Introduction
Obesity is a major health concern in developed
countries, and is caused by an imbalance between energy intake and
expenditure. Obesity is also known to be associated with various
metabolic diseases, including hypertension, type 2 diabetes,
cardiovascular diseases, atherosclerosis and various cancers
(1-4). Adipocytes are the major cellular
components of fat tissue, and excessive fat tissue growth,
hypertrophy and adipocyte differentiation are fundamental
characteristics of obesity (5).
The accumulation of lipid droplets in adipocytes has an important
role in lipid metabolism and regulation (6), and the differentiation of
preadipocytes is regulated by transcription factors, such as
peroxisome proliferator-activated receptor-γ (PPARγ) and
CCAAT/enhancer-binding protein-α (C/EBPα) (7,8).
Numerous methods, including diet control, exercise
and medication, have been suggested to prevent and treat obesity
(9). However, many clinically
available drugs have side effects such as constipation, anorexia,
dizziness, and insomnia, which remain issues of concern (10). To avoid the side effects of
pharmacological agents, there is a need for substitute therapies
with minimum side-effects, perhaps based on herbal or natural
products (11).
Euphorbia supina (E. supina) belongs
to the Euphorbiaceae family and is used in traditional medicine to
treat a variety of diseases, such as bronchitis, jaundice,
hemorrhage, and several gastrointestinal diseases (12,13). It has also been reported that
E. supina contains various biologically active compounds
like tannins, terpenoids and polyphenols (14), and polyphenols with antioxidant
properties (15). Furthermore, a
recent study revealed that E. supina polyphenol mixtures
inhibit the invasion and metastasis of breast cancer cells
(16). However, to the best of
our knowledge, no scientific evidence has yet been presented
regarding the anti-obesity effect of E. supina. The purpose
of the present study was to investigate the anti-obesity effect of
E. supina and the mechanism underlying its inhibitory effect
on adipogenesis.
Materials and methods
Materials and reagents
TRIzol reagent and SuperScript III kit were obtained
from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Mouse primary antibodies against PPARγ (sc-7273), C/EBPα
(sc-166258) and β-actin (sc-47778), and goat-anti-mouse horseradish
peroxidase (HRP)-conjugated secondary antibodies (sc-2030) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Primary antibodies were diluted to 1:1,000 and secondary antibodies
at 1:5,000. Garcinia cambogia (GC) extract was purchased
from Prakruti Products Pvt. Ltd. (Karnataka, India).
Preparation of the ethanol extract of E.
supina (ESEE)
E. supina was obtained from Gungangbogam
(Jechon, Korea). The dried and powdered fruit of E. supina
(500 g) were extracted using 50% ethanol for 2 h under
mantle-reflux at 80°C. Following filtration, and solvent removal on
a rotary vacuum evaporator (N-000; EYELA; Tokyo Rikakikai Co.,
Ltd., Tokyo, Japan) ESEE was obtained as a powder (17). The yield of ESEE obtained using
this procedure was 83.5 g (16.7% wt/wt). ESEE was stored at 4°C
until required.
Animal treatment
Male C57BL/6 mice (n=36; weight, 20.4±1.03 g; age, 5
weeks) were purchased from Samtako Bio Korea (Samtako Bio Korea,
Osan, Korea). Experimental procedures were conducted according to a
protocol approved by the institutional animal care committee of
Chonbuk National University. Mice were housed at 22±2°C, 50±5%
humidity and provided a normal diet and water ad libitum.
Following a 1-week acclimation period, mice were divided into six
groups (n=6 each), as follows: i) the normal diet (ND) group, fed
with normal standard diet containing 14% fat, 21% protein and 65%
carbohydrate (5L79; Orient Bio, Inc., Seongnam, Korea); ii) the
high-fat diet (HFD) group, fed with HFD containing 60% fat, 20%
protein and 20% carbohydrate (D12492; Research Diets, Inc., New
Brunswick, NJ, USA); iii) the HFD+ESEE (2) group, fed with HFD and treated with
ESEE (2 mg/kg/daily); iv) the HFD+ESEE (10) group, fed with HFD and treated with
ESEE (10 mg/kg/daily); v) the HFD+ESEE (50) group, fed with HFD and
treated with ESEE (50 mg/kg/daily); vi) the HFD+GC group, fed with
HFD and treated with GC (200 mg/kg/daily). ESEE or GC was
administered daily via oral gavage for 6 weeks. Body weights and
amounts of food consumed were determined weekly.
Determination of abdominal fat volume by
micro-computed tomography (micro-CT)
Following the 6-week treatment period, mice were
starved for 6 h prior to sacrifice and anesthetized via
intraperitoneal injection of ketamine (75 mg/kg; Yuhan Corporation,
Seoul, Korea) and rompun (15 mg/kg; Bayer Korea Ltd., Seoul,
Korea). Images were acquired using a Skyscan-1076 micro-CT scanner
(Bruker microCT, Kontich, Belgium). CT was performed using a pixel
size of 35 μm, a source voltage of 50 kVp, and a source
current of 200 μA. The X-ray detector contained a 12-bit,
water-cooled charge-coupled device camera with a resolution of
4,000×2,300 pixels and a scintillator. Images were acquired at 0.6
degrees and an exposure time of 0.46 sec using a 1-mm aluminum
energy filter. Mice were sacrificed following micro-CT scanning and
abdominal fat volumes were measured using an Olympus SP-500 UZ
camera (Olympus Corporation, Tokyo, Japan). Following micro-CT,
under anesthesia, mice were euthanized via intracardiac puncture.
Blood was collected (0.8-0.9 ml) via cardiac puncture followed by
removal of organs such as liver, kidney and spleen. Following
exsanguination, mortality was confirmed by incising the heart and
ensuring that no respiratory movement occurred for ≥3 min.
Hematoxylin and eosin (H&E)
staining
Liver tissue and epididymal white adipose tissue
(eWAT) were fixed using 10% neutral buffered formalin at room
temperature for 8 h, embedded in paraffin wax, and cut serially
into 10-μm sections. Sections were stained with H&E at
room temperature (hematoxylin, 4 min; eosin, 2 min) and histologic
alterations were observed and photographed under a light microscope
(magnification, ×100; Olympus CX21; Olympus Corporation) (18).
Biochemical analysis
To evaluate hepatic steatosis, levels of hepatic
total triglycerides (TG) and cholesterol (TC) were determined by
homogenizing liver tissues in a chloroform/methanol mixture (2:1,
v/v). TG and TC concentrations were then measured using
commercially available kits (TG; AM 1575-K; TC; AM 202-K; Asan
Pharmaceutical Co., Ltd., Seoul, Korea) as detailed previously
(19). In addition, rapid
enzymatic assay kits were used to measure serum levels of TC, TG
and high-density lipoprotein cholesterol (HDL-c; AM 203-K; Asia
Pharmaceutical Co., Ltd., Seoul, Korea), and low density
lipoprotein cholesterol (LDL-c) was calculated using Friedewald's
formula (20) as follows:
LDL-c=TC-(HDL-c+TG/5).
