Introduction
Preeclampsia is a hypertensive pregnancy disorder
that affects 2-7% of pregnancies, resulting in notable maternal and
fetal morbidities and mortalities, as well as greater
susceptibility and earlier onset of future cardiovascular diseases
in mothers and infants (1-4).
This condition is characterized by elevated blood pressure and
proteinuria, developing after 20 weeks of gestation (5).
There are multiple theories resulting in little
agreement concerning the ultimate cause of preeclampsia. However,
all forms of preeclampsia are characterized by disruption of
vascular remodeling and a systemic anti-angiogenic response in the
placenta (6). Trophoblast cells
of the placenta invade the uterus, together with blood vessels
(7), in order to establish
efficient fetomaternal exchange of molecules, including nutrients
and oxygen. In the placenta of a patient with preeclampsia,
interstitial invasion by trophoblast cells is shallow and limited,
resulting in the generation of an insufficient blood flow and
sustained hypoxic conditions (8).
Due to this, hypoxia is used to mimic preeclampsia conditions in
vitro and in vivo (9).
Despite numerous clinical trials (10,11), the only standard and effective
treatment for preeclampsia is preterm delivery of the infant, which
is the major cause of perinatal morbidity and mortality associated
with preeclampsia (12). Beyond
the delivery, the only successful medication used in the treatment
of preeclampsia is magnesium sulfate for prevention of convulsions
associated with eclampsia (13).
Estradiol and progesterone are critical female sex
steroid hormones that regulate growth, differentiation and function
in a broad range of target tissues, including the breasts, uterus
and placenta (14).
Concentrations of estrogen and progesterone, which are major
pregnancy hormones produced by the placenta, increase throughout
pregnancy, peaking at the end of gestation (15). These hormones potentially exist in
placental tissue at considerably higher concentrations than in
maternal blood (15). Notably,
estrogen concentrations are significantly lower in placental tissue
from pregnancies complicated by preeclampsia (16), and this evidence supports the
hypothesis that estrogen serves an important role in the
development of preeclampsia (17). It is well known that estrogen
stimulates expression of vascular endothelial growth factor (VEGF)
and angiogenesis in the uterus during the normal reproductive cycle
(18). Furthermore, estrogen has
been demonstrated to enhance uteroplacental blood flow (18-20) and microvascular volume in sheep
(21), presumably as a result of
enhanced angiogenesis and vasodilation. In addition, a previous
study has indicated that estrogen serves an important role in
regulating these processes in the primate placenta (18). This stimulatory action of estrogen
requires estrogen receptors (ESRs), as chronic estrogen treatment
has been demonstrated to induce angiogenesis in uterus tissue of
normal but not ESR-null mice (18). The specific receptors of estrogen,
ERα (ESR1) and ERβ (ESR2), act as ligand-activated transcription
factors. The classical mechanism of ESR activation involves
estrogen binding to receptors in the nucleus, after which the
receptors dimerize and bind to specific response elements, known as
estrogen response elements, located in the promoters of target
genes (14).
During pregnancy, progesterone stimulates growth and
differentiation of the endometrium for implantation, induces
immunological tolerance to the fetus, inhibits uterine
contractions, and maintains pregnancy (22). Research has suggested that
progesterone at least partially induces vascular adaptations during
normal pregnancy by reducing responsiveness of blood vessels to
vasoconstrictors and by inducing vasodilatation (23-26). Progesterone has been proposed to
prevent preeclampsia in previous studies, although evidence that
supports this hypothesis is relatively weak (27-29). Although the estrogen and
progesterone signaling pathways are assumed to be associated with
placental vascularization and preeclampsia, expression and
regulation of ESRs and progesterone receptor (PGR) in the placenta
have not been well studied. Therefore, the present study examined
the expression patterns of ESRs and PGR in placentas from normal
patients and patients with preeclampsia, and in a preeclampsia-like
in vitro system. In addition, the localization of the
receptors in a preeclampsia-like condition was examined.
Materials and methods
Tissue collection and processing
The biospecimens and data used for the present study
were provided by the Biobank of Pusan National University Hospital
(Busan, Korea), a member of the Korea Biobank Network. The present
study was approved by the Institutional Review Board of the Pusan
National University Hospital Clinical Trials Center
(H-1302-005-015), and all participants from Pusan National
University Hospital provided written informed consent. Placental
tissues (n=41) were obtained from patients, between January and
December of 2015, with hypertension (systolic blood pressure of
≥140 mmHg and diastolic blood pressure of ≥90 mmHg at least 6 h
apart) and proteinuria (≥300 mg/24 h or >1+ by dipstick). The
placental samples were divided into normal (n=21) and preeclampsia
(n=20) placentas, which were obtained from 29-40-week gestation.
The clinical characteristics of the patients are demonstrated in
Table I.
 | Table IClinical characteristics of
preeclamptic pregnancies. |
Table I
Clinical characteristics of
preeclamptic pregnancies.
| Pregnancy type
|
|---|
| Normal | Preeclampsia |
|---|
|
Characteristics | (n=21) | (n=20) |
| Gestational age,
weeks | 35.1±1.8 | 35.6±2.6 |
| Birth weight,
g | 2,485.7±439.3 | 2,130.0±686.2 |
| Systolic BP,
mmHg | 107.1±7.8 | 147.9±12.7a |
| Diastolic BP,
mmHg | 67.1±7.2 | 96.8±10.0a |
| Parity | 0.8±0.7 | 0.6±0.7 |
| Gravidity | 2.6±1.4 | 2.3±1.3 |
| Maternal body mass
index, kg/m2 | 25.8±3.0 | 28.6±5.8 |
Cell culture and in vitro model of
preeclampsia
The BeWo human choriocarcinoma-derived cell line was
purchased from the Korean Cell Line Bank (Seoul, Korea), cultured
in Dulbecco's modified Eagle's medium (DMEM; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (Hyclone; GE Healthcare Life Sciences), 100 IU/ml penicillin
and 100 μg/ml streptomycin, and grown in 5% CO2
at 37°C. The BeWo human choriocarcinoma cell line was used as a
model for trophoblast. The BeWo cell line is frequently used as a
trophoblast model as it maintains several characteristics of
cytotrophoblasts, including human chorionic gonadotropin secretion,
preservation of cytotrophoblast morphology and cytokeratin-7
expression (30). To establish an
in vitro model of preeclampsia, hypoxic conditions were
generated in a Modular Incubator Chamber (Billups-Rothenberg, Inc.,
San Diego, CA, USA), according to the manufacturer's protocol.
