Introduction
Approximately 20–40% of diabetic patients develop
diabetic nephropathy (DN), a clinical syndrome that comprises renal
failure and increased risk of cardiovascular disease (1,2).
DN is characterized by persistent albuminuria, development of
hyperfiltration and histopathological lesions including
extracellular matrix deposition, glomerular basement membrane
thickening, glomerular and mesangial expansion (3). As DN progresses to end-stage chronic
kidney disease, patients would require hemodialysis and even kidney
transplant in the end (4).
Multiple factors may contribute to the development
and outcomes of DN. For instance, interactions between genetic and
environmental factors are likely to determine the susceptibility of
DN. Hyperglycemia is the major driving force, and the progressive
renal hemodynamic changes may be the leading cause for DN
development. Essentially, inflammatory pathways are critical to its
progression (5,6). During the last decade, the findings
that macrophages infiltrate the kidney and produce a
pro-inflammatory microenvironment have drawn more attentions in DN
research. It is common knowledge that macrophages are central
mediators of inflammatory responses (7,8).
In most types of human kidney diseases, macrophage accumulation in
both glomeruli and interstitial tissues correlates closely with the
degree of renal structural injury and renal dysfunction, such as DN
(9–11). Additionally, migrated macrophages
release multiple cytokines under pathological stimuli, such as
TNF-α, interleukin-1β (IL-1β), and IL-6, provoking inflammatory
responses through autocrine and paracrine manners, resulting in
inflammatory cascades and accelerated renal injury (12,13).
Current treatments, involving glycemic and blood
pressure control, can delay the development of DN, but do not stop
the progression to end-stage renal failure (14). Novel and effective therapeutic
approaches are in serious demand to prevent against nephropathy in
patients with early-stage diabetes mellitus (DM).
Cell therapy with mesenchymal stem cells (MSCs) has
become an attractive therapeutic strategy with which to regulate
the immune response invoked in pathologies, such as tissue injury,
transplantation, and autoimmunity (15). The primary characteristics of MSCs
are their immunomodulatory ability, capacity for self-renewal, and
ability to differentiate into mesodermal tissues (16). Additionally, autologous MSCs are
easily harvested and expanded in culture, and are free of
immunorejection, implying the promising application in clinical
treatment (17). Several studies
have shown that MSCs lead to amelioration of acute or chronic renal
injury, caused by ischemia reperfusion injury, 5/6 nephrectomy,
unilateral ureteral ligation or even DN (18–20). In these studies, the immune
modulation and anti-apoptotic effects of MSCs through paracrine
mechanisms have been associated with the therapeutic effect
(21–23). More importantly, it has been
demonstrated that functional interactions occur between MSCs and
macrophages (8). The
immunomodulatory capacity to inhibit macrophage infiltration is
likely to be a critical mechanism in MSC-mediated amelioration of
inflammation-related diseases (24).
Based on the previous findings, we transplanted bone
marrow-derived MSCs in rats with streptozotocin (STZ)-induced
diabetes to explore the therapeutic potential of MSCs on renal
dysfunction in the early stage of diabetes and disclose its
immunoregulatory role on macrophage activity and the inflammatory
environment.
Materials and methods
Rat model of STZ-induced diabetes
Male SD rats (n=30; age, 10 weeks old; weight,
280–300 g) were purchased from Chengdu Dashuo Laboratory Animal
Technology Co. (Chengdu, China) and housed in an animal facility
under controlled temperature (23±1°C), humidity (45–65%), and a
12-h light/dark cycle with free access to water and chow. Diabetes
was induced by a single intraperitoneal injection of 55 mg/kg STZ
(Sigma Chemical, St. Louis, MO, USA) dissolved in sodium citrate
buffer (pH 4.5) after overnight fasting. Three days later, the rats
with a fasting glucose level >16.7 mM for 3 consecutive days
were identified as diabetic rats (14). None of the rats received insulin
treatment during the entire course of the experiment. Afterwards,
the development of renal injury was evaluated via biochemical and
histopathological assessment. All experimental protocols and
studies were approved by the Animal Ethics Committee of the Sichuan
University, which are consistent with the National Institutes of
Health Guide for the Care and Use of Laboratory Animals.
Isolation, culture and characterization
of MSCs and macrophages
Bone marrow-derived MSCs were isolated from femurs
and tibias of 25 3-week-old male SD rats (100–120 g; purchased from
Chengdu Dashuo Laboratory Animal Technology Co.); the rats were
sacrificed prior to the isolation of the MSCs. Cells were then
cultured in low-glucose Dulbecco's Modified Eagle's Medium (DMEM)
containing 10% fetal bovine serum (FBS) (Gibco), 100 U/ml
penicillin, and 100 mg/ml streptomycin (25,26). The surface markers of MSCs were
identified by flow cytometric analysis using fluorophore conjugated
antibodies: anti-rat CD29-FITC, CD44-FITC, CD34-PE and CD45-PE (BD
Biosciences, Franklin Lakes, NJ, USA). For multiple
differentiation, MSCs were induced to differentiate into adipocytes
and osteocytes for 14 and 21 days in adipogenic and osteogenic
media (Cyagen Biosciences Inc., Santa Clara, CA, USA),
respectively. Oil Red stain for lipid droplets and Alizarin Red for
calcium deposition were then used. Furthermore,
5-ethynyl-2′-deoxy-uridine (EdU) staining was performed to confirm
the proliferation capacity of MSCs before transplantation. The
method of collecting rat peritoneal macrophages via intraperitoneal
injection of phosphate-buffered saline (PBS) was carried out
according to a previous study (27). Briefly, 10 healthy 12-week-old
rats (400–450 g; purchased from Chengdu Dashuo Laboratory Animal
Technology Co.) were administered a lethal dose of sodium
pentobarbital (Nembutal, 100 mg/kg) to induce deep anesthesia;
subsequently, 20 ml of the cold harvest medium was injected into
the peritoneal wall via syringe needle (21-G). Under sterile
condition, a small incision was made on the abdomen and abdominal
lavage fluid was collected. The peritoneal exudate cells (PECs)
were then centrifuged at 400 × g for 10 min and washed twice with
sterile PBS. To determine the purification of isolated macrophages,
cells were stained with PE-conjugated anti-mouse CD68 antibody,
F4/80 and flow cytometry was performed via a Coulter®
Epics® XL™ cytometer (FC500; Beckman Coulter, Brea, CA,
USA).
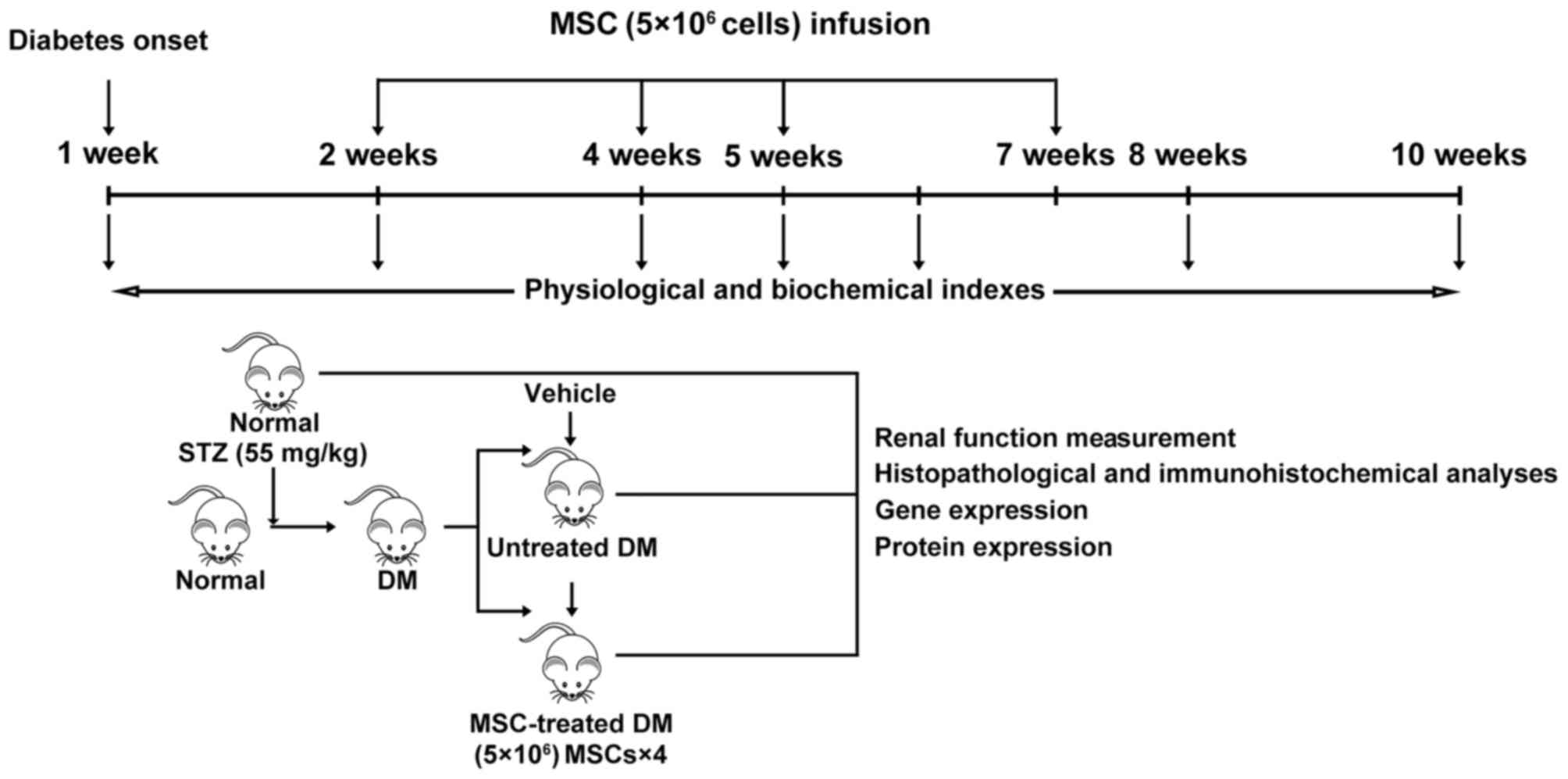
In vivo experiments
Experimental design and MSC
transplantation
A total of 30 rats were randomly assigned into 3
groups as follows: i) normal control group (NC, n=5), healthy rats
without treatment; ii) DN group (n=14), diabetic rats receiving
injection of 0.5 ml 0.9% saline instead of MSCs; iii) DN-MSC group
(n=11), diabetic rats with MSC transplantation. Subsequently, 2, 4,
5 and 7 weeks after successful establishment of the diabetes model,
MSCs were transplanted via the tail vein at a concentration of
5×106 cells in 0.5 ml 0.9% saline.