ELISA kits were used to measure leptin
(ADI-900-019A; Enzo Life Sciences Inc., Farmingdale, NY, USA) and
adiponectin (47-ADPMS-E01; R&D Systems, Inc., Minneapolis, MN,
USA). HFD-induced liver damage was assessed by measuring the serum
enzyme activities of alanine transaminase (ALT) and aspartate
aminotransferase (AST) using the ALT/AST cassette test kit (Alere
Cholestech LDX® System; Alere, Inc., Waltham, MA, USA)
(18).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from eWAT and liver tissues
using TRIzol RNA Isolation Reagent, according to the manufacturer's
instructions. The synthesis of cDNA was performed with 2 μg
RNA using the SuperScript III First Strand Synthesis kit protocol.
qPCR was performed using the ABI Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using SYBR Green PCR
Master Mix (Thermo Fisher Scientific, Inc.). PCR was performed
using the following conditions: 95°C for 10 min followed by 40
cycles of denaturation at 95°C for 15 sec, and annealing at 60°C
for 1 min, as detailed previously (21). Primer sequences were as follows:
GAPDH, forward 5′-CATGGCCTTCCGTGTTC-3′ and reverse
5′-CCTGGTCCTCAGTGTAGC-3′; PPARγ, forward
5′-GATGGAAGACCACTCGCATT-3′) and reverse 5′-AACCATTGGGTCAGCTCTTG-3′;
and C/EBPα, forward 5′-TTGTTTGGATTTATCTCGGC-3′ and reverse
5′-CCAAGAAGTCGGTGGACAAG-3′. The comparative quantification cycle
method was used to measure the relative quantitation of each gene
and mRNA levels were normalized with GAPDH, as detailed previously
(6).
Western blot analysis
eWAT and liver tissues were lysed in ice-cold
radioimmunoprecipitation assay buffer (sc-24948; Santa Cruz
Biotechnology, Inc., CA, USA) for 40 min and centrifuged (12,000 ×
g) for 20 min at 4°C, as detailed previously (21). Protein quantification was
determined via bicinchoninic acid assay and protein samples (20
μg/lane) were separated by 8% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (GE Healthcare, Chicago, IL,
USA), which were then blocked with 5% skimmed milk in tris-buffered
saline containing 0.1% Tween-20 (TBST) for 1 h at room temperature.
Membranes were then probed with primary antibodies at 4°C
overnight, washed with TBST 4 times, incubated at room temperature
with HRP-conjugated secondary antibody for 45 min, and rewashed
with TBST 3 times. Proteins were visualized using an enhanced
chemiluminescence detection kit (EMD Millipore, Bedford, MA, USA)
and a Fusion FX7 imaging system (Vilber Lourmat, Marne-la-Vallée,
France).
Ultra-pressure liquid chromatography
(UPLC)
UPLC was performed using an ACQUITY UPLC BEH C18
column (2.1×50 mm, 1.7 μm) and a photodiode array detector
(Waters Corporation, Milford, MA, USA), as detailed previously
(17). Elution was performed at a
flow rate of 0.15 ml/min using distilled water containing 0.1%
formic acid (solvent A) and acetonitrile containing 0.1% formic
acid (solvent B) in gradient mode (B 5% from 0-1 min, B 5-95% from
1-16 min, B 95-100% from 16-18 min, and B 100% from 18-26 min at a
flow rate of 0.15 ml/min at 25°C. Detection was performed at a
wavelength of 330 nm.
Statistical analysis
Data are presented as the mean + or ± standard error
of the mean, and were analyzed using GraphPad Prism software
(version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). One-way
analysis of variance was used to measure the significant difference
