Introduction
Subarachnoid hemorrhage (SAH) is a fatal and
disabling disease that accounts for ~6% of stroke cases; annually,
~10/100,000 patients develop aneurysmal SAH worldwide (1). The interval between the occurrence
of SAH and the time when patients are admitted to hospital for
treatment is associated with a mortality rate of ~12%, whereas the
mortality rate increases tô40% after patients are admitted to
hospital for treatment, and the disability rate for survivors of
SAH is ~30%. SAH places a heavy burden on patients themselves,
their families and society; therefore, the dangers of SAH cannot be
underestimated (2,3). Although surgical technology,
radiographic techniques and anesthesia have attained considerable
achievements with regards to time, the mortality and disability
rates associated with SAH have exhibited no marked alterations in
recent years (4). A previous
study has mainly focused on vasospasm and the resulting injury, and
have considered it the major cause of SAH-associated mortality and
disability (3). However, it has
been reported that the prognosis for patients with SAH is not
markedly improved following the prevention of cerebral vasospasm
(CVS) generation. In addition, patients with no vasospasm also
develop ischemic injury in the late stage of SAH; consequently,
doubts have begun to surface regarding the importance of CVS in
injury following SAH, and it is now considered one of numerous
pathogenic factors, rather than the previously believed priority
(4). Therefore, further
etiological and therapeutic research is required; and the research
focus has shifted to concentrate on all types of injury mechanisms
during early brain injury (EBI). Previous studies have indicated
that relieving all types of injury during EBI may improve the
prognosis of patients (5,6).
Notably, p53 has been reported to not only serve an
important role in the mechanisms underlying CVS and post-SAH
apoptosis, but to also participate in the development of
hydrocephalus; the reason for this is that the increased p53
following SAH can upregulate the expression and activity of matrix
metalloproteinase-9, thus leading to the destruction of blood-brain
barrier integrity (7). Therefore,
p53 is considered to serve a role in the pathophysiological
mechanism underlying SAH, and it has been suggested that targeting
p53 may relieve nerve injury following SAH. Notably, progress has
been achieved regarding the mode of post-transcriptional regulation
of p53 (8). The
post-transcriptional regulation of p53 can be divided into
acetylation and phosphorylation; it has been confirmed that the
protein levels of acetylated p53, which has a proapoptotic role,
are notably increased in the hippocampal region of rats following
cerebral ischemia (9). In
addition, it has been reported that the proapoptotic effects of
acetylated p53 may be relieved by deacetylation by the regulatory
factor sirtuin 1 (SIRT1); p53 is a non-histone substrate of SIRT1
and SIRT1 may inhibit apoptosis via deacetylation of p53 (10).
MicroRNAs are endogenous non-coding RNAs,
approximately 18-25 nucleotides in length, which regulate
transcriptional gene expression through binding the 3'-untranslated
region of mRNAs and the non-translation region in the d-terminus
(11). The majority of microRNA
genes are located in the exonic, intronic and non-coding regions of
the genome, and are transcribed into the microRNA primary
transcript by RNA polymerase II, which results in the addition of a
poly(A) tail (12). MicroRNAs
serve important roles in numerous pathophysiological processes,
including oxidative stress, the inflammatory response and cell
apoptosis (13). It is well known
that microRNAs depend on two principles of sequence complementarity
to negatively regulate target gene expression: i) The microRNA is
completely complementary to the target gene mRNA, thus resulting in
its degradation; ii) the microRNA is not completely complementary
to the target gene mRNA, thus inhibiting target gene mRNA
expression at the protein translation level (14). At present, >1,000 microRNAs
have been discovered, which regulate ≥30% of gene expression and
form a complex regulatory network; however, to the best of our
knowledge, there is no information regarding the regulation of
vascular smooth muscle cell (VSMC) apoptosis by microRNAs after SAH
(15). Therefore, investigating
the effects of microRNAs on the apoptotic mechanism of VSMCs
post-SAH is promising and may result in the generation of novel
knowledge. The present study aimed to determine the role of
p53/microRNA-22 in the regulation of inflammation and apoptosis in
SAH.
Materials and methods
Animals and SAH model
C57BL/6J male mice (weight, 19-20 g; age, 5-6 weeks
old; n=12) were purchased from Chongqing Medical University
(Chongqing, China), and were maintained at 23°C and 55% humidity
under a 12-h light/dark cycle with free access to food and water.
The present study was approved by the Animal Care and Use Committee
of Chongqing Medical University.
Mice were injected with pentobarbital sodium (30
mg/kg) and were positioned in a stereotactic frame. Subsequently,
mice were disinfected and a midline scalp incision was made; a hole
(1×1 mm) was made in the midline 7.5 mm anterior to the bregma.
Subsequently, 300 µl blood was collected from the femoral artery;
the blood was then injected into the prechiasmatic cistern. The
wound was sutured and sterilized, after which 50 µl 0.9%
NaCl was subcutaneously injected into the mice, which were
transferred to a recovery cage. After 30-60 min of recovery, the
mice were returned to clean cages and were housed at 23±1°C.
Experimental groups
SAH model mice were randomly assigned into the
following two groups: Control and pifithrin-α groups (n=8
mice/group). In the control group, mice were injected with
pentobarbital sodium (30 mg/kg) and were positioned in a
stereotactic frame without subarachnoid hemorrhage. Pifithrin-α was
purchased from Beyotime Institute of Biotechnology (Haimen, China),
and was dissolved in dimethyl sulfoxide to 2 mg/kg and diluted with
normal saline at 100 µl/10 g. Pifithrin-α was injected
intraperitoneally 12 h after SAH and the mice were sacrificed by
decapitation under pentobarbital sodium anesthesia (30 mg/kg) 36 h
after SAH.
Cell culture and cell transfection
The HEB human normal glial cell line was purchased
from Chongqing Medical University. The cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (both from Invitrogen,; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C in an incubator containing 5%
CO2.
Transfection
MicroRNA-22 (5'-CTCAACTGGTGTCGTGGAGTCGG-3' and
5'-CAATTCAGTTGAGACAGTTCT-3'), microRNA-22 inhibitor
(5'-GCGAAAGCATTTGCCAAGAA -3' and 5'-CATCACAGACCTGTTATTGC-3'), small
interfering RNA (si)-p53 (5'-ctcgagctatggttgccttgaaattatc-3' and
5'-gcggccgctgtaactctgggcagtgcaa-3') and negative controls
(5'-CAATTCAGTTGAGACAGTTCT-3' and 5'-ACGUGACACGUUCGGAGAATT-3') were
synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The
forward primer of microRNA-22 mimics was
5'-CTCAACTGGTGTCGTGGAGTCGG-3' and the reverse primer was
5'-CAATTCAGTTGAGACAGTTCT-3'; the forward primer of anti-microRNA-22
was 5'-UUCUCCGAACGUGUCACGUTT-3' and the reverse primer was
5'-ACGUGACACGUUCGGAGAATT-3'. HEB cells were treated with
lipopolysaccha-ride (LPS) and were seeded into 6-well plates at a
density of 1.5-2.0×105 cells/well. Subsequently, the
cells were transfected with the oligonucleotides using Lipofecta
mine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, the cells were transfected with microRNA-22,
microRNA-22 inhibitor, small interfering (si)RNA-p53 and negative
controls using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 4 h. After treatment for 4 h, old
medium was removed and new Dulbecco's Modified Eagle's Medium was
added at 37°C. HEB cells were treated with 50 ng/ml LPS (Beyotime
Institute of Biotechnology) for 2 h.
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted from tissue and cell samples
using RNAsimple Total RNA kit (DP419; Tiangen Biotech Co., Ltd.,
Beijing, China). cDNA was synthesized using Super M-MLV reverse
transcriptase (RP6502; BioTeke Corporation, Beijing, China). qPCR
was performed to measure the expression levels of microRNA-22 and
interleukin (IL)-6 mRNA using SYBR Premix kit (Takara Biotechnology
Co., Ltd., Dalian, China) on an ABI 7300 real-time PCR machine
(Applied Biosystems; Thermo Fisher Scientific, Inc.). cDNA was
synthesized using Super M-MLV reverse transcriptase (RP6502;
BioTeke Corporation) according to the manufacturer's protocol.
Amplification parameters were as follows: Denaturation at 95°C for
5 min, followed by 40 cycles at 95°C for 30 sec, 60°C for 30 sec
and 72°C for 30 sec. The primer sequences used were as follows:
miR-22 forward, 5'-TGCGCAGTTCTTCAGTGGCAAG-3' and reverse,
5'-CCAGTGCAGGGTCCGAGGTATT-3'; U6 forward,
5'-CGCTTCGGCAGCACATATAC-3' and reverse, 5'-AAATATGGAACGCTTCACGA-3'.
