Introduction
Tanshinone IIA (Tan-IIA; C19H18O3), is one of the
active components in Radix Salviae miltiorrhizae (1,2),
with antioxidant properties (3,4)
and anti-inflammatory activities (5,6).
Tan-IIA can inhibit many human cancer cell lines through different
molecular mechanisms, such as colon cancer colo205 cells (7), breast cancer MDA-MB-231 cells
(8), non-small cell lung cancer
A549 cells (9), small cell lung
cancer H146 cells (10),
hepatocellular carcinoma Hep-J5 cells (11), breast cancer BT-20 cells (12) and pancreatic cancer BxPC-3 cells
in vitro (13,14). The transmembrane tyrosine kinase
has been strongly implicated in the growth, survival, and
metastasis of a wide variety of human tumors (15,16). The PI3K/AKT/mTOR and
RAS/RAF/MEK/extracellular signal-regulated kinase (ERK) pathways
are two of the most frequently dysregulated kinase cascades in
human cancer are well documented (17,18). Both pathways represent important
signal transduction mechanisms that facilitate the proliferation
and survival of cancers driven by growth factor receptors, such as
human epidermal growth factor receptor 2 (HER2) or epidermal growth
factor receptor (EGFR). The individual downstream components of
these signaling cascades either through somatic mutation or
epigenetic modification, are also known to be frequently altered in
cancer, thus contributing to tumorigenesis and resistance to
anticancer therapies (19). It is
well documented that Tan-IIA can inhibit the proliferation of AGS
cells through inducing ER stress and intrinsic pathway to induce
apoptosis (20). Tan-IIA also can
inhibit human gastric cancer AGS cells through inducing G2/M phase
arrest, and extrinsic pathway to induce apoptosis (21). These indicate that Tan-IIA may be
one of the complementary medicines for gastric cancer. But the
molecular mechanisms of Tan-IIA in gastric cancer cells remain
unclear. In the present study, we investigated the protein
expression levels of VEGFR, HER2, Ras, Raf, MEK, ERK, PARP and
caspase-3 in human gastric cancer AGS cells treated with
Tan-IIA.
Materials and methods
Tan-IIA was obtained from Sigma-Aldrich (St. Louis,
MO, USA) (CAS no. 568-72-9); The HER2 (#2165, MW 185 kDa), Ras
(#3339, MW 21 kDa), Raf (#12552, MW 75 kDa), MEK (#9126, MW 45
kDa), and caspase-3 (#9661, MW 17 kDa) antibodies were all obtained
from Cell Signaling Technology, Inc. (Beverly, MA, USA). VEGFR
(N100-527, MW 150 kDa) antibodies were both obtained from Novus
Biologicals (Littleton, CO, USA). ERK (sc-94, MW 44 kDa) and PARP
(sc-7150, MW 116, 89 kDa) antibodies were all obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). F-12K medium, fetal
bovine serum (FBS), penicillin-streptomycin and glutamine were
obtained from Gibco-BRL (Grand Island, NY, USA). Potassium
phosphate and 0.2 mm PVFD membranes were purchased from Merck Co.
(Darmstadt, Germany); BioMax film was from Kodak (Rochester, NY,
USA). Sodium deoxycholate, leupeptin, Triton X-100, Tris-HCl,
ribonuclease-A, sodium pyruvate, HEPES, dimethyl sulfoxide (DMSO),
3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT)
and Tween-20, mouse anti-β-actin were from Sigma-Aldrich. The AGS
human gastric adenocarcinoma cell line (BCRC no. 60102) was
obtained from the Food Industry Research and Development Institute
(Hsinchu, Taiwan).
Cell culture
The human gastric adenocarcinoma AGS cells were
obtained from the Food Industry Research and Development Institute.
The cell culture procedure was as previously described (20,21). Briefly, the AGS cells were placed
into 75-cm2 tissue culture flasks and maintained in
F-12K contained with 10% heat-inactivated FBS (Gibco-BRL, Grand
Island, NY, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were grown at 37°C in a humidified atmosphere
of 95% air and 5% CO2. All data presented are from at
least three independent experiments.
Cytotoxicity assay
The cytotoxicity of Tan-IIA for AGS cells was
evaluated by MTT assay in triplicate as previously described
(20,21). Briefly, The AGS cells were plated
in 96-well plates at a density of 2×104 cells/well for
16–20 h. Thereafter, the cells were treated with various
concentrations (0, 1, 3, 9, 15, 30 and 60 µg/ml) of Tan-IIA
for 24, 48 and 72 h. Subsequently, the cells were incubated with 1
mg/ml of MTT in fresh complete F-12K medium for 1 h. The surviving
cells converted MTT to formazan by forming a blue-purple color when
dissolved in dimethyl sulfoxide. The intensity of formazan was
measured at 590 nm using a microplate reader. The relative
percentage of cell viability was calculated by dividing the
absorbance of treated cells by that of the control in each
experiment, using the following formula: proliferation rate (%) =
(OD test − OD blank) ×100, where OD test and OD blank are the
optical density of the test substances and the blank control,
respectively.