followed by Tukey's post hoc test for comparison of means.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ESEE reduces body weight gain in HFD-fed
mice
To investigate the anti-obesity effect of ESEE, mice
were fed HFD with or without ESEE (2, 10 and 50 mg/kg, oral gavage
daily) or with GC (200 mg/kg, oral gavage daily) for 6 weeks. Food
intake and body weights were recorded once weekly. At the end of
the 6-week treated period, HFD mice had a mean body weight gain of
41.2%, whereas ND mice exhibited a gain of 18.7%. Mean percentage
body weight gain of HFD mice was significantly reduced by ESEE (50
mg/kg) supplementation (Fig. 1A and
B). In addition, no significant difference in dietary intake
was observed among the HFD-fed and ESEE administered groups
(Fig. 1C), but 50 mg/kg ESEE
significantly reduced the food conversion efficiency as compared
with HFD-fed mice (Fig. 1D).
These results indicate that ESEE induced adipose weight loss, and
reduced body weight gain and the percentage of food efficiency.
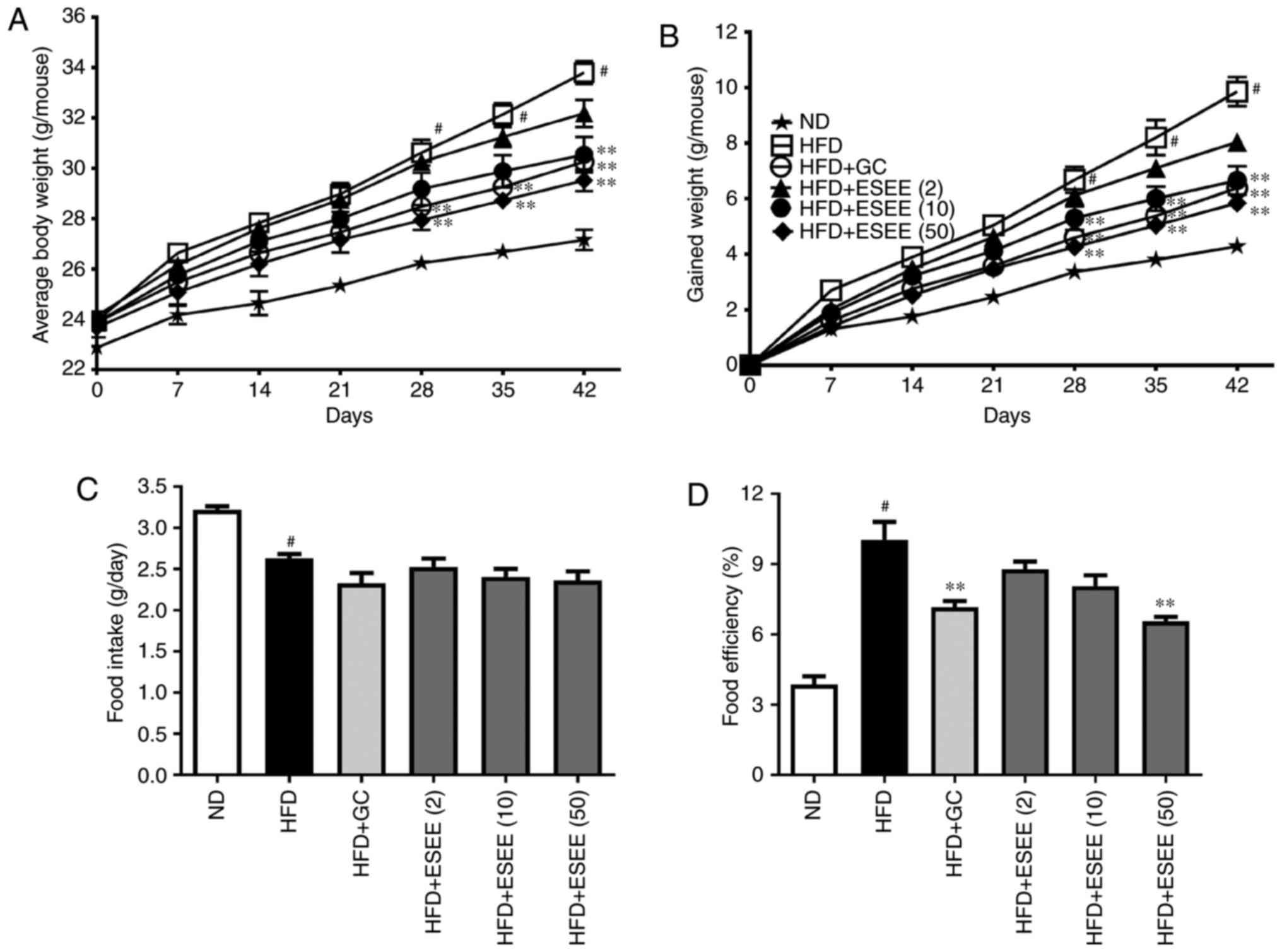 | Figure 1Effects of ESEE on body weight, food
intake and food conversion efficiency in HFD-fed mice. (A) Mean
body weight, (B) gained body weight, (C) mean food intake per day
and (D) food conversion efficiency were measured weekly. Results
are presented as the mean + or ± the standard error of the mean
(n=6). #P<0.05 vs. ND; *P<0.05 and
**P<0.01 vs. HFD. ESEE, ethanol extract of
Euphorbia supine; HFD, high-fat diet; ND, normal diet;
(2), 2 mg/kg; (10), 10 mg/kg; (50), 50 mg/kg; GC,
Garcinia cambogia. |
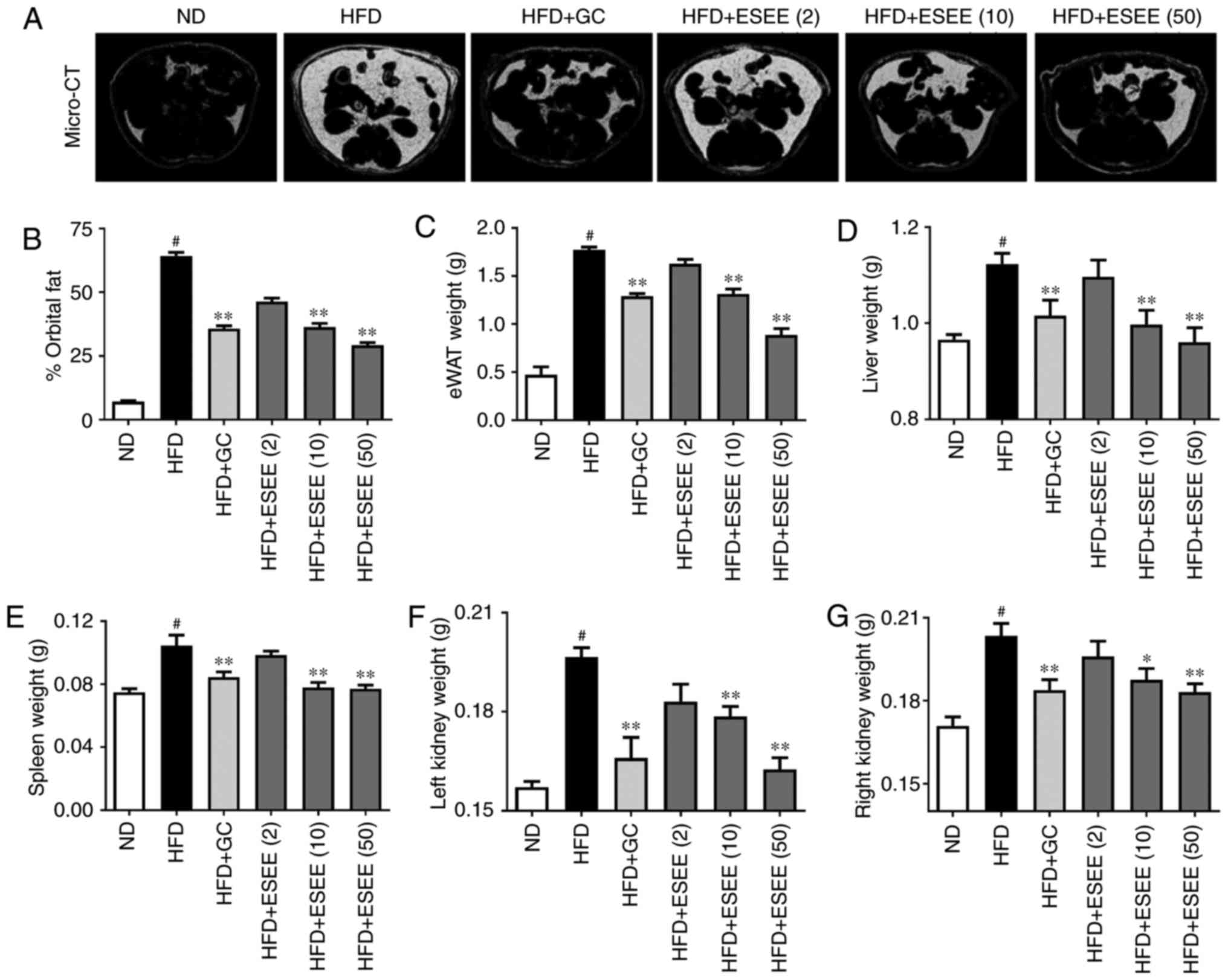
ESEE reduces fat deposition in organs in
HFD-fed mice
Micro-CT analysis results demonstrated that orbital
fat volume percentage was significantly lower in ESEE (10 and 50
mg/kg) treated mice than in HFD-fed mice (Fig. 2A and B). Furthermore, the effects
of ESEE supplementation on fat accumulation in eWAT, liver, spleen
and kidneys were investigated. The results demonstrated that these
organs were significantly heavier in HFD mice than in ND mice, and
that ESEE treatment significantly reduced fat deposition in these
organs, compared with HFD-fed mice (Fig. 2C–G).
ESEE suppresses histological changes in
the eWAT and liver tissues of HFD-fed mice
Increases in lipid accumulations in the liver and
eWAT are commonly observed in HFD-fed obese mice (17). H&E staining revealed enlarged
or hyper-trophic eWAT in the HFD group, and smaller eWAT sizes in
ESEE supplemented mice (Fig. 3A).
In addition, the histological study revealed enlarged hepatocytes,
as evidenced by excessive vacuolation in the HFD group as compared
with the ND group. As presented in Fig. 3B, ESEE markedly reduced
vacuolization and lipid droplet numbers in the liver tissues of HFD
mice. Hepatic steatosis was determined by measuring hepatic TG and
TC levels. The present results demonstrated that ESEE (10 and 50
mg/kg) supplementation significantly reduced HFD-induced levels of
TG and TC in liver tissues (Fig. 3C
and D). Serum ALT and AST levels were also measured, which are
clinical markers of liver damage. The results indicated that ESEE
significantly reduced the HFD-induced serum levels of AST and ALT
compared with the HFD group (Fig. 3E
and F). These data indicate that ESEE inhibited HFD-induced
hepatic steatosis and serum liver enzymes.
