Introduction
Colon cancer is one of the most common
gastrointestinal tumors; it is the second most common cancer in
women and third in men worldwide (1). In recent years, diagnosis,
treatments and prognosis of patients with colon cancer have been
improved (2). Systematic review
has provided various targeted therapies for the treatment of
advanced colorectal cancer and explored the potential of predictive
biomarkers (3). However, no
satisfactory therapies for colon cancer have been developed
clinically due to local migration and long distance metastasis
(4). Colon cancer metastasis and
invasion is a major issue for clinicians and detrimental for
patients with colon cancer (5,6).
The underlying molecular mechanisms of colorectal cancer metastasis
and invasion have attracted research to develop targeted therapies
for suppressing metastasis and invasion (7–9).
Given that regulation of tumor cell growth and metastasis is
imperative for patients with colon cancer and for future
development of clinical strategies, molecular bioinformatics has
enabled biopharmaceutical researchers to screen for targeted
molecules that could be useful for diagnosis and therapy protocols,
and offer the possibility of individual tailored medicine for
patients with cancer or other human diseases (10,11).
Resistant starch is widely present in carbohydrate
starch material and has miscellaneous effects in colon metabolism
(12). The
structure-physiological function of resistant starch is associated
with the extent of digestion and absorption in the colon (13). Systematic review and meta-analysis
of randomized controlled trials have demonstrated the beneficial
effects of resistant starch supplementation on bowel function in
healthy adults by increasing fecal wet weight, butyrate
concentration, fecal pH and defecation frequency (14). Dronamraju et al (15) investigated the effects of
resistant starch on cell kinetics and gene expression changes in
patients with colorectal cancer given resistant starch in a
randomized controlled trial (15). Studies have suggested that
dynbiotic intervention of Bifidobacterium lactis and
resistant starch are protective against colorectal cancer
development in a ratazoxymethane model, and long-term consumption
of resistant starch markedly decreased the risk of colorectal
cancer in a randomized controlled trial (16,17).
Increasing apoptosis of tumors cells has benefits
for prevention and treatment of colon cancer through regulation of
the expression of apoptosis-associated proteins (18). It has been reported that
endoplasmic reticulum (ER) stress is associated with apoptosis of
colon cancer cells (19). A
previous study has reported that upregulation of the ER stress
pathway can reduce γ-tocotrienol-induced apoptosis in mammary tumor
cells (20). Edagawa et al
(21) investigated the function
of activating transcription factor-3 (ATF-3) in ER stress-induced
apoptosis in human colon cancer cells. These studies indicated that
ER stress-mediated apoptosis may be associated with tumorigenesis
and development of colon cancer.
The current study investigated the anticancer
effects and potential mechanisms of resistant starch in the
tumorigenesis, formation and development
1,2-dimethylhydrazine-induced colon cancer. The colon physiological
functions of experimental mice were analyzed following consumption
of a diet containing resistant starch. Notably, this analysis
investigated whether resistant starch induces apoptosis of colon
tumor cells following treatment with 1,2-dimethylhydrazine.
Materials and methods
Ethics statement
This study was performed in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals (22). All experimental
protocols were performed in accordance with National Institutes of
Health and approved by the Committee on the Ethics of Animal
Experiments Defence Research (Northeast Agricultural University,
Harbin, China).
Animal study
A total of 20 C57BL/6 mice, 6–8 weeks old, were
purchased from Jackson Laboratory (Bar Harbor, ME, USA) and housed
in a temperature-controlled room (25±1°C) with artificial 12/12 h
light/dark cycle. All mice could access water containing
1,2-dimethylhydrazine (3 mg/kg) to induce colon cancer. The
incidence of colon tumor induced by 1,2-dimethylhydrazine was
calculated by histopathology as described in immunohistochemistry
assay. Experimental mice were divided into two groups (n=10/group)
with free access to a regular diet (5 mg/kg) or a resistant starch
diet (5 mg/kg). All mice were sacrificed for further analysis on
day 120.
Analysis of ammonia, pH and short chain
fatty acids
Ammonia in experimental mice was measured using
ionization constant. pH was determined by pH meter (Mettler). Short
chain fatty acids were analyzed using High Performance Liquid
Chromatography (Takara, Tokyo, Japan).
Cells culture and reagents
Colon epithelial cells and colon tumor cells were
isolated from experimental mice and cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS; Sigma-Aldrich; Merck KgaA, Darmstadt, Germany). Cells were
cultured at 37°C and 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was reverse transcribed into cDNA at 42°C for 2
h using the High Capacity cDNA Reverse Transcription kit (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. PCR amplification had preliminary
denaturation at 94°C for 2 min, followed by 45 cycles of 95°C for
30 sec; the annealing temperature was reduced to 56.8°C for 30 sec
and 72°C for 10 min. The reaction volume was a total of 20
μl containing 50 ng genomic cDNA, 200 μM dNTPs, 200
μM primers, and Taq DNA polymerase and SYBR-Green (both 2.5
U; Thermo Fisher Scientific, Inc.). Total RNA was extracted from
colon epithelial cell and colon tumor cells by using RNAeasy mini
kit (Qiagen, Inc., Valencia, CA, USA). mRNA expression levels of
heat shock protein 25 (HSP25), protein kinase C-d (PKC-d),
gastrointestinal glutathione peroxidase (GI-GPx), c-myc, Ras, p53,
proliferating cell nuclear antigen (PCNA), claudin 1, claudin 2,
mechanistic target of rapamycin kinase (mTOR), hexokinase-2
(HK-II), caspase-3, caspase-9, p53, Bcl-2 apoptosis regulator
(Bcl-2), superoxide dismutase (SOD) and glutathione synthetase
(GSH) in colon epithelial cell and/or colon tumor cells were
measured by RT-qPCR with β-actin as an endogenous control (23) (Invitrogen; Thermo Fisher
Scientific, Inc.). All the forward and reverse primers were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.)
(Table I). Relative mRNA
expression changes were calculated by 2−ΔΔCq (24). The results are expressed as the
n-fold change compared with control.
 | Table IPrimer sequences for RT-qPCR. |
Table I
Primer sequences for RT-qPCR.
| Gene name
Sequences |
|---|
| HSP25 |
| F:
5′-ATCGAGATCTAATGGAGCCAGGGGAGGCG-3′ |
| R:
5′-ATCGAGATCTGGAGAAGGCGGAGGGCGCGG-3′ |
| PKC-d |
| Forward:
5′-GCATCCTCTTCAGTTACGTCC -3′ |
| Reverse:
5′-AAGAGAGCTTCCGTAAGGCG-3′ |
| GI-GPx |
| Forward:
5′-GAGGATATTTCGTGCCGCGC-3′ |
| Reverse:
5′-GGAAGCTCCTGAAGATCTGT-3′ |
| c-myc |
| Forward:
5′-ATTGGGACAGCTTGGATCAC-3′ |
| Reverse:
5′-AGTCACACGTCATCGACACC-3′ |
| Ras |
| Forward:
5′-CGCATCCTCAAAGGAGACATTCC-3′ |
| Reverse:
5′-CACATCGAGGTGAACGGGAGTAAG-3′ |
| p53 |
| Forward:
5′-AAGGATCCTCAGTCTGAGTCAGGCC-3′ |
| Reverse:
5′-ACCACCATCCACTACAACTAC-3′ |
| PCNA |
| Forward:
5′-AGCACGGTAACGTAGGGTGT-3′ |
| Reverse:
5′-CATTGGAGGCATAAGCTG-3′ |
| Claudin1 |
| Forward:
5′-AGAACCAGTGAGCCTGATACATACAG-3′ |
| Reverse:
5′-GCAACTCCATCGGCCTTTCCTACCAG-3′ |
| Claudin2 |
| Forward:
5′-GGAGTAGAAGTCCCGCAGGAT-3′ |
| Reverse:
5′-AGGCCTCCTGGGCTTCAT-3′ |
| m-TOR |
| Forward:
5′-CGTACATGTCAGCCAGCTTC-3′ |
| Reverse:
5′-TGGAGGAATTCTTGCTTTGC-3′ |
| HK |
| Forward:
5′-GACGCAATCAATGTTTACTCG-3′ |
| Reverse:
5′-TATTTGGTTGGTCAGCACAGG-3′ |
| Caspase-3 |
| Forward:
5′-TTGAGGTAGCTGCACTGTGG-3′ |
| Reverse:
5′-GGGCGTGTTTCTGTTTTGTT-3′ |
| Caspase-9 |
| Forward:
5′-CCAACCAAATGAAGCCAAGT-3′ |
| Reverse:
5′-GCCCTTGCCTCTGAGTAGTG-3′ |
| CPI |
| Forward:
5′-GGAACACCTCGCTCTCCA-3′ |
| Reverse:
5′-GGGATTCCCTGGACCTAAAG-3′ |
| Bcl-2 |
| Forward:
5′-CGTCTTCAGAGACAGCCAGGAG-3′ |
| Reverse:
5′-TGAACCGGCATCTGCACAC-3′ |
| SOD |
| Forward:
5′-TTTGCCAGCAGTCACATTGC-3′ |
| Reverse:
5′-GTACCAGTGCAGGTCCTCAC-3′ |
| GSH |
| Forward:
5′-CCGATCCAATCTGTTCTGGT-3′ |
| Reverse:
5′-CCAGGGCTTTTCAAAAATGA-3′ |
| β-actin |
| Forward:
5′-AGCCTTCTCCATGGTCGTGA-3′ |
| Reverse:
5′-CGGAGTCAACGGATTTGGTC-3′ |
Western blot analysis
Colon tumor cells were homogenized in a
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KgaA)
and centrifuged at 6,000 × g at 4°C for 10 min. Protein
concentration was measured with a bicinchoninic acid protein assay
kit (Thermo Fisher Scientific, Inc.). A total of 10 μg/lane
protein was were separated in a 12% SDS assay and then transferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billierica,
MA, USA). Membranes were blocked in 5% BSA (Sigma-Aldrich; Merck
KgaA) for 1 h at 37°C and subsequently incubated with the following
primary antibodies: HSP25 (ab202846), PKC-d (ab182126), GI-GPx
(ab137431), c-myc (ab32071), Ras (ab52939), P53 (ab1431), PCNA
(ab18197), claudin1 (ab15098), claudin2 (ab53032), mTOR (ab2732)
and HK-II (ab24937), caspase-3 (ab13847), caspase-9 (ab202068),
Bcl-2 (ab59348), SOD (ab13533), GSH (ab26255), DDIT3 (ab179823),
Beclin1 (ab62557), CHOP (ab10444), BIP (ab108615), caspase-12
(ab62484), ATF-4 (ab23760), BACE1 (ab2077), eIF2α (ab5369) and
β-actin (ab8227) for 12 h at 4°C. All primary antibodies were used
at a dilution of 1:1,000 and purchased from Abcam (Cambridge, UK).
