Introduction
Colorectal cancer (CRC) is one of the most common
types of cancer in the Western world, and is the second most common
cause of cancer-associated mortality in the world (1–3).
It is estimated that >1.3 million people worldwide are affected
by CRC annually (4). The
worldwide threat posed by CRC is increasing, which is largely due
to an aging population and the increased adoption of Westernized
diets in developed and developing countries (5–7).
At present, surgery, chemotherapy and radiotherapy
are applied as the main therapeutic approaches for the treatment of
CRC in clinical practice (8–10).
Among them, systemic chemotherapy is regarded as a promising
therapeutic approach, due to its ability to elicit a good
therapeutic response, improve quality of life and prolong survival
(9). Cisplatin (CDDP) is one of
the most frequently used chemotherapy drugs, which exerts a strong
therapeutic effect; however, some tumor types, including colon,
ovarian and lung cancer, have not exhibited satisfactory results in
response to CDDP (11).
Therefore, the enhancement of efficacy by specific compounds may
provide a valuable contribution to the treatment of cancer based on
CDDP chemotherapy. For this purpose, the development of combined
application of CDDP with other safe and effective agents has been
the focus of research.
Currently, herbal medicines or natural compounds,
either used as a monotherapy or combined with conventional
chemotherapeutic agents, have been reported to exert beneficial
effects on the treatment of various types of cancer (12). Bruceae Fructus refers to the fruit
of Brucea javanica (L.) Merr. ('Ya-Dan-Zi' in Chinese), and
was initially recorded in Supplementations to the Compendium of
Chinese Materia Medica. Bruceae Fructus has been applied to treat
various ailments, including cancer, amoebic dysentery and malaria,
since the Ming Dynasty (1364-1644 AD) (13,14). The antitumor activity of Bruceae
Fructus is regarded as one of the most important biological
activities of this plant, and it has been commonly prescribed to
treat various types of cancer in China. In previous years, emerging
evidence has been provided with regards to the antitumor activity
of Bruceae Fructus (13).
B. javanica is rich in quassinoids, which are
considered the predominant ingredients responsible for its marked
antitumor activity (15).
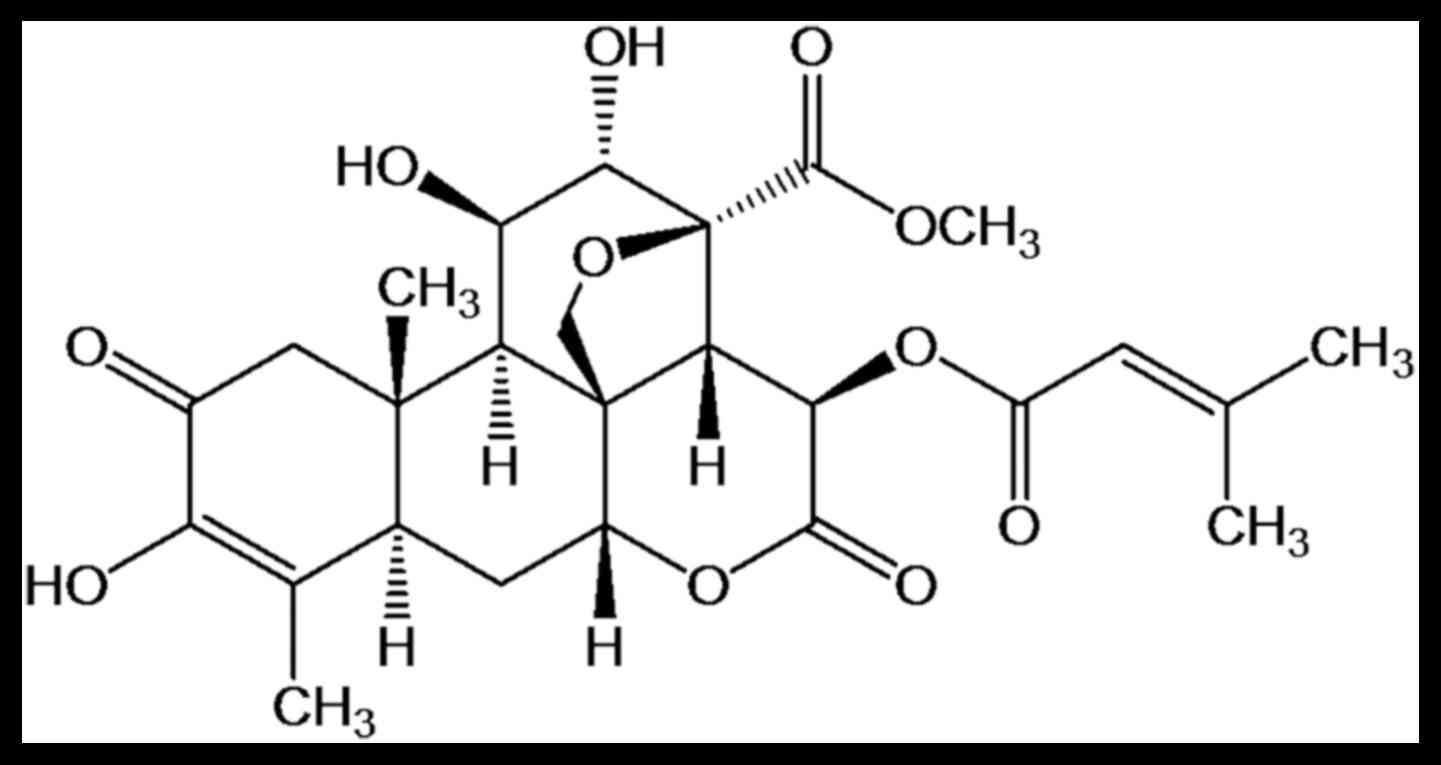
Brusatol (BR; C26H32O11), the
chemical structure of which is presented in Fig. 1, is one of the major quassinoids
isolated from B. javanica. This compound has been reported
to exert marked anti-inflammatory (16), antimalarial (17) and antitumor activities (18–21). In addition, BR has been
demonstrated to uniquely block the nuclear factor erythroid
2-related factor 2 pathway, thus sensitizing various cancer cells
in vitro and A549 mouse xenografts to chemotherapeutic
agents, including CDDP. These findings suggested that BR may be
considered a promising candidate for combating chemoresistance and
for further development into an effective adjuvant for chemotherapy
drugs (22). However, whether
CDDP combined with BR exerts synergistic antitumor activity on
CT-26 CRC cells remains unclear. Therefore, the present study aimed
to investigate the possible effects of BR alone, and in combination
with CDDP, on CT-26 CRC cells, and to evaluate the potential
mechanism.