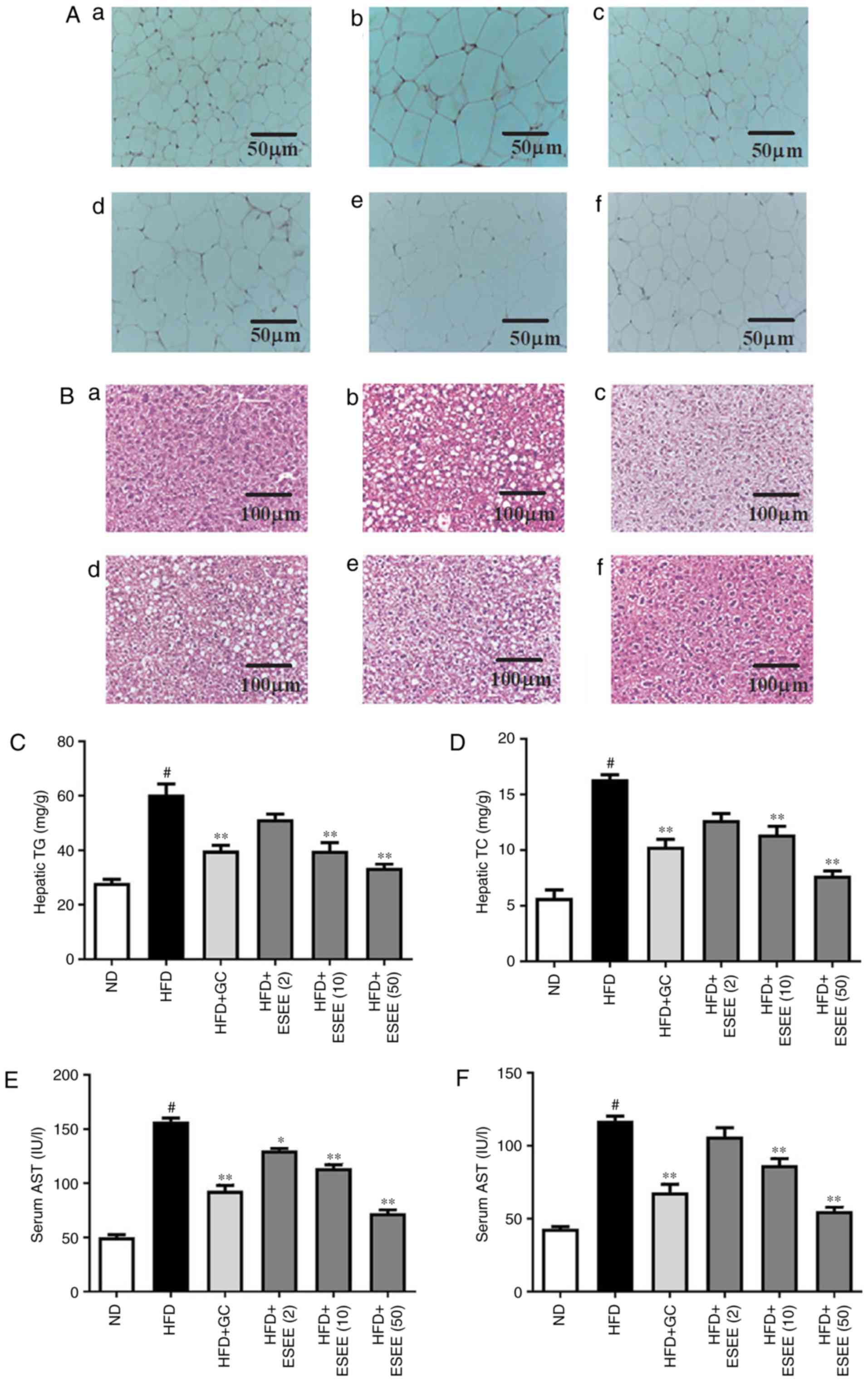 | Figure 3Effect of ESEE on histological
changes and lipid accumulations in eWAT and liver tissues of
HFD-induced obese mice. Histological changes were determined via
H&E staining in (A) eWAT and (B) liver tissue sections
(magnification, ×100) of mice from the (a) ND, (b) HFD, (c) HFD+GC,
(d) HFD+ESEE (2), (e) HFD+ESEE
(10) and (f) HFD+ESEE (50)-fed
mice. Lipid accumulation in liver was assessed by measuring hepatic
(C) TG and (D) TC levels. Serum levels of (E) AST and (F) ALT were
measured using commercial kits. Results are presented as the mean +
the standard error of the mean (n=6). #P<0.05 vs. ND;
*P<0.05 and **P<0.01 vs. HFD. ESEE,
ethanol extract of Euphorbia supine; HFD, high-fat diet;
eWAT, epididymal white adipose tissue; ND, normal diet; (2), 2 mg/kg; (10), 10 mg/kg; (50), 50 mg/kg; GC,
Garcinia cambogia; TG, total triglycerides; TC, total
cholesterol; AST, aspartate aminotransferase; ALT, alanine
transaminase. |
ESEE improves biochemical parameters of
blood in HFD-fed mice
HFD-fed mice exhibited significantly higher serum
levels of TC, low-density lipoprotein cholesterol (LDL-c) and TG,
and significantly lower levels of high-density lipoprotein
cholesterol (HDL-c) than mice in the ND group. ESEE significantly
inhibited TC (50 mg/kg), LDL-c (10 and 50 mg/kg) and TG (50 mg.kg)
increases, and increased HDL-c levels (10 and 50 mg/kg) vs. HFD-fed
mice (Fig. 4A–D). Furthermore,
ESEE significantly inhibited leptin levels and increased
adiponectin levels. At a dose of 50 mg/kg, ESEE decreased mean
leptin levels by 64.7% (Fig. 4E)
and increased mean adiponectin levels by 51.1% compared with the
HFD group (Fig. 4F).