The final extension step was as follows: Denaturation at 95°C for 5
min, followed by 40 cycles at 95°C for 30 sec, 60°C for 30 sec and
72°C for 30 sec; 4°C for 10 min. The comparative Cq method
(2−ΔΔCq) was used for relative quantification (16).
Western blot analysis
Proteins were extracted from tissue and cell samples
using radioimmunoprecipitation assay (RIPA) buffer (Beyotime
Institute of Biotechnology) at 4°C for 15-30 min. Protein content
was measured using the bicinchoninic acid (BCA) assay and 50
µg total protein was separated by 10% SDS-PAGE.
Subsequently, proteins were transferred onto polyvinylidene
fluoride membranes. The membranes were blocked with 5% nonfat milk
in Tris-buffered saline containing 0.1% Tween (TBST) for 1 h at
37°C and were then incubated with the following primary antibodies:
p53 (1:1,000), cysteine rich angiogenic inducer 61 (Cyr61;
1:1,000), B-cell lymphoma 2 (Bcl-2)-associated X protein (Bax;
1:1,000) and GAPDH (5174) (all Cell Signaling Technology, Inc.,
Danvers, MA, USA) at 4°C overnight. After four washes with TBST,
the membranes were incubated with horseradish peroxidase-conjugated
immuno-globulin G secondary antibodies (1:5,000; 7074; Cell
Signaling Technology, Inc.) for 45 min at 37°C, and blots were
developed using Enhanced Chemiluminescence Plus reagent (Beyotime
Institute of Biotechnology) and analyzed using Image-ProPlus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Caspase-3 activity assay
Proteins were extracted from tissue and cell samples
using RIPA buffer at 4°C for 15-30 min. Protein content was
measured using the BCA assay and 10 µg total protein was
incubated with the caspase-3 assay kit (BioVision, Inc., Milpitas,
CA, USA) for 1 h at 4°C. The absorbance was measured at 405 nm
using a multi-well spectrophotometer (BioTek Instruments, Inc.,
Winooski, VT, USA).
Statistical analysis
Data are presented as the means ± standard deviation
(n=3) using SPSS 19.0. Data were analyzed by one-way analysis of
variance followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
MicroRNA-22 expression in a mouse model
of SAH
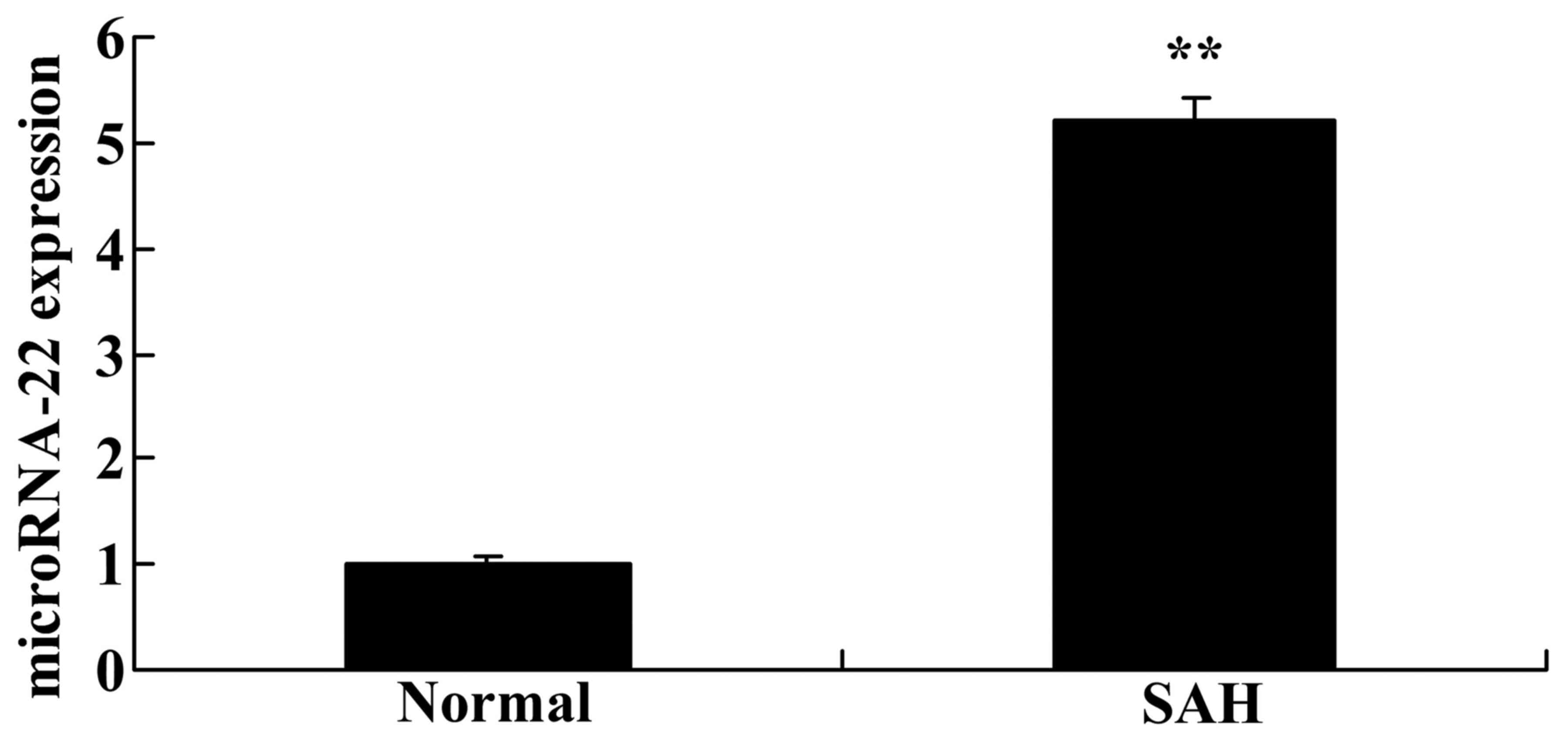
The present study demonstrated that microRNA-22
expression was significantly higher in SAH mice compared with in
normal mice without SAH (Fig. 1).
These results suggested that microRNA-22 expression may be
associated with SAH.
Effects of microRNA-22 on IL-6 mRNA
expression in HEB cells
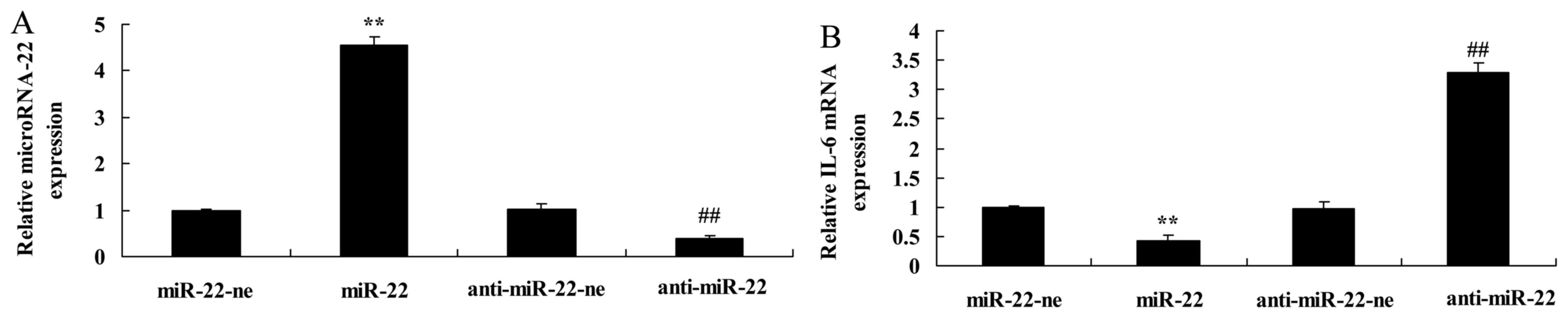
The present study transfected microRNA-22 and
microRNA-22 inhibitor plasmids into HEB cells, which were treated
with LPS. Transfection with the microRNA-22 plasmid increased
microRNA-22 expression, whereas the microRNA-22 inhibitor plasmid
inhibited microRNA-22 expression in LPS-treated HEB cells compared
with in the negative control group (Fig. 2A). Furthermore, microRNA-22
overexpression significantly inhibited IL-6 mRNA expression,
whereas downregulation of microRNA-22 significantly increased IL-6
mRNA expression in LPS-treated HEB cells compared with in the
negative control group (Fig.
