Introduction
Fungus is a member of eukaryotic organisms, also
including multicellular fungi, for example, all kinds of mushrooms
(1). Fungi is separate from the
animals, plants, protists and bacteria (2). The human use of fungi for food
preparation has a long history. Mushroom farming and mushroom
gathering are large industries in many countries (3,4).
The ethnomycology is the study of sociological impact and
historical uses of fungi (5,6).
Because of the capacity of this group to produce an enormous range
of natural products with antibacterial and other biological
activities, many species have long been used or are being developed
for industrial production of antibiotics, vitamins, and anticancer
and anticholesterol drugs (7–9).
Many species produce metabolites that are major sources of
pharmacological drugs (10).
Particularly important are the antibiotics, including penicillins,
a structurally related group of β-lactam antibiotics that are
synthesized from small peptides.
Polysaccharides are long sugar chains which are
combined with the glucosidic bond, and they are polymeric
carbohydrate molecules consisting of more than 10 monosaccharide
units (11,12). Cell-surface polysaccharides play a
great role in the procedure of metabolism (13). They are one of the four basic
substances of life (14).
Polysaccharide is a composite of nucleotide-activated precursor
(15,16). Lipopolysaccharide is the most
imperative of cell-surface polysaccharides. It has an impossibly
key effect on cell membrane structures, and is momentous material
of the cell-membrane receptor.
Cantharellus cibarius Fr. is a fungi which
grows in Aba country of Sichuan province in China at an elevation
of 3,600 m. In this study, the polysaccharide was got from the
fruiting bodies of Cantharellus cibarius Fr. by using
DEAE-cellulose column. Its chemical structures were characterized
for the first time. The structural analysis of the fraction was
done by using chemical methods, high performance liquid
chromatography (HPLC), infrared (IR) spectroscopy, and nuclear
magnetic resonance (NMR) spectroscopy. The antioxidant activity and
proliferation impact of immune cells of CC-1 was evaluated in
vitro. The result of this study introduced Cantharellus
cibarius Fr. as a possible valuable source which is helpful to
exhibit unique antioxidant and immune regulation properties.
Materials and methods
Chemicals
Cantharellus cibarius Fr. were collected in
Xiaojing Country of Sichuan province, China, and were authenticated
by Professor Xiang Ding (College of Life Sciences, China West
Normal University, Nanchong, China). A voucher specimen has been
deposited in Key Laboratory for Biological Resource and Ecological
Environment of Education Ministry, College of Life Sciences,
Sichuan University. Monosaccharides were from Beijing Biodee
Biotechnology Co., Ltd. (Beijing, China). DEAE-Cellulose-52 was
from Sigma-Aldrich (Mainland, China). The other reagents used were
of analytical grade. The other reagent
2-(2-methoxy-4-nitrophenyl-)-3-(4-nitrophenyl)-5-(2,4-disulfonic
acid benzene)-2H-tetrazolium monosodium salt (CCK-8) was purchased
from Dojindo Molecular Technologies, Inc. (Tokyo, Japan); D-Hanks
solution, RPMI-1640 medium, fetal calf serum, and dimethyl
sulfoxide were purchased from Gibco (Grand Island, NY, USA).
Penicillin G and streptomycin were from Sigma-Aldrich (17,18).
Extraction of polysaccharides from
Cantharellus cibarius Fr
Cantharellus cibarius Fr. fruiting bodies
(400 g), soaked with 95% EtOH (6 h) to remove lipids by filtration
(19–22). The residue was dried and extracted
with boiling water three times (6 h each). Refined polysaccharides
were obtained by concentrated filtrate, dialysis (MWCO 7000; Sigma)
centrifugation to remove impurity substance and small molecule
compounds. Adding 95% EtOH at 3 times volume in the supernatant
liquid precipitation crude polysaccharides, then drying in vacuo at
45°C, yielding the crude Cantharellus cibarius Fr.
polysaccharide (CC-1) (12.7 g, recovery 3.175%). DEAE-Sepharose
fast flow column was used to extract and purify polysaccharides of
Cantharellus cibarius Fr. polysaccharide, named CC-1.
Molecular weight determination of
polysaccharide CC-1
Molecular weight of polysaccharides were obtained by
high-performance gel permeation chromatography (HPGPC) (23). Deionized water was used to make
dextran standards and CC-1 dissolve at a concentration of 2.0 mg/ml
and then analyzed on an Agilent 1100 series HPLC system to
determine the retention time of standards and samples (24). The column and detector compartment
were maintained at 30 and 35°C, respectively. Distilled water was
used in mobile phase, and detection rate was 1.0 ml/min and tested
volume was 10 µl. The molecular weight of CEC-A was
calculated by constructing a calibration curve, in which the
logarithm of the molecular weight of the Dextran standards ranged
from 10,000–500,000 kDa as standard of the retention time using
Agilent ChemStation GPC Data Analysis Software (Millennium 32
software) (18).
Fourier transform infrared spectrometer
(FT-IR) analysis
Infrared spectroscopy is based on the fact that when
molecules absorb energy, undergo a transition to a state of higher
energy or excited state, and only vibrational energy transitions
occur in the mid-infrared region. The vibrations induced by
infrared radiation include strains and tensions of interatomic
bonds and changes of bonds angles. Thus, the vibration frequency
can be associated with a particular bond type (25). In this study, FT-IR spectra of
CC-1 was measured by grinding a mixture of polysaccharide with dry
KBr and then pressing in a mold. Spectra was collected using a
Thermo Nicolet 6700 FT-IR Spectrophotometer (Thermo Fisher
Scientific, Grand Island, NY, USA) in the coverage of 400–4000
cm−1 at resolution ratio of 4 cm−1 (26).
NMR experiment
The polysaccharide was dissolved in deuteroxide
accompanied with ultraonic wave precessing for 20 min. The varian
unity INOVA 400/45 (Varian Technologies, Palo Alto, CA, USA) was
used to perform the 1H NMR spectra and 13C
NMR spectra analysis with tetramethylsilane as internal standard
(27).
Monosaccharide composition analysis of
CC-1
CC-1 (10 mg) was hydrolyzed in 2 mol/l
trifluoroacetic acid at 100°C for 6 h on the mechanism of
acid-catalyzed hydrolysis (18).
The residual acid was removed with methyl alcohol (MeOH) and taking
to dryness three times. After the hydrolysis was completed, samples
were dissolved with distilled water for analyzing monosaccharide
composition. The hydrolyzates of CC-1 were analyzed by HPLC on an
Agilent 1100 series HPLC (Agilent Technologies, Palo Alto, CA, USA)
equipped with a RID (28). The
injection volume of mixed monosaccharide standards and CC-1
hydrolyzates was 10 µl. The temperature of the column was
set at 35°C. D-glucose, D-xylose, D-fructose, D-galactose,
L-arabinose and D-mannose were used as standard sugars.
Methylation analysis and gas
chromatography-mass spectrometry (GC-MS)
According to literature, we can use methyl iodide to
make polysaccharide methylation (29). Then the permethylated product was
depolymerized with 90% formic acid at 100°C for 4 h and further
hydrolysed with 2 M TFA at 100°C for 6 h. The resulting products
were derivatized using reagent and analyzed using Agilent
Technologies 7890A GC-MS system (Agilent Technologies) (29).
DPPH− radical scavenging
activity
The DPPH− radical scavenging activity of
the polysaccharide sample was measured by a decrease in absorbance
at 517 nm of a solution of purplecoloured DPPH− in
methanol brought about by the sample (30). The degree of free radical
scavenging rate can be judged by the size of the absorbance. The
higher the absorbance, the weaker the free radical scavenging
ability. Absorbance at 517 nm is measured after 30 min using
UV-visible Spectrometer. According to the formula to calculate the
free radical scavenging rate of DPPH:
DPPH scavenge(%)=[1−AtestAcontrol]×100%
A control represents the blank control group, A test
represents the absorbance in the presence of the polysaccharide
sample. In the study, the antioxidant activity of the extract was
compassed with vitamin C (Vc).
ABTS radical scavenging activity
ABTS+ radical scavenging activity of the
polysaccharide extracts and fractions was measured by the
ABTS+ cation decolorization assay (31). The ABTS+ radical cation
was confected by reaction of 7 mM stock solution of
ABTS+ with 2.45 mM ammonium persulphate (APS) and then
admixture at room temperature in the dark for 16 h. Then 2 ml of
various concentrations of the sample and 2 ml of ABTS+
radical solution (0.7 mM) were added. A control reaction was
carried out without the polysaccharide extracts. The absorbance was
measured immediately at 734 nm. The percentage of scavenging of
hydrogen radicals was calculated as follows:
Scavenging effect(%)=[1−(Asample−Asample+blank)Acontrol]×100%
where A control represents the absorbance of the control group in
the ABTS+ radicals generation system, A sample was the
absorbance of the test group and A sample blank was the absorbance
of the samples only. Vc was used as a positive control in the
study.
Cell lines and reagents
The T cell line and B cell line (Raji) were cultured
in RPMI-1640 medium containing 10% fetal bovine serum (FBS), 1%
penicillin (100 IU/ml) and streptomycin (100 mg/l) in a humidified
atmosphere with 5% CO2 at 37°C before use.
