Introduction
Osteoarthritis (OA) is the most prevalent
degenerative disease in joints characterized by progressive loss of
articular cartilage and a major cause of disability. OA has been
estimated to impact 5% of adults world wide (1). The number of sufferers is believed
to being increasing within the aging population with longer working
lives (2). Despite its high
prevalence, the underlying molecular mechanisms are still not fully
understood. OA is a multifactorial disease caused by both
environmental factors and genetic predisposition. It was previously
reported that the influence of genetic factors on OA ranged from
39–65% (3). It has been reported
that multiple genes are involved in OA (4,5),
however, few results have been firmly replicated across multiple
populations. Accumulating evidence demonstrates that multiple genes
contribute to OA susceptibility, such as asporin (ASPN), bone
morphogenetic protein 5 (BMP5) and growth and differentiation
factor 5 (GDF5) (6).
The functional integrity of the joint is maintained
by a delicate balance between degradation and synthesis of the
cartilage extracellular matrix (ECM) through mechanisms controlled
by chondrocytes (7). Anabolic and
catabolic factors, including dysregulation of the main cartilage
ECM degrading enzymes matrix metalloproteinase-13 (MMP-13) and
aggrecanases, have crucial roles in the molecular mechanisms
involved (8). Studies revealed
that GDF5, a member of the BMP family and the transforming growth
factor-β super-family, reduced MMP-13 expression in human
chondrocytes and acted extracellularly to promote the development,
maintenance and repair of synovial joint tissues, particularly bone
and cartilage (9,10). In addition, a causative
single-nucleotide polymorphism (SNP) rs143383 located in the
5′-untraslated region (5'-UTR) of GDF5 was demonstrated to affect
transcription factor binding, and consequently inactivated GDF5
expression (11). This research
indicated that mutant GDF5 gene could be exploited to overcome the
OA genetic deficit.
Stem cell-based tissue engineering for cartilage
regeneration is a promising alternative pathway in the regeneration
of damaged or diseased articular cartilage tissues or both.
However, the regeneration of articular cartilage tissues remains a
significant clinical challenge. Current medical treatments are
effective at reducing pain but ineffective at reversing the course
of musculoskeletal degeneration often caused by the low cellularity
and vascularity in these tissues. Cartilage tissue engineering
using human mesenchymal stem cells (hMSCs) has demonstrated the
potential to enhance cartilage healing (12).
Several novel genome-editing technologies have
emerged from which the four most studied methods are zinc-finger
nucleases (ZFNs) (13),
transcription activator-like effector nucleases (TALENs) (14), clustered regularly interspaced
short palindromic repeat (CRISPR) (15) and NgAgo (16). Each technique has demonstrated
highly efficient and locus-specific genome editing in numerous
species. Furthermore, these genome-editing tools have been
successfully applied in the correction of genetic mutation causing
hereditary tyrosinemia (17).
In this study, MSCs were obtained from a patient
with OA carrying a homozygous T allele of polymorphism rs143383 in
the GDF5 gene. The mutated locus was genetically corrected
using a pair of TALENs and a short single stranded DNA (ssDNA) in
cells in vitro. Following differentiation, chondrocytes
derived from the modified MSC colony exhibited normal cell
morphology and viability. Enzymes responsible for ECM metabolism
and the expression of associated genes were returned to normal
levels, suggesting the functional recovery of these corrected
chondrocytes.
Materials and methods
Isolation and culture of adipose
tissue-derived MSCs
The Ethics Committee of Southern Medical University
(Guangzhou, China) approved this study and written informed
consents were obtained from the patient/volunteer. Adipose tissue
was obtained from discharged fat tissue from a male 23-year-old
volunteer with OA carrying a homozygous T allele of polymorphism
rs143383 in the GDF5 gene confirmed by Sanger sequencing.
Additionally, adipose tissue from a 27-year-old healthy volunteer
was collected as the control and his genotype was confirmed by
sequencing as well. The methods for isolating and culturing stromal
stem cells from human adipose tissue was performed according to
previously described procedures with minor modifications (18). Briefly, the tissue was digested
with collagenase A type I (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C for 6 h. Cells were collected and then
plated at a density of ~1×106 cells/dish in 5 ml of
low-glucose Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), and
maintained at 37°C in a maximum humidity atmosphere containing 5%
CO2.
Design and construction of TALENs
vector
Candidate TALENs were identified using the online
tool TAL Effector Nucleotide Targeter 2.0 (https://tale-nt.cac.cornell.edu/node/add/talen)
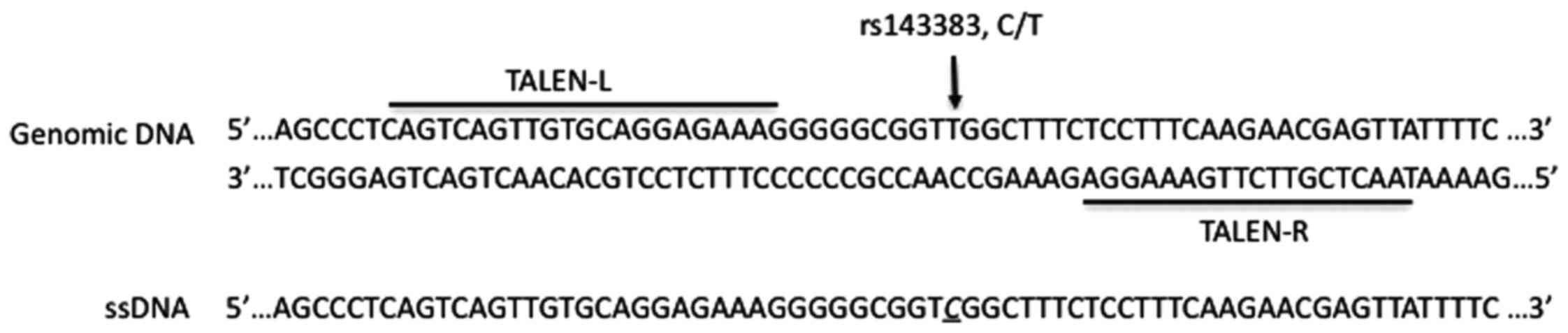
(19). Construction of a TALEN
pair targeting rs143383 is shown in Fig. 1. RVD modules were assembled into
the pTAL3 vector (Addgene, Cambridge, MA,USA) using the Golden Gate
method (20), and were
subsequently cloned into the modified backbone-vector pTAL-3.1
(Addgene), in which a cassette encoding a 152aa TALEN-terminal and
a 63aa C-terminal segment fused to an enhanced FokI nuclease
flanking either side of the insert (21). Additionally, a ssDNA of 160 nt in
length spanning the target site was chemically synthesized, in
which a C was incorporated at rs143383.
Electrotransfection of MSCs
MSCs were trypsinized, centrifuged at 300 × g for 10
min at room temperature, resuspended in modified minimal essential
medium (MEM; ref. 11380037; Invitrogen; Thermo Fisher Scientific,
Inc.). Electrotransfection was performed according to the previous
description with some modifications (22). Briefly, ~1×106 cells in
50 µl S-MEM containing 10 µg TALEN mRNA and 100 ng
ssDNA were then transferred to a 4 mm electroporation cuvette, and
electroporated using Gene Pulser Xcell electroporation system (both
from Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
electroporation was performed by applying a pulse magnitude ranging
from 80–120 V at a 1 Hz repetition frequency. After
electrotransfection, the cells were incubated at room temperature
for 5 min and transferred to the tissue culture dishes with 10 ml
culture medium.
Mutation identification of the cell
colonies
Seven to ten days after electroporation DNA was
extracted from the colonies by the phenol-chloroform method.
