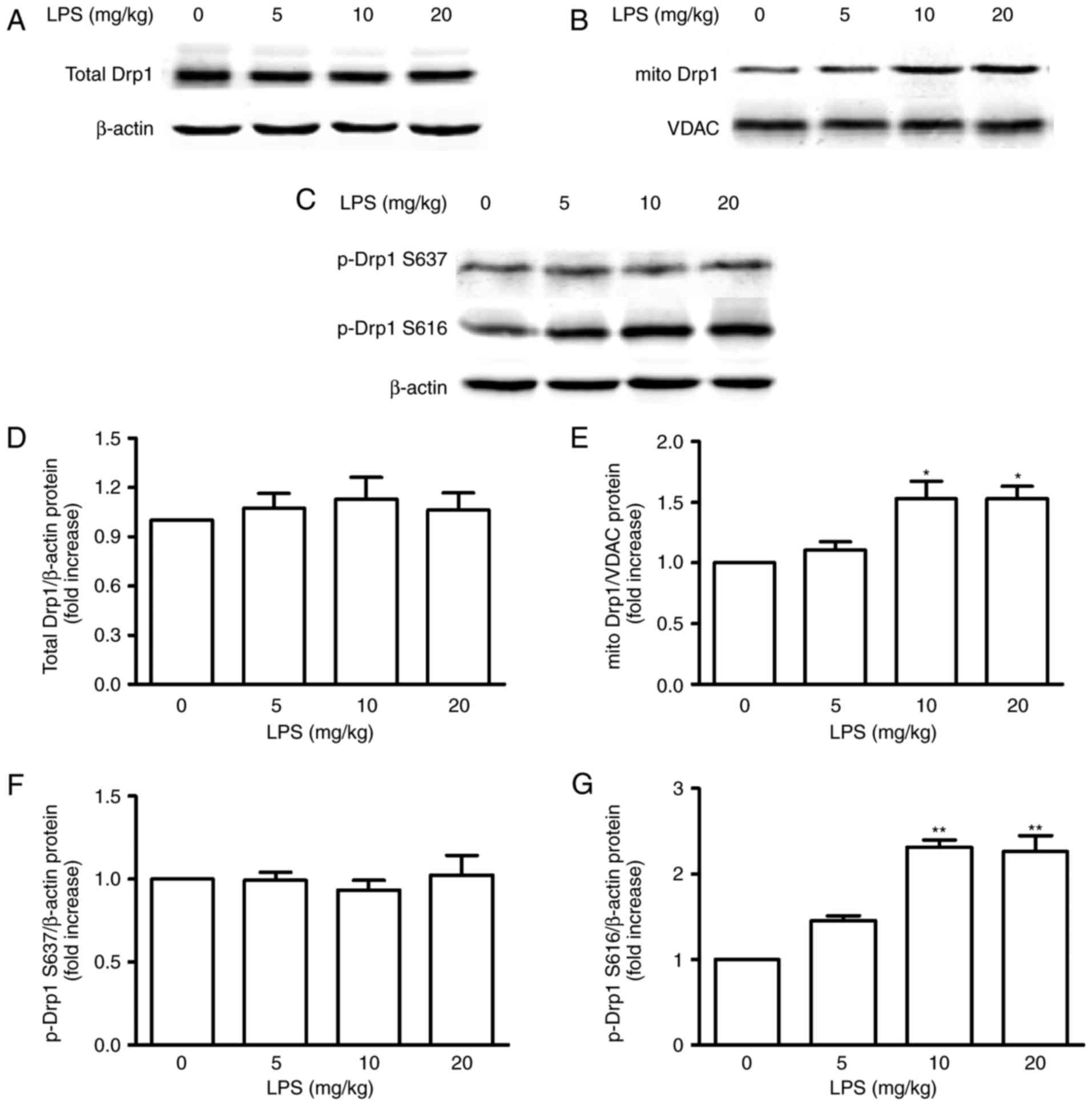
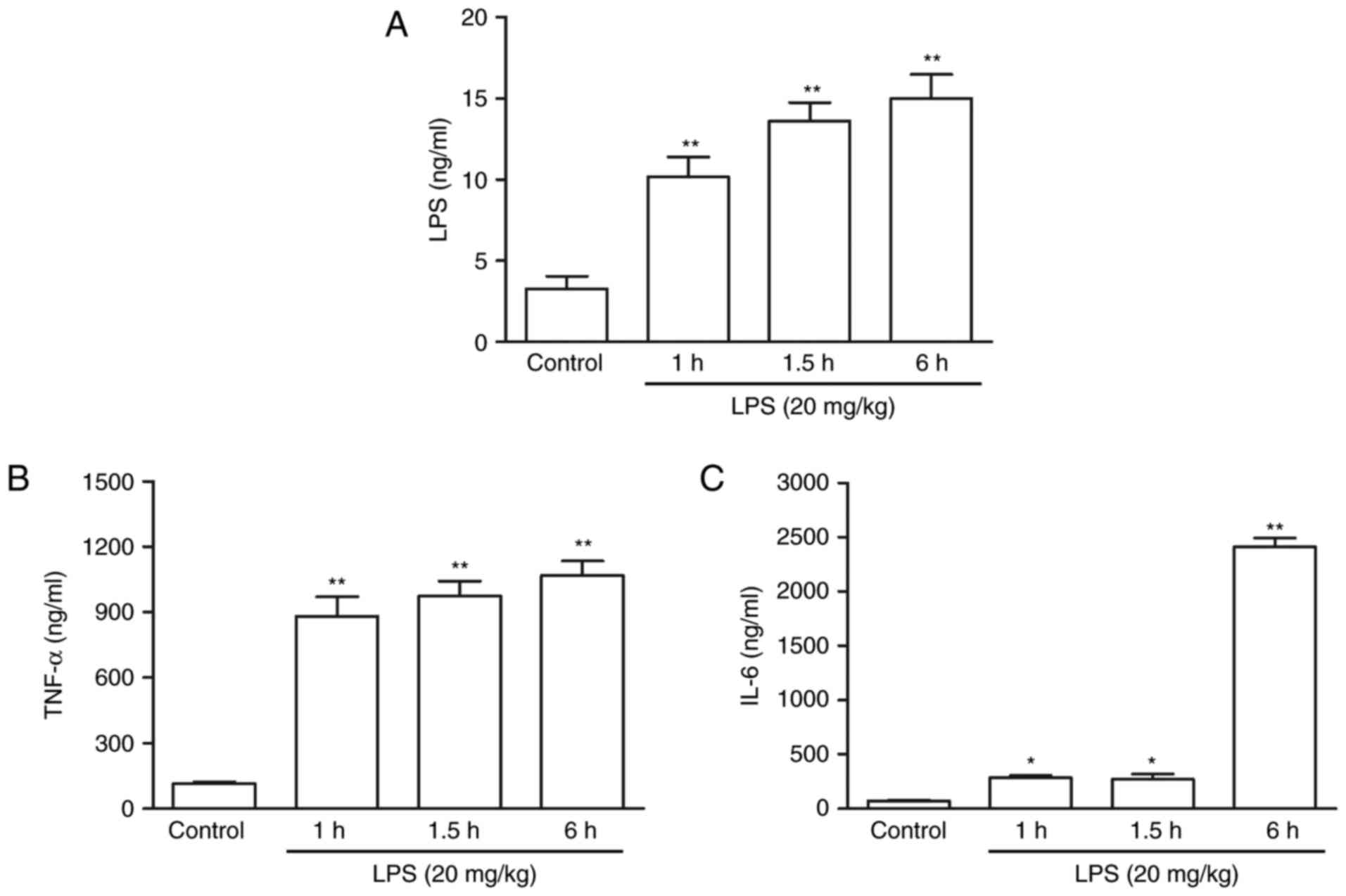
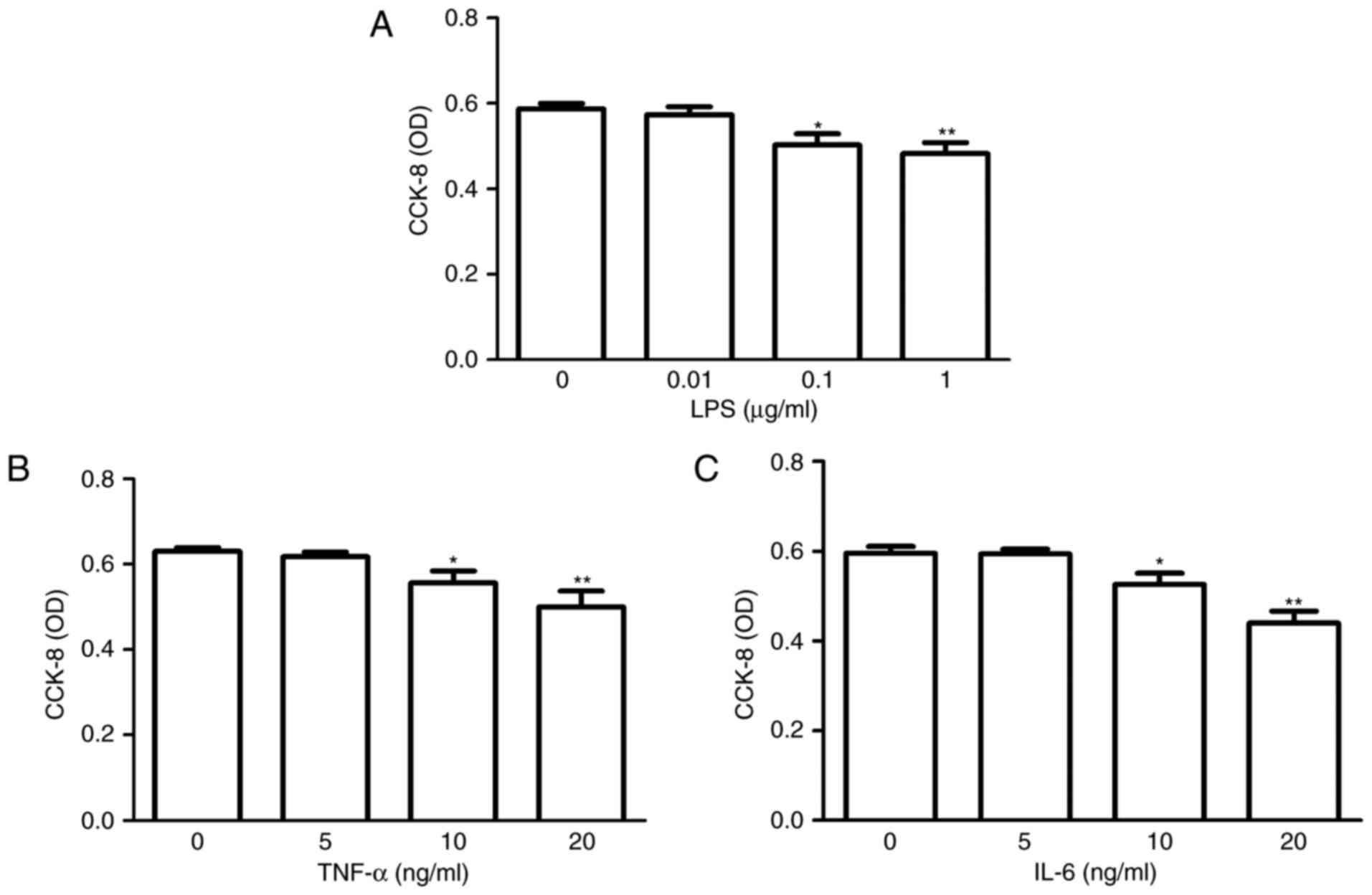
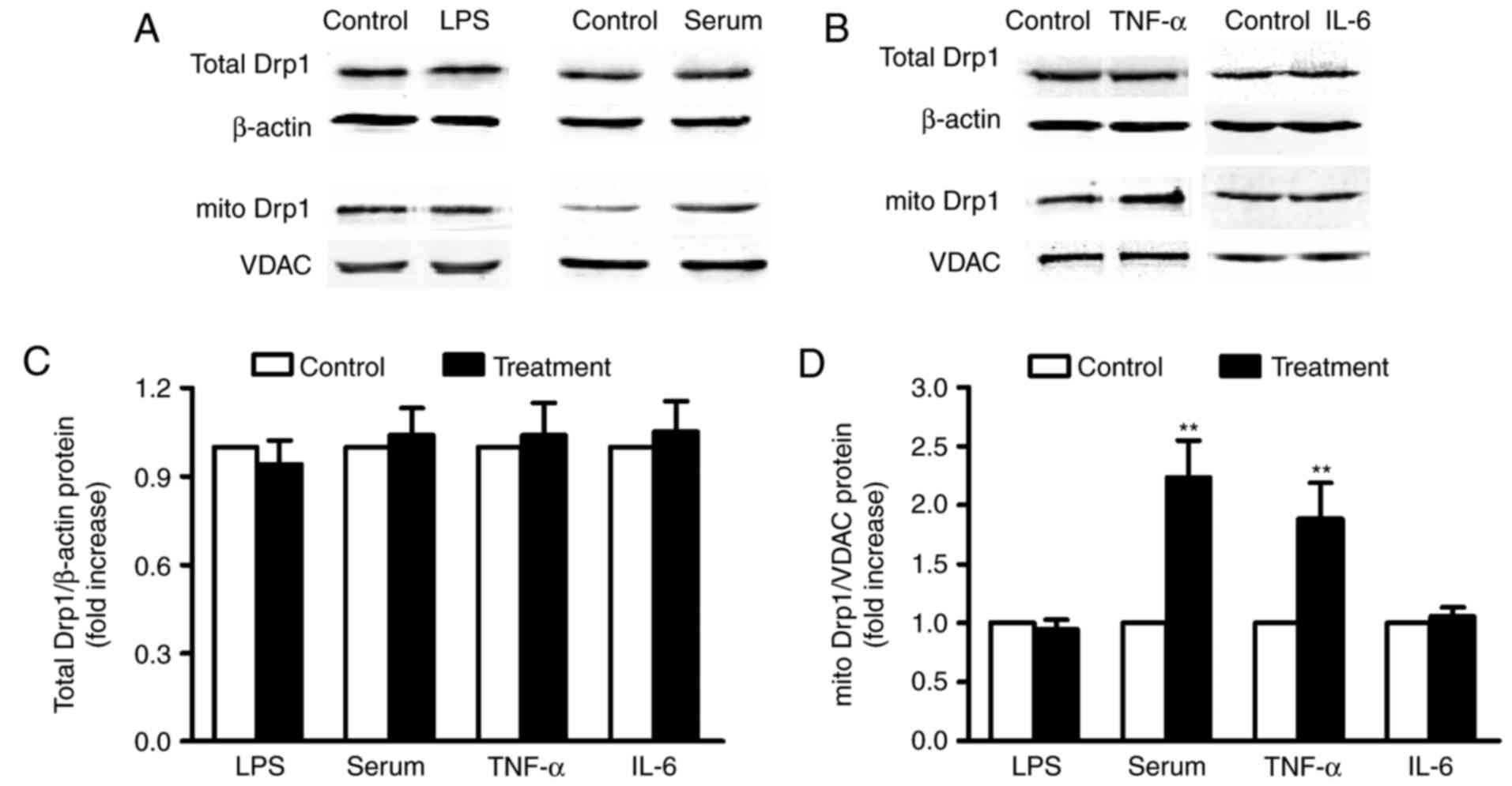
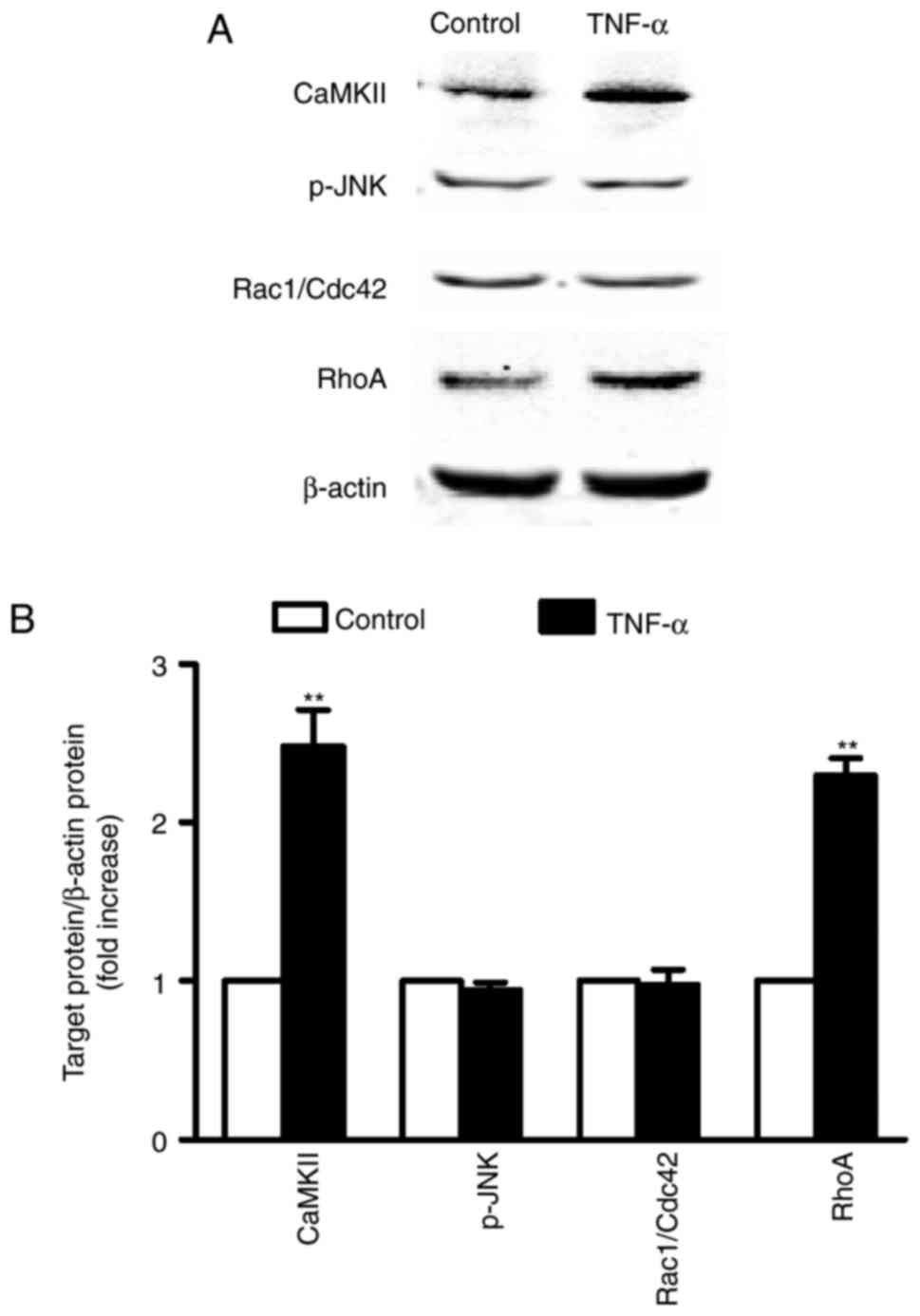
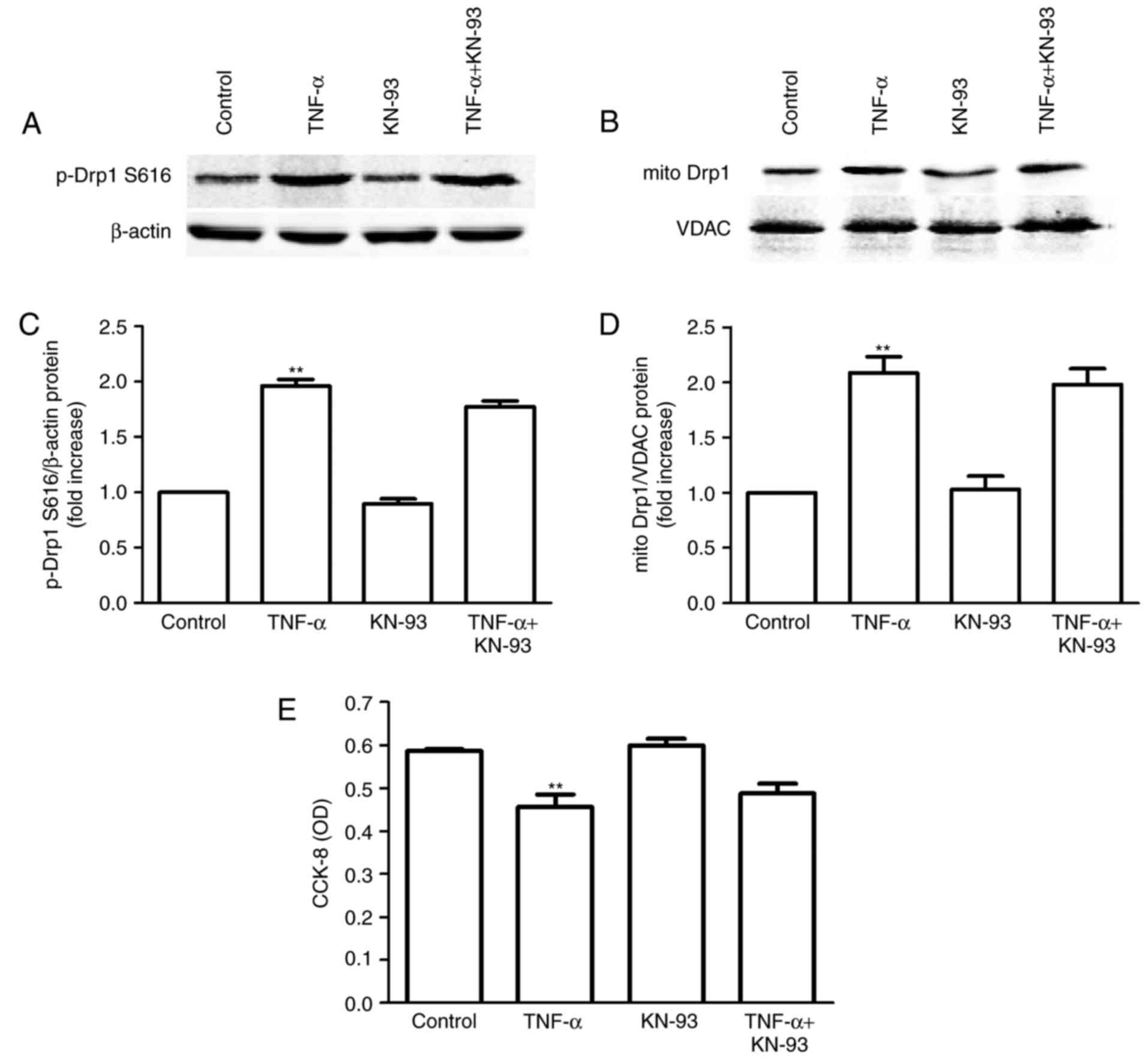
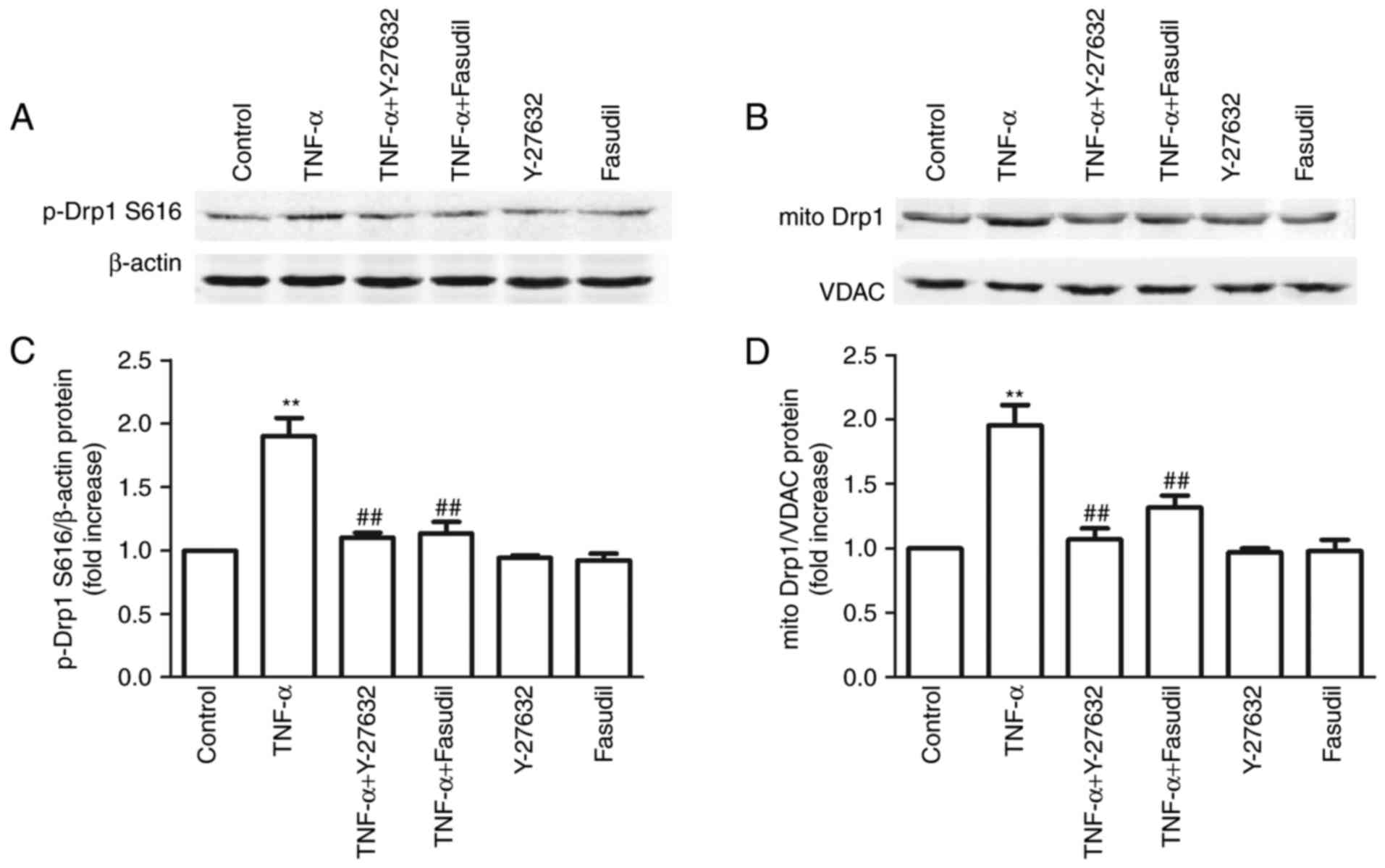
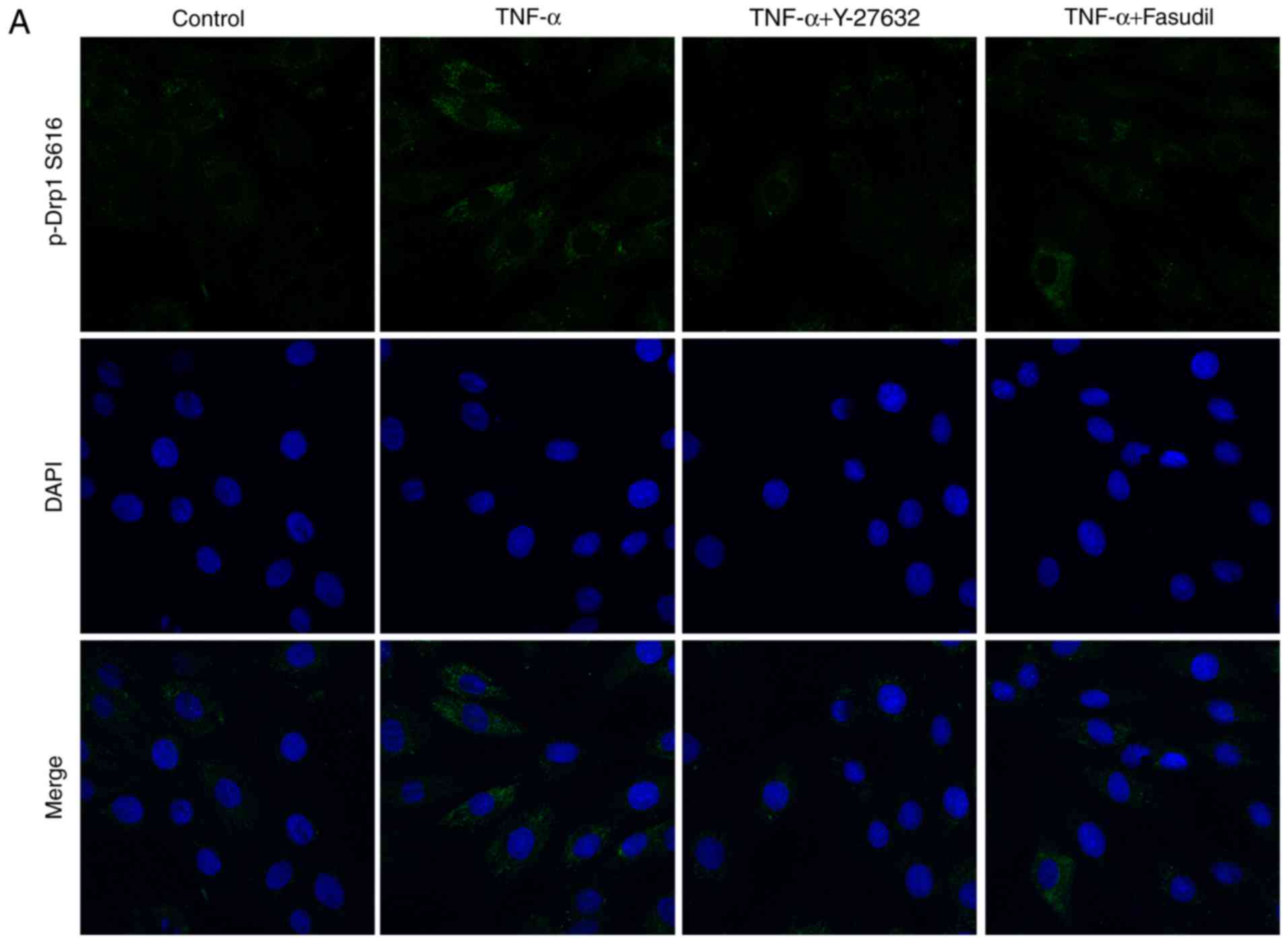
|
1
|
Ong SB and Hausenloy DJ: Mitochondrial
morphology and cardiovascular disease. Cardiovasc Res. 88:16–29.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ong SB, Kalkhoran SB, Cabrera-Fuentes HA
