Introduction
Burn injury disrupts the protective skin barrier by
inducing various reactions, including increased metabolism,
moisture loss and immune system malfunction, with the majority of
severely burned patients exhibiting dysfunction of the immune
system (1,2). The proliferation of keratinocytes,
vascular endothelial cells and dermal fibroblasts serves an
important role in wound healing (3,4).
In addition, the proliferation and growth of these cells is
regulated by numerous factors associated with immune system
malfunction, cell proliferation and cell apoptosis.
Long non-coding RNAs (lncRNAs) have been
demonstrated be essential for the immune response, cell
proliferation and the apoptosis of dermal fibroblasts (5–7).
Zhu et al (7) demonstrated
that the expression of lncRNA-activated by transforming growth
factor (TGF)-β and its target zinc finger protein 217 in keloid
fibroblasts promoted the autocrine secretion of TGF-β2, and thereby
promoted scar formation. In addition, the detection of the lncRNA
CACNA1G-AS1 in keloids indicated that lncRNAs are crucial for
keloid formation (8).
The lncRNA low expression in tumor (LET), a recently
identified lncRNA, is suppressed by enhancer of zeste homolog 2
(EZH2) (5). EZH2, the catalytic
subunit of polycomb repressive complex 2 (PRC2), is responsible for
epigenetic silencing through histone H3 lysine 27 trimethylation
(H3K27me3) (9). EZH2 and H3K27me3
are expressed in several human tumors and are positively associated
with high cancer cell proliferation rates and poor clinical
outcomes (9–11), suggesting that EZH2-mediated
H3K27me3 has oncogenic activity (10,12). Furthermore, the effect of EZH2 on
cell proliferation has been demonstrated in numerous studies, in
which the downregulation or suppression of EZH2 expression was
essential for the enhancement of cell apoptosis (13–16). In addition, the expression of EZH2
and H3K27me3 has been detected in inflammatory disorders, including
rheumatoid arthritis (17) and
colitis (18). Although the
elevated expression of EZH2 and downregulation of lncRNA LET have
been detected in invasive cancer tissues and cancer cells,
apoptosis and disease pathogenesis (19–21), studies focusing on the interaction
of EZH2 and lncRNA LET in the modulation of cell proliferation and
apoptosis are lacking.
The present study aimed to investigate the
interaction of EZH2 and lncRNA LET, and the mechanism underlying
its effect on in human dermal fibroblast (HDF) proliferation and
apoptosis. Tissue samples were collected from burn patients for
analysis. Isolated primary HDFs were transfected with LET
overexpression vectors to explore the effect of LET on cell
proliferation and cell apoptosis. The interactions of LET with
EZH2, and of EZH2 with H3K27me3 were determined in order to
elucidate the roles of lncRNA LET, EZH2 H3K27me3, and cell cycle-
and apoptosis-related proteins in the regulatory mechanism of HDFs.
This study may for the first time, to the best of our knowledge,
provide information on the interaction of EZH2 and lncRNA LET in
the modulation of cell proliferation and apoptosis, and its
potential application to burn wound-healing therapy.
Materials and methods
Tissue sample collection
A total of 33 samples were collected from 21 male
and 12 female patients (mean age, 31.4 years) at the Department of
Burns and Plastic Surgery of the First Affiliated Hospital of Henan
University of Science and Technology (Luoyang, China) between
January 10, 2014 and February 20, 2015. Patients with any of the
following exclusion criteria were not included in the present
study: Children under the age of 12 years, individuals older than
80 years, severe complications of burns, diagnosis with or history
of diabetes, long-term hormone intake, malignant tumors, and
receipt of chemoradiotherapy. All burns documented in this study
were second- and third-degree burns from patients admitted <24 h
after the time of burn injury (22). Patients were then classified into
three classes of burn injury: Slight (n=16), moderate (n=20) and
severe (n=7), according to the severity of the burn injury
(23). Eight normal control skin
tissues were collected from healthy volunteers who had undergone
plastic surgery procedures at the same department and hospital.
Informed consent from the 43 patients and approval from the Ethics
Committee of the College of Clinical Medicine of Henan University
were obtained prior to the study.
Primary HDF culture
Primary HDFs were derived from normal human skin
collected from patients who had undergone plastic surgery
procedures in the Department of Burns and Plastic Surgery, as
previously described (24).
Informed consent was obtained from the participants and approval
was provided by the Ethics Committee of the College of Clinical
Medicine of Henan University. In brief, the normal skin tissues
were dissected and cut into small pieces, washed with
phosphate-buffered saline, and then digested with 0.05%
trypsin-EDTA solution (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 30 min. Subsequently, isolated cells were cultured in
standard culture conditions comprising Dulbecco's modified Eagle's
medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
combined with penicillin-streptomycin (100 U; Invitrogen; Thermo
Fisher Scientific, Inc.) and 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2. In
addition, cell cultures were placed into a water heater tank and
incubated at 52°C for 30 sec to obtain heat-treated HDFs. The HDFs
were maintained under the standard culture conditions and used at
passages 5–8 throughout the experiments in the present study. 293
cells (CRL-1573; ATCC, Manassas, VA, USA) were cultured in DMEM
(10% FBS, penicillin-streptomycin) at 37°C with 5%
CO2.
Cell transfection
To observe the effects of lncRNA-LET overexpression
on HDF functions, an lncRNA-LET overexpression plasmid vector
(pc-LET; 50 nM) was constructed using cDNA with a functional
region. Pc-EZH2 and short interfering RNA (siRNA)-EZH2 plasmids (50
nM) were constructed for cell transfection. Plasmids were
transfected into HDFs using Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.). Cells (density, 2×105) were
then cultured for 24–48 h for use in the experiments. Cells without
any vector transfection were used as control. The primer for
silenced LET (si-LET) is as follows:
5′-TGGGAGTAAAGGGAAAGAGTT-3′.
Cell viability assay
Primary HDFs were seeded at a density of
1×104 cells/well into 96-well plates and incubated for
24–48 h for cell transfection. The viability of the transfected and
untransfected cells was determined using an MTT assay at 0, 12, 24
and 48 h after transfection (25). The optical density at 540 nm was
detected and used to plot a proliferation curve.
Clonogenic assay
A clonogenic assay of the primary HDFs was performed
using a method based on that described by Jiao et al
(26). Cells were maintained in
DMEM, transfected with pc-LET, seeded in 6-well plates at a final
density of 1,000 cells/well, and cultured for 14 days at 37°C. The
HDFs were then fixed with 4% paraformaldehyde at room temperatire
for 2 h, stained with Diff-Quick (Diff-Quick, Protocol HEMA 3
stain; Thermo Fisher Scientific, Inc.,), and air dried. Colonies
with ≥30 cells were counted using a microscope (IX83; Olympus
Corporation, Tokyo, Japan). All experiments were performed in
triplicate.