Biodistribution of transplanted
MSCs
A total of 8 10-week-old male SD rats (280–300 g)
were purchased from Chengdu Dashuo Laboratory Animal Technology
Co., and T1D models were obtained by a single intraperitoneal
administration of 55 mg/kg of STZ. To track the MSCs after
transplantation, MSCs were labeled with CM-DiI (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. Briefly, the third passage of MSCs were incubated
with CM-DiI in a working solution (1 µg/ml) for 5 min at 37°C,
followed by a further 15 min incubation at 4°C. After washing twice
with 0.9% saline, the CM-DiI-labeled MSCs were harvested in 0.9%
saline. Approximately 5×106 MSCs (1×107/ml,
0.5 ml)/rat were injected via the tail vein of 4 normal and 4
diabetic rats respecitvely, at 8 weeks after STZ injection. At 24
and 48 h after infusion of MSCs, rats were sacrificed and their
kidney, thymus, spleen, lung, heart, liver and lymph node tissues
were collected and processed to frozen sections. The labeled cells
were observed under confocal microscopy (Nikon A1S1; Nikon, Tokyo,
Japan).
Physical and biochemical analyses
The urine and serum samples were collected and renal
function was monitored before treatment and at 3, 4, 6 and 8 weeks
after initial MSC treatment. A total of 30 rats in three groups
[(i) normal control group (NC; n=5); (ii) DN group (n=14) ; (iii)
DN-MSC group (n=11)] were kept in metabolic cages to collect 24 h
urine and serum samples that were obtained from the tail vein with
a 26-G venous in-dwelling needle. Serum and urine samples were then
centrifuged at 800 × g for 10 min, and the supernatant was
subjected to biochemical measurements including serum creatinine
(Scr), blood urea nitrogen (BUN), glycosuria (GLU), microalbumin
(MAU) and albumin to creatinine ratio (ACR). In addition, fasting
blood glucose (FBG) of the rats was monitored each week. During the
experiment, some of the rats, particularly those in the DN group
were euthanized as certain humane endpoints were reached, such as
inability to partake of food and water due to inability to move, or
loss of appetite or severe diarrhea for >3 days, little or no
reaction to the stimulation, etc.) At the end of the experiment,
the surviving rats (14 rats in the DN group remained alive at the
end of the experiment and 9 of the 11 rats in the MSCs group
survived; rats in the normal control group all survived) were
sacrificed and kidneys were harvested, weighed, and processed for
histopathological analysis (Fig.
1).
Renal histology and
immunohistochemistry
Kidney sections were stained with hematoxylin and
eosin (H&E), periodic acid-Schiff (PAS), and masson trichrome
(MT; Richard-Allan Scientific, Kalamazoo, MI, USA) to assess the
renal pathology. For immunohistochemical staining, rabbit anti-rat
MCP-1 (ab25124; Abcam, Cambridge, MA, USA), IL-1β (ab82558; Abcam),
IL-6 (ab6672; Abcam), mouse anti-rat TNF-α (ab1793; Abcam), ICAM-1
(ab2213; Abcam) and CD68 (ab74704; Abcam), transforming growth
factor-β (TGF-β; BS1361; Bioworld Technology, Inc., St. Louis Park,
MN, USA), and fibronectin (FN; ab23751; Abcam) were used as the
primary antibodies and biotinylated goat anti-rabbit or mouse IgG
as the secondary antibody (Dako, Glostrup, Denmark). Standard
immunohistochemical procedures were then used (28). The expression levels of the above
factors were quantified by Image Pro-Plus v.6.0 software via
analyzing the integral optical density in 10 non-overlapping
cortical fields (magnification, ×400).
Quantitative reverse
transcription-PCR
Total RNA was extracted from renal cortical tissue
or macrophage cells with TRIzol reagent (Invitrogen) according to
the manufacturer's instructions, and then reverse transcribed into
cDNA using Transcriptor First Strand cDNA synthesis kit (Roche,
Indianapolis, IN, USA) and subjected to quantitative PCR using the
iQ SYBR-Green Supermix with the iCycleriQ real-time PCR detection
system (both from Bio-Rad Laboratories, Hercules, CA, USA). All the
primers were designed and generated by Shenggong Biotechnology
(Shanghai, China). Detailed primer information is presented in
Table I. Each sample was tested
in triplicates. The relative mRNA expression was determined as
2−ΔΔCT and normalized to the controls.
 | Table IPrimer sequences used for
quantitative PCR. |
Table I
Primer sequences used for
quantitative PCR.
| Target | Primer sequences
(5′→3′) |
|---|
| Rat FN | F:
GACACTATGCGGGTCACTTG |
| R:
CCCAGGCAGGAGATTTGTTA |
| Rat IFN-γ | F:
TCATCGAATCGCACCTGAT |
| R:
GGATCTGTGGGTTGTTCACC |
| Rat IL-1β | F:
GCCAACAAGTGGTATTCTCCA |
| R:
TGCCGTCTTTCATCACACAG |
| Rat IL-6 | F:
GTCAACTCCATCTGCCCTTC |
| R:
TGTGGGTGGTATCCTCTGTG |
| Rat TNF-α | F:
GCTCCCTCTCATCAGTTCCA |
| R:
GCTTGGTGGTTTGCTACGAC |
| Rat MCP-1 | F:
TGTTCACAGTTGCTGCCTGT |
| R:
AGTTCTCCAGCCGACTCATT |
| Rat TGF-β | F:
CCAACTACTGCTTCAGCTCCA |
| R:
GTGTCCAGGCTCCAAATGT |
| Rat IL-8 | F:
CTTTCAGAGACAGCAGAG |
| R:
CTAAGTTCTTTAGCACTCC |
| Rat β-actin | F:
CACCCGCGAGTACAACCTTC |
| R:
CCCATACCCACCATCACACC |
Serum cytokine expression
A 9-Plex rat cytokine/chemokine magnetic bead panel
assay (Millipore Corp., St. Charles, MO, USA) was used to determine
the cytokines in serum samples, including IL-1α, IL-1β, IL-2, IL-6,
epidermal growth factor (EGF), IL-10, TNF-α, interferon-γ (IFN-γ),
GRO and VEGF. The samples were 1:2 diluted in the serum matrix.
Beads were read on the Luminex 200 system (Luminex Corp., Austin,
TX, USA) following the protocol and data were analyzed using
Exponent 3.1 software.
Co-culture of MSCs and
lipopolysaccharide (LPS)-stimulated macrophages
The isolated rat peritoneal macrophages were seeded
into a 24-well plate with or without 14-mm-diameter plastic
coverslips at 1.5×105 cells/well. Two hours later, the
medium was removed and the attached macrophages were co-cultured
with 3×104 MSCs for 6 h with or without the stimulation
of 100 ng/ml LPS. Afterward, cells were collected and the mRNA
expression of inflammatory cytokines: MCP-1, IL-1β, TNF-α and IL-6
were detected by qPCR. For immunofluorescence staining, coverslips
with cells were fixed with 4% formaldehyde at room temperature for
15 min and then permeabilized with 1% Triton X-100 in Tris buffer
(Gibco) for another 15 min. Rabbit anti-rat MCP-1 (ab25124) and
IL-6 (ab6672) (both from Abcam) were used as the primary antibodies
and goat anti-rabbit IgG conjugated with FITC as the secondary
antibody. To visualize nuclei, the cells were counter-stained with
4′,6-diamidino-2-phenylindole (DAPI; Sigma Chemical) for 15 min at
room temperature. The slides were examined under inverted
fluorescence microscopy (Leica DM4000B; Leica, Wetzlar,
Germany).
Statistical analysis
All values are presented as the means ± SD.
Statistical analysis was performed using SPSS 17.0 statistical
software (SPSS Inc., Chicago, IL, USA). The statistical
significance was analyzed by one-way ANOVA method with Turkey
multiple comparison test. For survival experiments, a log-rank test
was conducted. A level of p<0.05 was considered statistically
significant.