Polymerase chain reaction (PCR) was conducted on the extracted DNA
using rTaq polymerase (Takara Biotechnology Co., Ltd., Dalian,
China) and primers spanning the target site, generating a 443 bp
amplicon (GDF5-forward, 5′-TGT GTG TGT GTG TGT GAA GTA-3′ and
GDF-reverse, 5′-TCA GCG GCT GGC CAG AGG-3′; Table I) under the following thermal
conditions: 5 min at 95°C followed by 35 cycles of 30 sec at 95°C,
30 sec at 95°C, 30 sec at 72°C, and a final extension of 3 min at
72°C, generating a 443-bp amplicon. Genotyping was executed by T7
endonuclease I (T7EI) assay as described by Sakurai et al
(23) with minor modifications
(23). Initially, 10 µl
PCR product from the tested colonies was mixed with 10 µl
PCR product from the healthy control. Subsequently, a mix
containing 10 µl PCR product and 2 µl of 10X NEB
buffer 2 (New England BioLabs, Inc., Ipswich, MA, USA) was
incubated at 95°C for 10 min and then incubated at room temperature
for ~30 min. Following the annealing reaction, 0.5 µl T7EI
(2.5 U/µl; New England BioLabs, Inc.) was added to the
sample and the mixture was incubated at 37°C for 1 h. Finally, the
digested product was electrophoresed on a 2.0% agarose gel.
Colonies producing cleaved bands at ~192 and 251 bp were subjected
to Sanger sequencing to confirm the editing.
 | Table IPrimers sequences. |
Table I
Primers sequences.
| Gene name | Forward
(5′-3′) | Reverse
(5′-3′) | Tm (°C) | Product (bp) |
|---|
| GDF5 |
TGTGTGTGTGTGTGTGAAGTA |
TCAGCGGCTGGCCAGAGG | 60 | 443 |
| Off-target 1 |
GCAAGTACTTACTCCAGA |
CTACCAGCCTGCCAGCAGT | 60 | 613 |
| Off-target 2 |
TCCTCACGTGTTCATTTCCTCA |
CCCATCGGTACCTATTAGGA | 59 | 421 |
| CD73 |
CGCAACAATGGCACAATTAC |
CAGGTTTTCGGGAAAGATC | 60 | 196 |
| COL2A1 |
GGGAGTAATGCAAGGACCAA |
ATCATCACCAGGCTTTCCAG | 62 | 175 |
| ACAN |
GACATCAGGGTGGCGACTCT |
GGGTTGAGGTATCAGAGGT | 60 | 152 |
| SOX9 |
CTGACCCAGTACCCCTTTGA |
CAGCTGGACTGGTTGTCTCA | 62 | 213 |
| MMP-13 |
GCACTGAAGCCAGGTCT |
GGGCCTTTTCTCCAGGTAAC | 60 | 177 |
| TIMP1 |
CCTGCAAGACTATCGACATGGA |
CCTCAGCAGACGCAGCTCTG | 60 | 283 |
| IL-1 |
CCCAGTGAAGATGCAGGT |
CAGCCTGAGAGGGTCTTG | 60 | 183 |
| TNF-α |
TCTTGGGACTGATGCTGGTG |
CATTTCCACGATTTCCCAGA | 58 | 155 |
| TRAIL |
ATCCACGAAACTACCTTCAA |
TCTTGATCTTCATTGTGCTG | 60 | 166 |
| FZD5 |
TGGGGGTACTGTGGAAATGC |
CCTTCCATTGCCCACTCTGT | 60 | 162 |
| DKK1 |
AACTTTGCTTCCCAGATGTCC |
GCCTCGGTGTCCCTTCATT | 58 | 233 |
| CTNNB1 |
CCAGGACGGTCATTTACGAG |
CGATGGTCTGGGTTCAGGTT | 60 | 217 |
| GAPDH |
GAGTCAACGGATTTGGTCGT |
GACAAGCTTCCCGTTCTCAG | 60 | 185 |
MSC differentiation into
chondrocytes
Chondrogenic differentiation of MSCs was induced
using MSC chondrogenic differentiation medium (PromoCell GmbH,
Heidelberg, Germany) according to manufacturer's instructions. MSCs
(1×105 per 24-well) were plated and incubated for 2 h in
MSC growth medium. The medium was changed to MSC chondrogenic
differentiation medium (PromoCell) to induce chondrogenesis. The
medium was changed every third day and chondrocytes were induced
(24). Three chondrocyte lines
were obtained: GDF5con/con, chondrocytes from the
healthy subject with wildtype GDF5 gene; GDF5mut/mut,
chondrocytes from the patient with OA with mutant GDF5 gene;
GDF5cor/cor, chondrocytes from the patient with OA with
genetically corrected GDF5 gene.
Cell proliferation assay
The methyl thiazolyl tetrazolium (MTT;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) colorimetric assay
was used to detect cell proliferation ability. Formazan was
dissolved in dimethyl sulphoxide (DMSO) in the MTT assay. The cells
were seeded in 96-well plates at a density of 1×103
cells/well. The cells were trypsinized after 1–12 days of seeding,
and suspended in PBS. Cell number was counted every two days for 12
days by measuring the absorbance at 490 nm. The assay was performed
in triplicate.
Assessment of apoptosis
Chondrocytes were stained with an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(R&D Systems, Inc., Minneapolis, MN, USA) to determine whether
cells are undergoing apoptosis. Propidium iodide (PI) staining was
used as a control to differentiate cells undergoing necrosis.
Chondrocytes were seeded in tissue culture slides and allowed to
attach for 24 h. Then the cells were resuspended in 500 µl
binding buffer, 5 µl Annexin V-FITC and 5 µl PI were
added and incubated for 5 min at 37°C in the dark. Flow cytometry
analysis was performed to evaluate the apoptosis (25). Chondrocytes were washed with PBS
and stained with Annexin V-FITC and PI according to the
manufacturer's instruction. Data was processed with FlowJo software
version 7.6.1.
Detection of MMPs in culture medium
An aliquot of 50 µl medium supernatant was
collected and human MMP-1, MMP-13 and TIMP metallopeptidase
inhibitor 1 (TIMP1) ELISA kits (cat. nos. DY901B, DM1300 and
DTM100, respectively; R&D Systems, Inc.) were used to quantify
the MMPs concentration strictly in accordance with the
manufacturer's instructions. Briefly, samples and standards were
diluted in 96-well plates and 50 µl conjugate solution was
added into each well. After an incubation of 2 h at room
temperature, the wells were washed three times. Subsequently, 200
µl substrate solution was added. The plate was incubated for
10–15 min and the color development was stopped. Absorbance of each
well was determined at 450 nm in a microplate reader (BioTeke
Corporation, Beijing, China). A standard curve was constructed by
plotting the absorbance of standards against the known
concentration and the sample content was deduced from the standard
curve.
Transcription analysis
Total RNA was extracted from the three cell lines
GDF5con/con, GDF5mut/mut and
GDF5cor/cor chondrocytes using TRIzol reagent according
to manufacturer's instructions. Subsequently, 1 µg RNA was
reverse transcribed into cDNA using a First-Strand cDNA synthesis
kit (Takara Biotechnology Co., Ltd.). cDNA (1 µl) was used
for quantification on an ABI 7500 real-time machine (Thermo Fisher
Scientific, Inc.) using SYBR-Green (Takara Biotechnology Co., Ltd.)
and gene expression primer pairs (Table I) for the target genes of GDF5,
cell viability-associated genes and genes in Wnt/β-catenin
signaling. The thermocycling parameters were 5 min at 95°C followed
by 40 cycles of 30 sec at 95°C, 30 sec at annealing temperature of
each primer pair (Table I), 30
sec at 72°C, and a final extension of 3 min at 72°C. GAPDH was
employed as internal control and gene expression was analyzed by
the the comparative ΔΔCt method (26).
