72 h, KFs were fixed with 4%
paraformaldehyde at 4°C overnight and permeabilized using 0.3%
Triton X-100 at room temperature for 1 h. Nonspecific binding sites
were blocked with normal goat serum (Sigma-Aldrich; Merck KGaA) at
37°C for 30 min. Subsequently, the KFs were incubated overnight at
4°C with primary rabbit anti-human antibodies against type Ⅰ
collagen (1:500; ab34710), α-SMA (1:100; ab5694) and Ki-67
(1:1,000; ab15580) (Abcam, Cambridge, MA, USA). Subsequently, the
cells were incubated with appropriate fluorescent goat anti-rabbit
secondary antibodies (111-035-003; Jackson Immunoresearch
Laboratories, Inc., West Grove, PA, USA) at room temperature for 1
h. DAPI was used to stain the nuclei prior to image acquisition.
Images of the positive cells (green) and DAPI nuclear staining
(blue) were captured using a fluorescence microscope (Olympus
Corporation, Tokyo, Japan). The incorporation ratio of
Ki-67-positive cells was determined using the following equation:
Number of Ki-67-positive cells/number of total cells. The results
were counted in five randomly selected fields.
Western blot analysis
Western blot analysis was performed as described
previously (19), using primary
antibodies specific for type I collagen, type III collagen,
fibronectin, phosphorylated (p)-Smad2, p-Smad3, total-Smad2/3,
Smad7, p-extracellular signal-regulated kinase (eRK)1/2 and
total-eRK1/2 (all purchased from Cell Signaling Technology, Inc.,
Danvers, MA, USA). Briefly, tissues were collected, and total
cellular protein was extracted in 100 μl RIPA lysis buffer
(Beyotime, Shanghai, China) supplemented with 1 mM
phenylmethylsulfonyl fluoride (PMSF) (Beyotime) and 50 μl/ml
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) at 4°C for
30 min. Protein concentration was measured using the bicinchoninic
acid (BCA) method. Samples containing 20 μg protein (2
μg/μl) were boiled, subjected to SDS-PAGE on 10%
Tris-Glycine gels, and then electrophoretically transferred to
polyvinylidene fluoride membranes. The membranes were blocked with
5% fat-free milk for 1 h at room temperature, and the blots were
incubated with appropriate primary antibodies [type I collagen
(1:2,000; ab34710; Abcam); type III collagen (1:2,000; ab7778;
Abcam); fibronectin (1:2,000; ab32419; Abcam); p-Smad3 (1:1,000;
8828; Cell Signaling Technology, Inc.); Smad2/3 (1:1,000; 8685;
Cell Signaling Technology, Inc.); Smad7 (1:2,000; ab190987; Abcam);
p-eRK1/2 (1:1,000; 4370; Cell Signaling Technology, Inc.); eRK1/2
(1:1,000; 4695; Cell Signaling Technology, Inc.)] overnight at 4°C.
The membranes were then incubated with horseradish
peroxidase-linked secondary antibodies (Beyotime) for 1 h at room
temperature. The goat anti-rabbit secondary antibodies were
purchased from Beyotime (cat. no. A0208) with a dilution of 1:1,000
for 1 h at room temperature. The blots were visualized using a
Super-GL enhanced chemiluminescence reagent (Novland, Shanghai,
China) and were exposed onto KodAK X-omat BT Film (Kodak,
Rochester, NY, USA). The results were analyzed using a digital
imaging system equipped with AlphaEaseFC software V (Alpha Imager
2000; ProteinSimple, San Jose, CA, USA).
Cell invasion assay
Transwell invasion chambers (membrane pore size, 8
μm) coated with Matrigel (BD Biosciences) were placed into
24-well plates. Following an overnight culture in serum-free
medium, KFs (1×105 cells/well) were added to the upper
chambers and were incubated with or without various Rg3
concentrations (50 or 100 μg/ml) for 24 and 48 h. Normal
medium containing serum was placed into the lower chambers. After
24 or 48 h, cells that remained on the upper surface of the
membrane were completely removed using a cotton swab. Cells that
crossed the Matrigel and migrated to the lower side of the
Transwell insert were fixed with 4% paraformaldehyde for 5 min at
room temperature and stained with DAPI. The number of cells that
invaded across the membrane was counted in five random fields under
an Olympus CX40 fluorescence microscope (olympus Corporation).
Immunohistochemical analysis of types I
and III collagen, cluster of differentiation (CD)31 and CD34 in
keloid explant cultures
Keloid tissues removed from the patients were cut
into 1-mm (1×2×5 mm) tissue explants. Once the tissue sections
adhered to the bottom of the dish, medium with or without various
Rg3 concentrations (50 or 100 μg/ml) was added to the wells.
Explants were added to 3.5-cm culture dishes. After 6 days, the
tissue blocks were fixed with 4% paraformaldehyde for 24 h at 4°C
and were embedded in paraffin. Immunohistochemical staining was
performed using a peroxidase-labeled streptavidin-biotin technique.
Briefly, tissues embedded in paraffin were cut into 4-μm
sections and placed onto glass slides. After antigen retrieval, the
sections were incubated overnight at 4°C with primary antibodies.
Subsequently, each section was incubated with an appropriate
secondary antibody and then detected by the formation of a
streptavidin-biotin-horseradish peroxidase complex
(Zhongshan-Jinqiao, Beijing, China). Immunostaining was considered
positive when the cells were stained brown after the addition of 3%
3,3′-diaminobenzidine reagent. Sections stained by isotype-matched
IgG instead of primary antibody were used as negative controls.
Goat anti-rabbit antibody (SPN-9001) was purchased from
Zhongshan-Jinqiao. Paraffin-embedded sections were used for
histological staining. Slides were then incubated overnight at 4°C
with primary antibodies against human type I collagen (1:200;
ab34710), type III collagen (1:200; ab7778), CD31 (1:100; ab28364)
and CD34 (1:100; ab81289) (Abcam). Subsequently, each section was
incubated with the appropriate secondary antibody
(Zhongshan-Jinqiao) and with DAB. All the tissue sections were
observed under a microscope (Nikon Corporation) at a ×400
magnification and images of five random views were captured for
each group. The relative density of collagen was analyzed using
Image Pro Plus 6.0 software. The number of microvessels was counted
in five random fields under a microscope (olympus Corporation).