Results
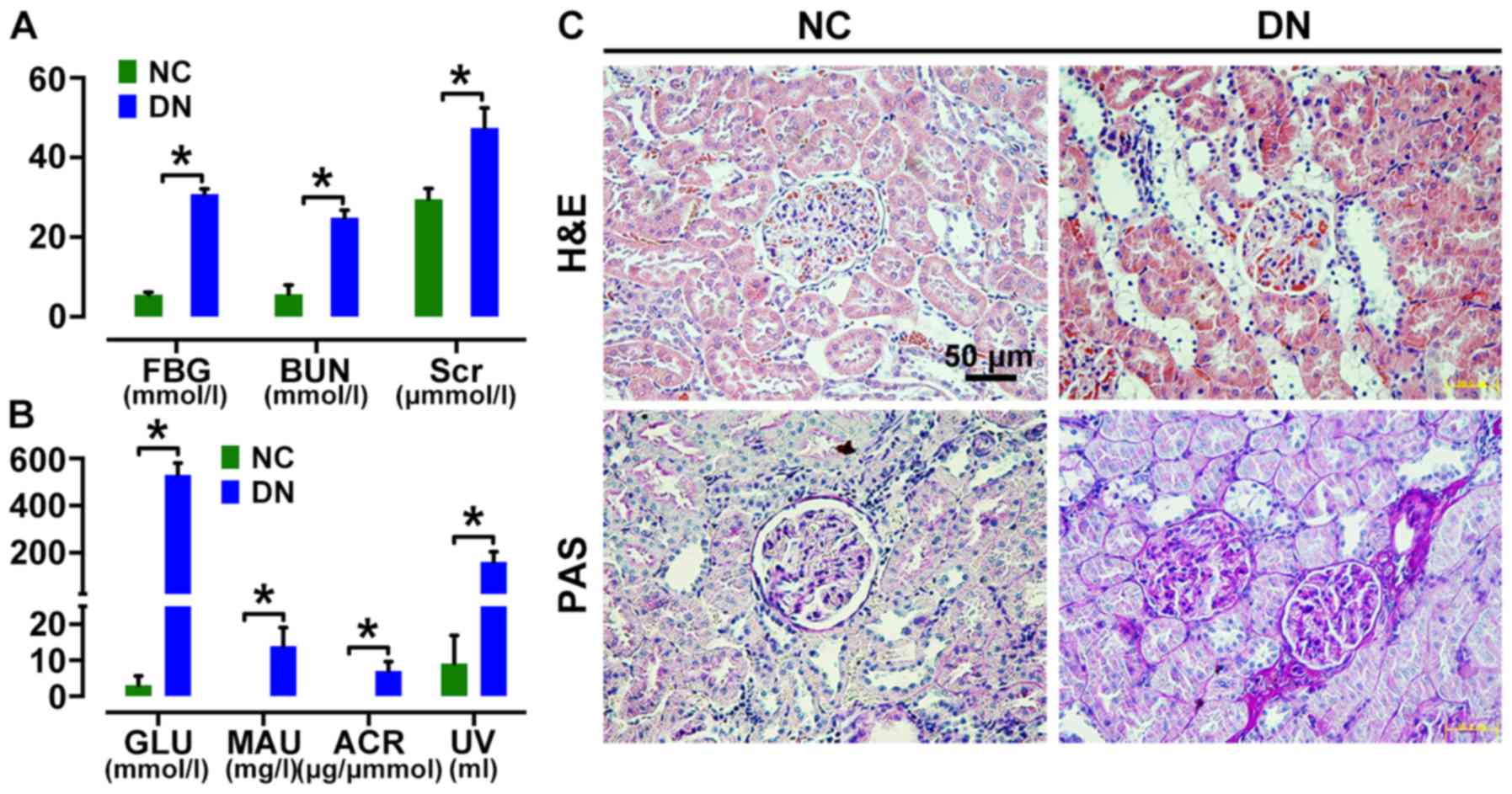
Assessment of renal injury in a DN rat
model
Rats with fasting glucose level >16.7 mM were
considered diabetic. Urine analyses revealed the onset of
microalbuminuria 2 weeks after STZ induction. Diabetic rats then
showed a significant increase in serum levels of FBG, Scr and BUN,
as well as MAU and ACR in urine (Fig.
2A and B) compared to those in age-matched control rats at 4
weeks after STZ injection. H&E and PAS staining showed renal
morphologic abnormalities in DN rats, including renal glomerular
hypertrophy, increased collagen fibers, expansion of the mesangium,
tubular dilatation and protein cylinders (Fig. 2C) at 8 weeks after STZ injection,
confirming diabetic renal injury.
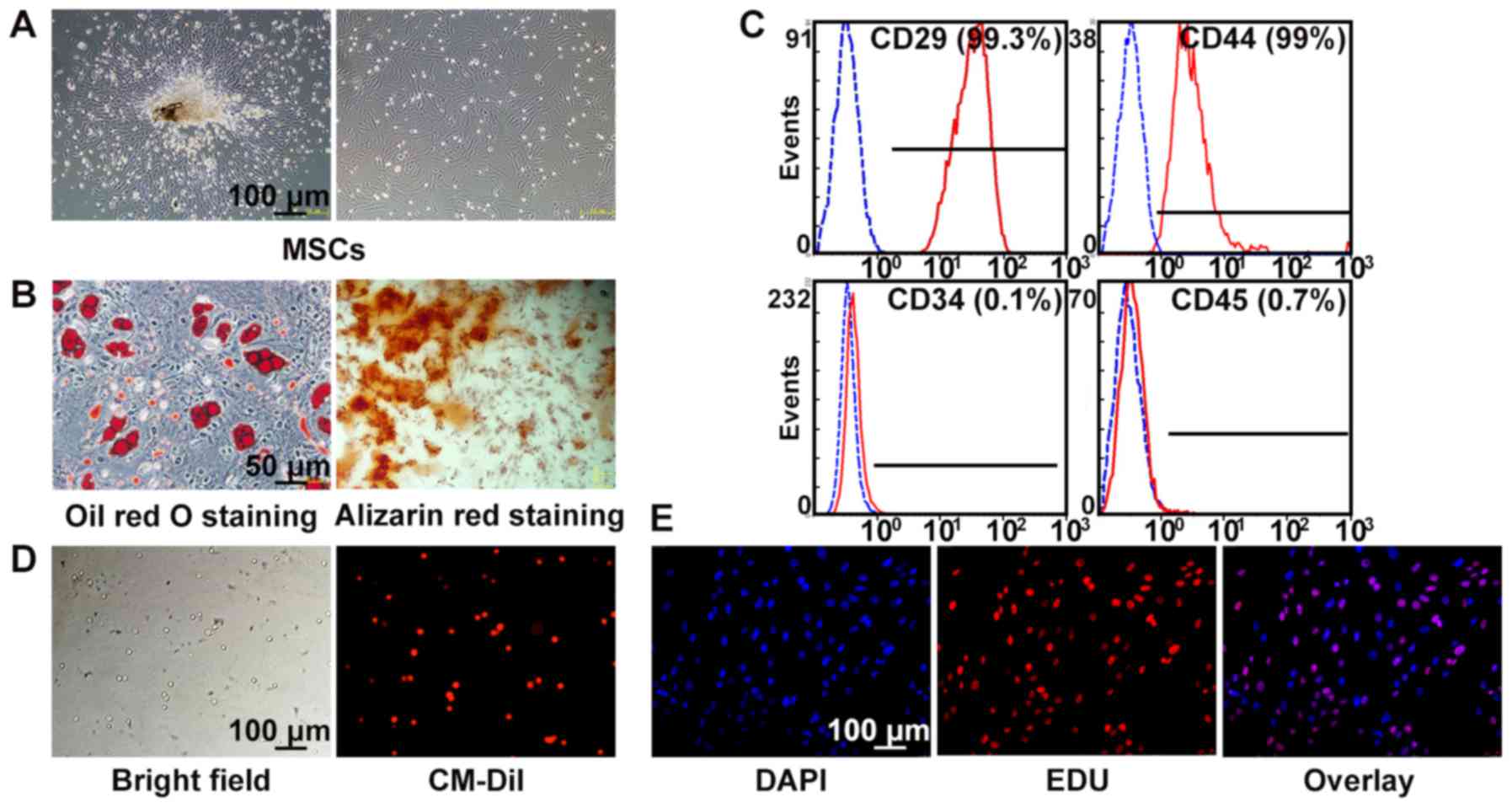
Characterization of rat MSCs
Bone marrow-derived MSCs from SD rats exhibited a
typical fibroblast-like morphology (Fig. 3A) and were capable of
differentiating into adipocytes after 14 days of culture and
osteocytes after 21 days of culture (Fig. 3B), using histochemical staining
for lineage-specific markers. Flow cytometry showed that MSCs at
passage 3 were positive for CD29 (99.3%) and CD44 (99%), and
negative for CD34 (0.1%) and CD45 (0.7%) (Fig. 3C). MSCs labeled by CM-DiI showed
red fluorescence, and the labeling efficiency was almost 100%
(Fig. 3D). In addition, MSCs
cultured with EdU ex vivo for 2 h showed more than 60%
positive staining, indicating a high proliferative activity
(Fig. 3E).