Materials and methods
Reagents and chemicals
BR (CAS: 14907-98-3; PubChem CID: 73432) was
isolated from Bruceae Fructus in our laboratory. Briefly, the seeds
of B. javanica were extracted twice with 95% EtOH for 2 h,
concentrated to give a crude extract and suspended in
H2O. The aqueous layer was further extracted with EtOAc
and evaporated under vacuum to afford extracts and subjected to
silica gel column chromatography eluted with a gradient of
CH2Cl2-MeOH (100:0-100:20). The
CH2Cl2-MeOH (100:1) eluate was evaporated to
yield a residue, which was further purified by repeated
recrystallization to obtain a white powder. The chemical structure
of BR was confirmed and purity was determined to be >98%
(21). CDDP and MTT were obtained
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Antibodies
against caspase-3 (sc-113427) and caspase-9 (sc-56073), cytochrome
c (sc-13156), B-cell lymphoma 2 (Bcl-2)-associated X protein
(Bax; sc-20067), Bcl-2 (sc-509) and β-actin (sc-47778) were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All
other chemicals and reagents were of analytical grade.
Cell culture
The murine CT-26 CRC cell line was purchased from
the American Type Culture Collection (Manassas, VA, USA). CT-26
cells were routinely grown in RPMI-1640 medium supplemented with
10% fetal bovine serum (both from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 1% penicillin-streptomycin at 37°C in a
humidified atmosphere containing 5% CO2.
In vitro cytotoxicity assays
In vitro BR and CDDP cytotoxic effects
against the CRC cell line were measured using an MTT assay.
Briefly, CT-26 cells in logarithmic growth were seeded onto a
96-well plate at a density of 4×103 cells/well. After 24
h of incubation at 37°C, fresh medium containing a series of
concentrations of BR (0.05, 0.15, 0.45, 1.35, 4.05 and 12.15
μg/ml) and CDDP (0.05, 0.15, 0.45, 1.35, 4.05 and 12.15
μg/ml) was added at 100 μl/well; each concentration
was used to treat six replicate wells. After 48 h of incubation at
37°C, the cells were further incubated with MTT (10 mg/ml) at 37°C
for 4 h. The supernatant was then removed and the precipitate was
dissolved with 100 μl dimethyl sulfoxide. Absorbance was
measured using a microplate reader (EXL808; BioTek Instruments,
Inc., Winooski, VT, USA) at a wavelength of 490 nm. Cytotoxicity
was expressed as the concentration of BR and CDDP that inhibited
cell growth by 50% [half maximal inhibitory concentration
(IC50) value]. The inhibitory rate was calculated
according to the following formula: Inhibitory rate (%) =
(1-ODexperiment group/ODcontrol group) ×
100%; where OD refers to optical density. The possible synergistic
effect of BR combined with CDDP was investigated by exposing CT-26
cells to various concentrations of each agent alone or in
combination for 48 h. The synergistic effect was assessed using
CalcuSyn software 2.0 (Biosoft, Cambridge, UK), which determines
the combination index (CI) based on that described by Chou and
Talalay (23,24). CI=1, CI<1 and CI>1
represented an additive effect, synergism and antagonism,
respectively (23).
Morphological observation of nuclear
alterations
CT-26 cancer cells were grown on coverslips placed
in 6-well plates and were treated with a single drug (BR or CDDP)
or combination for 48 h (incubation at 37°C). After washing twice,
Hoechst 33342 (Hoechst staining kit; Beyotime Institute of
Biotechnology, Beijing, China) was used to stain the cells for 1 h
at room temperature. Subsequently, cell morphology was observed and
images were captured from random visual fields using a fluorescence
microscopy (Zeiss GmbH, Jena, Germany).
Flow cytometric analysis of
apoptosis
The Annexin V-fluorescein isothiocyanate (FITC) kit
(Thermo Fisher Scientific, Inc.) was used to determine cellular
apoptosis. After exposure to BR (0.27 μg/ml), CDDP (1.44
μg/ml), or their combination for 48 h in 37°C, CT-26 cells
were collected, washed twice with PBS and subjected to
centrifugation at 180 × g for 5 min at room temperature.
Subsequently, the cell pellet was resuspended and treated with
Annexin V-FITC and propidium iodide (PI) solutions. After
incubating for 15 min at room temperature in the dark, additional
Annexin V binding buffer (400 μl) was added to each tube and
the cells were analyzed using a Cytomics™ FC500 flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA) and disposed with FlowJo
7.6.5. (FlowJo, LLC, Ashland, OR, USA).
Western blot analysis
After treatment, the cells were harvested and lysed
with radioimmunoprecipitation assay buffer (Cell Signaling
Technology, Inc., Boston, MA, USA) supplemented with cocktail
(Roche, Penzberg, Germany). Protein concentration was determined
using a BCA Protein Assay kit (cat. no. 23225, Thermo Fisher
Scientific, Inc.) and about 40 μg protein were separated
with 10% SDS-PAGE by electrophoresis and were transferred to
polyvinylidene fluoride (PVDF) membranes (Immobilon; EMD Millipore,
Billerica, MA, USA) using trans-blotting apparatus (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Non-fat milk (5%, w/v)
dissolved in Tris-buffered saline containing 0.1% Tween-20 (TBS-T)
was used to block the PVDF membranes. The membranes were then
incubated with the following primary antibodies: Procaspase-3
(1:200), procaspase-9 (1:200), cytochrome c (1:200), Bcl-2
(1:200), Bax (1:200) and β-actin (Santa Cruz Biotechnology, Inc.)
overnight at 4°C. After washing with TBS-T three times, the
membranes were incubated with the appropriate secondary antibodies
(1:1,000; sc-2350, sc-2005 and sc-2370; Santa Cruz Biotechnology,
Inc.). Finally, the protein bands were developed using enhanced
chemiluminescence western blot detection reagents (GE Healthcare,
Chicago, IL, USA) and were analyzed using ImageJ software 1.51s
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to
conduct all statistical analyses. Data are presented as the means ±
standard deviation. One-way analysis of variance was used for
multiple group comparisons, followed by Dunnett's test to detect
intergroup differences. P<0.05 was considered to indicate a
statistically significant difference.
Results
Synergistic cytotoxic effects of BR in
combination with CDDP on CT-26 CRC cells
To explore the possible synergistic cytotoxicity of
BR in combination with CDDP, the present study investigated the
effects of BR and CDDP cotreatment on CT-26 cell viability using an
MTT assay. CT-26 cells were treated with various concentrations of
BR (0.05, 0.15, 0.45, 1.35, 4.05 and 12.15 μg/ml) and CDDP
(0.05, 0.15, 0.45, 1.35, 4.05 and 12.15 μg/ml) for 48 h,
either alone or in combination.