and Hausenloy DJ: Mitochondrial fusion and fission proteins as
novel therapeutic targets for treating cardiovascular disease. Eur
J Pharmacol. 763:104–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marzetti E, Csiszar A, Dutta D, Balagopal
G, Calvani R and Leeuwenburgh C: Role of mitochondrial dysfunction
and altered autophagy in cardiovascular aging and disease: From
mechanisms to therapeutics. Am J Physiol Heart Circ Physiol.
305:H459–H476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Givvimani S, Pushpakumar SB, Metreveli N,
Veeranki S, Kundu S and Tyagi SC: Role of mitochondrial fission and
fusion in cardiomyocyte contractility. Int J Cardiol. 187:325–333.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frank S, Gaume B, Bergmann-Leitner ES,
Leitner WW, Robert EG, Catez F, Smith CL and Youle RJ: The role of
dynamin-related protein 1, a mediator of mitochondrial fission, in
apoptosis. Dev Cell. 1:515–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ong SB, Subrayan S, Lim SY, Yellon DM,
Davidson SM and Hausenloy DJ: Inhibiting mitochondrial fission
protects the heart against ischemia/reperfusion injury.
Circulation. 121:2012–2022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee Y, Lee HY, Hanna RA and Gustafsson AB:
Mitochondrial autophagy by Bnip3 involves Drp1-mediated
mitochondrial fission and recruitment of Parkin in cardiac