Statistical analysis
All the data were presented as the mean ± standard
deviation. ANOVA analysis and Dunnett test were performed to
evaluate the statistical significance between two means of equal
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Generation of corrected MSCs
MSCs were collected from the patient with OA and the
healthy volunteer, then cultured in cell plates. When the cells
reached a confluence of ~70%, they were transfected with 10
µg TALEN mRNA and 100 ng ssDNA. After 2 weeks, cell colonies
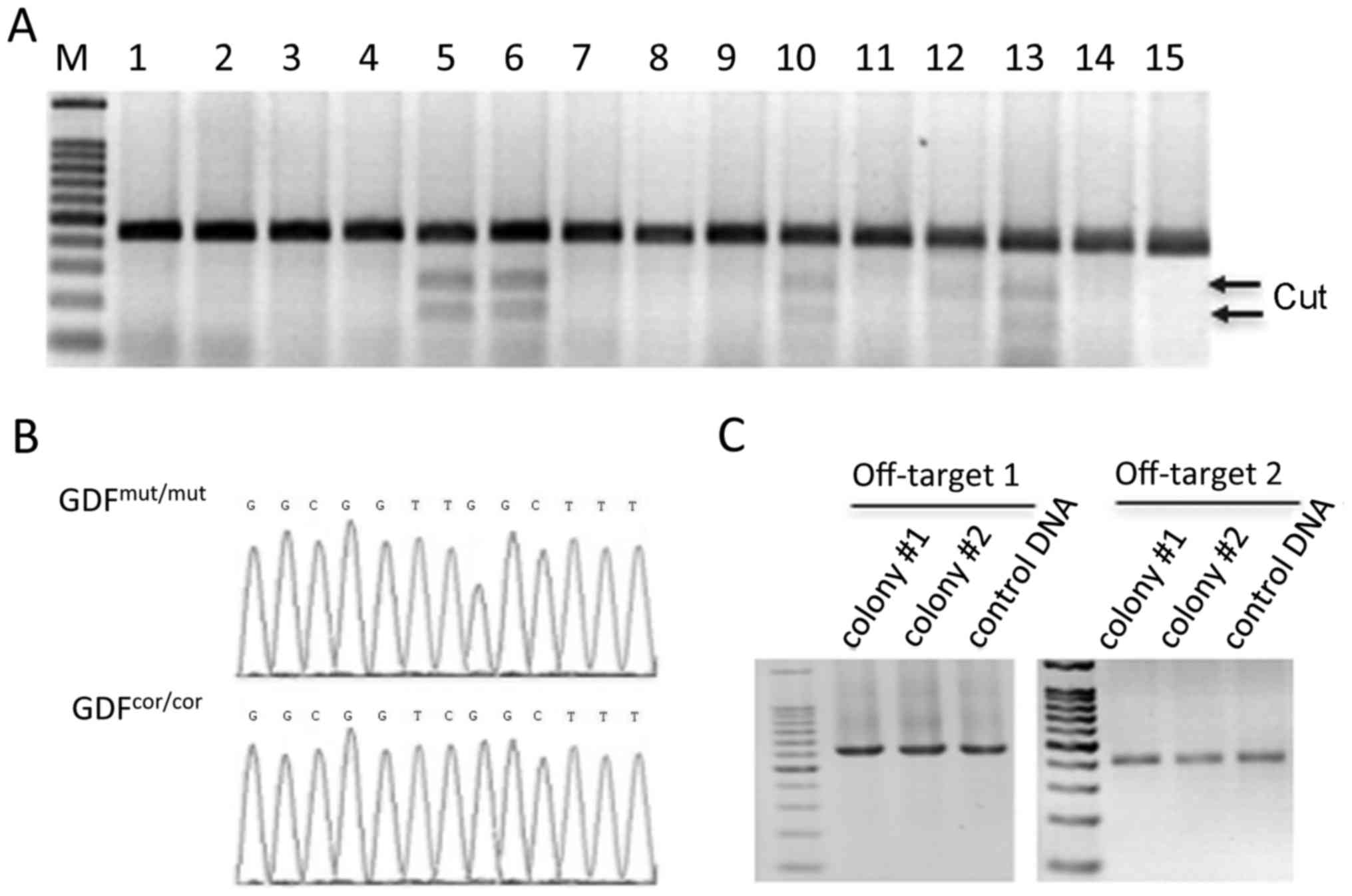
formed. T7EI assay was performed to screen for cells with the
mutation induced by TALENs (Fig.
2A). In all, 142 colonies were tested. Results here showed that
34 colonies were genetically edited: 26 colonies were modified to
heterozygous (one allele was indeled and the other was unchanged),
and 8 were homozygous. Fortunately, 2 colonies were identified as
completely corrected at rs143383 in the GDF5 gene, which was
confirmed by Sanger sequencing (Fig.
2B).
Although inherently site-specific, unsolicited
cleavage is a perpetual issue of genome-editing tools. To
interrogate possible off-target cleavage by the GDF5 TALENs, BLAST
was used to compare the targeting sequence against the human
genome, and picked the second and third most complimentary hits to
be analyzed for mutations. DNA from the corrected colonies was
amplified by two pairs of primers spanning the two off-target sites
(Table I). Off-target mutations
were then detected by T7EI assay as previously described (23). No cleaved bands were detected,
indicating that the TALENs did not target these two sites and
presumably no other sites predicted by the primary sequence alone
(Fig. 2C). Therefore, these
colonies were chosen for chondrocytic differentiation.