Pharmacological evaluation for B cells
and T cells stimulation
The cytotoxic effects of CC-1 on B cells and T cells
were determined by CCK-8-based colorimetric method (32). Briefly, B cells and T cells
suspended in RPMI-1640 medium at a density of 1×105
cells/ml were pipetted into a 96-well plate (100 µl/well)
and inoculated at 37°C in a humidified 5% CO2. After
incubation for 24 h, 100 µl of test sample with different
concentrations (0.625–80 µg/ml in fresh growth medium) was
added into each well, respectively, in an incubator at 37°C in a
humidified 5% CO2 for 48 h. RPMI-1640 and 5 µg/ml
lipopolysaccharide (LPS) was used as negative and positive
controls, respectively. Then, 10 µl of CCK-8 reagent was
added to each well, then the cells were cultured in the incubator
for 3 h. Absorbance of the cells in 96-well microplate was
evaluated by ELISA (Bio-Rad, Tokyo, Japan) at 490 nm.
Cell morphology observation
The morphology of T cell line and B cell line (Raji)
was observed under an inverted microscope (Olympus IX71; Olympus,
Tokyo, Japan).
Statistical methods
The data in this study were analyzed as the standard
deviation (SD) of three replications. Data processing was by One
way analysis of variance and Student's t-test. P<0.05 represents
a significant difference between the data.
Results
Determination of molecular weight
Molecular weight of CC-1 was evaluated by HPLC-GPC.
HPGPC of the polysaccharide fraction shows that each fraction was
represented by a broad and symmetrical peak on the chromatograms.
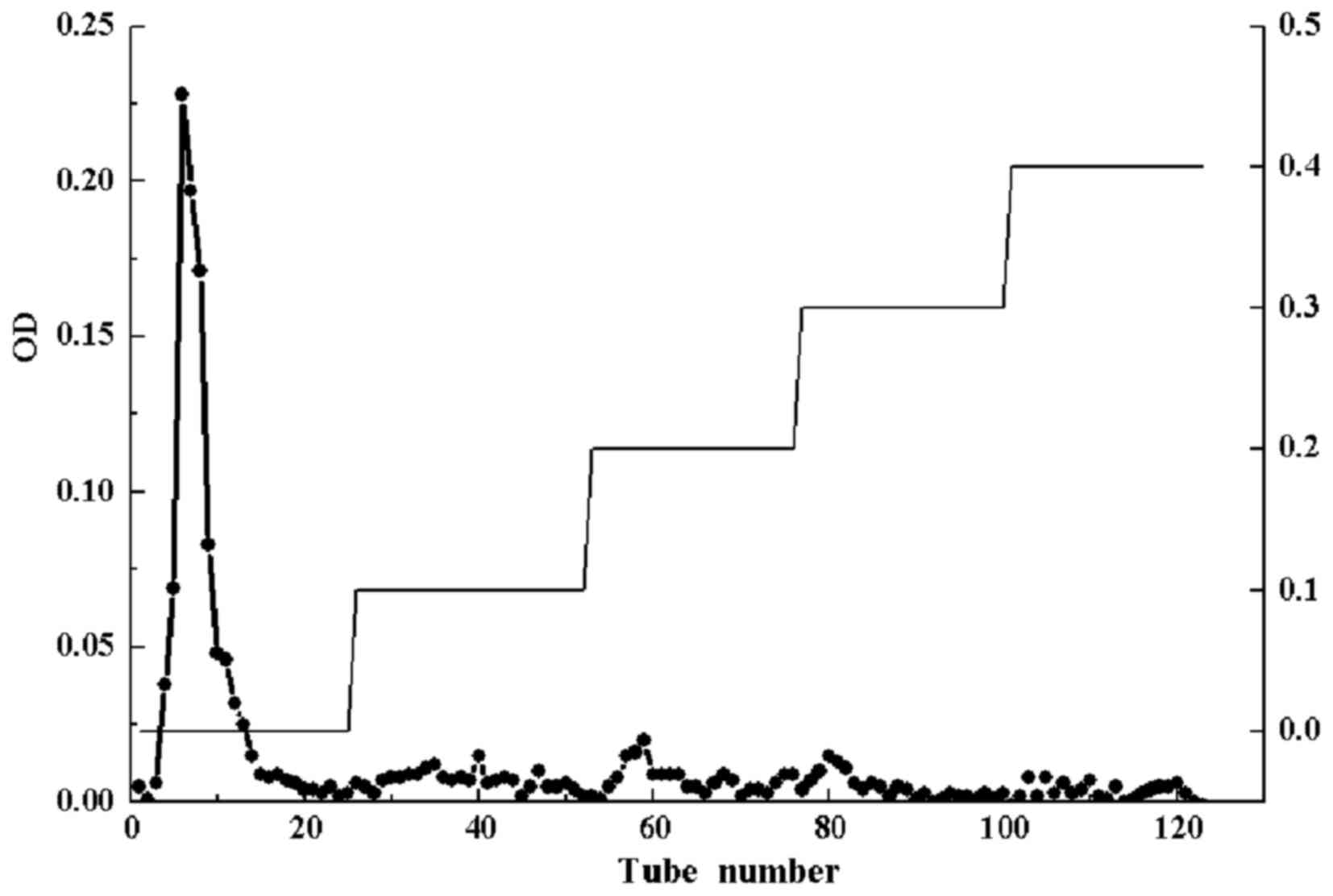
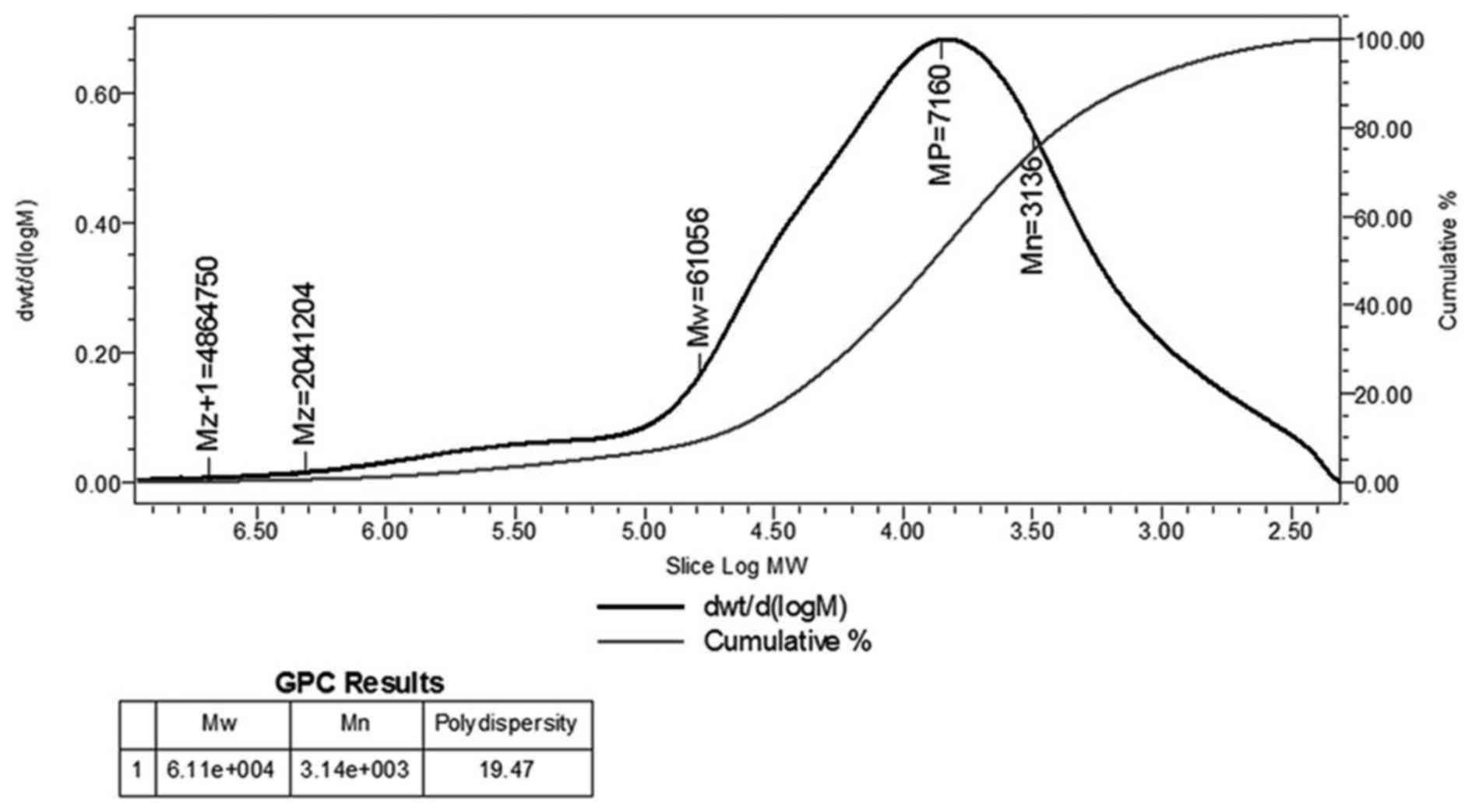
Fig. 1 shows DEAE cellulose-52
column chromatography and high performance gel permeation
chromatogram of CC-1, respectively. The molecular weight (Mw) of
CC-1 was 61,056 kDa, the peak molecular weight was 7,160 kDa, the
average molecular weight was 3,136 kDa, and the polydispersity was
19.47 (Fig. 2). The
polydispersity indicates the polymer molecular weight distribution.
The greater the polydispersity is the wider the molecular weight
distribution. Generally, the range of polydispersity value of the
polymer is 1.5–2.0, sometimes as high as 20–50. The polydispersity
of CC-1 was 19.47, which indicated a good molecular weight
distribution.