The membranes were then incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) monoclonal
secondary antibodies (1:2,000; cat. no. PV-6001; OriGene
Technologies, Inc., Beijing, China) for 24 h at 4°C. An enhanced
chemiluminescence substrate ECL Select™ (Roche Diagnostics, Basel,
Switzerland) was used to analyze the protein expression (Olympus
BX51; Olympus Corporation, Tokyo, Japan).
Transfection of small interfering RNA
(siRNA)
Colon tumor cells (1×106) were
transfected with 100 pmol of siRNA targeting eukaryotic translation
initiation factor 2α (eIF2α) with siRNA-vector as control (both
from Applied Biosystems; Thermo Fisher Scientific, Inc.) using a
Cell Line Nucleofector kit L. (Lonza Group, Ltd., Basel,
Switzerland). Cells were cultured in 2.5 ml DMEM containing 10% FBS
6-well plates for 24 h. All siRNAs were synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.) including siRNA-eIF2α (eIF2α,
L-015389) or siRNA-vector (scramble, D-001810). Cells were used for
the subsequent assays after 48-h transfection.
Cell differentiation
Colon tumor cells isolated from experimental mice
and cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck
KgaA) at a 37°C humidified atmosphere of 5% CO2. Cell
colonies growing on Matrigel® were loosely detached by
dispase treatment for 5 min, washed 3 times with PBS. Cells were
resuspended in DMEM medium containing 20% FBS. Cells were
maintained on 1% agar-coated and allowed to differentiate for
another 18 days. Cells were then fixed with 10% formalin for 1 h at
37°C. Next following stained with the 60% Oil Red O in isopropanol
as working solution for 10 min. The proportion of Oil Red
O-positive cells was determined by counting stained cells under a
light microscope.
Activity assays
Eukaryotic translation initiation factor 2-α kinase
3 (PERK) activity in colon tumor cells was analyzed by recombinant
glutathione S-transferase-PERK (536-1,116 amino acids) with
6-His-full-length human eIF2α as a substrate (25). eIF2α activity was analyzed by
stimulation of eIF-2-α kinase GCN2 in colon tumor cells (26). AMPK activity was determined by
transient transfection assays as described previously (27).
Immunohistochemistry and
immunofluorescent staining
Immunohistochemistry and immunofluorescent staining
were performed according to the standard procedures (28). Paraffin-embedded colon tumor
tissues sections were prepared and epitope retrieval was performed
for further analysis. The paraffin sections were treated with
hydrogen peroxide (3%) for 10–15 min, which subsequently blocked by
a regular blocking solution for 10–15 min 37°C for
immunohistochemistry. Colon tumor cells were cultured and stained
with microtubule associated protein 1 light chain 3 α (MAP1LC3A),
translocase of outer mitochondrial membrane 20 (TOMM20) for
observation of microtubules and/or microvessels, Neo (Nase), and
calreticulin (Invitrogen; Thermo Fisher Scientific, Inc.), NRP-2
(ab129050; Abcam), Apaf-1 (ab2001; Abcam), Bad (ab32445; Abcam) for
immunofluorescent staining. All antibodies were used at a dilution
of 1:1,000. Cells were then incubated with goat anti-rabbit IgG
H&L (HRP) (1:2,000; ab205718; Abcam) for 1 h at 37°C. All
fluorescent samples were visualized with a confocal fluorescence
microscope (Leica TCS SP8; Leica Corporation, Wetzlar,
Germany).
Cell cycle analysis
Cells (1×107) were collected from the
experimental mice, and fixed with 70% ethanol for 2 h at 30°C.
Fixed cells were rehydrated in PBS for 5 min and incubated in RNase
A (1 mg/ml) for 30 min at 37°C. The cells were then subjected to
PI/RNase staining followed by flow cytometric analysis using a
FACScan instrument (Becton Dickinson, Mountain View, CA, USA) and
Cell Quest software (Becton Dickinson).
Apoptosis assay
Colon tumor cells were isolated from experimental
mice and trypsinized and collected for apoptosis analysis. The
cells were adjusted to 5×106 cells/ml with
phosphate-buffered saline, labeled with Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) using an Annexin
V-FITC kit, and analyzed with a FACScan flow cytometer (both from
BD Biosciences, Franklin Lakes, NJ, USA). The treatments were
performed in triplicate, and the percentage of labeled cells
undergoing apoptosis in each group was determined and calculated
using FCS Express™ 4 IVD software 1.0 (De Novo Software, Glendale,
CA, USA).