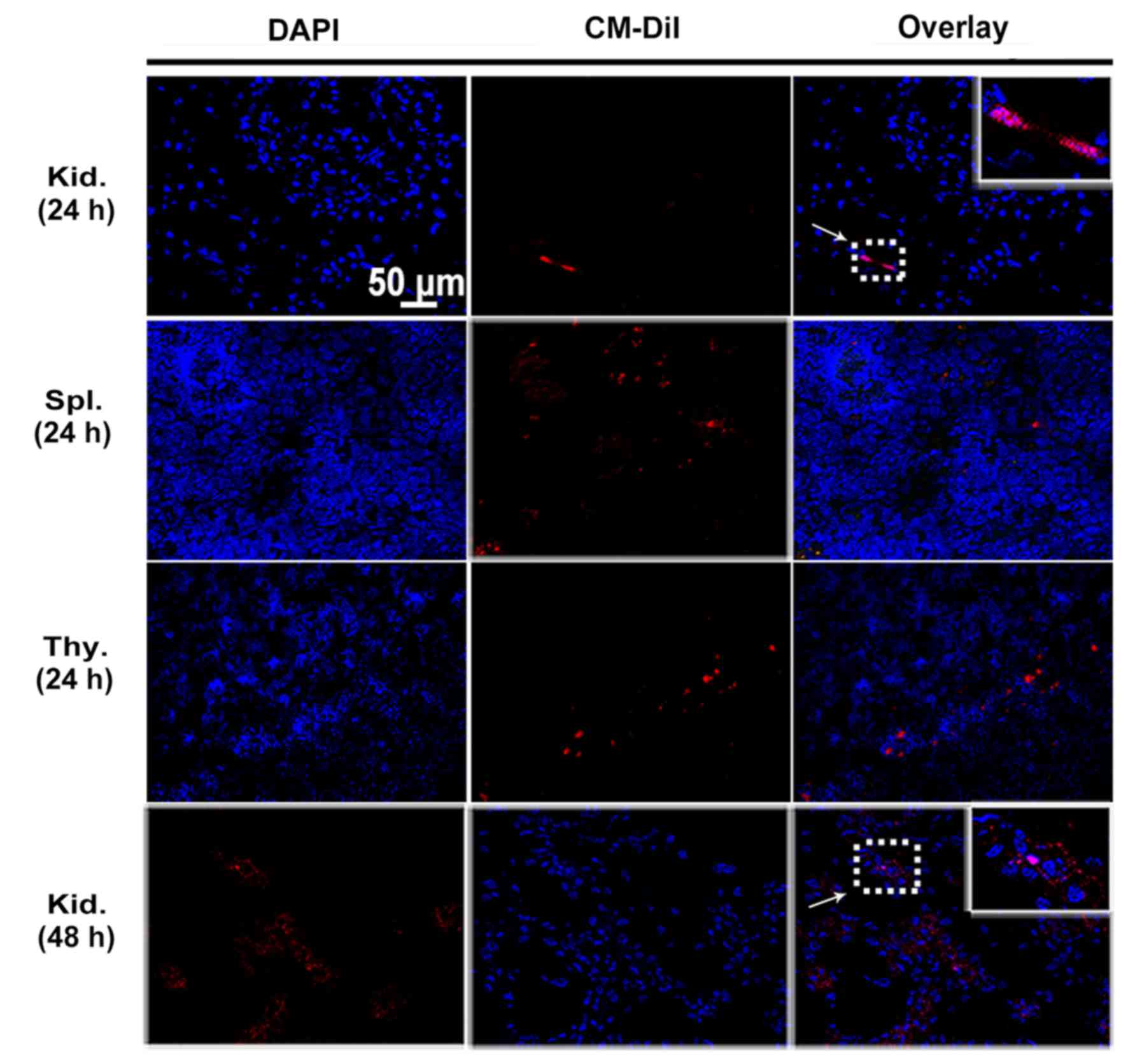
Intravenously injected MSCs localize in
the kidney and immune organs of DN rats
MSCs were labeled with CM-DiI to track their homing
after being transplanted to peripheral circulation of rats.
Engrafted MSCs with red fluorescence were detected in the glomeruli
as well as tubulointerstitium of diabetic kidneys by confocal
microscopy at 24 and 48 h after cell infusion (Fig. 4). Cell engraftment was also
observed in the immune organs such as spleen and thymus until 24 h
(Fig. 4). Interestingly, more
CM-DiI-labeled cells were found in the thymuses. However, the
number of MSCs positive for CM-DiI declined over time. When MSCs
were intravenously injected in normal control rats, no
CM-DiI-labeled cells were found in the kidney or immune organs
(data not shown).
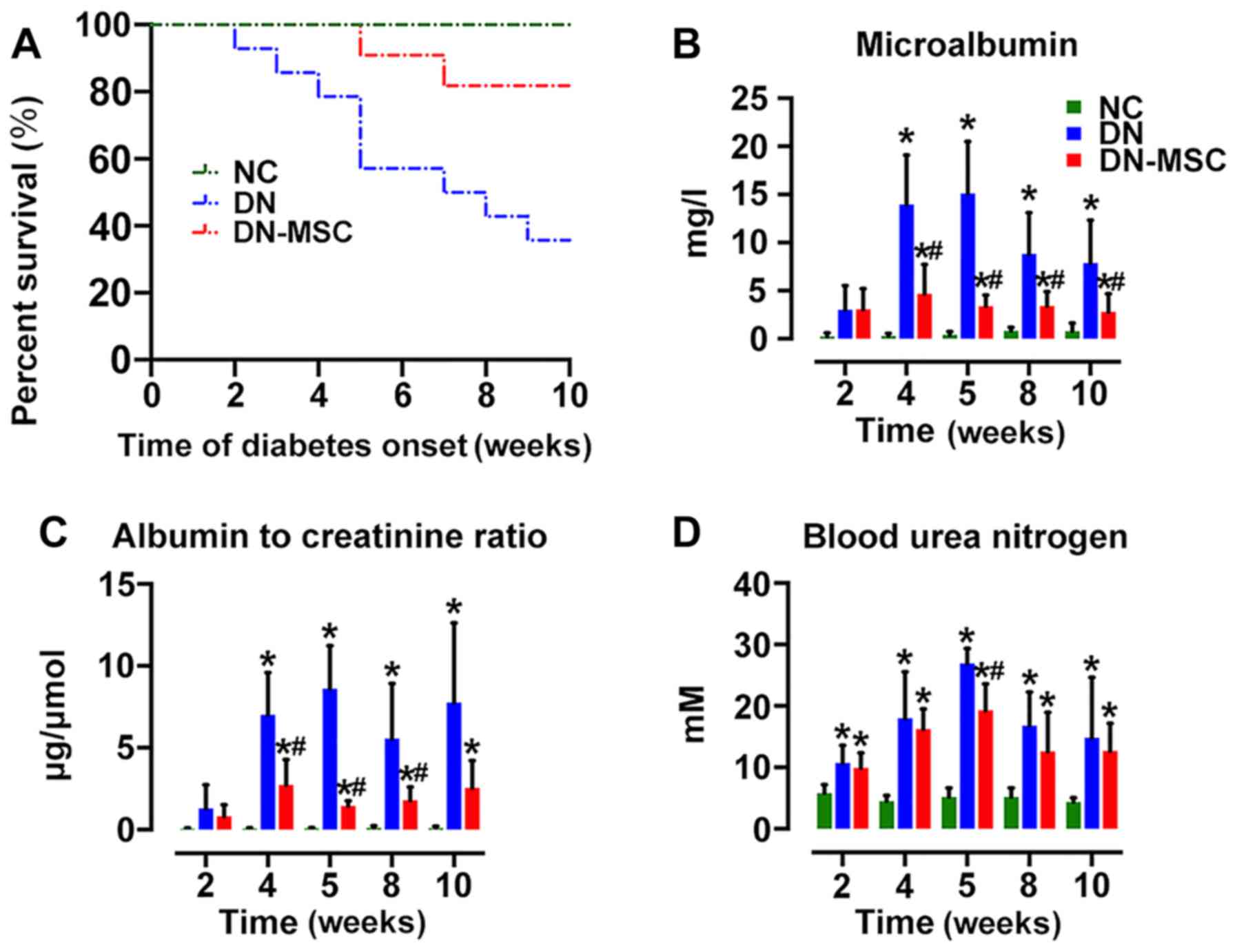
MSCs extend the survival of DN rats and
ameliorate renal function
At 10 weeks after diabetes onset, the survival of
the DN group was markedly decreased to 35.7% (5/14) in comparison
with 100% survival of the NC group. MSC transplantation
significantly extended the survival of DN rats, exhibiting 81.8%
survival (9/11) in the DN-MSC group (Fig. 5A). Although diabetic rats showed
marked elevations in MAU, ACR and BUN, treatment with MSCs resulted
in the suppression of STZ-induced detrimental impact on the above
parameters at 4, 5, 8 and 10 weeks after diabetes onset (Fig. 5B–D). However, only a slight
decrease in serum glucose level was observed in the DN-MSC rats in
comparison with that in the DN animals.
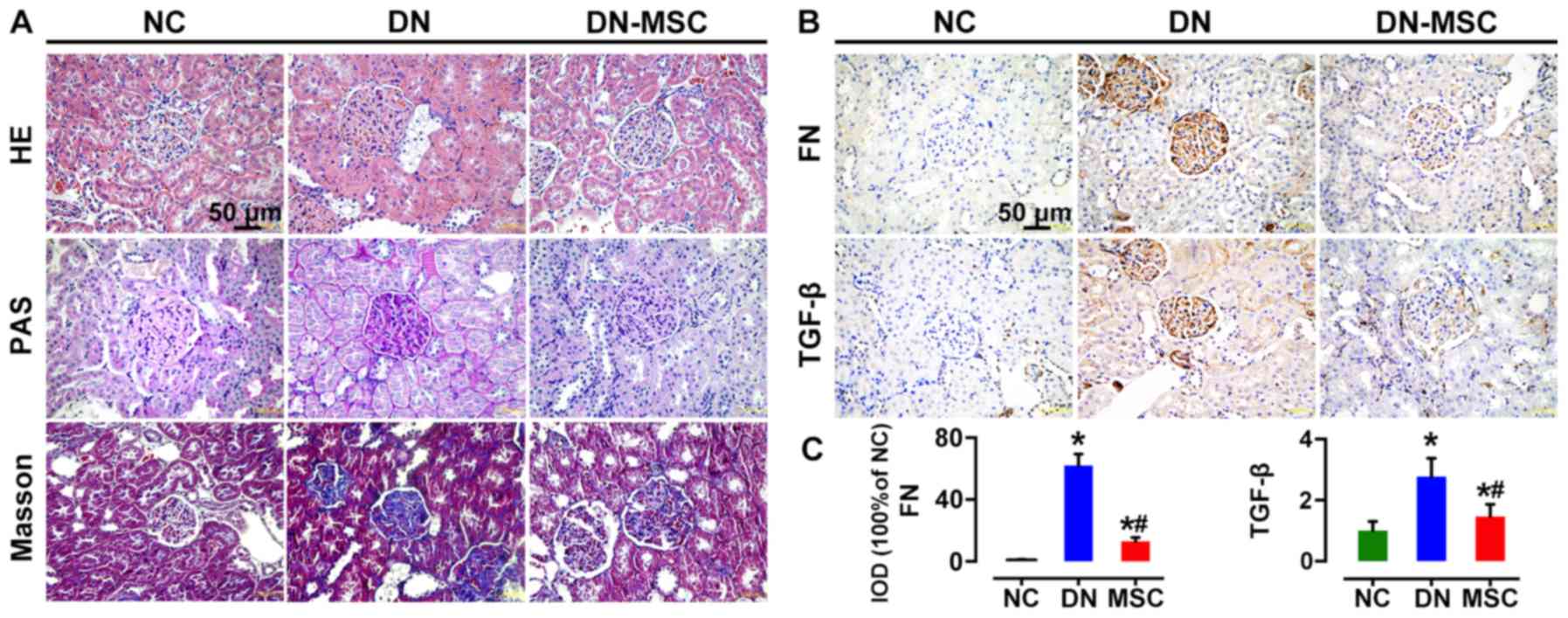
MSCs preserve renal structure and inhibit
fibrosis
Ten weeks after diabetes onset, H&E staining of
renal tissues from the DN rats showed typical chronic kidney
injury, including glomerular hypertrophy, epithelial flattening and
dilated tubules. Notable, histology of the DN-MSC rats was similar
to that of the control group with normal glomerular and tubular
morphology. In addition, kidneys of the DN group rats exhibited
profound extracellular matrix deposition and frequent fibrin cap
formation inside the glomeruli as revealed by PAS staining,
whereas, distinct decrease in mesangial matrix deposition and
glomerulosclerosis was observed in the MSC-treated group. Masson's
trichrome staining also demonstrated a significant reduction in
fibrosis in the DN-MSC rats (Fig.