Briefly, cells were placed in a hypoxia chamber with inflow and
outflow connectors, and hypoxic gas consisted of 2% O2,
5% CO2 and 93% N2. Experiments were conducted
in a constant 37°C environment by placing the chambers in an
incubator. In order to create oxidative stress, cells were either
treated with catechol-O-methyl transferase inhibitor (COMT-in; 10
μM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or
L-NG-nitroarginine methyl ester (L-NAME; 100 μM;
Sigma-Aldrich; Merck KGaA), respectively, for 24 h in a 37°C
incubator. All in vitro experiments were performed at least
three times in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from BeWo cells and placenta tissues was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. The concentration of total RNA was
measured using a spectrophotometer. First-strand cDNA was prepared
from total RNA (3 μg) by reverse transcription at 37°C using
a M-MLV reverse transcriptase kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and random primers (9-mers; Takara Bio, Inc.,
Otsu, Japan), according to the manufacturer's protocol. qPCR was
performed with cDNA template (2 μl) and 2X Power SYBRGreen
(6 μl; Toyobo Life Sciences, Osaka, Japan) containing
specific primers. Primer sequences for cytochrome c1 (CYC1),
β-actin, VEGF, soluble fms-like tyrosine kinase-1 (sFlt1),
ESR1, ESR2 and PGR are demonstrated in Table II. qPCR was conducted for 40
cycles using the following parameters: Denaturation at 95°C for 15
sec, followed by annealing and extension at 70°C for 60 sec.
Fluorescence intensity was measured at the end of the extension
phase of each cycle. The threshold value for the fluorescence
intensity of all samples was set manually. The reaction cycle at
which PCR products exceeded this fluorescence intensity threshold
during the exponential phase of PCR amplification was considered to
be the threshold cycle. Expression of the target gene was
quantified relative to that of β-actin and CYC1, which are
housekeeping genes, according to the 2−ΔΔCq method
(31).
 | Table IIPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analyses. |
Table II
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analyses.
| Gene name | Primer | Sequence
(5′-3′) | Fragment, bp |
|---|
| β-actin | Forward |
GGACTTCGAGCAAGAGATGG | 234 |
| Reverse |
AGCACTGTGTTGGCGTACAG | |
| Cytochrome c1 | Forward |
CCAGCTACCATGTCCCAGAT | 185 |
| Reverse |
TATGCCAGCTTCCGACTCTT | |
| Vascular
endothelial growth factor | Forward |
GGCCAGCACATAGGAGAGAT | 216 |
| Reverse |
ACGCTCCAGGACTTATACCG | |
| Soluble fms-like
tyrosine kinase-1 | Forward |
GTCGTGTAAGGAGTGGACCA | 211 |
| Reverse |
GCAGATTTCTCAGTCGCAGG | |
| ESR1 | Forward |
AGCACCCTGAAGTCTCTGGA | 153 |
| Reverse |
GATGTGGGAGAGGATGAGGA | |
| ESR2 | Forward |
AAGAAGATTCCCGGCTTTGT | 173 |
| Reverse |
TCTACGCATTTCCCCTCATC | |
| Progesterone
receptor | Forward |
AAATCATTGCCAGGTTTTCG | 209 |
| Reverse |
TGCCACATGGTAAGGCATAA | |
Western blot analysis
Protein samples were extracted with Pro-prep
solution (Intron Biotechnology, Inc., Seongnam, Korea), following
the manufacturer's protocol. The concentration of the protein was
determined using a bicinchoninic acid assay. A total of 20
μg protein sample was loaded and separated by 8-10% SDS-PAGE
and then transferred to nitrocellulose membranes (Daeil Lab
Services Co., Ltd., Seoul, Korea). Membranes were subsequently
blocked for 2 h with 5% skim milk in PBS with 0.05% Tween-20
(PBS-T) at room temperature. Following blocking, membranes were
incubated with hypoxia-inducible factor 1-α (HIF1A; 1:500; cat. no.
sc-53546; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-ESR1 (1:500; cat. no. sc-542; Santa Cruz Biotechnology, Inc.),
anti-ESR2 (1:500; cat. no. sc-8974; Santa Cruz Biotechnology, Inc.)
and anti-PGR (1:500; cat. no. sc-538; Santa Cruz Biotechnology,
Inc.) antibodies overnight, followed by incubation with horseradish
peroxidase-conjugated secondary antibodies (1:2,000; cat. no.
sc-2313; Santa Cruz Biotechnology, Inc.) in 5% skim milk with PBS-T
for 1 h at room temperature. Luminol reagent (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) was used to visualize antibody binding.
Each blot was then stripped by incubation with 2% SDS and 100 mM
mercaptoethanol in 62.5 mM Tris-HCl (pH 6.8) for 30 min at 50-60°C.
Membranes were subsequently probed with antibody against β-actin
(1:3,000; cat. no. 4967; Cell Signaling Technology, Inc., Danvers,
MA, USA) as an internal control for the same duration and
temperature as with the other primary antibodies. Blots were
scanned using Gel Doc 1000 version 1.5 (Bio-Rad Laboratories,
Inc.), and band intensities were normalized to β-actin levels.
Immunocytochemical analysis
BeWo cells were seeded at 5×104 cells
with 200 μl DMEM in 8-well chamber slides (Thermo Fisher
Scientific, Inc.). When the cells attained 70-80% confluence, they
were either exposed to hypoxic conditions or treated with COMT-in
or L-NAME for 24 h in a 37°C incubator. Following three washes with
1X PBS, the cells were fixed with 4% formaldehyde solution (200
μl) for 10 min at room temperature, permeated with 1% Triton
X-100 (Biosesang, Inc., Seonam, Korea) for 10 min at room
temperature, and subsequently washed with 1X PBS three times. The
cells were then blocked in 2% bovine serum albumin (GenDEPOT, Inc.,
Barker, TX, USA), in PBS for 1 h at room temperature, and
subsequently specific primary antibodies [anti-ERα (cat. no.
sc-542), anti-ERβ (cat. no. sc-8974) and anti-PGR (cat. no.
sc-538); Santa Cruz Biotechnology, Inc.] were added at a dilution
of 1:50 overnight at 4°C. Following washing, cells were incubated
with secondary antibody (goat anti-rabbit immunoglobulin G-Texas
red; 1:100; cat. no. sc-2313; Santa Cruz Biotechnology, Inc.) for 1
h in the dark at room temperature. Following one wash with 1X PBS,
the fluorochrome DAPI (2 μg/ml; Santa Cruz Biotechnology,
Inc.) was then applied to each well, after which samples were
incubated for 10 min in the dark at room temperature. Finally, the
cells were washed three times with 1X PBS and mounted in
Vectashield mounting medium (Vector Laboratories, Inc., Burlingame,
CA, USA). All cells were observed and analyzed using a fluorescent
microscope (Eclipse TX100; Nikon Corporation, Tokyo, Japan) at a
magnification of ×400.