Statistical analysis
All data were presented as mean + standard error
with three independent experiments. Statistical significance was
analyzed using two tailed Student's t-test between groups. Unpaired
data was analyzed by variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Resistant starch diet improves body
weight and the metabolic characteristics of colon tissues
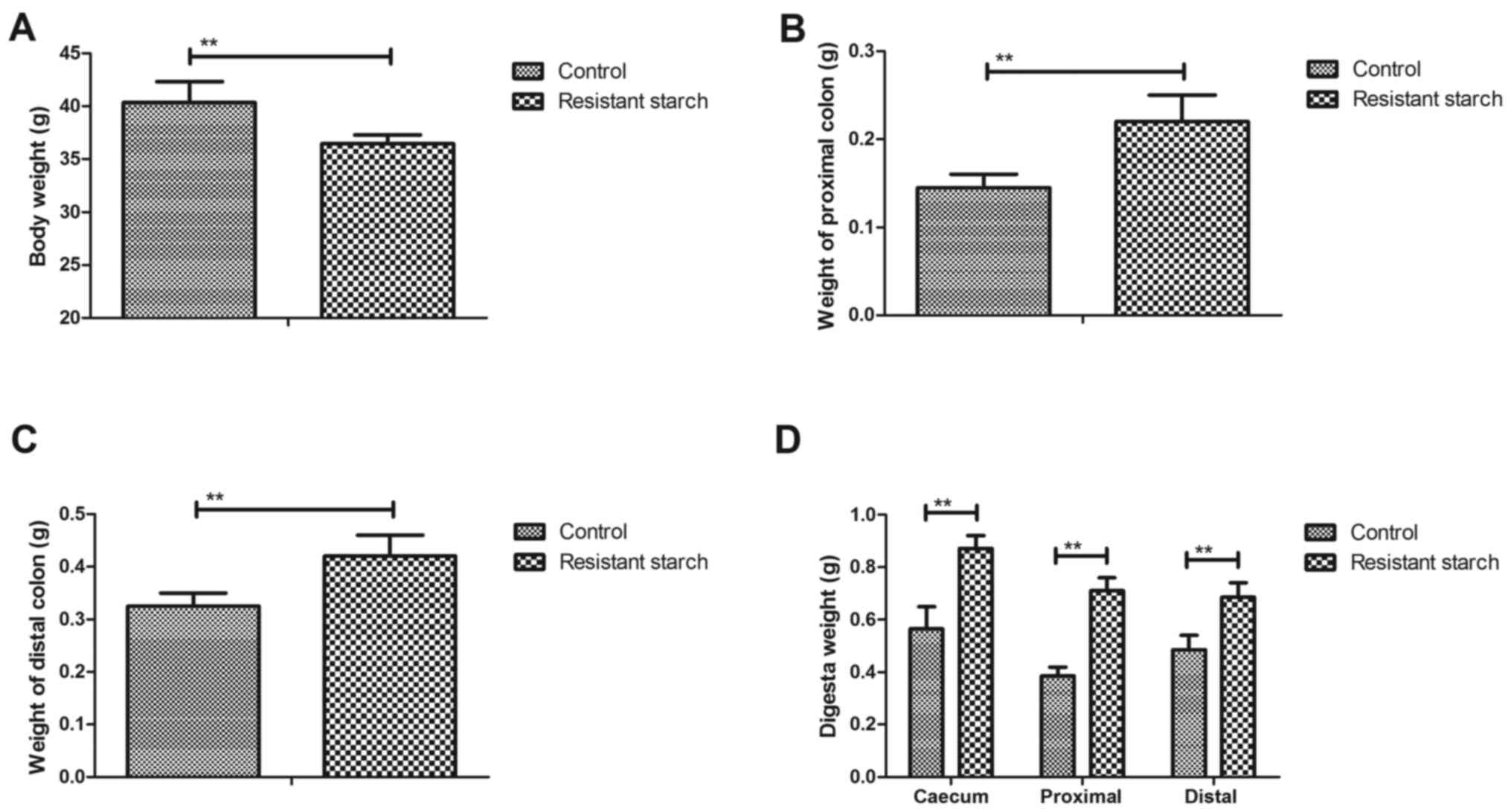
In order to investigate the benefits of resistant
starch diets for metabolism of experimental mice, body weight and
metabolic characteristic of colon tissues were analyzed. A
resistant starch diet decreased body weight compared with a regular
diet (Fig. 1A). Weight of the
proximal and distal colon was also increased by resistant starch
diets in experimental mice (Fig. 1B
and C). The results demonstrated that digesta weight in the
caecum, proximal colon and distal colon was significantly increased
by the resistant starch diet compared with a regular diet (Fig. 1D). The digesta pH in the caecum,
proximal colon and distal colon was downregulated in mice fed with
resistant starch compared with a normal diet (Fig. 1E). Free ammonia, pH and short
chain fatty acids (SCFA) in feces were decreased in mice fed with
resistant starch (Fig. 1F–H).
These results suggest that a resistant starch diet improves body
weight and metabolic characteristics of colon tissues.
Resistant starch diet inhibits
tumorigenesis in colon tissues induced by
1,2-dimethylhydrazine
Anti-tumorigenesis efficacy of resistant starch was
investigated in colon tissues induced by 1,2-dimethylhydrazine.
Results showed that resistant starch suppressed tumor formation in
experimental mice treated by 1,2-dimethylhydrazine (Fig. 2A). Histopathology demonstrated
that resistant starch significantly inhibited incidence of colon
tumor in mice model induced by 1,2-dimethylhydrazine (Fig. 2B). The gene and protein expression
levels of carcinogenesis-associated genes, HSP25, PKC-d and GI-GPx,
were downregulated in colon epithelial cells from mice receiving
resistant starch compared with the standard starch group (Fig. 2C and D). However, the gene and
protein expression levels of oncogenes c-myc, Ras and pro-apoptosis
gene p53 were upregulated by resistant starch in colon epithelial
cells (Fig. 2E and F).
Immunofluorescence demonstrated that microtubule and tumor vessels
were inhibited in colon tissues in mice in the resistant starch
group compared with the control group (Fig. 2G). Immunohistochemistry
demonstrated that the expression levels of MAT-1 and NRP-2 were
downregulated by resistant starch in colon tumor tissues (Fig. 2H). These results indicated that a
resistant starch diet inhibits tumorigenesis in colon tissues
induced by 1,2-dimethylhydrazine.
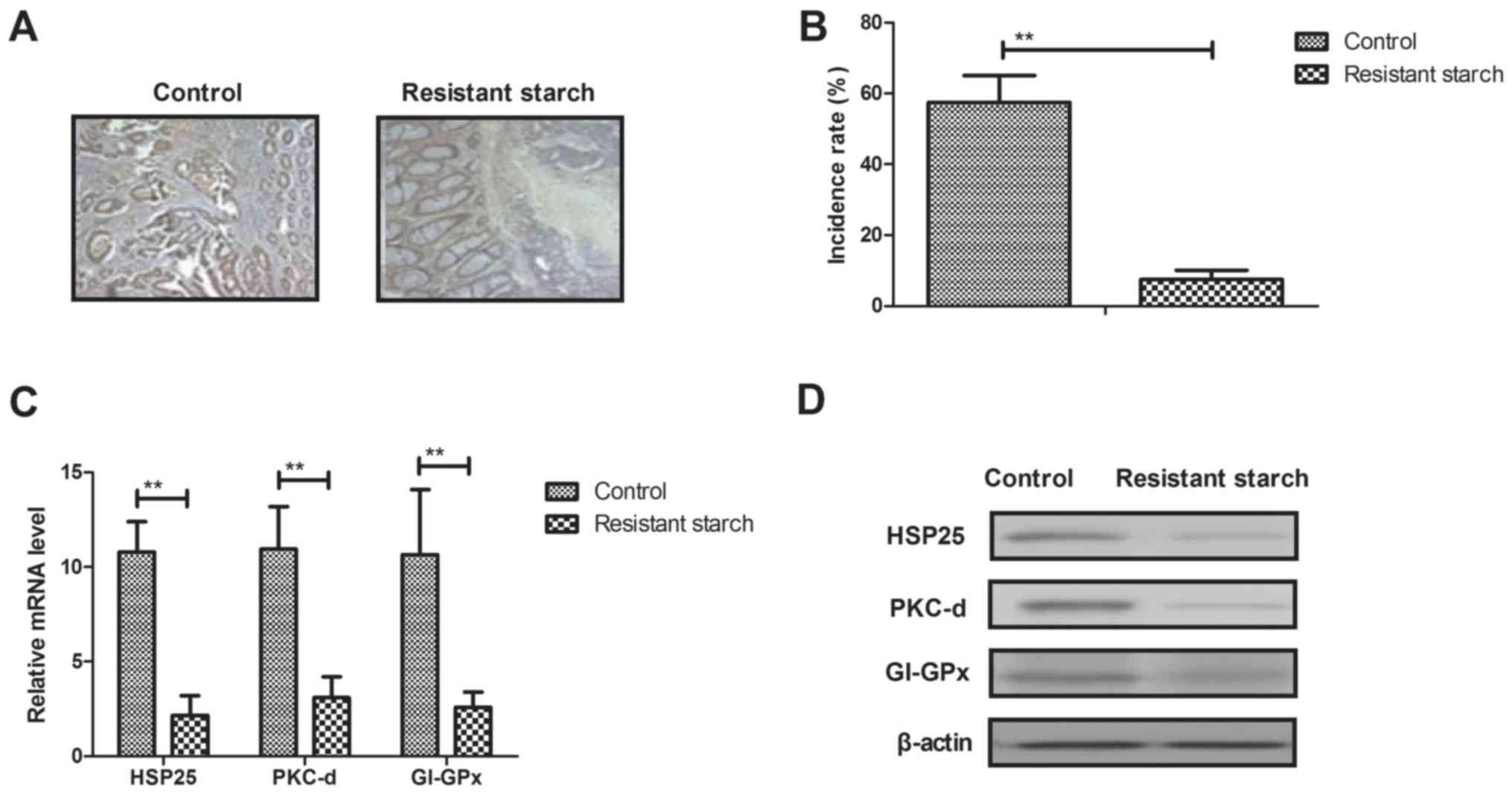 | Figure 2Effects of resistant starch on
tumorigenesis in colon tissues induced by 1,2-dimethylhydrazine.