Statistical analysis
All assays were performed in triplicate and all
results are presented as the means ± standard deviation. The
differences among the three groups were measured using one-way
analysis of variance and differences between two groups were
determined using Turkey's post-hoc statistical method. SPSS 21.0
software (IBM Corp., Armonk, NY, USA) was used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
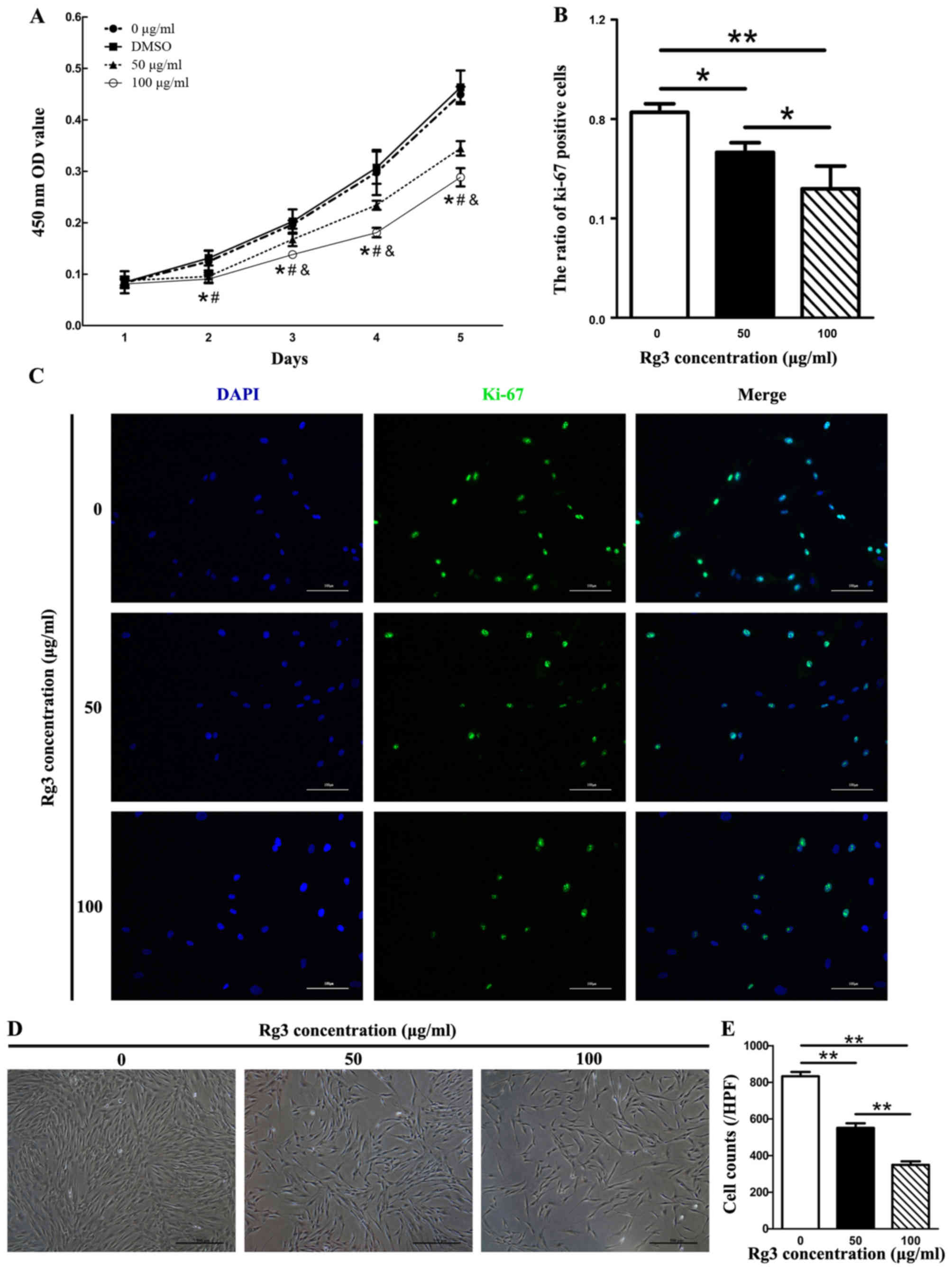
Rg3 suppresses KF proliferation
Since excessive and abnormal proliferation of
fibroblasts has been reported in keloids, the present study
investigated the regulatory function of Rg3 on the proliferation of
KFs using a cell viability assay (CCK-8 assay). In the Rg3-treated
groups, cell proliferation was inhibited from the second day (after
48 h of drug incubation) compared with in the control group
(Fig. 1A). There were significant
differences between the Rg3-treated groups and the 0
μg/ml-Rg3-treated group (P<0.05). In addition, an
apparent difference in cell proliferation between the 50 and 100
μg/ml Rg3-treated groups was detected from the third day
(after 72 h of drug incubation, P<0.05). There was also a marked
difference detected between the DMSO and Rg3-treated groups
(P<0.05); however, no obvious difference was observed between
the 0 μg/ml-Rg3-treated and DMSO groups. No statistical
differences were detected in cell proliferation rate among the
groups on day 1. The present study also investigated the
suppressive effects of Rg3 towards fibroblast growth using Ki-67
immu-nofluorescence in combination with dAPI nuclear staining
(Fig. 1B and C). The expression
of the proliferation marker Ki-67 was markedly decreased in
Rg3-treated groups, as fewer Ki-67-positive KFs were detected
compared with in the control group (Fig. 1C). The ratio of Ki-67-positive
cells was significantly different between the untreated and
Rg3-treated groups (P<0.05; Fig.
1B). The effects of various concentrations of Rg3 (50 or 100
μg/ml) on KF morphology were investigated by microscopy
after 72 h. KF density was gradually decreased as the
concentrations of Rg3 increased (P<0.01; Fig. 1D and E). These results indicated
that KF proliferation was effectively inhibited by certain
concentrations of Rg3.
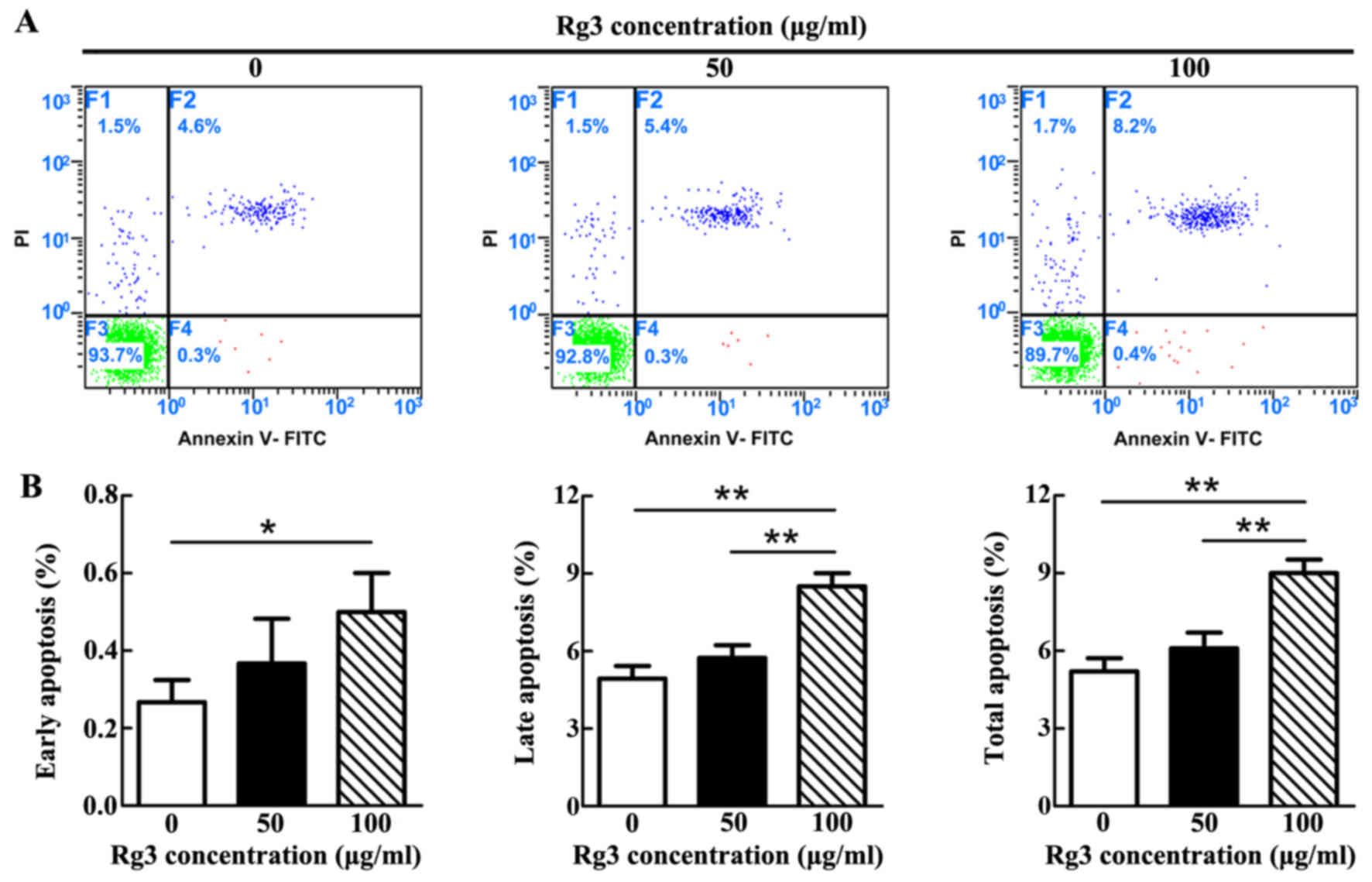
High concentration of Rg3 induces KF
apoptosis
The apoptotic rate was gradually increased as the
concentration of Rg3 increased (Fig.