Statistical analysis
Results were presented as the mean ± standard
deviation. Data were analyzed using one-way analysis of variance
followed by Tukey's multiple comparison test using SPSS version
10.10 for Windows (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Confirmation of preeclampsia conditions
by biomarker genes
To confirm preeclampsia conditions, the expression
levels of VEGF and sFlt1, which are well known
biomarkers of preeclampsia in the human placenta, were examined
(32). As expected, transcription
levels of VEGF and sFlt1 were altered in the
preeclamptic placenta. Expression levels of VEGF were
significantly reduced up to 2.2-fold in preeclampsia samples
compared with the levels in normal placentas (Fig. 1A). The expression of sFlt1
significantly increased up to 1.5-fold in the preeclamptic placenta
samples compared with the level in normal samples (Fig. 1B). These results suggest that the
normal and preeclamptic placentas were properly separated in the
present study.
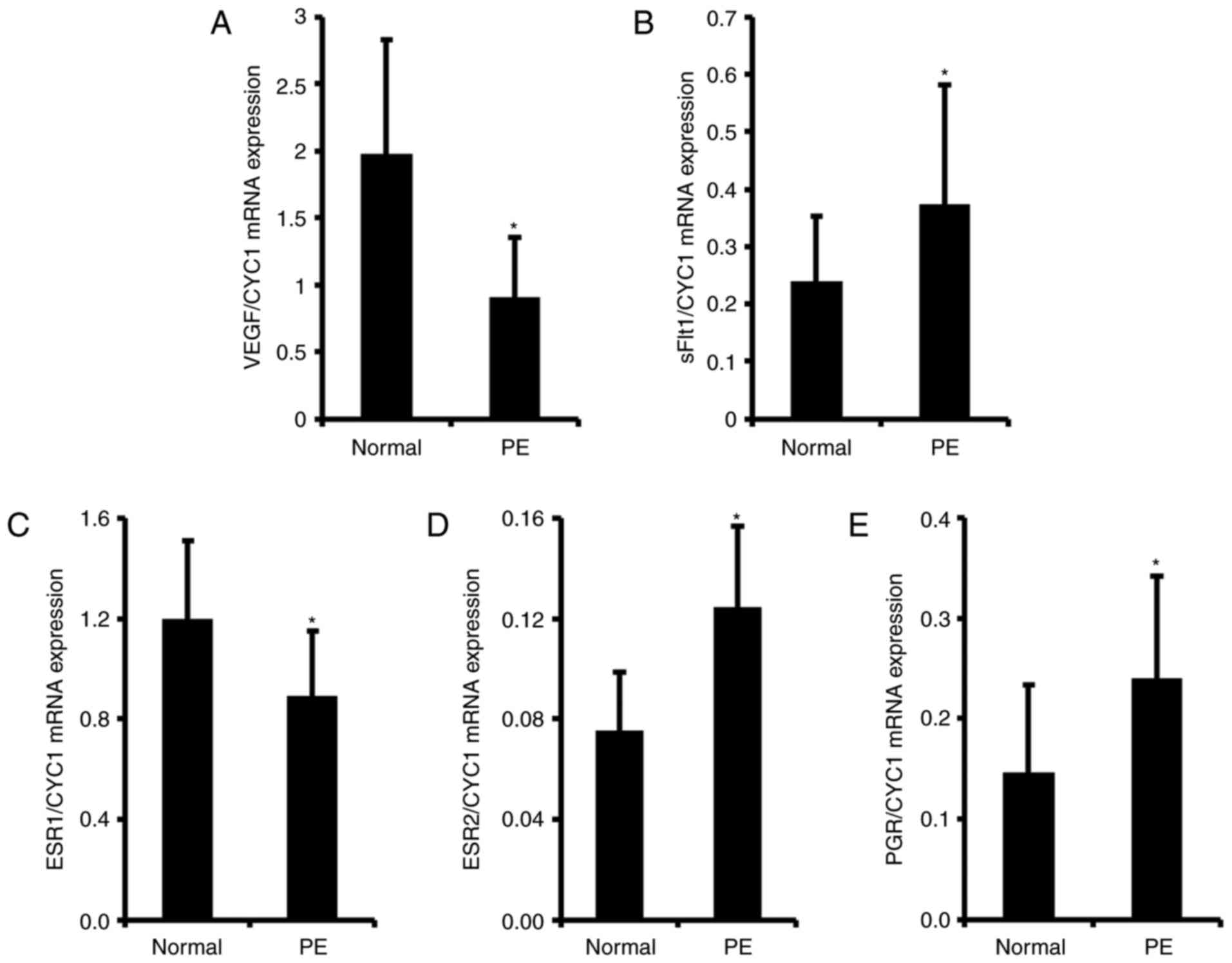 | Figure 1mRNA expression levels of VEGF,
sFlt1, ESR1, ESR2 and PGR in human normal and preeclamptic
placenta. Total mRNA was harvested from human normal and
preeclamptic placentas following the onset of labor.
Transcriptional levels of (A) VEGF, (B) sFlt1, (C)
ESR1, (D) ESR2 and (E) PGR were analyzed by
reverse transcription-quantitative polymerase chain reaction. Total
mRNA expression levels were normalized to that of CYC1. Data
are expressed as the mean ± standard deviation.