(A) Resistant starch inhibits tumor formation in experimental mice
treated by 1,2-dimethylhydrazine. (B) Effects of resistant starch
decreases incidence of colon tumor in a mouse model induced by
1,2-dimethylhydrazine. (C) Gene and (D) protein expression levels
of HSP25, PKC-d and GI-GPx in colon epithelial cells in
experimental mice fed with resistant starch after induced by
1,2-dimethylhydrazine. Effects of resistant starch on (E) gene and
(F) protein expression levels of c-myc, Ras and p53 in colon
epithelial cells. (G) Resistant starch diet inhibits formation of
microtubule and tumor vessel in colon tissues in experimental mice.
(H) Resistant starch diet inhibits protein express levels of MAT-1
and NRP-2 in colon tissues in experimental mice.
**P<0.01. HSP25, heat shock protein 25; PKC-d,
protein kinase C-d; GI-GPx, gastrointestinal glutathione
peroxidase; MAT-1, CDK-activating kinase assembly factor MAT1;
NRP-2, neuropilin-2 (magnification, ×40). |
Resistant starch diet inhibits the
proliferation and differentiation of colon cells
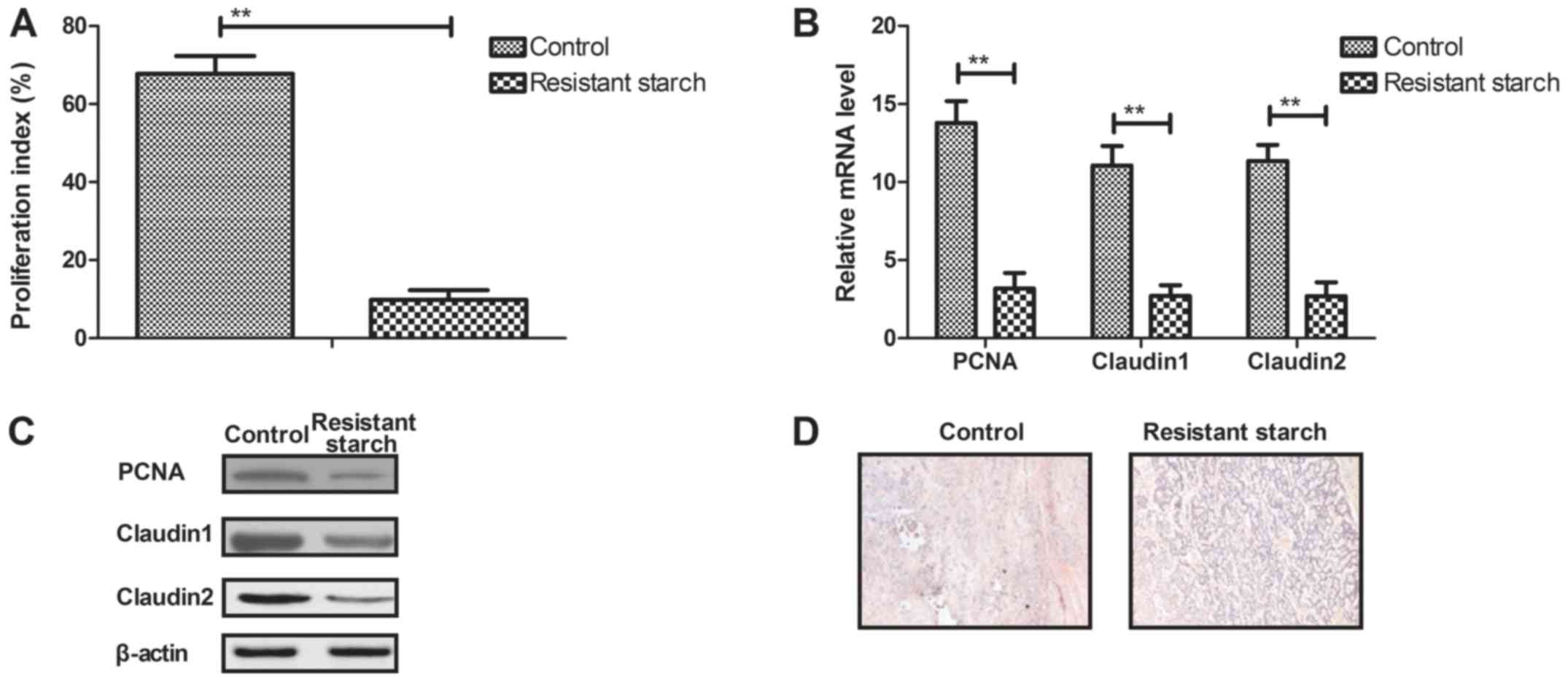
Tumor cell proliferation and differentiation has a
vital role in tumorigenesis. Thus, the effect of resistant starch
on colon tumor cell proliferation and differentiation was
determined. Colon tumor cell proliferation was suppressed by
resistant starch compared with the control group in
1,2-dimethylhydrazine-induced mice (Fig. 3A). RT-qPCR and western blot
analysis demonstrated that the expression levels of PCNA, claudin 1
and claudin 2 were decreased in colon tumor cells in resistant
starch-fed mice compared with the control group (Fig. 3B and C). The results also
demonstrated that resistant starch inhibited colon tumor cell
differentiation in colon tissues (Fig. 3D). RT-qPCR and western blot
analysis demonstrated that expression levels of mTOR and HK-II were
reduced by resistant starch compared with the control diet in colon
tumor cells (Fig. 3E and F). The
resistant starch diet increased S phase arrest of colon tumor
cells, and downregulation of mTOR and HK-II expression levels
compared with the normal diet group (Fig. 3G). Long-term survival of
experimental mice was prolonged by the inclusion of resistant
starch in the diet compared with the control diet group (Fig. 3H). These results suggest that
resistant starch diets can inhibit the proliferation and
differentiation of colon tumor cells by arresting the cell
cycle.
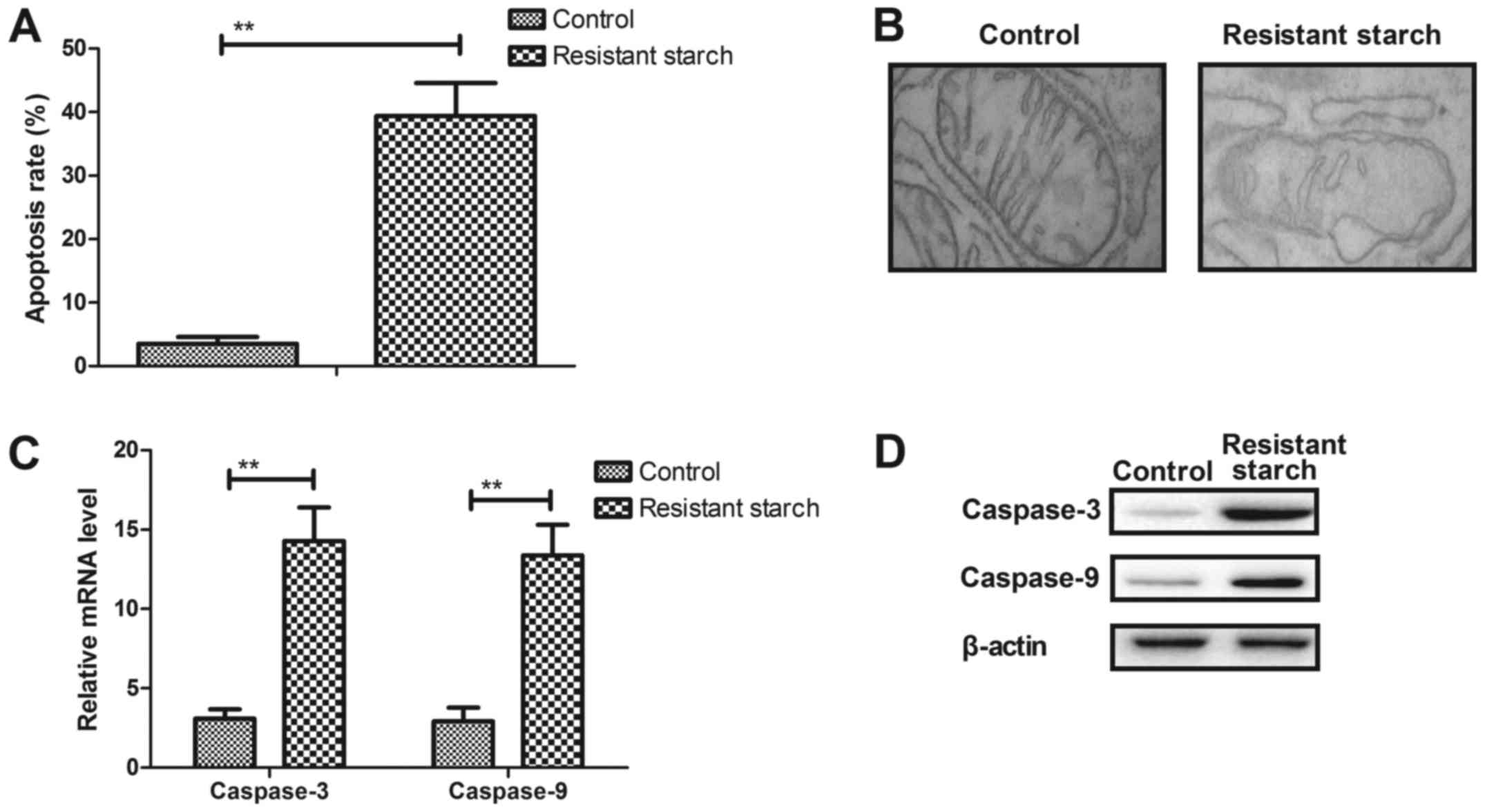
Resistant starch promotes apoptosis of
colon tumor cells through the mitochondrial pathway
The anti-apoptosis effects of resistant starch were
also analyzed in experimental mice with
1,2-dimethylhydrazine-induced colon tumors. The resistant starch
diet promoted apoptosis of colon tumor cells (Fig. 4A). Cellular structure demonstrated
that mitochondria exhibited different degrees of damage in colon
tumor cells (Fig. 4B).