Fourier transform infrared spectrometer
(FT-IR) analysis
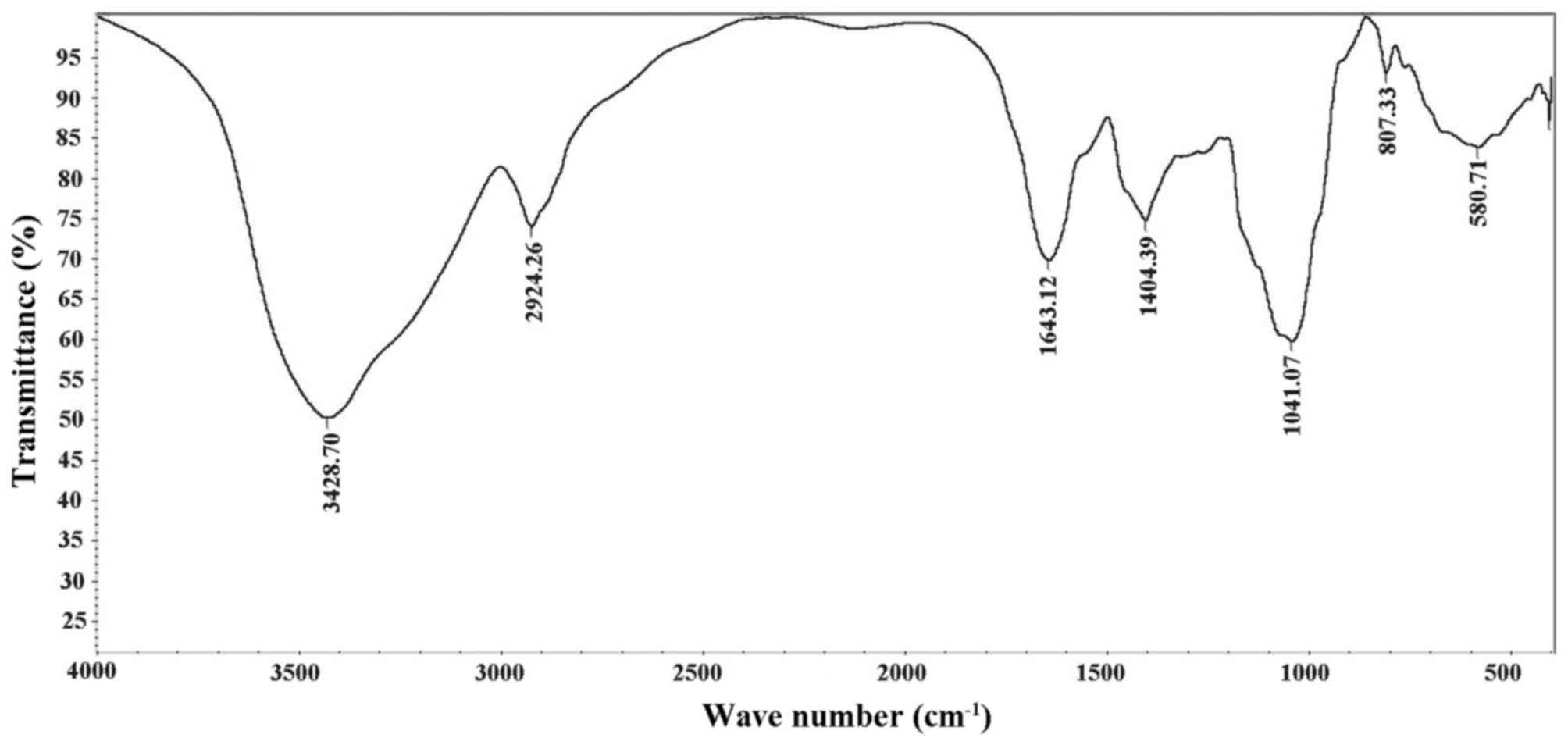
FT-IR was used for structure analysis of CC-1
(Fig. 3). The bands at 3428.70
cm−1 were detection results of OH bond stretching. The
absorption peak at 2924.26 cm−1 was C-H stretching of
vibration absorption peak of CC-1. The strong absorption band at
1643.12 cm−1 was caused by OH deformation vibration. The
bands at 1404.39 cm−1 arose from bending modes of
CH2, CH and OH.
The absorption peaks at 1041.07 cm−1 in
the range of 1200–1000 cm−1 in the IR spectrum suggested
that the monosaccharides in the samples had a pyranose-ring.
Partially, the bands at 1041.07 cm−1 were associated
with the ordered and amorphous structures in CC-1. The bands in the
region of 800–300 cm−1 correspond to C=C stretching and
C-OH bending modes. The bands at 580.71 cm−1 were due to
C-H rocking vibration.
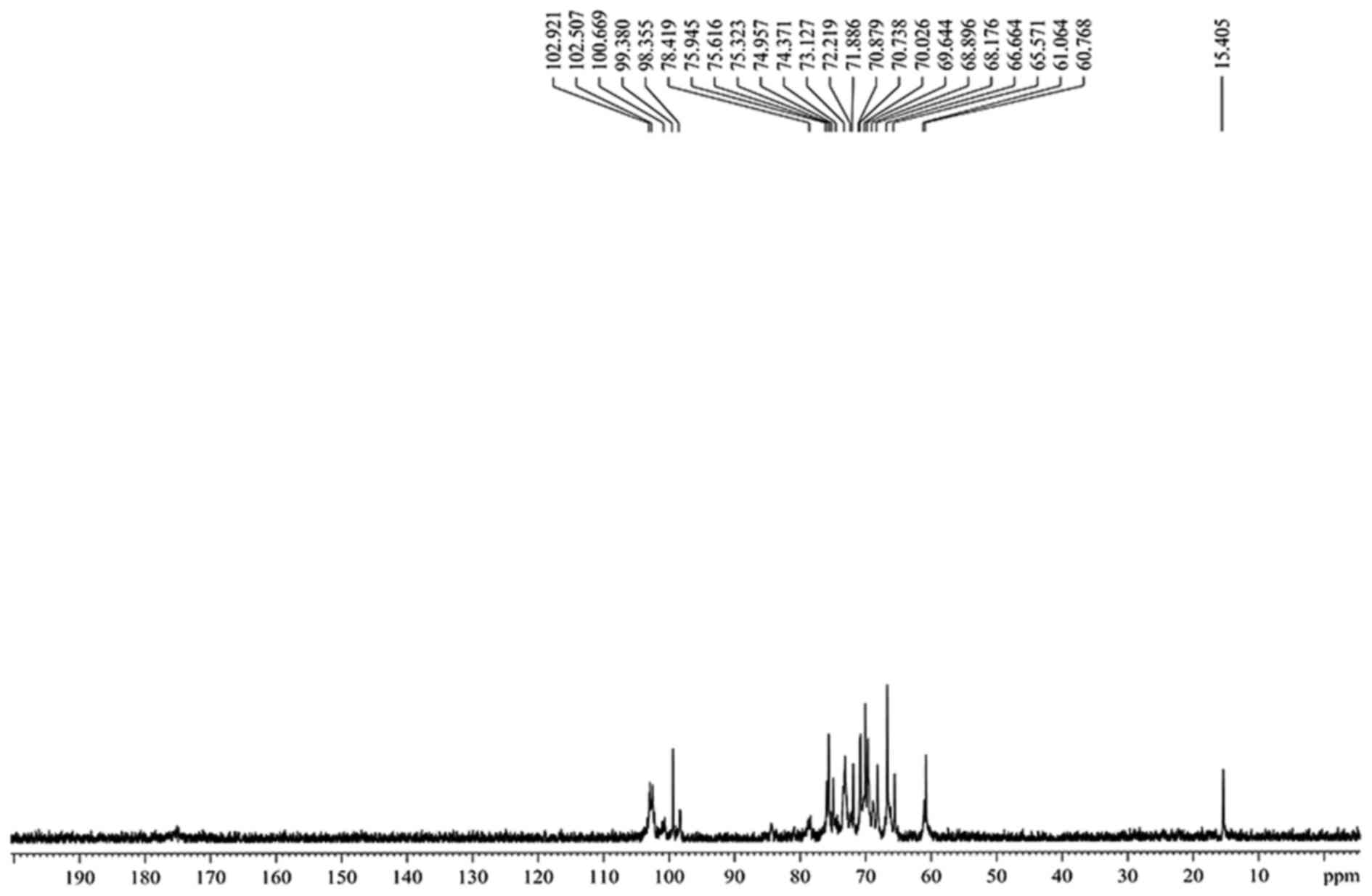
Analysis of the nuclear magnetic
resonance (NMR) experiment results
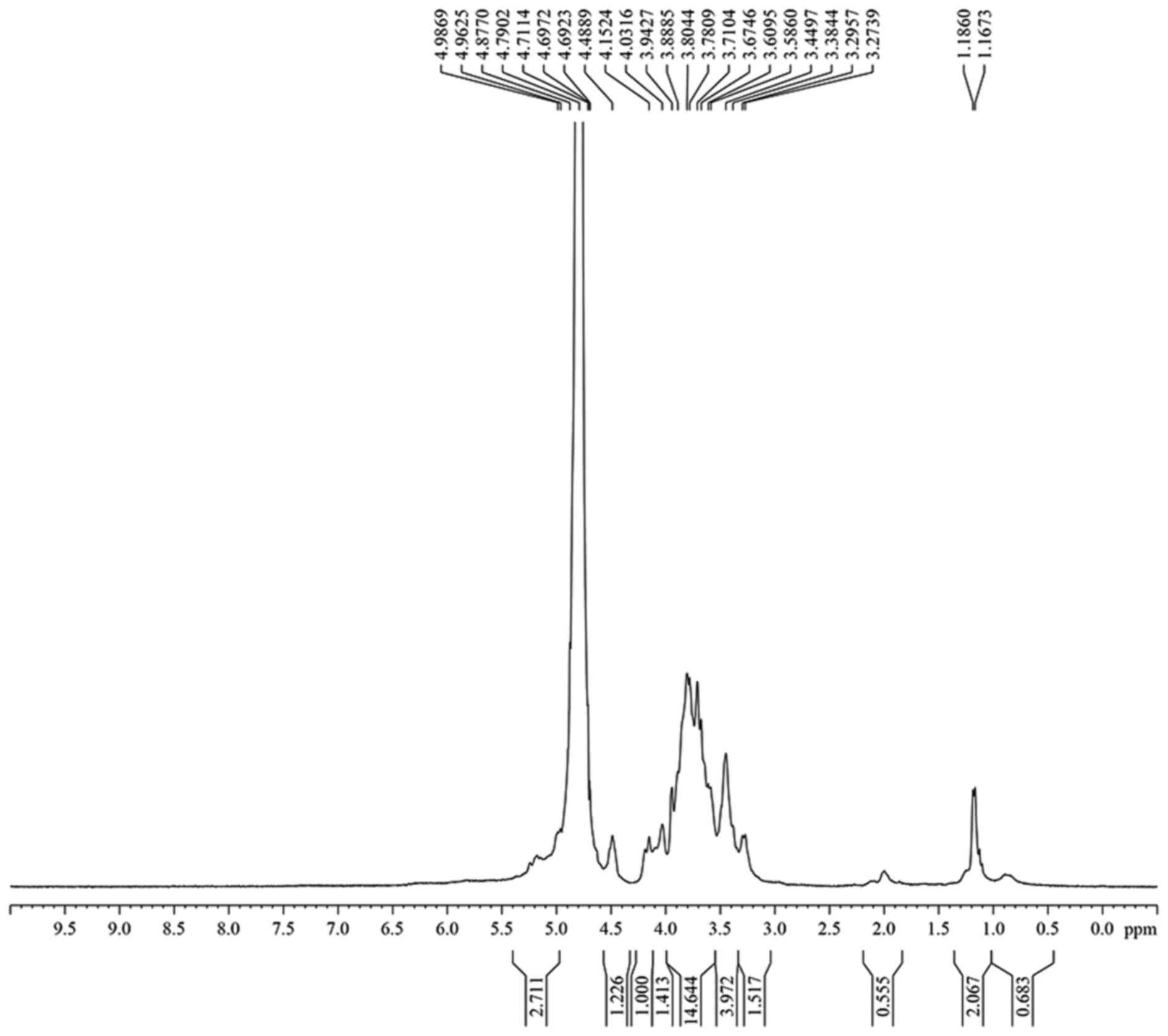
The hydrogen spectrum of CC-1 is shown in Fig. 4. In the 1H NMR(400HZ)
spectrum, δ 4.99 and δ 4.96 indicate there were two anomeric
hydrogen existing in CC-1, suggesting that CC-1 was composed of two
monosaccharides. δ 4.79 was the hydrogen signal of water. The
signals at δ 3.27–δ4.49 are the signal peak of remaining proton
which mostly formed by a number of signal peaks overlapping. The
13C-NMR spectrum of CC-1 (Fig. 5) showed the anomeric peaks were
centralised in δ 99.38-δ 102.92 ppm, indicating there was α
anomeric configuration of monomer in CC-1. The presence of CC-1