Western blot analysis
The western blot procedures followed previous
reports (20,21). Briefly, AGS cells were treated
with various concentrations of Tan-IIA for different durations, and
then the cells were lysed in ice-cold whole cell extract buffer
containing the protease inhibitors. The lysate was vibrated for 30
min at 4°C and centrifuged at 12,281 × g for 10 min. Protein
concentration was measured by BCA protein assay kit (Pierce,
Rockford, IL, USA). Equal amounts of proteins were subjected to
electrophoresis using 12% sodium dodecyl sulfate-polyacrylamide
gels. To verify equal protein loading and transfer, proteins were
then transferred to polyvinylidene difluoride membranes and the
membranes were blocked for 1 h at 4°C using blocking buffer (5%
non-fat dried milk in solution containing 50 mM Tris/HCl (pH 8.0),
2 mM CaCl2, 80 mM sodium chloride, 0.05% Tween-20 and
0.02% sodium azide). The membranes were then incubated for 2 h at
room temperature with specific primary antibody followed by
anti-rabbit or anti-mouse immunoglobulin G-horseradish peroxidase
conjugated secondary antibodies. The membranes were washed three
times for 10 min with washing solution. Finally, the protein bands
were visualized on the X-ray film using the enhanced
chemiluminescence detection system (PerkinElmer Life and Analytical
Sciences, Boston, MA, USA).
Statistical analysis
Values are presented as the means ± SD. The
Student's t-test was used to analyze statistical significance.
P-value <0.05, was considered to indicate a statistically
significant difference for all the tests, at P<0.05, P<0.01
and P<0.001.
Results
Effects of Tan-IIA in the viability of
AGS cells
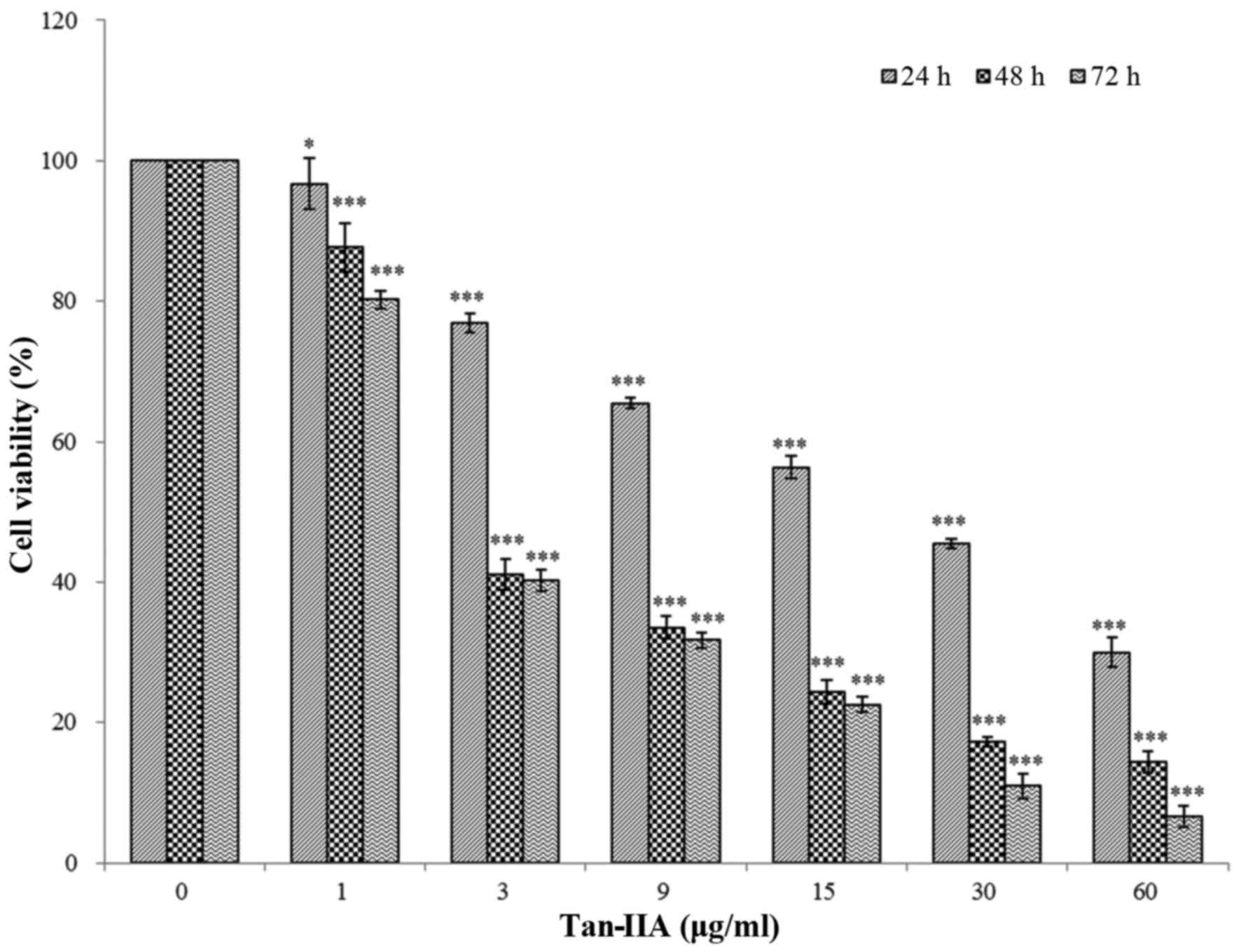
The results revealed that Tan-IIA can inhibit AGS
cells in a time- and dose-dependent manner. The half-maximal
inhibitory concentration (IC50) was 5.5, 3.7 and 3.5
µg/ml at 24, 48 and 72 h, respectively (Fig. 1), this is in agreement with our
previous studies (20,21).