Cell cycle evaluation
Trypsin-digested primary HDFs were seeded into
6-well plates at a density of 5×104 cells/well and
incubated for 24 h. The HDFs were then harvested, centrifuged and
fixed with ethanol at 4°C for 24 h. The fixed HDFs were treated
with propidium iodide (PI; Clontech Laboratories, Inc., Mountain
View, CA, USA). Finally, the cell cycle was analyzed using a
FACSCanto flow cytometer and CellQuest software version 5.1 (both
BD Biosciences, San Jose, CA, USA). All detections were performed
in three triplicates. The proportion of proliferative cells was
calculated as follows: Proliferative proportion = (S + G1/M) cells
/ total cells stained with PI.
Cell apoptosis assay
HDFs (density, 1×105) seeded on 24-well
plates were maintained for 24 h. The cell cultures were
supplemented with Annexin V-fluorescein isothiocyanate (FITC) and
PI (Clontech Laboratories, Inc.) for 10 min in the dark. Finally,
the FACSCanto flow cytometer was used to analyze cell apoptosis.
Annexin V-positive and PI-negative cells were considered to be
apoptotic cells.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the burn tissues and HDF cells was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and purified with RNase-free Dnase I (Promega
Corporation, Madison, WI, USA). The purified RNA was then used for
cDNA synthesis. The expression of certain genes in the burn tissues
or HDF cells was detected using a SYBR ExScript qRT-PCR kit (Takara
Biotechnology Co., Ltd, Dalian, China) on an ABI 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Primer
sequences are listed in Table I.
The LET primers were synthesized by Shanghai Sangon Biological
Engineering Technology and Services Co., Ltd. (Shanghai, China). A
final 20-µl reaction mixture was amplified using the
following reaction conditions: Initial desaturation at 95°C for 5
min, followed by denaturation at 95°C for 30 sec, annealing at 60°C
for 40 sec and extension at 72°C for 10 sec for 40 cycles. GAPDH
was used as an internal control for mRNA or lncRNAs. All reactions
were run in triplicate. PCR products were resolved on 2% agarose,
visualized with ethidium bromide staining, and analyzed using a
FluorChem 8900 imager (ProteinSimple, San Jose, CA, USA). The
relative mRNA expression level was calculated by the
2−ΔΔCq method (27).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Primer sequences
(5′-3′) |
|---|
| LET | F:
5′-GGAGTAAAGGGAAAGAGTTGC-3′ |
| R:
5′-GTGTCGTGGACTGGCAAAAT-3′ |
| EZH2 | F:
5′-CAGCCTTGTGACAGTTCGT-3′ |
| R:
5′-AGATGGTGCCAGCAATAGA-3′ |
| Bax | F:
5′-TGCCCGAAACTTCTAAAA-3′ |
| R:
5′-CGTGACTGTCCAATGAGC-3′ |
| Bcl-2 | F:
5′-GCAGAAGTCTGGGAATCG-3′ |
| R:
5′-GCATAAGGCAACGATCC-3′ |
| Caspase-3 | F:
5′-ACCGATGTCGATGCAGCTAA-3′ |
| R:
5′-AGGTCCGTTCGTTCCAAAAA-3′ |
| Cyclin D1 | F:
5′-GCTGCTCCTGGTGAACAAGC-3′ |
| R:
5′-CACAGAGGGCAACGAAGGTC-3′ |
| CDK4 | F:
5′-TGCCAATTGCATCGTTCACCGAG-3′ |
| R: 5′-TGCCCA
ACTGGTCGGCTTCA-3′ |
| Cyclin A1 | F:
5′-CGCACAGAGACCCTGTACTT-3′ |
| R:
5′-TTGGAACGGTCAGATCAAAT-3′ |
| Cyclin E1 | F:
5′-GTTATAAGGGAGACGGGGAG-3′ |
| R:
5′-TGCTCTGCTTCTTACCGCTC-3′ |
| GAPDH | F:
5′-GGAGCGAGATCCCTCCAAAAT-3′ |
| R:
5′-GGCTGTTGTCATACTTCTCATGG-3′ |
Semi-quantitative RT-PCR
The expression of si-EZH2 and IgG were analyzed
using semi-quantitative RT-PCR. Total RNA was extracted with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and RT-PCR
was performed with a PCR kit from Fermentas (Hanover, MD, USA). The
primers used for amplification were shown in Table I. The PCR profile was listed as
follows: 94°C for 2 min followed by 35 cycles of 94°C for 30 sec,
58°C for 30 sec, and 72°C for 30 sec, with a final extension at
72°C for 10 min.
Western blot assay
Transfected primary HDFs were seeded into 6-well
plates at a density of 1.0×106 in DMEM and harvested at
48 h after transfection. Cells were then lysed using
radioimmunoprecipitation assay buffer (Beijing Solarbio Bioscience
and Technology Co., Ltd., Beijing, China) and protein was
quantified using a BCA protein assay kit (Thermo Fisher Scientific,
Inc.). Equal amounts of protein (20 µg) were separated using
10% SDS-PAGE gels and transferred onto polyvinylidene difluoride
(PVDF) membranes (Invitrogen; Thermo Fisher Scientific, Inc.). The
PVDF membranes were then blocked with 5% skimmed milk (BD
Biosciences) and incubated with primary antibody against cyclin D1
(dilution 1:1,000; ab134175), cyclin-dependent kinase 4 (CDK4;
dilution 1:1,000; ab199728), cyclin E1 (dilution 1:1,000; ab33911),
cyclin A1 (dilution 1:1,000; ab118897), B-cell lymphoma 2 (Bcl-2;
dilution 1:1,000; ab59348), Bcl-2-associated X protein (Bax;
dilution 1:2,000; ab53154), cleaved caspase-3 (dilution 1:1,000;
ab32042), EZH2 (dilution 1:500; ab186006) and GAPDH (dilution
1:2,000; ab181602) at 4°C overnight. The membranes were then
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:1,000 dilution; ab9482) for 1 h at room temperature.
All antibodies were purchased from Abcam (Cambridge, UK). The bands
of the target proteins were visualized and analyzed with Enhanced
Chemiluminescence (ECL) reagent (GE Healthcare Life Sciences) using
the Tanon-5200 Chemiluminescent Imaging system (Tanon Science and
Technology Co., Ltd., Shanghai, China).
Chromatin immunoprecipitation (ChIP)
assay
As described by Luo et al (16), a ChIP assay was performed using an
EZ-ChIP™ Chromatin Immunoprecipitation kit (EMD Millipore,
Billerica, MA, USA). Firstly, cross-linked chromatin was sonicated
into fragments in the size range 200–1,000 bp, and the fragments
were immunoprecipitated using H3K27me3 antibody (dilution 1:500;
SAB4800015; Sigma-Aldrich; Merck KGaA). Normal human immunoglobulin
G (IgG; dilution 1:500; ab7461; Abcam) was chosen as the negative
control. Semi-quantitative RT-PCR was conducted as described
above.
RNA immunoprecipitation (RIP) assay
An RIP assay was performed using a Magna RIP™
RNA-Binding Protein Immunoprecipitation kit (EMD Millipore) as
described by Luo et al (16). Anti-EZH2 antibody and IgG
(control) were used for RIP [anti-EZH2 (dilution 1:1,000; 4905),
anti-IgG (dilution 1:1,000; 147008); Cell Signaling Technology,
Inc., Danvers, MA, USA]. Coprecipitated RNAs were purified and cDNA
was synthesized for analysis by RT-qPCR. Total RNAs (input
controls) and isotype controls were detected simultaneously to
demonstrate that the detected signals were the results of RNAs
specifically binding to EZH2.