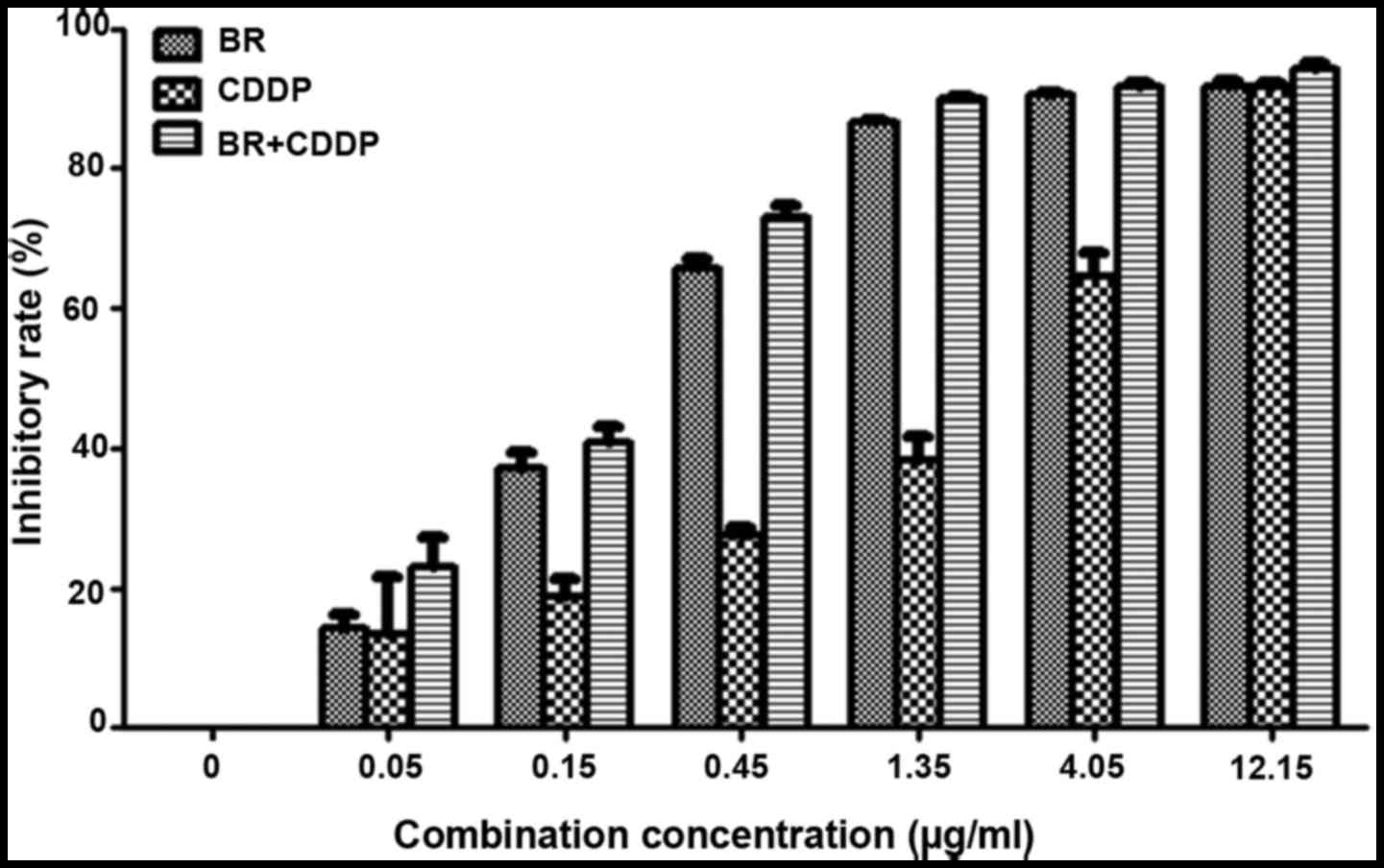
The inhibitory effects on the proliferation of CT-26
cells and IC50 values are presented in Fig. 2 and Table I, respectively. Following
treatment with BR and CDDP for 48 h, the viability of CT-26 cells
was reduced in a dose-dependent manner, with IC50 values
of 0.27±0.01 and 1.44±0.22 μg/ml, respectively. When BR was
combined with CDDP at a constant concentration ratio of 1:1, cell
growth inhibition was markedly enhanced compared with single-agent
treatment; the IC50 value of BR and CDDP cotreatment was
0.19±0.02 μg/ml.
 | Table IIC50 values of BR and CDDP
either alone or in combination on CT-26 cells. |
Table I
IC50 values of BR and CDDP
either alone or in combination on CT-26 cells.
| Agent | IC50
value (μg/ml) |
|---|
| BR | 0.27±0.01 |
| CDDP | 1.44±0.22 |
| BR + CDDP | 0.19±0.02 |
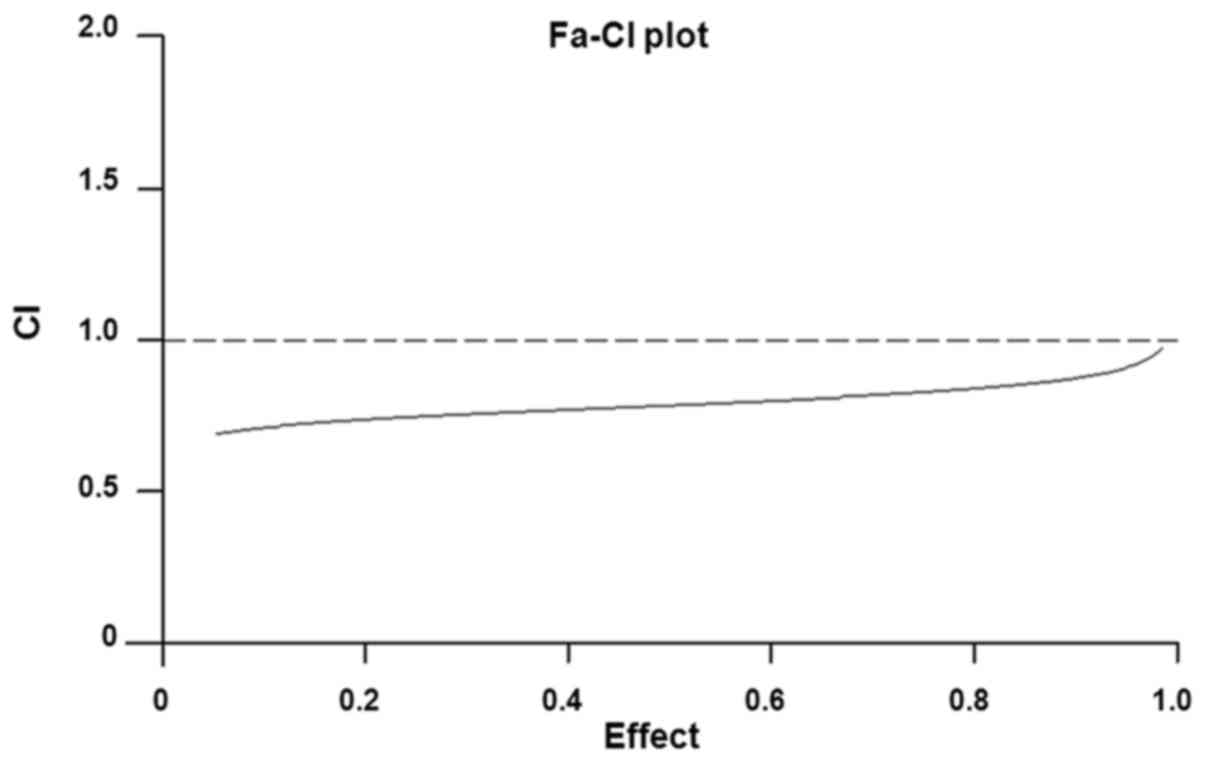
The effects of CDDP and BR cotreatment on cell
proliferation were revealed to be synergistic, as determined by
calculating the CI values (Fig.
