Introduction
Osteosarcoma, which is also known as osteogenic
sarcoma, is a malignant form of bone tumor that more commonly
occurs in adolescents and children <20 years old (1); osteosarcoma accounts for ~5% of
pediatric tumors (2).
Osteosarcoma is associated with high malignancy and rapid progress,
and can induce early lung metastasis. Furthermore, osteosarcoma has
a high recurrence rate and poor prognosis; the 5-year survival rate
of patients with osteosarcoma is only 20%, and it is associated
with a heavy burden to family members and society (3–5).
In recent years, with the development of neoadjuvant
chemotherapy/postoperative adjuvant chemotherapy, and the
combination of surgical treatment and radiotherapy, the prognosis
of patients with osteosarcoma has improved; however, the 5-year
survival rate could only be increased to ~60% (6–8).
Numerous studies have been conducted by researchers with regards to
the mechanisms underlying the occurrence and development of
osteosarcoma; however, the pathogenesis of osteosarcoma remains to
be elucidated. Therefore, the pathogenesis of osteosarcoma requires
further study, in order to provide scientific evidence for the
diagnosis, treatment and prognosis of osteosarcoma. Elucidation of
the pathogenesis of osteosarcoma may result in more effective
control of the pathogenic process of osteosarcoma, and may improve
patient prognosis, and reduce the burden on patients, families and
society (9,10).
Tumor occurrence and development are the results of
numerous gene mutations, including activation of oncogenes and
inactivation of tumor suppressor genes (11,12). At present, the majority of studies
have suggested that activation of oncogenes serves an important
role in tumor occurrence and development (13,14). During the occurrence and
development of tumors, tumor necrosis factor receptor-associated
factor 6 (TRAF6) functions as a cancer gene (15,16). TRAF6 belongs to the TRAF family;
TRAF is a cytoplasmic adapter protein, which comprises a family of
seven members (TRAF 1–7). These members serve important roles in
various signaling pathways, including the tumor necrosis factor
receptor superfamily and interleukin-1 receptor superfamily, which
are involved in regulating cell survival and cell death (17–19). TRAF6 is closely associated with
the maturation and differentiation of osteoclasts in vitro
(20). Recent studies have
reported that TRAF6 serves an important role in the development,
invasion and metastasis of tumors by activating nuclear factor
(NF)-κB (21,22). However, studies regarding the
expression of TRAF6 in osteosarcoma and its biological role are
rare.
Previous studies have revealed that TRAF6 is highly
expressed in lung cancer, glioma and esophageal cancer, and it is
associated with tumor invasion and metastasis (15,23–25). In addition, it has been suggested
that TRAF6 may promote tumor angiogenesis (26). Our previous study indicated that
TRAF6 mRNA and protein was highly expressed in osteosarcoma tissues
(27). This finding was
consistent with the expression pattern of TRAF6 in other tumors.
Numerous studies have been conducted in the field of osteosarcoma;
however, studies regarding the mechanism underlying the effects of
TRAF6 on the pathogenesis of osteosarcoma are rare, and why TRAF6
expression is upregulated in osteosarcoma remains unclear.
Therefore, research regarding the regulation of TRAF6 expression
may have important theoretical significance and practical
value.
In recent years, a novel method of gene expression
regulation, namely microRNA (miRNA/miR) regulation, has been
discovered (28,29). The present study aimed to
determine whether the high expression of TRAF6 in osteosarcoma
cells was due to miRNA regulation. Therefore, databases, including
TargetScan, miRanda and PicTar, were analyzed using online
software, so as to predict the possible regulatory effects of miRNA
on TRAF6, thus resulting in the identification of miR-124. It has
previously been reported that miR-124 exhibits low expression in
breast cancer tissues, whereas overexpression of miR-124 could
inhibit the proliferation and migratory ability of breast cancer
cells (30). In addition,
overexpression of miR-124 was able to inhibit the invasion and
migration of ovarian cancer cells (31). Therefore, the present study
hypothesized that the biological effects of TRAF6 in osteosarcoma
were regulated by miR-124. The present study confirmed that TRAF6
was a target gene of miR-124 using a luciferase reporter gene
system, after which the biological effects of miR-124 on human
osteosarcoma cells were determined according to a series of
experiments. The present study further tested and verified the
biological role of miR-124 via the regulation of TRAF6 using an
overexpression vector.
Materials and methods
Specimens
A total of 16 fresh osteosarcoma specimens and
normal bone tissues were collected from patients with osteosarcoma
(n=16; 8 male and 7 female; 11 younger than 40 years old and 5
older than 40 years old) by surgical excision; specimens were
immediately stored in liquid nitrogen. None of the patients had a
history of chemotherapy, radiotherapy or other treatments, and did
not have other inflammatory diseases. The present study was
approved by the Ethics Committee of Yancheng City No. 1 People's
Hospital (Yancheng, China; approval no. 2015YCYY-0001). The
patients provided written informed consent prior to sample
collection.
Main reagents
The miR-124 reverse transcription (RT) primer
(5′-GATACTCATAAGGCACGCGG-3′ and 5′-GTGCAGGGTCCGAGGT-3′), miR-124
mimic and non-specific control (mimic control) were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China).
The TaqMan miRNA isolation kit, TaqMan miRNA assay
and TaqMan Universal polymerase chain reaction (PCR) master mix
were purchased from Applied Biosystems; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA).
Fetal bovine serum (FBS), Dulbecco's modified
Eagle's medium (DMEM), Eagle's Minimum Essential medium (EMEM),
L-glutamine and HEPES were purchased from Gibco; Thermo Fisher
Scientific, Inc., and Lipofectamine® 2000 was purchased
from Invitrogen; Thermo Fisher Scientific, Inc. Nunc™ cell culture
plates and culture dishes were obtained from Thermo Fisher
Scientific, Inc., and the Transwell invasion chamber was purchased
from Corning Incorporated (Corning, NY, USA).
MTT and radioimmunoprecipitation assay (RIPA) lysis
buffer were purchased from Beyotime Institute of Biotechnology
(Shanghai, China). Trypsin, PBS and Hoechst 33342 were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Matrigel was
purchased from BD Biosciences (Franklin Lakes, NJ, USA). Annexin V
and propidium iodide (PI) were purchased from Roche Diagnostics
(Indianapolis, IN, USA). Rabbit anti-human TRAF6 polyclonal
antibody and mouse anti-human β-actin monoclonal antibody were
purchased from Abcam (Cambridge, MA, USA). Horseradish peroxidase
(HRP)-conjugated affinity-purified goat anti-mouse and goat
anti-rabbit immunoglobulin (Ig)G secondary antibodies were
purchased from Sigma-Aldrich; Merck KgaA. A protein extraction and
quantification kit (BCA kit) was purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA).
The carrier pcDNA3.1, pGEM-T and pGL3-Basic vectors,
competent cells (DH5α) and TRIzol® total RNA extraction
kit were purchased from Invitrogen; Thermo Fisher Scientific, Inc.
BamHI and XhoI restriction enzymes, and DNA Marker
were purchased from Takara Bio, Inc. (Otsu, Japan). T4 DNA ligase
was purchased from Promega Corporation (Madison, WI, USA). Taq DNA
polymerase and prestained protein marker were purchased from
Fermentas; Thermo Fisher Scientific, Inc. Transcriptor First Strand
cDNA synthesis kit was purchased from Qiagen GmbH (Hilden,
Germany).
Cell treatment
MG-63 cells were purchased from Shanghai Cell Bank
of Chinese Academy of Sciences (Shanghai, China), and were cultured
in EMEM (2 mM L-glutamine, 1.0 mM sodium pyruvate, 0.1 mM
non-essential amino-acid) containing 10% FBS. 293 cells were
purchased from American Type Culture Collection (Manassas, VA,
USA), and were cultured in DMEM containing 10% FBS.