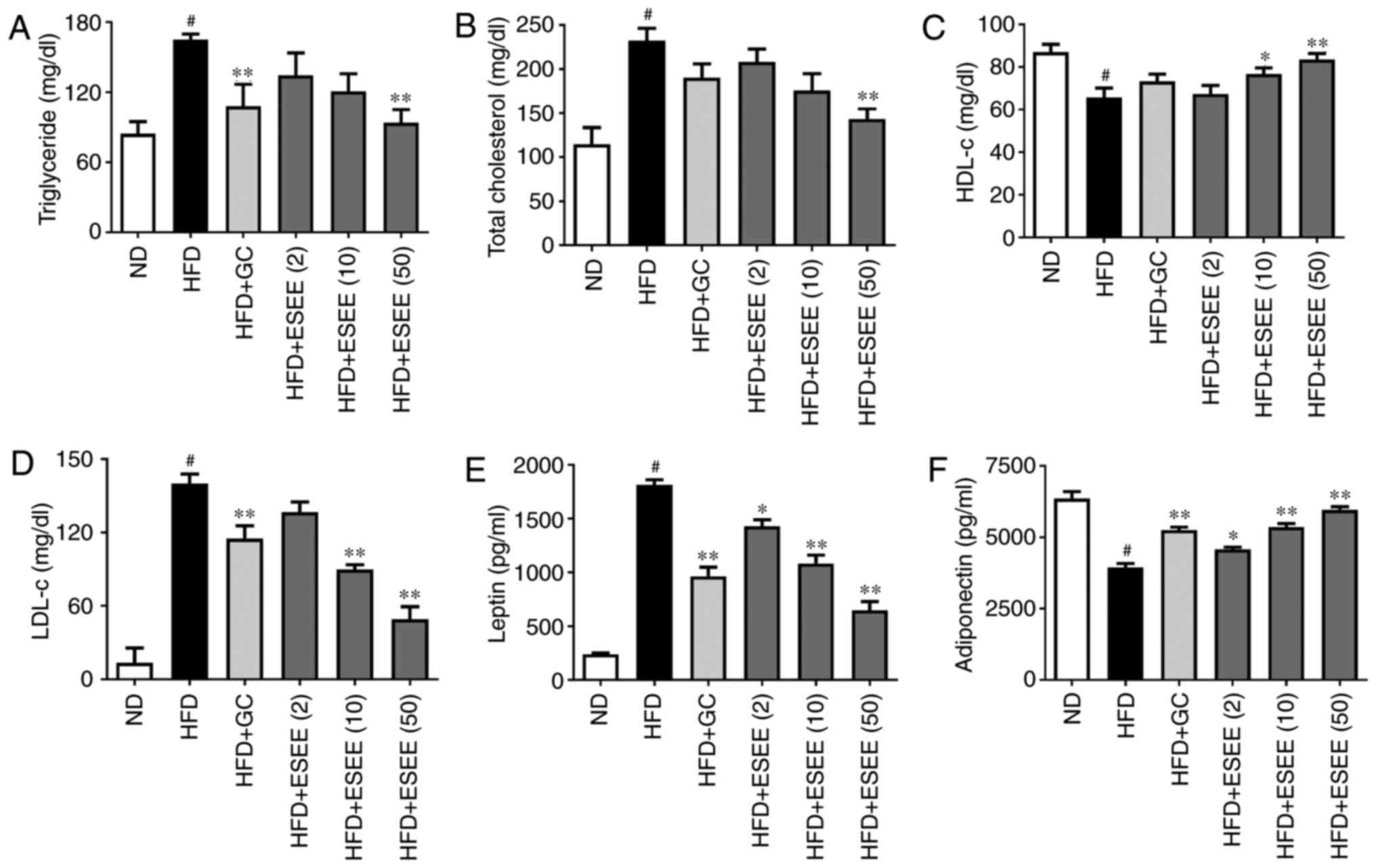 | Figure 4Effect of ESEE on blood biochemical
parameters in HFD-fed mice. Enzymatic methods were used to measure
serum levels of (A) triglyceride (B) total cholesterol (C) HDL-c,
and (D) LDL-c. ELISA was used to measure serum levels of (E) leptin
and (F) adiponectin. Results are presented as the mean + the
standard error of the mean (n=6). #P<0.05 vs. ND;
*P<0.05 and **P<0.01 vs. HFD. ESEE,
ethanol extract of Euphorbia supine; HFD, high-fat diet;
HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density
lipoprotein cholesterol; ND, normal diet; (2), 2 mg/kg; (10), 10 mg/kg; (50), 50 mg/kg; GC,
Garcinia cambogia. |
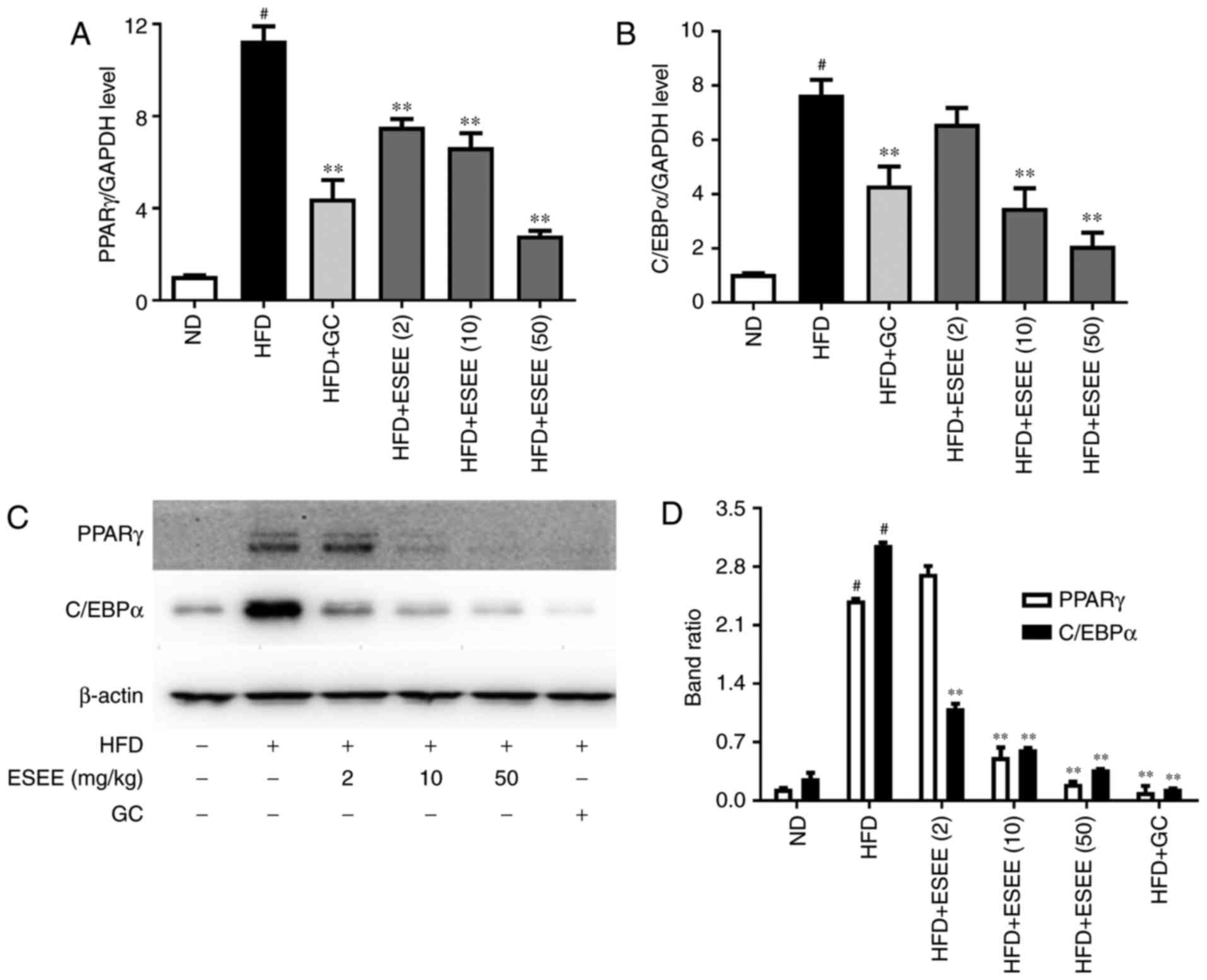