signal confirmed that all monomers should be pyran ring, as furan
ring signals should be around δ 107–109 ppm. According to the
literature, the resonance in the region of 98–102 ppm in the
13C NMR (400 MHz) spectrum of CC-1 was attributed to the
anomeric carbon atoms of β-D-glucose and α-D-xylopyranose. The
assignment of the carbon atom signals is shown in Table I.
 | Table I13C chemical shift data
(δ, ppm) for polysaccharide CC-1. |
Table I
13C chemical shift data
(δ, ppm) for polysaccharide CC-1.
| Sugar residues | Chemical shift, δ
(ppm)
|
|---|
| C1 | C2 | C3 | C4 | C5 | C6 |
|---|
|
→4)-α-D-Glcp-(1→ | 98.36 | 66.66 | 70.88 | 73.13 | 68.90 | 65.57 |
|
→3,6)-α-D-Glcp-(1→ | 99.38 | 68.18 | 71.89 | 74.96 | 69.90 | 70.03 |
| α-D-lyx-(1→ | 102.51 | 69.64 | 72.22 | 78.42 | 70.76 | 61.06 |
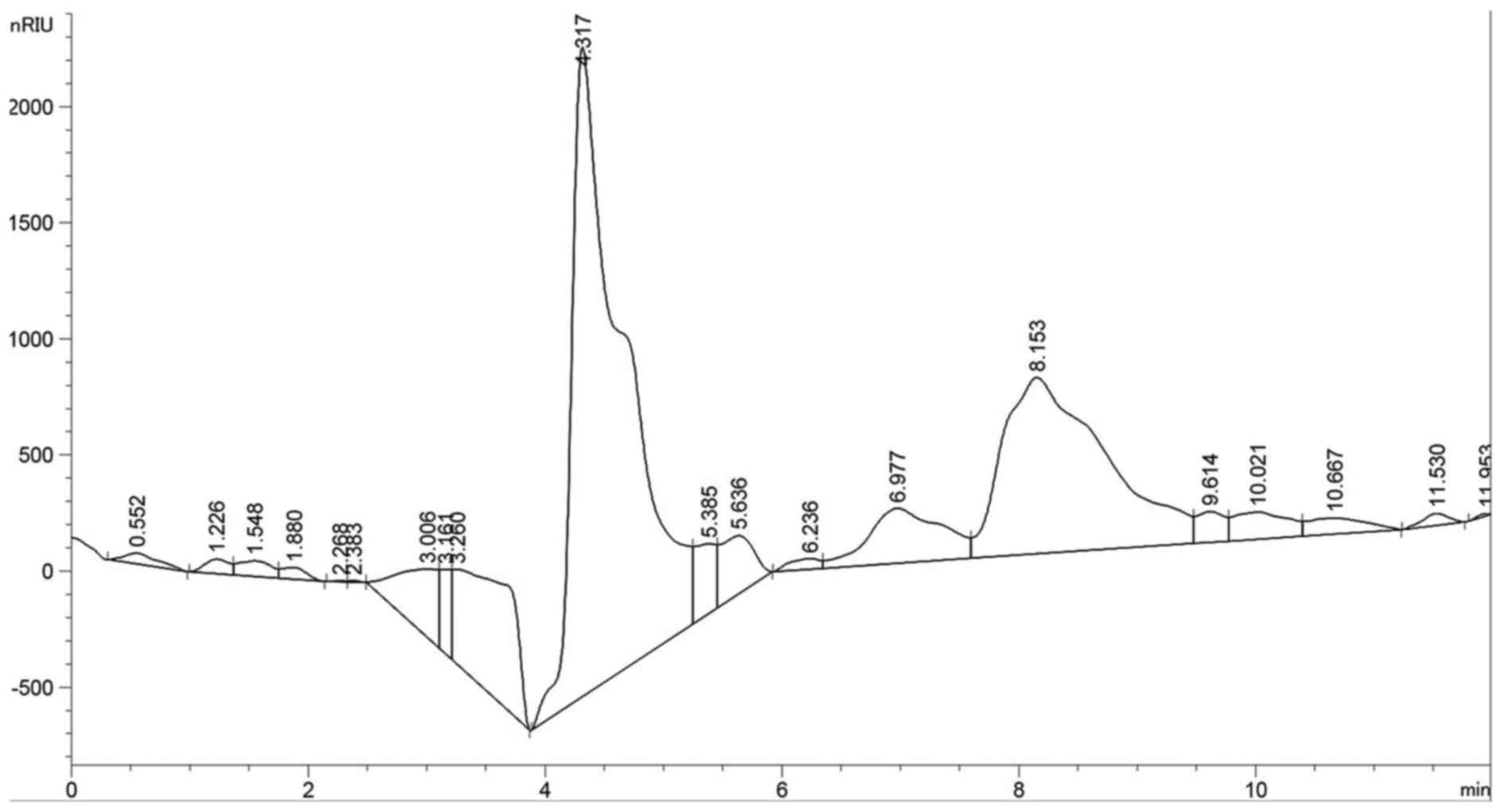
Monosaccharide composition analysis
The composition analysis of polysaccharides is an
important step to control the quality and to obtain basic
information on the polysaccharides. In this study, the CC-1
polysaccharide samples were hydrolyzed with TFA and then the
component monosaccharides were analyzed by HPLC with Agilent
refractive index detector (Fig.
6). Compared with the retention time of the standard
monosaccharide, the peak at retention time of 8.135 min represents
the β-D-glucose and the peak at retention time of 6.977 min
represents the α-D-xylopyranose, at ratio of 5:1. The chromatogram
using an HPLC-RID method shows that CC-1 was composed of two
monosaccharides, β-D-glucose and α-D-xylopyranose, which was in
good agreement with the D-configuration monosaccharide according to
GC-MS analysis.