Construction of recombinant TRAF6
expression plasmids
According to the human TRAF6 mRNA sequence in
GenBank (GI: 1732425; https://www.ncbi.nlm.nih.gov/genbank/) and enzyme
cleavage site analysis, a pair of primers was designed using Primer
Premier 5 software (Premier Biosoft International, Palo Alto, CA,
USA) in the two flanks of the open reading frame of TRAF6. The
primer sequences were as follows: Upstream primer P1,
5′-CGGGATCCATGAGTCTGCTAAACTGTGA-3′,
which contained the BamHI enzyme cleavage site; and
downstream primer P2, 5′-CCCTCGAGTACCCCTGCATCAGTACTTC-3′,
which contained the XhoI enzyme cleavage site (the
underlined sections of the primer sequences indicate the
restriction endonuclease site). The primers were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China).
Cultured MG-63 cells were collected and treated with
1 ml TRIzol® reagent; total RNA was extracted according
to manufacturer's protocol. RT was conducted using a first strand
cDNA synthesis kit, and the first chain was produced according to
the manufacturer's protocol. The TRAF6 primer was dissolved in
ddH2O and used to amplify TRAF6 by PCR. The PCR product
was then ligated into the pGEM-T carrier under T4 DNA ligase, so as
to screen positive clones. The positive clone was verified by
sequencing, and the subclone was merged into the pcDNA3.1
expression vector to construct the recombinant expression vector,
pcDNA3.1-TRAF6. The RT thermocycling conditions were as follows:
16°C for 30 min, 42°C for 30 min, 85°C for 5 min; in the end hold
at 4°C. The PCR conditions were as follows: 95°C for 10 min, 95°C
for 10 sec, 60°C for 60 sec (40 cycles).
Cell transfection
Cultured MG-63 cells were uniformly inoculated into
6-well culture plates at 3×105/ml; the volume of each
well was 1 ml. Following growth of adherent cells, transfection
with miR-124 mimic, mimic control, pcDNA3.1-TRAF6 recombinant
plasmid or empty plasmid control (pcDNA3.1) was conducted using
Lipofectamine® 2000 according to the manufacturer's
protocol. In addition, a normal control group (normal;
untransfected cells served as the normal control) was generated.
Briefly, miR-124 mimic, mimic control, pcDNA3.1-TRAF6 and pcDNA3.1
were diluted in minimum essential medium (MEM; Thermo Fisher
Scientific, Inc.). Subsequently, Lipofectamine® 2000 was
diluted in MEM, and was incubated for 5 min at room temperature
after gentle agitation. The diluted Lipofectamine® 2000
was mixed with the diluted miR-124 mimic or mimic control, and was
incubated for 20 min at room temperature after gentle agitation, so
as to form a compound. The compound was then added to the culture
plate containing MG-63 cells, and was incubated at 37°C in an
atmosphere containing 5% CO2. After 5 h, the medium was
replaced with MEM containing 10% FBS for a further 48 h.
Luciferase reporter gene system
The analysis result of TargetScan (http://www.targetscan.org) demonstrated that TRAF6 was
one of target gene of miR-124. Luciferase plasmids were
constructed, as follows: A sequence containing a fragment of the
wild-type 3′-untranslated region (3′-UTR) of the target gene,
TRAF6, was chemically synthesized, which was complementary to the
corresponding miR-124. Subsequently, the sequence fragment of the
TRAF6 3′-UTR (wild-type) containing the predicted miR-124 binding
sites, and a mutated sequence fragment of the TRAF6 3′-UTR (mutant)
were cloned into the XbaI site of a pGL3-Basic carrier. The
PCR primer with mutant site was synthesized and mutant 3′-UTR was
generated by PCR amplification.
Cultured 293 cells were uniformly inoculated into
6-well culture plates at 3×105/ml; the volume of each
well was 1 ml. Subsequently, the cells were transfected with the
luciferase plasmids containing TRAF6 3′-UTR (wild-type) or mutated
TRAF6 3′-UTR (mutant), alongside the miR-124 mimic or mimic
control. In addition, a normal control group was generated. A total
of 48 h post-transfection, cells were collected and detected using
a luciferase kit; a microplate reader was used for numerical
readings at a wavelength of 560 nm.
Western blot analysis
Proteins were extracted from MG-63 cells using RIPA
lysis buffer post-transfection with a miR-124 mimic or mimic
control for 48 h. The concentration in the supernatant was then
measured using the bicinchoninic acid method. Proteins (50
µg) were separated by 5–15% SDS-PAGE and were transferred
onto a polyvinylidene fluoride membrane via Wet Transfer (Bio-Rad
Laboratories, Inc.). The membrane was blocked for 1 h at room
temperature in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl and 0.1%
Tween-20) containing 5% skimmed milk powder. Subsequently, the
membrane was incubated with rabbit anti-human TRAF6 polyclonal
antibody (1:500 dilution; ab181622; Abcam) and rabbit anti-human
β-actin monoclonal antibody (1:1,000 dilution; ab8227; Abcam)
overnight at 4°C, and was washed three times with TBST (5
min/wash). The membrane was then incubated with HRP-conjugated goat
anti-rabbit (A6154; Sigma-Aldrich; Merck KgaA) or goat anti-mouse
(A4416; Sigma-Aldrich; Merck KgaA) IgG secondary antibodies
(dilution, 1:2,000) at 37°C for 1 h, after which it was washed
three times with TBST (5 min/wash). Blots were visualized using an
enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.). Relative protein expression levels of TRAF6 were expressed
as the gray value ratio of TRAF6/β-actin; relative expression
alterations were analyzed by PDQuest software (version 8.0; Bio-Rad
Laboratories, Inc.).
MTT assay
After MG-63 cells were transfected with a miR-124
mimic or mimic control for 48 h, RNA was extracted from the cells
in each group using the TaqMan miRNA isolation kit. Alterations in
the expression levels of miR-124 were detected in each group using
reverse transcription-quantitative PCR (RT-qPCR).
After MG-63 cells were transfected with a miR-124
mimic or mimic control for 48 h, they were incubated with 100
µl MTT (0.5 mg/ml) solution/well at 37°C in an atmosphere
containing 5% CO2 for 4 h. Subsequently, 100 µl
20% SDS (auxiliary solvent, 50% dimethylfomamide) was added to each
well for 24 h at 37°C. A microplate reader (Bio-Tek Instruments,
Inc., Winooski, VT, USA) was used to detect the optical density
values at 570 nm. In addition, the normal control group was
analyzed. A total of 10 complex holes were set up in each
experimental group, and the experiment was repeated three
times.
Flow cytometric detection of cell cycle
progression
MG-63 cells were transfected with a miR-124 mimic or
mimic control for 48 h. In addition, a normal control group
(normal) was generated. Subsequently, the cells were washed 1–2
times with PBS and trypsin was used to digest cells, after which
cells were washed a further 1–2 times with PBS and the cells were
collected by centrifugation. PI was added to the cells, which were
incubated in the dark for 30 min at 4°C. Finally, cells were
filtered and underwent flow cytometric analysis (BD Biosciences).
CellQuest software (BD Biosciences) was used to count cells, and
FACsuite software (BD Biosciences) was used to analyze the
data.
Flow cytometric analysis of
apoptosis
MG-63 cells were transfected with a miR-124 mimic or
mimic control for 48 h. In addition, a normal control group
(normal) was generated. Subsequently, the cells were washed 1–2
times with PBS, and were incubated with Annexin V-fluorescein
isothiocyanate and PI in the dark for 15 min at room temperature.