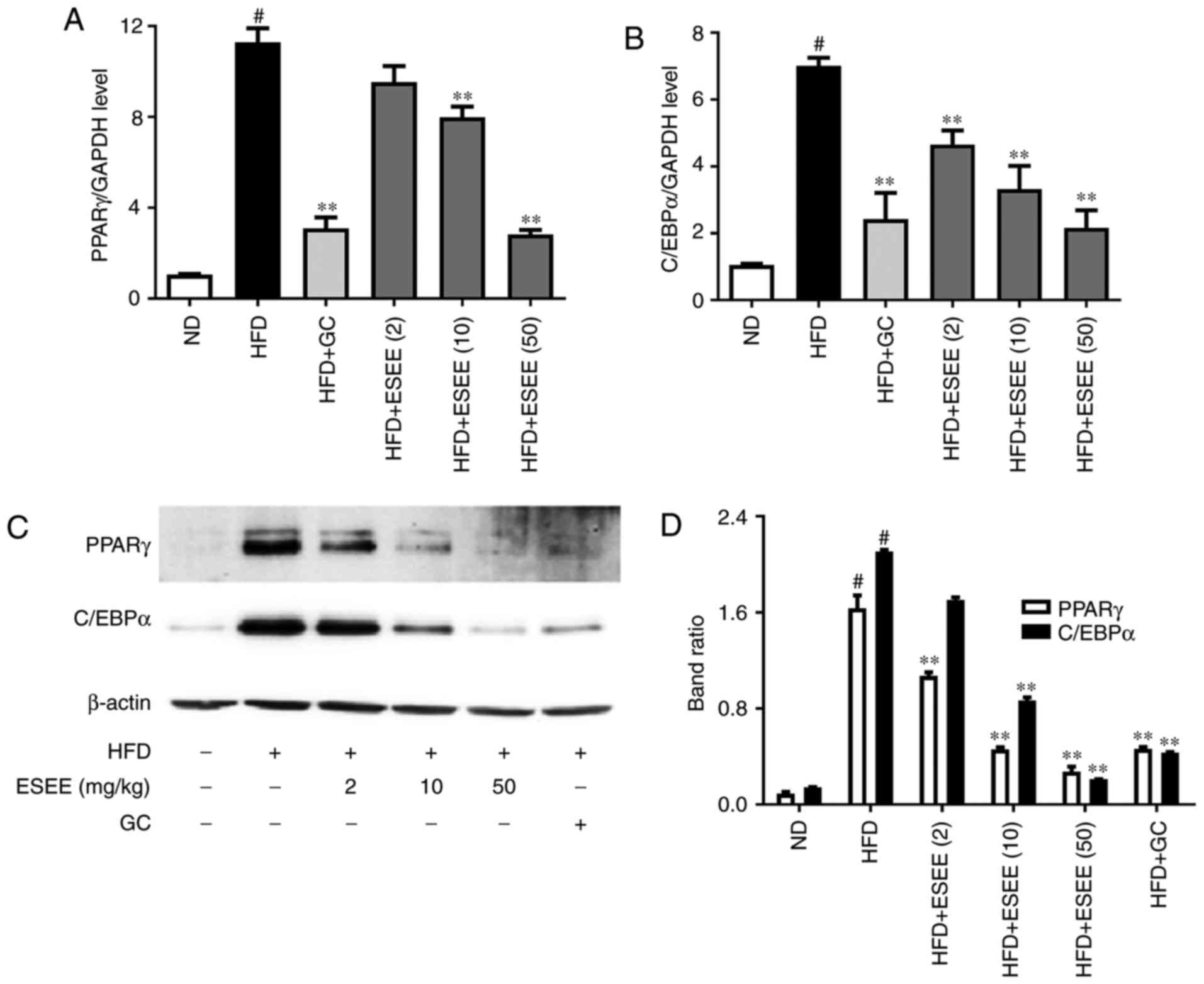
ESEE inhibits transcription factors of
adipogenesis in HFD-fed mice
RT-qPCR and western blotting were performed to
measure the mRNA and protein expressions, respectively, of
transcription factors of adipogenesis, such as PPARγ and C/EBPα.
The results demonstrated that ESEE (10 and 50 mg/kg) significantly
inhibited the mRNA and protein expressions of PPARγ and C/EBPα in
eWAT tissues (Fig. 5) and liver
tissues (Fig. 6), as compared
with the HFD group.
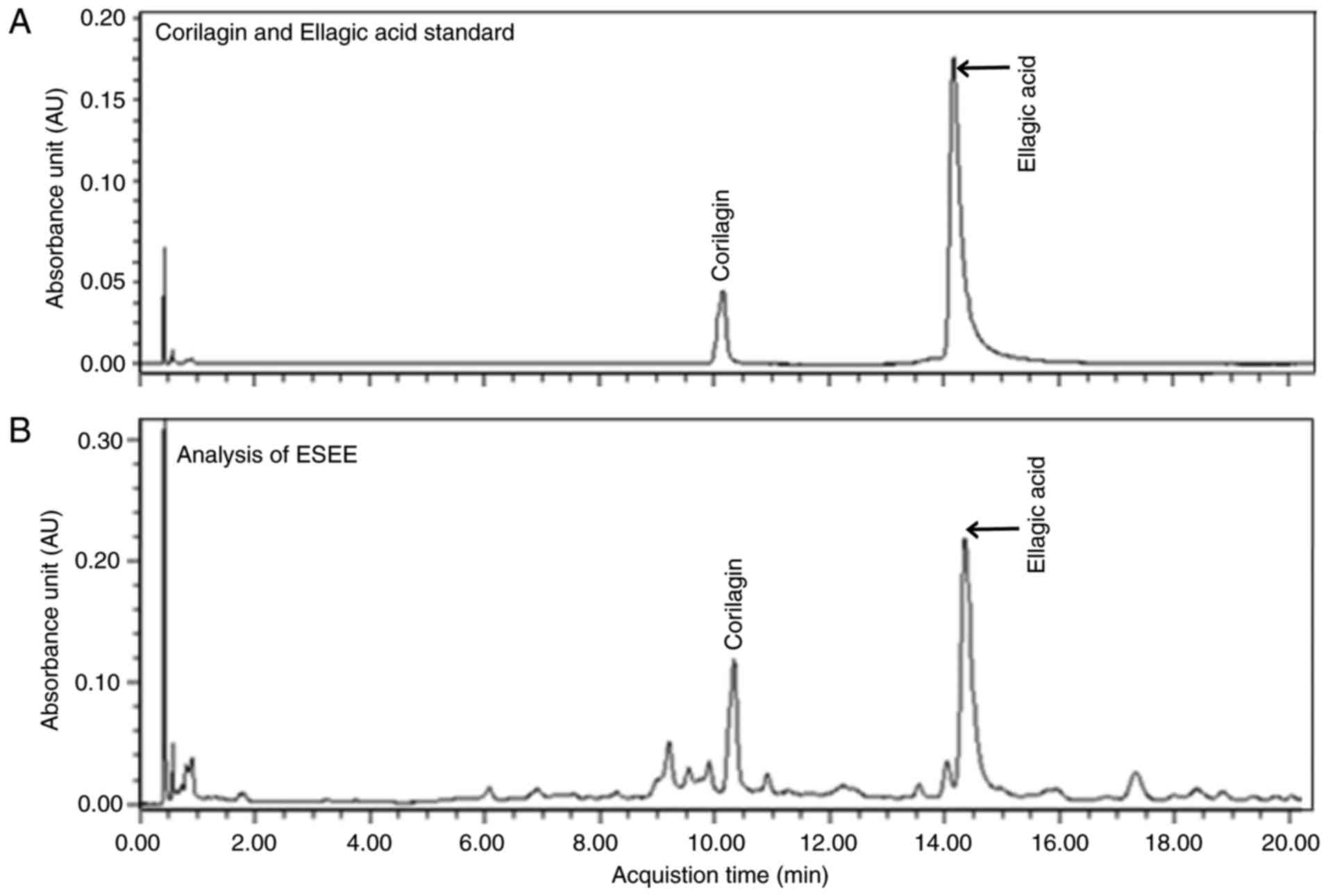
Chemical components of ESEE by UPLC
UPLC assay demonstrated that corilagin and ellagic
acid were the main components of ESEE (Fig. 7). However, other peaks observed
were unidentified and require further study.