2B).
Effects of microRNA-22 on Cyr61 and Bax
protein expression in HEB cells
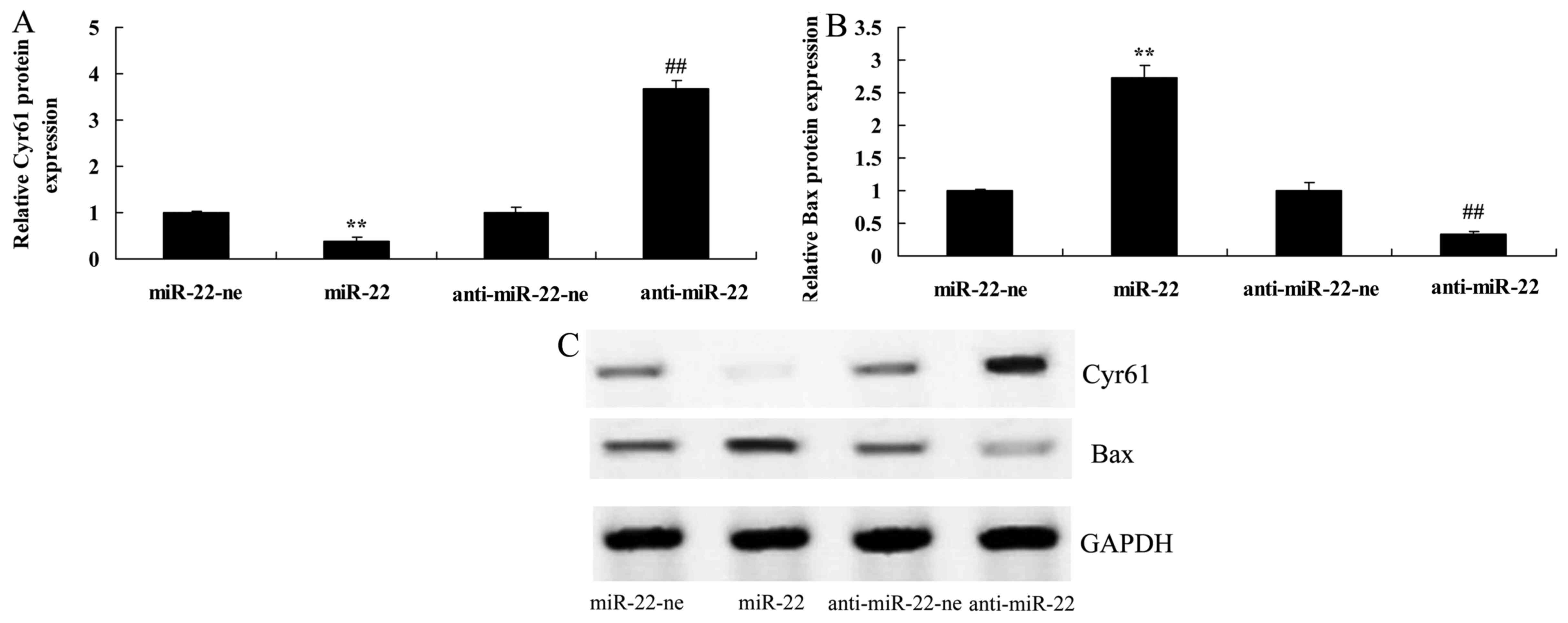
The present study aimed to determine whether
microRNA-22 affects Cyr61 expression in LPS-treated HEB cells. As
shown in Fig. 3, overexpression
of microRNA-22 significantly suppressed Cyr61 protein expression,
whereas downregulation of microRNA-22 significantly increased the
protein expression levels of Cyr61 and decreased Bax in LPS-treated
HEB cells compared with in the negative control group.
Effects of microRNA-22 on caspase-3
activity in HEB cells
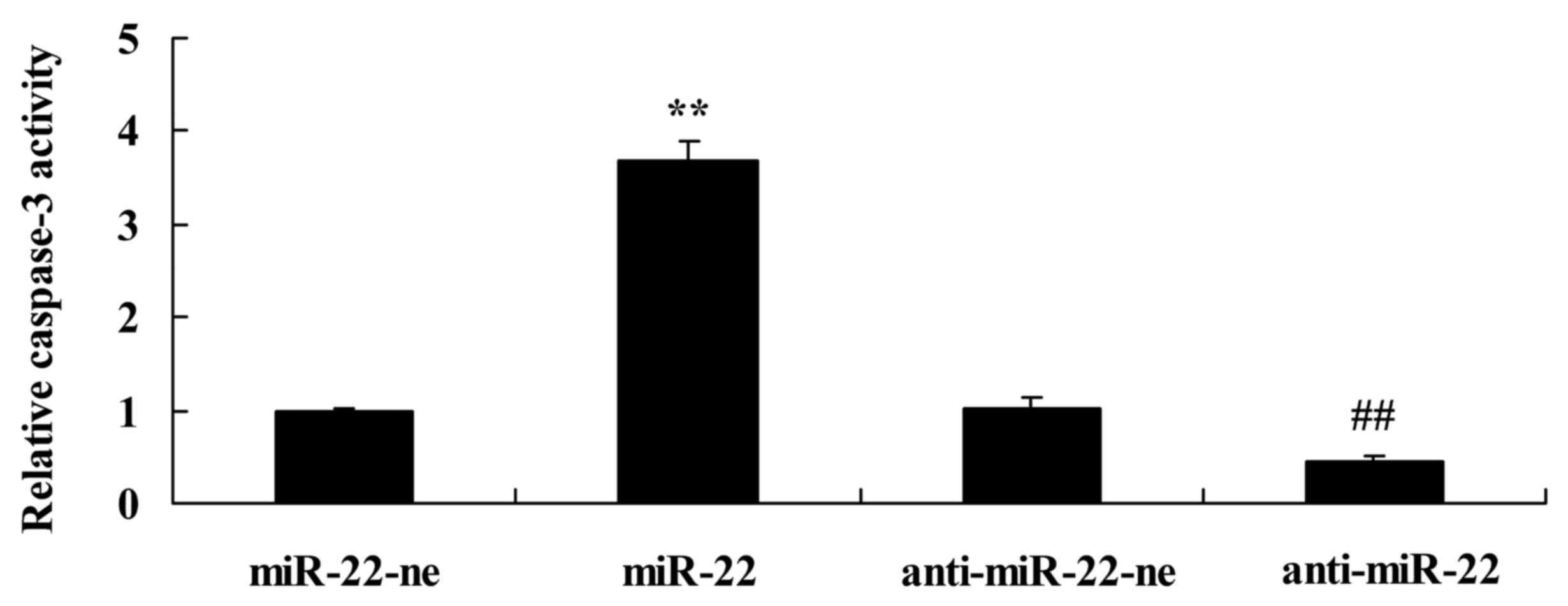
In order to analyze the apoptotic mechanism in the
present study, caspase-3 activity was detected. As presented in
Fig. 4, overexpression of
microRNA-22 significantly increased caspase-3 activity, whereas
downregulation of microRNA-22 significantly suppressed caspase-3
activity compared with in the negative control group.