myocytes. Am J Physiol Heart Circ Physiol. 301:H1924–H1931. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Li Y, Qin D, von Harsdorf R and Li
P: Mitochondrial fission leads to Smac/DIABLO release quenched by
ARC. Apoptosis. 15:1187–1196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borowiec A, Kontny E, Smolis-Bak E,
Kowalik I, Majos E, Załucka L, Plaziński K, Maśliński W, Szwed H
and Dabrowski R: Prospective assessment of cytokine IL-15 activity
in patients with refractory atrial fibrillation episodes. Cytokine.
74:164–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dunlay SM, Weston SA, Redfield MM, Killian
JM and Roger VL: Tumor necrosis factor-alpha and mortality in heart
failure: A community study. Circulation. 118:625–631. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alturfan AA, Basar I, Emekli-Alturfan E,
Ayan F, Koldas L and Emekli N: Galectin-3 and plasma cytokines in
patients with acute myocardial infarction. Lab Med. 45:336–341.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maeda A and Fadeel B: Mitochondria
released by cells undergoing TNF-α-induced necroptosis act as
danger signals. Cell Death Dis. 5:e13122014. View Article : Google Scholar
|
|
13
|
Preau S, Delguste F, Yu Y, Remy-Jouet I,
Richard V, Saulnier F, Boulanger E and Neviere R: Endotoxemia
engages the RhoA kinase pathway to impair cardiac function by
altering cytoskeleton, mitochondrial fission, and autophagy.