*P<0.05 vs. normal placenta. PE, preeclampsia; VEGF,
vascular endothelial growth factor; sFlt1, soluble fms-like
tyrosine kinase-1; ESR1, estrogen receptor 1; ESR2, estrogen
receptor 2; PGR, progesterone receptor; CYC1, cytochrome c1. |
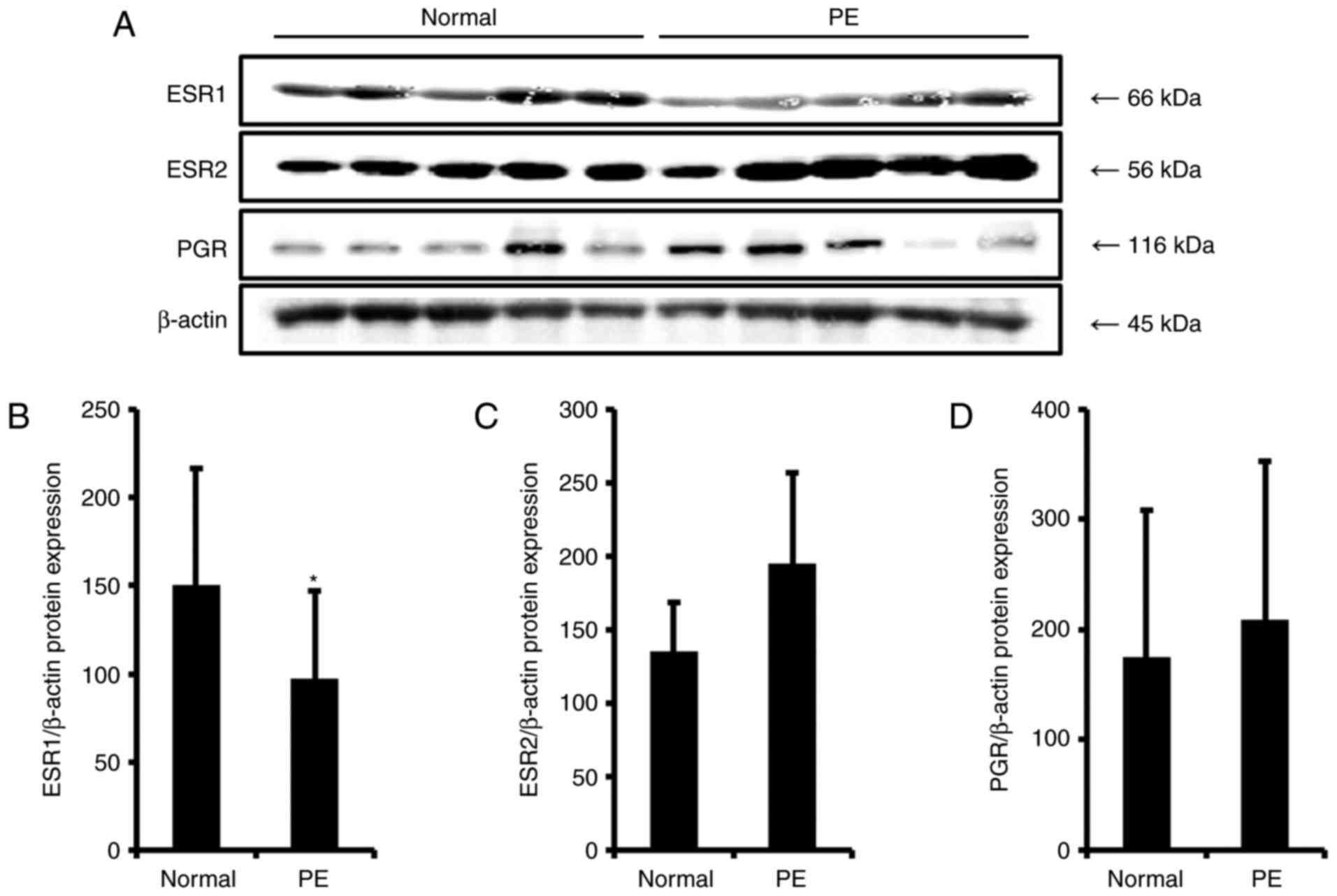
Expression of ESR1 is reduced in
preeclamptic placentas, and ESR2 and PGR expression is
increased
For the next experiment, the expression levels of
ESR1, ESR2 and PGR in normal and preeclamptic
placenta tissues were evaluated by RT-qPCR. As demonstrated in
Fig. 1C, the mRNA expression
level of ESR1 was significantly reduced by 1.4-fold, whereas
that of ESR2 was significantly elevated by 1.5-fold in
preeclamptic placenta samples, compared with the expression levels
in the normal group (Fig. 1D).
Expression of PGR, another steroid hormone receptor, was
significantly elevated up to 1.6-fold in the preeclamptic placenta
samples compared with the level in the normal group (Fig. 1E). To examine protein expression
levels of ESRs and PGR, western blotting was performed, and their
photographic figures and schematic graphs are represented in
Fig. 2. It was demonstrated that
protein expression levels of all steroid receptors were similar to
their mRNA expression level patterns in the preeclampsia group;
however, the only significant result obtained was for ESR1.
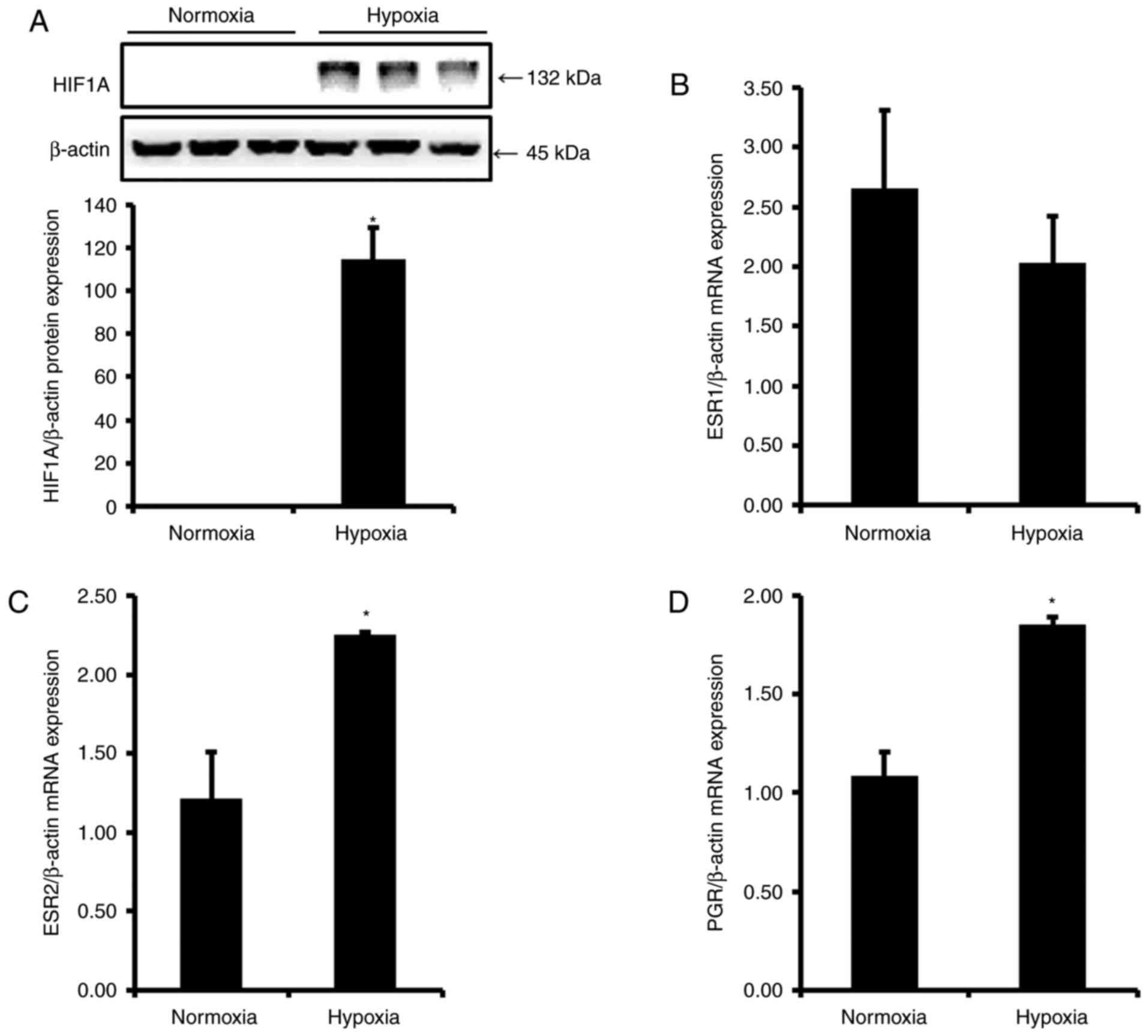
Induction of preeclamptic conditions in
vitro
To generate an in vitro preeclamptic
environment, human placenta-derived BeWo cells were incubated in a
hypoxia chamber, or under oxidative stress, induced by treatment
with COMT-in and L-NAME for 24 h. As HIF1A is a well-known
biomarker of hypoxia, HIF1A protein expression was examined to test
the present experimental conditions. As expected, HIF1A was not
expressed in the normoxia group, and the expression level increased
significantly in the hypoxia group compared with the level in the
normoxia group (Fig. 3A).