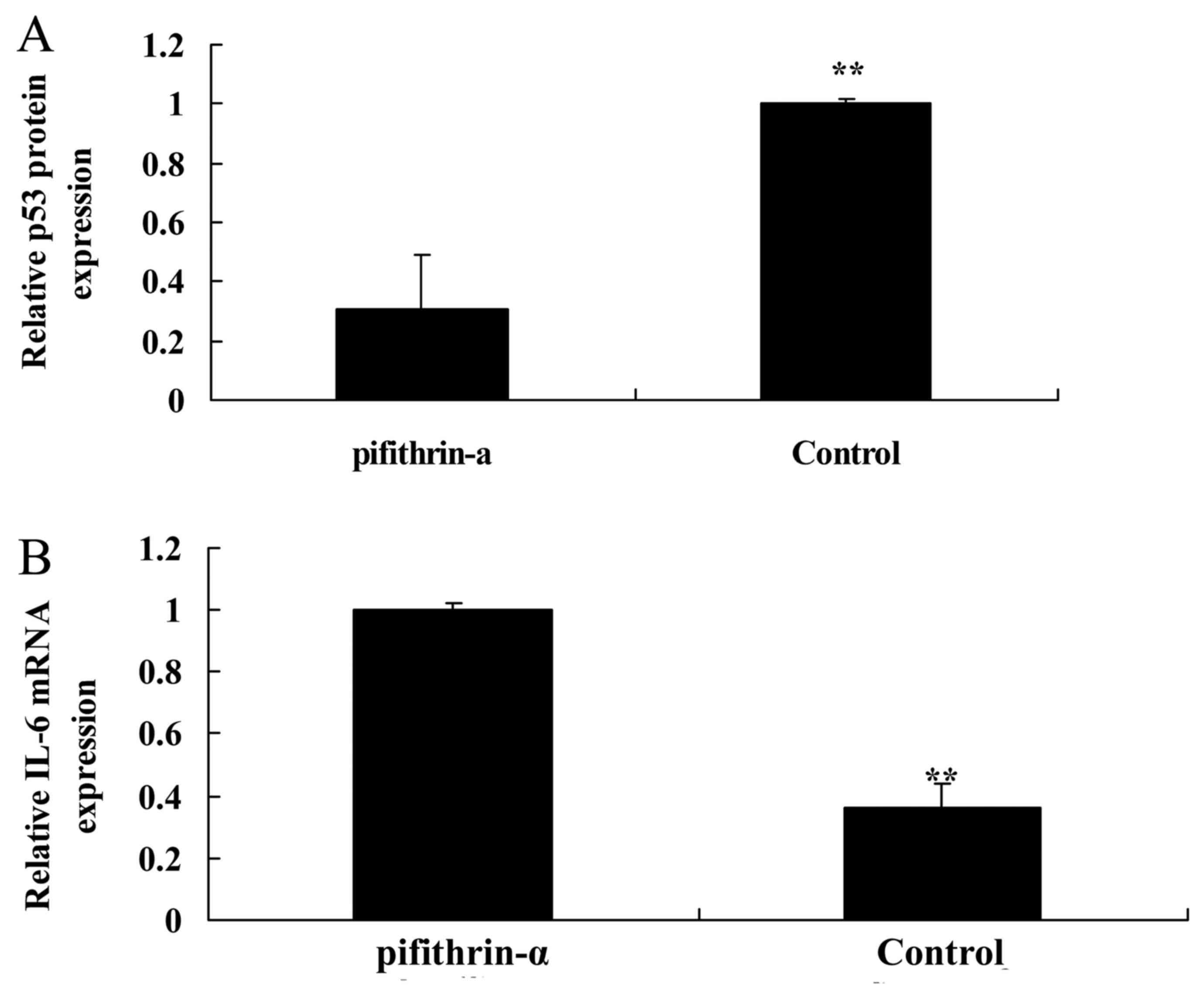
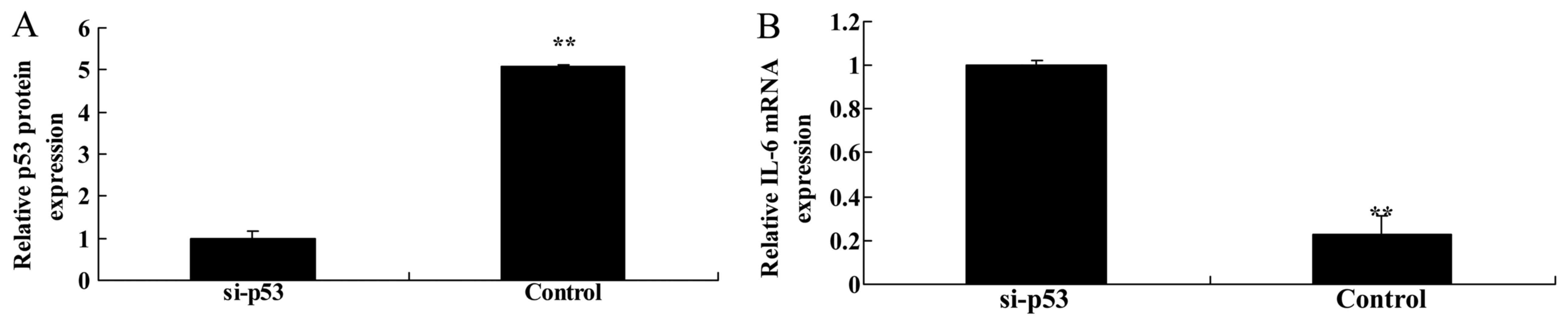
p53 inhibitor suppresses p53 protein
expression and induces IL-6 mRNA expression in SAH mice
To analyze apoptosis, a p53 inhibitor was used to
suppress p53 protein expression in SAH mice. Pifithrin-α was able
to suppress the protein expression levels of p53 and increase IL-6
mRNA expression in SAH mice compared with in the control group
(Fig. 5).
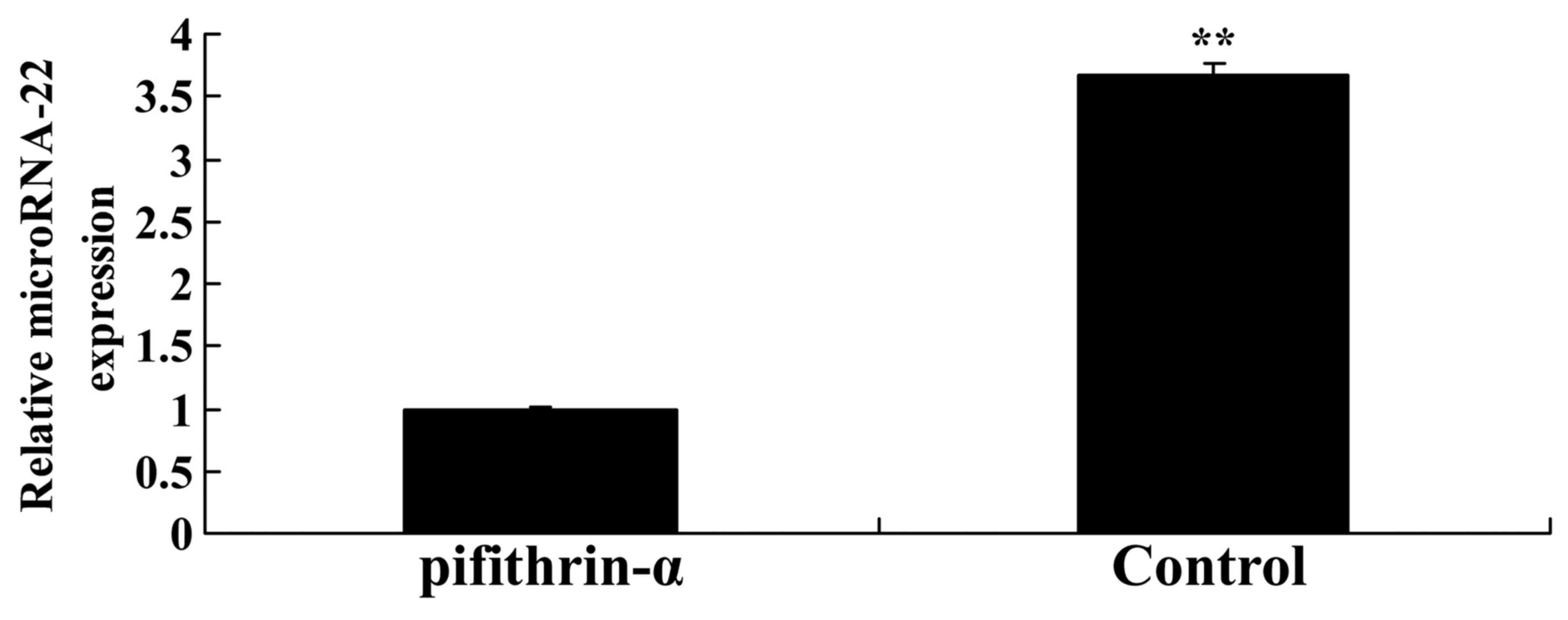
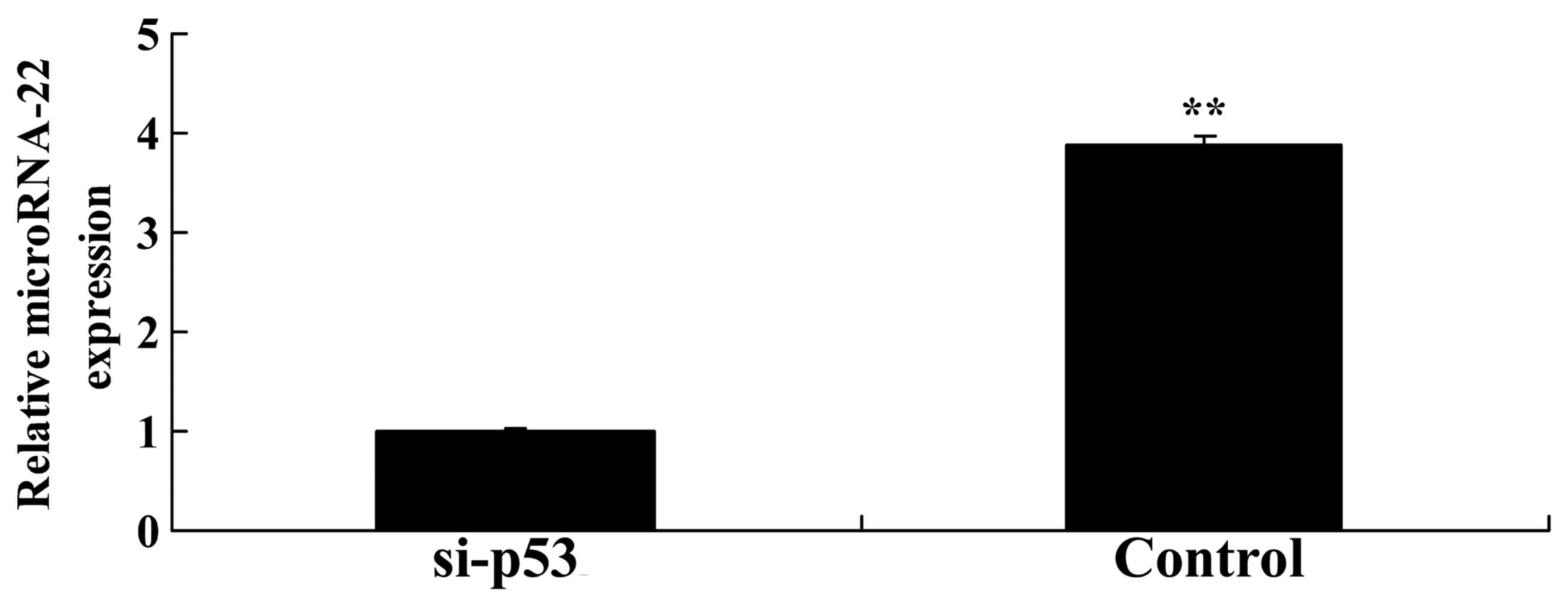
p53 inhibitor suppresses microRNA-22
expression in SAH mice
The present study explored whether suppression of