3). Isobologram analysis indicated that the CI value was <1,
thus suggesting that there was a synergistic effect of BR in
combination with CDDP on CT-26 cell inhibition.
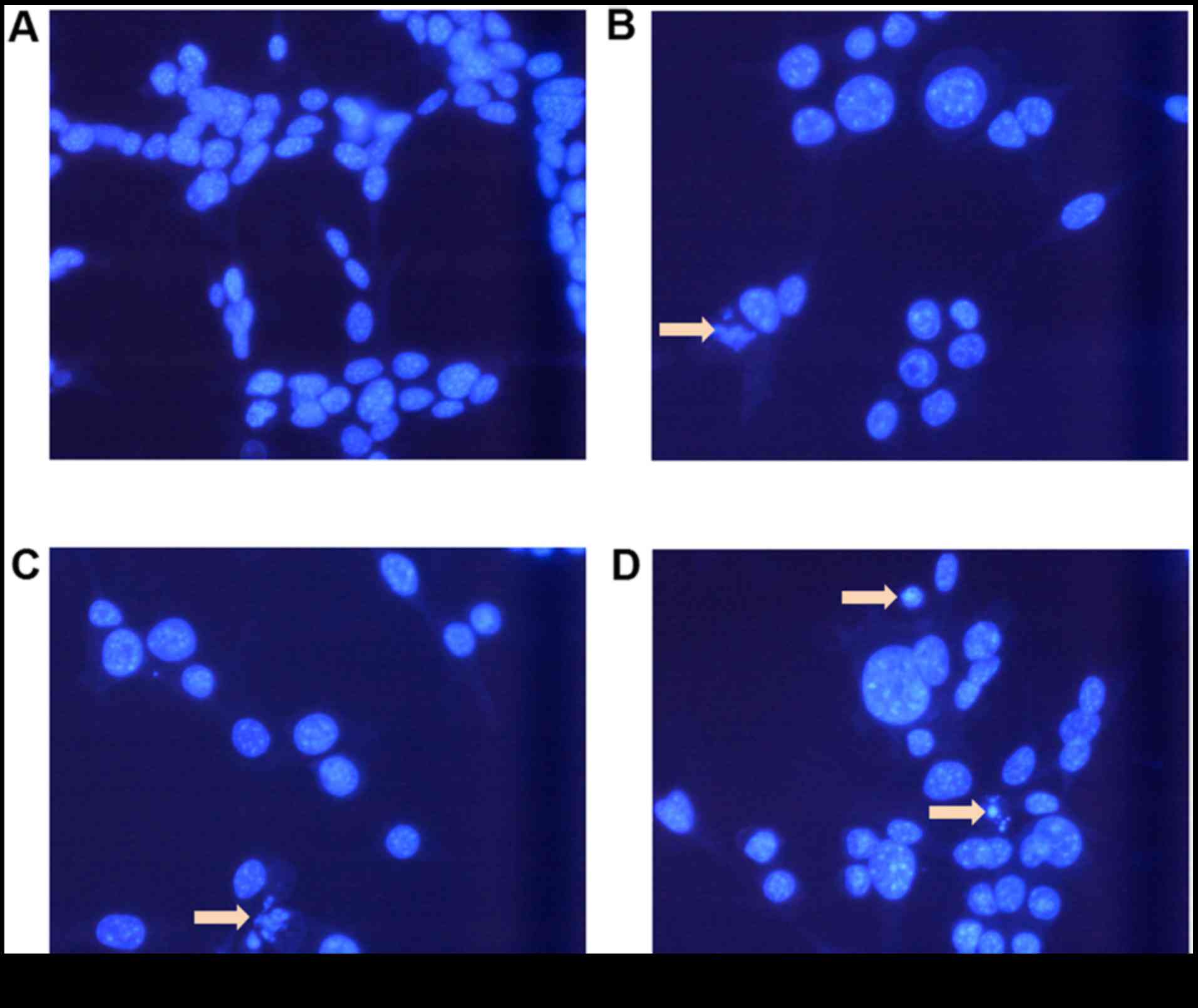
Morphological alterations in CT-26
cells
To investigate whether cellular apoptosis was
involved in the cytotoxic effects of BR and CDDP cotreatment on
CT-26 CRC cells, morphological alterations were observed using
Hoechst 33342 nuclear staining (Fig.
4). Compared with the control cells, cells treated with BR and
CDDP underwent chromatin condensation, and nuclear fragmentation
and shrinkage, which are characteristics of apoptosis. Compared
with in the BR and CDDP single treatment groups, cells treated with
a combination of BR and CDDP exhibited a more obvious increase in
the levels of apoptotic chromatin condensation and the number of
dead cells.
Synergistic induction of apoptosis of
CT-26 cells by BR and CDDP
To further confirm whether the antitumor effects of
BR and CDDP cotreatment on CT-26 cells were associated with the
induction of apoptosis, Annexin V/PI double staining was used to
detect apoptosis of CT-26 cells, which were treated with BR, CDDP
and their combination. The proportions of early and late apoptotic
cells were quantified using flow cytometric analysis, after
labeling cells with PI and Annexin V. As shown in Fig. 5, there was a marked increase in
the number of apoptotic cells when CT-26 cells were treated with BR
or CDDP. The results indicated that BR and CDDP, either
individually or in combination, were able to generate a significant
increase in the apoptotic population of CT-26 cells (P<0.01;
Fig. 5B). Compared with the BR or
CDDP groups, a significantly greater apoptotic rate was observed in
the BR and CDDP cotreatment group (P<0.01; Fig. 5B).