Pro-apoptosis gene and protein expression levels of caspase-3 and
caspase-9 were upregulated in colon tumor cells (Fig. 4C and D). However, anti-apoptosis
gene and protein expression levels of p53 and Bcl-2 were
downregulated in colon tumor cells in resistant starch-fed
experimental mice compared with the control treatment (Fig. 4E and F). Immunohistochemistry and
immunofluorescence demonstrated that resistant starch increased
Apaf-1 and Bad expression levels in colon tumor tissues in
experimental mice compared with control mice (Fig. 4G and H). These results suggest
that resistant starch in the diet can enhance apoptosis of colon
tumors through mitochondrial apoptotic pathway in mice with colon
tumors induced by 1,2-dimethylhydrazine.
Resistant starch diet enhances oxidative
stress in colon tumors through regulation of autophagy
progression
Changes of oxidative stress in colon tumors and
colon epithelial cells was investigated. Resistant starch diets
reduced the mRNA and protein levels of SOD and GSH in colon tumor
cells compared with mice that received the control diet (Fig. 5A and B). However, mRNA and protein
levels of SOD and GSH were increased by resistant starch in colon
epithelial cells compared to regular diet (Fig. 5C and D). AMP-activated protein
kinase (AMPK) activity was increased in colon tumor cells from mice
fed with resistant starch compared with the regular diet (Fig. 5E). Potential mechanisms were
demonstrated as resistant starch increased the expression levels of
DNA damage-inducible transcript 3 protein and Beclin 1 in colon
tumor cells (Fig. 5F). The
results also demonstrated that resistant starch promoted mitophagy
and reticulophagy of colon tumor cells (Fig. 5G and H). Collectively, the results
indicated that the resistant starch diet enhanced-activated
autophagy in colon tumors through upregulation of autophagy
genes.
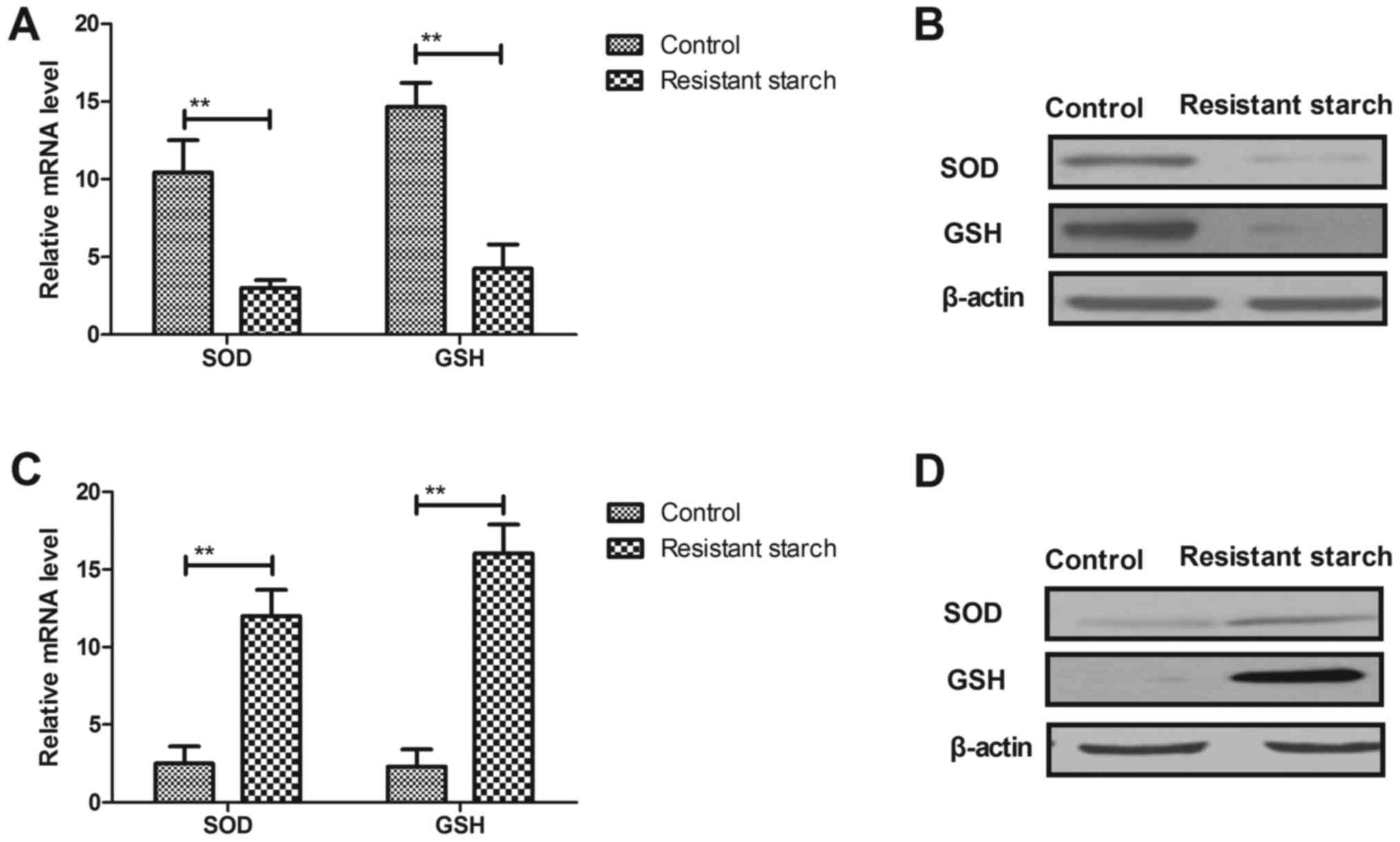 | Figure 5Resistant starch diet increases
oxidative stress in colon tumors through regulation of autophagy
progression. Effects of resistant starch on (A) mRNA and (B)
protein levels of SOD and GSH in colon tumor cells. Effects of
resistant starch on (C) mRNA and (D) protein levels of SOD and GSH
in colon epithelial cells. (E) Resistant starch upregulates AMPK
activity in colon tumor cells. (F) Resistant starch upregulates
expression levels of DDIT3 and Beclin 1 in colon tumor cells in
mice treated by 1,2-dimethylhydrazine. Resistant starch diet
promotes (G) mitophagy and (H) reticulophagy of colon tumor cells
in mice treated by 1,2-dimethylhydrazine (magnification, ×40).
**P<0.01. SOD, superoxide dismutase; GSH, glutathione
synthetase; AMPK, AMP-activated protein kinase; DDIT3, DNA
damage-inducible transcript 3 protein; MAP1LC3A, microtubule
associated protein 1 light chain 3 α; TOMM20, translocase of outer
mitochondrial membrane 20; Neo (Nase), 5′-Nase-ALPase. |
Resistant starch diet alters ER
stress-dependent PERK activity through upregulation of eIF2α
phosphorylation in colon tumor cells in
1,2-dimethylhydrazine-induced mice
The effect if resistant starch on ER stress and its
potential mechanism in inhibition of tumor cells growth was
investigated. The resistant starch diet increased the expression
levels of eIF2α, ATF-4 and secretase-β (BACE1) in colon tumor cells
compared with cells from control mice (Fig. 6A). Total protein expression levels
of eIF2α and PERK were increased by resistant starch in colon tumor
cells (Fig. 6B). eIF2α and PERK
activity was increased by the resistant starch diet compared with
the regular diet in mice induced by 1,2-dimethylhydrazine (Fig. 6C). The resistant starch diet
increased the expression levels of DNA damage-inducible transcript
3 protein (CHOP), binding immunoglobulin protein (BIP) and
caspase-12 in colon tumor cells (Fig.