p53 expression may affect microRNA-22 expression in SAH mice.
Pifithrin-α significantly suppressed microRNA-22 expression in SAH
mice compared with in the control group (Fig. 6).
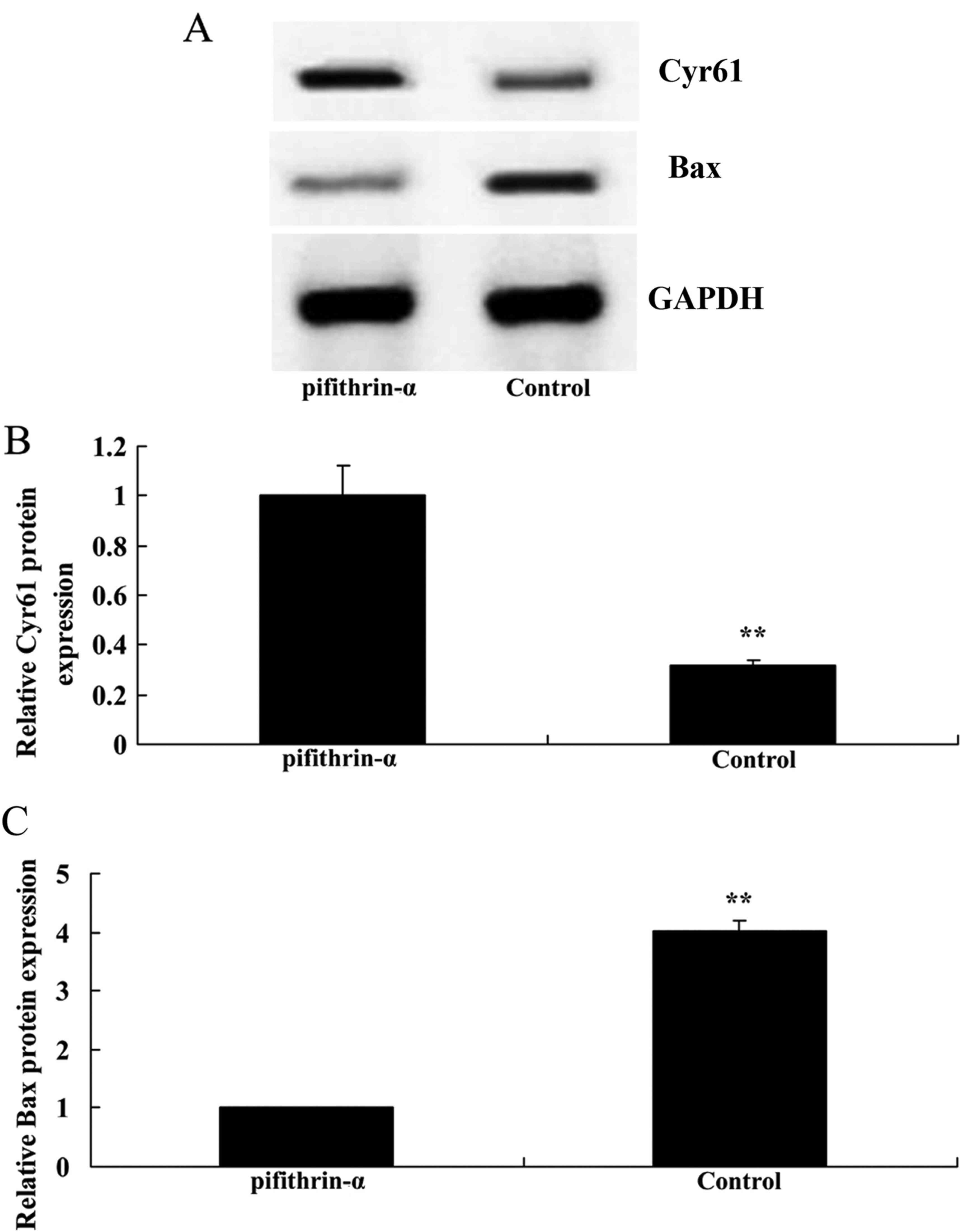
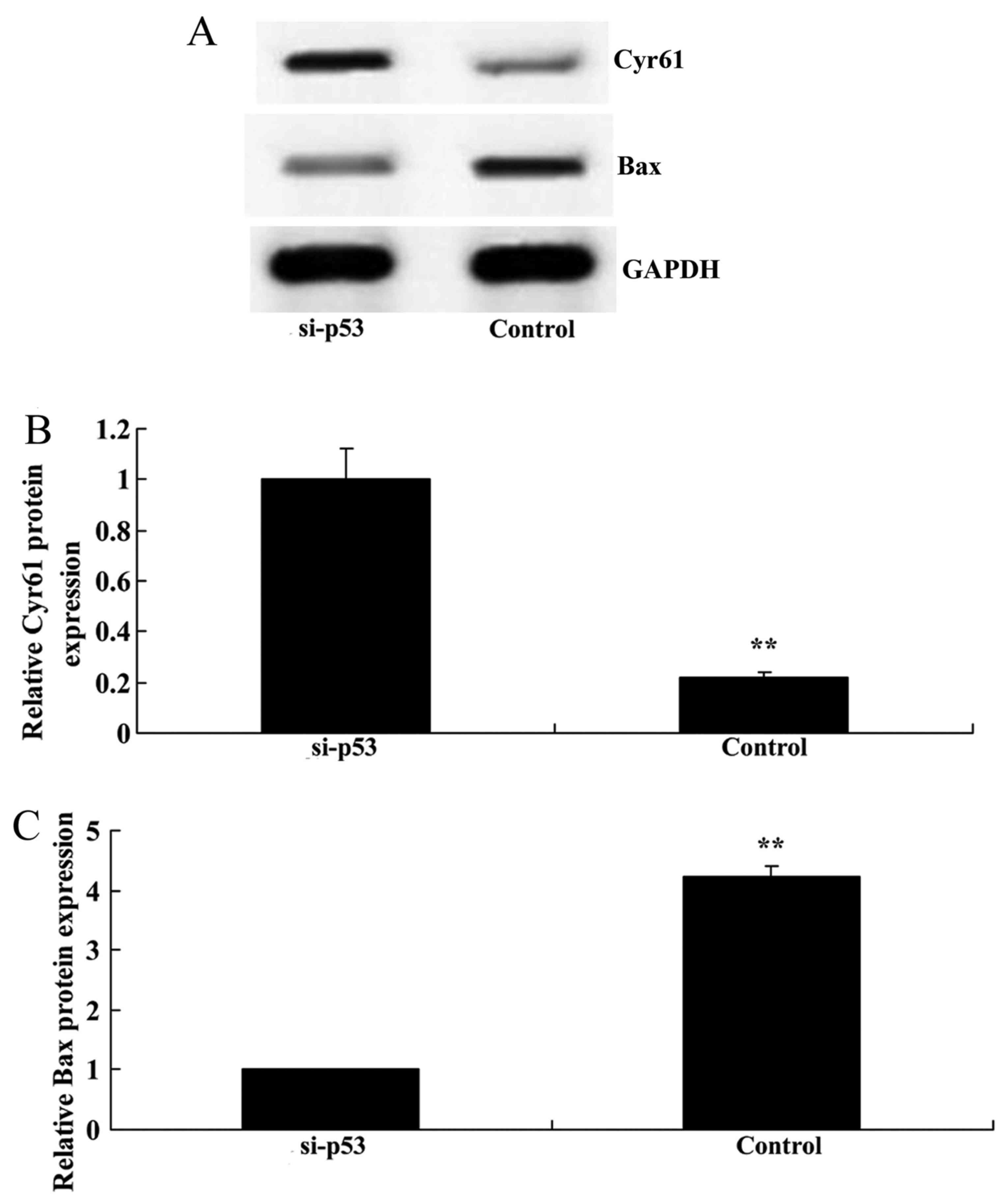
p53 inhibitor induces Cyr61 protein
expression and suppresses Bax protein expression in SAH mice
The present study aimed to determine whether p53
regulates the Cyr61 intrinsic pathway in SAH mice. As shown in
Fig. 7, suppression of p53
significantly enhanced Cyr61 protein expression and suppressed Bax
protein expression in SAH mice compared with in the control
group.