Finally cells were filtered and underwent flow cytometric analysis
(BD Biosciences) for the detection of apoptotic cells. CellQuest
software (BD Biosciences) was used to count cells, and Mac quit
software was used to analyze the data.
Transwell invasion chamber assay
Transfection of MG-63 cells with a miR-124 mimic and
mimic control was conducted using Lipofectamine® 2000
according to the manufacturer's protocol. In addition, a normal
control group (normal) was generated. MG-63 cells were transfected
with the specified mimics for 24 h; after transfection, the cells
were seeded in a Transwell invasion chamber and incubated for
another 24 h, and after cultivation under normal conditions for 24
h, the cells were washed 1–2 times with PBS, and the cells were
stained with Hoechst 33342. Eight visual fields were observed at
random, and the number of cells that passed through the Transwell
polycarbonate membrane was counted under a microscope (Leica DM IL;
Leica, Wetzlar, Germany). Cells that passed through the Transwell
polycarbonate membrane were considered invasive cells. The
Transwell chamber wells were coated in Matrigel.
Further validation of the role of TRAF6
in cell biology via miR-124 regulation
In order to confirm that TRAF6 serves a role in cell
biology via miR-124 regulation, a TRAF6 recombinant expression
vector, pcDNA3.1-TRAF6, was constructed. The cells were divided
into the following five groups: Normal control group (normal),
empty vector control group (pcDNA3.1), pcDNA3.1-TRAF6 transfection
group and miR-124 mimic + pcDNA3.1-TRAF6 transfection group. The
miR-124 mimic and pcDNA3.1-TRAF6 were simultaneously transfected
into MG-63 cells using Lipofectamine® 2000. For MTT
assay, cultured MG-63 cells were uniformly inoculated into 96-well
culture plates at 3×105/ml; the volume of each well was
0.1 ml. A total of 300 ng pcDNA3.1-TRAF6 and 50 nM mimic were then
diluted into 25 µl medium without serum. And 1 µl
Lipofectamine® 2000 was diluted into 25 µl
OptiMEM Medium and incubate for 5 min at room temperature. The
DNA-mimic-Lipofectamine® 2000 complexes (50 µl)
were directly added to each well of the plates containing cells and
were then mixed gently. To further validate the role of TRAF6 in
cell biology via miR-124 regulation, MTT, flow cytometry and
Transwell analytical methods were used.
Statistical analysis
The data are presented as mean ± SD. The experiments
were repeated 3 times. Statistical processing of experimental data
was conducted using SPSS 17.0 statistical analysis software (SPSS,
Inc., Chicago, IL, USA), and it was used to compare the differences
between two groups. One-way analysis of variance was used to
analyze the data and Tukey's post hoc test was used to analyze the
differences between more than two groups. Student's t-test was used
to analyze the differences between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Identification of TRAF6 as a target gene
of miR-124
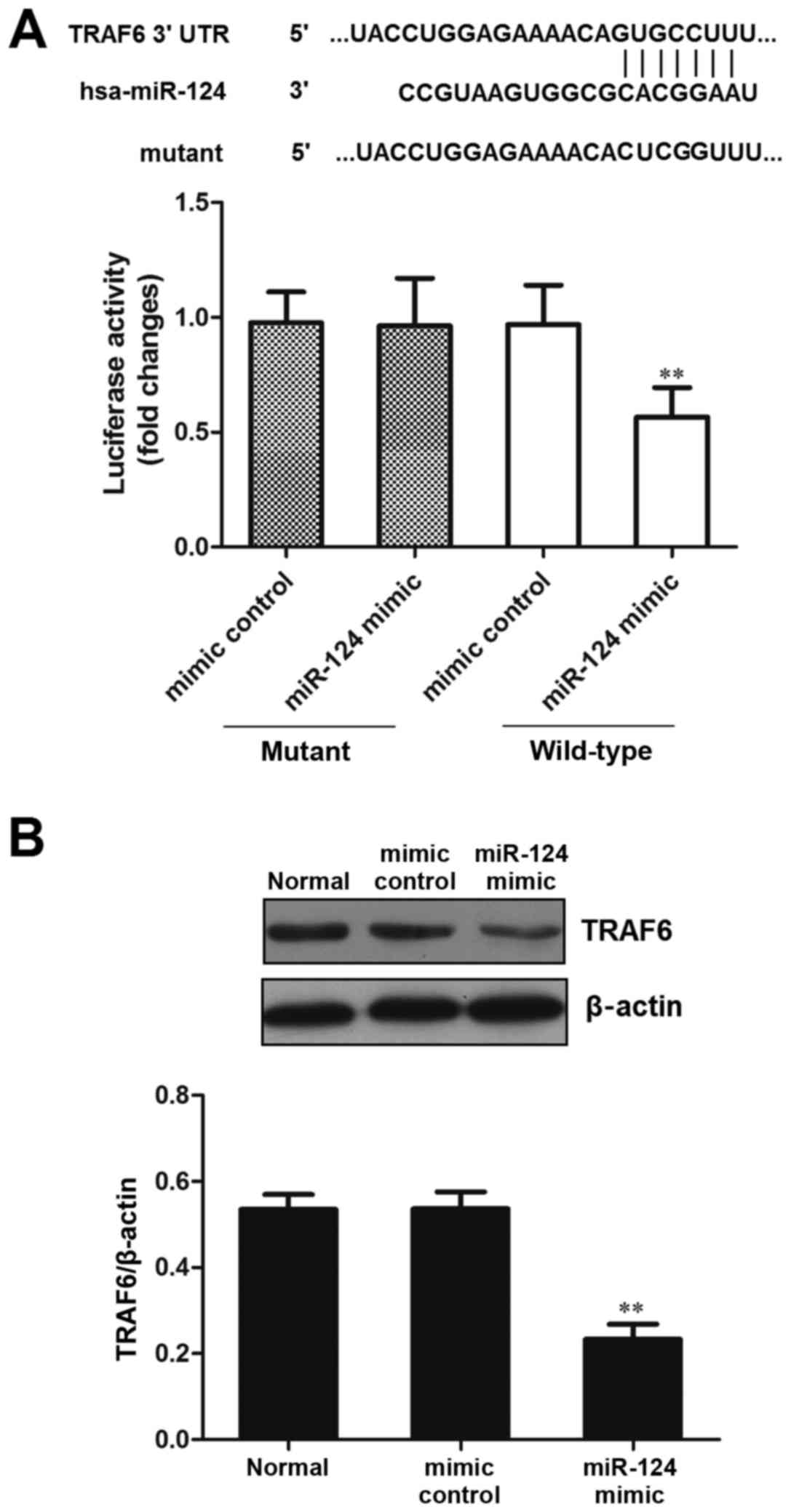
In order to verify that the predicted miR-124
binding site was located in the TRAF6 3′-UTR, luciferase plasmids
containing TRAF6 3′-UTR (wild-type) and mutated TRAF6 3′-UTR
(mutant) were constructed, and were transfected into 293 cells
alongside a miR-124 mimic or mimic control. The results
demonstrated that compared with in the control mimic group,
luciferase activity was significantly decreased in the miR-124
mimic and TRAF6 3′-UTR (wild-type) group (P<0.01; Fig. 1A). These results indicated that
miR-124 may directly act on the predicted target site of TRAF6
3′-UTR.