2A). However, statistical analysis demonstrated that a
significant difference only existed between the control and 100
μg/ml-Rg3-treated groups, with regards to early apoptosis
(P<0.05; Fig. 2B). With
regards to late and total apoptosis, significant differences
existed not only between the control and 100
μg/ml-Rg3-treated groups (P<0.01), but also between the
two Rg3-treated groups. Notably, there was no significant
difference between the control and 50 μg/ml-Rg3-treated
groups in each phase of apoptosis. The majority of apoptotic KFs in
each group were revealed to be undergoing late apoptosis.
Rg3 reduces collagen production and ECM
accumulation
The expression levels of types I and III collagen,
fibronectin, α-SMA, connective tissue growth factor (CTGF),
interferon (IFN)-γ and transforming growth factor (TGF)-β3 were
detected in KFs using qPCR, western blot analysis,
immunofluorescence and immunohistochemical analysis (Fig. 3). The mRNA expression levels of
types I and III collagen, fibronectin, α-SMA and CTGF
were significantly decreased in the Rg3-treated groups compared
with in the control group (P<0.05), thus indicating that Rg3 may
exert antifibrogenic effects in KFs (Fig. 3A). In addition, there were marked
differences between the 50 μg/ml and 100
μg/ml-Rg3-treated groups (P<0.05) with regards to the
mRNA expression levels of type I collagen, fibronectin and
α-SMA; however, no significant difference was detected
between these groups with regards to type III collagen and
CTGF. Furthermore, the mRNA expression levels of
IFN-γ and TGF-β3 were significantly increased in the
Rg3-treated groups compared with in the control group (P<0.05),
thus indicating that Rg3 may strengthen antifibrotic effects in KFs
(Fig. 3A). Intracellular
localization of type I collagen and α-SMA was examined by
immunofluorescence microscopy using the corresponding antibodies.
The expression of type I collagen and α-SMA was visibly weak in the
Rg3-treated groups; the most obvious effects were detected in the
100 μg/ml-Rg3-treated group (Fig. 3B). Western blot analysis indicated
that types I and III collagen, and fibronectin protein expression
levels were decreased in KFs as the concentration of Rg3 increased
(Fig. 3C). This tendency was
particularly obvious with regards to type I collagen and
fibronectin. The immunohistochemical analysis of types I and III
collagen in keloid explant cultures indicated that Rg3 suppressed
types I and III collagen synthesis compared with in the control
group (Fig. 3D). In addition, a
marked difference was detected among the three groups with regards
to the relative density of collagen I (P<0.05; Fig. 3E). With regards to type III
collagen, a statistical difference existed between the untreated
group and the treated groups, but not between the two treated
groups (Fig. 3E). These results
suggested that Rg3 may downregulate the expression of profibrotic
genes and proteins (types I and III collagen, fibronectin, α-SMA
and CTGF) and upregulate the expression of antifibrotic genes
(IFN-γ and TGF-β3) in KFs.
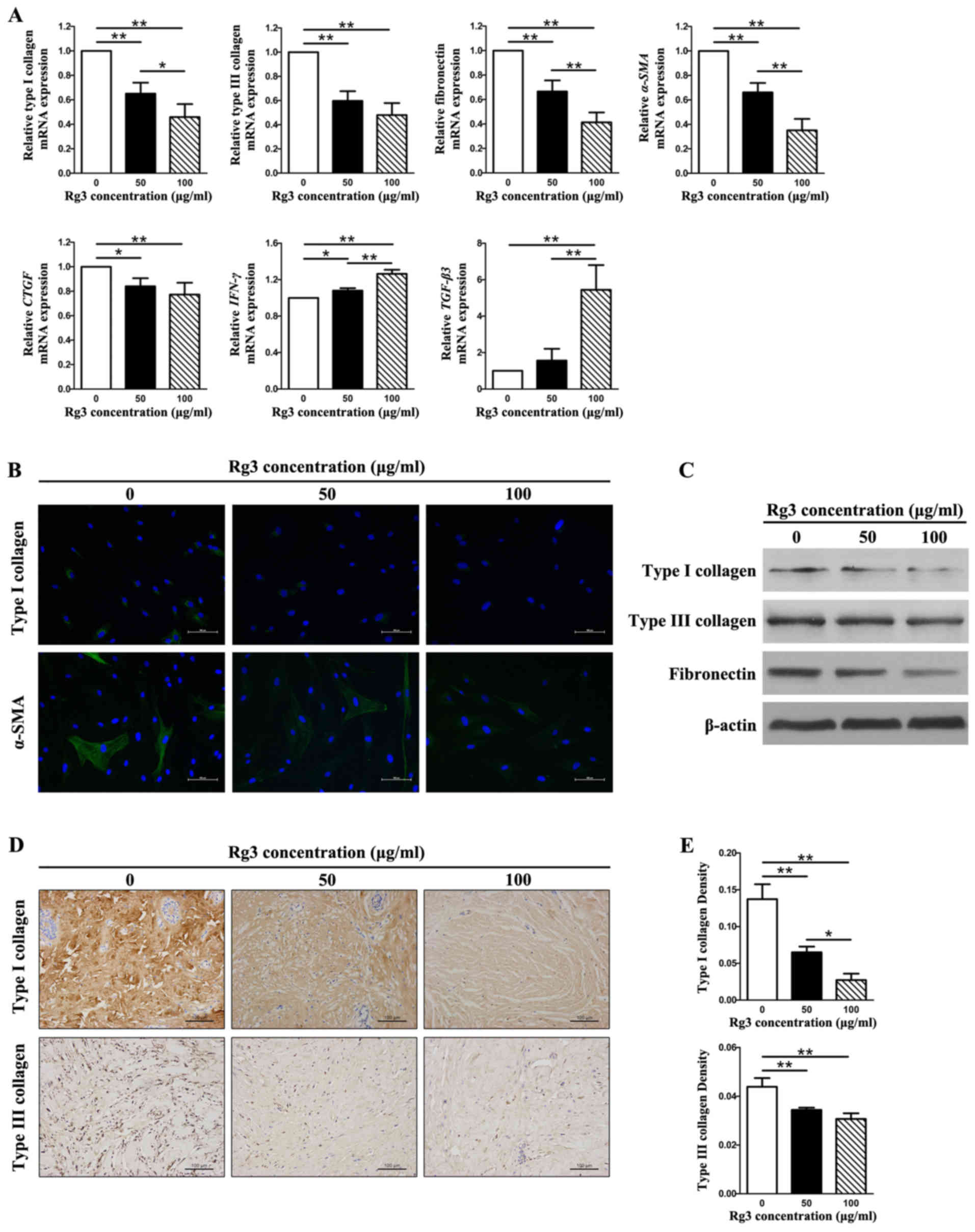 | Figure 3Fibrosis-associated gene and protein
expression in KFs treated with or without various Rg3
concentrations (50 or 100 μg/ml). (A) Quantitative
polymerase chain reaction results of fibrosis-associated genes. The
expression levels of types I and III collagen, fibronectin,
α-SMA and CTGF were markedly decreased in the
Rg3-treated groups; however, the expression levels of IFN-γ
and TGF-β3 were increased. *P<0.05 and
**P<0.01. (B) Immunofluorescence analysis of type I
collagen and α-SMA. The expression of type I collagen and α-SMA was
visibly weak in the Rg3-treated groups (scale bar, 100 μm).