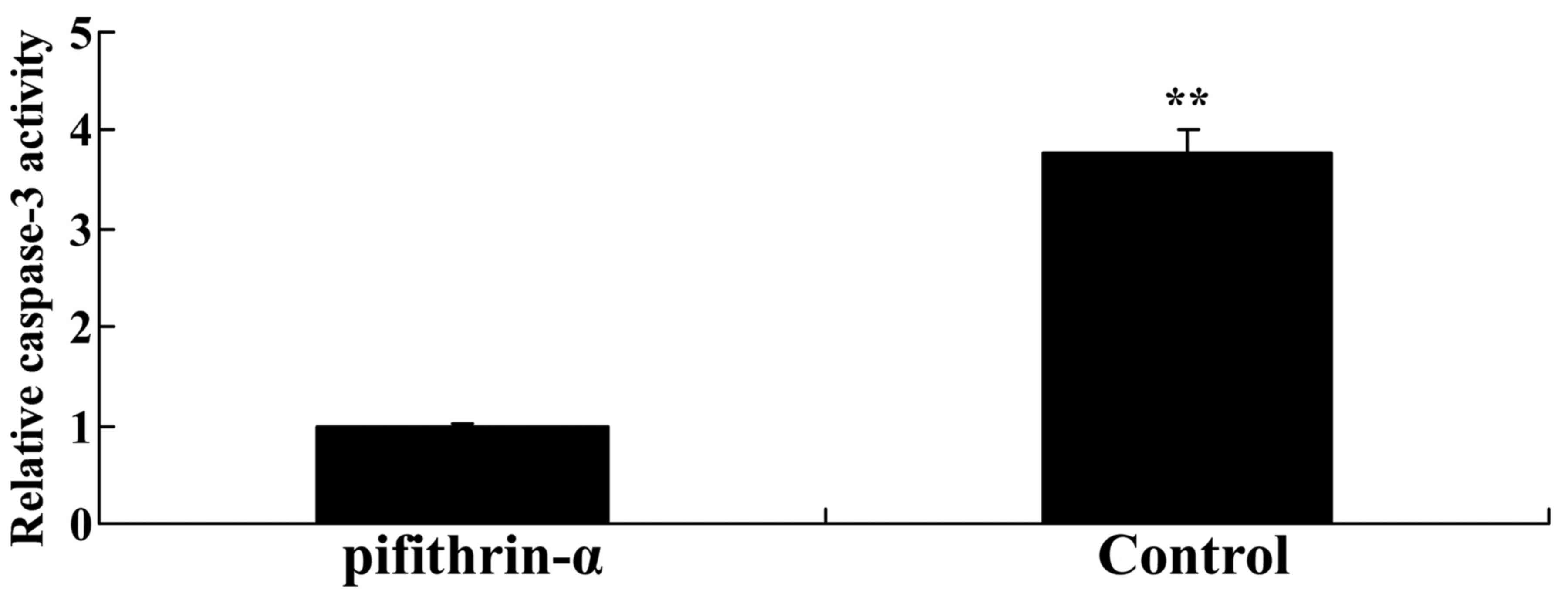
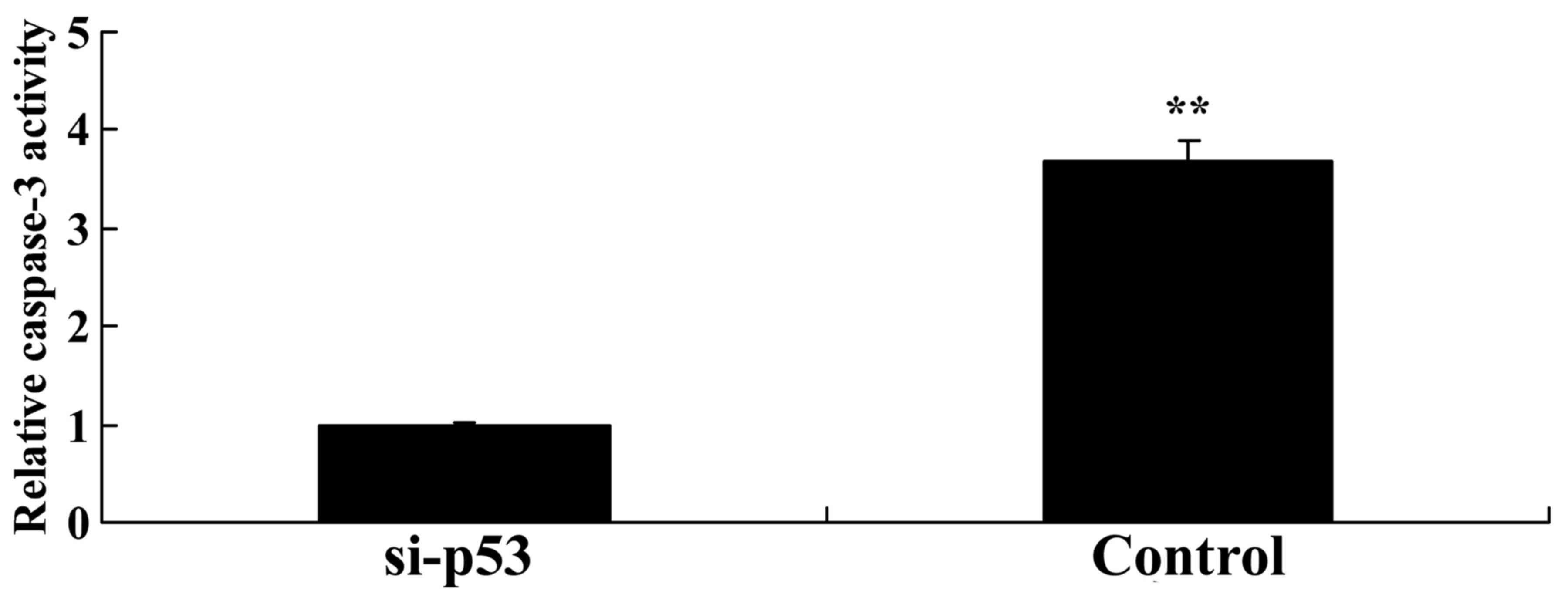
p53 inhibitor suppresses caspase-3
activity in SAH mice
To explore whether p53/microRNA-22 affects the
apoptotic mechanism in SAH mice, caspase-3 activity was measured
using a commercial kit. As shown in Fig. 8, caspase-3 activity was
significantly reduced by suppression of p53 expression in SAH mice
compared with in the control group.