MSCs differentiating into
chondrocytes
MSCs derived from the healthy control with normal
GDF5 gene, and from the OA cells with mutant and with corrected
GDF5 gene were cultured for chondrocytic differentiation,
designated as GDF5con/con GDF5mut/mut and
GDF5cor/cor, respectively. The three cell lines
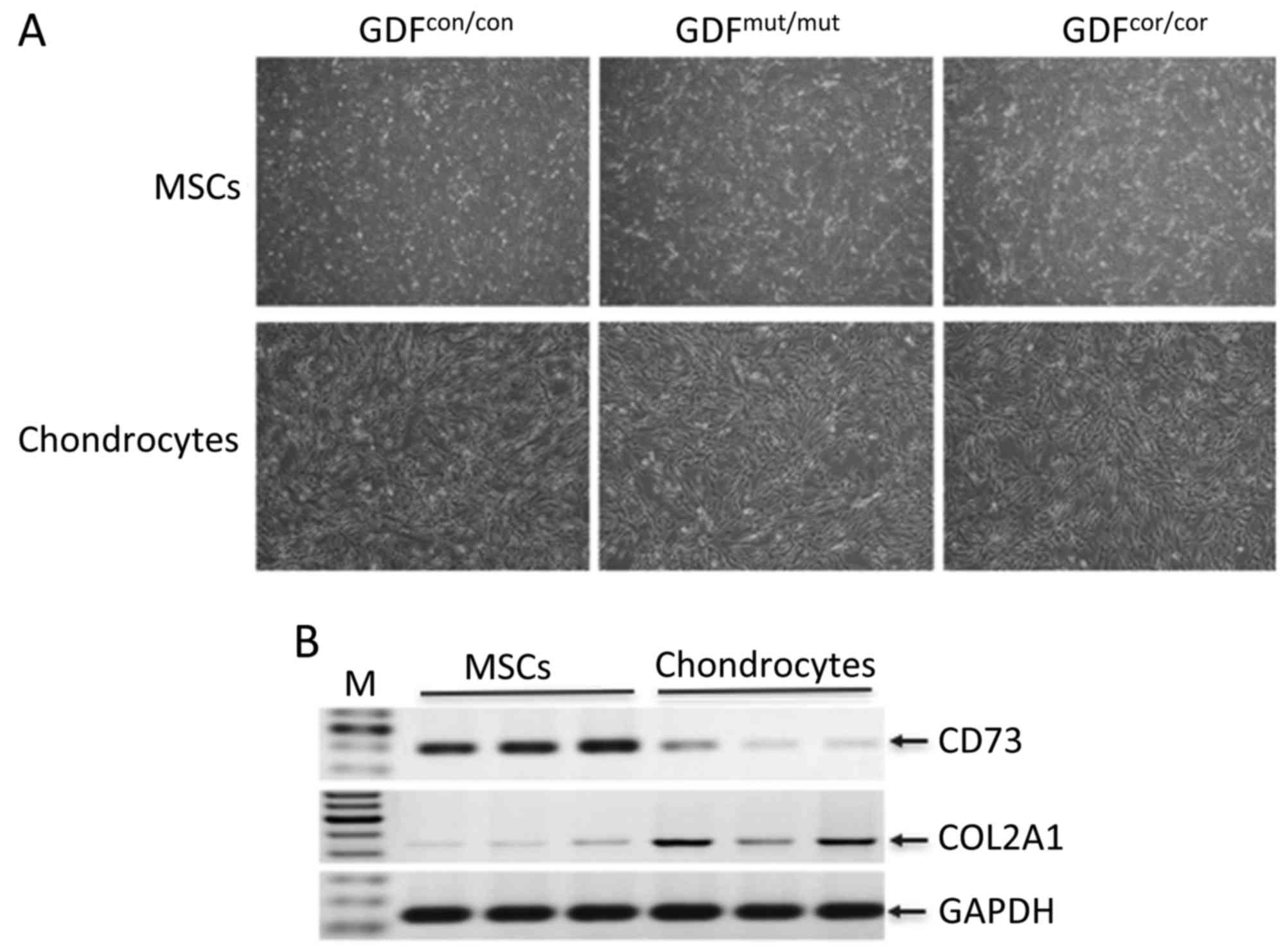
exhibited fibroblast-like morphology as classic MSCs (Fig. 3A). Following differentiation,
similar cell morphology was observed among these cells (Fig. 3A). To verify the differentiation,
these cells were characterized by lineage-specific markers, CD73
for MSCs and COL2A1 for chondrocytes (27). As shown by reverse transcription
PCR, CD73 was highly expressed in MSCs. Following differentiation,
chondrocyte-specific marker COL2A1 was observed to be expressed at
higher level in differentiated cells compared with in
undifferentiated cells (Fig. 3B).
These data demonstrated that the MSCs were successfully induced
into chondrocytes.
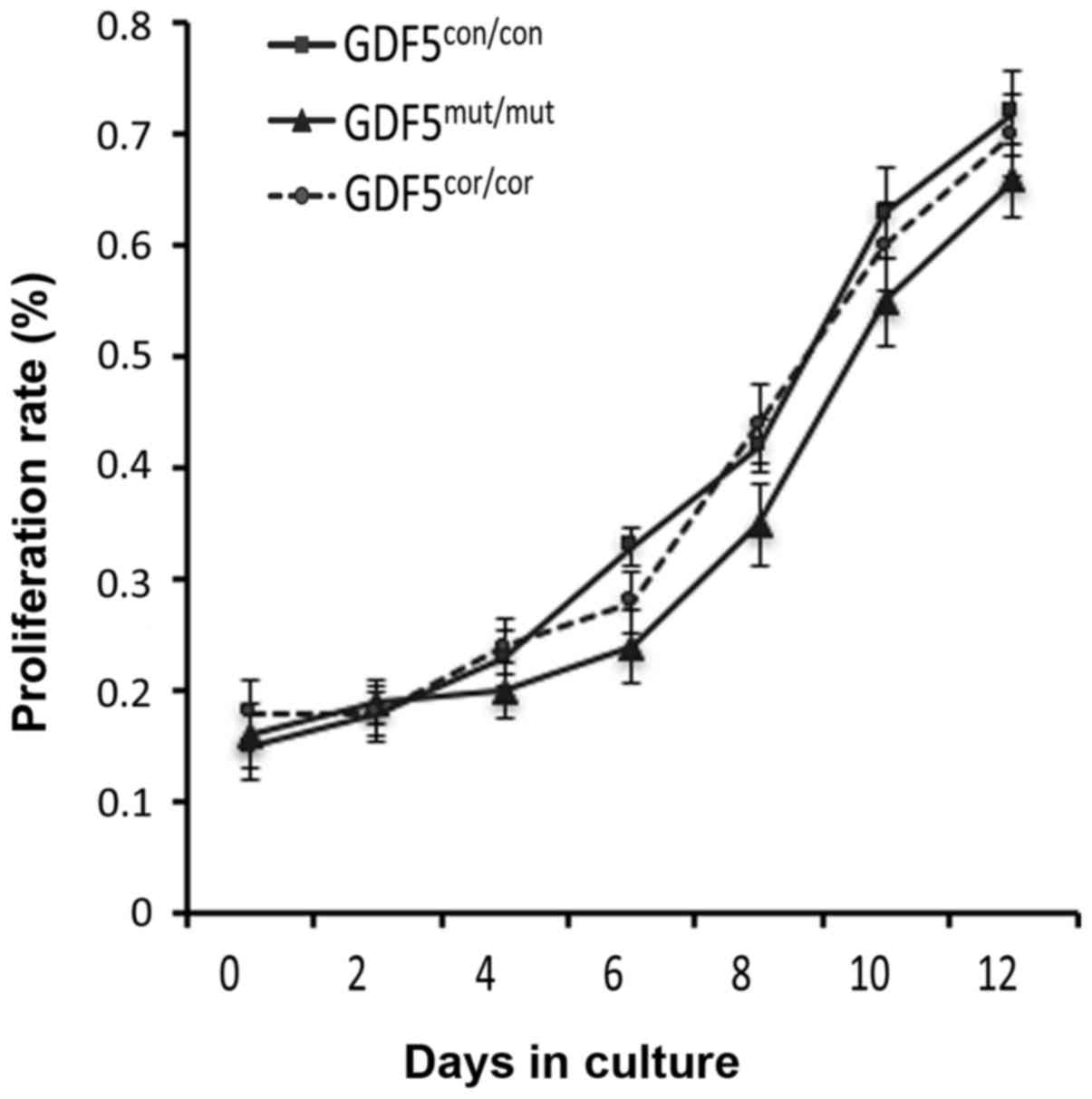
Proliferation of chondrocytes with the
corrected GDF5 gene
The defective of chondrocyte function and
proliferation would lead to the failure of the articular cartilage
and consequently to the progression of OA, thus the proliferation
of chondrocytes derived from the cells with mutant
(GDF5mut/mut) and corrected (GDF5cor/cor)
GDF5 was determined. The MTT assay showed that the
GDF5cor/cor cells propagated at a comparable rate with
the GDF5mut/mut cells. In the meantime, no significant
difference was observed between these two cell lines
(GDF5mut/mut and GDF5cor/cor) and the cells
from the healthy donor (GDF5con/con; Fig. 4).