Effects of Tan-IIA on the protein
expression of VEGFR, HER2, Ras, Raf, MEK, ERK, PARP, caspase-3 and
β-actin in AGS cells
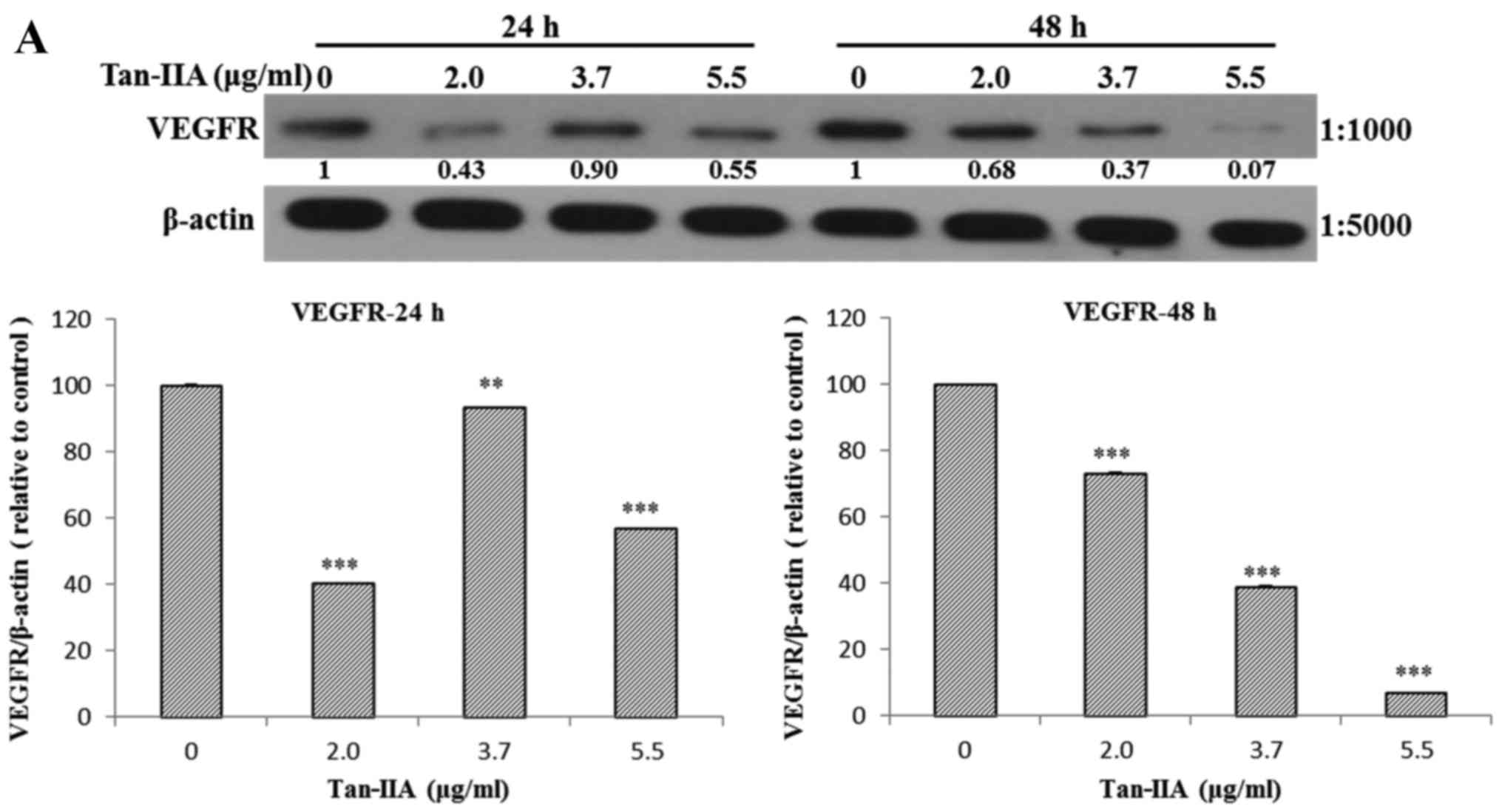
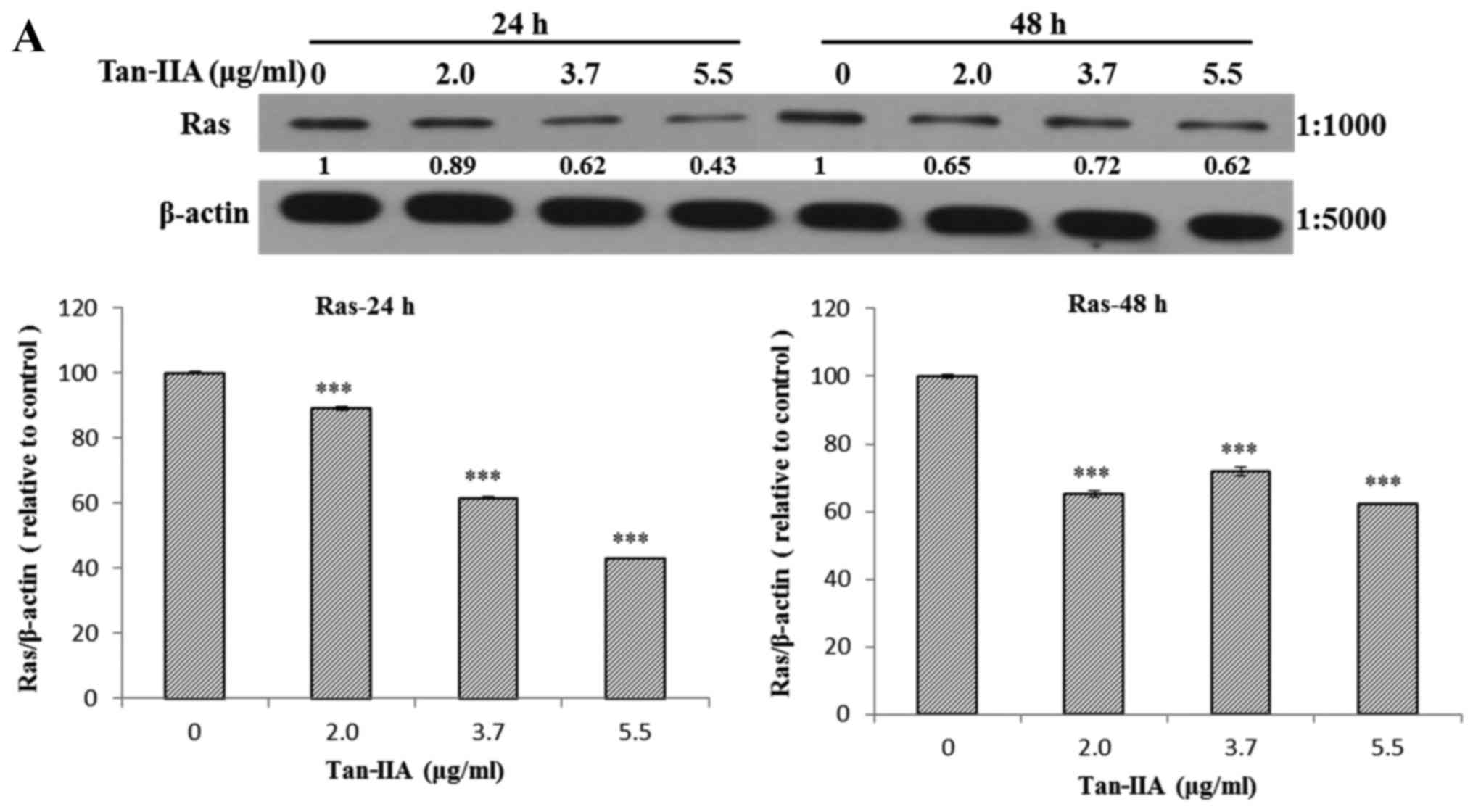
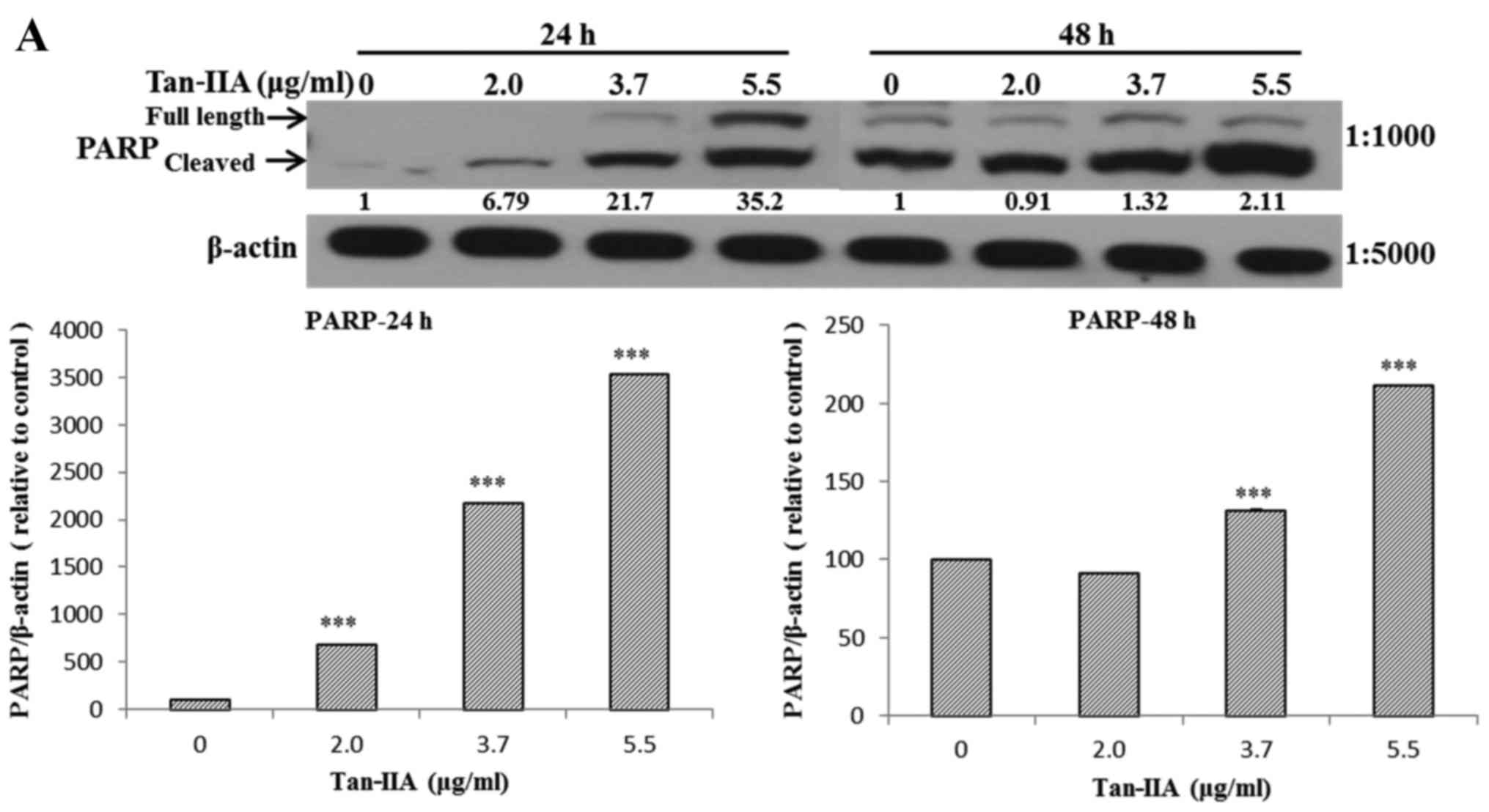
The AGS cells were treated with various
concentrations of Tan-IIA (0, 2.0, 3.7 and 5.5 µg/ml) for 24
or 48 h and then the protein expression levels of VEGFR, HER2, Ras,
Raf, MEK, ERK, PARP, caspase-3 and β-actin were evaluated by
western blot analysis. The results showed that Tan-IIA can decrease
the protein expression levels of VEGFR (Fig. 2A), HER2 (Fig. 2B), Ras (Fig. 3A), Raf (Fig. 3B), MEK (Fig. 3C) and ERK (Fig. 3D), but increase PARP (Fig. 4A) and caspase-3 (Fig. 4B) levels significantly.
Effects of Tan-IIA on the protein
expression of VEGFR, HER2, Ras, Raf, MEK, ERK, PARP, caspase-3 and
β-actin in AGS cells
The AGS cells were treated with Tan-IIA (3.7
µg/ml) for different durations (0, 24 and 48 h) and then the
protein expression levels of VEGFR, HER2, Ras, Raf, MEK, ERK, PARP,
caspase-3 and β-actin were evaluated by western blot analysis. The
results showed that Tan-IIA can decrease the protein expression
levels of VEGFR (Fig. 5A), HER2