6D). In vitro assays demonstrated that downregulation of
expression levels of eIF2α using siRNA suppressed PERK activity in
colon tumor cells (Fig. 6E).
Expression levels of ATF-4 and BACE1 were reduced by knockdown of
eIF2α compared with control siRNA in colon tumor cells (Fig. 6F). Knockdown of eIF2α exhibited
inhibitory effects on CHOP, BIP and caspase-12 expression in colon
tumor cells isolated from mice fed by resistant starch (Fig. 6G). Result also demonstrated that
knockdown of eIF2α inhibited resistant starch-induced apoptosis of
colon tumor cells (Fig. 6H).
Collectively, these results suggest that the resistant starch diet
regulated ER stress-dependent PERK activity through upregulation of
eIF2α activity in colon tumor cells in mice induced by
1,2-dimethylhydrazine.
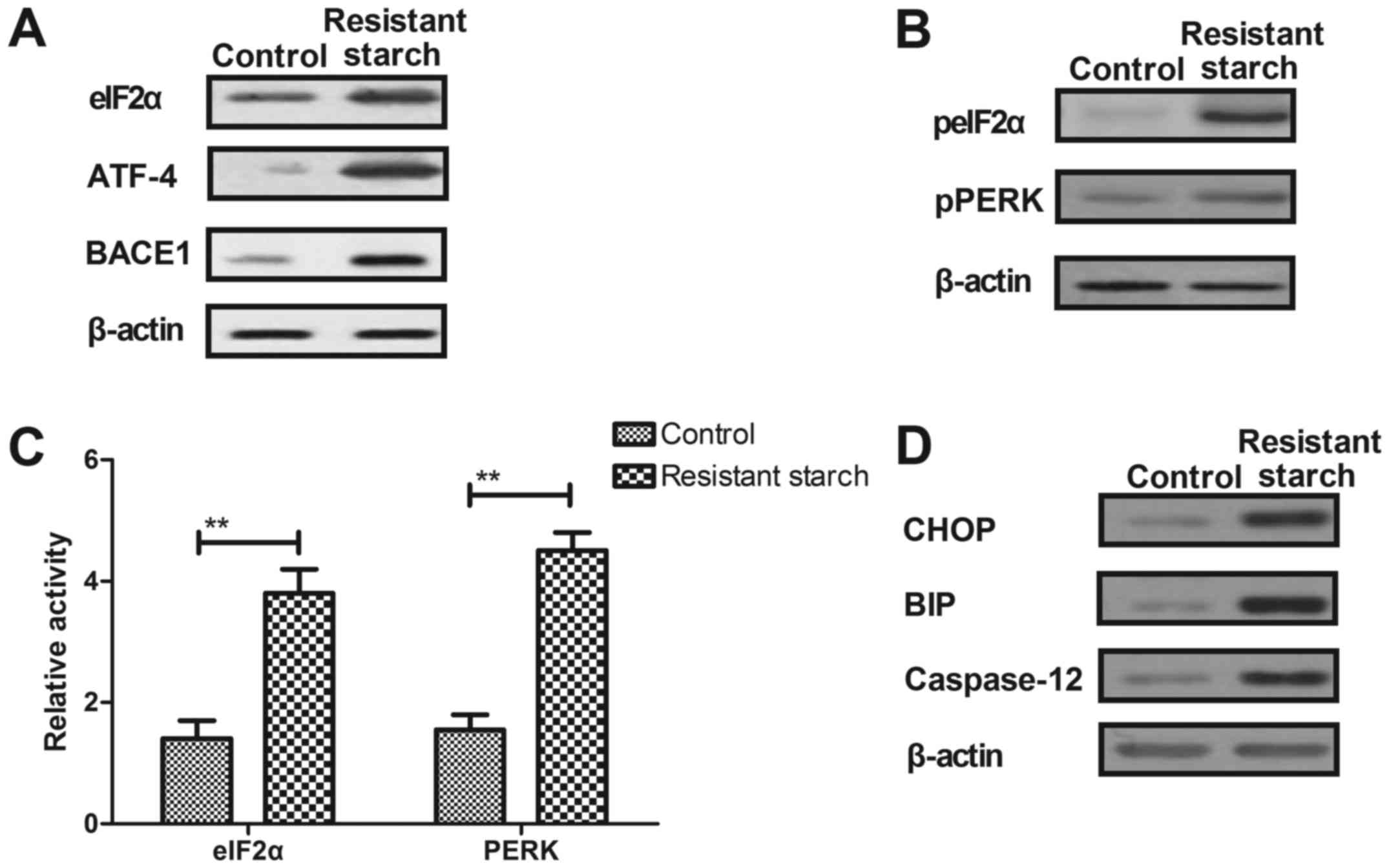 | Figure 6Resistant starch diet regulates
endoplasmic reticulum stress-dependent PERK activity through
upregulation of eIF2α phosphorylation in colon tumor cells. (A)
Resistant starch diet increases expression levels of eIF2α, ATF-4
and BACE1 in colon tumor cells. (B) Effects of resistant starch
diet on phosphorylation levels of eIF2α and PERK in colon tumor
cells in mice treated by 1,2-dimethylhydrazine. (C) Effects of
resistant starch diet on activity of eIF2α and PERK in colon tumor
cells in mice treated by 1,2-dimethylhydrazine. (D) Resistant
starch diet increases expression levels of CHOP, BIP and caspase-12
in colon tumor cells in mice treated by 1,2-dimethylhydrazine. (E)
Effects of DReIF2α on PERK activity in colon tumor cells in mice
induced by 1,2-dimethylhydrazine. (F) eIF2α knockdown inhibits
resistant starch-suppressed ATF-4 and BACE1 expression levels in
colon tumor cells in mice fed with resistant starch. (G) Knockdown
of eIF2α suppresses expression levels of CHOP, BIP and caspase-12
in colon tumor cells in mice fed with resistant starch. (H)
Knockdown of eIF2α inhibits resistant starch-induced apoptosis of
colon tumor cells isolated from mice fed with resistant starch.
**P<0.01. eIF2α, eukaryotic translation initiation
factor 2α; ATF-4, activating transcription factor-4; BACE1,
secretase-β; p, phospho; PERK, eukaryotic translation initiation
factor 2-α kinase 3; CHOP, DNA damage-inducible transcript 3
protein; BIP, binding immunoglobulin protein; AMPK, AMP-activated
protein kinase; DReIF2α, downregulation of eIF2α. |
Discussion
Colon cancer is one of the most common
gastrointestinal tumors, with highly invasive ability characterized
by rapid invasion of lymphatics, flow transfer and local invasion
(29,30). Various studies have indicated that
most cases of sporadic colon cancer can be attributed to diet
(31,32). Studies have suggested that
resistant starch fermentation modulated colonic bacterial
metabolism and reduced the risk of colon cancer tumorigenesis
(33). The current study
investigated the benefits and potential mechanism of resistant
starch-mediated anti-cancer efficacy in experimental mice induced
by 1,2-dimethylhydrazine. Compared with a regular diet, the
resistant starch diet inhibited tumorigenesis, proliferation and
differentiation in colon tissues induced by 1,2-dimethylhydrazine,
and increased the animal body weight and improved the metabolic
characteristics of the colon tissues. Analysis of the molecular
mechanisms indicated that the resistant starch diet
promotedapoptosis of colon tumor cells through the mitochondrial
apoptotic pathway, enhanced oxidative stress through regulation of
autophagy progression, and regulated ER stress-dependent PERK
activity through upregulation of eIF2α activity in the colon tumor
cells of mice induced by 1,2-dimethylhydrazine.
Tumorigenesis mechanisms are crucial for initiation
and progression of tumor formation (34). Previous studies have reported that
elevation of mRNA expression levels of PKC-d, HSP25 and GI-GPx are
associated with tumorigenesis (35). The resistant starch diet
downregulated expression levels of PKC-d, HSP25 and GI-GPx in colon
epithelial cells that contributed to inhibition of tumor lesions
formation. Studies have clearly demonstrated that tumor blood
vessels and higher expression levels of MAT-1 and NRP-2 hindered
the therapeutic efficacy of anti-cancer drugs (36–38). The data of the current study
revealed that the structure of microtubules and tumor vessels in
colon tumor cells were altered and gene expression of MAT-1 and
NRP-2 were downregulated in resistant starch-fed mice induced by
1,2-dimethylhydrazine.