Expression of ESR1 is reduced in an in
vitro model of preeclampsia, and ESR2 and PGR expression is
increased
To evaluate expression levels of ESRs and
PGR in hypoxic conditions, BeWo cells were grown in the
absence and presence of hypoxia stress, and mRNA levels were
examined by RT-qPCR. Notably, expression levels of ESR1,
ESR2 and PGR after hypoxic stress in BeWo cells
demonstrated similar regulation to those in placentas from patients
with preeclampsia. As indicated in Fig. 3, mRNA expression levels of
ESR1 were reduced by 1.3-fold following hypoxia (Fig. 3B), whereas ESR2 and
PGR levels were significantly elevated by 1.9- and 1.7-fold
(Fig. 3C and D), respectively,
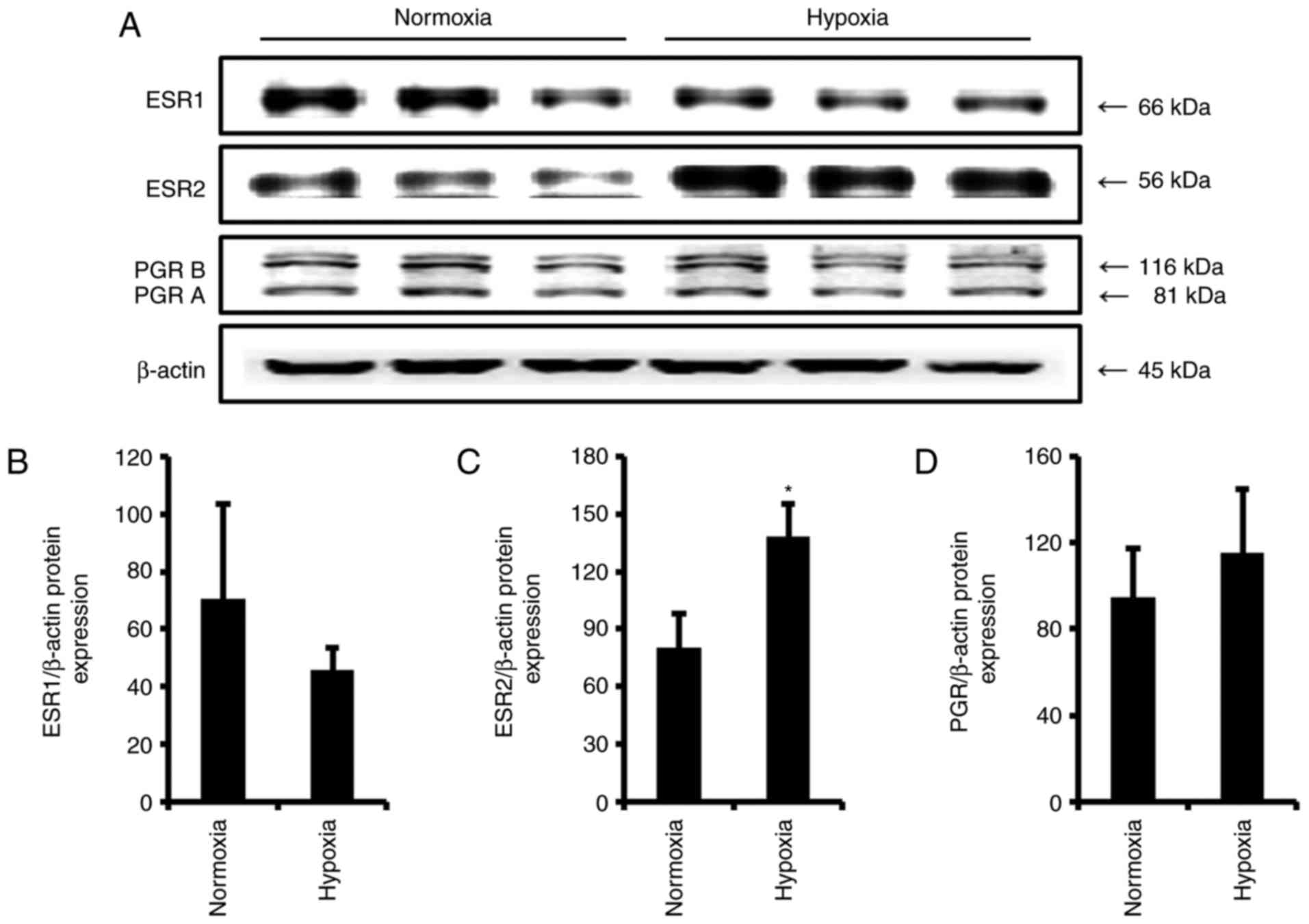
compared with the levels in the normoxia group. Protein expression
levels of ESRs and PGR were also analyzed (Fig. 4A). ESR1 protein expression
following hypoxia was reduced (Fig.
4B), while ESR2 and PGR expression levels were evaluated
compared with the levels in the normoxia group (Fig. 4C and D). These results were
consistent with the transcriptional results. In other in
vitro preeclampsia conditions, the expression levels of ESRs
and PGR were not significantly altered by COMT-in or L-NAME (data
not shown).
Immunofluorescent localization of ESR1,
ESR2 and PGR proteins
To analyze the spatial localization of ESR1, ESR2
and PGR in BeWo cells according to in vitro preeclamptic
conditions, immunocytochemistry was performed (Fig. 5). First, the localization of ESR1
was examined and it was revealed that the protein was predominantly
localized in the nucleus in the control group, and the localization
was not changed by hypoxia, COMT-in or L-NAME (Fig. 5A). In the case of ESR2, the
localization was limited to the nucleus in the control, hypoxia and
L-NAME groups. However, when the cells were treated with COMT-in,
the ESR2 protein was observed to be located in the cytoplasm
(Fig. 5B). The expression pattern
of PGR was similar to that of ESR2, indicating that PGR was present
in the nucleus in the control, hypoxia and L-NAME groups, and in
the cytoplasm in the COMT-in-treated group (Fig. 5C).