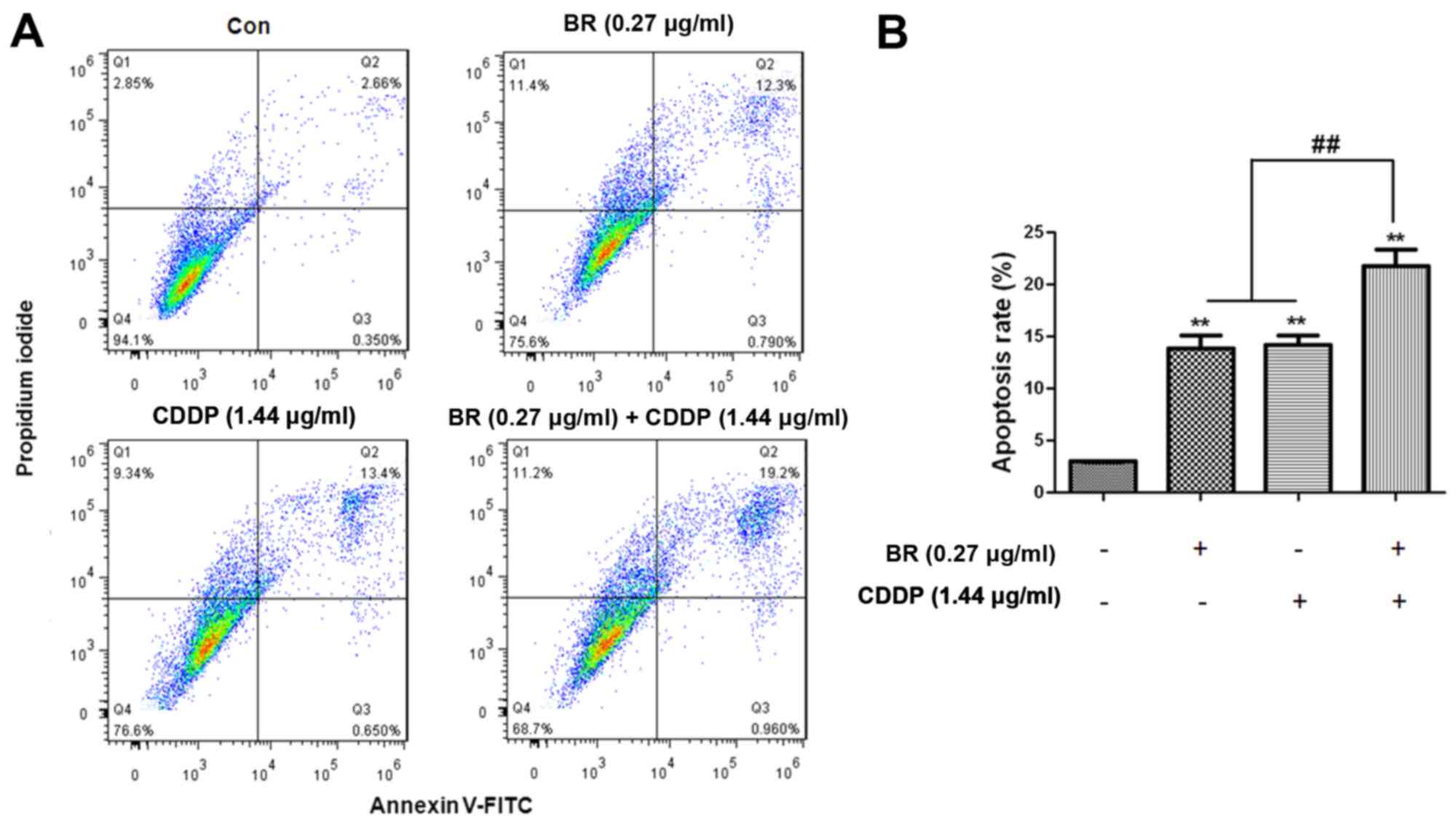 | Figure 5Apoptosis of CT-26 cells mediated by
BR and CDDP, alone or in combination. (A) Apoptosis was measured by
flow cytometry after PI/Annexin V-FITC staining. Q1, PI+
(cells undergoing necrosis); Q2, Annexin V-FITC+
PI+ (cells in the late period of apoptosis and
undergoing secondary necrosis); Q3, Annexin V-FITC+
PI− (cells in the early period of apoptosis); Q4,
Annexin V-FITC− PI− (living cells). Total
apoptotic rate was calculated as Q2 + Q3. (B) Apototic rates were
calculated. The proportion of early and late apoptotic cells
stained with Annexin V and PI is presented for each group. Data are
presented as the means ± standard deviation of three independent
experiments. **P<0.01 compared with the Con group;
##P<0.01 compared with the BR and CDDP monotherapy
groups. BR, brusatol; CDDP, cisplatin; Con, control; FITC,
fluorescein isothiocyanate; PI, propidium iodide |
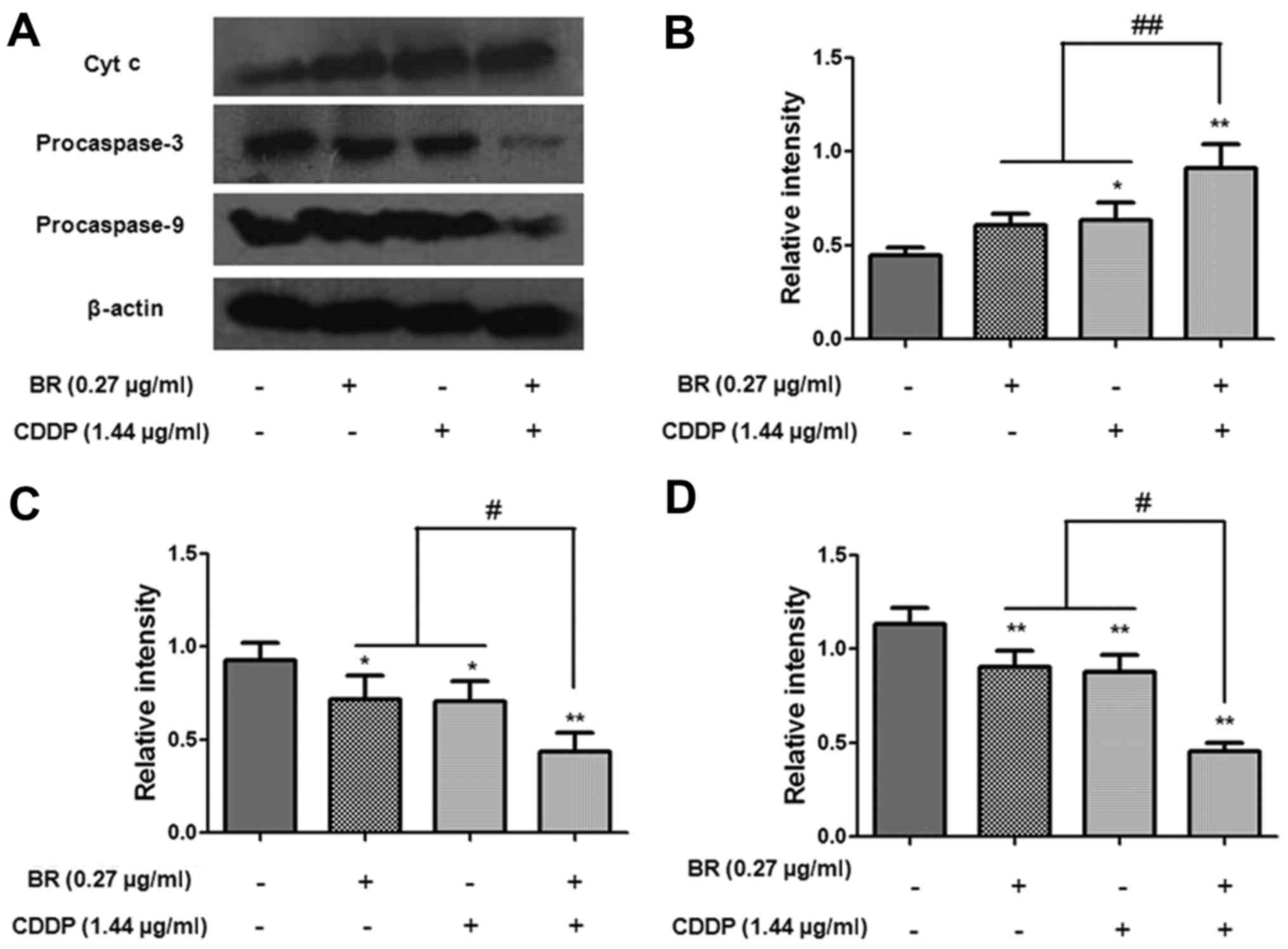
Effects of BR and CDDP on the expression
levels of apoptosis-associated proteins in CT-26 cells
According to the aforementioned results, the present
study aimed to further determine the mechanisms underlying the
synergistic antitumor effects of BR and CDDP. Since BR and CDDP
cotreatment markedly induced synergistic regulation of apoptosis,
the present study focused on the molecular mechanisms underlying
apoptosis. In the present study, western blot analysis was used to
detect the protein expression levels of procaspase-3, procaspase-9,
cytochrome c, Bax and Bcl-2. As shown in Fig. 6, the expression levels of
procaspase-3 and procaspase-9 were markedly decreased following
treatment with BR or CDDP alone. Compared with in the monotherapy
groups, BR and CDDP cotreatment significantly downregulated the
protein expression levels of procaspase-3 and procaspase-9
(P<0.05) and upregulated the protein expression levels of
cytosolic cytochrome c (P<0.01). The present study also