(Fig. 5B), Ras (Fig. 5C), Raf (Fig. 5D), MEK (Fig. 5E) and ERK (Fig. 5F), but increased PARP (Fig. 5G) and caspase-3 (Fig. 5H) levels significantly.
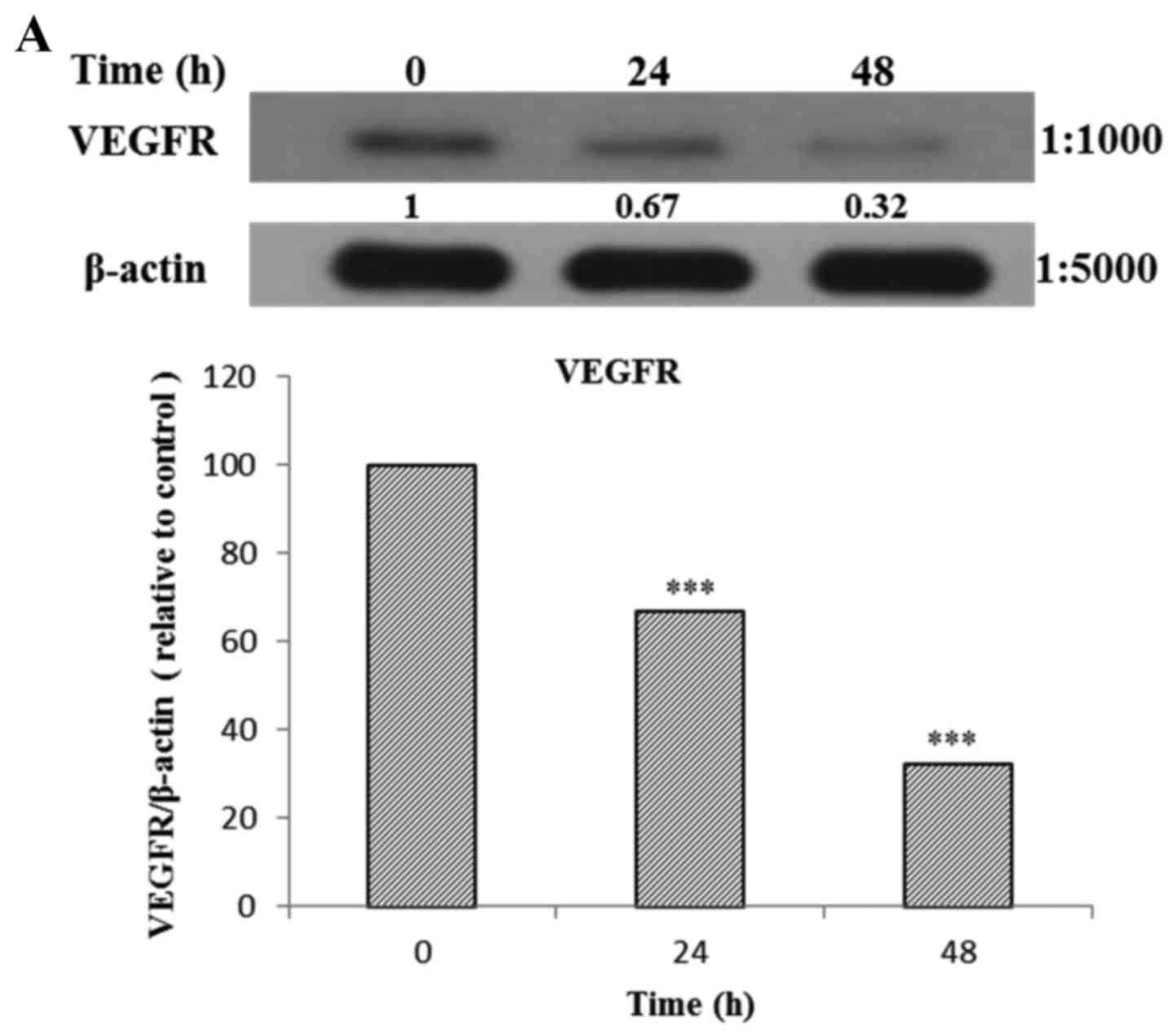 | Figure 5(A–F) Protein expression of vascular
epidermal growth factor receptor (VEGFR), human epidermal growth
factor receptor 2 (HER2), Ras, Raf, MEK, extracellular
signal-regulated kinase (ERK), PARP, caspase-3 and β-actin in AGS
cells. The AGS cells were treated with tanshinone IIA (Tan-IIA)
(3.7 µg/ml) for different durations (0, 24 and 48 h) and
then the protein expression levels of VEGFR, HER2, Ras, Raf, MEK,
ERK, PARP, caspase-3 and β-actin were evaluated by western blot
analysis. The results showed that Tan-IIA can decrease the protein
expression levels of (A) VEGFR, (B) HER2, (C) Ras, (D) Raf, (E) MEK
and (F) ERK levels significantly. *P<0.05;
**P<0.01; ***P<0.001. The results
showed that Tan-IIA can increase (G) PARP and (H) caspase-3 levels
significantly. |
Discussion
It is well documented that Tan-IIA inhibits the
proliferation of gastric cancer SGC7901 cells time- and
dose-dependently through inducing apoptosis and G0/G1 phase arrest
(22,23). Tan-IIA also triggered the
intrinsic apoptotic signaling pathway and arrested in G2/M phase to