6A). Meanwhile, the expression levels of TGF-β and FN in
kidney, which are typical biomarkers of epithelial-mesenchymal
transition (EMT) and fibrosis, were substantially downregulated in
the MSC-treated rats compared with DN rats as detected by
immunohistochemistry (Fig. 6B and
C).
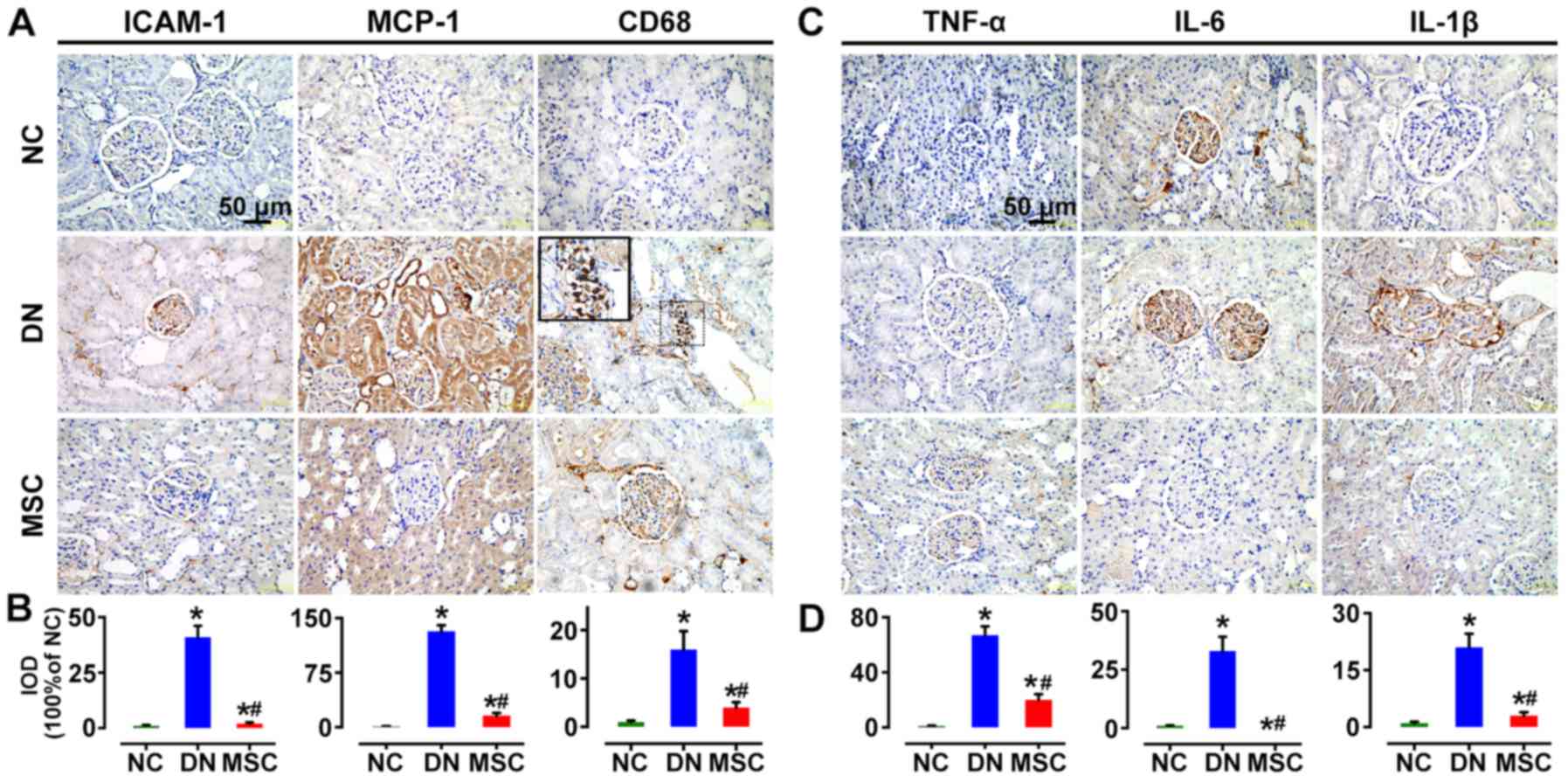
Regulatory effect of MSCs on the immune
microenvironment MSCs reduced renal CD68-positive macrophage
infiltration and inflammatory cytokine expression
MCP-1, and ICAM-1 have been known as essential
signals for macrophage activation and recruitment. In the present
study, the immunostaining for MCP-1 and ICAM-1 was significantly
increased in the DN group in glomeruli and interstitium compared
with the non-diabetic rats, but markedly reduced by MSC treatment.
CD68-positive cells infiltrated in the glomeruli of the diabetic
rats at 10 weeks after STZ injection. But MSC treatment markedly
suppressed the macrophage infiltration into the glomeruli (Fig. 7A and B). Consistently, the
markedly induced expression of pro-inflammatory cytokines TNF-α,
IL-6 and IL-1β in the kidneys of DN rats was abrogated by MSC
treatment in the DN-MSC group (Fig.
7C and D).
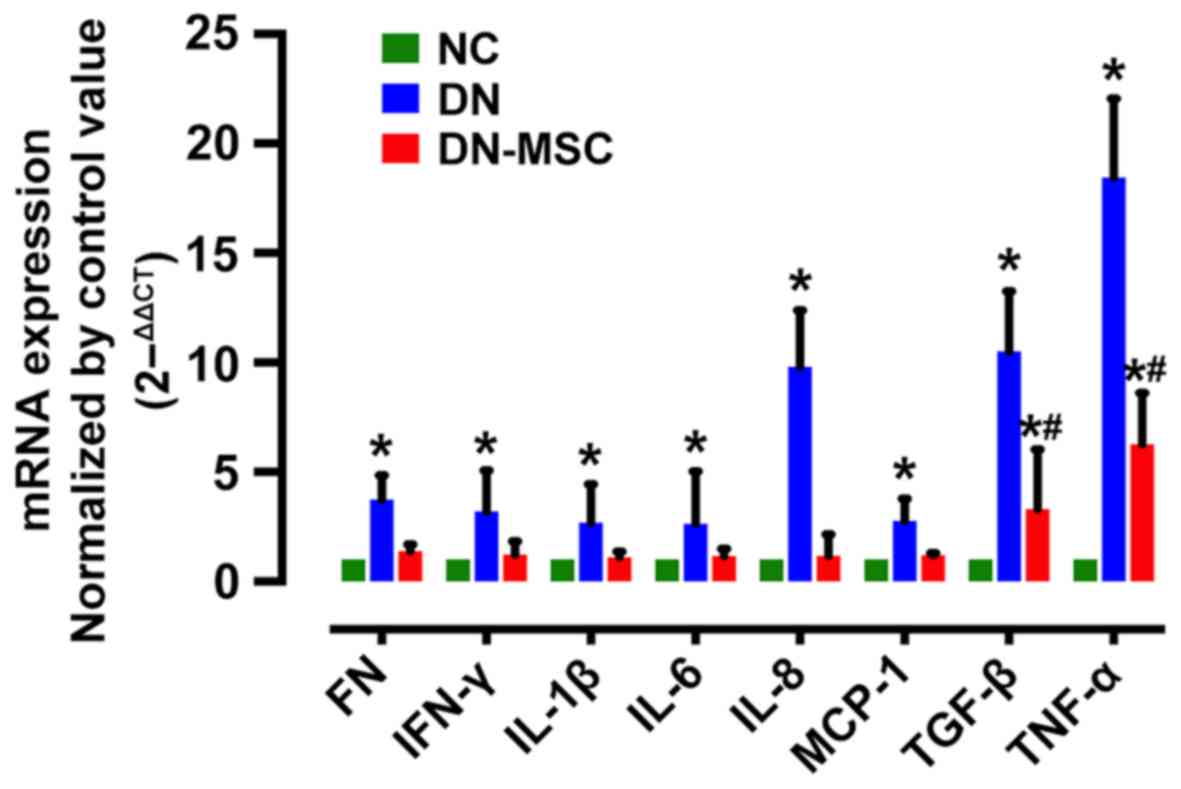
MSCs decrease renal inflammatory gene
expression
Diabetic rats exhibited increased gene expression of
pro-inflammatory cytokines TNF-α, IL-6, IL-8, IL-1β and IFN-γ,
chemokine MCP-1, and fibrosis markers TGF-β and FN compared with
the non-diabetic animals. However, treatment with MSCs resulted in
the notable suppression in gene expression of the above factors 10
weeks after diabetes onset (Fig.