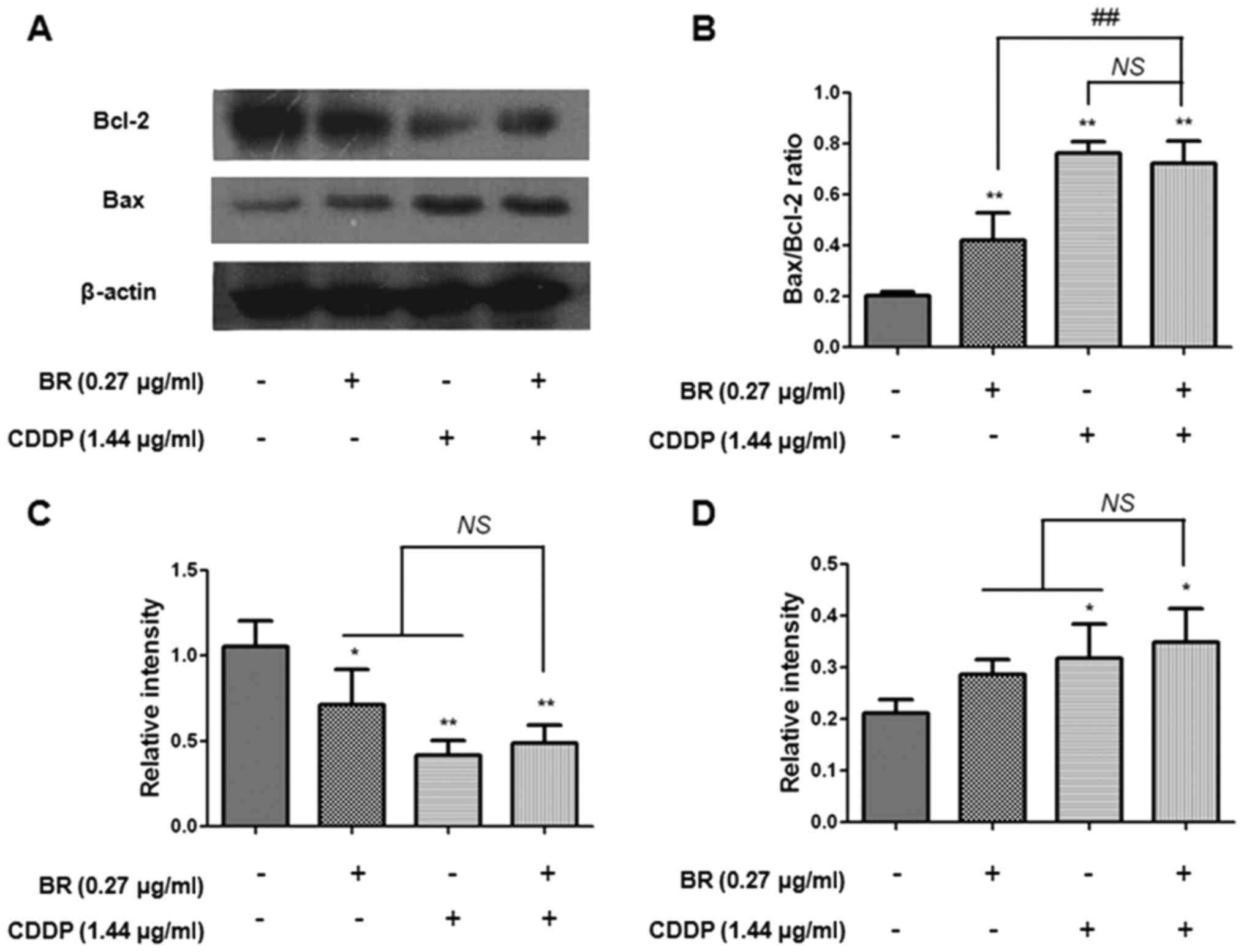
measured the expression levels of Bax and Bcl-2 (Fig. 7). Treatment with CDDP was able to
markedly increase the expression levels of Bax (P<0.05) and
decrease Bcl-2 expression (P<0.01). In addition, BR monotherapy
significantly decreased the expression levels of Bcl-2 (P<0.05).
However, there was no significant difference between BR or CDDP
monotherapy and cotreatment on the expression levels of Bax and
Bcl-2. Furthermore, the Bax/Bcl-2 ratio was also increased
(P<0.01) following monotherapy or cotreatment. These results
indicated that BR and CDDP induced cellular apoptosis via a
caspase-dependent signaling pathway.
Discussion
The present study aimed to evaluate the synergistic
effects of BR and CDDP on CT-26 CRC cells and to evaluate the
possible underlying mechanism. BR is the major active constituent
of B javanica, and has been reported to exert potent
anti-inflammatory (16),
antimalarial (17) and antitumor
activities (18–21). CDDP is a chemotherapy drug
commonly used in cancer therapy; however, the side effects,
including digestive tract reactions, renal toxicity, bone marrow
suppression and auditory neurotoxicity, cannot be ignored (25). Furthermore, long-term use of CDDP
can induce drug resistance (26,27). Compared with single-drug therapy,
it has been reported that combination therapy offers numerous
advantages, including several critical molecular targets, lower
dose and toxicity, and increased sensitivity (28). Therefore, the present study
investigated the synergistic effects of BR and CDDP on CT-26
cells.
The proliferative capacity of tumor cells is deemed
vital for the growth and development of tumors (29). The present study demonstrated that
a series of concentrations of BR and CDDP dose-dependently
suppressed the proliferation and growth of CT-26 cells (Fig. 2). In addition, the results
revealed that BR may exhibit a synergistic effect with CDDP on
CT-26 cells, with a CI value <1.