Green is staining α-SMA and type I collagen staining; blue staining
is nuclear staining. (C) Western blot analysis of types I and III
collagen, and fibronectin. Protein expression levels decreased in
KFs as the Rg3 concentration increased. (d and e)
Immunohistochemical analysis of types I and III collagen in keloid
explant cultures. Rg3 suppressed types I and III collagen synthesis
compared with in the control group. *P<0.05 and
**P<0.01 (scale bar, 100 μm). α-SMA, α-smooth
muscle actin; CTGF, connective tissue growth factor;
IFN-γ, interferon-γ; KFs, keloid fibroblasts; Rg3,
ginsenoside Rg3; TGF-β3, transforming growth factor-β3. |
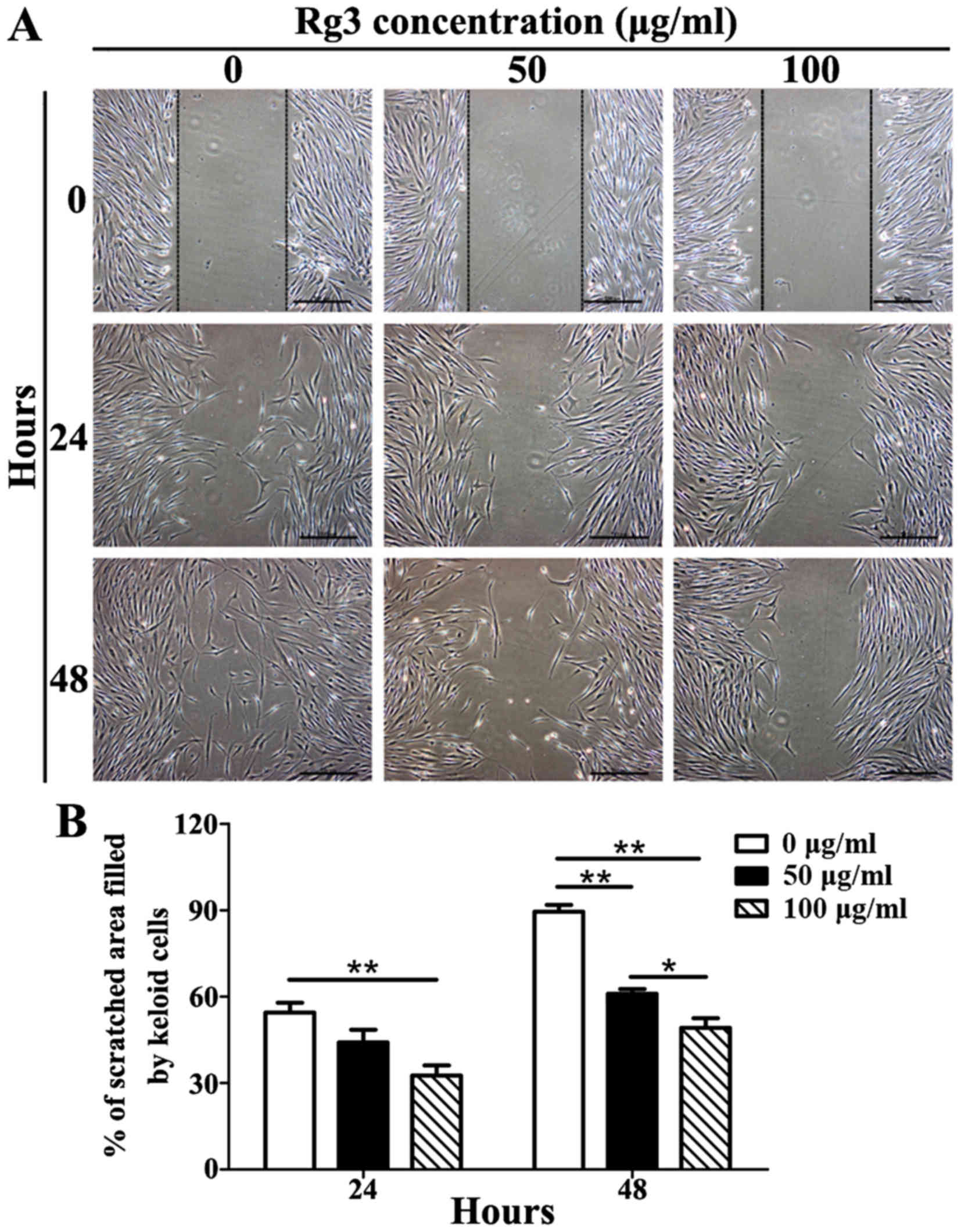
Rg3 inhibits cell migration
The results of a scratch wound assay indicated that
Rg3 was able to markedly inhibit KF migration (Fig. 4A). After 24 h, KFs in the control
group had migrated 55.43±6.03% (mean ± standard deviation) of the
scratched area, whereas 50 μg/ml-treated KFs had migrated
44.08±7.66% and 100 μg/ml-treated KFs had migrated only
32.60±6.13%. There were significant differences between the 100
μg/ml-Rg3-treated and control groups (P<0.01), but not
between the 50 μg/ml-Rg3-treated and control groups
(Fig. 4B). After 48 h, KFs in the
control group had migrated 89.46±4.12% of the scratched area,
whereas 50 μg/ml-treated KFs had migrated 60.96±2.85% and
100 μg/ml-treated KFs had migrated only 49.15±5.79%.
Significant differences were detected among the three groups
(P<0.05; Fig. 4B). These
results indicated that Rg3 may markedly suppress the migration of
KFs.