Proliferation and differentiation of colon tumor
cells contribute to tumor migration and invasion mediated by
molecular signaling through effector pathways (39). In the present study, the resistant
starch diet inhibited proliferation and differentiation of colon
tumor cells by decreasing PCNA, claudin 1 and claudin 2 gene and
protein expression levels. The results also indicated that
resistant starch arrested the cell cycle of colon tumor cells and
downregulated mTOR and HK-II expression levels, which may have
contributed to the increased long-term survival of experimental
mice in the resistant starch group compared with the control diet
group.
Induction of apoptosis by tumor therapeutic agents
is essential for cancer therapy (40). In this study, the anti-cancer
efficacy of resistant starch was investigated in mice induced with
1,2-dimethylhydrazine. The results demonstrated that resistant
starch promoted apoptosis via the mitochondrial apoptotic pathway,
and also increased the expression of tumor suppressor genes in
colon epithelial cells. These results suggest that resistant starch
contributes to apoptosis of colon tumor cells and anti-apoptosis
efficacy of colon epithelial cells in experimental mice induced by
1,2-dimethylhydrazine. However, we observed that p53 was reduced by
resistant starch, which is one of the most important tumor
suppressor gene. Therefore, further study should perform to
identify the inhibitory effects of resistant starch on tumor cells.
Additionally, we found that oncogenes c-myc and Ras were
upregulated by resistant starch, which also need to further
analyzed in our future work.
Autophagy is a cellular progression of materials
conversion that promotes the obliteration of metabolic precursors
(41,42). A previous study demonstrated that
autophagy contributes to the inhibition of oncogenesis and
suppression of tumor growth in colon cancer (43). Jang et al (44) reported that promotion of autophagy
can inhibit tumorigenesis and induce apoptosis of human cancer
cells by modulating sphingolipids, and suppress colon tumor
development in mice. In the current study, the resistant starch
diet increased oxidative stress and increased SOD and GSH levels in
colon tumors. AMPK activity, mitophagy, reticulophagy and Beclin 1
expression were also increased by resistant starch in colon tumor
cells (45,46).
Studies have indicated that ER stress is associated
with apoptosis of colon tumor cells (47). PERK activation has an important
role in promoting tumorigenesis by attenuating apoptosis of
premalignant granule cell precursors (48). The current study demonstrated that
the resistant starch diet promoted the expression levels of eIF2α,
ATF-4 and BACE1, and increased eIF2α activity in colon tumor cells.
Mechanistic analysis indicated that knockdown of eIF2α exhibited
inhibitory effects on CHOP, BIP and caspase-12 expression, and
apoptosis in colon tumor cells isolated from mice fed with the
resistant starch diet. The results of the present study indicated
that the resistant starch diet promoted apoptosis by regulating ER
stress-dependent PERK activity via upregulation of eIF2α activity
in colon tumor cells from mice induced by 1,2-dimethylhydrazine,
which contributes to tumor suppression.
In conclusion, resistant starch improved bowel
function, and the outcomes indicated that the resistant starch diet
improved body weight and the metabolic characteristic of colon
tissues. The results indicated that resistant starch inhibited
tumorigenesis in the colon by promoting apoptosis and autophagy by
upregulation of ER stress-dependent PERK activity, which mediated
by eIF2α in colon tumor cells from mice induced by
1,2-dimethylhydrazine. These investigations indicated that
resistant starch prevents tumorigenesis of colon tumors induced by
dimethylhydrazine via regulation of an ER stress-mediated
mitochondrial apoptosis pathway, which may useful in the future for
the prevention of tumorigenesis of colon tissues.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Lopez NE, Weiss AC, Robles J, Fanta P and
Ramamoorthy SL: A systematic review of clinically available gene
expression profiling assays for stage II colorectal cancer: Initial
steps toward genetic staging. Am J Surg. 212:700–714. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burness CB and Duggan ST:
Trifluridine/tipiracil: A review in metastatic colorectal cancer.
Drugs. 76:1393–1402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moriarity A, O'Sullivan J, Kennedy J,
Mehigan B and McCormick P: Current targeted therapies in the
treatment of advanced colorectal cancer: A review. Ther Adv Med
Oncol. 8:276–293. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chibaudel B, Bonnetain F, Tournigand C and
de Gramont A: Maintenance treatment in metastatic colorectal
cancer. Lancet Oncol. 16:e583–e584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moilanen JM, Kokkonen N, Löffek S,
Väyrynen JP, Syväniemi E, Hurskainen T, Mäkinen M, Klintrup K,
Mäkelä J, Sormunen R, et al: Collagen XVII expression correlates
with the invasion and metastasis of colorectal cancer. Hum Pathol.
46:434–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang
L, Han B, Meng J, Yan Z, Yan X, et al: MiR-125a suppresses tumor
growth, invasion and metastasis in cervical cancer by targeting
STAT3. Oncotarget. 6:25266–25280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang XB, Song L, Wen HJ, Bai XX, Li ZJ
and Ma LJ: Upregulation of microRNA-31 targeting integrin alpha5
suppresses tumor cell invasion and metastasis by indirectly
regulating PI3K/AKT pathway in human gastric cancer SGC7901 cells.
Tumour Biol. 37:8317–8325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Yu X, Gu J, Lin Z, Zhao G, Xu F, Lu
C and Ge D: Regulation of CXCR4/AKT-signaling-induced cell invasion
and tumor metastasis by RhoA, Rac-1, and Cdc42 in human esophageal
cancer. Tumour Biol. 37:6371–6378. 2016. View Article : Google Scholar
|
|
9
|
Jiang N, Deng JY, Liu Y, Ke B, Liu HG and
Liang H: Incorporation of perineural invasion of gastric carcinoma
into the 7th edition tumor-node-metastasis staging system. Tumour
Biol. 35:9429–9436. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bukurova IuA, Khankin SL, Krasnov GS,
Grigor'eva ES, Mashkova TD, Lisitsin NA, Karpov VL and Beresten'
SF: Comparison of 2D analysis and bioinformatics search efficiency
for colon cancer marker identification. Mol Biol (Mosk).
44:375–381. 2010.In Russian. View Article : Google Scholar
|
|
11
|
Thompson BA, Goldgar DE, Paterson C,
Clendenning M, Walters R, Arnold S, Parsons MT, Michael DW,
Gallinger S, Haile RW, et al: Colon Cancer Family Registry: A
multifactorial likelihood model for MMR gene variant classification
incorporating probabilities based on sequence bioinformatics and
tumor characteristics: A report from the Colon Cancer Family
Registry. Hum Mutat. 34:200–209. 2013. View Article : Google Scholar
|
|
12
|
Ma Z and Boye JI: Research advances on
structural characterization of resistant starch and its
structure-physiological function relationship: A review. Crit Rev
Food Sci Nutr. Sep 19–2016.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raigond P, Ezekiel R and Raigond B:
Resistant starch in food: A review. J Sci Food Agric. 95:1968–1978.
2015. View Article : Google Scholar
|
|
14
|
Shen D, Bai H, Li Z, Yu Y, Zhang H and
Chen L: Positive effects of resistant starch supplementation on
bowel function in healthy adults: A systematic review and
meta-analysis of randomized controlled trials. Int J Food Sci Nutr.
68:149–157. 2017. View Article : Google Scholar
|
|
15
|
Dronamraju SS, Coxhead JM, Kelly SB, Burn
J and Mathers JC: Cell kinetics and gene expression changes in
colorectal cancer patients given resistant starch: A randomised
controlled trial. Gut. 58:413–420. 2009. View Article : Google Scholar
|
|
16
|
Le Leu RK, Hu Y, Brown IL, Woodman RJ and
Young GP: Synbiotic intervention of Bifidobacterium lactis and
resistant starch protects against colorectal cancer development in
rats. Carcinogenesis. 31:246–251. 2010. View Article : Google Scholar
|
|
17
|
Mathers JC, Movahedi M, Macrae F, Mecklin
JP, Moeslein G, Olschwang S, Eccles D, Evans G, Maher ER, Bertario
L, et al: CAPP2 Investigators: Long-term effect of resistant starch
on cancer risk in carriers of hereditary colorectal cancer: An
analysis from the CAPP2 randomised controlled trial. Lancet Oncol.