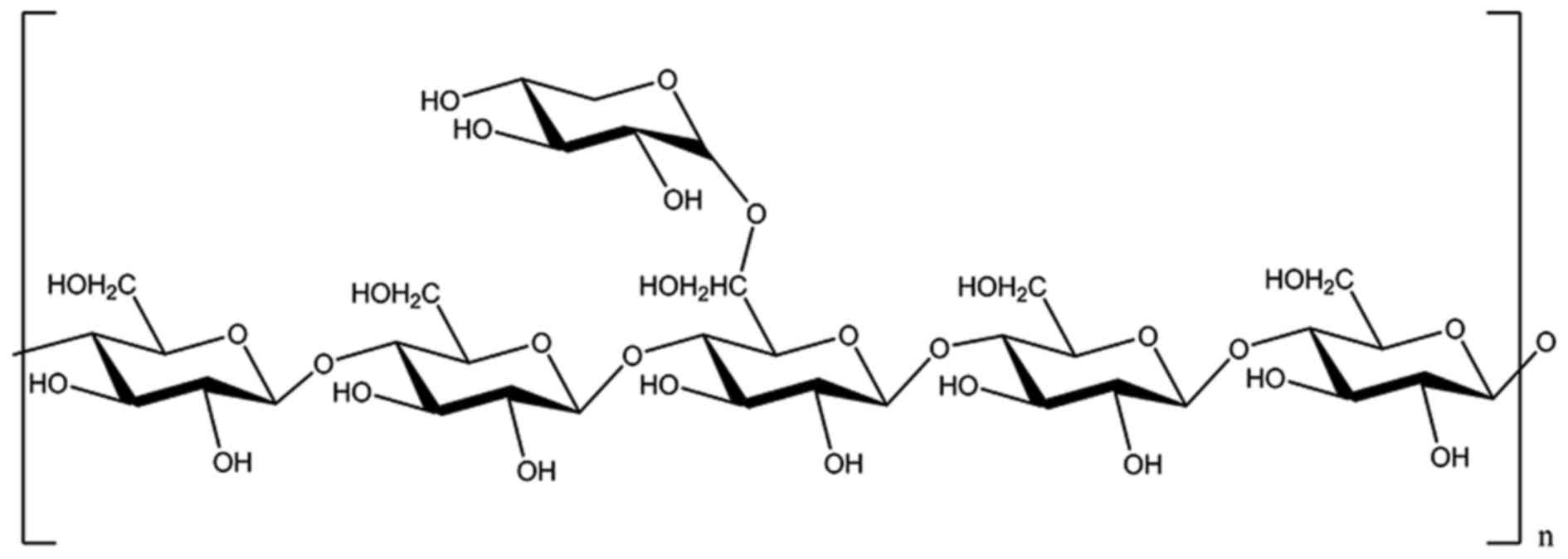
Methylation analysis
The methylated products of CC-1 were hydrolysed with
acid, converted into alditol acetate and analysis by GC-MS. The
experimental data are listed in Table II. The information in MS showed
that fragment ion peaks were consistent with data of
D-configuration monosaccharide fragment ion peaks and it can be
concluded that the xylose and glucose residues were
D-configurations, respectively. The GC-MS spectrum (Fig. 7), results of the silane
experiments need to be discussed further because of the incomplete
methylation, so after the comprehensive analysis of the data, we
inferred that the design feature of the CC-1 may be as follows: the
branched residue was (1→4)-linked-D-glucosepyranose and
(4→6)-linked-D-xylopyranose revealing that
(1→4)-linked-D-glucosepyranose possible form the backbone
structure. Residues of branch structure were terminated with
α-D-xylopyranose resides. It is concluded that a repeating unit of
CC-1 has a backbone of 1,4-linked-β-D-glucose which branched at O-6
and the branches were mainly composed of a →1)-α-D-xylopyranose
residue (Fig. 8).
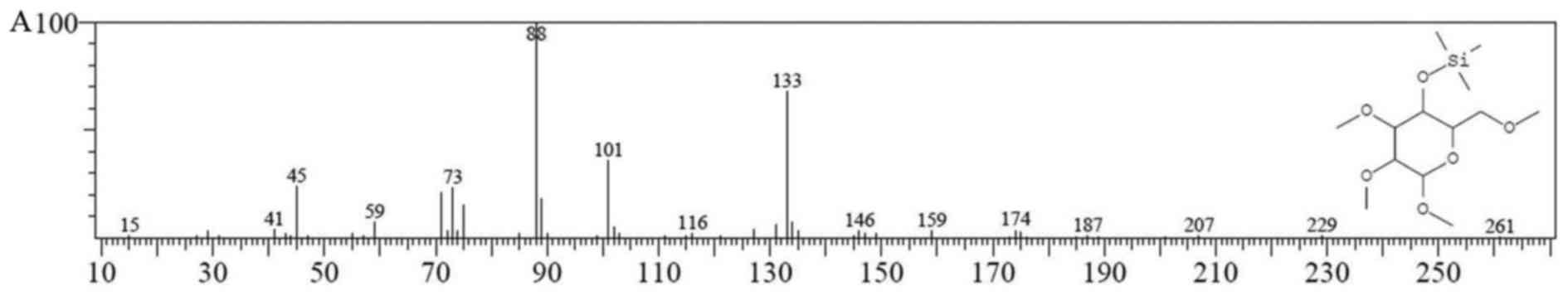 | Figure 7GC-MS chromatogram of Cantharellus
cibarius Fr. polysaccharide (CC-1). The horizontal coordinate
represents the retention time. (A) The fragment ion peaks of
2,3,6-Me 4-Glu; (B) the fragment ion peaks of 2,3-Me 1,4,6-Glu; (C)
the fragment ion peaks of 2,3,6-Me 1,4-Glu; (D) the fragment ion
peaks of 2,3,4-Me 1-Xyl. |
 | Table IIGC-MS results of methylation analysis
of CC-1. |
Table II
GC-MS results of methylation analysis
of CC-1.
| Methylated
sugar | Linkage | m/z |
|---|
| 2,3,6-Me-4-Glu | 4- | 15, 41, 45, 59, 73,
88, 101, 116, 133, 146, 159, 174, 187, 207, 229 |
|
2,3-Me-1,4,6-Glu | 1,4,6- | 59, 73, 89, 103,
117, 133, 147, 159, 175, 191, 205, 217, 232, 243, 259, 287,
377 |
|
2,3,6-Me-1,4-Glu | 1,4- | 29, 45, 59, 73, 88,
101, 113, 133, 146, 159, 175, 185, 201, 217, 232 |
| 2,3,4-Me-1
-Xyl | 1- | 15, 41, 45, 58, 73,
88, 101, 115, 133, 149, 159, 174, 185 |
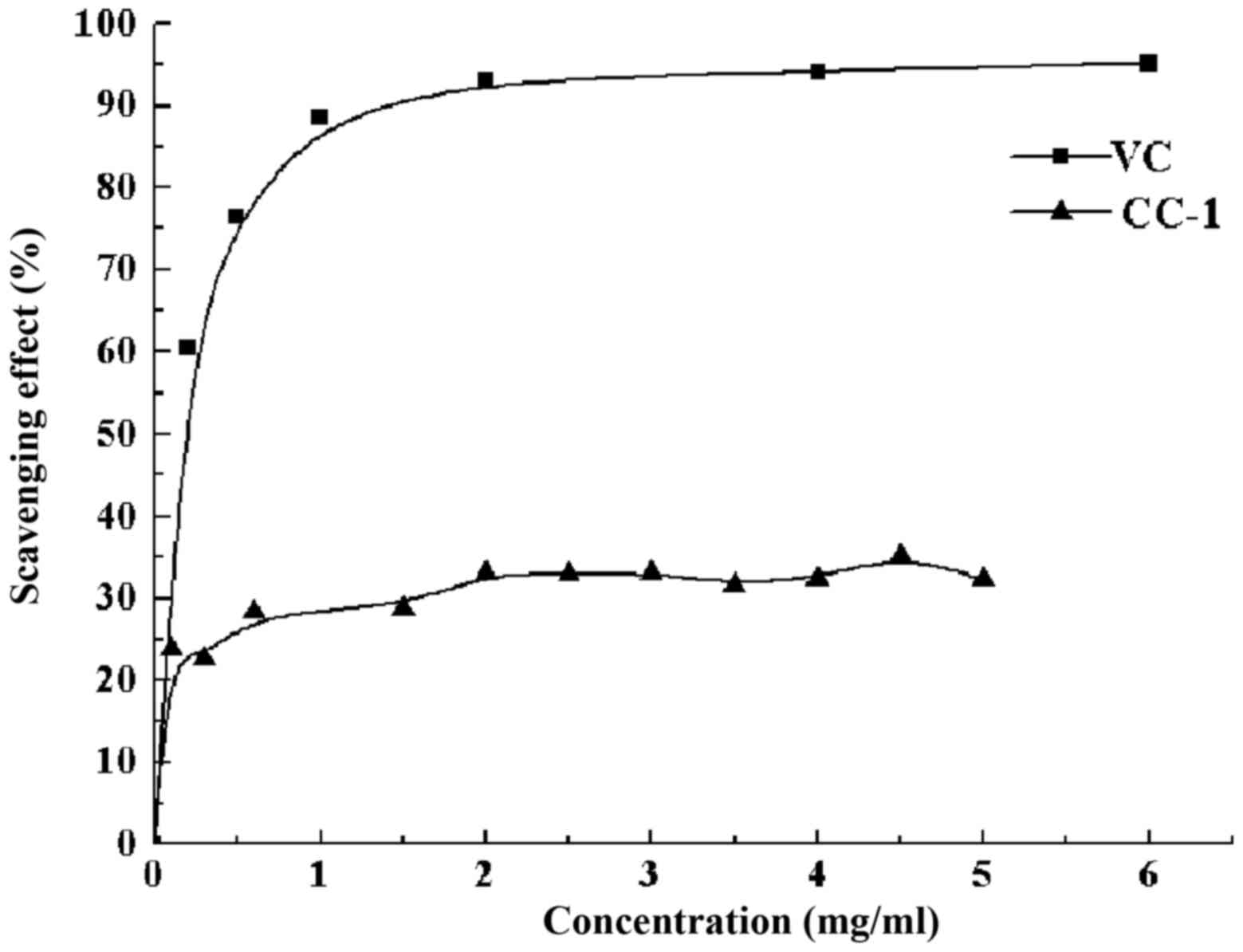
DPPH− free radical scavenging
activity of CC-1
The decrease of the absorbance of the resultant
solution is caused by the removal of the DPPH free radical.