Discussion
The induction of obesity in animals via HFD
supplementation is the most popular means of mimicking obesity in
human, and a number of studies have been conducted using
HFD-induced obese mouse models, in which HFD supplementation
increases abdominal fat and body weights (22). Previous studies have reported
crude plant extracts and natural compounds isolated from them
induce weight loss and prevent diet-induced obesity (17,23). Although, E. supina is known
to have potent anti-oxidant, anti-aging and anti-cancer effects,
its effects on obesity have not been previously studied. Therefore,
the present study was designed to investigate the effects of ESEE
in HFD-fed obese mice.
To the best of our knowledge, this is the first
study to report the anti-obesity effects of ESEE in an HFD-fed
obese mice model. ESEE was observed to reduce body weight gain and
fat accumulation in organs versus HFD-fed mice. Furthermore, ESEE
did not affect food intake levels but did reduce the food
conversion efficiencies, which indicated that ESEE treatment
reduced the abilities of animals to convert food into body mass.
ESEE also suppressed weight increases of eWAT, and of organs
including the liver, spleen and kidneys versus HFD-fed mice. These
findings are in accordance with those of a previous study, in which
a capsicoside G-rich fraction from pepper exhibited anti-obesity
effects in HFD-induced obese mice (23).
Obesity is associated with the development of fatty
liver (24), and an imbalance
between food consumption and combustion leads to hepatic steatosis
(25). Therefore, H&E
staining was performed to measure the histological morphologies of
adipose and liver tissues. ESEE was observed to inhibit adipocyte
hypertrophy and the accumulation of lipid droplets in the liver.
Furthermore, ESEE reduced hepatic steatosis as evidenced by
reductions in the levels of hepatic TG and TC. In addition, ESEE
reduced HFD-induced serum markers of fatty liver, including AST and
ALT. These findings were similar to those reported for the
anti-obesity effect of Gomisin N in HFD-induced obese mice
(26). ESEE reduced HFD-induced
increases in serum lipid profiles, such as TC, TG and LDLc, and
increased serum levels of HDLc, which were in accordance with its
observed suppressive effect on HFD-induced fatty liver and
hyperlipidemia in HFD-fed mice. WAT synthesizes leptin, which
serves important roles in the suppression of food intake and the
control of body weight (27), and
adiponectin, which regulates glucose levels and serves an important
role in hepatic fatty acid oxidation (28,29). Therefore, the levels of leptin and
adiponection in ESEE treated HFD-fed mice were examined. The
present results demonstrated that ESEE significantly reduced serum
leptin levels and increased serum adiponectin levels in HFD-fed
mice. These findings concur with those of a previous study, which
demonstrated that Triticum aestivum sprouts have an
anti-obesity effect in HFD-fed mice (17).
PPARγ and C/EBP-α are important transcription
factors of adipogenesis (30).
PPARγ is expressed predominantly in adipose tissues and serves an
important role in adipocyte differentiation, lipid storage, and
glucose homeostasis (31,32). PPARγ binds with C/EBP-α promoter
region and this binding induces the expression of C/EBP-α, which
directly controls adipocyte differentiation (33). In the present study, ESEE
significantly inhibited the mRNA and protein expressions of PPARγ
and C/EBP-α, which was previously detected in green tomato
extract-treated HFD-induced obese mice (34).
Chemical analysis by UPLC in the present study
showed that corilagin and ellagic acid were the main components of
ESEE, but other observed peaks were not identified. Many studies
have reported the anti-inflammatory, anti-cancer and
hepatoprotective effects of corilagin (35-37). Previous studies have also reported
that ellagic acid improved hepatic steatosis and suppressed serum
resistin levels in obese mice (38,39), and that ellagic acid suppresses
lipid accumulation by inhibiting adipogenesis and cell
proliferation (40). In a
previous study, it was demonstrated that the anti-obesity effect of
Geranium thunbergii was due to the presence of components
including corilagin and ellagic acid (41). According to the present findings,
corilagin and ellagic acid are the major components if ESEE, and
therefore, it is hypothesized that these compounds were at least
partially responsible for the observed anti-obesity effects of
ESEE.
The present study has some limitations that should
be noted. First, ESEE was administered at various doses to HFD-fed
mice but not ND-fed mice. Second, the effects of corilagin and
ellagic acid on HFD-induced obesity were not investigated.
To the best of our knowledge, the present study was
the first to demonstrate that ESEE effectively inhibits
adipogenesis, and that ESEE contains anti-adipogenic molecules that
downregulate transcription factors for adipogenesis, such as C/EBPα
and PPARα/γ. These findings suggest that ESEE possesses
anti-obesity effects, and that it may be a promising agent for the
treatment of obesity and its metabolic syndrome.
Acknowledgments
The present study was supported by a 2017 grant from
Wonkwang University (Jeonbuk, Korea; grant no. WK-2017-2).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Dorresteijn JA, Visseren FL and Spiering
W: Mechanisms linking obesity to hypertension. Obes Rev. 13:17–26.
2012. View Article : Google Scholar
|
|
2
|
McCarthy MI: Genomics, Type 2 diabetes,
and obesity. N Engl J Med. 363:2339–2350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tzotzas T, Evangelou P and Kiortsis DN:
Obesity, weight loss and conditional cardiovascular risk factors.
Obes Rev. 12:e282–e289. 2011. View Article : Google Scholar
|
|
4
|
Rocha VZ and Libby P: Obesity,
inflammation, and atherosclerosis. Nat Rev Cardiol. 6:399–409.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yeh WC, Cao Z, Classon M and McKnight SL:
Cascade regulation of terminal adipocyte differentiation by three
members of the C/EBP family of leucine zipper proteins. Genes Dev.
9:168–181. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poudel B, Nepali S, Xin M, Ki HH, Kim YH,
Kim DK and Lee YM: Flavonoids from Triticum aestivum inhibit
adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol
Med Rep. 12:3139–3145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han J, Farmer SR, Kirkland JL, Corkey BE,
Yoon R, Pirtskhalava T, Ido Y and Guo W: Octanoate attenuates
adipogenesis in 3T3-L1 preadipocytes. J Nutr. 132:904–910. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poudel B, Lim SW, Ki HH, Nepali S, Lee YM
and Kim DK: Dioscin inhibits adipogenesis through the AMPK/MAPK
pathway in 3T3-L1 cells and modulates fat accumulation in obese
mice. Int J Mol Med. 34:1401–1408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanif MW and Kumar S: Pharmacological
management of obesity. Expert Opin Pharmacother. 3:1711–1718. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Padwal RS and Majumdar SR: Drug treatments
for obesity: Orlistat, sibutramine, and rimonabant. Lancet.
369:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song MY, Lv N, Kim EK, Kwon KS, Yoo YB,
Kim JH, Lee SW, Song JH, Lee JH, Lee SK, et al: Antiobesity
activity of aqueous extracts of Rhizoma Dioscoreae Tokoronis on
high-fat diet-induced obesity in mice. J Med Food. 12:304–309.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nugroho A, Rhim TJ, Choi MY, Choi JS, Kim
YC, Kim MS and Park HJ: Simultaneous analysis and
peroxynitrite-scavenging activity of galloylated flavonoid
glycosides and ellagic acid in Euphorbia supina. Arch Pharm Res.