Statistical analysis
All data are expressed as mean ± standard deviation
from three triplicates. Data were analyzed using Graph Prism 6.0
software (GraphPad Prism, Inc., La Jolla, CA, USA). Student's
t-test and Tukey's test were used to analyze differences between
and among groups, respectively. Correlation between lncRNA LET and
EZH2 was analyzed using Pearson correlation coefficient. P<0.05
was considered to indicate a statistically significant result.
Results
Heat treatment inhibits the expression of
LET
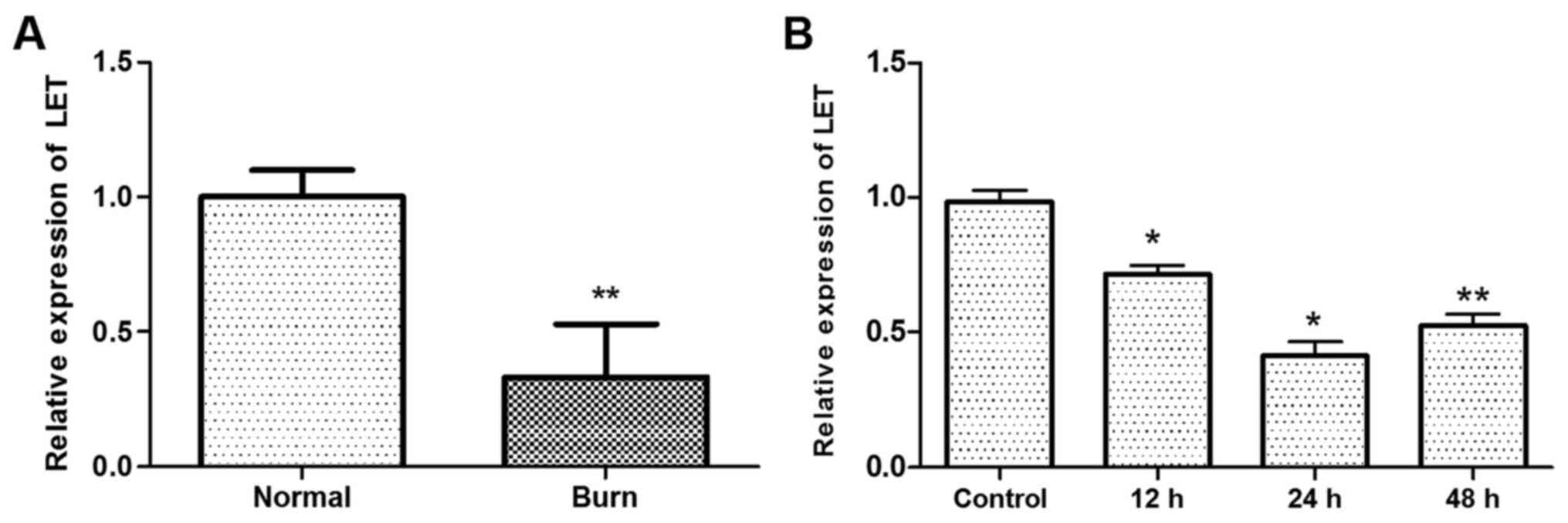
The expression of lncRNA LET was downregulated in
tissues from burn injury compared with normal HDFs (P<0.01;
Fig. 1A). In addition, the
expression of LET was lower in primary HDFs treated with heat
compared with the control (P<0.05 at 12 h and 24 h, P<0.01 at
48 h; Fig. 1B). The lowest
expression of LET in the HDFs was observed at 24 h following the
heat treatment. These results demonstrate that LET expression is
inhibited by heat.
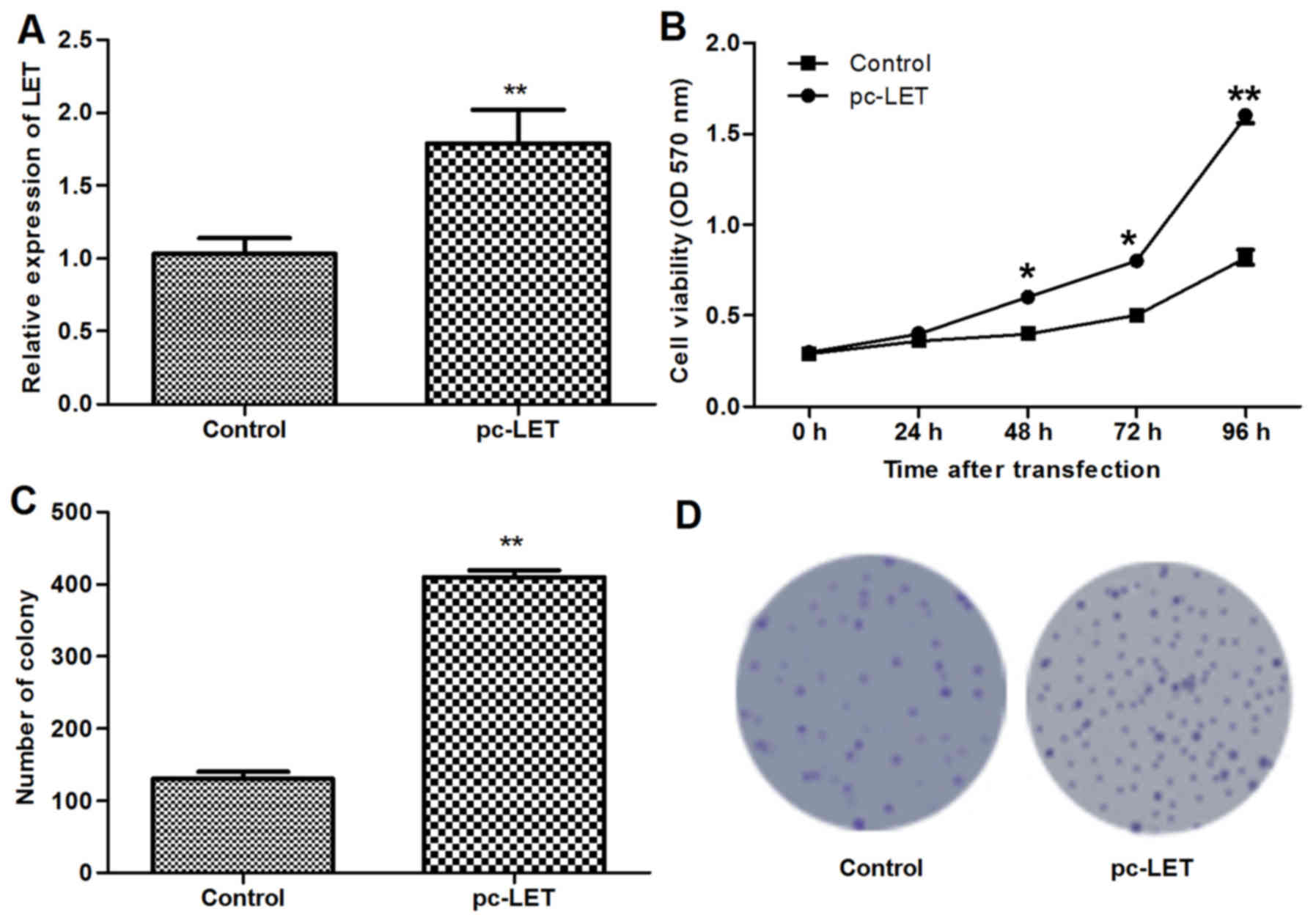
LET overexpression promotes cell
proliferation
The LET overexpression plasmid pc-LET was
constructed and transfected into heat-treated HDFs for 24 h.