Obviously, the color changed from purple to yellow. CC-1 exhibited
an antioxidant activity compared with that of standard ascorbic
acid at varying concentration tested. There was a dose-dependent
increase in the percentage of antioxidant activity for all
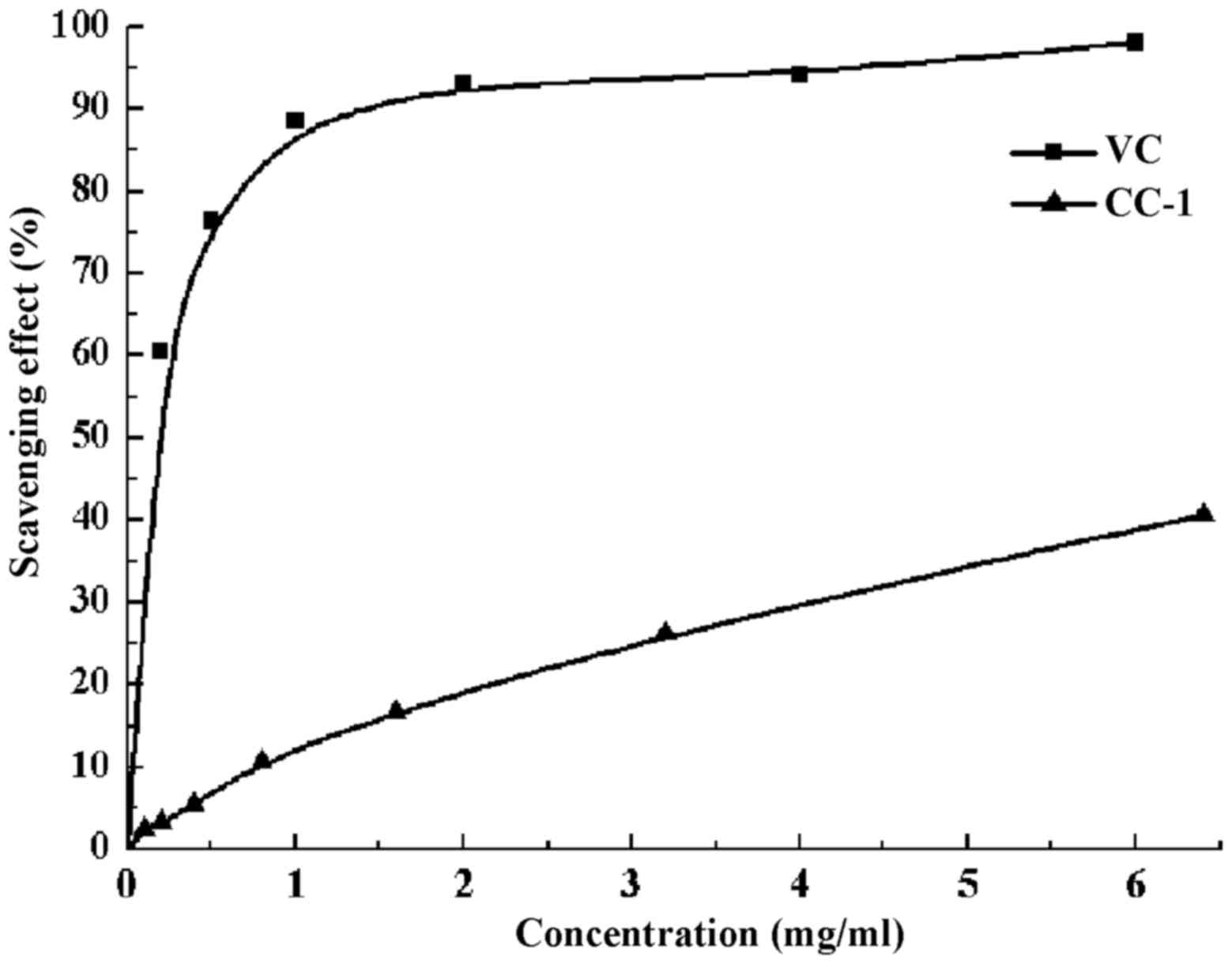
concentrations tested (Fig. 9).
The CC-1 at a concentration of 0.1 mg/ml showed a percentage
inhibition of 23.81% and for 4.5 mg/ml it achieved maximum of
35.23%. The clearance rate stabilized at around 23–36% when the
concentration of CC-1 is in the range of 0.1–5 mg/ml without
significant change. However, the scavenging ability was lower than
that of Vc.
ABTS+ radical scavenging
activity of CC-1
ABTS+ is used to mensurate total
antioxidant capacity. ABTS+ will change into green under
the appropriate oxidation, and the oxidation will be suppressed in
the presence of antioxidants. The ABTS+ radical
scavenging activity of CC-1 was tested spectrophotometrically at
734 nm. The results of antioxidant activity of CC-1 was expressed
as shown in Fig. 10. The
scavenging ability on ABTS+ radical of CC-1 is
positively correlated when its concentration is 0.1–6.4 mg/ml. When
the concentration of CC-1 is 6.4 mg/ml, the scavenging rate of
ABTS+ free radical can reach 40.70%, and the
IC50 value of CC-1 was 7.8624 mg/ml.
Effect of CC-1 on B cell activation in
vitro
B cells, also known as B lymphocytes, are a subtype
of white blood cells of the lymphocytes. They function in the
humoral immunity component of the adaptive immune system by
secreting antibodies. The stimulation of CC-1 on B cells is shown
in Fig. 11A. Cell proliferation
activity was very low when B cells were exposed to medium alone,
whereas incubation of these cells with increasing concentrations of
CC-1 was associated with a dose-dependent increase in cell
proliferation activity. Compared with the control group, the low
concentration of CC-1 significantly promoted B cells proliferation
(0.625–80 µg/ml, p<0.01). Furthermore, cell proliferation
activity at 5 µg/ml concentrations of CC-1 were comparable
to or even greater than that elicited by 5 µg/ml LPS. It is
worth noting that, the optimal concentration of CC-1 is 20
µg/ml, when more than this concentration, the cell growth
rate decline, but it is still very significant.
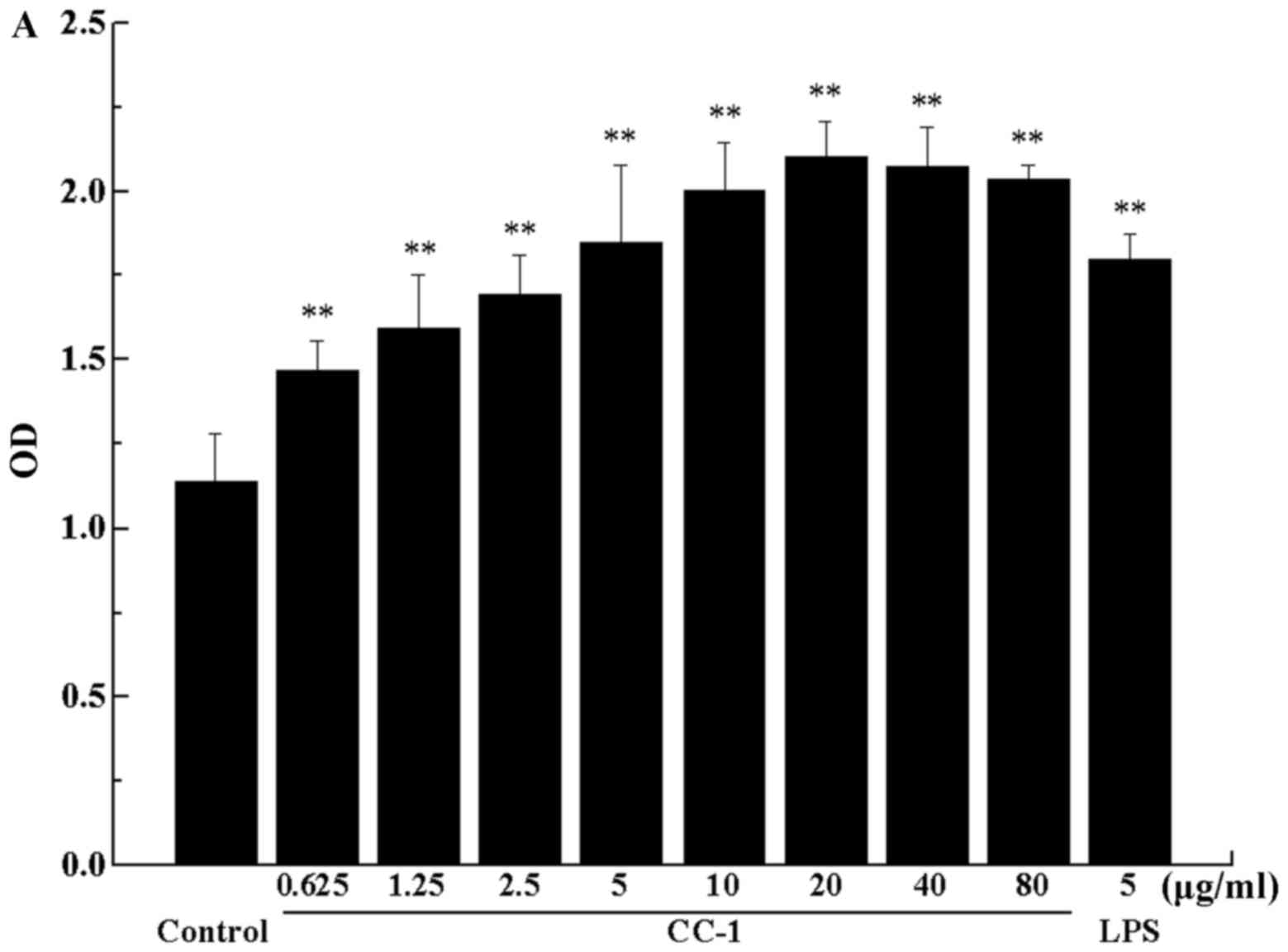 | Figure 11(A) The effect of Cantharellus
cibarius Fr. polysaccharide (CC-1) on the proliferation of B
cells. The horizontal coordinate indicates the concentration of
CC-1 and the vertical coordinate indicates the OD value of the B
cells. (B) The cell morphology effect of CC-1 on the proliferation
of B cells. When the concentration of 20 µg/ml of CC-1 was
used to stimulate the B cell, the B cell clusters up most
obviously. a, the blank group; b-i, the CC-1 experimental groups,
cells treated with 0.625, 1.25, 2.5, 5, 10, 20, 40 and 80
µg/ml CC-1; j, the lipopolysaccharide (LPS) group (5
µg/ml). Control, the blank group. Each value is presented as
the mean ± SD (n=5). *P<0.05 and
**P<0.01 compared with the control group. |
Effect of CC-1 on T cell activation in
vitro
T cells are a type of lymphocytes which play a
central role in cell-mediated immunity. They are called T cells
because they mature in the thymus from thymocytes. The stimulation
of CC-1 on T cells are shown in Fig.