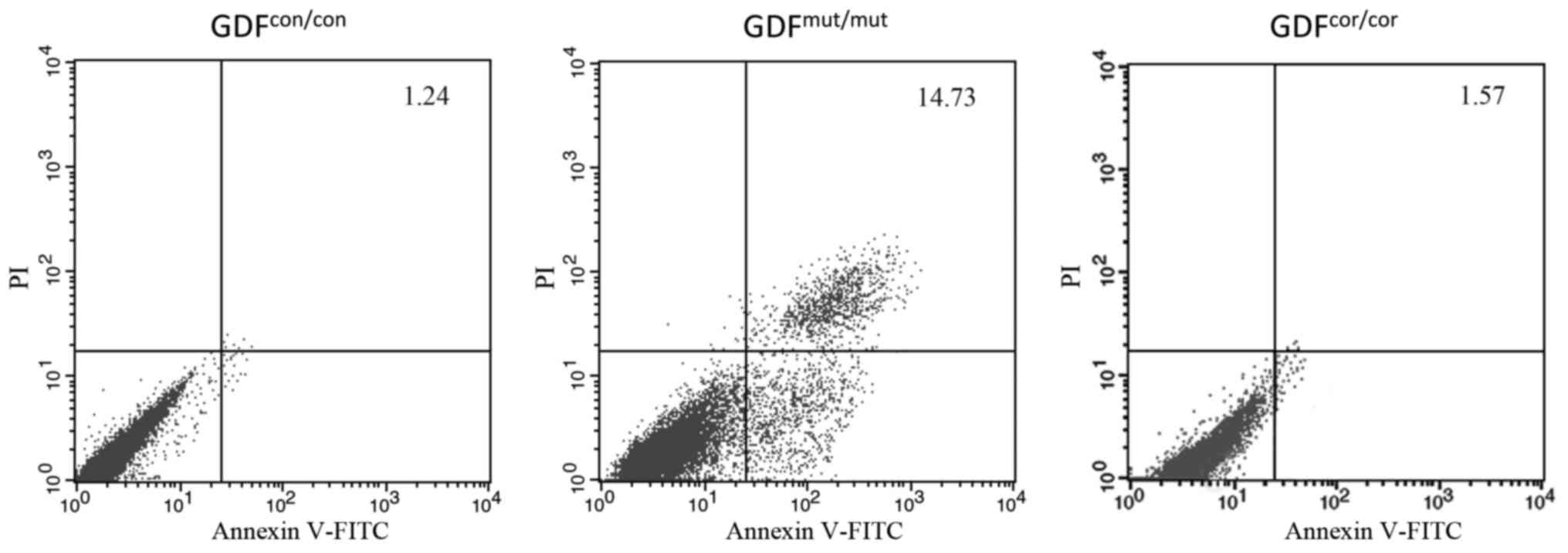
Decreased apoptosis rate in
GDF5cor/cor chondrocytes
As chondrocyte apoptosis has a crucial role in
maintaining the homeostasis of articular cartilages (28,29) and GDF5 modulates the apoptosis of
the chondrogenic cell line (30),
it was determined whether reversing the mutation of the functional
SNP rs143383 could restore the cell viability. The chondrocytes
were stained with Annexin V-FITC and flow cytometry was performed
to assess the proportion of cells undergoing apoptosis. Compared
with the control cells, a significant increase of apoptotic cells
was detected in the group GDF5mut/mut (1.31±0.12 vs.
14.25±0.68; Fig. 5). Importantly,
the number of apoptotic GDF5cor/cor chondrocytes was
reduced compared with the GDF5mut/mut group, and the
proportions of apoptotic cells were almost equal between the
GDF5cor/cor and GDF5con/con groups (Fig. 5). Results here indicated that
genetic correction of GDF5 prevented chondrocyte apoptosis.
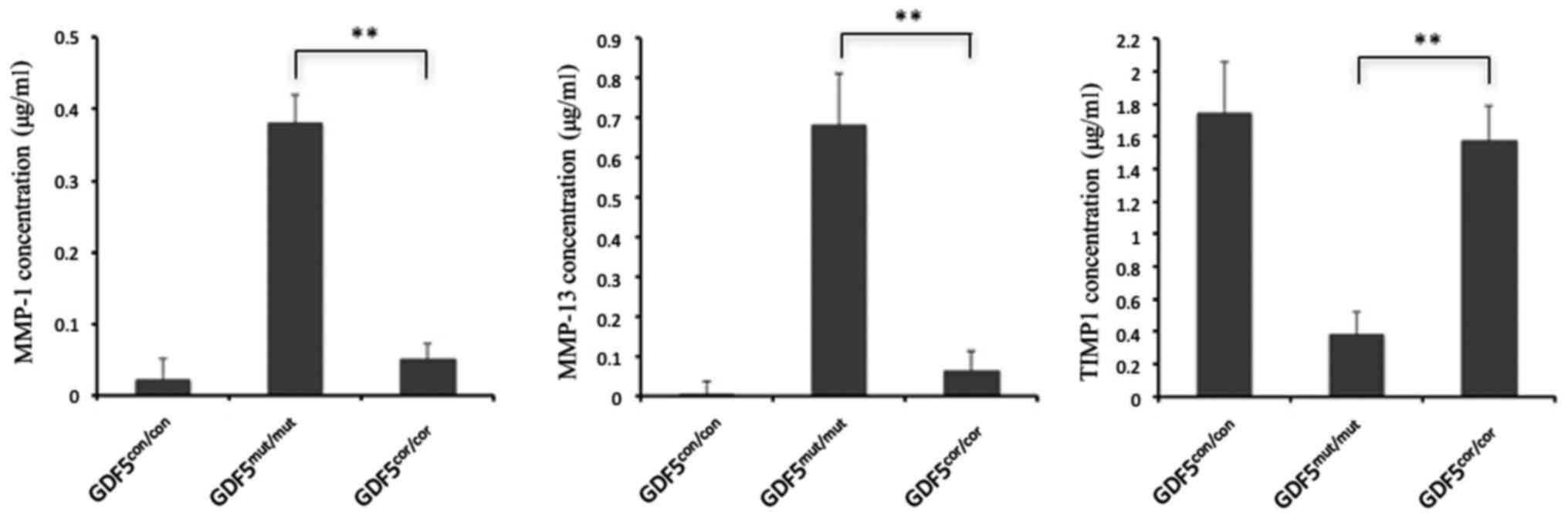
Decreased MMPs secretion in
GDF5cor/cor chondrocytes
MMPs functioning in the degradation of ECM were
documented as the most important candidates for the progression of
OA, including MMP-1, MMP-2 and MMP-9 (31). Particularly, MMP-13 was reported
to be the major collagenase in OA cartilage and had the highest
activity (32). In the current
study, the secretion of MMP-1, MMP-13 and TIMP1 was measured in the
supernatant of culture medium of GDF5con/con,
GDF5mut/mut and GDF5cor/cor chondrocytes.
Generally, the MMPs were at relatively low levels in
GDF5con/con cells. However, the concentrations of MMP-1
and MMP-13 were found to be higher in GDF5mut/mut
chondrocytes, while in the GDF5cor/cor chondrocytes, the
production of these MMPs was reduced compared with in
GDF5mut/mut chondrocytes to varying degrees. Maximum
suppression was observed for MMP-13, with a 7.2-fold decrease
compared with GDF5mut/mut chondrocytes. In addition, the
secretion of MMP suppressor TIMP1 was elevated in
GDF5cor/cor chondrocytes compared with
GDF5mut/mut chondrocytes (Fig. 6). Inhibition of MMP production and
increased expression of TIMP indicates that correction of the GDF5
mutation has the potential to inhibit the degradation of ECM.