Knockdown of p53 suppresses p53 protein
expression and increases IL-6 mRNA expression in HEB cells
In an in vitro model, si-p53 was transfected
into LPS-treated HEB cells to suppress p53 protein expression. As
shown in Fig. 9A, si-p53
significantly suppressed p53 protein expression in HEB cells
treated with LPS compared with in the negative control group.
Furthermore, knockdown of p53 significantly increased IL-6 mRNA
expression in HEB cells treated with LPS compared with in the
negative control group (Fig.
9B).
Knockdown of p53 suppresses microRNA-22
expression in HEB cells
Using an in vitro model, the present study
explored whether p53 knockdown affects microRNA-22 expression.
Knockdown of p53 significantly inhibited microRNA-22 expression in
HEB cells treated with LPS compared with in the negative control
group (Fig. 10).
Knockdown of p53 increases Cyr61 protein
expression and suppresses Bax expression in HEB cells
The present study evaluated whether p53 knockdown
affects Cyr61 protein expression in HEB cells treated with LPS. As
presented in Fig. 11, p53
knockdown significantly increased Cyr61 protein expression and
suppressed Bax expression in LPS-treated HEB cells compared with in
the negative control group.
Knockdown of p53 reduces caspase-3
activity in HEB cells
To further detect the apoptotic effects of
p53/microRNA-22 on HEB cells, caspase-3 activity was measured in
the in vitro model. As shown in Fig. 12, p53 knockdown significantly
inhibited caspase-3 activity in LPS-treated HEB cells compared with
in the negative control group.
Discussion
SAH is characterized by bleeding into the
subarachnoid space following the rupture of cerebral vessels or
superficial cerebral vessels (17). The occurrence rate of SAH is low;
however, it is associated with severe symptoms. According to
statistics, ~12.4% patients directly succumb to SAH (2). In addition, 40-60% patients succumb
within 48 h as a result of a second hemorrhage (18). Patients with SAH are often
accompanied with severe neurological symptoms and cognitive
impairment. Through examining the brain tissue of patients
following SAH-induced mortality, severe ischemic injuries are
commonly detected (2). The
occurrence of these symptoms is associated with EBI and CVS
following SAH. EBI not only induces fatal injury to patients,
including hydrocephalus, but is also closely associated with
sequelae (19). The present study
indicated that microRNA-22 expression was upregulated in SAH mice.
Therefore, targeting microRNA-22 expression may be considered a
therapeutic approach for SAH.
It is well known that numerous proteins, including
p53, Bcl-2 and caspases, are involved in vascular endothelial cell
apoptosis in CVS; all of these proteins serve important roles in
vascular endothelial cell and neuron apoptosis post-SAH (20). The present study demonstrated that
downregulation of microRNA-22 in HEB cells increased IL-6 mRNA
expression, induced Cyr61 expression, and suppressed Bax protein
expression and caspase-3 activity. Zhang et al reported that
microRNA-22 suppressed the growth of renal cell carcinoma cells via
p53 (21). Therefore, the present
study hypothesized that microRNA-22-mediated apoptosis may be
mediated by targeting proapoptotic genes, including p53. It was
hypothesized in the present study that microRNA-22 induced
apoptosis through p53 in subarachnoid hemorrhage.
Following SAH, intracellular p53 is activated under
the action of numerous factors, including hypoxia. Activated p53 is
able to upregulate the target gene Bax, inhibit the expression of
Bcl-2, and thus promote cell apoptosis (9). In a dog model of cerebellomedullary
cistern CVS, CVS is induced by introducing blood twice into the
cerebellomedullary cistern; the expression levels of p53 and
cytochrome c (cyto C) are markedly increased in rat basilar
artery endothelial cells following SAH, and apoptosis can be
detected (22). p53 is an
important tumor inhibiting factor that serves numerous roles in
inhibiting cell cycle progression, promoting genome repair and
inducing cell apoptosis (23).
Following SAH, p53 is activated under the action of several
factors, including hypoxia, and thus exerts its proapoptotic
function on vascular endothelial cells and neurons in the brain
through its target Bcl-2 and caspase family proteins, this results
in the generation of EBI and vasospasm post-SAH (22). In the present study, the p53
inhibitor, pifithrin-α, suppressed p53 protein expression and
increased IL-6 mRNA expression, decreased microRNA-22 expression
and suppressed Bax protein expression in SAH mice.
Caspase-3 has been verified to serve an essential
role in p53-mediated endothelial cell apoptosis after SAH (24). In bovine cerebral microvascular
endothelial cells, oxyhemoglobin increased the expression of
caspase-8, caspase-9, caspase-2 and caspase-3 in endothelial cells,
and apoptotic cells could be detected (25). In addition, it has been reported
that a caspase-3 inhibitor can effectively prevent CVS (26). Taken together, the present study
indicated that the p53 inhibitor, pifithrin-α, suppressed caspase-3
activity and induced Cyr61 expression in SAH mice.
The Bcl-2 family is particularly important for
p53-mediated endothelial cell apoptosis and the generation of CVS
after SAH. p53 acts on the mitochondria via the Bcl-2 family
proteins, thus leading to apoptosis. The Bcl-2 family is well known
to participate in cell apoptosis (20). The Bcl-2 family comprises four
homologous peptides, which are known as Bcl-2 homeodomains (BHs).
All of the family members possess one of the four BHs (BH1-BH4) as
the homologous region. The Bcl-2 protein family can be divided into
two categories: i) The anti-apoptotic family, which consists of
Bcl-2, Bcl-extra large and Bcl-w; and ii) the proapoptotic family,
which can be further divided into two types dependent on structure:
The first type is the Bax family, which is composed of Bax and BOK,
Bcl-2 family apoptosis regulator; the other type is the BH3 protein
family, which includes p53 upregulated modulator of apoptosis,
NOXA, Bcl-2 interacting killer, BLK proto-oncogene, Src family
tyrosine kinase, Bcl-2-associated agonist of cell death, Bcl-2
antagonist/killer 1 and BH3 interacting domain death agonist
(8,20). High expression of intracellular
Bax induces the release of cyto C by the mitochondria, which gives
rise to the activation of caspase-9 and -3 in succession, thus
inducing apoptosis. In addition, activated BH3-type proteins can
induce or promote the activation of Bax, thus indirectly inducing
the release of cyto C (24).
Among the Bcl-2 family, the relative Bax/Bcl-2 ratio may serve a
key role in determining cell survival or death (24). In the present study, p53
expression was knocked down using si-p53; transfection with si-p53
suppressed microRNA-22 expression and Bax protein expression in HEB
cells. These results suggested that p53/microRNA-22 may regulate
inflammation and apoptosis and may be considered a therapeutic
target for the treatment of SAH.
In recent years, increasing importance has been
attached to the role of the immunoinflammatory response in the
pathological mechanism underlying vasospasm. Clinical research has
provided a large amount of evidence to demonstrate that a series of
inflammatory responses are induced by blood clot stimulation
following SAH, including adhesion molecules, cytokines,
granulocytes, immunoglobulin and complement, which may serve
important roles in the pathogenesis of CVS (27). Inflammatory factors, including
adhesion molecules such as intercellular adhesion molecule-1 and
nuclear factor-κB, and cytokines such as IL-1β, IL-6 and IL-8, have
been reported to be associated with the pathogenesis of vasospasm;
IL-6 in particular is markedly increased in the cerebrospinal fluid
following SAH, thus indicating its important role in CVS (28). The results of the present study
indicated that downregulated p53/microRNA-22 expression increased
IL-6 mRNA expression, inhibited caspase-3 activity and induced
Cyr61 expression in HEB cells. Lin et al revealed that the
downregulation of microRNA-22 increased inflammation in rheumatoid
arthritis (29). The results of
the present study indicated that microRNA-22 promoted IL-6 mRNA
expression and Cyr61 protein expression to induce HEB cell
apoptosis. Therefore, it may by hypothesized that p53/microRNA-22
regulates Cyr61 and affects SAH-induced inflammation and
apoptosis.