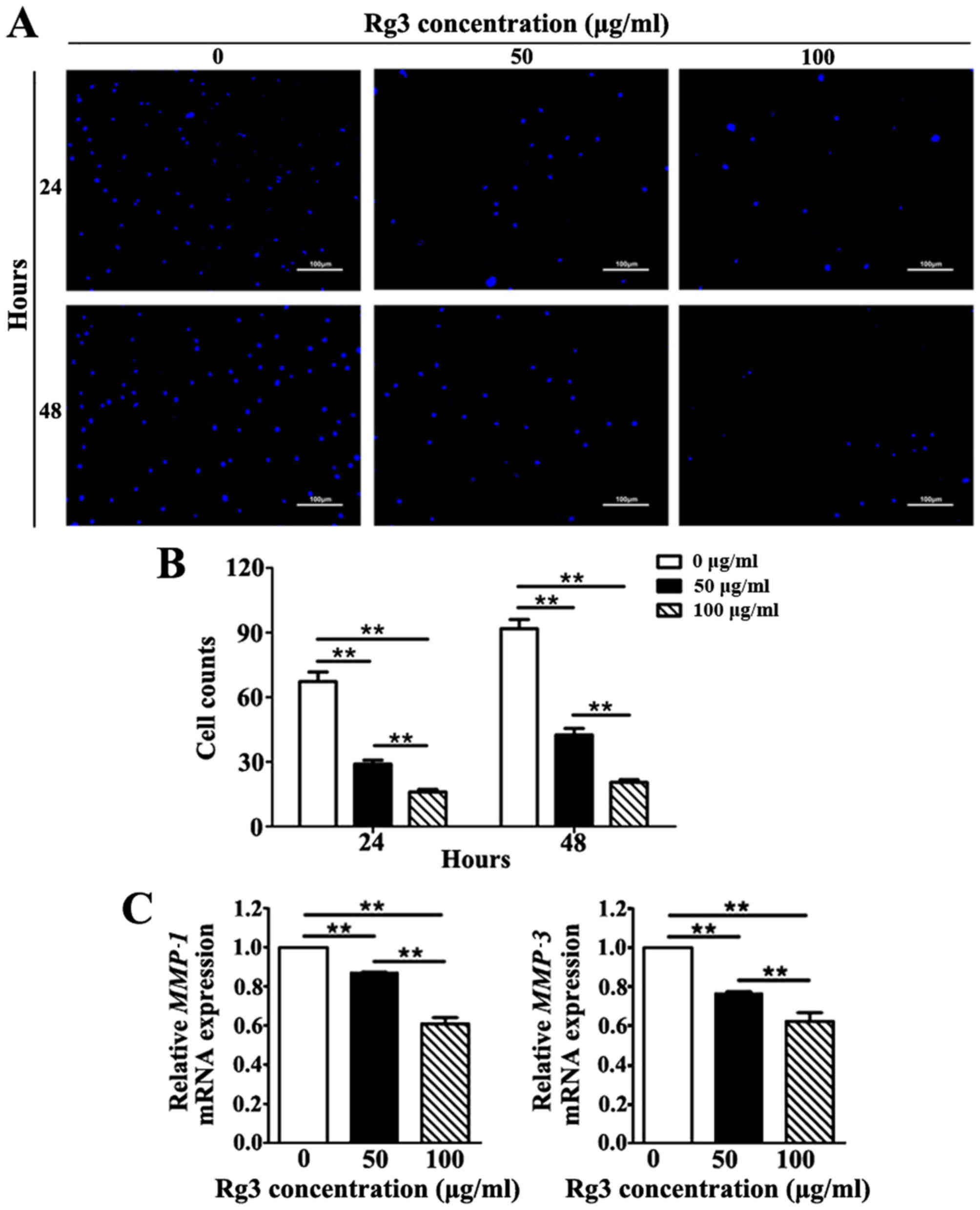
Rg3 suppresses KF invasion
The invasive capability of KFs was investigated
using a Transwell invasion assay. The number of KFs that migrated
across the Matrigel-coated polycarbonate membrane to the lower
chambers was markedly increased in the untreated group compared
with in the Rg3-treated groups at 24 and 48 h (Fig. 5A). Rg3 diminished the invasive
capability of KFs by reducing the number of cells that migrated
across the Matrigel-coated membrane in a concentration-dependent
manner; a significant difference was detected among the three
groups at both time-points (P<0.01; Fig. 5B). Furthermore, to validate the
inhibitory effects of Rg3 towards the invasive capability of KFs,
the mRNA expression levels of matrix metal-loproteinase
(MMP)−1 and MMP-3, which serve a pivotal role in cell
invasion during wound healing and cancer metastasis, were detected
(20). The results indicated that
the mRNA expression levels of MMP-1 and MMP-3 were
significantly downregulated in Rg3-treated groups (P<0.01;
Fig. 5C). These results revealed
that Rg3 may suppress the invasive capability of KFs.
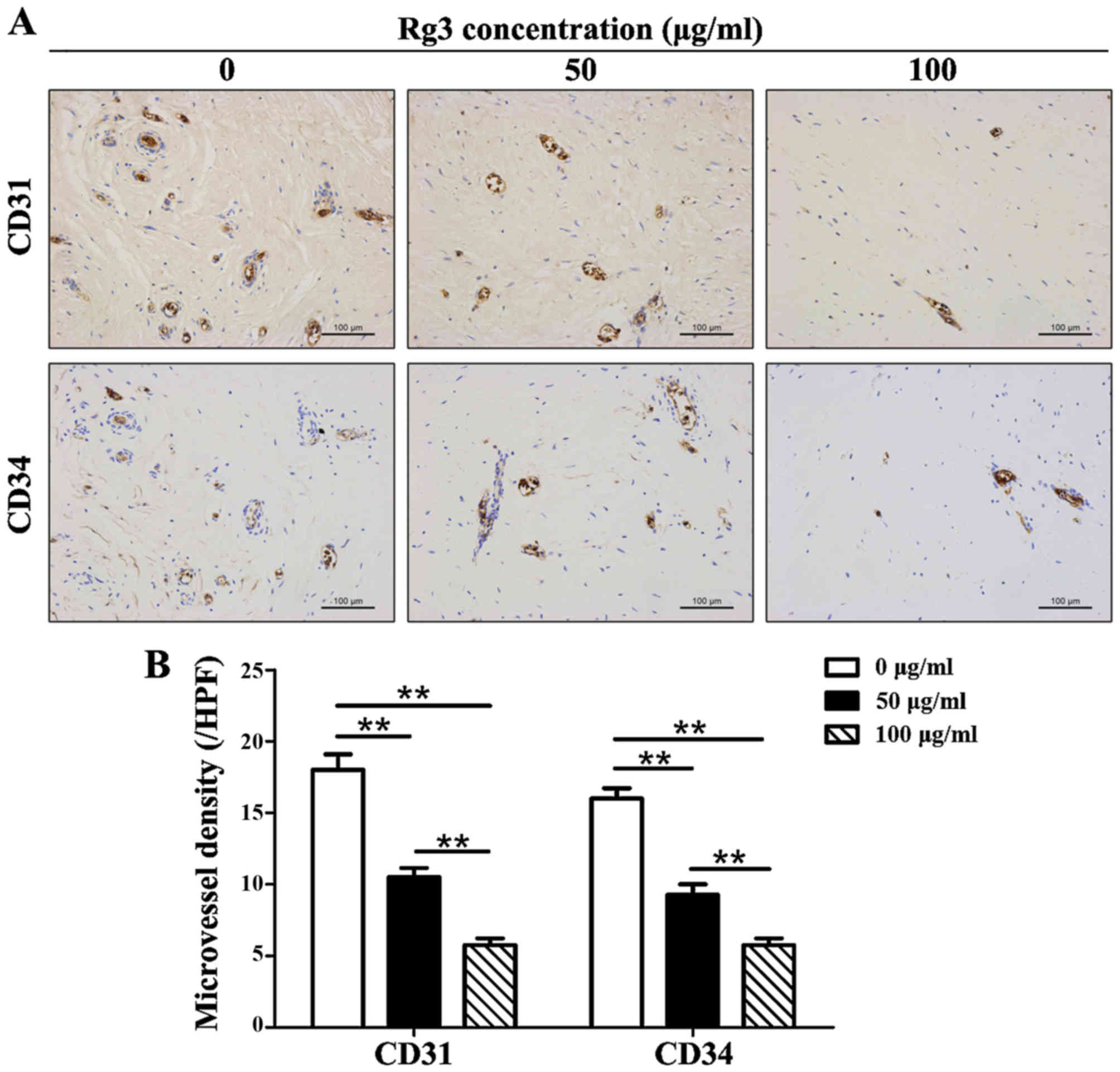
GS-Rg3 suppresses angiogenesis in keloid
explant cultures
The number of CD31 and CD34 positively stained
microvessels was decreased in Rg3-treated groups (Fig. 6A). The results indicated that
microvessel density was reduced by ~1/2 in the 50
μg/ml-treated group and by ~2/3 in the 100
μg/ml-treated group compared with in the control group
(Fig. 6B). Significant
differences existed among the three groups (P<0.01) with regards
to CD31+ and CD34+ microvessels.
Rg3 inhibits the biological behavior of
KFs through TGF-β/Smad and ERK pathways
The mRNA expression levels of TGF-β1, which
has been reported to be highly expressed in KFs (21), VEGF, which is associated
with malignant diseases (22),
and plasminogen activator inhibitor-1 (PAI-1), which is
strongly increased by TGF-β1, were significantly decreased
in the Rg3-treated groups compared with in the control group
(P<0.05; Fig. 7A). Whereas,
the mRNA expression levels of Smad7, which is a negative
feedback regulator in the TGF-β1/Smad pathway, was markedly
increased in response to Rg3 compared with in the control group
(P<0.01), thus indicating that TGF-β1-induced decreases
in Smad7 expression were reversed by Rg3 in a
concentration-dependent manner (Fig.