inhibit the proliferation of gastric cancer MKN-45 cells (24). Our results also showed that
Tan-IIA inhibited human gastric cancer AGS cells in a time- and
dose-dependent manner in vitro.
It is well documented that Ras/Raf/MAPK pathway
regulates a variety of cellular functions, and is also involved in
the formation of new blood vessels, wound healing and tissue
repair, cell cycle regulation, and cell migration. Ras is the most
frequently mutated oncogene in human cancer. Dysregulation of
Ras/Raf/MAPK pathway is a common event in cancer, signaling through
this pathway is important for tumorigenesis (25–28). MAPK, which in mammalians is also
called MEK, is a serine/threonine kinase activated in response to
growth factors and cytokines to promote cell apoptosis and survival
(29). ERK belongs to
mitogen-activated protein kinase (MAPK) pathway, and is activated
via phosphorylation in response to cytokines, growth factors and
stress. Phospho-ERK (p-ERK) can inhibit apoptosis (30,31). Our results demonstrated that AGS
cells treated with Tan-IIA can decrease the protein expression
level of VEGFR, HER2, Ras, Raf, MEK, ERK, time- and
dose-dependently. Our results also demonstrated that the treatment
of AGS cells with Tan-IIA can increase the protein expression of
PARP and caspase-3. These findings indicate Tan-IIA can induce
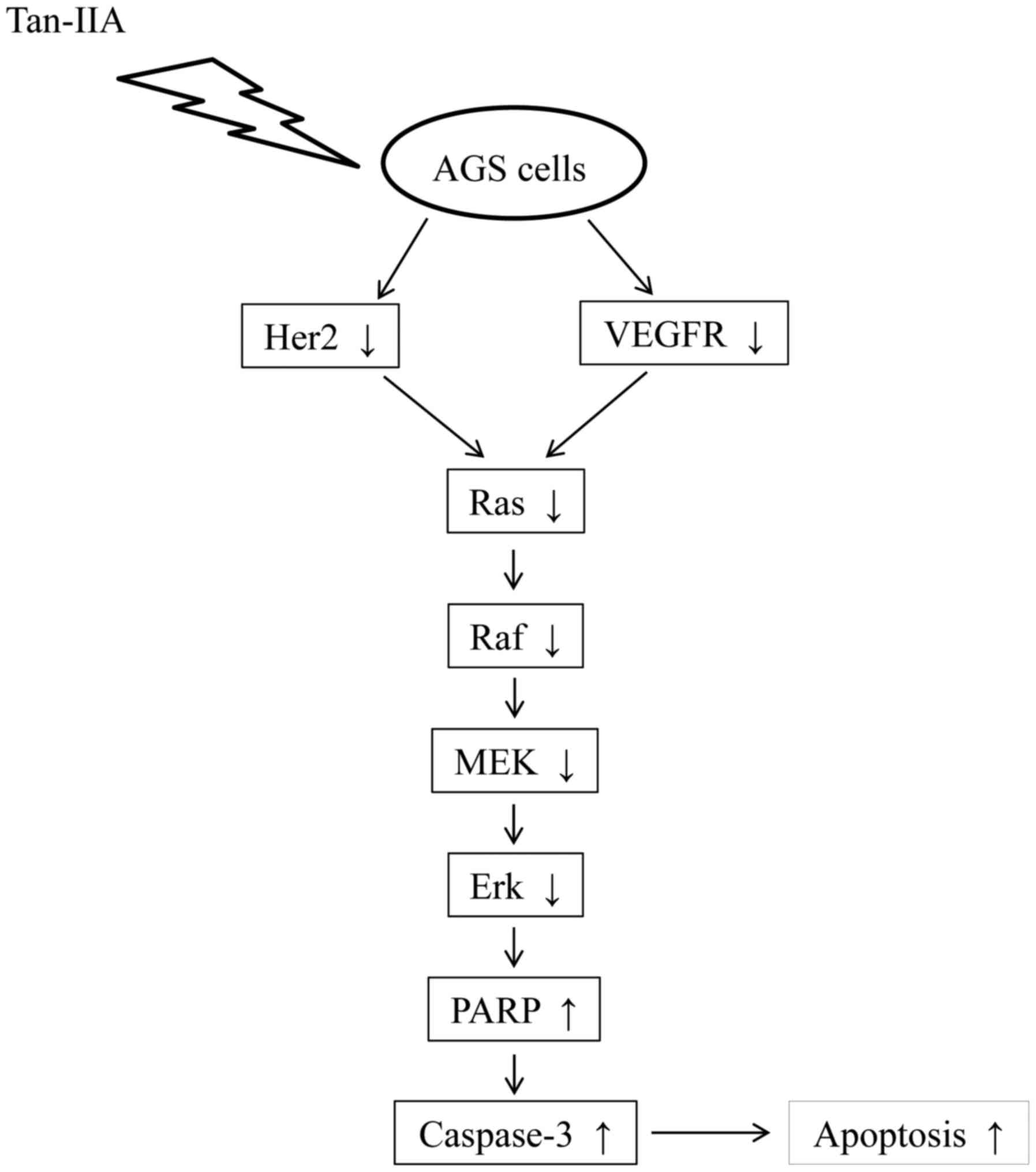
apoptosis to inhibit the proliferation of AGS cells. The proposed
model for tan-IIA to inhibit the proliferation of AGS cells is
shown in Fig. 6. This is the
first report that Tan-IIA could inhibit gastric carcinoma AGS cells
by decreasing the protein expression of VEGFR, Her2 and blocking
the Ras/Raf/MEK/ERK pathway to induce apoptosis. The
chemotherapeutic potential of Tan-IIA for human gastric cancer
warrants further study.
Acknowledgments
This study was supported by grant 102-CCH-IRP-066
from the Changhua Christian Hospital from the Research Section of
the Changhua Christian Hospital, Changhua, Taiwan, R.O.C.
Notes
[1] Competing
interests
The author declares there is no competing
interest.
References
|
1
|
Che AJ, Zhang JY, Li CH, Chen XF, Hu ZD
and Chen XG: Separation and determination of active components in
Radix Salviae miltiorrhizae and its medicinal preparations by
nonaqueous capillary electrophoresis. J Sep Sci. 27:569–575. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin R, Wang WR, Liu JT, Yang GD and Han
CJ: Protective effect of tanshinone IIA on human umbilical vein
endothelial cell injured by hydrogen peroxide and its mechanism. J
Ethnopharmacol. 108:217–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang AM, Sha SH, Lesniak W and Schacht J:
Tanshinone (Salviae miltiorrhizae extract) preparations attenuate
aminoglycoside-induced free radical formation in vitro and
ototoxicity in vivo. Antimicrob Agents Chemother. 47:1836–1841.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jang SI, Kim HJ, Kim YJ, Jeong SI and You
YO: Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW
264.7 cells: Possible involvement of the NIK-IKK, ERK1/2, p38 and
JNK pathways. Eur J Pharmacol. 542:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Li J, Ashok M, Wu R, Chen D, Yang L,
Yang H, Tracey KJ, Wang P, Sama AE, et al: A cardiovascular drug
rescues mice from lethal sepsis by selectively attenuating a
late-acting proinflammatory mediator, high mobility group box 1. J
Immunol. 178:3856–3864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su CC and Lin YH: Tanshinone IIA
downregulates the protein expression of ErbB-2 and upregulates
TNF-α in colon cancer cells in vitro and in vivo. Int J Mol Med.