Western blot analysis was used to detect TRAF6
expression and the effects of miR-124 on its regulation. The
results indicated that the protein expression levels of TRAF6 were
significantly reduced in the miR-124 mimic group compared with in
the normal and mimic control groups (P<0.01; Fig, 1B). These results suggested that
overexpression of miR-124 inhibited the protein expression levels
of TRAF6 protein.
miR-124 expression in human osteosarcoma
tissues and cell lines
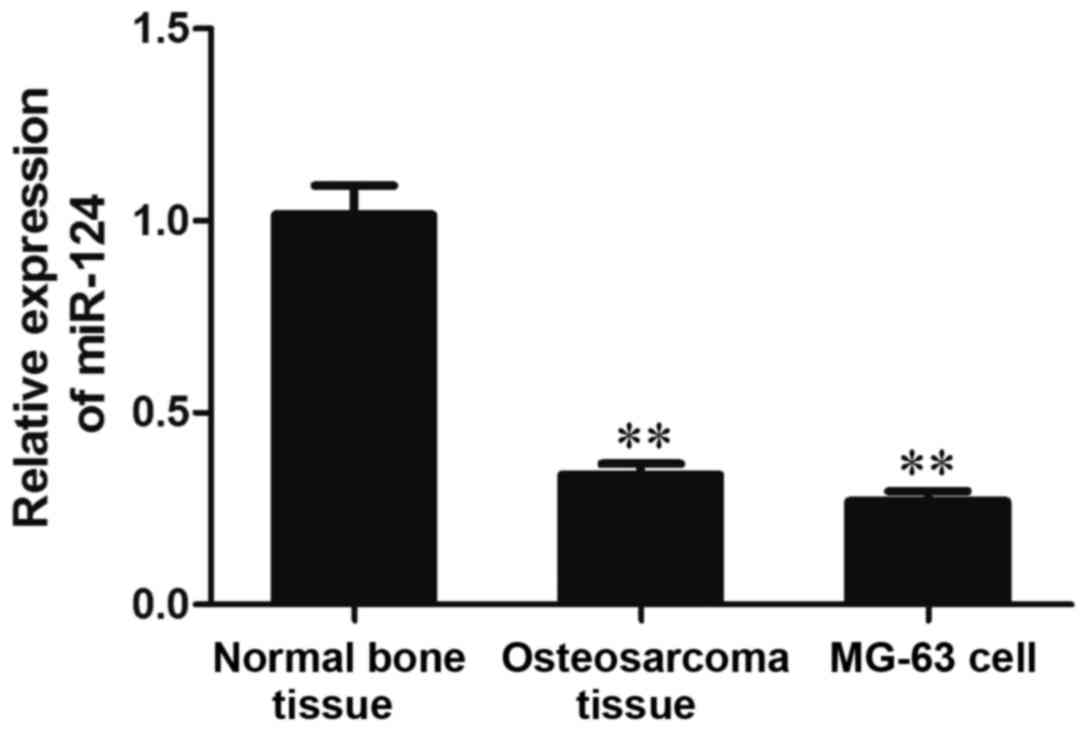
The results of RT-qPCR analysis demonstrated that
the expression levels of miR-124 were significantly reduced in
osteosarcoma tissues and MG-63 cells compared with in normal bone
tissue (P<0.01; Fig. 2). These
results suggested that miR-124 expression may be decreased in human
osteosarcoma tissues and cell lines.
Effects of miR-124 on cell viability, as
detected by MTT assay
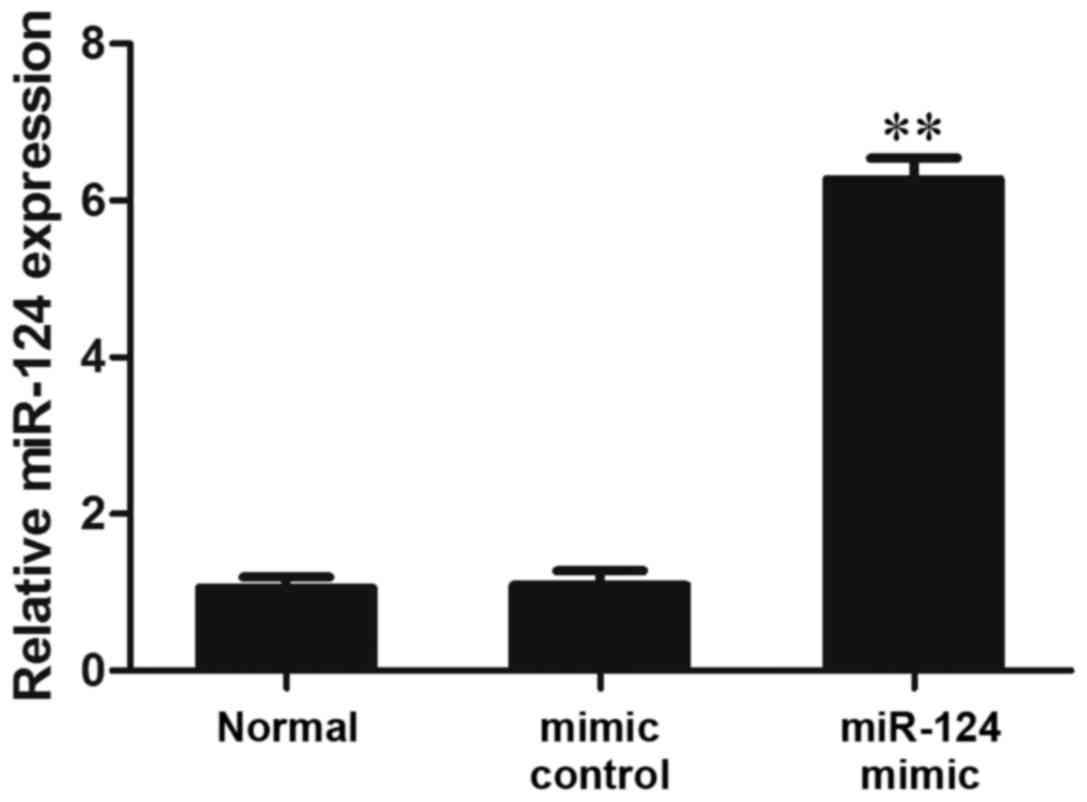
Transfection of MG-63 cells with a miR-124 mimic
induced miR-124 overexpression. The results of RT-qPCR analysis
demonstrated that in the miR-124 mimic group, the expression levels
of miR-124 were significantly increased compared with in the normal
and mimic control groups (P<0.01; Fig. 3).
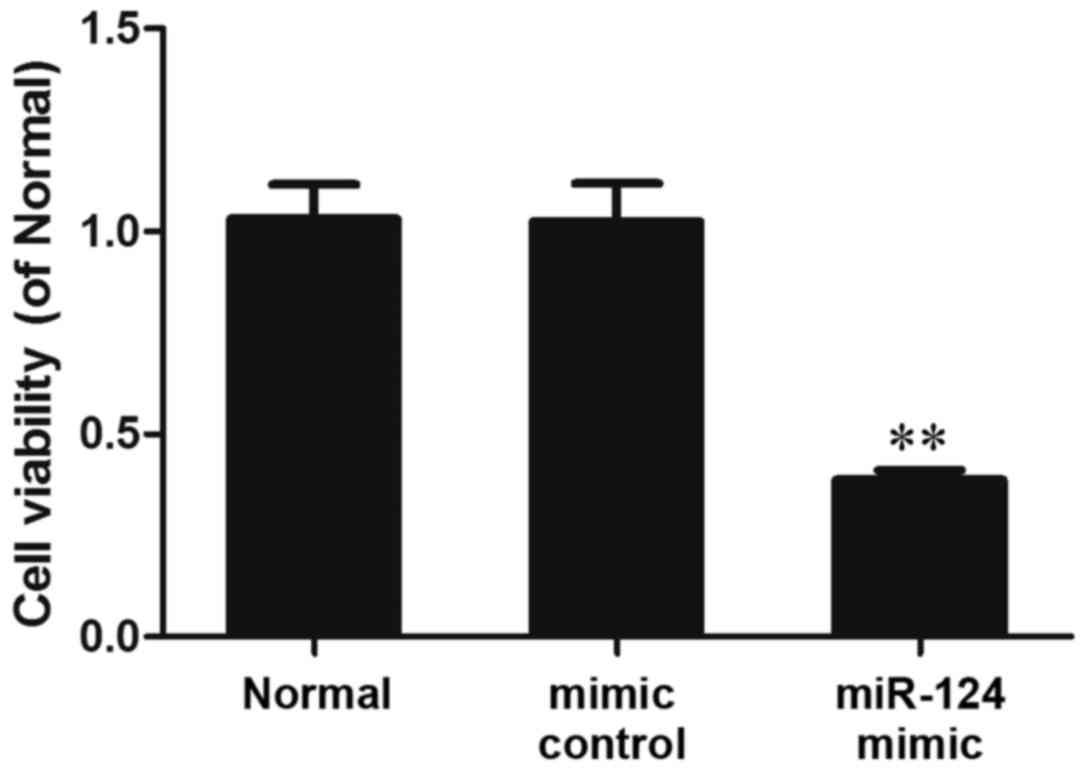
The results of the MTT analysis demonstrated that
following transfection with a miR-124 mimic for 48 h, cell
viability was significantly reduced compared with in the normal and
mimic control groups (P<0.01; Fig.