7A). Statistical analysis indicated that there were significant
differences in the expression levels of all genes among the three
groups (P<0.05). The protein expression levels of p-Smad2 and
p-Smad3, which are enhanced by TGF-β1, were markedly decreased in
Rg3-treated KFs (Fig. 7B). In
addition, p-eRK1/2 expression was suppressed by Rg3 treatment in
KFs (Fig. 7B). However, the
protein expression levels of Smad7 were increased in the
Rg3-treated groups compared with in the control group, which was
similar to the findings of the qPCR analysis (Fig. 7A). The protein expression levels
of total Smad2/3 and total eRK1/2 remained almost unchanged in the
three groups.
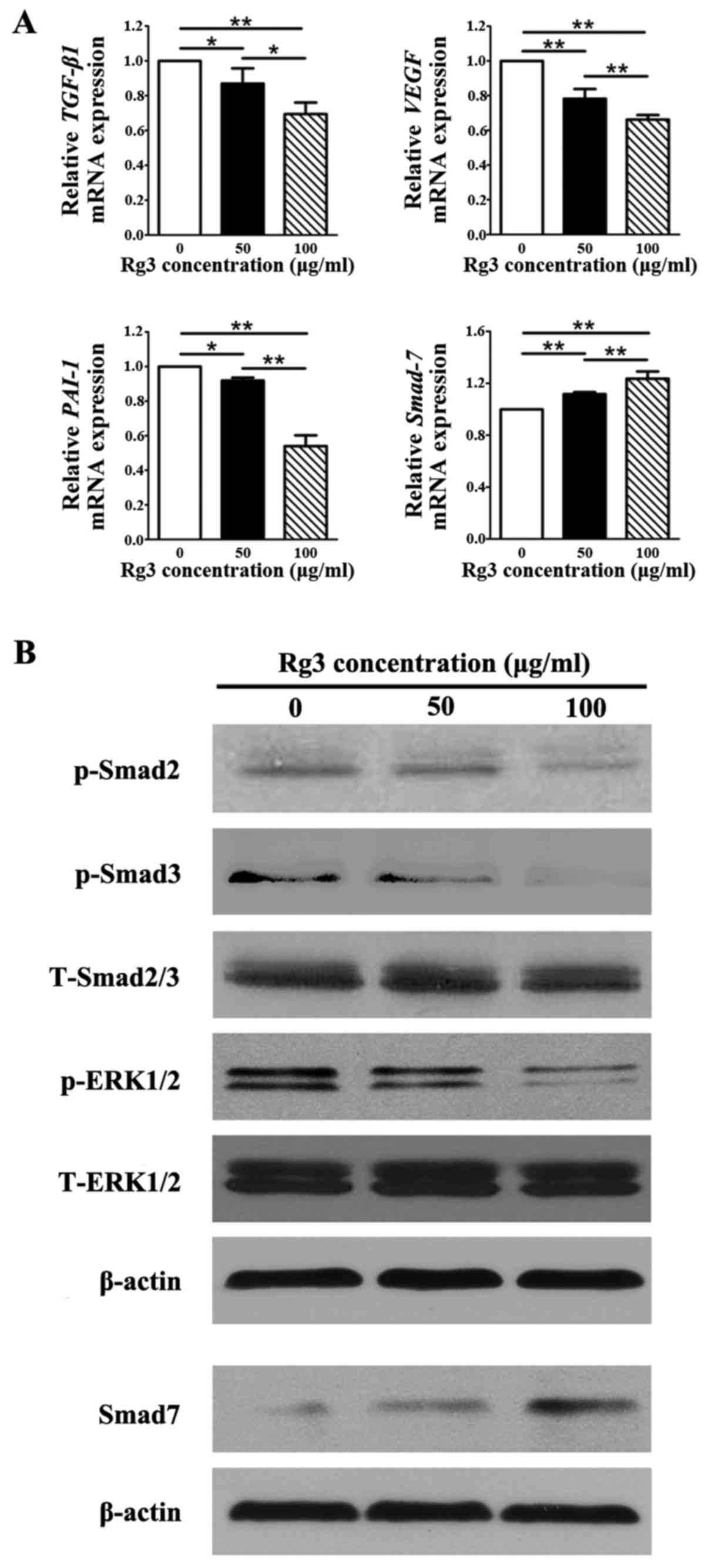 | Figure 7Expression of genes and proteins
associated with the signaling pathways underlying the effects of
Rg3 on KFs. (A) Quantitative polymerase chain reaction analysis of
TGF-β1/Smad-associated and vascularization-associated molecules.
mRNA levels of TGF-β1, VEGF and PAI-1 were
decreased in the Rg3-treated groups; however, the mRNA expression
levels of Smad7 were increased. *P<0.05 and
**P<0.01. (B) Western blot analysis of proteins in
the TGF-β1/Smad and eRK signaling pathways. The protein expression
levels of p-Smad2, p-Smad3 and p-eRK1/2 were suppressed by Rg3 in
KFs, whereas the protein levels of Smad7 were increased in the
Rg3-treated groups. eRK, extracellular-signal regulated kinase;
KFs, keloid fibroblasts; p-, phosphory-lated; PAI-1,
plasminogen activator inhibitor-1; Rg3, ginsenoside Rg3; t-, total;
TGF-β1, transforming growth factor-β3; VEGF, vascular
endothelial growth factor. |
Discussion
Rg3 has been acknowledged as a biologically active
component of Panax ginseng. In a previous study, the effects
of Rg3 on tumor inhibition were thoroughly investigated (23). Keloids are regarded as benign
tumors, but behave in part like malignant tumors, due to their
abilities to extend beyond the original wound margins and invade
into adjacent tissues. A single effective therapy for keloids is
not yet available. The present study is the first, to the best of
our knowledge, to indicate that Rg3 exerts effective therapeutic
outcomes in the field of keloid treatment. The results of the
present study demonstrated that Rg3 could inhibit the
proliferation, angiogenesis and collagen synthesis of KFs in
vitro via the TGF-β/Smad and eRK signaling pathways.
The present results suggested that Rg3 exerts marked
antiproliferative effects on KFs. This was verified by the
decreased expression of the proliferative marker Ki-67, which was
detected in Rg3-treated KFs in a concentration-dependent manner. In
addition, the results of the cell proliferation assay indicated
that Rg3 exerted inhibitory effects after 48 h, thus suggesting
that Rg3 could effectively suppress the growth of keloids. The FCM
analysis demonstrated that treatment with a relatively low
concentration of Rg3 could not obviously elevate the rate of
apoptosis, whereas a high Rg3 concentration could markedly increase
the percentage of cells undergoing apoptosis. These results
indicated that the relatively low concentration of Rg3 was able to
markedly inhibit KF proliferation, whereas the high concentration
of Rg3 not only inhibited cell proliferation but also induced cell
apoptosis.
The abnormal reaction of fibroblasts is a pivotal
factor in the process of keloid formation. Therefore, excessive
collagen and aberrant ECM deposition, potentially caused by
increased proliferation of fibroblasts, are distinct features in
keloids (24). In the process of
keloid formation, numerous profibrogenic molecules serve important
functions. In the dermis of keloids, type I collagen, elastin and
fibronectin all exhibit increased levels (25). In the process of wound repair, the
appropriate appearance and subsequent disappearance of
myofibroblasts is important, and ensures normal healing.