22:847–851. 2008.PubMed/NCBI
|
|
8
|
Su CC and Lin YH: Tanshinone IIA inhibits
human breast cancer cells through increased Bax to Bcl-xL ratios.
Int J Mol Med. 22:357–361. 2008.PubMed/NCBI
|
|
9
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
10
|
Cheng CY and Su CC: Tanshinone IIA may
inhibit the growth of small cell lung cancer H146 cells by
up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial
membrane potential. Mol Med Rep. 3:645–650. 2010.
|
|
11
|
Cheng CY and Su CC: Tanshinone IIA
inhibits Hep-J5 cells by increasing calreticulin, caspase 12 and
GADD153 protein expression. Int J Mol Med. 26:379–385.
2010.PubMed/NCBI
|
|
12
|
Yan MY, Chien SY, Kuo SJ, Chen DR and Su
CC: Tanshinone IIA inhibits BT-20 human breast cancer cell
proliferation through increasing caspase 12, GADD153 and
phospho-p38 protein expression. Int J Mol Med. 29:855–863.
2012.PubMed/NCBI
|
|
13
|
Huang CY, Chiu TL, Kuo SJ, Chien SY, Chen
DR and Su CC: Tanshinone IIA inhibits the growth of pancreatic
cancer BxPC3 cells by decreasing protein expression of TCTP, MCL1
and Bcl-xL. Mol Med Rep. 7:1045–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su CC: Tanshinone IIA could inhibit
pancreatic cancer BxPC-3 cells through increasing PERK, ATF6,
caspase-12 and CHOP expression to induce apoptosis. J Biomed Sci
Eng. 8:149–159. 2015. View Article : Google Scholar
|
|
15
|
Yuen JS and Macaulay VM: Targeting the
type 1 insulin-like growth factor receptor as a treatment for
cancer. Expert Opin Ther Targets. 12:589–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chitnis MM, Yuen JS, Protheroe AS, Pollak
M and Macaulay VM: The type 1 insulin-like growth factor receptor
pathway. Clin Cancer Res. 14:6364–6370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCubrey JA, Steelman LS, Kempf CR,
Chappell WH, Abrams SL, Stivala F, Malaponte G, Nicoletti F, Libra
M, Bäsecke J, et al: Therapeutic resistance resulting from
mutations in Raf/MEK/ERK and I3K/PTEN/Akt/mTOR signaling pathways.
J Cell Physiol. 226:2762–2781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su CC: Tanshinone IIA inhibits human
gastric carcinoma AGS cell growth by decreasing BiP, TCTP, Mcl 1
and Bcl xL and increasing Bax and CHOP protein expression. Int J
Mol Med. 34:1661–1668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su CC: Tanshinone IIA inhibits gastric
carcinoma AGS cells through increasing p-p38, p-JNK and p53 but
reducing p-ERK, CDC2 and cyclin B1 expression. Anticancer Res.
34:7097–7110. 2014.PubMed/NCBI
|
|
22
|
Hou J, He J, Jin X, Hu T and Zhang Y:
Study on optimisation of extraction process of tanshinone IIA and
its mechanism of induction of gastric cancer SGC7901 cell
apoptosis. Afr J Tradit Complement Altern Medicines. 10:456–458.
2013. View Article : Google Scholar
|
|
23
|
Xu M, Cao FL, Li NY, Liu YQ, Li YP and Lv
CL: Tanshinone IIA reverses the malignant phenotype of SGC7901
gastric cancer cells. Asian Pac J Cancer Prev. 14:173–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong X, Dong J, Peng G, Hou X and Wu G:
Growth-inhibiting and apoptosis-inducing effects of Tanshinone II A
on human gastric carcinoma cells. J Huazhong Univ Sci Technolog Med
Sci. 27:706–709. 2007. View Article : Google Scholar
|
|
25
|
Kranenburg O, Gebbink MF and Voest EE:
Stimulation of angiogenesis by Ras proteins. Biochim Biophys Acta.
1654:23–37. 2004.PubMed/NCBI
|
|
26
|
Stacey DW: Cyclin D1 serves as a cell
cycle regulatory switch in actively proliferating cells. Curr Opin
Cell Biol. 15:158–163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boonstra J, Rijken P, Humbel B, Cremers F,
Verkleij A and van Bergen en Henegouwen P: The epidermal growth
factor. Cell Biol Int. 19:413–430. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cary LA, Han DC and Guan JL:
Integrin-mediated signal transduction pathways. Histol Histopathol.
14:1001–1009. 1999.PubMed/NCBI
|
|
29
|
Schlesinger TK, Fanger GR, Yujiri T and
Johnson GL: The TAO of MEKK. Front Biosci. 3:D1181–D1186. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rubinfeld H and Seger R: The ERK cascade:
A prototype of MAPK signaling. Mol Biotechnol. 31:151–174. 2005.
View Article : Google Scholar : PubMed/NCBI
|