12A. Compared with the control group, the low concentration of
CC-1 significantly promote T cell proliferation (0.625–2.5
µg/ml, p<0.05; 5–20 µg/ml, p<0.01). Cell
proliferation activity at 5 µg/ml concentration of CC-1 was
comparable to or even greater than that elicited by 5 µg/ml
LPS, and the proliferation effect of T cells reached the maximum
value. When the concentration was further increased, the rate of
the increase in T cells declined.
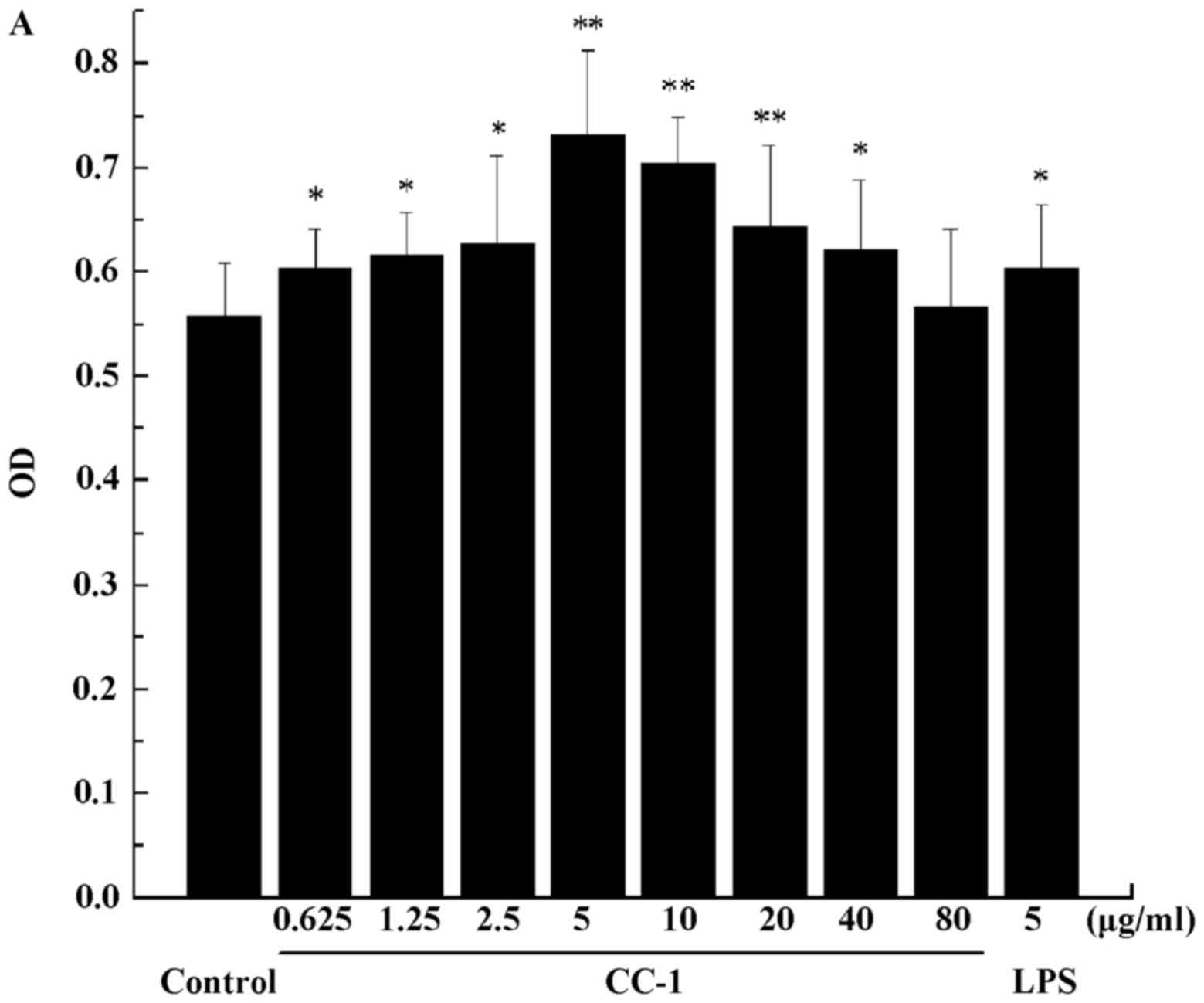 | Figure 12(A) The effect of Cantharellus
cibarius Fr. polysaccharide (CC-1) on the proliferation of T
cells. The horizontal coordinate indicates the concentration of
CC-1 and the vertical coordinate indicates the OD value of the T
cells. (B) The cell morphology effect of CC-1 on the proliferation
of T cells. When the concentration of 5 µg/ml of CC-1
wasused to stimulate the T cell, the T cell has the most vigorous
proliferative capacity. a, the blank group; b-i, the CC-1
experiment groups, cells treated with 0.625, 1.25, 2.5, 5, 10, 20,
40 and 80 µg/ml CC-1; j, the lipopolysaccharide (LPS) group
(5 µg/ml). Control, the blank group. Each value is presented
as the mean ± SD (n=5). *P<0.05 and
**P<0.01 compared with the control group. |
Cell morphology observation
The cell morphology of B cells and T cells is
revealed in Figs. 11B and
12B, respectively. With the
increase of the concentration of CC-1, the cells accelerated
division and became larger and larger. When the concentration of 20
µg/ml of CC-1 was used to stimulate the B cell, the B cells
clustered up most obviously. When the concentration of 5
µg/ml of CC-1 was used to stimulate the T cells, the T cells
showed the most vigorous proliferative capacity.
Conclusions and discussion
Villares et al (33) previously reported the structural
characterization of two polysaccharides isolated from the fruiting
bodies of the wild edible mushroom Cantharellus cibarius.
The polysaccharide from the boiling water fraction (PsCcib-I) was a
glucan-type carbohydrate with a molecular weight of 15,002 kDa. The
methylation analysis and NMR experiments showed that PsCcib-I was
composed of a main chain consisting of α-(1→6)-Glc units with
β-(1→4)-linked branches every third glucose residue. The present
study revealed that the polysaccharide obtained from
Cantharellus cibarius Fr., is a heteropolysaccharide, namely
CC-1. The purified polysaccharide (CC-1) prepared was confirmed of
high purity. Structure analysis indicated CC-1 consists of a
backbone of 1,4-linked-β-D-glucose which branched at O-6 and the
branches were mainly composed of a →1)-α-D-xylopyranose
residue.
In addition, in the experiments of DPPH free radical
scavenging activity, under the same experimental condition, the
scavenging ability of CC-1 was lower than that of Vc. DPPH is a
stable nitrogen centered free radical and its stability is from the
three benzene ring resonance stabilization, and steric hindrance,
cliping on the nitrogen atom of the intermediate unpaired
electrons, thus cannot function as electron pairs. As a stable free
radical, DPPH can capture other free radicals. According to the
respective structure characteristics of the polysaccharide
molecules, we can infer that the average molecular weight and the
degree of polymerization of polysaccharide extracted are greater,
while the amount of isolated hydroxyl are less. Therefore, the
scavenging activity of CC-1 through the direct reduction of the
electron and proton depend on the isolated hydroxyl, which make it
possible to decease the capacity of the N=N double bond in DPPH by
oxidation-reduction reaction. This may be the cause of DPPH free
radical scavenging rate being lower than the rate of the
ABTS+ free radical scavenging under the action of
CC-1.