Myofibroblasts are characterized by α-SMA expression. In response
to the aberrant accumulation of eCM components, myofibroblasts do
not disappear as usual and persistently express α-SMA (26). Therefore, α-SMA is often detected
at higher levels in keloids than in normal fibroblasts (27). CTGF is generated by fibroblasts,
serves an important role in cell proliferation and is involved in
numerous mechanisms, including regulation of the TGF-β1 signaling
pathway, positive feedback for fibroblast proliferation, epidermal
regeneration, and accumulation and rebuilding of the ECM, as well
as development of granulation tissue (28,29). Increased levels of CTGF have been
detected in keloids compared with in normal skin (30). Compared with the aforementioned
profibrogenic molecules, IFN-γ and TGF-β3 are antifibrogenic
molecules, which are associated with the inhibition of
proliferation and reduced fibrosis (31,32); they often exhibit low expression
levels in keloids. In the present study, the mRNA and protein
expression levels of types I and III collagen, fibronectin and
α-SMA, and the mRNA expression levels of CTGF, were
decreased following treatment with Rg3, whereas the mRNA expression
levels of IFN-γ and TGF-β3 were elevated, thus
indicating that Rg3 could effectively reduce collagen production
and ECM accumulation.
Keloids are regarded as benign tumors; however, they
are sometimes identified as malignant tumors, due to their ability
to invade surrounding tissues (33). The results of scratch wound and
Transwell invasion assays demonstrated that Rg3 was able to reduce
KF migratory and invasive capabilities, which are important
indicators of keloid progression. The Transwell invasion assay
simulates the invasive process and was used to detect the
therapeutic effects of Rg3 towards KF invasion in vitro.
Furthermore, numerous members of the MMP family are able to degrade
the basement membrane, and thus mediate the migratory and invasive
activity of KFs (34). It has
been reported that MMP-1 and MMP-3 are active in the process of ECM
degradation, and are highly expressed in keloids, particularly
during the active stage in order to facilitate the invasive action
of KFs (35,36). In the present study, the mRNA
expression levels of MMP-1 and MMP-3 were markedly
decreased in the Rg3-treated groups, thus indicating that Rg3 may
suppress the migration and invasion of KFs in keloid disease.
The key role that the TGF-β signaling pathway serves
in the formation of keloids has been reported in numerous studies
(37,38). The TGF-β family is involved in
numerous physiological activities, including cell proliferation,
migration, differentiation, deposition and ECM remodeling, as well
as the modulation of other signaling pathways. It is well accepted
that TGF-β1 transmits signals via the Smad family (e.g.
p-Smad2/Smad3) inside the cell. Smad family members then relay
signals in turn and finally act on target genes, particularly
fibrosis-associated genes (39).
However, Smad7 serves as a negative feedback regulator that is able
to obstruct p-Smad2 and -Smad3, and their polymer with Smad4
(40). Therefore, the present
study detected the expression of crucial molecules or proteins in
the TGF-β/Smad pathway, in order to determine the underlying
mechanisms of the effects of Rg3 on keloids. The present study
demonstrated that Rg3 markedly reduced the mRNA expression levels
of TGF-β1, the protein expression levels of p-Smad2 and
p-Smad3, and increased Smad7 at both the mRNA and protein
levels.
VEGF is a critical factor that is predominantly
involved in angiogenesis, inflammatory response and granulation
tissue formation. The expression levels of VEGF are higher in
keloid tissues and fibroblasts compared with in associated normal
skin (41). Angiogenesis is
essential for tumor growth; therefore, VEGF is pivotal for keloid
and malignant tumor progression. After combining to its specific
receptors (veGFR-1 or veGFR-2), veGF activates the eRK1/2 signaling
pathway in KFs (42). The present
study demonstrated that Rg3 could markedly reduce the mRNA
expression levels of VEGF and the protein expression levels
of p-eRK1/2. Furthermore, previous studies have demonstrated that
PAI-1 may be upregulated by TGF-β1 and VEGF (42–44). PAI-1 has been reported to be
intrinsically highly expressed in KFs (44). It has also been suggested that
elevated levels of PAI-1 may increase ECM deposition in keloids
(42). Consequently, Rg3-induced
down-regulation of PAI-1 detected in the present study may
decrease collagen accumulation and suppress keloid formation.
Due to the lack of a keloid animal model, keloid
explant culture has been extensively applied in the study of
pathophysiological processes. This method results in the
maintenance of cellular processes, vasculature and perhaps some
interactions, which may not be observed in in vitro assays.
In the present study, Rg3 significantly inhibited collagen
synthesis and reduced the quantity of
CD31+/CD34+ microvessels within keloid tissue
sections, further corroborating the inhibitory and antiangiogenic
effects of Rg3.
In conclusion, the present study clearly
demonstrated that Rg3 may inhibit KF proliferation, invasion,
angiogenesis and collagen accumulation. In addition, the present
study indicated that these effects were involved in the TGF-β⁄Smad
and eRK1/2 signaling pathways. These findings provide information
to suggest that Rg3 may be considered a potential therapeutic agent
used to suppress keloid formation. However, in vivo studies
and prospective clinical trials are required to further confirm the
therapeutic effects of Rg3.
Acknowledgments
The authors would like to thank the Shanghai Key
laboratory of Tissue Engineering for technical assistance. The
present study was supported in part by the National Natural Science
Foundation of China (grant nos. 81372073 and 81772099).
References
|
1
|
Trace AP, Enos CW, Mantel A and Harvey VM:
Keloids and Hypertrophic Scars: A Spectrum of Clinical Challenges.
Am J Clin Dermatol. 17:201–223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
English RS and Shenefelt Pd: Keloids and
hypertrophic scars. Dermatol Surg. 25:631–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang R, Chen J, Zhang Z and Cen Y: Role of
chymase in the local renin-angiotensin system in keloids:
Inhibition of chymase may be an effective therapeutic approach to
treat keloids. Drug Des Devel Ther. 9:4979–4988. 2015.PubMed/NCBI
|
|
4
|
Lu WS, Zheng XD, Yao XH and Zhang LF:
Clinical and epidemiological analysis of keloids in Chinese
patients. Arch Dermatol Res. 307:109–114. 2015. View Article : Google Scholar
|
|
5
|
Butler PD, Longaker MT and Yang GP:
Current progress in keloid research and treatment. J Am Coll Surg.
206:731–741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bijlard E, Steltenpool S and Niessen FB:
Intralesional 5-fluorouracil in keloid treatment: A systematic
review. Acta Derm Venereol. 95:778–782. 2015.PubMed/NCBI
|
|
7
|
Ud-Din S and Bayat A: Strategic management
of keloid disease in ethnic skin: A structured approach supported
by the emerging literature. Br J Dermatol. 169(Suppl 3): 71–81.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu T, Peng YF, Jia C, Yang BH, Tao X, Li
J and Fang X: Ginsenoside Rg3 improves erectile function in
streptozotocin-induced diabetic rats. J Sex Med. 12:611–620. 2015.
View Article : Google Scholar
|
|
9
|
Yuan HD, Quan HY, Zhang Y, Kim SH and
Chung SH: 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon
cancer cells is associated with AMPK signaling pathway. Mol Med
Rep. 3:825–831. 2010.
|
|
10
|
He BC, Gao JL, Luo X, Luo J, Shen J, Wang
L, Zhou Q, Wang YT, Luu HH, Haydon RC, et al: Ginsenoside Rg3
inhibits colorectal tumor growth through the downregulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011. View Article : Google Scholar
|
|
11
|
Wang JH, Nao JF, Zhang M and He P:
20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer
HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol.
35:11985–11994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joo SS, Yoo YM, Ahn BW, Nam SY, Kim YB,
Hwang KW and Lee DI: Prevention of inflammation-mediated
neurotoxicity by Rg3 and its role in microglial activation. Biol
Pharm Bull. 31:1392–1396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen QJ, Zhang MZ and Wang LX: Gensenoside
Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells.
Cell Physiol Biochem. 26:849–858. 2010. View Article : Google Scholar
|
|
14
|
Pazyar N, Omidian M and Jamshydian N:
Ginseng as a potential novel addition to the antikeloid weaponry.
Phytother Res. 26:1579–1580. 2012.PubMed/NCBI
|
|
15
|
Liu JP, Lu D, Nicholson RC, Zhao WJ, Li PY
and Wang F: Toxicity of a novel anti-tumor agent 20(S)-ginsenoside
Rg3: A 26-week intramuscular repeated administration study in rats.