37:890–898. 2014. View Article : Google Scholar
|
|
13
|
Han MH, Lee WS, Nagappan A, Kim HJ, Park
C, Kim GY, Hong SH, Kim ND, Kim G, Ryu CH, et al: Polyphenols from
Korean prostrate spurge Eurphorbia supine induce apoptosis through
the Fas-associated extrinsic pathway and activation of ERK in human
leukemic U937 cells. Oncol Rep. 36:99–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhanalakshmi C, Manivasagam T, Nataraj J,
Justin Thenmozhi A and Essa MM: Neurosupportive role of vanillin, a
natural phenolic compound, on rotenone induced neurotoxicity in
SH-SY5Y neuroblastoma cells. Evid Based Complement Alternat Med.
2015:6260282015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Y, Jeong SW, Lee WS, Park S, Kim YH,
Kim GS, Lee SJ, Jin JS, Kim CY, Lee JE, et al: Determination of
polyphenol components of Korean prostrate spurge (Euphorbia supina)
by using liquid chromatography-tandem mass spectrometry: Overall
contribution to antioxidant activity. J Anal Methods Chem.
2014:4186902014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ko YS, Lee WS, Joo YN, Choi YH, Kim GS,
Jung JM, Ryu CH, Shin SC and Kim HJ: Polyphenol mixtures of
Euphorbia supina the inhibit invasion and metastasis of highly
metastatic breast cancer MDA-MB-231 cells. Oncol Rep. 34:3035–3042.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Im JY, Ki HH, Xin M, Kwon SU, Kim YH, Kim
DK, Hong SP, Jin JS and Lee YM: Anti-obesity effect of Triticum
aestivum sprouts extract in high-fat-diet-induced obese mice.
Biosci Biotechnol Biochem. 79:1133–1140. 2015. View Article : Google Scholar
|
|
18
|
Nepali S, Ki HH, Lee JH, Lee HY, Lee YM
and Kim DK: Wheatgrass derived polysaccharide has
anti-inflammatory, anti-oxidative and anti-apoptotic effects on
LPS-induced hepatic injury in mice. Phytother Res. 31:1107–1116.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nepali S, Ki HH, Lee JH, Cha JH, Lee YM
and Kim DK: Triticum aestivum sprout-derived polysaccharide exerts
hepatoprotective effects against ethanol-induced liver damage by
enhancing the antioxidant system in mice. Int J Mole Med.
40:1243–1252. 2017. View Article : Google Scholar
|
|
20
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
21
|
Nepali S, Son JS, Poudel B, Lee JH, Lee YM
and Kim DK: Luteolin is a bioflavonoid that attenuates
adipocyte-derived inflammatory responses via suppression of nuclear
factor-κB/mitogen-activated protein kinases pathway. Pharmacogn
Mag. 11:627–635. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perez Gutierrez RM, Madrigales Ahuatzi D,
Horcacitas Mdel C, Garcia Baez E, Cruz Victoria T and Mota-Flores
JM: Ameliorative effect of hexane extract of Phalaris canariensis
on high fat diet-induced obese and streptozotocin-induced diabetic
mice. Evid Based Complement Alternat Med. 2014:1459012014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sung J, Jeong HS and Lee J: Effect of the
Capsicoside G-rich Fraction from Pepper (Capsicum annuum L.) seeds
on high-fat diet-induced obesity in mice. Phytother Res.
30:1848–1855. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haque M and Sanyal AJ: The metabolic
abnormalities associated with non-alcoholic fatty liver disease.
Best Pract Res Clin Gastroenterol. 16:709–731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reddy JK and Rao MS: Lipid metabolism and
liver inflammation. II. Fatty liver disease and fatty acid
oxidation. Am J Physiol Gastrointest Liver Physiol. 290:G852–G858.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jang MK, Yun YR, Kim JH, Park MH and Jung
MH: Gomisin N inhibits adipogenesis and prevents high-fat
diet-induced obesity. Sci Rep. 7:403452017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maffei M, Halaas J, Ravussin E, Pratley
RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al:
Leptin levels in human and rodent: Measurement of plasma leptin and
ob RNA in obese and weight-reduced subjects. Nat Med. 1:1155–1161.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Combs TP, Berg AH, Obici S, Scherer PE and
Rossetti L: Endogenous glucose production is inhibited by the
adipose-derived protein Acrp 30. J Clin Invest. 108:1875–1881.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pajvani UB and Scherer PE: Adiponectin:
Systemic contributor to insulin sensitivity. Curr Diab Rep.
3:207–213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soukas A, Socci ND, Saatkamp BD, Novelli S
and Friedman JM: Distinct transcriptional profiles of adipogenesis
in vivo and in vitro. J Biol Chem. 276:34167–34174. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schoonjans K, Staels B and Auwerx J: The
peroxisome proliferator activated receptors (PPARS) and their
effects on lipid metabolism and adipocyte differentiation. Biochim
Biophys Acta. 1302:93–109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rosen ED, Walkey CJ, Puigserver P and
Spiegelman BM: Transcriptional regulation of adipogenesis. Genes
Dev. 14:1293–1307. 2000.PubMed/NCBI
|
|
33
|
Wu Z, Xie Y, Bucher NL and Farmer SR:
Conditional ectopic expression of C/EBP beta in NIH-3T3 cells
induced PPAR gamma and stimulates adipogenesis. Genes Dev.
9:2350–2363. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi KM, Lee YS, Shin DM, Lee S, Yoo KS,
Lee MK, Lee JH, Kim SY, Lee YM, Hong JT, et al: Green tomato
extract attenuates high-fat-diet-induced obesity through activation
of the AMPK pathway in C57BL/6 mice. J Nutr Biochem. 24:335–342.
2013. View Article : Google Scholar
|
|
35
|
Li HR, Liu J, Zhang SL, Luo T, Wu F, Dong
JH, Guo YJ and Zhao L: Corilagin ameliorates the extreme
inflammatory status in sepsis through TLR4 signaling pathways. BMC
Complement Alter Med. 17:182017. View Article : Google Scholar
|
|
36
|
Attar R, Cincin ZB, Bireller ES and
Cakmakoglu B: Apoptotic and genomic effects of corilagin on SKOV3
ovarian cancer cell line. Oncol Targets Ther. 10:1941–1946. 2017.
View Article : Google Scholar
|
|
37
|
Liu FC, Chaudry IH and Yu HP:
Hepatoprotective effects of corilagin following hemorrhagic shock
are through Akt-dependent pathway. Shock. 47:346–351. 2017.
View Article : Google Scholar :
|
|
38
|
Yoshimura Y, Nishii S, Zaima N, Moriyama T
and Kawamura Y: Ellagic acid improves hepatic steatosis and serum
lipid composition through reduction of serum resistin levels and
transcriptional activation of hepatic ppara in obese, diabetic
KK-A(y) mice. Biochem Biophys Res Commun. 434:486–491. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Panchal SK, Ward L and Brown L: Ellagic
acid attenuates high-carbohydrate, high-fat diet-induced metabolic
syndrome in rats. Eur J Nutr. 52:559–568. 2013. View Article : Google Scholar
|
|
40
|
Woo MS, Choi HS, Seo MJ, Jeon HJ and Lee
BY: Ellagic acid suppresses lipid accumulation by suppressing early
adipogenic events and cell cycle arrest. Phytother Res. 29:398–406.
2015. View Article : Google Scholar
|
|
41
|
Sung YY, Yoon T, Yang WK, Kim SJ and Kim
HK: Anti-obesity effects of Geranium thunbergii extract via
improvement of lipid metabolism in high-fat diet-induced obese
mice. Mol Med Rep. 4:1107–1113. 2011.PubMed/NCBI
|