RT-qPCR analysis demonstrated that LET mRNA was upregulated in the
transfected HDFs compared with the untransfected HDFs (P<0.01;
Fig. 2A). The MTT assay indicated
that the LET overexpression induced in the HDFs following
transfection with pc-LET significantly increased cell viability
compared with that of untransfected HDFs (P<0.01; Fig. 2B). In addition, the number of
colonies formed by the transfected HDFs was significantly increased
compared with the control (P<0.01; Fig. 2C and D). These results demonstrate
that pc-LET increased the proliferation of the HDFs.
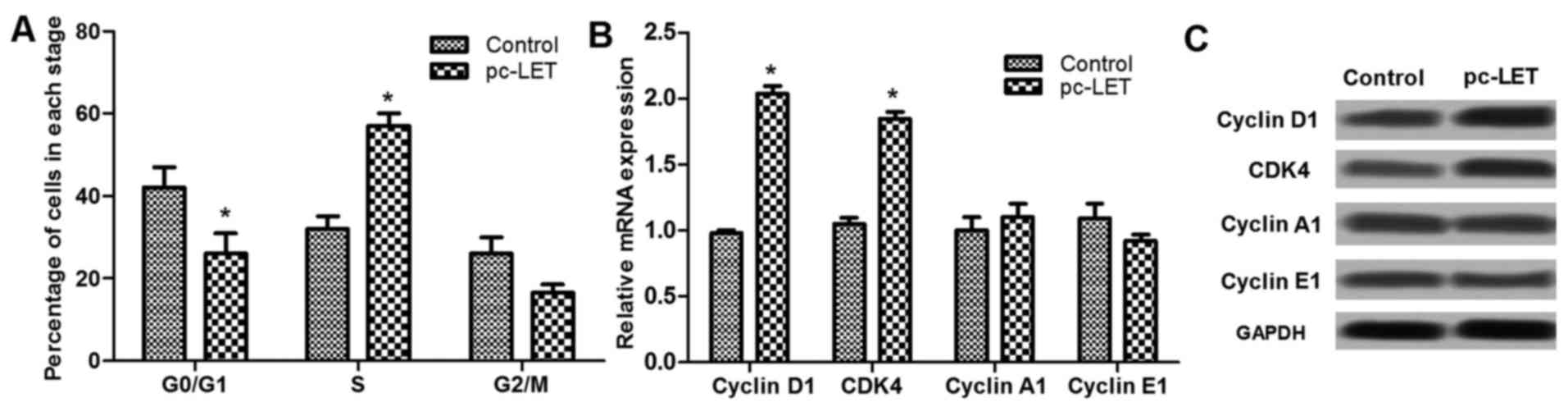
LET overexpression regulates the cell
cycle at the G1 stage
Analysis of the cell cycle using flow cytometry
revealed that pc-LET transfection significantly reduced the
percentage of cells in the G0/G1 stage, and upregulated the
percentage of cells in the S stage (P<0.05; Fig. 3A). Analysis of cell cycle-related
factors, including cyclin D1, CDK4, cyclin A1 and cyclin E1 by
RT-qPCR demonstrated that the expression of some of these factors
was dysregulated by pc-LET transfection. Cyclin D1 and CDK4 mRNA
levels were significantly increased by pc-LET transfection,
compared with those in the control HDFs (P<0.05; Fig. 3B). However, cyclin A1 and cyclin
E1 expression were not markedly regulated by pc-LET transfection.
In addition, the dysregulated expression levels of cyclin D1, CDK4,
cyclin A1 and E1 were shown in Fig.
3C. These results show that LET promotes HDF proliferation via
cell cycle arrest at the S stage through the upregulation of cyclin
D1 and CDK4.
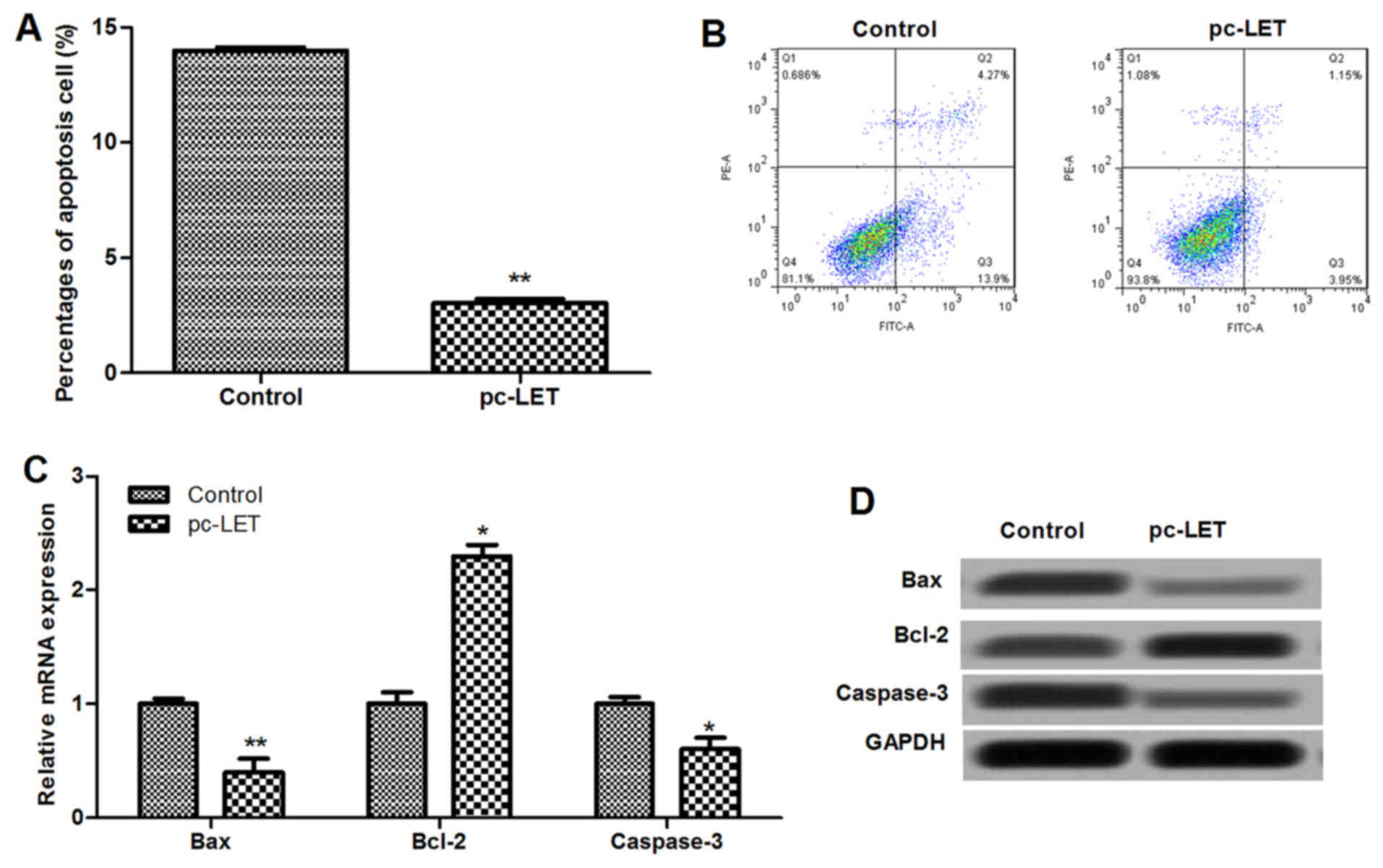
LET overexpression inhibits cell
apoptosis
Flow cytometry with Annexin V-FITC and PI dual
staining indicated that LET overexpression significantly inhibited
the apoptosis of HDFs (P<0.01; Fig. 4A and B). Results showed that the
mRNA expression of cell apoptosis-associated proteins, including
Bax, Bcl-2 and cleaved caspase-3, were dysregulated by pc-LET
transfection compared with those in the control (P<0.05;
Fig. 4C). The protein levels of
the cell apoptosis-related proteins were also analyzed using
western blotting (Fig. 4D). The
expression levels of Bax and cleaved caspase-3 in the pc-LET
transfected HDFs were significantly lower than those in the control
(P<0.05). However, the expression of Bcl-2 was upregulated in
the pc-LET transfected HDFs compared with the control (P<0.05).