Food Chem Toxicol. 50:3388–3396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SM, Lee SY, Cho JS, Son SM, Choi SS,
Yun YP, Yoo HS, Yoon DY, Oh KW, Han SB, et al: Combination of
ginsenoside Rg3 with docetaxel enhances the susceptibility of
prostate cancer cells via inhibition of NF-kappaB. Eur J Pharmacol.
631:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
18
|
Lan CC, Liu IH, Fang AH, Wen CH and Wu CS:
Hyperglycaemic conditions decrease cultured keratinocyte mobility:
Implications for impaired wound healing in patients with diabetes.
Br J Dermatol. 159:1103–1115. 2008.PubMed/NCBI
|
|
19
|
Shin JU, Lee WJ, Tran TN, Jung I and Lee
JH: Hsp70 knockdown by siRNA decreased collagen production in
keloid fibroblasts. Yonsei Med J. 56:1619–1626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gill SE and Parks WC: Metalloproteinases
and their inhibitors: Regulators of wound healing. Int J Biochem
Cell Biol. 40:1334–1347. 2008. View Article : Google Scholar
|
|
21
|
Liang CJ, Yen YH, Hung LY, Wang SH, Pu CM,
Chien HF, Tsai JS, Lee CW, Yen FL and Chen YL: Thalidomide inhibits
fibronectin production in TGF-β1-treated normal and keloid
fibroblasts via inhibition of the p38/Smad3 pathway. Biochem
Pharmacol. 85:1594–1602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schäfer M and Werner S: Cancer as an
overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell
Biol. 9:628–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin YM, Jung HJ, Choi WY and Lim CJ:
Antioxidative, anti-inflammatory, and matrix metalloproteinase
inhibitory activities of 20(S)-ginsenoside Rg3 in cultured
mammalian cell lines. Mol Biol Rep. 40:269–279. 2013. View Article : Google Scholar
|
|
24
|
Dong X, Mao S and Wen H: Upregulation of
proinflammatory genes in skin lesions may be the cause of keloid
formation (Review). Biomed Rep. 1:833–836. 2013. View Article : Google Scholar
|
|
25
|
Lee WJ, Ahn HM, Roh H, Na Y, Choi IK, Lee
JH, Kim Yo, Lew DH and Yun CO: Decorin-expressing adenovirus
decreases collagen synthesis and upregulates MMP expression in
keloid fibroblasts and keloid spheroids. Exp Dermatol. 24:591–597.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rao KB, Malathi N, Narashiman S and Rajan
ST: Evaluation of myofibroblasts by expression of alpha smooth
muscle actin: A marker in fibrosis, dysplasia and carcinoma. J Clin
Diagn Res. 8:ZC14–ZC17. 2014.
|
|
27
|
Chipev CC, Simman R, Hatch G, Katz AE,
Siegel DM and Simon M: Myofibroblast phenotype and apoptosis in
keloid and palmar fibroblasts in vitro. Cell death differ.
7:166–176. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mun JH, Kim YM, Kim BS, Kim JH, Kim MB and
Ko HC: Simvastatin inhibits transforming growth factor-β1-induced
expression of type I collagen, CTGF, and α-SMA in keloid
fibroblasts. Wound Repair Regen. 22:125–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu R, Yue B, Yang Q, Ma Y, Huang G, Guan
M, Avram MM and Lu Z: The effect of 595 nm pulsed dye laser on
connective tissue growth factor (CTGF) expression in cultured
keloid fibroblasts. Lasers Surg Med. 47:203–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khoo YT, Ong CT, Mukhopadhyay A, Han HC,
Do DV, Lim IJ and Phan TT: Upregulation of secretory connective
tissue growth factor (CTGF) in keratinocyte-fibroblast coculture
contributes to keloid pathogenesis. J Cell Physiol. 208:336–343.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duncan MR and Berman B: Differential
regulation of glycosaminoglycan, fibronectin, and collagenase
production in cultured human dermal fibroblasts by
interferon-alpha, -beta, and -gamma. Arch Dermatol Res. 281:11–18.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Y, Peng Y, Gao D, Feng C, Yuan X, Li H,
Wang Y, Yang L, Huang S and Fu X: Mesenchymal stem cells suppress
fibroblast proliferation and reduce skin fibrosis through a
TGF-β3-dependent activation. Int J Low Extrem Wounds. 14:50–62.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshimoto H, Ishihara H, Ohtsuru A, Akino
K, Murakami R, Kuroda H, Namba H, Ito M, Fujii T and Yamashita S:
Overexpression of insulin-like growth factor-1 (IGF-I) receptor and
the invasiveness of cultured keloid fibroblasts. Am J Pathol.
154:883–889. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma H, Cai H, Zhang Y, Wu J, Liu X, Zuo J,
Jiang W, Ji G, Zhang Y, Liu C, et al: Membrane palmitoylated
protein 3 promotes hepatocellular carcinoma cell migration and
invasion via upregulating matrix metalloproteinase 1. Cancer Lett.
344:74–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujiwara M, Muragaki Y and Ooshima A:
Keloid-derived fibroblasts show increased secretion of factors
involved in collagen turnover and depend on matrix
metalloproteinase for migration. Br J Dermatol. 153:295–300. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uchida G, Yoshimura K, Kitano Y, Okazaki M
and Harii K: Tretinoin reverses upregulation of matrix
metalloproteinase-13 in human keloid-derived fibroblasts. Exp
dermatol. 12(Suppl 2): 35–42. 2003. View Article : Google Scholar
|
|
37
|
Lee CH, Hong CH, Chen YT, Chen YC and Shen
MR: TGF-beta1 increases cell rigidity by enhancing expression of
smooth muscle actin: Keloid-derived fibroblasts as a model for
cellular mechanics. J Dermatol Sci. 67:173–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song R, Li G and Li S: Aspidin PB, a novel
natural anti-fibrotic compound, inhibited fibrogenesis in
TGF-β1-stimulated keloid fibroblasts via PI-3K/Akt and Smad
signaling pathways. Chem Biol Interact. 238:66–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Branton MH and Kopp JB: TGF-β and
fibrosis. Microbes Infect. 1:1349–1365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakao A, Afrakhte M, Morén A, Nakayama T,
Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH,
et al: Identification of Smad7, a TGFbeta-inducible antagonist of
TGF-beta signalling. Nature. 389:631–635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fujiwara M, Muragaki Y and Ooshima A:
Upregulation of transforming growth factor-beta1 and vascular
endothelial growth factor in cultured keloid fibroblasts: relevance
to angiogenic activity. Arch Dermatol Res. 297:161–169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Y, Zhang Q, Ann DK, Akhondzadeh A,
Duong HS, Messadi DV and Le AD: Increased vascular endothelial
growth factor may account for elevated level of plasminogen
activator inhibitor-1 via activating eRK1/2 in keloid fibroblasts.
Am J Physiol Cell Physiol. 286:C905–C912. 2004. View Article : Google Scholar
|
|
43
|
He S, Yang Y, Liu X, Huang W and Zhang X,
Yang S and Zhang X: Compound Astragalus and Salvia miltiorrhiza
extract inhibits cell proliferation, invasion and collagen
synthesis in keloid fibroblasts by mediating transforming growth
factor-β/Smad pathway. Br J Dermatol. 166:564–574. 2012. View Article : Google Scholar
|
|
44
|
Tuan TL, Wu H, Huang EY, Chong SS, Laug W,
Messadi D, Kelly P and Le A: Increased plasminogen activator
inhibitor-1 in keloid fibroblasts may account for their elevated
collagen accumulation in fibrin gel cultures. Am J Pathol.
162:1579–1589. 2003. View Article : Google Scholar : PubMed/NCBI
|