4). These results indicated that miR-124 overexpression may
reduce the viability of MG-63 cells.
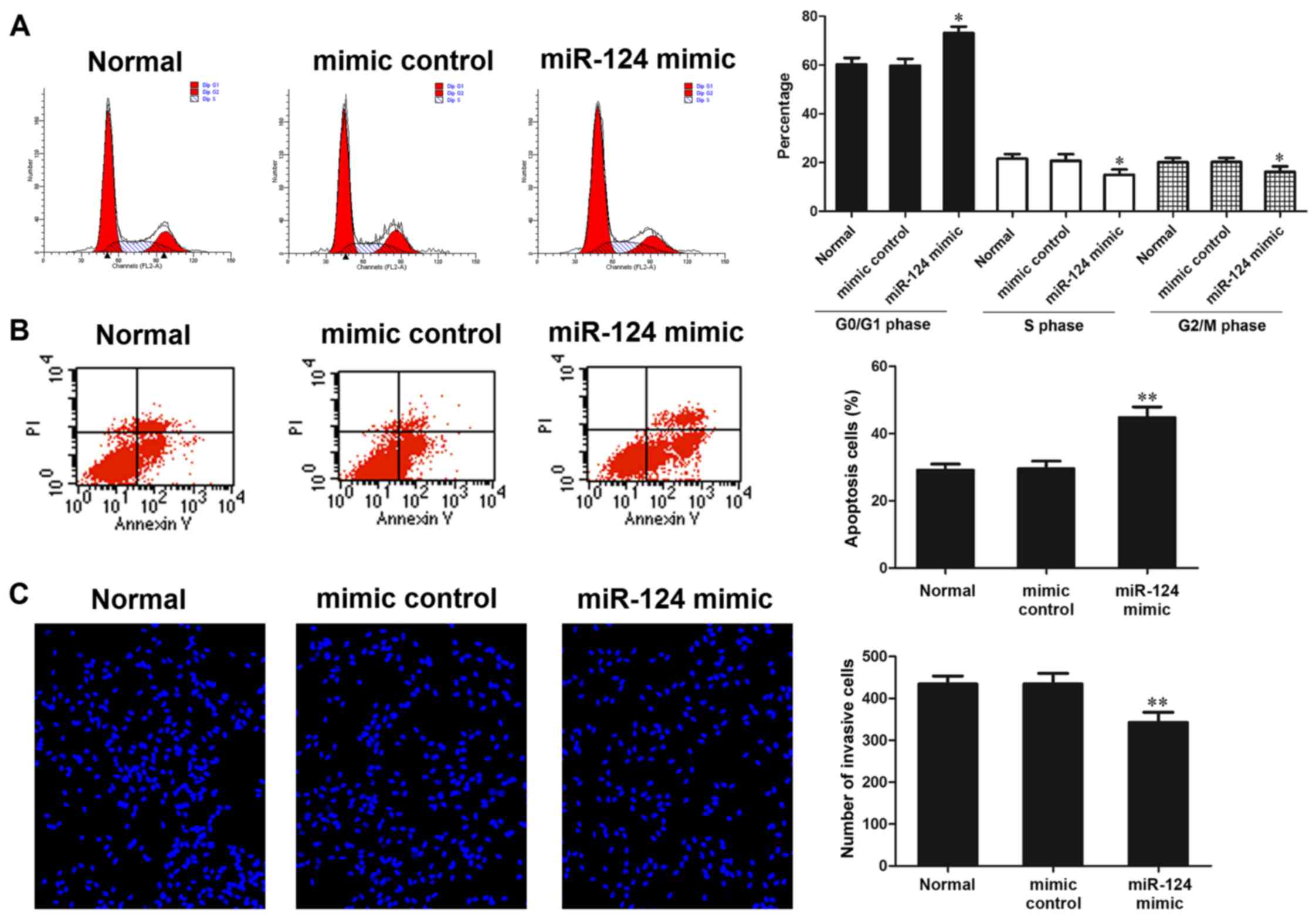
Effects of miR-124 on cell cycle
progression and apoptosis, as detected by flow cytometry
After MG-63 cells were transfected with a miR-124
mimic and mimic control for 48 h, flow cytometry was used to detect
the proportion of cells in each phase of the cell cycle. The
results indicated that in the miR-124 mimic group, the proportion
of S phase cells and G2/M phase cells was significantly
reduced compared with in the normal and mimic control groups
(P<0.05), whereas the proportion of G0/G1
phase cells was significantly increased compared with in the normal
and mimic control groups (P<0.05; Fig. 5A). These results indicated that
miR-124 overexpression may inhibit cell cycle progression.
Flow cytometry was also used to determine the
effects of miR-124 on cell apoptosis. The results demonstrated that
in the miR-124 mimic group, the proportion of apoptotic cells was
significantly higher compared with in the normal and mimic control
groups (P<0.01; Fig. 5B).
These results suggested that miR-124 overexpression may promote
cell apoptosis.
Effects of miR-124 on cell invasion, as
detected by Transwell invasion assay
After MG-63 cells were transfected with a miR-124
mimic and mimic control for 48 h, the number of cells that passed
through the polycarbonate membrane of a Transwell invasion chamber
was determined. Hoechst 33342 staining demonstrated that in the
miR-124 mimic group, the number of cells that passed through the
Transwell membrane was significantly decreased (P<0.01). In
addition, the migration of MG-63 cells was inhibited
post-transfection with a miR-124 mimic (Fig. 5C).