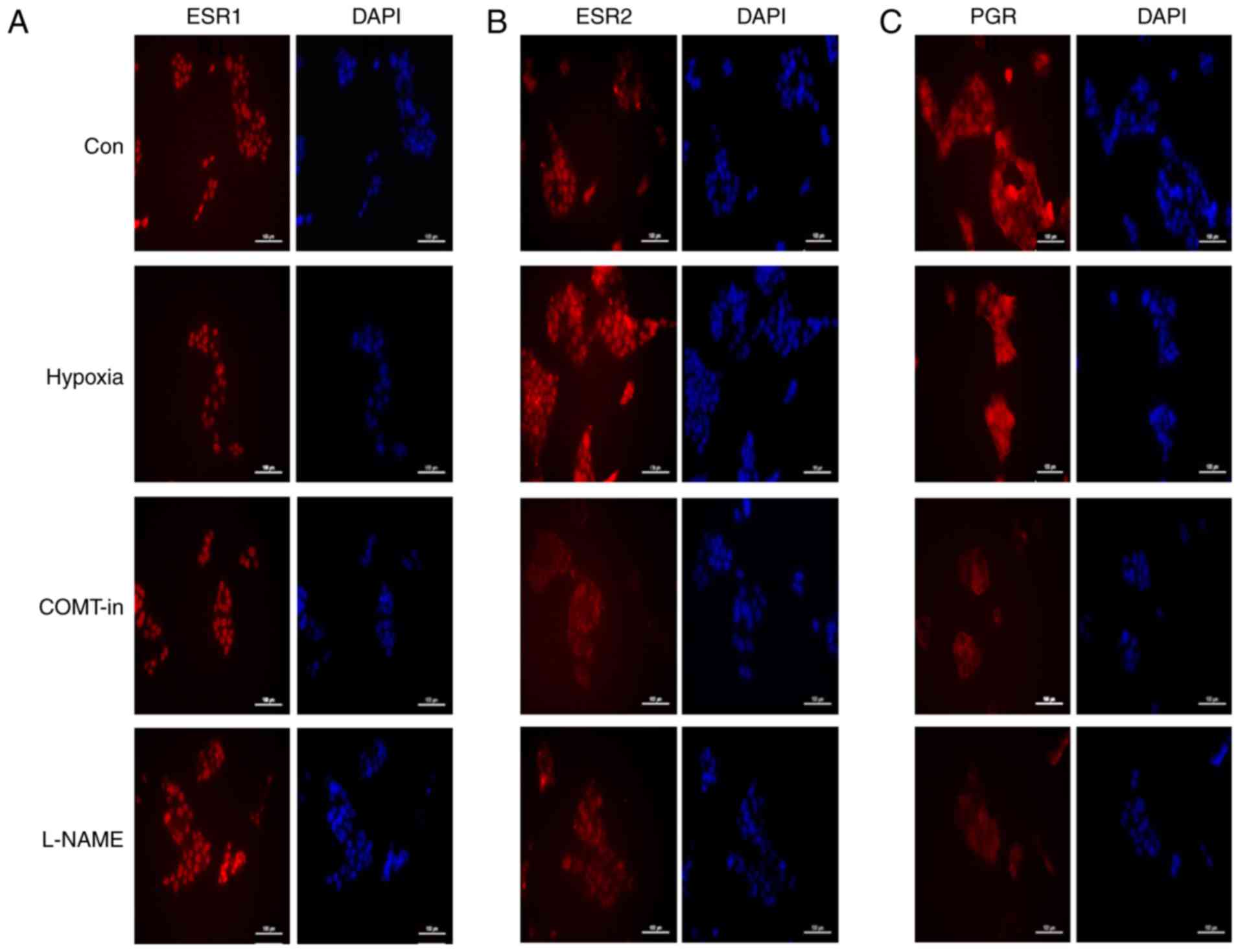 | Figure 5Immunocytochemistry analyses for
ESR1, ESR2 and PGR proteins in BeWo cells under hypoxic conditions
and following treatment with COMT-in and L-NAME. Cells were
cultivated under hypoxic conditions, or treated with COMT-in
(10−5 M) or L-NAME (10−4 M) for 24 h. Cells
were stained with primary antibodies against total (A) ESR1, (B)
ESR2 and (C) PGR. DAPI served as a counter stain. Scale bar, 100
μm. ESR1, estrogen receptor 1; ESR2, estrogen receptor 2;
PGR, progesterone receptor; COMT-in, catechol-O-methyl transferase
inhibitor; L-NAME, L-NG-nitroarginine methyl ester; Con,
control. |
Discussion
Preeclampsia is a common pregnancy-specific syndrome
char-acterized by hypertension and proteinuria (2). Despite the fact that preeclampsia is
regarded as a major disorder in pregnant women, its actual cause
and physiological characteristics are not clearly defined. Although
it is assumed that sex steroid hormones, including estrogen and
progesterone, may be associated with preeclampsia, expression and
regulation of steroid receptors in the preeclamptic placenta have
not been well studied. The present study examined the expression
levels of sex steroid receptors, including ESR1, ESR2 and
PGR, in the preeclamptic placenta. Initially, to confirm
that the placenta samples were derived from normal and preeclamptic
placentas, mRNA expression levels of VEGF and sFlt1,
biomarker genes of preeclampsia, were examined. Angiogenesis
requires complex interplay between VEGF and its cognate receptor,
VEGF receptor 1 (VEGFR1), which is alternatively named fms-like
tyrosine kinase (Flt1) (33).
Placental cells also secrete a soluble isoform of Flt1 (sFlt1),
which is generated through alternative splicing of Flt1
mRNA, and acts as an anti-angiogenic factor by interacting with,
and thereby neutralizing, VEGF (34). There is strong evidence to suggest
increased placental expression of sFlt1 and reduced
expression of free bioactive VEGF in patients with
preeclampsia (35). In the
present results, VEGF expression was decreased while
sFlt1 expression was increased in the preeclampsia group
compared with the levels in the normal group, as expected,
suggesting that the present placental samples were classified
correctly.