Apoptosis serves a central role in regulating normal
tissue equilibrium, and dysregulation of apoptosis presents a key
factor in the growth of cancer (30). Therefore, strategies that target
the apoptotic process may inhibit CRC development. Apoptotic cells
exhibit characteristics, including cell shrinkage, and chromatin
and nuclear condensation (31,32). To determine whether the inhibition
of CT-26 cellular proliferation induced by BR and CDDP was
associated with apoptosis, morphological alterations were detected
by Hoechst 33342 staining following treatment with BR and CDDP for
48 h. The treated cells displayed marked apoptotic characteristics,
including cell shrinkage, formation of small vesicles, cytoplasmic
condensation, pyknotic chromatin and nuclear fragmentation
(Fig. 4). The nuclei of CT-26
cells in the cotreatment group appeared to be slightly smaller with
brighter fluorescence compared with those of the monotherapy and
control groups.
During early apoptosis, phospholipid asymmetry takes
place prior to disintegration of the cellular membrane (33,34). Phosphatidylserine (PS) may
translocate to the outer layer of the plasma membrane from the
inner layer, where it is finally exposed to the external surface of
the cell. Therefore, surface exposure of PS is regarded as a
sensitive marker for assessing cellular membrane function and
apoptosis. Annexin V is a type of calcium-dependent
phospholipid-binding protein with a high affinity for PS; its
application with PI (a supravital fluorescent dye) is commonly used
to detect apoptotic and/or necrotic cells (34,35). To further quantify the apoptotic
rate of CT-26 cells following various treatments, cells were
stained with Annexin V and PI, and were subjected to flow
cytometry. Compared with the percentage of apoptotic cells in the
control group (3.00%), BR, CDDP and cotreatment significantly
increased the percentage of apoptotic cells to 13.88, 14.21 and
21.81%, respectively; apoptotic rate was relatively higher in the
BR and CDDP cotreatment group.
Activation of caspase cascades is vital for the
initiation of apoptosis (27,36). It has been reported that the
initiation of apoptosis involves the participation of at least two
distinct apoptotic pathways, including the intrinsic mitochondrial
apoptotic pathway, which is associated with caspase-9 activation,
and the extrinsic apoptotic pathway, which is associated with
caspase-3 activation (37,38).
To elucidate the molecular mechanism underlying the apoptosis of
CT-26 cells induced by BR and CDDP cotreatment, the present study
further investigated the possible activation of intrinsic and
extrinsic caspase cascades. In the present study, the protein
expression levels of procaspase-3 and procaspase-9 in CT-26 cells
treated with BR or CDDP monotherapy, or with a combination of BR
and CDDP, were significantly decreased compared with in the
untreated cells (P<0.05), whereas the expression of cytosolic
cytochrome c was significantly upregulated (P<0.05). BR
combined with CDDP led to synergistic regulation of the protein
expression of initiator and effector caspases in CT-26 cells
(Fig. 6). Therefore, it may be
suggested that apoptosis of CT-26 cells is induced by BR and CDDP
via downregulation of procaspase-3 and procaspase-9, and
upregulation of cytochrome c, which may be associated with
both intrinsic and extrinsic mitochondrial pathway.
The Bcl-2 family proteins, including Bcl-2 and Bax,
also serve an important role in regulation of the mitochondrial
apoptotic pathway (39). Released
cytochrome c binds with cytosolic apoptosis protease
activating factor, and induces the activation of caspase-9
(38). Bcl-2 suppresses the
initiation of apoptosis and promotes cell survival by inhibiting
the release of cytochrome c. Conversely, Bax elicits
apoptosis and evoked cell death through its promotion of cytochrome
c release from the mitochondria (40). Positive modulation of the
Bax/Bcl-2 ratio can lead to decreased mitochondrial membrane
potential and the release of cytochrome c, thereby contributing to
activation of the intrinsic apoptotic pathway. Therefore, the ratio
of Bax/Bcl-2 is commonly employed as an important index for the
assessment of mitochondria-mediated apoptosis (39). The present study detected the
protein expression levels of proapoptotic Bax, anti-apoptotic Bcl-2
and the Bax/Bcl-2 ratio in CT-26 cells by western blotting.
Compared with in the control cells, the expression levels of Bax
were significantly enhanced in the treated cells, whereas the
protein expression levels of Bcl-2 were markedly decreased
(Fig. 7). This observation may
result in the release of cytochrome c from the mitochondria,
which may further induce apoptosis.
In conclusion, the present study demonstrated that
BR could synergistically enhance the antitumor effects of CDDP on
CT-26 cells via the intrinsic and extrinsic apoptotic pathways, as
indicated by activation of Bax and cytochrome c, and
negative modulation of procaspase-3, procaspase-9 and Bcl-2. These
findings suggested that BR and CDDP cotreatment may be a beneficial
option to enhance the antitumor effects of CDDP on the treatment of
CRC.
Abbreviations:
|
BR
|
brusatol
|
|
CDDP
|
cisplatin
|
|
CRC
|
colorectal cancer
|
|
PI
|
propidium iodide
|
|
PS
|
phosphatidylserine
|
|
TBS-T
|
Tris-buffered saline containing 0.1%
Tween-20
|
Acknowledgments
The present study was supported by grants from the
Hong Kong, Macao and Taiwan Science and Technology Cooperation
Program of China (grant no. 2014DFH30010), the Chinese Medicinal
Scientific Research Project of Guangdong Province (grant no.
20161096), the Chinese Medicinal Scientific Research and Technology
Research Projects of Guangdong Provincial Hospital of Chinese
Medicine and the Key Discipline Construction Projects of
Integrative Medicine of High Level University of Guangdong Province
(grant no. YN2015QN05), the Science and Technology Planning Project
of Guangdong Province (grant no. 2017A050506044), the Natural
Science Foundation of Guangdong Province (grant no.
2017A030310124), the Science and Technology Planning Project of
Guangzhou of Guangdong Province (grant no. 201704030028), and the
China Postdoctoral Science Foundation (grant nos. 2016M600649 and
2017T100622).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Weitz J, Koch M, Debus J, Höhler T, Galle
PR and Büchler MW: Colorectal cancer. Lancet. 365:153–165. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lindblom A, Zhou XL, Liu T, Liljegren A,
Skoglund J, Djureinovic T and Swedish Low-Risk; Colorectal Cancer
Group: Colorectal cancer as a complex disease: Defining at-risk
subjects in the general population - a preventive strategy. Expert
Rev Anticancer Ther. 4:377–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zauber AG, Lansdorp-Vogelaar I, Knudsen
AB, Wilschut J, van Ballegooijen M and Kuntz KM: Evaluating test
strategies for colorectal cancer screening: A decision analysis for
the U.S. Preventive Services Task Force. Ann Intern Med.