Validation of the role of miR-124 in cell
biology via TRAF6 regulation
Our preliminary study confirmed that TRAF6 served a
role as a proto-oncogene in osteosarcoma (27). In addition, the results of the
present study indicated that miR-124 may be considered a tumor
suppressor gene in osteosarcoma, and TRAF6 was confirmed as a
target gene regulated by miR-124. Therefore, the present study
aimed to further validate the role of miR-124 in cell biology via
TRAF6 regulation. Therefore, a recombinant expression plasmid of
TRAF6 was constructed, after which MG-63 cells were cotransfected
with a miR-124 mimic and the pcDNA3.1-TRAF6 recombinant expression
vector using Lipofectamine® 2000. Subsequently, the
cells were evaluated using MTT, flow cytometry and Transwell
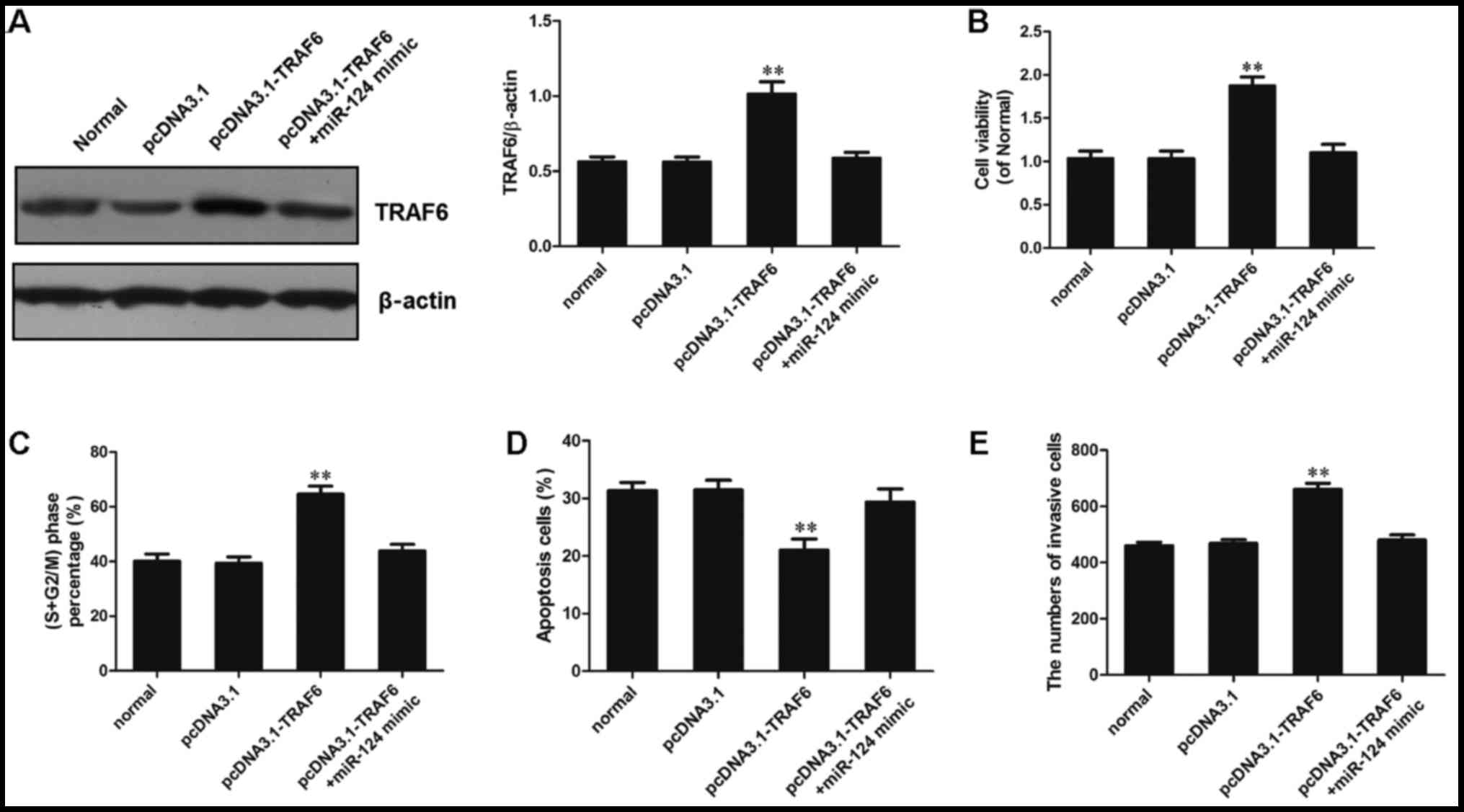
analytical methods (Fig. 6).
There were no significant differences between the miR-124 +
pcDNA3.1-TRAF6 group and the pcDNA3.1-TRAF6 group.
Firstly, western blot analysis was used to detect
the protein expression levels of TRAF6 in each group. The results
revealed that transfection of MG-63 cells with pcDNA3.1-TRAF6
significantly promoted TRAF6 expression (P<0.01). However, in
the miR-124 mimic + pcDNA3.1-TRAF6 transfection group, TRAF6
expression was significantly reduced compared with in the
pcDNA3.1-TRAF6 transfection group (P<0.01), and was slightly
higher than that in the normal and pcDNA3.1 groups (P>0.05;
Fig. 6A).
Cell viability was increased in the pcDNA3.1-TRAF6
transfection group, and the proportion of cells in the S +
G2/M phase was increased. Furthermore, the number of
cells that passed through the polycarbonate membrane was
significantly higher compared with in the other groups (P<0.01).
These results suggested that TRAF6 may be considered a
proto-oncogene in osteosarcoma. Cell viability in the miR-124 mimic
+ pcDNA3.1-TRAF6 transfection group was decreased, and the numbers
of cells in S + G2/M phase and that passed through the
polycarbonate membrane were significantly reduced compared with in
the pcDNA3.1-TRAF6 transfection group (P<0.01). Viability, cell
cycle progression and invasion was slightly higher in the miR-124
mimic + pcDNA3.1-TRAF6 transfection group than in the normal and
pcDNA3.1 groups (P>0.05; Fig. 6B,
C and E). Furthermore, apoptosis was analyzed by flow
cytometry, and the results revealed that in the miR-124 mimic +
pcDNA3.1-TRAF6 transfection group, the number of apoptotic cells
was significantly higher than in the pcDNA3.1-TRAF6 transfection
group, and was slightly lower than in the normal and pcDNA3.1
groups (P>0.05; Fig. 6D).
These results indicated that miR-124 overexpression could inhibit
TRAF6-induced cell cycle progression of osteosarcoma cells and
initiate apoptosis.
Discussion
Osteosarcoma often occurs in children and
adolescents, and is one of the most common forms of primary
malignant bone tumor (32,33).
Osteosarcoma is associated with a high degree of malignancy and
early blood metastasis; in addition, the incidence of pulmonary
metastasis is high, disease development is rapid and it is
associated with high levels of mortality (34,35). Increased information regarding the
molecular mechanism underlying the occurrence and metastasis of
osteosarcoma is required to serve a guiding role in gene-targeted
therapy (36,37).
During tumor occurrence and development, TRAF6 has a
functional role as a cancer gene (15,16). The TRAF6 protein is composed of
four domains; a coiled-coil structural domain (also known as TRAF-N
structural domain) and a conservative TRAF-C structural domain are
contained in its C-terminal, which can interact with upstream
receptors or other signaling proteins through self-association,
thus resulting in exertion of its biological function (38,39). The N-terminal of the TRAF6 protein
contains a RING finger structural domain (E3 ubiquitin ligase) and
multiple zinc finger domains (bound with specific DNA sequences);
these structures have important roles in the activation of
downstream signals (40). In
recent years, studies have revealed that TRAF6 has an important
role in the development, invasion and metastasis of tumors by
activating NF-κB (41,42). Our preliminary results indicated
that TRAF6 expression is increased in osteosarcoma tissues;
therefore it may be considered a proto-oncogene. These findings
were validated in the present study. The present study aimed to
explore the possible mechanism underlying the regulation of TRAF6
in osteosarcoma.
miRNAs are a class of evolutionarily conserved,
non-protein coding RNAs (43–45), which are widely expressed in
numerous organisms. Through the transcriptional regulation of
target genes, miRNAs are involved in the regulation of several
types of tumorigenesis and metastasis (46,47). In the present study, the miRNA
that may regulate TRAF6 expression was analyzed using a series of
online software, thus resulting in the identification of miR-124.