In conclusion, the present study revealed that the
neuro-protective effects of p53/microRNA-22 are associated with
regulation of IL-6 mRNA expression and the caspase-3/Bax signaling
pathway in SAH. These results support the perspective that
p53/microRNA-22 may be a rational therapeutic strategy for the
clinical treatment of SAH.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Gathier CS, Dankbaar JW, van der Jagt M,
Verweij BH, Oldenbeuving AW, Rinkel GJ, van den Bergh WM and
Slooter AJ; HIMALAIA Study Group: Effects of induced hypertension
on cerebral perfusion in delayed cerebral ischemia after aneurysmal
subarachnoid hemorrhage: A randomized clinical trial. Stroke.
46:3277–3281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong GK, Liang M, Tan H, Lee MW, Po YC,
Chan KY and Poon WS: High-dose simvastatin for aneurysmal
subarachnoid hemorrhage: A multicenter, randomized, controlled,
double-blind clinical trial protocol. Neurosurgery. 72:840–844.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong GK, Lam SW, Ngai K, Wong A, Mok V and
Poon WS: Quality of Life after Brain Injury (QOLIBRI) overall scale
for patients after aneurysmal subarachnoid hemorrhage. J Clin
Neurosci. 21:954–956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bulters DO, Birch AA, Hickey E, Tatlow I,
Sumner K, Lamb R and Lang D: A randomized controlled trial of
prophylactic intra-aortic balloon counterpulsation in high-risk
aneurysmal subarachnoid hemorrhage. Stroke. 44:224–226. 2013.
View Article : Google Scholar
|
|
5
|
de Oliveira Manoel AL, Jaja BN, Germans
MR, Yan H, Qian W, Kouzmina E, Marotta TR, Turkel-Parrella D,
Schweizer TA and Macdonald RL; SAHIT collaborators: The VASOGRADE:
A simple grading scale for prediction of delayed cerebral ischemia
after subarachnoid hemorrhage. Stroke. 46:1826–1831. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong GK, Lam SW, Ngai K, Wong A, Poon WS
and Mok V: Development of a short form of Stroke-Specific Quality
of Life Scale for patients after aneurysmal subarachnoid
hemorrhage. J Neurol Sci. 335:204–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou C, Yamaguchi M, Colohan AR and Zhang
JH: Role of p53 and apoptosis in cerebral vasospasm after
experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab.
25:572–582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cahill J, Calvert JW, Marcantonio S and
Zhang JH: p53 may play an orchestrating role in apoptotic cell
death after experimental subarachnoid hemorrhage. Neurosurgery.
60:531–545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan JH, Khatibi NH, Han HB, Hu Q, Chen CH,
Li L, Yang XM and Zhou CM: p53-induced uncoupling expression of
aquaporin-4 and inwardly rectifying K+ 4.1 channels in cytotoxic
edema after subarachnoid hemorrhage. CNS Neurosci Ther. 18:334–342.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan J, Chen C, Hu Q, Yang X, Lei J, Yang
L, Wang K, Qin L, Huang H and Zhou C: The role of p53 in brain
edema after 24 h of experimental subarachnoid hemorrhage in a rat
model. Exp Neurol. 214:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stylli SS, Adamides AA, Koldej RM, Luwor
RB, Ritchie DS, Ziogas J and Kaye AH: miRNA expression profiling of
cerebro-spinal fluid in patients with aneurysmal subarachnoid
hemorrhage. J Neurosurg. 126:1131–1139. 2017. View Article : Google Scholar
|
|
12
|
Liu D, Han L, Wu X, Yang X, Zhang Q and
Jiang F: Genome-wide microRNA changes in human intracranial
aneurysms. BMC Neurol. 14:1882014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Müller AH, Povlsen GK, Bang-Berthelsen CH,
Kruse LS, Nielsen J, Warfvinge K and Edvinsson L: Regulation of
microRNAs miR-30a and miR-143 in cerebral vasculature after
experimental subarachnoid hemorrhage in rats. BMC Genomics.
16:1192015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bache S, Rasmussen R, Rossing M, Hammer
NR, Juhler M, Friis-Hansen L, Nielsen FC and Møller K: Detection
and quantification of microRNA in cerebral microdialysate. J Transl
Med. 13:1492015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su XW, Chan AH, Lu G, Lin M, Sze J, Zhou
JY, Poon WS, Liu Q, Zheng VZ and Wong GK: Circulating microRNA
132-3p and 324-3p profiles in patients after acute aneurysmal
subarachnoid hemorrhage. PLoS One. 10:e01447242015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Rasmussen R, Juhler M and Wetterslev J:
Effects of continuous prostacyclin infusion on regional blood flow
and cerebral vasospasm following subarachnoid haemorrhage:
Statistical analysis plan for a randomized controlled trial.
Trials. 15:2282014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gonzalez NR, Connolly M, Dusick JR, Bhakta
H and Vespa P: Phase I clinical trial for the feasibility and
safety of remote ischemic conditioning for aneurysmal subarachnoid
hemorrhage. Neurosurgery. 75:590–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soppi V, Karamanakos PN, Koivisto T, Kurki
MI, Vanninen R, Jaaskelainen JE and Rinne J: A randomized outcome
study of enteral versus intravenous nimodipine in 171 patients
after acute aneurysmal subarachnoid hemorrhage. World Neurosurg.
78:101–109. 2012. View Article : Google Scholar
|
|
20
|
Li Y, Tang J, Khatibi NH, Zhu M, Chen D,
Zheng W and Wang S: Ginsenoside Rbeta1 reduces neurologic damage,
is anti-apoptotic, and downregulates p53 and BAX in subarachnoid
hemorrhage. Curr Neurovasc Res. 7:85–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang S, Zhang D, Yi C, Wang Y, Wang H and
Wang J: MicroRNA-22 functions as a tumor suppressor by targeting
SIRT1 in renal cell carcinoma. Oncol Rep. 35:559–567. 2016.
View Article : Google Scholar
|
|
22
|
Li J, Chen J, Mo H, Chen J, Qian C, Yan F,
Gu C, Hu Q, Wang L and Chen G: Minocycline protects against NLRP3
inflammasome-induced inflammation and p53-associated apoptosis in
early brain injury after subarachnoid hemorrhage. Mol Neurobiol.
53:2668–2678. 2016. View Article : Google Scholar
|
|
23
|
Cheng G, Chunlei W, Pei W, Zhen L and
Xiangzhen L: Simvastatin activates Akt/glycogen synthase
kinase-3beta signal and inhibits caspase-3 activation after
experimental subarachnoid hemorrhage. Vascul Pharmacol. 52:77–83.
2010. View Article : Google Scholar
|
|
24
|
He J, Ji X, Li Y, Xue X, Feng G, Zhang H,
Wang H and Gao M: Subchronic exposure of benzo(a)pyrene interferes
with the expression of Bcl-2, Ki-67, C-myc and p53, Bax, caspase-3
in sub-regions of cerebral cortex and hippocampus. Exp Toxicol
Pathol. 68:149–156. 2016. View Article : Google Scholar
|
|
25
|
Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen
J and Yang X: Inhibition of transforming growth factor beta/SMAD
signal by MiR-155 is involved in arsenic trioxide-induced
anti-angiogenesis in prostate cancer. Cancer Sci. 105:1541–1549.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sahu U, Sidhar H, Ghate PS, Advirao GM,
Raghavan SC and Giri RK: A novel anticancer agent,
8-methoxypyrimido[4',5':4,5] thieno(2,3-b) quinoline-4(3H)-one
induces neuro 2a neuroblastoma cell death through p53-dependent,
caspase-dependent and -independent apoptotic pathways. PLoS One.
8:e664302013. View Article : Google Scholar
|
|
27
|
Zhang T, Su J, Guo B, Zhu T, Wang K and Li
X: Ursolic acid alleviates early brain injury after experimental
subarachnoid hemorrhage by suppressing TLR4-mediated inflammatory
pathway. Int Immunopharmacol. 23:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lucke-Wold BP, Logsdon AF, Manoranjan B,
Turner RC, McConnell E, Vates GE, Huber JD, Rosen CL and Simard JM:
Aneurysmal subarachnoid hemorrhage and neuroinflammation: A
comprehensive review. Int J Mol Sci. 17:492016. View Article : Google Scholar
|
|
29
|
Lin J, Huo R, Xiao L, Zhu X, Xie J, Sun S,
He Y, Zhang J, Sun Y, Zhou Z, et al: A novel p53/microRNA-22/Cyr61
axis in synovial cells regulates inflammation in rheumatoid
arthritis. Arthritis Rheumatol. 66:49–59. 2014. View Article : Google Scholar : PubMed/NCBI
|