Restored gene expression in
GDF5cor/cor chondrocytes
Having identified the impacts of mutation correction
on cell survival and MMP production, the alterations in
OA-associated gene expressions caused by GDF5 editing were also
determined. In this study, GDF5 expression was observed to be
decreased in GDF5cor/cor chondrocytes compared with
GDF5mut/mut chondrocytes (Fig. 7A). This was in accordance with the
previous findings that the T allele of rs148833 reduces the mRNA
expression of GDF5 (33,34). Activation of GDF5 signaling
transduced by BMP receptor and downstream Smad1/5/8 regulated the
expression of target genes, including COL2A1, ACAN, SOX9, MMP-13,
MMP-1 and TIMP1, which encode key catabolic and anabolic proteins
in chondrocytes (35). The
relative expression of COL2A1, ACAN, SOX9 and TIMP1 were
significantly enhanced, while MMP-13 and MMP-1 were suppressed in
the GDF5cor/cor chondrocytes compared with
GDF5mut/mut chondrocytes. There was no difference in
mRNA expression of these targets between GDF5cor/cor and
GDF5con/con chondrocytes (P>0.05; Fig. 7A). The restoration of mRNA
production of GDF5 itself and its targets indicated that
appropriate signaling was rescued by gene correction.
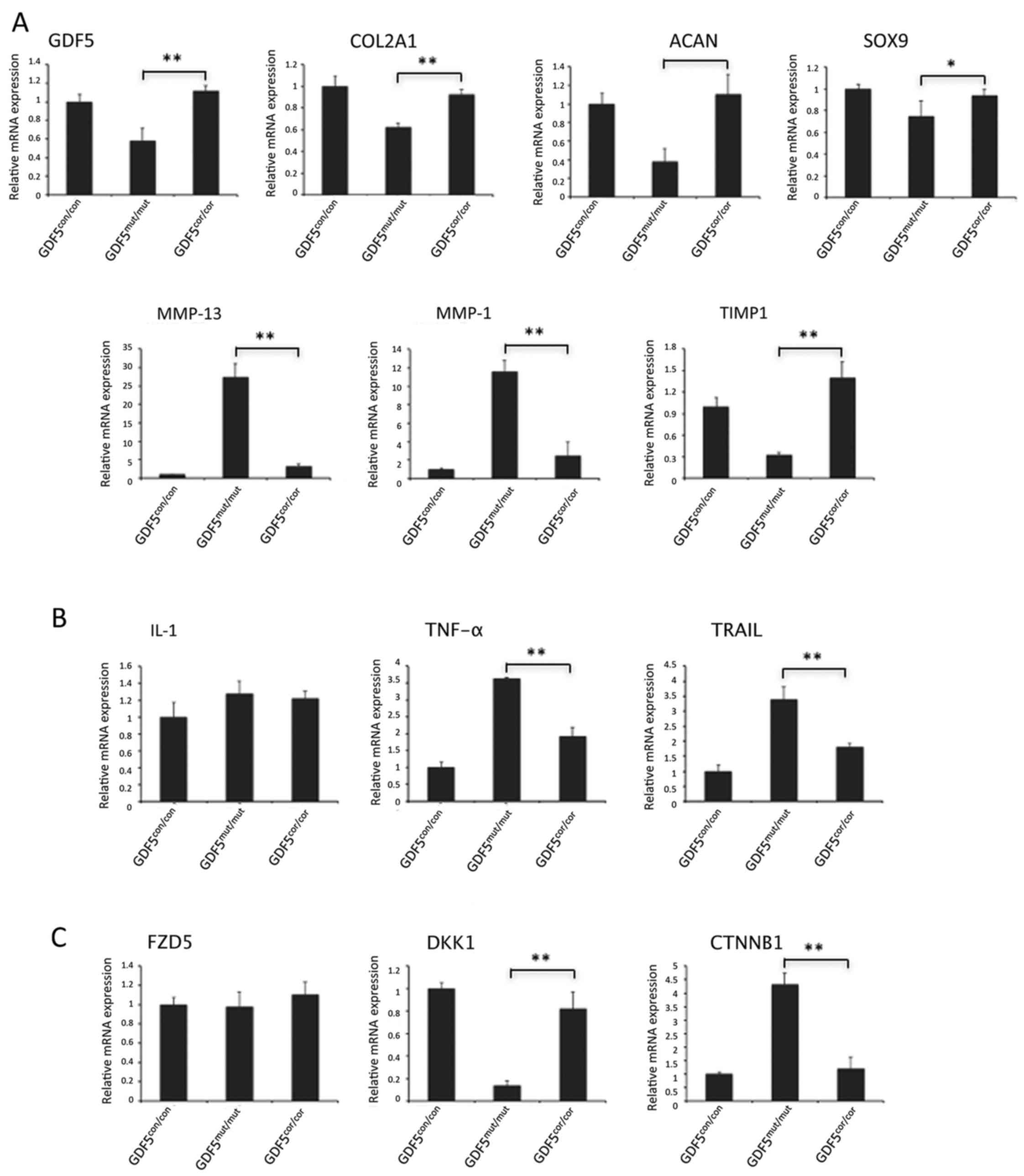 | Figure 7Expression profiling of target genes
of (A) GDF5, (B) cell viability-associated genes and (C) genes in
canonical Wnt/β-catenin signaling. GAPDH was used as internal
control and gene expression of the healthy control was set as 1.
*P<0.05, **P<0.01. GDF5, growth and
differentiation factor 5; con, control; mut, mutated; cor,
corrected mutation; COL2A1, collagen type II α 1 chain; ACAN,
aggrecan; SOX9, SRY-box 9; MMP, matrix metalloproteinase; TIMP1,
TIMP metallopeptidase inhibitor 1; IL-1, interleukin-1; TNF-α,
tumor necrosis factor-α; TRAIL, TNF superfamily member 10; FZD5,
frizzled class receptor 5; DKK1, dickkopf WNT signaling pathway
inhibitor 1; CTNNB1, catenin β 1. |
Interleukin-1 (IL-1), tumor necrosis factor-α
(TNF-α) and TNF superfamily member 10 (TRAIL) have critical
functions in chondrocyte viability (36,37). Expression of TNF-α and TRAIL in
GDF5mut/mut cells were significantly higher than that in
control cells. By contrast, GDF5cor/cor chondrocytes
displayed downregulated gene expressions compared with
GDF5mut/mut cells. There was no difference in IL-1
expression among these cells (Fig.
7B).
The canonical Wnt/β-catenin pathway, which is
mediated by frizzled (FZD) proteins, inducing nuclear translocation
of β-catenin, has important roles in ECM degradation (9). In this cascade, the main Wnt
receptor, FZD5, was expressed at similar levels in
GDF5mut/mut and GDF5cor/cor cells. Dickkopf
WNT signaling pathway inhibitor 1 (DKK1), which acts as a Wnt
antagonist (38), was upregulated
in corrected cells compared with the mutant cells, reaching the
same expression level as the controls. Accordingly, the expression
of the gene CTNNB1, encoding the core regulator of Wnt pathway, was
observed to be notably decreased in GDF5cor/cor
chondrocytes (Fig. 7C). The
altered gene expression of key regulators in Wnt signaling
suggested the inactivation of the pathway.
Discussion
OA is the most common degenerative disease in joints
and is predicted to be the single greatest cause of disability by
2030. Its prevalence after the age of 65 years is ~60% in men and
70% in women (39). The etiology
of OA is multifactorial, with inflammatory, metabolic and
mechanical causes. There is no cure, and current therapeutic
strategies are primarily aimed at reducing pain and improving joint
function (40). It is now
generally accepted that the chondrocyte is the target of these
aforementioned causative factors, and genetic factors also
contribute to the alterations in the functional activities of these
cells (41). Chondrocytes reside
in articular cartilages and are responsible for the maintenance of
ECM. The compromising of their function and survival would lead to
the failure of the articular cartilages (29). Therefore, cell-based functional
restore of chondrocytes could be a promising approach to improve
OA.