Existing research indicated that through the downregulation of
Rho-associated protein kinase 1, miR-124 may inhibit the
proliferation and migration of colon cancer cells (48). In astrocytoma, miR-124 has been
reported to regulate cell proliferation, apoptosis and migration
via proto-oncogene serine/threonine-protein kinase Pim-1 (49). In the present study, the results
of a luciferase reporter gene system demonstrated that in the
miR-124 mimic group, luciferase activity was significantly
decreased, and western blotting revealed that the overexpression of
miR-124 inhibited the expression of TRAF6. These findings verified
TRAF6 as a target gene regulated by miR-124.
In the present study, RT-qPCR was used to detect the
expression levels of miR-124 in osteosarcoma tissues; the results
revealed that miR-124 expression was downregulated in osteosarcoma
tissues and cells. Subsequently, miR-124 was overexpressed in MG-63
cells via transfection with a miR-124 mimic. The biological effects
of miR-124 overexpression were then examined using MTT, flow
cytometry and Transwell invasion assay analytical methods. The
results demonstrated that miR-124 overexpression decreased cell
viability, inhibited cell cycle progression and invasion, and
promoted apoptosis. Therefore, it may be hypothesized that miR-124
participates in the occurrence and metastasis of the osteosarcoma
malignant phenotype by regulating TRAF6 and associated downstream
signaling pathways. In the present study, miR-124 overexpression in
osteosarcoma cells reduced TRAF6 expression, thus inhibiting cell
cycle progression and invasion of osteosarcoma cells, and promoting
osteosarcoma cell apoptosis.
In order to further verify the role of TRAF6 in cell
biology via miR-124 regulation, the present study constructed a
TRAF6 recombinant expression vector, pcDNA3.1-TRAF6. Subsequently,
MG-63 cells were cotransfected with pcDNA3.1-TRAF6 and a miR-124
mimic, and MTT, flow cytometry and Transwell invasion assay
analytical methods were used to analyze the effects. The results
suggested that miR-124 overexpression could reverse the effects of
TRAF6 overexpression on osteosarcoma cells, and promote
apoptosis.
In conclusion, downregulation of miR-124 in human
osteosarcoma tissues and cell lines may increase the expression of
TRAF6 protein. miR-124 may inhibit cell cycle progression and
invasion of osteosarcoma cells, and promote apoptosis by regulating
TRAF6. Therefore, therapeutic strategies that enhance the
expression of miR-124 or inhibit the expression of TRAF6 may
benefit patients with osteosarcoma.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402226).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
QM and WZ designed the research and wrote the
manuscript. XX, JL, HM, XL, LQ, XZ and MZ collected the data,
analyzed and interpreted the data. All authors read and approved
the final manuscript.
[4] Ethics
approval and consent to participate
The present study was approved by the Ethics
Committee of Yancheng City No. 1 People's Hospital (Yancheng,
China; approval no. 2015YCYY-0001). The patients provided written
informed consent prior to sample collection.
[5] Consent for
publication
The patients provided written informed consent for
the use of data in this study.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Lugowska I, Mierzejewska E, Lenarcik M,
Klepacka T, Koch I, Michalak E and Szamotulska K: The clinical
significance of changes in ezrin expression in osteosarcoma of
children and young adults. Tumour Biol. 37:12071–12078. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JA, Jeon DG, Cho WH, Song WS, Yoon HS,
Park HJ, Park BK, Choi HS, Ahn HS, Lee JW, et al: Higher
gemcitabine dose was associated with better outcome of osteosarcoma
patients receiving gemcitabine-docetaxel chemotherapy. Pediatr
Blood Cancer. 63:1552–1556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang H, Liu W, Li G, Fan T and Mao B:
Chinese medicinal herbs in the treatment of upper airway cough
syndrome: A systematic review of randomized, controlled trials.
Altern Ther Health Med. 22:38–51. 2016.PubMed/NCBI
|
|
4
|
Wang L, Yang L, Lu Y, Chen Y, Liu T, Peng
Y, Zhou Y, Cao Y, Bi Z, Liu T, et al: Osthole induces cell cycle
arrest and inhibits migration and invasion via PTEN/Akt pathways in
osteosarcoma. Cell Physiol Biochem. 38:2173–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ali Gumustas S, Isyar M, Topuk S, Yilmaz
I, Oznam K, Onay T, Ofluoglu O and Mahirogullari M: Systematic
evaluation of drug-loaded hydrogels for application in osteosarcoma
treatment. Curr Pharm Biotechnol. 17:866–872. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kebudi R, Ozger H, Kızılocak H, Bay SB and
Bilgiç B: Osteosarcoma after hematopoietic stem cell
transplantation in children and adolescents: Case Report and Review
of the Literature. Pediatr Blood Cancer. 63:1664–1666. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng L, Wang C and Jing J: Cell cycle
kinases in osteosarcoma: Potential for therapeutic intervention.
Curr Pharm Des. 22:4830–4834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maximov VV and Aqeilan RI: Genetic factors
conferring metastasis in osteosarcoma. Future Oncol. 12:1623–1644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Zhang Y, Wan S, Li H, Li D, Xia J,
Yuan Z, Ren M, Yu S, Li S, et al: A comparative study between
limb-salvage and amputation for treating osteosarcoma. J Bone
Oncol. 5:15–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan J and Zhang X, Liu T and Zhang X:
Strategies and developments of immunotherapies in osteosarcoma.
Oncol Lett. 11:511–520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Durham BH, Diamond EL and Abdel-Wahab O:
Histiocytic neoplasms in the era of personalized genomic medicine.
Curr Opin Hematol. 23:416–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cagle PT, Raparia K and Portier BP:
Emerging biomarkers in personalized therapy of lung cancer. Adv Exp
Med Biol. 890:25–36. 2016. View Article : Google Scholar
|
|
13
|
Krishna A, Singh S, Kumar V and Pal US:
Molecular concept in human oral cancer. Natl J Maxillofac Surg.
6:9–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
De P, Carlson JH, Leyland-Jones B and Dey
N: Role of 'oncogenic nexus' of CIP2A in breast oncogenesis: How
does it work? Am J Cancer Res. 5:2872–2891. 2015.
|
|
15
|
Luo Z, Zhang X, Zeng W, Su J, Yang K, Lu
L, Lim CB, Tang W, Wu L, Zhao S, et al: TRAF6 regulates melanoma
invasion and metastasis through ubiquitination of Basigin.
Oncotarget. 7:7179–7192. 2016.PubMed/NCBI
|
|
16
|
Han F, Zhang L, Qiu W and Yi X: TRAF6
promotes the invasion and metastasis and predicts a poor prognosis
in gastric cancer. Pathol Res Pract. 212:31–37. 2016. View Article : Google Scholar
|
|
17
|
Lin FT, Lin VY, Lin VT and Lin WC: TRIP6
antagonizes the recruitment of A20 and CYLD to TRAF6 to promote the
LPA2 receptor-mediated TRAF6 activation. Cell Discov. 2:150482016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao T, Tang X, Umeshappa CS, Ma H, Gao H,
Deng Y, Freywald A and Xiang J: Simulated microgravity promotes
cell apoptosis through suppressing Uev1A/TICAM/TRAF/NF-κB-regulated
anti-apoptosis and p53/PCNA- and ATM/ATR-Chk1/2-controlled
DNA-damage response pathways. J Cell Biochem. 117:2138–2148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Panda S, Nilsson JA and Gekara NO:
Deubiquitinase MYSM1 regulates innate immunity through inactivation
of TRAF3 and TRAF6 complexes. Immunity. 43:647–659. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomomura M, Suzuki R, Shirataki Y,
Sakagami H, Tamura N, Tomomura A and Rhinacanthin C: Rhinacanthin C
inhibits osteoclast differentiation and bone resorption: Roles of
TRAF6/TAK1/MAPKs/NF-κB/NFATc1 signaling. PLoS One. 10:e01301742015.