8).
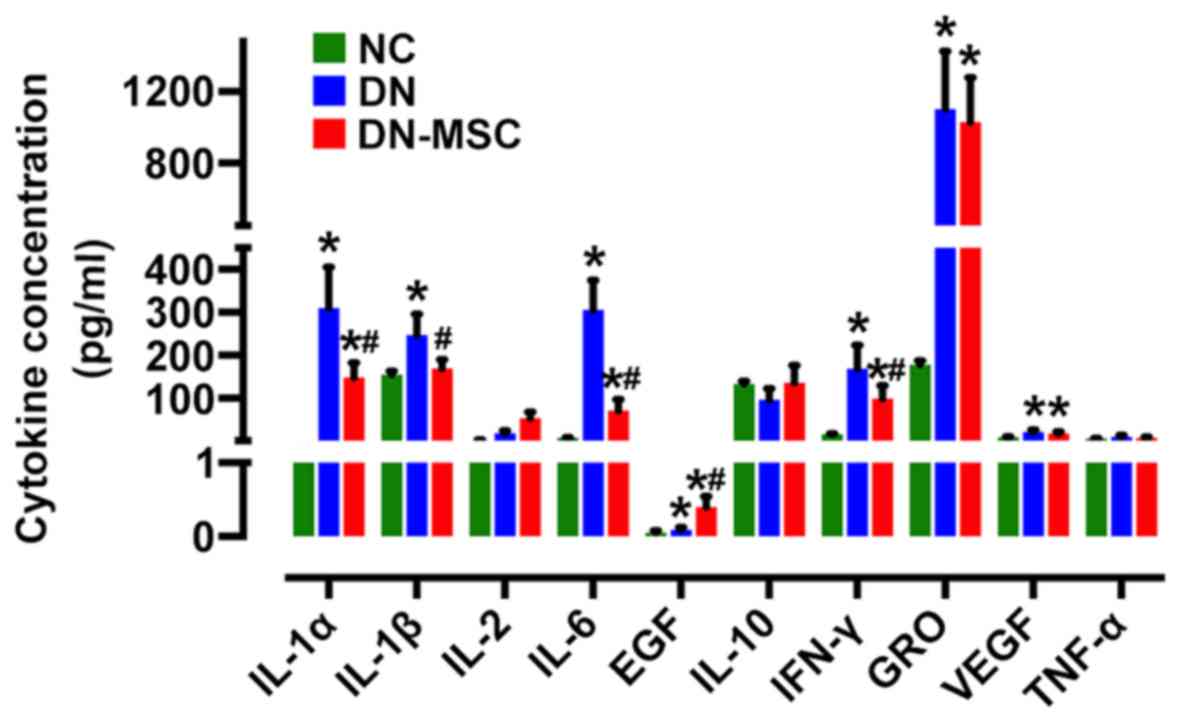
MSCs suppress serum inflammatory
cytokines and enhance anti-inflammatory cytokines
In regards to the immunosuppressive properties of
MSCs, we measured the serum levels of cytokines to further explore
the impact of MSCs on the immune environment of DN 10 weeks after
diabetes onset. Diabetic rats exhibited increased serum levels of
IL-1α, IL-1β, IL-6, IFN-γ and GRO (CXCL1) in comparison with the
non-diabetic control rats. Treatment with MSCs significantly
attenuated the expression of IL-1α, IL-1β, IL-6 and IFN-γ (Fig. 9). Notably, the level of EGF was
obviously higher in the MSC-treated rats than in both the control
and diabetic rats. However, no obvious change was observed in serum
levels of IL-10, VEGF and TNF-α after MSC treatment.
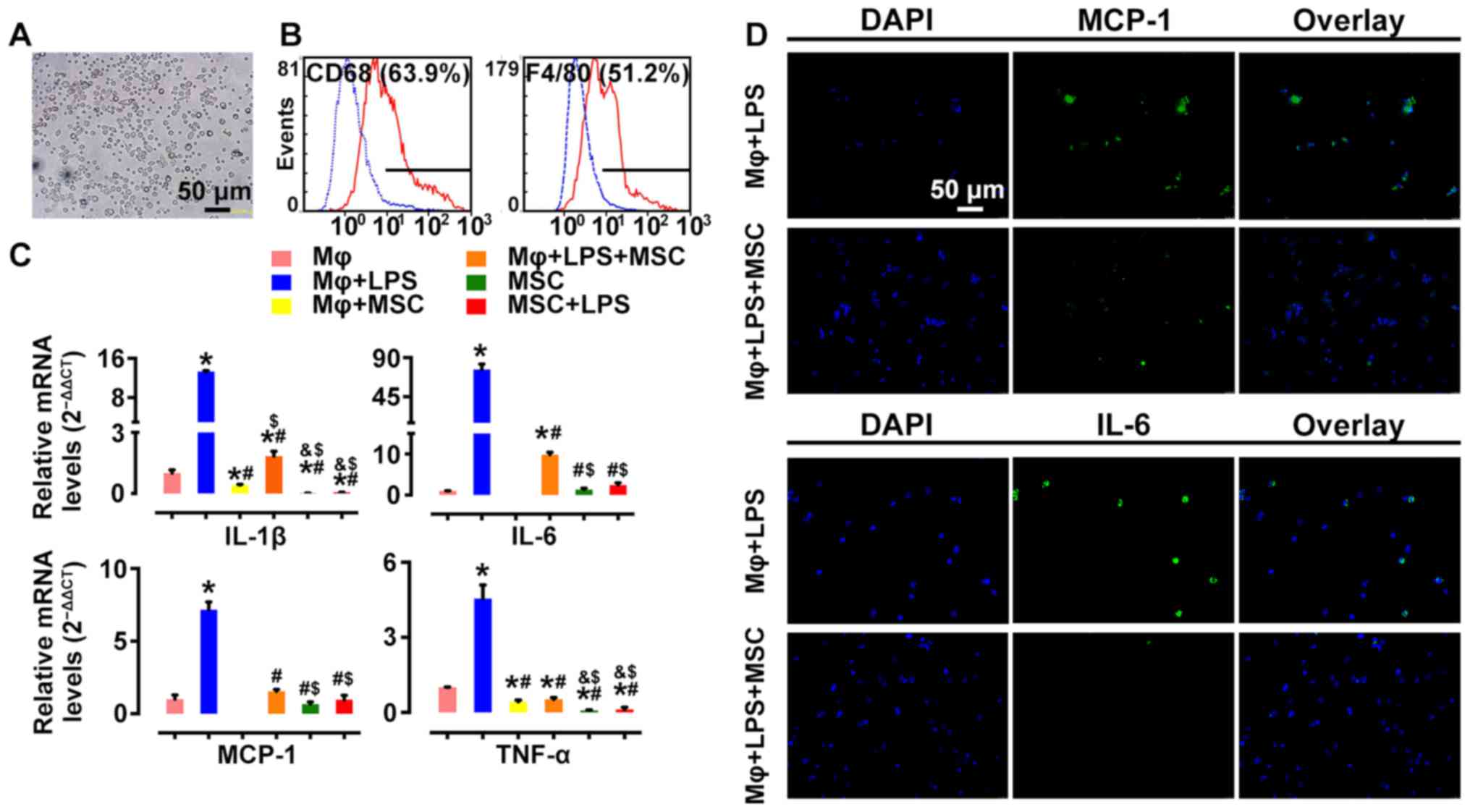
MSCs suppress pro-inflammatory cytokine
profile in activated macrophages ex vivo
To further assess the effect of MSCs on macrophage
activation, we examined the alteration of mRNA profiles of
pro-inflammatory cytokines in LPS-stimulated rat peritoneal
macrophages before and after MSC treatment. Macrophages were
identified as having positive expression of CD68 (63.9%) and F4/80
(51.2%) by flow cytometry (Fig. 10A
and B), markers of activated macrophages. Pro-inflammatory
cytokines, such as IL-6, IL-1β, TNF-α and MCP-1 were highly
expressed in LPS-stimulated macrophages. However, their mRNA
expression levels were significantly downregulated following
co-culture with MSCs (Fig. 10C).
In addition, immunofluorescence showed consistent results.
LPS-induced MCP-1 and IL-6 expression was decreased by MSC
intervention (Fig. 10D). These
results demonstrated that co-culturing with MSCs led to inhibition
of macrophage activation, which was relevant to the
immunomodulatory characteristics of MSCs.
Discussion
To date, diabetic nephropathy (DN) is still
incurable and is a big challenge for clinical practice. Previous
experimental studies of DN suggest that inflammation contributes to
its pathogenesis (9). Increasing
evidence has revealed that interactions of MSCs with macrophages
are likely to play a significant role in their
anti-inflammatory/immune modulatory effects (29), which may be a critical mechanism
in MSC-mediated amelioration of inflammation-related diseases
(24). In the present study, we
found that the early intervention of diabetes by MSCs preserved
renal function and ameliorated histopathological alterations in a
rat model of diabetes mellitus. The possible underlying mechanisms
are suggested to be the inhibition of macrophage activation and the
trigger for a pro-regenerative microenvironment, including the
production of protective trophic factors and the attenuation of the
pro-inflammatory response in the kidney, resulting in improved
renal dysfunction.