Antioxid Redox Signal. 24:529–542. 2016. View Article : Google Scholar
|
|
14
|
Gonzalez AS, Elguero ME, Finocchietto P,
Holod S, Romorini L, Miriuka SG, Peralta JG, Poderoso JJ and
Carreras MC: Abnormal mitochondrial fusion-fission balance
contributes to the progression of experimental sepsis. Free Radic
Res. 48:769–783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Y, Yan JB, Zheng MZ, Song XH, Wang LL,
Shen YL and Chen YY: Mitochondrial aldehyde dehydrogenase activity
protects against lipopolysaccharide-induced cardiac dysfunction in
rats. Mol Med Rep. 11:1509–1515. 2015. View Article : Google Scholar
|
|
16
|
Pennanen C, Parra V, López-Crisosto C,
Morales PE, Del Campo A, Gutierrez T, Rivera-Mejías P, Kuzmicic J,
Chiong M, Zorzano A, et al: Mitochondrial fission is required for
cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin
signaling pathway. J Cell Sci. 127:2659–2671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park J, Choi H, Min JS, Park SJ, Kim JH,
Park HJ, Kim B, Chae JI, Yim M and Lee DS: Mitochondrial dynamics
modulate the expression of pro-inflammatory mediators in microglial
cells. J Neurochem. 127:221–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motori E, Puyal J, Toni N, Ghanem A,
Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B, Conzelmann
KK, Götz M, et al: Inflammation-induced alteration of astrocyte
mitochondrial dynamics requires autophagy for mitochondrial network
maintenance. Cell Metab. 18:844–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hall AR, Burke N, Dongworth RK and
Hausenloy DJ: Mitochondrial fusion and fission proteins: Novel
therapeutic targets for combating cardiovascular disease. Br J
Pharmacol. 171:1890–1906. 2014. View Article : Google Scholar :
|
|
20
|
Rosdah AA, K Holien J, Delbridge LM,
Dusting GJ and Lim SY: Mitochondrial fission -a drug target for
cytoprotection or cytodestruction? Pharmacol Res Perspect.
4:e002352016. View
Article : Google Scholar
|
|
21
|
Cho DH, Nakamura T, Fang J, Cieplak P,
Godzik A, Gu Z and Lipton SA: S-nitrosylation of Drp1 mediates
beta-amyloid-related mitochondrial fission and neuronal injury.
Science. 324:102–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karbowski M, Neutzner A and Youle RJ: The
mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1
dependent mitochondrial division. J Cell Biol. 178:71–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zunino R, Schauss A, Rippstein P,
Andrade-Navarro M and McBride HM: The SUMO protease SENP5 is
required to maintain mitochondrial morphology and function. J Cell
Sci. 120:1178–1188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godoy JA, Arrazola MS, Ordenes D,
Silva-Alvarez C, Braidy N and Inestrosa NC: Wnt-5a ligand modulates
mitochondrial fission-fusion in rat hippocampal neurons. J Biol
Chem. 289:36179–36193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong Z, Kutty S, Toth PT, Marsboom G,
Hammel JM, Chamberlain C, Ryan JJ, Zhang HJ, Sharp WW, Morrow E, et
al: Role of dynamin-related protein 1 (Drp1)-mediated mitochondrial
fission in oxygen sensing and constriction of the ductus
arteriosus. Circ Res. 112:802–815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernandez-Cobo M, Gingalewski C, Drujan D
and De Maio A: Downregulation of connexin 43 gene expression in rat
heart during inflammation. The role of tumour necrosis factor.
Cytokine. 11:216–224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu M, Lei L, Zhu Z, Li Q, Guo D, Xu J,
Chen J, Sha H, Zhang X, Yang X, et al: Excess TNF-α in the blood
activates monocytes with the potential to directly form cholesteryl
ester-laden cells. Acta Biochim Biophys Sin. 47:899–907. 2015.
View Article : Google Scholar
|
|
28
|
Sugishita K, Kinugawa K, Shimizu T, Harada
K, Matsui H, Takahashi T, Serizawa T and Kohmoto O: Cellular basis
for the acute inhibitory effects of IL-6 and TNF-alpha on
excitation-contraction coupling. J Mol Cell Cardiol. 31:1457–1467.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu S, Wang P, Zhang H, Gong G, Gutierrez
Cortes N, Zhu W, Yoon Y, Tian R and Wang W: CaMKII induces
permeability transition through Drp1 phosphorylation during chronic
β-AR stimulation. Nat Commun. 7:131892016. View Article : Google Scholar
|
|
30
|
Ramachandran A and Jaeschke H: Mechanisms
of acetaminophen hepatotoxicity and their translation to the human
pathophysiology. J Clin Transl Res. 3(Suppl 1): S157–S169.
2017.
|
|
31
|
Gao X, Mi Y, Guo N, Hu Z, Hu F, Liu D, Gao
L, Gou X and Jin W: Disrupted in schizophrenia 1 (DISC1) inhibits
glioblastoma development by regulating mitochondria dynamics.
Oncotarget. 7:85963–85974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yin M, Lu Q, Liu X, Wang T, Liu Y and Chen
L: Silencing Drp1 inhibits glioma cells proliferation and invasion
by RHOA/ROCK1 pathway. Biochem Biophys Res Commun. 478:663–668.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wirth A: Rho kinase and hypertension.
Biochim Biophys Acta. 1802:1276–1284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai A, Li L and Zhou Y: Pathophysiological
effects of RhoA and Rho-associated kinase on cardiovascular system.
J Hypertens. 34:3–10. 2016. View Article : Google Scholar
|