View Article : Google Scholar
|
|
21
|
Kong X, Yang Y, Wu W, Wan H, Li X, Zhong
M, Su X, Jia S and Lin N: Triterpenoid saponin W3 from Anemone
flaccida suppresses osteoclast differentiation through inhibiting
activation of MAPKs and NF-κB pathways. Int J Biol Sci.
11:1204–1214. 2015. View Article : Google Scholar :
|
|
22
|
Kong X, Wu W, Yang Y, Wan H, Li X, Zhong
M, Zhao H, Su X, Jia S, Ju D, et al: Total saponin from Anemone
flaccida Fr. Schmidt abrogates osteoclast differentiation and bone
resorption via the inhibition of RANKL-induced NF-κB, JNK and p38
MAPKs activation. J Transl Med. 13:912015. View Article : Google Scholar
|
|
23
|
He Z, Huang C, Lin G and Ye Y:
siRNA-induced TRAF6 knockdown promotes the apoptosis and inhibits
the invasion of human lung cancer SPC-A1 cells. Oncol Rep.
35:1933–1940. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng Z, Shuangzhu Y, Yongjie J, Xinjun Z
and Ying L: Retraction note to: TNF receptor-associated factor 6
regulates proliferation, apoptosis, and invasion of glioma cells.
Mol Cell Biochem. 415:2072016. View Article : Google Scholar
|
|
25
|
Han Q, Yao F, Zhong C and Zhao H: TRAF6
promoted the metastasis of esophageal squamous cell carcinoma.
Tumour Biol. 35:715–721. 2014. View Article : Google Scholar
|
|
26
|
Sun H, Li XB, Meng Y, Fan L, Li M and Fang
J: TRAF6 upregulates expression of HIF-1α and promotes tumor
angiogenesis. Cancer Res. 73:4950–4959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng Q, Zheng M, Liu H, Song C, Zhang W,
Yan J, Qin L and Liu X: TRAF6 regulates proliferation, apoptosis,
and invasion of osteosarcoma cell. Mol Cell Biochem. 371:177–186.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng S, Gao D, Gao C, Wei P, Niu M and
Shuai C: MicroRNAs regulate signaling pathways in osteogenic
differentiation of mesenchymal stem cells (Review). Mol Med Rep.
14:623–629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeh CH, Moles R and Nicot C: Clinical
significance of microRNAs in chronic and acute human leukemia. Mol
Cancer. 15:372016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng T, Shao F, Wu Q, Zhang X, Xu D, Qian
K, Xie Y, Wang S, Xu N, Wang Y, et al: miR-124 downregulation leads
to breast cancer progression via LncRNA-MALAT1 regulation and
CDK4/E2F1 signal activation. Oncotarget. 7:16205–16216.
2016.PubMed/NCBI
|
|
31
|
Zhang H, Wang Q, Zhao Q and Di W: MiR-124
inhibits the migration and invasion of ovarian cancer cells by
targeting SphK1. J Ovarian Res. 6:842013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Denduluri SK, Wang Z, Yan Z, Wang J, Wei
Q, Mohammed MK, Haydon RC, Luu HH and He TC: Molecular pathogenesis
and therapeutic strategies of human osteosarcoma. J Biomed Res. Aug
30–2015.Epub ahead of print. PubMed/NCBI
|
|
33
|
Gu X, Ding J, Zhang Z, Li Q, Zhuang X and
Chen X: Polymeric nanocarriers for drugdelivery in osteosarcoma
treatment. Curr Pharm Des. 21:5187–5197. 2015. View Article : Google Scholar
|
|
34
|
Sampson VB, Yoo S, Kumar A, Vetter NS and
Kolb EA: MicroRNAs and potential targets in osteosarcoma: Review.
Front Pediatr. 3:692015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim HJ and Yang JL: Regulatory roles and
therapeutic potential of microRNA in sarcoma. Crit Rev Oncol
Hematol. 97:118–130. 2016. View Article : Google Scholar
|
|
36
|
Morrow JJ and Khanna C: Osteosarcoma
genetics and epigenetics: Emerging biology and candidate therapies.
Crit Rev Oncog. 20:173–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu H, Tamashiro S, Baritaki S, Penichet
M, Yu Y, Chen H, Berenson J and Bonavida B: TRAF6 activation in
multiple myeloma: A potential therapeutic target. Clin Lymphoma
Myeloma Leuk. 12:155–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar A, Bhatnagar S and Paul PK: TWEAK
and TRAF6 regulate skeletal muscle atrophy. Curr Opin Clin Nutr
Metab Care. 15:233–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landström M: The TAK1-TRAF6 signalling
pathway. Int J Biochem Cell Biol. 42:585–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan J, Yin Y, Zhong W, Wang C and Wang Z:
CD137 Regulates NFATc1 expression in mouse VSMCs through
TRAF6/NF-kappaB p65 signaling pathway. Mediators Inflamm.
2015:6397802015. View Article : Google Scholar
|
|
42
|
Shi Z, Zhang Z, Zhang Z, Wang Y, Li C,
Wang X, He F, Sun L, Jiao S, Shi W, et al: Structural Insights into
mitochondrial antiviral signaling protein (MAVS)-tumor necrosis
factor receptor-associated factor 6 (TRAF6) signaling. J Biol Chem.
290:26811–26820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tian X and Zhang X: A single nucleotide
polymorphism (rs1056629) in 3′-UTR of MMP-9 is responsible for a
decreased risk of metastatic osteosarcoma by compromising its
interaction with microRNA-491-5p. Cell Physiol Biochem.
38:1415–1424. 2016. View Article : Google Scholar
|
|
44
|
Zhu Z, Tang J, Wang J, Duan G, Zhou L and
Zhou X: MiR-138 acts as a tumor suppressor by targeting EZH2 and
enhances cisplatin-induced apoptosis in osteosarcoma cells. PLoS
One. 11:e01500262016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu S, Du X, Wu M, Du H, Shi X and Zhang T:
MicroRNA-409-3p inhibits osteosarcoma cell migration and invasion
by targeting catenin-δ1. Gene. 584:83–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bhattacharya S, Chalk AM, Ng AJ, Martin
TJ, Zannettino AC, Purton LE, Lu J, Baker EK and Walkley CR:
Increased miR-155-5p and reduced miR-148a-3p contribute to the
suppression of osteosarcoma cell death. Oncogene. 35:5282–5294.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pu Y, Zhao F, Cai W, Meng X, Li Y and Cai
S: MiR-193a-3p and miR-193a-5p suppress the metastasis of human
osteosarcoma cells by downregulating Rab27B and SRR, respectively.
Clin Exp Metastasis. 33:359–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou L, Xu Z, Ren X, Chen K and Xin S:
MicroRNA-124 (MiR-124) inhibits cell proliferation, metastasis and
invasion in colorectal cancer by downregulating Rho-associated
protein kinase 1 (ROCK1). Cell Physiol Biochem. 38:1785–1795. 2016.
View Article : Google Scholar
|
|
49
|
Deng D, Wang L, Chen Y, Li B, Xue L, Shao
N, Wang Q, Xia X, Yang Y and Zhi F: miR-124-3p regulates cell
proliferation, invasion, apoptosis and bioenergetics by targeting
PIM1 in astrocytoma. Cancer Sci. 107:899–907. 2016. View Article : Google Scholar : PubMed/NCBI
|