STZ-induced rat type 1 diabetes was used in this
study to mimic clinical DN, as it is a well documented type 1
diabetic model to quickly and stably develop hyperglycemia and
renal injury (30). We observed
that 8 weeks after STZ injection, diabetic rats were severely
hyperglycemic and developed typical alterations of DN. Serum MAU,
Scr and urine ACR were increased, as well as significant
pathological lesions were observed, including glomerular, tubular
epithelial hypertrophy, profound extracellular matrix deposition
and fibrosis, suggesting successful establishment of the DN rat
model (31) (Fig. 2). In terms of the timing of MSC
intervention, previous studies mostly conducted MSC infusion after
the animals already established DN (32,33). In this study, given that it is
difficult to reverse the advanced stage of nephropathy, we chose 2
weeks after diabetes onset as the timing for MSC therapy, due to
the fact that rats in the very early stage of DM are more sensitive
to MSC intervention. As expected, our results showed a better
preventive effect on renal injury than studies conducting MSC
transplantation in severe DN (32,33). Most importantly, in this study,
MSC-treated diabetic rats did not develop serious renal
histopathologic alterations, suggesting that MSC administration
hindered further renal impairment. However, previous studies only
observed mild microalbuminuria reversion as well as amelioration of
glomeruli structural damage. On the other hand, the diabetic rats
received 4 consecutive MSC infusions in the present study, which
was similar with the strategy reported by Semedo et al. They
conducted a 6-week MSC treatment of 3 consecutive infusions every
other week, showing obvious amelioration of kidney function, which
was better than single dose treatment (22). Parameters used for evaluation of
renal function are variable in the literature. Although in many
studies, functional parameters were only measured at the end of the
experiment (14,32), we monitored the dynamic changes of
serum and urine biochemistry with the purpose of assessing diabetic
status and progression of renal injury. We found that the
administration of multiple doses of MSCs was capable of preventing
albuminuria development regardless of the persistence of
hyperglycemia at all time points. MSCs effectively restored renal
function from 4 weeks after diabetes onset (2 weeks after MSC
transplantation), strikingly improving MAU and ACR (Fig. 5). Notably, the above renal indices
were increased in the DN group rats from 2 weeks after diabetes
onset and peaked at 5 weeks. Afterwards, BUN and MAU in the DN
group displayed a gradual decline. This may be due to the
auto-repair potential of kidneys after high glucose damage.
Previous studies have also shown that the
development of diabetes leads to increased innate immune responses,
which are predominantly characterized by the accumulation of kidney
macrophages (34). Activated
macrophages secrete a series of pro-inflammatory, pro-fibrogenic
and anti-angiogenesis cytokines, which are responsible for a
pro-inflammatory microenvironment (35) and their infiltrations are strongly
associated with proteinuria and declined renal function (10,13). In addition, this finding was
supported by in vitro studies indicating that activated
macrophages can promote renal cell death and stimulation of bone
marrow-derived macrophages by LPS resulting in the secretion of
substances known to induce apoptosis in cultured mesangial and
tubular epithelial cells (36).
In addition, kidney cells produce ICAM-1 and MCP-1 in response to a
variety of pro-inflammatory stimuli (9). In patients with DN, soluble forms of
ICAM are elevated during the progression of the disease and
experimental studies suggest that ICAM-1 facilitates kidney
macrophage recruitment during type 1 diabetes (37). Furthermore, MCP-1 is a specific
chemokine to recruit and activate monocytes from the circulation to
the inflammatory site (38),
especially contributing to infiltration of macrophages into both
mesangial and tubulointerstitial lesions in DN (39). In the present study, treatment
with MSCs markedly attenuated MCP-1 and ICAM-1 expression, and
diminished the accumulation of macrophages in glomeruli (Figs. 7 and 8), which is consistent with previous
studies (40,41). Additionally, expression levels of
classical markers of activated macrophages, such as IL-1β, TNF-α
and IL-6 as well as fibrosis factors TGF-β and FN in the MSC group
were significantly declined at the mRNA and/or protein levels
(Figs. 7 and 8), which implied that the inflammatory
environment of DN was substantially improved by MSCs. Furthermore,
our ex vivo study also provided more direct evidence that
MSCs were able to diminish the LPS driven activation of macrophages
(Fig. 10). Summarily, these
results suggested that the anti-inflammatory action of MSCs in the
DN rat model was related to the modulation of macrophages.
Recently, it has been shown that MSC administration reduces
macrophage infiltration into the target tissues (29) and switches macrophages from an M1
to M2 phenotype, benefiting tissue homeostasis in different animal
models (42). The in-depth study
of the mechanism involved in the MSC-mediated modulation of
macrophages in DN is warranted for better understanding of its
therapeutic effects.
It is undoubted that MSCs have the capability to
modulate the local inflammatory environment, and perturb local
interactions with inflammatory cells in diabetic kidney. We aimed
to ascertain whether MSCs alter systemic inflammation in this
scenario. The evaluation of specific cytokines in the serum
demonstrated that MSC administration led to a reduction in serum
pro-inflammatory cytokines, including IL-6, IL-1α, IL-1β and IFN-γ.
Notably, we also observed a systemic increase in EGF and IL-10
(Fig. 8). This immunosuppressive
property of MSCs has been described in a great number of literature
studies, including inhibition of the proliferation of
CD8+ and CD4+ T lymphocytes and natural
killer (NK) cells, inhibition of maturation of dendritic cells
(DCs), and stimulation of the proliferation of regulatory T cells
(16). This immune modulation is
thought to be due to the paracrine action of soluble factors. The
secretion of prostaglandin E2 (PGE2),
inducible nitric oxide synthase (iNOS), indoleamine-2,3-dioxygenase
(IDO), TGF-β, leukemia-inhibitory factor (LIF), and IL-10
contributes to this effect (15).
Moreover, trophic factors, such as VEGF, HGF, IGF-1 and EGF have
been shown to reduce tubular injury or mediate epithelial cell
proliferation (43).
To fully understand the mechanism underlying the
effect of MSCs, how MSCs are localized in deteriorated kidneys must
be determined (Fig. 4). Although
the exact mechanism involved in stem cell homing still remains
elusive, inflammation, hypoxia, and hyperglycemia, which are
ongoing in the diabetic kidney, may be the driving factors of MSC
migration (44–46). In addition, MSCs may be able to
both sense and respond to their immediate environment, which makes
them ideal cells to tune the response to injury and inflammation
(15). Notably, cell engraftments
were found in the immune organs such as thymus and spleen (Fig. 4). Previous studies have shown that
activated immunocytes secrete IFN-γ, promoting neighboring MSCs to
secrete PGE2, which could regulate the functions of a
variety of immune cells (47).
Meanwhile, it has been shown that inhibition of allogeneic T cell
responses by MSCs is mediated by indoleamine-2,3-dioxygenase (IDO)
(48). IDO is responsible for
catabolizing tryptophan through the kyneurinine pathway, thus
limiting the availability of L-tryptophan to T cells resulting in
nutrition deficiency-induced cell death (49). Taken together, this implies that
the obvious tendency of MSCs to engraft in immune organs is also a
determining factor for the MSC-mediated immunosuppressive effects
and alleviation of inflammation in the damaged kidney via systemic
immunoregulation.
In conclusion, we presented evidence that the early
intervention by MSCs effectively suppressed renal macrophage
infiltration and inflammatory cytokine expression in diabetic rats
via immunoregulation and paracrine actions, which restored the
homeostasis of the immune microenvironment and led to the
amelioration of kidney function and glomerulosclerosis. In
addition, MSCs infused via the tail vein were able to home to
deteriorated kidney and immune organs. Ex vivo, MSCs
inhibited activation of rat peritoneal macrophages after LPS
stimulation. We suggest that the prevention of DN with MSCs may
provide substantial promise for the development of novel MSC-based
interventions.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81370824) and the National Key
Clinical Project.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Dronavalli S, Duka I and Bakris GL: The
pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol
Metab. 4:444–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maisonneuve P, Agodoa L, Gellert R,
Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney
APS, Briggs D, et al: Distribution of primary renal diseases
leading to end-stage renal failure in the United States, Europe,
and Australia/New Zealand: Results from an international
comparative study. Am J Kidney Dis. 35:157–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chavers BM, Bilous RW, Ellis EN, Steffes
MW and Mauer SM: Glomerular lesions and urinary albumin excretion
in type I diabetes without overt proteinuria. N Engl J Med.
320:966–970. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCrary EB: The road to renal failure: An
overview of diabetic nephropathy. Adv Nurse Pract. 16:61–63.
2008.
|
|
5
|
Schena FP and Gesualdo L: Pathogenetic
mechanisms of diabetic nephropathy. J Am Soc Nephrol. 16(Suppl 1):
S30–S33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Navarro-González JF, Mora-Fernández C,
Muros de Fuentes M and García-Pérez J: Inflammatory molecules and
pathways in the pathogenesis of diabetic nephropathy. Nat Rev
Nephrol. 7:327–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Navarro-González JF and Mora-Fernández C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong
MH, Yoon SH, Kim YS and Ahn Y: Mesenchymal stem cells reciprocally
regulate the M1/M2 balance in mouse bone marrow-derived
macrophages. Exp Mol Med. 46:e702014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen D, Ping F, Mu W, Hill P, Atkins RC
and Chadban SJ: Macrophage accumulation in human progressive
diabetic nephropathy. Nephrology (Carlton). 11:226–231. 2006.