149:659–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lambert R, Sauvaget C and Sankaranarayanan
R: Mass screening for colorectal cancer is not justified in most
developing countries. Int J Cancer. 125:253–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH,
Liu JH, Lou QY and Gan AH: Colorectal cancer in Guangdong Province
of China: A demographic and anatomic survey. World J Gastroenterol.
16:960–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folkesson J, Martling A and Kodeda K:
Current considerations in colorectal cancer surgery. Colorectal
Cancer. 4:167–174. 2015. View Article : Google Scholar
|
|
9
|
Gustavsson B, Carlsson G, Machover D,
Petrelli N, Roth A, Schmoll HJ, Tveit KM and Gibson F: A review of
the evolution of systemic chemotherapy in the management of
colorectal cancer. Clin Colorectal Cancer. 14:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dagoglu N, Mahadevan A, Nedea E, Poylin V
and Nagle D: Stereotactic body radiotherapy (SBRT) reirradiation
for pelvic recurrence from colorectal cancer. J Surg Oncol.
111:478–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marin JJ, Sanchez de Medina F, Castaño B,
Bujanda L, Romero MR, Martinez-Augustin O, Moral-Avila RD and Briz
O: Chemoprevention, chemotherapy, and chemoresistance in colorectal
cancer. Drug Metab Rev. 44:148–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin SY, Wei WC, Jian FY and Yang NS:
Therapeutic applications of herbal medicines for cancer patients.
Evid Based Complement Alternat Med. 2013:3024262013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao M, Lau ST, Leung PS, Che CT and Lin
ZX: Seven quassinoids from Fructus Bruceae with cytotoxic effects
on pancreatic adenocarcinoma cell lines. Phytother Res.
25:1796–1800. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Commission CP: Pharmacopoeia of People's
Republic of China. 1. Chinese Medical Science and Technology Press;
Beijing: pp. 174–175. 2010
|
|
15
|
Fukamiya N, Okano M, Miyamoto M, Tagahara
K and Lee KH: Antitumor agents, 127. Bruceoside C, a new cytotoxic
quassinoid glucoside, and related compounds fro. Brucea javanica J
Nat Prod. 55:468–475. 1992. View Article : Google Scholar
|
|
16
|
Turpaev K and Welsh N: Brusatol inhibits
the response of cultured beta-cells to pro-inflammatory cytokines
in vitro. Biochem Biophys Res Commun. 460:868–872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen D, Toth I, Wright C, Kirby G,
Warhurst D and Phillipson J: In vitro antimalarial and cytotoxic
activities of semisynthetic derivatives of brusatol. Eur J Med
Chem. 28:265–269. 1993. View Article : Google Scholar
|
|
18
|
Cuendet M, Gills JJ and Pezzuto JM:
Brusatol-induced HL-60 cell differentiation involves NF-kappaB
activation. Cancer Lett. 206:43–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hitotsuyanagi Y, Kim IH, Hasuda T,
Yamauchi Y and Takeya K: A structure-activity relationship study of
brusatol, an antitumor quassinoid. Tetrahedron. 62:4262–4271. 2006.
View Article : Google Scholar
|
|
20
|
Mata-Greenwood E, Cuendet M, Sher D,
Gustin D, Stock W and Pezzuto JM: Brusatol-mediated induction of
leukemic cell differentiation and G(1) arrest is associated with
downregulation of c-myc. Leukemia. 16:2275–2284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren D, Villeneuve NF, Jiang T, Wu T, Lau
A, Toppin HA and Zhang DD: Brusatol enhances the efficacy of
chemotherapy by inhibiting the Nrf2-mediated defense mechanism.
Proc Natl Acad Sci USA. 108:1433–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Olayanju A, Copple IM, Bryan HK, Edge GT,
Sison RL, Wong MW, Lai ZQ, Lin ZX, Dunn K, Sanderson CM, et al:
Brusatol provokes a rapid and transient inhibition of Nrf2
signaling and sensitizes mammalian cells to chemical
toxicity-implications for therapeutic targeting of Nrf2. Free Radic
Biol Med. 78:202–212. 2015. View Article : Google Scholar :
|
|
23
|
Chou TC and Talalay P: Analysis of
combined drug effects: A new look at a very old problem. Trends
Pharmacol Sci. 4:450–454. 1983. View Article : Google Scholar
|
|
24
|
Ashton JC: Drug combination studies and
their synergy quantification using the Chou-Talalay method -
letter. Cancer Res. 75:2400. 2015. View Article : Google Scholar
|
|
25
|
Florea AM and Büsselberg D: Cisplatin as
an antitumor drug: Cellular mechanisms of activity, drug resistance
and induced side effects. Cancers (Basel). 3:1351–1371. 2011.
View Article : Google Scholar
|
|
26
|
Li B, Gao Y, Rankin GO, Rojanasakul Y,
Cutler SJ, Tu Y and Chen YC: Chaetoglobosin K induces apoptosis and
G2 cell cycle arrest through p53-dependent pathway in
cisplatin-resistant ovarian cancer cells. Cancer Lett. 356:418–433.
2015. View Article : Google Scholar :
|
|
27
|
Galluzzi L, López-Soto A, Kumar S and
Kroemer G: Caspases connect cell-death signaling to organismal
homeostasis. Immunity. 44:221–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin Y, Yang X, Lu M, Zheng W, Zhang J,
Zhuang H and Hua ZC: Herbal compound triptolide synergistically
enhanced antitumor activity of vasostatin 120–180. Anticancer
Drugs. 24:945–957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao W, Li XQ, Wang X, Fan HT, Zhang XN,
Hou Y, Liu SB and Mei QB: A novel polysaccharide, isolated from
Angelica sinensis (Oliv.) Diels induces the apoptosis of cervical
cancer HeLa cells through an intrinsic apoptotic pathway.
Phytomedicine. 17:598–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Doonan F and Cotter TG: Morphological
assessment of apoptosis. Methods. 44:200–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fadok VA1, de Cathelineau A, Daleke DL,
Henson PM and Bratton DL: Loss of phospholipid asymmetry and
surface exposure of phosphatidylserine is required for phagocytosis
of apoptotic cells by macrophages and fibroblasts. J Biol Chem.
276:1071–1077. 2001. View Article : Google Scholar
|
|
34
|
Vermes I, Haanen C, Steffens-Nakken H and
Reutelingsperger C: A novel assay for apoptosis. Flow cytometric
detection of phosphatidylserine expression on early apoptotic cells
using fluorescein labelled Annexin V. J Immunol Methods. 184:39–51.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Engeland M, Nieland LJ, Ramaekers FC,
Schutte B and Reutelingsperger CP: Annexin V-affinity assay: A
review on an apoptosis detection system based on phosphatidylserine
exposure. Cytometry. 31:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES, Wang X and Li P1: Cytochrome c
and dATP-dependent formation of Apaf-1/caspase-9 complex initiates
an apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao K, Jin M, Chen Q and Zheng PS:
Polysaccharides produced by Enterobacter cloacae induce apoptosis
in cervical cancer cells. Int J Biol Macromol. 72:960–964. 2015.
View Article : Google Scholar
|