MSCs are multipotent precursor cells originating
from several adult connective tissues. MSCs possess the ability to
self-renew and differentiate into several lineages, including
osteogenic, chondrogenic, cardiogenic and hepatogenic
differentiation (42). There has
been an increasing interest in the clinical use of MSCs in
regenerative medicine. MSCs can be obtained from various sources
such bone marrow, umbilical cord, periodontal ligament and adipose
tissue. Adipose-derived MSCs, especially autologous cells, are
believed to be one of the most promising cell populations
identified thus far, because adipose tissue is ubiquitous and
easily obtained in large quantities with minimal patient discomfort
(43). Therefore, MSCs were
isolated from the adipose tissue of a patient with OA and induced
these cells into chondrocytes in the present study.
Despite its high prevalence, the etiology of OA is
still not fully elucidated. Genetic factors have important roles
with estimated heritability ranging from 40–65% (44). In recent years, numerous candidate
genes associated with OA have been identified, such as nuclear
receptor coactivator 3, ASPN, GDF5 and cadherin 2 (45). A causative SNP rs143383 located in
the 5′-UTR of GDF5 alters transcription factor binding and
consequently reduces GDF5 expression (11). In chondrocytes, GDF5 was
previously reported to reduce MMP-13 expression via DKK1 (9). Thus, genetic correction of the
causative mutation may be important for MSCs-based therapy.
In recent years, great advances have been achieved
in the ability to make site-specific modifications to the human
genome. Currently, the major techniques to mediate genome
modification are the ZFN, TALENs and CRISPR/Cas9, all of which are
reported to be highly efficient. Each of these techniques has their
own potential advantages and disadvantages. It is important to
consider the off-target activity when editing the genome, and among
the platforms TALENs are highly specific (46). In this study, a pair of TALENs
were constructed to target the functional SNP rs143383 in GDF5 and
synthesized an ssDNA as the donor template to correct the mutant
site. Consequently, a total of 34 colonies were modified out of 142
MSC colonies, and 2 colonies were corrected in both alleles. When
determining the potential off-target activity in these 2 colonies,
no unpredicted mutations were observed.
Recovery of functionality and viability of
chondrocytes has a crucial role in maintaining the homeostasis of
articular cartilages (28,29).
In corrected cells, the proliferation rate was not changed compared
with control and mutation cells, while apoptosis was markedly
decreased compared with GDF5-mutant chondrocytes. These results
indicated that the TALENs caused enhanced cell viability. MMPs are
the major component involved in the degradation of ECM, which is an
important factor involved in the progression of OA (31). MMP-1 and MMP-13 secretion was
reduced, and TIMP1 was increased, in corrected cells compared with
mutation cells, suggesting that ECM degradation was inhibited in
modified cells. Expression of GDF5 target genes and genes affecting
cell viability and ECM metabolism, including TNF-α, TRAIL and
canonical Wnt/β-catenin signaling, were at similar levels in the
control and corrected cells. The data suggested that cell viability
and functionality were restored by correction of the GDF5
mutation.
In conclusion, the dysfunctional gene GDF5 was
successfully corrected in adipose tissue-derived MSCs using a pair
of TALENs. Modified MSCs were induced and differentiated into
chondrocytes. These cells exhibited normal morphology and enhanced
cell vitality. In corrected cells, the secretion of MMPs was
suppressed and TIMP1 was increased compared with the mutated cells.
The expressions of target genes of GDF5, cell vitality-associated
genes and ECM degrading genes were returned to normal levels by
correction of the GDF5 SNP, suggesting the functional recovery of
these corrected chondrocytes. To the best of our knowledge, this
study was the first attempt to generate functional chondrocytes by
editing a mutant gene in MSCs, and the results demonstrated that
TALEN-mediated genetic correction has the potential to be applied
in regenerative medicine. However, further work is required.
Acknowledgments
This study was supported by the Natural Science
Foundation of Guangdong Province, China (grant no.
2015A030313611).
Notes
[1] Competing
interests
The authors declare there is no competing
interest.
References
|
1
|
Felson DT, Naimark A, Anderson J, Kazis L,
Castelli W and Meenan RF: The prevalence of knee osteoarthritis in
the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum.
30:914–918. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oztürkmen Y, Uzümcügil O, Karamehmetoğlu
M, Leblebici C and Caniklioğlu M: Total knee arthroplasty for the
management of joint destruction in tuberculous arthritis. Knee Surg
Sports Traumatol Arthrosc. 22:1076–1083. 2014. View Article : Google Scholar
|
|
3
|
Spector TD and MacGregor AJ: Risk factors
for osteoarthritis: Genetics. Osteoarthritis and cartilage/OARS.
Osteoarthritis Res Soc. 12:S39–S44. 2004. View Article : Google Scholar
|
|
4
|
arcOGEN Consortium, arcOGEN Collaborators;
Zeggini E, Panoutsopoulou K, Southam L, Rayner NW, Day-Williams AG,
Lopes MC, Boraska V, Esko T, et al: Identification of new
susceptibility loci for osteoarthritis (arcOGEN): A genome-wide
association study. Lancet. 380:815–823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castaño Betancourt MC, Cailotto F, Kerkhof
HJ, Cornelis FM, Doherty SA, Hart DJ, Hofman A, Luyten FP,
Maciewicz RA, Mangino M, et al: Genome-wide association and
functional studies identify the DOT1L gene to be involved in
cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci USA.
109:8218–8223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bijsterbosch J, Kloppenburg M, Reijnierse
M, Rosendaal FR, Huizinga TW, Slagboom PE and Meulenbelt I:
Association study of candidate genes for the progression of hand
osteoarthritis. Osteoarthritis Cartilage. 21:565–569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grimaud E, Heymann D and Rédini F: Recent
advances in TGF-beta effects on chondrocyte metabolism. Potential
therapeutic roles of TGF-beta in cartilage disorders. Cytokine
Growth Factor Rev. 13:241–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enochson L, Stenberg J, Brittberg M and
Lindahl A: GDF5 reduces MMP13 expression in human chondrocytes via
DKK1 mediated canonical Wnt signaling inhibition. Osteoarthritis
Cartilage. 22:566–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mikic B: Multiple effects of GDF-5
deficiency on skeletal tissues: Implications for therapeutic
bioengineering. Ann Biomed Eng. 32:466–476. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dodd AW, Syddall CM and Loughlin J: A rare
variant in the osteoarthritis-associated locus GDF5 is functional
and reveals a site that can be manipulated to modulate GDF5
expression. Eur J Hum Genet. 21:517–521. 2013. View Article : Google Scholar :
|
|
12
|
Kuo CK, Li WJ, Mauck RL and Tuan RS:
Cartilage tissue engineering: Its potential and uses. Curr Opin
Rheumatol. 18:64–73. 2006. View Article : Google Scholar
|
|
13
|
Kim YG, Cha J and Chandrasegaran S: Hybrid
restriction enzymes: Zinc finger fusions to Fok I cleavage domain.
Proc Natl Acad Sci USA. 93:1156–1160. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun N and Zhao H: Transcription
activator-like effector nucleases (TALENs): A highly efficient and
versatile tool for genome editing. Biotechnol Bioeng.
110:1811–1821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang T, Wei JJ, Sabatini DM and Lander ES:
Genetic screens in human cells using the CRISPR-Cas9 system.