These results indicate that pc-LET transfection inhibits cell
apoptosis through downregulation of the Bax/Bcl-2 ratio and
caspase-3.
Association of LET with EZH2 is involved
in cell growth and apoptosis
lncRNAs have been shown to serve crucial roles in
the proliferation of skin fibroblasts (28). EZH2 is indicated to be a
transcription factor of H3K27me3, and a combination of these two
factors is essential for the pathogenesis and development of
various diseases (28,29). To investigate the association of
EZH2 and H3K27me3 with LET in primary HDFs derived from burned
skin, RIP and ChIP analyses were conducted. The results
demonstrated that EZH2 was upregulated in heat-treated HDF cells at
12–48 h after heat exposure (Fig.
5A). The expression of EZH2 mRNA was significantly upregulated
by heat treatment, and the highest expression was observed at 24 h
post-treatment (P<0.01 vs. control). The RIP analysis revealed
that EZH2 was enriched in the HDFs by binding to LET (Fig. 5B). Furthermore, following
transfection with siRNA-EZH2 (Fig.
5C), ChIP analysis revealed an interaction between H3K27me3 and
EZH2 (Fig. 5D). Analysis of LET
and EZH2 expression in the HDFs (Fig.
5E) and burn tissues from patients (Fig. 5F) revealed that there was a
negative correlation between LET and EZH2 expression. These results
suggest that the elevated expression of EZH2 in burned HDFs
inhibits LET by binding to it, thus suppressing H3K27me3 expression
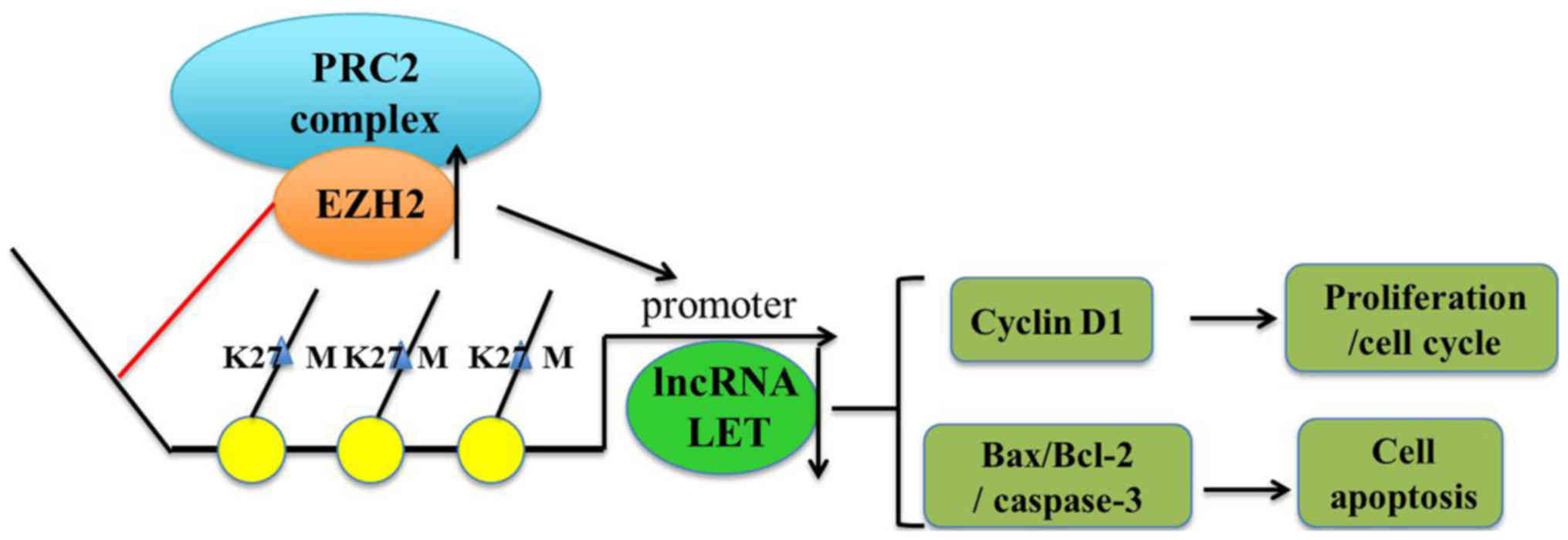
and modulating cell proliferation and apoptosis (Fig. 6).