View Article : Google Scholar
|
|
10
|
Chow F, Ozols E, Nikolic-Paterson DJ,
Atkins RC and Tesch GH: Macrophages in mouse type 2 diabetic
nephropathy: Correlation with diabetic state and progressive renal
injury. Kidney Int. 65:116–128. 2004. View Article : Google Scholar
|
|
11
|
Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee
VWS, Zheng G, Tan TK, Ince J, Alexander SI, et al:
IL-10/TGF-β-modified macrophages induce regulatory T cells and
protect against adriamycin nephrosis. J Am Soc Nephrol. 21:933–942.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prockop DJ and Oh JY: Mesenchymal
stem/stromal cells (MSCs): Role as guardians of inflammation. Mol
Ther. 20:14–20. 2012. View Article : Google Scholar :
|
|
13
|
Chow FY, Nikolic-Paterson DJ, Atkins RC
and Tesch GH: Macrophages in streptozotocin-induced diabetic
nephropathy: potential role in renal fibrosis. Nephrol Dial
Transplant. 19:2987–2996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Li Y, Zhao J, Zhang J and Huang Y:
Mesenchymal stem cells ameliorate podocyte injury and proteinuria
in a type 1 diabetic nephropathy rat model. Biol Blood Marrow
Transplant. 19:538–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singer NG and Caplan AI: Mesenchymal stem
cells: Mechanisms of inflammation. Annu Rev Pathol. 6:457–478.
2011. View Article : Google Scholar
|
|
16
|
Volarevic V, Arsenijevic N, Lukic ML and
Stojkovic M: Concise review: Mesenchymal stem cell treatment of the
complications of diabetes mellitus. Stem Cells. 29:5–10. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee RH, Seo MJ, Reger RL, Spees JL, Pulin
AA, Olson SD and Prockop DJ: Multipotent stromal cells from human
marrow home to and promote repair of pancreatic islets and renal
glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA.
103:17438–17443. 2006. View Article : Google Scholar :
|
|
18
|
Tögel F, Hu Z, Weiss K, Isaac J, Lange C
and Westenfelder C: Administered mesenchymal stem cells protect
against ischemic acute renal failure through
differentiation-independent mechanisms. Am J Physiol Renal Physiol.
289:F31–F42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tögel F, Weiss K, Yang Y, Hu Z, Zhang P
and Westenfelder C: Vasculotropic, paracrine actions of infused
mesenchymal stem cells are important to the recovery from acute
kidney injury. Am J Physiol Renal Physiol. 292:F1626–F1635. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ezquer FE, Ezquer ME, Parrau DB, Carpio D,
Yañez AJ and Conget PA: Systemic administration of multipotent
mesenchymal stromal cells reverts hyperglycemia and prevents
nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant.
14:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Semedo P, Palasio CG, Oliveira CD, Feitoza
CQ, Gonçalves GM, Cenedeze MA, Wang PMH, Teixeira VPA, Reis MA,
Pacheco-Silva A, et al: Early modulation of inflammation by
mesenchymal stem cell after acute kidney injury. Int
Immunopharmacol. 9:677–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semedo P, Correa-Costa M, Antonio Cenedeze
M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu
MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO and Olsen N:
Mesenchymal stem cells attenuate renal fibrosis through immune
modulation and remodeling properties in a rat remnant kidney model.
Stem Cells. 27:3063–3073. 2009.PubMed/NCBI
|
|
23
|
Park JH, Hwang I, Hwang SH, Han H and Ha
H: Human umbilical cord blood-derived mesenchymal stem cells
prevent diabetic renal injury through paracrine action. Diabetes
Res Clin Pract. 98:465–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakajima H, Uchida K, Guerrero AR,
Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT,
Johnson WEB, et al: Transplantation of mesenchymal stem cells
promotes an alternative pathway of macrophage activation and
functional recovery after spinal cord injury. J Neurotrauma.
29:1614–1625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang TS, Cai L, Ji WY, Hui YN, Wang YS,
Hu D and Zhu J: Reconstruction of the corneal epithelium with
induced marrow mesenchymal stem cells in rats. Mol Vis.
16:1304–1316. 2010.PubMed/NCBI
|
|
26
|
Mafi P: Adult mesenchymal stem cells and
cell surface characterization - a systematic review of the
literature. Open Orthop J. 5(Suppl 2): 253–260. 2011. View Article : Google Scholar
|
|
27
|
Zhang X, Goncalves R and Mosser DM: The
isolation and characterization of murine macrophages. Curr Protoc
Immunol. Chapter: Unit-14.1. 2008. View Article : Google Scholar
|
|
28
|
Wang Y, Wang Y, Feng X, Bao S, Yi S,
Kairaitis L, Tay YC, Rangan GK and Harris DC: Depletion of CD4(+) T
cells aggravates glomerular and interstitial injury in murine
adriamycin nephropathy. Kidney Int. 59:975–984. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim J and Hematti P: Mesenchymal stem
cell-educated macrophages: A novel type of alternatively activated
macrophages. Exp Hematol. 37:1445–1453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tesch GH and Allen TJ: Rodent models of
streptozotocin-induced diabetic nephropathy. Nephrology (Carlton).
12:261–266. 2007. View Article : Google Scholar
|
|
31
|
Liu CX, Hu Q, Wang Y, Zhang W, Ma ZY, Feng
JB, Wang R, Wang XP, Dong B, Gao F, et al: Angiotensin-converting
enzyme (ACE) 2 overexpression ameliorates glomerular injury in a
rat model of diabetic nephropathy: A comparison with ACE
inhibition. Mol Med. 17:59–69. 2011.
|
|
32
|
Lv SS, Liu G, Wang JP, Wang WW, Cheng J,
Sun AL, Liu HY, Nie HB, Su MR and Guan GJ: Mesenchymal stem cells
transplantation ameliorates glomerular injury in
streptozotocin-induced diabetic nephropathy in rats via inhibiting
macrophage infiltration. Int Immunopharmacol. 17:275–282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv S, Cheng J, Sun A, Li J, Wang W, Guan
G, Liu G and Su M: Mesenchymal stem cells transplantation
ameliorates glomerular injury in streptozotocin-induced diabetic
nephropathy in rats via inhibiting oxidative stress. Diabetes Res
Clin Pract. 104:143–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tesch GH: MCP-1/CCL2: A new diagnostic
marker and therapeutic target for progressive renal injury in
diabetic nephropathy. Am J Physiol Renal Physiol. 294:F697–F701.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brady HR: Leukocyte adhesion molecules and
kidney diseases. Kidney Int. 45:1285–1300. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duffield JS, Erwig L-P, Wei X, Liew FY,
Rees AJ and Savill JS: Activated macrophages direct apoptosis and
suppress mitosis of mesangial cells. J Immunol. 164:2110–2119.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chow FY, Nikolic-Paterson DJ, Ozols E,
Atkins RC and Tesch GH: Intercellular adhesion molecule-1
deficiency is protective against nephropathy in type 2 diabetic
db/db mice. J Am Soc Nephrol. 16:1711–1722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Banba N, Nakamura T, Matsumura M, Kuroda
H, Hattori Y and Kasai K: Possible relationship of monocyte
chemoattractant protein-1 with diabetic nephropathy. Kidney Int.
58:684–690. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santos JC, de Brito CA, Futata EA, Azor
MH, Orii NM, Maruta CW, Rivitti EA, Duarte AJS and Sato MN:
Upregulation of chemokine C-C ligand 2 (CCL2) and C-X-C chemokine 8
(CXCL8) expression by monocytes in chronic idiopathic urticaria.
Clin Exp Immunol. 167:129–136. 2012. View Article : Google Scholar :
|
|
40
|
Li W, Zhang Q, Wang M, Wu H, Mao F, Zhang
B, Ji R, Gao S, Sun Z, Zhu W, et al: Macrophages are involved in
the protective role of human umbilical cord-derived stromal cells
in renal ischemia-reperfusion injury. Stem Cell Res (Amst).
10:405–416. 2013. View Article : Google Scholar
|
|
41
|
Villanueva S, Ewertz E, Carrión F, Tapia
A, Vergara C, Céspedes C, Sáez PJ, Luz P, Irarrázabal C, Carreño
JE, et al: Mesenchymal stem cell injection ameliorates chronic
renal failure in a rat model. Clin Sci (Lond). 121:489–499. 2011.
View Article : Google Scholar
|
|
42
|
Ezquer F, Giraud-Billoud M, Carpio D,
Cabezas F, Conget P and Ezquer M: Proregenerative microenvironment
triggered by donor mesenchymal stem cells preserves renal function
and structure in mice with severe diabetes mellitus. Biomed Res
Int. 2015:1647032015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bi B, Schmitt R, Israilova M, Nishio H and
Cantley LG: Stromal cells protect against acute tubular injury via
an endocrine effect. J Am Soc Nephrol. 18:2486–2496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang A, Wang Y, Ye Z, Xie H, Zhou L and
Zheng S: Mechanism of TNF-α-induced migration and hepatocyte growth
factor production in human mesenchymal stem cells. J Cell Biochem.
111:469–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim YH, Ryu JM, Lee YJ and Han HJ:
Fibronectin synthesis by high glucose level mediated proliferation
of mouse embryonic stem cells: Involvement of ANG II and TGF-β1. J
Cell Physiol. 223:397–407. 2010.PubMed/NCBI
|
|
46
|
Wise AF and Ricardo SD: Mesenchymal stem
cells in kidney inflammation and repair. Nephrology (Carlton).
17:1–10. 2012. View Article : Google Scholar
|
|
47
|
Rasmusson I: Immune modulation by
mesenchymal stem cells. Exp Cell Res. 312:2169–2179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meisel R, Zibert A, Laryea M, Göbel U,
Däubener W and Dilloo D: Human bone marrow stromal cells inhibit
allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated
tryptophan degradation. Blood. 103:4619–4621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tipnis S, Viswanathan C and Majumdar AS:
Immunosuppressive properties of human umbilical cord-derived
mesenchymal stem cells: Role of B7-H1 and IDO. Immunol Cell Biol.
88:795–806. 2010. View Article : Google Scholar : PubMed/NCBI
|