Science. 343:80–84. 2014. View Article : Google Scholar
|
|
16
|
Gao F, Shen XZ, Jiang F, Wu Y and Han C:
DNA-guided genome editing using the Natronobacterium gregoryi
Argonaute. Nat Biotechnol. 34:768–773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pankowicz FP, Barzi M, Legras X, Hubert L,
Mi T, Tomolonis JA, Ravishankar M, Sun Q, Yang D, Borowiak M, et
al: Reprogramming metabolic pathways in vivo with CRISPR/Cas9
genome editing to treat hereditary tyrosinaemia. Nat Commun.
7:126422016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katz AJ, Tholpady A, Tholpady SS, Shang H
and Ogle RC: Cell surface and transcriptional characterization of
human adipose-derived adherent stromal (hADAS) cells. Stem Cells.
23:412–423. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doyle EL, Booher NJ, Standage DS, Voytas
DF, Brendel VP, Vandyk JK and Bogdanove AJ: TAL effector-nucleotide
targeter (TALE-NT) 2.0: Tools for TAL effector design and target
prediction. Nucleic Acids Res. 40:W117–W122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cermak T, Doyle EL, Christian M, Wang L,
Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ and Voytas
DF: Efficient design and assembly of custom TALEN and other TAL
effector-based constructs for DNA targeting. Nucleic Acids Res.
39:e822011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller JC, Tan S, Qiao G, Barlow KA, Wang
J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al: A TALE
nuclease architecture for efficient genome editing. Nat Biotechnol.
29:143–148. 2011. View Article : Google Scholar
|
|
22
|
Liew A, André FM, Lesueur LL, De Ménorval
MA, O'Brien T and Mir LM: Robust, efficient, and practical
electrogene transfer method for human mesenchymal stem cells using
square electric pulses. Hum Gene Ther Methods. 24:289–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakurai T, Watanabe S, Kamiyoshi A, Sato M
and Shindo T: A single blastocyst assay optimized for detecting
CRISPR/Cas9 system-induced indel mutations in mice. BMC Biotechnol.
14:692014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ibrahim AM, Elgharabawi NM, Makhlouf MM
and Ibrahim OY: Chondrogenic differentiation of human umbilical
cord blood-derived mesenchymal stem cells in vitro. Microsc Res
Tech. 78:667–675. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Darzynkiewicz Z, Sharpless T,
Staiano-Coico L and Melamed MR: Subcompartments of the G1 phase of
cell cycle detected by flow cytometry. Proc Natl Acad Sci USA.
77:6696–6699. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Floris I, Billard H, Boquien CY,
Joram-Gauvard E, Simon L, Legrand A, Boscher C, Rozé JC,
Bolaños-Jiménez F and Kaeffer B: MiRNA analysis by quantitative PCR
in preterm human breast milk reveals daily fluctuations of
hsa-miR-16-5p. PLoS One. 10:e01404882015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roszek K, Porowińska D, Bajek A, Hołysz M
and Czarnecka J: Chondrogenic differentiation of human mesenchymal
stem cells results in substantial changes of ecto-nucleotides
metabolism. J Cell Biochem. 116:2915–2923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Héraud F, Héraud A and Harmand MF:
Apoptosis in normal and osteoarthritic human articular cartilage.
Ann Rheum Dis. 59:959–965. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hwang HS and Kim HA: Chondrocyte apoptosis
in the pathogenesis of osteoarthritis. Int J Mol Sci.
16:26035–26054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itoh S, Kanno S, Gai Z, Suemoto H,
Kawakatsu M, Tanishima H, Morimoto Y, Nishioka K, Hatamura I,
Yoshida M, et al: Trps1 plays a pivotal role downstream of Gdf5
signaling in promoting chondrogenesis and apoptosis of ATDC5 cells.
Genes Cells. 13:355–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murphy G and Nagase H: Reappraising
metalloproteinases in rheumatoid arthritis and osteoarthritis:
Destruction or repair? Nat Clin Pract Rheumatol. 4:128–135. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baragi VM, Becher G, Bendele AM, Biesinger
R, Bluhm H, Boer J, Deng H, Dodd R, Essers M, Feuerstein T, et al:
A new class of potent matrix metalloproteinase 13 inhibitors for
potential treatment of osteoarthritis: Evidence of histologic and
clinical efficacy without musculoskeletal toxicity in rat models.
Arthritis Rheum. 60:2008–2018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyamoto Y, Mabuchi A, Shi D, Kubo T,
Takatori Y, Saito S, Fujioka M, Sudo A, Uchida A, Yamamoto S, et
al: A functional polymorphism in the 5′ UTR of GDF5 is associated
with susceptibility to osteoarthritis. Nat Genet. 39:529–533. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reynard LN, Bui C, Syddall CM and Loughlin
J: CpG meth-ylation regulates allelic expression of GDF5 by
modulating binding of SP1 and SP3 repressor proteins to the
osteoarthritis susceptibility SNP rs143383. Hum Genet.
133:1059–1073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ratnayake M, Plöger F, Santibanez-Koref M
and Loughlin J: Human chondrocytes respond discordantly to the
protein encoded by the osteoarthritis susceptibility gene GDF5.
PLoS One. 9:e865902014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schuerwegh AJ, Van Offel JF, Stevens WJ,
Bridts CH and De Clerck LS: Influence of therapy with chimeric
monoclonal tumour necrosis factor-alpha antibodies on intracellular
cytokine profiles of T lymphocytes and monocytes in rheumatoid
arthritis patients. Rheumatology (Oxford). 42:541–548. 2003.
View Article : Google Scholar
|
|
37
|
Pettersen I, Figenschau Y, Olsen E,
Bakkelund W, Smedsröd B and Sveinbjörnsson B: Tumor necrosis
factor-related apop-tosis-inducing ligand induces apoptosis in
human articular chondrocytes in vitro. Biochem Biophys Res Commun.
296:671–676. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thomas E, Peat G and Croft P: Defining and
mapping the person with osteoarthritis for population studies and
public health. Rheumatology (Oxford). 53:338–345. 2014. View Article : Google Scholar
|
|
40
|
Sarzi-Puttini P, Cimmino MA, Scarpa R,
Caporali R, Parazzini F, Zaninelli A, Atzeni F and Canesi B:
Osteoarthritis: An overview of the disease and its treatment
strategies. Semin Arthritis Rheum. 35(Suppl 1): 1–10. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goldring MB: The role of the chondrocyte
in osteoarthritis. Arthritis Rheum. 43:1916–1926. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Visweswaran M, Pohl S, Arfuso F, Newsholme
P, Dilley R, Pervaiz S and Dharmarajan A: Multi-lineage
differentiation of mesenchymal stem cells - To Wnt, or not Wnt. Int
J Biochem Cell Biol. 68:139–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gimble JM, Katz AJ and Bunnell BA:
Adipose-derived stem cells for regenerative medicine. Circ Res.
100:1249–1260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kraus VB, Jordan JM, Doherty M, Wilson AG,
Moskowitz R, Hochberg M, Loeser R, Hooper M, Renner JB, Crane MM,
et al: The Genetics of Generalized Osteoarthritis (GOGO) study:
Study design and evaluation of osteoarthritis phenotypes.
Osteoarthritis Cartilage. 15:120–127. 2007. View Article : Google Scholar
|
|
45
|
Tsezou A: Osteoarthritis year in review
2014: Genetics and genomics. Osteoarthritis Cartilage.
22:2017–2024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hendel A, Fine EJ, Bao G and Porteus MH:
Quantifying on- and off-target genome editing. Trends Biotechnol.
33:132–140. 2015. View Article : Google Scholar : PubMed/NCBI
|