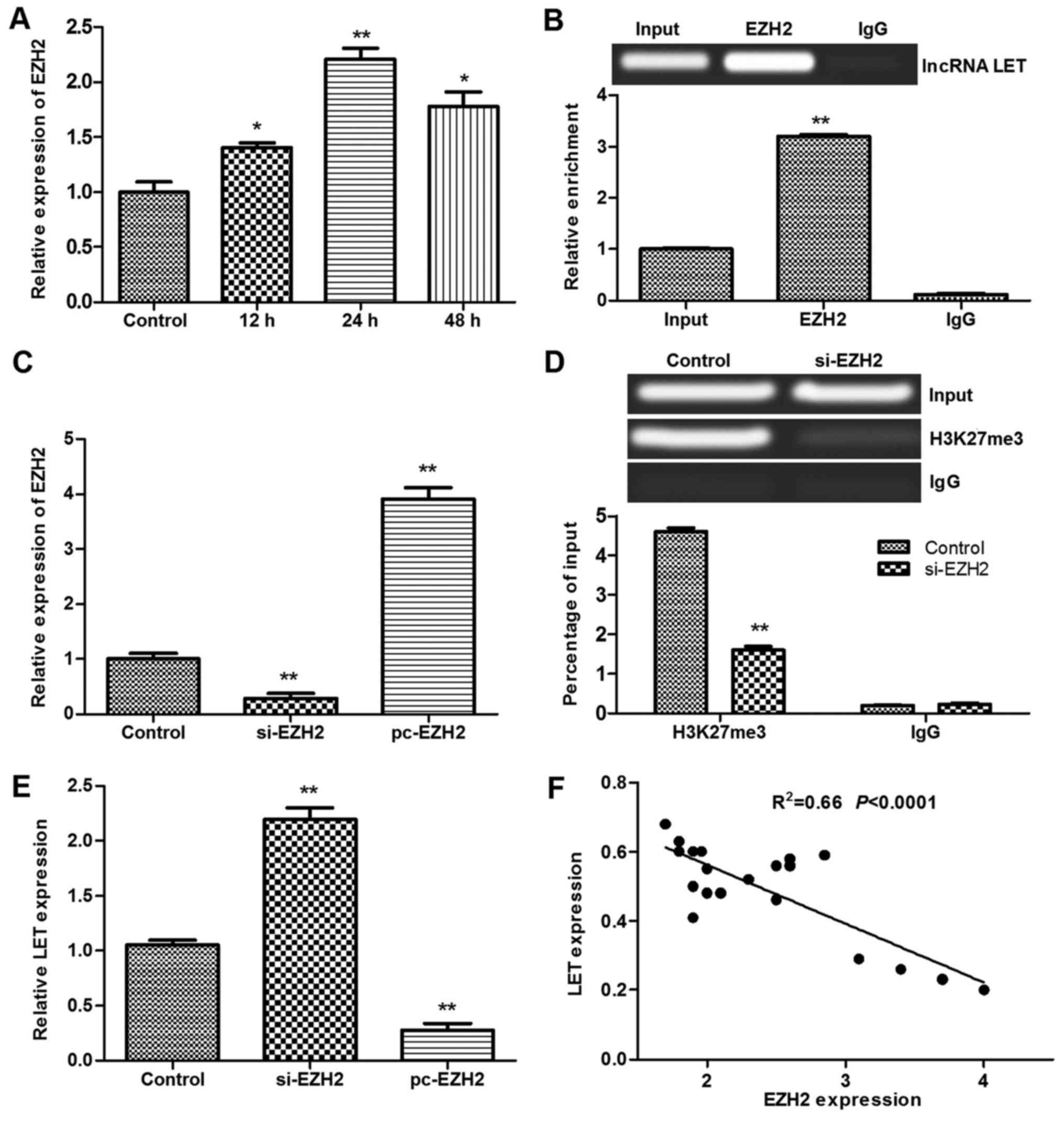 | Figure 5Results of RIP and ChIP assays for
primary HDFs. (A) Relative expression of EZH2 in heat-treated HDFs.
(B) RIP analysis was performed using antibodies against EZH2 and
IgG. (C) pc-EZH2 and si-EZH2 were transfected into HDFs. (D) ChIP
analysis was performed using antibodies against H3K27me3 and IgG.
(E) Relative expression of LET in HDFs transfected with pc-EZH2 or
si-EZH2. (F) Correlation of LET and EZH2 expression in burn tissues
from patients. *P<0.05 and **P<0.01 vs.
control or input, respectively. RIP, RNA immunoprecipitation; ChIP,
chromatin immunoprecipitation; HDF, human dermal fibroblast; EZH2,
enhancer of zeste homolog 2; IgG, immunoglobulin G; pc-EZH2, EZH2
overexpression plasmid; si-EZH2, EZH2, small interfering RNA; LET,
low expression in tumor; lncRNA, long non-coding RNA; H3K27me3,
histone H3 lysine 27 trimethylation. |
Discussion
Previous studies have shown that the interaction of
lncRNA with EZH2 is associated with cancer cell metastasis
(16,30), proliferation (14,31) and apoptosis (13,14) by target modulation, implicating
the role of EZH2 and lncRNA in cell proliferation. The present
study aimed to investigate the interaction of EZH2 and lncRNA LET,
and its involvement in the mechanism of HDF proliferation and
apoptosis. The results demonstrated that there was a negative
correlation between EZH2 and lncRNA LET expression in burned skin
tissues and HDFs subjected to heat treatment. Transfection with
pc-LET promoted HDF proliferation, arrested the cell cycle at the S
stage, and inhibited cell apoptosis via modulation of the cell
cycle and apoptosis-associated proteins.
EZH2, the catalytic subunit of PRC2, is a core
epigenetic regulator that has a crucial role in cell proliferation,
cell fate decisions, cancer initiation and the response of cells to
inflammation (9,32). EZH2 and H3K27me3 expression has
been observed to be positively associated with high cancer cell
proliferation rates and poor clinical outcomes (9–11),
which indicates that the EZH2-mediation of H3K27me3 is oncogenic
(10,12). In addition, the high expression of
EZH2 and H3K27me3 has been detected in synovial fibroblasts in
rheumatoid arthritis and fibroblasts associated with renal
fibrogenesis (17), in accordance
with studies indicating that EZH2-mediated H3K27me3 increase is
involved in the inflammatory response (32–34). DuPage et al demonstrated a
critical role for EZH2 in the maintenance of regulatory T (Treg)
cell identity during cellular activation, with EZH2-deficient Treg
cells being destabilized and lacking the ability to prevent
autoimmunity (32). In the
present study, the expression of EZH2 in primary HDFs was increased
following heat treatment compared with that in the control.
Furthermore, the positive association of H3K27me3 expression with
EZH2 was revealed by ChIP analysis. These results indicate that the
upregulation of EZH2 and EZH2-mediated H3K27me3 serve major roles
in the fate decision of HDF cells from burned skin, which may
benefit autoimmunity and the cell response to inflammation.
In the present study, analysis of the association
between lncRNA LET downregulation and EZH2 overexpression in burned
human skin tissues revealed a negative correlation. In addition, in
lncRNA-LET gain-of-function experiments in which pc-LET
overexpression plasmids were transfected into HDFs, increased cell
viability and clone formation, and a reduced proportion of
apoptotic cells were observed. These results reveal that the
expression of lncRNA LET in primary HDF cells promoted cell
proliferation and inhibited cell apoptosis. This was in accordance
with the negative association of lncRNA LET and EZH2 expression in
hepatocellular carcinoma observed by Sun et al (5). Sun et al (5) reported that the loss of expression
of lncRNA-LET in nasopharyngeal carcinoma (NPC) tissues was
negatively associated with risk factors and clinical features
including advanced clinical stage and poor patient survival, and
EZH2 expression. Furthermore, lncRNA-LET gain- and loss-of-function
experiments conducted in that study demonstrated that LET
overexpression inhibited EZH2 expression and NPC cell
proliferation, and promoted NPC cell apoptosis. By contrast, the
silencing of LET using siRNA promoted cell proliferation and
inhibited cell apoptosis. An association of low lncRNA-LET with
poor prognosis was also reported in gastric cancer by Zhou et
al (21). Together, these
results demonstrate that the decreased expression of lncRNA LET is
a poor prognosis factor for tumors and burn-injured patients.