T lymphocytes, referred to as T cells, originate
from bone marrow, migrate to the thymus for differentiation and
mature. Mature T cells can specifically bind with target cells,
directly kill the target cells, or release the lymphatic factors,
which enhances the immune effect mainly in the body's cellular
immunity. B cells are from the auxe of hematopoietic stem cells of
mammalian bone marrow or bird bursa. In antigen stimulation, B
cells differentiate into plasma cells that synthesize and secrete
antibodies, and perform humoral immune function. In this study,
CC-1 could effect the proliferation and cell morphology of T and B
cells. The LPS as the positive control. The results show that, T
and B cells can promote proliferation effect and CC-1 concentration
(<5 µg/ml) was positively related and cell morphology was
not changed, with good state of cells. Polysaccharides with high
molecular weight in different concentrations will lead to different
aggregation degrees of molecules, which will eventually affect the
immune activity in B cells and T cells in different concentrations
of CC-1 since B cells and T cells have different receptors on the
surface. But the specific molecular mechanism needs further study.
Cantharellus cibarius Fr. may be used in nutritional or
pharmaceutical fields.
Acknowledgments
The authors would like to thank all the
participants, who provided feedback so hat the research on the
programme could be achieved.
Notes
[1]
Funding
This study was supported by the National Natural
Science Foundation of China (31400016 and 31200012), the Cultivate
Major Projects of Sichuan Province (16CZ0018), the Nanchong Science
and Technology Bureau of Sichuan Province (16YFZJ0043), the Talent
Program of China West Normal University (17YC328, 17YC136 and
17YC329), the National Training Project of China West Normal
University (17c039) and the Innovative Team Project of China West
Normal University (CXTD 2017-3).
[2] Availability
of data and material
The data supporting the findings can be found in the
Key Laboratory of Southwest China Wildlife Resources Conservation,
College of Life Sciences, China West Normal University, Nanchong,
China.
[3] Authors'
contributions
YH conceived the presented idea. DZ and XD carried
out the experiment. DZ and YH wrote the manuscript. All authors
discussed the results and implications and commented on the
manuscript at all stages.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown MW, Kolisko M, Silberman JD and
Roger AJ: Aggregative multicellularity evolved independently in the
eukaryotic supergroup Rhizaria. Curr Biol. 22:1123–1127. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pawlowski J, Audic S, Adl S, Bass D,
Belbahri L, Berney C, Bowser SS, Cepicka I, Decelle J, Dunthorn M,
et al CBOL Protist Working Group; CBOL protist working group:
Barcoding eukaryotic richness beyond the animal, plant, and fungal
kingdoms. PLoS Biol. 10:e10014192012. View Article : Google Scholar
|
|
3
|
Mansour-Benamar M, Savoie JM and Chavant
L: Valorization of solid olive mill wastes by cultivation of a
local strain of edible mushrooms. C R Biol. 336:407–415. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang ST: The World Mushroom Industry:
Trends and technological development. Int J Med Mushrooms.
8:297–314. 2006. View Article : Google Scholar
|
|
5
|
Pacheco L: Sex differences in mushroom
gathering: Men expend more energy to obtain equivalent benefits.
Evol Hum Behav. 31:289–297. 2010. View Article : Google Scholar
|
|
6
|
Leonti M: The future is written: Impact of
scripts on the cognition, selection, knowledge and transmission of
medicinal plant use and its implications for ethnobotany and
ethnopharmacology. J Ethnopharmacol. 134:542–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hussain H, Krohn K, Schulz B, Draeger S,
Nazir M and Saleem M: Two new antimicrobial metabolites from the
endophytic fungus, Seimatosporium sp. Nat Prod Commun. 7:293–294.
2012.PubMed/NCBI
|
|
8
|
Yang WT, Shi SH, Jiang YL, Zhao L, Chen
HL, Huang KY, Yang GL and Wang CF: Genetic characterization of a
densovirus isolated from great tit (Parus major) in China. Infect
Genet Evol. 41:107–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kievit FM, Florczyk SJ, Leung MC, Wang K,
Wu JD, Silber JR, Ellenbogen RG, Lee JS and Zhang M: Proliferation
and enrichment of CD133(+) glioblastoma cancer stem cells on 3D
chitosan-alginate scaffolds. Biomaterials. 35:9137–9143. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Obach RS: Pharmacologically active drug
metabolites: Impact on drug discovery and pharmacotherapy.
Pharmacol Rev. 65:578–640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo H, Yi W, Song JK and Wang PG: Current
understanding on biosynthesis of microbial polysaccharides. Curr
Top Med Chem. 8:141–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferreira SS, Passos CP, Madureira P,
Vilanova M and Coimbra MA: Corrigendum to 'Structure-function
relationships of immunostimulatory polysaccharides: A review'
[Carbohydr Polym. 132 (2015) 378–396]. Carbohydr Polym.
147:557–558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeung MK: Molecular and genetic analyses
of Actinomyces spp. Crit Rev Oral Biol Med. 10:120–138. 1999.
View Article : Google Scholar
|
|
14
|
Ganeshapillai J, Vinogradov E, Rousseau J,
Weese JS and Monteiro MA: Clostridium difficile cell-surface
polysaccharides composed of pentaglycosyl and hexaglycosyl
phosphate repeating units. Carbohydr Res. 343:703–710. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ebert B, Rautengarten C, Guo X, Xiong G,
Stonebloom S, Smith-Moritz AM, Herter T, Chan LJ, Adams PD, Petzold
CJ, et al: Identification and characterization of a golgi-localized
UDP-xylose transporter family from arabidopsis. Plant Cell.
27:1218–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hadley B, Maggioni A, Ashikov A, Day CJ,
Haselhorst T and Tiralongo J: Structure and function of nucleotide
sugar transporters: Current progress. Comput Struct Biotechnol J.
10:23–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dubois M, Gibbs KA, Hamilton JK, Rebers PA
and Smith F: Colorimetric methods for the determination of sugars
and related substances. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
18
|
Wang F, Hou Y, Ding X, Hou W, Song B, Wang
T, Li J and Zeng Y: Structure elucidation and antioxidant effect of
a polysaccharide from Lactarius camphoratum (Bull.) Fr. Int J Biol
Macromol. 62:131–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YT, Kim EH, Cheong C, Williams DL, Kim
CW and Lim ST: Structural characterization of beta-D-(1→3,
1→6)-linked glucans using NMR spectroscopy. Carbohydr Res.
328:331–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao W, Li XQ, Liu L, Wang M, Fan HT, Li C,
Lv Z, Wang X and Mei Q: Structural analysis of water-soluble
glucans from the root of Angelica sinensis (Oliv.) Diels. Carbohydr
Res. 341:1870–1877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Luo D and Liang Z: Structure of
polysaccharides from the fruiting body of Hericium erinaceus Pers.
Carbohydr Polym. 57:241–247. 2004. View Article : Google Scholar
|
|
22
|
Ding X, Hou Y, Hou W, Zhu Y, Fu L and Zhu
H: Structure elucidation and anti-tumor activities of water-soluble
oligosaccharides from Lactarius deliciosus (L. ex Fr.) Gray.
Pharmacogn Mag. 11:716–723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding X, Hou Y, Zhu Y, Wang P, Fu L, Zhu H,
Zhang N, Qin H, Qu W, Wang F, et al: Structure elucidation,
anticancer and antioxidant activities of a novel polysaccharide
from Gomphus clavatus Gray. Oncol Rep. 33:3162–3170. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gentili A, Caretti F, D'Ascenzo G,
Marchese S, Perret D, Di Corcia D and Rocca LM: Simultaneous
determination of water-soluble vitamins in selected food matrices
by liquid chromatography/electrospray ionization tandem mass
spectrometry. Rapid Commun Mass Spectrom. 22:2029–2043. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo A and Fan Y: In vitro antioxidant of a
water-soluble polysaccharide from Dendrobium fimhriatum
Hook.var.oculatum Hook. Int J Mol Sci. 12:4068–4079. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Auddy B, Ferreira M, Blasina F, Lafon L,
Arredondo F, Dajas F, Tripathi PC, Seal T and Mukherjee B:
Screening of antioxidant activity of three Indian medicinal plants,
traditionally used for the management of neurodegenerative
diseases. J Ethnopharmacol. 84:131–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sudo A, Uenishi K and Endo T: Anionic
copolymerization of epoxide with bifunctional aromatic lactone
derived from 2-methylresorcinol. J Polym Sci Pol Chem.
46:3447–3451. 2008. View Article : Google Scholar
|
|
28
|
Bradford PA, Petersen PJ, Young M, Jones
CH, Tischler M and O'Connell J: Tigecycline MIC testing by broth
dilution requires use of fresh medium or addition of the
biocatalytic oxygen-reducing reagent oxyrase to standardize the
test method. Antimicrob Agents Chemother. 49:3903–3909. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding X, Hou Y and Hou W: Structure feature
and antitumor activity of a novel polysaccharide isolated from
Lactarius deliciosus Gray. Carbohydr Polym. 89:397–402. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou Y, Ding X and Hou W: Composition and
antioxidant activity of water-soluble oligosaccharides fro Hericium
erinaceus. Mol Med Rep. 11:3794–3799. 2015. View Article : Google Scholar
|
|
31
|
Luo D: Identification of structure and
antioxidant activity of a fraction of polysaccharide purified from
Dioscorea nipponica. Makino. Carbohydr Polym. 71:544–549. 2008.
View Article : Google Scholar
|
|
32
|
Hou Y, Ding X, Hou W, Song B, Wang T, Wang
F, Li J, Zeng Y, Zhong J, Xu T, et al: Pharmacological evaluation
for anticancer and immune activities of a novel polysaccharide
isolated from Boletus speciosus Frost. Mol Med Rep. 9:1337–1344.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Villares A, García-Lafuente A, Guillamón E
and Mateo-Vivaracho L: Separation and characterization of the
structural features of macromolecular carbohydrates from wild
edible mushrooms. Bioac Carbohyd Diet Fibr. 2:15–21. 2013.
View Article : Google Scholar
|