13:1242–1249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JS, Jung WK, Jeong MH, Yoon TR and Kim
HK: Sanguinarine induces apoptosis of HT-29 human colon cancer
cells via the regulation of Bax/Bcl-2 ratio and caspase-9-dependent
pathway. Int J Toxicol. 31:70–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crespo I, San-Miguel B, Prause C, Marroni
N, Cuevas MJ, González-Gallego J and Tuñón MJ: Glutamine treatment
attenuates endoplasmic reticulum stress and apoptosis in
TNBS-induced colitis. PLoS One. 7:e504072012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wali VB, Bachawal SV and Sylvester PW:
Endoplasmic reticulum stress mediates gamma-tocotrienol-induced
apoptosis in mammary tumor cells. Apoptosis. 14:1366–1377. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edagawa M, Kawauchi J, Hirata M, Goshima
H, Inoue M, Okamoto T, Murakami A, Maehara Y and Kitajima S: Role
of activating transcription factor 3 (ATF3) in endoplasmic
reticulum (ER) stress-induced sensitization of p53-deficient human
colon cancer cells to tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL)-mediated apoptosis through
upregulation of death receptor 5 (DR5) by zerumbone and celecoxib.
J Biol Chem. 289:21544–21561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hawkins P, Morton DB, Burman O, Dennison
N, Honess P, Jennings M, Lane S, Middleton V, Roughan JV, Wells S,
et al: A guide to defining and implementing protocols for the
welfare assessment of laboratory animals: eleventh report of the
BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab
Anim. 45:1–13. 2011. View Article : Google Scholar
|
|
23
|
Xiao S, Wang J and Xiao N: MicroRNAs as
noninvasive biomarkers in bladder cancer detection: a diagnostic
meta-analysis based on qRT-PCR data. Int J Biol Markers.
31:e276–e285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
25
|
Atkins C, Liu Q, Minthorn E, Zhang SY,
Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, et al:
Characterization of a novel PERK kinase inhibitor with antitumor
and antiangiogenic activity. Cancer Res. 73:1993–2002. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaveroux C, Carraro V, Canaple L, Averous
J, Maurin AC, Jousse C, Muranishi Y, Parry L, Mesclon F, Gatti E,
et al: In vivo imaging of the spatiotemporal activity of the
eIF2α-ATF4 signaling pathway: Insights into stress and related
disorders. Sci Signal. 8:rs52015. View Article : Google Scholar
|
|
27
|
Yuan W, Ahmad S and Najar A: Morin, a
plant derived flavonoid, modulates the expression of peroxisome
proliferator-activated receptor-gamma coactivator-1alpha mediated
by AMPK pathway in hepatic stellate cells. Am J Translat Res.
9:5662–5670. 2017.
|
|
28
|
Christova I: Enzyme-linked immunosorbent
assay, immunofluorescent assay, and recombinant immunoblotting in
the serodiagnosis of early Lyme borreliosis. Int J Immunopathol
Pharmacol. 16:261–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamaoka Y, Yamaguchi T, Kinugasa Y, Shiomi
A, Kagawa H, Yamakawa Y, Numata M, Sugimoto S, Imai K, Hotta K, et
al: Adenocarcinoma arising from jejunal ectopic pancreas mimicking
peritoneal metastasis from colon cancer: a case report and
literature review. Surg Case Rep. 1:1142015. View Article : Google Scholar :
|
|
30
|
Hirai HW, Tsoi KK, Chan JY, Wong SH, Ching
JY, Wong MC, Wu JC, Chan FK, Sung JJ and Ng SC: Systematic review
with meta-analysis: Faecal occult blood tests show lower colorectal
cancer detection rates in the proximal colon in
colonoscopy-verified diagnostic studies. Aliment Pharmacol Ther.
43:755–764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Birt DF and Phillips GJ: Diet, genes, and
microbes: Complexities of colon cancer prevention. Toxicol Pathol.
42:182–188. 2014. View Article : Google Scholar :
|
|
32
|
Ou J, Carbonero F, Zoetendal EG, DeLany
JP, Wang M, Newton K, Gaskins HR and O'Keefe SJ: Diet, microbiota,
and microbial metabolites in colon cancer risk in rural Africans
and African Americans. Am J Clin Nutr. 98:111–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ridlon JM and Hylemon PB: A potential role
for resistant starch fermentation in modulating colonic bacterial
metabolism and colon cancer risk. Cancer Biol Ther. 5:273–274.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yueh AE, Payne SN, Leystra AA, Van D Hey
DR, Foley TM, Pasch CA, Clipson L, Matkowskyj KA and Deming DA:
Colon cancer tumorigenesis initiated by the H1047R mutant PI3K.
PLoS One. 11:e01487302016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang C, Qu S, Wei X, Feng Y, Zhu H, Deng
J, Wang K, Liu K, Liu M, Zhang H, et al: HSP25 downregulation
enhanced p53 acetylation by dissociation of SIRT1 from p53 in
doxorubicin-induced H9c2 cell apoptosis. Cell Stress Chaperones.
21:251–260. 2016. View Article : Google Scholar
|
|
36
|
Molinari AJ, Pozzi EC, Monti Hughes A,
Heber EM, Garabalino MA, Thorp SI, Miller M, Itoiz ME, Aromando RF,
Nigg DW, et al: Tumor blood vessel 'normalization' improves the
therapeutic efficacy of boron neutron capture therapy (BNCT) in
experimental oral cancer. Radiat Res. 177:59–68. 2012. View Article : Google Scholar
|
|
37
|
Marín M, Muskus C, Ramírez JR, Arbelaez
LF, Alzate JF and Berberich C: The gene encoding the
metacyclogenesis-associated transcript Mat-1 is conserved in the
genus Leishmania and shows a tendency to form dimers upon protein
expression. Parasitol Res. 86:431–435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stanton MJ, Dutta S, Zhang H, Polavaram
NS, Leontovich AA, Hönscheid P, Sinicrope FA, Tindall DJ, Muders MH
and Datta K: Autophagy control by the VEGF-C/NRP-2 axis in cancer
and its implication for treatment resistance. Cancer Res.
73:160–171. 2013. View Article : Google Scholar :
|
|
39
|
Haigis KM, Kendall KR, Wang Y, Cheung A,
Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A,
Sebolt-Leopold J, Shannon KM, et al: Differential effects of
oncogenic K-Ras and N-Ras on proliferation, differentiation and
tumor progression in the colon. Nat Genet. 40:600–608. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hino M, Ichihara H, Matsumoto Y and Ueoka
R: Anti-tumor effects of cationic hybrid liposomes against colon
carcinoma along with apoptosis in vitro. Biol Pharm Bull.
35:2097–2101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nabizadeh A, Bamdad T, Arefian E and
Razavi Nikoo SH: Autophagy gene activity may act as a key factor
for sensitivity of tumor cells to oncolytic vesicular stomatitis
virus. Iran J Cancer Prev. 9:e39192016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dagistanli FK, Ozkaya HM, Kucukyoruk B,
Biceroglu H, Metin D, Gazioglu N, Oz B, Kadioglu P and Ozturk M:
Preoperative somatostatin analogue treatment might trigger
apoptosis and autophagy in tumor tissues of patients with
acro-megaly: A pilot study. Exp Clin Endocrinol Diabetes:. Jun
20–2016.Epub ahead of print. View Article : Google Scholar
|
|
43
|
Zhai H, Song B, Xu X, Zhu W and Ju J:
Inhibition of autophagy and tumor growth in colon cancer by
miR-502. Oncogene. 32:1570–1579. 2013. View Article : Google Scholar
|
|
44
|
Jang Y, Park NY, Rostgaard-Hansen AL,
Huang J and Jiang Q: Vitamin E metabolite 13′-carboxychromanols
inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in
human cancer cells by modulating sphingolipids and suppress colon
tumor development in mice. Free Radic Biol Med. 95:190–199. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baskaran R, Poornima P, Priya LB, Huang CY
and Padma VV: Neferine prevents autophagy induced by hypoxia
through activation of Akt/mTOR pathway and Nrf2 in muscle cells.
Biomed Pharmacother. 83:1407–1413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu W, Shang G, Yang S, Huang J, Xue X,
Lin Y, Zheng Y, Wang X, Wang L, Lin R, et al: Electroacupuncture
protects against ischemic stroke by reducing autophagosome
formation and inhibiting autophagy through the mTORC1-ULK1
complex-Beclin1 pathway. Int J Mol Med. 37:309–318. 2016.
View Article : Google Scholar :
|
|
47
|
Khan I, Paul S, Jakhar R, Bhardwaj M, Han
J and Kang SC: Novel quercetin derivative TEF induces ER stress and
mitochondria-mediated apoptosis in human colon cancer HCT-116
cells. Biomed Pharmacother. 84:789–799. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ho Y, Li X, Jamison S, Harding HP,
McKinnon PJ, Ron D and Lin W: PERK activation promotes
medulloblastoma tumorigenesis by attenuating premalignant granule
cell precursor apoptosis. Am J Pathol. 186:1939–1951. 2016.
View Article : Google Scholar : PubMed/NCBI
|