Furthermore, the upregulation of lncRNA LET may be regarded as a
therapeutic intervention option for cancer and burns.
Since lncRNA LET expression contributes to the
proliferation and apoptosis of cancer cells and human primary HDFs,
the expression of cell cycle- and apoptosis-related proteins in
pc-LET transfected cells was investigated in the present study. The
cell cycle and apoptosis are complicated processes involving
numerous genes and signaling pathways (35–38). Bcl-2, Bax and caspase-3 are
important factors for cell apoptosis, and an increase in the
Bax/Bcl-2 ratio may promote cell apoptosis (35,36,39,40). Furthermore, cyclin D1 and CDK4/6
are essential for maintaining cell cycle progression, and
inhibition of the cyclin D1-CDK4/6 pathway may arrest the cell
cycle with cell accumulation at the G0/G1 stage (41,42). The observation that enhanced
lncRNA LET expression significantly upregulated the expression of
cyclin D1 and CDK4, altered the progression of cells from the G0/G1
stage to the S stage, and inhibited cyclin E1, an oncogene
(43), indicates that lncRNA LET
modulates the cell cycle via the cyclin D1-CDK4 signaling
pathway.
The observation that increased lncRNA LET expression
significantly inhibited cell apoptosis and downregulated the
Bax/Bcl-2 ratio and cleaved caspase-3 expression indicate that
lncRNA LET inhibits cell apoptosis through the Bax/Bcl-2/caspase-3
signaling pathway. Thus, the present study demonstrates that lncRNA
LET inhibits cell apoptosis and promotes cell proliferation via
Bax/Bcl-2/caspase-3 and cyclin D1-CDK4 signaling pathways,
respectively, in HDFs.
In conclusion, the results of the present study
indicate that in burns, the reduced expression of lncRNA LET and
upregulation of EZH2 promote cell apoptosis and inhibit the
proliferation of HDFs. Furthermore, a negative correlation between
lncRNA LET and EZH2 expression was detected in burn-injured
tissues. RIP and ChIP analysis confirmed the interaction of EZH2
with lncRNA LET, and EZH2 with H3K27me3. The LET gain-of-function
experiment using primary HDFs suggests that increased lncRNA-LET
expression promoted cell proliferation and inhibited cell apoptosis
via the cyclin D1-CDK4 and Bax/Bcl-2/caspase-3 signaling pathways,
respectively. From these observations, it may be speculated that
the upregulation of lncRNA LET is a potential therapeutic
intervention for burn wound healing. However, the mechanism of
action of lncRNA LET in cell apoptosis and proliferation, as well
as the direct target proteins of lncRNA LET, require further
exploration.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Patil NK, Bohannon JK, Luan L, Guo Y,
Fensterheim B, Hernandez A, Wang J and Sherwood ER: FLT3 ligand
treatment attenuates T cell dysfunction and improves survival in a
murine model of burn wound sepsis. Shock. 47:40–51. 2017.
View Article : Google Scholar
|
|
2
|
Xiu F and Jeschke MG: Perturbed
mononuclear phagocyte system in severely burned and septic
patients. Shock. 40:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chigurupati S, Mughal MR, Okun E, Das S,
Kumar A, McCaffery M, Seal S and Mattson MP: Effects of cerium
oxide nanoparticles on the growth of keratinocytes, fibroblasts and
vascular endothelial cells in cutaneous wound healing.
Biomaterials. 34:2194–2201. 2013. View Article : Google Scholar :
|
|
4
|
Nauta A, Seidel C, Deveza L, Montoro D,
Grova M, Ko SH, Hyun J, Gurtner GC, Longaker MT and Yang F:
Adipose-derived stromal cells overexpressing vascular endothelial
growth factor accelerate mouse excisional wound healing. Mol Ther.
21:445–455. 2013. View Article : Google Scholar :
|
|
5
|
Sun Q, Liu H, Li L, Zhang S, Liu K, Liu Y
and Yang C: Long noncoding RNA-LET, which is repressed by EZH2,
inhibits cell proliferation and induces apoptosis of nasopharyngeal
carcinoma cell. Med Oncol. 32:2262015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Jinnin M, Nakamura K, Harada M,
Kudo H, Nakayama W, Inoue K, Nakashima T, Honda N, Fukushima S, et
al: Long non-coding RNA TSIX is upregulated in scleroderma dermal
fibroblasts and controls collagen mRNA stabilization. Exp Dermatol.
25:131–136. 2016. View Article : Google Scholar
|
|
7
|
Zhu HY, Bai WD, Li C, Zheng Z, Guan H, Liu
JQ, Yang XK, Han SC, Gao JX, Wang HT, et al: Knockdown of
lncRNA-ATB suppresses autocrine secretion of TGF-β2 by targeting
ZNF217 via miR-200c in keloid fibroblasts. Sci Rep. 6:247282016.
View Article : Google Scholar
|
|
8
|
Liang X, Ma L and Longxand Wang X: lncRNA
expression profiles and validation in keloid and normal skin
tissue. Int J Oncol. 47:1829–1838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schult D, Hölsken A, Siegel S, Buchfelder
M, Fahlbusch R, Kreitschmann-Andermahr I and Buslei R: EZH2 is
highly expressed in pituitary adenomas and associated with
proliferation. Sci Rep. 5:169652015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu F, Gu L, Cao Y, Fan X, Zhang F and
Sang M: Aberrant overexpression of EZH2 and H3K27me3 serves as poor
prognostic biomarker for esophageal squamous cell carcinoma
patients. Biomarkers. 50:1–11. 2015.
|
|
11
|
Bae WK, Yoo KH, Lee JS, Kim Y, Chung IJ,
Park MH, Yoon JH, Furth PA and Hennighausen L: The
methyltransferase EZH2 is not required for mammary cancer
development, although high EZH2 and low H3K27me3 correlate with
poor prognosis of ER-positive breast cancers. Mol Carcinog.
54:1172–1180. 2015. View
Article : Google Scholar :
|
|
12
|
Li CP, Cai MY, Jiang LJ, Mai SJ, Chen JW,
Wang FW, Liao YJ, Chen WH, Jin XH, Pei XQ, et al: CLDN14 is
epigenetically silenced by EZH2-mediated H3K27ME3 and is a novel
prognostic biomarker in hepatocellular carcinoma. Carcinogenesis.
37:557–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Appelmann I, Scuoppo C, Thapar V, Ledezma
D, Lujambio A, Lowe SW and Chicas A: Suppression of EZH2
accelerates MYC-driven lymphomagenesis by inhibition of apoptosis.
Blood. 124:30092014.
|
|
14
|
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie
Y, Li S, Tang G, Tang H and He X: MicroRNA-124 inhibits
proliferation and induces apoptosis by directly repressing EZH2 in
gastric cancer. Mol Cell Biochem. 392:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu L and Xu PC: Downregulated lncRNA-ANCR
promotes osteoblast differentiation by targeting EZH2 and
regulating Runx2 expression. Biochem Biophys Res Commun.