To the best of our knowledge, expression of PGR has
not been previously evaluated in the placentas of women with
preeclampsia, while there have been a few studies that have
examined the expression of ESRs in the preeclamptic placenta
(36). A study by Yin et
al (36) reported a
significant increase of ESR1 mRNA in preeclamptic placentas
compared with that in normal pregnancies. However, Schiessl et
al (37) reported that ESR
protein levels were not significantly altered in preeclamptic
placentas, although only immunostaining was performed in their
study. In contrast to the report by Yin et al (36), the present study demonstrated
reduced ESR1 expression in the preeclampsia group compared
with the normal group, whereas ESR2 and PGR
expression were increased at the mRNA and protein levels. It is
well established that ESR1 and ESR2 are
differentially expressed in various human tissues. Significantly
elevated ESR1 expression was observed in macroadenomas
compared with microadenomas, and in non-invasive compared to
invasive tumors (38).
ESR1 expression significantly increased while ESR2
decreased in human non-functional pituitary adenomas, suggesting
that the balance between ESR1 and ESR2 may affect the invasiveness
of these tumors (38).
To study the association between steroid receptors
and preeclampsia, the present study generated in vitro
preeclamptic conditions. Although the accurate cause of
preeclampsia remains unclear, lowered placental vascularization and
hypoxia are the most common model for preeclampsia (39,40). Therefore, placental cells were
incubated in a hypoxic chamber and the expression levels of steroid
receptors were examined. Although the effects of hypoxia were not
robust, the expression pattern of steroid receptors was similar to
those in the placenta of women with preeclampsia, with decreased
ESR1 and increased ESR2 and PGR expression
levels. In addition to hypoxia, preeclampsia models may be
constructed by stimulating the cells with L-NAME and COMT-in
(39). L-NAME is a potent
inhibitor of nitric oxide synthase and induces oxidative stress by
reducing nitric oxide production (41). COMT is a key enzyme in the
metabolism of estrogen, and generates 2-methoxyestradiol (2ME) that
is increased during pregnancy and is related to cytotrophoblast
invasion and preeclampsia (42).
A study by Kanasaki et al (43) demonstrated that the plasma
concentration of 2ME and placental COMT activity were reduced in
women with preeclampsia (43). In
addition, other previous studies have suggested that treatments
with COMT and L-NAME may be used as models of preeclampsia
(43,44). However, the mechanism of 2ME has
not been sufficiently studied. In the present study, L-NAME and
COMT-in did not significantly alter the expression of the steroid
receptors.
Steroid receptors, including ESR and PGR, are
classified as nuclear receptors, which directly bind to DNA and
regulate the expression of target genes (45). Therefore, the localization of the
steroid receptors is critical for their activation and function. In
normal conditions, the ESR1, ESR2 and PGR proteins were present in
the nucleus when the cells were immunostained in the present study.
The hypoxia and L-NAME treatment did not alter the localization of
the receptors; however, COMT-in translocated ESR2 and PGR from the
nucleus to the cytoplasm, suggesting that ESR2 and PGR may be
inactivated when the action of 2ME is blocked in placental cells.
It is also possible that ESR2 and PGR expression was
enhanced in the preeclamptic placenta to compensate for their
inactivation caused by reduction of 2ME.
Taken together, the present study suggested that
ESRs and PGR were dynamically associated with preeclampsia. In
preeclamptic placenta samples and hypoxic conditions, the mRNA and
protein expression levels of ESR1 were reduced. Furthermore, in
vitro study indicated that the activation of ESR2 and PGR was
blocked by treatment with COMT-in. The reduced ESR1 signals and
inactivation of ESR2 and PGR may be associated with the
physiological symptoms of preeclampsia.
Acknowledgments
The present study was supported by a Biomedical
Research Institute Grant (grant no. 2014-09) from Pusan National
University Hospital.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Barker DJ: The fetal origins of coronary
heart disease. Acta Paediatr Suppl. 422:78–82. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cockell AP and Poston L: Flow-mediated
vasodilatation is enhanced in normal pregnancy but reduced in
preeclampsia. Hypertension. 30:247–251. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jobe SO, Tyler CT and Magness RR: Aberrant
synthesis, metabolism, and plasma accumulation of circulating
estrogens and estrogen metabolites in preeclampsia implications for
vascular dysfunction. Hypertension. 61:480–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine RJ, Maynard SE, Qian C, Lim KH,
England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein
FH, et al: Circulating angiogenic factors and the risk of
preeclampsia. N Engl J Med. 350:672–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
VanWijk MJ, Kublickiene K, Boer K and
VanBavel E: Vascular function in preeclampsia. Cardiovasc Res.
47:38–48. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Furuya M, Kurasawa K, Nagahama K, Kawachi
K, Nozawa A, Takahashi T and Aoki I: Disrupted balance of
angiogenic and antiangiogenic signalings in preeclampsia. J
Pregnancy. 2011:1237172011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Staun-Ram E and Shalev E: Human
trophoblast function during the implantation process. Reprod Biol
Endocrinol. 3:562005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pennington KA, Schlitt JM, Jackson DL,
Schulz LC and Schust DJ: Preeclampsia: Multiple approaches for a
multifacto-rial disease. Dis Model Mech. 5:9–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Genbacev O, Joslin R, Damsky CH, Polliotti
BM and Fisher SJ: Hypoxia alters early gestation human
cytotrophoblast differentiation/invasion in vitro and models the
placental defects that occur in preeclampsia. J Clin Invest.
97:540–550. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dekker GA and Sibai BM: Low-dose aspirin
in the prevention of preeclampsia and fetal growth retardation:
Rationale, mechanisms, and clinical trials. Am J Obstet Gynecol.
168:214–227. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Altman D, Carroli G, Duley L, Farrell B,
Moodley J, Neilson J and Smith D; Magpie Trial Collaboration Group:
Do women with pre-eclampsia, and their babies, benefit from
magnesium sulphate? The Magpie Trial: A randomised
placebo-controlled trial. Lancet. 359:1877–1890. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sibai B, Dekker G and Kupferminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Witlin AG and Sibai BM: Magnesium sulfate
therapy in preeclampsia and eclampsia. Obstet Gynecol. 92:883–889.
1998.PubMed/NCBI
|
|
14
|
Björnström L and Sjöberg M: Mechanisms of
estrogen receptor signaling: Convergence of genomic and nongenomic
actions on target genes. Mol Endocrinol. 19:833–842. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JZ, Sheehan PM, Brennecke SP and
Keogh RJ: Vessel remodelling, pregnancy hormones and extravillous
trophoblast function. Mol Cell Endocrinol. 349:138–144. 2012.
View Article : Google Scholar
|
|
16
|
Açıkgöz Ş, Bayar UO, Can M, Güven B,
Mungan G, Doğan S and Sümbüloğlu V: Levels of oxidized LDL,
estrogens, and progesterone in placenta tissues and serum
paraoxonase activity in preeclampsia. Mediators Inflamm.
2013:8629822013. View Article : Google Scholar
|
|
17
|
Feng Y, Zhang P, Zhang Z, Shi J, Jiao Z
and Shao B: Endocrine disrupting effects of triclosan on the
placenta in pregnant rats. PLoS One. 11:e01547582016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albrecht ED and Pepe GJ: Estrogen
regulation of placental angiogenesis and fetal ovarian development
during primate pregnancy. Int J Dev Biol. 54:397–408. 2010.