432:612–617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trenkmann M, Brock M, Bertoncelj MF, Gay
RE, Michel BA, Huber LC and Gay S: THU0115 epigenetic repression of
the long noncoding Rna hotair regulates NF-κB signalling and the
expression of matrix metalloproteases in synovial fibroblasts. Ann
Rheum Dis. 72(Suppl 3)2013. View Article : Google Scholar
|
|
18
|
Sarmento O1, Xiong Y, Sun Z, Svingen P,
Bamidele A, Smyrk T, Nair A, Baheti S, McGovern D, Friton J, et al:
O-015 YI Alterations in the FOXP3 EZH2 pathway associates with
increased susceptibility to colitis in both mice and human. Inflamm
Bowel Dis. 22(Suppl 1): S5–S6. 2016. View Article : Google Scholar
|
|
19
|
Varambally S, Cao Q, Mani RS, Shankar S,
Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, et al:
Genomic loss of microRNA-101 leads to overexpression of histone
methyltransferase EZH2 in cancer. Science. 322:1695–1699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coe BP, Thu KL, Aviel-Ronen S, Vucic EA,
Gazdar AF, Lam S, Tsao MS and Lam WL: Genomic deregulation of the
E2F/Rb pathway leads to activation of the oncogene EZH2 in small
cell lung cancer. PLoS One. 8:e716702013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou B, Jing XY, Wu JQ, Xi HF and Lu GJ:
Down-regulation of long non-coding RNA LET is associated with poor
prognosis in gastric cancer. Int J Clin Exp Pathol. 7:8893–8898.
2014.
|
|
22
|
Kalantar Motamedi MH, Heydari M, Heydari M
and Ebrahimi A: Prevalence and pattern of facial burns: A 5-year
assessment of 808 patients. J Oral Maxillofac Surg. 73:676–682.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cassidy JT, Phillips M, Fatovich D, Duke
J, Edgar D and Wood F: Developing a burn injury severity score
(BISS): Adding age and total body surface area burned to the injury
severity score (ISS) improves mortality concordance. Burns.
40:805–813. 2014. View Article : Google Scholar
|
|
24
|
Jiang T, Wang X, Wu W, Zhang F and Wu S:
Let-7c miRNA Inhibits the proliferation and migration of
heat-denatured dermal fibroblasts through down-regulating HSP70.
Mol Cells. 39:345–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boncler M, Różalski M, Krajewska U,
Podsędek A and Watala C: Comparison of PrestoBlue and MTT assays of
cellular viability in the assessment of anti-proliferative effects
of plant extracts on human endothelial cells. J Pharmacol Toxicol
Methods. 69:9–16. 2014. View Article : Google Scholar
|
|
26
|
Jiao P, Zhou YS, Yang JX, Zhao YL, Liu QQ,
Yuan C and Wang FZ: MK-2206 induces cell cycle arrest and apoptosis
in HepG2 cells and sensitizes TRAIL-mediated cell death. Mol Cell
Biochem. 382:217–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real- time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Yang KY and Chen DL: Shikonin inhibits
inflammatory response in rheumatoid arthritis synovial fibroblasts
via lncRNA-NR024118. Evid Based Complement Alternat Med.
2015:6317372015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chinaranagari S, Sharma P and Chaudhary J:
EZH2 dependent H3K27me3 is involved in epigenetic silencing of ID4
in prostate cancer. Oncotarget. 5:7172–7182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Au SL, Wong CC, Lee JM, Wong CM and Ng IO:
EZH2-Mediated H3K27me3 is involved in epigenetic repression of
deleted in liver cancer 1 in human cancers. PLoS One. 8:e68226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan Qi W, Teng H, Li L, Chuai L, Zhang S,
Zeng R, Li J, Fan M, Lin HY, et al: Selective inhibition of Ezh2 by
a small molecule inhibitor blocks tumor cells proliferation. Proc
Natl Acad Sci USA. 109:21360–21365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
DuPage M, Chopra G, Quiros J, Rosenthal
WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A and Bluestone
JA: The chromatin-modifying enzyme Ezh2 is critical for the
maintenance of regulatory T cell identity after activation.
Immunity. 42:227–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hui T, A P, Zhao Y, Wang C, Gao B, Zhang
P, Wang J, Zhou X and Ye L: EZH2, a potential regulator of dental
pulp inflammation and regeneration. J Endod. 40:1132–1138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dreger H, Ludwig A, Weller A, Baumann G,
Stangl V and Stangl K: Epigenetic suppression of iNOS expression in
human endothelial cells: A potential role of Ezh2-mediated
H3K27me3. Genomics. 107:145–149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu G, Wang T, Wang T, Song J and Zhou Z:
Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on
cerebral ischemia rats. Biomed Rep. 1:861–867. 2013. View Article : Google Scholar
|
|
36
|
Liang K, Ye Y, Wang Y, Zhang J and Li C:
Formononetin mediates neuroprotection against cerebral
ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2
ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci.
344:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Liu S, Tang Y, Liu Q and Yao Y:
MPT64 protein from Mycobacterium tuberculosis inhibits apoptosis of
macrophages through NF-kB-miRNA21-Bcl-2 pathway. PLoS One.
9:e100949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu M, Chen X, Han Y, Ma C, Ma L and Li S:
Clusterin silencing sensitizes pancreatic cancer MIA-PaCa-2 cells
to gmcitabine via regulation of NF-kB/Bcl-2 signaling. Int J Clin
Exp Med. 8:12476–12486. 2015.PubMed/NCBI
|
|
39
|
Yap JL, Cao X, Vanommeslaeghe K, Jung K-Y,
Peddaboina C, Wilder PT, Nan A, MacKerell AD Jr, Smythe WR and
Fletcher S: Relaxation of the rigid backbone of an
oligoamide-foldamer-based α-helix mimetic: Identification of potent
Bcl-xL inhibitors. Org Biomol Chem. 10:2928–2933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yue J, Ben Messaoud N and López JM:
Hyperosmotic shock engages two positive feedback loops through
caspase-3-dependent proteolysis of JNK1-2 and Bid. J Biol Chem.
290:30375–30389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abraham RT, Vanarsdale T, Shields DV, Lee
V, Koehler M and Arndt K: Abstract SY34-03: Braking the cycle:
Inhibition of the cyclin D-Cdk4/6 pathway in breast cancer. Cancer
Res. 74(19 Supplement): 772–779. 2014. View Article : Google Scholar
|
|
42
|
Rader J, Russell MR, Hart LS, Nakazawa MS,
Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin
SJ, et al: Dual CDK4/CDK6 inhibition induces cell-cycle arrest and
senescence in neuroblastoma. Clin Cancer Res. 19:6173–6182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pils D, Bachmayr-Heyda A, Auer K, Svoboda
M, Auner V, Hager G, Obermayr E, Reiner A, Reinthaller A, Speiser
P, et al: Cyclin E1 (CCNE1) as independent positive prognostic
factor in advanced stage serous ovarian cancer patients - a study
of the OVCAD consortium. Eur J Cancer. 50:99–110. 2014. View Article : Google Scholar
|