View Article : Google Scholar :
|
|
19
|
Magness RR: Maternal cardiovascular and
other physiologic responses to the endocrinology of pregnancy.
Endocrinol Pregnancy. 9:507–539. 1998. View Article : Google Scholar
|
|
20
|
Magness RR and Rosenfeld CR: Local and
systemic estradiol-17 beta: Effects on uterine and systemic
vasodilation. Am J Physiol. 256:E536–E542. 1989.PubMed/NCBI
|
|
21
|
Reynolds LP, Kirsch JD, Kraft KC, Knutson
DL, McClaflin WJ and Redmer DA: Time-course of the uterine response
to estradiol-17beta in ovariectomized ewes: Uterine growth and
microvascular development. Biol Reprod. 59:606–612. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szekeres-Bartho J: Immunosuppression by
progesterone in pregnancy. CRC Press; pp. 1–155. 1992
|
|
23
|
Buhimschi I, Yallampalli C, Chwalisz K and
Garfield RE: Pre-eclampsia-like conditions produced by nitric oxide
inhibition: Effects of L-arginine, D-arginine and steroid hormones.
Hum Reprod. 10:2723–2730. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ito M, Nakamura T, Yoshimura T, Koyama H
and Okamura H: The blood pressure response to infusions of
angiotensin II during normal pregnancy: Relation to plasma
angiotensin II concentration, serum progesterone level, and mean
platelet volume. Am J Obstet Gynecol. 166:1249–1253. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao QP, Buhimschi IA, Saade G, Chwalisz K
and Garfield RE: Regulation of vascular adaptation during pregnancy
and post-partum: Effects of nitric oxide inhibition and steroid
hormones. Hum Reprod. 11:2777–2784. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radwanska E: The role of reproductive
hormones in vascular disease and hypertension. Steroids.
58:605–610. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dodd JM, Jones L, Flenady V, Cincotta R
and Crowther CA: Prenatal administration of progesterone for
preventing preterm birth in women considered to be at risk of
preterm birth. Cochrane Database Syst Rev. CD0049472013.PubMed/NCBI
|
|
28
|
Hass DM and Ramsey PS: Progestogen for
preventing miscarriage. Cochrane Database Syst Rev.
CD0035112008.
|
|
29
|
Meher S and Duley L: Interventions for
preventing pre-eclampsia and its consequences: Generic protocol.
Cochrane Libr. CD0053012005.
|
|
30
|
Jones HN, Ashworth CJ, Page KR and McArdle
HJ: Cortisol stimulates system A amino acid transport and SNAT2
expression in a human placental cell line (BeWo). Am J Physiol
Endocrinol Metab. 291:E596–E603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Carty DM, Delles C and Dominiczak AF:
Novel biomarkers for predicting preeclampsia. Trends Cardiovasc
Med. 18:186–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hiratsuka S, Maru Y, Okada A, Seiki M,
Noda T and Shibuya M: Involvement of Flt-1 tyrosine kinase
(vascular endothelial growth factor receptor-1) in pathological
angiogenesis. Cancer Res. 61:1207–1213. 2001.PubMed/NCBI
|
|
34
|
Ahmed A, Dunk C, Ahmad S and Khaliq A:
Regulation of placental vascular endothelial growth factor (VEGF)
and placenta growth factor (PlGF) and soluble Flt-1 by oxygen-a
review. Placenta. 21(Suppl A): S16–S24. 2000. View Article : Google Scholar
|
|
35
|
McKeeman GC, Ardill JE, Caldwell CM,
Hunter AJ and McClure N: Soluble vascular endothelial growth factor
receptor-1 (sFlt-1) is increased throughout gestation in patients
who have preeclampsia develop. Am J Obstet Gynecol. 191:1240–1246.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin G, Zhu X, Guo C, Yang Y, Han T, Chen
L, Yin W, Gao P, Zhang H, Geng J, et al: Differential expression of
estradiol and estrogen receptor α in severe preeclamptic
pregnancies compared with normal pregnancies. Mol Med Rep.
7:981–985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schiessl B, Mylonas I, Hantschmann P, Kuhn
C, Schulze S, Kunze S, Friese F and Jeschke U: Expression of
endothelial NO synthase, inducible NO synthase, and estrogen
receptors alpha and beta in placental tissue of normal,
preeclamptic, and intrauterine growth-restricted pregnancies. J
Histochem Cytochem. 53:1441–1449. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Manoranjan B, Salehi F, Scheithauer BW,
Rotondo F, Kovacs K and Cusimano MD: Estrogen receptors alpha and
beta immunohistochemical expression: Clinicopathological
correlations in pituitary adenomas. Anticancer Res. 30:2897–2904.
2010.PubMed/NCBI
|
|
39
|
Nevo O, Soleymanlou N, Wu Y, Xu J, Many A,
Zamudio S and Caniggia I: Increased expression of sFlt-1 in in vivo
and in vitro models of human placental hypoxia is mediated by
HIF-1. Am J Physiol Regul Integr Comp Physiol. 291:R1085–R1093.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Soleymanlou N, Jurisica I, Nevo O, Ietta
F, Zhang X, Zamudio S, Post M and Caniggia I: Molecular evidence of
placental hypoxia in preeclampsia. J Clin Endocrinol Metab.
90:4299–4308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Babbedge RC, Bland-Ward PA, Hart SL and
Moore PK: Inhibition of rat cerebellar nitric oxide synthase by
7-nitro indazole and related substituted indazoles. Br J Pharmacol.
110:225–228. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pertegal M, Fenoy FJ, Hernández M,
Mendiola J, Delgado JL, Bonacasa B, Corno A, López B, Bosch V and
Hernández I: Fetal Val108/158Met catechol-O-methyltransferase
(COMT) polymorphism and placental COMT activity are associated with
the development of preeclampsia. Fertil Steril. 105:134–143. e1–e3.
2016. View Article : Google Scholar
|
|
43
|
Kanasaki K, Palmasten K, Sugimoto H, Ahmad
S, Hamano Y, Xie L, Parry S, Augustin SH, Gattone VH, Folkman J, et
al: Deficiency in catechol-O-methyltransferase and
2-methoxyoestradiol is associated with pre-eclampsia. Nature.
453:1117–1121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yallampalli C and Garfield RE: Inhibition
of nitric oxide synthesis in rats during pregnancy produces signs
similar to those of preeclampsia. Am J Obstet Gynecol.
169:1316–1320. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Beato M: Gene regulation by steroid
hormones. Cell. 56:335–344. 1989. View Article : Google Scholar : PubMed/NCBI
|